Abstract
Objective
To determine whether intranasal oxytocin, alone or in combination with instructed mimicry of facial expressions, would augment neural activity in patients with frontotemporal dementia (FTD) in brain regions associated with empathy, emotion processing, and the simulation network, as indexed by blood oxygen–level dependent (BOLD) signal during fMRI.
Methods
In a placebo-controlled, randomized crossover design, 28 patients with FTD received 72 IU intranasal oxytocin or placebo and then completed an fMRI facial expression mimicry task.
Results
Oxytocin alone and in combination with instructed mimicry increased activity in regions of the simulation network and in limbic regions associated with emotional expression processing.
Conclusions
The findings demonstrate latent capacity to augment neural activity in affected limbic and other frontal and temporal regions during social cognition in patients with FTD, and support the promise and need for further investigation of these interventions as therapeutics in FTD.
ClinicalTrials.gov identifier
Classification of evidence
This study provides Class III evidence that a single dose of 72 IU intranasal oxytocin augments BOLD signal in patients with FTD during viewing of emotional facial expressions.
A hallmark symptom of frontotemporal dementia (FTD) is the progressive loss of empathy.1 For those with FTD, there are currently no approved treatments for their symptoms, and only a few off-label treatments of limited efficacy are available. Impairments in insight and reporting of emotional experience further complicate the assessment of treatments for these symptoms. At present, the lack of treatments targeting the early loss of empathy and social dysfunction in FTD renders physicians unable to effectively manage the symptoms that are most difficult for families and caregivers.
The hormone and neuropeptide oxytocin has been implicated in augmenting prosocial behavior and empathy. Oxytocin receptors are expressed in the amygdala, medial prefrontal cortex (PFC), insula, and nucleus accumbens,2,3 regions involved in emotion and reward processing and affected by FTD pathology. In patients with behavioral variant FTD (bvFTD), a single dose or short courses of oxytocin have been associated with reduced recognition of anger and fear, improved neuropsychiatric symptoms, and reduced social apathy/indifference.4,5
Abnormal processing of emotional facial expressions is considered a key factor in the empathy deficits observed in FTD in both bvFTD and the semantic dementia subtype with right temporal atrophy.6,7 Patients with bvFTD show consistent impairments in negative facial expression recognition, with preserved nonemotional feature processing. Humans also show an unconscious drive to imitate others' facial expressions.8 Viewing emotional reactions in others activates neural regions in the observer similar to those activated when one experiences that emotion9; such activity is correlated with trait measures of empathy10 and is deficient in patients with FTD.11,12
One's emotional experience is influenced by the facial expressions they adopt, even when their emotional expressions are manipulated without their awareness (e.g., having participants hold a pen in their teeth to produce a smile).13 Emotional mirroring differentially engages areas such as the amygdala, anterior insula, and anterior cingulate cortex (ACC), as well as simulation network regions such as the inferior frontal gyrus (IFG).14,15 Thus, mimicry has been considered a potentially potent means of arousing empathy.16,17 In FTD, recent studies have confirmed abnormal automatic mimicry of emotional facial expressions.18,19 Furthermore, oxytocin administration to healthy adults has been associated with increased automatic motor imitation20 and mimicry of emotional facial expressions.21
The objective of this study was to determine whether 2 potential interventions, oxytocin and emotional mimicry, alone or in combination, augment neural activation related to social cognition in the compromised nervous system of patients with bvFTD. We predicted that oxytocin and instructed mimicry would modulate neural activity in brain regions affected in bvFTD and implicated in emotional facial expression processing.
Methods
Participants
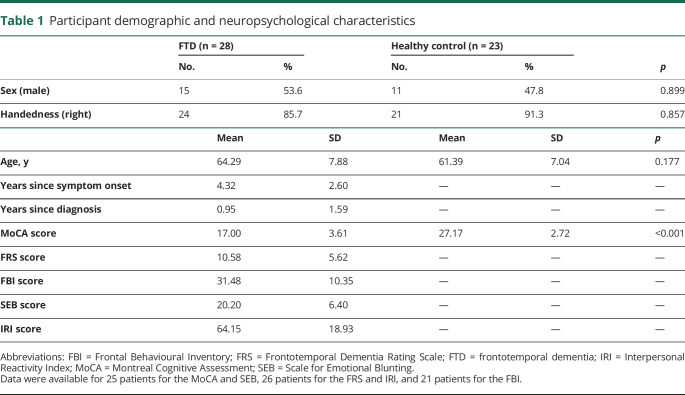
Fifty-one participants met eligibility criteria and took part in this study between 2013 and 2017, including 28 patients with FTD and 23 healthy volunteers. Twenty-six participants in the FTD group met the revised international consensus diagnostic criteria for bvFTD.22 Twenty had frontal or frontal and temporal atrophy, while 6 had predominant right temporal atrophy. Two patients initially had symptoms of semantic variant primary progressive aphasia23 and at the time of the study also displayed the behavioral features of bvFTD, with bitemporal atrophy, left greater than right. Three participants were known to have the C9orf72 repeat expansion, and 1 participant carried a TBK1 mutation associated with FTD. All patients completed cognitive testing assessing attention, memory, executive functioning, language, and visuospatial skills and had MRI, CT, or SPECT imaging consistent with the diagnosis (table 1). Exclusion criteria included a history of stroke or other neurological disorder excluding FTD, a diagnosis of bipolar disorder or schizophrenia that was not accounted for by the diagnosis of FTD, or cognitive impairment that precluded comprehension of task instructions (CONSORT flowchart available from Dryad: doi.org/10.5061/dryad.59zw3r254). Patients were recruited through the Cognitive Neurology and Alzheimer Research Centre at Parkwood Hospital in London, Ontario, Canada. Age- and sex-matched control participants with no history of a neurologic or psychiatric disorder were recruited through advertisements to caregivers at local FTD family support groups and volunteer databases of the center.
Table 1.
Participant demographic and neuropsychological characteristics
Standard protocol approvals, registrations, and patient consents
All participants and caregivers provided written informed consent according to the Declaration of Helsinki. This study was registered at ClinicalTrials.gov (NCT01937013) and approved by the Health Sciences Research Ethics Board at the University of Western Ontario, London, Ontario, Canada.
Procedure
Baseline symptoms and cognition
At the first visit, before the fMRI procedure, participants completed the Montreal Cognitive Assessment.24 Caregivers of patients with FTD completed the Frontotemporal Dementia Rating Scale25 to determine disease severity and third-person questionnaires assessing behavior, personality, and empathy changes, including the Frontal Behavioural Inventory,26 the Scale for Emotional Blunting,27 and the Interpersonal Reactivity Index,28 a multidimensional empathy questionnaire.
Oxytocin administration and fMRI
In a double-blind, placebo-controlled, randomized crossover design (see Dryad for details: doi.org/10.5061/dryad.59zw3r254), patients received 72 IU placebo saline mist (Salinex; Markham, Ontario, Canada) or oxytocin (Syntocinon; Novartis, Bern, Switzerland) nasal spray, for which our group has previously shown safety and tolerability in FTD.5 The nasal spray was administered via 3 sprays per nostril at 10-minute intervals. Participants began the fMRI View and Imitate Task ≈45 minutes after their last spray. While direct knowledge of the CNS pharmacokinetics of oxytocin in humans is limited, in a study of human volunteers, CSF oxytocin levels increased by 64% 75 minutes after 24 IU intranasal oxytocin.29 In macaques, elevated CSF oxytocin levels have been observed at 15 to 60 minutes after intranasal oxytocin administration.30–33 After the fMRI procedure, participants completed 3 behavioral tasks outside of the scanner: Behavioural View and Imitate Task, the Multifaceted Empathy Test (MET), and the Postural Knowledge Test (PKT; detailed below).
Two weeks later, patients returned and completed the fMRI and behavioral battery again under the alternative treatment condition. The order of behavioral task completion was randomized across patients, but the order of presentation for both fMRI blocks and behavioral tasks was the same on both visits for each patient. Healthy controls completed the same fMRI procedure and battery of testing as patients but came for 1 visit and received only placebo saline mist, although they believed they could be receiving oxytocin or placebo. After completion of their sole visit, healthy individuals were debriefed regarding the administration of only saline mist and reconsented accordingly. Healthy control participants were matched with patients on the basis of age and sex and completed the fMRI task blocks and behavioral tasks in the same order as their matched case participant.
fMRI View and Imitate Task
Participants underwent the above fMRI while completing a View and Imitate Task developed by the laboratory (programmed in Eprime) and based on previous work exploring the simulation network in relation to empathy.34 During the task, participants were presented with short dynamic videos of 20 actors demonstrating facial expressions and hand actions (button presses) and were asked to either view or imitate each. Facial expressions included emotional (anger, disgust, fear, happiness, sadness) and neutral (calm, nonemotional movements [NEMs] such as pursing the lips) ones. Hand actions involved button presses using the same button box used in the scanner (index finger, middle finger). Each video was presented once in each condition (view, imitate). Inclusion of the button pressing trials allowed for the potential discrimination between motor mimicry and facial mimicry. Before scanning, participants completed a practice version of the task to ensure comprehension.
The task was presented in 4 runs, each comprising a view and an imitate block. The order of runs and blocks was counterbalanced across patients, and trials within runs were randomly presented. Each block included 45 trials (5 per expression action, 2500 milliseconds each) preceded by an intertrial instruction (view or imitate; 1,000 milliseconds) and 15 jitter trials displaying a fixation cross (3,000 milliseconds). Each block began with an 8,000-millisecond instruction screen indicating the condition (view or imitate) and was followed by a 15,500-millisecond interblock interval. Button presses were recorded, allowing for accuracy assessments of motor imitation. Participants' faces were recorded throughout the task with an MRI-compatible camera to ensure task compliance. Videos were coded using an adaptation of the Facial Expressions Coding System.35 For each participant video, a blinded rater coded the onset of each trial, the participant’s attentiveness (eyes open/eyes closed), and whether an attempt to imitate an expression was made (yes/no).
MRI data acquisition
Participants were scanned with a 3T Siemens Prisma scanner (Malvern, PA) with a 32-channel head coil at Robarts Research Institute. fMRI images were taken with a T2*-gradient echo-planar imaging sequence (repetition time 3,000 milliseconds, echo time 30 milliseconds, field of view 240 mm, matrix 120 × 120). Parameters were chosen to optimize the signal-to-noise ratio for regions of interest with high signal dropout such as the ventromedial PFC and the amygdala while maintaining coverage. For functional scans, 45 contiguous slices of 2.0 × 2.0 mm in-plane with a slice thickness of 2.5 mm were obtained. A high-resolution, T1-weighted, anatomic scan was acquired with whole-brain coverage (repetition time 2,300 milliseconds, echo time 2.98 milliseconds, field of view 256 mm, matrix 256 × 240, 192 axial slices, 1.0 × 1.0 × 1.0–mm voxels).
fMRI analysis
Analyses of fMRI data were conducted with the Analysis of Functional NeuroImages (AFNI) software.36 All volumes were slice-time corrected, and anatomic data were registered to the functional volume with the minimal outlier fraction. Echo-planar imaging data were registered to the anatomic scan, followed by nonlinear registration into Montreal Neurological Institute 152 space. Data were spatially smoothed with a 4-mm full width at half-maximum isotropic gaussian kernel and normalized such that each time point within a voxel was represented as a percent change from the mean voxel intensity.
Within task runs, volumes and the preceding volume were censored if the derivatives of the 6 generated motion parameters had a euclidean norm >2.0 mm. Regressors were created for each condition by convolving the stimulus events with a gamma-variate hemodynamic response function. Nuisance regressors for the motion parameters and their derivatives were included in the model, with linear and quadratic detrending to account for baseline drift. The blood oxygen–level dependent (BOLD) response was fitted to each regressor to conduct linear regression modeling for each participant visit. This produced a β coefficient and t statistic at each voxel for each regressor. Regression coefficients represented the percentage signal change from the mean activity.
Regressors were created for each expression action (anger, disgust, fear, happiness, sadness, NEM, button press [index or middle finger]) for each condition (view, imitate), including only trials in which participants appropriately viewed or imitated according to the video coding and button-pressing responses. Regressors were also created to model trials of obstinate viewing or imitation (i.e., errors) and a regressor of no interest for trials in which participants were not attending to the stimuli (eyes closed).
Five patients and 1 control were excluded from the fMRI analyses due to artifacts or inability to complete the task (details and sample sizes by condition in table e-1 from Dryad: doi.org/10.5061/dryad.59zw3r254).
Oxytocin versus placebo in FTD
Whole-brain analyses were used to investigate the within-group neural effects of oxytocin and emotional mimicry in participants with FTD. A 2 treatment (oxytocin, placebo) × 2 condition (view correct, imitate correct) × 7 expression action (anger, disgust, fear, happiness, sadness, NEM, button press) analysis of covariance (ANCOVA) using 3dLME in AFNI was conducted. Oxytocin order (visit 1, visit 2) and sex were included as nuisance between-participant factors to control for order effects and given that the distribution of oxytocin receptors is known to vary by sex. Mean framewise displacement (FD) was also included as a covariate. Pairwise contrasts were modeled to delineate the nature of significant effects. Whole-brain contrasts were thresholded at p < 0.001 and family-wise error (FWE) corrected for multiple comparisons to p < 0.05 (45 contiguous voxels) using 3dFWHMx and 3dClustSim in AFNI.
Healthy controls versus participants with FTD
Whole-brain analyses were used to determine whether between-group differences in neural responding while viewing emotional expressions in FTD could be replicated with dynamic stimuli11 and to explore the neural effects of emotional mimicry in healthy controls vs participants with FTD. Given that healthy controls received only placebo saline mist and completed 1 visit, only participants with FTD who received placebo on their first visit were included in this analysis (n = 11). A 2 group (control, FTD) × 2 condition (view correct, imitate correct) × 7 expression action (anger, disgust, fear, happiness, sadness, NEM, button press) ANCOVA using 3dLME in AFNI was conducted. Sex was included as a nuisance between-participant factor, and age and mean FD were included as covariates. There was no significant difference in mean FD between groups, t (11.98) = −1.11, p = 0.288. Pairwise contrasts were modeled to delineate the nature of significant effects. Whole-brain contrasts were thresholded at p < 0.001 and FWE corrected for multiple comparisons to p < 0.05 (42 contiguous voxels).
Behavioral imaging analysis
Analyses of errors conducted with the video coding and button pressing response data from the fMRI task are available from Dryad (doi.org/10.5061/dryad.59zw3r254).
Behavioral measures
View and Imitate Task
To further assess the accuracy of participant's imitation of the various facial expressions and the relationship between expression imitation and recognition, outside the scanner, participants completed a shortened version of the View and Imitate Task performed in the scanner while their facial expressions were video recorded. The task was identical to the one performed in the scanner with the following modifications: only facial expressions were included; 2 runs (70 trials each) were presented consisting of 1 view and 1 imitate block each; each video was presented twice consecutively per trial; and participants were asked to identify the emotion from 6 choices presented in random order (angry, disgusted, fearful, happy, sad, neutral). Participants responded aloud, and the tester inputted their response via button press. Participant videos were coded with an adaptation of the Facial Expressions Coding System.35 For each video, independent blinded raters coded the duration, valence, intensity, and emotion for each expression. Data were excluded from 2 patients due to comprehension issues, 5 patients due to a lack of imitation before responding, and 1 patient due to the presence of obstinate imitation.
Multifaceted Empathy Test
The MET is a performance-based multidimensional measure of empathy, previously used to index cognitive and emotional empathy in FTD.7,37 During the MET, participants answer questions that dissociably tap cognitive and emotional empathy in response to naturalistic emotionally charged images (see Dryad for details: doi.org/10.5061/dryad.59zw3r254). MET data were excluded from analyses for 2 patients due to comprehension issues and 1 control due to abnormal performance (>2 SDs from control mean on 7 of 12 measures).
Postural Knowledge Test
The PKT is a picture-matching task that evaluates nonemotional action understanding and motor representation, which is thought to be an indirect assessment of simulation network, or mirror neuron system, function in neurodegenerative disorders.38 During the task, participants are presented with a partially drawn cartoon of a person performing an action and select the correct gesture among 3 options presented below to complete the cartoon. The task consists of 4 training cartoons and 20 randomly presented test cartoons, including 10 transitive actions (object related; e.g., ironing, cutting hair) and 10 intransitive actions (non–object related; e.g., waving goodbye, saluting).
Behavioral task analysis
We conducted χ2 analyses and independent t tests to identify any group differences in demographics or standardized neuropsychological test performance (table 1). For the View and Imitate Task recognition accuracy data, for the FTD group only, a 2 treatment (oxytocin, placebo) × 2 condition (view, imitate) × 6 expression (anger, disgust, fear, happiness, sadness, NEM) ANCOVA was conducted. For healthy controls vs participants with FTD who received placebo on their first visit, a 2 group (control, FTD) × 2 condition × 6 expression ANCOVA was conducted.
MET performance for each of the cognitive and emotional empathy measures, as well as context-only stimuli ratings, was analyzed with 2 treatment × 2 valence (positive, negative) ANCOVAs for the FTD group only and 2 group × 2 valence ANCOVAs for the healthy controls and the FTD group subset. Lastly, PKT accuracy data were analyzed with 2 treatment × 2 action type (transitive, intransitive) ANCOVAs for the FTD group only and 2 group × 2 action type ANCOVAs for the healthy controls and the FTD group subset.
For within-group FTD treatment comparisons, oxytocin order and sex were included as nuisance between-participant factors, whereas age and sex were included as covariates for healthy control vs FTD group comparisons. Follow-up independent and paired t tests, with Bonferroni correction and corrected values according to the Levene test when appropriate, were conducted to delineate the nature of significant effects.
Data availability
The data are not publicly available because written consent for data sharing was not obtained and data contain information (face videos) that could compromise the privacy of research participants.
Results
Behavioral results: Oxytocin versus placebo in FTD
There were no significant effects of treatment or condition for the Behavioral View and Imitate Task in FTD (n = 20) (table e-2 from Dryad: doi.org/10.5061/dryad.59zw3r254). However, there was a main effect of expression, driven by patients showing greater accuracy across conditions for happy expressions vs all other expressions (all p < 0.001).
For the MET in FTD (n = 26), the only effect of treatment was a main effect for empathic concern, with patients providing slightly higher ratings on placebo (7.49 ± 0.237) than oxytocin (7.11 ± 0.284, p = 0.013). There were also significant effects of valence, with greater values for positive vs negative images for cognitive empathy accuracy, empathic concern, and affective sharing intensity, and greater ratings for negative vs positive stimuli for affective sharing valence, affective sharing arousal, context-only valence, and context-only arousal.
A main effect of treatment was found for the PKT in FTD (n = 28), characterized by greater accuracy on oxytocin (0.712 ± 0.026) than placebo (0.656 ± 0.028, p = 0.006). There was also a main effect of action type, driven by greater accuracy for intransitive (0.807 ± 0.028) vs transitive (0.562 ± 0.030, p < 0.001) actions, but no treatment × action type interaction.
Healthy controls versus FTD
The ANCOVA interrogating recognition accuracy on the View and Imitate Task outside the scanner (FTD n = 11, control n = 23) demonstrated a main effect of group, driven by patients (0.646 ± 0.032) performing worse than controls (0.795 ± 0.021, p = 0.001) regardless of condition.
For the MET (FTD n = 14, control n = 22), a main effect of group was revealed for cognitive empathy accuracy, driven by controls (0.951 ± 0.012) showing greater accuracy than patients (0.887 ± 0.016, p = 0.003). For empathic concern and affective sharing intensity, there was a main effect of valence driven by greater ratings for positive vs negative images. In addition, significant group × valence interactions were identified for affective sharing valence, affective sharing arousal, context-only valence, and context-only arousal, characterized by patients providing lower ratings than controls for negative images but not positive images.
A main effect of group was found for the PKT (FTD n = 14, control n = 23), with controls (0.839 ± 0.018) showing greater accuracy across action types than patients (0.705 ± 0.024, p < 0.001).
Supplementary material from Dryad (table e-3; doi.org/10.5061/dryad.59zw3r254) provides behavioral imaging results.
Neuroimaging
Oxytocin versus placebo in FTD (p < 0.001, cluster correction = 45)
Main effects of treatment, condition, and expression
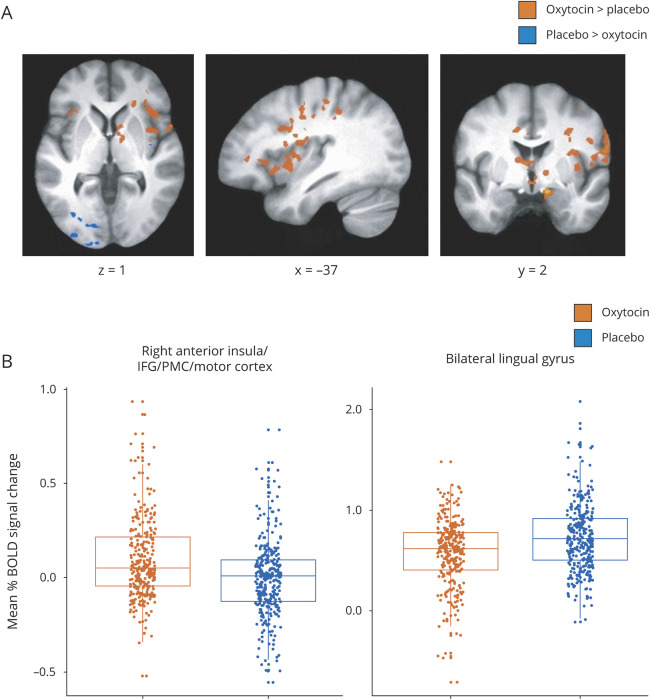
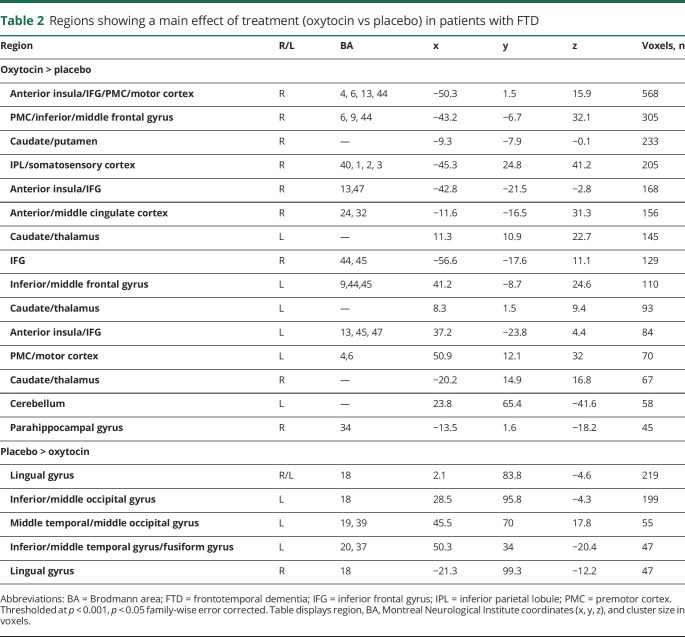
Whole-brain fMRI analyses (n = 23; see table e-1 for numbers by condition from Dryad: doi.org/10.5061/dryad.59zw3r254) revealed a main effect of treatment in multiple regions (figure 1 and table 2). Most regions showed greater activation on oxytocin vs placebo, including the right anterior insula into IFG, premotor cortex (PMC), and motor cortex, the right inferior parietal lobule (IPL) into somatosensory cortex, right anterior/middle cingulate, the left anterior insula into IFG, and bilateral caudate. Areas demonstrating greater activation on placebo vs oxytocin included the left inferior/middle occipital gyri, bilateral lingual gyrus, and fusiform gyrus. A significant main effect of condition was also observed, with greater activation during imitation vs viewing in bilateral IFG, PMC, motor cortex, somatosensory cortex, IPL, and supplementary motor area (SMA); a greater response to viewing vs imitation was observed in bilateral caudate, cingulate, medial PFC, superior temporal sulcus (STS), and cerebellum (table e-4 from Dryad). In addition, a main effect of expression action was observed in multiple regions, including greater activation in the precuneus/posterior cingulate and lingual/fusiform gyrus for button pressing and reduced activation in medial PFC and ACC for anger and disgust compared to other action expressions.
Figure 1. Main effect of oxytocin on blood oxygen level–dependent (BOLD) signal during facial expression processing.
(A) Regions showing increased BOLD signal after oxytocin treatment compared to placebo and regions showing greater BOLD signal during placebo treatment compared to oxytocin. Whole-brain analyses were conducted at p < 0.001, corrected to family-wise error p < 0.05. (B) Example distributions of mean percent BOLD signal change during oxytocin and placebo treatments across conditions and expressions in clusters showing a main effect of treatment with opposing activation patterns. IFG = inferior frontal gyrus; PMC = premotor cortex.
Table 2.
Regions showing a main effect of treatment (oxytocin vs placebo) in patients with FTD
Treatment interactions
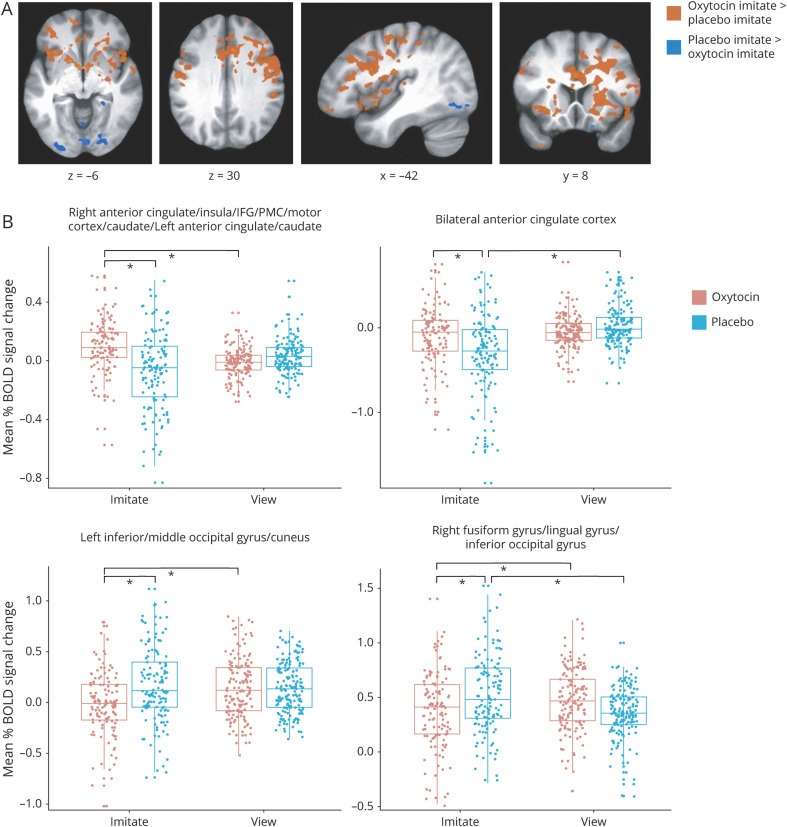
A treatment × condition interaction was significant in multiple regions (figure 2 and table 3). Brain areas that were more active during imitation on oxytocin compared to both viewing on oxytocin and imitation on placebo included bilateral ACC, anterior insula, IFG, PMC, caudate, bilateral SMA, and right globus pallidus/amygdala. In contrast, regions showing greater activation during imitation on placebo than both viewing on placebo and imitation on oxytocin included visual areas such as bilateral fusiform and lingual gyri. Table e-5 from Dryad (doi.org/10.5061/dryad.59zw3r254) provides results from pairwise comparisons in functionally defined regions, including areas hypothesized to be influenced by oxytocin or instructed mimicry (anterior insula, amygdala, and IFG).
Figure 2. Interaction of treatment and condition on blood oxygen level–dependent (BOLD) signal during facial expression processing.
(A) Regions showing increased BOLD signal after oxytocin compared to placebo treatment during imitate compared to view and regions showing greater BOLD signal during placebo treatment compared to oxytocin for imitate in comparison to view. Whole-brain analyses were conducted at p < 0.001, corrected to family-wise error p < 0.05. (B) Example distributions of mean percent BOLD signal change (beta weights) during imitate and view conditions on oxytocin and placebo treatments across expressions in clusters identified in the treatment × condition interaction showing different activation patterns. Bars indicate where significant differences exist. IFG = inferior frontal gyrus; PMC = premotor cortex.
Table 3.
Regions showing a treatment (oxytocin vs placebo) × condition (imitate vs view) interaction in patients with FTD
Condition × expression interaction
A significant condition × expression action interaction was demonstrated with greater activation observed in the right cerebellum, left cingulate gyrus and SMA, and the left posterior insula into IPL during imitation of button pressing vs the other action expressions. Compared to imitation of the other expression actions, decreased BOLD responses were also observed during imitation of button pressing in right PMC, during anger and disgust in bilateral ACC and medial PFC, and during fear in right posterior middle temporal gyrus (table e-6 from Dryad: doi.org/10.5061/dryad.59zw3r254).
Healthy controls versus FTD (p < 0.001, cluster correction = 42 voxels)
Main effects of group, condition, and expression
Whole-brain ANCOVAs exploring the neural effects of emotional mimicry in healthy controls compared to individuals with FTD (FTD n = 11, control n = 22; see table e-1 for numbers by condition from Dryad: doi.org/10.5061/dryad.59zw3r254) revealed main effects of condition (imitate vs view; table e-7 from Dryad) and expression action.
Interactions
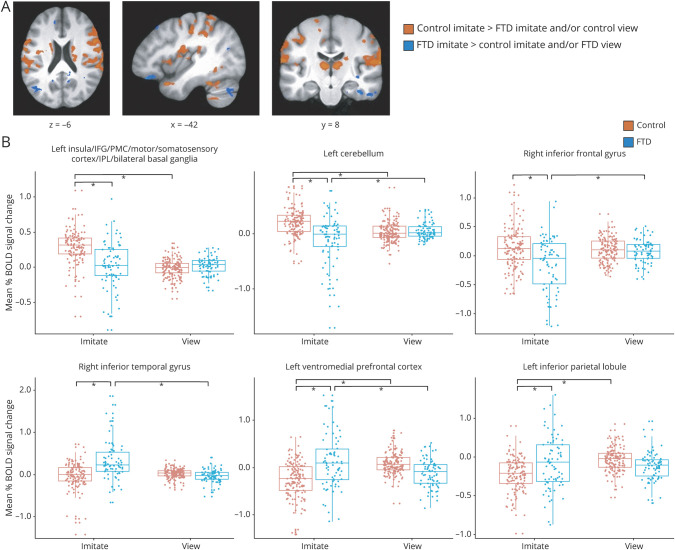
There was a significant group × condition interaction in multiple areas (figure 3 and table e-8 from Dryad: doi.org/10.5061/dryad.59zw3r254). Regions that showed greater activation during imitation in controls vs both imitation in patients and viewing in controls included the left insula, and bilateral IFG, PMC, primary motor and somatosensory cortices, IPL, basal ganglia, and SMA. However, there were also areas that showed greater activity during imitation in patients than controls such as the bilateral middle/inferior temporal gyrus and left IPL and ventromedial PFC. Patients and controls showed no significant differences in activation during viewing across expression actions. Table e-5 from Dryad gives results from pairwise comparisons in functionally defined regions of particular interest.
Figure 3.
(A) Regions showing increased BOLD signal in healthy controls during imitate vs patients with frontotemporal dementia (FTD) during imitate or controls during view and regions showing greater BOLD signal in patients with FTD during imitate vs controls during imitate or patients during view. Whole-brain analyses were conducted at p < 0.001, corrected to family-wise error p < 0.05. (B) Example distributions of mean percent BOLD signal change (β weights) during imitate and view conditions in controls and patients across expressions in clusters identified in the group × condition interaction showing different activation patterns. Bars indicate where significant differences exist. IFG = inferior frontal gyrus; IPL = inferior parietal lobule; PMC = premotor cortex.
A significant condition × expression action interaction was also present, with several areas of frontal and parietal cortex differentially responding to button pressing (table e-9 from Dryad: doi.org/10.5061/dryad.59zw3r254).
Classification of evidence
This study provides Class III evidence that a single dose of 72 IU intranasal oxytocin increases neural activity as measured by BOLD signal during facial expression viewing in frontotemporal regions in patients with FTD on the basis of the completion rate of <80% of FTD patients enrolled (78%).
Discussion
Patients with FTD show impairments in empathy and its key components, including impaired facial expression recognition and pathophysiologic responses to emotional stimuli.39 Using fMRI and an emotional expression viewing and imitation task, we examined the effects of oxytocin and instructed mimicry on neural activity associated with internal emotional experience and empathy in patients with FTD. Using whole-brain analysis, we observed robust effects of oxytocin on BOLD signal in regions associated with emotional facial and action-expression processing and the simulation network. Specifically, increased activity was observed after oxytocin compared to placebo in frontal and limbic regions, including bilateral anterior insula and IFG, caudate, and right ACC and IPL. The combination of oxytocin treatment and instructed mimicry was associated with increased responses in these regions and in the right amygdala. During instructed mimicry alone, patients and controls showed greater activation in bilateral IFG and IPL. Reduced BOLD activity after oxytocin compared to placebo treatment was observed in posterior visual regions, including fusiform and lingual gyri. These findings demonstrate that oxytocin and imitation, alone or in combination, activate frontal and other limbic brain regions in patients with FTD. This provides evidence that latent capacity is present, even in patients with significant neurodegeneration, in brain regions affected early in the disease course. In the context of the functional neural models of empathy and social cognition, the augmented BOLD signal in these neural regions and networks supports the potential promise of oxytocin and mimicry to improve empathy and related social cognitive deficits in patients with FTD.
In healthy humans, fMRI studies of oxytocin have highlighted effects in the regions identified in the present study, including the amygdala, insula, ACC, and caudate. The majority of these studies have found that oxytocin increases activation of these regions; however, some studies have reported decreases. Potential reasons for this variability include differences in tasks, region-of-interest vs whole-brain analytic approaches, and sex-specific effects.40 In this cohort of patients with FTD, we conducted a placebo-controlled, crossover design with whole-brain analysis to reduce confounds from interindividual heterogeneity and potential biases of region-of-interest approaches. No sex-specific effects were observed. Meta-analyses of intranasal oxytocin studies in healthy adults or in other neuropsychiatric disorders have identified the STS, insula, amygdala, ACC, and caudate as most commonly showing modulation by oxytocin,41,42 even when restricted to studies using whole-brain analysis.40 Although less commonly reported, changes in occipital region BOLD signal in response to oxytocin have been described, including decreased activity in the fusiform gyrus in healthy adults.43 It is interesting to note that oxytocin receptor expression and binding are evident in multiple early areas of the visual system, indicating a role in modulating basic sensory processes that is still poorly understood.44
Our findings of increased activity in the IFG and IPL support models of oxytocin modulation of the simulation network. In healthy populations, emotional empathy is consistently associated with greater activation in the anterior insula and ACC, amygdala, and simulation network regions, including IFG and IPL.45,46 In particular, lesion studies indicate that the insula and IFG are especially critical hubs in the empathy network.47,48 Training healthy individuals in elements of emotional empathy has been associated with increased activity in the insula, ACC, and dorsal striatum.49 Enhanced activity in these regions likely augments the processing of socially relevant cues, including facial expression, gesture/body position (IPL/STS), gaze and related eye feature processing (amygdala), and integration of somatosensory component signals of emotion (insula, caudate, and ACC).12,50 We also found that oxytocin improved gesture recognition accuracy on the PKT and increased BOLD signal during imitation of both emotional and nonemotional actions in patients with FTD. This raises the possibility, also suggested in healthy adults, that oxytocin may also modulate nonemotional forms of social communication. Together, these findings and the synergistic effects of imitation and oxytocin observed on activity in key regions implicated in emotional empathy support the potential promise of these interventions to restore emotional empathy-related processing in patients with FTD. An ongoing phase 2 randomized clinical trial of oxytocin in patients with FTD (Intranasal Oxytocin for Frontotemporal Dementia [FOXY]) will provide further data regarding whether and how repeated dosing of oxytocin modulates empathy, expression recognition, and related social behavior (ClinicalTrials.gov).51
While fMRI changes induced by oxytocin and imitation in this cohort were significant and in line with models of enhancing facial expression and empathy-related processing, mimicry or a single dose of 72 IU oxytocin did not significantly improve expression labeling or self-report of empathic feelings when viewing emotional pictures. Patients with FTD showed cognitive empathy deficits relative to controls on the MET. They also rated negative pictures as less negative, although did not show significant differences in empathic concern ratings, replicating our prior findings.7 The lack of a measurable benefit of instructed mimicry or oxytocin on expression recognition accuracy in patients with FTD indicates that although fMRI changes were robust and serve as an objective index of oxytocin effects, additional ecologic assessments, particularly of daily behaviors, are needed to determine whether these neural signals will translate into improved symptoms and behavior in real-world situations. In addition, evidence suggests that brain atrophy can confound BOLD responses.52,53 Although the present sample of participants with FTD exhibited heterogeneity in clinical presentation and brain atrophy, the within-participant design for the FTD oxytocin vs placebo analyses ensures that our findings are not being driven by between-participant factors such as atrophy, age, medication, or disease duration. It should also be noted that histopathologic or genetic confirmation is required for a diagnosis of definite FTD.
We found that both oxytocin and instructed mimicry increase BOLD activity in limbic and frontal regions involved in emotion and in simulation of other's emotional and physical states in patients with FTD. The results support the merit of further investigation of oxytocin and other pharmacologic and behavioral approaches to augment empathy and related social cognitive processing to ameliorate key symptoms of FTD.
Acknowledgment
The authors acknowledge Julia MacKinley for assistance with data collection and Gabrielle Brook, Kaitlyn Helou, Ian Jones, Jessica Jung, Amber McCallum, Mika Ohtsuka, Darren Pankoff, Marwan Syed, Mathura Thiyagarajah, and Sophia Wen for assistance with video coding. Special thanks to all participants and caregivers for their contribution to this work.
Glossary
- ACC
anterior cingulate cortex
- ANCOVA
analysis of covariance
- AFNI
Analysis of Functional NeuroImages
- BOLD
blood oxygen–level dependent
- bvFTD
behavioral variant FTD
- FD
framewise displacement
- FOXY
Intranasal Oxytocin for Frontotemporal Dementia
- FTD
frontotemporal dementia
- FWE
family-wise error
- IFG
inferior frontal gyrus
- IPL
inferior parietal lobule
- MET
Multifaceted Empathy Test
- NEM
nonemotional movement
- PFC
prefrontal cortex
- PKT
Postural Knowledge Test
- PMC
premotor cortex
- SMA
supplementary motor area
- STS
superior temporal sulcus
Appendix. Authors
Footnotes
Editorial, page 849
Class of Evidence: NPub.org/coe
Study funding
This research was supported by funding from the Canadian Institutes of Health Research to E. Finger and D. Mitchell (286763), the Ministry of Research and Innovation of Ontario (E. Finger), and Canada First Research Excellence Fund BrainsCAN.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol 2005;18:28–36. [DOI] [PubMed] [Google Scholar]
- 2.Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain: an autoradiographic study. Brain Res 1991;555:220–232. [DOI] [PubMed] [Google Scholar]
- 3.Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA. Immunohistochemical localization of oxytocin receptors in human brain. Neuroscience 2013;253:155–164. [DOI] [PubMed] [Google Scholar]
- 4.Jesso S, Morlog D, Ross S, et al. The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain 2011;134:2493–2501. [DOI] [PubMed] [Google Scholar]
- 5.Finger EC, MacKinley J, Blair M, et al. Oxytocin for frontotemporal dementia: a randomized dose-finding study of safety and tolerability. Neurology 2015;84:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamminga J, Kumfor F, Burrell JR, Piguet O, Hodges JR, Irish M. Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: an examination of clinical characteristics and emotion processing. J Neurol Neurosurg Psychiatry 2015;86:1082–1088. [DOI] [PubMed] [Google Scholar]
- 7.Oliver LD, Mitchell DG, Dziobek I, et al. Parsing cognitive and emotional empathy deficits for negative and positive stimuli in frontotemporal dementia. Neuropsychologia 2015;67:14–26. [DOI] [PubMed] [Google Scholar]
- 8.Dimberg U, Thunberg M. Empathy, emotional contagion, and rapid facial reactions to angry and happy facial expressions. Psych J 2012;1:118–127. [DOI] [PubMed] [Google Scholar]
- 9.Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage 2005;25:312–319. [DOI] [PubMed] [Google Scholar]
- 10.Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage 2007;34:1744–1753. [DOI] [PubMed] [Google Scholar]
- 11.Virani K, Jesso S, Kertesz A, Mitchell D, Finger E. Functional neural correlates of emotional expression processing deficits in behavioural variant frontotemporal dementia. J Psychiatry Neurosci 2013;38:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall CR, Hardy CJD, Russell LL, et al. The functional neuroanatomy of emotion processing in frontotemporal dementias. Brain 2019;142:2873–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soussignan R. Duchenne smile, emotional experience, and autonomic reactivity: a test of the facial feedback hypothesis. Emotion 2002;2:52–74. [DOI] [PubMed] [Google Scholar]
- 14.Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA 2003;100:5497–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron 2003;40:655–664. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman ML. Empathy and Moral Development Implications for Caring and Justice. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 17.Bavelas JB, Black A, Lemery CR, Mullett J, editors. Motor Mimicry as Primitive Empathy. New York: Cambridge University Press; 1987. [Google Scholar]
- 18.Marshall CR, Hardy CJD, Russell LL, et al. Motor signatures of emotional reactivity in frontotemporal dementia. Sci Rep 2018;8:1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua AY, Sible IJ, Perry DC, et al. Enhanced positive emotional reactivity undermines empathy in behavioral variant frontotemporal dementia. Front Neurol 2018;9:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Coster L, Mueller SC, T'Sjoen G, De Saedeleer L, Brass M. The influence of oxytocin on automatic motor simulation. Psychoneuroendocrinology 2014;50:220–226. [DOI] [PubMed] [Google Scholar]
- 21.Korb S, Malsert J, Strathearn L, Vuilleumier P, Niedenthal P. Sniff and mimic: intranasal oxytocin increases facial mimicry in a sample of men. Horm Behav 2016;84:64–74. [DOI] [PubMed] [Google Scholar]
- 22.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 25.Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR. Clinical staging and disease progression in frontotemporal dementia. Neurology 2010;74:1591–1597. [DOI] [PubMed] [Google Scholar]
- 26.Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci 1997;24:29–36. [DOI] [PubMed] [Google Scholar]
- 27.Mendez MF, McMurtray A, Licht E, Shapira JS, Saul RE, Miller BL. The scale for emotional blunting in patients with frontotemporal dementia. Neurocase 2006;12:242–246. [DOI] [PubMed] [Google Scholar]
- 28.Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog Selected Documents Psychol 1980;10:85. [Google Scholar]
- 29.Striepens N, Kendrick KM, Hanking V, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep 2013;3:3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proc Natl Acad Sci USA 2012;109:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PLoS One 2014;9:e103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman SM, Samineni S, Allen PC, et al. Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology 2016;66:185–194. [DOI] [PubMed] [Google Scholar]
- 33.Lee MR, Scheidweiler KB, Diao XX, et al. Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay. Mol Psychiatry 2018;23:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leslie KR, Johnson-Frey SH, Grafton ST. Functional imaging of face and hand imitation: towards a motor theory of empathy. NeuroImage 2004;21:601–607. [DOI] [PubMed] [Google Scholar]
- 35.Kring AM, Sloan DM. The Facial Expression Coding System (FACES): development, validation, and utility. Psychol Assess 2007;19:210–224. [DOI] [PubMed] [Google Scholar]
- 36.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 37.Dziobek I, Rogers K, Fleck S, et al. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET). J Autism Dev Disord 2008;38:464–473. [DOI] [PubMed] [Google Scholar]
- 38.Mozaz M, Rothi LJ, Anderson JM, Crucian GP, Heilman KM. Postural knowledge of transitive pantomimes and intransitive gestures. J Int Neuropsychol Soc 2002;8:958–962. [DOI] [PubMed] [Google Scholar]
- 39.Sturm VE, Sible IJ, Datta S, et al. Resting parasympathetic dysfunction predicts prosocial helping deficits in behavioral variant frontotemporal dementia. Cortex 2018;109:141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grace SA, Rossell SL, Heinrichs M, Kordsachia C, Labuschagne I. Oxytocin and brain activity in humans: a systematic review and coordinate-based meta-analysis of functional MRI studies. Psychoneuroendocrinology 2018;96:6–24. [DOI] [PubMed] [Google Scholar]
- 41.Wang D, Yan X, Li M, Ma Y. Neural substrates underlying the effects of oxytocin: a quantitative meta-analysis of pharmaco-imaging studies. Soc Cogn Affect Neurosci 2017;12:1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wigton R, Radua J, Allen P, et al. Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. J Psychiatry Neurosci 2015;40:E1–E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci 2008;28:6607–6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grinevich V, Stoop R. Interplay between oxytocin and sensory systems in the orchestration of socio-emotional behaviors. Neuron 2018;99:887–904. [DOI] [PubMed] [Google Scholar]
- 45.Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev 2011;35:903–911. [DOI] [PubMed] [Google Scholar]
- 46.Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist 2011;17:18–24. [DOI] [PubMed] [Google Scholar]
- 47.Leigh R, Oishi K, Hsu J, et al. Acute lesions that impair affective empathy. Brain 2013;136:2539–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 2009;132:617–627. [DOI] [PubMed] [Google Scholar]
- 49.Klimecki OM, Leiberg S, Ricard M, Singer T. Differential pattern of functional brain plasticity after compassion and empathy training. Soc Cogn Affect Neurosci 2014;9:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Said CP, Moore CD, Norman KA, Haxby JV, Todorov A. Graded representations of emotional expressions in the left superior temporal sulcus. Front Syst Neurosci 2010;4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finger E, Berry S, Cummings J, et al. Adaptive crossover designs for assessment of symptomatic treatments targeting behaviour in neurodegenerative disease: a phase 2 clinical trial of intranasal oxytocin for frontotemporal dementia (FOXY). Alzheimers Res Ther 2018;10:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu X, Gerraty RT, Grinband J, Parker D, Razlighi QR. Brain atrophy can introduce age-related differences in BOLD response. Hum Brain Mapp 2017;38:3402–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pur DR, Eagleson RA, de Ribaupierre A, Mella N, de Ribaupierre S. Moderating effect of cortical thickness on BOLD signal variability age-related changes. Front Aging Neurosci 2019;11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available because written consent for data sharing was not obtained and data contain information (face videos) that could compromise the privacy of research participants.