Abstract
Background:
Results from the Systolic Blood Pressure Intervention Trial (SPRINT) showed that intensive systolic blood pressure (SBP) control significantly reduced the occurrence of mild cognitive impairment, but not dementia. This planned analysis of a subgroup of participants examined the effects of intensive SBP lowering on specific cognitive functions.
Methods:
SPRINT was an open-label randomized clinical trial at 102 sites in the United States and Puerto Rico of adults aged 50 years or older with SBP > 130 mmHg, but without diabetes, history of stroke or dementia. (ClinicalTrials.gov, NCT01206062). Participants were randomized (1:1) to an SBP goal of <120 mmHg (intensive treatment) versus <140 mmHg (standard treatment). All major classes of antihypertensive agents were included. A subgroup of the randomly assigned participants (target 30%) was then randomly selected, subject to study design constraints, for a concurrent cognitive function sub-study. Each individual was administered concurrently both a screening and extended cognitive battery at baseline and during the planned 4-year follow-up. The primary outcomes for this sub-study were standardized composite scores for memory (consisting of Logical Memory tests I and II, Modified Rey-Osterreith Complex Figure test immediate recall and Hopkins Verbal Learning Test-Revised delayed recall) and processing speed (consisting of the Trail Making Test and Digit Symbol Coding test).
Findings:
From November 23, 2010 through December 28, 2012, 2921 participants (mean age 68·4 years [SD 8·6], 1080 [37%] women) who had been randomly assigned in the SPRINT study were enrolled in the sub-study (1448 intensive treatment, 1473 standard treatment). Over a median follow-up of 4·1 years (interquartile range 3·7 to 5·8 years), there were no between-group differences in memory, with annual mean standardized domain score declines of −0·005 (95% CI −0·010, 0·001) and −0·001 (95% CI −0·006, 0·005) in the intensive and standard treatment groups, respectively (between-group difference −0·004, 95% CI −0·012, 0·004, p=0·33). Declines for the standardized processing speed domain scores were slightly greater with intensive treatment (between-group difference −0·010, 95% CI −0·017, −0·002, p=0·02), with annual declines of −0·025 (95% CI −0·030, −0·019) and −0·015 (95% CI −0·021, 0·009) for the intensive and standard treatment groups, respectively.
Interpretation:
Intensive SBP treatment, as compared to standard treatment, did not result in clinically relevant change for memory or processing speed in a subgroup of SPRINT participants. The impact of blood pressure lowering may not be evident in particular domains of cognitive function, but rather distributed across multiple domains.
BACKGROUND
Hypertension affects over 1 billion people worldwide1 and is a risk factor for cognitive decline, cerebrovascular disease and dementia.2,3 Reducing vascular risk factors and hypertension, in particular, could markedly reduce the incidence of cognitive impairment.4 Results from the Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that targeting a systolic blood pressure (SBP) of <120 mmHg (intensive treatment) did not significantly reduce the occurrence of probable dementia, the primary outcome, but did significantly reduce the occurrence of mild cognitive impairment (MCI), a risk factor for dementia (secondary outcome),5 and was associated with smaller increases in white matter lesion volumes, compared with a standard treatment target of <140 mmHg (standard treatment).6 It is unknown whether intensive SBP control preferentially impacts particular cognitive functions such as memory, processing speed, executive function, or language as compared to higher SBP targets. Poorer processing speed and executive function have been associated with hypertension,7,8 but, randomized trials examining the effects of blood pressure lowering with antihypertensive medications on cognitive function have produced inconsistent, even contradictory results.9,10 Understanding whether intensive SBP control has specific cognitive effects could reveal important preclinical markers and help elucidate neuropathological mechanisms underlying cognitive impairment. The present analysis focuses on a subgroup of SPRINT participants that was administered comprehensive cognitive assessments throughout follow-up. We evaluated the effect of intensive treatment, as compared to standard treatment, on key cognitive domains including memory and processing speed (primary outcomes), individual tests (secondary outcomes) and language, executive function, and global cognitive function domains (exploratory outcomes).
METHODS
Study Design
The design and methodology of SPRINT have been described previously.11 Briefly, it was a multicenter randomized clinical trial that compared intensive SBP lowering treatment (target of <120 mmHg) to a standard treatment group (target SBP of <140 mmHg) in older adults with hypertension who were at high risk for cardiovascular disease without a history of stroke, diabetes, or heart failure. The primary outcome was first occurrence of cardiovascular events or death. Secondary outcomes included all-cause mortality, decline in kidney function or development of end-stage renal disease, incident dementia and mild cognitive impairment, decline in cognitive function, and small-vessel cerebral ischemic disease. All participants were to be administered a cognitive screening battery (described below) at baseline, year 2, year 4, and study closeout if it occurred >1 year after the year 4 assessment (Supplemental Figure 1, page 19). Participants screening positive for potential cognitive impairment then received an extended cognitive battery (described below) and additional assessments which were used to adjudicate cognitive impairment (probable dementia or mild cognitive impairment) by experienced clinicians masked to treatment assignment.5 The cognitive function subgroup, the focus of the present analysis, received both the screening and extended cognitive batteries at each time point regardless of screening results, but were only adjudicated for cognitive impairment on the basis of a positive screening result.
On August 20, 2015, the Director of the National Heart, Lung, and Blood Institute accepted the Data and Safety Monitoring Board recommendation to inform the investigators and participants of the cardiovascular results after analyses of the primary outcome (composite of cardiovascular events) exceeded the monitoring boundary at two consecutive time points, thus initiating the process to end the protocol-driven BP intervention. Many of the planned year 4 cognitive assessments had not been completed as of this date, and so were completed at a final study closeout visit while the trial was still providing medication at no cost to the participant. After the closeout visit, medications were no longer provided by the study. An extended follow-up visit, which included another cognitive assessment, was conducted between October 10, 2017 and June 18, 2018.
Participants
Participants were ≥50 years of age and had a systolic BP between 130 and 180 mmHg at the screening visit. They were considered to have an increased cardiovascular risk if they had clinical or subclinical cardiovascular disease, chronic kidney disease (estimated glomerular filtration rate <60 ml/min/1·73m2) or a Framingham CVD risk score ≥ 15%, or if they were ≥75 years old. Individuals residing in a nursing home, or with a diagnosis of dementia (based on medical record review) or treated with medications primarily used for dementia were excluded. Race and ethnicity were collected via self-report using fixed categories prescribed by the National Institutes of Health Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research (https://grants.nih.gov/grants/funding/women_min/guidelines.htm). All participants provided written informed consent.
At the SPRINT randomization visit, a subset of participants (target size = 2800) was randomly selected by a statistician at the Data Coordinating Center for participation in the cognitive function sub-study to assess effects of treatment assignment on specific cognitive functions. It was not a simple random sampling scheme in that the probability of selection varied by clinic site, with higher probabilities of selection set for clinic sites that had access to a magnetic resonance imaging (MRI) scanner and participants enrolled in a nested MRI sub-study6 to facilitate relating cognitive function to imaging results. Remaining participants were selected without additional inclusion or exclusion criteria based on selection probabilities that varied both by site and across time during the course of recruitment in order to meet the target of 2800 participants. For example, selection probabilities were set generally higher at the beginning of recruitment, and then were reduced over time as target sample size was approached. Since the sampling scheme was not a simple random one, the cognitive function subgroup was not presumed to be representative of the full trial cohort.
Randomization and Masking
All participants in the trial were randomized by a statistician at the Data Coordinating Center at Wake Forest University School of Medicine (1:1) to either intensive treatment or standard treatment. The randomization used permuted random blocks (random selection of block lengths of 2, 4, and 6) and was stratified by clinic site. Because the trial was open-label, cognitive assessors were not masked to treatment group assignment.
Procedures
The treatment algorithms and formulary for SPRINT are listed in the study protocol (SPRINT Protocol. SPRINT Protocol. 2012. Available from: URL: https://www.sprinttrial.org/public/Protocol_Current.pdf). Briefly, all major classes of antihypertensive agents were included in the formulary and were provided at no cost to participants. Following randomisation, participants baseline antihypertensive regimens were adjusted per study group assignment. Dose adjustment was based on the mean of three standardized blood pressure measurements. Participants were seen monthly for the first 3 months and every 3 months thereafter. Enrollment occurred between November 23, 2010 and December 28, 2012, and follow-up continued through June 18, 2018. The trial (which included participation in this subgroup) was approved by the institutional review board at each participating site and each participant provided informed consent prior to randomisation.
At baseline and during follow-up, all participants were administered a cognitive screening battery: Montreal Cognitive Assessment (MoCA),12 Logical Memory (LM) I and II,13 and Digit Symbol Coding (DSC).14 All participants in the cognitive sub-study also were administered concurrently an extended battery that included the Hopkins Verbal Learning Test-Revised (HVLT-R),15 Modified Rey-Osterreith Complex Figure (mROCF),16 the 15-item Boston Naming Test (BNT-15),17 Category Fluency–Animals (CF-A),18 Trail Making Test (TMT, Parts A and B),19 and Digit Span (DS),20 (Supplemental Table 1, page 12). Centrally trained and certified examiners administered all cognitive tests following standardized procedures. They were administered in either English or Spanish, depending on the participant’s preferred language. All exams were audio-recorded and at least one case from each of the 102 sites was selected by coordinating center staff and reviewed for quality control.
Outcomes
For all tests except the TMT, higher scores indicate better performance, therefore, TMT scores were transformed to speeds (score = 1/time in seconds). Each test was standardized by subtracting the baseline median from the raw score and then dividing by the baseline interquartile range. Composite domain scores were calculated by averaging the standardized scores of component tests, followed by a further standardization so that all domain scores had similar scales. Two domains were pre-specified in the trial protocol: Memory, composed of LM I and II, mROCF immediate recall, and HVLT-R delayed recall; and Processing Speed, composed of TMT-Parts A and B and DSC. We also examined individual test scores as part of secondary analyses. We conducted exploratory analyses of three domains: Language, composed of BNT-15 and CF-A; Executive Function, composed of TMT – Part B minus Part A and DS; and Global Cognitive Function (GCF), composed of all tests included in all domain scores and separately the MoCA total (see Supplemental Table 1, page 12).
Sex, age, education, race/ethnicity, smoking history, and comorbidities were assessed with standardized questions asked at the randomisation visit. Study staff documented medications at each clinic visit. Depressive symptoms were measured with the Patient Health Questionnaire (PHQ-9),21 administered with the cognitive tests.
Statistical Analysis
Sample size considerations for the cognitive function sub-study were informed by data from the Action to Control Cardiovascular Risk in Diabetes22 and Gingko Evaluation of Memory23 trials included tests similar to several of the cognitive tests in the SPRINT battery. Assuming 3% loss to follow-up per year, 2800 total participants was estimated to provide 90% power to detect a standardized effect size of 0.132 (between-group difference / SD). Linear mixed-effect models were used to compare longitudinal change in domain scores between the treatment groups. Models included random effects for participant and clinic site to account for longitudinal assessments and correlations between participants at the same clinic site. Primary analyses quantified the change in each domain score assuming a linear annual slope. For graphical purposes and for evaluating the suitability of the assumption of linear (group) change over time, we also fit models that flexibly modeled the effect of time since randomization using B-splines with two internal knots at 2 and 4 years of follow-up. Analyses of secondary outcomes (individual cognitive tests) were based on robust linear mixed models that are fully described in the Supplemental Files (page 10). We conducted subgroup analyses by age (<65 years, 65 to <75 years, 75 to <80 years, and ≥80 years) for primary and exploratory cognitive domains. Finally, we used multiple imputation to examine the influence of missing data and used robust linear mixed models to examine the effect of intensive treatment on the individual cognitive tests, the details of which are described in the Appendix. No adjustment was made for multiple comparisons. All analyses were performed using SAS version 9.4 (SAS Institute Inc.) and the R Statistical Computing Environment (http://www.r-project.org).
Role of the Funding Source
SPRINT was funded by the National Institutes of Health (including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke), which contributed to the design and conduct of the study, interpretation of the data, and review of the manuscript, but not data collection or analysis or the decision to submit the manuscript for publication. The Alzheimer’s Association provided financial support, but participated in no decisions related to the study. Dr. Pajewski had full access to all the data in the study and takes final responsibility for the integrity of the data and the accuracy of the data analysis.
RESULTS
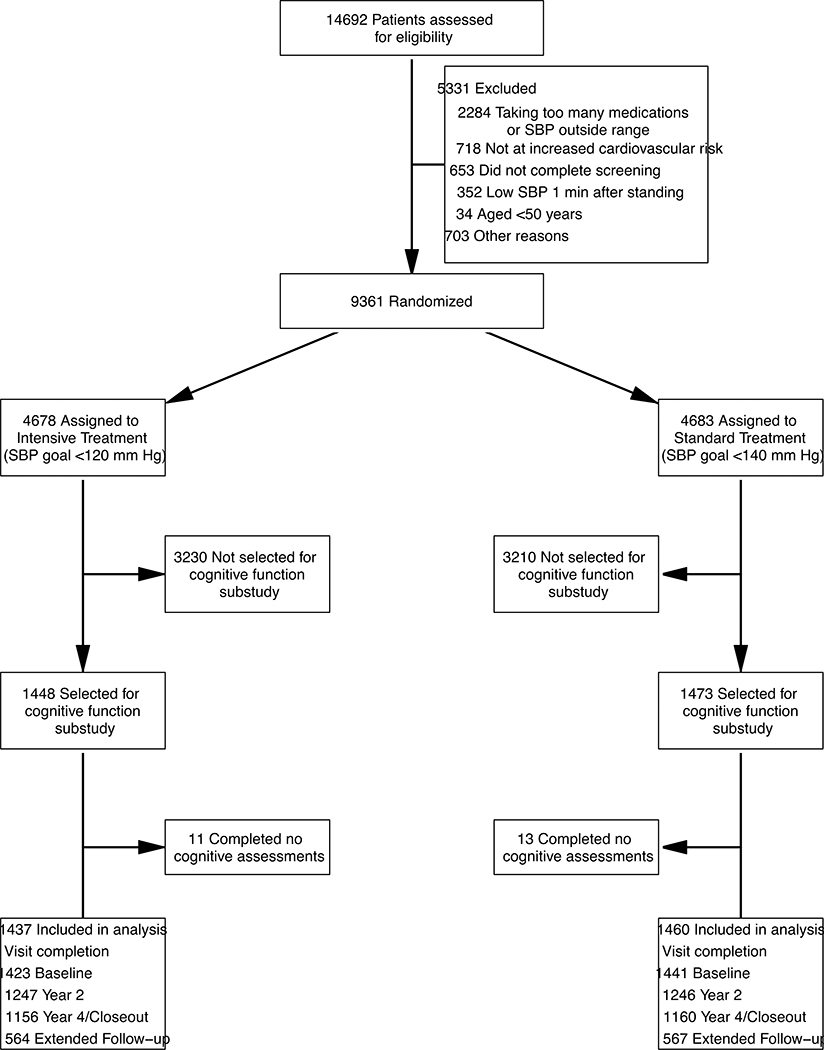
Of 9361 randomized participants, 2921 (31%) (Figure 1) were included in the cognitive function sub-study. Baseline demographics and characteristics for this subgroup are shown in Table 1. The mean age was 68·4 years (SD, 8·6 years), with 27% of participants aged 75 years or older. Participants were 37% female, 30% black, and 9% Hispanic. The mean SBP at baseline was 138·7 mmHg (SD: 16·0 mmHg). Median scores on the MoCA, LM I/II, and DSC were 23, 20/8, and 51, respectively (Table 2). Compared with remaining participants in the trial, cognitive function sub-study participants were on average slightly older, though fewer were ≥ 75 years of age, more likely to be white, had lower baseline BP, and lower estimated glomerular filtration rate. There were no differences on the MoCA, LM I/II or DSC (Supplemental Table 2, page 13).
FIGURE 1.
Eligibility, Randomization, and Follow-up for Participants in the Cognitive Function Sub-study
TABLE 1.
Baseline Characteristics of Participants in the Cognitive Function Sub-study
| Characteristics | Intensive Treatment No.=1448 | Standard Treatment No.=1473 |
|---|---|---|
| Age, mean (SD), years | 68·6 (8·5) | 68·3 (8·7) |
| Age ≥ 75 years | 398 (27%) | 383 (26%) |
| Female sex | 537 (37%) | 543 (37) |
| Race/ethnicity | ||
| White | 863 (60%) | 864 (59%) |
| Black | 418 (29%) | 460 (31%) |
| Hispanic | 132 (9%) | 121 (8%) |
| Other | 35 (2%) | 28 (2%) |
| Education | ||
| <High school education | 123 (9%) | 132 (9%) |
| High school graduate | 239 (17%) | 245 (17%) |
| Post high-school training | 526 (37%) | 497 (34%) |
| College graduate or higher | 555 (39%) | 598 (41%) |
| PHQ-9 score≥10a | 109 (8%) | 113 (8%) |
| Smoking status | ||
| Current smoker | 189 (13%) | 169 (12%) |
| Former smoker | 615 (43%) | 661 (45%) |
| Never smoker | 639 (44%) | 639 (44%) |
| Systolic blood pressure, mean (SD), mmHg | 138·6 (16·1) | 138·8 (15·9) |
| Diastolic blood pressure, mean (SD), mmHg | 77·3 (11·6) | 77.4 (11·8) |
| Orthostatic hypotensionb | 97 (6·7%) | 97 (6·6%) |
| History of cardiovascular disease | 283 (20%) | 301 (20%) |
| Estimated glomerular filtration rate (eGFR), mean (SD), ml/min/1.73 m2 c | 70·5 (20·7) | 71·3 (21·0) |
| eGFR<60 ml/min/1.73 m2 c | 442 (30·8%) | 439 (30·0%) |
| Urine albumin to creatinine ratio, median [IQR], mg/g | 9·9 [6·0 to 24·1] | 9·5 [5·6 to 21·7] |
| Body Mass Index, mean (SD), kg/m2 | 29·9 (5·7) | 29·8 (5·6) |
| No. of antihypertensive agents, mean (SD) | 1·9 (1·1) | 1·9 (1·1) |
| Use of statins | 609 (42%) | 660 (45%) |
| Use of aspirin | 758 (53%) | 744 (51%) |
SD denotes standard deviation, PHQ-9 Patient Health Questionnaire 9-item depression scale, IQR interquartile range.
Scores on the PHQ-9 range from 0 to 27, with higher scores indicating greater severity of depressive symptoms. Score of 10 or higher suggest moderate-to-severe depressive symptoms.
Defined as a standing systolic BP minus seated systolic BP ≤ −20 mmHg or a standing diastolic BP minus seated diastolic BP ≤ −10 mmHg
Based on the 4-variable Modification of Diet in Renal Disease equation.
Table 2.
Baseline cognitive test scores (secondary outcomes) and domain scores (primary and exploratory outcomes) by treatment group
| Domain or Test Score | Intensive Treatment | Standard Treatment |
|---|---|---|
| Primary Outcomes | Mean (SD) | Mean (SD) |
| Memory Domain | −0·07 (0·71) | −0·04 (0·71) |
| Processing Speed Domain | 0·01 (0·75) | 0·04 (0·74) |
| Secondary Outcomes | Median [IQR] | Median [IQR] |
| Montreal Cognitive Assessment (0 to 30) | 23 [21 to 26] | 23 [21 to 26] |
| Logical Memory I (0 to 28) | 20 [16 to 23] | 20 [16 to 23] |
| Logical Memory II (0 to 14) | 8 [6 to 11] | 8 [6 to 11] |
| Digit Symbol Coding (0 to 135) | 51 [41 to 60] | 51 [41 to 61] |
| HVLT-R Delayed Recall (0 to 12) | 6 [1 to 9] | 6 [2 to 9] |
| mROCF Immediate Recall (0 to 24) | 14·5 [11·0 to 18·0] | 14·5 [11·0 to 18·0] |
| Boston Naming-15 (0 to 15) | 13 [10 to 14] | 13 [10 to 14] |
| Category Fluency – Animals (0 to No Limit) | 17 [14 to 21] | 17 [14 to 21] |
| Trail Making Test - Part A (0 to 300 seconds) | 38 [30 to 50] | 38 [30 to 49] |
| Trail Making Test - Part B (0 to 300 seconds) | 99 [73 to 146] | 97 [71 to 140] |
| Trail Making Test – Part B minus Part A | 59 [37 to 98] | 55 [36 to 93] |
| Digit Span (0 to 32) | 16 [14 to 19] | 17 [14 to 20] |
| Exploratory Outcomes | Mean (SD) | Mean (SD) |
| Language Domain | −0·12 (0·76) | −0·08 (0·78) |
| Executive Function Domain | 0·07 (0·68) | 0·01 (0·69) |
| Global Cognitive Function-Composite | −0·07 (0·73) | −0·02 (0·73) |
Numbers in parenthesis denote maximum score on each test. IQR denotes interquartile range, SD standard deviation, HVLT-R Hopkins Verbal Learning Test – Revised and mROCF modified Rey-Osterreith Complex Figure. The memory composite outcome includes the Logical Memory I and II, mROCF Immediate Recall, and the HVLT-R Delayed Recall. The processing speed composite includes Trail Making Test Parts A and B and the Digit Symbol Coding. The language composite includes the Boston Naming Test and Category Fluency – Animals. The executive function composite includes the Trail Making Test Part B minus Part A and the Digit Span. The global cognitive function composite includes all tests except the Montreal Cognitive Assessment.
There was a sustained mean between-group difference in SBP (Supplemental Figure 2, page 20) of 15·2 mmHg (95% CI 12·8, 17·7 mmHg from randomization through the decision to stop the trial intervention (August 20, 2015), with a mean SBP of 120·1 mmHg (95% CI 118·5, 121·8 mmHg) in the intensive treatment group and 135·4 mmHg (95% CI 133·6, 137·2 mmHg) in the standard treatment group. This was slightly larger than the mean between-group difference for participants not in the sub-study (12·8 mmHg, 95% CI 11·6, 13·9). The between-group difference in SBP attenuated over time following cessation of study treatment. For example, during the extended follow-up visits (October 10, 2017 to June 18, 2018), the mean between-group SBP difference was reduced to 3·1 mmHg (95% CI, −2·5, 8·7 mmHg), attributable mainly to an increase in the mean SBP in the intensive treatment group to 132·2 mmHg (95% CI 128·4, 136·0 mmHg). (Supplemental Figure 2, page 20)
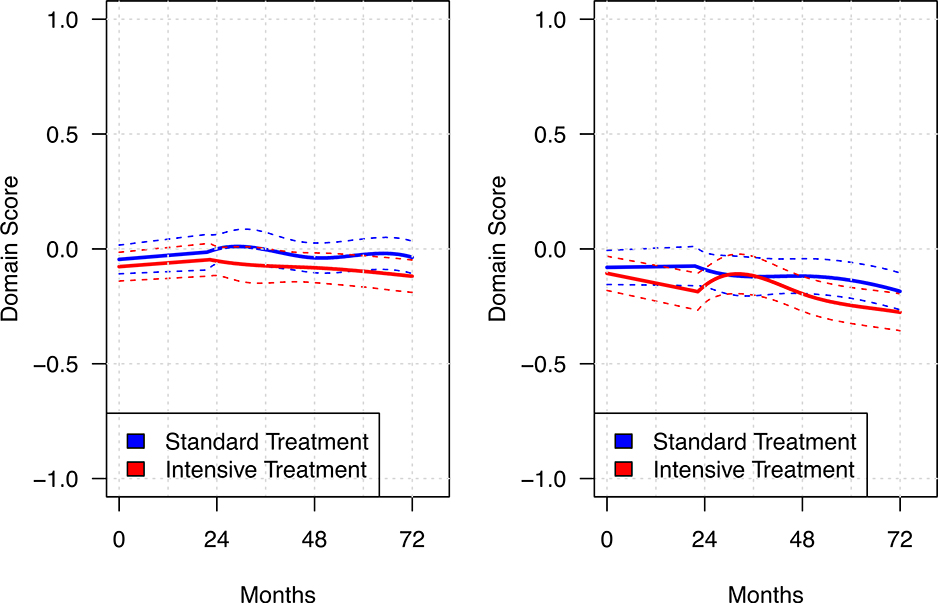
Figure 2 shows the estimated mean trajectories for the memory and processing speed, estimated using B-splines within a linear mixed model. Over a median follow-up of 4·1 years (interquartile range 3·7 – 5·8 years), there was very little change in either group for memory, with annual declines of −0·005 (95% CI −0·010, 0·001) and −0·001 (95% CI −0·006, 0·005) in the intensive and standard treatment groups, respectively (p = 0·33, Table 3). Declines for processing speed, though slightly greater compared to memory (confidence intervals for the slopes by group in each domain do not overlap), were small. Declines in the processing speed were statistically greater with intensive treatment (p = 0·02), with annual slopes of −0·025 (95% CI −0·030, −0·019) and −0·015 (95% CI −0·021, 0·009) for the intensive and standard treatment groups, respectively. Secondary analyses of the individual cognitive tests (Supplemental Table 3, page 15) indicated that only TMT-A showed significant between group differences. Mean times (untransformed) on the TMT-A increased (indicating poorer performance) by 0·43 seconds per year (95% CI 0·30, 0·57) in the intensive treatment group versus 0·12 seconds (95% CI 0·12, 0·26) per year in the standard treatment group. Thus, differences for the processing speed domain score appear largely attributable to scores on the TMT-A, with non-significant differences on DSC. Finally, there were small declines in the exploratory domains (Table 3), but no significant between-group differences for language (p = 0·84), executive function (p = 0·40), or global cognitive function-composite (p = 0·09).
FIGURE 2.
Change in memory and processing speed performance over the course of follow-up
Solid lines denote estimated mean for each treatment group based on B-splines within a linear mixed model. Dashed lines represent 95% confidence intervals.
TABLE 3.
Annual Change in Primary, Secondary and Exploratory Outcomes by Treatment Group
| Intensive Treatment Estimate (95% CI) | Standard Treatment Estimate (95% CI) | Mean Difference (95% CI) | P Value | |
|---|---|---|---|---|
| Primary Outcomes | ||||
| Memory Domain | −0·005 (−0·010, 0·001) | −0·001 (−0·006, 0·005) | −0·004 (−0·012, 0·004) | 0·33 |
| Processing Speed Domain | −0.025 (−0.030, −0.019) | −0·015 (−0·021, −0·009) | −0·010 (−0·017, −0·002) | 0·02 |
| Secondary Outcomes | ||||
| Montreal Cognitive Assessment (0 to 30) | −0·04 (−0·07, −0·01) | −0·04 (−0·07, −0·01) | 0·00 (−0·05, 0·04) | 0·95 |
| Logical Memory I (0 to 28) | 0·13 (0·09, 0·17) | 0·12 (0·08, 0·16) | 0·01 (−0·05, 0·06) | 0·79 |
| Logical Memory II (0 to 14) | 0·12 (0·09, 0·15) | 0·11 (0·08, 0·14) | 0·00 (−0·04, 0·05) | 0·84 |
| Digit Symbol Coding (0 to 135) | −0·43 (−0·51, −0·35) | −0·32 (−0·40, −0·24) | −0·11 (−0·22, 0·00) | 0·06 |
| HVLT-R Delayed Recall (0 to 12) | 0·08 (0·04, 0·12) | 0·12 (0·09, 0·16) | −0·05 (−0·10, 0·01) | 0·10 |
| mROCF Immediate Recall (0 to 24) | −0·47 (−0·52, −0·43) | −0·40 (−0·45, −0·36) | −0·07 (−0·13, −0·01) | 0·02 |
| Boston Naming Test-15 (0 to 15) | −0·01 (−0·02, 0·01) | −0·02 (−0·04, 0·00) | 0·01 (−0·01, 0·04) | 0·27 |
| Category Fluency - Animals (0 to No Limit) | −0·12 (−0·17, −0·08) | −0·13 (−0·17, −0·08) | 0·00 (−0·06, 0·07) | 0·95 |
| Trail Making Test - Part A (seconds) | 0·43 (0·30, 0·57) | 0·12 (−0·01, 0·26) | 0·31 (0·12, 0·50) | 0·001 |
| Trail Making Test - Part B (seconds) | 1·28 (0·80, 1·76) | 0·94 (0·46, 1·42) | 0·34 (−0·34, 1·02) | 0·33 |
| Trail Making Test - Part B minus Part A (seconds) | 0·84 (0·35, 1·33) | 0·62 (0·13, 1·10) | 0·23 (−0·46, 0·92) | 0·52 |
| Digit Span (0 to 32) | −0·02 (−0·05, 0·01) | −0·01 (−0·05, 0·02) | 0·00 (−0·05, 0·04) | 0·84 |
| Exploratory Outcomes | ||||
| Language | −0·014 (−0·019, −0·001) | −0·015 (−0·020, −0·009) | 0·001 (−0·007, 0·009) | 0·84 |
| Executive Function | −0·010 (−0·016, −0·003) | −0·006 (−0·012, −0·006) | −0·004 (−0·013, 0·005) | 0·40 |
| Global Cognitive Function | −0·016 (−0·021, −0·012) | −0·011 (−0·015, −0·006) | −0·006 (−0·012, 0·001) | 0·09 |
Estimates for primary and exploratory outcomes represent annual slope assuming linear change over time based on a linear mixed model. Estimates for secondary outcomes similarly assume linear change over time but are based on a robust formulation of a linear mixed model (See Supplemental Methods, page 4). CI denotes confidence interval, HVLT-R Hopkins Verbal Learning Test – Revised, and mROCF modified Rey-Osterreith Complex Figure.
To examine whether cessation of the study intervention influenced the findings, we compared groups sat the end of close-out when medications stopped being provided by the study in a post-hoc analysis. Results were largely unchanged with the exclusion of the extended follow-up visits (Supplemental Table 4, page 16). Results based on multiple imputation were largely consistent with results based on observed data (Supplemental Table 5, page 17). Subgroup analyses by age did not reveal consistent indications of differential treatment effects (Supplemental Table 6, page 18). We did observe nominally significant interactions (uncorrected for multiple comparisons) with respect to language (p = 0·03) and global cognitive function (p = 0·05), though there was not a clear pattern of effect with respect to increasing age. We also compared the occurrence of previously reported adjudicated outcomes (probable dementia or mild cognitive impairment) for participants in the cognitive function subgroup versus the remaining trial participants (Supplemental Figure 3, page 21). There was no evidence of differential effects with respect to probable dementia (interaction p-value = 0·96); both subgroups had fewer cases in the intensive treatment group (48 v. 56, respectively; Hazard Ratio = 0·85, 95t% CI 0·57 – 1·26) compared to the standard treatment group (101 v. 120; Hazard Ratio = 0·84, 95% CI 0·64, 1·10). However, cognitive function sub-study participants randomized to intensive treatment had a similar rate of MCI as compared to those randomized to standard treatment (107 v. 105 cases, respectively; Hazard Ratio = 1·08, 95% CI 0·82, 1·42), whereas, participants not in the cognitive function sub-study had fewer cases of MCI with intensive treatment (180 v 248 cases; Hazard Ratio = 0·72, 95% CI 0·59, 0·87) (interaction p-value = 0·03). A similar finding was seen with a composite outcome of either MCI or probable dementia (interaction p-value = 0·04). There was no evidence of a differential effect of treatment with respect to the incidence of cardiovascular events, all-cause mortality, and declines in kidney function between the cognitive function subgroup and those not in the cognitive function subgroup (Supplemental Figure 3, page 21).
DISCUSSION
In a subgroup of SPRINT participants administered a comprehensive cognitive battery repeatedly over a median follow-up of 4·1 years, there was no evidence that intensive SBP control had a beneficial or detrimental effect on memory, language, executive function, or global cognitive function. We did observe a slightly greater decline in processing speed in the intensive treatment group compared to the standard treatment group. This difference, while nominally significant, was small and largely attributable to a mean 0·31 second per year slower speed on the TMTA; a difference of doubtful clinical significance when one considers that TMT-A norms for older adults (69–71 years) have an interquartile range spanning 15 seconds (31 to 46).24 Across all domains, there was generally little change in the cognitive scores during follow-up for both treatment groups. Our results did not substantively change when we stratified analyses by age, or when follow-up was restricted to the timeframe when participants were being provided antihypertensive medications by the study.
Midlife hypertension, particularly untreated, is associated with an increased risk of cognitive decline and impairment in late life7. However, evidence on the impact of late-life hypertension on cognitive function has been inconsistent,7,25 with some arguing that it may be protective.26 A recent cohort study showed early and midlife hypertension and increases in BP measured prospectively over 33 years were positively associated with white matter hyperintensities, smaller total-brain volumes, and smaller hippocampal volumes, but not with amyloid-β status or cognitive function measured at ages 69–71 years.27 Randomized trials of antihypertensive treatment to improve cognitive function have also produced conflicting results.9,25,28 One review of sixteen studies of BP reduction and cognition found small improvements on measures of global cognitive function and memory, but a small detrimental effect on perceptual processing and learning.29 A Cochrane review of the effects of BP lowering in older patients without prior cerebrovascular disease on incident dementia and cognitive decline revealed no benefit when restricted to randomized trials,28 a result replicated in a more recent meta-analysis.30 In the Heart Outcomes Prevention Evaluation-3 randomized clinical trial, a subgroup of participants (mean age 74 yrs., 6% with diabetes mellitus) with ≥1 cardiovascular risk factor but without CVD or known cognitive impairment received either candesartan plus hydrochlorothiazide or placebo to lower BP (mean BP = 140/79). Despite a mean group difference in SBP at the end of treatment (median follow-up = 5·7 yrs.) of 6·0 mmHg favoring the candesartan/hydrochlorothiazide group, there were no group differences for cognitive measures of processing speed, executive function or global cognitive function.31 Thus, the present results are largely consistent with prior antihypertensive treatment studies in showing no clinically meaningful beneficial or detrimental impact of BP lowering on particular cognitive functions when measured as group means.
How well do the present findings align with the previously reported SPRINT results where intensive treatment resulted in a significantly lower incidence of MCI and a composite of MCI or probable dementia as compared to standard treatment?5 While finding a domain(s)-specific treatment effect in the present analysis might have been expected, there are several reasons why the present results need not be considered discordant with the adjudicated results. First, it is important to distinguish carefully adjudicated cognitive impairment outcomes from individual surrogates of cognitive function. In the trial, MCI and probable dementia were determined by an adjudication process in which experienced clinicians, masked to treatment group, reviewed cognitive test scores; information regarding health status, mood, sleep, functional abilities, and medications; hospitalizations; and informant-provided information on participant’s functional status. Moreover, to qualify as an occurrence of MCI for analyses, a participant had to be adjudicated as impaired (MCI or probable dementia) on two consecutive assessments.5 A single cognitive test or domain score is an incomplete surrogate for adjudicated cognitive impairment, much like left ventricular thickening or coronary calcification are surrogates for cardiovascular disease. Next, a classification of MCI or all-cause dementia can derive from deficits in any cognitive domain(s) and does not require a deficit in a particular cognitive domain. With multiple and diverse cognitive functions measured at each assessment point, one would be more likely to detect a deficit leading to an adjudication of MCI or dementia than if only a single function were assessed. Lastly, the adjudication process is highly selective of only individuals demonstrating a likely cognitive deficit, while the present analysis included all participants’ test scores. Thus, a treatment effect observed in participants experiencing cognitive deficit or decline could be diluted if the majority of participants did not show a deficit or decline. The present results suggest that cognitive deficits required for the previously reported adjudicated outcomes did not concentrate within a particular domain. This may be because hypertension affects multiple regions and systems in the brain and therefore produces heterogeneous cognitive effects.29
We also found evidence that the cognitive function sub-study participants were different from the remaining participants when we compared the effect of intensive treatment on incident MCI, with the protective effect of intensive treatment being driven by participants not in the cognitive function sub-study. It is not clear what may account for these subgroup differences as there were no baseline differences in traditional risk factors for cognitive decline including sex, education, depressive symptoms or cognitive test scores. Moreover, cognitive tests were administered to all participants following identical procedures and using an identical process for adjudication procedures. A ‘healthy volunteer’ effect is suggested by the fact that sub-study group at baseline had fewer participants aged ≥75 years, more women, more whites and fewer Hispanics, lower BP and only slightly worse kidney function than the rest of the trial cohort. The selection of participants in the cognitive function sub-study was not entirely random, as it was influenced by proximity to study sites with an MRI scanner and other selection priorities which may have affected its composition. And, we cannot rule out the influence of unmeasured factors.
It has been argued that lowering SBP in older adults could result in poorer cognitive function, because of resulting cerebral hypoperfusion.32 While we cannot address the hypoperfusion question directly, we found no evidence of a clinically meaningful detrimental effect of lower SBP on cognitive function compared to standard treatment. As noted, the nominally significant decline in processing speed in the intensive treatment group compared to standard treatment was small and not clinically meaningful and could be due to chance as our analyses were not adjusted for multiple comparisons.
This study is not without limitations. The cognitive function subgroup was not selected in a completely random fashion, which may have influenced our results. Subtle undetected differences in administration of tests cannot be ruled out, since cognitive assessors were not masked to treatment assignment. Early termination of the intervention was associated with some attenuation of the mean SBP group difference which could have reduced group differences. However, when we restricted the analysis to the period when treatment was provided, results did not change. Strengths of the study include a large sample size, the length of treatment and follow-up, the use of a comprehensive battery of validated cognitive measures, and the inclusion of multiple major domains of cognitive function each with multiple component measures.10
Supplementary Material
Research in Context.
Evidence before this study
PUBMED was searched between 1999 and August, 2019 with the terms ‘blood pressure’, ‘blood pressure lowering’ ‘cognitive’ and ‘cognition,’ alone and in combination and restricted to English. Considered were meta-analyses, systematic reviews, and individual reports of randomized clinical trials in which anti-hypertensive medications were compared to placebo or other antihypertensive meds to reduce blood pressure in older adults and that reported primary or secondary cognitive function endpoints. Prospective observational studies examining effect of antihypertensive medications on specific cognitive function(s) were also considered.
Studies of the effects of hypertension on cognitive functioning in older adults have produced convincing evidence of a negative association, particularly for midlife and uncontrolled hypertension. There is a lack of clarity about the effects of anti-hypertensive medication treatments on specific cognitive functions in later life, however, due in part to methodological limitations of prior studies such as small size, short treatment duration, and a limited range and depth cognitive measures. The Systolic Blood Pressure Intervention Trial produced results showing that adjudicated mild cognitive impairment is reduced in older patients treated with medications intensively to a goal of <120 mmHg compared to those treated to goal of <140 mmHg. That study enrolled a large number of participants between November 23, 2010 and December 28, 2012, who were treated over four years and a cognitive function sub-group was administered a comprehensive cognitive test battery repeatedly during follow-up through June 18, 2018. This provided the opportunity to determine whether the intensive treatment produced a protective or deleterious effect in specific cognitive domains (memory, processing speed, language, executive function, global cognitive function).
Added value of the study
In addition to examining global cognitive function, the most common cognitive outcome in prior studies, the SPRINT study measured specific treatment effects on memory, processing speed, language, executive function, and global cognitive function with at least two measures per domain measured three to four times over a mean follow-up of approximately four years in sub-group of SPRINT participants.
Implications of all the available evidence
Intensive pharmacological antihypertensive treatment to a goal of systolic blood pressure <120 mmHg may not produce change in a particular cognitive domain when compared with standard treatment (goal of <140 mmHg). This suggests hypertension affects multiple regions and systems in the brain and therefore produces heterogeneous cognitive effects.
Acknowledgments
Funding: National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Aging, National Institute of Neurological Disorders and Stroke and the Alzheimer’s Association.
Conflicts of Interest
All authors have received support for this work from the National Institutes of Health. Dr. Rapp reports grants from the National Institutes of Health during the conduct of this study; Ms. Gaussoin reports grants from NIH, during the conduct of the study; Dr. Auchus reports grants from University of Mississippi Medical Center, during the conduct of the study; Dr. Beddhu reports grants from Novo Nortis, grants from Bayer, grants from Boeringher Inghelheim, grants from NIDDK, grants from NHLBI, grants from VA, outside the submitted work; Dr. Chelune reports grants from University of Utah, during the conduct of the study, personal fees from International Neuropsychological Society (INS), personal fees from Psychological Assessment Resources, personal fees from Kessler Foundation, outside the submitted work, and he has a patent Copyright - Wisconsin Card Sorting Test with royalties paid to Psychological Assessment Resources; Dr. Krousel-Wood reports grants from National Institutes of Health, during the conduct of the study; Dr. Lerner reports grants from NIH during the conduct of the study; Dr. Martindale-Adams reports grants from National Institutes of Health, during the conduct of the study; Dr. Nichols reports grants from NIH, during the conduct of the study; Dr. Reboussin reports grants from NIH, during the conduct of the study; Dr. Rifkin reports grants from NIH, during the conduct of the study; Dr. Tamariz reports grants from NIH, during the conduct of the study; Dr. Wadley reports grants from NIH, during the conduct of the study; Dr. Whittle reports grants from National Institutes of Health, non-financial support from Department of Veterans Affairs, non-financial support from Takeda Pharmaceuticals International Inc, during the conduct of the study; Dr. Supiano reports grants from National Institutes of Health, grants from the Department of Veteran’s Affairs, during the conduct of the study; Dr. Miller reports grants from National Institutes of Health, during the conduct of the study; Dr. Williamson reports grants from National Institutes of Health, during the conduct of the study; Dr. Pajewski reports grants from National Institutes of Health, grants from Alzheimer’s Association, during the conduct of the study.
FINANCIAL DISCLOSURE
The Systolic Blood Pressure Intervention Trial was funded by the National Institutes of Health (including the National Heart, Lung, and Blood Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke) under contracts HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C and interagency agreement A-HL-13-002-001. It was also supported in part with resources and use of facilities through the Department of Veterans Affairs. Azilsartan and chlorthalidone (combined with azilsartan) were provided by Takeda Pharmaceuticals International Inc. Additional support was provided through the following National Center for Advancing Translational Sciences clinical and translational science awards: UL1TR000439 (awarded to Case Western Reserve University); UL1RR025755 (Ohio State University); UL1RR024134 and UL1TR000003 (University of Pennsylvania); UL1RR025771 (Boston University); UL1TR000093 (Stanford University); UL1RR025752, UL1TR000073, and UL1TR001064 (Tufts University); UL1TR000050 (University of Illinois); UL1TR000005 (University of Pittsburgh); 9U54TR000017-06 (University of Texas Southwestern Medical Center); UL1TR000105-05 (University of Utah); UL1 TR000445 (Vanderbilt University); UL1TR000075 (George Washington University); UL1 TR000002 (University of California, Davis); UL1 TR000064 (University of Florida); and UL1TR000433 (University of Michigan); and by National Institute of General Medical Sciences, Centers of Biomedical Research Excellence award NIGMS P30GM103337 (awarded to Tulane University). Additional support also provided by R01AG055606, K01HL133468 (Dr. Bress), K23NS107645 (Dr. Miller), the Wake Forest Claude Pepper Center (P30AG021332), and the Alzheimer’s Association. The authors not named individually in this section have nothing to declare beyond the support from NIH.
Footnotes
DATA SHARING
De-identified participant data and associated data dictionaries will be available in BioLinCC (https://biolincc.nhlbi.nih.gov/studies/sprint). Beginning 7/1/2020 data will be available for investigators providing an IRB/Ethics approval or certification of exemption from IRB/Ethics review, and also agreeing to the terms and conditions of a data use agreement.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017; 135(10): e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alber J, Alladi S, Bae HJ, et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement (N Y) 2019; 5: 107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003; 348(13): 1215–22. [DOI] [PubMed] [Google Scholar]
- 4.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014; 13(8): 788–94. [DOI] [PubMed] [Google Scholar]
- 5.SPRINT Research Group, Williamson JD, Pajewski NM, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019; 321(6): 553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SPRINT Research Group, Nasrallah IM, Pajewski NM, et al. Association of Intensive vs Standard Blood Pressure Control With Cerebral White Matter Lesions. JAMA 2019; 322(6): 524–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iadecola C, Yaffe K, Biller J, et al. Impact of Hypertension on Cognitive Function: A Scientific Statement From the American Heart Association. Hypertension 2016; 68(6): e67–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker KA, Sharrett AR, Wu A, et al. Association of Midlife to Late-Life Blood Pressure Patterns With Incident Dementia. JAMA 2019; 322(6): 535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birns J, Kalra L. Cognitive function and hypertension. J Hum Hypertens 2009; 23(2): 86–96. [DOI] [PubMed] [Google Scholar]
- 10.Elias MF, Torres RV, Davey A. Clinical Trials of Blood Pressure Lowering and Antihypertensive Medication: Is Cognitive Measurement State-of-the-Art? Am J Hypertens 2018; 31(6): 631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11(5): 532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53(4): 695–9. [DOI] [PubMed] [Google Scholar]
- 13.Wechsler D Wechsler Memory Scale-IV. Fourth ed. Hoboken, NJ: Pearson; 2009. [Google Scholar]
- 14.Wechsler D Wechsler Adult Intelligence Scale-IV. Fourth ed. Hoboken, NJ: Pearson; 2008. [Google Scholar]
- 15.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test Revised: Normative data and analysis of inter-form and test-retest reliability. Clinical Neuropsychologist 1998; 12(1): 43–55. [Google Scholar]
- 16.Becker JT, Boller F, Saxton J, Gonigle-Gibson KL. Normal rates of forgetting of verbal and non-verbal material in Alzheimer’s disease. Cortex 1987; 23(1): 59–72. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test, 2nd Ed. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 18.Strauss E, Sherman EMS, Spreen O, Spreen O. A compendium of neuropsychological tests : administration, norms, and commentary. 3rd ed. Oxford; New York: Oxford University Press; 2006. [Google Scholar]
- 19.Reitan R Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills 1958; 8: 271–6. [Google Scholar]
- 20.Wechsler D Wechsler Adult Intelligence Scale-III (WAIS-III). New York: Psychological Corporation/Harcourt, Inc; 1996. [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16(9): 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson JD, Launer LJ, Bryan RN, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med 2014; 174(3): 324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeKosky ST, Williamson JD, Fitzpatrick AL, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA 2008; 300(19): 2253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE token, WRAT-R reading, AMNART, STROOP, TMT, and JLO. Clinical Neuropsychologist 1996; 10(3): 262–78. [Google Scholar]
- 25.Walker KA, Power MC, Gottesman RF. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: a Review. Curr Hypertens Rep 2017; 19(3): 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corrada MM, Hayden KM, Paganini-Hill A, et al. Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study. Alzheimers Dement 2017; 13(2): 103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane CA, Barnes J, Nicholas JM, et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): an epidemiological study. Lancet Neurol 2019; 18(10): 942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev 2009; (4): CD004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birns J, Morris R, Donaldson N, Kalra L. The effects of blood pressure reduction on cognitive function: a review of effects based on pooled data from clinical trials. J Hypertens 2006; 24(10): 1907–14. [DOI] [PubMed] [Google Scholar]
- 30.Levi Marpillat N, Macquin-Mavier I, Tropeano AI, Bachoud-Levi AC, Maison P. Antihypertensive classes, cognitive decline and incidence of dementia: a network meta-analysis. J Hypertens 2013; 31(6): 1073–82. [DOI] [PubMed] [Google Scholar]
- 31.Bosch J, O’Donnell M, Swaminathan B, et al. Effects of blood pressure and lipid lowering on cognition: Results from the HOPE-3 study. Neurology 2019; 92(13): e1435–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu C, von Strauss E, Fastbom J, Winblad B, Fratiglioni L. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch Neurol 2003; 60(2): 223–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.