Highlights
-
•
Carotenoids present anti-inflammatory effects in healthy and overweight adults.
-
•
Nanotechnology can enhance carotenoid's bioactive potential.
-
•
Nanoparticles loaded with carotenoids from Cantaloupe melon were used in obese rats.
-
•
Animals receiving the nanoparticles showed no signs of toxicity.
-
•
Animals treated with nanoparticles had organs better aspect compared to untreated.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate transferase; BSD, Bowman’s space dilation; CEUA, Ethics Committee on the Use of Animals; CE, crude carotenoid extract; EI, efficiency of incorporation; EPG, crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin; FTIR, Fourier Transform Infrared Spectroscopy; GGT, gamma-glutamyl transferase; HGLI, high glycemic index and high glycemic load; IIF, inflammatory infiltrate foci; OECD, Organization for Economic Co-operation and Development; PVA, percentage of villous absence; PIGI, percentage of intestinal gland integrity; PVI, percentage of villus integrity; PVN, percentage of villous necrosis; PHT, presence of hypertrophic tubules; PUV, percentage of ulcerated villi; SEM, Scanning Electron Microscope; THC, tubular hyaline cylinders
Keywords: β-carotene, Curcubitaceae, Nanotechnology, Toxicity, Obesity
Abstract
The safety and bioactive potential of crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin (EPG) were evaluated in a chronic inflammatory experimental model. Animals were fed a high glycemic index and high glycemic load (HGLI) diet for 17 weeks and treated for ten days with 1) HGLI diet, 2) standard diet, 3) HGLI diet + crude carotenoid extract (CE) (12.5 mg/kg), and 4) HGLI diet + EPG (50 mg/kg). General toxicity signals were investigated, considering body weight, food intake, hematological, biochemical parameters, relative weight, morphology, and histopathology of organs. The biochemical parameters indicated the low toxicity of EPG. Acute hepatitis was observed in animals' livers, but CE and EPG groups presented improved tissue appearance. Chronic enteritis was observed in animals, with villi and intestinal glands preservation in the EPG group. The results suggest the safety and the bioactive effect of EPG, possibly related to its anti-inflammatory potential.
1. Introduction
Carotenoids are lipophilic compounds with several functional properties, such as antioxidant [1], anti-inflammatory, and, some of them, vitamin A precursors [[2], [3], [4]]. Among the different plant sources of carotenoids, orange pulp melons stand out, as the commercial Cantaloupe type, produced in Northeast Brazil, mainly presents β-carotene and lutein [5].
Studies highlight the potent anti-inflammatory action of carotenoids administered in the short and medium-term in healthy and overweighted individuals or animals [2,[6], [7], [8]]. However, interestingly, individuals with chronic inflammation showed better results. Many of these findings can be attributed to the antioxidant effects beneficial to health, and other mechanisms such as altering the cascades of intracellular signaling and gene expression and reducing the formation of inflammatory cytokines [9].
In this context, according to the review study by Bonet et al. [6], carotenoids with pro-vitamin A activity (β-carotene, α-carotene, and β-cryptoxanthin), vitamin A and its derivatives may play specific roles in mature adipocytes, such as controlling metabolism, the production of inflammatory mediators and, consequently, oxidative stress. Therefore, being of fundamental importance in the nutritional status of obesity. Thereby, Bonet et al. [10] highlighted that epidemiological studies demonstrate a beneficial effect of carotenoid supplementation (with pro-vitamin A activity) in decreasing abdominal adiposity accumulation.
On the other hand, carotenoids present instability in the presence of factors involved in the processing and storing of food, such as the presence of light, acids, oxygen, heat, and undesirable reactions with other food compounds. Thus, the use of techniques that preserve the beneficial properties to health is fundamental [11].
Encapsulation is a technique that aims to retain bioactive and phytochemical compounds in a wall material, which acts as a barrier against processing and storage conditions [12]. In this context, nanotechnology can play an essential role in preserving or enhancing the features of bioactive compounds, increasing bioavailability, solubility, bioaccessibility in the intestine, and promoting controlled release [11].
Studies involving nanoencapsulation of crude carotenoid extract from Cantaloupe melons (CE) are scarce in the literature. Medeiros et al. [13] observed that crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin (EPG) showed greater incorporation efficiency and solubility in water in addition to better color stability in the food matrix (yogurt). However, considering the functional properties and possible bioactive and biotechnological applications of EPG, safety must be assessed to define potential risks of use [14]. Thus, the present study aims to assess the safety and bioactive potential of nanoparticles loaded with a crude carotenoid extract from Cantaloupe melon and porcine gelatin in Wistar rats.
2. Materials and methods
2.1. Obtaining and characterizing the crude carotenoid extract from Cantaloupe melon (CE)
Commercial Cantaloupe melons registered in the National System for the Management of Genetic Heritage and associated traditional knowledge (SISGEN) under number A5A85DF were purchased in the city of Natal/RN. The processing of the pulp and the obtaining of CE were performed according to Medeiros et al. [13].
2.1.1. UV–vis absorption spectrophotometry
The determination of the total carotenoid content of the CE was performed according to Biehler et al. [15] in the maximum absorption wavelength obtained by scanning spectrophotometry using the following equation:
| C (mol/L) = (A450 × FD)/2592 | (1) |
Where A450 = average absorbance obtained at the maximum absorption wavelength; FD = dilution factor adjusted for the absorbance determinations of the dry CE solubilized in hexane; 2592 = molar absorption coefficient (ε) of β-carotene.
The result was expressed in micrograms of carotenoids/g of fresh melon pulp (μg/g).
2.1.2. Ultra high performance liquid chromatography (UHPLC)
The analysis was performed in a chromatograph (Shimadzu) coupled to a diode array detector (SPD-M20A) for identification and quantification of β-carotene at 450 nm, according to Rodriguez-Amaya [16] with modifications proposed by Medeiros et al. [13].
2.2. Encapsulation of carotenoids
The crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin (EPG) were produced using the O/W emulsification technique, with subsequent dispersion of the aqueous phase containing Tween 20 (Sigma-Aldrich®, St. Louis, MO, USA) and porcine gelatin (Sigma-Aldrich®, St. Louis, MO, USA) in the obtained emulsion. Then, freeze-drying was used to obtain the material powder, according to Medeiros et al. [13].
2.2.1. Nanoparticles characterization
2.2.1.1. Scanning electron microscope (SEM)
The EPGs were suspended in acetone and dripped onto silicon plates fixed with carbon tape in the stub. The analyses were performed in high vacuum, 2–3 kV tension, without metallization, using different magnifications in SEM-FEG ZEISS (AURIGA), according to Medeiros et al. [13].
2.2.1.2. Laser diffraction
The methodology was standardized, according to Medeiros et al. [13], with modifications. The EPG was previously de-agglomerated to facilitate laser diffraction analysis, as proposed by Medeiros et al. [13]. The final dispersion for measuring was in distilled water.
The dispersion was arranged in a glass cuvette and read at 10 runs/min on the NanoBrookZetaPlusZeta Potential Analyzer, software BrookhavenInstruments - ZetaPALSParticleSizing Software. The experiment was performed in triplicate.
2.2.1.3. Fourier transform infrared spectroscopy (FTIR)
EPG, CE, porcine gelatin, and Tween 20 were homogenized separately with potassium bromide (KBr), macerated, and pressed to form tablets. Subsequently, they were submitted to FTIR analysis using a spectrometer (SHIMADZU IR tracer-100) in the range of 400 to 4000 cm−1, with a scan of 32 and resolution of 4 cm−1 [13].
2.2.2. Efficiency of incorporation of carotenoids
EPG (150 mg) solubilized in hexane (1 mL/extraction cycle) was used. Subsequently, the dispersion was placed in an ultrasound bath (Ultra Cleaner 1650 Unique®) for 3 min. It was then centrifuged (Micro Centrifuge 6000 RPM HT) at 947 x g for 20 min to promote the separation of the supernatant containing the carotenoids. The procedure was performed in triplicate and repeated until color exhaustion, based on Medeiros et al. [13].
The absorbance of the supernatant was measured on a spectrophotometer at 450 nm to determine the concentration of crude carotenoid extract incorporated in the particles. The efficiency of incorporation (%) was done using the equation of the line previously obtained for the CE, based on the construction of the calibration curve in the concentration range of 0.002 at 0.36 mg/mL.
The amount of CE inside the particles was obtained from the equation proposed by Hu et al. [17]:
| Efficiency of incorporation (%) = (crude carotenoid extract in the nanoparticles / total crude carotenoid extract used) × 100 | (2) |
2.3. In vivo safety of the bioactive dose of nanoencapsulated carotenoids
The safety evaluation of the bioactive dose of the EPG was performed according to the experimental protocol of the OECD Guideline for Testing of Chemical - Acute Oral Toxicity - Acute Toxic Class Method 423 [18], following Guide for the Care and Use of Laboratory Animals [19].
The CE concentration was estimated based on previous tests to assess the amount of extract that prevented or inhibited oxidation by 50 % (IC50). In this evaluation, 50 mg/kg EPG showed the same radical inhibition effect observed for 12.5 mg/kg EC, and these doses were considered bioactive. When calculating the dose, the CE ratio: porcine gelatin present in EPG (1:80 w/w) and the encapsulation efficiency were also considered. Besides, the EPG concentration administered in Wistar rats was in agreement with the study by Pinzón-García et al. [20].
2.3.1. Experimental design
Adult male Wistar rats with chronic inflammation-induced for 17 weeks by consuming a high glycemic index and high glycemic load (HGLI) diet [21] were obtained at the bioterium of Potiguar University (UnP). This study was conducted following the ethical standards of experimental animals and approved by the Ethics Committee on the Use of Animals of Potiguar University (CEUA-UnP), protocol number 019/2017.
The animals were divided by simple randomization (random allocation) into four groups containing five animals for safety evaluation of the EPG bioactive dose. Initially, they were adapted for five days and kept under standard light, temperature, humidity, water, and food ad libitum.
Then, the groups were evaluated for ten days of treatment as follows: (1) untreated rats receiving HGLI diet and water by gavage; (2) rats receiving standard diet and water by gavage; (3) rats receiving HGLI diet and 12.5 mg of CE/kg in water by gavage; and (4) rats receiving HGLI diet and 50 mg of EPG/kg in water by gavage.
On the 11th day of the experiment, after 1 h of the gavage, rats were anesthetized with 250 mg of tiletamine hydrochloride and 250 mg of Zolazepam hydrochloride. Then, the blood was collected through a cardiac puncture, and organs were removed for weight measurement and biochemical, macroscopic, and histopathological analyses. Immediately after removal, the blood was centrifuged (500×g/10 min at 4 °C) to separate the serum for subsequent biochemical analyses. Trained and qualified veterinarians performed all anesthesia, euthanasia, and blood and organ collection procedures.
2.3.2. Assessment of food intake, weight and general signs of toxicity
The Wistar rats were distributed individually in polypropylene cages, and before the experiment, they passed through an adaptation period of five days to establish the frequent intake of each animal. During this period, all animals received procedures similar to those that would occur during the experimental period, including gavage. After the intake pattern was established, the animals were treated as described previously in 2.3.1.
On the first and last day of the experiment, before administering the treatments, animals were weighed using a digital semi-analytical scale (Filizola, MF-3) in the bioterium. The results were expressed as the mean and standard deviation to assess the change in total weight.
Before, of the free access to the diet, the animals fasted for 8 h. Food intake was assessed based on the following calculation:
| Food intake (g) = Feed offered (g) - Leftover feed (g) | (3) |
The results were expressed as the mean and standard deviation at the beginning and end of the experiment to assess changes in food intake.
The animals were evaluated daily for general signs of toxicity: piloerection, locomotor, and behavioral changes such as apathy, increased/decreased touch response, constipation, diarrhea, shedding, and death.
2.3.3. Hematological and biochemical analyses
The safety assessment of the bioactive dose was performed using hematological and biochemical parameters. For hematological parameters (hemoglobin, hematocrit, total leukocyte count, and platelet count), the animals' blood was collected in tubes with EDTA. For the other parameters were used the serum collect in tubes separately. Glucose, triglycerides, total cholesterol, liver function were assessed by determining liver enzymes (aspartate aminotransferase - AST, alanine aminotransferase - ALT, gamma-glutamyl transferase - GGT, alkaline phosphatase - ALP) and renal function by creatinine, urea, total proteins, and albumin. Independent blinded evaluators conducted this analysis in automated colorimetric-enzymatic tests (Labtest®, Natal, RN, BR).
Table 1 presents reference values for the hematological and biochemical parameters studied in male, adult, eutrophic Wistar rats (320–380 g), acclimated under this study's same conditions, consuming a standard Labina® diet. These same reference values have been used in other studies developed in the same bioterium [22].
Table 1.
Blood reference parameters for male, adult, eutrophic Wistar rats acclimated in the bioterium of Potiguar University/Brazil, in January 2019.
| Biochemical parameters | Reference values |
|---|---|
| Hemoglobin (g/dL) | 11.50–41.30 |
| Hematocrit (%) | 33.00–46.00 |
| Total leukocyte count (x103/μL) | 5.40–7.10 |
| Platelet (x105/μL) | 2.58–4.23 |
| Fasting blood glucose (mg/dL) | 84.00–113.00 |
| Triglycerides (mg/dL) | 55.00–73.00 |
| Total cholesterol (mg/dL) | 82.00–184.00 |
| AST (U/L) | 34.00–67.00 |
| ALT (U/L) | 35.00–49.00 |
| GGT (U/L) | 29.80–38.70 |
| ALP (U/L) | 55.80–71.10 |
| Urea (mg/dL) | 22.80–41.00 |
| Creatinine (mg/dL) | 0.60–1.00 |
| Albumin (mg/dL) | 1.90–2.60 |
| Total proteins (mg/dL) | 6.00–7.10 |
2.3.4. Determination of the liver, small intestine, kidneys, stomach, and spleen relative weight
The determination of the relative weight of the organs (liver, small intestine, kidneys, stomach, and spleen) was performed based on the measurement of fresh weight using an analytical scale (BIOPRECIOSA, FA-2104 N, Paraná, Brazil) with an accuracy of 0.0001 g. The determination of the relative weight of the organs was performed according to the following formula [23]:
| Relative weight (%) = (Weight of the organ (g)/Weight of the mouse (g)) × 100 | (4) |
2.3.5. Histopathological analysis of the liver, small intestine, kidneys, stomach, and spleen
The collected organs were fixed in 4% formaldehyde, packed individually, in plastic pots with lids, cleaved, and dehydrated in different percentages of alcohol (70, 80, and 90 %). The sections were then diaphanized in xylol (PA), embedded in histological paraffin, sectioned in a microtome (Leica RM2235, Buffalo Grove, USA) at 5 μm, and stretched on a glass slide. Finally, they were stained with routine staining (hematoxylin and eosin) and covered with a coverslip [24]. Independent blinded evaluators and experienced pathologists conducted this analysis.
The histological slides were obtained from the left lobe of the liver (four slides with three sections per slide), duodenum, jejunum, and ileum of the small intestine (three slides with three sections each), right and left kidney (two slides with three sections each), cardiac stomach region, bottom and body (two slides with three sections each), and spleen (one slide with three sections). The examination of the slides was performed using the B800 Optical microscope (Optika, Pontenarica, Italy), and digital images of the sections were obtained using the Optikam PRO6 digital camera (Optika, Pontenarica, Italy) coupled to the microscope mentioned above, using increases of 40×, 100× and 400×.
According to criteria such as organ presentation, sufficient amount of specimen on the slide, and quality of the section, a detailed histopathological description of the liver, stomach, and spleen was chosen. The establishment of scores (0–4) was performed according to Silva et al. [25] with modifications for the semiquantitative analysis of the changes found in the small intestine, considering the percentage of villus integrity (PVI), the percentage of ulcerated villi (PUV), the percentage of villous absence (PVA), the percentage of villous necrosis (PVN), and the percentage of intestinal gland integrity (PIGI).
In addition to the kidneys, considering the presence of atrophic and/or hypertrophic tubules (PHT), were showed alteration of proximal contorted tubule, amyloid, glomerular hyalinization (GH), tubular hyaline cylinders (THC), glomeruli with nodular sclerosis, Bowman's space dilation (BSD), inflammatory infiltrate foci (IIF), and necrosis (Table 2).
Table 2.
Semiquantitative evaluation scores of percent change in small intestine and kidneys of Wistar rats groups submitted to different treatments for ten days.
| Score | Classification |
|---|---|
| 0 | Absent |
| 1 | >0 to 25 % |
| 2 | >25 % to 50 % |
| 3 | >50 % to 75 % |
| 4 | Above 75 % |
2.4. Statistical analysis
The sample size was calculated considering simple and random sampling (Cochran model) and according to the 3Rs principle (reduction, refinement and replacement). The result was an n of 4.36 animals, which was rounded up to five animals per group. The 10 % anticipated variation coefficient was adopted, with a probability of error of less than 5 % and a power of 90 %.
Data are expressed as the means and standard deviation. The Kolmogorov-Smirnov test was used to evaluate the distributions of the studied variables. When the distribution was normal, parametric ANOVA tests and Tukey post-tests were used to compare the studied groups. For non-parametric variables, the Kruskal-Wallis and Dunn’s post-tests were used.
For all analyses, a p-value less than 0.05 was considered significant. To compare the initial and final weight and food consumption of animals from the same group, the Kolmogorov-Smirnov test was also used to assess distribution, and because it assumed a normal distribution, the two-tailed paired Student’s t-test (p < 0.05) was used. The statistical analysis was performed using GraphPad Prism software, version 5.0 (GraphPad Software, San Diego, CA).
For the semiquantitative histopathological analysis, the data distribution was also verified using the Kolmogorov-Smirnov test. Posteriorly, the Kruskal-Wallis test (p < 0.05) and Student-Newman-Keuls post hoc test were used. The Spearman correlation was performed in SPSS (Version 20.0, IBM, Chicago, USA).
3. Results and discussion
A previous study by Medeiros et al. [13] with EPG solved the technical barriers associated with using carotenoids in food (stability and solubility) through nanotechnology. It was then necessary to evaluate the safety and bioactive potential of EPG in an animal model. A new batch of EPG was then obtained in the same standard conditions as before, and the material was characterized to ensure the same physical and chemical characteristics. Also, in this current study the highlight will be related about the safety of this nanoparticle.
3.1. Characterization of crude carotenoid extract from Cantaloupe melon
Foods with total carotenoids above 20 μg/g have more significant health benefits [26]. In the present study, the Cantaloupe melon pulp presented 44.92 (0.001) μg of total carotenoids/g and 26.16 (0.001) μg of β-carotene/g. These results are in accordance to those found by Medeiros et al. [13]. Fleshman et al. [27] found lower values of β-carotene in Cantaloupe melon (12.5 μg of β-carotene/g). This variation can be explained by different soil, climate, solar radiation, incidence of rain, and other favors that involve the cultivation of fruits [28,29].
3.2. EPG characterization
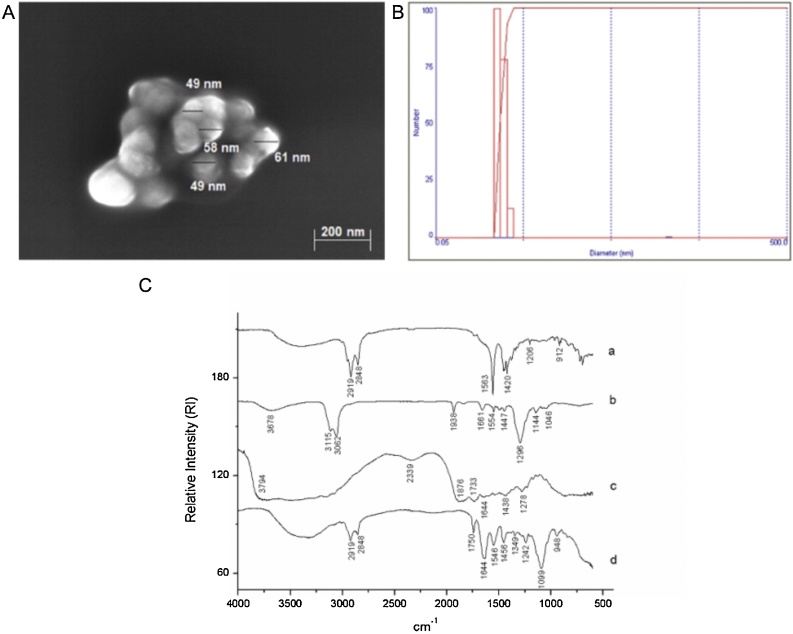
The characterization of EPG using SEM, laser diffraction, and FTIR were presented in Fig. 1. For SEM (Fig. 1A), spherical particles were observed, with a smooth surface, without cracks or depressions, and homogeneous sizes on the nanometric scale (<100 nm). A mean diameter of 74 (12.7) nm and a mean polydispersity index of 0.53 (0.12) were observed (Fig. 1B), indicating that the EPG was similar to that found by Medeiros et al. [13], presenting the desired characteristics. These results also confirmed the reproducibility of the encapsulation technique used by Medeiros et al. [13] to obtain EPG.
Fig. 1.
Characterization of EPG and encapsulating agents. A. Scanning Electron Microscopy (SEM). B. Laser diffraction. C. Fourier Transform Infrared Spectroscopy (FTIR): a. Crude carotenoid extract from Cantaloupe melon (Cucumis melo L.); b. Tween 20; c. porcine gelatin; d. EPG: crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin. (2-column fitting image).
The spectra obtained by FTIR (Fig. 1C) for the CE, Tween 20, and porcine gelatin were typical, as seen by Medeiros et al. [13]. In the EPG spectrum, the crude carotenoid extract's vibrational bands in 2919 cm−1 and 2848 cm−1 were attenuated, and the bands in 920 cm−1 and 1206 cm−1 were not observed, indicating that the CE was protected by gelatin. Besides, the appearance of new bands in 3008 cm−1, 1750 cm−1, and 1349 cm−1 was observed, which might indicate that the non-polar amino acids of gelatin performed hydrophobic interactions with the carbon chain of the CE.
The incorporation efficiency was 98 % (1.78), confirming that a new batch of EPG with the same physical and chemical characteristics presented by Medeiros et al. [13]. This result is a good, considering the difficulties related to reproducibility in obtaining EPG [11].
3.3. In vivo safety assessment
It has been show that carotenoids can present anti-inflammatory effects in 10 days or less [7]. Based on this, in the present study, toxicity and bioactive potential were evaluated considering the use of nanoparticles in the context of a highly inflammatory environment promoted by feeding with the HGLI diet, which induced chronic inflammation in the studied animals.
Therefore, the main signs of toxicity, such as changes in food intake, weight, coat, relative organ mass, and hematological and biochemical blood parameters, were evaluated. The morphology and histopathology of critical organs, which help visualize macroscopic and cellular damage caused by the ingested molecules, were also used. Studies with encapsulated carotenoids that evaluate these signals and parameters are scarce in the literature, and these evaluations strengthen our study.
3.3.1. Assessment of body weight, food intake and general signs of toxicity
Although there was a reduction in body weight in the groups treated with carotenoids (CE and EPG), there was no statistically significant difference (p > 0.05) between the initial and final weight of each group of animals (Table 3).
Table 3.
Initial and final body weight of the four groups of Wistar rats submitted to different treatments during ten days of the experiment.
| Group | Body weight |
Food intake |
||
|---|---|---|---|---|
| Initial (g) | Final (g) | Initial (g) | Final (g) | |
| HGLI + water | 362.80 (27.36)a | 364.20 (24.31)a | 16.90 (3.19)a | 20.80 (1.48)b |
| Standard diet + water | 378.80 (88.50)a | 375.80 (83.90)a | 20.00 (2.86)a | 24.10 (5.38)a |
| HGLI + CE | 452.00 (9.35)a | 446.80 (9.41)a | 21.06 (3.34)a | 22.00 (2.06)a |
| HGLI + EPG | 501.50 (36.62)a | 490.50 (33.42)a | 22.50 (0.94)a | 19.50 (2.37)a |
Data expressed as mean (standard deviation), n = 5. Equal lowercase letters on the same line indicate that there was no statistically significant difference. CE: crude carotenoid extract from Cantaloupe melon (Cucumis melo L.) (12.5 mg/kg of weight). EPG: crude carotenoid extract from Cantaloupe melon (Cucumis melo L.) nanoencapsulated in porcine gelatin (50 mg/kg of weight). HGLI: diet with a high glycemic index and high glycemic load. The statistical difference between the initial and final weight and food consumption of the animals was calculated using the paired t-Student test (p < 0.05).
In general, studies with nanoparticles based on carotenoids have not consistently shown changes in animals' body weight, considering all possible outcomes, such as increase, reduction, or maintenance. These data may be associated with the type of bioactive compound used, wall materials, in addition to variations in animal species. According to Pinzón-García et al. [20], rats with obesity, triggered by a diet rich in fat, treated with bixin encapsulated with cyclodextrins, showed significant weight reduction. For Wu et al. [30], mice with alcoholic liver fibrosis treated for four weeks with liposome-encapsulated astaxanthin increased body weight.
The change in body weight is one of the parameters most used in toxicological assessments to indicate the appearance of toxic effects of a specific substance in the animal organism [18]. Therefore, the absence of this body alteration indicates the low toxicity of the EPG.
Studies with encapsulated carotenoids to assess food intake are also not unanimous about the results. Pinzón-García et al. [20] observed a significant reduction in food intake than the control group with a standard diet. There was no statistically significant difference in the food intake of Wistar rats treated with CE and EPG in the present study.
3.3.2. In vivo safety of the bioactive dose of EPG
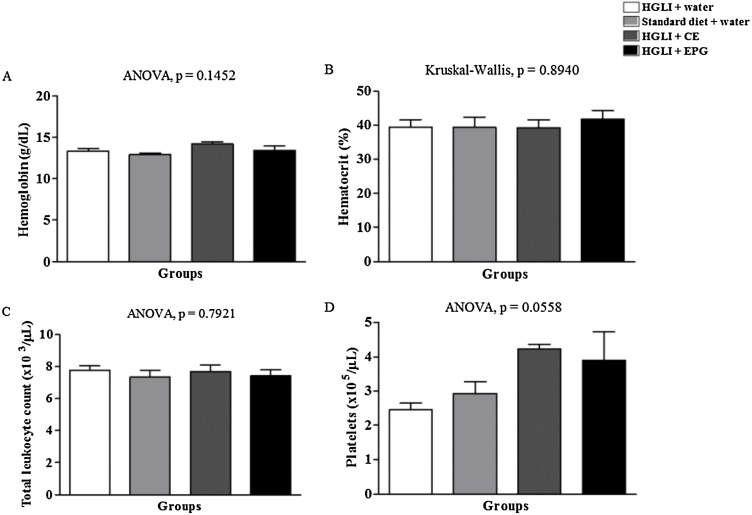
Hemoglobin, hematocrit, total leukocyte, and platelet count showed no statistically significant difference (Fig. 2), and the values were within the reference range (Table 1). There is no consensus regarding reference values for hematological parameters in Wistar rats. However, changes in these parameters can be associated with toxicity in a compound [18]. The present study demonstrated that there was no significant change for hematological parameters.
Fig. 2.
Toxicity of the bioactive dose of EPG (50 mg/kg of weight) under hematological parameters in Wistar rats. CE: crude carotenoid extract from Cantaloupe melon (Cucumis melo L.). EPG: crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin. HGLI: diet with a high glycemic index and high glycemic load. The significant difference between the groups was assessed using Kruskal-Wallis and Dunn's post-test for non-parametric variables, according to the Kolmogorov-Smirnov normality test, with p < 0.05. Moreover, ANOVA (p < 0.05) and Tukey's post-test were used for parametric variables. (1,5-column fitting image).
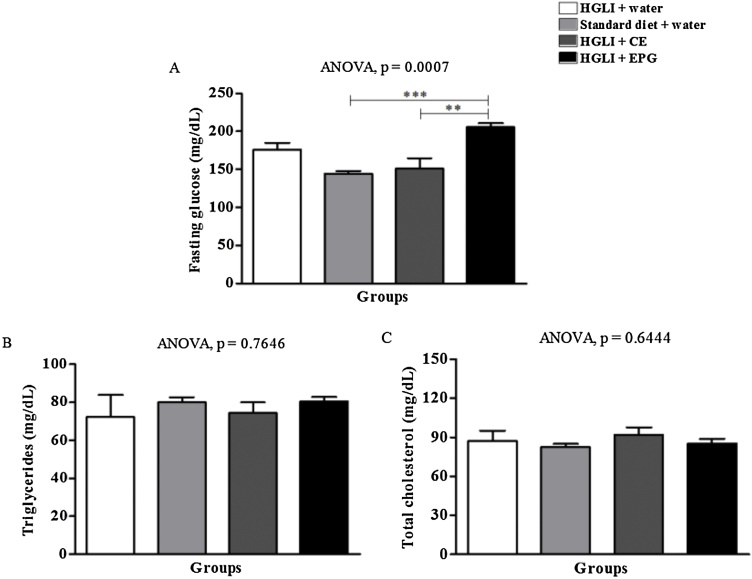
Serum glucose (Fig. 3A) was above the mean reference values in all studied groups (Table 1). This increase was expected as a response to the ingestion for 17 weeks of the HGLI diet, which rich in carbohydrates [21]. Although EPG treated animals presented higher blood glucose than those receiving CE or standard diet, these values were not different from those of untreated animals (Fig. 3A). Triglycerides (Fig. 3B) and total cholesterol (Fig. 3C) were not different between the studied groups, and the mean values were within the normal range.
Fig. 3.
Toxicity of the bioactive dose of EPG (50 mg/g of weight) under parameters of fasting blood glucose and lipid profile in Wistar rats. CE: crude carotenoid extract from Cantaloupe melon (Cucumis melo L.). EPG: crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin. HGLI: diet with a high glycemic index and high glycemic load. The significant difference between the groups was assessed using ANOVA (p < 0.05) and Tukey's post-test. ***: p < 0.001. **: p < 0.01. (1,5-column fitting image).
Changes in hematological parameters, significant variations for fasting blood glucose, triglycerides, and total cholesterol indicate toxicity [18]. However, EPG even showed a significant increase in glucose than the groups treated with the standard diet. CE presented low toxicity, considering there were no significant differences compared to the untreated group.
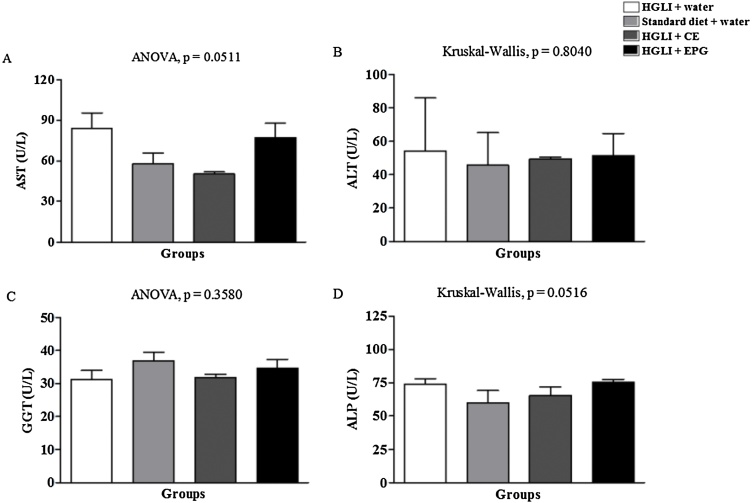
Concerning liver parameters, including AST, ALT, GGT, and alkaline phosphatase (ALP) (Fig. 4), no statistical differences were observed between the groups of Wistar rats analyzed, and the mean values found were within the reference range for eutrophic animals.
Fig. 4.
Toxicity of the bioactive dose of EPG (50 mg/kg in weight) under biochemical parameters in Wistar rats. CE: crude carotenoid extract from Cantaloupe melon (Cucumis melo L.). EPG: crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin. HGLI: diet with a high glycemic index and high glycemic load. AST: aspartate transferase. ALT: alanine aminotransferase. GGT: gamma-glutamyl transferase. ALP: alkaline phosphatase. The Kruskal-Wallis test and Dunn's post-test were used to compare the groups for non-parametric variables, according to the Kolmogorov-Smirnov normality test, with p < 0.05. ANOVA and Tukey's post-test were used to compare the studied groups for the parametric variables (p < 0.05). (1,5-column fitting image).
Studies have demonstrated that carotenoids when administered free or encapsulated by different techniques and encapsulating agents, alter liver transaminases [20,31]. Accumulation of carotenoids in the liver can occur because it is the reserve organ of this bioactive component, causing liver enzymes' elevation even before the appearance of tissue lesions and, thus, indicating a possible hepatotoxic potential [32]. However, the isolated increase in this parameter cannot be considered a standard for diagnosing liver damage. It is necessary to perform histopathological studies to observe the compounds' action against liver tissue cells. The EPG did not significantly change the transaminases in the present study, indicating low liver toxicity of this nanoencapsulated compound.
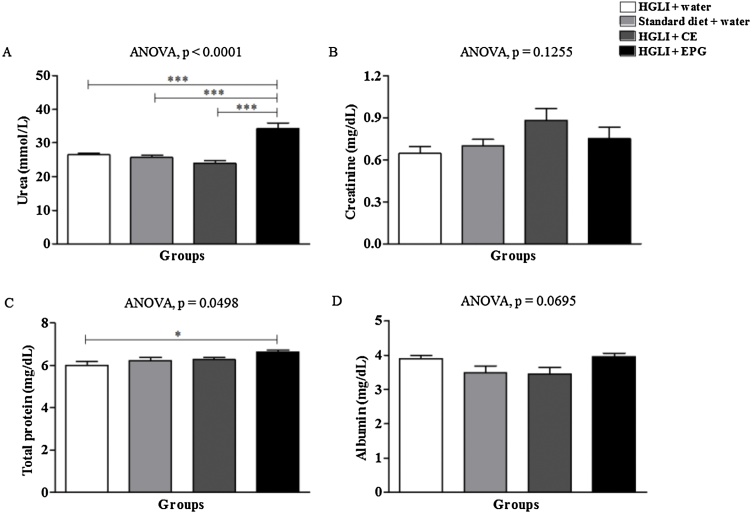
Plasma urea concentrations were significantly higher in the group receiving EPG than the other studied groups, although all mean values were within the normal range (Fig. 5A). Creatinine (Fig. 5B) did not differ between the studied groups, and the mean values were also within the reference parameters. Total protein (Fig. 5C) was also significantly higher in the group receiving EPG than the untreated group, but all mean values were within the normal range (Table 1).
Fig. 5.
Toxicity of the bioactive dose of EPG (50 mg/kg in weight) under renal and protein biochemical parameters in Wistar rats. CE: crude carotenoid extract from Cantaloupe melon (Cucumis melo L.). EPG: crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin. HGLI: diet with a high glycemic index and high glycemic load. ANOVA and Tukey's post-test were used to compare the studied groups (p < 0.05). ***: p < 0.001. *: p < 0.05. (1,5-column fitting image).
Albumin concentrations were higher than the reference values (Table 1) in all studied groups and did not change as a response to the tested treatments (Fig. 5D). These high albumin concentrations might be explained by the fact that the HGLI diet influenced the inflammatory process characterized by increased albumin [33].
Studies are not unanimous regarding the increase or reduction of renal and protein parameters in response to the use of carotenoids encapsulated. Stojiljkovic et al. [34], when administering lycopene encapsulated in liposomes to Wistar rats, with kidney damage caused by methotrexate, found a significant reduction in serum urea and creatine concentrations. The present study was conducted with a crude carotenoid extract different from the previously mentioned. Besides, the method and wall material used for encapsulation in the present study are different. The blood parameters demonstrated low toxicity of the EPG, despite the significant changes in fasting blood glucose, urea, and total proteins presented by the evaluated groups of Wistar rats.
3.3.3. Assessment of relative weight, organ morphology and histopathology
These analyses help identify changes in the cell's organs and even in the presence of tumors seen under microscopy. In the present study, evaluations of the liver, small intestine, kidneys, stomach, and spleen were performed. When macroscopically evaluating the organs, no apparent changes were observed.
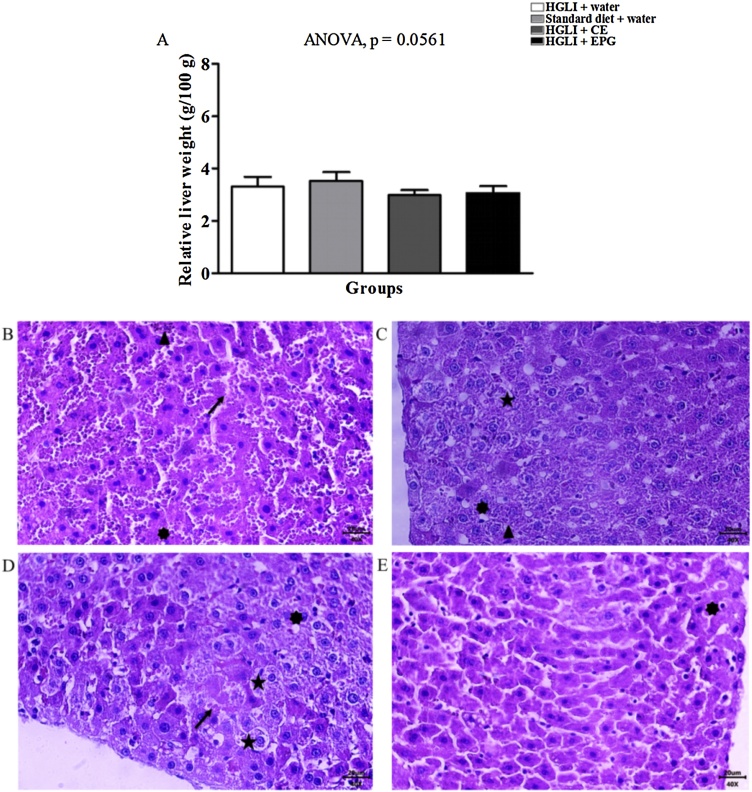
The relative liver weight ranged from 2.99 to 3.52 % and did not differ between the studied groups (Fig. 6A), demonstrating that the liver did not change in weight due to the EPG treatment. Other studies with different carotenoids and encapsulation techniques also indicated that there was no significant difference in the relative weights of the livers in response to the use of the particles [31,35].
Fig. 6.
Relative liver weight and liver histological sections stained with H&E revealing acute hepatitis in Wistar rats. Bar scale: 40x (20 μm). (A) Relative liver weight of the groups studied. (B) HGLI diet + water by gavage (n = 5). (C) Standard diet + water by gavage (n = 5). (D) CE (12.5 mg/kg of weight) and HGLI diet (n = 5). (E) EPG (50 mg/kg of weight) and HGLI diet (n = 5). CE: crude carotenoid extract from Cantaloupe melon (Cucumis melo L.). EPG: crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin. HGLI: diet with a high glycemic index and high glycemic load.  : hyperemia.
: hyperemia.  : ballooning degeneration.
: ballooning degeneration.  : microvesicular steatosis.
: microvesicular steatosis.  : necrosis. ANOVA and Tukey's post-test were used to compare the studied groups (p < 0.05). (1,5-column fitting image).
: necrosis. ANOVA and Tukey's post-test were used to compare the studied groups (p < 0.05). (1,5-column fitting image).
The main histopathological findings for the liver in all groups evaluated were the presence of hyperemia, ballooning degeneration, microvesicular steatosis, and hepatocytes suggestive of necrosis. The lesions with worsened aspects were verified for the untreated group (Fig. 6B). However, the group treated with the standard diet presented an improved aspect concerning ballooning degeneration and reduced areas with necrosis (Fig. 6C). The rats treated with CE (Fig. 6D) showed an improved aspect of the parenchyma and stroma and a few hepatocyte necrosis areas. Moreover, the group that received EPG showed similar results to the previous group (Fig. 6E).
Histological data indicated a diagnosis of hepatic steatosis for all groups evaluated, probably due to the HGLI diet's consumption, rich in carbohydrates with a predominance of ultra-processed food offered during the induction of inflammation for 17 days. This same effect had already been observed in another study, using this same HGLI diet [21].
The consumption of a diet rich in ultra-processed foods associated with a sedentary lifestyle represents possible risk factors for developing fatty liver [36], obesity, type 2 diabetes, metabolic syndrome, dietary disorders, among other diseases [37]. The liver transaminases' results reinforce the histological data of the groups treated with CE and EPG, which showed better aspects of the liver, especially of the parenchyma and stroma. Thus, these two treatments conditioned the scarce areas of necrosis in hepatocytes differently from the untreated group or treated with the standard diet.
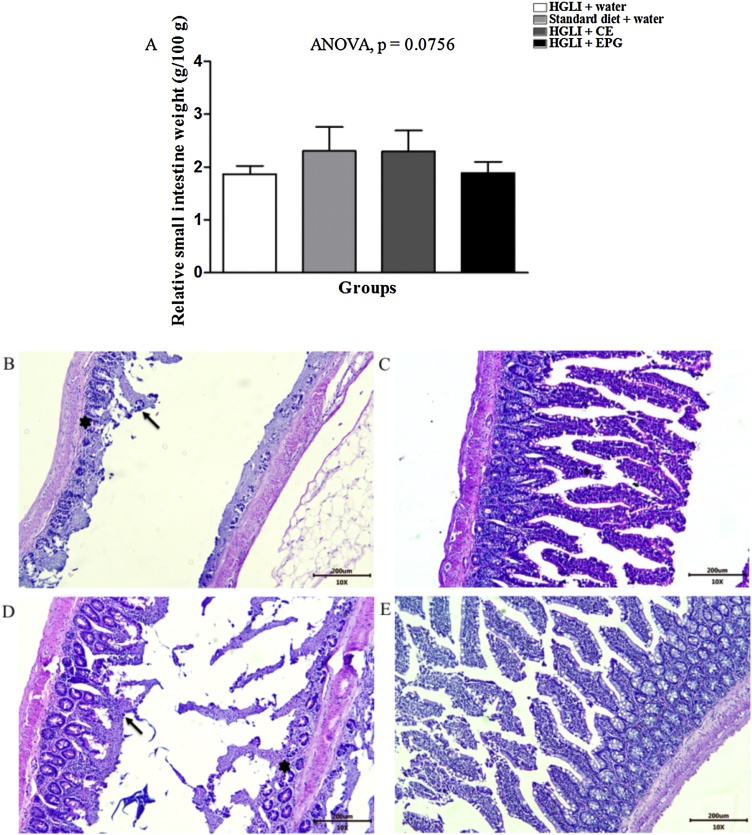
Histological data of the small intestine (Fig. 7) showed that all groups presented chronic enteritis, classified as mild (rats treated with standard diet and EPG), moderate (untreated group), and severe (rats treated with CE) (Supplementary Fig. S1). This result was mainly attributed to the HGLI diet consumption, which is highly inflammatory [21].
Fig. 7.
Small intestine relative weight and histological sections stained with HE revealing chronic enteritis in Wistar rats. Bar scale: 10x (200 μm). (A) Small intestine relative weight in the studied groups. (B) HGLI diet + water by gavage (n = 5). (C) Standard diet + water by gavage (n = 5). (D) CE (12.5 mg/kg of weight) and HGLI diet (n = 5). (E) EPG (50 mg/kg of weight) and HGLI diet (n = 5). CE: crude carotenoid extract from Cantaloupe melon (Cucumis melo L.). EPG: crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin. HGLI: diet with a high glycemic index and high glycemic load. : intestinal villus necrosis.
: intestinal villus necrosis. : destruction of intestinal glands. ANOVA and Tukey's post-test were used to compare the studied groups (p < 0.05). (1,5-column fitting image).
: destruction of intestinal glands. ANOVA and Tukey's post-test were used to compare the studied groups (p < 0.05). (1,5-column fitting image).
For the relative weight of the small intestine, there was no statistically significant difference (p > 0.05) between the groups evaluated (Fig. 7A). The results showed considerable lesions in untreated animals and treated with the CE (Fig. 7B and D). For untreated Wistar rats, there was an absence of a large part of the intestinal villi, necrosis in the few remaining villi. In addition to a significant destruction of the intestinal glands compromising the normal functions of this organ. In animals treated with CE, intestinal glands and villi were found, although a large part was ulcerated or necrotic.
On the other hand, for the group treated with a standard diet (Fig. 7C), improved integrity of the intestinal glands and villi was found, even though ulcerated areas were evident. The group treated with EPG (Fig. 7E) also showed improved integrity for the villi and intestinal glands. Therefore, nanoencapsulation potentiated the crude extract rich in carotenoids, considering that the small intestines analyzed presented aspects related to this organ's integrity and functionality compared to the other groups.
Semiquantitative assessments of intestinal sections (Supplementary Fig. S1) showed no statistically significant difference (p > 0.05). On the other hand, there were significant negative correlations (p < 0.05) for PIGI and PVN (r = −0.786, p < 0.01), PVI and PVN (r = −0.481, p = 0.043) (Supplementary Table S1). These results were expected, considering that the morphological parameters evaluated refer to necrosis and the intestinal sections' integrity, which justifies this high and moderate negative correlation, respectively. Considering the presence of intact intestinal glands and the absence of villi, between PIGI and PVA (r = −0.566, p = 0.09), there was also a statistically significant difference and a moderate negative correlation (Fig. 7B and D). Besides, there was a moderate positive correlation between PVA and PVN (r = 0.506, p = 0.032) and PIGI and PVI (r = 0.520, p = 0.027), both showing a statistically significant difference.
Therefore, necrosis exhibited a positive correlation with the absence of villi in all groups of animals evaluated, which could be seen in the intestinal histological sections stained with H&E (Fig. 7). Besides, the intestinal glands' integrity depends on the integrity of the villi. Thus, the group treated with EPG for ten days showed improved aspects of the intestinal structures evaluated (Fig. 7D).
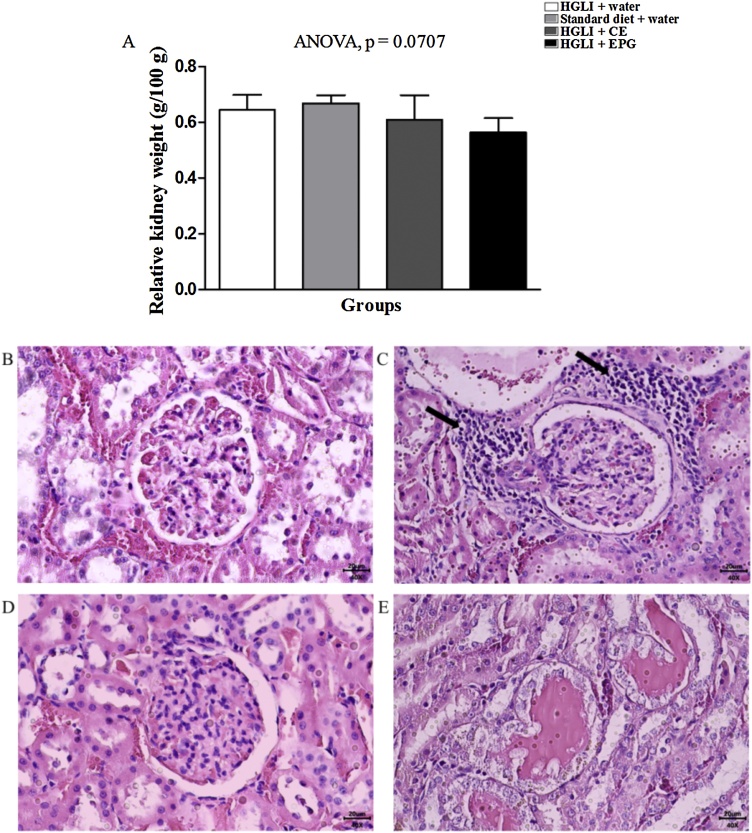
Regarding the relative weight of the kidneys (Fig. 8), there was a variation from 0.56 to 0.67 g/100 g (Fig. 8A), with no statistically significant difference (p > 0.05) between the groups evaluated. Other studies with encapsulated carotenoids also did not identify a significant difference in the kidneys' relative weight [35]. As for histopathological findings, at least one animal belonging to each evaluated group had, to a small extent, an inflammatory infiltrate focus (Fig. 8). The results of the increased albumin dosage already indicated inflammation, being in line with the histological analysis.
Fig. 8.
Kidney relative weight and renal histological sections stained with HE revealed inflammatory infiltrate in Wistar rats. Bar scale: 40x (20 μm). (A) Kidney relative weight of the studied groups. (B) HGLI diet + water by gavage (n = 5). (C) Standard diet + water by gavage (n = 5). (D) CE (12.5 mg/kg of weight) and HGLI diet (n = 5). (E) EPG (50 mg/kg of weight) and HGLI diet (n = 5). CE: crude carotenoid extract from Cantaloupe melon (Cucumis melo L.). EPG: crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin. HGLI: diet with a high glycemic index and high glycemic load.  : Mononuclear inflammatory infiltrate focus. ANOVA and Tukey's post-test were used to compare the studied groups (p < 0.05). (1,5-column fitting image).
: Mononuclear inflammatory infiltrate focus. ANOVA and Tukey's post-test were used to compare the studied groups (p < 0.05). (1,5-column fitting image).
Considering the analyzed criteria, such as the percentage of hypertrophic renal tubules, glomerular hyalinization, and dilation of Bowman's space, an aspect of normality was verified and, therefore, they were classified with a score of 0 (Supplementary Fig. S2). Besides, there was no significant difference (p > 0.05) for semiquantitative assessments of the renal sections of the evaluated groups. Therefore, the observed change, related to the foci of inflammatory infiltrates and tubular hyaline cylinders (p > 0.05), is probably associated with the consumption of the HGLI diet for 17 weeks to induce chronic inflammation. Besides, Spearman's correlation (Table 2 supplementary material) did not show any significant difference (p > 0.05) for the analyzed data, which may suggest that the changes in the evaluated parameters are independent.
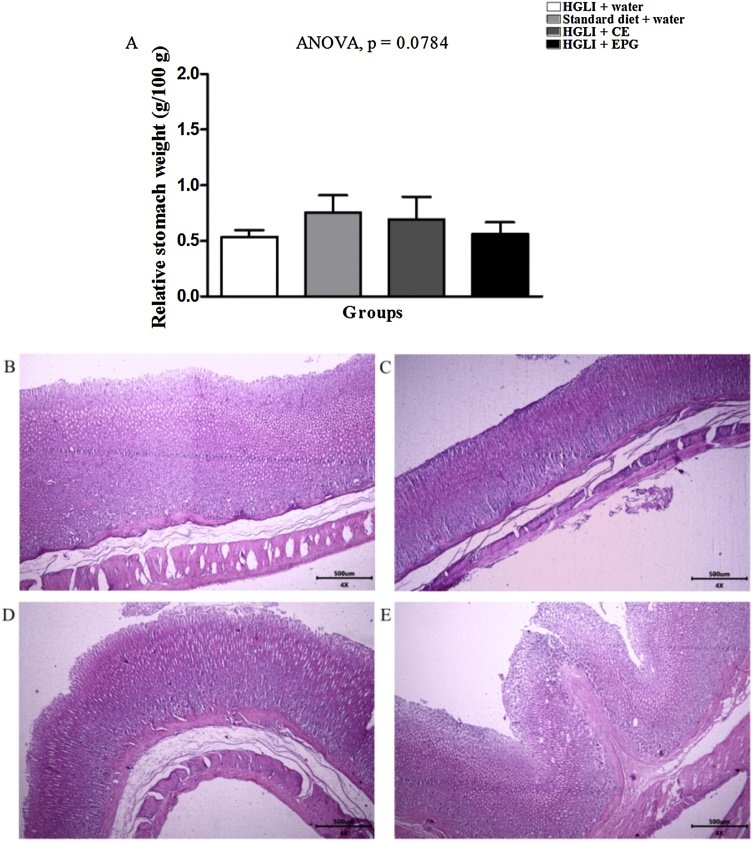
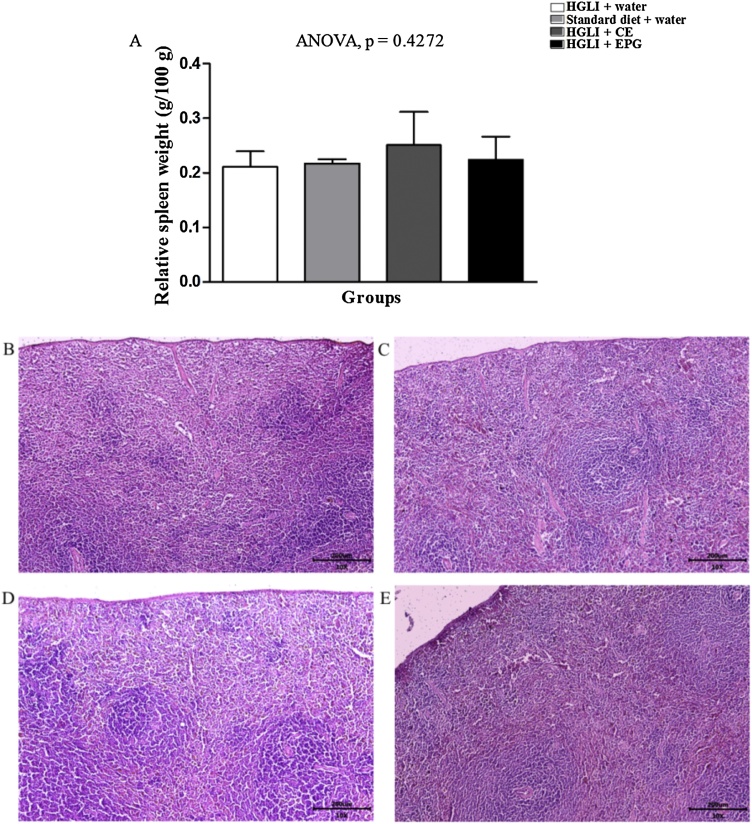
The stomach’s relative weight also did not differ between the studied groups, ranging from 0.52 to 0.94 g/100 g (Fig. 9A). All animals presented normal aspect stomachs with preserved stomach layers and sublayers. The same results were observed for the spleen, with normal relative weights (0.21 to 0.25 g/100 g) and well-defined areas of white and red flesh with aspects of normality (Fig. 10A).
Fig. 9.
Stomach relative weight and histopathological sections stained with HE revealed aspects of normality in Wistar rats. Bar scale: 4x (500 μm). (A) Stomach relative weight in the groups studied. (B) HGLI diet + water by gavage (n = 5). (C) Standard diet + water by gavage (n = 5). (D) CE (12.5 mg/kg of weight) and HGLI diet (n = 5). (E) EPG (50 mg/kg of weight) and HGLI diet (n = 5). CE: crude carotenoid extract from Cantaloupe melon (Cucumis melo L.). EPG: crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin. HGLI: diet with a high glycemic index and high glycemic load. ANOVA and Tukey's post-test were used to compare the studied groups (p < 0.05). (1,5-column fitting image).
Fig. 10.
Spleen relative weight and histopathological sections stained with HE revealed aspects of normality in Wistar rats. Bar scale: 10x (200 μm). (A) Relative spleen weight of the studied groups. (B) HGLI diet + water by gavage (n = 5). (C) Standard diet + water by gavage (n = 5). (D) CE (12.5 mg/kg of weight) and HGLI diet (n = 5). (E) EPG (50 mg/kg of weight) and HGLI diet (n = 5). CE: crude carotenoid extract from Cantaloupe melon (Cucumis melo L.). EPG: crude carotenoid extract from Cantaloupe melon nanoencapsulated in porcine gelatin. HGLI: diet with a high glycemic index and high glycemic load. ANOVA and Tukey's post-test were used to compare the studied groups (p < 0.05). (1,5-column fitting image).
In the present study, different results showed inflammation in the liver, small intestine, and kidneys of Wistar rats fed the HGLI diet, rich in ultra-processed food. The EPG presented anti-inflammatory potential, as revealed by the histological aspect in the liver and intestine. However, we did not evaluate inflammatory markers, which is a limitation of the study, that should be explored in future studies.
The results have shown that there was no toxicological evidence related to the administration of EPG. Therefore, this study is essential since a previous study [13] showed that nanoencapsulation provided the improvement of technological factors, limiting the application of carotenoids in food (water solubility and color stability in food matrix).
Another interesting finding is that the animals treated with CE or EPG did not show the classic signs of hypervitaminosis A, such as enlarged spleen and liver, elevated serum concentrations of alkaline phosphatase, AST, ALT, GGT or lymphocytic infiltrates. This finding is of great importance, considering that carotenoids are also precursors of vitamin A. Once nanoencapsulated, this vitamin A function could be enhanced, and the related risks of hypervitaminosis A would be evident.
4. Conclusion
Nanoparticles with crude carotenoid extract from Cantaloupe melon (Cucumis melo L.) presented low toxicity and better appearance in the liver and intestine in a chronic inflammatory experimental model. The results suggest EPG is safe for application and presents a bioactive effect, possibly related to the anti-inflammatory potential of the carotenoid extract, demonstrating the safety and potential for use in clinical studies.
Funding
This work was supported by the Coordination for the Improvement of Higher Education Personnel (Finance Code 001 - CAPES) and National Council for Scientific and Technological Development (CNPq) for the Universal Project number: 422405/2016-7.
CRediT authorship contribution statement
Isaiane Medeiros: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Grazielle Louise Ribeiro de Oliveira: Methodology, Investigation. Jaluza Luana Carvalho de Queiroz: Methodology, Investigation. Camila de Carvalho Gomes: Methodology, Investigation. Fabiana Maria Coimbra de Carvalho: Resources, Supervision. Maíra Conceição Jerônimo de Souza Lima: Resources, Supervision. Alexandre Coelho Serquiz: Resources. Pedro Paulo de Andrade Santos: Methodology, Resources. Christina da Silva Camillo: Methodology, Resources. Bruna Leal Lima Maciel: Formal analysis, Writing - review & editing. Ana Heloneida de Araújo Morais: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing - review & editing. Thaís Souza Passos: Conceptualization, Methodology, Software, Formal analysis, Investigation, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We thank Prof. Dr. Cícero Flávio Soares Aragão of the Laboratory of Quality Control of Medicines of the Department of Pharmacy of UFRN; Nicolau Apoena Castro of the Laboratory of Structural Characterization of Materials of the Department of Materials Engineering of UFRN; Professor Dra. Raquel Brandt Giordani of the Laboratory of Pharmacognosy at the Pharmacy Department of UFRN; and Professor Dr. Daniel Pontes of the Analytical Center at the Institute of Chemistry of UFRN.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00567.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Rao A., Rao L. Carotenoids and human health. Pharmacol. Res. 2007;55:207–216. doi: 10.1016/j.phrs.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Ezzat S.M., Raslan M., Salama M.M., Menze E.T., Hawary S.S.E. In vivo anti-inflammatory activity and UPLC-MS/MS profiling of the peels and pulps of Cucumis melo var. cantalupensis and Cucumis melo var. reticulatus. J. Ethnopharmacol. 2019;237:245–254. doi: 10.1016/j.ped.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Leão D.P., Franca A.S., Oliveira L.S., Bastos R., Coimbra M.A. Physicochemical characterization, antioxidant capacity, total phenolic and proanthocyanidin content of flours prepared from pequi (Caryocar brasilense Camb.) fruit by-products. Food Chem. 2017;225:146–153. doi: 10.1016/j.foodchem.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 4.Murillo E., Giuffrida D., Menchaca D., Dugo P., Torre G., Meléndez-Martinez A.J. Native carotenoids composition of some tropical fruits. Food Chem. 2013:825–836. doi: 10.1016/j.foodchem.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Laur L.M., Tian L. Provitamin A and vitamin C contents in selected California-grown cantaloupe and honeydew melons and imported melons. J. Food Compos. Anal. 2011;24(2):194–201. doi: 10.1016/j.jfca.2010.07.009. [DOI] [Google Scholar]
- 6.Bonet M.L., Canas J.A., Ribot J., Palou A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 2015;572:112–125. doi: 10.1016/j.abb.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Kaulmann A., Bohn T. Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014;34(11):907–929. doi: 10.1016/j.nutres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Tripathi D.N., Jena G.B. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: role of Nrf2, p53, p38 and phase-II enzymes. Mutat. Res. – Genet. Toxicol. Environ. Mutagen. 2010;696(1):69–80. doi: 10.1016/j.mrgentox.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Rubin L.P., Ross A.C., Stephensen C.B., Bohn T., Tanumihardjo S.A. Metabolic effects of inflammation on vitamin A and carotenoids in humans and animal models. Adv. Nutr. 2017;8(2):197–212. doi: 10.3945/an.116.014167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonet M.L., Ribot J., Galmés S., Serra F., Palou A. Carotenoids and carotenoid conversion products in adipose tissue biology and obesity : pre-clinical and human studies. BBA – Mol. Cell Biol. Lipids. 2020;1865(11):1–23. doi: 10.1016/j.bbalip.2020.158676. [DOI] [PubMed] [Google Scholar]
- 11.Soukoulis C., Bohn T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2018;58(1):1–36. doi: 10.1080/10408398.2014.971353. [DOI] [PubMed] [Google Scholar]
- 12.Augustin M.A., Sanguansri L. Encapsulation of bioactives. In: Aguilera J.M., Lillford Pj., editors. Food Materials Science Principles and Practice. 1st ed. Springer; New york: 2008. pp. 1–611. [DOI] [Google Scholar]
- 13.Medeiros A.K.D.O.C., Gomes C.D.C., Amaral M.L.Q.D.A., Medeiros L.D.G.D., Medeiros I., Porto D.L. Nanoencapsulation improved water solubility and color stability of carotenoids extracted from Cantaloupe melon (Cucumis melo L.) Food Chem. 2019:270. doi: 10.1016/j.foodchem.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 14.European Food Safety Authorit (EFSA) Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: part 1, human and animal health. EFSA J. 2018;16(7):95. doi: 10.2903/j.efsa.2018.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biehler E., Bohn T. Methods for assessing aspects of carotenoid bioavailability. Curr. Nutr. Food Sci. 2010;6(1):44–69. doi: 10.1111/j.1750-3841.2009.01417.x. [DOI] [Google Scholar]
- 16.Rodriguez-Amaya D.B. In: A Guide to Carotenoid Analysis in Foods. 1th ed. Life Sciences, editor. ILSI Human Nutrition Institute; Washington: 2001. [Google Scholar]
- 17.Hu K., Huang X., Gao Y., Huang X., Xiao H., Mcclements D.J. Core – shell biopolymer nanoparticle delivery systems: synthesis and characterization of curcumin fortified zein – pectin nanoparticles. Food Chem. 2015;182:275–281. doi: 10.1016/j.foodchem.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 18.OECD (Organisation for Economic Co-operation and Development) OECD Publishing; 2001. OECD Guideline for Testing of Chemicals. Test No. 423: Acute Oral Toxicity – Acute Toxic Class Method; p. 14. [DOI] [Google Scholar]
- 19.Committee for the Update of the Guide for the Care and Use of Laboratory And Animals; Institute for Laboratory Animal Research; Division on Life Studies . 8th ed. The National Academies Press; Washington: 2011. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals; p. 246. [DOI] [Google Scholar]
- 20.Pinzón-García A.D., Orellano L.A.A., Lazari M.G.T., Campos P.P., Cortes M.E., Sinisterra R.D. Evidence of hypoglycemic, lipid-lowering and hepatoprotective effects of the Bixin and Bixin: β-CD inclusion compound in high-fat-fed obese mice. Biomed. Pharmacother. 2018;106:363–372. doi: 10.1016/j.biopha.2018.06.144. [DOI] [PubMed] [Google Scholar]
- 21.Luz A.B.S., dos Figueredo J.B.S., Salviano B.D.P.D., Aguiar A.J.F.C., Pinheiro L.G.S.D., Krause M.F.D. Adipocytes and intestinal epithelium dysfunctions linking obesity to inflammation induced by high glycemic index pellet-diet in Wistar rats. Biosci. Rep. 2018;38(3) doi: 10.1042/BSR20180304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matias L.L.R., Costa R.O.A., Passos T.S., Queiroz J.L.C., Serquiz A.C., Maciel B.L.L. Tamarind trypsin inhibitor in chitosan–whey protein nanoparticles reduces fasting blood glucose levels without compromising insulinemia: a preclinical study. Nutrients. 2019:11. doi: 10.3390/nu1112770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva R.O., Andrade V.M., Rêgo E.S.B., Dória G.A.A., Lima B.S., Silva F.A. Acute and sub-acute oral toxicity of Brazilian red propolis in rats. J. Ethnopharmacol. 2015;170:66–71. doi: 10.1016/j.jep.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Martins L.B., de Oliveira M.C., Menezes-Garcia Z., Rodrigues D.F., Lana J.P., Vieira L.Q. Paradoxical role of tumor necrosis factor on metabolic dysfunction and adipose tissue expansion in mice. Nutrition. 2018;50:1–7. doi: 10.1016/j.nut.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Silva P.F., Mcgurk C., Knudsen D.L., Adams A., Thompson K.D., Bron J.E. Histological evaluation of soya bean-induced enteritis in Atlantic salmon (Salmo salar L.): quantitative image analysis vs. semi-quantitative visual scoring. Aquaculture. 2015;445:42–56. doi: 10.1016/j.aquaculture.2015.04.002. [DOI] [Google Scholar]
- 26.Rodriguez-Amaya D.B., Kimura M., Godoy H.T., Amaya-Farfan J. Updated Brazilian database on food carotenoids: factors affecting carotenoid composition. J. Food Compos. Anal. 2008;21(6):445–463. doi: 10.1016/j.jfca.2008.04.001. [DOI] [Google Scholar]
- 27.Fleshman M.K., Lester G.E., KENM Riedl, Kopec R.E., Narayanasamy S., Curley R.W., Jr. Carotene and novel apocarotenoid concentrations in orange-fleshed Cucumis melo Melons: determinations of β-carotene bioaccessibility and bioavailability. J. Agric. Food Chem. 2011;59(9):4448–4454. doi: 10.1021/jf200416a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernández V., Hellín P., Fenoll J., Flores P. Increased temperature produces changes in the bioactive composition of tomato, depending on its developmental stage. J. Agric. Food Chem. 2015;63(9):2378–2382. doi: 10.1021/jf200416a. [DOI] [PubMed] [Google Scholar]
- 29.Moura F.F., Miloff A., Boy E. Retention of provitamin A carotenoids in staple crops targeted for biofortification in Africa: cassava, maize and sweet potato. Crit. Rev. Food Sci. Nutr. 2015;55(9):1246–1269. doi: 10.1080/10408398.2012.724477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y.C., Huang H.H., Wu Y.J., Manousakas I., Yang C.C., Kuo S.M. Therapeutic and protective effects of liposomal encapsulation of astaxanthin in mice with alcoholic liver fibrosis. Int. J. Mol. Sci. 2019;20(16) doi: 10.3390/ijms20164057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lima F.F., Traesel G.K., Menegati S.E.L.T., Santos A.C., Souza R.I.C., Oliveira V.S. Acute and subacute oral toxicity assessment of the oil extracted from Attalea phalerata Mart ex Spreng. pulp fruit in rats. Food Res. Int. 2017;91:11–17. doi: 10.1016/j.foodres.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Farhangi M.A., Keshavarz S.A., Eshraghian M., Ostadrahimi A., Saboor-Yaraghi A.A. Vitamin A supplementation, serum lipids, liver enzymes and C-reactive protein concentrations in obese women of reproductive age. Ann. Clin. Biochem. 2013;50(1):25–30. doi: 10.1258/acb.2012.012096. [DOI] [PubMed] [Google Scholar]
- 33.Cray C., Zaias J., Altman N.H. Acute phase response in animals: a review. Comp. Med. 2009;59(6):517–526. [PMC free article] [PubMed] [Google Scholar]
- 34.Stojiljkovic N., Ilic S., Jakovljevic V., Stojanovic N., Stojnev S., Kocic H. The encapsulation of lycopene in nanoliposomes enhances its protective potential in methotrexate-induced kidney injury model. Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/2627917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu C.H., Chang C.C., Lin S.T., Chyau C.C., Peng R.Y. Improved hepatoprotective effect of liposome-encapsulated astaxanthin in lipopolysaccharide-induced acute hepatotoxicity. Int. J. Mol. Sci. 2016;17(7) doi: 10.3390/ijms17071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro G.S.F., Almeida B.B., Leonardi D.S., Ovídio P.P., Jordão A.A. Association between hepatic cholesterol and oleic acid in the liver of rats treated with partially hydrogenated vegetable oil. Rev. Nutr. 2012;25(1):45–56. doi: 10.1590/S1415-52732012000100005. [DOI] [Google Scholar]
- 37.Guillén N., Navarro M.A., Arnal C., Noone E., Arbonés-Mainar J.M., Acín S. Microarray analysis of hepatic gene expression identifies new genes involved in steatotic liver. Physiol. Genomics. 2009;37(3):187–198. doi: 10.1152/physiolgenomics.90339.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.