Abstract
Chronic ethanol exposure induces impairments in CNS excitatory and inhibitory activity. These impairments are associated with glutamatergic dysfunction, including altered neuroplasticity. This study examined the effects of 6-week ethanol (15% and 30% v/v) consumption, by male alcohol-preferring P rats, on protein expression associated with neuroplasticity and glutamate transporter-1 (GLT-1) function. The latter regulates intra- and extra-synaptic glutamate levels. We focused on the shell and core subregions of the nucleus accumbens (Acb); i.e., shell (AcbSh) and core (AcbCo), for these measures. Chronic ethanol exposure increased the expression of BDNF, Arc and phosphorylated (p)-post-synaptic density protein-95 (p-PSD-95) in the AcbSh of P rats. Moreover, the ratio of phospho-neuronal nitric oxide synthase (p-nNOS) to total nNOS was also increased in the AcbSh. These changes in BDNF, Arc and p-nNOS/nNOS ratio were not observed in the AcbCo. Furthermore, chronic ethanol exposure reduced GLT-1 expression in the AcbSh. Alternatively, treatment with ceftriaxone (CEF), a known GLT-1 upregulator, abolished the effect of chronic ethanol exposure on BDNF expression in the AcbSh. Overall, the present findings confirm that chronic ethanol consumption modulates activity-associated synaptic proteins, including BDNF, Arc and nNOS in a subregion-specific (i.e., in the AcbSh but not AcbCo) manner. Thus, alterations in mesocorticolimbic glutamatergic homeostasis and neuroplasticity are possible functional targets for the treatment of alcohol use disorders.
Keywords: Ethanol dependence, glutamate, GLT-1, BDNF, Arc, nNOS, nucleus accumbens
Introduction
Ample evidence indicates that chronic ethanol exposure induces alterations in central neurotransmitter function, including that of glutamate, acetylcholine (Ach), and dopamine (DA). A primary reward circuit includes subregions of the mesocorticolimbic reward system [e.g., ventral tegmental area, VTA; nucleus accumbens, Acb; prefrontal cortex, PFC) (Ehrlich et al., 2012; He et al., 2005; Jeanes et al., 2011; Spiga et al., 2014; Uys et al., 2016). The Acb has been extensively studied for its crucial role in alcohol use disorders (AUDs) and substance use disorders (SUDs) (Heinze et al., 2009; Müller et al., 2016; Neasta et al., 2011). Its subregions, the core (AcbCo) and shell (AcbSh), control rewarding behavior such as the inhibition, or promotion, of motivated reward-seeking behavior via distinct neurocircuits (Augur et al., 2016; Keistler et al., 2015; Stefanik et al., 2016). For instance, glutamatergic projections extend from the prelimbic/cingulate and infralimbic subregions of the mPFC to the AcbCo and AcbSh, respectively, with the former promoting and the latter inhibiting drug seeking behavior (Kalivas, 2009; Scofield et al., 2016). Nevertheless, the functional role of these neurocircuits are also drug-specific. For example, the dorsal mPFC (dmPFC: prelimbic/cingulate) facilitates cocaine- and heroin-seeking behavior, whereas the ventral mPFC (vmPFC: infralimbic) inhibits cocaine- but facilitates heroin-seeking behavior (Peters et al., 2013).
Within the AcbSh, ethanol induces neuroplastic changes by remodeling dendritic spines, including loss of long thin spines [for review see ref. (Chandler et al., 2006)]. Ethanol also impairs long-term depression (LTD), and induces Acb metaplasticity by switching from LTD to long-term potentiation (LTP) (Jeanes et al., 2011; Spiga et al., 2014). Ethanol-induced alterations in neurotransmitter systems are often associated with abnormal neuroplasticity and neuropathology (Ji et al., 2015; Ji et al., 2017; Shillinglaw et al., 2018). Brain-derived neurotrophic factor (BDNF) is implicated in the regulation of synaptic activity, neuroplasticity and connectivity (Grande et al., 2010). Studies suggested that glutamate may alter BDNF function, which can regulate synaptic transmission and neuroplasticity (Martin and Finsterwald, 2011). BDNF upregulates both mRNA and subsequent protein expression of vesicular glutamate transporters, suggesting that glutamatergic neurotransmission might be regulated by BDNF (Melo et al., 2013). Both BDNF and glutamate transporter expression and/or function are altered differentially by ethanol exposure. For example, chronic ethanol exposure downregulates GLT-1 (its human homolog is excitatory amino acid transporter 2, EAAT2) and this effect was associated with increased extracellular glutamate in the Acb (Alhaddad et al., 2014b; Das et al., 2015). However, acute ethanol exposure increases BDNF mRNA expression and its downstream signaling molecule activity-regulated cytoskeleton-associated protein (Arc) in the central amygdala (CeA) and medial amygdala (MeA), while ethanol withdrawal induced downregulation of the BDNF-Arc signaling pathway (Pandey et al., 2008). Arc protein expression is also differentially altered by exposure to drugs of addiction such as morphine; specifically, morphine-induced conditioned place preference (CPP) upregulated Arc expression in the AcbSh, while reinstatement of morphine-induced CPP increased Arc expression in the AcbCo (Lv et al., 2011).
The expression of BDNF and Arc are regulated by multiple signaling pathways, including intracellular and extracellular nitric oxide (NO) pathways (Riccio et al., 2006). Nitric oxide synthase (NOS), specifically the neuronal isoform (nNOS), is highly implicated in the development of tolerance (Khanna et al., 1993; Khanna et al., 1995) and sensitization (Itzhak and Martin, 2000; Santos-Rocha et al., 2018) to ethanol. Additionally, chronic ethanol exposure stimulates N-methyl-D-aspartate (NMDA) receptors, which are linked to nNOS via the post-synaptic density protein-95 (PSD-95) (Sattler et al., 1999). This activates nNOS and subsequently the production of NO (Chandler et al., 1997; Spanagel et al., 2002). Moreover, nNOS knock-out mice have much higher voluntary ethanol intake than wild-type mice, indicating a role for nNOS in mediating the neurobehavioral effects of ethanol consumption (Spanagel et al., 2002). Together, these studies implicate the glutamatergic system, BDNF-Arc signaling as well as NO pathways in the development and maintenance of AUDs. The current study examined the effects of ethanol intake on neuroplasticity-related proteins (BDNF, Arc, nNOS, and PSD-95) and determined the association between these proteins and changes in GLT-1 expression within the AcbSh and AcbCo.
Materials and methods
Animal model
Male P rats were housed in a room that was maintained at 21°C on a 12/12 h light/dark cycle. Rats had free access to water and food. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Indiana University School of Medicine (Indianapolis, IN, USA), in accordance with the guidelines of the IACUC of the National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals.
Ethanol-intake paradigm
Rats were randomly assigned to groups at 90 days of age: water-control and ethanol-exposed groups. For the ethanol naïve group (n = 8), rats were exposed to only water and food throughout the exposure procedures, and was considered as the water-control group. For the ethanol group (n = 8), rats were exposed to continuous free-choice access to ethanol (15% and 30%, v/v, available concurrently) for six weeks; this results in pharmacologically relevant blood ethanol concentration (50–200 mg%) [For review see ref. (Bell et al., 2006)]. Measurement of ethanol intake was performed daily (g of ethanol intake/kg of body weight/day). The average ethanol intake during week 6 was 6.87 ± 0.41 g/kg/day (Figure 1). Rats whose average ethanol intake ≤ 4 g/kg/day were excluded from the study following the criteria for the development of ethanol dependence (Bell et al., 2012; Li et al., 1987; Sari and Sreemantula, 2012).
Figure 1. Ethanol consumption in P-rats.
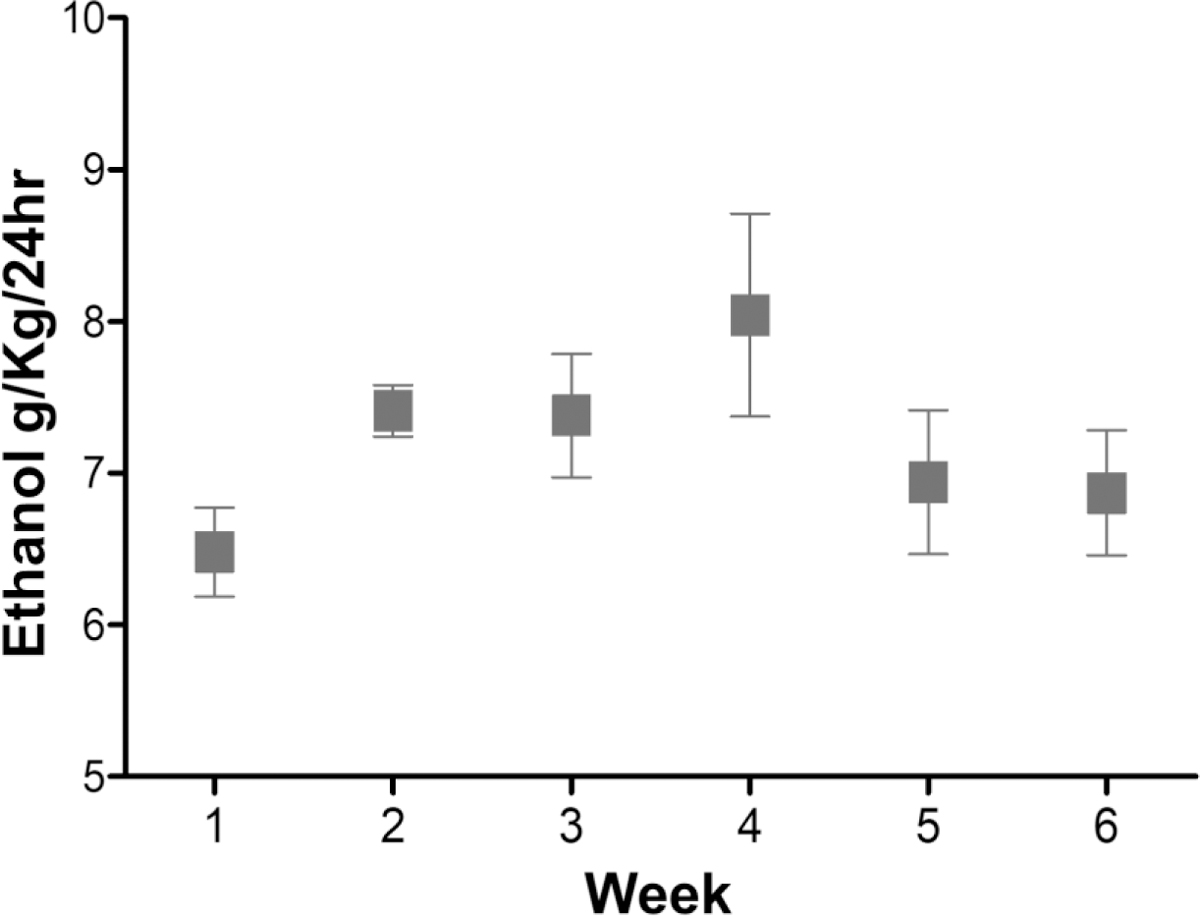
Average ethanol intake (g/kg/24hours) over 6 weeks in male P rats (n=8) with continuous free access to 0, 15% and 30% ethanol [with permission from the publisher (Alhaddad et al., 2020)]. P rats with intake of < 4 g/kg/day did not meet the criteria for the development of ethanol dependence and were excluded from the study.
Brain tissue extraction
At the last day of week 6, all P rats were removed from the cage around mid-day and rapidly euthanized by CO2 inhalation followed by rapid decapitation with a guillotine, so there was no ethanol withdrawal period. Brains were isolated and immediately frozen on dry ice and stored at −80°C. The AcbCo and AcbSh were dissected using a cryostat apparatus (−20°C). We used surgical blades to micropunch and isolate the brain regions following visualized landmarks using the stereotaxic coordinates provided by Paxinos and colleague’s rat brain stereotactic atlas (Paxinos et al., 2007). The AcbCo and AcbSh were kept at −80°C for later western blot analyses.
Western blot procedure
Western blot was used to determine protein expression of GLT-1, BDNF, Arc, phospho nNOS (p-nNOS), total nNOS (t-nNOS), Phospho PSD-95 (p-PSD-95), PSD-95, and β-tubulin in the AcbCo and AcbSh as previously published (Alasmari et al., 2017; Hammad et al., 2017; Sari et al., 2009). AcbCo and AcbSh homogenates were generated using a lysis buffer containing protease and phosphatase inhibitors. The amount of protein in each tissue sample was quantified using a detergent compatible protein assay (Bio-Rad, Hercules, CA, USA). The polyacrylamide gels (10%) were loaded with an equal amount of protein from each lysate; and the proteins were then separated using electrophoresis. Proteins were transferred electrophoretically from the gels onto Polyvinylidene difluoride (PVDF) membranes. Membranes were incubated in 5% free-fat milk in Tris-buffered saline with Tween-20 (TBST) for one hour at room temperature. Membranes were then incubated overnight at 4°C with appropriate primary antibodies: anti-GLT-1 (1:5000, Abcam, ab41621), anti-BDNF antibody (1:500; Abcam, ab108319), anti-Arc antibody (1:1000; Abcam, ab183183), anti-nNOS (1:1000; Abcam, ab76067), anti-p-nNOS (1:1000; Abcam, ab16650), anti-p-PSD-95 (1:1000; Abcam ab172628), and anti-PSD-95 (1:1000; Abcam ab76115). Anti-β-tubulin was used as a loading control antibody (1:1000; BioLegend). Membranes were incubated with the matched secondary antibody (1:5000) on the next day at room temperature for 90 minutes. The membranes were washed with TBST and dried for further analysis. The dried membranes were incubated with chemiluminescent reagents (Super Signal West Pico, Pierce Inc.) for 1–2 minutes. The digitized blot images were developed using the GeneSys imaging system. ImageJ software was used to quantify and analyze the expression of GLT-1, BDNF, Arc, p-nNOS, nNOS, p-PSD-95, PSD-95, and β-tubulin. The water-control group (Ethanol-naïve group) data served as 100% (relative to water-control) to assess alterations in the expression of these proteins, of interest, as performed in previous studies (Alasmari et al., 2018; Devoto et al., 2013; Koehler et al., 2019; Li et al., 2003; Raval et al., 2003; Zhang and Tan, 2011).
Statistical analysis
Two-way ANOVA followed by Bonferroni post-hoc tests were conducted for the statistical analyses. Two-factor ANOVA with treatment (ethanol vs water) and location (AcbCo vs AcbSh) was used to detect main effects for each factor and the interaction between factors, using Graph Pad Prism software. For ceftriaxone (CEF) experiment, two-way repeated measures ANOVA test followed by Bonferroni post-hoc multiple comparison test was used to compare ethanol drinking behavior between ethanol-saline and ethanol-CEF groups. Finally, we used one-way ANOVA followed by Newman-Keuls multiple comparison test to compare protein expression of BDNF and Arc between water-control, ethanol-saline and ethanol-CEF200 groups. p-values of 0.05 or less are presented as statistically significant.
Results:
Effects of chronic ethanol consumption on the expression of BDNF in the AcbSh and AcbCo
We further measured the effects of 6-weeks of continuous ethanol consumption on the expression of BDNF in the AcbSh and AcbCo (Figure 2). The two-way ANOVA analysis revealed a significant main effect of treatment [F(1,26) = 5.97, p = 0.021]. Bonferroni post-hoc tests revealed a significant increase in the expression of BDNF (p < 0.05) in the AcbSh of the ethanol group compared to the water-control group, whereas there was no significant change in the expression of BDNF in the AcbCo (Figure 2B).
Figure 2. Effect of six weeks ethanol consumption on BDNF protein expression in the AcbCo and AcbSh.
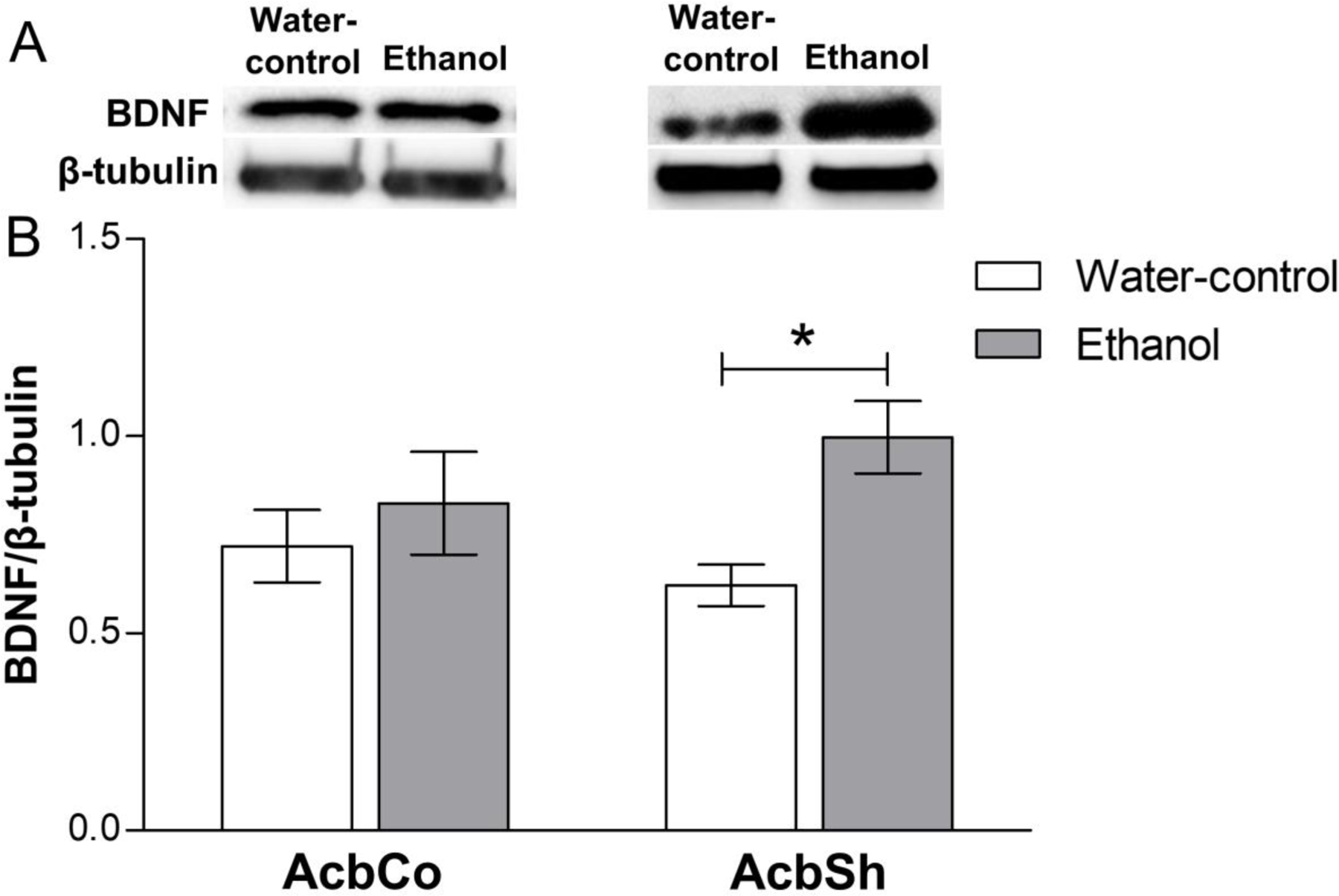
(A) Representative immunoblots for BDNF and β-tubulin of water-control vs ethanol groups in AcbCo (left panel) and AcbSh (right panel). (B) Statistical analyses revealed no significant change in BDNF protein expression in the AcbCo of the ethanol group, as compared to the water-control group. A significant upregulation of BDNF protein expression in the AcbSh was observed following chronic ethanol drinking. Data are shown as mean ± SEM; (*p < 0.05); (n = 7–8 for each group).
Effects of chronic ethanol consumption on the expression of Arc in the AcbSh and AcbCo
We also determined the effects of 6-weeks of continuous ethanol consumption on the expression of Arc in the AcbSh and AcbCo (Figure 3). Statistical analysis showed that there was a trend towards significance for treatment [F(1,28) = 3.78, p = 0.06] with a clear nonsignificant effect for location [F(1,28) = 0.01, p = 0.89] on the expression of Arc. Following the trend towards significance of treatment, Bonferroni post hoc analyses revealed a significant increase in Arc expression by ethanol in the AcbSh (p < 0.05), but not the AcbCo, relative to water control values (Figure 3B).
Figure 3. Effect of six weeks ethanol consumption on Arc protein expression in the AcbCo and AcbSh.
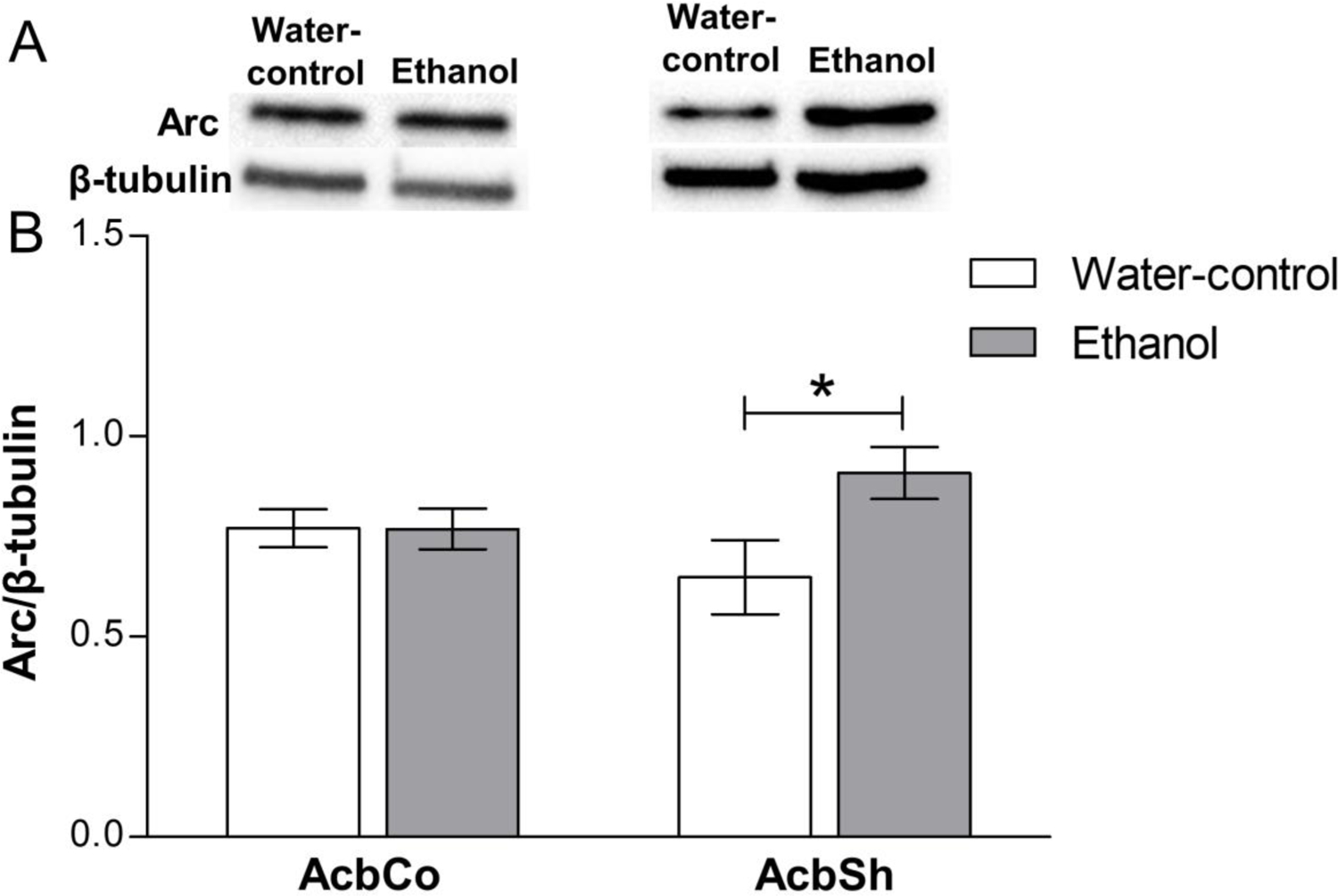
(A) Representative immunoblots for Arc and β-tubulin of water-control and ethanol groups in the AcbCo (left panel) and the AcbSh (right panel). (B) Statistical analyses revealed no significant change in Arc protein expression in the AcbCo of the ethanol group, as compared to the water-control group. A significant upregulation of Arc protein expression in the AcbSh was observed after chronic ethanol intake. Data are shown as mean ± SEM; (*p < 0.05); (n = 8 for each group).
Effects of chronic ethanol consumption on the expression of p-nNOS and t-nNOS in the AcbSh and AcbCo
We investigated the expression of phosphorylated-nNOS (p-nNOS) and total-nNOS (t-nNOS) in the AcbSh and AcbCo of P rats that had continuous access to ethanol (15% and 30%, v/v, available concurrently) for six weeks (Figure 4). The two-way ANOVA revealed a significant main effect of location on the expression of both p-nNOS [F(1,28) = 6.58, p = 0.016] and t-nNOS [F(1,28) = 4.30, p = 0.047] (Figure 4A, B). Post hoc tests showed that nNOS expression was significantly decreased by ethanol (p < 0.05) in the AcbSh, but not in the AcbCo, relative to the water-control group (Figure 4B), while there was no significant change in p-NOS expression in both Acb regions. Accordingly, the p-nNOS/nNOS ratio was significantly increased (p < 0.05) in the AcbSh, but not AcbCo, of the ethanol group vs the water-control group.
Figure 4. Effect of six weeks ethanol consumption on p-nNOS and t-nNOS protein expression in the AcbCo and AcbSh.
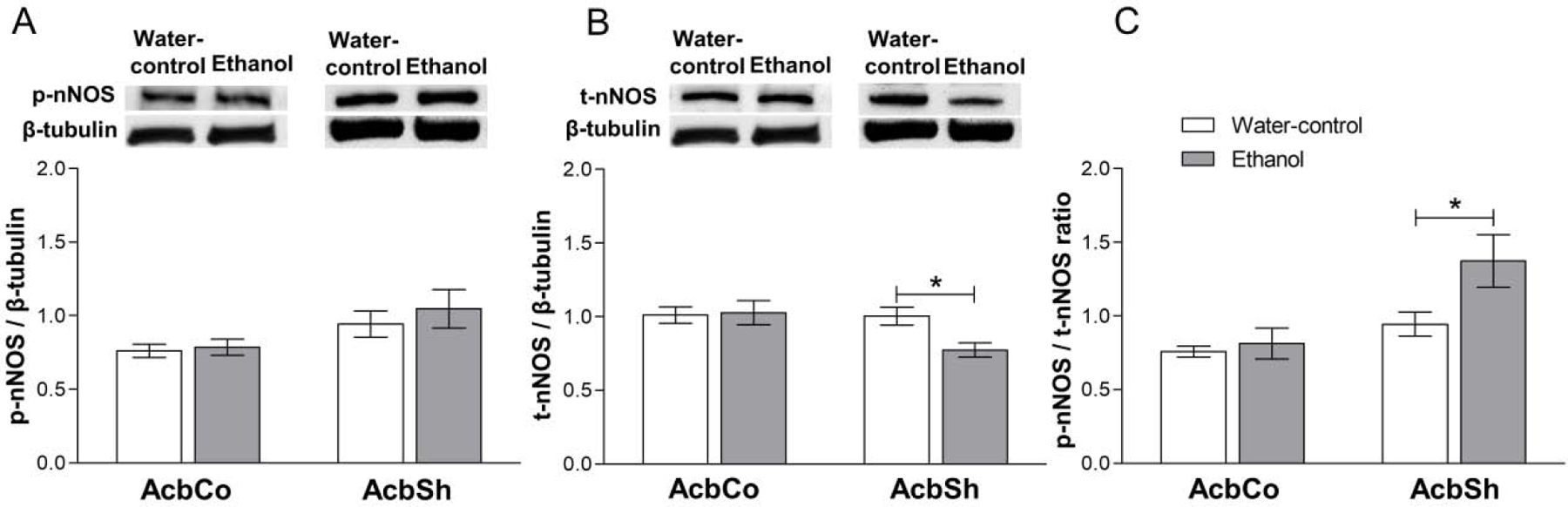
(A) Representative immunoblots for p-nNOS and β-tubulin in AcbCo and AcbSh respectively (upper panel) of the water-control and ethanol groups. Statistical analyses revealed no significant change in p-nNOS protein expression within either the AcbCo or AcbSh of the ethanol group, as compared to the water-control group (lower panel). (B) Representative immunoblots for t-nNOS and β-tubulin in the AcbCo and AcbSh, respectively (upper panel), of the water-control and ethanol groups. Statistical analyses revealed no significant change in t-nNOS protein expression in the AcbCo of the ethanol group, whereas t-nNOS protein expression in the AcbSh was significantly downregulated by chronic ethanol (lower panel). (C) Statistical analyses showed a significant increase in the p-nNOS/t-nNOS ratio following ethanol drinking in the AcbSh, but not AcbCo. The ratio is calculated by dividing the expression of p-nNOS by t-nNOS from the same western blot membranes. Data are shown as mean ± SEM; (*p < 0.05); (n = 8 for each group).
Effects of chronic ethanol consumption on the expression of p-PSD-95 and total t-PSD-95 in the AcbSh and AcbCo
The two-way ANOVA revealed a significant effect of location [F(1,28) = 9.15, p = 0.005] on p-PSD-95 expression in the Acb, but only a trend towards significance for treatment [F(1,28) = 3.78, p = 0.06] (Figure 5A). Post hoc tests showed that p-PSD-95 expression was significantly increased (p < 0.05) in the AcbSh of the ethanol exposed group as compared to the water-control group, while there were no difference in the AcbCo (Figure 5A). Total t-PSD-95 was not altered in either the AcbSh or AcbCo (Figure 5B). Although, the p-PSD-95/PSD-95 ratio was not significantly changed by ethanol in either Acb subregion (Figure 5C).
Figure 5. Effect of six weeks ethanol consumption on p-PSD-95 and t-PDS-95 protein expression in the AcbCo and AcbSh.
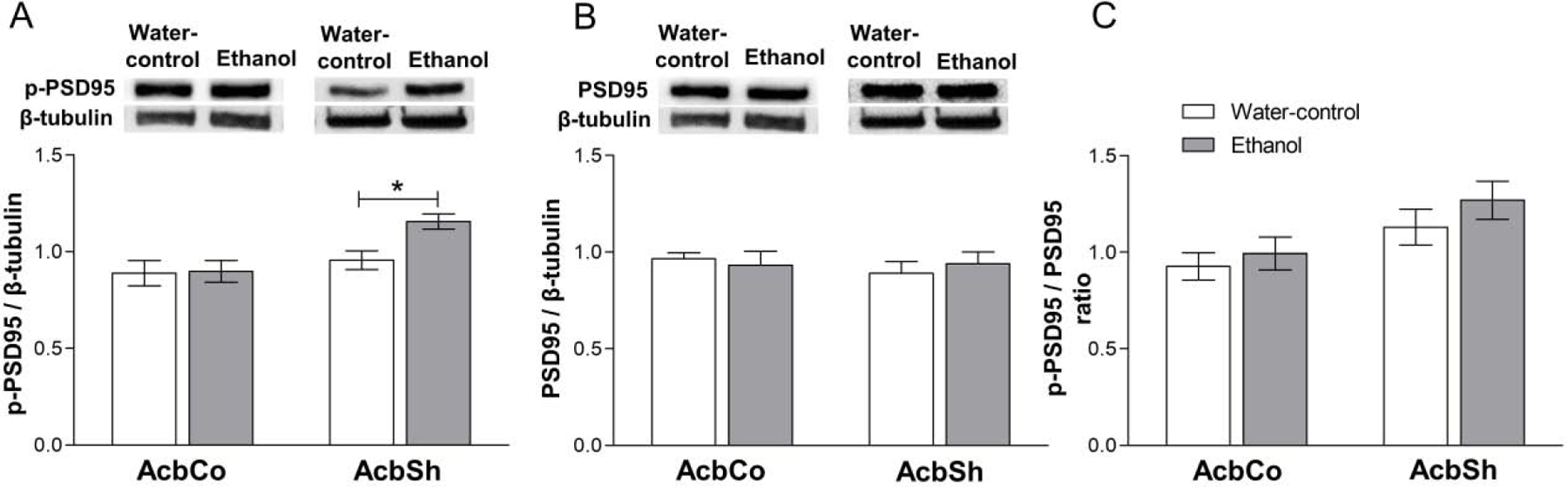
(A) Representative immunoblots for p-PSD-95 and β-tubulin in AcbCo and AcbSh respectively (upper panel) of the water-control and ethanol groups. Statistical analyses revealed no significant change in the p-PSD-95 protein expression within the AcbCo, whereas there was a significant upregulation of p-PSD-95 protein expression in the AcbSh (lower panel). (B) Representative immunoblots for PSD-95 and β-tubulin in the AcbCo and AcbSh respectively (upper panel) of the water-control and ethanol groups. Statistical analyses revealed no significant change in PSD-95 protein expression in either the AcbCo or AcbSh following ethanol drinking (lower panel). (C) Statistical analyses revealed no significant change in the p-PSD-95/PSD-95 ratio of the ethanol group compared to water-control group in either the AcbCo or AcbSh. The ratio is calculated by dividing the expression of p-PSD-95 by PSD-95 from the same western blot membranes. Data are shown as mean ± SEM; (*p < 0.05); (n = 8 for each group).
Effects of chronic ethanol consumption on the expression of GLT-1 in the AcbSh and AcbCo
We measured the expression of GLT-1 in the AcbCo and AcbSh of P rats given 6-weeks of free-choice access to 15% and 30% ethanol as well as water (available concurrently) vs water only (Figure 6). The two-way ANOVA analysis revealed significant main effects of treatment [F(1,26) = 4.86, p = 0.036] and location [F(1,26) = 6.09, p = 0.020] on GLT-1 expression. Bonferroni tests revealed a significant decrease in GLT-1 expression (p < 0.05) in the AcbSh of the ethanol group compared to the water-control group, whereas there was no significant difference within the AcbCo (Figure 6B).
Figure 6. Effect of six weeks ethanol consumption on GLT-1 protein expression in the AcbCo and AcbSh.
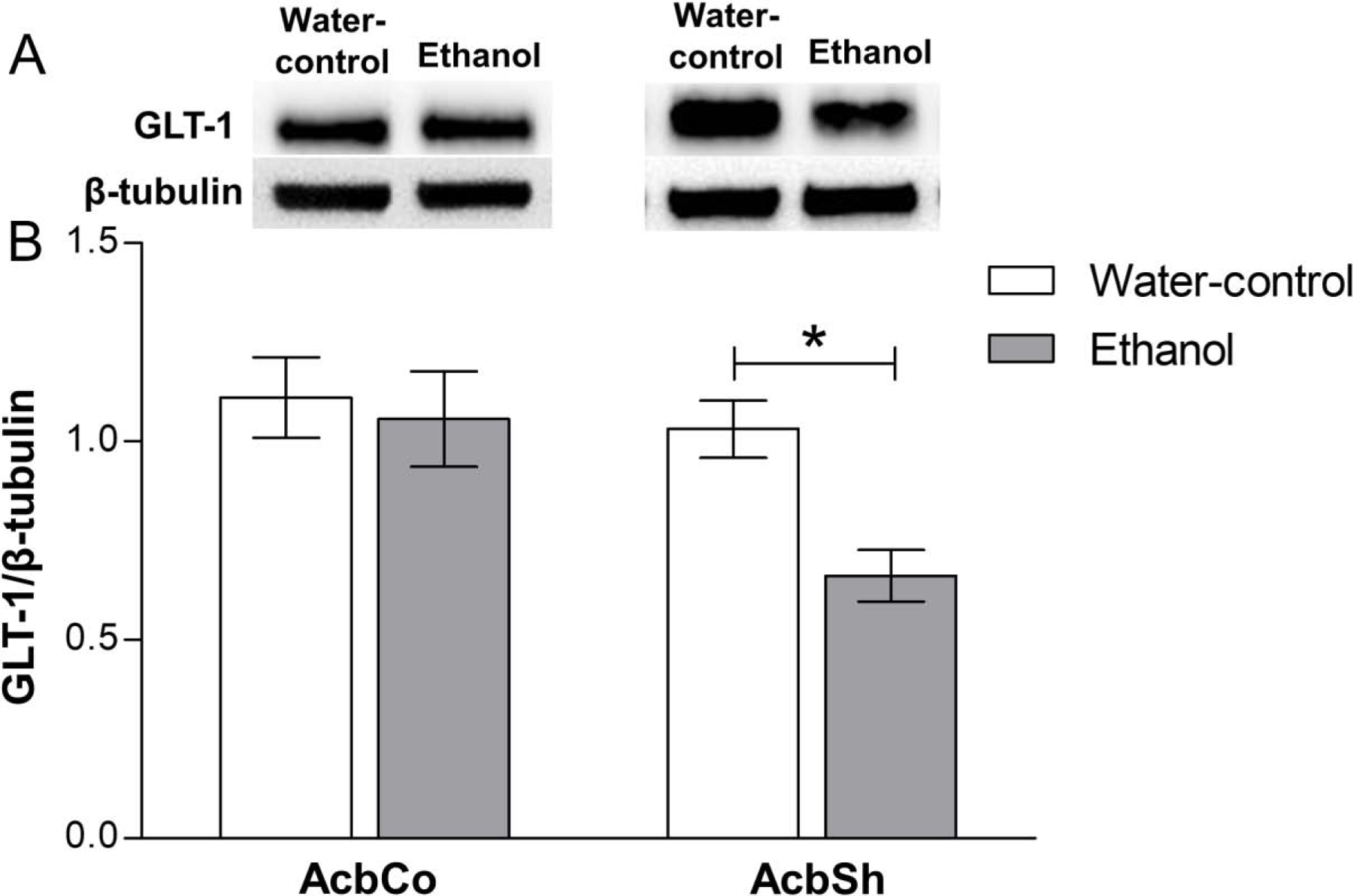
(A) Representative immunoblots for GLT-1 and β-tubulin of water-control and ethanol groups in the AcbCo (left panel) and AcbSh (right panel). (B) Statistical analyses revealed no significant change in the GLT-1 protein expression in the AcbCo of the ethanol group as compared to the water-control group, while there was a significant downregulation of GLT-1 protein expression in the AcbSh of the ethanol group, as compared to the water-control group. Data are shown as mean ± SEM; (*p < 0.05); (n = 6–8 for each group).
Effects of ceftriaxone treatment on ethanol and water consumption and protein expression of BDNF and Arc in the AcbSh
It has been proposed that CEF at dose of 100 or 200 mg/kg attenuates ethanol drinking behavior via, at least in part, upregulation of GLT-1 in P rats (Das et al., 2015; Qrunfleh et al., 2013; Sari et al., 2011). Therefore, we aimed here to investigate the effect of CEF treatment (200 mg/kg) on ethanol-induced changes in neuroplasticity proteins. Two groups of P rats (n=5–6/group) had 24 hours free access to 0, 15% and 30% ethanol in water for 5 weeks. At Day 1 of Week 6, one group received daily CEF (200 mg/kg, i.p.) injections for 5 days. The other group received equivolume i.p. injections of saline for 5 days. Another group of P rats (n=6/group) exposed to water only and served as water-control group. All rats were euthanized at Day 6. Ethanol and water consumption were calculated as described previously (Alhaddad et al., 2014b; Sari et al., 2011). Statistical analysis using two way RM ANOVA followed by bonferroni multiple comparison test showed that CEF (200 mg/kg, i.p.) treatment significantly reduced ethanol drinking as compared to ethanol-saline group [F(6,54) = 7.99, p< 0.01] at Day 3 through Day 6 as shown in Figure 7A. This effect was accompanied by significant increase in water intake in ethanol-CEF group compared to ethanol-saline group [F(6,54) = 7.99, p< 0.001] at Day 3 through Day 6 (Figure 7B). Importantly, BDNF protein expression in ethanol-CEF group was not significantly different compared to ethanol-saline or water-control group (Figure 7C), although there was significantly higher BDNF expression in ethanol-saline compared to water-control groups [F(2,17) = 4.05, p< 0.05], Figure 7D. However, CEF treatment did not affect Arc protein expression compared to ethanol-saline group [F(2,17) = 4.13, p< 0.05]. These data suggest that CEF may be useful in normalization of certain protein involved in neuroplasticity that might be affected by chronic ethanol exposure in AcbSh.
Figure 7. Effects of ceftriaxone treatment on ethanol and water drinking behavior and expression of BDNF and Arc in the AcbSh.
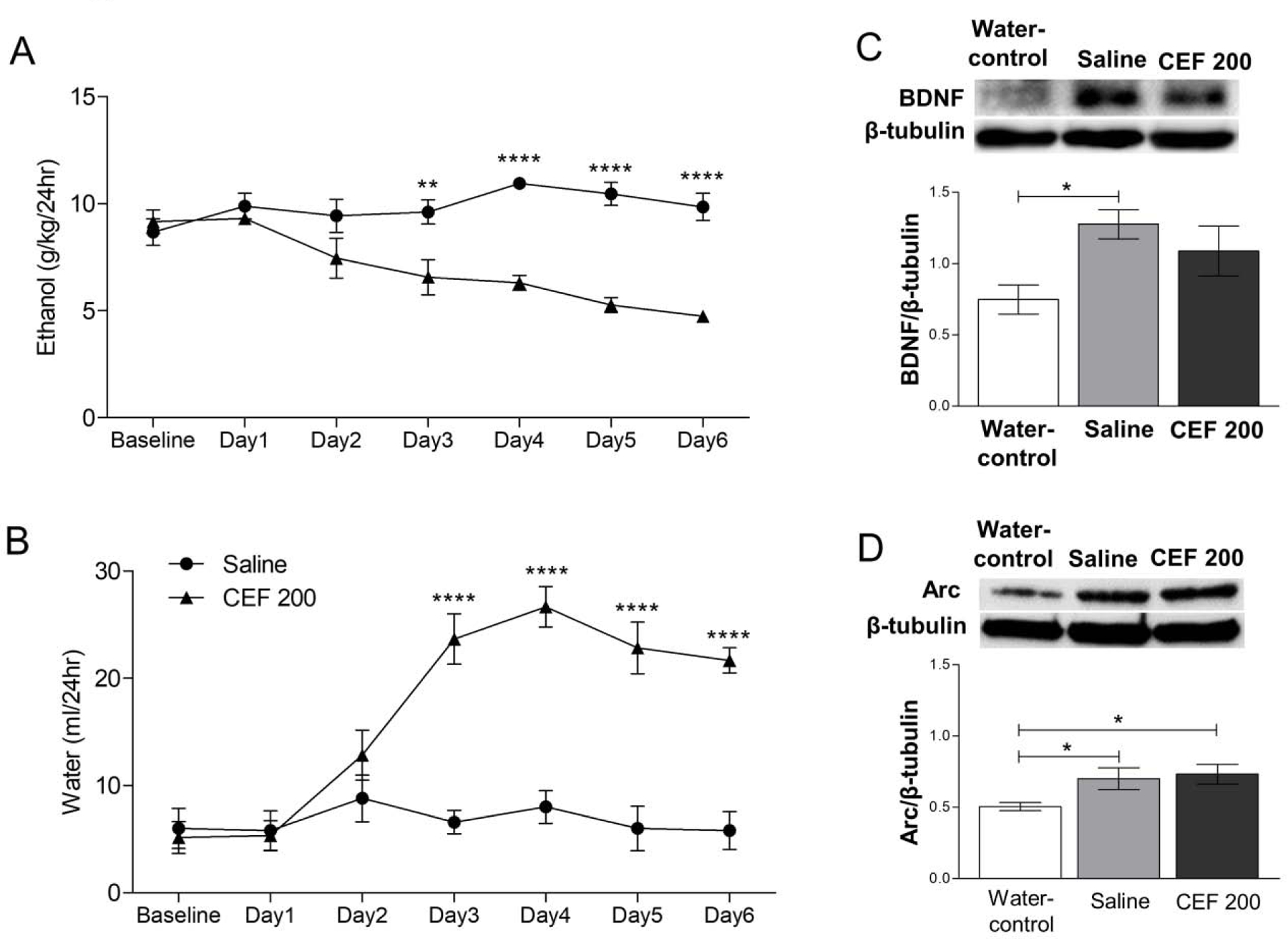
(A) CEF (200 mg/kg, i.p.) treatment significantly reduced ethanol consumption in Day 3 through Day 6 as compared to saline treated group (n=5–6/group). (B) Water consumption was significantly increased in CEF group compared to saline treated group in Day 3 through Day 6 (n=5–6/group). (C) Representative immunoblots for BDNF and β-tubulin of water-control, ethanol-saline (saline) and ethanol-CEF 200 (CEF 200) groups in the AcbSh (upper panel). Statistical analyses revealed that BDNF expression was significantly increased in ethanol-saline group compared to water-control group. There was no significant change in BDNF expression in CEF 200 group compared to ethanol-saline and water-control groups (lower panel). (D) Representative immunoblots for Arc and β-tubulin of water-control, saline and CEF 200 groups in the AcbSh (upper panel). Statistical analyses revealed that Arc expression was significantly increased in ethanol-saline and ethanol-CEF 200 group compared to water-control group. There was no significant change in the expression between CEF 200 and saline groups (lower panel). Data are shown as mean ± SEM; (*p < 0.05, **p < 0.01 and ****p<0.0001); (n = 6 for each group).
Discussion
This study revealed that chronic ethanol drinking has differential effects in the AcbSh and AcbCo of P rats. Specifically, the results showed that 6 weeks of voluntary ethanol consumption induced upregulation of BDNF, Arc, and p-PSD-95 protein expression, as well as increasing the p-nNOS/nNOS ratio, while downregulating GLT-1 expression in the AcbSh. However, we did not find any changes in the expression of these proteins within the AcbCo. These results further support a discrete role for Acb subregions in regulating ethanol consumption. Downregulation of GLT-1 in the AcbSh is in agreement with previous studies showing that chronic ethanol exposure reduced GLT-1 expression in the Acb (Alhaddad et al., 2014b; Das et al., 2015), and increased Acb extracellular glutamate concentrations (Das et al., 2015; Melendez et al., 2005). We have also recently reported that chronic ethanol consumption induced downregulation of GLT-1 in the AcbSh, but not AcbCo, of young (21–30 days old) P rats (Althobaiti et al., 2019) and high-alcohol-drinking (HAD) rats (Alasmari et al., 2020). The present results and previous studies suggest that chronic ethanol induces dysregulation of glutamate homeostasis in the mesocorticolimbic brain regions.
Additionally, the data showed that BDNF protein expression was increased in the AcbSh, but not in the AcbCo, following chronic ethanol drinking. At the glutamatergic synapse, BDNF exert synthesis-dependent regulation of synaptic modulation and neuroplasticity-inducing processes through NMDAR activation (Kojima et al., 2002). It is noteworthy that P rats have lower protein expression of BDNF in the Acb compared to alcohol-non-preferring NP rats (Yan et al., 2005). In addition, other studies have shown that BDNF expression is differentially changed based on brain region assessed and length of ethanol exposure. For instance, a single dose of ethanol induced an increase in BDNF mRNA expression in the dorsal striatum, while 6-week of ethanol drinking was associated with decreased BDNF protein expression in the cortex, but neither the dorsal nor ventral striatum, of mice (Logrip et al., 2009). Moreover, chronic ethanol self-administration increased BDNF expression in the dorsolateral, but not dorsomedial, striatum of outbred rats (Jeanblanc et al., 2009). Furthermore, the mRNA expression of BDNF in the dorsal striatum of male C57BL/6 mice was increased following exposure to ethanol (McGough et al., 2004). Interestingly, it has been shown that activation of glutamate receptors, via kainic acid or NMDA, increased the expression and synthesis of BDNF, using both in vitro and in vivo assays (Zafra et al., 1991). We suggest here that increase in glutamatergic activity, due to decrease in GLT-1 expression in the AcbSh may underlie, at least partly, increase in BDNF expression. This was confirmed with our finding (Figure 7C) that revealed CEF, which is known to upregulate GLT-1, might be associated with normalization of BDNF expression upon chronic ethanol exposure in the AcbSh.
It is important to note that BDNF was found to regulate Arc expression via the Erk1/2-CREB-Elk-1 pathways (Ramanan et al., 2005; Waltereit et al., 2001; Ying et al., 2002). Arc is an immediate early gene that is involved in neuroplasticity within the soma and dendrites (Guzowski et al., 2006). Studies have revealed that Arc expression is increased by stressful conditions, which can lead to the consolidation of neuroplasticity and long-term memory (Guzowski et al., 2000; Ons et al., 2004; Plath et al., 2006). BDNF also induced an increase in Arc expression via tyrosine kinase receptor- (TRKB)-mediated phosphorylation and activation of Erk1/2 (Davis et al., 2000; Waltereit et al., 2001; Yin et al., 2002). (Pandey et al., 2008) suggested that increases in BDNF-Arc signaling are associated with the anxiolytic effects of ethanol, possibly via neuropeptide Y (NPY) activity, whereas decreases in BDNF and Arc signaling are associated with the anxiogenic effects of ethanol withdrawal (Pandey et al., 2008). In other studies, the increase in Arc protein level, within the AcbSh, indicated activation of synapses in that region (Steward et al., 1998). Moreover, the activation of NMDA receptors appears to be required for the synthesis and targeting of Arc mRNA to stimulated synaptic regions, within dendrites, which suggests on-site (i.e., dendritic/synaptic) translation (Steward et al., 1998; Steward and Worley, 2001). Likewise, integrated signals from NMDA and AMPA glutamatergic receptors are crucial for Arc production (Rao et al., 2006). Therefore, present and previous findings suggest that increases in glutamate neurotransmission are associated with increased BDNF-Arc signaling activity in the AcbSh, which may modulate anxiety-like behavior induced by ethanol consumption and subsequent withdrawal (Pandey et al., 2008).
In the present study, the activity of nNOS was increased in the AcbSh after 6 weeks of ethanol intake as a result of increasing in the p-NOS/nNOS ratio, which suggests increases in NO production (Hinchee-Rodriguez et al., 2013; Kar et al., 2015). nNOS-derived NO is known to regulate synaptic plasticity by induction of protein involved in synaptic changes through cGMP, protein kinase G and ERK pathways (Gallo and Iadecola, 2011a). Studies have revealed contradictory roles for nNOS regarding ethanol dependence and withdrawal. For example, although consumption of ethanol is higher in nNOS KO mice compared to wild type mice (Spanagel et al., 2002), intracerebroventricular of nNOS antisense oligonucleotides resulted in decreased ethanol drinking by rats (Naassila et al., 2000) and an ethanol-associated CPP in mice (Itzhak et al., 2009). Interestingly, nNOS-derived NO production is critical for the downregulation of GLT-1 in vitro (Yamada et al., 2006). Moreover, cue-induced amphetamine- and cocaine-seeking behaviors were associated with increased glutamate release, which also was critical for the production of NO through activation of nNOS in the Acb (Siemsen et al., 2020; Smith et al., 2017). These studies supported our finding that reduction in GLT-1 expression is associated with an increase in nNOS activity (i.e., nNOS is phosphorylated resulting in a higher p-nNOS/nNOS ratio) in the AcbSh of chronically drinking P rats. Additionally, a recent study from our laboratory found that 6-weeks of ethanol consumption decreased glucocorticoid receptor (GR)-α mRNA expression in the AcbSh, while GR-β mRNA expression was reduced in the AcbCo (Alhaddad et al., 2020). Alternatively, chronic ethanol exposure was associated with inflammatory response in AcbSh but not AcbCo in HAD rats, and treatment with β-lactam antibiotic (ampicillin/sulbactam) was able to restore ethanol-induced inflammatory responses probably by modulating GLT-1 expression (Alasmari et al., 2020). These findings with nNOS expression alterations in our study suggest that chronic ethanol consumption may be associated with neuroinflammation specifically in the AcbSh.
The present results revealed that BDNF, Arc and p-PSD-95 protein expression were increased in the AcbSh after 6 weeks of ethanol intake. PSD-95 involved in synaptic remodeling, stabilization and regulation of activity-dependent synaptic plasticity (Maletic-Savatic et al., 1999; Migaud et al., 1998). It has been shown that PSD-95 mediates several ethanol drinking behaviors; such as reduced ethanol drinking and hypersensitivity to some of ethanol’s acute intoxicating effects after functional deletion of PSD-95 (Camp et al., 2011). Others have shown that stimulation of NMDA receptors (an ethanol-associated effect) results in activation of nNOS and the production of NO (Bredt and Snyder, 1990; Brenman et al., 1996). This activation may be mediated through PSD-95 activity, since the nNOS-PSD-95 pathway mediates post-synaptic nNOS activity (Zhou and Zhu, 2009). In addition, nNOS-derived NO increased the expression of BDNF and Arc in an in vitro cortical neuronal culture (Gallo and Iadecola, 2011b), which confirms a role for NO signaling in neuroplasticity (Lu et al., 1999; O’Dell, 1991; Schuman and Madison, 1991). It has been suggested that BDNF and NO are crucial for synaptic plasticity (Biojone et al., 2015). By its action at TRKB, BDNF upregulates nNOS expression and increased NO production (Biojone et al., 2015) as well as increased CREB-dependent gene expression, which results in enhancement of synaptic potentiation (Hardingham et al., 2013; Nott et al., 2008). These studies suggested that post-translational modifications of BDNF, through a NO-dependent pathway, reduced the effects of BDNF on synaptic strength. Similarly, NO decreases the efficacy of BDNF at its receptor (TRKB), by NO-derived nitration of BDNF and/or TRKB, as a negative feedback mechanism. By extension, NO and BDNF act together to induce nitration or phosphorylation of TRKB, which activates or deactivates specific downstream cellular pathways of TRKB (Biojone et al., 2015). Thus, increases in BDNF and Arc expression as well as nNOS activity, in the AcbSh, may modulate neuroplasticity associated with chronic ethanol consumption (Figure 8). However, a prior study showed that ceftriaxone did not induce any cganges on the level of BDNF in the hippocampus and frontal cortex in animals had developed pneumococcal meningitis (Barichello et al., 2014). More research is needed to study the effects of ceftriaxone on BDNF in the AcbSh and AcbCo.
Figure 8.
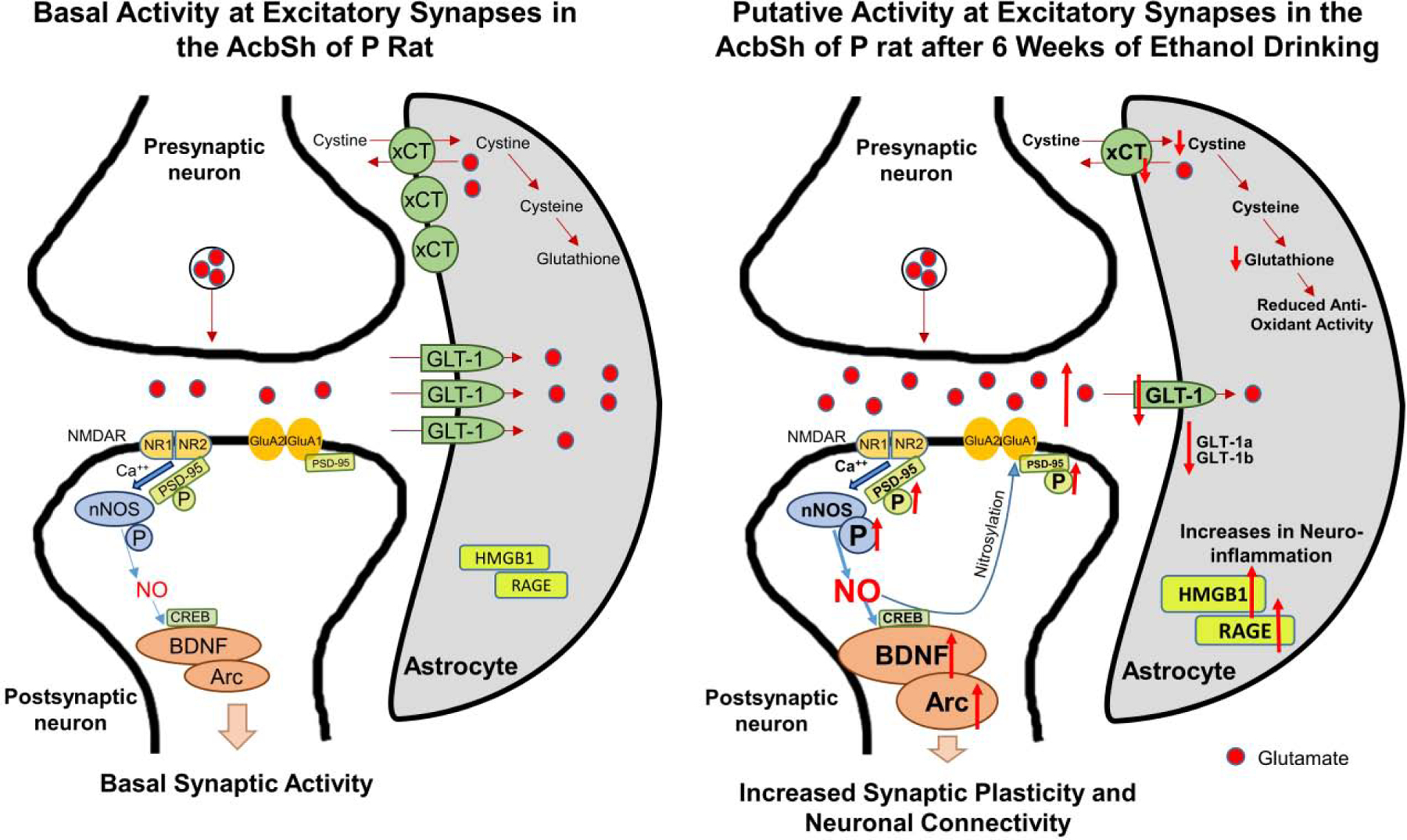
Schematic diagram summarizes the effects of chronic ethanol consumption on GLT-1, NO pathway, BDNF, and Arc expression in the AcbSh. Chronic ethanol consumption increases synaptic glutamate concentration mainly due to downregulation of GLT-1 (both GLT-1a and GLT-1b isoforms) and cystine/glutamate exchanger transporter (xCT) expression (Alhaddad et al., 2014a; Das et al., 2015). Chronic ethanol consumption also increases NO system activity, BDNF-Arc expression and PSD-95. NO regulates the incorporation of postsynaptic GluA1 subunit of AMPA receptors probably through CREB-BDNF-Arc pathway and ubiquitination of PSD-95. In addition, chronic ethanol exposure was associated with increases in the inflammatory response through upregulation of inflammatory mediators such as high mobility group box 1 (HMGB1), a receptor for advanced glycation end products (RAGE) and TNF-α (Alasmari et al., 2020).
Conclusion and Future Direction
In conclusion, chronic ethanol consumption by P rats caused reductions in GLT-1 expression in the AcbSh with probable increases in extracellular glutamate levels, which can lead to overstimulation of NMDA receptors and increased PSD-95-nNOS activity. This activity can lead to increases in the expression of BDNF and Arc, as observed in the present study. This BDNF-Arc signaling pathway, in turn, regulates synaptic plasticity and neuronal connectivity. However, this neuroplasticity may lead to incongruous patterns of connections within the AcbSh, which results in a disruption of glutamate homeostasis, as it was shown in previous work as well this present study. However, treatment with CEF was associated with tendency to normalize BDNF expression. In accordance to our previous findings, these alterations were not observed in the AcbCo. Therefore, the maintenance of ethanol drinking might be maintained by dysregulated neuronal pathways in the AcbSh, which is a critical brain region of the mesocorticolimbic reward neurocircuitry (Augur et al., 2016; Keistler et al., 2015). Future studies are warranted to investigate the effects of long-term CEF treatment on the expression of BDNF, Arc, p-nNOS, nNOS, and p-PSD-95 in the AcbSh in animals exposed to alcohol.
Highlights.
Chronic ethanol exposure increased BDNF and Arc, and reduced GLT-1 in Acb-shell in P rats.
Chronic ethanol exposure increased phospho-neuronal NO synthase in Acb-shell.
Ceftriaxone normalized BDNF expression against the effect of ethanol intake in Acb-shell
Funding
The presented research was supported by grant AA019458 (Y. Sari) and AA013522 and AA015512 (R.L. Bell) from. The authors extend their appreciation to the National Institutes on Alcohol Abuse and Alcoholism [AA019458 (Y. Sari) and AA013522 and AA015512 (R.L. Bell)] and International Scientific Partnership Program ISPP at King Saud University [ISPP-146] for funding this research work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Alasmari F, Alexander LEC, Nelson JA, Schiefer IT, Breen E, Drummond CA, Sari Y, 2017. Effects of chronic inhalation of electronic cigarettes containing nicotine on glial glutamate transporters and α−7 nicotinic acetylcholine receptor in female CD-1 mice. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 77, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Bell RL, Rao PSS, Hammad AM, Sari Y, 2018. Peri-adolescent drinking of ethanol and/or nicotine modulates astroglial glutamate transporters and metabotropic glutamate receptor-1 in female alcohol-preferring rats. Pharmacol Biochem Behav 170, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Alhaddad H, Wong W, Bell RL, Sari Y, 2020. Ampicillin/Sulbactam Treatment Modulates NMDA Receptor NR2B Subunit and Attenuates Neuroinflammation and Alcohol Intake in Male High Alcohol Drinking Rats. Biomolecules. 10, 1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y, 2014a. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 231, 4049–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, Wei Y, Sari Y, 2014b. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Frontiers in Behavioral Neuroscience. 8, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Gordon DM, Bell RL, Jarvis EE, Kipp ZA, Hinds TD, Sari Y, 2020. Chronic ethanol consumption alters glucocorticoid receptor isoform expression in stress neurocircuits and mesocorticolimbic brain regions of alcohol-preferring rats. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althobaiti YS, Alshehri FS, Hakami AY, Hammad AM, Sari Y, 2019. Effects of clavulanic acid treatment on reinstatement to methamphetamine, glial glutamate transporters, and mGluR 2/3 expression in P rats exposed to ethanol. Journal of Molecular Neuroscience. 67, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augur IF, Wyckoff AR, Aston-Jones G, Kalivas PW, Peters J, 2016. Chemogenetic activation of an extinction neural circuit reduces cue-induced reinstatement of cocaine seeking. Journal of Neuroscience. 36, 10174–10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barichello T, Carlos Nepomuceno Goncalves J, da Silva Generoso J, Roque Simoes L, Hikaru Tashiro M, de Assis Goularte J, Vuolo F, Henrique Rodrigues D, Carvalho Vilela M, Petronilho F, 2014. Protection of blood brain barrier integrity and modulation of inflammatory mediators during treatment of pneumococcal meningitis with daptomycin or ceftriaxone. Current Neurovascular Research. 11, 210–222. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ, 2006. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addiction Biology. 11, 270–288. [DOI] [PubMed] [Google Scholar]
- Bell RL, Sable HJ, Colombo G, Hyytia P, Rodd ZA, Lumeng L, 2012. Animal models for medications development targeting alcohol abuse using selectively bred rat lines: neurobiological and pharmacological validity. Pharmacol Biochem Behav 103, 119–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biojone C, Cabrera Casarotto P, Regiane Joca S, Castren E, 2015. Interplay between nitric oxide and brain-derived neurotrophic factor in neuronal plasticity. CNS Neurological Disorders-Drug Targets. 14, 979–987. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH, 1990. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proceedings of the National Academy of Sciences. 87, 682–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, 1996. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell. 84, 757–767. [DOI] [PubMed] [Google Scholar]
- Camp MC, Feyder M, Ihne J, Palachick B, Hurd B, Karlsson RM, Noronha B, Chen YC, Coba MP, Grant SG, 2011. A novel role for PSD-95 in mediating ethanol intoxication, drinking and place preference. Addiction Biology. 16, 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G, Norwood D, Sumners C, Crews FT, 1997. Chronic Ethanol IncreasesN-Methyl-D-Aspartate-Stimulated Nitric Oxide Formation but Not Receptor Density in Cultured Cortical Neurons. Molecular Pharmacology. 51, 733–740. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Carpenter-Hyland E, Hendricson AW, Maldve RE, Morrisett RA, Zhou FC, Sari Y, Bell R, Szumlinski KK, 2006. Structural and functional modifications in glutamateric synapses following prolonged ethanol exposure. Alcoholism: Clinical and Experimental Research. 30, 368–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y, 2015. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 97, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y, 2015. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 97, 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto VP, Bogetti ME, de Plazas SF, 2013. Developmental and hypoxia-induced cell death share common ultrastructural and biochemical apoptotic features in the central nervous system. Neuroscience. 252, 190–200. [DOI] [PubMed] [Google Scholar]
- Ehrlich D, Pirchl M, Humpel C, 2012. Effects of long-term moderate ethanol and cholesterol on cognition, cholinergic neurons, inflammation, and vascular impairment in rats. Neuroscience. 205, 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo EF, Iadecola C, 2011a. Neuronal nitric oxide contributes to neuroplasticity-associated protein expression through cGMP, protein kinase G, and extracellular signal-regulated kinase. Journal of Neuroscience. 31, 6947–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande I, Fries GR, Kunz M, Kapczinski F, 2010. The role of BDNF as a mediator of neuroplasticity in bipolar disorder. Psychiatry Investigations. 7, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA, 2000. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. Journal of Neuroscience. 20, 3993–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Miyashita T, Chawla MK, Sanderson J, Maes LI, Houston FP, Lipa P, McNaughton BL, Worley PF, Barnes CA, 2006. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proceedings of the National Academy of Sciences. 103, 1077–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad AM, Alasmari F, Althobaiti YS, Sari Y, 2017. Modulatory effects of Ampicillin/Sulbactam on glial glutamate transporters and metabotropic glutamate receptor 1 as well as reinstatement to cocaine-seeking behavior. Behavioural Brain Research. 332, 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham N, Dachtler J, Fox K, 2013. The role of nitric oxide in pre-synaptic plasticity and homeostasis. Frontiers in Cellular Neuroscience. 7, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT, 2005. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. European Journal of Neuroscience. 21, 2711–2720. [DOI] [PubMed] [Google Scholar]
- Heinze H-J, Heldmann M, Voges J, Hinrichs H, Marco-Pallares J, Hopf J-M, Müller U, Galazky I, Sturm V, Bogerts B, 2009. Counteracting incentive sensitization in severe alcohol dependence using deep brain stimulation of the nucleus accumbens: clinical and basic science aspects. Frontiers in Human Neuroscience. 3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchee-Rodriguez K, Garg N, Venkatakrishnan P, Roman MG, Adamo ML, Masters BS, Roman L, 2013. Neuronal nitric oxide synthase is phosphorylated in response to insulin stimulation in skeletal muscle. Biochemical Biophysical Research Communications. 435, 501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL, 2000. Blockade of alcohol-induced locomotor sensitization and conditioned place preference in DBA mice by 7-nitroindazole. Brain Research. 858, 402–407. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Roger-Sánchez C, Anderson KL, 2009. Role of the nNOS gene in ethanol-induced conditioned place preference in mice. Alcohol. 43, 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanblanc J, He D-Y, Carnicella S, Kharazia V, Janak PH, Ron D, 2009. Endogenous BDNF in the dorsolateral striatum gates alcohol drinking. Journal of Neuroscience. 29, 13494–13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA, 2011. In vivo chronic intermittent ethanol exposure reverses the polarity of synaptic plasticity in the nucleus accumbens shell. Journal of Pharmacology Experimental Therapeutics. 336, 155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Saha S, Martin GE, 2015. The origin of glutamatergic synaptic inputs controls synaptic plasticity and its modulation by alcohol in mice nucleus accumbens. Frontiers in Synaptic Neuroscience. 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Saha S, Kolpakova J, Guildford M, Tapper AR, Martin GE, 2017. Dopamine receptors differentially control binge alcohol drinking-mediated synaptic plasticity of the core nucleus accumbens direct and indirect pathways. Journal of Neuroscience. 37, 5463–5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, 2009. The glutamate homeostasis hypothesis of addiction. Nature Reviews Neuroscience. 10, 561–572. [DOI] [PubMed] [Google Scholar]
- Kar R, Kellogg DL III, Roman L, 2015. Oxidative stress induces phosphorylation of neuronal NOS in cardiomyocytes through AMP-activated protein kinase (AMPK). Biochemical Biophysical Research Communications. 459, 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keistler C, Barker JM, Taylor JR, 2015. Infralimbic prefrontal cortex interacts with nucleus accumbens shell to unmask expression of outcome-selective Pavlovian-to-instrumental transfer. Journal of Learning and Memory. 22, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna J, Morato G, Shah G, Chau A, Kalant H, 1993. Inhibition of nitric oxide synthesis impairs rapid tolerance to ethanol. Brain Research Bulletin. 32, 43–47. [DOI] [PubMed] [Google Scholar]
- Khanna J, Morato G, Chau A, Shan G, 1995. Influence of nitric oxide synthase inhibition on the development of rapid tolerance to ethanol. Brain Research Bulletin. 37, 599–604. [DOI] [PubMed] [Google Scholar]
- Koehler D, Shah ZA, Williams FE, 2019. The GSK3β inhibitor, TDZD-8, rescues cognition in a zebrafish model of okadaic acid-induced Alzheimer’s disease. Neurochemistry International. 122, 31–37. [DOI] [PubMed] [Google Scholar]
- Kojima M, Klein RL, Hatanaka H, 2002. Pre-and post-synaptic modification by neurotrophins. Neuroscience Research. 43, 193–199. [DOI] [PubMed] [Google Scholar]
- Li J, Olinger A, Dassow M, Abel M, 2003. Up-regulation of GABA B receptor mRNA and protein in the hippocampus of cocaine-and lidocaine-kindled rats. Neuroscience. 118, 451–462. [DOI] [PubMed] [Google Scholar]
- Li TK, Lumeng L, McBride WJ, Murphy JM, 1987. Rodent lines selected for factors affecting alcohol consumption. Alcohol and Alcoholism. 1, 91–96. [PubMed] [Google Scholar]
- Logrip ML, Janak PH, Ron D, 2009. Escalating ethanol intake is associated with altered corticostriatal BDNF expression. Journal of Neurochemistry. 109, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-F, Kandel ER, Hawkins RD, 1999. Nitric oxide signaling contributes to late-phase LTP and CREB phosphorylation in the hippocampus. Journal of Neuroscience. 19, 10250–10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv X-F, Xu Y, Han J-S, Cui C-L, 2011. Expression of activity-regulated cytoskeleton-associated protein (Arc/Arg3. 1) in the nucleus accumbens is critical for the acquisition, expression and reinstatement of morphine-induced conditioned place preference. Behavioural Brain Research. 223, 182–191. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Malinow R, Svoboda K, 1999. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 283, 1923–1927. [DOI] [PubMed] [Google Scholar]
- Martin JL, Finsterwald C, 2011. Cooperation between BDNF and glutamate in the regulation of synaptic transmission and neuronal development. Commun Integr Biol 4, 14–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough NN, He D-Y, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, Ron D, 2004. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. Journal of Neuroscience. 24, 10542–10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW, 2005. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism: Clinical and Experimental Research. 29, 326–333. [DOI] [PubMed] [Google Scholar]
- Melo CV, Mele M, Curcio M, Comprido D, Silva CG, Duarte CB, 2013. BDNF regulates the expression and distribution of vesicular glutamate transporters in cultured hippocampal neurons. PloS one. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, 1998. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 396, 433–439. [DOI] [PubMed] [Google Scholar]
- Müller U, Sturm V, Voges J, Heinze H-J, Galazky I, Büntjen L, Heldmann M, Frodl T, Steiner J, Bogerts B, 2016. Nucleus accumbens deep brain stimulation for alcohol addiction–safety and clinical long-term results of a pilot trial. Pharmacopsychiatry. 49, 170–173. [DOI] [PubMed] [Google Scholar]
- Naassila M, Beauge F, Sebire N, Daoust M, Behavior, 2000. Intracerebroventricular injection of antisense oligos to nNOS decreases rat ethanol intake. Pharmacology, Biochemistry & Behavior. 67, 629–636. [DOI] [PubMed] [Google Scholar]
- Neasta J, Hamida SB, Yowell QV, Carnicella S, Ron D, 2011. AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biological Psychiatry. 70, 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A, 2008. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 455, 411–415. [DOI] [PubMed] [Google Scholar]
- O’Dell T, Hawkins R, Kandel ER, Arancio O, 1991. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proceedings of the National Academy of Sciences. 88, 11285–11289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ons S, Martí O, Armario A, 2004. Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-fos mRNA. Journal of Neurochemistry. 89, 1111–1118. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K, 2008. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. Journal of Neuroscience. 28, 2589–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2007. The rat brain in stereotaxic coordinates. Academic Press/Elsevier:Boston, MA. [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, 2006. Arc/Arg3. 1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 52, 437–444. [DOI] [PubMed] [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y, 2013. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. Journal of Psychopharmacology. 27, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan N, Shen Y, Sarsfield S, Lemberger T, Schütz G, Linden DJ, Ginty DD, 2005. SRF mediates activity-induced gene expression and synaptic plasticity but not neuronal viability. Nature Neuroscience. 8, 759–767. [DOI] [PubMed] [Google Scholar]
- Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S, 2006. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nature Neuroscience. 9, 887–895. [DOI] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Mochly-Rosen D, Sick TJ, Pérez-Pinzón MA, 2003. εPKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. Journal of Neuroscience. 23, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Alvania RS, Lonze BE, Ramanan N, Kim T, Huang Y, Dawson TM, Snyder SH, Ginty DD, 2006. A nitric oxide signaling pathway controls CREB-mediated gene expression in neurons. Molecular Cell. 21, 283–294. [DOI] [PubMed] [Google Scholar]
- Santos-Rocha JB, Rae M, Teixeira AMA, Teixeira SA, Munhoz CD, Muscará MN, Marcourakis T, Szumlinski KK, Camarini R, 2018. Involvement of neuronal nitric oxide synthase in cross-sensitization between chronic unpredictable stress and ethanol in adolescent and adult mice. Alcohol. 68, 71–79. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV, 2009. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci 29, 9239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL, 2011. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol and Alcoholism. 46, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN, 2012. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 227, 327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler R, Xiong Z, Lu W-Y, Hafner M, MacDonald JF, Tymianski M, 1999. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 284, 1845–1848. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Madison DV, 1991. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 254, 1503–1506. [DOI] [PubMed] [Google Scholar]
- Scofield M, Heinsbroek J, Gipson C, Kupchik Y, Spencer S, Smith A, Roberts-Wolfe D, Kalivas P, 2016. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacological Reviews. 68, 816–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillinglaw JE, Morrisett RA, Mangieri RA, 2018. Ethanol modulates glutamatergic transmission and NMDAR-mediated synaptic plasticity in the agranular insular cortex. Frontiers in Pharmacology. 9, 1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemsen B, McFaddin J, Haigh K, Brock A, Leath MN, Hooker K, McGonegal L, Scofield M, 2020. Amperometric measurements of cocaine cue and novel context-evoked glutamate and nitric oxide release in the nucleus accumbens core. Journal of Neurochemistry. e14952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, Spencer S, Garcia-Keller C, Stankeviciute NM, Smith RJ, 2017. Accumbens nNOS interneurons regulate cocaine relapse. Journal of Neuroscience. 37, 742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Siegmund S, Cowen M, Schroff K-C, Schumann G, Fiserova M, Sillaber I, Wellek S, Singer M, Putzke J, 2002. The neuronal nitric oxide synthase gene is critically involved in neurobehavioral effects of alcohol. Journal of Neuroscience. 22, 8676–8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Talani G, Mulas G, Licheri V, Fois GR, Muggironi G, Masala N, Cannizzaro C, Biggio G, Sanna E, 2014. Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats. Proceedings of the National Academy of Sciences. 111, E3745–E3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Kalivas PW, 2016. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Structure and Function. 221, 1681–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF, 1998. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 21, 741–751. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF, 2001. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 30, 227–240. [DOI] [PubMed] [Google Scholar]
- Uys JD, McGuier NS, Gass JT, Griffin WC III, Ball LE, Mulholland PJ, 2016. Chronic intermittent ethanol exposure and withdrawal leads to adaptations in nucleus accumbens core postsynaptic density proteome and dendritic spines. Addiction Biology. 21, 560–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, Bundman M, Kuhl D, 2001. Arg3. 1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. Journal of Neuroscience. 21, 5484–5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Kawahara K, Kosugi T, Tanaka M, 2006. Nitric oxide produced during sublethal ischemia is crucial for the preconditioning-induced down-regulation of glutamate transporter GLT-1 in neuron/astrocyte co-cultures. Neurochemical Research. 31, 49–56. [DOI] [PubMed] [Google Scholar]
- Yan Q-S, Feng M-J, Yan S-E, 2005. Different expression of brain-derived neurotrophic factor in the nucleus accumbens of alcohol-preferring (P) and-nonpreferring (NP) rats. Brain Research. 1035, 215–218. [DOI] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW, 2002. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proceedings of the National Academy of Sciences. 99, 2368–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S-W, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TV, Bramham CR, 2002. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. Journal of Neuroscience. 22, 1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Castren E, Thoenen H, Lindholm D, 1991. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proceedings of the National Academy of Sciences. 88, 10037–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Tan Y, 2011. Nerve growth factor augments neuronal responsiveness to noradrenaline in cultured dorsal root ganglion neurons of rats. Neuroscience. 193, 72–79. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhu D-Y, 2009. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 20, 223–230. [DOI] [PubMed] [Google Scholar]


