Abstract
Objective
To characterise studies which have used Demographic and Health Survey (DHS) datasets to evaluate vaccination status.
Design
Scoping review.
Data sources
Electronic databases including PubMed, EBSCOhost and POPLINE, from 2005 to 2018.
Study selection
All English studies with vaccination status as the outcome and the use of DHS data.
Data extraction
Studies were selected using a predetermined list of eligibility criteria and data were extracted independently by two authors. Data related to the study population, the outcome of interest (vaccination) and commonly seen predictors were extracted.
Results
A total of 125 articles were identified for inclusion in the review. The number of countries covered by individual studies varied widely (1–86), with the most published papers using data from India, Nigeria, Pakistan and Ethiopia. Many different definitions of full vaccination were used although the majority used a traditional schedule recommended in the WHO’s Expanded Programme on Immunisation. We found studies analysed a wide variety of predictors, but the most common were maternal education, wealth, urbanicity and child’s sex. Most commonly reported predictors had consistent relationships with the vaccination outcome, outside of sibling composition.
Conclusions
Researchers make frequent use of the DHS dataset to describe vaccination patterns within one or more countries. A clearer idea of past use of DHS can inform the development of more rigorous studies in the future. Researchers should carefully consider whether a variable needs to be included in the multivariable model, or if there are mediating relationships across predictor variables.
Keywords: paediatric infectious disease & immunisation, public health, international health services
Strengths and limitations of this study.
The Demographic and Health Surveys (DHSs) are some of the most used sources of national-level vaccination data.
Most DHS studies find consistent relationships between sociodemographic variables and vaccination outcomes.
There are large variations in how often a country’s DHS dataset is used.
A limitation is the use only of English language material.
Studies using other national-level vaccination surveys were not included.
Introduction
Vaccinations have been a cost-effective method to control and achieve elimination and eradication of common and sometimes deadly infectious diseases.1 The introduction of routine vaccinations in the USA, for example, has led to a >90% decline in cases of diphtheria, measles, mumps, pertussis, polio, rubella, smallpox and tetanus since the prevaccine era.2 Nevertheless, every year, more than 2.7 million individuals die from acute illnesses caused by common vaccine-preventable diseases.3 The overwhelming majority of vaccine-preventable deaths among children <5 years occur in low-income and middle-income countries.4
Based on the prevalence and severity of disease and on the availability of a safe and effective vaccine, WHO recommends that countries include nine vaccines on their publicly funded vaccine schedule for young children.5 Referred to as the Expanded Programme on Immunisation (EPI), the schedule initially recommended vaccination with BCG, diphtheria–tetanus–pertussis vaccine (DTP), polio vaccine and a measles-containing vaccine (MCV). Since 2004, five additional paediatric vaccines have been added to the WHO EPI: hepatitis B vaccine (HepB), Haemophilus influenzae type b vaccine (Hib), rubella vaccine, pneumococcal conjugate vaccine (PCV) and rotavirus vaccine. Individual countries decide which vaccines to publicly fund and also to make available on the private market resulting in wide variation globally in the adoption of these vaccines. For example, in 2015, 194 countries included three doses of DTP and polio in their immunisation schedule whereas only 84 included rotavirus.6 Many countries now use a pentavalent vaccine, which includes DTP, HepB and Hib vaccines in one vial. Substantial efforts on the part of Gavi The Vaccine Alliance and other international agencies are devoted to logistically and financially supporting the introduction of new and underused vaccines.7 These efforts are particularly important because a discouragingly high number of children consistently do not receive some or all of the vaccines that were first recommended by the WHO. According to WHO, 19.4 million children have not received three doses of DTP, with a majority (11.7 million) living in just 10 countries: Nigeria, India, Pakistan, Indonesia, Ethiopia, Philippines, the Democratic Republic of the Congo, Brazil, Angola and Vietnam.8 With the exception of Brazil, all of these countries have vaccination coverage regularly assessed as part of the Demographic and Health Survey (DHS) programme.
Nationally representative surveys, like those of the DHS programme, have been essential to evaluating country-specific and region-specific vaccination programmes over time. DHS programmes are funded and facilitated by the US Agency for International Development (USAID). The DHS programme was launched in 1984 with a goal of advancing global understanding of health and population trends in low-income and middle-income countries (LMICs). Since its inception it has provided technical assistance for over 300 surveys in 93 developing countries across the globe. Today, the programme is known for collecting and disseminating accurate, nationally representative data on a variety of topics including fertility, family planning, maternal and child health, gender, HIV/AIDS, malaria and nutrition. Host countries have ownership of data collection, analysis, presentation and use and the data are designed to ultimately be used in policy formation, programme planning and monitoring and evaluation.9
A large number of prior studies have amalgamated data from several different DHS datasets, or have included data from many countries, but none has systematically evaluated how these past studies have actually used the vaccination data provided by DHS.10–12 Given that DHS has had widespread use over several decades in evaluating vaccination programmes through identification of undervaccinated groups, and characterising systematic barriers to vaccination, a clearer idea of past use of DHS can inform the development of more rigorous studies in the future. The purpose of this scoping review was to characterise studies which have used DHS datasets to evaluate childhood vaccination status. Specifically, we report on the global distribution of studies, list the predictors used in multivariable regression models, and examine the different definitions of ‘full vaccination’ across studies and how these relate to the WHO EPI recommendations.
Methods
This scoping review was completed by following the steps outlined by the Preferred Reporting Items of Systematic Reviews and Meta-Analyses Extension for Scoping Reviews.13
Search strategies
Searches were performed in three different electronic databases: PubMed/MEDLINE, PopLine and EBSCOhost’s Africa-Wide Information, Global Health, Global Health Archives and Health Policy Reference Center databases. The search terms used were: “Vaccine” (and its variations such as vaccination and vaccinate), “Immunization” (and its variations such as immunize), “demographic and health surveys”, “demographic and health survey”, “DHS”, “National Family Health Survey”, and “NFHS”. Within PubMed the exact search was the following:
(“demographic and health surveys” OR “demographic and health survey” OR “DHS” OR “National Family Health Survey” OR “NFHS”) AND (immuniz* OR Vaccin*) AND (“2000/01/01”[PDAT]: “3000/12/31”[PDAT]).
In addition, the searches were limited to only return papers published between 1 January 2005 and 31 December 2018. References from articles found to be relevant were searched in order to identify additional articles.
Eligibility criteria
The titles of all papers returned through use of the search terms were initially screened for relevance. The abstracts of all remaining papers were then accessed with specific inclusion and exclusion criteria in mind. Abstracts and manuscripts were included if they met all inclusion criteria: (1) studies were conducted using DHS data from LMICs; (2) studies looked at routine vaccination coverage as the primary outcome; (3) studies were cross-sectional in design; (4) studies used either the DHS or the National Family Health Survey (NFHS), a similar study conducted only in India; (5) studies looked specifically at the vaccination outcome of children (usually aged between 0 and 60 months). A set of exclusion criteria was also created: (1) studies published before 2005 or after 2018 (though studies with an online publication in 2018 but print publication in 2019 were included); (2) studies that looked only at the vaccination outcome of adults; (3) studies that looked at population in high income countries; (4) studies that used modelling or projections instead of just analysing the data provided or (5) systematic reviews.
Study selection
LS removed all duplicates and assessed all titles for relevance. Then three reviewers (LS/BFC/AW) independently assessed all abstracts and full-text publications for eligibility using the eligibility criteria laid out. All disagreements were resolved by discussion between reviewers.
Data extraction
In addition to assessment for relevance, data were also extracted independently by three reviewers (LS/BFC/AW). A data extraction form was designed using Google Sheets and was piloted before beginning data extraction. Data from three main categories were gathered during data extraction. The first area was the study population, including the countries of interest, the subpopulation of children being examined, years of the survey administration and whether any surveys besides DHS or NFHS were used. The second category was the outcome of interests: which individual vaccines were assessed, whether full or under vaccination was examined, and if full or under vaccination was examined how were they defined. Lastly, data on vaccination predictors were gathered. We tabulated whether a given study included the most common predictors found in a previous systematic review of vaccination timeliness14: maternal education, wealth index, urbanicity, sex of child, age of mother, birth order, birth delivery location, number of antenatal care (ANC) visits, media exposure and paternal education.
Study methodological quality evaluation
We modified the Downs and Black checklist15 for assessing biases in systematic reviews because all eligible studies used a similar data source. The checklist included the following criteria:
Introduction/study population
Is the hypothesis/aim/objective of the study clearly described? (1=yes, 0=no).
Are the main outcomes (including defining full vaccination, if applicable) to be measured clearly described in the introduction or methods? (1=yes, 0=no).
Are the characteristics of study population eligibility criteria (including age range) clearly described? (1=yes, 0=no).
Descriptive statistics
Does the paper use weighting and clustering? (1=yes, 0=no).
Does the paper provide estimates of random variability (eg, 95% CI of weighted estimates or SE) for the main outcomes? (1=yes, 0=no).
Analytical statistics
Does the paper use do a multivariable analysis? (1=yes, 0=no).
Does the paper show distribution of confounders/covariates? (1=yes, 0=no).
Does the paper describe how the researchers arrived at the final list of confounders? (2=a priori knowledge or used directed acyclic graph (DAG), 1=used p values from crude analysis or used stepwise technique, 0=did not describe or did not use multivariable analysis).
Does the paper write out p values under 0.05? (1=yes, or provided 95% CIs, 0=no).
The quality score could range from 0 to 10, and we describe the average values with a mean and median quality score among all studies.
Synthesis of study findings
Given the heterogeneity of outcomes, predictors and study populations of the included studies it was not possible to combine the results into a meta-analysis. Instead, we present a narrative summary of the data. We describe the distribution of studies by population, what predictor variables are used (and what direction of association they have with outcome), and how full vaccination is defined. In the discussion, we provide recommendations for future analyses of DHS data.
A choropleth map was created using freely available shapefiles from Natural Earth16 in QGIS V.3.6 (QGIS Development Team). The map shows how many studies using data from only one country were published by country. We also show if a country’s data was part of a multicountry study, and we identify countries which had a standard DHS dataset administered between 2003 and 2016 but which did not have a published study. The years 2003–2016 were chosen as a lag time of 2 years compared with the scoping review inclusion criteria to account for delays in publishing the data and writing up a manuscript.
Patient and public involvement
This research was done without public involvement. Members of the public were not invited to comment on the study design and were not consulted, nor were they invited to contribute to this document to improve accessibility.
Results
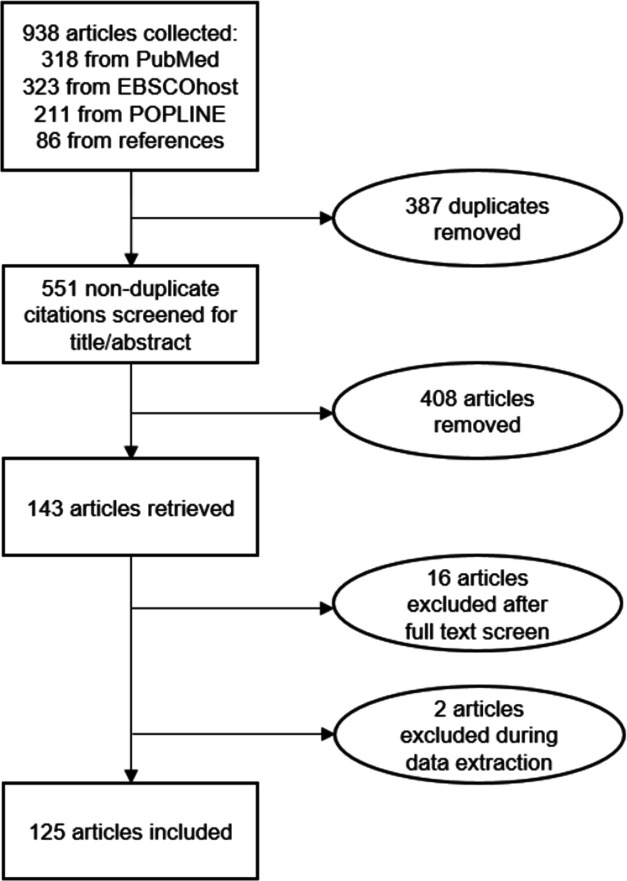
Our search terms initially yielded 938 papers; 318 from PubMed, 323 from EBSCOhost and 211 from POPLINE. An additional 86 papers were identified through searching the references of selected papers. After removing duplicates, 551 papers remained. These papers’ abstracts were screened using the inclusion and exclusion criteria to narrow down the study pool to 143 papers. However, during full-text screen and data extraction another 18 studies were removed, which left 125 (figure 1).
Figure 1.
Diagram of studies’ selection into a scoping review of vaccination studies using the Demographic and Health Surveys.
The quality sum score (possible range from 0 to 10) was on average 6.48 with a median of 7. The most commonly missed items contributing to a lower quality sum score were absence of exact p values or CIs (64% did not), not including estimates of random variability for the outcome (52%), and failure to account for appropriate use of clustering and weights (44%).
DHS has operated in a total of 92 countries since its inception, and between 2003 and 2016, has conducted surveys in 71 different countries.
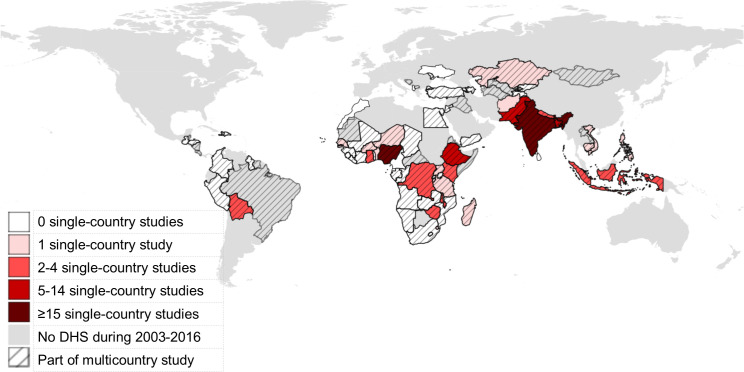
Overall, 23 (18%) studies used DHS datasets from multiple countries, ranging from 217–19 to 86 countries.11 Seven studies used data from multiple African countries,20–26 4 from just Asian countries,17 18 27 28 1 from the Americas19 and the remainder (11) used data from multiple continents.10–12 29–36 For one study, we were unable to determine what exact countries were included in the analysis.36
Figure 2 is a choropleth map showing which countries’ DHS dataset have been used for vaccination studies. The most frequently represented country is India (26 studies, 21%), followed by Nigeria (17, 14%), Ethiopia and Pakistan (seven each, 6%), and Bangladesh (6, 5%). Notably, there are many countries (44) in the Americas, Europe and Africa, which had one or more DHS conducted between 2003 and 2016 yet for which there are no corresponding single-country papers published using DHS data in this scoping review. However, most of these countries were a part of multicountry studies. Only five countries’ DHS datasets were not part of any (single country or multicountry) DHS study: Cabo Verde, Maldives, Morocco, Sri Lanka and Ukraine.
Figure 2.
Map of countries by the number of published studies using Demographic and Health Survey (DHS) datasets. Shading corresponds to number of studies using DHS data from only one country; hash marks indicate a study using multiple countries.
Characteristics of the papers are shown in table 1. About half (51%) of studies included children 12–23 or 24 months of age, and the two next most common age ranges were 12–59 or 60 months of age (11%) and 0–59 months of age (8%).
Table 1.
List of papers included in a scoping review of studies assessing vaccination status using the Demographic and Health Survey (DHS)
| Author | Year | Countries | Age of child | Vaccination outcome | Quality score |
| Bowie et al55 | 2006 | Malawi | 12–23 months | BCG, OPV, DTP, MCV, Full (BCG +3 OPV+3 DTP+MCV) | 4 |
| Choi and Lee56 | 2006 | India | 12–48 months | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Gaudin andYazbeck57 | 2006 | India | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 3 |
| Akmatov et al58 | 2007 | Kazakhstan | 12–60 months | Full (BCG +4 OPV+3 DTP+MCV) | 8 |
| Anand and Bärnighausen29 | 2007 | Multicountry | Not specified | OPV, DTP, MCV | 3 |
| Bhandari et al59 | 2007 | Nepal | 12–23 months | BCG, OPV, DTP, MCV, Full (BCG +3 OPV+3 DTP+MCV) | 5 |
| Datar et al60 | 2007 | India | 2–35 months | OPV, Full (BCG +3 OPV+3 DTP+MCV) | 5 |
| Minh Thang et al61 | 2007 | Vietnam | 11–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 5 |
| Munthali62 | 2007 | Malawi | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 3 |
| Ntenda et al63 | 2007 | Malawi | 12–23 months | BCG, OPV, DTP, MCV, Full (BCG +3 DTP+3 OPV+1 MCV) | 6 |
| Chidiebere et al64 | 2008 | Nigeria | 0–23 months | Full (BCG +4 OPV+3Penta+1 MCV+YF) | 7 |
| Gatchell et al65 | 2008 | India | 1–3 years | Full (BCG +3 OPV+3 DTP+MCV) | 4 |
| Halder and Kabir66 | 2008 | Bangladesh | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Meheus and Van Doorslaer30 | 2008 | Multicountry | 12–23 months | MCV | 4 |
| Patra67 | 2008 | India | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Antai68 | 2009 | Nigeria | Older than 12 months | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Antai69 | 2009 | Nigeria | Older than 12 months | Full (BCG +3 OPV+3 DTP+MCV) | 8 |
| Bondy et al70 | 2009 | Philippines | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 5 |
| Corsi et al71 | 2009 | India | Under 5 years | BCG, OPV, DTP, MCV, Full (age dependent after 9 months) | 3 |
| Osaki et al72 | 2009 | Indonesia | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 3 |
| Sia et al73 | 2009 | Burkina Faso | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV + YF) | 6 |
| Antai74 | 2010 | Nigeria | 12 months and older | BCG, OPV, DTP, MCV, Full (BCG +3 OPV+3 DTP+MCV) | 8 |
| Hong and Chhea75 | 2010 | Cambodia | 12–59 months | DTP | 8 |
| Rahman and Obaida-Nasrin76 | 2010 | Bangladesh | 12–59 months | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Sahu et al77 | 2010 | India | Preceding two births in last 3 years | Full (BCG +3 OPV+3 DTP+MCV) | 5 |
| Semali78 | 2010 | Tanzania | 12–23 months | Full (BCG +4 OPV+3 DTP+MCV) | 6 |
| Abuya79 | 2011 | Kenya | 12–35 months | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Antai80 | 2011 | Nigeria | 12 months and older | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Fernandez et al81 | 2011 | Indonesia | 0–59 months | BCG, OPV, DTP, MCV, HepB | 9 |
| Fernandez et al82 | 2011 | Indonesia | 0–59 months | MCV | 8 |
| Kumar and Mohanty83 | 2011 | India | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 5 |
| Lauridsen et al84 | 2011 | India | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 5 |
| Pandey and Lee85 | 2011 | Nepal | 12–23 months | BCG, OPV, DTP, MCV, Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Singh86 | 2011 | India | 12–48 months | Full (BCG +3 OPV+3 DTP+MCV) | 8 |
| Afzal and Zainab87 | 2012 | Bangladesh | Under 5 years | Full (BCG +3 OPV+3 DTP+MCV) | 5 |
| Antai88 | 2012 | Nigeria | 12–59 months | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Rammohan et al31 | 2012 | Multicountry | Not specified | MCV | 5 |
| Sabarwal et al89 | 2012 | India | 12–24 months | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Singh90 | 2012 | India | 12–59 months | Full (BCG +3 OPV+3 DTP+MCV) | 5 |
| Wiysonge20 | 2012 | Multicountry | 12–23 months | Full (DTP3) | 6 |
| Barman and Dutta91 | 2013 | India | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Bbaale92 | 2013 | Uganda | 0–36 months (12–36 for full) | BCG, OPV, DTP, MCV, Full (BCG +3 OPV+3 DTP+MCV) | 8 |
| Haque and Bari93 | 2013 | Bangladesh | 9–59 months | MCV | 8 |
| Kumar and Ram94 | 2013 | India | 0–59 months | Full (BCG +3 OPV+3 DTP+MCV) | 5 |
| Moyer et al95 | 2013 | Ethiopia | 12–24 months | BCG, OPV, DTP, MCV, Full (BCG +3Penta+4 OPV+1 MCV) | 6 |
| Singh et al96 | 2013 | India | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 8 |
| Singh et al97 | 2013 | Nigeria | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Singh98 | 2013 | India | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Van Malderen et al99 | 2013 | Kenya | 12–23 months | MCV | 6 |
| Adegboye et al100 | 2014 | Nigeria | 12–59 months | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Bonfrer et al101 | 2014 | Burundi | Older than 1 year | BCG, OPV, DTP, MCV | 7 |
| Bugvi et al102 | 2014 | Pakistan | 12–23 months | Full (BCG +3 DTP+4 OPV+3 HepB+1 MCV) | 9 |
| Canavan et al21 | 2014 | Multicountry | 12–23 months | Full (BCG +4 OPV+1 MCV+3Penta) | 9 |
| Clouston et al103 | 2014 | Madagascar | 0–59 months | BCG, OPV, DTP, MCV, Hib | 7 |
| Ebot104 | 2014 | Ethiopia | 12–30 months | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Grundy et al27 | 2014 | Multicountry | Not specified | DTP | 3 |
| Heaton et al105 | 2014 | Bolivia | Not specified | Full (BCG +3 OPV+3 DTP+MCV) | 4 |
| Helleringer et al32 | 2014 | Multicountry | 12–23 months | OPV, SIA participation | 4 |
| Javed et al106 | 2014 | Pakistan | 12–28 months | BCG, OPV, DTP, MCV, Full (BCG +3 OPV+3 DTP+MCV) | 8 |
| Luqman107 | 2014 | Nigeria | 12–23 months | BCG, OPV, DTP, MCV, Full (BCG +4 OPV+3 DTP+MCV) | 6 |
| Malhotra et al108 | 2014 | India | Older than 12 months | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Neupane and Nwaru109 | 2014 | Nepal | Not specified | Full (BCG +1 DTP+1 OPV) | 8 |
| Prusty and Kumar110 | 2014 | India | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Rai et al111 | 2014 | Niger | 12–59 months | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Singh and Parsuraman17 | 2014 | Multicountry | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 5 |
| Singh et al112 | 2014 | India | 12–36 months | Full (BCG +3 OPV+3 DTP+MCV) | 8 |
| Ushie et al113 | 2014 | Nigeria | Under 5 years | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Wagner et al22 | 2014 | Multicountry | 0–59 months | BCG | 8 |
| Zaidi et al49 | 2014 | Pakistan | 0–5 years | OPV, DTP, MCV | 9 |
| Abadura et al114 | 2015 | Ethiopia | 12–59 months | Full (BCG +3 OPV+3 DTP+MCV) | 8 |
| Ebot115 | 2015 | Ethiopia | 12–30 months | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Hajizadeh et al33 | 2015 | Multicountry | Under 59 months | BCG, OPV, DTP | 8 |
| Lakew et al116 | 2015 | Ethiopia | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 8 |
| McGlynn et al117 | 2015 | Ghana | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 9 |
| Mukungwa118 | 2015 | Zimbabwe | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Onsomu et al119 | 2015 | Kenya | 12–23 months | BCG, OPV, DTP, MCV | 8 |
| Osetinsky et al120 | 2015 | Bolivia | 24 months - 5 years | Full (BCG +3 Polio +3 DTP+1 MMR+YF) | 6 |
| Prusty and Keshri121 | 2015 | India | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Rossi122 | 2015 | Zimbabwe | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 9 |
| Schweitzer et al18 | 2015 | Multicountry | 12–59 months | DTP, MCV | 6 |
| Shrivastwa et al123 | 2015 | India | 12–36 months | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Singh et al23 | 2015 | Multicountry | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 7 |
| Smith-Greenaway and Madhavan124 | 2015 | Benin | 1–59 months | Ever received any vaccine | 6 |
| Tsawe et al125 | 2015 | eSwatini | Not specified | Ever received any vaccine | 9 |
| Arsenault et al34 | 2016 | Multicountry | 12–23 months | DTP, MCV | 5 |
| Chima and Franzini126 | 2016 | Nigeria | 12–59 months | BCG, OPV, DTP, MCV | 6 |
| Gurmu and Etana127 | 2016 | Ethiopia | 12–23 months | Full (BCG +3 OPV+3 DTP+MCV) | 6 |
| Hosseinpoor et al35 | 2016 | Multicountry | 12–23 months in most | DTP | 5 |
| Kriss et al128 | 2016 | Zimbabwe | 12–23 months | BCG, OPV, DTP, MCV, Full (BCG +3 OPV+3Penta+1MCV | 9 |
| Kumar et al129 | 2016 | India | 12–23 months | Full (BCG +3 DTP+3 OPV+1 MCV) | 9 |
| Restrepo-Méndez et al11 | 2016 | Multicountry | 12–23 months in most | Full (BCG +3 DTP+3 OPV+1 MCV) | 6 |
| Restrepo-Méndez et al12 | 2016 | Multicountry | 12–23 months | BCG, OPV, DTP, MCV, Full (BCG +3 DTP+3 OPV+1 MCV) | 4 |
| Schweitzer et al19 | 2016 | Multicountry | Birth - 250 weeks | DTP | 5 |
| Adedokun et al130 | 2017 | Nigeria | 12–23 months | Full (BCG +3 OPV+3Penta+MCV) | 7 |
| Aghaji131 | 2017 | Nigeria | 12–23 months | MCV | 4 |
| Ambe et al132 | 2017 | Ethiopia | 12–23 months | MCV, Full (BCG +3 DTP+3 OPV+1MCV | 4 |
| Arsenault et al10 | 2017 | Multicountry | 12–23 months | DTP, MCV | 8 |
| Delprato and Akyeampong36 | 2017 | Multicountry | Not specified | Full (BCG +DTP + OPV+MCV (no. unspecified)) | 5 |
| Herliana and Douiri133 | 2017 | Indonesia | 12–59 months | Full (BCG +3 DTP+4 OPV+1 MCV+1HepB | 9 |
| Kazungu and Adetifa24 | 2017 | Multicountry | 12–23 months | Full (BCG +3 DTP+3 OPV+1 MCV) | 7 |
| Kc et al134 | 2017 | Nepal | Not specified | BCG, OPV, DTP, MCV, Full (BCG +3 DTP+3 OPV+1 MCV) | 6 |
| Khan et al135 | 2017 | Pakistan | Under 5 years | OPV | 7 |
| Mbengue et al136 | 2017 | Senegal | 12–23 months | Full (BCG +3Penta+3 OPV+1 MCV) | 8 |
| Oleribe et al137 | 2017 | Nigeria | 12–24 months | BCG, OPV, DTP, MCV, Full (BCG +3 DTP+3 OPV dose +1 MCV) | 5 |
| Singh and Patel138 | 2017 | India | 12–13 months | Full (Not defined) | 6 |
| Uthman et al139 | 2017 | Nigeria | 12–23 months | OPV | 9 |
| Zuhai and Roy140 | 2017 | India | Not specified | BCG, OPV, DTP, MCV | 7 |
| Acharya et al141 | 2018 | DRC | 12–23 months | Full (BCG +3 DTP+3 OPV+1 MCV) | 9 |
| Adetokunboh et al25 | 2018 | Multicountry | 12–23 months | DTP | 6 |
| Adetokunboh et al26 | 2018 | Multicountry | 12–23 months | DTP | 4 |
| Ashbaugh et al142 | 2018 | DRC | 6–59 months | MCV | 9 |
| Asuman et al143 | 2018 | Ghana | 12–59 months | Full (BCG +3 DTP+3 OPV+1 MCV) | 8 |
| Boulton et al144 | 2018 | Bangladesh | 12–24 months | BCG, OPV, DTP, MCV, Full (BCG +3Penta+3 OPV+1 MCV) | 7 |
| Burroway and Hargrove145 | 2018 | Nigeria | 12–24 months | Full (BCG +3 DTP+4 OPV+1 MCV) | 7 |
| Imran et al146 | 2018 | Pakistan | 12–23 months | OPV | 7 |
| Khan et al147 | 2018 | India | 12–23 months | BCG, OPV, DTP, MCV, Full (BCG +3 DTP+3 OPV+1 MCV) | 9 |
| Kols et al148 | 2018 | Pakistan | 12–23 months | BCG, OPV, DTP, MCV, Full (BCG +3 DTP+3 OPV+1 MCV) | 9 |
| McGavin et al149 | 2018 | Nigeria | 12–24 months | Full (BCG +3 DTP+4 OPV+1 MCV) | 9 |
| Raza et al150 | 2018 | Pakistan | 12–23 months | Full (BCG +3 DTP+3 OPV+3 HepB+3 Hib+1 MCV) | 5 |
| Shenton et al151 | 2018 | Afghanistan | 12–60 months | Full (BCG +3Penta+3 OPV+1 MCV) | 10 |
| Shenton et al152 | 2018 | India | 12–48 months | Full (BCG +3 OPV+3 DTP+MCV) | 8 |
| Sohn et al28 | 2018 | Multicountry | Not specified | BCG, OPV, DTP, MCV | 7 |
| Lungu et al153 | 2019 | Malawi | Not specified | Full (not specified) | 1 |
| Masters et al154 | 2019 | Kenya | 12–23 months | BCG, OPV, DTP, MCV, Full (BCG +3Penta+3 OPV+1 MCV) | 10 |
| Vyas et al155 | 2019 | Bangladesh | Not specified | BCG, DTP, MCV | 3 |
DRC, democratic republic of the Congo; DTP, diphtheria –tetanus-pertussis vaccine; HepB, hepatitis B vaccine; Hib, Haemophilus influenzae type b vaccine; MCV, measles-containing vaccine; MMR, measles-mumps-rubella vaccine; OPV, oral polio vaccine; Penta, pentavalent vaccine; SIA, supplementary immunisation activity; YF, yellow fever.
Full vaccination was assessed in three-fourths (94, 75%) of papers; otherwise, the four most common vaccines assessed one at a time were MCV (39, 31%), DTP (36, 29%), polio (33, 26%) and BCG (27, 22%). There were at least 12 different definitions of full vaccination used in the papers including in this scoping review. Of the 94 papers which evaluated full vaccination coverage, most (66, 70%) used a traditional schedule based off of the four vaccines first recommended for the WHO’s EPI in 1974: one dose BCG, three doses polio, three doses DTP and one dose MCV. Five (5%) papers modified this traditional definition to include a birth dose of polio, and 11 others used a pentavalent vaccine instead of DTP (of these, three had a four-dose polio schedule, and eight had a three-dose polio schedule). Other papers modified the traditional definition in order to include yellow fever (in a total of 4 four papers), measles–mumps–rubella vaccine (in one paper), or to exclude certain vaccine series, like measles, polio or BCG. Some measure of DTP was included in all definitions of full vaccination. No papers included information about PCV or rotavirus vaccine as an outcome in a multivariable regression model, although one used rotavirus vaccine as a predictor variable.19
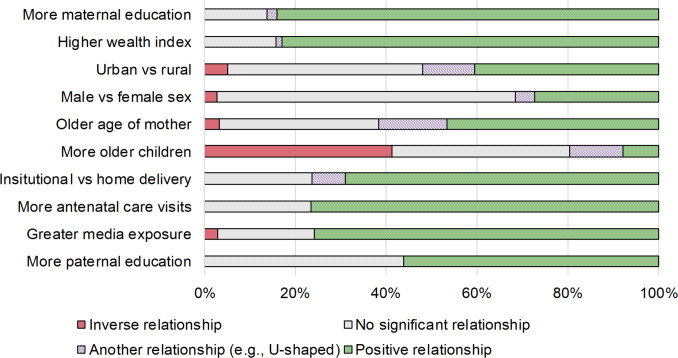
Four variables were used in a majority of studies. The top 10 variables used in a study (with their relationship shown in a model) are maternal education (in 94, or 75% of studies), wealth index (88, 70%), urbanicity (79, 63%), child’s sex (73, 58%), mother’s age (60, 48%), birth order (51, 41%), delivery location (42, 34%), ANC visits (34, 27%), media exposure (33, 26%) and paternal education (32, 26%).
The relationship between the most commonly used predictor and vaccination outcomes is shown in figure 3. For most predictors, there is a relatively clear relationship to vaccination outcome. For a majority of studies, greater vaccination coverage (across any vaccination outcome considered) was related to maternal education (in 84% of studies that considered the variable), higher wealth index (83%), more ANC visits (76%), greater media exposure (76%), an institutional birth (69%) and more paternal education (56%). For several predictors, a large proportion of studies found no significant relationship. This was especially true for child’s sex (66% of studies), more paternal education (44%) and urbanicity (43%). Sibling composition was one variable for which there was no clear relationship with the outcome: in 41% of studies, having more older siblings was associated with lower vaccination coverage, in 8% it was associated with higher vaccination coverage, and for the rest of studies, there was no significant relationship (35%) or there was a significant, non-monotonic relationship (12%).
Figure 3.
Commonly reported predictors of vaccination status used in studies using the Demographic and Health Survey.
Discussion
Vaccination programmes enjoy wide support from many international health organisations and national governments. Vaccination has achieved the sole instance of human disease eradication—smallpox, while polio, measles and rubella have been eliminated in some regions of the world.1 37 Global vaccination coverage has increased in recent years but 12.8 million children in 2015 still had not yet received DTP dose 1,6 a common marker of routine immunisation initiation. Regularly conducted studies on vaccination uptake are necessary to assessing population-level susceptibility and immunisation programme reach while also ensuring that countries are on track with international guidelines for maintaining high vaccination coverage and the control or elimination of certain vaccine-preventable diseases. The DHS datasets tend to be very large, both in number of variables looked at and number of participants surveyed. This allows the examination of many possible associations with sufficient statistical power and the ability to control for a number of possible confounders.
DHS is not conducted in all LMICs, only in certain countries with a USAID presence, and it is conducted at irregular intervals. However, it is one of the most widely available surveys for assessing vaccinations globally. This systematic review found wide variation in how full vaccination was defined across 125 studies using DHS data between 2005 and 2018. However, the majority of studies did look at full vaccination and defined it according to the WHO’s EPI schedule; one dose BCG, three doses polio, three doses DTP and one dose MCV. Additionally, studies looked at similar subpopulations (children <5) and very similar predictors, with the most common being maternal education, wealth, urbanicity and child’s sex.
The vaccines commonly evaluated reflect priorities of international efforts. For example, polio was targeted for elimination by 2018.38 Measles is also subject to an international elimination effort,39 40 and all six WHO regional offices have established target dates for elimination.41 BCG was one of the first vaccines ideally administered shortly after birth (joined more recently in certain locations with HepB and polio birth doses). And DTP dose three has long been used as a proxy for adherence to repeat visits to immunisation appointments.42 43 As more vaccines are added to the vaccine schedule, not only does it become more complicated, but it likely introduces the potential for greater diversity among countries in their respective EPI schedules. Over the past few decades, DHS has operated in 92 countries. However, a significant number of papers came from a relatively small number of countries. We note the most commonly used countries (India, Nigeria, Ethiopia, Pakistan and Bangladesh) are among the 12 most populous countries in the world, and, with the exception of Bangladesh, are among the five countries with the most number of unvaccinated children.8 Given that countries have control over their own vaccine policies and use a wide variety of socioeconomic variables across individual countries, more country-specific analyses of DHS vaccination data is important.
Recommendations for future analyses
This study identified the variables commonly used as explanatory variables in multivariable regression models. Many studies appeared to use the DHS datasets to test the significance and estimate the strength of association for many explanatory variables concomitantly. Since DHS is a cross-sectional study, it cannot be used to investigate the effect of an exposure which could vary across time, such as education or urbanicity. However, a strength of DHS is its ability to be used as a hypothesis generating device. Associations can subsequently be examined in other types of studies, such as cohort studies.
However, given consistent relationships between commonly used predictors and outcomes, it is worth revisiting the use of DHS datasets in multivariable analyses. First, given this consistency, it is more important than ever to consider the plausible causal relationships across all variables used in a model. An approach widely used in epidemiology is to chart the directionality of relationships among variables through DAGs.44 Online software, like dagitty.net, can be used to build these models and assess which variables should be included in the final multivariable model. A potential problem is inclusion of so many variables in one model can obscure the mediating effects of certain variables.45 For example, researchers examining the relationship between media exposure and vaccination status may include maternal age as a confounder. However, the parameter estimate for maternal age in this multivariable model includes the mediator media exposure. Theoretically, a model with age as the main predictor and with media exposure as a main predictor would have different sets of covariates. Although the potential impact of inappropriately controlling for mediation is context-specific, one study suggests parameter estimates may change up to 10%–25%.46
Evolving immunisation schedules mean that future studies will likely take local programmatic considerations into account. However, to make cross-country comparisons, studies could still provide an estimate of full vaccination using the traditional BCG, three doses polio, three doses DTP and one-dose MCV schedule.
Timeliness has also emerged as an important dimension of vaccination uptake within the past two decades.47 48 Measures of timeliness require vaccination dates,14 information missing from many individuals in the DHS datasets. For example, in the 2006–2007 Pakistan DHS EPI immunisation cards, and thus data on vaccination dates, were available for just 10% of cases.49
Finally, researchers analysing DHS data should be aware of its structure and limitations. Most DHS samples are stratified and based on clusters. Studies should use survey procedures and weights to ensure that estimates are representative of the national population and that standard errors are honest reflections of the sampling structure. Additionally, because DHS includes so many individuals with unknown vaccination age, any study should account for this substantial left censoring, through Turnbull estimation methods50 or accelerated failure time models. A substantial minority of studies examined did not specify the age range of the study population. This has implications for timeliness but should be presented in studies calculating more traditional measures of vaccine uptake that do not incorporate timing or age.
The DHS provides national estimates from politically neutral sources over time, in countries where USAID operates. Its continued existence ensures that reliable, comparable and nationally representative data sources are publicly available. Other surveys, like the District Level Household Survey and the Annual Health Survey in India and the Multiple Indicators Cluster Survey (MICS) in over 100 countries, are developed in close collaboration with DHS.51 52
Limitations
There are several limitations to this study. Because the study populations, use of explanatory variables and definitions of outcomes differed among studies, we were unable to conduct a meta-analysis to compare the association of various explanatory variables on outcomes. We did not examine the grey literature or non-English language papers as part of this review, nor did we review reports which may have listed vaccination coverage, but did not include some statistical analysis. Inclusion of these types of articles could have included data from more countries. Vaccination data from the DHS is limited in that it partially comes from information contained on vaccination cards,53 and partially from parental recall—with its obvious potential for errors. However, some countries, such as Ethiopia, have attempted to combat this problem in recent years through the introduction of a Health Facility Questionnaire. This questionnaire is used to record vaccination information for all children, who were discovered to not have a vaccination card during administration of the Woman’s Questionnaire.54 In addition, since the DHS is a standardised questionnaire there is limited opportunity to modify the survey to be locally relevant and take predictors into account that may only be relevant in parts of the country. However, overall the DHS programmes are widely available surveys providing researchers, policy-makers and the public with nationally representative data. These data provide a basis for evaluation of immunisation programmes that would either not exist or not be as robust in their absence.
Conclusions
This scoping review of papers about vaccination published using DHS data found diversity in analyses and qualities of studies. Although certain countries—like India, Nigeria, Pakistan and Ethiopia—have had ≥7 vaccination studies published using DHS data, there are dozens of countries whose vaccination data have not yet been published within single-country studies. Studies find consistent relationships between greater vaccination uptake and more maternal education, higher wealth index, more ANC visits, greater media exposure, and institutional delivery. The relationship between birth order and vaccination status is more varied across countries. Researchers using the DHS datasets should understand the limitations of using recorded vaccination dates, and should clarify the interpretation of estimates from multivariable analyses given the potential for mediation.
Supplementary Material
Acknowledgments
We are grateful to the DHS personnel who diligently carry out their work and to USAID for sponsoring the DHS projects.
Footnotes
Twitter: @abramwagner
LMS and ALW contributed equally.
Contributors: MB conceived of the study design, helped interpret the data and revised the manuscript critically for important intellectual content. LS and BFC downloaded manuscripts, assessed their fit for this systematic review, abstracted data from the manuscripts, completed qualitative synthesis and helped revise the manuscript critically for important intellectual content. MJ abstracted data from the manuscripts and helped revise the manuscript critically for important intellectual content. AW helped interpret the data, and drafted the article. All authors gave final approval of the manuscript to be published.
Funding: AW’s salary was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number K01AI137123.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Map disclaimer: The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. The data abstracted from these studies are publicly available: https://doi.org/10.6084/m9.figshare.12177135.
References
- 1.Centers for Disease Control and Prevention Ten great public health achievements, 1900– 1999: impact of vaccines universally recommended for children. Morb Mortal Wkly Rep 1999;48:243–8. [Google Scholar]
- 2.Roush SW, Murphy TV, Vaccine-Preventable Disease Table Working Group . Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 2007;298:2155–63. 10.1001/jama.298.18.2155 [DOI] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. The Lancet 2012;380:2095–128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. The Lancet 2010;375:1969–87. 10.1016/S0140-6736(10)60549-1 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization Summary of WHO position papers - recommendations for routine immunization, 2017. Available: http://www.who.int/immunization/policy/Immunization_routine_table1.pdf
- 6.Casey RM, Dumolard L, Danovaro-Holliday MC, et al. Global routine vaccination coverage, 2015. MMWR Morb Mortal Wkly Rep 2016;65:1270–3. 10.15585/mmwr.mm6545a5 [DOI] [PubMed] [Google Scholar]
- 7.GAVI Alliance Vaccine goal indicators. GAVI, 2020. Available: https://www.gavi.org/programmes-impact/our-impact/measuring-our-performance/2016-2020-indicators [Accessed 2020-11-30].
- 8.Peck M, Gacic-Dobo M, Diallo MS, et al. Global routine vaccination coverage, 2018. MMWR Morb Mortal Wkly Rep 2019;68:937–42. 10.15585/mmwr.mm6842a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The DHS Program The DHS program: demographic and health surveys, 2017. Available: https://dhsprogram.com/
- 10.Arsenault C, Johri M, Nandi A, et al. Country-level predictors of vaccination coverage and inequalities in Gavi-supported countries. Vaccine 2017;35:2479–88. 10.1016/j.vaccine.2017.03.029 [DOI] [PubMed] [Google Scholar]
- 11.Restrepo-Méndez MC, Barros AJD, Wong KLM, et al. Inequalities in full immunization coverage: trends in low- and middle-income countries. Bull World Health Organ 2016;94:794–805. 10.2471/BLT.15.162172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Restrepo-Méndez MC, Barros AJD, Wong KLM, et al. Missed opportunities in full immunization coverage: findings from low- and lower-middle-income countries. Glob Health Action 2016;9:30963–6. 10.3402/gha.v9.30963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 14.Masters NB, Wagner AL, Boulton ML. Vaccination timeliness and delay in low- and middle-income countries: a systematic review of the literature, 2007-2017. Hum Vaccin Immunother 2019;15:2790–805. 10.1080/21645515.2019.1616503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natural Earth Admin 0 - Countries, 2020. Available: https://www.naturalearthdata.com/downloads/50m-cultural-vectors/50m-admin-0-countries-2/
- 17.Singh PK, Parsuraman S. Sibling composition and child immunization in India and Pakistan, 1990–2007. World J Pediatr 2014;10:145–50. 10.1007/s12519-014-0483-z [DOI] [PubMed] [Google Scholar]
- 18.Schweitzer A, Krause G, Pessler F, et al. Improved coverage and timing of childhood vaccinations in two post-Soviet countries, Armenia and Kyrgyzstan. BMC Public Health 2015;15:798. 10.1186/s12889-015-2091-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schweitzer A, Pessler F, Akmatov MK. Impact of rotavirus vaccination on coverage and timing of pentavalent vaccination – experience from 2 Latin American countries. Hum Vaccin Immunother 2016;12:1250–6. 10.1080/21645515.2015.1127486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiysonge CS, Uthman OA, Ndumbe PM, et al. Individual and contextual factors associated with low childhood immunisation coverage in sub-Saharan Africa: a multilevel analysis. PLoS One 2012;7:e37905 10.1371/journal.pone.0037905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canavan ME, Sipsma HL, Kassie GM, et al. Correlates of complete childhood vaccination in East African countries. PLoS One 2014;9:e95709–7. 10.1371/journal.pone.0095709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner Z, Szilagyi PG, Sood N. Comparative performance of public and private sector delivery of BCG vaccination: evidence from sub-Saharan Africa. Vaccine 2014;32:4522–8. 10.1016/j.vaccine.2014.06.020 [DOI] [PubMed] [Google Scholar]
- 23.Singh K, Bloom S, Brodish P. Gender equality as a means to improve maternal and child health in Africa. Health Care Women Int 2015;36:57–69. 10.1080/07399332.2013.824971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazungu JS, Adetifa IMO. Crude childhood vaccination coverage in West Africa: trends and predictors of completeness. Wellcome Open Res 2017;2:12 10.12688/wellcomeopenres.10690.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adetokunboh OO, Uthman OA, Wiysonge CS. Non-Uptake of childhood vaccination among the children of HIV-infected mothers in sub-Saharan Africa: a multilevel analysis. Hum Vaccin Immunother 2018;14:2405–13. 10.1080/21645515.2018.1502524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adetokunboh OO, Uthman OA, Wiysonge CS. Effect of maternal HIV status on vaccination coverage among sub-Saharan African children: a socio-ecological analysis. Hum Vaccin Immunother 2018;14:2373–81. 10.1080/21645515.2018.1467204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grundy J, Annear P, Chomat AM, et al. Improving average health and persisting health inequities-towards a justice and fairness platform for health policy making in Asia. Health Policy Plan 2014;29:873–82. 10.1093/heapol/czt068 [DOI] [PubMed] [Google Scholar]
- 28.Sohn M, Lin L, Jung M. Effects of maternal decisional authority and media use on vaccination for children in Asian countries. Medicina 2018;54. 10.3390/medicina54060105. [Epub ahead of print: 07 Dec 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anand S, Bärnighausen T. Health workers and vaccination coverage in developing countries: an econometric analysis. The Lancet 2007;369:1277–85. 10.1016/S0140-6736(07)60599-6 [DOI] [PubMed] [Google Scholar]
- 30.Meheus F, Van Doorslaer E. Achieving better measles immunization in developing countries: does higher coverage imply lower inequality? Soc Sci Med 2008;66:1709–18. 10.1016/j.socscimed.2007.12.036 [DOI] [PubMed] [Google Scholar]
- 31.Rammohan A, Awofeso N, Fernandez RC. Paternal education status significantly influences infants’ measles vaccination uptake, independent of maternal education status. BMC Public Health 2012;12:336 10.1186/1471-2458-12-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helleringer S, Abdelwahab J, Vandenent M. Polio supplementary immunization activities and equity in access to vaccination: evidence from the demographic and health surveys. J Infect Dis 2014;210 Suppl 1:S531–9. 10.1093/infdis/jiu278 [DOI] [PubMed] [Google Scholar]
- 33.Hajizadeh M, Heymann J, Strumpf E, et al. Paid maternity leave and childhood vaccination uptake: longitudinal evidence from 20 low-and-middle-income countries. Soc Sci Med 2015;140:104–17. 10.1016/j.socscimed.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 34.Arsenault C, Harper S, Nandi A, et al. Monitoring equity in vaccination coverage: a systematic analysis of demographic and health surveys from 45 Gavi-supported countries. Vaccine 2017;35:951–9. 10.1016/j.vaccine.2016.12.041 [DOI] [PubMed] [Google Scholar]
- 35.Hosseinpoor AR, Bergen N, Schlotheuber A, et al. State of inequality in diphtheria-tetanus-pertussis immunisation coverage in low-income and middle-income countries: a multicountry study of household health surveys. The Lancet Global Health 2016;4:e617–26. 10.1016/S2214-109X(16)30141-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delprato M, Akyeampong K. The effect of early marriage timing on women’s and children’s health in sub-Saharan Africa and southwest Asia. Ann Glob Heal 2017;83:557–67. 10.1016/j.aogh.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention Achievements in public health: elimination of rubella and congenital rubella syndrome-US, 1969-2004. MMWR Morb Mortal Wkly Rep 2005;54:279–82. [PubMed] [Google Scholar]
- 38.Mwengee W, Okeibunor J, Poy A, et al. Polio eradication initiative: contribution to improved communicable diseases surveillance in who African region. Vaccine 2016;34:5170–4. 10.1016/j.vaccine.2016.05.060 [DOI] [PubMed] [Google Scholar]
- 39.Keegan R, Dabbagh A, Strebel PM, et al. Comparing measles with previous eradication programs: enabling and constraining factors. J Infect Dis 2011;204 Suppl 1:S54–61. 10.1093/infdis/jir119 [DOI] [PubMed] [Google Scholar]
- 40.Levin A, Burgess C, Garrison LP, et al. Global eradication of measles: an epidemiologic and economic evaluation. J Infect Dis 2011;204 Suppl 1:S98–106. 10.1093/infdis/jir096 [DOI] [PubMed] [Google Scholar]
- 41.Thapa A, Khanal S, Sharapov U, et al. Progress Toward Measles Elimination - South-East Asia Region, 2003-2013. MMWR Morb Mortal Wkly Rep 2015;64:613–7. [PMC free article] [PubMed] [Google Scholar]
- 42.Tsega A, Daniel F, Steinglass R. Monitoring coverage of fully immunized children. Vaccine 2014;32:7047–9. 10.1016/j.vaccine.2014.10.057 [DOI] [PubMed] [Google Scholar]
- 43.Hong R, Banta JE. Effects of extra immunization efforts on routine immunization at district level in Pakistan. East Mediterr Health J 2005;11:745-52. [PubMed] [Google Scholar]
- 44.Krieger N, Davey Smith G, Smith GD. The tale wagged by the DAG: broadening the scope of causal inference and explanation for epidemiology. Int J Epidemiol 2016;45:dyw114–808. 10.1093/ije/dyw114 [DOI] [PubMed] [Google Scholar]
- 45.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol 2013;177:292–8. 10.1093/aje/kws412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bandoli G, Palmsten K, Chambers CD, et al. Revisiting the table 2 fallacy: a motivating example examining preeclampsia and preterm birth. Paediatr Perinat Epidemiol 2018;32:390–7. 10.1111/ppe.12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bolton P, Hussain A, Hadpawat A, et al. Deficiencies in current childhood immunization indicators. Public Health Rep 1998;113:527–32. [PMC free article] [PubMed] [Google Scholar]
- 48.Laubereau B, Hermann M, Schmitt HJ, et al. Detection of delayed vaccinations: a new approach to visualize vaccine uptake. Epidemiol Infect 2002;128:185–92. 10.1017/S0950268801006550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaidi SMA, Khowaja S, Dharma VK, et al. Coverage, timeliness, and determinants of immunization completion in Pakistan: evidence from the demographic and health survey (2006-07). Hum Vaccines Immunother 2014;10:1712–20. 10.4161/hv.28621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shrivastwa N, Gillespie BW, Lepkowski JM, et al. Vaccination timeliness in children under India's universal immunization program. Pediatr Infect Dis J 2016;35:955–60. 10.1097/INF.0000000000001223 [DOI] [PubMed] [Google Scholar]
- 51.Dandona R, Pandey A, Dandona L. A review of national health surveys in India. Bull World Health Organ 2016;94:286–96. 10.2471/BLT.15.158493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.UNICEF Multiple indicator cluster surveys, 2017. Available: http://mics.unicef.org/
- 53.Wagner AL. The use and significance of vaccination cards. Hum Vaccin Immunother 2019;15:2844–6. 10.1080/21645515.2019.1625647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Central Statistical Agency, The DHS Program . Ethiopia demographic and health survey, 2016, 2016. Available: https://dhsprogram.com/pubs/pdf/FR328/FR328.pdf [Accessed 2017-10-03].
- 55.Bowie C, Mathanga D, Misiri H. Poverty, access and immunization in Malawi - a descriptive study. Malawi Med J 2006;18:19–27. 10.4314/mmj.v18i1.10902 [DOI] [Google Scholar]
- 56.Choi JY, Lee S-H. Does prenatal care increase access to child immunization? gender bias among children in India. Soc Sci Med 2006;63:107–17. 10.1016/j.socscimed.2005.11.063 [DOI] [PubMed] [Google Scholar]
- 57.Gaudin S, Yazbeck AS. Immunization in India 1993–1999: wealth, gender, and regional inequalities revisited. Soc Sci Med 2006;62:694–706. 10.1016/j.socscimed.2005.06.042 [DOI] [PubMed] [Google Scholar]
- 58.Akmatov MK, Kretzschmar M, Krämer A, et al. Determinants of childhood vaccination coverage in Kazakhstan in a period of societal change: implications for vaccination policies. Vaccine 2007;25:1756–63. 10.1016/j.vaccine.2006.11.030 [DOI] [PubMed] [Google Scholar]
- 59.Bhandari P, Shrestha SS, Ghimire DJ. Sociocultural and geographical disparities in child immunization in Nepal. Asia-Pacific Popul J 2007;22:43–64. 10.18356/1e441780-en [DOI] [Google Scholar]
- 60.Datar A, Mukherji A, Sood N. Health infrastructure & immunization coverage in rural India. Indian J Med Res 2007;125:31–42. [PubMed] [Google Scholar]
- 61.Minh Thang N, Bhushan I, Bloom E, et al. Child immunization in Vietnam: situation and barriers to coverage. J Biosoc Sci 2007;39:41–58. 10.1017/S0021932006001234 [DOI] [PubMed] [Google Scholar]
- 62.Munthali AC. Determinants of vaccination coverage in Malawi: evidence from the demographic and health surveys. Malawi Med J 2007;19:79–82. 10.4314/mmj.v19i2.10934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ntenda PAM, Chuang K-Y, Tiruneh FN, et al. Analysis of the effects of individual and community level factors on childhood immunization in Malawi. Vaccine 2017;35:1907–17. 10.1016/j.vaccine.2017.02.036 [DOI] [PubMed] [Google Scholar]
- 64.Chidiebere ODI, Uchenna E, Kenechi OS. Maternal sociodemographic factors that influence full child immunisation uptake in Nigeria. S Afr J CH 2014;8:138–42. 10.7196/sajch.661 [DOI] [Google Scholar]
- 65.Gatchell M, Thind A, Hagigi F. Informing state-level health policy in India: the case of childhood immunizations in Maharashtra and Bihar. Acta Paediatr 2008;97:124–6. 10.1111/j.1651-2227.2007.00569.x [DOI] [PubMed] [Google Scholar]
- 66.Halder AK, Kabir M. Inequalities in infant immunization coverage in Bangladesh. Health Serv Insights 2008;1:HSI.S927–11. 10.4137/HSI.S927 [DOI] [Google Scholar]
- 67.Patra N. Exploring the determinants of childhood immunisation. Econ Polit Wkly 2008;43:97–104. [Google Scholar]
- 68.Antai D. Inequitable childhood immunization uptake in Nigeria: a multilevel analysis of individual and contextual determinants. BMC Infect Dis 2009;9:181 10.1186/1471-2334-9-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antai D. Faith and child survival: the role of religion in childhood immunization in Nigeria. J Biosoc Sci 2009;41:57–76. 10.1017/S0021932008002861 [DOI] [PubMed] [Google Scholar]
- 70.Bondy JN, Thind A, Koval JJ, et al. Identifying the determinants of childhood immunization in the Philippines. Vaccine 2009;27:169–75. 10.1016/j.vaccine.2008.08.042 [DOI] [PubMed] [Google Scholar]
- 71.Corsi DJ, Bassani DG, Kumar R, et al. Gender inequity and age-appropriate immunization coverage in India from 1992 to 2006. BMC Int Health Hum Rights 2009;9:S3 10.1186/1472-698X-9-S1-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osaki K, Hattori T, Kosen S, et al. Investment in home-based maternal, newborn and child health records improves immunization coverage in Indonesia. Trans R Soc Trop Med Hyg 2009;103:846–8. 10.1016/j.trstmh.2009.03.011 [DOI] [PubMed] [Google Scholar]
- 73.Sia D, Fournier P, Kobiané J-F, et al. Rates of coverage and determinants of complete vaccination of children in rural areas of Burkina Faso (1998-2003). BMC Public Health 2009;9:416 10.1186/1471-2458-9-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Antai D. Migration and child immunization in Nigeria: individual- and community-level contexts. BMC Public Health 2010;10:116 10.1186/1471-2458-10-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hong R, Chhea V. Trend and inequality in immunization dropout among young children in Cambodia. Matern Child Health J 2010;14:446–52. 10.1007/s10995-009-0466-1 [DOI] [PubMed] [Google Scholar]
- 76.Rahman M, Obaida-Nasrin S. Factors affecting acceptance of complete immunization coverage of children under five years in rural Bangladesh. Salud Publica Mex 2010;52:134–40. 10.1590/S0036-36342010000200005 [DOI] [PubMed] [Google Scholar]
- 77.Sahu D, Pradhan J, Jayachandran V, et al. Why immunization coverage fails to catch up in India? a community-based analysis. Child Care Health Dev 2010;36:332–9. 10.1111/j.1365-2214.2009.01003.x [DOI] [PubMed] [Google Scholar]
- 78.Semali IA. Trends in immunization completion and disparities in the context of health reforms: the case study of Tanzania. BMC Health Serv Res 2010;10:299. 10.1186/1472-6963-10-299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abuya BA, Onsomu EO, Kimani JK, et al. Influence of maternal education on child immunization and stunting in Kenya. Matern Child Health J 2011;15:1389–99. 10.1007/s10995-010-0670-z [DOI] [PubMed] [Google Scholar]
- 80.Antai D. Rural-Urban inequities in childhood immunisation in Nigeria: the role of community contexts. Afr J Prim Health Care Fam Med 2011;3:a238 10.4102/phcfm.v3i1.238 [DOI] [Google Scholar]
- 81.Fernandez RC, Awofeso N, Rammohan A. Determinants of apparent rural-urban differentials in measles vaccination uptake in Indonesia. Rural Remote Health 2011;11:1–14. [PubMed] [Google Scholar]
- 82.Fernandez R, Rammohan A, Awofeso N. Correlates of first dose of measles vaccination delivery and uptake in Indonesia. Asian Pac J Trop Med 2011;4:140–5. 10.1016/S1995-7645(11)60055-2 [DOI] [PubMed] [Google Scholar]
- 83.Kumar A, Mohanty SK. Socio-economic differentials in childhood immunization in India, 1992–2006. J Popul Res 2011;28:301–24. 10.1007/s12546-011-9069-y [DOI] [Google Scholar]
- 84.Lauridsen J, Pradhan J, et al. Socio-economic inequality of immunization coverage in India. Health Econ Rev 2011;1:11 10.1186/2191-1991-1-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pandey S, Lee Hnim. Determinants of child immunization in Nepal: The role of women’s empowerment. Health Educ J 2012;71:642–53. 10.1177/0017896911419343 [DOI] [Google Scholar]
- 86.Singh A. Inequality of opportunity in Indian children: the case of immunization and nutrition. Popul Res Policy Rev 2011;30:861–83. 10.1007/s11113-011-9214-5 [DOI] [Google Scholar]
- 87.Afzal N, Zainab B. Determinants and status of vaccination in Bangladesh. Dhaka Univ. J. Sci. 2012;60:47–51. 10.3329/dujs.v60i1.10336 [DOI] [Google Scholar]
- 88.Antai D. Gender inequities, relationship power, and childhood immunization uptake in Nigeria: a population-based cross-sectional study. International Journal of Infectious Diseases 2012;16:e136–45. 10.1016/j.ijid.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 89.Sabarwal S, McCormick MC, Silverman JG, et al. Association between maternal intimate partner violence victimization and childhood immunization in India. J Trop Pediatr 2012;58:107–13. 10.1093/tropej/fmr052 [DOI] [PubMed] [Google Scholar]
- 90.Singh A. Gender based Within-Household inequality in childhood immunization in India: changes over time and across regions. PLoS One 2012;7:e33045 10.1371/journal.pone.0035045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barman D, Dutta A. Access and barriers to immunization in West Bengal, India: quality matters. J Heal Popul Nutr 2013;31:510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bbaale E. Factors influencing childhood immunization in Uganda. J Health Popul Nutr 2013;31:118–27. 10.3329/jhpn.v31i1.14756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haque SMR, Bari W. Positive role of maternal education on measles vaccination coverage in Bangladesh. Int J Psychol Behav Sci 2013;3:11–17. [Google Scholar]
- 94.Kumar A, Ram F. Influence of family structure on child health: evidence from India. J Biosoc Sci 2013;45:577–99. 10.1017/S0021932012000764 [DOI] [PubMed] [Google Scholar]
- 95.Moyer CA, Tadesse L, Fisseha S. The relationship between facility delivery and infant immunization in Ethiopia. Int J Gynaecol Obstet 2013;123:217–20. 10.1016/j.ijgo.2013.06.030 [DOI] [PubMed] [Google Scholar]
- 96.Singh A, Singh A, Mahapatra B. The consequences of unintended pregnancy for maternal and child health in rural India: evidence from prospective data. Matern Child Health J 2013;17:493–500. 10.1007/s10995-012-1023-x [DOI] [PubMed] [Google Scholar]
- 97.Singh K, Haney E, Olorunsaiye C. Maternal autonomy and attitudes towards gender norms: associations with childhood immunization in Nigeria. Matern Child Health J 2013;17:837–41. 10.1007/s10995-012-1060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singh PK. Trends in child immunization across geographical regions in India: focus on urban-rural and gender differentials. PLoS One 2013;8:10. 10.1371/journal.pone.0073102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Malderen C, Ogali I, Khasakhala A, et al. Decomposing Kenyan socio-economic inequalities in skilled birth attendance and measles immunization. Int J Equity Health 2013;12:3 10.1186/1475-9276-12-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adegboye OA, Kotze D, Adegboye OA. MULTI-YEAR trend analysis of childhood immunization uptake and coverage in Nigeria. J Biosoc Sci 2014;46:225–39. 10.1017/S0021932013000254 [DOI] [PubMed] [Google Scholar]
- 101.Bonfrer I, Van de Poel E, Van Doorslaer E. The effects of performance incentives on the utilization and quality of maternal and child care in Burundi. Soc Sci Med 2014;123:96–104. 10.1016/j.socscimed.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 102.Bugvi AS, Rahat R, Zakar R, et al. Factors associated with non-utilization of child immunization in Pakistan: evidence from the demographic and health survey 2006-07. BMC Public Health 2014;14:232 10.1186/1471-2458-14-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clouston S, Kidman R, Palermo T. Social inequalities in vaccination uptake among children aged 0–59 months living in Madagascar: an analysis of demographic and health survey data from 2008 to 2009. Vaccine 2014;32:3533–9. 10.1016/j.vaccine.2014.04.030 [DOI] [PubMed] [Google Scholar]
- 104.Ebot JO. Place matters: community level effects of women’s autonomy on Ethiopian children’s immunization status. African Population Studies 2014;28:1202–15. 10.11564/28-0-568 [DOI] [Google Scholar]
- 105.Heaton TB, Crookston B, Forste R, et al. Inequalities in child health in Bolivia: has Morales made a difference? Health Sociology Review 2014;23:208–18. 10.1080/14461242.2014.11081974 [DOI] [Google Scholar]
- 106.Javed SA, Imran W, Haider A, et al. Mothers related differentials in childhood immunization uptake in Pakistan. Res Humanit Soc Sci 2014;4:62–72. [Google Scholar]
- 107.Luqman B, Titus Kolawole O. Mothers’ health seeking behaviour and socio-economic differentials: A factor analysis of full childhood immunization in South-Western Nigeria. J Public Heal Epidemiol 2014;6:132–47. [Google Scholar]
- 108.Malhotra C, Malhotra R, Østbye T, et al. Maternal autonomy and child health care utilization in India: results from the National family health survey. Asia-Pacific J Public Heal 2014;26:401–13. [DOI] [PubMed] [Google Scholar]
- 109.Neupane S, Nwaru BI. Impact of prenatal care utilization on infant care practices in Nepal: a national representative cross-sectional survey. Eur J Pediatr 2014;173:99–109. 10.1007/s00431-013-2136-y [DOI] [PubMed] [Google Scholar]
- 110.Prusty RK, Kumar A. Socioeconomic dynamics of gender disparity in childhood immunization in India, 1992–2006. PLoS One 2014;9:e104598–2006. 10.1371/journal.pone.0104598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rai RK, Singh PK, Singh L, et al. Individual characteristics and use of maternal and child health services by adolescent mothers in niger. Matern Child Health J 2014;18:592–603. 10.1007/s10995-013-1276-z [DOI] [PubMed] [Google Scholar]
- 112.Singh PK, Parasuraman S, et al. ‘Looking beyond the male–female dichotomy’ – Sibling composition and child immunization in India, 1992–2006. Soc Sci Med 2014;107:145–53. 10.1016/j.socscimed.2014.02.017 [DOI] [PubMed] [Google Scholar]
- 113.Ushie BA, Fayehun OA, Ugal DB. Trends and patterns of under-5 vaccination in Nigeria, 1990-2008: what manner of progress? Child Care Health Dev 2014;40:267–74. 10.1111/cch.12055 [DOI] [PubMed] [Google Scholar]
- 114.Abadura SA, Lerebo WT, Kulkarni U, et al. Individual and community level determinants of childhood full immunization in Ethiopia: a multilevel analysis. BMC Public Health 2015;15:972 10.1186/s12889-015-2315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ebot JO. “Girl power!”: the relationship between women’s autonomy and children’s immunization coverage in Ethiopia. J Health Popul Nutr 2015;33:1–9. 10.1186/s41043-015-0028-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lakew Y, Bekele A, Biadgilign S. Factors influencing full immunization coverage among 12–23 months of age children in Ethiopia: evidence from the National demographic and health survey in 2011. BMC Public Health 2015;15:728 10.1186/s12889-015-2078-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McGlynn N, Wilk P, Luginaah I, et al. Increased use of recommended maternal health care as a determinant of immunization and appropriate care for fever and diarrhoea in Ghana: an analysis pooling three demographic and health surveys. Health Policy Plan 2015;30:895–905. 10.1093/heapol/czu090 [DOI] [PubMed] [Google Scholar]
- 118.Mukungwa T. Factors associated with full immunization coverage amongst children aged 12 – 23 months in Zimbabwe. APS 2015;29:1761–74. 10.11564/29-2-745 [DOI] [Google Scholar]
- 119.Onsomu EO, Abuya BA, Okech IN, et al. Maternal education and immunization status among children in Kenya. Matern Child Health J 2015;19:1724–33. 10.1007/s10995-015-1686-1 [DOI] [PubMed] [Google Scholar]
- 120.Osetinsky B, Gaydos LM, Leon JS. Predictors of completed childhood vaccination in Bolivia. Int J Child Adolesc health 2015;8:413–23. [PMC free article] [PubMed] [Google Scholar]
- 121.Prusty RK, Keshri K. Differentials in child nutrition and immunization among migrants and non-migrants in urban India. Int J Migr Health Soc Care 2015;11:194–205. 10.1108/IJMHSC-02-2014-0006 [DOI] [Google Scholar]
- 122.Rossi R. Do maternal living arrangements influence the vaccination status of children age 12–23 months? A data analysis of demographic health surveys 2010–11 from Zimbabwe. PLoS One 2015;10:e0132357–19. 10.1371/journal.pone.0132357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shrivastwa N, Gillespie BW, Kolenic GE, et al. Predictors of vaccination in India for children aged 12–36 months. Am J Prev Med 2015;49:S435–44. 10.1016/j.amepre.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 124.Smith-Greenaway E, Madhavan S. Maternal migration and child health: An analysis of disruption and adaptation processes in Benin. Soc Sci Res 2015;54:146–58.10.1016/j.ssresearch.2015.06.005. [DOI] [PMC free article] [PubMed]
- 125.Tsawe M, Moto A, Netshivhera T, et al. Factors influencing the use of maternal healthcare services and childhood immunization in Swaziland. Int J Equity Health 2015;14:32 10.1186/s12939-015-0162-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chima CC, Franzini L. Spillover effect of HIV-specific foreign aid on immunization services in Nigeria. Int Health 2016;8:108–15. 10.1093/inthealth/ihv036 [DOI] [PubMed] [Google Scholar]
- 127.Gurmu E, Etana D. Factors Influencing Children’s Full Immunization in Ethiopia. African Popul Stud 2016;30:2306–17. [Google Scholar]
- 128.Kriss JL, Goodson J, Machekanyanga Z, et al. Vaccine receipt and vaccine card availability among children of the apostolic faith: analysis from the 2010-2011 Zimbabwe demographic and health survey. Pan Afr Med J 2016;24:47 10.11604/pamj.2016.24.47.8663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kumar C, Singh PK, Singh L, et al. Socioeconomic disparities in coverage of full immunisation among children of adolescent mothers in India, 1990-2006: a repeated cross-sectional analysis. BMJ Open 2016;6:e009768. 10.1136/bmjopen-2015-009768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Adedokun ST, Uthman OA, Adekanmbi VT, et al. Incomplete childhood immunization in Nigeria: a multilevel analysis of individual and contextual factors. BMC Public Health 2017;17:236 10.1186/s12889-017-4137-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aghaji AE. Trends in measles vaccination in Nigeria and implications for childhood blindness. Int J Med Heal Dev 2017;22:82–8. [Google Scholar]
- 132.Ambel AA, Andrews C, Bakilana AM, et al. Examining changes in maternal and child health inequalities in Ethiopia. Int J Equity Health 2017;16:152 10.1186/s12939-017-0648-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herliana P, Douiri A. Determinants of immunisation coverage of children aged 12–59 months in Indonesia: a cross-sectional study. BMJ Open 2017;7:e015790 10.1136/bmjopen-2016-015790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kc A, Nelin V, Raaijmakers H, et al. Increased immunization coverage addresses the equity gap in Nepal. Bull World Health Organ 2017;95:261–9. 10.2471/BLT.16.178327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khan MT, Zaheer S, Shafique K. Maternal education, empowerment, economic status and child polio vaccination uptake in Pakistan: a population based cross sectional study. BMJ Open 2017;7:e013853 10.1136/bmjopen-2016-013853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mbengue MAS, Sarr M, Faye A, et al. Determinants of complete immunization among senegalese children aged 12–23 months: evidence from the demographic and health survey. BMC Public Health 2017;17:630 10.1186/s12889-017-4493-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oleribe O, Kumar V, Awosika-Olumo A, et al. Individual and socioeconomic factors associated with childhood immunization coverage in Nigeria. Pan Afr Med J 2017;26:220 10.11604/pamj.2017.26.220.11453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Singh A, Patel SK. Gender differentials in feeding practices, health care utilization and nutritional status of children in northern India. Int J Hum Rights Healthc 2017;10:323–31. 10.1108/IJHRH-05-2017-0023 [DOI] [Google Scholar]
- 139.Uthman OA, Adedokun ST, Olukade T, et al. Children who have received no routine polio vaccines in Nigeria: who are they and where do they live? Hum Vaccin Immunother 2017;13:2111–22. 10.1080/21645515.2017.1336590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zuhair M, Roy RB. Socioeconomic determinants of the utilization of antenatal care and child vaccination in India. Asia Pac J Public Health 2017;29:649–59. 10.1177/1010539517747071 [DOI] [PubMed] [Google Scholar]
- 141.Acharya P, Kismul H, Mapatano MA, et al. Individual- and community-level determinants of child immunization in the Democratic Republic of Congo: a multilevel analysis. PLoS One 2018;13:e0202742. 10.1371/journal.pone.0202742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ashbaugh HR, Hoff NA, Doshi RH, et al. Predictors of measles vaccination coverage among children 6–59 months of age in the Democratic Republic of the Congo. Vaccine 2018;36:587–93. 10.1016/j.vaccine.2017.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Asuman D, Ackah CG, Enemark U. Inequalities in child immunization coverage in Ghana: evidence from a decomposition analysis. Health Econ Rev 2018;8:9 10.1186/s13561-018-0193-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boulton ML, Carlson BF, Power LE, et al. Socioeconomic factors associated with full childhood vaccination in Bangladesh, 2014. Int J Infect Dis 2018;69:35–40. 10.1016/j.ijid.2018.01.035 [DOI] [PubMed] [Google Scholar]
- 145.Burroway R, Hargrove A. Education is the antidote: Individual- and community-level effects of maternal education on child immunizations in Nigeria. Soc Sci Med 2018;213:63–71. 10.1016/j.socscimed.2018.07.036 [DOI] [PubMed] [Google Scholar]
- 146.Imran W, Abbas F, Javed SA. What is causing high polio vaccine dropout among Pakistani children? Public Health 2018;164:16–25. 10.1016/j.puhe.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 147.Khan J, Shil A, Prakash R. Exploring the spatial heterogeneity in different doses of vaccination coverage in India. PLoS One 2018;13:e0207209. 10.1371/journal.pone.0207209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kols A, Gorar Z, Sharjeel M, et al. Provincial differences in levels, trends, and determinants of childhood immunization in Pakistan. East Mediterr Health J 2018;24:333–44. 10.26719/2018.24.4.333 [DOI] [PubMed] [Google Scholar]
- 149.McGavin ZA, Wagner AL, Carlson BF, et al. Childhood full and under-vaccination in Nigeria, 2013. Vaccine 2018;36:7294–9. 10.1016/j.vaccine.2018.10.043 [DOI] [PubMed] [Google Scholar]
- 150.Raza O, Lodhi FS, Morasae EK, et al. Differential achievements in childhood immunization across geographical regions of Pakistan: analysis of wealth-related inequality. Int J Equity Health 2018;17:122. 10.1186/s12939-018-0837-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shenton LM, Wagner AL, Carlson BF, et al. Vaccination status of children aged 1-4 years in Afghanistan and associated factors, 2015. Vaccine 2018;36:5141–9. 10.1016/j.vaccine.2018.07.020 [DOI] [PubMed] [Google Scholar]
- 152.Shenton LM, Wagner AL, Bettampadi D, et al. Factors associated with vaccination status of children aged 12–48 months in India, 2012–2013. Matern Child Health J 2018;22:1–10. [DOI] [PubMed] [Google Scholar]
- 153.Lungu EA, Biesma R, Chirwa M, et al. Is the urban child health advantage declining in Malawi?: evidence from demographic and health surveys and multiple indicator cluster surveys. J Urban Health 2019;96:131–43. 10.1007/s11524-018-0270-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Masters NB, Wagner AL, Carlson BF, et al. Childhood vaccination in Kenya: socioeconomic determinants and disparities among the Somali ethnic community. Int J Public Health 2019;64:313–22. 10.1007/s00038-018-1187-2 [DOI] [PubMed] [Google Scholar]
- 155.Vyas P, Kim D, Adams A. Understanding spatial and contextual factors influencing Intraregional differences in child vaccination coverage in Bangladesh. Asia Pac J Public Health 2019;31:51–60. 10.1177/1010539518813604 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.