Abstract
PURPOSE:
To compare the efficacy of intraoperative scleral application with subconjunctival injection of mitomycin C (MMC) in trabeculectomy.
DESIGN:
Prospective, randomized, interventional study.
METHODS:
This study took place in a single clinical practice in an academic setting. Patients had medically uncontrolled glaucoma as indicated by high intraocular pressure (IOP), worsening visual field, or optic nerve head changes in whom primary trabeculectomy was indicated. Patients were older than 18 years with medically uncontrolled glaucoma and no history of incisional glaucoma surgery. Patients were randomized to MMC delivered by preoperative subconjunctival injection or by intraoperative direct scleral application using surgical sponges during trabeculectomy. Comprehensive eye examinations were conducted at 1 day, 1 week, 6 weeks, 3 months, and 6 months postoperatively. Subconjunctival 5-fluorouracil injections were given postoperatively, as needed. The primary outcome was the proportion of patients who demonstrated IOP of <21 mm Hg and ≥30% reduction in IOP from baseline. Secondary outcome measures included the number of IOP-lowering medications, bleb morphology using the Indiana Bleb Appearance Grading Scale, and complication rates.
RESULTS:
Participants (n = 100) were randomized into groups matched for baseline demographics, glaucoma status, and baseline IOP. At 6 months, there were no significant differences between the injection (n = 38) and sponge (n = 40) groups in surgical success (P = .357), mean IOP (P = .707), number of glaucoma medications (P = 1.000), bleb height (P = .625), bleb extension (P = .216), bleb vascularity (P = .672), or complications rates.
CONCLUSION:
Both techniques of MMC delivery (subconjunctival injection and direct scleral application) resulted in comparable surgical outcomes and bleb morphologies.
Surgical management of glaucoma is aimed at lowering intraocular pressure (IOP) to preserve vision. Trabeculectomy has been used for >5 decades to lower IOP, and various modifications have been introduced throughout this period to presumably enhance its efficacy and safety. Numerous clinical studies have evaluated factors that influence surgical outcomes and complications with trabeculectomy, particularly when intra- or postoperative antifibrotic agents, such as 5-fluorouracil (5-FU)1–3 or mitomycin C (MMC),4–6 are used. These adjunctive agents have been demonstrated to modify scar formation and reduce the risk of surgical failure.7,8 However, trabeculectomy with an antimetabolite, particularly with the use of MMC, is associated also with complications, such as blebitis and hypotony.9,10
The methods used to apply MMC during surgery affect both rates of surgical success and complications. Increasing MMC concentration or the volume applied increases the intrascleral penetration of MMC.11 The duration of MMC exposure can be as important as MMC concentrations in determining outcomes and complications.4 Although the optimal exposure time has not been established in large-scale studies and can vary from patient to patient, reducing intraoperative exposure time from 5 to 1-2 minutes does not appear to significantly affect IOP control.12 In studies that compared MMC application before or after scleral flap dissection, overall surgical success was higher when MMC was applied after scleral flap dissection, but rates of hypotony were also higher.13 The method for delivering MMC also has been of interest.
Intra-Tenon MMC injection, rather than direct scleral application using MMC-soaked sponges, was proposed >10 years ago as an alternative method for drug delivery, and favorable results were reported.14 A retrospective study that compared intraoperative injections to conventional sponge application subsequently reported comparable rates of success, and fewer postoperative interventions with MMC injections.15 The present prospective study was designed to compare the outcomes of direct scleral application to subconjunctival injection of MMC.
METHODS
THE STUDY WAS CONDUCTED AT THE SHILEY EYE INSTITUTE and the Hamilton Glaucoma Center of the University of California San Diego (UCSD). Approval was obtained prospectively from the local governing institutional review board. Written informed consent was obtained from all subjects for participation in the research and treatment. The study adhered to the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act (HIPAA) and local patient privacy protection regulations. This study is registered with clinicaltrials.gov as NCT04352660.
ELIGIBILITY CRITERIA:
Patients older than 18 years with medically uncontrolled glaucoma and no previous incisional glaucoma surgery were enrolled in this study. Exclusion criteria included patients with no light perception vision, previous glaucoma surgery, pregnant or nursing women, iris neovascularization or proliferative retinopathy, iridocorneal endothelial syndrome, chronic or recurrent uveitis, steroid-induced glaucoma, pathologic myopia or myopia of ≥6.00 diopters, unwillingness or inability to give consent, or inability to return for scheduled protocol visits. Only 1 eye from eligible patients was included in the study.
RANDOMIZATION AND TREATMENT:
Study subjects were recruited at the UCSD Shiley Eye Institute from May 2016 to October 2018. Written informed consent was obtained before subjects were permitted to participate in the study and complied with HIPAA requirements. Subjects were randomized on the day of surgery to receive MMC delivered by preoperative subconjunctival injection or intraoperative direct scleral application with impregnated cellulose sponges using a predetermined random list of 100 numbers generated by a random number generator (www.graphpad.com). Subjects were assigned to a treatment group in the sequence and based on value of the number (ie, 0 or 1) from the predetermined random number list. The surgeon and patient were not masked to randomization assignment at the time of surgery. Trained glaucoma specialists, who were masked to the patient’s group assignment, performed the postoperative examination and collected the data.
SURGICAL TECHNIQUE:
All procedures and surgeries were performed and/or supervised by a single surgeon (R.N.W.). Patients randomized to the subconjunctival injection group received topical anesthesia with proparacaine 0.5% followed by a subconjunctival injection of MMC (0.15 mL, 0.2 mg/mL, 30 μg total) at least 8 mm posterior to the limbus and just temporal to the superior rectus muscle using a 30-gauge needle in the preoperative area immediately before transportation to the operating room. The injected fluid was distributed nasally, temporally, and toward the limbus by gentle digital massage applied over the eyelid.
Anesthesia was achieved with intravenous sedation. The eye was sterilized with povidone-iodine, rinsed with a normal saline solution, and then covered with a sterile drape. After a lid speculum was placed, a two-clock hour conjunctival peritomy was made in the superior conjunctiva at the 12 o’clock position. Blunt dissection of Tenon’s capsule was used to create a sub-Tenon space. For the direct scleral application group, approximately 2 × 2 × 4-mm pieces of Weck-Cel (Beaver-Visitec International, Waltham, MA) sponges were soaked in MMC (0.4 mg/mL). Three or 4 sponges were placed in a sub-Tenon’s pocket that was at least at the 4 o’clock position at the limbus and 6 mm posterior to the limbus. The duration of application was titrated based on the thickness and hyperemia of the conjunctiva and Tenon’s capsule, and ranged from 1-2 minutes. The sub-Tenon’s space was then irrigated with copious amounts of balanced salt solution. A 2.0 × 1.5-mm triangular half-thickness scleral flap was created using a scalpel blade and crescent knife. Lamellar dissection under the scleral flap was continued 1 mm into the cornea. A temporal paracentesis was made. The anterior chamber was entered underneath the scleral flap, and a Kelly punch was used to excise a block of cornea under the scleral flap. The scleral flap was secured with 3 10-0 nylon sutures, and the knots were buried. The conjunctiva was closed with 2 10-0 nylon wing sutures. Tobramycin/dexamethasone ointment was applied at the end of surgery.
Postoperative management consisted of ofloxacin 0.3% eye drops 4 times daily for 1 week and prednisolone acetate 1% eye drops every hour tapered over 8-12 weeks based on the clinician’s assessment of inflammation and bleb function. Sutures securing the scleral flap were lysed by argon laser as indicated based on bleb appearance, anterior chamber cells and flare, and IOP beginning after 1 week. Subconjunctival injections of 5-FU (0.10-0.15 mL, 50 mg/mL) were administered adjacent and posterior to the bleb using a 30 gauge needle. The number of injections were adjusted based on the degree of conjunctival hyperemia or evidence of scarring. If scarring was noted, a 27-gauge needle was used to deliver 2.5 mg of 5-FU into the sub-Tenon space adjacent to the bleb and to dissect subconjunctival adhesions.
DATA COLLECTION:
Baseline demographic and clinical information was collected for enrolled patients, including medical history, ocular history, glaucoma medications, best-corrected visual acuity, Goldmann applanation tonometry, gonioscopy, slit-lamp examination, fundus examination, standardized automated perimetry, optical coherence tomography, and IOP goal. Data were collected with a standardized form at visits 1 day, 1 week, 6 weeks, 3 months, and 6 months after surgery. Postoperative visits included best-corrected visual acuity measurements, slit-lamp examination, Goldmann applanation tonometry, number of glaucoma medications, bleb morphology, and complications.
Bleb morphology was graded by masked clinicians according to the Indiana Bleb Appearance Grading Scale. Bleb parameters were assessed and scored according to bleb height (H0-H3), extent (E0-E3), vascularity (V0-V4), and leakage (S0-S2).16
The primary outcome measure was the rate of surgical success at 6 months. Complete success was defined prospectively as IOP <21 mm Hg and ≥30% reduction in IOP from baseline, absence of hypotony maculopathy, no need for reoperation for glaucoma, and retention of at least light perception vision. Eyes that required supplemental glaucoma medical therapy to meet the criteria of complete success were categorized as qualified successes. Failure was defined as IOP >21 mm Hg or <30% reduction in IOP from baseline. Complete failure was defined as loss of light perception vision or necessity for further glaucoma surgical intervention.
Secondary outcomes measures included IOP, glaucoma medication use, visual acuity, surgical complications, postoperative interventions, and bleb morphology.
STATISTICAL ANALYSIS:
The study recruited 50 patients for each group based on a power analysis to detect a 3 mm Hg difference in IOP with >80% power, 0.05 significance level, assuming a 5 mm Hg SD in measurements, and a 10% rate of loss to follow-up. Patients who underwent glaucoma surgeries following the initial trabeculectomy were counted as surgical failures and were assigned outcome values from the last visit before additional surgery for subsequent visits. Continuous variables were reported as means and SDs or 95% confidence intervals (CIs); significance was determined by paired t-test within groups, 2-sample t-tests between groups, or the Mann-Whitney U test. Categorical variables were reported as counts and percentages; differences were tested using Fisher’s exact test. Analyses were conducted using RStudio (version 1.1.456 or higher; RStudio Team, 2016, Boston, Massachusetts).
RESULTS
DEMOGRAPHICS AND PREOPERATIVE BASELINES:
One hundred patients were enrolled in the study. Patients were randomized to receive MMC by either a preoperative, subconjunctival injection C (injection group; n = 50) or intraoperative direct scleral application (sponge group; n = 50). All patients received the treatment that was assigned. Table 1 presents the baseline demographics and clinical factors of the study groups.
TABLE 1.
Baseline Characteristics of Patients
| Treatment |
|||
|---|---|---|---|
| Injection (n = 50) | Sponge (n = 50) | P Value | |
| Age, y, mean ± SD | 69.9 ± 13.4 | 74.2 ± 9.5 | .208 |
| Sex, n (%) | .842 | ||
| Female | 26 (52.0) | 24 (48.0%) | |
| Male | 24 (48.0) | 26 (52.0%) | |
| Diagnosis, n (%) | >.99 | ||
| Primary open angle glaucoma | 45 (90.0) | 43 (86.0) | |
| Pseudoexfoliation glaucoma | 2 (4.0) | 3 (6.0) | |
| Pigmentary glaucoma | 0 (0.0) | 1 (2.0) | |
| Chronic angle closure glaucoma | 1 (2.0) | 0 (0.0) | |
| Other | 2 (4.0) | 3 (6.0) | |
| Ethnicity, n (%) | .737 | ||
| White | 27 (54.0) | 28 (56.0) | |
| Asian | 9 (18.0) | 7 (14.0) | |
| Hispanic | 9 (18.0) | 5 (10.0) | |
| Black | 2 (4.0) | 3 (6.0) | |
| Other | 3 (6.0) | 7 (14.0) | |
| Visual acuity, logMAR, mean ± SD | 0.31 ± 0.34 | 0.36 ± 0.52 | .798 |
| Preoperative MD, mean ± SD | −17.19 ± 7.90 | −15.87 ± 8.09 | .446 |
| Preoperative PSD, mean ± SD | 9.4 ± 3.6 | 8.9 ± 3.2 | .501 |
| Preoperative IOP, mean ± SD | 21.1 ± 7.2 | 21.8 ± 9.3 | .887 |
| IOP goal, mean ± SD | 11.8 ± 0.9 | 12.7 ± 2.2 | .045a |
| No. of medications, n (%) | .023a | ||
| None | 0 (0.0) | 4 (8.0) | |
| 1 | 4 (8.0) | 6 (12.0) | |
| 2 | 6 (12.0) | 12 (24.0) | |
| 3 | 21 (42.0) | 21 (42.0) | |
| 4 | 17 (34.0) | 7 (14.0) | |
| 5 | 2 (4.0) | 0 (0.0) | |
IOP = intraocular pressure; logMAR = logarithm of minimal angle of resolution.
Mean patient age was 69.9 ± 13.4 years and 74.2 ± 9.5 years in the injection and sponge groups, respectively (P = .208). There were 24 (48%) and 26 (52%) male patients in the injection and sponge groups, respectively (P = .842). There was no difference in glaucoma diagnosis between the injection or sponge groups (P > 0.99). The most common diagnosis was primary open angle glaucoma in both the injection (n = 44; 88%) and sponge (n = 43; 86%) groups. There were no differences in ethnicity between the injection and sponge groups (P = .737). The most common ethnicity was non-Hispanic Caucasian in both the injection (n = 27; 54%) and sponge (n = 28; 56%) groups. There were no statistically significant differences between groups with respect to preoperative visual acuity (P = .510), IOP (P = .665), mean deviation (P = .446), or pattern SD (P = .501).
Patients in the injection group were treated preoperatively with a greater number of IOP-lowering medications (3.1 ± 1.0) than patients in the sponge group (2.4 ± 1.1) (p = .023). The injection group had a lower mean IOP goal (n = 41; 11.7 ± 0.8 mm Hg) than the sponge group (n = 40; 12.7 ± 2.2 mm Hg) (P = .045).
At 6 months, 12 and 10 patients were lost to follow-up in the injection group and sponge group, respectively.
SURGICAL SUCCESS:
Table 2 presents the distribution of complete successes, qualified successes, failures, and complete failures of the study groups after 6 months. At 6 months, there was no statistical difference in treatment success, defined as IOP <21 mm Hg and ≥30% reduction in IOP from baseline, between the injection group or sponge group (p = .357). The reason for complete failures was a need for additional glaucoma surgery. No patients experienced loss of light perception vision in either group.
TABLE 2.
Treatment Outcomes After 6 Months Grouped by Success Criteria
| Treatment |
|||
|---|---|---|---|
| Injection (n = 38) | Sponge (n = 40) | P Value | |
| Criteria: IOP <21 mm Hg and ≥30% IOP reduction from baseline, n (%) | .357 | ||
| Complete success | 21 (63.6) | 21 (67.7) | |
| Qualified success | 1 (3.0) | 3 (9.7) | |
| Failure | 3 (9.1) | 0 (0.0) | |
| Complete failure | 8 (24.2) | 7 (22.6) | |
IOP = intraocular pressure.
IOP AND GLAUCOMA MEDICATIONS:
At 6 months, there were statistically significant changes in mean IOP from baseline in the injection group (−10.1 mm Hg; P < 0.001) and sponge group (−10.6 mm Hg; P < 0.001). There were no statistically significant differences at 6 months between the mean IOP of the injection group (10.9 mm Hg; 95% CI: 7.9-13.8) and sponge group (10.2 mm Hg; 95% CI: 8.5-12.0) (P = .707) or in the difference in change of IOP from baseline between groups (0.4 mm Hg; 95% CI: −4.8 to 5.6; P = .873). Over the course of the study, the mean IOP of the injection group was statistically different from the sponge group at postoperative day 1 (Figure 1).
FIGURE 1.
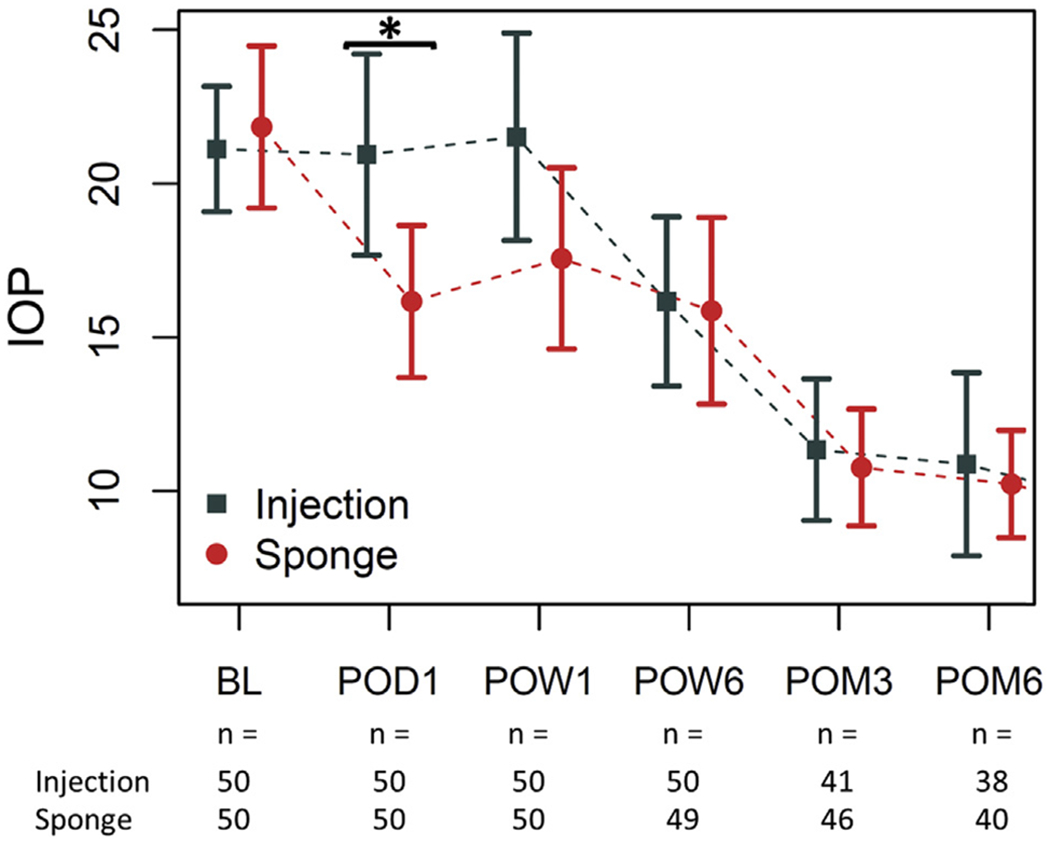
IOPs over time of the injection groups versus the sponge group. *P < 0.05 by 2-sample t-test. IOP = intraocular pressure.
The number of glaucoma medications did not differ between groups after 6 months (p = 1.000) (Table 3).
TABLE 3.
Glaucoma Medication Use of Treatment Groups at the Study Endpoint
| Treatment |
|||
|---|---|---|---|
| No. of glaucoma medications (%) | Injection (n = 38) | Sponge (n = 40) | P value |
| 0 | 33 (86.8) | 34 (85.0) | 1.000 |
| 1 | 3 (7.9) | 4 (10.0) | |
| 2 | 1 (2.6) | 1 (2.5) | |
| 3 | 1 (2.6) | 1 (2.5) | |
VISUAL ACUITY AND VISUAL FIELDS:
After 6 months, there was no difference in mean visual acuity between the injection group and the sponge group nor was there a difference in the change in visual acuity (Table 4). At 6 months, visual field mean deviations and pattern SDs were not statistically different between the injection group and the sponge group (Table 4).
TABLE 4.
Visual Acuity and Visual Field Parameters of Treatment Groups at Baseline and at the Study Endpoint
| Treatment |
|||
|---|---|---|---|
| Injection | Sponge | P value | |
| Visual acuity | |||
| Baseline | n = 50 | n = 50 | |
| Mean logMAR (95% CI) | 0.31 (0.22 to 0.41) | 0.36 (0.22 to 0.51) | .564 |
| POM6 | n = 38 | n = 40 | |
| Mean logMAR (95% CI) | 0.39 (0.26 to 0.52) | 0.36 (0.23 to 0.49) | .726 |
| Visual field | |||
| Baseline | n = 50 | n = 50 | |
| MD, mean (95% CI) | −17.19 (−19.5 to −14.9) | −15.87 (−18.4 to −13.4) | .446 |
| PSD, mean (95% CI) | 9.4 (8.3 to 10.5) | 8.9 (7.9 to 9.9) | .501 |
| POM6 | n = 38 | n = 40 | |
| MD, mean (95% CI) | −17.20 (−19.78 to −14.62) | −14.37 (−16.92 to −11.82) | .153 |
| PSD, mean (95% CI) | 9.2 (8.2 to 10.3) | 8.7 (7.7 to 9.8) | .538 |
CI = confidence interval; logMAR = logarithm of minimal angle of resolution.
BLEB MORPHOLOGY:
There were no differences in bleb morphology between groups after 6 months (Figure 2). Blebs in the injection group were similar to those in the sponge group with respect to bleb height, extension, and vascularity (Table 5).
FIGURE 2.
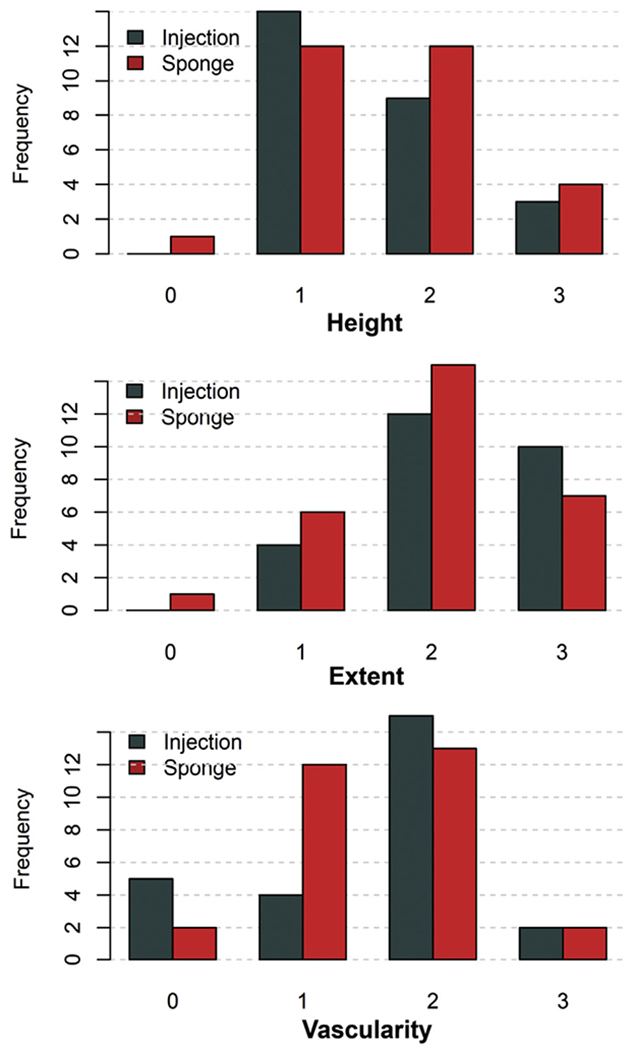
Bar plots of bleb appearance and morphology of treatment groups after 6 months graded according to the Indiana Bleb Appearance Grading Scale.
TABLE 5.
Bleb Morphology of the Treatment Groups After 6 Months Graded According to the Indiana Bleb Appearance Grading Scale
| Treatment |
|||
|---|---|---|---|
| Injection (n = 38) | Sponge (n = 40) | P Value | |
| Height, mean (95% CI) | 1.6 (1.3-1.9) | 1.7 (1.4-1.9) | .625 |
| Extent, mean (95% CI) | 2.2 (1.9-2.5) | 2.0 (1.7-2.3) | .216 |
| Vascularity, mean (95% CI) | 1.5 (1.2-1.9) | 1.5 (1.2-1.8) | .672 |
POSTOPERATIVE INTERVENTIONS:
Postoperative management did not differ between groups based on the MMC application method (Table 6). Both the injection group and sponge group underwent a similar number of subconjunctival 5-FU injections and laser suture lyses.
TABLE 6.
Cumulative Postoperative Interventions, Complications, and Operations of Treatment Groups Over the Entire Study Period
| Treatment |
|||
|---|---|---|---|
| Injection (n = 50) | Sponge (n = 50) | P Value | |
| Interventions, mean (95% CI) | |||
| 5-FU injections | 3.3 (2.6-4.0) | 3.2 (2.2-4.3) | .474 |
| Laser suture lysis | 2.0 (1.5-2.5) | 1.6 (1.1-2.1) | .249 |
| Complications, n (%) | |||
| Choroidal effusion | 2 (4.0) | 5 (10.0) | .436 |
| Hypotony (IOP ≤5 mm Hg) | 10 (20.0) | 7 (14.0) | .416 |
| Hypotony maculopathy | 3 (6.0) | 1 (2.0) | .617 |
| Secondary procedures, n (%) | |||
| Glaucoma operations | |||
| Trabeculectomy revision | 8 (16.0) | 5 (10.0) | .554 |
| Glaucoma drainage device | 2 (4.0) | 1 (2.0) | 1.000 |
| XEN Gel Stent | 0 (0.0) | 1 (2.0) | 1.000 |
| Trabectome | 0 (0.0) | 1 (2.0) | 1.000 |
| Cataract extraction | 8 (16.0) | 5 (10.0) | .554 |
| YAG capsulotomy | 0 (0.0) | 1 (2.0) | 1.000 |
CI = confidence interval; 5FU = 5-fluorouracil; IOP = intraocular pressure; YRAG = yttrium-aluminum-garnet.
COMPLICATIONS, REOPERATIONS, AND CATARACT PROGRESSION:
There were no statistical differences between groups with regard to postoperative complications (Table 6). Hypotony, defined as an IOP ≤5 mm Hg, and hypotony maculopathy were observed at similar rates in both the injection and sponge groups. The visual acuities of patients with hypotony but without hypotony maculopathy were within 1-2 Snellen lines of preoperative visual acuities. Choroidal effusion rates were also similar. Most of these resolved spontaneously, except for 1 patient in the injection group and 1 patient in the sponge group who had concurrent hypotony maculopathy; both patients were treated with revision of the trabeculectomy.
At 6 months, there were no statistically significant differences between groups in glaucoma reoperations or cataract progression as indicated by cataract extraction surgeries (Table 6).
DISCUSSION
THIS STUDY PROSPECTIVELY COMPARED THE EFFECTIVENESS and safety of trabeculectomy based on the delivery technique of MMC application. The success rate of trabeculectomy was similar between patients who received subconjunctival injections and those who received direct scleral application. After 6 months, the rates of success were 66.6% for the injection group (n = 38) and 77.4% for the sponge group (n = 40). The predominant reason for classifying a subject as a failure was a subsequent glaucoma surgery. After 6 months of follow-up, there was no difference in IOP, glaucoma medications, visual acuity, bleb morphology, postoperative interventions, postoperative complications, or glaucoma reoperations between patients who had subconjunctival injections and patients who received direct scleral application. Therefore, subconjunctival injection and direct scleral application of MMC during trabeculectomy are equally effective in lowering IOP, have similar risk profiles, and result in morphologically similar blebs.
Pakravan et al. recently reported trabeculectomy outcomes in 80 patients who underwent trabeculectomy in a prospective study with either sub-Tenon’s injection or sponge application of MMC.17 In contrast to our study, their patients were entirely from an Iranian population with Caucasian ethnicity and used MMC concentrations of 0.1 mg/mL for intra-Tenon injection and 0.2 mg/mL soaked sponges for direct scleral application.17,18 Differences in bleb morphologies were observed, with sub-Tenon injections resulting in more diffuse, less vascularized, and shallower blebs after 6 months. Extended follow-up after 3 years also attributed a more favorable bleb morphology to intra-Tenon injection.18 In contrast, differences in bleb morphology secondary to MMC delivery were not observed in this study. Several factors might account for the differences in their study compared with the present one. Racial and ethnic factors influence the conjunctival scarring response following trabeculectomy and contribute to higher risks of failure in Black patients compared with that in Caucasian counterparts.19 All subjects from the study by Pakravan et al. were Iranians of non-Hispanic Caucasian ethnicity. In contrast, only 55% of our study population included non-Hispanic Caucasian patients with the other patients being of Hispanic, Asian, or Black ethnicities. The concentration and duration of MMC application during direct scleral application also affects the degree of antifibrotic effects. The present study used MMC concentrations of 0.2 mg/mL for subconjunctival injections and 0.4 mg/mL for direct scleral applications over 1-2 minutes. In contrast, the study by Pakravan et al. used lower MMC concentrations of 0.1 mg/mL for sub-Tenon’s injections and 0.2 mg/mL for subconjunctival application for 1-3 minutes. Although these concentrations may be effective for non-Hispanic Caucasian populations, higher concentrations are necessary for patients with an increased likelihood of fibrosis to prevent bleb failure and are more in line with clinically relevant concentrations.20,21
There are advantages and disadvantages to direct scleral application and subconjunctival injection of MMC. Direct application permits exposure time to be adjusted. However, the area of application is limited to the area of dissection and may not extend as posterior as a subconjunctival injection. Conversely, although a subconjunctival injection applies MMC to a more diffuse area and reduces surgical inactivity, it is not possible to easily adjust the time of exposure. Furthermore, there is a risk of subconjunctival hemorrhage with a subconjunctival injection that may cause inflammation and fibrosis. Blood components such as red blood cells and plasma proteins also have the potential to bind MMC, reducing the effective MMC concentration and also stimulate a wound healing process.
There were limitations to our study. Although this was a prospective study in which patients were randomized to treatments, there were differences in the number of baseline glaucoma medications between the injection and sponge groups that were not significant after 6 months. The difference in the number of baseline medications was likely a reflection of lower IOP goals in the injection group. However, it should be noted that the baseline IOP, visual acuity, and visual field parameters were comparable between the groups despite the difference in the number of baseline glaucoma medications, which suggested the limitations of maximum medical therapy to reduce IOP at a certain point. Nonetheless, it was possible that there might be confounding variables that were not sufficiently controlled by randomization in this study. In addition, results from 6-month follow-up were reported. Although IOP stabilized, and there were no significant differences based on treatment, caution should be exercised when extrapolating beyond the study period. A longer follow-up period is necessary to determine long-term outcomes and complications. It was possible that some patients might have become qualified successes if medications were used. In contrast, if a patient had additional glaucoma surgery, they would be classified as a failure. 5-FU injections were administered based on presence of postoperative anterior chamber cells and conjunctival hyperemia. Although 5-FU is commonly used in clinical practice and the number of 5-FU injections did not differ between groups, it was a confounding variable in this study because of its antifibrotic activities. Lastly, although there were no statistically significant differences in surgical success between the groups based on our sample sizes, a much larger sample could demonstrate differences. Post hoc sample size calculations based on surgical success suggest that to detect a 10% difference between groups with a power of 80%, 90%, or 95%, the study would require 318, 415, or 526 subjects for each group, respectively.
CONCLUSION
IN SUMMARY, SUBCONJUNCTIVAL INJECTION AND DIRECT scleral application of MMC during trabeculectomy demonstrated comparable surgical outcomes, bleb morphologies, and complication at 6 months postoperatively. Based on these results, we suggest that ophthalmic surgeons use the MMC application technique with which they are familiar and can apply safely for patient care.
Acknowledgments
FUNDING/SUPPORT: SUPPORTED IN PART BY P30EY022589 CORE GRANT (NATIONAL EYE INSTITUTE) AND AN UNRESTRICTED grant from Research to Prevent Blindness (New York, NY).
Financial Disclosures: R.N.W. has been a consultant for Aerie Pharmaceuticals Allergan, Bausch & Lomb, Eyenovia, and Novartis; and has received research support (instruments) from Heidelberg Engineering, Carl Zeiss Meditec, Konan, Optovue, and Centervue. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Contributor Information
JIUN L. DO, Hamilton Glaucoma Center, Shiley Eye Institute and Viterbi Family Department of Ophthalmology, University of California, San Diego, California, USA
BENJAMIN Y. XU, USC Roski Eye Institute, Department of Ophthalmology, Keck School of Medicine at the University of Southern California, Los Angeles, California, USA
BRANDON WONG, USC Roski Eye Institute, Department of Ophthalmology, Keck School of Medicine at the University of Southern California, Los Angeles, California, USA.
ANDREW CAMP, Hamilton Glaucoma Center, Shiley Eye Institute and Viterbi Family Department of Ophthalmology, University of California, San Diego, California, USA.
PHILIP NGAI, Hamilton Glaucoma Center, Shiley Eye Institute and Viterbi Family Department of Ophthalmology, University of California, San Diego, California, USA.
CHRISTOPHER LONG, Hamilton Glaucoma Center, Shiley Eye Institute and Viterbi Family Department of Ophthalmology, University of California, San Diego, California, USA.
JAMES PROUDFOOT, Hamilton Glaucoma Center, Shiley Eye Institute and Viterbi Family Department of Ophthalmology, University of California, San Diego, California, USA.
SASAN MOGHIMI, Hamilton Glaucoma Center, Shiley Eye Institute and Viterbi Family Department of Ophthalmology, University of California, San Diego, California, USA.
DIYA YANG, Hamilton Glaucoma Center, Shiley Eye Institute and Viterbi Family Department of Ophthalmology, University of California, San Diego, California, USA.
DEREK S. WELSBIE, Hamilton Glaucoma Center, Shiley Eye Institute and Viterbi Family Department of Ophthalmology, University of California, San Diego, California, USA
ROBERT N. WEINREB, Hamilton Glaucoma Center, Shiley Eye Institute and Viterbi Family Department of Ophthalmology, University of California, San Diego, California, USA
REFERENCES
- 1.Weinreb RN. Adjusting the dose of 5-fluorouracil after filtration surgery to minimize side effects. Ophthalmology 1987;94(5):564–570. [DOI] [PubMed] [Google Scholar]
- 2.Heuer DK, Parrish RK, Gressel MG, et al. 5-fluorouracil and glaucoma filtering surgery: III. Intermediate follow-up of a pilot study. Ophthalmology 1986;93(12):1537–1546. [DOI] [PubMed] [Google Scholar]
- 3.Heuer DK, Parrish RK, Gressel MG, Hodapp E, Palmberg PF, Anderson DR. 5-fluorouracil and glaucoma filtering surgery: II. A pilot study. Ophthalmology 1984;91(4):384–394. [DOI] [PubMed] [Google Scholar]
- 4.Robin AL, Ramahrishnan R, Krishnadas R, et al. A long-term dose-response study of mitomycin in glaucoma filtration surgery. Arch Ophthalmol 1997;115(8):969–974. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JS, Greff LJ, Novack GD, Wind BE. A placebo-controlled, double-masked evaluation of mitomycin C in combined glaucoma and cataract procedures. Ophthalmology 1996;103(11):1934–1942. [DOI] [PubMed] [Google Scholar]
- 6.Carlson DW, Alward WLM, Barad JP, Zimmerman MB, Carney BL. A randomized study of mitomycin augmentation in combined phacoemulsification and trabeculectomy. Ophthalmology 1997;104(4):719–724. [DOI] [PubMed] [Google Scholar]
- 7.Cheng JW, Cai JP, Li Y, Wei RL. Intraoperative mitomycin C for nonpenetrating glaucoma surgery: a systematic review and meta-analysis. J Glaucoma 2011;20(5):322–326. [DOI] [PubMed] [Google Scholar]
- 8.Wilkins M, Indar A, Wormald R. Intra-operative mitomycin C for glaucoma surgery. Cochrane Database Syst Rev 2005; 20(4):CD002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jampel HD, Quigley HA, Kerrigan-Baumrind LA, Shields MB. Risk factors for late-onset infection following glaucoma filtration surgery. Evidence-Based Eye Care 2002;3(1):32–33. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann OJ, Bunce C, Matheson MM, et al. Risk factors for development of post-trabeculectomy endophthalmitis. Br J Ophthalmol 2000;12:1349–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vass C, Georgopoulos M, El Menyawi I, Radda S, Nimmerrichter P. Intrascleral concentration vs depth profile of mitomycin-C after episcleral application: impact of applied concentration and volume of mitomycin-C solution. Exp Eye Res 2000;70(5):571–575. [DOI] [PubMed] [Google Scholar]
- 12.Mégevand GS, Salmon JF, Scholtz RP, Murray ADN. The effect of reducing the exposure time of mitomycin C in glaucoma filtering surgery. Ophthalmology 1995;102(1):84–90. [DOI] [PubMed] [Google Scholar]
- 13.Yazdani S, Rezai S, Pakravan M, Afrouzifar M, Ghahari E. Mitomycin-C application before versus after scleral flap dissection in trabeculectomy; a randomized clinical trial. J Ophthalmic Vis Res 2015;10(4):391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee E, Doyle E, Jenkins C. Trabeculectomy surgery augmented with intra-Tenon injection of mitomycin C. Acta Ophthalmol 2008;86(8):866–870. [DOI] [PubMed] [Google Scholar]
- 15.Khouri AS, Huang G, Huang LY. Intraoperative injection vs sponge-applied mitomycin C during trabeculectomy: one-year study. J Curr Glaucoma Pract 2017;11(3):101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantor LB, Mantravadi A, WuDunn D, Swamynathan K, Cortes A. Morphologic classification of filtering blebs after glaucoma filtration surgery: The Indiana Bleb Appearance Grading Scale. J Glaucoma 2003;12(3):266–271. [DOI] [PubMed] [Google Scholar]
- 17.Pakravan M, Esfandiari H, Yazdani S, et al. Mitomycin C-augmented trabeculectomy: subtenon injection versus soaked sponges: a randomised clinical trial. Br J Ophthalmol 2017; 101(9):1275–1280. [DOI] [PubMed] [Google Scholar]
- 18.Esfandiari H, Pakravan M, Yazdani S, Doozandeh A, Yaseri M, Conner IP. Treatment outcomes of mitomycin C-augmented trabeculectomy, sub-Tenon injection versus soaked sponges, after 3 years of follow-up. Ophthalmol Glaucoma 2018;1(1):66–74. [DOI] [PubMed] [Google Scholar]
- 19.Taubenslag KJ, Kammer JA. Outcomes disparities between black and white populations in the surgical management of glaucoma. Semin Ophthalmol 2016;31(4):385–393. [DOI] [PubMed] [Google Scholar]
- 20.Broadway D, Grierson I, O’Brien C, Hitchings R. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol 1994;112(11):1446–1454. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen AH, Fatehi N, Romero P, et al. Observational outcomes of initial trabeculectomy with mitomycin C in patients of African descent vs patients of European descent: five-year results. JAMA Ophthalmol 2018;90095:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


