Abstract
BACKGROUND
Previous studies showed a higher incidence of lung cancer among young women than among young men in the United States. Whether this pattern has continued in contemporary birth cohorts and, if so, whether it can be fully explained by sex differences in smoking behaviors are unknown.
METHODS
We examined the nationwide population-based incidence of lung cancer according to sex, race or ethnic group, age group (30 to 34, 35 to 39, 40 to 44, 45 to 49, and 50 to 54 years), year of birth (1945 to 1980), and calendar period of diagnosis (1995–1999, 2000–2004, 2005–2009, and 2010–2014), and we calculated female-to-male incidence rate ratios. We also examined the prevalence of cigarette smoking, using data from the National Health Interview Survey from 1970 to 2016.
RESULTS
Over the past two decades, the age-specific incidence of lung cancer has generally decreased among both men and women 30 to 54 years of age in all races and ethnic groups, but the declines among men have been steeper. Consequently, among non-Hispanic whites, the female-to-male incidence rate ratios increased, exceeding 1.0 in the age groups of 30 to 34, 35 to 39, 40 to 44, and 45 to 49 years. For example, the female-to-male incidence rate ratio among whites 40 to 44 years of age increased from 0.88 (95% confidence interval [CI], 0.84 to 0.92) during the 1995–1999 period to 1.17 (95% CI, 1.11 to 1.23) during the 2010–2014 period. The crossover in sex-specific rates occurred among non-Hispanic whites born since 1965. Sex-specific incidence rates converged among non-Hispanic blacks, Hispanics, and non-Hispanic Asians and Pacific Islanders but crossed over from a higher incidence among men to a higher incidence among women only among Hispanics. The prevalence of cigarette smoking among women born since 1965 has approached, but generally not exceeded, the prevalence among men.
CONCLUSIONS
The patterns of historically higher incidence rates of lung cancer among men than among women have reversed among non-Hispanic whites and Hispanics born since the mid-1960s, and they are not fully explained by sex differences in smoking behaviors. Future studies are needed to identify reasons for the higher incidence of lung cancer among young women. (Funded by the American Cancer Society.)
LUNG CANCER CAUSES MORE PREVENTABLE deaths than any other cancer in the United States, and cigarette smoking contributes to about 80% of the 154,000 total deaths from lung cancer that occur each year.1,2 The age-standardized incidence and mortality rates associated with lung cancer continue to be lower among women than among men, because historically women were less likely to smoke, initiated smoking at older ages, and smoked fewer cigarettes per day.3,4 However, smoking behaviors have become increasingly similar between men and women in contemporary cohorts in the United States.3,4 Our previous report of a convergence of incidence rates of lung cancer among young men and women was consistent with this pattern.5 Subsequently, two studies showed higher incidence rates of lung cancer among young women than among young men,6,7 but they did not examine the extent to which the higher incidence among women could be explained by sex differences in smoking behaviors. We examined up-to-date data on the incidence of lung cancer and the prevalence of cigarette smoking in the United States according to sex and race or ethnic group to concurrently assess whether the incidence of lung cancer in contemporary cohorts is higher among women than among men and, if so, whether this pattern can be fully explained by sex differences in smoking behaviors.
METHODS
DATA SOURCE AND STUDY DESIGN
We analyzed data from the North American Association of Central Cancer Registries (NAACCR)8 on cases of invasive lung cancer (including lung and bronchus cancer) diagnosed from 1995 through 2014 in 46 states and the District of Columbia. We used data that met standards for completeness, accuracy, and timeliness of collection as stipulated by the NAACCR9; these data covered 96% of the U.S. population. (Kansas, Maryland, and Vermont did not consent to participate, and data from Minnesota did not meet the NAACCR data standards.) Complete data for all study years were available for 25 states, which covered 67% of the U.S. population. Race or ethnic group in the NAACCR database is categorized as non-Hispanic white (300,343 cases of lung cancer), non-Hispanic black (62,427 cases), non-Hispanic Asian or Pacific Islander (9920 cases), and Hispanic (19,328 cases).10 Hereafter we refer to the non-Hispanic groups as whites, blacks, and Asians or Pacific Islanders. The age at diagnosis was grouped according to 5-year age group (30 to 34, 35 to 39, 40 to 44, 45 to 49, and 50 to 54 years), and the year of diagnosis was grouped according to 5-year calendar period (1995 to 1999, 2000 to 2004, 2005 to 2009, and 2010 to 2014). Histologically confirmed cancer cases among whites were categorized in six main histologic types according to morphology codes in the International Classification of Diseases for Oncology, third edition, as adenocarcinoma, squamous-cell carcinoma, small-cell carcinoma, large-cell carcinoma, other specified carcinoma, and unspecified carcinomas (including non-small-cell carcinoma) (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org).7,11 The numbers of lung cancer cases in other races and ethnic groups were too small to allow the examination of trends according to histologic type.
STATISTICAL ANALYSIS
We calculated the age-specific incidence of lung cancer per 100,000 person-years according to sex, race or ethnic group, year of diagnosis, and histologic type using SEER*Stat software, version 8.3.4. We then calculated the female-to-male incidence rate ratios with 95% confidence intervals for each category of age, race or ethnic group, year of diagnosis, and histologic type with the Tiwari method; two-sided P values of less than 0.05 were considered to indicate statistical significance.12 We calculated the year of birth by subtracting the mid-year of age (the age halfway between the youngest and oldest age in each age category listed above) from the mid-year of diagnosis (the year halfway between the first and last year in each diagnosis calendar period listed above), which yielded eight birth cohorts corresponding to the mid-year of birth (1945, 1950, 1955, 1960, 1965, 1970, 1975, and 1980). We performed a sensitivity analysis that included only the 25 states that had data available for all study years (1995 through 2014) to assess whether the results were affected by the inclusion of registries with missing data for any year or years. In a supplementary analysis, we also examined the death rates from lung cancer in the contemporary birth cohort according to sex and race or ethnic group, using national mortality data from 1995 through 2014.13
We used data from the National Health Interview Survey (NHIS) from 1970 through 2016 to calculate the prevalence of current smoking and of current or former smoking (≥100 cigarettes smoked in a lifetime), as well as the average number of cigarettes smoked per day, as reported by the respondents, according to sex, age, race or ethnic group, year of survey, and birth cohort,14 on the basis of methods developed by Holford et al.4 In brief, the prevalence of current smoking was estimated as the product of the prevalence of current or former smoking and the cumulative probability of not quitting, which were obtained by means of age-period-cohort modeling of the probabilities of smoking initiation and cessation (defined as not having smoked for ≥2 years), with adjustment for differential mortality. Age-period-cohort modeling is a quantitative technique used to simultaneously examine the effects of age, calendar period, and year of birth. Similarly, the mean number of cigarettes smoked per day was estimated with the use of cumulative logistic regression on the basis of ordered categories of smoking intensity (<5, 5 to <15, 15 to <25, 25 to <35, 35 to <45, and ≥45 cigarettes per day), with the mid-number (3, 10, 20, 30, 40, and 60 cigarettes) of each category used in the calculation of means.
We also calculated the female-to-male ratios of the prevalence of current smoking and the prevalence of current or former smoking with 95% confidence intervals to assess whether the smoking prevalence differed significantly between men and women. We began our analysis with the 1970 NHIS because questions about smoking intensity were added that year. The collection of information in the NHIS according to Hispanic ethnic group began with the 1978 survey. We assumed that information on smoking behavior obtained from whites and blacks in the 1970 and 1974 surveys reflected that of non-Hispanic whites and non-Hispanic blacks because Hispanics represented less than 5% of the U.S. population before 1980.15 We could not calculate smoking prevalence among Asians and Pacific Islanders because of sparse data. Statistical analyses of smoking prevalence were conducted with SAS software, version 9.4, and the bootstrap method was used to calculate 95% confidence intervals, with 1000 replications. For comparability of the data within and across figures, we used log scales to plot patterns — according to birth cohort and calendar period — in lung cancer incidence, smoking prevalence, and incidence and prevalence ratios.16
RESULTS
INCIDENCE OF LUNG CANCER
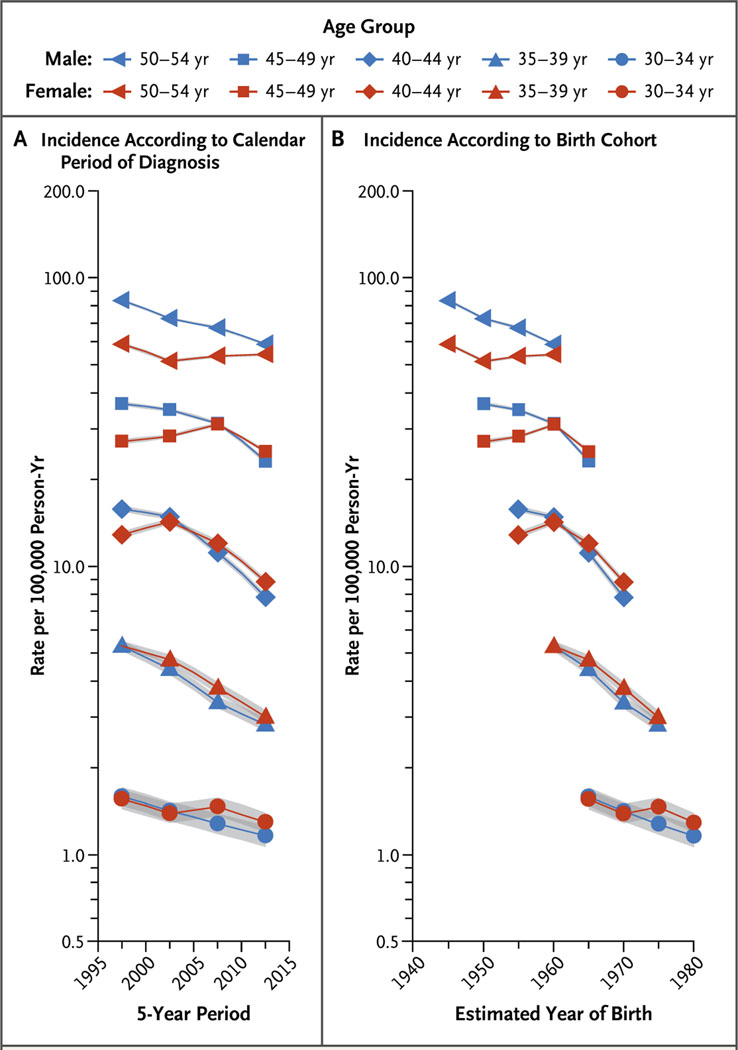
Figure 1A shows trends in the age-specific incidence of lung cancer (per 100,000 person-years) among men and women 30 to 54 years of age, including all races and ethnic groups, according to calendar period of diagnosis, from 1995 through 2014. Incidence generally decreased among both men and women, but the declines were greater among men. Consequently, the female-to-male incidence rate ratios increased, exceeding 1.0 in the age groups of 30 to 34, 35 to 39, 40 to 44, and 45 to 49 years. For example, the incidence rate ratio among persons 40 to 44 years of age increased from 0.82 (95% confidence interval [CI], 0.79 to 0.85) during the 1995–1999 period to 1.13 (95% CI, 1.08 to 1.18) during the 2010–2014 period.
Figure 1. Age-Specific Incidence Rates of Lung Cancer in All Races and Ethnic Groups According to Sex, Calendar Period of Diagnosis, and Birth Cohort.
Shading indicates 95% confidence intervals. The estimated year of birth was calculated by subtracting the mid-year of age (the age halfway between the youngest and oldest age in each age category) from the mid-year of diagnosis (the year halfway between the first and last year in each 5-year calendar period of diagnosis).
Figure 1B shows the age-specific incidence rates among men and women in all races and ethnic groups according to birth cohort. Among men, incidence rates generally decreased in successive birth cohorts, whereas among women, rates increased in the cohorts born around 1950 to around 1960 and decreased thereafter. As a result, incidence rates among women surpassed those among men in contemporary cohorts. For example, among persons 45 to 49 years of age, the incidence was 27.0 per 100,000 person-years among women and 36.5 per 100,000 person-years among men in the cohort born around 1950 (female-to-male incidence rate ratio, 0.74; 95% CI, 0.72 to 0.76), whereas the incidence was 24.9 per 100,000 person-years among women and 23.1 per 100,000 person-years among men in the cohort born around 1965 (incidence rate ratio, 1.08; 95% CI, 1.05 to 1.11).
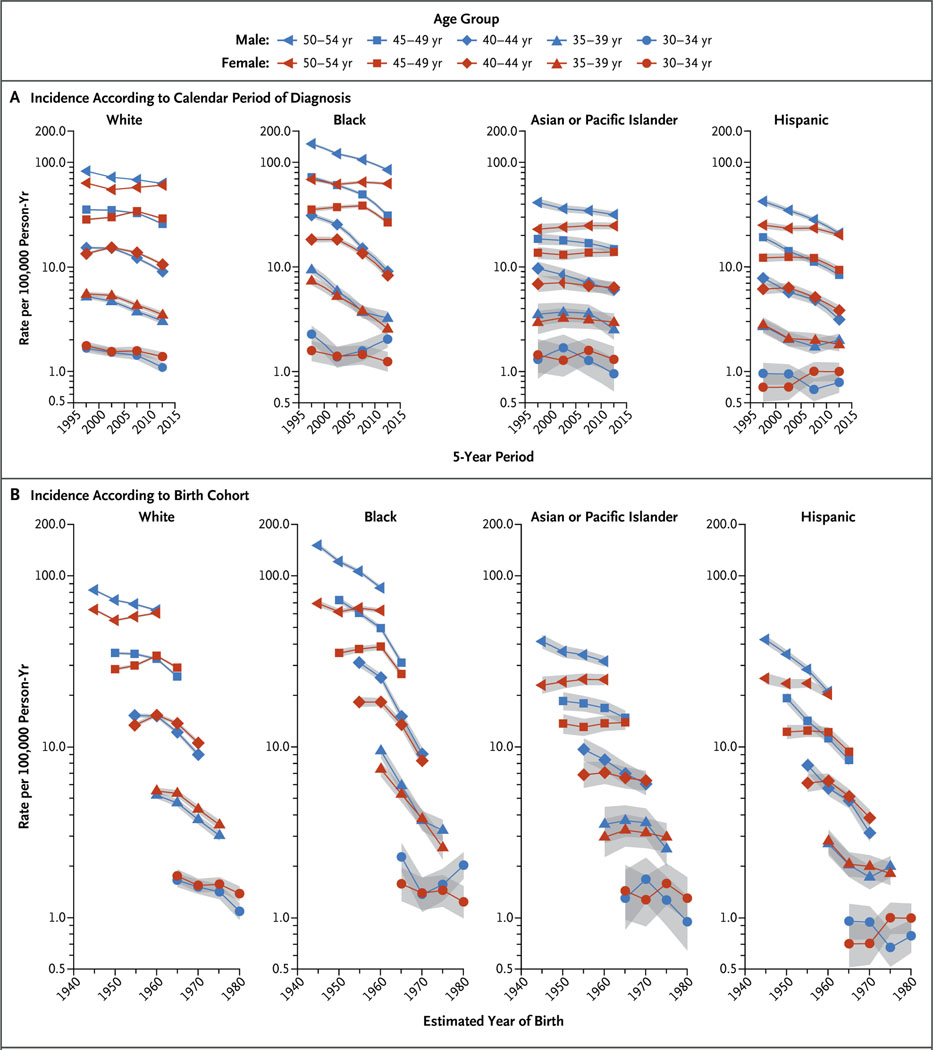
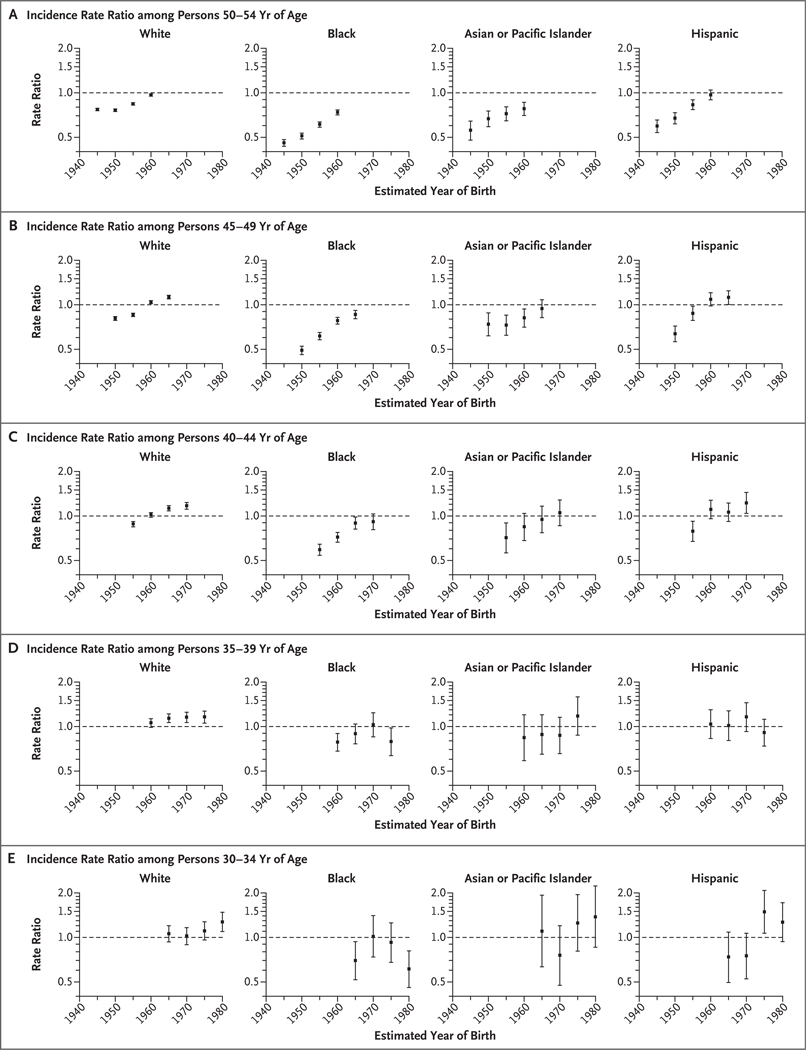
Figure 2 shows trends in age-specific incidence according to calendar period of diagnosis and birth cohort in four major races and ethnic groups, and Figure 3 shows the corresponding female-to-male incidence rate ratios according to birth cohort. The higher incidence among women is confined to whites and Hispanics. For example, among persons in the age group of 40 to 44 years, the incidence rate ratio increased from 0.88 (95% CI, 0.84 to 0.92) during the 1995–1999 period to 1.17 (95% CI, 1.11 to 1.23) during the 2010–2014 period among whites and from 0.79 (95% CI, 0.67 to 0.92) to 1.22 (95% CI, 1.04 to 1.44) among Hispanics. Similarly, among persons in the age group of 45 to 49 years, the incidence rate ratio increased among whites from 0.81 (95% CI, 0.78 to 0.83) in the cohort born around 1950 to 1.13 (95% CI, 1.09 to 1.16) in the cohort born around 1965, and among Hispanics from 0.64 (95% CI, 0.56 to 0.72) in the cohort born around 1950 to 1.12 (95% CI, 1.00 to 1.25) in the cohort born around 1965. Incidence rates for men and women converged in some age groups among blacks and Asians or Pacific Islanders but did not cross over to higher rates among women (Fig. 3). The overall incidence patterns according to sex, race or ethnic group, and birth cohort were generally similar when we limited the analysis to the 25 states with available data for all study years (1995 through 2014). Death rates from lung cancer among young white and Hispanic women approached or equaled those among men born since the mid-1960s but did not cross over.
Figure 2. Age-Specific Incidence Rates of Lung Cancer According to Race or Ethnic Group, Sex, and Calendar Period of Diagnosis and Birth Cohort.
Shading indicates 95% confidence intervals.
Figure 3 (facing page). Age-Specific Female-to-Male Incidence Rate Ratios for Lung Cancer According to Race or Ethnic Group and Birth Cohort.
Men served as the reference group, and incidence rate ratios are based on unrounded rates. I bars indicate 95% confidence intervals.
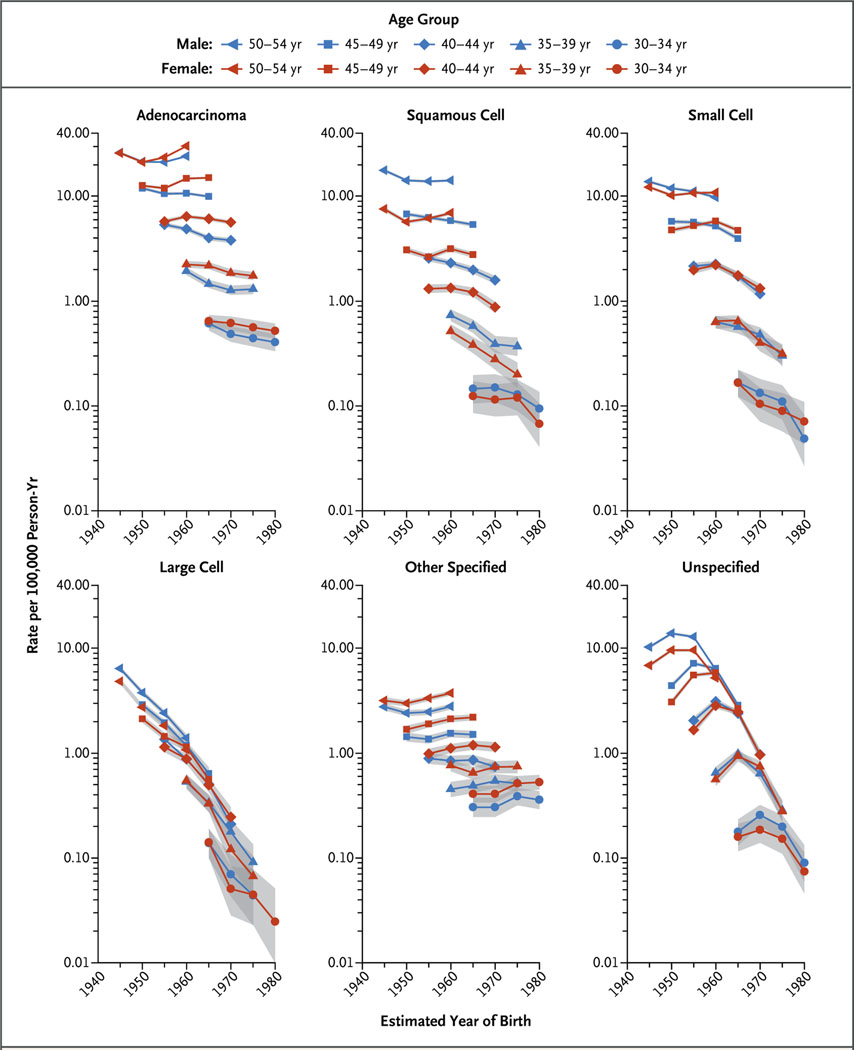
Figure 4 shows the age-specific incidence of lung cancer among whites according to histologic type of the cancer and birth cohort. The incidence trends for adenocarcinoma, squamous-cell carcinoma, and small-cell carcinoma generally followed the trends for all histologic types combined; rates among men continued to decrease in successively younger generations, whereas rates among women continued to increase in each birth cohort through the group born around 1960 and declined thereafter. Trends for the other histologic categories varied, with rates of large-cell carcinoma sharply decreasing and rates of other specified carcinomas increasing in successively younger generations. The female-to-male incidence rate ratios according to histologic types are shown in Figure S4 in the Supplementary Appendix. Most of the higher incidence among white women was confined to adenocarcinoma, whereas the incidence of squamous-cell carcinoma remained lower among women than among men. Actual values and more information are provided in the Supplementary Appendix.
Figure 4. Age-Specific Incidence Rates of Lung Cancer According to Histologic Type, Sex, and Birth Cohort among Non-Hispanic Whites.
The incidence rate for large-cell lung carcinoma among men 30 to 34 years of age born around 1980 could not be calculated because of sparse data (fewer than six cases). Shading indicates 95% confidence intervals.
PREVALENCE OF SMOKING
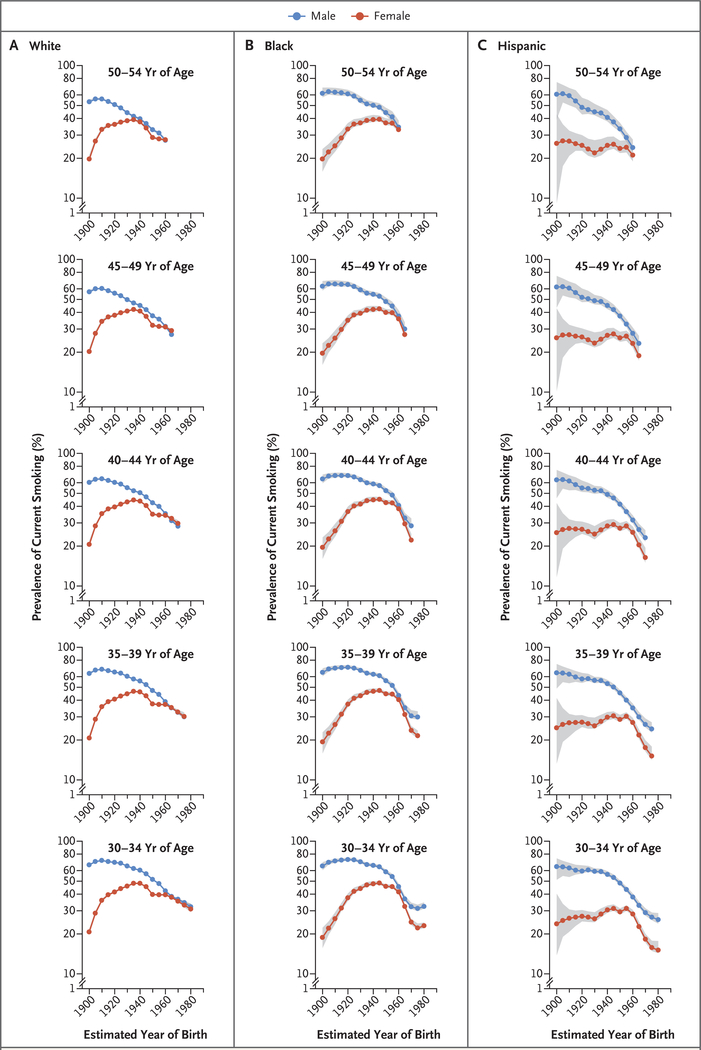
Figure 5 shows the prevalence of current smoking according to age, sex, race or ethnic group, and birth cohort. Historically, smoking prevalence was considerably higher among men than among women in all races and ethnic groups. The difference in prevalence became progressively smaller in successively younger birth cohorts because of the convergence of female and male initiation rates (prevalence among persons 25 to 29 years of age was used as a proxy) and lower cessation rates among women. Among whites, sex differences disappeared among persons born during the 1960s and afterward, and prevalence was minimally higher among women 40 to 49 years of age born around 1965. Smoking behaviors were generally similar among blacks and Hispanics, with the female-to-male ratio of smoking prevalence peaking among persons born during the 1960s and declining thereafter (Fig. 5). Similar sex differences occurred in the prevalence of smoking among persons who had ever smoked (≥100 cigarettes smoked in a lifetime).
Figure 5. Prevalence of Current Smoking According to Race or Ethnic Group, Age, Sex, and Birth Cohort.
Shading indicates 95% confidence intervals.
Figure S8 in the Supplementary Appendix shows the average number of cigarettes smoked per day among white and black men and women according to birth cohort. Daily cigarette use decreased among both men and women in successive generations born since 1930, but the difference between the sexes narrowed only among blacks, whereas among whites, daily cigarette use has remained substantially higher among men than among women, even in recent cohorts.
DISCUSSION
Among persons born since the mid-1960s, incidence rates of lung cancer have become significantly higher among young women than among young men, with the higher burden confined to whites and Hispanics. Except for a minimally higher smoking prevalence among white women than men 40 to 49 years of age born around the mid-1960s (due to delayed smoking cessation among women), sex differences in smoking behaviors do not explain this finding. The prevalence of smoking among white women born after the 1970s and among Hispanics born after the 1960s approached, but did not exceed, that among their male counterparts. Moreover, the average number of cigarettes smoked per day continues to be considerably lower among women than among men. Women are more likely than men to smoke menthol cigarettes,17 which are not associated with a higher risk of lung cancer than nonmenthol cigarettes.18 Furthermore, the use of tobacco products other than cigarettes, such as cigars and smokeless tobacco (e.g., chewing tobacco), has been much lower among women than among men.19 The crossover from a higher incidence of lung cancer among young men to a higher incidence among young women is especially remarkable among Hispanics, given that among young adults, smoking prevalence is substantially lower among Hispanic women than among Hispanic men.
In concert with the diminishing sex differences in smoking prevalence, it is possible that differences in the distribution of histologic subtypes, coupled with the differences in the speed of risk reduction associated with these subtypes after smoking cessation, may have contributed to the higher incidence of lung cancer among women than among men. The risk of adenocarcinoma, a lung cancer subtype that is more common among women than among men,7 decreases more slowly than the risk of other types of lung cancer after smoking cessation.20,21 The annual risk reduction after smoking cessation has been reported to be 8% for adenocarcinoma versus 17% for small-cell carcinoma.20
The controversial hypothesis that women may be more susceptible to the deleterious effects of tobacco carcinogens has been studied since the early 1990s, but the results have been mixed.22 Prospective studies22–24 have not replicated the results of several case-control studies25,26 that showed a higher risk of lung cancer among women than among men at comparable levels of exposure to cigarette smoking. However, all these studies involved persons 50 years of age or older who were born before the middle of the last century. The risk of lung cancer in this group may not be representative of the risks among smokers born later, because the design and composition of cigarettes continued to change through the 1990s, and the risk of lung cancer associated with smoking increased with more modern cigarettes.27 There has been some limited biologic and genetic evidence to support a higher susceptibility among women, including a higher frequency of mutations in critical driver genes, such as TP53 and the KRAS oncogene.28,29
Factors other than active tobacco use account for about 15% of cases of lung cancer in women and 10% in men. Occupational exposure to lung carcinogens, such as asbestos and arsenic, which have synergistic effects with smoking and were historically more common among men than among women, have decreased dramatically over the past several decades.30,31 This may have contributed to the steeper decline in lung cancer among men. Although exposure to secondhand smoke has also decreased substantially over the past several decades, the decline has not been shown to differ significantly between men and women.32 Similarly, changes in exposure to outdoor air pollution are not expected to differ according to sex. In contrast, results of pooled, large cohort studies have shown that the incidence of lung cancer among nonsmokers appears to be slightly higher among women than among men younger than 70 years of age.33,34 It will be difficult, however, to estimate how much of the excess risk of lung cancer among women is due to sex differences in temporal changes in exposure to nontobacco causes and in background rates.
More frequent detection of indolent lung tumors in women than in men through screening or diagnostic imaging is an alternative explanation for the higher rates of lung cancer among women. A study of baseline computed tomographic (CT) screening for lung cancer in North America showed that the prevalence of lung cancer among women was nearly twice as high as that among men of similar age and with similar smoking history, which raises the possibility that lung cancer may progress more slowly in women.35 Although the higher incidence rates of lung cancer among women 30 to 49 years of age largely involved early-stage disease (Table S5) in the Supplementary Appendix, neither men nor women in this age range typically undergo CT screening, nor did the percentage of cases diagnosed at a localized stage increase consistently among young women or young men during the study period, as would be expected from overdiagnosis.
A strength of our study is the use of nation-wide, high-quality population-based data on both lung cancer incidence and smoking prevalence. However, our study has several limitations. First, recent advances in the molecular characterization of lung cancer and the use of targeted therapies have led to improvements in histologic classifications, with unknown histologic types increasingly classified as adenocarcinoma or squamous-cell carcinoma.36 However, this would not affect overall lung cancer incidence rates or trends. Second, individual-level information on smoking behavior and other known risk factors for lung cancer except age are not routinely captured in medical records or in cancer registries, so they cannot be used to directly measure the contribution of these factors to the emerging higher risk among young women. Third, the data from the NHIS that we used to compare cigarette-smoking habits according to sex were reported by the respondents and may have been influenced by sex differences in social desirability bias. According to data from the National Health and Nutrition Examination Survey, however, no discrepancies were noted between biochemically assessed and respondent-reported smoking prevalence overall or according to sex.37,38 Unlike cancer registries, the NHIS excludes institutionalized persons, and it excluded military personnel until 1997. Because these cohorts disproportionately represent male smokers,39,40 their exclusions might have attenuated sex differences in smoking prevalence. Fourth, although we were unable to examine data on Hispanics according to country of birth, the influx of Hispanic immigrants to the United States is unlikely to explain the notably higher incidence of lung cancer among Hispanic women because smoking prevalence among foreign-born Hispanic women is considerably lower than that among Hispanic men. Finally, because of sparse data for certain groups, we used modeled data for cigarette-smoking prevalence — which introduce uncertainties both from survey samples and from the model — rather than observed prevalence. The model overestimated the prevalence among persons 30 to 39 years of age similarly according to sex and thus is unlikely to affect the interpretation of our main findings.
In conclusion, the historical patterns of higher incidence rates of lung cancer among men than among women have reversed among non-Hispanic whites and Hispanics born since the mid-1960s and are not fully explained by sex differences in smoking behaviors. This finding has important implications for public health. It may foreshadow a higher future burden of overall lung cancer among women than among men as younger cohorts age, which further underscores the need to intensify antitobacco measures to decrease smoking among young women. Our finding also calls for continued monitoring of sex-specific risks of lung cancer and for etiologic studies, including studies of sex differences in smoking-related susceptibility to lung cancer, to identify reasons for the higher rates of lung cancer among young women.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the American Cancer Society.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Ahmedin Jemal, Surveillance and Health Services Research, American Cancer Society, Atlanta
Kimberly D. Miller, Surveillance and Health Services Research, American Cancer Society, Atlanta
Jiemin Ma, Surveillance and Health Services Research, American Cancer Society, Atlanta
Rebecca L. Siegel, Surveillance and Health Services Research, American Cancer Society, Atlanta
Stacey A. Fedewa, Surveillance and Health Services Research, American Cancer Society, Atlanta
Farhad Islami, Surveillance and Health Services Research, American Cancer Society, Atlanta
Susan S. Devesa, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD
Michael J. Thun, Surveillance and Health Services Research, American Cancer Society, Atlanta
references
- 1.Siegel RL, Jacobs EJ, Newton CC, et al. Deaths due to cigarette smoking for 12 smoking-related cancers in the United States. JAMA Intern Med 2015;175:1574–6. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68: 7–30. [DOI] [PubMed] [Google Scholar]
- 3.Anderson CM, Burns DM, Dodd KW, Feuer EJ. Chapter 2: Birth-cohort-specific estimates of smoking behaviors for the U.S. population. Risk Anal 2012;32:Suppl 1:S14–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort-specific smoking histories, 1965–2009. Am J Prev Med 2014; 46(2):e31–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jemal A, Travis WD, Tarone RE, Travis L, Devesa SS. Lung cancer rates convergence in young men and women in the United States: analysis by birth cohort and histologic type. Int J Cancer 2003;105:101–7. [DOI] [PubMed] [Google Scholar]
- 6.Houston KA, Henley SJ, Li J, White MC, Richards TB. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004–2009. Lung Cancer 2014;86:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis DR, Check DP, Caporaso NE, Travis WD, Devesa SS. US lung cancer trends by histologic type. Cancer 2014; 120:2883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SEER*Stat database: CiNA public use data set (https://www.naaccr.org/cina-public-use-data-set/).
- 9.North American Association of Central Cancer Registries . Certification criteria for meeting NAACCR’s data standard for cancer registries (https://www.naaccr.org/certification-criteria/).
- 10.Clegg LX, Reichman ME, Hankey BF, et al. Quality of race, Hispanic ethnicity, and immigrant status in population- based cancer registry data: implications for health disparity studies. Cancer Causes Control 2007;18:177–87. [DOI] [PubMed] [Google Scholar]
- 11.Fritz A, Percy C, Jack A, et al. , eds. International classification of diseases for oncology 3rd ed ICD-O. Geneva: World Health Organization, 2000. [Google Scholar]
- 12.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res 2006;15: 547–69. [DOI] [PubMed] [Google Scholar]
- 13.SEER*Stat database: U.S. mortality data (https://seer.cancer.gov/seerstat/).
- 14.Centers for Disease Control and Prevention. National Health Interview Survey. 2017. (https://www.cdc.gov/nchs/nhis/tobacco/tobacco_changes.htm).
- 15.Pew Research Center. Facts on U.S. Latinos, 2015: statistical portrait of Hispanics in the United States. September 18, 2017. (http://www.pewhispanic.org/2017/09/18/facts-on-u-s-latinos/#growth-sources).
- 16.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol 1995;141:300–4. [DOI] [PubMed] [Google Scholar]
- 17.Giovino GA, Villanti AC, Mowery PD, et al. Differential trends in cigarette smoking in the USA: is menthol slowing progress? Tob Control 2015;24:28–37. [DOI] [PubMed] [Google Scholar]
- 18.Blot WJ, Cohen SS, Aldrich M, McLaughlin JK, Hargreaves MK, Signorello LB. Lung cancer risk among smokers of menthol cigarettes. J Natl Cancer Inst 2011;103:810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson DE, Mowery P, Tomar S, Marcus S, Giovino G, Zhao L. Trends in smokeless tobacco use among adults and adolescents in the United States. Am J Public Health 2006;96:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenfield SA, Wei EK, Stampfer MJ, Rosner BA, Colditz GA. Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control 2008;17:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khuder SA, Mutgi AB. Effect of smoking cessation on major histologic types of lung cancer. Chest 2001;120:1577–83. [DOI] [PubMed] [Google Scholar]
- 22.Thun MJ, Henley SJ, Calle EE. Tobacco use and cancer: an epidemiologic perspective for geneticists. Oncogene 2002; 21:7307–25. [DOI] [PubMed] [Google Scholar]
- 23.Bain C, Feskanich D, Speizer FE, et al. Lung cancer rates in men and women with comparable histories of smoking. J Natl Cancer Inst 2004;96:826–34. [DOI] [PubMed] [Google Scholar]
- 24.Freedman ND, Leitzmann MF, Hollenbeck AR, Schatzkin A, Abnet CC. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol 2008;9:649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Risch HA, Howe GR, Jain M, Burch JD, Holowaty EJ, Miller AB. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol 1993;138:281–93. [DOI] [PubMed] [Google Scholar]
- 26.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 1996;88:183–92. [DOI] [PubMed] [Google Scholar]
- 27.Office of the Surgeon General. The health consequences of smoking — 50 years of progress: a report of the Surgeon General. Rockville, MD: Department of Health and Human Services, January 2014. [Google Scholar]
- 28.Barrera-Rodriguez R, Morales-Fuentes J. Lung cancer in women. Lung Cancer (Auckl) 2012;3:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollerup S, Berge G, Baera R, et al. Sex differences in risk of lung cancer: expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer 2006; 119:741–4. [DOI] [PubMed] [Google Scholar]
- 30.Concha-Barrientos M, Nelson DI, Driscoll T, et al. Selected occupational risk factors In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, eds. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Vol. 1 Geneva: World Health Organization, 2004:1651–801 (http://www.who.int/publications/cra/chapters/volume2/1651-1802.pdf). [Google Scholar]
- 31.Samet JM, Avila-Tang E, Boffetta P, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res 2009;15:5626–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Disparities in secondhand smoke exposure — United States, 1988–1994 and 1999–2004. MMWR Morb Mortal Wkly Rep 2008;57:744–7. [PubMed] [Google Scholar]
- 33.Thun MJ, Hannan LM, Adams-Camp- bell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med 2008;5(9):e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol 2007;25:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Early Lung Cancer Action Program Investigators, Henschke CI, Yip R, Miettinen OS. Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA 2006;296:180–4. [DOI] [PubMed] [Google Scholar]
- 36.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243–60. [DOI] [PubMed] [Google Scholar]
- 37.Caraballo RS, Giovino GA, Pechacek TF, Mowery PD. Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2001;153:807–14. [DOI] [PubMed] [Google Scholar]
- 38.West R, Zatonski W, Przewozniak K, Jarvis MJ. Can we trust national smoking prevalence figures? Discrepancies between biochemically assessed and self-reported smoking rates in three countries. Cancer Epidemiol Biomarkers Prev 2007;16:820–2. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy SM, Sharapova SR, Beasley DD, Hsia J. Cigarette smoking among inmates by race/ethnicity: impact of excluding African American young adult men from national prevalence estimates. Nicotine Tob Res 2016;18:Suppl 1:S73–S78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson JP, Pederson LL. Military tobacco use: a synthesis of the literature on prevalence, factors related to use, and cessation interventions. Nicotine Tob Res 2008;10:775–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.