Abstract
Periodontitis is an inflammatory disease caused by host immune response, resulting in a loss of periodontium and alveolar bone. Immune cells, such as T cells and macrophages, play a critical role in the periodontitis onset. Halofuginone, a natural quinazolinone alkaloid, has been shown to possess anti-fibrosis, anti-cancer, and immunomodulatory properties. However, the effect of halofuginone on periodontitis has never been reported. In this study, a ligature-induced mice model of periodontitis was applied to investigate the potential beneficial effect of halofuginone on periodontitis. We demonstrated that the administration of halofuginone significantly reduced the expression levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in vivo, and markedly suppressed immune cell infiltration into the infected sites. Furthermore, we also observed that halofuginone treatment blocked the T-helper 17 (Th17) cell differentiation in vivo and in vitro. We demonstrated for the first time that halofuginone alleviated the onset of periodontitis through reducing immune responses.
Keywords: halofuginone, naive CD4+ T, osteoclast, periodontitis, T-helper 17
Introduction
Periodontitis is a periodontal disease that affects the alveolar bone and the supporting tissue of the teeth. It is characterized by the formation of deep periodontal spaces and gingival recession.1 Without appropriate treatment, the periodontitis can progress to teeth loosen, mastication impairment, and teeth loss.2,3 Studies have revealed that periodontitis development is a result of chronic inflammatory interaction between bacterial and host immune system.4 However, the underlying mechanism is unknown.
Subgingival pathogenic microorganisms, especially gram-negative bacteria, produce bacterial lipopolysaccharides (LPS) that stimulates the oral mucosa to release proinflammatory cytokines (e.g. TNF-α, IL-1β, and IL-6), osteoclast differentiation-related factors (e.g. macrophage colony-stimulating factor (M-CSF), and the receptor activator of nuclear factor-κB ligand (RANKL)).5 These factors then attract immune cells (e.g. T cells, B cell, macrophages, and neutrophils) to the site of infection, resulting in systemic inflammation and tissue destruction.6,7 Thus, a better comprehension of the relationship between host immune system and inflammatory reaction, as well as the underlying molecular basis and mechanism is important for the development of the potential therapeutic strategy for periodontitis.
Halofuginone is a low molecular weight natural quinazolinone alkaloid found in the Chinese herb medicine, Dichroa febrifuga, which has been used to against malaria parasite for centuries.8 As one of the most potent febrifugine analogs, it has gained much attention due to its various health-promoting properties and has been proposed to be used as a potential therapeutic reagent or supplement for specific diseases.9 For example, the Food and Drug Administration (FDA) approved halofuginone for the prevention of coccidiosis caused by Eimeria tenella and by Eimeria adenoeides in broiler chickens and growing turkeys, respectively.8 Moreover, halofuginone suppressed the increase of excess collagen synthesis, a hallmark of fibrosis, in various animal models, including the rat model of pulmonary fibrosis, and the mouse model of hepatic fibrosis.10–12 Furthermore, halofuginone exhibits anti-cancer ability. Administration of halofuginone has been shown to inhibit tumor growth and angiogenesis in pancreas cancer, bladder cancer, and melanoma.13–15 More importantly, halofuginone is a potent agent to regulate autoimmune system.16 It is possible that halofuginone may have beneficial effect on periodontitis.
In the current study, we aimed to investigate the biological function of halofuginone on naive CD4+ T cell to Th17 cell differentiation in vitro and the onset of periodontitis in vivo in a mouse model of periodontitis.
Materials and methods
Reagents and antibodies
Pure halofuginone (CAS No. 55837-20-2) was purchased from Sigma-Aldrich (St. Louis, MO, USA). BLZ945 (CAS# 953769-46-5) was purchased from APExBIO (Boston, MA, USA). Phospho-Smad2 (Ser465/467) (138D4), Smad2 (D43B4), phospho-Smad3 (Ser423/425) (C25A9) and Smad3 (C67H9) Rabbit mAb were purchased from Cell signaling technology (Danvers, MA, USA). PB-conjugated anti-CD4; PE-conjugated anti-IL-17a and PerCP5.5-conjugated anti-CD45; APC-conjugated anti-CD11b were purchased from Ebioscience (San Diego, CA, USA).
Animal model construction
Wild-type mice on C57BL/6 background at 8 weeks old were purchased from animal model research center of Nanjing University. All experiment protocols were approved by the ethical committee of the Hospital of Stomatology, the Fourth Military Medical University (#2019-042) and were performed conform to the China institutional guidelines for the care and use of animals.
C57BL/6 background mice were randomly divided into seven groups as follows: group 1, wild-type mice for normal control; group 2, chronic periodontitis (CP) mice with PBS treatment for vehicle control; group 3, CP mice with halofuginone (100 μg/mice) treatment; group 4, CP mice with halofuginone (500 μg/mice) treatment; group 5, CP mice with halofuginone (1 mg/mice) treatment; group 6, CP mice with BLZ945 (4 mg/mice) treatment; group 7, CP mice with BLZ945 (4 mg/mice)+ halofuginone (1 mg/mice) treatment.
The sterile silk suture ligature (SUT-15-1) (Roboz Surgical Instrument, MD, USA) was saturated in Porphyromonas gingivalis (Pg) solution prior to all experiments. Mice were anesthetized with 4% isoflurane flow during the whole procedure. The silk suture ligature was cut, and two knots were typed in the center. The mouth of mice was opened gently and the silk suture ligature was carefully placed into the subgingival of the first and second molars (M1-M2) interdental contact. After surgery, the mice were fed with high-sugar food. The ligation on the mice was observed three times weekly and was fixed if it was observed loosened or displaced. The ligation was removed 4 weeks after surgery. The mice were administered with PBS or halofuginone with different doses via i.p. injection for another 4 weeks. The distance of cemento-enamel-junction (CEJ) to alveolar bone crest (ABC) was measured by micro-computed tomography (CT) scans. Animals were euthanized via cervical dislocation or CO2 asphyxiation and then perfused via slow intracardiac injection with 10 mL of fresh fixing buffer (1% PFA in PBS, pH 7.4 (v/v) over 2–3 min. Injection can be done via manual or pump through the left ventricle.
Real-time quantitative PCR analyzes
Total RNAs from mice tissues or cells were extracted with the use of TRIZOL reagent (Invitrogen, USA) and treated with DNase I. The cDNA was generated from RNA using the SuperScript II Reverse Transcriptase Kit (Invitrogen, USA). For gene expression analysis, real-time quantitative PCR (RT-qPCR) was performed using SYBR Green PCR master mix (Applied Biosysyems, MD, USA). It was performed on an ABI 7500 instrument (Life Technology, USA). The relative expression levels of target genes were normalized to β-actin. The primer sequences are as following: Il6 sense 5′-ctgatgctggtgacaaccac-3′, Il6 antisense 5′-cagacttgccattgcacaac-3′; Il12a sense 5′-ccattgaactggcgttggaag-3′, Il12a antisense 5′-acttgagggagaagtaggaatgg-3′; Il1b sense 5′-aagcctcgtgctgtcggacc-3′, Il1b antisense 5′-tgaggcccaaggccacaggt-3′; Tnf sense 5′-catcttctcaaaattcgagtgacaa-3′, Tnf antisense 5′-ccagctgctcctccacttg-3′.
Flow cytometric analysis
Cells isolated from gingival tissues of all mice were counted, washed, and resuspend in 100 μL cold FACS buffer (PBS with 2% FBS and 1 mM EDTA). Appropriate antibodies combination (PerCP5.5-conjugated anti-CD45 and APC-conjugated anti-CD11b or PE-conjugated anti-CD4; PE-conjugated anti-IL-17α and APC-conjugated anti-CD11b) were added into the cell suspension for 30 min on ice. Cells were washed twice using cold FACS buffer. The IL-17α-positive and CD11b-positive cell population were analyzed using BD Accuri™ C6 Plus Cell Analyzer (BD Biosciences, San Jose, CA, USA).
Osteoclast generation
Mice bone marrow cells were isolated following a standard operation protocol, and cultured in DMEM medium without FBS for 1 h, then the non-adherent cells were washed away. The attached monocyte-macrophage progenitor cells were cultured with DMEM supplied with 20% FBS and 10 ng/mL M-CSF for 4 days. The osteoclast precursors were further treated with PBS or Halofuginone. For osteoclastogenesis, 50 ng/mL RANKL and 10 ng/mL M-CSF were used to treat osteoclast precursors which were cultured for another 4 days.
CD4+ T cells to Th17 differentiation and activation
CD4+ T Cell Isolation Kit (Miltenyi Biotech, Shanghai, China) was applied to isolated CD4+ from gingival tissues of the mice. The isolated CD4+ T cells were resuspended in Th17-inducing mix containing anti-CD28, IL6, and TGF-b, then seeded in the cell culture plate pre-coated with anti-CD3. After 5 days of culture, Th17 cells can be assessed by flow cytometric analysis. For Th17 cells activation, the Th17 cells were treated with Brefeldin-A (BFA) (10 μg/mL), ionomycin (1 μM), and phorbol myristate acetate (PMA) (50 ng/mL) for 4 h in the incubator.
Western blot
CD4+ cells from gingival tissues of the mice were used to extract total protein using RIPA buffer with protease inhibitor (Thermo Fisher Scientific, MA, USA). Protein samples were loaded on a sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gel, separated by electrophoresis, and transferred to a PVDF (polyvinylidene difluoride) membrane (Millipore, Billerica, MA, USA). After blocking with PBST containing 5% non-fat milk, the membrane was incubated with the primary antibodies against p-Smad2, Smad2, p-Smad3, or Smad3 either at room temperature for 2 h or in cold room overnight. After washing, the membrane was further probed with second antibodies. The protein signaling on the membrane was developed by the enhanced chemiluminescent substrate (Pierce, ThermoFisher Scientific, USA). The target protein bands were visualized using an iBind western system (ThermoFisher Scientific, MA, USA).
Statistical analysis
Sample size of each group was determined using established statistical power analysis. Differences between means of each compared group were divided by the standard deviation to determine the standardized effect size (>2.0), then 5% was used as significance level in Student t-test with 90% as the power, the minimum required sample size was calculated to be 6. Data were expressed as mean ± SEM. The difference between the two groups is determined by the paired t-test. One-way analysis of variance (ANOVA) followed by Tukey–Kramer test was used to analyze the difference between multiple groups. P < 0.05 was considered to be statistically significant.
Results
Halofuginone alleviated the onset of periodontitis
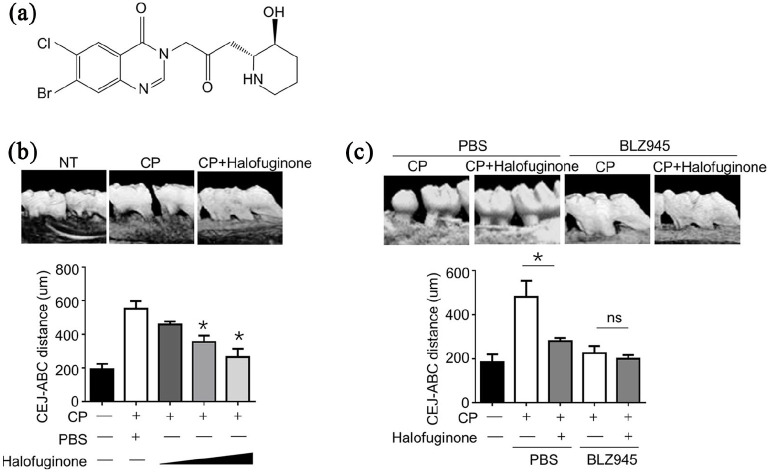
To investigate the effect of halofuginone on the onset of periodontitis, we applied a well-established ligature-induced mouse model of chronic periodontitis (CP). The chemic structure of halofuginone was shown in Figure 1a. As illustrated in Figure 1b, the CEJ-ABC distance was almost three times longer in mice with CP than that in normal control mice, suggesting a successful creation of the CP mice model. Of note, administration of halofuginone significantly decreased the distance of CEJ-ABC in a dose-dependent manner, suggesting that halofuginone can inhibit the development of periodontitis in vivo. More importantly, we found that administration of BLZ945, a potent and selective CSF-1R kinase inhibitor, completely abolished the development of periodontitis, and additional halofuginone treatment has no synergistic effect on reducing CEJ-ABC distance in BLZ945 treated CP mice group (Figure 1c). These results suggested that the innate immune cells may play an essential role in the development of periodontitis.
Figure 1.
Halofuginone ameliorated inflammation in mouse chronic periodontitis models. (a) Structure of Halofuginone. (b) The mice were i.p. pretreated with different doses of Halofuginone from 100 μg/mice to 1 mg/mice as indicated, and then induced chronic periodontitis model (n = 6 for each group) 1 day later. The cemento-enamel-junction–alveolar bone crest was analyzed micro-CT by and plotted (n = 10 for each group). (c) The mice were i.p. injected with Halofuginone (500 μg/mice) with or without BLZ945 (4 mg/mice) i.p. injection, and then induced chronic periodontitis model (n = 6 for each group) 1 day later.
Data are presented as mean ± SEM values and representative of at least three independent experiments. Statistical analyzes represent variations in experimental replicates.
*P < 0.05.
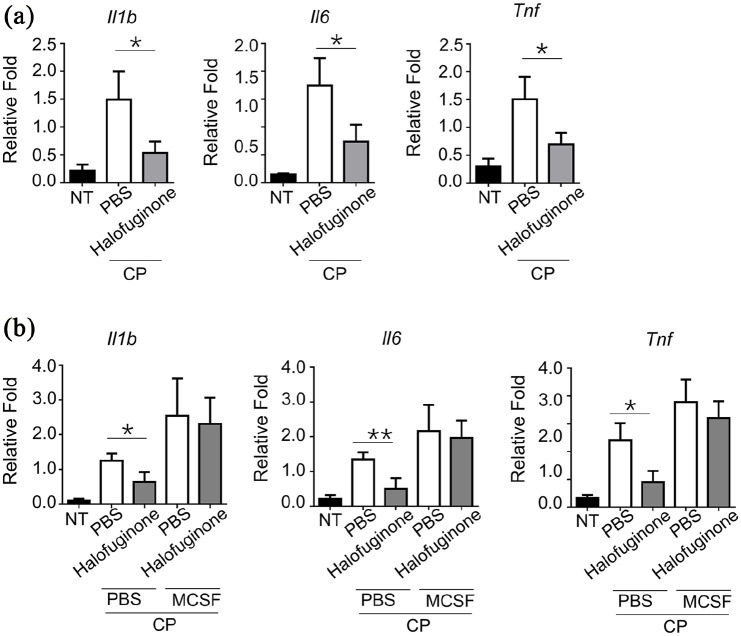
Halofuginone inhibited the pro-inflammatory cytokine expression
Various pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, are related to inflammatory cell infiltration and osteoclastogenesis.17 Indeed, we observed that the expression levels of IL-1β, IL-6, and TNF-α were dramatically higher in gingival tissues from CP mice compared to normal control mice (Figure 2a). Halofuginone treatment resulted in a significantly decreased of all three cytokines in gingival tissues when compared with those in PBS treated CP mice group (Figure 2a). Interestingly, we found that pre-treated mice with M-CSF can, on the one hand, enhanced the expression of three cytokines in CP mice compared to PBS-treated CP mice, and, on the other hand, can totally abolish the inhibition effect of halofuginone on the expression of three cytokines in the gingival tissues of CP mice (Figure 2b). Because the expression levels of IL-1β, IL-6, and TNF-α were comparable in gingival tissues from M-CSF-treated CP mice and M-CSF+ halofuginone-treated CP mice, these results suggested that M-CSF can rescue halofuginone-induced inhibition effect on pro-inflammatory cytokine (IL-1β, IL-6, and TNF-α) expression.
Figure 2.
Halofuginone suppressed the expression of proinflammatory cytokines in mouse chronic periodontitis models. (a) WT mice were i.p. pretreated with 500 μg/mice Halofuginone. 24 h later, a chronic periodontitis model was induced in these mice (n = 5 for each group) as indicated. mRNA levels in gingival tissues from mice with chronic periodontitis were examined by RT-qPCR. All data are presented as fold relative to the β-actin mRNA level. (b) The mice were i.p. treated with M-CSF (250 mg/kg) plus 500 μg/mice Halofuginone, and then induced chronic periodontitis model (n = 8 for each group) 1 day later. mRNA levels in gingival tissues from mice with chronic periodontitis were examined by RT-qPCR.
Data are presented as mean ± SEM values and representative of at least three independent experiments. Statistical analyzes represent variations in experimental replicates.
*P < 0.05, **P < 0.01.
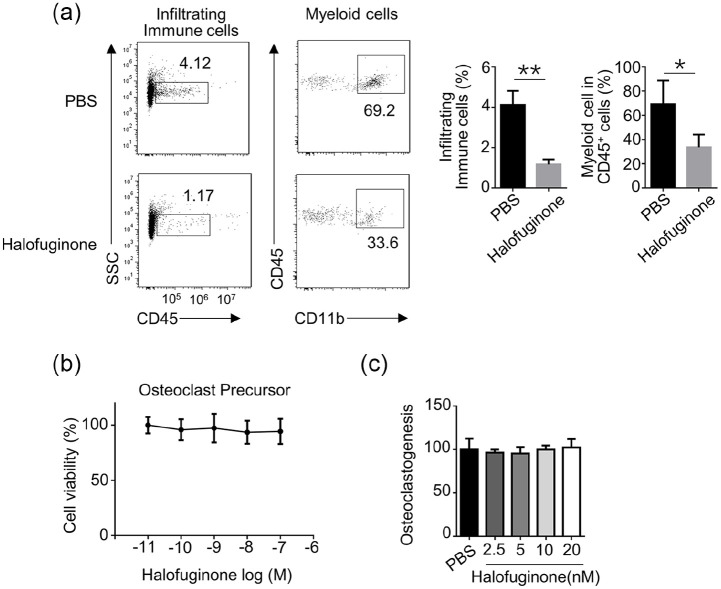
Halofuginone decreased the infiltrating immune cells in gingival tissues
To study the involvement of immune cells in halofuginone-mediated alleviation of periodontitis in vivo, we compared the total infiltrating immune cells in gingival tissues collected from PBS- or halofuginone- treated CP mice. The total immune cell population was identified as the CD45-positive population, as CD45, also known as leukocyte common antigen, is a marker for all hematolymphoid cells. The myeloid cell population was identified as CD45 and CD11b double-positive cell population. As shown in Figure 3a, the halofuginone-treated CP mice evidentially had less total infiltrating immune cells and myeloid cells in gingival tissues when compared with those in PBS-treated CP mice. Furthermore, we found that halofuginone treatment did not affect the cell viability of osteoclast precursors within the concentration ranged from 10−11 M to 10−6 M (Figure 3b). Similarly, halofuginone treatment had a negligible effect on osteoclastogenesis (Figure 3c).
Figure 3.
Halofuginone indirectly affected the frequency of infiltrating myeloid cells in gingival tissues. WT mice were pretreated with 500 μg/mL Halofuginone. 24 h later, a chronic periodontitis model was induced in these mice as indicated. (a) The frequency of indicated cell-types in gingival tissues was analyzed by flow cytometry 3 weeks after the treatment (n = 4). (b) BMs-derived osteoclast precursors were treated with various concentrations of Halofuginone for 24 h, and cell viability was determined by MTT assay. (c) BM were cultured in M-CSF (25 ng/mL) and RANKL (50 ng/mL) for 3 days. The differentiation of osteoclast was analyzed by TRAP staining.
Data are presented as mean ± SEM values and representative of at least three independent experiments. Statistical analyzes represent variations in experimental replicates.
*P < 0.05, **P < 0.01.
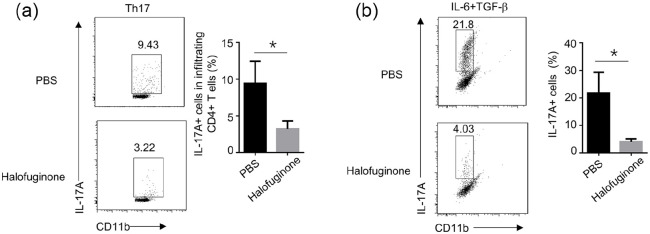
Halofuginone suppressed the Th17 differentiation
Recent studies revealed that a small CD4+ T cell subset named T-help 17 cells (Th17) plays a stimulatory role during osteoclastogenesis.18 T-help 17 cells are characterized by the secretion of IL-17. A 60% reduction of CD4+ and IL-17α+ T-help 17 cells was identified in gingival tissues from halofuginone-treated CP mice compared to PBS-treated CP mice (Figure 4a). We hypothesized that halofuginone treatment might impair Th17 cell differentiation. To verify our hypothesis, we isolated naive CD4+ T cells from PBS- or halofuginone- treated CP mice, and we performed CD4+ T cells to Th17 cells differentiation in vitro. We found that the halofuginone-treated naive CD4+ T cells exhibited markedly decreased ability to be differentiated into Th17 cells when compared to PBS-treated naive CD4+ T cells (Figure 4b).
Figure 4.
Halofuginone directly suppressed the differentiation of Th17. WT mice were pretreated with 500 μg/mL Halofuginone. 24 h later, a chronic periodontitis model was induced in these mice as indicated. (a) The cemento-enamel-junction–alveolar bone crest was analyzed micro-CT by and plotted (n = 10 for each group). The frequency of indicated cell-types in gingival tissues was analyzed by flow cytometry 3 weeks after the treatment. (b) PBS- or Halofuginone (20 nM)-treated naive CD4+ T cells that isolated from mice were stimulated for 4 days with aCD3/aCD28 under different polarization conditions. The frequency of T cell differentiation was analyzed by flow cytometry based on intracellular staining of the indicated cytokines or specific transcriptional factors (n = 4).
Data are presented as mean ± SEM values and representative of at least three independent experiments. Statistical analyses represent variations in experimental replicates.
*P < 0.05.
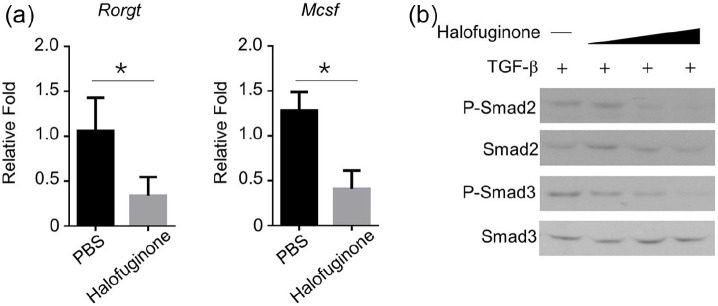
Halofuginone inhibited TGF-β signaling
RORgt and M-CSF are two signature genes that have been characterized in mouse Th17 cells.19 We further found that the expression levels of RORgt and M-CSF were significantly downregulated in Th17 cells originated from halofuginone-treated naive CD4+ T cells compared with these in Th17 cells derived from PBS-treated naive CD4+ T cells (Figure 5a). Smad signaling pathway is essential for TGF-β-induced Th17 cell differentiation. Halofuginone treatment decreased the expression levels of p-Smad2 and 3, but not total-Smad 2 and 3, in a dose-dependent manner in TGF-β-induced Th17 cells (Figure 5b).
Figure 5.
Halofuginone inhibited the signal transduction mediated by TGF-β receptor. WT mice were pretreated with 500 μg/mL Halofuginone. 24 h later, a chronic periodontitis model was induced in these mice as indicated. (a) PBS- or Halofuginone (20 nM)-treated naive CD4+ T cells that isolated from mice were stimulated for 4 days with aCD3/aCD28 under different polarization conditions (n = 3). mRNA levels in gingival tissues from mice with chronic periodontitis were examined by RT-qPCR. All data are presented as fold relative to the β-actin mRNA level. (b) Western blot analysis of the indicated total proteins in whole-cell lysates in TGF-b receptor (5 ng/mL)-mediated signals in CD4+ T cells pretreated with Halofuginone (20 nM) at 60 min.
Discussion
The development of periodontitis starts with plaque-a sticky film, and tartar-a hard calcified deposit, which is mainly composed of bacteria.4 The bacteria produce LPS or other factors that can cause infection and inflammation of the gingivitis.1 However, the activated host immune response that attracting various immune cells to fight against bacteria is the main cause of the degradation of periodontal tissues and bones.20 The protective aspect of host immune-defense includes the recruitment of both myeloid and lymphoid cell lineages to the infected areas. The myeloid cells, such as monocytes, macrophages, and neutrophils, play important roles in the coordination of the infection resolution.21,22 Upon bacterial infection, the neutrophils followed by macrophages were recruited to the microbial invasion sites. The overall functions of macrophages include phagocytosis of the pathogenic bacteria, production of cytokines or chemokines that attract other immune cells to the infection sites, and promotion of fibrosis and scar tissue healing.23,24 When the inflammatory process becomes chronic, the bacterial products such as LPS induce excessive production of two essential osteoclast differentiation essential cytokines (RANKL and M-CSF), promoting monocytes differentiate into osteoclasts.25 The excess osteoclasts cause bone resorption, leading to the development of periodontitis. BLZ945 is a highly specific small-molecule inhibitor of colony-stimulating factor 1 receptor (CSF-1R), also known as M-CSF receptor, and has been widely used for depletion of macrophage in vivo.26 We observed that BLZ945 treatment completely prevented the onset of periodontitis, suggesting that macrophages accumulation in the gingival tissues was essential for the development of periodontitis. In a parallel experiment, we also found that halofuginone treatment ameliorated the onset of periodontitis, indicating that halofuginone may also inhibit M-CSF signaling.
Cytokines play pivotal roles in the regulation of the amplitude and the duration of immune response. Cytokines mediate immune cell proliferation, differentiation, migration, and survival.27 In the case of periodontal pathogenesis, after bacterial infection, the pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, are secreted in the infected sites. IL-1β and IL-6 are reported to be associated with macrophage migration, polarization, and osteoclastogenesis.28 TNF-α is a multi-function cytokine that can induce cell apoptosis, migration, and differentiation as well as stimulate other cells to secrete cytokines, such as IL-1β, IL-6, and RANKL to promote osteoclasts formation.29 Our results showed that halofuginone treatment reduced the expression levels of IL-1β, IL-6, and TNF-α in the gingival tissue of CP mice. Besides, the addition of M-CSF can rescue the halofuginone-mediated inhibition effects on the expression of IL-1β, IL-6, and TNF-α. Collectively, these results suggested that halofuginone inhibited the expression of IL-1β, IL-6, and TNF-α through regulation of M-CSF signaling.
Th17 cells, known for the production of IL-17, are a subset of T helper cells involved in the regulation of autoimmune inflammation.19 Recent studies demonstrated that Th17 cells are vital mediators in the onset of periodontitis by affecting alveolar bone destruction in animal models. They are commonly observed in the chronic inflammatory sites of periodontitis and are reported to promote osteoclastogenesis.30 Interestingly, Sundrud et al.31 revealed that halofuginone is a potent and selective inhibitor of differentiation of Th17 by activation of the amino acid starvation response signaling pathway. Consistent with this finding, we also observed that halofuginone treatment partially blocked the naive T cell to Th17 cell differentiation.
Although our current data exhibiting the potential beneficial effects of halofuginone on ameliorating inflammation in mouse chronic periodontitis models, several important questions are needed to be addressed. For example, the detailed molecular mechanisms of how halofuginone affect the frequency of infiltrating myeloid cells in gingival tissues of CP mice need further investigation. Furthermore, the immunomodulatory effect of halofuginone should be tested in other animal models (e.g. dog, monkey, and pig) to confirm the safety and efficacy of halofuginone before it can be used on clinical trials. Finally, if halofuginone has been demonstrated to be safe and efficient on different animal models, finding the optimal doses of halofuginone for treating patients with chronic periodontitis is another major challenge. Thus, more substantial researches are warranted to explore the potential benefit of halofuginone on periodontitis treatment.
Conclusion
In the current study, we demonstrated that halofuginone exhibited an alleviation effect on periodontitis in vivo, probably via suppressing of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) and restraint of the Th17 cell differentiation.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Key R&D projects in Shaanxi Province (2019SF-140).
Ethics approval: Ethical approval for this study was obtained from the ethical committee of the Hospital of Stomatology, the Fourth Military Medical University (#2019-042).
Animal welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
ORCID iD: Yingjie Wang  https://orcid.org/0000-0002-7459-1179
https://orcid.org/0000-0002-7459-1179
References
- 1. Pihlstrom BL, Michalowicz BS, Johnson NW. (2005) Periodontal diseases. The Lancet 366: 1809–1820. [DOI] [PubMed] [Google Scholar]
- 2. Shaddox LM, Walker CB. (2010) Treating chronic periodontitis: Current status, challenges, and future directions. Clinical, Cosmetic and Investigational Dentistry 2: 79–91. [PMC free article] [PubMed] [Google Scholar]
- 3. Tariq M, Iqbal Z, Ali J, et al. (2012) Treatment modalities and evaluation models for periodontitis. International Journal of Pharmaceutical Investigation 2: 106–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saini R, Marawar PP, Shete S, et al. (2009) Periodontitis, a true infection. Journal of Global Infectious Diseases 1: 149–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cetinkaya B, Guzeldemir E, Ogus E, et al. (2013) Proinflammatory and anti-inflammatory cytokines in gingival crevicular fluid and serum of patients with rheumatoid arthritis and patients with chronic periodontitis. Journal of Periodontology 84: 84–93. [DOI] [PubMed] [Google Scholar]
- 6. Akram Z, Abduljabbar T, Abu Hassan MI, et al. (2016) Cytokine profile in chronic periodontitis patients with and without obesity: A systematic review and meta-analysis. Disease Markers 2016: 4801418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cekici A, Kantarci A, Hasturk H, et al. (2014) Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000 64: 57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pines M, Spector I. (2015) Halofuginone - the multifaceted molecule. Molecules 20: 573–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pines M. (2014) Halofuginone for fibrosis, regeneration and cancer in the gastrointestinal tract. World Journal of Gastroenterology 20: 14778–14786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nagler A, Firman N, Feferman R, et al. (1996) Reduction in pulmonary fibrosis in vivo by halofuginone. American Journal of Respiratory and Critical Care Medicine 154: 1082–1086. [DOI] [PubMed] [Google Scholar]
- 11. Pines M, Snyder D, Yarkoni S, et al. (2003) Halofuginone to treat fibrosis in chronic graft-versus-host disease and scleroderma. Biology of Blood and Marrow Transplantation 9: 417–425. [DOI] [PubMed] [Google Scholar]
- 12. Pines M, Knopov V, Genina O, et al. (1997) Halofuginone, a specific inhibitor of collagen type I synthesis, prevents dimethylnitrosamine-induced liver cirrhosis. Journal of Hepatology 27: 391–398. [DOI] [PubMed] [Google Scholar]
- 13. Lamora A, Mullard M, Amiaud J, et al. (2015) Anticancer activity of halofuginone in a preclinical model of osteosarcoma: Inhibition of tumor growth and lung metastases. Oncotarget 6: 14413–14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsuchida K, Tsujita T, Hayashi M, et al. (2017) Halofuginone enhances the chemo-sensitivity of cancer cells by suppressing NRF2 accumulation. Free Radical Biology & Medicine 103: 236–247. [DOI] [PubMed] [Google Scholar]
- 15. Chen G, Gong R, Shi X, et al. (2016) Halofuginone and artemisinin synergistically arrest cancer cells at the G1/G0 phase by upregulating p21Cip1 and p27Kip1. Oncotarget 7: 50302–50314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hou X, Zhou J, Yang R, et al. (2017) Effect of halofuginone on the pathogenesis of autoimmune thyroid disease in different mice models. Endocrine, Metabolic & Immune Disorders Drug Targets 17: 141–148. [DOI] [PubMed] [Google Scholar]
- 17. Jung SM, Kim KW, Yang CW, et al. (2014) Cytokine-mediated bone destruction in rheumatoid arthritis. Journal of Immunology Research 2014: 263625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sato K, Suematsu A, Okamoto K, et al. (2006) Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. Journal of Experimental Medicine 203: 2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sandquist I, Kolls J. (2018) Update on regulation and effector functions of Th17 cells. F1000Research 7: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nedzi-Gora M, Kowalski J, Gorska R. (2017) The immune response in periodontal tissues. Archivum Immunologiae et Therapiae Experimentalis 65: 421–429. [DOI] [PubMed] [Google Scholar]
- 21. Silva N, Abusleme L, Bravo D, et al. (2015) Host response mechanisms in periodontal diseases. Journal of Applied Oral Science 23: 329–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zelkha SA, Freilich RW, Amar S. (2010) Periodontal innate immune mechanisms relevant to atherosclerosis and obesity. Periodontology 2000 54: 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicholson LB. (2016) The immune system. Essays in Biochemistry 60: 275–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ragland SA, Criss AK. (2017) From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathogens 13: e1006512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mormann M, Thederan M, Nackchbandi I, et al. (2008) Lipopolysaccharides (LPS) induce the differentiation of human monocytes to osteoclasts in a tumour necrosis factor (TNF) alpha-dependent manner: A link between infection and pathological bone resorption. Molecular Immunology 45: 3330–3337. [DOI] [PubMed] [Google Scholar]
- 26. Lu X, Meng T. (2019) Depletion of tumor-associated macrophages enhances the anti-tumor effect of docetaxel in a murine epithelial ovarian cancer. Immunobiology 224: 355–361. [DOI] [PubMed] [Google Scholar]
- 27. Foster JR. (2001) The functions of cytokines and their uses in toxicology. International Journal of Experimental Pathology 82: 171–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang DH, Yang MY. (2019) The role of macrophage in the pathogenesis of osteoporosis. International Journal of Molecular Sciences 20: 2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalliolias GD, Ivashkiv LB. (2016) TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nature Reviews Rheumatology 12: 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Adamopoulos IE, Bowman EP. (2008) Immune regulation of bone loss by Th17 cells. Arthritis Research & Therapy 10: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sundrud MS, Koralov SB, Feuerer M, et al. (2009) Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science 324: 1334–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]