Abstract
Apple (Malus domestica Borkh) is rich in phenolic compounds, which may enhance resistance to scab disease caused by Venturia inaequalis. In present study, apple cv. Golden Delicious was used for estimation of total phenols, flavonoids and quantification of six individual phenolic compounds. between control vs inoculated samples at different inoculation stages. The relative gene expression of phenylalanine ammonia lyase, chalcone synthase and flavanone 3 hydroxylase increased and polyphenolic compounds were constitutively upregulated at different post-inoculation stages. Data suggest that synthesis and accumulation of polyphenols is closely related with disease resistance against Venturia inaequalis. This study may play a vital role in understanding and finding out the governing mechanisms of scab resistance.
Keywords: Apple, Venturia inaequalis, Polyphenols, Phenylalanine ammonia lyase, Resistance
1. Introduction
Apple scab caused by Venturia inaequalis, a filamentous fungus, is one of the most detrimental fungal pathogens that remain a major threat to apple production worldwide (Mansoor et al., 2019). It is among the most noteworthy threats to apple growing areas situated in the regions where climate is cool (MacHardy, 1996). Causal agent of apple scab Venturia inaequalis (Cke) belongs to subdivision Ascomycota, class Loculoascomycetes, order Pleosporales and family Venturiaceae and genus Venturia. Saprophytic (sexual state Venturia inaequalis (Cke) and parasitic (asexual state Spilocaea Pomi Fr) are the two states of this fungus. Damage caused by this pathogen are developed in between the cuticle and the cell wall of epidermal layers (MacHardy, 1996, Mansoor et al., 2019).
Phenolic compounds are vital factors which determine the sensory and nutritious quality of apple. These compounds have been suggested to be involved in plant defense against various biotic and abiotic stresses, Various different type of phenolic compounds has been reported to be present in apple tissues (Fünfgelder et al., 1993, Bazzi et al., 2003). Plant phenols arising from phenylpropanoid pathway is the secondary metabolites of several classes of structurally diverse products. They play a significant role in pigmentation, growth and especially resistance to pathogens (Lattanzio et al., 2006). These phenols deposits inside the cell wall and acts as first line of defense, thus prevents the fungal penetration and infection (Schwalb and Feucht, 1999). Polyphenols possess antibacterial, antiviral and antifungal properties (Martini et al., 2009). Flavonoids present in plant tissues are reported to play crucial role in plant resistance e.g phloretin found in apple fruits and leaves confer resistance against Venturia inaequalis (Treutter, 2005). Large number of fungi including Venturia inaequalis hydrolyses phloridzin in to phloretin thus induces defense response. Other compounds such as hydroxycinnamic acids also play a defensive role in apple against V. inaequalis among which flavonols (epicatechin, procyanidin B1, catechin) and dihydrochalcones play a key role (Slatnar et al., 2012). Phenolic acids, are reported to impact the healing by increasing lignification of damaged areas and their concentrations may increase at site of infection after pathogen attack (Michalek et al., 1998).
Keeping in view, the objective of this study was to determine the concentrations of different polyphenolic compounds at different time periods of infection as well to analyze the relative gene expression.
2. Materials and method
2.1. Plant material and inoculation
One-year old plants of apple cv. Golden Delicious grafted on M9 rootstock were kept in growth chamber where temperature and humidity were maintained (18 °C temperature and humidity (Fig. 1). The experimentation was carried out at Plant Biotechnology, ICAR-Central Institute of Temperate Horticulture Srinagar.
Fig. 1.
One year-old plants grafted on rootstock M9 grown in a greenhouse and shifted to growth chamber where temperature and humidity was maintained.
2.1.1. Preparation of inoculation load
Conidia of Venturia inaequalis pure culture were used for inoculation in a concentration of 5 × 105 spores/ml measured with the help of Neuberg hemacytometer.
2.1.2. Inoculation conditions and sample collection
The inoculation of plants was carried out by spraying the leaves with the spore solution, the plants were continuously irrigated. In addition, non-inoculated (control) plants were mock-sprayed with water under the same conditions. Pooled samples consisting of six to eight leaves from three to four plants were taken pre-inoculation, 1-day, 7-days and 24-days post inoculation, frozen in liquid nitrogen, and stored at −80 °C for gene expression analyses. Leaves from 24 dpi having highest percentage of infected area were used for relative gene expression studies.
2.2. Preparation of extracts
2.2.1. Extraction procedure
The Fragmentation of leaves was carried out in a motar-pestle and were immediately frozen with liquid nitrogen (1:2w/v) in order to avoid the oxidation of the phenolic compounds (Guyot et al., 2002). Homogenization and final extraction of freeze-dried material without midrib of leaves was carried out by method given by Shafi et al. (2019).
2.2.2. Determination of total polyphenolic content (TPC)
Total phenol contents were determined by the modified Folin-Ciocalteau method (Omoruyi et al., 2012), Absorbance was then measured at 765 nm using spectrophotometer. The results were expressed as mg of gallic acid equivalents/100 g fw.
2.2.3. Determination of total flavonoid content
Total flavonoid content was determined using the method of Chang et al. (2002). The absorbance was then measured at 415 nm using spectrophotometer. Results are expressed in terms of catechin equivalent (mg/100 g fw).
2.3. High performance liquid chromatography (HPLC) analysis
The HPLC analysis of samples was carried out in a Shimadzu HPLC (Kyoto, Japan) equipped with quaternary pumps, degasser coupled to a photo-diode-array detector and injection valve with a 20 µL loop. An injection volume of 20 μL, a flow rate of 1.0 ml min−1 with 1 h of run time was used for separation process. Thrice the analysis was carried out for each sample. Quercetin was detected at 262 nm and the retention time was 1.915 mins. Quercetin, catechin and rutin standard were obtained from Sigma Aldrich. Chromatographic separations were performed on C18 (250 mm × 4.6 mm), 5 µm column using a solvent system in gradient mode followed by isocratic run as represented in Table 1. The filtration of mobile phase was done through a 0.45 μm membrane filter (Millipore, Bedford, MA, USA) and was subjected to 40mins ultrasonication. Instrument control, data acquisition and data processing were done by using Class WP software (version 6.1) from Shimadzu. Quantitative determinations were made by taking into account the respective peak areas of standards at particular retention time versus concentration and expressed in µg/g of apple leaves (Shafi et al., 2019).
Table 1.
Mobile phase used for determining standard peak area at particular retention time for quantitative determination.
| Compound | Mobile Phase | Gradient | Lambda max |
|---|---|---|---|
| Catechin | Solvent A— 2.5% Acetic acid Solvent B- Aceto-nitrile |
3–9% B (0–5 min) 16–36.4% B (15–33 min) 100% B (5 min) |
280 nm |
| Phloridzin | 326 nm | ||
| Coumaric acid | 254 nm | ||
| Apigenin | 280 nm | ||
| Rutin | 254 nm | ||
| Ferulic acid | 322 nm |
2.4. RNA isolation
Prior to RNA isolation, microfuge tubes, tips, tip boxes, mortar and pestle, spatula, forceps were DEPC (Diethyl pyrocarbonate) treated (0.1%) overnight and then autoclaved followed by drying in hot air oven. Total RNA was extracted from frozen leaf samples of inoculated apple cultivars before inoculation and 1-day, 7-days and 24 days post inoculation using cetyltrimethylammonium bromide (CTAB) method (Asif et al., 2006).
2.4.1. First-strand cDNA synthesis, validation and PCR amplification
First-strand cDNA was synthesized from isolated RNA using oligo-dT and Superscript II (Invitrogen) in a reaction volume of total 20 μL. cDNA synthesis confirmation was determined using GAPDH (Glyceraldehyde 3-phosphate dehydrogenase) (Guerriero et al., 2012) and cDNA (50 ng) was further capitalized for PCR amplification
2.4.2. Relative gene expression studies
The relative gene expression was performed according to manufactures instructions (BIORAD, Californian, USA). The PCR conditions were same as specified (95 °C for 3 min, 40 cycles each at 95 °C for 30 s and 50–62 °C for 30 s). The reference gene used was actin to calculate relative gene expression of candidate PAL pathway genes. Actin gene is constitutively expressed in most of plant tissues, thus is commonly useful internal real-time PCR endogenous base line control in plants (Sukno et al., 2007). All the reactions were carried out in three technical triplicates and no-template controls (NTC). The quantification of target PAL pathway genes was determined relative to actin using the ΔΔCt method and expressed as 2 − ΔΔ CT for graphic representation (Livak and Schmittgen, 2001). Relative gene expressions were determined according to the Livak and Schmittgen (2001) formula.
[Δ]Ct sample -Ct value for any sample normalized to the endogenous housekeeping gene
[Δ]Ct reference – Ct value for the reference sample normalized to the endogenous housekeeping gene
3. Results
3.1. Estimation of total phenols and flavonoids using spectrophotometer
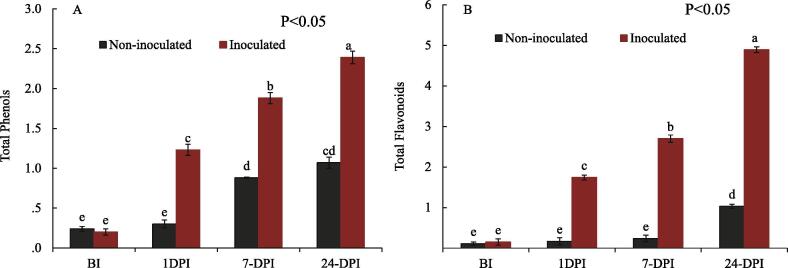
The Venturia inaequalis inoculated cultivar has demonstrated statistically differential levels of total polyphenolic content ranging between 0.205 and 2.395 mg GAE/100 g fw. There was a 4-fold increase in total phenolics post 1 day (24-hours) of inoculation and from 1 day to 7 days content increased by 1.3 times while as from pre-inoculation to post 24 day of inoculation phenolic content increased 12-fold. In control, no such increase in the phenolic levels at different stages post treatment was found, the highest level in control was found 24 days post inoculation as 1.871 mg GAE/100 g fw (Fig. 2). Total Flavonoids content was again highest in inoculated cultivar, where the content was found to be 4.89 mg CE/100 g fw at 24-day post inoculation. Before inoculation phenolic content was 0.15 mg CE/100 g fw, followed by 1.74, 2.70 & 4.89 mg CE/100 g fw and highest content reached at 24-DPI when symptoms in susceptible were clear visible. Pre-inoculation to post 1 day (24 h) the flavonoid content increased by 11.6 times and from 24 h to 7 days 1.6-time increase was found. While as from 7 day to 24 day there was 1.8-fold increase in flavonoid content. After 24- days post inoculation the flavonoid content was 1.03 mg CE/100 g fw (Fig. 2).
Fig. 2.
Effect of Venturia inaequalis on total phenolics and flavonoids of apple. Total phenols (mg GAE equivalent (gallic acid)/100 g fw) and flavonoids (mg CE equivalent (catechin) /100 g fw) were measured at different time-points (BI, 1, 7 and 24 Days) of pathogen inoculation and sterile water treatment (as the control). Data are shown as mean ± SD (Standard Deviation) of three replicates from one independent experiment.
3.2. HPLC based quantification of different phenolic compounds
In present investigation, the following phenolic compounds were quantified in the leaves of inoculated apple cultivars: apigenin, p-coumaric acid, (+) - catechin, phloridzin, rutin and ferulic acid.
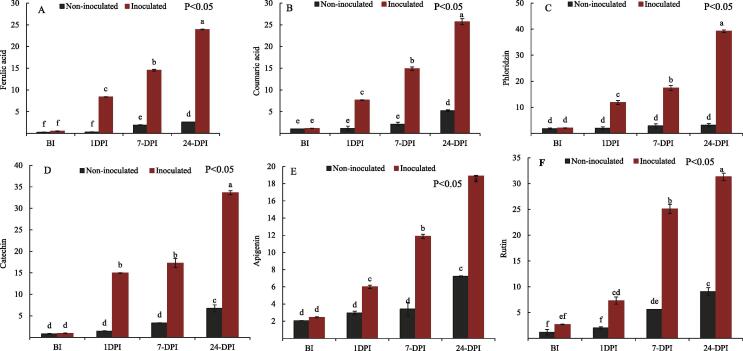
The current investigation has revealed that among selected apple cultivar, the highest ferulic acid content was showed by inoculated apple cultivar ranging between 0.50 and 23.94 µg/g fw with significant increase at each post treatment advanced stage. Pre-inoculation to post 1 day (24 h) in Venturia inaequalis treated cultivar showed 16-fold increase in ferulic acid level, while as in control (water sprayed) 1.3-fold increase was observed. Venturia inaequalis treated cultivar showed highest ferulic acid levels as compared to control in which lower levels of ferulic acid were found at each advance stage post treatment (Fig. 3).
Fig. 3.
Effect of Venturia inaequalis on (µg/g fw) ferulic acid, coumaric acid, catechin, apigenin, rutin and phloridzin content which were measured at different time-points (BI, 1, 7 and 24 Days) of pathogen inoculation and sterile water treatment (as the control). Data are shown as mean ± SD (Standard Deviation) of three replicates from one independent experiment.
Coumaric acid content was observed to be 1.15 µg/g fw before Venturia inaequalis inoculation and after 1 day (24 h) the content reached to 7.67 µg/g fw which shows a 1.6-fold increase in its content. While as in other stages a 2- fold increase was observed. In case of control this content remained in constant range till 7-day 1.02 µg/g fw to 1.91 µg/g fw. While as after 24-days the content reached up to 5.22 µg/g fw. Thus a 2-fold increase was observed from 7 to 24 days post inoculation in control (Fig. 3). In susceptible treated with Venturia inaequalis both of the hydroxycinnamic acids rapidly increased with time period.
Within dihydrochalcones phloridzin content ranged from 2.06 µg/g fw to 39.24 µg/g fw in Venturia inaequalis treated cultivar and in control the content was in range of 1.83 µg/g fw to 3.18 µg/g fw. In case of control phloridzin increased1.2-fold while as in Venturia inaequalis treated the increase way 5.7-fold after 1-day (24 h) of inoculation.
Flavanol catechin production showed drastic increases in Venturia inaequalis treated cultivar the content of catechin raised by 16-fold, apigenin 2.4-fold and rutin 2.7- fold after 1-day (24 h) of inoculation. While as, in control catechin level increased by 1.7-fold, apigenin 1.4-fold and rutin by 1.7-fold after 1-day (24 h) of inoculation. After 24 days of post inoculation the content of catechin was found highest in Venturia inaequalis treated cultivar 33.64 µg/g at this point of time infection was severe and spots were prominent. Isoflavonoid apigenin content before inoculation was 2.45 µg/g fw which increased after inoculation and had a peak value of 11.87 µg/g fw and 18.89 µg/g fw at 7-days and 24-day respectively. Rutin content increased from 2.70 µg/g fw (BI) to 31.31 µg/g fw (24-Days PI) in Venturia inaequalis treated cultivar. In control, all the three flavonoids showed a small increase from pre-inoculation stage to 24- days post inoculation stage (Fig. 3). The phenolic contents overall showed a marked increase in Venturia inaequalis treated susceptible cultivar than in control. In control, phenolic content either remained constant or increased only marginally along with time period.
3.3. RNA isolation and cDNA preparation
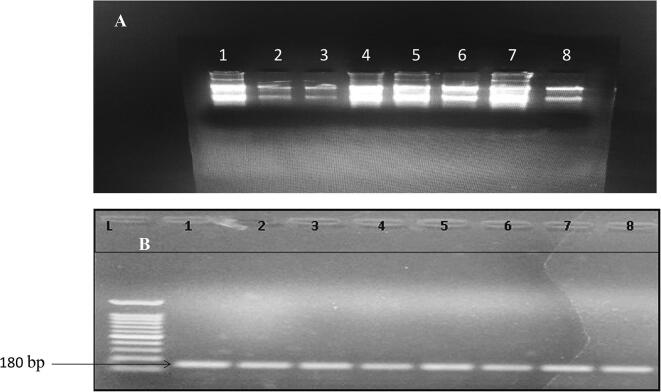
The total RNA was isolated from each of the mentioned samples, the intactness, size and quality of RNA extracted was checked on 1.5% agarose gel electrophoresis (Fig. 4A). The total RNA isolated was quantified having concentration between 200 and 717.2 ng/μl. cDNA synthesis confirmation was seen based on GAPDH amplification at 180 bp region (Fig. 4B).
Fig. 4.
RNA extracted at different stages after inoculation. (A) (1) Before inoculation (Treated), (2) Before inoculation (Control), (3) One day post inoculation (Treated), (4) One day post inoculation (Control), (5) 7- day post inoculation (Treated), (6) 7- day post inoculation (control), (7) 24-days post inoculation (Treated) and (8) 24- days post inoculation (control) and (B) cDNA confirmation of isolated RNA from leaves of apple cultivars using GAPDH primer (Band at 181 bp). L indicates marker DNA ladder starting from 100 bp.
3.4. Relative gene expression of PAL, CHS and F3Hgenes
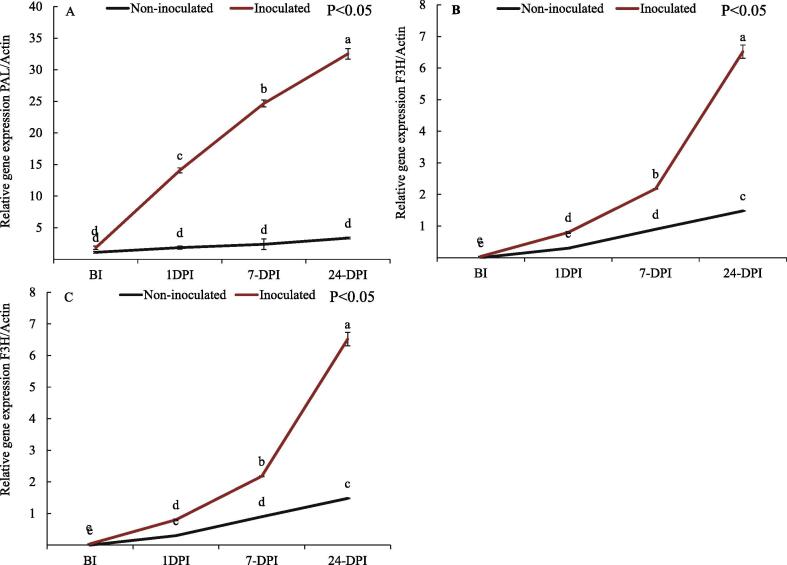
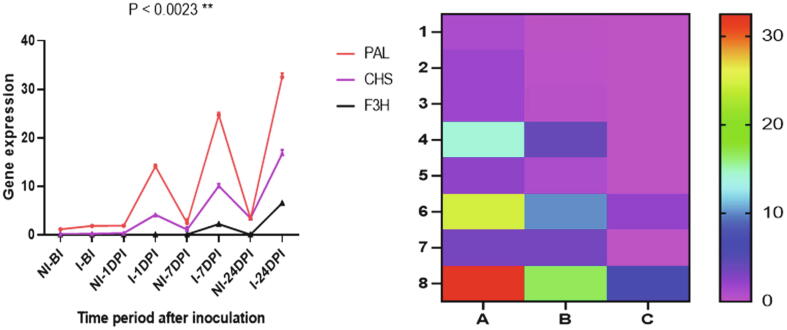
Relative levels of gene expression of polyphenolics biosynthetic genes i.e. PAL, CHS, C3H and F3H among apple cultivars, each in triplicates (n = 3) was detected by their mean Ct values. Sharp amplification, melt peaks and melting curves were observed post completion of qRT-PCR. The sharp amplification peaks rose up once cycle number reached near 20 cycles corresponding to relative fluorescence units (RFU) indicating correctness of PCR conditions (Fig. S1). Using Ct values of samples as well as housekeeping gene ACTIN, the relative gene expression levels of each sample was determined through ΔΔCT approach. The relative gene expression of PAL gene with respect to ACTIN among scab susceptible Venturia inaequalis treated apple cultivars was found statistically significantly higher as compared to control. Among susceptible cultivar, there was significant increase in the PAL expression levels i.e. 1.83 ± 0.25 pre-inoculation and 14.08 ± 0.39 post 1-day (24 h) of inoculation, which showed a 7.8-fold increase. While as in control no such significant change was found. The level of gene expression in control was in 1.11 ± 0.15 to 3.36 ± 0.13 range which demonstrated lower insignificant expression of PAL gene. Therefore, PAL gene expression levels were found highest in Venturia inaequalis treated after 24 days post inoculation 32.51 ± 0.83, thus indicating the highest PAL expression levels observed 24 days post inoculation (Fig. 5).
Fig. 5.
Gene Expression and Heatmap for (A) PAL (B) CHS and (C) F3H genes representing relative levels of gene expression at different stages before and post inoculation events when compared to corresponding house-keeping gene ACTIN. Where NI- Non inoculated, I- inoculated, BI-Before inoculation and DPI- Days Post inoculation. Each point represents the mean of three replicates and was expressed as mean ± SD (Standard deviation). Asterisks (*) indicate significant differences (P < 0.05) between Venturia inaequalis treatment (inoculated) and control (Non-inoculated).
Another important gene in the form of CHS was found up-regulated in Venturia inaequalis treated cultivar at different stages i.e. i.e. 4.11 ± 0.55 (1DPI), 10.1 ± 0.42 (7-Days PI) and 16.67 ± 0.82 (24 days PI). From pre-inoculation to post 1-day inoculation in Venturia inaequalis treated cultivar there was a 23-fold increase in gene expression level. While as from 1-day to 7-day 2.5-fold and 7-day to 24-day 1.7-fold increase in CHS expression level and the lowest insignificant relative gene expression was found in control samples (Fig. 5). The moderate levels of F3H gene expression was observed in Venturia inaequalis treated apple cultivar at different stages, which ranged from 0.04 to 6.52 from pre-inoculation to post 24-day inoculation. Before inoculation to post 1-day F3H was highly upregulated i.e. a 20- fold increase while as no such upregulation were observed in control. The heatmap was constructed indicating differential gene expression levels of all genes i.e. PAL, F3H, CHS, Different color codes of heatmap demonstrate diverse gene expression levels (Fig. 6). e.g. reddish (Highest expression)
Fig. 6.
Expression levels of all genes and heatmap indicating differential gene expression levels of PAL, F3H, CHS, color codes represent different gene expressions.
4. Discussion
Phenylpropanoids display large antimicrobial activities and are thus are assumed to help the plant act against various microbial diseases (VanEtten et al., 1994). The secondary metabolites play a role as antimicrobial in plant defense systems (Yang et al., 2018). Role of various phytoalexins or phytoanticipins have been potted in previous studies as providing widespread facts on the biological activities of phenylpropanoid compounds entangled in plant defence (Dixon and Paiva, 1995, Dixon, 2001, Tsao et al., 2003, Shafi et al., 2019). Dihydrochalcones have been found to accumulate in scab infected neighboring tissues of leaves and fruits compared to that of healthy apple peel (Slatnar et al., 2016).
Generally polyphenolic content increases with infection or attack of any pathogen. Phenols certainly are not only component responsible for contributing to resistance of apples towards scab but, as it can be observed from results that with increase in time period after pathogen treatment their synthesis increased. The production and distribution of defensive phenolics such as flavonoids, hydroxycinnamic acid and dihydrochalcones is enhanced in response to pathogen attack and infection. In the current study, phenolics were at the higher level in leaves after inoculation, this significant increase in polyphenols was observed in Venturia inaequalis inoculated cultivars at a different time point. The higher level of increase may be associated with defense process in order to avoid further spread of infection. In inoculated cultivar ferulic acid and coumaric acid accumulate at a faster rate in the Venturia inaequalis treated apple cultivar. According to previous studies, it was hypothesized that with infection the leaves accumulated more phenolic compounds compared with healthy leaves. The maximum part from the total content among observed hydroxycinnamic acids belonged to p-coumaric acid and the minimum part belonged to ferulic acid. These results may be explained in relation to the previous studies where the increased production of hydrocinnamic acid with infection can contribute as an outcome of the enhanced activity of phenylalanine ammonia lyase (PAL) (Petkovšek et al., 2008, Petkovšek et al., 2009).
Phloridzin is commonly anticipated for playing a defensive role against various kinds of pathogens as well as it is involved in resistance to various diseases. Ratio of flavanol/phloridzin are mostly debated with respect to resistance against apple scab (Picinelli et al., 1995, Petkovšek et al., 2008). In our study, the scab infected leaves had phloridzin content 39.246 µg/g after 24-days of post inoculation. In case of inoculated the infected leaves accumulated more phloridzin from first day post inoculation. This suggests that with pathogen (Venturia inaequalis) attack phloridzin accumulated faster as a defense response in infected leaves, this result was in concord with results observed by Leser and Treutter, 2005, Lattanzio et al., 2006. Another study reveals that exogenous. Epigallocatechin-3-Gallate (EGCG), application increased plant resistance to TMV as revealed by significantly decreased transcript levels of TMV-coat protein (CP) in tomato leaves and results suggest that EGCG maintains a delicate balance between ROS signaling and ROS scavenging to enhance tomato resistance to TMV (Zhang et al., 2020). As a result, it can be concluded that phloridzin is an active compound which is synthesized in response to fungi attack but the degree of resistance it will provide cannot correlated with different kind of apple cultivars (Gosch et al., 2009). We found that phloridzin levels increased in leaves of the Venturia inaequalis inoculated leaves where after 24 h of inoculation there was approximately 6 fold increase in phloridizin content and highest level was found after 24 days when symptoms were prominent, thus infected leaves accumulated higher phloridizin around the tissues, which specifies it is not the production of these phenolics only but the degree at which phloridzin is transformed may also likely play a role in resistance(Skłodowska et al., 2018). Flavonoids are reported to play a role in the disease resistance of apples to Venturia inaequalis. In treated cultivar, there was higher synthesis of catechin, apigenin and rutin on the entire infected leaf whereas in the control observed catechin, apigenin and rutin were 1.3 to 2.8 times lower than in infected leaves. Treutter and Feucht, 1990b, Picinelli et al., 1995, Petkovšek et al., 2009 had reported same kind of results where after the Venturia inaequalis attack flavonoids production was enhanced. Catechin accumulation after 24 h was more comparatively to apigenin and rutin.
Phenylpropanoid pathway is the main secondary metabolic pathway which is responsible for production of defence related compounds. The rate limiting enzyme and the key regulator of this pathway is PAL which catalyzes conversion of phenylalanine to trans-cinnamic acid, then producing the precursors for another metabolite/compound biosynthesis. Phenolic and flavonoid are the compounds antifungal compounds that get accumulated after the attack of any pathogen attack and act by inhibiting the spore germination and mycelia growth(Mayr et al., 1997). It has been observed that with the pathogen attack there is an up-regulation of genes that are responsible for production and accumulation of different defense related flavonoids compounds (Treutter and Feucht, 1990a, Mayr et al., 1993, Mayr et al., 1997, Picinelli et al., 1995, Leser and Treutter, 2005). This activation of the genes of PAL pathway and synthesis of polyphenolic compounds may explain the broad and unspecific role of these compounds in defense to disease like apple scab. Plants produce a number of metabolites through secondary metabolic pathways, which function mostly in plant defense responses to biotic and abiotic stresses. Flavonoids and phenols are the major groups of secondary metabolites that accumulate in response to pathogen attacks. Ahammed et al. (2020) found that the application of melatonin and/or inoculation with arbuscular mycorrhizal fungi AMF increased the content of phenols and flavonoids, which might enhance defense against Fusarium wilt in cucumber (Ahammed et al., 2020).
PAL, is main and F3H and CHS are among the key branch-point enzymes that help in controlling the regulation of the PAL pathway (Dixon et al., 2002, Vrancken et al., 2013). Our data presented that post inoculation there was noteworthy increase in activities of PAL, CHS and F3H genes, that accounted for the enhanced production of phenolic and flavonoid compounds. qRT-PCR results have demonstrated that PAL, CHS and F3H gene expression levels / gene fold change was found constitutively increasing post inoculation days in Venturia inaequalis infected cultivar. The results observed in our steady are in coherence with previous studies on apple, muskmelon, cherry and tomato fruit that revealed that with up regulation in gene expression levels of PAL, F3H and CHS the disease resistance also tends to get increased against pathogens (Liu et al., 2014, Wei et al., 2019). The observed early defense response of plant towards pathogen attack is quick accumulation of polyphenolic compounds (Treutter, 2005). Activation of the phenylpropanoid pathway contributes to plant defense against pathogenic fungi. Therefore, they analyzed the activity of PAL, the first key enzyme in the phenyl propanoid pathway. Time-course analysis of PAL activity revealed that inoculation with C. gloeosporioides caused an initial decline in the early few hours; however, PAL activity, then, gradually increased with post-inoculation time period. Expression analysis of genes involved in phenylpropanoid pathway showed that both exogenous EBR and C. gloeosporioides inoculation increased transcript levels of PAL, C4H, and 4CL; however, combined treatment with EBR and C. gloeosporioides resulted in a greater increase (Zhang et al., 2018) This was also observed in our results that after 24 h of inoculation the gene expression levels as well as production of polyphenols were much higher compared to control. This might be a strategy aimed to overcome pathogen and the spread of infection. It has been designated that these compounds possess antimicrobial potential which help the plant for structural defence against pathogen.
Similar kind of results that we observed were also reported in the poly e lysine treated apple fruits by Ge et al. (2018) where after one-week time the activity of PAL peaked in comparison to control which showed slow activity of PAL from 0 to 8 days. In the present study, it was observed that three enzymes (i.e., PAL, CHS, F3H) are involved in apple responses to Venturia inaequalis disease. These enzymes catalyze key reactions in the phenylpropanoid pathway, an increase in the activity of these enzymes upregulates the biosynthesis of phenolic acid, chalcones and flavonoids. There seems a positive correlation between levels of gene expression of PAL, CHS and F3H genes and production of different phenolics and flavonoids such as apigenin, p-coumaric acid, (+) - catechin, phloridzin, rutin, and ferulic acid. The amounts of phenolic acids and flavonoids were determined in the present study before and after inoculation of Venturia inaequalis and similar correlation has been observed in earlier studies as well (Petkovšek et al., 2008). Another study conducted by Xu et al. (2019) where fulvic acid treatment extremely enhanced production of polyphenolic compounds by increasing the activities of PAL, C4H and 4CL.
Our results are in consistent with previous studies in muskmelon, cherry, Chinese cabbage and tomato that displayed enhanced activity of PAL enzymes (Liu et al., 2014, Islam et al., 2018, Skłodowska et al., 2018, Wei et al., 2019). The up-regulation of PAL, CHS and F3H genes in Venturia inaequalis treated apple cultivar resulted in an enhanced production of phenylpropanoid compounds.
These results can provide new insights related to defense response of apple cultivar which may help to understand mechanisms of basal defense. The comparison of treated and non-treated Golden Delicious apple plants provided evidence that Venturia inaequalis up regulated genes in the inoculated cultivar. Our results demonstrate that scab infection caused a significant increase in hydroxycinnamic acids, catechin, epicatechin and phloridzin in infected tissue. The results observed signify a vital contribution to understand plant-pathogen interaction and may be useful for further investigations.
5. Conclusions
Our results are in support of the previous studies and hypothesis conducted on different fruits that phenolic compounds are involved in defense response and might play an important role in disease resistance and the mechanism of offering this resistance is directly linked to the host-pathogen interaction which involves different genes. Apart from PAL genes and secondary metabolites like phenols and flavonoids still pathogen overcomes the resistance in susceptible cultivar. So, detailed investigation on mechanism and time period of action of all PAL pathway genes, secondary metabolites and resistance genes may through a better light in understanding the plant pathogen interaction and defense related events as well. By overexpressing the genes related topolyphenols and introgressing them using cisgenic or intragenic approach can be used as an adjunct approach to reduce scab.
Funding
This study was funded by Science and Engineering Research Board, Department of Science and Technology, Government of India under SERB project: SERB/EMR/2016/005598.
Declaration of Competing Interest
No conflict of interest exists neither in the conduct of this research nor in preparation of this manuscript.
Acknowledgement
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2020/193), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2020.09.007.
Contributor Information
Vikas Sharma, Email: vikas.skuast@gmail.com.
Khalid Z. Masoodi, Email: masoodikz@skuastkashmir.ac.in.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ahammed G.J., Mao Q., Yan Y., Wu M., Wang Y., Ren J., Guo P., Liu A., Chen S. Role of melatonin in arbuscular mycorrhizal fungi-induced resistance to Fusarium wilt in cucumber. Phytopathology. 2020;110(5):999–1009. doi: 10.1094/PHYTO-11-19-0435-R. [DOI] [PubMed] [Google Scholar]
- Asif M., Trivedi P., Solomos T., Tucker M. Isolation of high-quality RNA from apple (Malus domestica) fruit. J. Agric. Food Chem. 2006;54:5227–5229. doi: 10.1021/jf053137n. [DOI] [PubMed] [Google Scholar]
- Bazzi C., Messina C., Tortoreto L., Bini F., Cecca G., Stefani E. Investigations on the possible use of abiotic and biotic elicitors in defence-related responses in plants. Eur. J. Horticul. Sci. 2003;68:115–122. [Google Scholar]
- Chang C.-C., Yang M.-H., Wen H.-M., Chern J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10 [Google Scholar]
- Dixon R.A. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- Dixon R.A., Achnine L., Kota P., Liu C.J., Reddy M.S., Wang L. The phenylpropanoid pathway and plant defence—a genomics perspective. Mol. Plant Pathol. 2002;3:371–390. doi: 10.1046/j.1364-3703.2002.00131.x. [DOI] [PubMed] [Google Scholar]
- Dixon R.A., Paiva N.L. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fünfgelder S., Mayr U., Treutter D., Feucht W. International Symposium on Natural Phenols in Plant Resistance. 1993. The activity of phenylalanine ammonia-lyase in apple leaves after wounding; pp. 474–478. [Google Scholar]
- Ge Y., Wei M., Li C., Chen Y., Lv J., Meng K., Wang W., Li J. Reactive oxygen species metabolism and phenylpropanoid pathway involved in disease resistance against Penicillium expansum in apple fruit induced by ∊-poly-l-lysine. J. Sci. Food Agric. 2018;98:5082–5088. doi: 10.1002/jsfa.9046. [DOI] [PubMed] [Google Scholar]
- Gosch C., Halbwirth H., Kuhn J., Miosic S., Stich K. Biosynthesis of phloridzin in apple (Malus domestica Borkh.) Plant Sci. 2009;176:223–231. [Google Scholar]
- Guerriero G., Giorno F., Ciccotti A.M., Schmidt S., Baric S. A gene expression analysis of cell wall biosynthetic genes in Malus× domestica infected by ‘Candidatus Phytoplasma mali. Tree Physiol. 2012;32:1365–1377. doi: 10.1093/treephys/tps095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot S., Le Bourvellec C., Marnet N., Drilleau J.F. Procyanidins are the most abundant polyphenols in dessert apples at maturity. LWT-Food Sci. Technol. 2002;35(3):289–291. [Google Scholar]
- Islam M.T., Lee B.-R., Das P.R., Jung H.-I., Kim T.-H. Characterization of p-Coumaric acid-induced soluble and cell wall-bound phenolic metabolites in relation to disease resistance to Xanthomonas campestris pv. campestris in Chinese cabbage. Plant Physiol. Biochem. 2018;125:172–177. doi: 10.1016/j.plaphy.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Lattanzio V., Lattanzio V.M., Cardinali A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem.: Adv. Res. 2006;661:23–67. [Google Scholar]
- Leser C., Treutter D. Effects of nitrogen supply on growth, contents of phenolic compounds and pathogen (scab) resistance of apple trees. Physiol. Plant. 2005;123:49–56. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ge Y., Bi Y., Li C., Deng H., Hu L., Dong B. Effect of postharvest acibenzolar-S-methyl dipping on phenylpropanoid pathway metabolism in muskmelon (Cucumis melo L.) fruits. Sci. Hortic. 2014;168:113–119. [Google Scholar]
- MacHardy W.E. American Phytopathological Society (APS Press); 1996. Apple Scab: Biology, Epidemiology, and Management. [Google Scholar]
- Mansoor S., Ahmed N., Sharma V., Jan S., Nabi S.U., Mir J.I., Mir M.A., Masoodi K.Z. Elucidating genetic variability and population structure in Venturia inaequalis associated with apple scab diseaseusing SSR markers. PLoS ONE. 2019;14 doi: 10.1371/journal.pone.0224300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini S., D’Addario C., Colacevich A., Focardi S., Borghini F., Santucci A., Figura N., Rossi C. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int. J. Antimicrob. Agents. 2009;34:50–59. doi: 10.1016/j.ijantimicag.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Mayr U., Batzdorfer R., Treutter D., Feucht W. International Symposium on Natural Phenols in Plant Resistance. 1993. Surfactant-induced changes in phenol content of apple leaves and fruit skins; pp. 479–487. [Google Scholar]
- Mayr U., Michalek S., Treutter D., Feucht W. Phenolic compounds of apple and their relationship to scab resistance. J. Phytopathol. 1997;145:69–75. [Google Scholar]
- Michalek S., Mayr U., Treutter D., Lux-Endrich A., Gutmann M., Feucht W., Geibel M. Eucarpia Symposium on Fruit Breeding and Genetics. 1998. Role of flavan-3-ols in resistance of apple trees to Venturia inaequalis; pp. 535–540. [Google Scholar]
- Omoruyi B.E., Bradley G., Afolayan A.J. Antioxidant and phytochemical properties of Carpobrotus edulis (L.) bolus leaf used for the management of common infections in HIV/AIDS patients in Eastern Cape Province. BMC Complement. Alternative Med. 2012;12:215. doi: 10.1186/1472-6882-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovšek M.M., Stampar F., Veberic R. Increased phenolic content in apple leaves infected with the apple scab pathogen. J. Plant Pathol. 2008:49–55. [Google Scholar]
- Petkovšek M.M., Štampar F., Veberič R. Accumulation of phenolic compounds in apple in response to infection by the scab pathogen, Venturia inaequalis. Physiol. Mol. Plant Pathol. 2009;74:60–67. [Google Scholar]
- Picinelli A., Dapena E., Mangas J.J. Polyphenolic pattern in apple tree leaves in relation to scab resistance. A preliminary study. J. Agric. Food Chem. 1995;43:2273–2278. [Google Scholar]
- Schwalb P., Feucht W. Changes in the concentration of phenolic substances in the bark during the annual development of the cherry tree (Prunus avium L.) Adv. Horticul. Sci. 1999:71–75. [Google Scholar]
- Shafi W., Mansoor S., Jan S., Singh D.B., Kazi M., Raish M., Alwadei M., Mir J.I., Ahmad P. Variability in catechin and rutin contents and their antioxidant potential in diverse apple genotypes. Molecules. 2019;24:943. doi: 10.3390/molecules24050943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skłodowska M., Mikiciński A., Wielanek M., Kuźniak E., Sobiczewski P. Phenolic profiles in apple leaves and the efficacy of selected phenols against fire blight (Erwinia amylovora) Eur. J. Plant Pathol. 2018;151:213–228. [Google Scholar]
- Slatnar A., Mikulic-Petkovsek M., Veberic R., Štampar F. Research on the involment of phenoloics in the defence of horticultural plants. Acta Agricul. Slovenica. 2016;107:183–189. [Google Scholar]
- Slatnar A., Petkovsek M.M., Halbwirth H., Stampar F., Stich K., Veberic R. Polyphenol metabolism of developing apple skin of a scab resistant and a susceptible apple cultivar. Trees. 2012;26:109–119. [Google Scholar]
- Sukno S.A., McCuiston J., Wong M.-Y., Wang X., Thon M.R., Hussey R., Baum T., Davis E. Quantitative detection of double-stranded RNA-mediated gene silencing of parasitism genes in Heterodera glycines. J. Nematol. 2007;39:145. [PMC free article] [PubMed] [Google Scholar]
- Treutter D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005;7:581–591. doi: 10.1055/s-2005-873009. [DOI] [PubMed] [Google Scholar]
- Treutter D., Feucht W. Accumulation of flavan-3-ols in fungus-infected leaves of Rosaceae/Synthese von Flavan-3-olen in pilz-infizierten Blättern von Rosaceen. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/J. Plant Dis. Protect. 1990;97:634–641. [Google Scholar]
- Treutter D., Feucht W. The pattern of flavan-3-ols in relation to scab resistance of apple cultivars. J. Hortic. Sci. 1990;65:511–517. [Google Scholar]
- Tsao R., Yang R., Young J.C., Zhu H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC) J. Agric. Food Chem. 2003;51:6347–6353. doi: 10.1021/jf0346298. [DOI] [PubMed] [Google Scholar]
- VanEtten H.D., Mansfield J.W., Bailey J.A., Farmer E.E. Two classes of plant antibiotics: phytoalexins versus “phytoanticipins“. Plant Cell. 1994;6:1191. doi: 10.1105/tpc.6.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrancken K., Holtappels M., Schoofs H., Deckers T., Treutter D., Valcke R. Erwinia amylovora affects the phenylpropanoid–flavonoid pathway in mature leaves of Pyrus communis cv. Conférence. Plant Physiol. Biochem. 2013;72:134–144. doi: 10.1016/j.plaphy.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Wei M., Ge Y., Li C., Han X., Qin S., Chen Y., Tang Q., Li J. G6PDH regulated NADPH production and reactive oxygen species metabolism to enhance disease resistance against blue mold in apple fruit by acibenzolar-S-methyl. Postharvest Biol. Technol. 2019;148:228–235. [Google Scholar]
- Xu D., Deng Y., Xi P., Yu G., Wang Q., Zeng Q., Jiang Z., Gao L. Fulvic acid-induced disease resistance to Botrytis cinerea in table grapes may be mediated by regulating phenylpropanoid metabolism. Food Chem. 2019;286:226–233. doi: 10.1016/j.foodchem.2019.02.015. [DOI] [PubMed] [Google Scholar]
- Yang L., Wen K.-S., Ruan X., Zhao Y.-X., Wei F., Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23:762. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ahammed G.J., Li X., Wei J.P., Li Y., Yan P., Zhang L.P., Han W.Y. Exogenous brassinosteroid enhances plant defense against Colletotrichum gloeosporioides by activating phenylpropanoid pathway in Camellia sinensis L. J. Plant Growth Regul. 2018;37(4):1235–1243. [Google Scholar]
- Zhang X.N., Wang X.R., Zhang L., Ahammed G.J., Li Q.Y., Li X. Epigallocatechin-3-gallate enhances tomato resistance to tobacco mosaic virus by modulating RBOH1-dependent H2O2 signaling. Plant Physiol. Biochem. 2020 doi: 10.1016/j.plaphy.2020.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.