Abstract
Available evidence provides arguments both for and against a primary pathogenic role for T cells in human type 1 diabetes. Genetic susceptibility linked to HLA Class II lends strong support. Histopathology documents HLA Class I hyperexpression and islet infiltrates dominated by CD8+ T cells. While both hallmarks are near absent in autoantibody-positive donors, the variable insulitis and residual beta cells of recent-onset donors suggests the existence of a younger-onset endotype with more aggressive autoimmunity and an older-onset endotype with more vulnerable beta cells. Functional arguments from ex vivo and in vitro human studies and in vivo ‘humanised’ mouse models are instead neutral or against a T cell role. Clinical support is provided by the appearance of islet autoantibodies before disease onset. The faster C-peptide loss and superior benefits of immunotherapies in individuals with younger-onset type 1 diabetes reinforce the view of age-related endotypes. Clarifying the relative role of T cells will require technical advances in the identification of their target antigens, in their detection and phenotyping in the blood and pancreas, and in the study of the T cell/beta cell crosstalk. Critical steps toward this goal include the understanding of the link with environmental triggers, the description of T cell changes along the natural history of disease, and their relationship with age and the ‘benign’ islet autoimmunity of healthy individuals.
Graphical abstract
Electronic supplementary material
The online version of this article (10.1007/s00125-020-05298-y) contains a slideset of the figures for download, which is available to authorised users.
Keywords: Autoantibodies, Autoimmunity, Beta cells, Endotypes, HLA, Immunotherapy, Insulitis, Islets, Pancreas, Review, T cells




Introduction
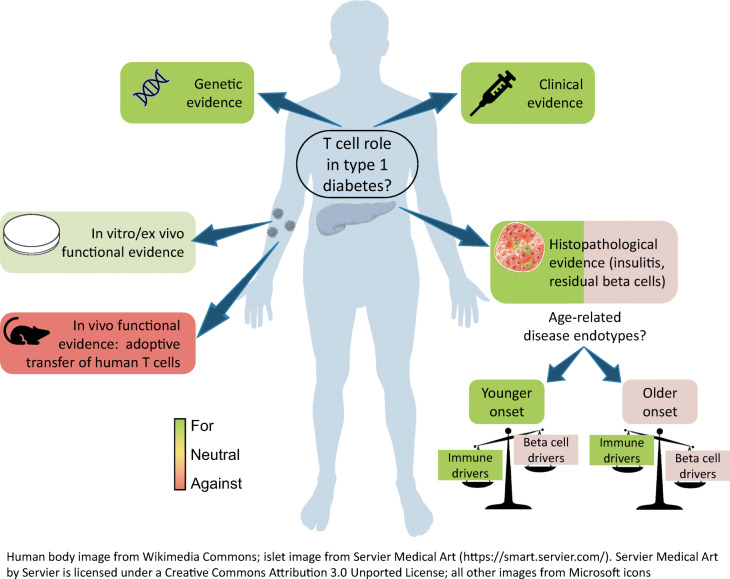
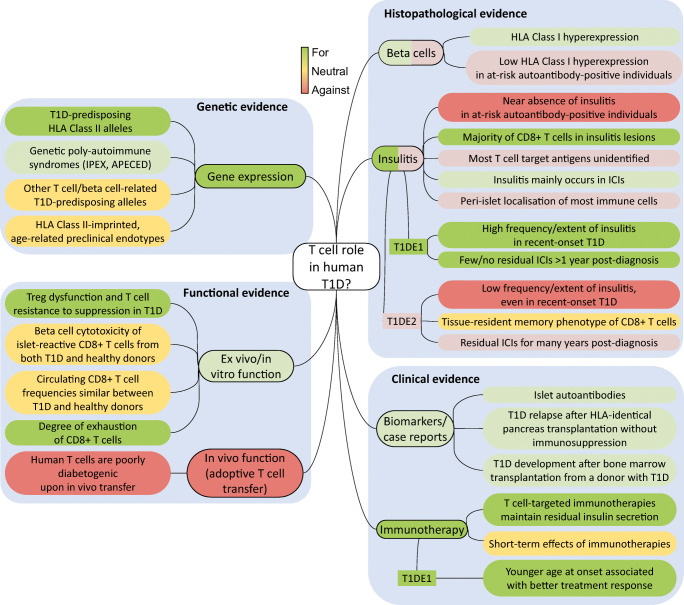
While type 1 diabetes is described as a T cell-mediated autoimmune disease, a more holistic view comprising the dialogue between T cell aggressors and beta cell targets is gaining credit. On one hand, this novel view underlines the active pathogenic role played by beta cells (reviewed in [1]) and on the other, it calls for a critical reappraisal of the role of T cells, which we address here. We contend that evidence for a primary or exclusive role of T cells is variable and probably reflects a balance with mechanisms of beta cell dysfunction that underlie different disease subtypes (endotypes) [2]. These endotypes may thus feature T cells as either ‘lead culprits’ or ‘partners in crime’ for beta cell destruction. We will review arguments for and against T cell-mediated mechanisms (summarised in Fig. 1), link them to age-related endotypes, and propose future directions to settle these questions.
Fig. 1.
Evidence for or against a primary pathogenic role for T cells in human type 1 diabetes (T1D). APECED, autoimmune polyendocrinopathy, candidiasis, ectodermal dystrophy (syndrome); IPEX, immune dysregulation, polyendocrinopathy, enteropathy, X-linked (syndrome); This figure is available as part of a downloadable slideset
Genetic evidence
For
Type 1 diabetes susceptibility and protection are strongly associated with HLA Class II (HLA-II) and, to lesser extent, HLA Class I (HLA-I) loci [3], with HLA-DQB1*06:02 exerting a dominant protection. Given the antigen-presenting function of HLA molecules, these associations support a role for T cells. Several other disease-associated gene variants regulate T cell responses (e.g. PTPN22, IL2RA, CTLA4) [3]. Moreover, disease-associated, and likely causal, genetic variants are enriched in open chromatin (i.e. accessible for transcription) specifically in immune cells, particularly in CD4+ effector T cells [4]. The rare genetic poly-autoimmune syndromes immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome (FOXP3 mutations) and autoimmune polyendocrinopathy, candidiasis, ectodermal dystrophy (APECED) syndrome (AIRE mutations) affect T cell tolerance mechanisms and can comprise type 1 diabetes. Genetically imprinted, age-related disease endotypes may exist, as younger (<13 months old) children more frequently carry HLA-DR4/DQ8 and first seroconvert for anti-insulin autoantibodies (IAA), while older children (>40 months old) more often carry HLA-DR3/DQ2 and present with anti-GAD at first seroconversion [5].
Against
Although several disease-predisposing alleles are expressed in beta cells and modulate islet inflammation [1], their contribution to disease predisposition is rather small, suggesting a modulatory rather than driving role. Interestingly, HLA-II-imprinted endotypes influence seroconversion [5] but do not strongly affect age of onset [6], clinical progression or C-peptide loss post-diagnosis [7], indicating that other genetic and/or acquired factors determine the progression rate of beta cell damage.
Histopathological evidence
For
HLA-I hyperexpression on beta cells [8, 9] suggests a role for CD8+ T cells. The second histopathological hallmark is the presence of immune infiltrates (insulitis) dominated by CD8+ T cells, followed by CD68+ macrophages, CD20+ B cells and CD4+ T cells [10–13]. Scattered neutrophils are also observed throughout the pancreas [14, 15] and evidence of dysregulated systemic innate immunity has been consistently reported, even before autoantibody seroconversion [14]. The relative under-representation of CD4+ T cells and the near absence of regulatory T cells (Tregs) may reflect an earlier, more peripheral role (e.g. in pancreatic lymph nodes), while the over-representation of CD8+ T cells may reflect a final effector role in beta cell destruction.
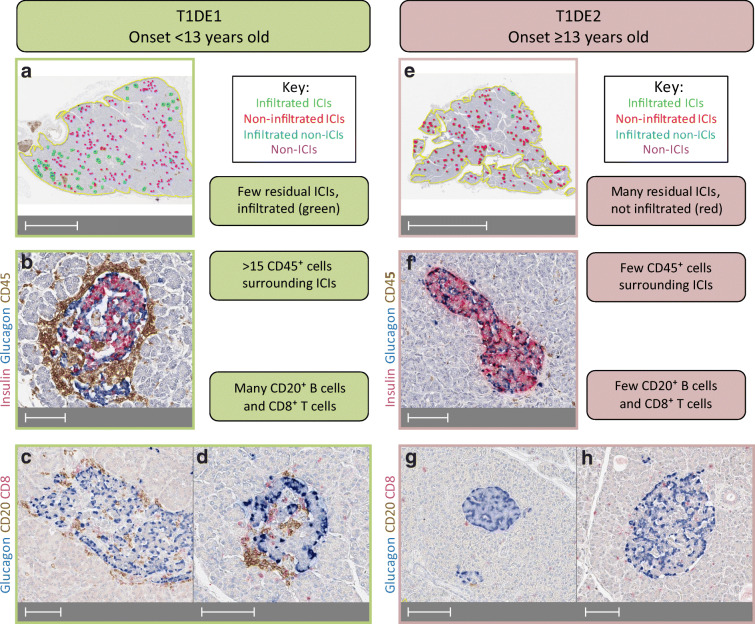
Studies on the Exeter Archival Diabetes Biobank (EADB) documented that insulitis of donors with recent-onset type 1 diabetes differs according to age [13, 16, 17], suggesting the existence of two endotypes with different pathogenic mechanisms (Fig. 2): type 1 diabetes endotype 1 (T1DE1), in individuals with onset at age <13 years; and type 1 diabetes endotype 2 (T1DE2), mostly occurring in individuals diagnosed at ≥13 years of age. T1DE1 is characterised by a CD20high insulitis rich in CD8+ T cells, few residual insulin-containing islets (ICIs; indicative of residual beta cells) and evidence of abnormal insulin processing in the remaining beta cells. In contrast, T1DE2 displays a CD20low insulitis with fewer CD8+ T cells, more residual ICIs and normal insulin processing. Recent-onset (<6 month) CD20high donors have a median of 78% of insulitis-positive ICIs (range 19–100%; n = 20), vs 14% (range 0–52%; n = 19) in CD20low donors (SJR, unpublished data). In line with this endotype view, the frequency of insulitis within 1 month of diagnosis is 73% in young donors with type 1 diabetes (<14 years old, likely to be mostly T1DE1) [18] and the parallel reduction in residual ICIs may indicate an aggressive beta cell destruction. In contrast, insulitis within 1 month from diagnosis is less frequent (29%) in older donors (15–40 years old, likely T1DE2) and long-term ICI preservation suggests reduced aggressiveness [19].
Fig. 2.
Key differences in insulitis and residual ICIs in type 1 diabetes endotypes T1DE1 and T1DE2. (a–d) T1DE1 donors (mostly with a disease onset <13 years old) have few residual ICIs (a; green and red circles) compared with non-ICIs (blue and pink circles). The majority of residual ICIs display significant immune infiltration (a; >15 CD45+ cells, green circles); a representative image is shown in (b). This infiltrate is enriched in CD20+ B cells and CD8+ T cells (c, d). (e–h) T1DE2 donors (mostly with a disease onset ≥13 years old) retain significant residual ICIs (e; red and green circles), the majority of which do not meet the criteria for insulitis (e; red circles). Most ICIs have limited infiltration with CD45+ cells (f), the majority of which are CD8+ T cells with few CD20+ B cells (g, h). Specimens from representative donors from the EADB biobank (https://foulis.vub.ac.be) were immunostained with antibodies against insulin/glucagon/CD45 or glucagon/CD20/CD8 using triple chromogen-based immunohistochemistry similar to that described in [78]. Scale bars, 5 mm (a), 100 μm (b–d, f–h) or 10 mm (e). This figure is available as part of a downloadable slideset
Against
If viewing type 1 diabetes as a single disease, the histopathological evidence for a critical T cell involvement is unconvincing. Immune infiltrates are limited, as exemplified by the debate on the diagnostic definition of insulitis being the presence of ≥15 immune (CD45+) cells/islet in ≥3 islets [20, 21]. This definition should be interpreted by considering the following points: (1) 15 immune cells/islet is only twice the number found in non-diabetic donors [10]; (2) it does not account for differences in islet size (i.e. the insulitis density); (3) infiltrated islets are typically few (<10%) and found in a minority (~20%) of donors [18, 22]; (4) peri-islet, non-invasive insulitis is more common [19]; and (5) lymphocytes are also found scattered in the exocrine tissue, even in autoantibody-positive non-diabetic donors [12]. Moreover, insulitis is mostly confined to ICIs (33% vs 2% in non-ICIs) and beta cell area and mass are higher in donors with type 1 diabetes with insulitis than in those without insulitis [22]. These patterns may indicate that immune cells leave the islets once beta cell destruction is complete.
These findings become more convincing when interpreted according to age-related endotype differences. However, a major bias is introduced by the specimens available, which are very limited for children with recent-onset type 1 diabetes because, fortunately, very few still die close to disease onset. For instance, the Network of Pancreatic Organ Donors with Diabetes (nPOD) has collected only one such specimen since 2007. In contrast, most tissues are available for T1DE2 donors with older-onset type 1 diabetes (i.e. those with weaker evidence for a major T cell role). In most studies, age-related endotype differences are further blurred by the analysis of specimens from individuals with overall long disease durations, which, together with age, influence the extent of insulitis and the number of residual ICIs. For instance, the frequency of insulitis in young donors with type 1 diabetes (<14 years old) drops from 73% within 1 month of diagnosis to 4% beyond 1 year [18]. Thus, even for the younger histopathological T1DE1 with stronger evidence for a major T cell role, key hallmarks have likely waned in most long-standing diabetes specimens available. Together with the focus of most studies on older-onset donors, these limitations may bias our view against T cells.
The bias of long disease duration is absent in the Diabetes Virus Detection Study (DiViD), which collected surgical pancreas specimens from living adults with new-onset disease [23]. In these donors, 11% of the islets displayed insulitis, a proportion comparable with that observed in similar age/duration-matched CD20low donors in both EADB and nPOD specimens. The fact that these samples came from living donors rules out perimortem or post-mortem changes as possible confounders. Collectively, the DiViD, EADB and nPOD findings with older-onset but short-duration disease rule out the possibility that the limited insulitis observed represents an extinguished phase of islet autoimmunity, as could be the case in donors with longer disease duration.
Even in individuals with recent-onset disease, the autoimmune process dates back several months or years and the situation could be different at the preclinical disease stages: stage 1, presence of ≥2 autoantibodies; stage 2, presence of autoantibodies and initial metabolic alterations (e.g. loss of first-phase insulin secretion); and stage 3, presence of autoantibodies and overt hyperglycaemia (i.e. clinical disease) [24]. One single autoantibody marks a low risk of progression, especially in adults, and is therefore not included in this staging system. Yet, most autoantibody-positive pancreas donors analysed to date are adults who are positive for a single autoantibody. It should be noted, however, that the low-risk definition of the single autoantibody status is based on longitudinal assessments in living humans and that the probability of appearance of a second autoantibody is approximately 7–20% at 5 years (depending on age), with a subsequent risk of clinical progression similar to those individuals who are positive for multiple autoantibodies from the start [25]. Thus, out of the reasonably large series of ~100 donors positive for a single autoantibody analysed to date, a fraction would have eventually progressed. Yet, insulitis was invariably absent in all autoantibody-positive donors, even in those with predisposing HLA haplotypes [19]. Additionally, in the relatively limited number of specimens from donors positive for multiple autoantibodies (i.e. stage 1 disease; ~20 analysed to date), little or no insulitis was observed (1–9% of islets) [10, 22, 26]. This picture may reflect a disease kinetics remaining stable for many years before sudden progression. Arguably, snap-shooting this ‘point of no-return’ may be difficult in histopathological studies.
HLA-I hyperexpression is also typically found in ICIs; expression decreases with disease duration and is often associated with CD8+ insulitis [27]. However, it is more readily apparent than insulitis and is present in most ICIs, probably reflecting a readout of beta cell stress. A possible interpretation of this discrepancy is that insulitis hallmarks may be more labile due to immune cells migrating between islets and the peri-islet and exocrine tissue, resulting in a variable pattern at any given time point compared with the more stable HLA-I hyperexpression. Nonetheless, even HLA-I hyperexpression was only found in 13% of islets from individuals positive for two autoantibodies, associated with higher yet mild CD8+ T cell infiltration [28].
Another point of controversy is that, despite the identification of several antigen specificities among pancreas-infiltrating T cells, a large fraction remains unassigned. Moreover, whether these T cells present an effector/memory phenotype compatible with an active autoimmune engagement is unknown. One study suggested a prevalent (46%) tissue-resident memory phenotype of peri-islet CD8+ T cells [29] that is enriched in non-diabetic donors [30] and, possibly, individuals who are T1DE2 [16, 29]. Although described as non-cytotoxic in insulitis lesions [29], tissue-resident T cells can proliferate locally, produce proinflammatory cytokines and recruit circulating cells, thus possibly contributing to perpetuate disease. Mouse studies [31, 32] suggest that a significant fraction of pancreas-infiltrating T cells are naive (also in humans [15]) and non-islet-reactive, possibly configuring insulitis as an ‘open’ lesion in which bystander T cell activation may contribute to beta cell damage.
Functional evidence
For
Although the NOD mouse model is imperfect, both CD4+ and CD8+ T cells are needed to transfer disease [33]. Moreover, NOD mice lacking MHC Class I (MHC-I) do not develop diabetes unless receiving splenocytes from diabetic animals [34]. Interestingly, beta cell-selective MHC-I knockout in mice [35] protects against diabetes but not insulitis, suggesting that MHC-I-interacting CD8+ T cells exert a late pathogenic role, consistent with their final involvement in beta cell cytotoxicity. The antigen specificity of these T cells has been explored with T cell receptor (TCR)-transgenic NOD mouse studies, which suggest a variable pathogenic potency of different T cell clonotypes. Indeed, diabetes development is accelerated by some TCRs, such as the CD4+ TCRs BDC-6.9 [36] (recognising a proinsulin/islet amyloid polypeptide hybrid peptide [37]) and 4.1 of unknown specificity [38] and the CD8+ islet-specific glucose-6-phosphatase catalytic-subunit related protein (IGRP)206–214-reactive 8.3 TCR [38]). Others do not accelerate diabetes development (i.e. the CD4+ TCR BDC-2.5 reactive to a proinsulin/chromogranin-A hybrid peptide [37]), unless on a NOD/scid immunodeficient background [39], and the CD8+ InsB15-23-reactive G9C8 TCR, which requires prior InsB15-23 immunisation [40]).
Reports analysing Tregs concluded that, while the frequency of circulating forkhead box P3 (FOXP3)+CD4+ Tregs is unaltered in type 1 diabetes, their regulatory activity is diminished [41]. This alteration reflects both a reduced suppressive function of Tregs [42], sometimes associated with increased secretion of proinflammatory cytokines (IFN-γ, IL-17) [41, 43], and an increased resistance of conventional T cells to suppression [44]. Treg dysfunction is partly genetically imprinted (e.g. through IL2RA polymorphisms leading to unstable FOXP3 expression under limiting IL-2 concentrations [41]). It is also highly heterogeneous across individuals and largely overlapping with non-diabetic control individuals, possibly pointing to Treg-driven disease endotypes. Similarly, IL-10-polarised islet-reactive CD4+ T cells were enriched in healthy donors and individuals with type 1 diabetes of later onset [45], who may be representative of T1DE2 [13].
Against
Animal models of autoimmune diseases were initially produced by immunisation with organ extracts or antigens (e.g. for multiple sclerosis (1933) [46], orchitis, thyroiditis [47], adrenalitis, rheumatoid arthritis [48]). Despite a better knowledge of target antigens, similar immunisations of non-transgenic animals never provided an equivalent model of experimental insulitis/diabetes, possibly reflecting the requirement for beta cell dysfunction. The BioBreeding rat provides another spontaneous animal model of human type 1 diabetes. Interestingly, it harbours a profound systemic T cell lymphopenia, including near absence of CD8+ T cells, which, despite some CD8+ T cell infiltration in islets [49], is required for spontaneous diabetes development [50].
CD8+ T cell clones recognising preproinsulin [51], IGRP [52] and zinc transporter 8 [53] epitopes can lyse beta cells in vitro. However, we documented that T cell clones from healthy donors display similar cytotoxic potency [53]. Although this similarity could reflect the long-term in vitro stimulation of these clones, erasing ex vivo differences, it demonstrates that islet-reactive CD8+ T cells from any individual can be differentiated into cytotoxic effectors. Albeit this does not negate a pathogenic role for T cells, it does not provide supportive evidence either, as frequently claimed. Moreover, most in vitro cytotoxic experiments [51, 52] employed high effector/target ratios (10/1 to 25/1) compared with those observed in situ in the pancreas [8].
These results mirror recent observations by us [53–55] and others [56–58] that islet-reactive CD8+ T cells circulate at similar frequencies in healthy individuals and individuals with type 1 diabetes. It is unclear whether the same applies to CD4+ T cells. These reports detected CD8+ T cells using HLA-I multimers (i.e. independent of their functional phenotype), while a functional ELISpot readout of IFN-γ secretion repeatedly documented disease specificity [51, 53, 59, 60]. Thus, islet-reactive CD8+ T cells may differ according to disease status in terms of functional profile (e.g. exhaustion) rather than frequency [57, 61] and in their capacity to home to the pancreas, where their density is enriched in donors with type 1 diabetes [53, 54]. This difference in homing may point to the heightened vulnerability of stressed/senescent beta cells as an important driver [1, 62, 63]. Overall, these results open key questions to better understand T cell autoimmunity and biomarkers but do not offer arguments either for or against a primary pathogenic role for T cells.
Human T cell adoptive transfer into immunodeficient mice was not diabetogenic (e.g. using an IGRP-reactive CD8+ T cell clone [52]). Although TCR-transgenic CD4+ T cells recognising the HLA-DQ8-restricted INSB9-23 epitope transferred into HLA-DQ8-transgenic mice reconstituted with human thymus and CD34+ cells induced diabetes [64], they required strong priming conditions (i.e. multiple low-dose streptozotocin and INSB9-23 peptide immunisation). Streptozotocin-induced beta cell death and antigen release may provide the beta cell dysfunction signal needed for efficient T cell priming.
Clinical evidence
For
Islet autoantibodies appear before disease onset [5] and are produced by B cells, thus implying a helper role provided by CD4+ T cells. Eight case reports documented disease relapse in non- or minimally immunosuppressed recipients with type 1 diabetes 6–12 weeks after pancreas transplantation from non-diabetic HLA-identical siblings, with no relapse observed under immunosuppression [65]. Conversely, a case report described disease development 4 years after non-T cell-depleted HLA-identical bone marrow transplantation from a donor with type 1 diabetes [66].
Among the panoply of immunotherapeutic agents trialled in individuals with new-onset type 1 diabetes, four have shown some effect on C-peptide preservation: LFA-3-Ig (alefacept) [67], CTLA-4-Ig (abatacept) [68], anti-CD20 (rituximab) [69] and anti-CD3 monoclonal antibodies [70–73]. Apart from the anti-B cell agent rituximab, all target mainly T cells, arguing for T cell-driven mechanisms targetable by drugs. Interestingly, superior clinical benefit is often observed in children [67, 69, 70, 72], who may represent the more T cell/B cell-driven endotype T1DE1. Concordantly, individuals diagnosed at younger ages generally lose C-peptide secretion more rapidly and the fast-progressing subgroup displays age-dependent blood gene expression and cell count profiles that are higher for B cells and lower for neutrophils [7]. Moreover, individuals with higher pre-treatment B cell counts achieve superior C-peptide preservation after rituximab treatment [7], exemplifying the relevance of disease endotypes to selection of more-targeted immunotherapies.
Against
The limited and partial improvements observed in immunotherapy trials starkly contrast with the more significant benefits achieved in other autoimmune diseases. Examples of mainstay disease-modifying therapies include the following: IFN-β, glatiramer, dimethyl fumarate, sphingosine-1-phosphate receptor modulators and antibodies to very late antigen-4 and CD20 for multiple sclerosis [74]; and methotrexate, TNF and IL-6 inhibitors, rituximab and abatacept for rheumatoid arthritis [75]. However, all these agents perform much better when given early in the disease course, at a stage that might correspond to the preclinical phase of type 1 diabetes.
A recurrent observation from immunotherapy trials in individuals with new-onset diabetes is that the effect on C-peptide preservation is limited to the first months of treatment and resumes its decline thereafter [76]. While this may indicate a need for prolonged treatment, it could also reflect targeting of only the T cell pathogenic component, leaving others, possibly related to beta cells [1], free to drive further disease progression. However, trials with putative beta cell-protective agents (e.g. glucagon-like peptide-1 agonists) have so far failed to deliver significant clinical benefits, or to convincingly ascribe any such benefit to beta cell protection [77].
Gaps in knowledge preventing conclusive evidence of the pathogenic role of T cells
The fulfilment of Koch’s postulates for a pathogenic role for T cells is currently incomplete, partly due to technical limitations hindering progress (see Text box: ‘Technical limitations in the study of islet-reactive T cells that hamper definition of their pathogenic role and fulfilment of Koch’s postulates’). Conceptually, our understanding remains limited at two levels. First, it is not clear how environmental factors trigger T cell engagement. Most candidates (e.g. enteroviral infections and nutrients disrupting the gut barrier and/or microbiota composition) could exert their effects on both T cells and beta cells. Thus, the relative weight and temporal sequence of these effects would clarify whether T cell engagement represents a primary causative event or a bystander consequence of beta cell dysfunction, and whether such engagement affects disease initiation and/or progression/amplification. We also need to better understand the intermediate steps (e.g. metabolic and inflammatory derangements) connecting environmental triggers with T cell recruitment, and the innate-adaptive immune crosstalk. Second, differences in the frequency and/or phenotype of circulating islet-reactive T cells between diabetic and healthy donors are rather subtle if not absent altogether. We need to better understand the features of this universal state of ‘benign’ autoimmunity, how it is lost and its relationship with age. To this end, a detailed description of T cell modifications along the natural history of disease is required but remains largely unexplored in peripheral blood and even more so in the pancreas. Very few organ donors analysed are positive for multiple autoantibodies (stage 1 disease), and none are representative of stage 2 with initial dysglycaemia. We can speculate on whether insulitis may represent a transient phase in disease progression. Its near absence in stage 1 donors may thus reflect a relatively late autoimmune acceleration during stage 2 that we are presently missing.
Conclusion
Figure 1 summarises the available evidence for or against a primary pathogenic role for T cells in type 1 diabetes. While most of the evidence favours such a role, some does not provide the support often given as granted. Histopathological evidence yields a more conflicting picture. Although biased by some limitations inherent to these studies, they suggest the existence of two age-related endotypes. Clinical evidence of faster beta cell loss and superior benefit of immunotherapeutic intervention in individuals with younger-onset diabetes further support this endotype view. Several technical limitations and gaps in knowledge need to be filled to gauge T cell involvement according to age and disease endotypes. Type 1 diabetes may be a case of one name but two diseases (i.e. younger-onset with primary T cell-driven mechanisms and older-onset with primary beta cell-driven mechanisms) leading to similar clinical presentations but requiring different treatments.
Electronic supplementary material
(PPTX 1.35 mb)
Acknowledgements
The authors would like to thank R. Wyatt (University of Exeter), I. Kusmartseva and M. Padilla (University of Florida) for the preparation and imaging of the pancreas sections.
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Abbreviations
- DiViD
Diabetes Virus Detection Study
- EADB
Exeter Archival Diabetes Biobank
- FOXP3
Forkhead box P3
- HLA-I
HLA Class I
- HLA-II
HLA Class II
- ICI
Insulin-containing islet
- IGRP
Islet-specific glucose-6-phosphatase catalytic-subunit related protein
- MHC-I
MHC Class I
- nPOD
Network for Pancreatic Organ Donors with Diabetes
- TCR
T cell receptor
- T1DE1
Type 1 diabetes endotype 1
- T1DE2
Type 1 diabetes endotype 2
- Treg
Regulatory T cell
Contribution statement
AC, SJR, EL and RM wrote the paper. All authors approved the version to be published.
Funding
RM is funded by grants from The Leona M. and Harry B. Helmsley Charitable Trust (Helmsley no. 1901-03689), JDRF (2-SRA-2016-164-Q-R), the Fondation Francophone pour la Recherche sur le Diabète, the Fondation pour la Recherche Médicale (EQU20193007831) and the Agence Nationale de la Recherche (ANR-19-CE15-0014-01). SJR is funded by grants from Diabetes UK (16/0005480), JDRF (5-CDA-2014-221-A-N; 2-SRA-2018-474-S-B) and the MRC (MR/P010695/1). RM and SJR are funded and by the Innovative Medicines Initiative 2 Joint Undertaking under grant agreements 115797 and 945268 (INNODIA and INNODIA HARVEST). These Joint Undertakings receive support from the Union’s Horizon 2020 research and innovation programme, European Federation of Pharmaceutical Industries Associations, JDRF and The Leona M. and Harry B. Helmsley Charitable Trust. Research in the laboratories of RM and SJR is performed with the support of the Network for Pancreatic Organ donors with Diabetes (nPOD; RRID:SCR_014641), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD: 5-SRA-2018-557-Q-R) and The Leona M. & Harry B. Helmsley Charitable Trust (Grant#2018PG-T1D053). The content and views expressed are the responsibility of the authors and do not necessarily reflect the official view of nPOD. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at http://www.jdrfnpod.org/for-partners/npod-partners/.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sarah J. Richardson, Email: s.richardson@exeter.ac.uk
Roberto Mallone, Email: roberto.mallone@inserm.fr.
References
- 1.Mallone R, Eizirik DL. Presumption of innocence in type 1 diabetes: why are beta cells such vulnerable autoimmune targets? Diabetologia. 2020;63:1999–2006. doi: 10.1007/s00125-020-05176-7. [DOI] [PubMed] [Google Scholar]
- 2.Battaglia M, Ahmed S, Anderson MS, et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care. 2020;43(1):5–12. doi: 10.2337/dc19-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noble JA, Valdes AM, Varney MD, et al. HLA Class I and genetic susceptibility to type 1 diabetes. Diabetes. 2010;59(11):2972–2979. doi: 10.2337/db10-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson CC, Inshaw JRJ, Onengut-Gumuscu S, et al (2020) Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells, genes and drug targets for type 1 diabetes. bioRxiv 158071 (preprint). 10.1101/2020.06.19.158071 [DOI] [PMC free article] [PubMed]
- 5.Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet. 2016;387(10035):2331–2339. doi: 10.1016/S0140-6736(16)30582-7. [DOI] [PubMed] [Google Scholar]
- 6.Valdes AM, Erlich HA, Carlson J, Varney M, Moonsamy PV, Noble JA. Use of class I and class II HLA loci for predicting age at onset of type 1 diabetes in multiple populations. Diabetologia. 2012;55(9):2394–2401. doi: 10.1007/s00125-012-2608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufort MJ, Greenbaum CJ, Speake C, Linsley PS. Cell type-specific immune phenotypes predict loss of insulin secretion in new-onset type 1 diabetes. JCI Insight. 2019;4(4):e125556. doi: 10.1172/jci.insight.125556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209(1):51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson SJ, Rodriguez-Calvo T, Gerling IC, et al. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia. 2016;59(11):2448–2458. doi: 10.1007/s00125-016-4067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.In’t Veld P, Lievens D, De Grijse J, et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 2007;56(9):2400–2404. doi: 10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- 11.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155(2):173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes. 2014;63(11):3880–3890. doi: 10.2337/db14-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arif S, Leete P, Nguyen V, et al. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes. 2014;63(11):3835–3845. doi: 10.2337/db14-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vecchio F, Buono NL, Stabilini A, et al. Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes. JCI Insight. 2018;3(18):e122146. doi: 10.1172/jci.insight.122146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damond N, Engler S, Zanotelli VRT, et al. A map of human type 1 diabetes progression by imaging mass cytometry. Cell Metab. 2019;29(3):755–768. doi: 10.1016/j.cmet.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leete P, Willcox A, Krogvold L, et al. Differential insulitic profiles determine the extent of β-cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65(5):1362–1369. doi: 10.2337/db15-1615. [DOI] [PubMed] [Google Scholar]
- 17.Leete P, Oram RA, McDonald TJ, et al. Studies of insulin and proinsulin in pancreas and serum support the existence of aetiopathological endotypes of type 1 diabetes associated with age at diagnosis. Diabetologia. 2020;63(6):1258–1267. doi: 10.1007/s00125-020-05115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.In’t Veld P. Insulitis in human type 1 diabetes. Islets. 2011;3(4):131–138. doi: 10.4161/isl.3.4.15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Calvo T, Richardson SJ, Pugliese A. Pancreas pathology during the natural history of type 1 diabetes. Curr Diab Rep. 2018;18(11):124. doi: 10.1007/s11892-018-1084-3. [DOI] [PubMed] [Google Scholar]
- 20.Campbell-Thompson ML, Atkinson MA, Butler AE, et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia. 2013;56(11):2541–2543. doi: 10.1007/s00125-013-3043-5. [DOI] [PubMed] [Google Scholar]
- 21.Donath MY, Hess C, Palmer E. What is the role of autoimmunity in type 1 diabetes? A clinical perspective. Diabetologia. 2014;57(4):653–655. doi: 10.1007/s00125-013-3153-0. [DOI] [PubMed] [Google Scholar]
- 22.Campbell-Thompson M, Fu A, Kaddis JS, et al. Insulitis and β-cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719–731. doi: 10.2337/db15-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krogvold L, Wiberg A, Edwin B, et al. Insulitis and characterisation of infiltrating T cells in surgical pancreatic tail resections from patients at onset of type 1 diabetes. Diabetologia. 2016;59(3):492–501. doi: 10.1007/s00125-015-3820-4. [DOI] [PubMed] [Google Scholar]
- 24.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964–1974. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bingley PJ, Boulware DC, Krischer JP, Type 1 Diabetes TrialNet Study Group The implications of autoantibodies to a single islet antigen in relatives with normal glucose tolerance: development of other autoantibodies and progression to type 1 diabetes. Diabetologia. 2016;59(3):542–549. doi: 10.1007/s00125-015-3830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiberg A, Granstam A, Ingvast S, et al. Characterization of human organ donors testing positive for type 1 diabetes-associated autoantibodies. Clin Exp Immunol. 2015;182(3):278–288. doi: 10.1111/cei.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson SJ, Morgan NG, Foulis AK. Pancreatic pathology in type 1 diabetes mellitus. Endocr Pathol. 2014;25(1):80–92. doi: 10.1007/s12022-014-9297-8. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Calvo T, Suwandi JS, Amirian N, et al. Heterogeneity and lobularity of pancreatic pathology in type 1 diabetes during the prediabetic phase. J Histochem Cytochem. 2015;63(8):626–636. doi: 10.1369/0022155415576543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuric E, Seiron P, Krogvold L, et al. Demonstration of tissue resident memory CD8 T cells in insulitic lesions in adult patients with recent-onset type 1 diabetes. Am J Pathol. 2017;187(3):581–588. doi: 10.1016/j.ajpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Radenkovic M, Uvebrant K, Skog O, et al. Characterization of resident lymphocytes in human pancreatic islets. Clin Exp Immunol. 2017;187(3):418–427. doi: 10.1111/cei.12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnuson AM, Thurber GM, Kohler RH, Weissleder R, Mathis D, Benoist C. Population dynamics of islet-infiltrating cells in autoimmune diabetes. Proc Natl Acad Sci U S A. 2015;112(5):1511–1516. doi: 10.1073/pnas.1423769112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christoffersson G, Chodaczek G, Ratliff SS, Coppieters K, von Herrath MG. Suppression of diabetes by accumulation of non-islet-specific CD8+ effector T cells in pancreatic islets. Sci Immunol. 2018;3(21):eaam6533. doi: 10.1126/sciimmunol.aam6533. [DOI] [PubMed] [Google Scholar]
- 33.Bendelac A, Carnaud C, Boitard C, Bach JF. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wicker LS, Leiter EH, Todd JA, et al. β2-Microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43(3):500–504. doi: 10.2337/diab.43.3.500. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton-Williams EE, Palmer SE, Charlton B, Slattery RM. Beta cell MHC class I is a late requirement for diabetes. Proc Natl Acad Sci U S A. 2003;100(11):6688–6693. doi: 10.1073/pnas.1131954100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pauza ME, Dobbs CM, He J, et al. T-cell receptor transgenic response to an endogenous polymorphic autoantigen determines susceptibility to diabetes. Diabetes. 2004;53(4):978–988. doi: 10.2337/diabetes.53.4.978. [DOI] [PubMed] [Google Scholar]
- 37.Delong T, Wiles TA, Baker RL, et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351(6274):711–714. doi: 10.1126/science.aad2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186(10):1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurrer MO, Pakala SV, Hanson HL, Katz JD. Beta cell apoptosis in T cell-mediated autoimmune diabetes. Proc Natl Acad Sci U S A. 1997;94(1):213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong FS, Khai Siew L, Scott G, et al. Activation of insulin-reactive CD8 T-cells for development of autoimmune diabetes. Diabetes. 2009;58(5):1156–1164. doi: 10.2337/db08-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hull CM, Peakman M, Tree TIM. Regulatory T cell dysfunction in type 1 diabetes: what’s broken and how can we fix it? Diabetologia. 2017;60(10):1839–1850. doi: 10.1007/s00125-017-4377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TIM. Defective suppressor function in CD4+CD25+ T-cells from patients with type 1 diabetes. Diabetes. 2005;54(1):92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 43.McClymont SA, Putnam AL, Lee MR, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186(7):3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+FOXP3+ regulatory T cells. J Immunol. 2008;181(10):7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arif S, Tree TI, Astill TP, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113(3):451–463. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivers TM, Sprunt DH, Berry GP. Observations on attempts to produce acute disseminated encephalomyelitis in monkeys. J Exp Med. 1933;58(1):39–53. doi: 10.1084/jem.58.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rose NR, Witebsky E. Studies on organ specificity. V. Changes in the thyroid glands of rabbits following active immunization with rabbit thyroid extracts. J Immunol. 1956;76(6):417–427. [PubMed] [Google Scholar]
- 48.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosszufalusi N, Chan E, Teruya M, Takei S, Granger G, Charles MA. Quantitative phenotypic and functional analyses of islet immune cells before and after diabetes onset in the BB rat. Diabetologia. 1993;36(11):1146–1154. doi: 10.1007/BF00401059. [DOI] [PubMed] [Google Scholar]
- 50.Mordes JP, Bortell R, Blankenhorn EP, Rossini AA, Greiner DL. Rat models of type 1 diabetes: Genetics, environment, and autoimmunity. ILAR J. 2004;45(3):278–291. doi: 10.1093/ilar.45.3.278. [DOI] [PubMed] [Google Scholar]
- 51.Skowera A, Ellis RJ, Varela-Calviño R, et al. CTLs are targeted to kill β cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118(10):3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Unger WWJ, Pearson T, Abreu JRF, et al. Islet-specific CTL cloned from a type 1 diabetes patient cause beta-cell destruction after engraftment into HLA-A2 transgenic NOD/SCID/IL2RG null mice. PLoS One. 2012;7(11):e49213. doi: 10.1371/journal.pone.0049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Culina S, Lalanne AI, Afonso G, et al. Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci Immunol. 2018;3(20):eaao4013. doi: 10.1126/sciimmunol.aao4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gonzalez-Duque S, Azoury ME, Colli ML, et al. Conventional and neo-antigenic peptides presented by β cells are targeted by circulating naïve CD8+ T cells in type 1 diabetic and healthy donors. Cell Metab. 2018;28(6):946–960. doi: 10.1016/j.cmet.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Azoury ME, Tarayrah M, Afonso G, et al (2020) Peptides Derived From Insulin Granule Proteins are Targeted by CD8+ T Cells Across MHC Class I Restrictions in Humans and NOD Mice. Diabetes 69(9). 10.2337/db20-0013 [DOI] [PubMed]
- 56.Skowera A, Ladell K, McLaren JE, et al. β-Cell-specific CD8 T cell phenotype in type 1 diabetes reflects chronic autoantigen exposure. Diabetes. 2015;64(3):916–925. doi: 10.2337/db14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiedeman AE, Muir VS, Rosasco MG, et al. Autoreactive CD8+ T cell exhaustion distinguishes subjects with slow type 1 diabetes progression. J Clin Invest. 2019;130(1):480–490. doi: 10.1172/JCI126595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeo L, Pujol-Autonell I, Baptista R, et al. Circulating β cell-specific CD8+ T cells restricted by high-risk HLA class I molecules show antigen experience in children with and at risk of type 1 diabetes. Clin Exp Immunol. 2020;199(3):263–277. doi: 10.1111/cei.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mallone R, Martinuzzi E, Blancou P, et al. CD8+ T-cell responses identify β-cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56(3):613–621. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- 60.Scotto M, Afonso G, Larger E, et al. Zinc transporter (ZnT)8186-194 is an immunodominant CD8+ T cell epitope in HLA-A2+ type 1 diabetic patients. Diabetologia. 2012;55(7):2026–2031. doi: 10.1007/s00125-012-2543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKinney EF, Lee JC, Jayne DRW, Lyons PA, Smith KGC. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature. 2015;523(7562):612–616. doi: 10.1038/nature14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson PJ, Shah A, Ntranos V, Van Gool F, Atkinson M, Bhushan A (2019) Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metab 29(5):1045–1060.e10. 10.1016/j.cmet.2019.01.021 [DOI] [PubMed]
- 63.Lee H, Lee Y-S, Harenda Q, et al (2020) Beta cell dedifferentiation induced by IRE1α deletion prevents type 1 diabetes. Cell Metab 31(4):822–836.e5. 10.1016/j.cmet.2020.03.002 [DOI] [PMC free article] [PubMed]
- 64.Tan S, Li Y, Xia J, et al. Type 1 diabetes induction in humanized mice. Proc Natl Acad Sci U S A. 2017;114(41):10954–10959. doi: 10.1073/pnas.1710415114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sutherland DE, Goetz FC, Sibley RK. Recurrence of disease in pancreas transplants. Diabetes. 1989;38(S1):85–87. doi: 10.2337/diab.38.1.s85. [DOI] [PubMed] [Google Scholar]
- 66.Lampeter EF, Homberg M, Quabeck K, et al. Transfer of insulin-dependent diabetes between HLA-identical siblings by bone marrow transplantation. Lancet. 1993;341(8855):1243–1244. doi: 10.1016/0140-6736(93)91148-f. [DOI] [PubMed] [Google Scholar]
- 67.Rigby MR, Harris KM, Pinckney A, et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest. 2016;125(8):3285–3296. doi: 10.1172/JCI81722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orban T, Bundy B, Becker DJ, et al. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37(4):1069–1075. doi: 10.2337/dc13-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keymeulen B, Walter M, Mathieu C, et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia. 2010;53(4):614–623. doi: 10.1007/s00125-009-1644-9. [DOI] [PubMed] [Google Scholar]
- 71.Aronson R, Gottlieb PA, Christiansen JS, et al. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase iii study in recent-onset human type 1 diabetes. Diabetes Care. 2014;37(10):2746–2754. doi: 10.2337/dc13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sherry N, Hagopian W, Ludvigsson J, et al. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378(9790):487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herold KC, Bundy BN, Long SA, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381(7):603–613. doi: 10.1056/NEJMoa1902226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cree BAC, Mares J, Hartung H-P. Current therapeutic landscape in multiple sclerosis: an evolving treatment paradigm. Curr Opin Neurol. 2019;32(3):365–377. doi: 10.1097/WCO.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 75.Giacomelli R, Afeltra A, Alunno A, et al. International consensus: what else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren’s syndrome)? The unmet needs and the clinical grey zone in autoimmune disease management. Autoimmun Rev. 2017;16(9):911–924. doi: 10.1016/j.autrev.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 76.Greenbaum CJ, Beam CA, Boulware D, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite type 1 diabetes TrialNet data. Diabetes. 2012;61(8):2066–2073. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual β-cell function. Diabetes. 2011;60(5):1599–1607. doi: 10.2337/db10-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kusmartseva I, Beery M, Hiller H, et al. Temporal analysis of amylase expression in control, autoantibody-positive, and type 1 diabetes pancreatic tissues. Diabetes. 2020;69(1):60–66. doi: 10.2337/db19-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PPTX 1.35 mb)