Abstract
While praziquantel mass drug administration is currently the most widely used method in the control of human schistosomiasis, it does not prevent subsequent reinfection hence persistent transmission. Towards schistosomiasis elimination, understanding the reinfection rate is crucial in planning for the future interventions. However, there is scarcity of information on the global reinfection rate of schistosomiasis. This systematic review and meta-analysis aimed at summarizing studies that estimated the reinfection rate of human schistosomiasis. Three data bases (PubMed, Hinari and Google Scholar) were thoroughly searched to retrieve original research articles presenting data on reinfection rate of human schistosomiasis. Study quality and risk of bias was assessed based on Joanna Briggs Institute critical appraisal checklist. Meta-analysis was conducted using statistical R version 3.6.2 and R Studio using “meta” and “metafor” packages. Random effect model was employed to estimate pooled reinfection rates. Heterogeneity was determined using Cochran’s Q (chi-square)–test and Higgins I2 statistics. A total of 29 studies met inclusion criteria to be included in this review. All studies had at least satisfactory (5–9 scores) quality. The overal mean and pooled reinfection rates of schistosomiasis were 36.1% (±23.3%) and 33.2% (95% CI, 26.5–40.5%) respectively. For intestinal schistosomiasis, the mean and pooled reinfection rates were 43.9% (±20.6%) and 43.4% (95% CI, 35.8–51.4%), and that for urogenital schistosomiasis were 17.6% (±10.8%) and 19.4% (95% CI, 12.3%– 29.2%) respectively. Cochran’s Q (chi-square)–test and Higgins I2 statistic indicated significant heterogeneity across studies (p-values < 0.001, I2 values > 95%). Results of subgroup analysis showed that, the type of Schistosoma species, participants’ age group, sample size and geographical area had influence on disparity variation in reinfection rate of schistosomiasis (p < 0.1). Despite the control measures in place, the re-infection rate is still high, specifically on intestinal schistosomiasis as compared to urogenital schistosomiasis. Achieving 2030 sustainable development goal 3 on good health and wellbeing intensive programmatic strategies for schistosomiasis elimination should be implemented. Among such strategies to be used at national level are repeated mass drug administration at least every six months, intensive snails control and health education.
Introduction
Human schistosomiasis is one of the neglected tropical diseases caused by trematodes of the genus Schistosoma. Human schistosomiasis occur in two forms known as intestinal schistosomiasis and urogenital schistosomiasis [1]. The main species causing intestinal schistosomiasis are; Schistosoma mansoni, Schistosoma japonicum, Schistosoma mekongi and Schistosoma intercalatum while urogenital schistosomiasis is caused by Schistosoma haematobium [2]. These species require fresh water snail for the development of an infective stage of the parasite which afterward infect people as they come into contact with water. Human acquire the infection during routine domestic, occupational, agricultural and recreational activities which expose them to infested water [3]. The groups at higher risk of acquiring schistosomiasis are pre-school aged children, school-aged children and people with occupations that involve contact with infested water such as irrigation workers, fishermen, farmers and women doing domestic chores in infested water [4].
The disease is more prevalent in tropical and subtropical countries particularly in poor communities where sanitation and water resources are of poor quality and inadequate [4, 5]. Poor water, sanitation and hygiene conditions such as the inadequate supply of clean and safe water, inadequate sanitation and unhygienic practices such as open defecation and urination play an important role in contamination of fresh water sources and therefore facilitate transmission of human schistosomiasis [6, 7].
Human schistosomiasis lead to hepatomegaly, splenomegaly, anaemia, kidney malfunction, stunting growth in children and reduce ability to learn in school/cognitive dysfunction in children [8, 9] The magnitude of human schistosomiasis varies across different areas with respect to time. In Sub-Saharan Africa alone, it was estimated that 436 million people in 78 countries live in endemic areas that put them at risk of urogenital schistosomiasis and over 112 million people were infected. For the case of intestinal schistosomiasis 393 million people were at the risk of infection and 54 million people were infected with more than 200,000 deaths [10].
The interventions such as preventive chemotherapy (praziquantel), snail control, health education and improvement of water supply and sanitation facilities have been going on in order to prevent and control schistosomiasis [11, 12]. Large scale use of preventive chemotherapy has been very useful in the reduction of morbidity and mortality due to schistosomiasis. However, schistosomiasis transmission is not yet interrupted by the current control measures [7, 12]. It has also been observed that treatment does not avert subsequent infection; thus, if contact with infested water is continued, re-infection can take place relatively quickly [13]. Several factors have been associated with the rapid reinfection rate of schistosomiasis such as age, gender, sex, pre-treatment intensity, level of immunity and water contact behaviour attributed to economic, domestic and recreational activities [14–16]. However the effects of these factors were shown to vary depending on the type of Schistosoma species and its geographical distribution. Studies on the reinfection rate of schistosomiasis have been documented in several parts of the world. Despite the available information on the factors contributing to the rapid reinfection rate of schistosomiasis, to our knowledge, there is no review which described the reinfection rate of schistosomiasis based on the evidence gathered from different parts of the world. The available article reviewed data on reinfection rate of Schistosoma japonicum only in China [16]. Hence, this study was designed to conduct a systematic review, with meta-analysis, of studies that estimated reinfection rate of human schistosomiasis globally. A clear understanding of the schistosomiasis reinfection rate is crucial for planning effective and sustainable strategies to control schistosomiasis transmission.
Materials and methods
Search strategy
The protocol for this systematic and meta-analysis was prepared to guide authors throughout the process (S1 File). However, registration was not sought. From 30th to 31th of August 2019, we conducted a thoroughly literature search of the following electronic databases; PUBMED, HINARI and Google Scholar. To obtain study articles containing information of our interest, the words Schistosomiasis, Schistosoma mansoni, Schistosoma haematobium, Schistosoma japonicum and reinfection joined using OR and AND booleans were used to create search query. Search string for each database is presented in S1 Table. We limited our search to journal articles only. There was no limitation of language of publication, year of publication and area where study was conducted. Search results from the three databases were combined together for selection process. The bibliographies of the selected articles were manually searched for the presence of other review related articles.
Study eligibility criteria
We included all studies presenting original research work with the following characteristics. Population: articles must have presented original research work conducted on human participants of any age, sex, race and from any geographical area. Intervention: the articles must have indicated that before follow-up, the study participants were diagnosed with any of human Schistosoma species and treated till cured. However there was no limitation on the type of drug, number of doses used and time interval from treatment to cure. Outcome: our outcome of interest was reinfection rate of human schistosomiasis. The included articles must have presented data on the reinfection rate with any Schistosoma species. According to our definition, reinfection rate means the proportion of egg-positive participants who get cured (egg-negative by microscopic techniques) and then become egg-positive after a certain specified period of time. We excluded articles on experimental and non human studies, review articles, letter to editors and articles which did not report on reinfection rate as per our definition.
Study selection and data extraction
After combining search results from the three databases, we removed all duplicates. Reviewer AZ screened all searched articles based on the titles and abstracts for their eligibility to be included in full text review. Articles eligible for full text review were retrieved. All accessed full text articles were reviewed by two independent reviewers (AZ and VM) for the eligibility to be included in data extraction. Matched articles selected by the two reviewers were subjected to data extraction; whenever there was mismatch the third reviewer (TM) was involved. Data were independently extracted by the same two reviewers (AZ and VM) using a designed data extraction form. Prepared data extraction form was pre-tested by three reviewers before it had been used. When there was difference in data extracted by the two reviewers from similar article, the third reviewer (TM) was invited to independently extract the data. Similar data extracted by either one of the first two reviewers and the third reviewer was taken. When the third reviewer came with different data, consensus was reached through discussion. Extracted data included publication details (first author’s name, journal name and year of publication), methodology (study design, country of study, year of data collection, description of study setting, characteristics of study population, diagnostic test, drug(s) and dose(s) used, and follow-up time) and results (identified Schistosoma species and reinfection rate)
Assessment of study quality and risk of bias
The study quality and risk of bias for selected studies were assessed based on 9 criteria as described in the Joanna Briggs Institute critical appraisal checklist for use in reviews of prevalence studies [17]. Each criterion was given either of the two options of YES if a criterion was met or NO if a criterion was not met. A YES option was graded as 1 and NO option as 0. The minimum score of 0 was given if all criteria were not met and maximum score of 9 was given if all criteria were met. Studies with overall grades ranging from 0–4 were considered of low quality, 5–7 moderate quality and 8–9 high quality. Studies with moderate to high quality were included in the review. Two reviewers (AZ and VM) independently assessed the quality of selected articles.
Data analysis
We used Statistical Package for Social Sciences version 24 (IBM Corp., Armonk, NY, USA) and statistical R version 3.6.2 (R Studio using “meta” and “metafor” packages) to perform descriptive statistical tests and meta-analysis respectively. The random effect model (with the options of log transformation and restricted maximum likelihood method) was used to calculate pooled reinfection rates of schistosomiasis and the degree of heterogeneity with their corresponding 95% confidence intervals. We used random effect model because our meta-analysis included large number of studies (large meta-analysis), therefore we expected between-study variances. Subsets of the data were used to calculate the pooled reinfection rates of schistosomiasis (with corresponding 95% confidence intervals) based on the two main forms of schistosomiasis (intestinal and urogenital schistosomiasis). Visual inspection of forest plots was used to assess any indicator of the presences of heterogeneity. We employed Cochran’s Q (chi-square) test to identify the presence of any significant heterogeneity (significant at p < 0.1); Higgins inconsistency statistic (I2) was used to estimate the proportion of between-studies variability. I2 value above 50% was considered to imply substantial heterogeneity [18].
Subgroup analysis and publication bias
Subgroup analysis was performed to assess the effect of different subgroups (factors) that were considered as a potential source of heterogeneity. The factors assessed were; type of Schistosoma species, follow-up time, study setting, sample size, geographical area and age groups. We could not include type of drug used as very few studies have not used praziquantel during treatment. These factors were categorized as follows; Schistosoma species into three categories (Schistosoma haematobium, Schistosoma mansoni and Schistosoma japonicum), follow-up time into two categories (less than 12 months and 12 months and above), study setting into two categories (school based studies and community based studies), geographical area into two categories (Africa and Non Africa), age groups into two categories (less than 16 years and all ages), and sample size (small; n < 100, moderate; n = 100 to 499 and large; n ≥ 500). Univariate subgroup analysis was performed for each factor. Factors with significant heterogeneity (p < 0.1) were subjected to multivariate subgroup analysis. The R2 (amount of heterogeneity accounted by each factor or the combination of all significant factors) were calculated. Summary reinfection rates of categories in each factor were also determined. Publication bias was evaluated using visual inspection of funnel plot and the degree of asymmetry of funnel plot was confirmed using Egger’s regression analysis.
Results
Search results and study selection
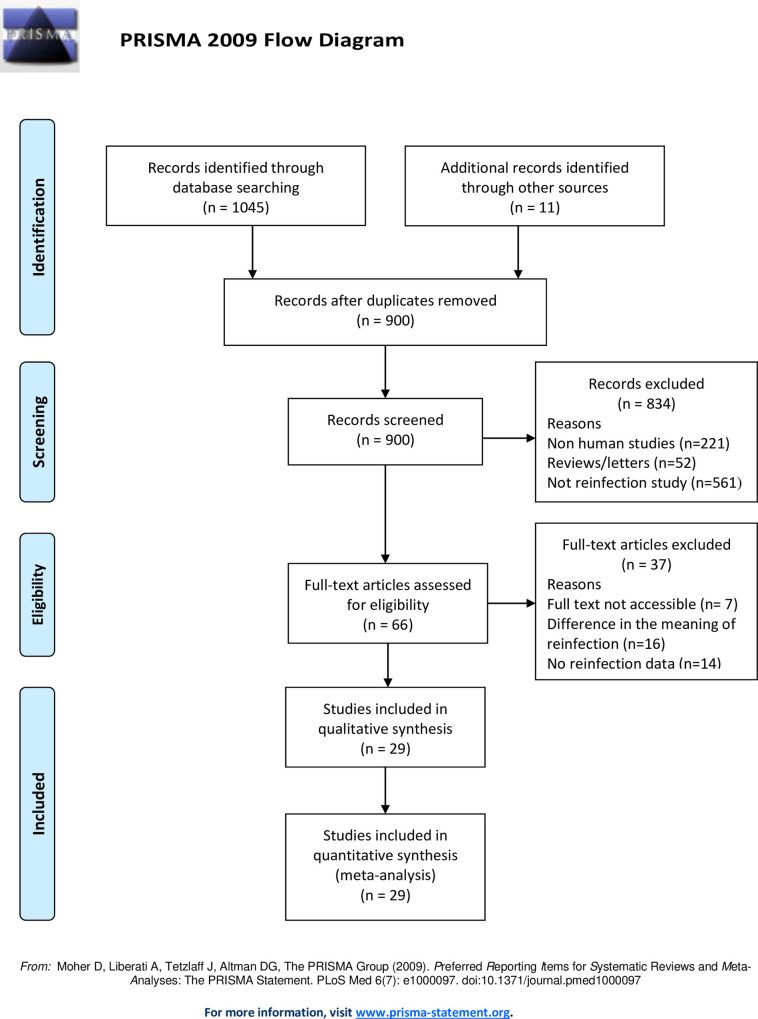
A total of 1045 search results were obtained from the three electronic databases. From these search results 145 duplicates were removed. After the primary screening of titles and abstracts, a total of 66 studies were eligible for full text review. Based on inclusion and exclusion criteria, 29 studies were found eligible for data synthesis process (Fig 1).
Fig 1. PRISMA flow diagram showing steps followed during study selection process for systematic review and meta-analysis.
Study quality and risk of bias assessment results
Study quality and risk of bias assessment results showed no low quality study, 9 studies scored 5–7 (moderate quality) and 20 studies scored 8–9 (high quality). The average score was 7.9 which indicate the overall moderate quality of included studies. According to the criteria used 21 (72.4%) studies had adequate sample sizes. All studies used valid laboratory diagnostic tests for the diagnosis of schistosomiasis (S2 Table).
Schistosomiasis reinfection
Studies characteristics
Twenty nine studies published between the year 1991 and 2019 were included for data synthesis. Out of 29 included studies 54 datasets on reinfection rate of schistosomiasis caused by three Schistosoma species were extracted. Of 29 studies, 10 studies reported on Schistosoma mansoni, 12 studies reported on Schistosoma haematobium, 4 studies reported on Schistosoma japonicum, 2 studies reported on Schistosoma mansoni and Schistosoma haematobium and 1 study reported on Schistosoma mansoni and Schistosoma japonicum. These studies were conducted in 14 countries. Eleven out of 14 countries are in Africa, 2/14 countries are in Asia and 1/14 countries is in South America. The follow-up time ranged from 3 to 156 months. The age of participants ranged from 1 to 67 years with all of studies involved both male and female populations. Praziquantel and Oxamniquine antischistosoma drugs were used to cure study participants prior to follow-up in 27 and 2 studies respectively (Table 1).
Table 1. Characteristics of included studies in systematic review and meta-analysis.
| Study | Country | Study design | Study Setting | Age (years) | Diagnostic Test | Treatment Drug | Follow up (months) |
|---|---|---|---|---|---|---|---|
| Schistosoma mansoni | |||||||
| de Lima e Costa et al., [19] | Brazil | PC | Community | ≥5 | Kato-katz | Oxamniquine | 156 |
| de Moira et al., [20] | Uganda | Longitudinal | Community | 5 to 66 | Kato-katz | Praziquantel | 12 |
| Egesa et al., [21] | Uganda | BF (no control) | Community | 6 to 40 | Kato katz | Praziquantel | 12 |
| Favre et al., [22] | Brazil | RCT | SC | 6 to 15 | Kato katz | Praziquantel | 12 |
| Garba et al., [23] | Niger | LC | Children | 6 to15 | Kato katz | Praziquantel | 6 and 12 |
| Gazzinelli et al., [24] | Brazil | Longitudinal | Children | 6 to 15 | Kato-katz | Praziquantel | 12 |
| Munishi et al., [25] | Tanzania | Longitudinal | SC | 6 to 16 | Kato katz | Praziquantel | 5 and 8 |
| Nalungwa et al., [26] | Uganda | RCT | PSAC | 1 to 5 | Kato katz | Praziquantel | 8 |
| Olliaro et al., [27] | Tanzania & Brazil | RCT | Children | 10 to 19 | Kato katz | Praziquantel | 6 and 12 |
| Reis et al., [28] | Brazil | RCT | Children | 7 to 18 | Kato-katz | Oxamniquine | 6 |
| Satti et al., [29] | Sudan | Longitudinal | Farmers | 25 to 55 | Kato-katz | Praziquantel | 12 |
| Woldegerima et al., [30] | Ethiopia | Follow-up | SC | 9 to 14 | Kato katz | Praziquantel | 6 |
| Schistosoma haematobium | |||||||
| Chandiwana et al., [31] | Zimbabwe | Cohort | Community | ≥2 | Filtration | Praziquantel | 3.5 |
| Friis et al., [32] | Zimbabwe | RCT | Children | 11 to 17 | Filtration | Praziquantel | 12 |
| Garba et al., [23] | Niger | LC | Children | 6 to15 | Filtration | Praziquantel | 6 and 12 |
| Gyoten et al., [33] | Kenya | BF (no control) | Community | All | Urinalysis | Praziquantel | 12 |
| Houmsou et al., [34] | Nigeria | Follow-up | Children | 1 to 15 | Filtration | Praziquantel | 12 |
| Kabuyaya et al., [35] | S. Africa | PC | SC | 10 to 15 | Filtration | Praziquantel | 5 and 7 |
| Lemos et al., [36] | Angola | Longitudinal | Children | 2 to 15 | Centrifugation | Praziquantel | 6 |
| Mutsaka-makuvaza et al., [37] | Zimbabwe | PC | PSAC | 0 to 5 | Filtration | Praziquantel | 12 |
| Mutapi et al., [38] | Zimbabwe | BF (no control) | Children | 6 to 15 | Filtration | Praziquantel | 9 |
| Mduluza et al., [39] | Zimbabwe | NA | Children | ≤5 | Filtration | Praziquantel | 24 |
| Ofoezie, [40] | Nigeria | NA | SC | 5 to 16 | Filtration | Praziquantel | 3, 6 and 12 |
| Senghor et al., [41] | Senegal | LC | SC | 5 to 15 | Filtration | Praziquantel | 6 |
| Senghor et al., [42] | Senegal | LC | Community | 5 to 60 | Filtration | Praziquantel | 7, 12 and 36 |
| Webster et al., [43] | Senegal | NA | Children | 3 to 15 | Filtration | Praziquantel | 6 and 12 |
| Schistosoma japonicum | |||||||
| Belizario et al., [44] | Philippines | RCT | SC | 10 to 19 | Kato katz | Praziquantel | 6 and 12 |
| Jiz et al., [45] | Philippines | Cohort | Community | 7 to 30 | Kato-katz | Praziquantel | 6 and 12 |
| Li et al., [46] | China | PC | Community | all | Kato-katz | Praziquantel | 24 |
| Olliaro et al., [27] | Philippines | RCT | Children | 10 to 19 | Kato katz | Praziquantel | 6 and 12 |
| Zhaosong et al., [47] | China | BF (no control) | Community | 3 to 67 | Kato-katz | Praziquantel | 9 |
Note: PC = prospective cohort, BF = before and after, RCT = randomised clinical trial, LC = longitudinal cohort, NA = not indicated, SC = school children and PSAC = preschool aged children
Reinfection rate of schistosomiasis
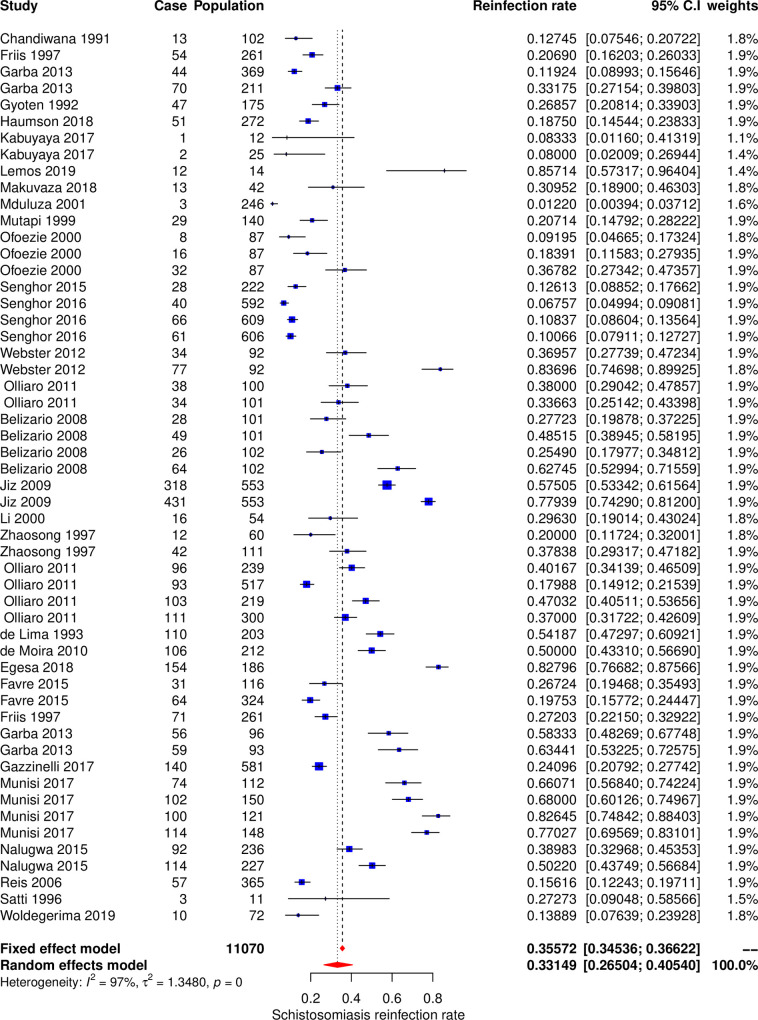
The overall mean reinfection rate of schistosomiasis was 36.1% (±23.3%). The lowest reinfection rate (1.2%) was recorded in a study conducted in Zimbabwe after follow-up time of 12 months. Participants were preschool aged children of less than 6 years old living in an endemic area. The study participants were cured from Schistosoma haematobium infection after being treated with multiple doses of 40 mg/kg of praziquantel (every 8 weeks for 2 years). The highest reinfection rate (85.7%) was reported in a study conducted in Angola after follow-up time of 6 months. In this study, participants were school aged children at the community aged between 2 and 15 years old. The study participants were cured from Schistosoma haematobium infection after being treated with a single dose of praziquantel (40 mg/kg).
Meta-analysis and subgroup analysis
The meta-analysis included 54 datasets extracted from 29 studies to calculate the pooled estimate of the reinfection rate of schistosomiasis (Fig 2). The pooled estimate of rate of reinfection was 33.2% (95% CI, 26.5–40.5%). The Cochran’s Q (chi-square) test indicated the presence of significant heterogeneity (p < 0.001). The degree of between-studies variances was substantially high as indicated by the Higgins I2 (95%CI) statistic of 97.0% (96.5% - 98.7%). Due to the presence of high heterogeneity, subgroup analysis was performed to check for the important contributing factors. The following factors were assessed for their contribution to the variance; type of Schistosoma species, geographical area, participants’ age group, follow up time, study settings and sample size. Univariate subgroup analysis results showed that the type of Schistosoma species and age groups of study participants were associated with the pooled reinfection rate. The amount of heterogeneity contributed by Schistosoma species was R2species = 20.2%, pspecies = 0.01 and age group was R2age = 4.4%, page = 0.07. The multivariate subgroup analysis shows that heterogeneity due to the combination of these two factors was R2species+age = 21%, pspecies+age = 0.002. The pooled reinfection rates of schistosomiasis according to the type of Schistosoma species were 44.3% (95% CI, 34.2%– 54.9%, I2 = 97%) for Schistosoma mansoni, 41.8% (95% CI, 30.9%– 53.5%, I2 = 96%) for Schistosoma japonicum and 19.4% (95% CI, 12.3%– 29.2%, I2 = 95%) for Schistosoma haematobium. The pooled reinfection rates were 25.7% (95% CI, 17/0%– 36.9%, I2 = 95%) for participants aged less than 16 years and 38.89% (95% CI, 30.17%– 48.38%, I2 = 95%) for participants of all ages.
Fig 2. Forest plot showing 54 datasets of reinfection rates of human schistosomiasis collected from 29 studies.
Intestinal schistosomiasis reinfection
Studies characteristics
Intestinal schistosomiasis due to Schistosoma mansoni was reported in studies conducted in Africa (8 studies), Brazil (4 studies) and Africa and Brazil (1 study), while Schistosoma japonicum was reported in Philippines (3 studies) and China (2 studies). From 18 studies, a total of 32 datasets showing reinfection rates of intestinal schistosomiasis were extracted. These datasets present reinfection rates after clients have been cured of the parasitic worm by praziquantel treatment (40 and/or 60 mg per kg body weight) as described in 17 studies and Oxamniquine (20 mg per kg body weight) as shown in 1 study. The study participants were people with age ranging from 1 to 67 years old. Ten studies were conducted at the communities, 7 studies were conducted at schools, and 2 studies involved both community and school settings. All studies used Kato-katz technique for the identification and quantification of Schistosoma mansoni and Schistosoma japonicum eggs in stool samples. Shortest and longest follow-up times were 5 and 156 months reported in Tanzania and Brazil respectively (Table 1).
Reinfection rate of intestinal schistosomiasis
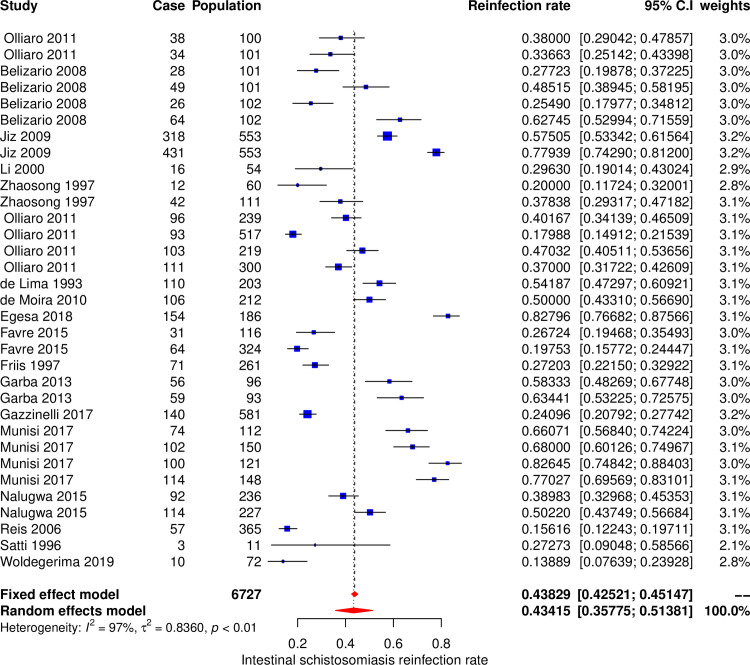
The mean reinfection rate of intestinal schistosomiasis was 43.9% (±20.6%). The lowest reinfection rate was recorded in a study conducted in Ethiopia after a follow-up time of 6 months. Participants were primary school children aged between 9 and 14 years. The study participants were cured from Schistosoma mansoni infection after being treated with a single dose of praziquantel (40 mg/kg). The highest reinfection rate was reported in a study conducted in Tanzania after follow-up time of 8 months. In this study, participants were school going children aged between 6 and 16 years leaving in an endemic area. The study participants were cured from Schistosoma mansoni infection after being treated with a single dose of praziquantel (40 mg/kg).
Meta-analysis and subgroup analysis for intestinal schistosomiasis
The meta-analysis included 32 datasets extracted from 18 studies to calculate the pooled estimate of the reinfection rate of intestinal schistosomiasis (Fig 3). The pooled estimate of reinfection rate was 43.4% (95% CI, 35.8–51.4%). The Cochran’s Q (chi-square) test indicated the presence of significant heterogeneity (p < 0.001). The degree of between-studies variances was substantially high as indicated by the Higgins I2 (95%CI) statistic of 97.0% (95.5% - 98.4%). Due to the presence of high heterogeneity, subgroup analysis was performed to check for the important contributing factors. The assessed factors included type of Schistosoma species, age groups, study settings, follow up time, sample size and geographical area. Univariate subgroup analysis results showed that geographical area and age groups of study participants were associated with the pooled reinfection rate. The amount of heterogeneity contributed by geographical area was R2area = 15.0%, p area = 0.02 and age group was R2age = 5.1%, page = 0.09. The multivariate subgroup analysis showed that heterogeneity due to the combination of these two factors was R2area+age = 24.7%, parea+age = 0.003. The pooled reinfection rates of intestinal schistosomiasis according to the geographic area was 53.7% (95% CI, 41.7%– 65.2%, I2 = 96.7%) for studies conducted in Africa, and 35.5% (95% CI, 27.5%– 44.4%, I2 = 96.5%) for studies conducted out of Africa. The pooled reinfection rates were 33.5% (95% CI, 22.6%– 46.4%, I2 = 96%) for participants aged less than 16 years and 47.4% (95% CI, 38.2%– 56.7%, I2 = 97.2%) for participants of all ages.
Fig 3. Forest plot showing reinfection rates of intestinal schistosomiasis.
Urogenital schistosomiasis reinfection
Studies characteristics
Urogenital schistosomiasis was reported in 14 studies conducted in Africa only (Table 1). From the 14 studies, a total of 22 datasets showing reinfection rates of Schistosoma haematobium were extracted. These datasets present reinfection rate after clients have been cured from this parasitic worm by praziquantel treatment (40 mg per kg body weight). The study participants were people with age ranging from 2 to 60 years. Eight studies were conducted at the community and 6 studies were conducted at school settings. Urine filtration (10 studies), centrifugation (1 study) and urinalysis (1 study) were the microscopic techniques employed for the identification and quantification of Schistosoma haematobium eggs in urine samples. Shortest and longest follow-up periods were 3 and 36 months reported in Nigeria and Senegal respectively.
Reinfection rate of urogenital schistosomiasis
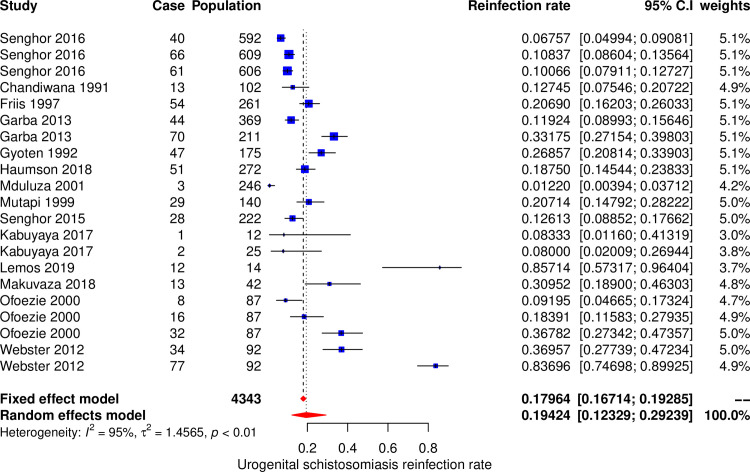
The mean reinfection rate of urogenital schistosomiasis was 17.6% (±10.8%). Generally urogenital schistosomiasis was detected at low and higher reinfection rate compared to intestinal schistosomiasis. Therefore, all information regarding reinfection rates of urogenital schistosomiasis were provided above (Reinfection rate of schistosomiasis section).
Meta-analysis and subgroup analysis for urogenital schistosomiasis
The meta-analysis included 22 datasets extracted from 14 studies to calculate the pooled estimate of the reinfection rate of urogenital schistosomiasis (Schistosoma haematobium) (Fig 4). The pooled estimate of reinfection rate was 19.4% (95% CI, 12.3%– 29.2%). The Cochran’s Q (chi-square) test indicated the presence of significant heterogeneity (p < 0.001). The degree of between-studies variances was substantially high as indicated by the Higgins I2 (95%CI) statistic of 95.2% (94.5% - 97.8%). Due to the presence of high heterogeneity, subgroup analysis was performed to check for the important contributing factors. The assessed factors included age groups, study settings, follow up time, sample size and geographical area. Univariate subgroup analysis results showed that sample size was associated with the pooled reinfection rate. The amount of heterogeneity contributed by sample size was R2sample size = 25.6%, p sample size = 0.014. The pooled reinfection rates of urogenital schistosomiasis according to the sample size were 32.3% (95% CI, 14.9%– 56.5%, I2 = 95.2%) for studies with small sample sizes, 15.5% (95% CI, 9.3%– 24.5%, I2 = 95.3%) for studies with medium sample sizes and 9.2% (95% CI, 6.9%– 12.0%, I2 = 71.5%) for studies with large sample sizes.
Fig 4. Forest plot showing reinfection rate of urogenital schistosomiasis.
Publication bias
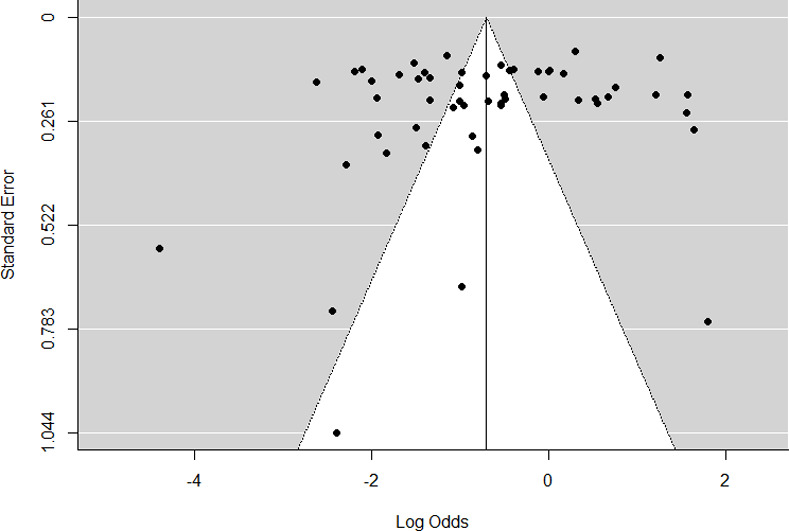
Funnel plot displaying graphical presentation of studies which show the absence of publication bias (Fig 5). Egger’s regression analysis for funnel plot asymmetry results confirmed the absence of significant level of publication bias (p = 0.483).
Fig 5. Funnel plot showing asymmetrical distribution for 29 (54 datasets) included studies.
Discussion
Schistosomiasis is a serious human disease of public health concern, commonly presented in two forms namely intestinal and urogenital. To date the most successful means of controlling the disease is through chemotherapy by Praziquantel [44, 48]. In this review, we assessed the reinfection rate after administration of schistosomiasis treatment and the results are presented in terms of mean and pooled estimates values. The mean value provides useful information at a population level to understand how many individuals were reinfected with Schistosoma species within a given population. Pooled estimates takes into account the study size so that the contribution of each study to the meta-analysis reflects the number of individuals involved in each study. To our knowledge this is a first systematic review and meta-analysis that provides information on the global reinfection rate of schistosomiasis caused by the three predominant species following drug administrations.
The overall mean reinfection rate of schistosomiasis was high (36.1%) with the minimum reinfection rate being 1.2% from Zimbabwe; as a result of intense repeated treatment in every eight weeks for a period of two years [39]. The minimum value reported from Zimbabwe suggests that intensive repeated treatment of the disease for every two month for a period of 2–5 years will significantly control schistosomiasis infections. The observed pooled reinfection rate of 33.2% suggests that the reinfection rate is still higher worldwide amid the existing control programs in place hence, calling for effective operational strategies for the control and elimination of the disease.
The reinfection rate was further analyzed based on the two forms of schistosomiasis manifestation. The mean reinfection rate of intestinal schistosomiasis was 43.9%, lowest value being 13.9% from Ethiopia [30]. Intestinal schistosomiasis had a pooled estimate of reinfection rate of 43.4%. Again the trivial differences between the calculated mean and pooled estimate of reinfection rates confirm the global high prevalence of intestinal schistosomiasis. The mean reinfection rate of urogenital schistosomiasis was 17.6% and pooled reinfection rate was 19.4%. The lowest reinfection rate of 1.2% from Zimbabwe was seen and is due to the factor explained above. Comparing the two forms of the disease urogenital schistosomiasis has the smallest reinfection rate comparing to intestinal schistosomiasis.
Several factors were found to influence high heterogeneity. Among six assessed factors the following contributed to the heterogeneity; type of Schistosoma species, age groups, sample size, geographical area and study settings. Generally, the reinfection rate of schistosomiasis was contributed by the type of Schistosoma species and age groups. Schistosoma mansoni and Schistosoma japonicum had higher pooled reinfection rates than Schistosoma haematobium, therefore contributed more to the overall pooled reinfection rate of schistosomiasis (Figs 3 and 4). Both Schistosoma mansoni and Schistosoma japonicum are transmitted from the human hosts to the environment (fresh water sources) through fecal contamination. High reinfection rate of Schistosoma mansoni and Schistosoma japonicum could be due to preferably defecation in or near fresh water sources because of privacy provided by vegetation growing around the surrounding the water sources and availability of water for washing after defecation. It was estimated that when a total of 991 people defecate near water source and go to clean in water, the amount of feacal particles deposited in is equivalent to amount of feaces contributed by 12 people who defecated directly in the water. Also, it was observed that eggs of Schistosoma mansoni and Schistosoma japonicum have high longevities than Schistosoma haematobium eggs [7]. Pooled reinfection rate of schistosomiasis for studies with participants aged less than 16 years was low compared to pooled reinfection rate for studies with participants of all ages. This is unexpected results as we expected young children (less than 16 years old) to have high reinfection rate due to high rate of exposure and low protective immunity [15]. Probably our findings could be due to fact that most of studies included in this meta-analysis involved adult populations who are occupationally exposed to high-risk of schistosomiasis infection (fishermen and rice paddy farmers). Additionally, 24 (77.4%) and 9 (39.1%) studies on intestinal schistosomiasis were conducted on participants of all ages and less than 16 years age groups respectively. The high number of studies on intestinal schistosomiasis conducted on participants of all ages could be the reason for high reinfection rate in this category compared to studies with participants aged less than 16 years as Schistosoma species causing intestinal schistosomiasis were observed to have higher reinfection rates.
The heterogeneity in reinfection rates of intestinal schistosomiasis was contributed by age group and geographical area. It was observed that studies with participants of all ages had higher reinfection rate compared to studies with participants aged less than 16 years. This is because studies with participants of all ages included adults who worked in routine occupations and domestic activities that exposed them to infested water. This is in contrast with other studies which showed children less than 16 years have higher reinfection rate compared to other ages [20]. However, studies with participants of all age groups included participants less than 16 years old. In the geographical area, studies conducted from Africa region had higher reinfection rate of schistosomiasis compared to studies from non-African region. It is known that Africa bears the large burden of schistosomiasis than other areas in the world [49]. This could be due to different socio-economic status, climatic and environmental factors. Most studies included in this review were from low-income African countries where people are involved in high-risk water contact occupations compared to studies outside Africa. It has been observed that in low-income countries, people engage in agricultural activities, fishing, irrigation canal cleaning, car washing, gold prospecting and tin-mining which results in subsequent exposure to water hence increase the risk of reinfection [50]. Also, climatic and environmental conditions play an important role in reinfection rate of schistosomiasis. Between the two identified intestinal Schistosoma species only Schistosoma mansoni is favoured with the climatic conditions of Africa region, while the climatic conditions of outside the Africa favour survival of both Schistosoma mansoni (South America) and Schistosoma japonicum (Asia). Based on our findings Schistosoma mansoni had high reinfection rate compared to Schistosoma japonicum and hence high reinfection rate in Africa region.
The variation in reinfection rate of urogenital schistosomiasis was attributed by sample size. It was observed that the smaller the sample size the higher the reinfection rate of urogenital schistosomiasis and vice versa. The reason for higher reinfection rate in studies with small sample sizes could be due to the intensive follow up compared to studies with large sample sizes. Furthermore, the observed variation might be due to different level of endemicity of urogenital schistosomiasis. For example all datasets contributed to large sample size category were from a single study, therefore presented only a reinfection rate at single area.
We expected study setting and follow-up time to contribute on the pooled reinfection rate of schistosomiasis [22, 44]. However, our findings revealed that there is no statistically significant association between these two factors and reinfection rate of schistosomiasis. Study setting was divided into two categories (school based and community) with the view that school based studies involved participants at higher risk than community based studies. Though this is not the case with our finding and might be due to inclusion of other high-risk groups in the community based studies.
Follow-up time was divided into two categories (< 12 months and ≥ 12 months). The findings showed that the difference in reinfection rates between the two follow-up time categories was not statistically significant as opposed to our expectation (higher reinfection rates in studies with follow-up time of ≥ 12 months). The results imply that reinfection occurs rapidly even before 12 months and remain steady.
Conclusion and recommendation
For the successful elimination of human schistosomiasis and attaining 2030 health and wellbeing for all (sustainable development goal number 3), intercept of reinfection is a core need. Global reinfection rate of human schistosomiasis is still higher as stipulated in this review, with African countries being a hotbed for reinfection. This review also confirms that intestinal schistosomiasis have higher reinfection rate than urogenital. Reinfection can be triggered by different factors; hence the understanding of the epidemiology patterns, biology of the parasite and social status in every society is essential. The existing control tool so far has not taken us to elimination goal. Is intensive repeated mass drug administration for a control of disease transmission feasible? That is yet still a myth. Swotting from Zimbabwe, this review suggests that, for the control of human schistosomiasis transmission biannual mass drug administration rounds for people of all age groups is essential, this strategy coupled up with robust snail control and health education campaigns will lead us to the elimination of schistosomiasis.
Strength and limitation
The strength of this study lies in the number of studies included. This review included 29 studies from different countries around the world, which increases the power to detect publication bias and heterogeneity.
The limitation of this review included diagnostic techniques used in the assessment of the rate of reinfection cannot differentiate reinfection from recrudescent. However, to minimize this, the authors included studies that determined the rate of reinfection at least three months from eggs clearance in urine or stool.
Another limitation is the criteria used to select the articles which could result in missing some important data from studies that were not selected. However, the criteria used were important because we wanted to have uniformities in some important issues such as the definition of reinfection rate and methodology used.
Supporting information
(DOCX)
(DOCX)
(DOC)
(DOC)
(CSV)
Acknowledgments
Authors would like to thank Ms Mary Joseph and Ms Eliza Lupenza for their assistance during review process.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Anto F, Asoala V, Adjuik M, Anyorigiya T, Oduro A, Akazili J, et al. Water contact activities and prevalence of schistosomiasis infection among school-age children in communities along an irrigation scheme in rural Northern Ghana. J Bacteriol Parasitol 2013;4:1–6. 10.4172/2155-9597.1000177 [DOI] [Google Scholar]
- 2.Sturrock R. The schistosomes and their intermediate hosts In: Mahmoud AAF, editor. Schistosomiasis, London: Imperial College Press; 2001. [Google Scholar]
- 3.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet, vol. 383, Lancet Publishing Group; 2014, p. 2253–64. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Key facts. Schistosomiasis 2019. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed May 5, 2020).
- 5.CDC. Parasite biology. Schistosomiasis 2019. https://www.cdc.gov/dpdx/schistosomiasis/index.html (accessed May 5, 2020).
- 6.Secor WE. Water-based interventions for schistosomiasis control. Pathog Glob Health 2014;108:246–54. 10.1179/2047773214Y.0000000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The roles of water, sanitation and hygiene in reducing schistosomiasis:A review. Parasites and Vectors 2015;8:1–16. 10.1186/s13071-014-0608-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bustinduy AL, Parraga IM, Thomas CL, Mungai PL, Mutuku F, Muchiri EM, et al. Impact of polyparasitic infections on anemia and undernutrition among Kenyan children living in a schistosoma haematobium-endemic area. Am J Trop Med Hyg 2013;88:433–40. 10.4269/ajtmh.12-0552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Key facts. Schistosomiasis 2016. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed May 5, 2020).
- 10.World Health Organization. Current estimated total number of individuals with morbidity and mortality due to Schistosomiasis haematobium and S. mansoni infection in Sub-Saharan Africa. Schistosomiasis 2017. https://www.who.int/schistosomiasis/epidemiology/table/en/ (accessed May 21, 2020).
- 11.Montresor A, Gabrielli AF, Chitsulo L, Ichimori K, Mariotti S, Engels D, et al. Preventive chemotherapy and the fight against neglected tropical diseases. Expert Rev Anti Infect Ther 2012;10:237–42. 10.1586/eri.11.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell SJ, Savage GB, Gray DJ, Atkinson JAM, Soares Magalhães RJ, Nery S V., et al. Water, Sanitation, and Hygiene (WASH): A Critical Component for Sustainable Soil-Transmitted Helminth and Schistosomiasis Control. PLoS Negl Trop Dis 2014;8 10.1371/journal.pntd.0002651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis 2008;21:659–67. 10.1097/QCO.0b013e328318978f [DOI] [PubMed] [Google Scholar]
- 14.Wilkins HA. Reinfection after treatme t of schistosome infections. Parasitol Today 1989;5:83–8. 10.1016/0169-4758(89)90008-2 [DOI] [PubMed] [Google Scholar]
- 15.Mbanefo EC, Huy NT, Wadagni AA, Eneanya CI, Nwaorgu O, Hirayama K. Host determinants of reinfection with schistosomes in humans:A systematic review and meta-analysis. PLoS Negl Trop Dis 2014;8:e3164 10.1371/journal.pntd.0003164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang A, Nie Z, Chen F, Cai S, Liu Q, Guo Y. Meta-analysis of Schistosoma japonicum reinfection and its risk factors in Chinese population. Chinese J Epidemiol 2015;36:181–5. [PubMed] [Google Scholar]
- 17.JBI. Critical appraisal checklist for prevalence studies. The Joanna Briggs Institute; 2017.
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lima e Costa MFF, Rocha RS, Filho PC, Katz N. A 13-year follow-up of treatment and snail control in an area endemic for Schistosoma mansoni in Brazil: Incidence of infection and reinfection. Bull World Health Organ 1993;2:197–205. [PMC free article] [PubMed] [Google Scholar]
- 20.de Moira AP, Fulford AJC, Kabatereine NB, Ouma JH, Booth M, Dunne DW. Analysis of complex patterns of human exposure and immunity to Schistosomiasis mansoni: The influence of age, sex, ethnicity and IgE. PLoS Negl Trop Dis 2010;4 10.1371/journal.pntd.0000820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egesa M, Lubyayi L, Jones FM, Diepen A Van, Chalmers IW, Tukahebwa EM, et al. Antibody responses to Schistosoma mansoni schistosomula antigens. Parasite Immunol 2018:1–11. 10.1111/pim.12591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favre TC, Pereira APB, Beck LCNH, Galvão AF, Pieri OS. School-based and community-based actions for scaling-up diagnosis and treatment of schistosomiasis toward its elimination in an endemic area of Brazil. Acta Trop 2015;149:155–62. 10.1016/j.actatropica.2015.04.024 [DOI] [PubMed] [Google Scholar]
- 23.Garba A, Lamine MS, Barkiré N, Djibo A, Sofo B, Gouvras AN, et al. Efficacy and safety of two closely spaced doses of praziquantel against Schistosoma haematobium and S. mansoni and re-infection patterns in school-aged children in Niger. Acta Trop 2013;128:334–44. 10.1016/j.actatropica.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 24.Gazzinelli A, Oliveira-Prado R, Matoso LF, Veloso M, Andrade G, Kloos H, et al. Schistosoma mansoni reinfection: Analysis of risk factors by classification and regression tree (CART) modeling 2017:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munisi DZ, Buza J, Mpolya EA, Angelo T, Kinung SM. The efficacy of single-dose versus double-dose praziquantel treatments on Schistosoma mansoni infections: Its implication on undernutrition andaAnaemia among primary schoolchildren in two on-shore communities, Northwestern Tanzania. Biomed Res Int 2017;2017 10.1155/2017/7035025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nalugwa A, Nuwaha F, Tukahebwa EM, Olsen A. Single versus double dose praziquantel comparison on efficacy and Schistosoma mansoni re-infection in preschool-age children in Uganda: A randomized controlled trial. PLoS Negl Trop Dis 2015;9:1–16. 10.1371/journal.pntd.0003796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olliaro PL, Vaillant MT, Belizario VJ, Lwambo NJS. A multicentre randomized controlled trial of the efficacy and safety of single-dose praziquantel at 40 mg / kg vs. 60 mg / kg for treating Intestinal Schistosomiasis in the Philippines, Mauritania, Tanzania and Brazil. PLoS Negl Trop Dis 2011;5:e1165 10.1371/journal.pntd.0001165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reis E, Reis M, Silya R, Carmo T, Assis A, Barreto M, et al. Biological and immunological predictors of efficacy of treatment or reinfection risk for Schistosoma mansoni. Am J Trop Med Hyg 2006;75:904–9. [PubMed] [Google Scholar]
- 29.Satti MZ, Sulaiman SM, Homeida MMA, Younis SA, Ghalib HW, Medicine T. Clinical, parasitological and immunological features of canal cleaners hyper-exposed to Schistosoma mansoni in the Sudan. Clin Exp Immunol 1996;104:426–31. 10.1046/j.1365-2249.1996.00051.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woldegerima E, Bayih AG, Tegegne Y, Aemero M, Zeleke AJ. Prevalence and reinfection rates of Schistosoma mansoni and praziquantel efficacy against the parasite among primary school children in Sanja Town, Northwest Ethiopia. J Parasitol Res 2019;2019 10.1155/2019/3697216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandiwana SK, Woolhouse MEJ, Bradley M. Factors affecting the intensity of reinfection with Schistosoma haematobium following treatment with praziquantel. Parasitology 1991;102:73–83. 10.1017/s0031182000060364 [DOI] [PubMed] [Google Scholar]
- 32.Friis H, Ndhlovu P, Kaondera K, Sandstrom B, Michaelsen K, Vennervald B, et al. The impact of zinc supplementation on Schistosoma mansoni reinfection rate and intensities: A randomized, controlled trial among rural Zimbabwean schoolchildren. Eur J Clin Nutr 1997;51:33–7. 10.1038/sj.ejcn.1600359 [DOI] [PubMed] [Google Scholar]
- 33.Gyoten J, Kimura E, Muhoho N, Katsumata T, Migwi D, Mutua W, et al. Schistosoma haematobium reinfection occurred shortly after treatment with praziquantel. J Japanese Soc Trop Med 1992;20:157–64. [Google Scholar]
- 34.Houmsou R, Wama B, Agere H, Uniga J, Amita E, Kela A. High efficacy of praziquantel in Schistosoma haematobium-infected children in Taraba State, Northeast Nigeria. Sultan Qaboos Univ Med J 2018;18:304–10. 10.18295/squmj.2018.18.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabuyaya M, Chimbari MJ, Manyangadze T, Mukaratirwa S. Efficacy of praziquantel on Schistosoma haematobium and re-infection rates among school-going children in the Ndumo area of uMkhanyakude district, KwaZulu-Natal, South Africa. BMC-Infectious Dis Poverty 2017;6:1–9. 10.1186/s40249-017-0293-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemos M, Fançony C, Mirante C, Sousa P De, Barros H. Integrated community-based intervention for urinary schistosomiasis and soil-transmitted helminthiasis in children from Caxito, Angola. Int Health 2019. 10.1093/inthealth/ihz055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mutsaka-makuvaza MJ, Matsena-zingoni Z, Tshuma C, Ray S, Zhou X, Webster B, et al. Reinfection of urogenital schistosomiasis in pre-school children in a highly endemic district in Northern Zimbabwe: a 12 months compliance study. BMC-Infectious Dis Poverty 2018;7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mutapi F, Ndhlovu PD, Hagan P, Woolhouse MEJ. A comparison of re-infection rates with Schistosoma haematobium following chemotherapy in areas with high and low levels of infection. Parasite Immunol 2000;21:253–9. [DOI] [PubMed] [Google Scholar]
- 39.Mduluza T, Ndhlovu PD, Madziwa TM, Midzi N, Zinyama R, Turner CMR, et al. The impact of repeated treatment with praziquantel of schistosomiasis in children under six years of age living in an endemic area for Schistosoma haematobium infection. Mem Inst Oswaldo Cruz 2001;96:157–64. 10.1590/s0074-02762001000900024 [DOI] [PubMed] [Google Scholar]
- 40.Ofoezie IE. Patterns of reinfection following praziquantel treatment of urinary schistosomiasis at a period of low transmission. Acta Trop 2000;75:123–6. 10.1016/s0001-706x(99)00078-9 [DOI] [PubMed] [Google Scholar]
- 41.Senghor B, Diaw OT, Doucoure S, Sylla SN, Seye M, Talla I, et al. Efficacy of praziquantel against urinary schistosomiasis and reinfection in Senegalese school children where there is a single well-defined transmission period. Parasit Vectors 2015;8:1–11. 10.1186/s13071-014-0608-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senghor B, Diaw OT, Doucoure S, Seye M. Impact of annual praziquantel treatment on urogenital schistosomiasis in a Seasonal transmission focus in Central Senegal. PLoS Negl Trop Dis 2016;10:e0004557 10.1371/journal.pntd.0004557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webster BL, Diaw OT, Seye M, Faye DS, Stothard JR, Sousa-Figueiredo JC, et al. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: Monitoring treatment success and re-infection patterns. Acta Trop 2013;128:292–3002. 10.1016/j.actatropica.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 44.Belizario VY, Amarillo MLE, Martinez RM, Mallari AO, Tai CMC. Efficacy and safety of 40 mg/kg and 60 mg/kg single doses of praziquantel in the treatment of schistosomiasis. J Pediatr Infect Dis 2008;3:27–34. 10.1055/s-0035-1556962 [DOI] [Google Scholar]
- 45.Jiz M, Friedman JF, Leenstra T, Jarilla B, Pablo A, Langdon G, et al. Immunoglobulin E (IgE) responses to paramyosin predict resistance to reinfection with Schistosoma japonicum and are attenuated by IgG4. Infect Immun 2009;77:2051–8. 10.1128/IAI.00012-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li YS, Sleigh AC, Ross AGP, Liz Y, Williams GM, Tanner M, et al. Two-year impact of praziquantel treatment for Schistosoma japonicum infection in China: re-infection, subclinical disease and fibrosis marker measurements. Trans R Soc Trop Med Hyg 2000;94:191–7. 10.1016/s0035-9203(00)90274-8 [DOI] [PubMed] [Google Scholar]
- 47.Zhaosong Z, Haiweil W, Suchen C, Linthen H. Association reinfection between IgE antibody against with Schistosoma japonicum soluble egg antigen and resistance to. Trans R Soc Trop Med Hyg 1997;91:606–8. 10.1016/s0035-9203(97)90047-x [DOI] [PubMed] [Google Scholar]
- 48.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. N Engl J Med 2007;357:1018–27. 10.1056/NEJMra064142 [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. Schistosomiasis 2020. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed October 28, 2020).
- 50.Sawyer SG, Roes A, Butera G. Occupational health risk factors for schistosomiasis: systematic review and analysis. Georg. Washingt. Univ. Res. Days, Washington DC, USA: GW Himmelfarb Health Science Labrary; 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOC)
(DOC)
(CSV)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.