Abstract
Cancer immunotherapy has veered the paradigm of cancer treatment. Despite recent advances in immunotherapy for improved antitumor efficacy, the complicated tumor microenvironment (TME) is highly immunosuppressive, yielding both astounding and unsatisfactory clinical successes. In this regard, clinical outcomes of currently available immunotherapy are confined to the varied immune systems owing in large part to the lack of understanding of the complexity and diversity of the immune context of the TME. Various advanced designs of nanomedicines could still not fully surmount the delivery barriers of the TME. The immunosuppressive TME may even dampen the efficacy of antitumor immunity. Recently, some nanotechnology-related strategies have been inaugurated to modulate the immunosuppressive cells within the tumor immune microenvironment (TIME) for robust immunotherapeutic responses. In this review, we will highlight the current understanding of the immunosuppressive TIME and identify disparate subclasses of TIME that possess an impact on immunotherapy, especially those unique classes associated with the immunosuppressive effect. The immunoregulatory cell types inside the immunosuppressive TIME will be delineated along with the existing and potential approaches for immunosuppressive cell modulation. After introducing the various strategies, we will ultimately outline both the novel therapeutic targets and the potential issues that affect the efficacy of TIME-based nanomedicines.
KEY WORDS: Cancer immunotherapy, Nanomedicine, Tumor immunosuppressive microenvironment, Drug delivery
Graphical abstract
Immunosuppressive cells in the tumor immune microenvironment (TIME) are potential targets for nanomedicine-based immunotherapy. Various regulation strategies have been harnessed to modulate the immunosuppressive TIME.

Highlights
-
•
Immunosuppressive tumor microenvironment dampens the efficacy of antitumor immunity.
-
•
Immunosuppressive cells in the tumor microenvironment are responsible for the negative regulation of immune responses.
-
•
TAMs, MDSCs, Treg cells, and CAFs are the main immunosuppressive cells with extensive research.
-
•
Nanomedicines remodel the TIME by sensitizing the immunosuppressive cells and facilitating the immune cell recruitment.
-
•
Combined nanomedicine-based immunotherapy interacts with the cancer–immunity cycle, yielding amplified therapeutic effect.
1. Introduction
Cancer immunotherapy, which was developed in the light of parsing the mechanism of tumor escape, manipulates the immune system to recognize and reactivate the antitumor immune responses, overcoming the correlated pathways leading to escape1. The past decade has witnessed a massive shift in cancer therapy by moving away from drugs that target tumors extensively (i.e., chemotherapy and radiotherapy) and toward the utilization of nanotechnology-based immunotherapies that modulate immune responses against tumors2,3. Nanomedicines, referred to drug-loaded nanoparticles (NPs) with a diameter of 1–1000 nm, can be harnessed to selectively deliver cytotoxic agents to tumors, increasing therapeutic efficacy and minimizing the systemic adverse reactions4. Although many nanotherapeutics have been approved for cancerous persons, they have modest effects on survival and, in some cases, are less effective than other approved treatments5. In addition, the clinical benefits of several U.S. Food and Drug Administration (FDA)-approved nanomedicines such as liposomal daunorubicin (DaunoXome) and NP albumin-bound paclitaxel (Abraxane) for the cancer therapy were testified to be non-ideal, posing great challenges for the translation development of novel nanomedicines6,7. In this regard, we suggest that the pathophysiology of the tumor microenvironment (TME) may limit the delivery of systemically administered and locally applied nanomedicines, hence compromising their efficacy, even if they could accumulate in tumors8,9.
The tumor lesion is usually surrounded by immune cells, fibroblasts, soluble signaling molecules, blood vessels, along with the extracellular matrix (ECM). These cells/components in the TME are severely resistant to currently available immunotherapy10. The therapeutic effect of antibody–drug conjugates and cancer vaccines can also be greatly attenuated by the immunosuppressive TME11. As a dynamic interaction arena, the TME posed various delivery barriers and is responsible for the modest therapeutic benefits of multiple nanomedicines12. Additionally, the blockage of immune checkpoint molecules with antibodies has shown great promise for shaping tumor immunogenicity, but the response rate toward immune checkpoint inhibitors prodigiously varies among disparate tumor types owing in large part to the intricate TME13. Revealing the complexity and diversity of TME using techniques such as high-resolution single-cell RNA sequencing, flow cytometry, and imaging, is important to understand immunosuppressive effect of the TME, and thereby inspiring the development of nanomedicines to advance cancer immunotherapy10.
For the purpose of improving the therapeutic efficacy of nanomedicines, various strategies such as active targeting ploy, tumor-responsive nanosystems, along with optimization of the physiochemical parameters of nanoparticles have been developed14, 15, 16, 17. Notwithstanding, these approaches hinge on the advanced development of nanomaterials themselves, and cannot overcome the immunosuppressive obstacles of the TIME (Fig. 1). Hence, the modulation of the TIME is of intense interest to serve as a pivotal tool to amplify or rewire the immune system. In this review, we first retrospect and summarize the current understanding of the composition, function, and different immune cells within the TIME. We will then provide a summary of the evidence unraveling how the immunosuppressive TIME confines the therapeutic efficacy of both tumor nanomedicines and immunotherapies, following with the discussion of how modulating the immunosuppressive cells may improve drug delivery and the therapeutic outcomes of cancerous persons upon immunotherapy. Several regulation strategies of the immunosuppressive cells in the TIME will be parsed out and nanomedicine-based ploys that may overcome the resistance to immunotherapies will be highlighted at length. Ultimately, we will propose potent tactics of reengineering and developing novel nanomedicines directing at modulating the tumor immunosuppressive cells while minimizing cytotoxicity and immune-associated adverse events, and we further explore the potential of the combined nanomedicine-based TIME remodeling as well as their clinical translation.
Figure 1.
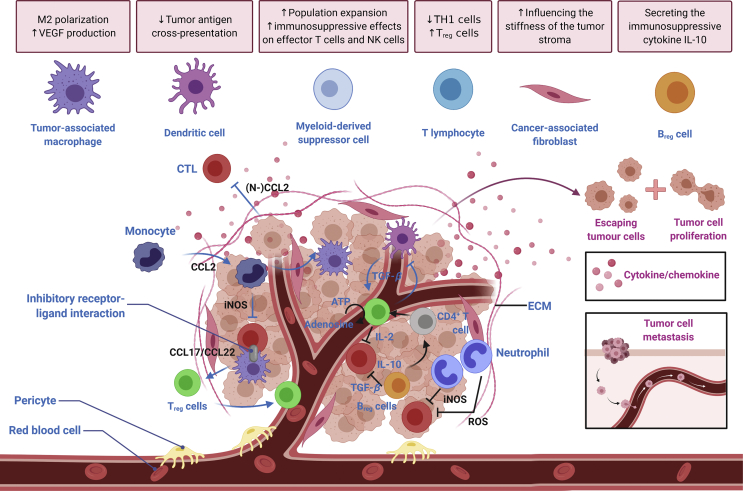
The immunosuppressive tumor immune microenvironment. Various mechanisms are involved in generating an immunosuppressive TIME to promote immune escape, thus facilitating tumor progression and metastasis formation.
2. Tumor immune microenvironment (TIME) and its composition
2.1. Classification of the TIME
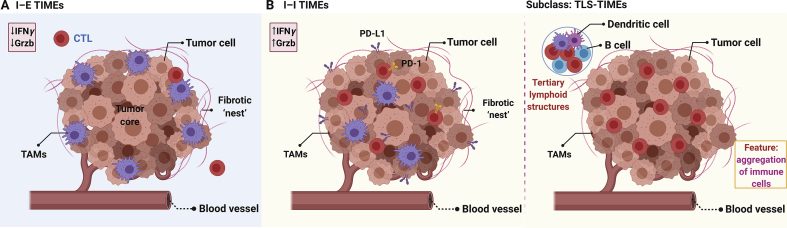
Accordingly, there are two broad categories of moderate-resolution TIME (Fig. 2). 1) “Cold” TIME: infiltrated-excluded (I–E) TIMEs18,19. This kind of TIMEs referred to TIMEs that are widely populated with immune cells but are comparatively void of cytotoxic lymphocytes in the tumor core. Of note, tumors categorized as I-E TIMEs are currently assumed to be poorly immunogenic, such as colorectal cancer, melanoma, and pancreatic ductal adenocarcinoma18,19. In comparison with more inflamed TIMEs, the infiltrated-excluded TIMEs have lower expression of activation markers and poor infiltration of cytotoxic lymphocytes into the tumor core, which can be regarded as features of immune ignorance. The adaptive immunity cannot recognize and respond to the malignancy of cancer20. 2) “Hot” TIME: Infiltrated-inflamed (I–I) TIMEs. The I–I TIMEs are characterized by an abundance of PD-L1 expression on tumor and myeloid cells as well as highly activated cytotoxic lymphocytes. This sort of TIMEs is defined to be immunologically ‘hot’ tumors, bringing about increased numbers of neoepitopes10. Notably, there is a subclass of “Hot” TIME, namely tertiary lymphoid structures (TLSs) TIMEs. This type of TIMEs exhibits histological evidence of encompassing TLSs and lymphoid aggregates with a component closely resembled that in lymph nodes, i.e., dendritic cells (DCs), B cells, and regulatory T (Treg) cells10,21.
Figure 2.
General classification of the TIME. A) and B) are infiltrated-excluded (I–E) TIMEs (“Cold”) and infiltrated-inflamed (I–I) TIMEs (“Hot”), respectively. The right image termed as tertiary lymphoid structures (TLSs) TIMEs is the subclass of this sort of TIME.
2.2. Interconnectivity of tumor phenotypes and the TIME
The tumor phenotype of the TIME affects the sensitivity to immunotherapy (i.e., immune-checkpoint inhibition) and can also be roughly divided into three staple phenotypes22. 1) The immune-desert phenotype lacking antitumor immune cells. It refers to immunological ignorance (lack of antigen or its presentation), tolerance (lack of responses toward antigen presentation), as well as insufficient T cell priming22. Tumors of this phenotype respond least to the immune-checkpoint inhibition. Further, the pathophysiology of TIME contributes to this tumor phenotype. For instance, VEGF, which is an angiogenic growth factor caused by hypoxia, can facilitate immunological ignorance via inhibiting the maturation of DCs, thus dialing down the degree of antigen presentation23. 2) The immune-excluded phenotype, which refers to the location of immune cells around the tumor or interstitial. These immune cells are hindered by immature blood vessels and ECM22, characterized by transforming growth factor-β (TGF-β), high-density cancer-associated fibroblasts (CAFs) along with unrestrained ECM24,25. Desmoplasia is a major reason for this phenotype, CAFs possess immunosuppressive subsets, generating and maintaining the dense desmoplasia that prevents CTL migration26. Besides, angiogenesis accounts for another main reason for this phenotype because angiogenesis signals regulate the expression of adhesion molecules on the vascular walls, thus impairing the degree of leukocyte binding and circumscribing their inlet to the tumor27. 3) The inflamed tumor phenotype with T cells in the parenchyma and the expression of pro-inflammatory cytokines, implicating the pre-existing antitumor immune response that may be prevented by the immunosuppressive TIME22. Actually, this phenotype is most sensitive to immune-checkpoint inhibition owing in large part to the presence of plentiful T cells, regardless of many hypoxic-suppressed immune cells24. Notably, this phenotype involves the recruitment of immunosuppressive cells along with the expression of immune checkpoint molecules. Hence, the reduced activity of immune cells may be the conspicuous attribute of this phenotype28,29. Even if T cells are present in the parenchyma of an inflamed phenotype, environmental factors such as the CAF, acidity and the collagen density may all minimize their cytotoxic level30, 31, 32.
2.3. Characteristics and composition of the immunosuppressive TIME
In general, tumors are composed of various stromal cells, such as immune cells, cells of mesenchymal origin (i.e., fibroblasts, myofibroblasts, and mesenchymal stromal cells), and vascular cells (i.e., pericytes and endothelial cells), along with the structural component, including ECM, and signaling components (i.e., chemokines, cytokines, and growth factors; Fig. 3). The TME contains both immunostimulatory cells [i.e., DCs, cytotoxic T lymphocytes (CTLs), T helper (Th) cells, natural killer (NK) cells, and macrophage type 1 cells] and immunosuppressive cells [i.e., macrophage type 2 cells, myeloid-derived suppressor cells (MDSCs), Treg cells, regulatory B (Breg) cells, and CAFs], possessing a complex and diverse of the immune context. A variety of immunosuppressive cells, as well as a large number of cytokines, chemokines and metabolites secreted by tumor cells, plays a central role in the generation of an inhibitory microenvironment. In this context, the irregular TME limits the efficacy of conventional cancer treatment modalities against advanced cancer and poses challenges such as off-target side effects derived from the immune checkpoint blockade33. However, it also creates unique opportunity for nanomedicine delivery to tumors. Nanomedicines with tunable and specific targeting capability can be exploited to enter tumors with an active process through endothelial cells34. Zhou et al.35 has recently unraveled that nanoconjugates might actively infiltrate throughout the tumor tissue through transcytosis. Of note, cationic nanoconjugate could undergo caveolae-mediated endocytosis and transcytosis, enabling transendothelial and transcellular transport along with a relatively uniform distribution throughout the tumor35. Accordingly, compared with traditional drugs, the TME opens up new opportunities—the nanoparticle–endothelial cell interactions allow nanomedicines to specifically target and aggregate more efficiently at the tumor site36. Moreover, small NPs (10–100 nm in diameter) can be taken up by the lymphatics and diffuse to the lymph node to target lymph node-resident immune cells37. Additionally, nanomedicines can reduce immune-related adverse events, and meanwhile, converting “cold” tumors into “hot” tumors, especially for TIME with a wealth of immunosuppressive cells38,39. Hence, to fully parse out the potential of cancer immunotherapy, we constrain our focus on the identification of the immunosuppressive TIME and introduce critical components associated with immunosuppression.
Figure 3.
Immunosuppressive cells in the TIME as potential targets for nanomedicine-based immunotherapy. Cold tumors are composed of immunosuppressive cells, such as Treg cells, myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), CAFs along with Breg cells, which inhibit the cytotoxic effects of T cells and natural killer (NK) cells through secreting immunosuppressive factors. Various regulation strategies can be harnessed to modulate the immunosuppressive TIME.
2.3.1. Main immune cells within the TIME
2.3.1.1. Tumor-associated macrophages
There are myeloid-derived macrophages and tissue-resident macrophages in the TME collectively referred to as TAMs. The TAM is the most abundant tumor-infiltrating immune cell group in TME40. Macrophages are extremely plastic cells. According to the cytokine type they are exposed to, activated macrophages possess two polarization states: classically activated M1 and alternatively activated M2 phenotypes41. The M1 macrophage, which generates pro-inflammatory cytokines with robust killing effects on pathogens invading the body, plays a significant role in human immune function42. In contrast, M2 macrophage exhibits anti-inflammatory activity by producing cytokines, such as IL-10, IL-13, and TGF-β to boost tumor progression (Fig. 3)43. TAMs can be expressed as the M1 phenotype with tumor-killing function and the M2 phenotype promoting tumor growth, but in the tumor microenvironment, the M2 type is usually dominant44. The M1 phenotype can convert to the M2 phenotype in response to the TIME signals (i.e., cytokines, growth factors, and other cell-derived molecules), and vice versa. Although the macrophage polarization may occur during the progression of the tumor, the dominant M2 phenotype contributes to tumor metastasis and may bring about poor clinical outcomes45.
2.3.1.2. Myeloid-derived suppressor cells
Myeloid-derived suppressor cells are a heterogeneous population of cells generated during cancer, inflammation and other pathologic conditions, and have a distinguished capacity of suppressing T-cell responses46. Indeed, accumulating evidence has demonstrated that MDSCs may contribute to the negative regulation of immune responses during the progression of cancer. Aside from their suppressive effects on adaptive immunity, MDSCs have also been revealed to modulate innate immunity via regulating the cytokine generation of the macrophage47. The activity of these myeloid cells is strictly governed by signal networks from pathogens in the form of Toll-like receptor (TLR) ligands, damage-associated molecular patterns (DAMPs) along with pathogen-associated molecular patterns (PAMPs)48. In cancer patients, MDSCs always coexist with normal monocytes and neutrophils, but there is an elevated and dominant number of MDSCs during tumor progression, thereby inhibiting adaptive immunity and promoting tumor progression and metastasis49. During the tumor progression, some of the obstacles to the differentiation of immature myeloid cells (IMCs) into mature myeloid cells have led to the expansion of this population. More important, the IMC activation in a pathological state causes up-regulation of the expression of its immunosuppressive factors [i.e., arginase 1 (ARG), inducible nitric oxide synthase (iNOS), and elevated generation of nitrogen oxide (NO) as well as the reactive oxygen species (ROS); Fig. 3]46. These myeloid cells also exert an immunosuppressive effect through up-regulating the inhibitory PD-L1 on the surface, releasing immunosuppressive cytokines, such as transforming growth factor (TGF-β) and IL-10, along with the generation of chemokines [i.e., C–C motif chemokine ligand (CCL)4 and CCL5] for regulatory T cells into tumors50.
MDSC is composed of two main subgroups, namely monocytic (M-MDSC)50 and granulocytic or polymorphonuclear (PMN-MDSC)51. In the context of cancer, neutrophils are generally referred to as PMN-MDSCs, which have been identified as the most adverse prognostic immune cell population in terms of a meta-analysis52. As for patients with pancreatic cancer, high neutrophil infiltration is correlated with the worst prognosis53. Also, according to the experimental data stemmed from pancreatic cancer murine models, genetic ablation of CXCR2 or inhibition of CXCR2, which may limit the intratumoral neutrophil accumulation, could reduce the metastasis progression and increase the infiltration of activated T cells, thereby improving responsiveness toward immune checkpoint blockade54,55. Hence, the accumulation of neutrophils may confine the infiltration of cytotoxic T lymphocytes (Fig. 1) along with increasing the capacity of tumor cells to metastasize.
2.3.1.3. Regulatory T cells
Treg cell plays a pivotal role in maintaining peripheral tolerance, restraining autoimmunity, and limiting the chronic inflammatory disease56. The most physiologically relevant Treg cell is characterized by the expression of surface markers CD4/CD25 as well as the transcription factor Forkhead box protein 3 (FoxP3)57. Although this kind of cell can modulate tumor homeostasis, it can also impede antitumor immune responses58,59. Up till now, there is increasing evidence that large numbers of CD4+CD25+FoxP3+ Treg cells infiltrate into the tumor and their presence is regarded as a staple barrier to effective immunotherapy59. Further, these cells are accumulated in the draining lymph nodes and peripheral blood of sufferers with diverse solid tumor types60,61. The increase in the number of Treg cells is usually related to the deterioration of the patient's condition62.
Treg-mediated inhibition mechanisms mainly through: 1) IL-2 consumption. They can express the high-affinity IL-2 receptor α chain (CD25) to strip this cytokine from neighboring cells, thus confining the effector T cell activation and proliferation63; 2) Hindering the co-stimulation of T cells. The CTLA-4 expressed on them possesses a higher affinity for the co-stimulatory molecules (i.e., CD80 and CD86) on dendritic cells than CD28 expressed on T cells64,65; 3) The generation of immunosuppressive cytokines (i.e., TGF-β and IL-10, Fig. 3), which may distort the functions of DCs and T cells66; 4) The induction of dysfunction in CTLs. According to intravital microscopy researches, Treg cells were observed to interplay with antigen-presenting cells (APCs), bringing about the downregulation of the co-stimulatory molecules on APCs and suppressed antitumor immunity of CTLs67,68. In this regard, Treg cells within the TIME can actively inhibit CTLs (Fig. 1), regardless of the activation of T cells in the draining lymph nodes; 5) The induction of NK cell apoptosis. These cells can also induce the apoptosis of NK cells (Fig. 1), resulting in increased lung metastasis of breast cancer cells in murine models69; 6) The expression of high levels of the ectonucleotidases CD39 and CD73. Treg cells can mediate the conversion of extracellular ATP into immunosuppressive adenosine (Fig. 1), thereby inhibiting the activation of CTLs70, 71, 72.
2.3.1.4. B cells
In solid tumors, B cells usually account for a large proportion of tumor-infiltrating leukocytes, yet their function remains controversial in virtue of the observed protumorigenic and antitumorigenic activities73. In this regard, B cells are diverse in phenotype and function. As far back as 1978, studies showed that B cells could promote the growth of certain tumors in murine models and that in B-cell-deficient mice, tumors develop more slowly than those in B-cell competent mice74,75. Aside from their well-established antitumor immunity (i.e., action as APCs, the formation of tertiary lymphoid structures, and generation of antibodies, cytokines, as well as chemokines)76, 77, 78, B cells may interact with immune complexes and myeloid cells, yielding the accumulation of circulating immune complexes in distant organs and recruiting myeloid cells to facilitate metastatic propagation79.
B cells can also affect the immune response by antigen presentation and cytokine generation. They are subverted toward Breg cells inside the TME, which can secrete the immunosuppressive cytokine IL-1080. Breg cells can negatively regulate antitumor immunity via: 1) Generation of immunosuppressive mediators (i.e., IL-10, IL-35, and TGF-β)77; 2) Inactivation of immunostimulatory cells through the expression of immune checkpoints81; 3) Induction the apoptosis of effector T cells (Fig. 1) by expressing the death-inducing molecule Fas ligand (FASL)82; 4) Promotion of the expression of suppressive markers via cell-to-cell contact83. Of note, several other researches have revealed that in the absence of B cells or Breg cells, the metastasis is reduced, and the myeloid compartment, which is also closely related to metastasis promotion, is still greatly expanded84. Bodogai et al.85 further uncovered that the enlarged myeloid compartment exhibited reduced inhibitory capacity under such a condition, implicating that Breg cells are “educating” these cells to form an inhibitory functional phenotype function.
2.3.2. Cancer-associated fibroblasts
Cancer-associated fibroblasts, as a bountiful and active stromal cell population in the TIME, can promote tumorigenicity through initiating the remodeling of ECM or secreting cytokines, conducing to the formation of a desmoplastic tumor niche86. CAFs can interact with the ECM, influencing the stiffness of the tumor stroma. This process can be described with the term “mechanoreciprocity”, which is composed of “inside-out” and “outside-in” signaling patterns87,88. The “inside-out” signal pattern refers to the regulation of integrin–ECM interplays via intracellular signals89. Moreover, the “outside-in” signal pattern is regarded as well-established signaling by which the ECM protein functions as ligands, binding to integrin receptors on the cell membrane90,91. For a long time, CAF-dependent tumor promotion has been attributed to the CAF secretome, but it is undeniable that direct cell contact may also play a critical role in CAF-mediated cancer cell migration and invasion92. These cells are involved in the generation and cross-linking of collagen, contributing to the stiffness of ECM86. Further, CAFs can talk with different stromal components in the desmoplastic TIME. By potentiating various pathways, such as TGF-β and WNT signaling, these cells can also impinge upon the angiogenesis and ECM of proliferative tumor stroma, as well as induce tumor cell drug resistance93. Of note, the cancer-associated fibroblast is responsible for the broad reciprocal signaling interplays with tumor cells and crosstalk with infiltrating leukocytes, and they are also correlated with the immune evasion process94.
2.4. Chronic inflammation and cancer development
Cancer is characterized by unresolved inflammatory responses. The chronic inflammation refers to a long-term, abnormal state of inflammation that cannot be resolved in the usual way, resulting in a progressive alteration in the immune cell types and polarization at the inflammation site79. In this process, the upregulation of immune checkpoint molecules, the release of immune-regulating cytokines, and the activation of immune-regulating cells along with the change of antigen expression can all restrict immunopathology95. In some cases of cancerous persons, the initiating immune trigger remains ambiguous, hence persistently activating inflammatory responses. Despite the use of immunomodulatory mechanisms to limit immunopathology, as a result, the function of CTLs is also suppressed, resulting in a chronically inflammatory but immunosuppressed state96. In addition, tumor cells may actively enhance immunosuppressive mechanisms by secreting and up-regulating immunoregulatory factors (Fig. 1)97. Therefore, malignant cells further generate inflammatory mediators through stimulating stromal cells and immune cells, thereby exacerbating the chronically inflamed microenvironment96,98. Notably, this chronic activation of inflammatory signals not only inhibits the adaptive immunity through mechanisms such as ECM remodeling, promotes angiogenesis, as well as the increased growth and release of immune regulatory factors, but also buoys up the tumor growth concurrently99.
3. Regulation strategies of modulating tumor immunosuppressive microenvironment
With respect to the modulation of the tumor immunosuppressive microenvironment, various regulation strategies (i.e., radiotherapy, photodynamic and photothermal therapies, ultrasound, sonodynamic therapy, magnetomechanical approach100, induction of immunogenic cell death, ferroptosis101, and remodeling of the ECM) have been deployed to reverse or alleviate the immunosuppression effect of TIME (Fig. 3). Remarkably, of these strategies, photothermal and photodynamic therapies, radiotherapy, sonodynamic therapy, and magnetomechanical therapy can be applied to target and attune the functions of immunosuppressive cells in the TIME mainly through sensitizing the immune cells to amplify the antitumor efficacy of combination immunotherapy. For instance, radiotherapy can not only bring about the elevated level of presented antigen peptides on the surface of dying cancer cells102,103 but also boost the recruitment and expansion of immunosuppressive cell types (Treg cells—less radiosensitive than other lymphocytes, Fig. 4)104,105. Besides, photodynamic therapy can elicit the recruitment of neutrophils at the site of treatment within a few minutes after irradiation106. Accordingly, either physical or chemobiological strategies can be deployed to rewire the immune system and reverse the immunosuppressive effects of various immune cells.
Figure 4.
Radiotherapy deployed to target and attune the functions of immunosuppressive cells within the TIME. In addition to directly kill tumor cells, this therapy can initiate antitumor immune responses due to immunogenic cell death and potentiate the release of DAMPs along with antigen peptides. In this context, alterations in chemokines may also remobilize T cells and repolarize the M2 macrophages to the M1 macrophages. Down-regulation of Treg cells can also be observed, thus modulating the TIME.
Nanomedicines were originally under development to improve the pharmacokinetics along with toxicity profiles of chemotherapeutics and to facilitate their accumulation in tumors. In addition, the capacity of concentrating agents within the TIME is of relevance for promoting potent immunotherapy. Of note, nanomedicines also permit neoteric mechanisms of action for immunotherapy drugs (i.e., displaying ligands to immune cells, regulating intracellular delivery of cell impenetrable agents, and controlling the drug release profile) and they can be further functionalized with targeting molecules to interact with the immunosuppressive cells107. Herein, we summarize and highlight the regulation strategies mainly associated with the targeting of specific immunosuppressive cells to reshape the TIME in vivo. Various strategies for nanomedicine-based TIME regulation to enhance immunotherapy are listed in the following Table 1108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141.
Table 1.
Miscellaneous strategies targeting different immunosuppressive cells for nanomedicine-based TIME regulation.
| Target cell | Nanomedicine platform | Payload | Tumor model | Efficacy | Ref |
|---|---|---|---|---|---|
| TAMs | Lipid-like nanoparticle | CCR2 siRNA | CT26, EL-4 | Reduced tumor volumes and the number of TAMs, resulting in advanced antitumor efficacy | 108 |
| SCNs | Platinum-prodrug and BLZ-945 | 4T1 | Reduced tumor volumes and depleted TAMs from tumor tissues. Highly favorable for cancer chemo-immunotherapy | 109 | |
| Lipid-coated calcium zoledronate NPs | Zoledronate | Sarcoma S180 | Decreased angiogenesis, reduced tumor volumes and direct depletion of TAMs. The exact delivery of Zoledronate may help to restore DOX chemosensitivity and immunogenic cell death in multidrug-resistant human cancer cells | 110,111 | |
| Poly(β-amino ester) nanoparticles | IL-12 | B16-F10 melanoma | Re-education of the tumor-infiltrated macrophages from M2 to M1 | 112 | |
| Clodronate liposomes modified with PEG | IFN-γ | B16-BL6 melanoma | Retarded the growth of B16-BL6 tumors and depleted TAMs from tumor tissues, improving the therapeutic effect of IFN-γ in TAM-repleted and IFN-γ-resistant tumors | 113 | |
| Bisphosphonate-glucomannan conjugate | Alendronate | Sarcoma S180 | Decreased angiogenesis, recovered local immune surveillance, and elimination of TAMs for cancer immunotherapy | 114 | |
| Sialic acid-cholesterol conjugate-modified liposomes | Epirubicin | Sarcoma S180 | The depletion of TAMs and improved survival rate of the mice models indicated the enhanced chemo-immunotherapy for epirubicin | 115 | |
| PEGylated Calcium bisphosphonate NPs | Calcium bisphosphonate | 4T1 | Depleted TAMs, suppressed angiogenesis, normalized tumor vasculatures, enhanced intratumoral perfusion, and relieved tumor hypoxia. Highly favorable for cancer radioisotope therapy | 116 | |
| Trastuzumab-modified mannosylated liposome | Gefitinib/Vorinostat | NCI-H1975 | Repolarize the protumor M2 phenotype to the antitumor M1 and cause the elevating ROS in the cancer cells, consequently modulating the intracellular redox balance, facilitating the EGFRT790M degradation, thus resensitizing the EGFRT790M-positive cells to gefitinib | 117 | |
| Mannosylated lactoferrin NPs | Shikonin and JQ1 | CT26 | Inducing immune cell death in the cancer cells, repressing glucose metabolism and repolarizing TAMs, consequently resulting in remodeling the TIME (e.g., promotion of DC maturation and CD8+ T cell infiltration, along with Treg cell suppression), producing synergistic PD-L1 blockage therapy with JQ1 | 118 | |
| MDSCs | High-density lipoprotein-like NPs | ‒ | B16-F10 melanoma | Suppressed MDSCs, increased CD8+ T cells and reduced Treg cells in the metastatic TME; Significantly reduced tumor growth, metastatic tumor burden, and increases survival | 119 |
| anti-Flt1 antibody-conjugated PEG-cored PAMAM | Gemcitabine | CFPAC-1 pancreatic cancer | Eliminating the tumor-induced myeloid cells, altering the immune infiltration levels in the tumor tissue, improving the anticancer efficacy | 120 | |
| Porous 3D scaffolds | Gemcitabine | 4T1 | The direct depletion of MDSCs and elimination of residual tumor cells resulted in 100% survival. It could also prevent tumor recurrence at the surgical site | 121 | |
| Micelles of propylene sulfide | 6-thioguanine | B16-F10 melanoma, E.G7-OVA thymoma | Depleting suppressive MDSCs and activating CD8+ T cells, promoting antitumor immunity when used in combination with T cell immunotherapies | 122 | |
| Micellar LMMW-tocopherol succinate NPs | DOX and α-galactosylceramide | B16-F10 melanoma, 4T1 breast cancer | Interference with the early recruitment of G-MDSCs along with the inhibition of the postoperative metastasis and the tumor recurrence | 123 | |
| Neutrophil membrane coated-PLGA NPs) | ‒ | B16-F10 melanoma, 4T1 breast cancer | Reducing the expansion of MDSCs and their enrichment in tumors, dramatically increasing the number of tumor-infiltrating T cells, restoring their antitumor functions, and synergistically inhibiting tumor progression when combined with the immune checkpoint blockade therapy | 124 | |
| Lipid-coated hollow mesoporous silica NPs | All-trans retinoic acids, DOX, and IL-2 | B16-F10 melanoma | Differentiation of immature MDSCs into mature cells and significant inhibition of tumor growth and metastasis. This nanomedicine could elicit synergistic antitumor effects for cancer immunotherapy | 125 | |
| PLGA NPs | CDDO-Me | B16-F10 melanoma | Remodeled TAFs, collagen and vessel in TME, enhanced the Fas signaling pathway and sensitized CTL-mediated killing of tumor cells. The combination with Trp2 nanovaccine resulted in an increase of antitumor efficacy and apoptotic tumor tissue than Trp2 vaccine alone | 126 | |
| Iron oxide NPs | ‒ | KP1 lung cancer | The induced pro-inflammatory macrophage polarization significantly inhibited growth of subcutaneous adenocarcinomas, prevented liver metastasis, and potentiated macrophage-modulating cancer immunotherapies | 127 | |
| Treg cells | PEG-modified SWCNTs | ‒ | B16 melanoma | Targeting intratumor immune populations to elicit the systemic depletion of Treg cells | 128 |
| Lipid-PLGA hybrid NPs modified with tLyp1 peptide | Imatinib, anti-CTLA-4 mAb | B16 melanoma | Prolonged survival rate, improved tumor inhibition, decreased intratumoral Treg cells, and elevated intratumoral CD8+ T cells when combined with anti-CTLA-4 immunotherapy | 129 | |
| RBC membrane-coated nanogels | Paclitaxel, IL-2 | B16-F10 melanoma | The selective reduction of Treg cells and the increased number of CD4+ T cells to reshape the TIME. An appealing chemo-immunotherapy strategy realized the combinatorial antitumor effect | 130 | |
| PLGA-NPs | 7-Acyl lipid A and JSI-124 (STAT3 inhibitor) | B16-F10 melanoma | A significant increase in the tumor infiltrated T cells and a significant decrease in the suppressive effects of Treg cells on T cell proliferation augmented the efficacy of cancer immunotherapy | 131 | |
| Layer-by-layer hybrid NPs | IR-780 and imatinib | B16-BL6 melanoma, MC-38 | The inhibition of intratumoral Treg cells and the decreased number of Treg cells favorable for boosting antitumor photoimmunotherapy | 132 | |
| Polymeric micelles | Sunitinib | B16-F10 melanoma | The increased cytotoxic T-cell infiltration and decreased the number and percentage of Treg and MDSC cells in the TIME enhanced vaccine therapy in advanced melanoma | 133 | |
| CAFs | Lipid bilayer-modified CaP NPs | Gemcitabine monophosphate and cisplatin | UMUC3 bladder cancer | Tumor stroma was 85% depleted and 87% of the remaining TAFs were TUNEL-positive after a combined gemcitabine monophosphate nanoparticles and cisplatin nanoparticles treatment | 134 |
| Lipid-coated protamine DNA complexes | sTRAIL | BXPC3-Luc2 pancreatic cancer | Induced apoptosis in tumor cells adjacent to CAFs and the reversion of residual fibroblasts to a quiescent state, further compromising tumor growth and remodeling the TME. It also increased tumor uptake of chemotherapeutic drugs and apoptosis of carcinoma cells | 135 | |
| Ferritin nanocage | FAP-specific scFv | 4T1 | Selectively killing CAFs and triggering the ECM deposition, yielding the reduced tumor volumes, highly favorable for boosting antitumor photoimmunotherapy | 136 | |
| Polymeric NPs | Docetaxel | 4T1 and MDA-MB-231 | Significant tumor CAF depletion occurred within 16 hours post injection. It had a significantly more enhanced antimetastatic effect than docetaxel and nab-paclitaxel treatments | 137 | |
| FAP-α antibody-modified cell-penetrating peptide-based NPs | CXCL12 siRNA | PC3 | Induced the inactivation of CAFs and the significant inhibition of tumor cell invasion, migration, tumor angiogenesis, and tumor metastasis | 138 | |
| Telmisartan-grafted glycolipid micelles | DOX | Human MCF-7 breast cancer | Induced CAFs apoptosis, reduced tumor volumes elevated the sensitivity of tumor cells to cytotoxic agents, favorable to reverse TMDR | 139 | |
| Tumor stroma-targeted nanovehicle | DOX | Human Hep G2 | Eradication of CAFs and downregulated ECM deposition, decreasing IFP and facilitating blood perfusion, favorable for chemoimmunotherapy | 140 | |
| Cell membrane-coated SPN | ‒ | 4T1 | Generating enhanced cytotoxic heat and singlet oxygen to eliminate CAFs and resulting in significant reduced tumor volumes | 141 |
CAF, cancer-associated fibroblasts; CDDO-Me, methyl-2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oate; CpG, Cytosine Guanine (linear dinucleotide); CTL, cytotoxic T lymphocyte; CXCL12, C−X−C motif chemokine ligand 12; DC, dendritic cell; DOX, doxorubicin; FAP, fibroblast-activation protein; IFP, interstitial fluid pressure; IL-2, interleukin-2; LMWH, low molecular weight heparin; mAb, monoclonal antibody; PAMAM, poly(amidoamine) dendrimers; PEG, poly(ethylene glycol);PLGA, poly-lactic-glycolic-acid; RBC, red blood cell; scFv, single chain variable fragment; SCNs, tumor microenvironment sensitive cluster nanoparticles; SPN, semiconducting polymer nanoparticle; STAT3, signal transducer and activator of transcription 3; SWCNTs, single-walled carbon nanotubes; TMDR, tumor microenvironment-mediated drug resistance; TRAIL, TNF-related apoptosis-inducing ligand; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
3.1. Modulating immunosuppressive cells in the TIME with nanoparticles and nanoconjugates
As set forth, tumor-related immunosuppressive cells exemplify negative regulatory cells that significantly dampen the immunity process in TIME, including TAMs, Treg cells, Breg cells, MDSCs, as well as CAFs. Once utilized by tumor cells in TIME, they are expected to suppress the antitumor immune responses through paracrine effects, including inhibiting the function and differentiation of APCs and the depletion and dysfunction of T cells, thus enabling tumor cells to evade the immune surveillance. With respect to the fact that antitumor immunity must be perpetuated for empowering durable immune responses toward immunotherapy, utilizing engineered NPs and nanoconjugates to program the immune system and overcome the immune tolerance, especially for the precise modulation of the immunosuppressive cells within TIME has come to the fore as a promising strategy for improving antitumor responses in vivo. The diverse methods and distinct approaches in which nanomedicines interplay with immunosuppressive cells will be retrospected and summarized in this section at length. Considering there is little research on nanomedicines regulating Breg cells to enhance cancer immunotherapy, this review mainly focuses on modulating TAMs, Treg cells, MDSCs, and CAFs in the TIME with nanomedicines for enhanced cancer immunotherapy.
3.1.1. Nanomedicines that target TAMs in the TIME
For the purpose of preventing the stage for metastatic development, it is critical to restrain the crosstalk between TAMs and adaptive immune cells to contain the immunosuppressive microenvironment. Currently, there are three strategies to target TAMs: (1) Suppressing or blocking the recruitment and localization of monocytes to tumor tissues; (2) Directly killing or depleting TAMs; (3) Repolarizing of TAMs into the antitumor M1 phenotype30,142.
Multiple studies had shown that the accumulation of TAMs and their progenitor cells were modulated by chemokine ligands released from the TIME143. Hence, macrophage-recruiting chemokines, complement components, colony-stimulating factor-1 (CSF-1), and vascular endothelial growth factor (VEGF) are potential therapeutic targets and can be deployed to contain the progression of the malignant tumor via disrupting the agglomeration of pro-metastatic TAMs143, 144, 145, 146. Shen et al.109 designed an immunostimulatory nanovesicle, namely BLZ-945SCNs/Pt, to spatially target the TAMs for chemoimmunotherapy. The BLZ-945 is a molecule inhibitor of the CSF-1 receptor of TAMs and its release can be preferentially taken up by TAMs to induce TAMs depletion from tumor tissues, while the nanocarrier carrying Pt-prodrug render deep tumor penetration along with intracellularly specific drug release to kill tumor cells109. Besides, another research sheds light on disturbing the CCL2-CCR2 signaling pathway to interrupt the monocyte recruitment process108. Based upon the idea that cationic NPs may possess a higher propensity to accumulate in monocytes than their neutral counterparts, Shen et al.147 also developed siCCR2-encapsulated cationic nanoparticles to modify immunosuppressive TIME in a more efficient manner.
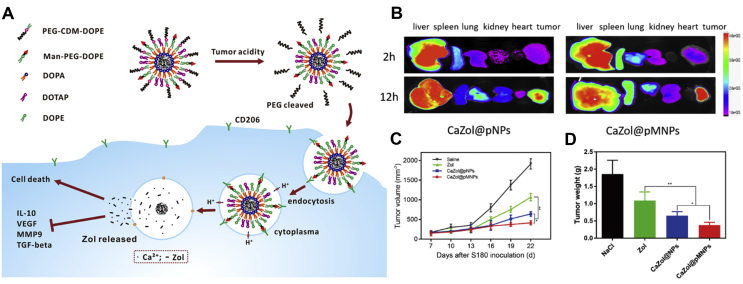
Various nanomedicines are also designed to deliver chemical agents that directly deplete TAMs. Zang et al.110 established a nanosystem of lipid-coated calcium zoledronate NPs (denoted as CaZol@pMNPs) encompassing conjugated mannose, which was under spatial shielding with an extracellular pH-sensitive material. The NPs showed enhanced cellular internalization mediated by mannose ligand following PEG detachment at lower pH conditions, bringing about considerable cytotoxicity to TAMs. The in vitro and in vivo data further indicated that this system can specifically target TAMs with the induction of their apoptosis (Fig. 5)110. Besides, several other studies laid emphasis on the bisphosphonate, which can induce the apoptosis of TAMs. Hence, nanomedicines with modified properties to tackle the limitation of the bisphosphonate (i.e., short half-life/high accumulation in the bone) are also in the good graces of researchers. Kiyota et al.113 functionalized clodronate liposomes with poly(ethylene glycol) (PEG) to deplete TAMs in tumor-bearing murine models. By establishing two types of solid tumors, they also found that TAM depletion can effectively improve the therapeutic effect of IFN-γ on TAMs-replete and IFN-γ-resistant tumors. To obtain the higher specific targeting effect, Zhan and colleagues reported a bisphosphonate-glucomannan nanoconjugate, which can specifically target and eliminate TAMs in the TIME114. Of note, they harnessed the glucomannan, which exhibited a high binding affinity for mannose receptors on macrophages, to conjugate alendronate (another bisphosphonate compound), thereby surmounting the intrinsic tumor susceptibility. Furthermore, recently, as unveiled by Tian et al. 116, a biocompatible and biodegradable nanoplatform based on calcium bisphosphonate NPs, was deployed for achieving effective in vivo depletion of TAMs. After intravenous injection, the NPs demonstrated effective tumor homing, as indicated by single-photon-emission computed tomography imaging. Accordingly, such modulation of TIME also seemed to be quite favorable for radioisotope therapy owing in large part to its synergistic therapeutic effect in suppressing tumor growth.
Figure 5.
Targeted delivery of zoledronate to TAMs for cancer immunotherapy. (A) Schematic illustration of the targeting and depletion mechanism of the lipid-coated calcium zoledronate nanoparticles in vivo. (B) Ex vivo images of dissected organs after injection of DiR-loaded NPs. (C) Tumor growth curves of the S180-bearing mouse models. (D) The tumor weight of excised tumors at 22 days. Reproduced with the permission from Ref. 110. Copyright © 2019, American Chemical Society.
In addition to the aforementioned aspects, the repolarization of TAMs toward the M1-like phenotype is in vogue. Peng et al.117 revealed a reprogramming strategy to break through the EGFRT790M-associated drug resistance via a dual-targeting codelivery system of gefitinib/vorinostat, which can simultaneously act on TAMs and the HER-2 positive non-small cell lung cancer (NSCLC) cells with overexpression of the mannose receptor. In this work, the trastuzumab-modified mannosylated liposome nanosystem can repolarize the M2 phenotype to the M1 as well as arouse the elevated level of ROS in TIME, thereby regulating intracellular redox balance, facilitating the EGFRT790M degradation, and resensitizing the EGFRT790M-positive cells to gefitinib. Interestingly, another work regarding the remodeling of TIME and metabolism was realized by constituting a mannosylated lactoferrin nanoparticulate system, namely Man-LF NPs. In the study of Wang et al.118, they brought the dual-targeting biomimetic codelivery of shikonin and JQ1 to fruition by the mannose receptor and LRP-1 that are overexpressed in tumor cells and TAMs. It was shown that Man-LF NPs induced immune cell death in the cancer cells, repressed glucose metabolism and repolarized TAMs, consequently resulting in remodeling the TIME and producing synergistic PD-L1 blockage therapy with JQ1. Additionally, Zanganeh et al.127 verified that Ferumoxytol, which is an FDA-approved agent (superparamagnetic iron oxide nanoparticles), could suppress the progression of breast cancer along with lung/liver metastasis through triggering the polarization of M1-like phenotype in the TIME. It is noteworthy that such a reprogramming process of TAMs might result in the production of ROS by iron oxide Fenton reactions and increased TNF-α level.
3.1.2. Nanomedicines that target MDSCs in the TIME
In terms of the previous description, another way to modulate the immunosuppressive TIME is to restore antitumor responses through blockade, regulation, or depletion of MDSCs in the tumor-bearing hosts.
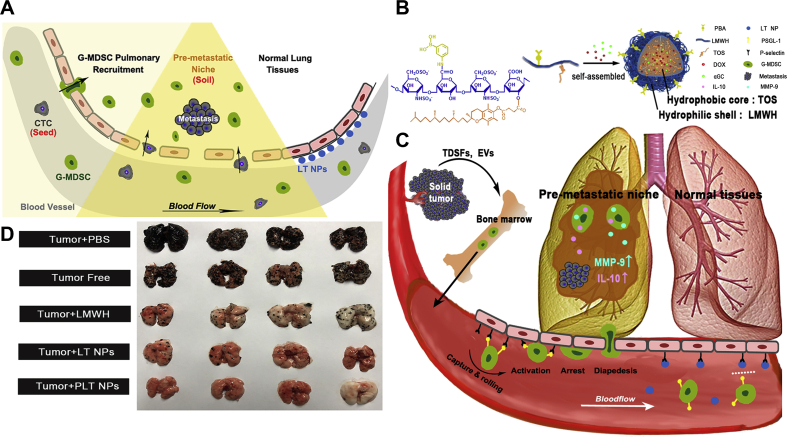
As for the depletion of MDSCs, Yoyen-Ermis et al.120 fabricated the anti-Flt1 antibody-conjugated PEG-cored poly(amidoamine) (PAMAM) dendrimers to improve the efficacy of gemcitabine against pancreatic tumor. Interestingly, when gemcitabine was loaded into the dendrimer conjugations, the number of myeloid cells decreased significantly, while the percentage distribution of granulocytes and mononuclear myeloid cells did not always alter remarkably. In addition, the immune infiltration level in the tumor tissue was also changed. In the study of Phuengkham et al.121, the research team designed implantable, engineered 3D porous scaffolds to elicit synergistic action between MDSC-depleting agents, counteracting the immunosuppression effect of TIME. Such local implantation of the synthetic immune niche (iCD) after surgery in the breast cancer model induced systemic anti-tumor immunity and prevented tumor recurrence, resulting in 100% survival. The promising therapeutic outcome was mainly due to the suppression of MDSCs with the delivery of gemcitabine along with the recruitment of DCs, thus elevating the generation of IFN-γ via the iCD cancer vaccine121. Besides, He's research group123 recently uncovered a study concerning the interference with the early recruitment and the depletion of MDSCs. They established a micellar hypotoxic low molecular weight heparin (LMWH)-tocopherol succinate NP (denoted as LT NP) and found that the hydrophilic segment LMWH in the NPs could suppress early pulmonary recruitment of MDSCs through suppressing P-selectin/PSGL-1-mediated adhesion between vascular endothelial cells and granulocytic MDSCs (Fig. 6)123. Moreover, LT NP could keep the normal microenvironment of lungs and effectively inhibit CTC implantation and colonization. When modified with phenylboronic acid and loaded with doxorubicin and an immunopotentiator α-galactosylceramide, this nanomedicine could effectively inhibit the postoperative metastasis and tumor recurrence simultaneously.
Figure 6.
Self-delivery micellar NPs restrain premetastatic niche formation through interfering with the early recruitment of granulocytic-MDSCs (G-MDSCs). (A) Schematic illustration of the premetastatic niche and the granulocytic-MDSCs recruitment. (B) Schematic illustration of the synthesis of the NPs. (C) Schematic illustration of the anti-G-MDSC recruitment mechanism of NPs. (D) The antimetastatic treatment analysis in terms of the images of harvested lungs. Reproduced with the permission from Ref. 123. Copyright © 2019, American Chemical Society.
Alternatively, inhibition of the proliferation and tumor trafficking of MDSCs has emerged as effective and safe tumor immunotherapy. Li et al.124 recently unveiled that neutrophil plasma membrane-coated NPs sponging cytokines could disrupt the expansion and tumor trafficking of MDSCs as well as reverse immune tolerance. In particular, this nanomedicine has a coat of the neutrophil plasma membrane, which is similar in phenotype and morphology to polymorphonuclear MDSCs (PMN-MDSCs), inheriting most membrane receptors from the “parental” neutrophils to neutralize cytokines correlated with MDSCs. The outcomes indicated that the expansion of MDSCs along with their enrichment in peripheral lymphoid organs and tumors were reduced without the compensatory influx of alternative myeloid subtypes after administration. Intriguingly, such a nanomedicine treatment could also dramatically up-regulate the level of tumor-infiltrating T lymphocytes and restore their antitumor functions124. In this regard, such a pseudocell nanoplatform may usher in new paths toward effective rewiring the immunosuppressive TIMEs.
Besides, several other studies revealed the possibility of blocking the development and function of MDSCs in the TIME. Huo et al.133 unveiled a potent mannose-modified lipid calcium phosphate (LCP) NPs-based Trp2 vaccine for melanoma treatment. Notably, a multitarget receptor tyrosine kinase inhibitor, sunitinib base, was efficiently encapsulated into polymeric micelles to decrease the number of MDSCs and Treg cells within the TIME. The encapsulated sunitinib worked synergistically with the anti-melanoma vaccine and the immune response was led to a shift from Th2 to Th1 mode, bringing about the tumor regression133. Further, this research team has also uncovered that intravenous delivery of CDDO-Me-harnessed PLGA NPs combined with the subcutaneous Trp2 vaccine led to an improved antitumor efficacy along with apoptotic tumor tissue than Trp2 vaccine alone in B16F10 melanoma126.
3.1.3. Nanomedicines that target or reprogram T cells
Nanomedicines-based delivery system has recently been applied to target immunotherapeutic agents directly to T cells or accurately program tumor-recognizing potential into circulating T cells148,149. Lower concentrations of immunostimulants had been utilized to galvanize or augment T cell responses. To this end, Schmid et al.148 reported antibody-targeted NPs that bind to CD8+ T cells in the blood, lymphoid tissues, as well as tumors in the mouse models. PEG and poly(lactic-co-glycolic acid) (PLGA) were employed to fabricate NPs encompassing either SD-208 or a TLR7/TLR8 agonist to the restoration of T cell function and recruitment of lymphocytes to non-inflamed tumors. Importantly, the delivery of inhibitors of TGF-β signaling to cells expressing PD-1 prolonged the survival of tumor-bearing mice, while free agents had no effect at this dose148. As for the reprogramming of T cells, synthetic DNA nanocarriers were designed to efficiently introduce leukemia-targeting CAR genes into the T-cell nuclei, thus leading to long-term cancer remission150. In this work, T-cell-targeting anti-CD3e f(ab')2 fragments were coupled to the surfaces of NPs [poly(β-amino ester)-based NPs] for the purpose of enabling receptor-mediated endocytosis. The NPs were further functionalized with microtubule-associated sequences along with nuclear localization signals to prompt fast-track nuclear import. Of note, the NPs were furnished with antitumor programming abilities via packing plasmid DNA encoding the leukemia-specific 194-1BBz CAR150. Accordingly, these polymeric nanocarriers are easy to manufacture in a stable form, providing oncologists with “on-demand” practical applications of antitumor immunity within TIME. In addition to polymeric nanocarriers, nanomedicines based upon biomimetic technology, such as nanoerythrosomes, can also obtain outstanding capacity in enhancing the tumor immunotherapy151.
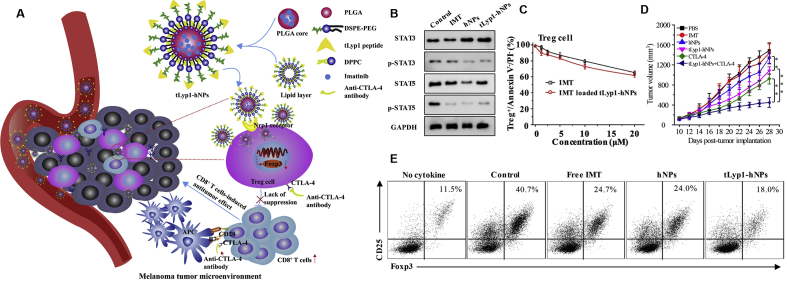
Besides, some other researches placed a greater focus on Treg cells, which are an immunosuppressive class of T cells152. In one approach, a biomimetic nanogel with TIME-responsive capacity was carried out for the combinatorial antitumor effects of chemoimmunotherapy. Song et al.130 synthesized nanogel with hydroxypropyl-β-cyclodextrin acrylate and two opposite charged chitosan derivatives to entrap paclitaxel and precisely control the pH-response ability. Of note, the erythrocyte membrane buoyed up by this nanosystem could deliver the immunotherapeutic molecule interleukin (IL)-2 without affecting its biological activity. Further, the combination of these drugs at low doses could reshape the TIME, which could be demonstrated by the enhancement of immune effector cell infiltration as well as the minimization of immunosuppressive factors130. With respect to the four well-known Treg cell-enriched markers (folate receptor 4 (FR4), CD39, CD103, and glucocorticoid-induced TNFR-related receptor (GITR))128, there are potential nanomedicines, which hammered at least one of these markers, to target the Treg cells in the TIME. For instance, Ou et al.132 fabricated layer-by-layer hybrid nanoparticles, which were pH-sensitive, to enable the release of IR-780 dye for photothermal and photodynamic effects, as well as the release of imatinib-loaded GTIR-targeting PLGA NPs to elicit antitumor immunotherapy. The complex successfully down-regulated the suppressive function of Treg cells and eliminated tumor growth in vivo. Additionally, this group also constructed tLyp1 peptide-conjugated hybrid NPs for targeting Treg cells in the TIME (Fig. 7)129. The in vivo outcomes unraveled that the synergistic effect of imatinib base (IMT)-loaded tLyp1-hNPs together with anti-CTLA-4 antibody can be leveraged to elicit a robust immune response against tumors through down-regulating immunosuppressive Treg cells (Fig. 7). This nanosystem exhibited good stability and could effectively target to Treg cells, and they improved the inhibition effect of imatinib on Treg cell function via inhibiting the STAT3 and STAT5 phosphorylation129.
Figure 7.
Treg cell-targeted hybrid NPs combined with immuno-checkpoint blockage for modulating TIMEs. (A) Schematic illustration of tLyp1 peptide-conjugated hybrid NPs (tLyp1-hNPs). (B) In vitro STAT3, p-STAT3, STAT5, and p-STAT5 protein expression in Treg cells after treatment with various NPs. (C) Effect of free imatinib base (IMT) and IMT-loaded tLyp1-hNPs on apoptosis of Treg cells. (D) Tumor volumes of the murine models treated with the indicated formulations. (E) Effect of free IMT, IMT-loaded hNPs, and IMT-loaded tLyp1-hNPs on Treg cell differentiation. Reproduced with the permission from Ref. 129. Copyright © 2018, Elsevier.
3.1.4. Nanomedicines that target CAFs in the TIME
Due to the abundance of the CAF in the TIME and its critical role of exerting a tumor-promoting effect, several nanomedicines have been developed for the reeducation of CAFs153. Fibroblast-activation protein (FAP), which is overexpressed on the surface of CAFs, has been harnessed as a potential target to eliminate the CAF154. Zhen et al.136 utilized ferritin (a compact NP protein cage) as a photosensitizer carrier to bind FAP-specific single-chain variable fragments (scFv) to the surface of ferritin. Through light irradiation, the activated nano-PIT can effectively eliminate CAFs in the TIME, and due to the local nature of the treatment, the damage to the healthy tissue is very small. In addition, there is an elevated level of macromolecules and NPs in the irradiated-side tumors, suggesting that the eradication of CAFs may induce the degradation of ECM and prompt the penetration of nanovesicles136. Importantly, in another work, a CAF-targeting siRNA delivery system was established through loading FAP-α antibody onto the cell-penetrating peptide (CPP)-based NPs, which specifically down-regulated C–X–C motif chemokine ligand 12 (CXCL12) expression in the CAF138. This nanosystem could effectively inactivate CAFs and inhibited the metastasis of orthotopic prostate cancer via silencing the CXCL12 gene expression138. Besides, in the study of Zhu et al.139, a biologically inspired Telmisartan grafting glycolipid micelles (Tel-CSOSA) was developed, which could sequentially target angiotensin II type I receptor (AT1R) overexpressed on CAFs. Notably, the micelles exhibited superior accumulation capacity in tumor tissues to bare CSOSA micelles, leading to the eradication of the ECM via DOX and inducing targeted apoptosis of CAFs. Collectively, these tactics of sequentially targeting CAFs could synergistically improve the therapeutic effect of cancer with reversed immunosuppressive TIME.
Another interesting research is associated with comprehensively priming the TIME through CAF-targeted liposomes for combined therapy with a cancer cell-targeted chemotherapeutic agent delivery system. According to previous research that the FH peptide (FHKHKSPALSPV) fragment could be leveraged as a specific ligand targeting CAFs155, Zhang's group140,156 unveiled that co-culturing CAFs and HepG2 cells might bring about a multidrug resistance to DOX. Hence, they conjugated FH peptide with DSPE-PEG2000 to prepare CAF-targeted navitoclax-loaded liposomes (FH-SSL-Nav). Consequently, the chemotherapeutic drug resistance elicited by the TIME was partially reversed by the FH-SSL-Nav. Other strategies regarding the targeting of CAFs may refer to the CAFs membrane-mediated nanosystem, Li et al.141 designed organic multimodal phototheranostic nanoagents to biomimetically target CAFs in the TIME for improved multimodal imaging-guided antitumor treatment. Biomimetic nanocamouflages comprised of a near-infrared (NIR) absorbing semiconducting polymer NP coated with the cell membranes of activated fibroblasts were realized to specifically target CAFs, resulting in advanced tumor accumulation relative to the uncoated and cancer cell membrane coated counterparts after the systemic administration141. Besides, some recent studies probed into nanomedicines based upon natural products (i.e., curcumin, silibinin, fraxinellone, astragaloside IV, resveratrol, etc.) which can influence the interaction between tumor cells and CAFs157, 158, 159, 160. Collectively, through influencing the crosstalk between CAFs and tumor cells, the proliferation, migration, invasion, and drug resistance of tumor cells elicited by CAFs can be considerably suppressed. By exploiting various nanomedicines, CAFs can be depleted or be reverted from the activated state into a quiescent state161. Notwithstanding, direct CAF depletion might enhance hypoxia in the tumors and thus trigger epithelial-to-mesenchymal transition in the desmoplastic TIME, which ultimately resulted in aggressive cancer progression93. Accordingly, nanomedicines designed to normalize CAFs would be a better way to inaugurate new opportunities for the high-performance modulation of TIME.
3.2. Combination nanomedicines and clinical translation
Given the miscellaneous agents needed to promote the advancement of the cancer–immune cycle in patients with solid tumors, a great deal of studies has further shifted to the invention of nanomedicines that amalgamate multiple immunotherapies (i.e., chemotherapy and immune-modulators; radiosensitizers, incorporating photothermal and photodynamic therapies and immune-modulators; RNA interference-based immune-modulators; RNA-based or other vaccines loaded with adjuvants)162. As a matter of fact, some functions of nanomedicines (i.e., the control of release chemistry of drug–polymer joints, the conjugation of a drug or antibody on the surface, and coordination of drug interactions within the core of nanomedicines) indicate the possibilities of the synchronous administration of multiple drugs163. In other words, multiple reagents (including nucleic acids) can be integrated into single nanomedicines. Of note, these functions can control pharmacokinetics, drug concentration, along with the release rate of bioactive payloads, thereby optimizing the synergistic effect164. Taking the use of self-assembled micelles as an example, in the study of Duan et al.165, micelles carrying oxaliplatin in the core and dihydroartemisinin in the shell could elicit strong antitumor immunity in addition to anticancer efficacy. Moreover, phagocytic cells took up fragments of dead cancer cells, resulting in T cell activation and antitumor vaccination. In a groundbreaking study, a nanomedicine featuring four components (including antibodies targeting tumor antigens, an anti-PD-1 antibody, a recombinant form of IL-29, and a T cell stimulatory vaccine) was developed and exhibited robust antitumor effects166. The overall outcomes indicated that the elicited endogenous immune response had the ability to destroy large established tumors and elucidated the crucial characteristics of combined immunotherapy. Such combination immunotherapy could cure most tumors in an experimental environment that is often considered refractory. Intriguingly, in another work, an intrinsically therapeutic fucoidan–dextran-based magnetic nanomedicine integrated with a checkpoint inhibitor and T-cell activators was applied to repair the immunosuppressive TIME through reinvigorating tumor-infiltrating lymphocytes, and meanwhile targeting the agents to the tumor through magnetic navigation so as to minimize off-target effects167. In addition, Phuengkham et al.168 constructed a designer scaffold loaded with both immune nanoconverters encompassed with resiquimod and DOX. This system provided the repolarization of immunosuppressive TAMs into tumoricidal antigen-presenting cells, rather than depleting them, along with in situ vaccination that can be produced in vivo without the demand to analyze and sequence tumor antigens to favor neoantigen-specific T cell responses in advance168. The use of a designer scaffold with long-term central and effector memory T cell responses might induce synergistic antitumor immunity to remodel the TIME, thus preventing recurrence and metastasis of postoperative tumors. In another study of Huang et al.169, they invented a nanoenabled means for indoleamine 2,3-dioxygenase-1 (IDO1) pathway intervention, which was realized through delivering IDO1 siRNA to both tumor-draining lymph nodes and tumor tissues with the help of cationic lipid-assisted NPs. Importantly, they further verified that contemporaneous administration of oxaliplatin and IDO1 siRNA could generate synergetic antitumor effects by facilitating DC maturation, increasing tumor-infiltrating T lymphocytes along with reducing the number of Treg cells in the tumor models169.
A range of nanomedicine-based targeting strategies to remodel the immunosuppressive TIME and educate the different immune cells have been discussed in this article. These technologies have also brought many challenges and opportunities for clinical translation. The specific clinical performance of these miscellaneous nanomedicine-based combinational treatment methods will hinge on whether these treatments should be performed concurrently, sequentially or are contraindicated. Selecting the right preclinical model is momentous, as is designing delivery techniques that can be effectively translated into cancerous persons. Some of the basic design criteria for clinical translation involve treatment stability, scalability, along with cost-effectiveness and intricacy170. The clinical translation of underlying nanomedicines is an expensive and time-consuming process owing in large part to the complexity in stark contrast to conventional formulation technology encompassing free agent dispersed in a base171. In addition, the choice of nanomaterials will impinge upon the timetable for clinical translation. For instance, a system developed using FDA-approved nanomaterials may be superior to enter the clinic in comparison with more complex unapproved nanomaterials. This is particularly useful for lipid-based and polymer-based materials, as FDA has approved several materials that can be designed as drug delivery platforms. This is especially pertinent for lipid- and polymer-based nanomaterials, some of which have been approved by the FDA as delivery platforms for various antitumor drugs172. Interestingly, a study of liposome-entrapped mitoxantrone hydrochloride injection in relapsed/refractory peripheral T-cell lymphoma and NK/T-cell lymphoma is under the recruiting status of the FDA173. Furthermore, an injectable stent for delivery of cancer vaccines, called WWDAX, has been approved by Novartis and is being assessed in clinical trials (NCT01753089)174. Despite these encouraging achievements, we must face up to a problem in terms of the potential adverse effects in certain patients that combinational nanomedicines and treatment plans containing more than one drug must be scrupulously designed to avert administering an unwarranted number of agents to patients.
4. Conclusions and perspectives
With the advanced understanding of cancer immunology and the gradual discovery of cellular and molecular mechanisms for innate and adaptive immunity175, the paradigm of cancer immunotherapy has undergone significant revolution176 and an array of novel antitumor immunotherapies regarding the remodeling of TIME is being exploited. As previously mentioned, nanomedicines possess the potential to advance cancer immunotherapy in multiple approaches, and preclinical evidence contributes to articulate motivation for clinical inspection of many ideas. Preliminary human trials of several of these methods are currently underway177, and the next few years should delineate a substantial understanding concerning their clinical potential.
Despite gratifying scientific research progress, this field still faces several pressing or intractable challenges, where solutions could have a momentous impact on clinical translation of the potential nanomedicines. Due to the possible immunosuppressive TIME, compressed vasculature, high interstitial fluid pressure, as well as dense fibrotic tissue around solid tumors (impeding T cell infiltration), it is still difficult to specifically target immune cells to entities through cell transfer or tumor vaccination. Hence, further researches may concentrate on targeting both the immune system and the TIME so as to trigger more effective immunotherapy. Specifically, given that the angiotensin inhibitor losartan has been leveraged in phase II clinical trials (NCT01821729) in pancreatic cancerous persons178, and has been verified to enhance the therapeutic efficacy of multiple solid tumor types179, the angiotensin inhibitor-associated nanosystem may play a promising role in priming the immunosuppressive TIME. Future studies exploiting other potential drug targets to reshape the TIME may spur the construction of new nanomedicine-based drug delivery platform to target solid tumors and minimize the adverse effects.
Besides, safe, and credible approaches of systemically targeting efficacious innate immune stimulators (i.e., TLR/STING agonists) to the TIME remain to be further developed. Although these drugs have proven potent antitumor activity when administered intratumorally in preclinical models and are under evaluation of intratumoral administration in early clinical trials, intratumoral administration cannot ensure the avoidance of systemic distribution along with ensuing cytotoxicity from such small molecule compounds, ruling out direct action of disease transmission180. In particular, the tropism of NPs utilized for MDSCs in circulation, spleen and liver makes it vague whether innate stimuli formulated with nanomedicine can be a safe solution180. Also, there is a need for a more comprehensive understanding of the molecular mechanisms that cause dysregulation of myeloid cell generation and functional polarization of MDSCs in the TIME181. Another notable challenge is to stably deliver genetic material encoding TCRs, CARs or support proteins to lymphocytes in vivo 182, 183, 184. Going a step further, the first trial to target RNA or DNA to T cells harnessing polymeric nanomaterials has been reported recently, but the efficiency of their transfection in vivo is still very low and it remains vague whether short-lived mRNAs or randomly integrating transposon DNAs are the explanation180. Nonetheless, the potential impact of safe and potent gene delivery toward various immune cells within the TIME should not be played down.
Additionally, the following studies should also focus on modifying the nanomedicines to explore novel delivery technologies to expand and engineer cell therapy in vitro. Recently, technologies based on microfluidic technology have been leveraged to advance and accelerate the intracellular delivery of large molecules to immune cells in vitro185,186. Such a carrier-free delivery system consists of cells that undergo rapid mechanical deformation which may temporarily destroy the immune cell membrane, thereby absorbing the large molecular cargo present in the buffer. Of note, this technique has been applied to swiftly deliver a series of nucleic acids and macromolecules to immune cells within the TIME. Accordingly, this nanomedicine-based delivery system may surmount the challenges with delivering electroporation- and carrier-based nucleic acids to immune cells in vitro187.
In addition to the improvement of delivery strategies, future work can implement an externally or internally elicited nanosystem in which therapeutic payloads can be triggered to stimulate the antitumor response required at the target tissue sites, thus minimizing the off-tissue effect. In fact, some studies in this field are now modifying the utilization of mechanical or pH188, light189, or ultrasound-triggered approaches190 for distantly controlled immunotherapies. In addition, in view of the fact that several biological nanomaterials and drug delivery tactics activate immune stimulation pathways in the absence of other immune signals, it is essential to conduct basic research on the interaction between nanomaterials and immune cells to actively guide the immune response in the TIME191,192.
In general, despite intense clinical studies, much more research remains in high demand so as to therapeutically induce trained immunity of immune cells in the immunosuppressive TIME. Notwithstanding, there is a critical issue we need to take into rigorous consideration, the TIME immune phenotype may alter with therapy, over time and can also vary by location. Notably, as noted earlier, even the metastatic TIME may differ from other metastatic tumors and primary tumors in the same patient. Aside from these uncertainties, we should also bear in mind that complex modifications to nanomedicines are expected to lead to multifunctional delivery formulations with steady groups, targeted ligands, along with bio-responsive ligands, which may also complicate the large-scale production. Moreover, the timing and sequence of administration of the combination drugs need to be carefully inspected in virtue of the unexpected cytotoxicity193,194. Nevertheless, with our continued efforts to fight cancer and our further exploration of the molecular mechanisms regarding the TIME, we believe that cancer immunotherapy will break the clinical bottleneck and amplify the innate immune attack against the immunosuppression.
Acknowledgments
The authors are grateful to the financial support from the National Natural Science Foundation of China (81773283 and 81701684).
Author contributions
Yuefei Zhu, Xiangrong Yu, and Zhiqing Pang conceived the manuscript. Yuefei Zhu wrote the manuscript and designed the figures. Yuefei Zhu, Xiangrong Yu, Soracha D. Thamphiwatana, Ying Zheng, and Zhiqing Pang edited the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Xiangrong Yu, Email: yxr00125040@126.com.
Zhiqing Pang, Email: zqpang@fudan.edu.cn.
References
- 1.Kennedy L.B., Salama A.K.S. A review of cancer immunotherapy toxicity. CA A Cancer J Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg S.A. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peer D., Karp J.M., Hong S., Farokhzad O.C., Margalit R., Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 4.Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Control Release. 2012;164:138–144. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Martin J.D., Cabral H., Stylianopoulos T., Jain R.K. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17:251–266. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gradishar W.J., Tjulandin S., Davidson N., Shaw H., Desai N., Bhar P. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 7.Buyse M., Sargent D.J., Grothey A., Matheson A., De Gramont A. Biomarkers and surrogate end points—the challenge of statistical validation. Nat Rev Clin Oncol. 2010;7:309–317. doi: 10.1038/nrclinonc.2010.43. [DOI] [PubMed] [Google Scholar]
- 8.Quesada J.R., Hersh E.M., Manning J., Reuben J., Keating M., Schnipper E. Treatment of hairy cell leukemia with recombinant alpha-interferon. Blood. 1986;68:493–497. [PubMed] [Google Scholar]
- 9.Alwan L.M., Grossmann K., Sageser D., Van Atta J., Agarwal N., Gilreath J.A. Comparison of acute toxicity and mortality after two different dosing regimens of high-dose interleukin-2 for patients with metastatic melanoma. Targeted Oncol. 2014;9:63–71. doi: 10.1007/s11523-013-0276-7. [DOI] [PubMed] [Google Scholar]
- 10.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Donnell J.S., Teng M.W.L., Smyth M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 12.Maman S., Witz I.P. A history of exploring cancer in context. Nat Rev Drug Discov. 2018;17:13–30. doi: 10.1038/s41568-018-0006-7. [DOI] [PubMed] [Google Scholar]
- 13.Fridman W.H., Zitvogel L., Sautès–Fridman C., Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B., Shi W., Jiang T., Wang L., Mei H., Lu H. Optimization of the tumor microenvironment and nanomedicine properties simultaneously to improve tumor therapy. Oncotarget. 2016;7:62607. doi: 10.18632/oncotarget.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu L., Kate P., Torchilin V.P. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6:3491–3498. doi: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang S., Shao K., Liu Y., Kuang Y., Li J., An S. Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis. ACS Nano. 2013;7:2860–2871. doi: 10.1021/nn400548g. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y., Yue C., Han Y., Zhang W., He A., Zhang C. Tumor-responsive small molecule self-assembled nanosystem for simultaneous fluorescence imaging and chemotherapy of lung cancer. Adv Funct Mater. 2016;26:8735–8745. [Google Scholar]
- 18.Beatty G.L., Winograd R., Evans R.A., Long K.B., Luque S.L., Lee J.W. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6Clow F4/80+ extratumoral macrophages. Gastroenterology. 2015;149:201–210. doi: 10.1053/j.gastro.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol. 2016;28:383–391. doi: 10.1093/intimm/dxw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans R.A., Diamond M.S., Rech A.J., Chao T., Richardson M.W., Lin J.H. Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkin S., Yuan D., Stein I., Taniguchi K., Weber A., Unger K. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol. 2015;16:1235–1244. doi: 10.1038/ni.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen D.S., Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 23.Veglia F., Gabrilovich D.I. Dendritic cells in cancer: the role revisited. Curr Opin Immunol. 2017;45:43–51. doi: 10.1016/j.coi.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chauhan V.P., Chen I.X., Tong R., Ng M.R., Martin J.D., Naxerova K. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc Natl Acad Sci U S A. 2019;116:10674–10680. doi: 10.1073/pnas.1819889116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen I.X., Chauhan V.P., Posada J., Ng M.R., Wu M.W., Adstamongkonkul P. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci U S A. 2019;116:4558–4566. doi: 10.1073/pnas.1815515116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukumura D., Kloepper J., Amoozgar Z., Duda D.G., Jain R.K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Facciabene A., Peng X., Hagemann I.S., Balint K., Barchetti A., Wang L.P. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T reg cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 29.Palazon A., Tyrakis P.A., Macias D., Veliça P., Rundqvist H., Fitzpatrick S. An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Canc Cell. 2017;32:669–683. doi: 10.1016/j.ccell.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calcinotto A., Filipazzi P., Grioni M., Iero M., De Milito A., Ricupito A. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Canc Res. 2012;72:2746–2756. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- 31.Costa A., Kieffer Y., Scholer-Dahirel A., Pelon F., Bourachot B., Cardon M. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Canc Cell. 2018;33:463–479. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Kuczek D.E., Larsen A.M.H., Thorseth M.L., Carretta M., Kalvisa A., Siersbæk M.S. Collagen density regulates the activity of tumor-infiltrating T cells. J Immunother Cancer. 2019;7:68. doi: 10.1186/s40425-019-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 34.Sindhwani S., Syed A.M., Ngai J., Kingston B.R., Maiorino L., Rothschild J. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19:566–575. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Q., Shao S., Wang J., Xu C., Xiang J., Piao Y. Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat Nanotechnol. 2019;14:799–809. doi: 10.1038/s41565-019-0485-z. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y.Y., Syed A.M., MacMillan P., Rocheleau J.V., Chan W.C.W. Flow rate affects nanoparticle uptake into endothelial cells. Adv Mater. 2020;32:1906274. doi: 10.1002/adma.201906274. [DOI] [PubMed] [Google Scholar]
- 37.Schudel A., Francis D.M., Thomas S.N. Material design for lymph node drug delivery. Nat Rev Mater. 2019;4:415–428. doi: 10.1038/s41578-019-0110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai L., Xu J., Yang Z., Tong R., Dong Z., Wang C. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35–46. doi: 10.1002/mco2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y., Song Y., Du W., Gong L., Chang H., Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26:1–13. doi: 10.1186/s12929-019-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ley K. M1 means kill; M2 means heal. J Immunol. 2017;199:2191–2193. doi: 10.4049/jimmunol.1701135. [DOI] [PubMed] [Google Scholar]
- 42.Zhou J., Tang Z., Gao S., Li C., Feng Y., Zhou X. Tumor-associated macrophages: recent insights and therapies. Front Oncol. 2020;10:188. doi: 10.3389/fonc.2020.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sica A., Schioppa T., Mantovani A., Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Canc. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Quail D.F., Joyce J.A. The microenvironmental landscape of brain tumors. Canc Cell. 2017;31:326–341. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang M., He Y., Sun X., Li Q., Wang W., Zhao A. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7:19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinha P., Clements V.K., Bunt S.K., Albelda S.M., Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 48.Gabrilovich D.I. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Condamine T., Gabrilovich D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marvel D., Gabrilovich D.I. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–3364. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandruzzato S., Brandau S., Britten C.M., Bronte V., Damuzzo V., Gouttefangeas C. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother. 2016;65:161–169. doi: 10.1007/s00262-015-1782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gentles A.J., Newman A.M., Liu C.L., Bratman S.V., Feng W., Kim D. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reid M.D., Basturk O., Thirabanjasak D., Hruban R.H., Klimstra D.S., Bagci P. Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol. 2011;24:1612–1619. doi: 10.1038/modpathol.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chao T., Furth E.E., Vonderheide R.H. CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunol Res. 2016;4:968–982. doi: 10.1158/2326-6066.CIR-16-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steele C.W., Karim S.A., Leach J.D.G., Bailey P., Upstill-Goddard R., Rishi L. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Canc Cell. 2016;29:832–845. doi: 10.1016/j.ccell.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vignali D.A.A., Collison L.W., Workman C.J. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 58.Josefowicz S.Z., Lu L.F., Rudensky A.Y. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka A., Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27:109–118. doi: 10.1038/cr.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ormandy L.A., Hillemann T., Wedemeyer H., Manns M.P., Greten T.F., Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Canc Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 61.Sasada T., Kimura M., Yoshida Y., Kanai M., Takabayashi A. CD4+ CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer Interdiscip Int J Am Cancer Soc. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 62.Nishikawa H., Sakaguchi S. Regulatory T cells in tumor immunity. Int J Canc. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 63.Chinen T., Kannan A.K., Levine A.G., Fan X., Klein U., Zheng Y. An essential role for the IL-2 receptor in T reg cell function. Nat Immunol. 2016;17:1322–1333. doi: 10.1038/ni.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakaguchi S., Takahashi T., Yamazaki S., Kuniyasu Y., Itoh M., Sakaguchi N. Immunologic self tolerance maintained by T-cell-mediated control of self-reactive T cells: implications for autoimmunity and tumor immunity. Microb Infect. 2001;3:911–918. doi: 10.1016/s1286-4579(01)01452-6. [DOI] [PubMed] [Google Scholar]
- 65.Qureshi O.S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E.M. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao X., Cai S.F., Fehniger T.A., Song J., Collins L.I., Piwnica-Worms D.R. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 67.Bauer C.A., Kim E.Y., Marangoni F., Carrizosa E., Claudio N.M., Mempel T.R. Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J Clin Invest. 2014;124:2425–2440. doi: 10.1172/JCI66375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jang J.E., Hajdu C.H., Liot C., Miller G., Dustin M.L., Bar-Sagi D. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep. 2017;20:558–571. doi: 10.1016/j.celrep.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olkhanud P.B., Baatar D., Bodogai M., Hakim F., Gress R., Anderson R.L. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Canc Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobie J.J., Shah P.R., Yang L., Rebhahn J.A., Fowell D.J., Mosmann T.R. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 71.Deaglio S., Dwyer K.M., Gao W., Friedman D., Usheva A., Erat A. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allard B., Beavis P.A., Darcy P.K., Stagg J. Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol. 2016;29:7–16. doi: 10.1016/j.coph.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 73.Flynn N.J., Somasundaram R., Arnold K.M., Sims-Mourtada J. The multifaceted roles of B cells in solid tumors: emerging treatment opportunities. Targeted Oncol. 2017;12:139–152. doi: 10.1007/s11523-017-0481-x. [DOI] [PubMed] [Google Scholar]
- 74.Brodt P., Gordon J. Anti-tumor immunity in B lymphocyte-deprived mice: I. Immunity to a chemically induced tumor. J Immunol. 1978;121:359–362. [PubMed] [Google Scholar]
- 75.Schwartz M., Zhang Y., Rosenblatt J.D. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer. 2016;4:40. doi: 10.1186/s40425-016-0145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nelson B.H. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185:4977–4982. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 77.Sarvaria A., Madrigal J.A., Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;14:662–674. doi: 10.1038/cmi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sautès Fridman C., Petitprez F., Calderaro J., Fridman W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Canc. 2019;19:307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 79.Garner H., de Visser K.E. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol. 2020;20:483–497. doi: 10.1038/s41577-019-0271-z. [DOI] [PubMed] [Google Scholar]
- 80.Rosser E.C., Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42:607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 81.Khan A.R., Hams E., Floudas A., Sparwasser T., Weaver C.T., Fallon P.G. PD-L1 hi B cells are critical regulators of humoral immunity. Nat Commun. 2015;6:1–16. doi: 10.1038/ncomms6997. [DOI] [PubMed] [Google Scholar]
- 82.Peng B., Ming Y., Yang C. Regulatory B cells: the cutting edge of immune tolerance in kidney transplantation. Cell Death Dis. 2018;9:1–13. doi: 10.1038/s41419-017-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kessel A., Haj T., Peri R., Snir A., Melamed D., Sabo E. Human CD19+ CD25 high B regulatory cells suppress proliferation of CD4+ T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev. 2012;11:670–677. doi: 10.1016/j.autrev.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 84.Bodogai M., Chang C.L., Wejksza K., Lai J., Merino M., Wersto R.P. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Canc Res. 2013;73:2127–2138. doi: 10.1158/0008-5472.CAN-12-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bodogai M., Moritoh K., Lee-Chang C., Hollander C.M., Sherman-Baust C.A., Wersto R.P. Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells. Canc Res. 2015;75:3456–3465. doi: 10.1158/0008-5472.CAN-14-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu T., Zhou L., Li D., Andl T., Zhang Y. Cancer-associated fibroblasts build and secure the tumor microenvironment. Front Cell Dev Biol. 2019;7:60. doi: 10.3389/fcell.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Helvert S., Storm C., Friedl P. Mechanoreciprocity in cell migration. Nat Cell Biol. 2018;20:8–20. doi: 10.1038/s41556-017-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shattil S.J., Kim C., Ginsberg M.H. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Erdogan B., Ao M., White L.M., Means A.L., Brewer B.M., Yang L. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol. 2017;216:3799–3816. doi: 10.1083/jcb.201704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benito Jardón M., Klapproth S., Gimeno LLuch I., Petzold T., Bharadwaj M., Müller D.J. The fibronectin synergy site re-enforces cell adhesion and mediates a crosstalk between integrin classes. Elife. 2017;6 doi: 10.7554/eLife.22264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R.P., Chaudhry S.I. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Labernadie A., Kato T., Brugués A., Serra Picamal X., Derzsi S., Arwert E. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat Cell Biol. 2017;19:224–237. doi: 10.1038/ncb3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen R., Huang L., Hu K. Natural products remodel cancer-associated fibroblasts in desmoplastic tumors. Acta Pharm Sin B. 2020 doi: 10.1016/j.apsb.2020.04.005. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Canc. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Virgin H.W., Wherry E.J., Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 96.Palucka A.K., Coussens L.M. The basis of oncoimmunology. Cell. 2016;164:1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kersten K., Coffelt S.B., Hoogstraat M., Verstegen N.J.M., Vrijland K., Ciampricotti M. Mammary tumor-derived CCL2 enhances pro-metastatic systemic inflammation through upregulation of IL1β in tumor-associated macrophages. OncoImmunology. 2017;6 doi: 10.1080/2162402X.2017.1334744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Voronov E., Dotan S., Krelin Y., Song X., Elkabets M., Carmi Y. Unique versus redundant functions of IL-1α and IL-1β in the tumor microenvironment. Front Immunol. 2013;4:177. doi: 10.3389/fimmu.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen M., Wu J., Ning P., Wang J., Ma Z., Huang L. Remote control of mechanical forces via mitochondrial-targeted magnetic nanospinners for efficient cancer treatment. Small. 2020;16:1905424. doi: 10.1002/smll.201905424. [DOI] [PubMed] [Google Scholar]
- 101.Li J., Cao F., Yin H., Huang Z., Lin Z., Mao N. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reits E.A., Hodge J.W., Herberts C.A., Groothuis T.A., Chakraborty M.K., Wansley E. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gupta A., Probst H.C., Vuong V., Landshammer A., Muth S., Yagita H. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–566. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 104.Qu Y., Jin S., Zhang A., Zhang B., Shi X., Wang J. Gamma-ray resistance of regulatory CD4+ CD25+ Foxp3+ T cells in mice. Radiat Res. 2010;173:148–157. doi: 10.1667/RR0978.1. [DOI] [PubMed] [Google Scholar]
- 105.Kachikwu E.L., Iwamoto K.S., Liao Y.P., DeMarco J.J., Agazaryan N., Economou J.S. Radiation enhances regulatory T cell representation. Int J Radiat Oncol Biol Phys. 2011;81:1128–1135. doi: 10.1016/j.ijrobp.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Korbelik M. Induction of tumor immunity by photodynamic therapy. J Clin Laser Med Surg. 1996;14:329–334. doi: 10.1089/clm.1996.14.329. [DOI] [PubMed] [Google Scholar]
- 107.Chauhan V.P., Jain R.K. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12:958–962. doi: 10.1038/nmat3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Leuschner F., Dutta P., Gorbatov R., Novobrantseva T.I., Donahoe J.S., Courties G. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–1010. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shen S., Li H.J., Chen K.G., Wang Y.C., Yang X.Z., Lian Z.X. Spatial targeting of tumor-associated macrophages and tumor cells with a pH-sensitive cluster nanocarrier for cancer chemoimmunotherapy. Nano Lett. 2017;17:3822–3829. doi: 10.1021/acs.nanolett.7b01193. [DOI] [PubMed] [Google Scholar]
- 110.Zang X., Zhang X., Hu H., Qiao M., Zhao X., Deng Y. Targeted delivery of zoledronate to tumor-associated macrophages for cancer immunotherapy. Mol Pharm. 2019;16:2249–2258. doi: 10.1021/acs.molpharmaceut.9b00261. [DOI] [PubMed] [Google Scholar]
- 111.Riganti C., Castella B., Kopecka J., Campia I., Coscia M., Pescarmona G. Zoledronic acid restores doxorubicin chemosensitivity and immunogenic cell death in multidrug-resistant human cancer cells. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060975. e60975–e60975. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Wang Y., Lin Y.X., Qiao S.L., An H.W., Ma Y., Qiao Z.Y. Polymeric nanoparticles promote macrophage reversal from M2 to M1 phenotypes in the tumor microenvironment. Biomaterials. 2017;112:153–163. doi: 10.1016/j.biomaterials.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 113.Kiyota T., Takahashi Y., Watcharanurak K., Nishikawa M., Ohara S., Ando M. Enhancement of anticancer effect of interferon-γ gene transfer against interferon-γ-resistant tumor by depletion of tumor-associated macrophages. Mol Pharm. 2014;11:1542–1549. doi: 10.1021/mp4007216. [DOI] [PubMed] [Google Scholar]
- 114.Zhan X., Jia L., Niu Y., Qi H., Chen X., Zhang Q. Targeted depletion of tumour-associated macrophages by an alendronate–glucomannan conjugate for cancer immunotherapy. Biomaterials. 2014;35:10046–10057. doi: 10.1016/j.biomaterials.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 115.Zhou S., Zhang T., Peng B., Luo X., Liu X., Hu L. Targeted delivery of epirubicin to tumor-associated macrophages by sialic acid-cholesterol conjugate modified liposomes with improved antitumor activity. Int J Pharm. 2017;523:203–216. doi: 10.1016/j.ijpharm.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 116.Tian L., Yi X., Dong Z., Xu J., Liang C., Chao Y. Calcium Bisphosphonate nanoparticles with chelator-free radiolabeling to deplete tumor-associated macrophages for enhanced cancer radioisotope therapy. ACS Nano. 2018;12:11541–11551. doi: 10.1021/acsnano.8b06699. [DOI] [PubMed] [Google Scholar]
- 117.Peng H., Chen B., Huang W., Tang Y., Jiang Y., Zhang W. Reprogramming tumor-associated macrophages to reverse EGFRT790M resistance by dual-targeting codelivery of gefitinib/vorinostat. Nano Lett. 2017;17:7684–7690. doi: 10.1021/acs.nanolett.7b03756. [DOI] [PubMed] [Google Scholar]
- 118.Wang H., Tang Y., Fang Y., Zhang M., Wang H., He Z. Reprogramming tumor immune microenvironment (TIME) and metabolism via biomimetic targeting codelivery of shikonin/JQ1. Nano Lett. 2019;19:2935–2944. doi: 10.1021/acs.nanolett.9b00021. [DOI] [PubMed] [Google Scholar]
- 119.Plebanek M.P., Bhaumik D., Bryce P.J., Thaxton C.S. Scavenger receptor type B1 and lipoprotein nanoparticle inhibit myeloid-derived suppressor cells. Mol Canc Therapeut. 2018;17:686–697. doi: 10.1158/1535-7163.MCT-17-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoyen-Ermis D., Ozturk Atar K., Kursunel M.A., Aydin C., Ozkazanc D., Gurbuz M.U. Tumor-induced myeloid cells are reduced by gemcitabine-loaded PAMAM dendrimers decorated with anti-Flt1 antibody. Mol Pharm. 2018;15:1526–1533. doi: 10.1021/acs.molpharmaceut.7b01075. [DOI] [PubMed] [Google Scholar]
- 121.Phuengkham H., Song C., Um S.H., Lim Y.T. Implantable synthetic immune niche for spatiotemporal modulation of tumor-derived immunosuppression and systemic antitumor immunity: postoperative immunotherapy. Adv Mater. 2018;30:1706719. doi: 10.1002/adma.201706719. [DOI] [PubMed] [Google Scholar]
- 122.Jeanbart L., Kourtis I.C., van der Vlies A.J., Swartz M.A., Hubbell J.A. 6-Thioguanine-loaded polymeric micelles deplete myeloid-derived suppressor cells and enhance the efficacy of T cell immunotherapy in tumor-bearing mice. Cancer Immunol Immunother. 2015;64:1033–1046. doi: 10.1007/s00262-015-1702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Long Y., Lu Z., Xu S., Li M., Wang X., Zhang Z. Self-delivery micellar nanoparticles prevent premetastatic niche formation by interfering with the early recruitment and vascular destruction of granulocytic myeloid-derived suppressor cells. Nano Lett. 2020;20:2219–2229. doi: 10.1021/acs.nanolett.9b03883. [DOI] [PubMed] [Google Scholar]
- 124.Li S., Wang Q., Shen Y., Hassan M., Shen J., Jiang W. Pseudoneutrophil cytokine sponges disrupt myeloid expansion and tumor trafficking to improve cancer immunotherapy. Nano Lett. 2020;20:242–251. doi: 10.1021/acs.nanolett.9b03753. [DOI] [PubMed] [Google Scholar]
- 125.Kong M., Tang J., Qiao Q., Wu T., Qi Y., Tan S. Biodegradable hollow mesoporous silica nanoparticles for regulating tumor microenvironment and enhancing antitumor efficiency. Theranostics. 2017;7:3276. doi: 10.7150/thno.19987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao Y., Huo M., Xu Z., Wang Y., Huang L. Nanoparticle delivery of CDDO-Me remodels the tumor microenvironment and enhances vaccine therapy for melanoma. Biomaterials. 2015;68:54–66. doi: 10.1016/j.biomaterials.2015.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zanganeh S., Hutter G., Spitler R., Lenkov O., Mahmoudi M., Shaw A. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sacchetti C., Rapini N., Magrini A., Cirelli E., Bellucci S., Mattei M. In vivo targeting of intratumor regulatory T cells using PEG-modified single-walled carbon nanotubes. Bioconjugate Chem. 2013;24:852–858. doi: 10.1021/bc400070q. [DOI] [PubMed] [Google Scholar]
- 129.Ou W., Thapa R.K., Jiang L., Soe Z.C., Gautam M., Chang J.-H. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J Control Release. 2018;281:84–96. doi: 10.1016/j.jconrel.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 130.Song Q., Yin Y., Shang L., Wu T., Zhang D., Kong M. Tumor microenvironment responsive nanogel for the combinatorial antitumor effect of chemotherapy and immunotherapy. Nano Lett. 2017;17:6366–6375. doi: 10.1021/acs.nanolett.7b03186. [DOI] [PubMed] [Google Scholar]
- 131.Molavi O., Ma Z., Hamdy S., Lavasanifar A., Samuel J. Immunomodulatory and anticancer effects of intra-tumoral co-delivery of synthetic lipid A adjuvant and STAT3 inhibitor, JSI-124. Immunopharmacol Immunotoxicol. 2009;31:214–221. doi: 10.1080/08923970802380452. [DOI] [PubMed] [Google Scholar]
- 132.Ou W., Jiang L., Thapa R.K., Soe Z.C., Poudel K., Chang J.H. Combination of NIR therapy and regulatory T cell modulation using layer-by-layer hybrid nanoparticles for effective cancer photoimmunotherapy. Theranostics. 2018;8:4574. doi: 10.7150/thno.26758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Huo M., Zhao Y., Satterlee A.B., Wang Y., Xu Y., Huang L. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. J Control Release. 2017;245:81–94. doi: 10.1016/j.jconrel.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang J., Miao L., Guo S., Zhang Y., Zhang L., Satterlee A. Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model. J Control Release. 2014;182:90–96. doi: 10.1016/j.jconrel.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miao L., Liu Q., Lin C.M., Luo C., Wang Y., Liu L. Targeting tumor-associated fibroblasts for therapeutic delivery in desmoplastic tumors. Canc Res. 2017;77:719–731. doi: 10.1158/0008-5472.CAN-16-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhen Z., Tang W., Wang M., Zhou S., Wang H., Wu Z. Protein nanocage mediated fibroblast-activation protein targeted photoimmunotherapy to enhance cytotoxic T cell infiltration and tumor control. Nano Lett. 2017;17:862–869. doi: 10.1021/acs.nanolett.6b04150. [DOI] [PubMed] [Google Scholar]
- 137.Murakami M., Ernsting M.J., Undzys E., Holwell N., Foltz W.D., Li S.D. Docetaxel conjugate nanoparticles that target α-smooth muscle actin–expressing stromal cells suppress breast cancer metastasis. Canc Res. 2013;73:4862–4871. doi: 10.1158/0008-5472.CAN-13-0062. [DOI] [PubMed] [Google Scholar]
- 138.Lang J., Zhao X., Qi Y., Zhang Y., Han X., Ding Y. Reshaping prostate tumor microenvironment to suppress metastasis via cancer-associated fibroblast inactivation with peptide-assembly-based nanosystem. ACS Nano. 2019;13:12357–12371. doi: 10.1021/acsnano.9b04857. [DOI] [PubMed] [Google Scholar]
- 139.Zhu Y., Wen L., Shao S., Tan Y., Meng T., Yang X. Inhibition of tumor-promoting stroma to enforce subsequently targeting AT1R on tumor cells by pathological inspired micelles. Biomaterials. 2018;161:33–46. doi: 10.1016/j.biomaterials.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 140.Chen B., Dai W., Mei D., Liu T., Li S., He B. Comprehensively priming the tumor microenvironment by cancer-associated fibroblast-targeted liposomes for combined therapy with cancer cell-targeted chemotherapeutic drug delivery system. J Control Release. 2016;241:68–80. doi: 10.1016/j.jconrel.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 141.Li J., Zhen X., Lyu Y., Jiang Y., Huang J., Pu K. Cell membrane coated semiconducting polymer nanoparticles for enhanced multimodal cancer phototheranostics. ACS Nano. 2018;12:8520–8530. doi: 10.1021/acsnano.8b04066. [DOI] [PubMed] [Google Scholar]
- 142.Cassetta L., Pollard J.W. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904. doi: 10.1038/nrd.2018.169. [DOI] [PubMed] [Google Scholar]
- 143.Argyle D., Kitamura T. Targeting macrophage-recruiting chemokines as a novel therapeutic strategy to prevent the progression of solid tumors. Front Immunol. 2018;9:2629. doi: 10.3389/fimmu.2018.02629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Halama N., Zoernig I., Berthel A., Kahlert C., Klupp F., Suarez-Carmona M. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Canc Cell. 2016;29:587–601. doi: 10.1016/j.ccell.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 145.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ries C.H., Cannarile M.A., Hoves S., Benz J., Wartha K., Runza V. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Canc Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 147.Shen S., Zhang Y., Chen K.-G., Luo Y.L., Wang J. Cationic polymeric nanoparticle delivering CCR2 siRNA to inflammatory monocytes for tumor microenvironment modification and cancer therapy. Mol Pharm. 2018;15:3642–3653. doi: 10.1021/acs.molpharmaceut.7b00997. [DOI] [PubMed] [Google Scholar]
- 148.Schmid D., Park C.G., Hartl C.A., Subedi N., Cartwright A.N., Puerto R.B. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat Commun. 2017;8:1–12. doi: 10.1038/s41467-017-01830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Labanieh L., Majzner R.G., Mackall C.L. Programming CAR-T cells to kill cancer. Nat Biomed Eng. 2018;2:377–391. doi: 10.1038/s41551-018-0235-9. [DOI] [PubMed] [Google Scholar]
- 150.Smith T.T., Stephan S.B., Moffett H.F., McKnight L.E., Ji W., Reiman D. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Han X., Shen S., Fan Q., Chen G., Archibong E., Dotti G. Red blood cell–derived nanoerythrosome for antigen delivery with enhanced cancer immunotherapy. Sci Adv. 2019;5 doi: 10.1126/sciadv.aaw6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miao L., Li J., Liu Q., Feng R., Das M., Lin C.M. Transient and local expression of chemokine and immune checkpoint traps to treat pancreatic cancer. ACS Nano. 2017;11:8690–8706. doi: 10.1021/acsnano.7b01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guo J., Zeng H., Chen Y. Emerging nano drug delivery systems targeting cancer-associated fibroblasts for improved antitumor effect and tumor drug penetration. Mol Pharm. 2020;17:1028–1048. doi: 10.1021/acs.molpharmaceut.0c00014. [DOI] [PubMed] [Google Scholar]
- 154.Garin Chesa P., Old L.J., Rettig W.J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim M.Y., Kim O.R., Choi Y.S., Lee H., Park K., Lee C.T. Selection and characterization of tenascin C targeting peptide. Mol Cell. 2012;33:71–77. doi: 10.1007/s10059-012-2214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen B., Wang Z., Sun J., Song Q., He B., Zhang H. A tenascin C targeted nanoliposome with navitoclax for specifically eradicating of cancer-associated fibroblasts. Nanomed Nanotechnol Biol Med. 2016;12:131–141. doi: 10.1016/j.nano.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 157.Wang Q., Qu C., Xie F., Chen L., Liu L., Liang X. Curcumin suppresses epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells by inhibiting cancer-associated fibroblasts. Am J Cancer Res. 2017;7:125–133. [PMC free article] [PubMed] [Google Scholar]
- 158.Ting H., Deep G., Kumar S., Jain A.K., Agarwal C., Agarwal R. Beneficial effects of the naturally occurring flavonoid silibinin on the prostate cancer microenvironment: role of monocyte chemotactic protein-1 and immune cell recruitment. Carcinogenesis. 2016;37:589–599. doi: 10.1093/carcin/bgw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hou L., Liu Q., Shen L., Liu Y., Zhang X., Chen F. Nano-delivery of fraxinellone remodels tumor microenvironment and facilitates therapeutic vaccination in desmoplastic melanoma. Theranostics. 2018;8:3781. doi: 10.7150/thno.24821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Suh J., Kim D.H., Surh Y.J. Resveratrol suppresses migration, invasion and stemness of human breast cancer cells by interfering with tumor-stromal cross-talk. Arch Biochem Biophys. 2018;643:62–71. doi: 10.1016/j.abb.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 161.Özdemir B.C., Pentcheva Hoang T., Carstens J.L., Zheng X., Wu C.C., Simpson T.R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Canc Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nam J., Son S., Park K.S., Zou W., Shea L.D., Moon J.J. Cancer nanomedicine for combination cancer immunotherapy. Nat Rev Mater. 2019;4:398–414. [Google Scholar]
- 163.Kinoh H., Miura Y., Chida T., Liu X., Mizuno K., Fukushima S. Nanomedicines eradicating cancer stem-like cells in vivo by pH-triggered intracellular cooperative action of loaded drugs. ACS Nano. 2016;10:5643–5655. doi: 10.1021/acsnano.6b00900. [DOI] [PubMed] [Google Scholar]
- 164.Cabral H., Miyata K., Osada K., Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118:6844–6892. doi: 10.1021/acs.chemrev.8b00199. [DOI] [PubMed] [Google Scholar]
- 165.Duan X., Chan C., Han W., Guo N., Weichselbaum R.R., Lin W. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat Commun. 2019;10:1–15. doi: 10.1038/s41467-019-09221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moynihan K.D., Opel C.F., Szeto G.L., Tzeng A., Zhu E.F., Engreitz J.M. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med. 2016;22:1402. doi: 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chiang C.S., Lin Y.J., Lee R., Lai Y.H., Cheng H.W., Hsieh C.H. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat Nanotechnol. 2018;13:746–754. doi: 10.1038/s41565-018-0146-7. [DOI] [PubMed] [Google Scholar]
- 168.Phuengkham H., Song C., Lim Y.T. A designer scaffold with immune nanoconverters for reverting immunosuppression and enhancing immune checkpoint blockade therapy. Adv Mater. 2019;31:1903242. doi: 10.1002/adma.201903242. [DOI] [PubMed] [Google Scholar]
- 169.Huang H., Jiang C.T., Shen S., Liu A., Gan Y.J., Tong Q.S. Nanoenabled reversal of Ido1-mediated immunosuppression synergizes with immunogenic chemotherapy for improved cancer therapy. Nano Lett. 2019;19:5356–5365. doi: 10.1021/acs.nanolett.9b01807. [DOI] [PubMed] [Google Scholar]
- 170.Hua S., De Matos M.B.C., Metselaar J.M., Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol. 2018;9:790. doi: 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sainz V., Conniot J., Matos A.I., Peres C., Zupanǒiǒ E., Moura L. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun. 2015;468:504–510. doi: 10.1016/j.bbrc.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 172.Caster J.M., Patel A.N., Zhang T., Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9:e1416. doi: 10.1002/wnan.1416. [DOI] [PubMed] [Google Scholar]
- 173.CSPC ZhongQi Pharmaceutical Technology Co. Ltd. A study of liposome-entrapped mitoxantrone hydrochloride injection in relapsed/refractory peripheral T-cell lymphoma and NK/T-cell lymphoma. Available from: https://clinicaltrials.gov/ct2/show/NCT03776279?term=liposome&cond=immune+cells&draw=2&rank=1.
- 174.Dana-Farber Cancer Institute. Dendritic cell activating scaffold in melanoma. Available from: https://clinicaltrials.gov/ct2/show/NCT01753089?term=NCT01753089.
- 175.Li X., Wenes M., Romero P., Huang S.C.C., Fendt S.M., Ho P.C. Navigating metabolic pathways to enhance antitumour immunity and immunotherapy. Nat Rev Clin Oncol. 2019;16:425–441. doi: 10.1038/s41571-019-0203-7. [DOI] [PubMed] [Google Scholar]
- 176.Sanmamed M.F., Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu Y., Guo J., Huang L. Modulation of tumor microenvironment for immunotherapy: focus on nanomaterial-based strategies. Theranostics. 2020;10:3099. doi: 10.7150/thno.42998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Massachusetts General Hospital. Proton w/FOLFIRINOX-losartan for pancreatic cancer. Available from: https://clinicaltrials.gov/ct2/show/NCT01821729.
- 179.Chauhan V.P., Martin J.D., Liu H., Lacorre D.A., Jain S.R., Kozin S.V. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:1–11. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Irvine D.J., Dane E.L. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20:321–334. doi: 10.1038/s41577-019-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Patel S., Fu S., Mastio J., Dominguez G.A., Purohit A., Kossenkov A. Unique pattern of neutrophil migration and function during tumor progression. Nat Immunol. 2018;19:1236–1247. doi: 10.1038/s41590-018-0229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yee C. Adoptive T cell therapy: points to consider. Curr Opin Immunol. 2018;51:197–203. doi: 10.1016/j.coi.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 183.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 184.Moffett H.F., Coon M.E., Radtke S., Stephan S.B., McKnight L., Lambert A. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nat Commun. 2017;8:389. doi: 10.1038/s41467-017-00505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sharei A., Trifonova R., Jhunjhunwala S., Hartoularos G.C., Eyerman A.T., Lytton-Jean A. Ex vivo cytosolic delivery of functional macromolecules to immune cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Szeto G.L., Van Egeren D., Worku H., Sharei A., Alejandro B., Park C. Microfluidic squeezing for intracellular antigen loading in polyclonal B-cells as cellular vaccines. Sci Rep. 2015;5:10276. doi: 10.1038/srep10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stewart M.P., Sharei A., Ding X., Sahay G., Langer R., Jensen K.F. In vitro and ex vivo strategies for intracellular delivery. Nature. 2016;538:183–192. doi: 10.1038/nature19764. [DOI] [PubMed] [Google Scholar]
- 188.Wilson J.T., Keller S., Manganiello M.J., Cheng C., Lee C.C., Opara C. pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7:3912–3925. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang C., Zhang J., Shi G., Song H., Shi S., Zhang X. A light responsive nanoparticle-based delivery system using pheophorbide a graft polyethylenimine for dendritic cell-based cancer immunotherapy. Mol Pharm. 2017;14:1760–1770. doi: 10.1021/acs.molpharmaceut.7b00015. [DOI] [PubMed] [Google Scholar]
- 190.Pan Y., Yoon S., Sun J., Huang Z., Lee C., Allen M. Mechanogenetics for the remote and noninvasive control of cancer immunotherapy. Proc Natl Acad Sci U S A. 2018;115:992–997. doi: 10.1073/pnas.1714900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Andorko J.I., Pineault K.G., Jewell C.M. Impact of molecular weight on the intrinsic immunogenic activity of poly(beta amino esters) J Biomed Mater Res. 2017;105:1219–1229. doi: 10.1002/jbm.a.35970. [DOI] [PubMed] [Google Scholar]
- 192.Andorko J.I., Hess K.L., Pineault K.G., Jewell C.M. Intrinsic immunogenicity of rapidly-degradable polymers evolves during degradation. Acta Biomater. 2016;32:24–34. doi: 10.1016/j.actbio.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhu Y., Liu Y., Jin K., Pang Z. Polysaccharide carriers for drug delivery. Woodhead Publishing; 2019. Polysaccharide nanoparticles for cancer drug targeting; pp. 365–396. [Google Scholar]
- 194.Li C., Wang J., Wang Y., Gao H., Wei G., Huang Y. Recent progress in drug delivery. Acta Pharm Sin B. 2019;9:1145–1162. doi: 10.1016/j.apsb.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]