Keywords: crush injury, defensin 1, gait analysis, intraoperative administration, nerve conduction velocity, nervous system, neutrophil peptide 1, peripheral nerve injury, peripheral nerve regeneration, sciatic nerve, tibialis anterior muscle, trauma
Abstract
Neutrophil peptide 1 belongs to a family of peptides involved in innate immunity. Continuous intramuscular injection of neutrophil peptide 1 can promote the regeneration of peripheral nerves, but clinical application in this manner is not convenient. To this end, the effects of a single intraoperative administration of neutrophil peptide 1 on peripheral nerve regeneration were experimentally observed. A rat model of sciatic nerve crush injury was established using the clamp method. After model establishment, a normal saline group and a neutrophil peptide 1 group were injected with a single dose of normal saline or 10 μg/mL neutrophil peptide 1, respectively. A sham group, without sciatic nerve crush was also prepared as a control. Sciatic nerve function tests, neuroelectrophysiological tests, and hematoxylin-eosin staining showed that the nerve conduction velocity, sciatic functional index, and tibialis anterior muscle fiber cross-sectional area were better in the neutrophil peptide 1 group than in the normal saline group at 4 weeks after surgery. At 4 and 8 weeks after surgery, there were no differences in the wet weight of the tibialis anterior muscle between the neutrophil peptide 1 and saline groups. Histological staining of the sciatic nerve showed no significant differences in the number of myelinated nerve fibers or the axon cross-sectional area between the neutrophil peptide 1 and normal saline groups. The above data confirmed that a single dose of neutrophil peptide 1 during surgery can promote the recovery of neurological function 4 weeks after sciatic nerve injury. All the experiments were approved by the Medical Ethics Committee of Peking University People’s Hospital, China (approval No. 2015-50) on December 9, 2015.
Chinese Library Classification No. R459.9; R363; R364
Introduction
Peripheral nerve injury leads to loss of sensory and motor functions and causes a heavy burden on individuals, families and society (Yuce et al., 2015). Compared with the central nervous system, peripheral nerves have the ability to partially regenerate after injury (Gong et al., 2016; Panagopoulos et al., 2017). However, the regeneration ability is extremely limited because of the pathophysiological characteristics of the nerve. In addition to anatomically restoring the integrity of nerve tissue by different surgical means (Zhang et al., 2010; Trehan et al., 2016), physicians have made extensive explorations into drugs and biological products that can promote the recovery of peripheral nerve function, such as nerve growth factor (Sang et al., 2018), Schwann cells (Gomez-Sanchez et al., 2017), stem cells (Jiang et al., 2017), macrophages (Kobiela Ketz et al., 2017), and lithium chloride (Chen et al., 2016). In recent decades, some progress has been made in the field of peripheral nerve repair; however, clinical outcomes are still largely unsatisfactory (Terzis and Kokkalis, 2009).
Neutrophil peptide 1 (or defensin 1, NP-1) belongs to a class of immunoregulatory polypeptides with stable physicochemical properties that are secreted by neutrophils and that are widely present in mammals (Lehrer et al., 1993). Their biological effects are dose-dependent (Kanmura et al., 2009). NP-1 is strongly associated with tissue repair, and can promote wound healing of pulmonary epithelial tissue (Zhou et al., 2007), proliferation of pulmonary epithelial cells (Aarbiou et al., 2002), fibroblasts (Murphy et al., 1993) and retinal epithelial cells (Muller et al., 2002). In the field of neuroscience, Nozdrachev et al. (2006) showed that NP-1 has the ability to accelerate regeneration of peripheral nerves after denervation. A similar effect was also observed in a model of peripheral nerve denervation. One week after continuous intramuscular injection of NP-1, the regeneration ability of the injured nerve was enhanced (Xu et al., 2016).
However, in the clinic, patients are reluctant to undergo invasive treatment for several consecutive days, and it is often difficult to achieve treatment effects similar to those of a laboratory experiment. In this study, NP-1 was applied around the affected nerve in a single dose to imitate immediate intraoperative administration to explore the clinical application of the immunoregulatory polypeptide.
Materials and Methods
Experimental animals
Thirty-six female specific-pathogen-free Sprague-Dawley rats aged 6 weeks and weighing 200–230 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., China (license No. SCXK (Jing) 2016-0006) and housed in a barrier environment with a 12-hour light/dark cycle and humidity of 40%. All experiments were conducted in strict accordance with the animal welfare rules and were approved by the Medical Ethics Committee of the People’s Hospital of Peking University, China (approval No. 2015-50) on December 9, 2015.
Drug preparation
Solutions of 10 μg/mL NP-1 (Shanghai Qiangyao Biological Technology Co., Ltd., Shanghai, China) and physiological saline were prepared and all samples were numbered. To eliminate subjective bias, the personnel in charge of the experiment and data analysis did not know the relationship between number and drug.
Establishment of the animal model
All rats were randomly divided into a sham group (n = 12), a normal saline (NS) group (n = 12), and a NP-1 group (n = 12). Rats were anesthetized with isoflurane (2.5%, 100 mL/min) and then the right hindlimb was shaved. The skin, fascia and muscle were cut sequentially to expose the sciatic nerve. The sciatic nerve was wrapped with a moist rubber strip 5–7 mm proximal to the sciatic nerve bifurcation. Ten-centimeter hemostatic forceps were used to crush the nerve for 30 seconds with three clasps locked, and the length of the crush zone was approximately 4 mm (Dun and Parkinson, 2018). The 12 rats of the NS group were intermuscularly injected with 0.5 mL saline at the site where the sciatic nerve is located. The rats of the NP-1 group were injected with an equal volume of 10 μg/mL NP-1 at the same site. A muscle space bulge was seen after the injection, but no fluid leaked from the suture zone. When palpating the outside muscles, the undulation could be felt. The muscle and fascia were sutured, and the skin was sutured and sterilized with alcohol. In the sham group, muscle, fascia and skin were sutured and disinfected after sciatic nerve exposure. At 4 and 8 weeks after surgery, six rats from each group were used for detection of sciatic functional index (SFI) and neuroelectrophysiology.
SFI detection
At 4 and 8 weeks after operation, six rats from each group were taken for gait analysis using the CatWalk XT 10.5 system (Noldus, Wageningen, Netherlands). The width of the walking platform was adjusted to accommodate the size of the rats (Kappos et al., 2017). The camera position and focal length were adjusted, and the software parameters were chosen. Before the gait test, the rats in each group were trained in running. After defecating and urinating, the rats from each group were placed on the left side of the walking platform of the CatWalk XT small animal gait analyzer (Noldus), so that they spontaneously ran to the right end. The following parameters were automatically recorded: print length (PL), the greatest length of one footprint; toe spread (TS), the distance between the first toe and the fifth toe; and intermediary toe spread (IT), the distance between the second toe and the fourth toe. Using the right foot as experimental (E) data and the left foot as normal (N) data, three factors were calculated: print length factor (PLF) = (EPL – NPL)/NPL; toe spread factor (TSF) = (ETS – NTS)/NTS; and intermediary toe spread factor (ITF) = (EIT – NIT)/NIT. SFI was equal to –38.3 (PLF) + 109.5 (TSF) + 13.3 (ITF) –8.8 (Timotius et al., 2019).
Nerve conduction velocity recording
After gait analysis, rats were anesthetized with isoflurane gas (2.5%, 100 mL/min). The skin was cut along the original surgical incision and a stimulating electrode was placed approximately 5 mm from the distal and proximal ends of the crush zone. The sensing electrode was placed on the tibialis anterior muscle. A ground wire was inserted into the gluteus maximus. A rectangular pulse was generated with a duration of 0.1 ms, current of 0.09 mA and frequency of 1 Hz (Synergy electrophysiology instrument, Oxford, UK). After a pulse was transmitted once, the difference in conduction time between adjacent distal and proximal ends was recorded and calculated. The conduction velocity of the common peroneal nerve was measured as above. The difference between the tibial nerve conduction velocity and the common peroneal nerve conduction velocity was calculated (Yuan et al., 2019).
Muscle wet weight
After rats were euthanized, the tibialis anterior muscle was severed from the ankle joint, dissected along its length and dissociated at its proximal attachment. After wiping the muscle surface with gauze, the weight was immediately recorded using an electronic balance to two decimal places.
Hematoxylin-eosin staining of muscles
After the measurement of muscle wet weight, the ventral part of the middle 1/3 of the right tibialis anterior muscle was fixed with 4% paraformaldehyde at 4°C overnight, dehydrated with gradient sucrose-based solution, and embedded in OCT compound (Sakura, Tokyo, Japan) at –80°C. The sample was sectioned into 8-μm sections with a freezing microtome (Leica, Germany). The sections were stained with hematoxylin and eosin. Cross-section muscle images were captured at 100× magnification, and five fields were randomly selected from each sample. Cross-section area and density of muscle fibers were measured using Image Pro Plus 4.5 software (Media Cybernetics, Silver Spring, MD, USA).
Scanning electron microscopy
Rats were euthanized at 4 and 8 weeks and the nerve 1 mm distal to the clamp was obtained, pre-fixed in 2.5% glutaraldehyde for 6 hours, washed with phosphate-buffered saline, dehydrated through a graded alcohol series, embedded in embedding medium, and sectioned with a semi-thin microtome. The specimen embedded in each semi-thin section was trimmed under an anatomical microscope (Xingming Optical Instrument Co., Ltd., Shenzhen, China). The cross-section was then cut into 70 nm ultrathin sections. The sections were collected with a copper screen, and stained with 3% uranium acetate and lead citrate, and then observed and recorded using a transmission electron microscope (Olympus, Tokyo, Japan).
The semi-thin slices prepared above were baked for 30 minutes in a 60°C oven, then dewaxed in a gradient solution of xylene and anhydrous ethanol. After washing in water, sections were immersed in 1% toluidine blue dye solution for 10 minutes, and washed with clear water. All sections were treated with a mixture of xylene and ethanol, and mounted with neutral resin. Five images from different parts of each section were analyzed under a microscope (Olympus, Tokyo, Japan), and data from five nerve sections were quantified. Finally, the average diameter of the axons and the total number of myelinated axons were evaluated using ImageJ software 1.8.0 (National Institutes of Health, Bethesda, MD, USA) (Lu et al., 2019; Rao et al., 2019).
Statistical analysis
All data are expressed as the mean ± SD. All data were analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance was used to check the differences among groups. The least significant difference test was utilized for intergroup comparison. The Games-Howell method was used for heterogeneity of variance. P values < 0.05 were considered statistically significant.
Results
General observations
All rats survived. At 4 and 8 weeks after surgery, the hindlimbs in the NP-1 and NS groups were atrophied to varying degrees compared with the sham group, especially the plantar muscles causing the feet to curl up (Figure 1). There was no obvious toe autophagy in any group. Adhesion of the sciatic nerve to surrounding tissues was minimal; only a few connective tissues adhered to surrounding tissue at the crush site. At 4 weeks, there was edema in the distal nerve of the sham group. At 8 weeks, the edema had subsided.
Figure 1.
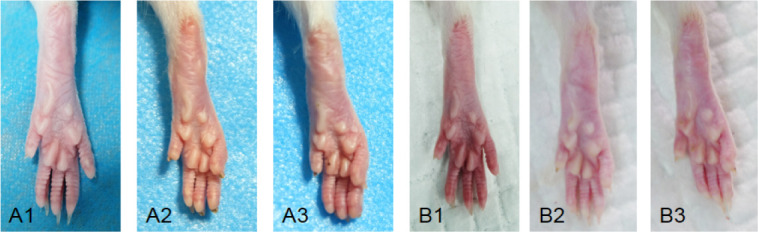
General observation of rat limbs in each group.
(A, B) General observation of hindlimbs in different groups at 4 and 8 weeks, respectively. 1: Sham group; 2: NS group; 3: NP-1 group. Compared with the sham group, NP-1 and NS groups showed toe flexion deformity at 4 weeks. Plantar muscle atrophy was noticeable in NS and NP-1 groups at 8 weeks. NP-1: Neutrophil peptide 1; NS: normal saline.
Changes in SFI
The absolute value of SFI was significantly lower in the sham group than in the NS and NP-1 groups (P < 0.05). At postoperative week 4, SFI was significantly lower in the NP-1 group than in the NS group (P < 0.05). At postoperative week 8, SFI was slightly lower in the NP-1 group than in the NS group (P = 0.63; Figure 2).
Figure 2.
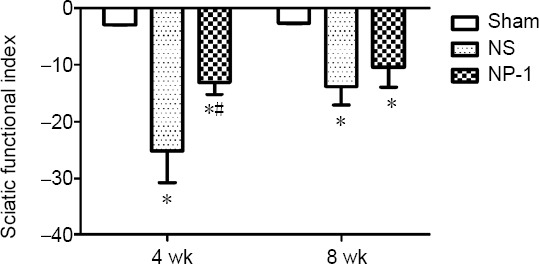
Sciatic functional index among the three groups.
Each group contained six rats. The SFI of the NP-1 group was much lower than that of the NS group. One-way analysis of variance was used to check the differences among groups. *P < 0.05, vs. sham group; #P < 0.05, vs. NS group. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance followed by least significant difference post hoc test). NP-1: Neutrophil peptide 1; NS: normal saline; SFI: sciatic functional index.
Changes in muscle wet weight
Compared with the sham group, the wet weight of the atrophic tibialis anterior muscle was reduced by different amounts in the NP-1 and NS groups at postoperative 4 and 8 weeks (P < 0.05; Figure 3). At postoperative weeks 4 and 8, the wet weights of the tibialis anterior muscle were slightly higher in the NP-1 group than in the NS group (P > 0.05; Figure 3).
Figure 3.

Tibialis anterior muscles and muscle wet weight.
(A, B) Images of the tibialis anterior muscle at 4 and 8 weeks, respectively. 1, 2, 3: Sham, NS, and NP-1 groups, respectively. At the same time point, the muscle wet weight of the NP-1 group and NS group was significantly lower than that of the sham group. In the same group, muscle wetness was greater at 8 weeks than at 4 weeks. There was no difference in muscle wet weight between NP-1 and NS groups at the same time point. *P < 0.05, vs. sham group. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance followed by the least significant difference post hoc test). NP-1: Neutrophil peptide 1; NS: normal saline.
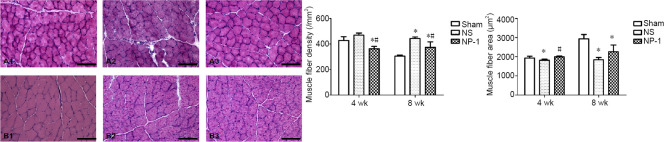
Changes in muscle histology
Fiber diameter was larger at 8 weeks than at 4 weeks after surgery in all three groups. Compared with the sham group, muscle fibers were atrophied to varying degrees in the NS and NP-1 groups after surgery (Figure 4). At 4 weeks post-surgery, the density of muscle fibers was slightly lower in the sham group compared with that in the NS group (P = 0.135), but the diameter of muscle fibers was significantly larger in the sham group than in the NS group (P < 0.05). The number of muscle fibers was significantly more in the sham group than in the NP-1 group (P < 0.05). The density of muscle fibers was smaller in the NP-1 group than in the NS group (P < 0.05), but cross-sectional area was larger in the NP-1 group than in the NS group (P < 0.05). At 8 weeks post-surgery, the density of muscle fibers was significantly smaller in the sham group than in the NS and NP-1 groups (P < 0.05). Mean cross-sectional area of muscle fibers was significantly higher in the sham group than in the NS and NP-1 groups (P < 0.05). The density of muscle fibers was smaller in the NP-1 group than in the NS group (P < 0.05), but cross-sectional area was slightly larger in the NP-1 group than in the NS group (P = 0.09).
Figure 4.
Histological images of tibialis anterior muscle and muscle fiber density and area.
(A, B) Tibialis anterior muscle at 4 and 8 weeks, respectively. 1, 2, 3: Sham, NS and NP-1 groups, respectively. The gap between muscle fibers was increased to varying degrees at 4 and 8 weeks. Scale bars: 100 μm. The number of muscle fibers was less in the NP-1 group than in the NS group, but the diameter of the muscle fibers was larger in the NP-1 group than in the NS group at four weeks. *P < 0.05, vs. sham group; #P < 0.05, vs. NS group. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance followed by the least significant difference post hoc test). NP-1: Neutrophil peptide 1; NS: normal saline.
Changes in neuroelectrophysiology
At 4 weeks post-surgery, the conduction velocity was significantly lower in the NS and NP-1 groups compared with that in the sham group (P < 0.05; Figure 5). Conduction velocity of common peroneal nerves was significantly higher in the NP-1 group compared with that in the NS group (P < 0.05). At 8 weeks post-surgery, the conduction velocity was significantly lower in the NS and NP-1 groups compared with that in the sham group. The conduction velocity was slightly higher in the NP-1 group than in the NS group (P = 0.08; Figure 5).
Figure 5.
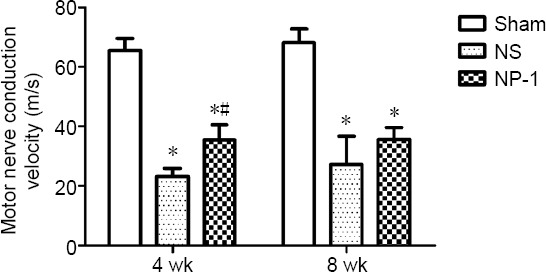
Motor nerve conduction velocity (MNCV).
At four weeks, the nerve conduction velocity was significantly higher in the NP-1 group than in the NS group. At eight weeks, there was no significant difference in nerve conduction velocity between the NS and NP-1 groups. *P < 0.05, vs. sham group; #P < 0.05, vs. NS group. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance followed by the least significant difference post hoc test). NP-1: Neutrophil peptide 1; NS: normal saline.
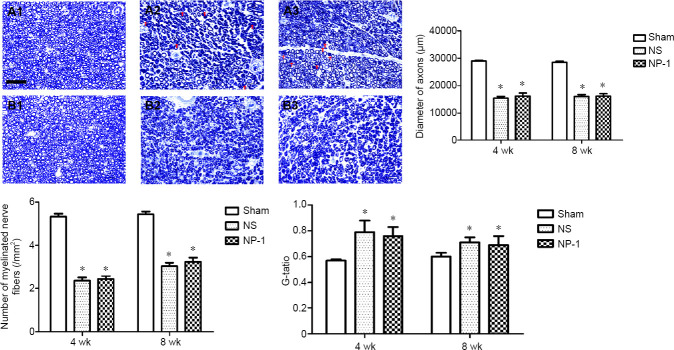
Changes in neurohistology
The number and axon diameter of myelinated nerve fibers were significantly smaller in the NS and NP-1 groups compared with those in the sham group (P > 0.05). No significant difference in number, axon diameter and G-ratio of myelinated nerve fibers was seen between the NP-1 and NS groups at various time points (P > 0.05; Figures 6 and 7).
Figure 6.
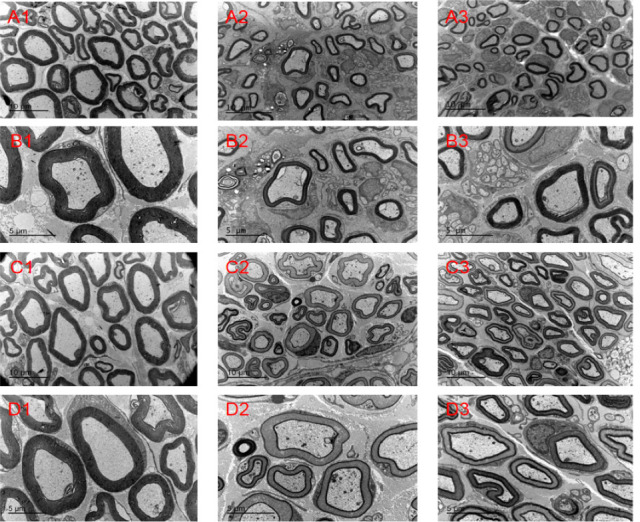
Nerve morphology in each group.
(A, B) Electron microscopy images of nerves in each group at 4 weeks post-surgery. (C, D) Electron microscopy images of nerves in each group at 8 weeks post-surgery. 1, 2, 3: Sham, NS and NP-1 groups, respectively. Compared with the sham group, the myelinated nerve fibers in the NS and NP-1 groups were sparsely arranged at the same time point. Atrophic axons of varying numbers were seen in both the NP-1 and NS groups. Original magnification, 2550× in A and C and 4200× in B and D. Scale bars: 10 μm in A and C, 5 μm in B and D. NP-1: Neutrophil peptide 1; NS: normal saline.
Figure 7.
Toluidine blue images and fiber number, diameter and G-ratio.
(A, B) Toluidine blue images at 4 and 8 weeks post-surgery. 1, 2, 3: Sham, NS and NP-1 groups, respectively. Blue ring: myelin sheath. At 4 weeks post-surgery, a large number of immature myelin sheaths are visible in the NS and NP-1 groups. The number of myelinated nerve fibers and the diameter of axons were smaller in the NP-1 and the NS groups compared with those in the sham group at the same time point. Red arrows indicate immature myelin sheaths. Scale bar: 50 μm. *P < 0.05, vs. sham group. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance followed by the least significant difference post hoc test). NP-1: Neutrophil peptide 1; NS: normal saline.
Discussion
There are many kinds of animal models to study peripheral nerve repair. The rat sciatic nerve crush injury is such a model that is stable and commonly used (Savastano et al., 2014; Yi et al., 2016). Crush injury can cause a different pathophysiological response compared with a cut injury, resulting in permanent damage to the function of the innervated area (Ousman et al., 2017). To explore the characteristics of NP-1 in promoting peripheral nerve regeneration and to lay the foundation for its clinical application, this study used this model to simulate treatment with a single administration during surgery.
Wallerian degeneration occurs after peripheral nerve crush. This is a neurodegenerative change discovered by Waller in 1851 that occurs at the distal end of the damaged axon. Gilley and Coleman (2010) believe that the degradation of axon skeletons that maintain neuromorphism may be the origin of degeneration. The degradation of axons and myelin are mechanically different processes (Gamage et al., 2017). According to the traditional view, Wallerian degeneration is the pathological change after peripheral nerve injury. Axons and myelin disintegrate and release a large number of low molecular mass substances that inhibit nerve regeneration, start an inflammatory chain reaction, and block nerve regeneration (Chen et al., 2015). Recent findings provide direct evidence that Wallerian degeneration is a conservative axonal disintegration program driver rather than a passive response to axonal injury (Osterloh et al., 2012; Pellegatta and Taveggia, 2019). It is considered to be a complex, immune-related pathophysiological process regulated by multiple-factor networks (DeFrancesco-Lisowitz et al., 2015; Ding and Hammarlund, 2019). After nerve injury, Schwann cells and macrophages are activated by damage signals, and began to remove the debris produced by distal tissue disintegration. Simultaneously, neurotrophic factors and inflammatory factors are released, which lay the foundation for axon regeneration. Wallerian degeneration is strongly associated with nerve regeneration (Wang et al., 2018). The speed of Wallerian degeneration determines the speed of nerve regeneration to a certain extent (Gaudet et al., 2011; Mietto et al., 2015).
NP-1 is a small-molecule polypeptide with immunomodulatory effects on many processes of the inflammatory response (Chan and Gallo, 1998; Jenssen et al., 2006; Klotman and Chang, 2006). NP-1 can activate ERK and JNK signaling pathways in lung endothelial cells and monocytes (Aarbiou et al., 2004), thereby regulating the expression of downstream pro-inflammatory response factors (Roman-Blas and Jimenez, 2006; Shiozawa and Tsumiyama, 2009). Recent studies have found that NP-1 is inextricably linked to macrophages. Among several neutrophil peptide subtypes, NP-1 has the strongest chemotactic effect on monocytes and can recruit macrophages and T cells to accumulate at the site of inflammation. The peak concentration for macrophage chemotaxis was approximately 5 × 10–9 m (Territo et al., 1989). NP-1 can also stimulate macrophages and enhance their phagocytic ability (Ganz, 2003). In a recent study, NP-1 was called the valve of the macrophage-dominated inflammatory response (Brook et al., 2016). NP-1 at low concentrations (5–15 μg/mL) can bind to RNA in macrophages to block mRNA translation of inflammatory factors (such as tumor necrosis factor-α and interleukin-1β) and regulate the expression of Th1 inflammatory factors. NP-1 is also the first cell-synthesized peptide that has been demonstrated to directly enter a target cell to affect translation and regulate protein expression (Brook et al., 2016). In a series of studies on the pathogenesis of colitis, Maeda et al. (2016) found that the regulatory effect of NP-1 on macrophages may present a “bipolar effect”. High dose NP-1 (100 ug/d) can enhance the expression of inflammatory factors. In contrast, a physiological dose of NP-1 (5 ug/d) can remarkably reduce the expression of tumor necrosis factor-α, interleukin-1β, interleukin-6 and other strong inflammatory factors, and reduce the inflammatory response. In intestinal inflammation caused by inactivated Escherichia coli, when the concentration of NP-1 was 10 μg/mL, interleukin-10 expression was markedly increased and cell viability peaked. When the concentration was increased to 100 μg/mL, cell viability and interleukin-10 expression were dramatically decreased (Maeda et al., 2016).
In this study, NP-1, as a small-molecule that can be mass-produced, was used to treat peripheral nerve crush injury. After eliminating interference from researcher’s choice bias, NP-1 can promote the early recovery of peripheral nerve crush injury to a certain extent, but NP-1 cannot enhance the quality of nerve regeneration. The nerve conduction velocity was better in the NP-1 group than in the NS group in all the experimental animals at 4 weeks post-surgery. However, there was no significant difference in nerve conduction velocity, number of new myelinated nerve fibers, diameter of nerve fibers, thickness of myelin sheath or wet weight of muscle between NP-1 and NS groups.
We speculate on the one hand that NP-1 may activate macrophages in the injured sciatic nerve, increase the concentration of some macrophages in the Wallerian degeneration area, and then accelerate the clearance of substances that hinder peripheral nerve regeneration produced by tissue disintegration during Wallerian degeneration, thus speeding up peripheral nerve regeneration. On the other hand, based on its immunoregulation, NP-1 may play a “trigger role”, which shifts the immunological process of Wallerian degeneration in a direction conducive to nerve regeneration. However, NP-1 cannot directly interact with the peripheral nerve, so NP-1 cannot enhance the quality of new nerve fibers.
In the methodology of this study, the researchers performing the experiments and the related data analysis were blind to the animal grouping. This effectively excluded subjective bias while the experiments were conducted and during analysis of the data to achieve relatively objective results.
Imitating spinal cord shock therapy after spinal cord injury, NP-1 was given directly to the injured site in a single high-dose to play an immunomodulatory role in Wallerian degeneration, which is proven to promote the early recovery of neurological function after peripheral nerve crushing. It is suggested that NP-1, as a kind of mass-produced biological preparation (Bai et al., 2013), is a drug with the potential for wide application in the treatment of peripheral nerve crush injury.
In this study, a method of intraoperative administration was modeled because it is easier to implement in the clinic. This mode of administration allows NP-1 to regulate only in the early period of Wallerian degeneration. Our results showed that this kind of NP-1 administration can promote early nerve regeneration. However, this study does not reveal the mechanism by which NP-1 promotes peripheral nerve regeneration. The mode of administration was single administration, which may result in an insufficient effective dose of the drug. However, based on the ability of rodents to recover strongly, the observation time of 8 weeks after surgery may enable the rats to recover and cover up the effect of the drug, resulting in similar long-term effects between the experimental group and the control group.
In summary, NP-1 effectively promoted peripheral nerve regeneration, even via a single intraoperative injection. Although this method limits the amount of NP-1 administered, it still improved the nerve conduction speed in the first four weeks post-surgery. This protective effect might be caused by NP-1 altering the Wallerian degeneration environment in the early stages of neural regeneration. The mechanism by which NP-1 exerts its ability to promote peripheral nerve regeneration remains to be further studied in the future.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This research was funded by the National Natural Science Foundation of China, No. 31571236 (to YHK); the Key Laboratory of Trauma and Neural Regeneration (Peking University), Ministry of Education, China, No. BMU2019XY007-01 (to YHK); the Ministry of Education Innovation Program of China, No. IRT_16R01 (to YHK); the Research and Development Funds of Peking University People’s Hospital, China, Nos. RDH2017-01 (to YHK), RDY2018-09 (to HL). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: All the experiments were conducted in strict accordance with the animal welfare rules and approved by the Medical Ethics Committee of Peking University People’s Hospital, China (approval No. 2015-50) on December 9, 2015. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This research was funded by the National Natural Science Foundation of China, No. 31571236 (to YHK); the Key Laboratory of Trauma and Neural Regeneration (Peking University), Ministry of Education, China, No. BMU2019XY007-01 (to YHK); the Ministry of Education Innovation Program of China, No. IRT_16R01 (to YHK); the Research and Development Funds of Peking University People’s Hospital, China, Nos. RDH2017-01 (to YHK), RDY2018-09 (to HL).
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Allen J, Norman C, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Aarbiou J, Ertmann M, van Wetering S, van Noort P, Rook D, Rabe KF, Litvinov SV, van Krieken JH, de Boer WI, Hiemstra PS. Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J Leukoc Biol. 2002;72:167–174. [PubMed] [Google Scholar]
- 2.Aarbiou J, Verhoosel RM, Van Wetering S, De Boer WI, Van Krieken JH, Litvinov SV, Rabe KF, Hiemstra PS. Neutrophil defensins enhance lung epithelial wound closure and mucin gene expression in vitro. Am J Respir Cell Mol Biol. 2004;30:193–201. doi: 10.1165/rcmb.2002-0267OC. [DOI] [PubMed] [Google Scholar]
- 3.Bai LL, Yin WB, Chen YH, Niu LL, Sun YR, Zhao SM, Yang FQ, Wang RR, Wu Q, Zhang XQ, Hu ZM. A new strategy to produce a defensin: stable production of mutated NP-1 in nitrate reductase-deficient Chlorella ellipsoidea. PLoS One. 2013;8:e54966. doi: 10.1371/journal.pone.0054966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brook M, Tomlinson GH, Miles K, Smith RW, Rossi AG, Hiemstra PS, van ‘t Wout EF, Dean JL, Gray NK, Lu W, Gray M. Neutrophil-derived alpha defensins control inflammation by inhibiting macrophage mRNA translation. Proc Natl Acad Sci U S A. 2016;113:4350–4355. doi: 10.1073/pnas.1601831113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caillaud M, Richard L, Vallat JM, Desmouliere A, Billet F. Peripheral nerve regeneration and intraneural revascularization. Neural Regen Res. 2019;14:24–33. doi: 10.4103/1673-5374.243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan YR, Gallo RL. PR-39, a syndecan-inducing antimicrobial peptide, binds and affects p130 (Cas) J Biol Chem. 1998;273:28978–28985. doi: 10.1074/jbc.273.44.28978. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Piao X, Bonaldo P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015;130:605–618. doi: 10.1007/s00401-015-1482-4. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Weng J, Han D, Chen B, Ma M, Yu Y, Li M, Liu Z, Zhang P, Jiang B. GSK3beta inhibition accelerates axon debris clearance and new axon remyelination. Am J Transl Res. 2016;8:5410–5420. [PMC free article] [PubMed] [Google Scholar]
- 9.DeFrancesco-Lisowitz A, Lindborg JA, Niemi JP, Zigmond RE. The neuroimmunology of degeneration and regeneration in the peripheral nervous system. Neuroscience. 2015;302:174–203. doi: 10.1016/j.neuroscience.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding C, Hammarlund M. Mechanisms of injury-induced axon degeneration. Curr Opin Neurobiol. 57:171–178. doi: 10.1016/j.conb.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dun XP, Parkinson DB. Transection and crush models of nerve injury to measure repair and remyelination in peripheral nerve. Methods Mol Biol. 2018;1791:251–262. doi: 10.1007/978-1-4939-7862-5_20. [DOI] [PubMed] [Google Scholar]
- 12.Gamage KK, Cheng I, Park RE, Karim MS, Edamura K, Hughes C, Spano AJ, Erisir A, Deppmann CD. Death receptor 6 promotes wallerian degeneration in peripheral axons. Curr Biol. 27:890–896. doi: 10.1016/j.cub.2017.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz T. The role of antimicrobial peptides in innate immunity. Integr Comp Biol. 2003;43:300–304. doi: 10.1093/icb/43.2.300. [DOI] [PubMed] [Google Scholar]
- 14.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez-Sanchez JA, Pilch KS, van der Lans M, Fazal SV, Benito C, Wagstaff LJ, Mirsky R, Jessen KR. After nerve injury, lineage tracing shows that myelin and remak Schwann cells elongate extensively and branch to form repair Schwann cells, which shorten radically on remyelination. J Neurosci. 2017;37:9086–9099. doi: 10.1523/JNEUROSCI.1453-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong L, Wu J, Zhou S, Wang Y, Qin J, Yu B, Gu X, Yao C. Global analysis of transcriptome in dorsal root ganglia following peripheral nerve injury in rats. Biochem Biophys Res Commun. 2016;478:206–212. doi: 10.1016/j.bbrc.2016.07.067. [DOI] [PubMed] [Google Scholar]
- 18.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang L, Jones S, Jia X. Stem cell transplantation for peripheral nerve regeneration: current options and opportunities. Int J Mol Sci. 2017;18:E94. doi: 10.3390/ijms18010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanmura S, Uto H, Numata M, Hashimoto S, Moriuchi A, Fujita H, Oketani M, Ido A, Kodama M, Ohi H, Tsubouchi H. Human neutrophil peptides 1-3 are useful biomarkers in patients with active ulcerative colitis. Inflamm Bowel Dis. 2009;15:909–917. doi: 10.1002/ibd.20854. [DOI] [PubMed] [Google Scholar]
- 21.Kappos EA, Sieber PK, Engels PE, Mariolo AV, D’Arpa S, Schaefer DJ, Kalbermatten DF. Validity and reliability of the CatWalk system as a static and dynamic gait analysis tool for the assessment of functional nerve recovery in small animal models. Brain Behav. 2017;7:e00723. doi: 10.1002/brb3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 23.Kobiela Ketz A, Byrnes KR, Grunberg NE, Kasper CE, Osborne L, Pryor B, Tosini NL, Wu X, Anders JJ. Characterization of macrophage/microglial activation and effect of photobiomodulation in the spared nerve injury model of neuropathic pain. Pain Med. 2017;18:932–946. doi: 10.1093/pm/pnw144. [DOI] [PubMed] [Google Scholar]
- 24.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Yan X, Sun X, Shen X, Yin H, Wang C, Liu Y, Lu C, Fu H, Yang S, Wang Y, Sun X, Zhao L, Lu S, Mikos AG, Peng J, Wang X. Synergistic effects of dual-presenting VEGF- and BDNF-mimetic peptide epitopes from self-assembling peptide hydrogels on peripheral nerve regeneration. Nanoscale. 2019;11:19943–19958. doi: 10.1039/c9nr04521j. [DOI] [PubMed] [Google Scholar]
- 26.Maeda T, Sakiyama T, Kanmura S, Hashimoto S, Ibusuki K, Tanoue S, Komaki Y, Arima S, Nasu Y, Sasaki F, Taguchi H, Numata M, Uto H, Tsubouchi H, Ido A. Low concentrations of human neutrophil peptide ameliorate experimental murine colitis. Int J Mol Med. 2016;38:1777–1785. doi: 10.3892/ijmm.2016.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mietto BS, Mostacada K, Martinez AM. Neurotrauma and inflammation: CNS and PNS responses. Mediators Inflamm. 2015;2015:251204. doi: 10.1155/2015/251204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller CA, Markovic-Lipkovski J, Klatt T, Gamper J, Schwarz G, Beck H, Deeg M, Kalbacher H, Widmann S, Wessels JT, Becker V, Muller GA, Flad T. Human alpha-defensins HNPs-1, -2, and -3 in renal cell carcinoma: influences on tumor cell proliferation. Am J Pathol. 2002;160:1311–1324. doi: 10.1016/s0002-9440(10)62558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy CJ, Foster BA, Mannis MJ, Selsted ME, Reid TW. Defensins are mitogenic for epithelial cells and fibroblasts. J Cell Physiol. 1993;155:408–413. doi: 10.1002/jcp.1041550223. [DOI] [PubMed] [Google Scholar]
- 30.Nozdrachev AD, Kolosova LI, Moiseeva AB, Ryabchikova OV. The role of defensin NP-1 in restoring the functions of an injured nerve trunk. Neurosci Behav Physiol. 2006;36:313–315. doi: 10.1007/s11055-006-0018-8. [DOI] [PubMed] [Google Scholar]
- 31.Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou YJ, Nathan C, Ding A, Brown RH, Jr, Conforti L, Coleman M, Tessier-Lavigne M, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ousman SS, Frederick A, Lim EF. Chaperone proteins in the central nervous system and peripheral nervous system after nerve injury. Front Neurosci. 2017;11:79. doi: 10.3389/fnins.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panagopoulos GN, Megaloikonomos PD, Mavrogenis AF. The present and future for peripheral nerve regeneration. Orthopedics. 2017;40:e141-156. doi: 10.3928/01477447-20161019-01. [DOI] [PubMed] [Google Scholar]
- 34.Pellegatta M, Taveggia C. The complex work of proteases and secretases in Wallerian degeneration: beyond neuregulin-1. Front Cell Neurosci. 2019;13:93. doi: 10.3389/fncel.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao F, Zhang D, Fang T, Lu C, Wang B, Ding X, Wei S, Zhang Y, Pi W, Xu H, Wang Y, Jiang B, Zhang P. Exosomes from human gingiva-derived mesenchymal stem cells combined with biodegradable chitin conduits promote rat sciatic nerve regeneration. Stem Cells Int. 2019;2019:2546367. doi: 10.1155/2019/2546367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roman-Blas JA, Jimenez SA. NF-kappa B as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Sang Q, Sun D, Chen Z, Zhao W. NGF and PI3K/Akt signaling participate in the ventral motor neuronal protection of curcumin in sciatic nerve injury rat models. Biomed Pharmacother. 2018;103:1146–1153. doi: 10.1016/j.biopha.2018.04.116. [DOI] [PubMed] [Google Scholar]
- 38.Savastano LE, Laurito SR, Fitt MR, Rasmussen JA, Gonzalez Polo V, Patterson SI. Sciatic nerve injury: a simple and subtle model for investigating many aspects of nervous system damage and recovery. J Neurosci Methods. 2014;227:166–180. doi: 10.1016/j.jneumeth.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Shiozawa S, Tsumiyama K. Pathogenesis of rheumatoid arthritis and c-Fos/AP-1. Cell Cycle. 2009;8:1539–1543. doi: 10.4161/cc.8.10.8411. [DOI] [PubMed] [Google Scholar]
- 40.Territo MC, Ganz T, Selsted ME, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terzis JK, Kokkalis ZT. Selective contralateral c7 transfer in posttraumatic brachial plexus injuries: a report of 56 cases. Plast Reconstr Surg. 2009;123:927–938. doi: 10.1097/PRS.0b013e31819ba48a. [DOI] [PubMed] [Google Scholar]
- 42.Timotius IK, Canneva F, Minakaki G, Moceri S, Plank AC, Casadei N, Riess O, Winkler J, Klucken J, Eskofier B, von Horsten S. Systematic data analysis and data mining in CatWalk gait analysis by heat mapping exemplified in rodent models for neurodegenerative diseases. J Neurosci Methods. 2019;326:108367. doi: 10.1016/j.jneumeth.2019.108367. [DOI] [PubMed] [Google Scholar]
- 43.Trehan SK, Model Z, Lee SK. Nerve repair and nerve grafting. Hand Clin. 2016;32:119–125. doi: 10.1016/j.hcl.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Waller A. Experiments on the section of the glosso-pharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Edinb Med Surg J. 1851;76:369–376. [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Song M, Song F. Neuronal autophagy and axon degeneration. Cell Mol Life Sci. 2018;75:2389–2406. doi: 10.1007/s00018-018-2812-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu C, Bai L, Chen Y, Fan C, Hu Z, Xu H, Jiang B. Effect of mutated defensin NP-1 on sciatic nerve regeneration after transection: a pivot study. Neurosci Lett. 2016;617:283–287. doi: 10.1016/j.neulet.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Yi J, Puyang Z, Feng L, Duan L, Liang P, Backman V, Liu X, Zhang HF. Optical detection of early damage in retinal ganglion cells in a mouse model of partial optic nerve crush injury. Invest Ophthalmol Vis Sci. 2016;57:5665–5671. doi: 10.1167/iovs.16-19955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan YS, Niu SP, Yu YL, Zhang PX, Yin XF, Han N, Zhang YJ, Zhang DY, Xu HL, Kou YH, Jiang BG. Reinnervation of spinal cord anterior horn cells after median nerve repair using transposition with other nerves. Neural Regen Res. 2019;14:699–705. doi: 10.4103/1673-5374.247474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuce S, Cemal Gokce E, Iskdemir A, Koc ER, Cemil DB, Gokce A, Sargon MF. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann Plast Surg. 2015;74:684–692. doi: 10.1097/SAP.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 50.Zhang B, Yang C, Wang W, Li W. Repair of ocular-oral synkinesis of postfacial paralysis using cross-facial nerve grafting. J Reconstr Microsurg. 2010;26:375–380. doi: 10.1055/s-0030-1249603. [DOI] [PubMed] [Google Scholar]
- 51.Zhou L, Beuerman RW, Huang L, Barathi A, Foo YH, Li SF, Chew FT, Tan D. Proteomic analysis of rabbit tear fluid: defensin levels after an experimental corneal wound are correlated to wound closure. Proteomics. 2007;7:3194–3206. doi: 10.1002/pmic.200700137. [DOI] [PubMed] [Google Scholar]