Abstract
Cordyceps militaris has been reported to the diverse pharmaceutical effects including cancer, inflammatory diseases, and bacteria or virus infection. However, the effect of C. militaris on exercise performance has not yet been elucidated. In this study, we investigated the beneficial effect of C. militaris on exercise performance. To evaluate exercise performance, we prepared C. militaris ethyl acetate extract (CMEE) and conducted grip strength tests every week after administration. Additionally, blood samples were collected at the end of the experiment for biochemical analysis. The administration of CMEE slightly increased grip strength, and this result was similar to the red ginseng treated group. According to the result of biochemical analysis, CMEE had an effect on the biomarkers related to ATP generation pathway but had little influence on the muscle fatigue related biomarkers. Therefore, C. militaris has the possibility of improving exercise performance, which could be associated with the increase in ATP production rather than the decrease in muscle fatigue during exercise.
Keywords: Cordyceps militaris, exercise performance, ATP, AMPK
1. Introduction
Health promotion is a positively related to reducing the risk factors for cardiovascular diseases, metabolic disorders, and bone diseases; whereas an inability to maintain exercise is well known to be associated with increase in these diseases [1]. Recently, the emergence of the concept of well-being has resulted in the increasing interest in the maintenance or enhancement of exercise performance [2,3]. For improving exercise performance, there are many dietary supplements, including amino acids, vitamins, minerals, and botanicals or mushrooms [4]. Among numerous medicinal mushrooms, Cordyceps militaris is considered as the valuable mushroom because of their various health benefits, including anti-cancer [5], immune modulating [6], anti-aging [7], anti-viral and anti-bacterial [8], and anti-fatigue effects [9]. However, the influence of C. militaris on enhancing exercise performance as well as its underlying mechanism has yet to be proven in animal models.
The exercise performance is correlated with recovery of muscle fatigue, endurance, and activating the neuromuscular system [10,11]. To evaluating of muscle fatigue or endurance in animals, there is usually the measurement of the specific enzymatic activity and/or biochemical analysis in blood samples. These biomarkers are related to muscle damage or fatigue and energy metabolism, such as creatine kinase (CK), lactate dehydrogenase (LDH), blood urea nitrogen (BUN), insulin-responsive glucose transporter 4 (GLUT4), pyruvate dehydrogenase (PDH), AMP-activated protein kinase (AMPK), and TCA cycle, lipid metabolism, and electron transport chain-involved oxidative enzymes [12–14].
The purpose of present study is to evaluate the effect on improving exercise performance of C. militaris ethanol extract (CMEE) in mice during the grip strength test. In addition, we have analyzed the several biochemical biomarkers, such as blood concentrations of LDH, aspartate aminotransferase (AST), alanine aminotransferase (ALT), BUN, creatine, phosphocreatine, adenosine-5′-thriphosphate (ATP), GLUT4, PDH, AMPK, and peroxisome proliferator-activated receptor-γ (PPAR-γ), involved in the muscle fatigue and the energy production or metabolism pathway during exercise.
2. Materials and methods
2.1. Reagents
The Korean Red Ginseng Powder Special was purchased from Geumsan Red Ginseng Land (Geumsan-gun, Chungcheongnam-do, Republic of Korea).
2.2. Preparation of C. militaris ethanol extract (CMEE)
Artificial cultured C. militaris was provided from Mushtech Co., Ltd. (Hoengseong, Korea). Cultivated whole fruiting bodies of C. militaris, containing 2.33 mg/g of cordycepin, were dried at 50 °C and crushed in a blender and then the crude powder was extracted with ethanol at 85 °C for 6 hr. The ethanol extract was vacuum filtered using a filter paper (Whatman No. 2) and then was evaporated at 65 °C by an evaporator (N-1000; Eyela, Tokyo, Japan) under reduced pressure. The concentrated extract was frozen at −80 °C and then lyophilized using a freeze-dryer.
2.3. Animals and treatment
Male CrljOri:CD1 (ICR) mouse (6 weeks) were purchased from Orientbio, Inc. (Gapyeong, Korea). The animals were housed in groups of 5 per cage under standard laboratory conditions (temperature 23 ± 3 °C, relative humidity 53 ± 15%, and 12 hr light/dark cycle of 150–300 Lux). Food and sterilized water were available ad libitum. Animals were divided equally into 5 groups: G1, vehicle-treated normal control group; G2, red ginseng 100 mg/kg treated comparative group (orally once daily for 12 weeks); G3–G5, C. militaris treated groups (50, 150, and 300 mg/kg, orally once daily for 12 weeks). All animals were closely monitored, and there were no clinical symptoms observed during the entire experimental period. All experiment protocols were approved by the Institutional Animal Care and Use Committee at the KNOTUS Co. Ltd. (Guri, Korea; Certificate No: IACUC 17-KE-296).
2.4. Grip strength test
Grip strength was measured with a computerized grip strength meter (47200; Ugo-Basile, Varese, Italy). When the animals (n = 10 per group per test) grasped the transducer metal bar with their forepaws, the experimenter pulled the animals backwards by the tail until grip was lost. Basal grip strength values were recorded for each animal as once a week for 12 weeks.
2.5. Biochemical analysis
Animals were anesthetized with isoflurane at 12 weeks and blood was collected from the postcaval vein of the subjects into a vacutainer tube containing a clot activator. After the serum was solidified at room temperature, then allowed to stand for about 15 min, the serum was separated by centrifuged for 10 min at 3,000 rpm. The quantitative determination of contents of lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatine were analyzed by Hitachi Chemistry Analyzer (7180; Hitachi, Tokyo, Japan), and ELISA assay was allowed for the quantitative determination of phosphocreatine (Cat. No. MBS2700694; Mybiosource, San Diego, CA, USA), adenosine triphosphate (ATP) (Cat. No. Ab83355; Abcam, Cambridge, UK), insulin induces glucose transporter 4 (GLUT4) (Cat. No. MBS727326; Mybiosource), pyruvate dehydrogenase (PDH) (Cat. No. MAK183; Sigma-Aldrich, St. Louis, MO, USA), AMP-activated protein kinase (AMPK) (Cat. No. MBS2505028; Mybiosource), and peroxisome proliferator-activated receptor-γ (PPAR-γ) (Cat. No. MBS2501353; Mybiosource).
2.6. Statistical analysis
All data are expressed as the mean ± standard error (SE). Statistical analysis was performed using Prism 5.04 program (GraphPad Software Inc., San Diego, CA, USA). The statistical comparisons were performed using one-way analysis of variance (ANOVA) followed by Dunnett’s test. A significant difference was defined as p < 0.05.
3. Results
3.1. Effect of CMEE on grip strength
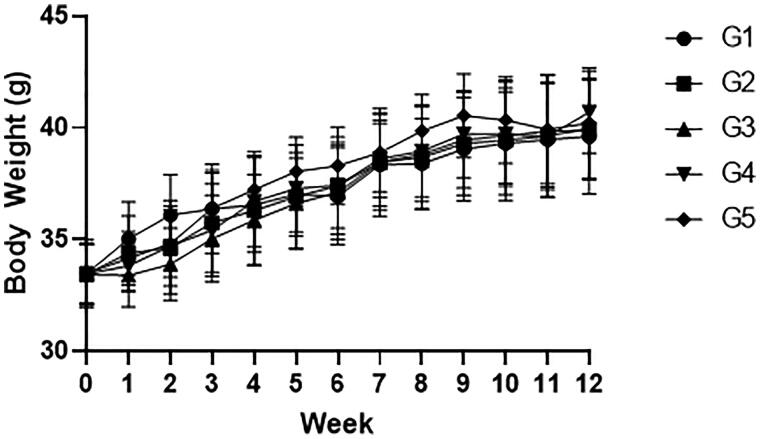
To evaluate the influence of the CMEE on exercise, we employed the grip strength test model and used the red ginseng as positive control. The red ginseng is one of the most famous medicinal herb and has been reported to the various health beneficial effects including the protection of muscle damage, the relief of fatigue, and the improvement of exercise endurance [15]. There were no significant changes in body weight during the experiment (Figure 1). Despite the exercise improving effect of CMEE was not in dose-dependent manner, CMEE (G3–G5) and red ginseng (G2) increased grip strength by approximately 10 gf compared to the control group (G1) at 11 and 12 weeks after administration (Table 1).
Figure 1.
Body weight change during the experiment. Mice were fed with vehicle (G1), 100 mg/kg of red ginseng (G2), or 50, 150, and 300 mg/kg of CMEE (C3–C5) for 12 weeks. Data were expressed as mean ± S.D. (n = 10 mice in each group). *p < 0.05, compared with normal control (G1).
Table 1.
Effect of CMEE on grip strength (gf, grams of force).
| Week | Grip strength (gf) |
||||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | |
| 0 | 110.9 ± 14.8 | 114.4 ± 13.7 | 125.2 ± 16.0 | 125.3 ± 14.5 | 119.1 ± 14.2 |
| 1 | 120.8 ± 14.9 | 125.8 ± 11.2 | 117.0 ± 16.0 | 127.1 ± 16.8 | 123.5 ± 13.5 |
| 2 | 121.7 ± 16.0 | 124.2 ± 12.3 | 124.3 ± 12.1 | 124.0 ± 17.6 | 117.0 ± 8.4 |
| 3 | 121.2 ± 9.7 | 131.9 ± 10.8 | 127.8 ± 11.4 | 129.7 ± 9.0 | 123.2 ± 6.9 |
| 4 | 124.8 ± 4.1 | 132.9 ± 10.1 | 126.8 ± 15.4 | 128.3 ± 9.3 | 123.4 ± 8.0 |
| 5 | 126.2 ± 7.3 | 133.8 ± 9.5 | 130.7 ± 13.9 | 130.9 ± 10.5 | 129.8 ± 8.4 |
| 6 | 129.5 ± 4.3 | 134.6 ± 7.4 | 131.6 ± 13.6 | 134.1 ± 9.9 | 136.5 ± 12.8 |
| 7 | 132.1 ± 6.4 | 136.9 ± 8.5 | 135.5 ± 13.3 | 135.9 ± 9.2 | 140.0 ± 13.5 |
| 8 | 136.1 ± 5.6 | 142.6 ± 8.8 | 140.1 ± 11.3 | 140.7 ± 8.6 | 142.3 ± 11.4 |
| 9 | 137.8 ± 7.3 | 145.0 ± 5.4 | 143.7 ± 9.5 | 143.8 ± 7.4 | 144.3 ± 6.3 |
| 10 | 138.2 ± 5.9 | 145.8 ± 6.9 | 144.4 ± 8.1 | 145.6 ± 5.9 | 145.7 ± 6.7 |
| 11 | 136.3 ± 4.5 | 146.3 ± 4.6*** | 143.0 ± 5.1** | 148.9 ± 3.5*** | 145.9 ± 3.2*** |
| 12 | 136.4 ± 1.5 | 147.2 ± 3.6*** | 142.6 ± 3.3*** | 148.8 ± 2.8*** | 146.7 ± 3.4*** |
Mice were fed with vehicle (G1), 100 mg/kg of red ginseng (G2), or 50, 150, and 300 mg/kg of CMEE (C3–C5) for 12 weeks. Data were expressed as mean ± S.D. (n = 10 mice in each group). ***p < 0.001, **p < 0.01, compared with normal control (G1).
3.2. Effect of CMEE on muscle fatigue and ATP production
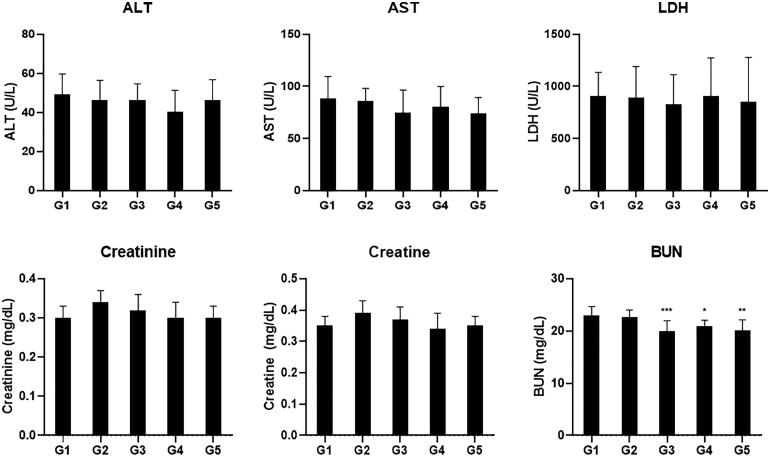
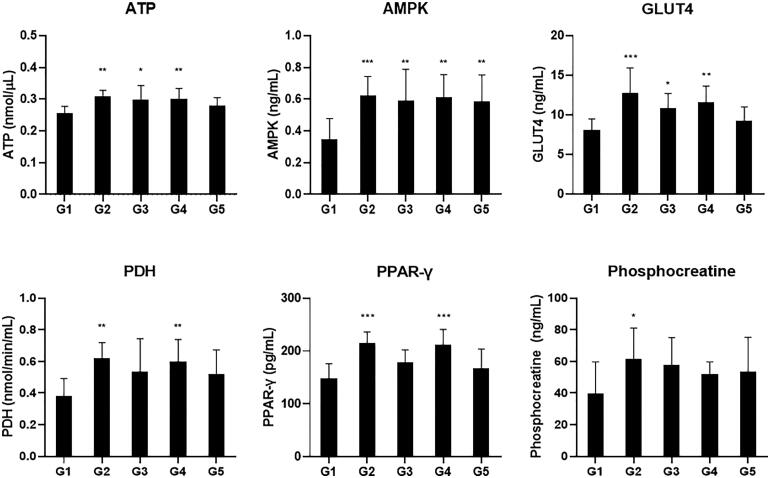
To elucidate the molecular mechanism of improving exercise performance of CMEE, we performed biochemical and ELISA analysis on blood samples collected at the end of the experiment. The results of biochemical analysis showed that there were no significant changes in the concentration of AST, ALT, LDH, creatinine, and creatine in all groups (Figure 2). The level of BUN was decreased in CMEE and red ginseng treated groups. Especially, the levels of BUN both G3 and G5 groups were significantly reduced compared G1 and G2 groups (Figure 3(A)). In addition, the results of ELISA analysis demonstrated red ginseng showed dramatically increased the levels of ATP, AMPK, PPAR-γ, GLUT4, PDH, and phosphocreatine, which were related to cellular energy generation (Figure 3). In the case of CMEE-treated groups, the level of AMPK were significantly elevated all groups (G3–G5), and the levels of ATP, GLUT4, PDH, PPAR-γ, and phosphocreatine were also increased compared to G1 control group but there were no statistical significance, except for G4 group (Figure 3).
Figure 2.
Serum biochemical analysis results of the muscle fatigue related biomarkers. Mice were fed with vehicle (G1), 100 mg/kg of red ginseng (G2), or 50, 150, and 300 mg/kg of CMEE (C3–C5) for 12 weeks. Data are expressed as mean ± S.D. (n = 10 mice in each group). ***p < 0.001, **p < 0.01, *p < 0.05, compared with normal control (G1).
Figure 3.
ELISA analysis results of the energy production related biomarkers. Mice were fed with vehicle (G1), 100 mg/kg of red ginseng (G2), or 50, 150, and 300 mg/kg of CMEE (C3–C5) for 12 weeks. Data are expressed as mean ± S.D. (n = 10 mice in each group). ***p < 0.001, **p < 0.01, *p < 0.05, compared with normal control (G1).
4. Discussion
Although it has been well known the various pharmaceutical benefits of C. militaris on the health, the precision mechanism of enhancing exercise performance remains poorly understood. In the present study, we evaluated whether the administration of C. militaris has an influence on increasing exercise performance in an animal model. To explain the effect of exercise performance by C. militaris, we analyzed peripheral blood biomarkers, related to muscle fatigue and ATP generation.
To measurement of muscle strength in rodent models, grip strength test is general used due to convenient and noninvasive method [16]. During the experiment, we found no significant difference in body weight between normal control group (G1) and experimental groups (G2–G5) (Figure 1). As a result of grip strength test, all CMEE-treated groups (G3–G5) were slightly higher than that of normal control group (G1) at 11 and 12 weeks. These results were similar to red ginseng-treated group (G2), a positive control (Table 1). These observations suggest that CMEE could contribute to improve exercise performance.
The alleviation of exercise-induced muscle fatigue and the increase on energy metabolism can be associated with enhancing exercise performance [17–19]. We investigated whether the beneficial effect CMEE on exercise performance is in consequence of the reduction of muscle fatigue and the revitalization of energy production. The results of biochemical analysis showed that the levels of fatigue-related biomarkers such as ALT, AST, LDH, creatinine, and creatine were no change except for BUN (Figure 2).
In contrast, the results of ELISA analysis showed that the concentrations of AMPK, GLUT4, PDH, PPAR-γ, and phosphocreatine involved in energy production as well as ATP increased (Figure 3). AMPK is a sensor of intracellular ATP level and is activated by ATP depletion. During the exercise, ATP-consuming process is accelerated, as a result AMP/ATP or ADP/ATP ratio increased. AMPK activation leads to decrease ATP-consuming process and increase ATP generation, which maintains the energy homeostasis. Interestingly, AMPK promotes the transcription of GLUT4 gene and regulates lipogenesis homeostasis [20]. GLUT4 plays an important role in glucose homeostasis in skeletal muscle, and transports glucose from blood to skeletal muscle. Glucose in muscle cells is phosphorylated by hexokinase and then enters glycolysis process or stored as glycogen [21]. Glycolysis is the metabolic process, which produces pyruvate from glucose. Pyruvate is converted to acetyl-CoA, an important metabolic intermediate of TCA cycle by PDH [22]. In addition, the muscle contractile activity is dependent on phosphocreatine activity that is involved in ATP regeneration [23], and PPAR-γ is a metabolic regulator and is related to glucose and lipid metabolism [24]. PPAR-γ activation upregulates PPAR-γ-controlled genes including mitochondrial biogenesis and aerobic respiration, consequently, has beneficial effects in skeletal muscle. Because these factors are involved in ATP generation and exercise endurance, the increased expression and activity of them is associated with enhancing exercise performance. Therefore, the administration of CMEE is influenced on ATP production pathway, and consequently to assist the improvement of exercise performance.
5. Conclusion
We investigated the effect of the administration of CMEE on exercise performance in grip strength test and analyzed biochemical biomarkers for elucidating the mechanism of improving exercise performance. Our results demonstrated that the administration of CMEE enhances exercise performance by upregulating the ATP generation pathway rather than alleviating muscle fatigue. Taken together, our finding suggests that C. militaris could be useful material for an adjuvant and a functional food improving exercise performance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Warburton DE, Nicol CW, Bredin SS.. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loureiro A, Veloso S.. Green exercise, health and well-being In: Fleury-Bahi G, Pol E, Navarro O, editors. Handbook of environmental psychology and quality of life research. Cham (Switzerland): Springer; 2017. p. 149–169. [Google Scholar]
- 3.Penedo FJ, Dahn JR.. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18:189–193. [DOI] [PubMed] [Google Scholar]
- 4.Jagim AR, Harty PS, Camic CL.. Common ingredient profiles of multi-ingredient pre-workout supplements. Nutrients. 2019;11:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JG, Son YJ, Lee TH, et al. Anticancer efficacy of Cordyceps militaris ethanol extract in a xenografted leukemia model. Evid Based Complement Alternat Med. 2017;2017:8474703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin S, Kwon J, Lee S, et al. Immunostimulatory effects of Cordyceps militaris on macrophages through the enhanced production of cytokines via the activation of NF-kappaB. Immune Netw. 2010;10:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li XT, Li HC, Li CB, et al. Protective effects on mitochondria and anti-aging activity of polysaccharides from cultivated fruiting bodies of Cordyceps militaris. Am J Chin Med. 2010;38:1093–1106. [DOI] [PubMed] [Google Scholar]
- 8.Dong CH, Yang T, Lian T.. A comparative study of the antimicrobial, antioxidant, and cytotoxic activities of methanol extracts from fruit bodies and fermented mycelia of caterpillar medicinal mushroom Cordyceps militaris (Ascomycetes). Int J Med Mushrooms. 2014;16:485–495. [DOI] [PubMed] [Google Scholar]
- 9.Song J, Wang Y, Teng M, et al. Studies on the antifatigue activities of Cordyceps militaris fruit body extract in mouse model. Evid Based Complement Alternat Med. 2015;2015:174616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan JJ, Qin Z, Wang PY, et al. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017;49:e384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang WC, Chiu WC, Chuang HL, et al. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Nutrients. 2015;7:905–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H, Park S, Han DS, et al. Octacosanol supplementation increases running endurance time and improves biochemical parameters after exhaustion in trained rats. J Med Food. 2003;6:345–351. [DOI] [PubMed] [Google Scholar]
- 13.Dalla Corte CL, de Carvalho NR, Amaral GP, et al. Antioxidant effect of organic purple grape juice on exhaustive exercise. Appl Physiol Nutr Metab. 2013;38:558–565. [DOI] [PubMed] [Google Scholar]
- 14.Ping FW, Keong CC, Bandyopadhyay A.. Effects of acute supplementation of Panax ginseng on endurance running in a hot & humid environment. Indian J Med Res. 2011;133:96–102. [PMC free article] [PubMed] [Google Scholar]
- 15.Shin EJ, Jo S, Choi S, et al. Red ginseng improves exercise endurance by promoting mitochondrial biogenesis and myoblast differentiation. Molecules. 2020;25:865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeshita H, Yamamoto K, Nozato S, et al. Modified forelimb grip strength test detects aging-associated physiological decline in skeletal muscle function in male mice. Sci Rep. 2017;7:42323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vollestad NK, Sejersted OM.. Biochemical correlates of fatigue. A brief review. Eur J Appl Physiol Occup Physiol. 1988;57:336–347. [DOI] [PubMed] [Google Scholar]
- 18.Tung YT, Hsu YJ, Liao CC, et al. Physiological and biochemical effects of intrinsically high and low exercise capacities through multiomics approaches. Front Physiol. 2019;10:1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Layzer RB. Muscle metabolism during fatigue and work. Baillieres Clin Endocrinol Metab. 1990;4:441–459. [DOI] [PubMed] [Google Scholar]
- 20.Ke R, Xu Q, Li C, et al. Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol Int. 2018;42:384–392. [DOI] [PubMed] [Google Scholar]
- 21.Richter EA, Hargreaves M.. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017. [DOI] [PubMed] [Google Scholar]
- 22.Morales-Alamo D, Guerra B, Santana A, et al. Skeletal muscle pyruvate dehydrogenase phosphorylation and lactate accumulation during sprint exercise in normoxia and severe acute hypoxia: effects of antioxidants. Front Physiol. 2018;9:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guimaraes-Ferreira L. Role of the phosphocreatine system on energetic homeostasis in skeletal and cardiac muscles. Einstein (Sao Paulo). 2014;12:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas AW, Davies NA, Moir H, et al. Exercise-associated generation of PPARgamma ligands activates PPARgamma signaling events and upregulates genes related to lipid metabolism. J Appl Physiol. 2012;112:806–815. [DOI] [PubMed] [Google Scholar]