Abstract
The muscle-tendon interface is an anatomically specialized region that is involved in the efficient transmission of force from muscle to tendon. Due to constant exposure to loading, the interface is susceptible to injury. Current treatment methods do not meet the socioeconomic demands of reduced recovery time without compromising the risk of reinjury, requiring the need for developing alternative strategies. The extracellular matrix (ECM) present in muscle, tendon and at the interface of these tissues consists of unique molecules that play significant roles in homeostasis and repair. Better, understanding the function of the ECM during development, injury and aging has the potential to unearth critical missing information that is essential for accelerating the repair at the muscle-tendon interface. Recently, advanced techniques have emerged to explore the ECM for identifying specific roles in musculoskeletal biology. Simultaneously, there is a tremendous increase in the scope for regenerative medicine strategies to address the current clinical deficiencies. Advancements in ECM research can be coupled with the latest regenerative medicine techniques to develop next generation therapies that harness ECM for treating defects at the muscle-tendon interface. The current work provides a comprehensive review on the role of muscle and tendon ECM to provide insights about the role of ECM in the muscle-tendon interface, and discusses the latest research techniques to explore the ECM to gathered information for developing regenerative medicine strategies.
Keywords: extracellular matrix, muscle-tendon interface, clinical significance, mass spectrometry, protein labeling, ECM visualization, regenerative medicine
Introduction
The musculoskeletal system, which is comprised of bone, connective tissue and skeletal muscle, provides the body with structure and facilitates movement. The central nervous system triggers signals via motor neurons to muscle, which results in a transmission of force from contracting muscle fibers through tendon to bone, enabling motion 1. The force is transmitted from muscle to tendon across an anatomically specialized interface called the myotendinous junction (MTJ) 2. Due to constant exposure of force during physical activity, the MTJ is susceptible to injury. Eccentric stretching of the muscle beyond the normal range of motion can result in injury, and the severity depends on the extent of force experienced at the muscle-tendon interface. Traditional treatment methods for acute injuries include PRICE (protection, rest, ice, compression and elevation), NSAID (non-steroidal anti-inflammatory drugs) administration and physical therapy, which assist in the repair and recovery process. However, the persistent socioeconomic burden these injuries present necessitates a need for alternate treatment options that reduce recovery time without comprising the risk of injury recurrence. Moreover, severe injuries resulting in a complete tear at the MTJ lack standardized clinical treatment methods.
Understanding the assembly of MTJ during development, repair and aging has the potential to provide essential information for the development of new strategies that address injuries at the muscle-tendon interface. Structurally, the MTJ consists of the subsarcolemma of the muscle cell membrane and transmembrane, intercellular and extracellular proteins that connect myofibers to the collagen-rich tendon matrix 3. The overall force transmission at the MTJ is dependent on the relationship between the structural units present in muscle and tendon 3. Challenges in the clinical translation of engineering technologies targeted towards the muscle-tendon interface arise due to the difficulty in integrating two disparate tissues to form a seamless MTJ 4. The extracellular matrix (ECM), representing the three-dimensional (3D) network of molecules within the tissue, forms a continuum that integrates muscle and tendon. Developmental studies identified that some ECM components play a role in cell migration, tissue formation, alignment and guidance at the MTJ 5; however, there is limited knowledge regarding the role and overall composition of the ECM at the MTJ either during morphogenesis or in adults during repair and aging. Understanding the role and composition of the ECM present in muscle and tendon can provide information regarding the ECM at the muscle-tendon interface. Skeletal muscle ECM provides chemical, physical and biological cues that modulate tissue homeostasis 6, tissue remodeling 7, and cell-ECM interactions that regulate myoblast migration8, alignment9, proliferation10 and differentiation11. Similarly, tendon ECM plays a role in adaptation to mechanical loading 12, modulation of the tendon stem cell niche 13, and cell-ECM interactions leading to tenocyte migration14, proliferation14 and differentiation13. Therefore, better understanding the roles of the ECM that integrates muscle and tendon at the MTJ can unearth important information that is beneficial for developing novel regenerative medicine strategies. This review focuses on describing the existing knowledge on the structure of the MTJ, clinical relevance to injuries at the MTJ, and the role and distribution of ECM in the muscle and tendon during development, homeostasis, repair and aging to provide insights about the ECM at the muscle-tendon interface. Finally, the review highlights the latest techniques employed for exploring ECM composition and dynamics that have the potential to deliver guidance for developing novel regenerative therapies.
The Myotendinous Junction
The MTJ is a highly specialized and architecturally complex structure, where the sarcolemma of the muscle meets the ECM of the tendon (Figure 1). At the junction, muscle fibers break into subdivisions of several sarcomeres by the inside-out folding of the sarcolemma. Myofilaments of these subdivisions insert into the plasma membrane at the subsarcolemmal layer 15. Structurally, the MTJ comprises of actin microfilaments and actin binding proteins that extend from the last z-band to the sarcolemma, the transmembrane proteins that connect the cytoskeletal components to the basement membrane, and the proteins that link the basement membrane with the tendon ECM 16. The finger-like processes at the MTJ are critical in force transmission and are hypothesized to reduce stress at the interface by increasing the surface area by 10–20 fold 15. In addition, the angle of incident stress is lowered in such a way that shear stress is experienced, which leads to force transmission parallel to the basement membrane, improving the strength at the MTJ and preventing failure 17,18.
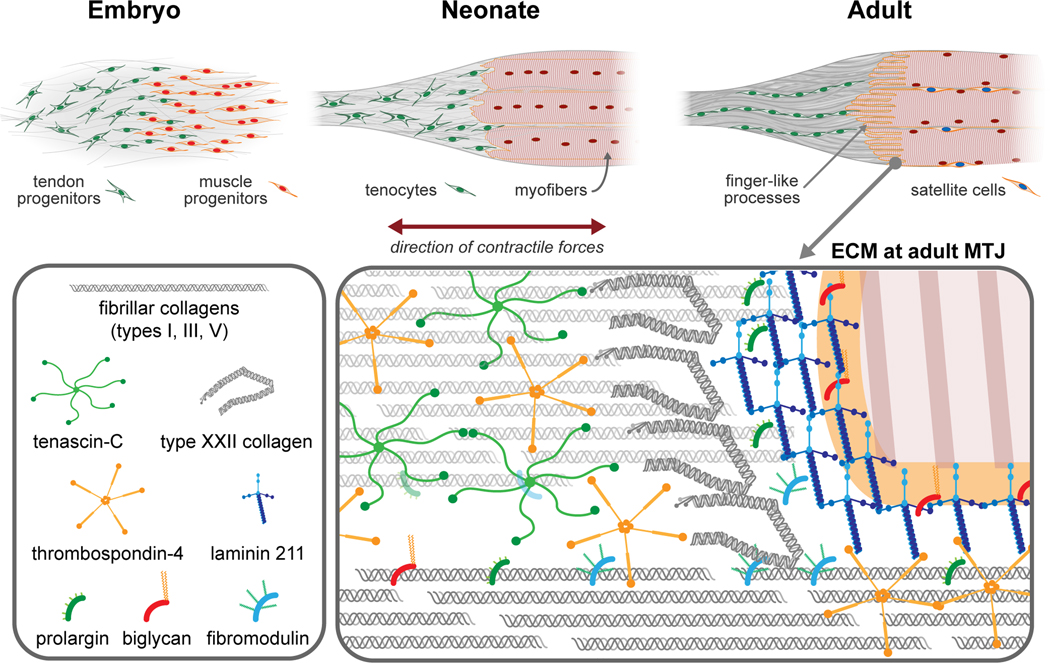
Figure 1:
Development of the MTJ (top). During embryogenesis, tendon and muscle progenitors condense at the site of future MTJ. In the neonate, the muscle - tendon interface appears smooth and there are very few finger-like processes present. In the adult, muscle fibers are mature and perfectly aligned at the MTJ. Finger-like extensions of the myofibers are well established and integrated with the tendon structure. The tendon ECM and cells are aligned along the direction of the load. Distribution of ECM discussed in this review within MTJ (bottom).
MTJ Assembly During Development
MTJ formation begins during embryogenesis and continues to develop and mature through the neonatal period (Figure 1). During embryogenesis, tendon and muscle progenitors coexist together at the site of the future MTJ 19. Then, tendon progenitors and ECM components are thought to align along the direction of the contractile force experienced during muscle development 19. Cross-talk between muscle and tendon progenitors, via cell-cell interactions, endocrine signaling and paracrine signaling, activate key signaling pathways that are critical for the proper maturation and functioning of the MTJ. For example, the neuroregulatory ligand vein is secreted by muscle progenitors in Drosophila, and activates the EGF signaling pathway in tendon progenitors, which in turn determines the fate of tendon cell differentiation 20. Similarly, FGF4 signaling from muscle progenitors induces expression of scleraxis and tenascin-C in tendon progenitors, which modulates tendon maturation 21. In addition, retinoic acid signaling between these two populations was previously established to play an important role in muscle cell apoptosis and myotendinous assembly 22. Several other proteins (e.g. Moleskin, slowdown, thrombospondin-4) and microRNAs (e.g. miR-9a) are also thought to be involved in the cross talk between muscle and tendon progenitors to regulate proper assembly of the MTJ during development 23–26. Further details on the role of cell communication during the assembly and maturation of the MTJ is reviewed elsewhere 3, 5.
Interactions between muscle and tendon progenitors are not limited to cellular and molecular crosstalk during MTJ assembly. Several studies indicate that the assembly of the MTJ is also mediated by factors autonomous from the muscle and tendon progenitors. For instance, when the transcription factor Pax3 is knocked out, the embryos develop muscle-less limbs, but with appropriate tendon formation 27. Similarly, when the transcription factor Scleraxis is knocked out, disrupting tendon differentiation, condensation of skeletal muscles still occurs in the correct locations 28. These studies indicate that there could be factors such as other cell populations and/or the ECM that direct the tendon and muscle progenitors during the assembly of the MTJ.
Adaptation of MTJ to Mechanical Loading
The MTJ adapts to changes in homeostasis such as in response to exercise and aging. Previous studies demonstrated that eccentric strength training, aerobics and running can shift the position of the MTJ and change the angle of both the finger-like processes and muscle fascicles, promoting increased force transmission and muscle hypertrophy 29. In addition, a recent study showed that a fast running training regime upregulated the expression of TGF-β and type III betaglycan receptor genes in tendon proximal to the interface, which are correlated with an increase in type I collagen deposition, suggesting the ECM in the MTJ is adapting to the increase in loading 30. On the contrary, studies on the aging MTJ revealed shortening and atypical sarcolemma invagination and alterations in collagen fiber arrangement and deposition, leading to conditions similar to muscle atrophy 31, indicating a compromise in force transmission and stabilization due to muscle inactivity 31. Moreover, prolonged bed rest led to muscle atrophy and significant decrease in muscle-tendon stiffness 32. These studies suggest that exercise can induce changes in the ultrastructure of the MTJ and is hypothesized to be a countermeasure for muscle atrophy. Understanding the communication between muscle and tendon when there is an injury at the MTJ could help identify factors that influence biomechanics post repair. Additionally, comprehending the changes in muscle and tendon biomechanics post MTJ injury could help in prognosis. Several studies indicate that muscle and tendon homeostasis is altered when there is an injury in either of the tissues. In a recent study, significant changes in tendon ECM was demonstrated in response to injury in the gastrocnemius muscle indicating that muscle and tendon affect each other due to change in tissue mechanics 33. Conversely, a clinical study provided evidence for changes in the gastrocnemius muscle post Achilles tendon injury 34.
Together, these studies indicate that muscle and tendon communicate with each other bidirectionally with functional consequences. Therefore, exploring the changes in the ECM at the interface between muscle and tendon during development and post injury at the MTJ could aid in advancing clinical prognosis.
Clinical Challenges for Repairing Injuries at the Muscle-Tendon Interface
MTJ injuries can arise from diverse events, including daily activities, physical work, sports and trauma. These typically occur due to excessive eccentric force that results in muscle strains 35. The strain localizes to the tapering fibers of the interface leading to microscopic and macroscopic damage at the MTJ 36 (Figure 1). Notably, eccentric forces greater than 20% of the average maximum isometric force are sufficient to induce a rupture at the muscle-tendon interface, which is why this type of injury predominantly occurs during physical activities 37. This presents a significant challenge for sports medicine, where one-third of all sports-related injuries are due to muscle strains 38. In modern day economics, sport is a profession and is recognized as an industry 39; therefore, MTJ injuries preventing an athlete from training and competing impose a considerable economic and psychological influence on the athlete and the team 40. In addition, aging presents a tremendous impact as studies demonstrate that the recovery time for muscle injuries are significantly increased with advanced age 41, and the economic burden is skewed towards the aging population 42. A recent report indicated that by 2050, 25% of the population will be 65 years or older 43, suggesting that demographic shifts will challenge the health care infrastructure. Taken together, muscle strain injuries, and the effect on the MTJ, will continue to be a significant contributor to the long term functional deficit, pain and economic burden on the world scale 44, 45.
Unfortunately, there is no definitive time frame for treating muscle strain injuries as scientific reports are contradictory. In general, the PRICE protocol is adopted immediately after injury to reduce the bleeding at the injury site 46. As far as medication is concerned, NSAIDs and glucocorticoids are prescribed to aid in the initial phase of the recovery process. However, recent studies demonstrated that anti-inflammatory drugs hinder muscle regeneration and may pose detrimental effects 47. After the initial treatment phase, isometric, isotonic and isokinetic training exercises are advised to regain the range of motion and functionally condition the injured muscle 48. Surgical interventions are very rare for muscle strain injuries 49. Currently, there are no standard clinical surgical intervention methods for a complete tear at the MTJ, and suturing techniques are typically employed to treat these occurrences in the clinic 50.
Regenerative Medicine Strategies for the Muscle-Tendon Interface
Regenerative medicine is an interdisciplinary strategy that focuses on repair, replacement and regeneration of cells and tissues to reverse the effects of genetic defects, injury and aging and restore functionality 51. Complete rupture of the muscle-tendon interface is an uncommon occurrence; yet there are instances of clinically reported cases for a complete rupture 49, 50. Unfortunately, surgical suturing methods are currently the only available clinical options. Notably, the efficacy of these studies is still questionable as there are no long-term data available to provide conclusive evidence that they work. Scaffold-based regenerative medicine strategies offer an alternative to address the current limitations in circumstances of a complete rupture at the muscle-tendon interface. Engineered scaffolds (acellular and synthetic) have the potential to be tailored based on the tissue type and are successfully used clinically for bone 52 and muscle 53. However, there are no clinically available scaffolds for treating injuries at the muscle-tendon interface. This can be attributed to the complexity of the physical, chemical and biological properties at the interface. Understanding of the mechanical properties at the muscle-tendon interface is inadequate and is limited to mathematical models predicting the strain 36. Moreover, it is challenging to design and develop scaffolds that have properties tailored to satisfy three distinct regions; muscle, tendon and the MTJ. To this end, electrospinning was employed to design a scaffold with three distinct materials within a continuous system and the results demonstrated that the designed scaffold had properties mimicking the native muscle-tendon interface 54. Similarly, a decellularized muscle-tendon interface ECM scaffold was evaluated for exhibiting biphasic structural and mechanical properties to mimic the natural MTJ microenvironment 55. Both the studies provide critical information about the rational design of scaffold-based regenerative medicine strategies to restore the muscle-tendon interface. However, the current lack of a successful intervention indicates that the appropriate cues are not being incorporated. Taken together, there are limited regenerative strategies addressing the muscle-tendon interface and there is an immediate need to improve the current available methods for treating MTJ injuries.
From the current literature, it is evident that injuries at the muscle-tendon interface impose physical, mental and financial burdens on society, apart from posing a challenge for the medical community. There is space for improvement in injury prevention and repair/recovery strategies, which open up multiple research avenues. Limitations in treatment options are due to the difference in the tissue properties between muscle and tendon at the interface. The ECM presents a unique niche of molecules that form a continuous material that bridges the MTJ and plays a critical role during development, homeostasis, repair and aging. Therefore, understanding the role and composition of the ECM at the MTJ has the potential to provide critical information that can be used to develop biomimetic constructs that accelerate repair at the muscle-tendon interface.
Role of ECM at the Muscle-Tendon Interface
The ECM is a complex, molecular network that is present in all tissues and organs. There are three major molecular classes of ECM: glycosaminoglycans (GAGs) such as hyaluronic acid (HA) and chondroitin sulfate; proteoglycans (PGs) such as perlecan and decorin, and fibrillar/network forming proteins such as collagens, elastin, fibronectin and laminins 56, 57. These components act as scaffolds on which cells reside, and provide physical, chemical and biological cues for modulating cell behavior, migration, proliferation, differentiation, growth and homeostasis 58. Importantly, the ECM plays a pivotal role in regulating development, repair, aging and disease progression 59.
In the adult muscle-tendon unit, type XXII collagen is thought to be restricted to the MTJ 60, and play a role in strengthening the MTJ by interacting with the muscle basal lamina via integrin α7β1 61. Knockdown of col22a1 in the zebrafish caused a reduction in the MTJ folds and resulted in contraction-induced muscle fiber detachment, leading to muscle weakness and a muscular dystrophy-like phenotype 61. A study that used laser-capture microdissection to isolate the murine MTJ from cryosections found several other ECM proteins such as collagens, biglycan, fibromodulin, prolargin and laminin were located in the MTJ 62. While their specific roles in the MTJ have yet to be determined, understanding the roles of these ECM molecules in muscle and tendon can potentially inform about their roles in the muscle-tendon interface (Figure 1). Several fibril-forming collagens are present in skeletal muscle, and are oriented both along the axis of muscle contraction and surrounding the muscle fibers 63. Collagen fibrils provide mechanical stability to skeletal muscle and regulate cell adhesion and differentiation 64. The majority of the tendon ECM is comprised of fibril-forming collagens (~60–85% of dry weight) 65, which are organized into different hierarchical levels aligned along the axis of mechanical loading to provide structural integrity 66. Assembly of collagen fibrils is modulated by small leucine rich proteoglycans (SLRPs), such as biglycan, fibromodulin and decorin, which also regulate cellular functions 67. Biglycan modulates myofiber anchorage to the surrounding ECM by binding with dystrophin-glycoprotein complex. Moreover, biglycan regulates utrophin expression to compensate for the disruption of dystrophin protein complex in Duchene muscular dystrophy 68. In tendon, biglycan is essential for maintaining collagen fibril structure, realignment and mechanical properties in the adult 69. Fibromodulin modulates myogenesis of skeletal muscle by inhibiting the activity of myostatin, a negative regulator for muscle growth 70. In tendon, fibromodulin binds to collagen fibrils and upregulates fibrillogenesis by mediating the stabilization and growth of fibrils. During collagen fibril maturation, fibromodulin displaces another SLRP, lumican, and facilitates fusion of individual collagen fibrils; thus, increasing the fibril diameter 71. Prolargin functions as a basement membrane anchor by interacting with perlecan present in the basement membrane and type I collagen in the interstitial connective tissue 72. In tendon, prolargin was reported to be involved in collagen fibrillogenesis 73; however, the role has yet to be established. Laminin-211 is predominantly observed in the basement membrane of skeletal muscle and its interaction with α-dystroglycan is essential for maintaining the structural integrity and force transmission 74. Along with other ECM proteins such as nidogens, perlecan, agrin and type IV collagen, laminin-211 forms the niche for muscle specific stem cells called satellite cells in the basal lamina, a layer of basement membrane adjacent to the cell surface 75. Similar to satellite cells, tendon progenitor cells reside within a unique ECM niche, comprised of biglycan and fibromodulin, that regulates their function 13. A basement membrane consisting of laminin, nidogen-1, perlecan and type IV collagen is also found surrounding the flexor tendon and is essential for preventing tendon adhesions between tendon and synovial sheath 76. Therefore, enrichment of laminin at the muscle-tendon interface suggests that an ECM niche that supports satellite cells and tendon-specific stem cell could be present. This would provide a local source of cells that can immediately contribute to repair at the interface. Indeed, prior reports demonstrated that satellite cells were localized at the remodeling muscle-tendon interface in individuals undergoing heavy resistance training 77.
Besides the ECM identified at the MTJ, there are many other matrix molecules that play important roles in muscle and tendon. Several GAGs such as HA and heparan sulfate are present in the interstitial matrix of skeletal muscle. HA helps facilitate tissue hydration and regulates the viscoelasticity of the tissue 78. In addition, HA levels were found to be significantly increased during development and hypertrophy, suggesting potential roles in modulating myogenesis 79, 80. Heparan sulfate binding PGs are also involved in regulating several key processes during myogenesis. For instance, glypican-1 binds with fibroblast growth factor 2 (FGF-2), a negative regulator for myoblast proliferation and differentiation 81, and sequesters FGF-2 from its receptors. Glypican-1 is also involved in hepatocyte growth factor mediated signaling, which is essential for myoblast migration 8. Furthermore, skeletal muscle ECM consists of various other PGs such as decorin, and lumican that interact with the collagens to maintain the ECM structural organization 63. In addition, these PGs play other important roles in skeletal muscle homeostasis. For example, decorin binds transforming growth factor beta (TGFβ), an inhibitor for myogenesis 82, sequestering its bioavailability 83. Decorin also plays a role in maintaining collagen fibril structure realignment in the direction of load and mechanical properties in the mature tendon 69. Furthermore, decorin is differentially upregulated during exercise training; thus, modulating collagen assembly and leading to increased tendon strength 84. Additionally, glycoproteins (tenascin-C, lubricin and tenomodulin) and elastic fibers (elastin, fibrillin-1 and −2) are also present within the tendon ECM 66 and play key roles. Lubricin plays an important role in tendon gliding, a process that dictates motion, and knockout of lubricin significantly increases tendon gliding resistance 85. Tenomodulin localizes to collagen type I fibrils and promotes collagen assembly during adaptation 86. Elastic fibers are widely distributed within the tendon ECM and provide elastic recoil and resilience 87. Further, they are part of the pericellular matrix surrounding the tenocytes and promotes their cell attachment by forming an intermediate link by binding to integrin α5β3 and type VI collagen 88.
Understanding the roles of the ECM at the muscle-tendon interface during various biological processes is essential in providing information for developing regenerative medicine strategies based on the type of injury repair. The following sub-sections detail the roles played by the ECM during development, repair, and aging.
ECM in the Developing MTJ
The ECM is secreted by cells from the early stages of embryogenesis. It is thought that ECM deposition and assembly in the embryonic spaces provide structural integrity and binding cues, as well as modulate cell migration 89. Studies in developing zebrafish showed that fibronectin and laminin α2 are involved in the proper attachment of muscle fibers to the intersegmental boundaries (ISB), an anatomical location where the myoblasts and tenocytes condense to form the MTJ 90. A recent study demonstrated that laminin-111 and fibronectin are reciprocally expressed by somites during the development of MTJ in the zebrafish 91. Fibronectin is involved in the early assembly of somites during development. After somite formation, basement membrane consisting of laminin-111, type IV collagen, nidogen-1, nidogen-2 and perlecan is deposited 92. Laminin-111 polymerization initiates signaling cascades that promote localization of matrix metalloprotease 11 (MMP11), which in turn downregulates fibronectin; thereby, leading to a laminin-rich basement membrane at the MTJ during development 91. Similarly, thrombospondin-4 plays a role in the assembly of ECM at the MTJ and promotes muscle attachment to the ISB during zebrafish development 25. As the MTJ matures, connective tissue cells secrete PGs and collagen that assemble into collagen fibrils, which are essential for providing structural stability to the MTJ and the tendon 5. ECM proteins such as tenascin-C 93 and decorin 94 are involved in the maturation of the collagen fibrils, which dictate the mechanical properties of the muscle-tendon interface.
Further understanding on the role of ECM during the MTJ formation and maturation has the potential to provide crucial information to develop regenerative strategies that aid in the recovery from injuries that occur in the muscle tendon interface. For example, investigations into the basic biology of the ECM during development led to the generation of novel regenerative strategies for cardiac 95, 96 and neural 97–99 engineering. Fibronectin was shown to promoted cardiac precursor cell migration during development 100. Using this information, scaffolds functionalized with fibronectin were constructed and shown to improve migration of cardiomyocytes to improve cardiac function in vivo 95. Similarly, tenascin-C and laminin promote neural crest cell migration during development 101, 102. Hydrogels that incorporated tenascin-C- and laminin-111 were designed and shown to facilitate migration of neuroblasts 103 and improve survival of neural stem cells 99, respectively, within the mouse brain.
ECM Changes in the Injured MTJ
Injury at the MTJ is characterized by damage to the ECM surrounding the muscle fibers at the interface with tendon, and the muscle fibers partially separate from the surrounding matrix 104. However, in extreme cases, muscle fibers are completely detached from the surrounding matrix and the tendon aponeurosis, creating a complete rupture of the muscle-tendon interface. Throughout the repair process, the ECM in the muscle and tendon undergoes characteristic changes to regulate different stages. After injury, the basal lamina enclosing the muscle fibers gets damaged and disintegrates. Then, proteases, including MMPs, digest type IV collagen and laminin-211 in the basal lamina, releasing fragments that play a role in modulating inflammatory cell migration 75. Studies indicate that MMP9 induced by the inflammatory response leads to activation of satellite cells 105, due to the breakdown of their ECM niche. Several reports demonstrate that satellite cells secrete MMP2 and MMP9 to breakdown type IV collagen and laminin-211 present in the basal lamina 106. Furthermore, migrating satellite cells express MMP13, which is involved in the degradation of collagens and fibronectin, to facilitate their migration 107. Then, the satellite cells undergo asymmetric cell division, which is regulated by many factors including fibronectin, into myogenic precursor cells that trigger the muscle repair process, and daughter satellite cells that maintain the satellite cell pool 108. Simultaneously, new ECM is deposited, predominantly by fibroblasts, to provide instructive cues for the newly regenerating muscle fibers. However, the deposited ECM must be remodeled during the repair to prevent fibrotic tissue formation, which restricts overall regeneration 109. Fibrotic tissue presents a mechanical barrier and reduces the extensibility of the tissue, which renders the tissue susceptible for reinjury 110. Vascular endothelial growth factor (VEGF) was previously demonstrated to reduce fibrosis in acute skeletal muscle injuries 111. However, the factors responsible for VEGF-induced angiogenesis and reduced scar tissue formation in damaged skeletal muscle still remain elusive 112. In the later stages of repair, heparan sulfate PGs play a role in regulating the differentiation of the myogenic precursor cells and fiber formation 113, 114. Interestingly, tenascin-C, which is not typically expressed in uninjured adult skeletal muscle tissue was shown to be highly expressed in response to compensatory overloading in the plantaris muscle 79. Furthermore, tenascin-C rich ECM deposition coincided with new myofiber formation during newt forelimb regeneration post amputation, indicating a potential role for tenascin-C in de novo muscle regeneration by facilitating myoblast migration and proliferation 115.
Similar to skeletal muscle, the ECM plays important roles during repair in tendon. Post injury, there is a significant increase in type III collagen deposition by the fibroblasts that migrated to the injury site 116. Additionally, several SLRPs (e.g. decorin, biglycan) that play a role in collagen fiber organization are secreted during the proliferation stage of repair 117. The final stage of repair is characterized by ECM remodeling and is subdivided into two stages. In the first phase, there is a decrease in ECM secretion and there is a gradual replacement of type III collagen by type I collagen, which aligns along the tendon axis of mechanical loading. ECM remodeling in tendon is orchestrated by MMPs (particularly MMP3), which mediate degradation of collagens deposited in the early stages of repair 118. During the second phase of ECM remodeling, the collagen fibrils begin to cross-link; thereby, providing maturity to the recovering tendon 116.
Similar to development, cues from remodeling post-injury were used to develop scaffolds that elicited favorable tissue remodeling. For example, a recent study demonstrated that an acellular biological scaffold derived from porcine urinary bladder epithelial basement membrane (MatriStem UMB™, Acell Inc.) facilitated constructive tissue remodeling by promoting migration of satellite cells at the site of injury when treated for volumetric muscle loss in a preclinical trial 119. Similarly, an acellular dermal matrix scaffold containing skin epithelial basement membrane generated a pro-regenerative microenvironment by favoring M2 macrophage cells that are responsible for secreting MMPs to remodel the ECM during a full thickness cutaneous wound healing 120. Therefore, future studies focusing on the ECM remodeling post injury at the muscle-tendon interface have the potential to contribute in developing regenerative medicine strategies.
Imaging techniques such as MRI reveal the location of the injury and help to distinguish if the injury is within the free tendon or at the ECM present in the muscle-tendon junction 104. Depending on the location of the injury (tendon, MTJ or muscle), the architecture of the underlying ECM varies, which in turn determines the nature of the tissue repair and occurrence of reinjury. In addition to aiding diagnosis, these imaging techniques are also frequently employed for the prognosis of muscle strain injuries; however, they do not provide adequate and reliable information on the extent of the ECM damage at the muscle-tendon interface. Recent studies indicate that MRI can be used to understand the ECM damage and changes in tendon 121, articular cartilage 122, metastatic cancer 123, and ruptured aneurysms 124. Furthermore, development of a GAG-based contrast agent led to high quality in vivo MRI of the cartilage in knee joints for diagnosing osteoarthritis 125. Therefore, clinical imaging techniques directed towards the specialized ECM present in the muscle-tendon interface have the potential to significantly improve the diagnosis and prognosis of muscle strain injuries.
Additionally, the treatment procedures employed to address muscle strains influence ECM changes at the site of injury. Cryotherapy (topical application of ice) upregulated type I collagen and type III collagen gene expression levels in an injured rat tibialis anterior muscle but, it did not alter collagen deposition or ECM remodeling 126. Interestingly, NSAID administration increased fibrosis in murine muscle repair studies 127, 128; whereas, it improved recovery from acute muscle injuries in human clinical studies 129. Furthermore, a proteomics study indicated that NSAID administration following acute skeletal muscle injury decreased laminin β2 protein levels 130, which plays an important role in regulating the presynaptic active zone formation in the neuromuscular junction 131. Eccentric contraction exercises prescribed as a part of physical therapy significantly increased tenascin-C, COL1A1, COL2A1, and COL4A1 gene expression 132, which correlates with the observed increase of these ECM proteins during repair. Understanding the muscle-tendon interface ECM remodeling during repair has the potential to aid in the development of clinical treatment procedures that promote favorable ECM remodeling. Recently, a novel treatment based on low-level laser therapy was explored, and preliminary studies in rat models indicate that the therapy induced beneficial ECM remodeling by modulating type I collagen and type III collagen 133; however, there are no clinical trials to date that validate the efficacy of this method.
ECM Remodeling in the Aging MTJ
As tissues age, the composition and physical properties of the ECM changes. There is an elevated MMP-assisted degradation and decreased synthesis of basement membrane proteins 134. Simultaneously, there is an elevated expression of reactive oxygen species, interleukins, cytokines and other inflammatory markers that lead to chronic inflammatory responses 135, 136. The elevated MMP, plasminogen activator inhibitor and reactive oxygen species levels disrupt the integrity of elastin networks and modify the collagen matrix 59. Taken together with the reduced production of GAGs 137, the overall integrity of the ECM is compromised as the tissues age 138, 139. Several reports indicate that with aging there is an increase in the stiffness of skeletal muscle ECM due to modification of collagen networks and advanced glycation end-products 140, 141. Furthermore, changes in the mechanical properties of the ECM with aging impairs satellite cell activation and renewal, as well as chemotactic and inflammatory responses, which are essential during skeletal muscle regeneration 142. Acute resistance training in elderly men decreased gene expression levels of MMPs such as MMP3, MMP9 and MMP15 compared to young men; thereby demonstrating age-related ECM remodeling 143. Age-related ECM gene expression was also observed in rat tendon, where differential expression of Col1a1, Col3a1 and Col5a1, elastin and lubricin were observed 144. Additionally, elevated gene expression of Mmp2 and Mmp9 were also reported in rat tendons; whereas, Timp1 and Timp2 were downregulated with aging 145. Similarly, a recent study demonstrated significant decrease of GAG content in aged rat tendon compared to young tendon 146. Interestingly, tendon cell proliferation was not affected during repair in aged mice; however, significant loss in fibrillar collagen deposition was observed 147. While these studies provide some insight into how the ECM changes with time, additional studies on how aging affects the role, composition and 3D architecture of the tendon ECM are necessary for developing effective strategies to counteract the consequences of aging.
These studies indicate that the ECM plays a critical role in the development, homeostasis, repair and aging of muscle and tendon tissue. However, there remains a knowledge gap in understanding the specific roles played by the ECM at the muscle-tendon interface. Therefore, identifying how the composition, organization and function of the ECM at the muscle-tendon interface change during various biological process has the potential to provide better understanding of the repair process. The following sections detail the latest methods employed for understanding the composition, structure and role of ECM.
Techniques to Explore ECM
Traditionally, the in vivo roles of ECM molecules during development and repair were deciphered using knockout models148, site-specific mutations149, and rescue studies150. Over the years, several ECM loss of function mouse models were generated to elucidate the role of specific ECM molecules 89. Moreover, techniques such as secondary harmonic generation microscopy and scanning electron microscopy were used to characterize the morphology of collagens and other ECM proteins 151. However, these studies focused on the role and function of the ECM of interest based on the cellular response and are typically limited to the characterization of a few ECM components at a time. Nevertheless, these experimental models still hold a lot of promise to unearth new insights into the function of specific ECM molecules148 when combined with recent advances in technology, including novel proteomics methods based on mass spectrometry, protein labeling and decellularization. The following sections will describe how these methods have the potential to open new research avenues to explore and better understand the roles played by the ECM in development, homeostasis, repair and aging.
Mass Spectrometry
Mass spectrometry (MS) is a powerful and sensitive technique that measures the mass to charge ratio (m/z ratio) of ions. The instrument ionizes the sample molecules and segregates the corresponding ions to characterize the signal intensity based on the m/z ratio. For proteomics analyses, mass spectrometry is combined with chromatography techniques to improve the overall resolution. Typically, samples are enzymatically digested into peptides and loaded onto a liquid chromatography (LC) column, which separates the sample based on molecular specificity. Then the separated samples go through two rounds of MS, which sequentially measures the m/z ratio of the peptides then after peptide fragmentation. Over the years, different analysis methods/strategies such as metabolic labelling (SILAC) 152and isobarically labelled peptides (iTRAQ) 153 were developed to quantify peptides characterized through LC-MS/MS. Subsequently, several software programs such as MaxQuant and MASCOT daemon were developed to identify peptides via in silico databases 154 and provide label free quantification. Detailed reviews on the principles behind LC-MS/MS proteomics are discussed elsewhere 155.
Mass spectrometry-based proteomics recently emerged as a leading candidate to characterize and analyze ECM composition in normal and diseased conditions 156. Furthermore, ECM proteomics evolved into a strategy to unearth novel targets, therapeutic markers for diseases and as a tool to identify molecular mechanisms 157. ECM molecules are usually large in size, covalently bound, cross-linked and glycosylated, thereby making them relatively insoluble and difficult to analyze using traditional biochemical methods 158. However, researchers took advantage of the insoluble nature of the ECM molecules and developed fractionation techniques in combination with mass spectrometry to identify the ECM protein composition of various tissues 158, 159. Tissue fractionation works on the principle that intracellular and soluble factors can be removed using buffers of increasing stringency; thus, leaving behind the ECM 158. These studies resulted in the development of a bioinformatics platform called the Matrisome Database, which consists of an ensemble of genes that encode for the ECM and the associated proteins in different tissue types and disease conditions 156, 160.
As mentioned in a previous section, laser-capture microdissection was combined with mass spectrometry was employed to specifically isolate the MTJ in mouse limbs, and identify unique proteins localized at the muscle-tendon interface 62. However, the tissues were not processed to enrich or characterize the ECM proteins localized to the MTJ. To date, there are no mass spectrometry-based proteomics studies conducted to identify tissue specific ECM in the MTJ under normal and injury/repair conditions. Critical information on the ECM at the muscle-tendon interface can be gathered by analyzing the ECM present in muscle and tendon through mass spectrometry-based proteomics. Analysis of equine forelimb tendon demonstrated that several key ECM proteins such as COL7A1, COL22A1, heparin proteoglycan-2, tenascin-X, dermatopontin were localized to the interfascicular matrix (softer outer region), whereas COL17A1, COL18A1, fibrillin-2 and thrombospondin-1 were identified in the tendon core. Moreover, ECM molecules such as biglycan, COMP, decorin, lumican, COL6A1, and COL6A3 were present in injured equine superficial digital flexor tendon 161. Studies on human tendon indicated that COMP was a major glycoprotein present in the insoluble ECM 162 and was differentially expressed during injury 163. In addition, several ECM molecules were differentially regulated with age 164. Mass spectrometry-based differential quantification of skeletal muscle ECM in response to injury has not been addressed to date; although, a recent study characterized skeletal muscle ECM proteins in adult mice 165.
Protein Labeling
Over the years, protein labeling methods provided researchers a way to monitor biological processes, and to quantify and detect protein modifications. Traditional protein labeling techniques involve the use of isotopes, mass tags and fluorescent labels 166. Unfortunately, these methods cannot adequately detect or enrich for proteins that are in low abundance 167. In addition, in vivo protein labeling methods require prolonged feeding time to enable proteome labeling 168. To address these limitations, collaborative efforts in the fields of chemistry and biology led to the development of a protein labeling platform that uses non-canonical amino acids (ncAAs). For example, the methionine analogs homopropargylglycine (Hpg) and azidohomoalanine (Aha), contain a functional groups that can be modified via click chemistry to enrich for newly synthesized proteins that are otherwise difficult to identify and quantify as they are diluted by the unlabeled proteins from the same tissue 169. The ncAAs are bioorthogonal, which means they are integrated into the biological system without reacting with the naturally occurring functional groups. In addition, the amino acid analogs are incorporated into the newly synthesized proteins using the native cellular machinery. This bioorthognal non-canonical amino acid tagging, or BONCAT 170, is used to enrich for and characterize the nascent proteome in various in vitro and in vivo systems using LC-MS/MS. Furthermore, the functional groups on ncAAs enables the labeling of newly synthesized proteins with fluorophores via click chemistry for visualization (fluorescent noncanonical amino acid tagging, FUNCAT) 171.
Previous studies demonstrated that the enriched biorthogonal ncAA labeled proteins can be analyzed via mass spectrometry-based analysis to map the nascent proteome of a range of model organisms 172. The overall enrichment process involves the extraction of proteins, intracellular/extracellular or both, followed by a click reaction with biotinylated alkyne to tag the ncAA incorporated nascent proteins. The tagged proteins are then pulled down using streptavidin beads, which are further washed and cleaved to release the enriched ncAA labeled proteins that can be processed for mass spectrometry analysis 173. This method of ncAA label assisted mass spectrometry-based proteomic analysis was utilized to understand protein turnover depending on the rate of nascent protein degradation during development 173 and cellular function 174. Given the limited knowledge on the ECM at the muscle-tendon interface, ncAA protein labeling can be utilized to understand the intracellular and extracellular protein turn over in the MTJ during homeostasis, repair and aging. Prior research indicates that these methods have the potential to advance knowledge of the ECM composition and roles. For instance, a recent study demonstrated that nascent ECM secreted by mesenchymal stem cells and chondrocytes undergoing chondrogenesis can be visualized in vitro by fluorescently labeling Hpg and Aha present in the ECM 175. Moreover, Aha labeling was used to understand how mesenchymal stromal cells interacted in a 3D microenvironment with the nascent ECM proteins that are involved in cell adhesion; thereby providing information on cues that can used for designing 3D scaffold systems to enhance cell-material interactions that facilitates cell adhesion 176. Furthermore, Aha and Hpg were successfully used to label newly synthesized protein in the developing mouse embryo with minimal impact on the developing proteome 172. Additionally, a Aha labeling method was recently described to identify and quantify nascent ECM protein turnover in the different tissue fractions during development 173.
Visualization of the ECM
Traditional 2D imaging techniques do not resolve the architectural and surface topographical properties of the ECM, which are critical in understanding the ECM function. High-resolution 3D visualization provides structural details about the native ECM 177; however, it is hindered by the opaque nature of the biological tissues. Hence, it is important to develop decellularization techniques that preserve the 3D organization of the ECM proteins while simultaneously removing the intracellular contents of a tissue. In general, decellularization involves the process of removing native cells, lipids and nuclear content from the tissue, while retaining the native 3D organization. The overall process utilizes various chemical, physical and enzymatic agents to effectively decellularize the tissue, as explained in detail elsewhere 178. Perfusion-based decellularization techniques, a process where detergents are perfused through the blood vessels, is commonly used to decellularize organs with well-defined vasculature such as heart, liver, lungs and kidney 179. Unfortunately, the muscle-tendon interface has limited vasculature; therefore, novel decellularization methods must be designed to achieve whole tissue decellularization efficiently without compromising the native ECM properties. To address this, a method was recently developed to decellularize soft tissues, like murine embryos, using a hydrogel that retained the native 3D conformation of the ECM 180. Currently, confocal and multiphoton microscopy methods are employed to visualize the 3D spatial distribution of the ECM networks in the decellularized tissues 179, 180. Furthermore, efforts are made to optimize antibody staining methods for the decellularized tissues to enable visualization of tissue specific ECM proteins 179. Efficient decellularization techniques in combination with high-resolution 3D visualization can provide new information about the spatial distribution of tissue-specific ECM.
Implications for Regenerative Medicine
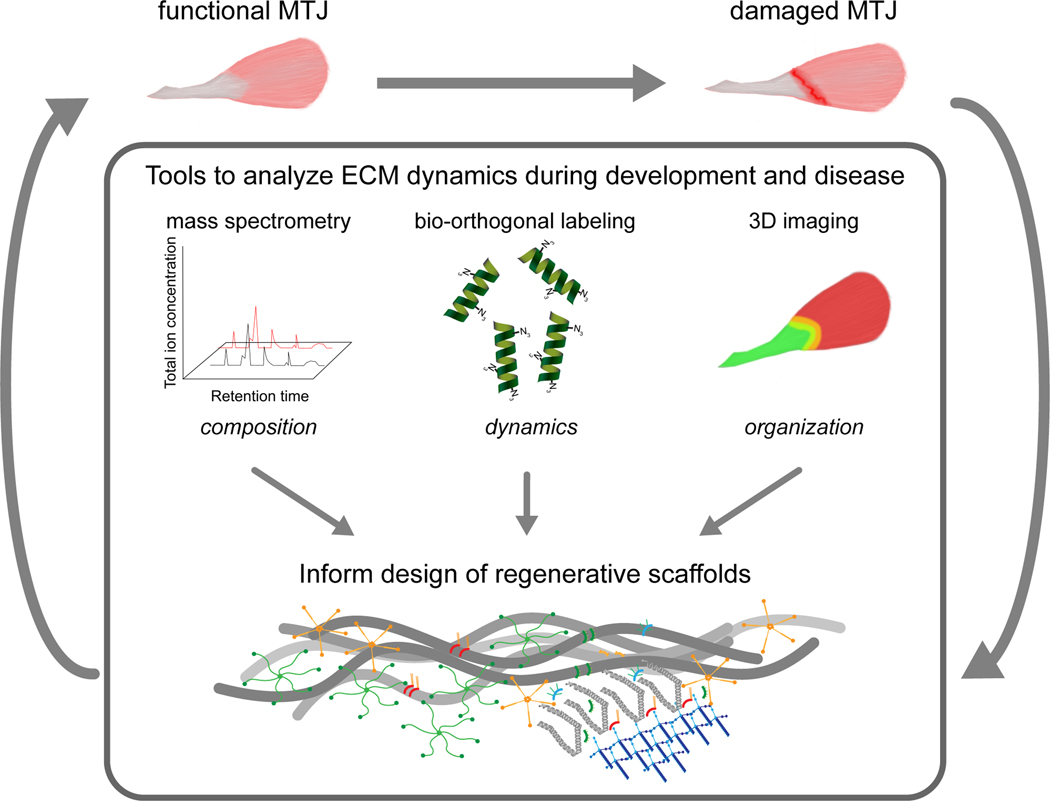
The techniques described above have the potential to provide important information about the composition, turnover and organization of ECM molecules across tendon and skeletal muscle that can be utilized while developing biomimetic constructs to restore the muscle – tendon interface. For instance, comparative analyses of the homeostatic and damaged/aging MTJ using mass spectrometry has the potential to inform about the overall composition of the ECM at the muscle-tendon interface, as well as identify key molecules that are upregulated and downregulated that compromise functionality and identify targets for intervention (Figure 2). Similarly, protein labeling methods have the capacity to present in depth details on the dynamics of newly synthesized ECM molecules that are critical for remodeling the interface during development, repair and aging. While these methods provide information about the identity of specific ECM it is critical for scaffold design to know the spatial distribution of components in 3D. Antibody staining combined with high resolution 3D imaging has the ability to deliver knowledge regarding the organization of an ECM molecule of interest. Finally, the mechanical properties of the designed scaffold should ideally match the surrounding microenvironment at the muscle-tendon interface. Efficient decellularization methods provide an opportunity to characterize the mechanical properties of the ECM networks as previously reported 181. However, new instrumentation to test the mesoscale mechanics of tissues needs to be developed. Taken together, better understanding of the structural, biophysical and biochemical properties of the ECM can provide essential information to guide the construction of scaffolds that can repair damaged muscle-tendon interfaces (Figure 2).
Figure 2:
The implication of ECM-based research in developing regenerative medicine for muscle-tendon interface. Knowledge regarding the composition and role of the ECM at the muscle-tendon interface will be advanced using techniques such as mass spectrometry, protein labeling and 3D imaging. This information will provide critical design information for developing ECM instructive scaffolds, which can be used alone or in combination with cells and growth factors. It is hypothesized that regenerative medicine strategies that involve the use of ECM instructive scaffolds will facilitate scar-free repair at the muscle-tendon interface.
Limitations and Future Directions
Major challenges lie in the establishment of suitable research techniques that provide essential information about the ECM during repair and development. Advances in technology and novel techniques such as mass spectrometry-based proteomics analysis, ECM protein labeling and decellularization will help in exploring the role, composition and spatial distribution of the ECM proteins. However, obstacles remain in tissue processing, ECM protein extraction, visualization of ECM, and scaling up of decellularization for higher mammals. Therefore, continued research in the development and optimization of these areas will help advance the identification of the specific roles played by ECM present in the muscle-tendon interface during development, repair and aging.
Another hurdle lies in the efficient translation of engineering information obtained from ECM-based research to develop regenerative engineering strategies. Substantial advances have been made in bioengineering methods to characterize, design, develop and fabricate scaffolds for tissue engineering. However, biomimetic scaffolds developed for the MTJ repair do not recapitulate the architectural complexity and composition of the native ECM. Even though considerable strides were made in translating preclinical treatment methods, there remains a void in translating regenerative medicine strategies due to complications in the formulation, fabrication and scale-up of ECM based scaffolds. Therefore, future studies focused on preclinical and clinical translation of ECM based regenerative medicine treatment could significantly progress clinical treatment methods available for the muscle-tendon interface.
In conclusion, the ECM plays various roles in the development, repair, homeostasis and aging of the muscle-tendon interface. Therefore, understanding ECM function during repair and development has the potential to unearth important information that provides critical insight for developing regenerative medicine strategies aimed towards restoring the muscle-tendon interface.
Acknowledgements and Funding Information
The authors thank the members of the Calve lab for helpful comments and edits. This work was supported by NIH R01 AR063712 and NIH DP2 AT009833.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Maas H, Sandercock TG. Force transmission between synergistic skeletal muscles through connective tissue linkages. J Biomed Biotechnol. 2010;2010:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tidball JG. The geometry of actin filament‐membrane associations can modify adhesive strength of the myotendinous junction. Cell Motil. 1983;3:439–447. [DOI] [PubMed] [Google Scholar]
- 3.Charvet B, Ruggiero F, Le Guellec D. The development of the myotendinous junction. A review. Muscles Ligaments Tendons J. 2012;2:53. [PMC free article] [PubMed] [Google Scholar]
- 4.VanDusen K, Larkin L. Muscle–tendon interface In. Muscle–tendon interface. Regenerative Engineering of Musculoskeletal Tissues and Interfaces: Elsevier; 2015. [Google Scholar]
- 5.Subramanian A, Schilling TF. Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development. 2015;142:4191–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad K, Lee EJ, Moon JS, Park S-Y, Choi I. Multifaceted interweaving between extracellular matrix, insulin resistance, and skeletal muscle. Cells. 2018;7:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franchi MV, Reeves ND, Narici MV. Skeletal muscle remodeling in response to eccentric vs. concentric loading: morphological, molecular, and metabolic adaptations. Front Physiol. 2017;8:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González MN, De Mello W, Butler-Browne GS, Silva-Barbosa SD, Mouly V, Savino W, Riederer I. HGF potentiates extracellular matrix-driven migration of human myoblasts: involvement of matrix metalloproteinases and MAPK/ERK pathway. Skelet Muscle. 2017;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao A, Moerk CT, Penland N, Perla M, Kim J, Smith AS, Murry CE, Kim DH. Regulation of skeletal myotube formation and alignment by nanotopographically controlled cell‐secreted extracellular matrix. J Biomed Mater Res A. 2018;106:1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi V, Dye DE, Kinnear BF, Van Kuppevelt TH, Grounds MD, Coombe DR. Interactions between skeletal muscle myoblasts and their extracellular matrix revealed by a serum free culture system. PloS one. 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melo F, Carey DJ, Brandan E. Extracellular matrix is required for skeletal muscle differentiation but not myogenin expression. J Cell Biochem. 1996;62:227–239. [DOI] [PubMed] [Google Scholar]
- 12.Kjær M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. [DOI] [PubMed] [Google Scholar]
- 13.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo B-M, Zhang L. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Zhu J, Zhou Y, Thampatty BP, Wang JH. Tendon Stem/Progenitor Cells and Their Interactions with Extracellular Matrix and Mechanical Loading. Stem Cells Int. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudsen A, Larsen M, Mackey A, Hjort M, Hansen K, Qvortrup K, Kjaer M, Krogsgaard M. The human myotendinous junction: An ultrastructural and 3 D analysis study. Scand J Med Sci Sports. 2015;25:e116–e123. [DOI] [PubMed] [Google Scholar]
- 16.Charvet B, Malbouyres M, Pagnon-Minot A, Ruggiero F, Le Guellec D. Development of the zebrafish myoseptum with emphasis on the myotendinous junction. Cell Tissue Res. 2011;346:439–449. [DOI] [PubMed] [Google Scholar]
- 17.Kojima H, Sakuma E, Mabuchi Y, Mizutani J, Horiuchi O, Wada I, Horiba M, Yamashita Y, Herbert DC, Soji T. Ultrastructural changes at the myotendinous junction induced by exercise. J Orthop Sci. 2008;13:233–239. [DOI] [PubMed] [Google Scholar]
- 18.Tidball JG. Force transmission across muscle cell membranes. J Biomech. 1991;24:43–52. [DOI] [PubMed] [Google Scholar]
- 19.Kostrominova TY, Calve S, Arruda EM, Larkin LM. Ultrastructure of myotendinous junctions in tendon-skeletal muscle constructs engineered in vitro. Histol Histopathol. 2009;24:541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yarnitzky T, Min L, Volk T. The Drosophila neuregulin homolog Vein mediates inductive interactions between myotubes and their epidermal attachment cells. Genes Dev. 1997;11:2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edom-Vovard F, Bonnin M-A, Duprez D. Misexpression of Fgf-4 in the chick limb inhibits myogenesis by down-regulating Frek expression. Dev Biol. 2001;233:56–71. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Guzman M, Montero JA, Santesteban E, Gañan Y, Macias D, Hurle JM. Tendon-muscle crosstalk controls muscle bellies morphogenesis, which is mediated by cell death and retinoic acid signaling. Dev Biol. 2007;302:267–280. [DOI] [PubMed] [Google Scholar]
- 23.Gilsohn E, Volk T. Slowdown promotes muscle integrity by modulating integrin-mediated adhesion at the myotendinous junction. Development. 2010;137:785–794. [DOI] [PubMed] [Google Scholar]
- 24.Liu ZC, Geisbrecht ER. Moleskin is essential for the formation of the myotendinous junction in Drosophila. Dev Biol. 2011;359:176–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian A, Schilling TF. Thrombospondin-4 controls matrix assembly during development and repair of myotendinous junctions. Elife. 2014;3:e02372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yatsenko AS, Shcherbata HR. Drosophila miR-9a targets the ECM receptor Dystroglycan to canalize myotendinous junction formation. Dev Cell. 2014;28:335–348. [DOI] [PubMed] [Google Scholar]
- 27.Schweitzer R, Zelzer E, Volk T. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development. 2010;137:2807–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pryce BA, Watson SS, Murchison ND, Staverosky JA, Dünker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFβ signaling are essential for tendon formation. Development. 2009;136:1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franchi MV, Atherton PJ, Reeves ND, Flück M, Williams J, Mitchell WK, Selby A, Beltran Valls R, Narici M. Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol (Oxf). 2014;210:642–654. [DOI] [PubMed] [Google Scholar]
- 30.Curzi D, Sartini S, Guescini M, Lattanzi D, Di Palma M, Ambrogini P, Savelli D, Stocchi V, Cuppini R, Falcieri E. Effect of different exercise intensities on the myotendinous junction plasticity. PloS one. 2016;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciena AP, De Almeida SRY, De Sousa Bolina C, De Sousa Bolina‐Matos R, Grassi Rici RE, Da Silva MCP, Miglino MA, Watanabe IS. Ultrastructural features of the myotendinous junction of the sternomastoid muscle in Wistar rats: from newborn to aging. Microsc Res Tech. 2012;75:1292–1296. [DOI] [PubMed] [Google Scholar]
- 32.Koryak YA. Influence of simulated microgravity on mechanical properties in the human triceps surae muscle in vivo. I: effect of 120 days of bed-rest without physical training on human muscle musculo-tendinous stiffness and contractile properties in young women. Eur J Appl Physiol. 2014;114:1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barin FR, Neto IVDS, Ramos GV, Szojka A, Ruivo AL, Mota Anflor CT, Hurtado Agualimpia JD, Domingues AC, Franco OL, Adesida A. Calcaneal tendon plasticity following gastrocnemius muscle injury in rat. Front Physiol. 2019;10:1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng W-C, Chang Y-P, Chao Y-H, Fu S, Rolf C, Shih TT, Su S-C, Wang H-K. Morphomechanical alterations in the medial gastrocnemius muscle in patients with a repaired Achilles tendon: associations with outcome measures. Clin Biomech (Bristol, Avon). 2017;43:50–57. [DOI] [PubMed] [Google Scholar]
- 35.Crema MD, Yamada AF, Guermazi A, Roemer FW, Skaf AY. Imaging techniques for muscle injury in sports medicine and clinical relevance. Curr Rev Musculoskelet Med. 2015;8:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharafi B, Ames EG, Holmes JW, Blemker SS. Strains at the myotendinous junction predicted by a micromechanical model. J Biomech. 2011;44:2795–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tidball J, Chan M. Adhesive strength of single muscle cells to basement membrane at myotendinous junctions. J Appl Physiol. 1989;67:1063–1069. [DOI] [PubMed] [Google Scholar]
- 38.Ekstrand J, Hägglund M, Waldén M. Epidemiology of muscle injuries in professional football (soccer). Am J Sports Med. 2011;39:1226–1232. [DOI] [PubMed] [Google Scholar]
- 39.Öztürk S, Kılıç D. What is the economic burden of sports injuries. Eklem Hastalik Cerrahisi. 2013;24:108–111. [DOI] [PubMed] [Google Scholar]
- 40.Brukner P, Khan K. Clinical Sports Medicine. 2009. Australia: McGraw-Hill. [Google Scholar]
- 41.Rader EP, Faulkner JA. Effect of aging on the recovery following contraction-induced injury in muscles of female mice. J Appl Physiol. 2006;101:887–892. [DOI] [PubMed] [Google Scholar]
- 42.Manini T. Development of physical disability in older adults. Curr Aging Sci. 2011;4:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harper S. Economic and social implications of aging societies. Science. 2014;346:587–591. [DOI] [PubMed] [Google Scholar]
- 44.Murray CJ, Barber RM, Foreman KJ, Ozgoren AA, Abd-Allah F, Abera SF, Aboyans V, Abraham JP, Abubakar I, Abu-Raddad LJ. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Widanarko B, Legg S, Stevenson M, Devereux J, Eng A, Cheng S, Douwes J, Ellison-Loschmann L, McLean D, Pearce N. Prevalence of musculoskeletal symptoms in relation to gender, age, and occupational/industrial group. Int J Ind Ergo. 2011;41:561–572. [Google Scholar]
- 46.Fernandes TL, Pedrinelli A, Hernandez AJ. Muscle injury: physiopathology, diagnostic, treatment and clinical presentation. Rev Bras Orthop. 2011;46:247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gumucio JP, Flood MD, Phan AC, Brooks SV, Mendias CL. Targeted inhibition of TGF-β results in an initial improvement but long-term deficit in force production after contraction-induced skeletal muscle injury. J Appl Physiol. 2013;115:539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Järvinen TA, Järvinen TL, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33:745–764. [DOI] [PubMed] [Google Scholar]
- 49.Sonnery-Cottet B, Daggett M, Gardon R, Pupim B, Clechet J, Thaunat M. Surgical management of recurrent musculotendinous hamstring injury in professional athletes. Orthop J Sports Med. 2015;3:2325967115606393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chernchujit B, Parate PH. Surgical Technique to Repair Musculotendinous Junction Tear of Supraspinatus Using Lateral-Row Anchors to Avoid Cut-Through. Arthrosc Tech. 2017;6:e65–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mason C, Dunnill P. A brief definition of regenerative medicine. 2008. [DOI] [PubMed] [Google Scholar]
- 52.Calori G, Tagliabue L, Gala L, d’Imporzano M, Peretti G, Albisetti W. Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions: a prospective randomised clinical study on 120 patients. Injury. 2008;39:1391–1402. [DOI] [PubMed] [Google Scholar]
- 53.Dziki J, Badylak S, Yabroudi M, Sicari B, Ambrosio F, Stearns K, Turner N, Wyse A, Boninger ML, Brown EH. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. NPJ Regen Med. 2016;1:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ladd MR, Lee SJ, Stitzel JD, Atala A, Yoo JJ. Co-electrospun dual scaffolding system with potential for muscle–tendon junction tissue engineering. Biomaterials. 2011;32:1549–1559. [DOI] [PubMed] [Google Scholar]
- 55.Zhao C, Wang S, Wang G, Su M, Song L, Chen J, Fan S, Lin X. Preparation of decellularized biphasic hierarchical myotendinous junction extracellular matrix for muscle regeneration. Acta Biomater. 2018;68:15–28. [DOI] [PubMed] [Google Scholar]
- 56.Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339:237. [DOI] [PubMed] [Google Scholar]
- 58.Watt FM, Huck WT. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol. 2013;14:467. [DOI] [PubMed] [Google Scholar]
- 59.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koch M, Schulze J, Hansen U, Ashwodt T, Keene DR, Brunken WJ, Burgeson RE, Bruckner P, Bruckner-Tuderman L. A novel marker of tissue junctions, collagen XXII. J Biol Chem. 2004;279:22514–22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Charvet B, Guiraud A, Malbouyres M, Zwolanek D, Guillon E, Bretaud S, Monnot C, Schulze J, Bader HL, Allard B. Knockdown of col22a1 gene in zebrafish induces a muscular dystrophy by disruption of the myotendinous junction. Development. 2013;140:4602–4613. [DOI] [PubMed] [Google Scholar]
- 62.Can T, Faas L, Ashford DA, Dowle A, Thomas J, O’Toole P, Blanco G. Proteomic analysis of laser capture microscopy purified myotendinous junction regions from muscle sections. Proteome Sci. 2014;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44:318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gosselin LE. Skeletal muscle collagen: age, injury and disease In. Skeletal muscle collagen: age, injury and disease. Sarcopenia–Age-Related Muscle Wasting and Weakness: Springer; 2011. [Google Scholar]
- 65.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. [DOI] [PubMed] [Google Scholar]
- 66.Screen HR, Berk DE, Kadler KE, Ramirez F, Young MF. Tendon functional extracellular matrix. J Orthop Res. 2015;33:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen S, Birk DE. The regulatory roles of small leucine‐rich proteoglycans in extracellular matrix assembly. The FEBS journal. 2013;280:2120–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amenta AR, Yilmaz A, Bogdanovich S, McKechnie BA, Abedi M, Khurana TS, Fallon JR. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci U S A. 2011;108:762–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson KA, Sun M, Barnum CE, Weiss SN, Huegel J, Shetye SS, Lin L, Saez D, Adams SM, Iozzo RV. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol. 2017;64:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee EJ, Jan AT, Baig MH, Ashraf JM, Nahm S-S, Kim Y-W, Park S-Y, Choi I. Fibromodulin: a master regulator of myostatin controlling progression of satellite cells through a myogenic program. FASEB J. 2016;30:2708–2719. [DOI] [PubMed] [Google Scholar]
- 71.Ezura Y, Chakravarti S, Oldberg Å, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bengtsson E, Mörgelin M, Sasaki T, Timpl R, Heinegård D, Aspberg A. The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J Biol Chem. 2002;277:15061–15068. [DOI] [PubMed] [Google Scholar]
- 73.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. [DOI] [PubMed] [Google Scholar]
- 74.Holmberg J, Durbeej M. Laminin-211 in skeletal muscle function. Cell Adh Migr. 2013;7:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas K, Engler AJ, Meyer GA. Extracellular matrix regulation in the muscle satellite cell niche. Connect Tissue Res. 2015;56:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor SH, Al-Youha S, Van Agtmael T, Lu Y, Wong J, McGrouther DA, Kadler KE. Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PloS one. 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jakobsen JR, Jakobsen N, Mackey AL, Koch M, Kjaer M, Krogsgaard MR. Remodeling of muscle fibers approaching the human myotendinous junction. Scand J Med Sci Sports. 2018;28:1859–1865. [DOI] [PubMed] [Google Scholar]
- 78.Itano N. Simple primary structure, complex turnover regulation and multiple roles of hyaluronan. J Biochem. 2008;144:131–137. [DOI] [PubMed] [Google Scholar]
- 79.Calve S, Isaac J, Gumucio JP, Mendias CL. Hyaluronic acid, HAS1, and HAS2 are significantly upregulated during muscle hypertrophy. Am J Physiol Cell Physiol. 2012;303:C577–C588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leng Y, Abdullah A, Wendt MK, Calve S. Hyaluronic acid, CD44 and RHAMM regulate myoblast behavior during embryogenesis. Matrix Biol. 2019;78:236–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Velleman SG, Liu C, Coy CS, McFarland DC. Effects of glypican‐1 on turkey skeletal muscle cell proliferation, differentiation and fibroblast growth factor 2 responsiveness. Dev Growth Differ. 2006;48:271–276. [DOI] [PubMed] [Google Scholar]
- 82.Florini JR, Ewton DZ, Magri KA. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991;53:201–216. [DOI] [PubMed] [Google Scholar]
- 83.Droguett R, Cabello-Verrugio C, Riquelme C, Brandan E. Extracellular proteoglycans modify TGF-β bio-availability attenuating its signaling during skeletal muscle differentiation. Matrix Biol. 2006;25:332–341. [DOI] [PubMed] [Google Scholar]
- 84.Xu SY, Liu SY, Xu L, Deng SY, He YB, Li SF, Ni GX. Response of decorin to different intensity treadmill running. Mol Med Rep. 2018;17:7911–7917. [DOI] [PubMed] [Google Scholar]
- 85.Hayashi M, Zhao C, Thoreson AR, Chikenji T, Jay GD, An K-N, Amadio PC. The effect of lubricin on the gliding resistance of mouse intrasynovial tendon. PloS one. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dex S, Alberton P, Willkomm L, Söllradl T, Bago S, Milz S, Shakibaei M, Ignatius A, Bloch W, Clausen-Schaumann H. Tenomodulin is required for tendon endurance running and collagen I fibril adaptation to mechanical load. EBioMedicine. 2017;20:240–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giusti B, Pepe G. Fibrillins in tendon. Frontiers in aging neuroscience. 2016;8:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grant TM, Thompson MS, Urban J, Yu J. Elastic fibres are broadly distributed in tendon and highly localized around tenocytes. J Anat. 2013;222:573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Snow CJ, Peterson MT, Khalil A, Henry CA. Muscle development is disrupted in zebrafish embryos deficient for fibronectin. Dev Dyn. 2008;237:2542–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jenkins MH, Alrowaished SS, Goody MF, Crawford BD, Henry CA. Laminin and Matrix metalloproteinase 11 regulate Fibronectin levels in the zebrafish myotendinous junction. Skelet Muscle. 2016;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thorsteinsdóttir S, Deries M, Cachaço AS, Bajanca F. The extracellular matrix dimension of skeletal muscle development. Dev Biol. 2011;354:191–207. [DOI] [PubMed] [Google Scholar]
- 93.Riley GP, Harrall RL, Cawston TE, Hazleman BL, Mackie EJ. Tenascin-C and human tendon degeneration. Am J Pathol. 1996;149:933. [PMC free article] [PubMed] [Google Scholar]
- 94.Mochida Y, Parisuthiman D, Pornprasertsuk-Damrongsri S, Atsawasuwan P, Sricholpech M, Boskey AL, Yamauchi M. Decorin modulates collagen matrix assembly and mineralization. Matrix Biol. 2009;28:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang B-J, Kim H, Lee SK, Kim J, Shen Y, Jung S, Kang K-S, Im SG, Lee SY, Choi M. Umbilical-cord-blood-derived mesenchymal stem cells seeded onto fibronectin-immobilized polycaprolactone nanofiber improve cardiac function. Acta Biomater. 2014;10:3007–3017. [DOI] [PubMed] [Google Scholar]
- 96.McDevitt TC, Angello JC, Whitney ML, Reinecke H, Hauschka SD, Murry CE, Stayton PS. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J Biomed Mater Res. 2002;60:472–479. [DOI] [PubMed] [Google Scholar]
- 97.Stabenfeldt SE, García AJ, LaPlaca MC. Thermoreversible laminin‐functionalized hydrogel for neural tissue engineering. J Biomed Mater Res A. 2006;77:718–725. [DOI] [PubMed] [Google Scholar]
- 98.Suri S, Schmidt CE. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng Part A. 2010;16:1703–1716. [DOI] [PubMed] [Google Scholar]
- 99.Tate CC, Shear DA, Tate MC, Archer DR, Stein DG, LaPlaca MC. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. J Tissue Eng Regen Med. 2009;3:208–217. [DOI] [PubMed] [Google Scholar]
- 100.Sakaguchi T, Kikuchi Y, Kuroiwa A, Takeda H, Stainier DY. The yolk syncytial layer regulates myocardial migration by influencing extracellular matrix assembly in zebrafish. Development. 2006;133:4063–4072. [DOI] [PubMed] [Google Scholar]
- 101.Bronner‐Fraser M. Distribution and function of tenascin during cranial neural crest development in the chick. J Neurosci Res. 1988;21:135–147. [DOI] [PubMed] [Google Scholar]
- 102.Coles EG, Gammill LS, Miner JH, Bronner-Fraser M. Abnormalities in neural crest cell migration in laminin α5 mutant mice. Dev Biol. 2006;289:218–228. [DOI] [PubMed] [Google Scholar]
- 103.Motalleb R, Berns EJ, Patel P, Gold J, Stupp SI, Kuhn HG. In vivo migration of endogenous brain progenitor cells guided by an injectable peptide amphiphile biomaterial. J Tissue Eng Regen Med. 2018;12:e2123–e2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Balius R, Alomar X, Pedret C, Blasi M, Rodas G, Pruna R, Peña-Amaro J, Fernández-Jaén T. Role of the extracellular matrix in muscle injuries: histoarchitectural considerations for muscle injuries. Orthop J Sports Med. 2018;6:2325967118795863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fukushima K, Nakamura A, Ueda H, Yuasa K, Yoshida K, Takeda Si, Ikeda S-i. Activation and localization of matrix metalloproteinase-2 and-9 in the skeletal muscle of the muscular dystrophy dog (CXMD J). BMC Musculoskelet Disord. 2007;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferré PJ, Liaubet L, Concordet D, SanCristobal M, Uro-Coste E, Tosser-Klopp G, Bonnet A, Toutain P-L, Hatey F, Lefebvre HP. Longitudinal analysis of gene expression in porcine skeletal muscle after post-injection local injury. Pharm Res. 2007;24:1480–1489. [DOI] [PubMed] [Google Scholar]
- 107.Lei H, Leong D, Smith LR, Barton ER. Matrix metalloproteinase 13 is a new contributor to skeletal muscle regeneration and critical for myoblast migration. Am J Physiol Cell Physiol. 2013;305:C529–C538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H, Rudnicki MA. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell stem cell. 2013;12:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Järvinen TA, Järvinen TL, Kääriäinen M, Äärimaa V, Vaittinen S, Kalimo H, Järvinen M. Muscle injuries: optimising recovery. Best Pract Res Clin Rheumatol. 2007;21:317–331. [DOI] [PubMed] [Google Scholar]
- 110.Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002;84:822–832. [PubMed] [Google Scholar]
- 111.Frey SP, Jansen H, Raschke MJ, Meffert RH, Ochman S. VEGF improves skeletal muscle regeneration after acute trauma and reconstruction of the limb in a rabbit model. Clin Orthop Relat Res. 2012;470:3607–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Latroche C, Gitiaux C, Chrétien F, Desguerre I, Mounier R, Chazaud B. Skeletal muscle microvasculature: a highly dynamic lifeline. Physiology. 2015;30:417–427. [DOI] [PubMed] [Google Scholar]
- 113.Casar JC, Cabello-Verrugio C, Olguin H, Aldunate R, Inestrosa NC, Brandan E. Heparan sulfate proteoglycans are increased during skeletal muscle regeneration: requirement of syndecan-3 for successful fiber formation. J Cell Sci. 2004;117:73–84. [DOI] [PubMed] [Google Scholar]
- 114.Langsdorf A, Do A-T, Kusche-Gullberg M, Emerson CP Jr, Ai X. Sulfs are regulators of growth factor signaling for satellite cell differentiation and muscle regeneration. Dev Biol. 2007;311:464–477. [DOI] [PubMed] [Google Scholar]
- 115.Calve S, Odelberg SJ, Simon H-G. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev Biol. 2010;344:259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Docheva D, Müller SA, Majewski M, Evans CH. Biologics for tendon repair. Adv Drug Deliv Rev. 2015;84:222–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leyden J, Kaizawa Y, Chang J. Principles of Tendon Regeneration In. Principles of Tendon Regeneration. Regenerative Medicine and Plastic Surgery: Springer; 2019. [Google Scholar]
- 118.Ireland D, Harrall R, Curry V, Holloway G, Hackney R, Hazleman B, Riley G. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20:159–169. [DOI] [PubMed] [Google Scholar]
- 119.Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, Turner NJ, Weber DJ, Simpson TW, Wyse A. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med. 2014;6:234ra258–234ra258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He C, Yang Z, Jin Y, Qi X, Chu J, Deng X. ADM scaffolds generate a pro-regenerative microenvironment during full-thickness cutaneous wound healing through M2 macrophage polarization via Lamtor1. Front Physiol. 2018;9:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Quantitative analysis of tendon ECM damage using MRI. 2010. IEEE International Symposium on Biomedical Imaging: From Nano to Macro: IEEE; date. 1365–1368. [Google Scholar]
- 122.Zheng S, Xia Y, Bidthanapally A, Badar F, Ilsar I, Duvoisin N. Damages to the extracellular matrix in articular cartilage due to cryopreservation by microscopic magnetic resonance imaging and biochemistry. Magn Reson Imaging. 2009;27:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pathak AP, McNutt S, Shah T, Wildes F, Raman V, Bhujwalla ZM. In vivo “MRI phenotyping” reveals changes in extracellular matrix transport and vascularization that mediate VEGF-driven increase in breast cancer metastasis. PloS one. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brangsch J, Reimann C, Kaufmann JO, Adams LC, Onthank DC, Thöne-Reineke C, Robinson SP, Buchholz R, Karst U, Botnar RM. Concurrent molecular magnetic resonance imaging of inflammatory activity and extracellular matrix degradation for the prediction of aneurysm rupture. Circ Cardiovasc Imaging. 2019;12:e008707. [DOI] [PubMed] [Google Scholar]
- 125.Brinkhof S, Nizak R, Khlebnikov V, Prompers JJ, Klomp DW, Saris DB. Detection of early cartilage damage: feasibility and potential of gagCEST imaging at 7T. Eur Radiol. 2018;28:2874–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ramos GV, Pinheiro CM, Messa SP, Delfino GB, de Cássia Marqueti R, de Fátima Salvini T, Durigan JLQ. Cryotherapy reduces inflammatory response without altering muscle regeneration process and extracellular matrix remodeling of rat muscle. Sci Rep. 2016;6:18525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol. 2004;287:C475–C483. [DOI] [PubMed] [Google Scholar]
- 128.Bondesen BA, Mills ST, Pavlath GK. The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am J Physiol Cell Physiol. 2006;290:C1651–C1659. [DOI] [PubMed] [Google Scholar]
- 129.Morelli KM, Brown LB, Warren GL. Effect of NSAIDs on recovery from acute skeletal muscle injury: a systematic review and meta-analysis. Am J Sports Med. 2018;46:224–233. [DOI] [PubMed] [Google Scholar]
- 130.Bryant AE, Aldape MJ, Bayer CR, Katahira EJ, Bond L, Nicora CD, Fillmore TL, Clauss TR, Metz TO, Webb-Robertson B-J. Effects of delayed NSAID administration after experimental eccentric contraction injury–A cellular and proteomics study. PloS one. 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rogers RS, Nishimune H. The role of laminins in the organization and function of neuromuscular junctions. Matrix Biol. 2017;57:86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hyldahl RD, Nelson B, Xin L, Welling T, Groscost L, Hubal MJ, Chipkin S, Clarkson PM, Parcell AC. Extracellular matrix remodeling and its contribution to protective adaptation following lengthening contractions in human muscle. FASEB J. 2015;29:2894–2904. [DOI] [PubMed] [Google Scholar]
- 133.Alves A, Fernandes K, Melo C, Yamaguchi R, França C, Teixeira D, Bussadori SK, Nunes FD, Mesquita-Ferrari R. Modulating effect of low level-laser therapy on fibrosis in the repair process of the tibialis anterior muscle in rats. Laser Med Sci. 2014;29:813–821. [DOI] [PubMed] [Google Scholar]
- 134.Callaghan T, Wilhelm KP. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part 2: Clinical perspectives and clinical methods in the evaluation of ageing skin. Int J Cosmet Sci. 2008;30:323–332. [DOI] [PubMed] [Google Scholar]
- 135.Callaghan T, Wilhelm KP. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: Cellular and molecular perspectives of skin ageing. Int J Cosmet Sci. 2008;30:313–322. [DOI] [PubMed] [Google Scholar]
- 136.Campisi J, Di Fagagna FDA. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. [DOI] [PubMed] [Google Scholar]
- 137.Collin EC, Carroll O, Kilcoyne M, Peroglio M, See E, Hendig D, Alini M, Grad S, Pandit A. Ageing affects chondroitin sulfates and their synthetic enzymes in the intervertebral disc. Signal Transduct Target Ther. 2017;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kular JK, Basu S, Sharma RI. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J Tissue Eng. 2014;5:2041731414557112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lacraz G, Rouleau A-J, Couture V, Söllrald T, Drouin G, Veillette N, Grandbois M, Grenier G. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PLoS One. 2015;10:e0136217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stearns‐Reider KM, D’Amore A, Beezhold K, Rothrauff B, Cavalli L, Wagner WR, Vorp DA, Tsamis A, Shinde S, Zhang C. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell. 2017;16:518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kragstrup TW, Kjaer M, Mackey A. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports. 2011;21:749–757. [DOI] [PubMed] [Google Scholar]
- 143.Wessner B, Liebensteiner M, Nachbauer W, Csapo R. Age-specific response of skeletal muscle extracellular matrix to acute resistance exercise: A pilot study. Eur J Sport Sci. 2019;19:354–364. [DOI] [PubMed] [Google Scholar]
- 144.Kostrominova TY, Brooks SV. Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. Age. 2013;35:2203–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu T-Y, Pang J-HS, Wu KP-H, Chen MJ, Chen C-H, Tsai W-C. Aging is associated with increased activities of matrix metalloproteinase-2 and-9 in tenocytes. BMC Musculoskelet Disord. 2013;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Marqueti RC, Durigan JL, Oliveira AJS, Mekaro MS, Guzzoni V, Aro AA, Pimentel ER, Selistre-de-Araujo HS. Effects of aging and resistance training in rat tendon remodeling. FASEB J. 2018;32:353–368. [DOI] [PubMed] [Google Scholar]
- 147.Ackerman JE, Bah I, Jonason JH, Buckley MR, Loiselle AE. Aging does not alter tendon mechanical properties during homeostasis, but does impair flexor tendon healing. J Orthop Res. 2017;35:2716–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aszodi A, Legate KR, Nakchbandi I, Fässler R. What mouse mutants teach us about extracellular matrix function. Annu. Rev. Cell Dev. Biol. 2006;22:591–621. [DOI] [PubMed] [Google Scholar]
- 149.Otten C, Wagener R, Paulsson M, Zaucke F. Matrilin-3 mutations that cause chondrodysplasias interfere with protein trafficking while a mutation associated with hand osteoarthritis does not. J Med Genet. 2005;42:774–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hall T, Wood A, Ehrlich O, Li M, Sonntag C, Cole N, Huttner I, Sztal T, Currie P. Cellular rescue in a zebrafish model of congenital muscular dystrophy type 1A. NPJ Regen Med. 2019;4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leonard AK, Loughran EA, Klymenko Y, Liu Y, Kim O, Asem M, McAbee K, Ravosa MJ, Stack MS. Methods for the visualization and analysis of extracellular matrix protein structure and degradation In. Methods for the visualization and analysis of extracellular matrix protein structure and degradation. Methods in cell biology: Elsevier; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lanucara F, Eyers CE. Mass spectrometric-based quantitative proteomics using SILAC In. Mass spectrometric-based quantitative proteomics using SILAC. Methods in Enzymology: Elsevier; 2011. [DOI] [PubMed] [Google Scholar]
- 153.Wiese S, Reidegeld KA, Meyer HE, Warscheid B. Protein labeling by iTRAQ: a new tool for quantitative mass spectrometry in proteome research. Proteomics. 2007;7:340–350. [DOI] [PubMed] [Google Scholar]
- 154.Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10:1794–1805. [DOI] [PubMed] [Google Scholar]
- 155.Nahnsen S, Bielow C, Reinert K, Kohlbacher O. Tools for label-free peptide quantification. Mol Cell Proteomics. 2013;12:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shao X, Taha IN, Clauser KR, Gao YT, Naba A. MatrisomeDB: the ECM-protein knowledge database. Nucleic Acids Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tian Y, Li H, Gao Y, Liu C, Qiu T, Wu H, Cao M, Zhang Y, Ding H, Chen J. Quantitative proteomic characterization of lung tissue in idiopathic pulmonary fibrosis. Clin Proteomics. 2019;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Naba A, Clauser KR, Hynes RO. Enrichment of extracellular matrix proteins from tissues and digestion into peptides for mass spectrometry analysis. J Vis Exp. 2015:e53057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Naba A, Clauser KR, Mani D, Carr SA, Hynes RO. Quantitative proteomic profiling of the extracellular matrix of pancreatic islets during the angiogenic switch and insulinoma progression. Sci Rep. 2017;7:40495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016;49:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Peffers MJ, Thorpe CT, Collins JA, Eong R, Wei TK, Screen HR, Clegg PD. Proteomic analysis reveals age-related changes in tendon matrix composition, with age-and injury-specific matrix fragmentation. J Biol Chem. 2014;289:25867–25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sato N, Taniguchi T, Goda Y, Kosaka H, Higashino K, Sakai T, Katoh S, Yasui N, Sairyo K, Taniguchi H. Proteomic analysis of human tendon and ligament: solubilization and analysis of insoluble extracellular matrix in connective tissues. J Proteome Res. 2016;15:4709–4721. [DOI] [PubMed] [Google Scholar]
- 163.Hakimi O, Ternette N, Murphy R, Kessler BM, Carr A. A quantitative label-free analysis of the extracellular proteome of human supraspinatus tendon reveals damage to the pericellular and elastic fibre niches in torn and aged tissue. PloS one. 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thorpe CT, Peffers MJ, Simpson D, Halliwell E, Screen HR, Clegg PD. Anatomical heterogeneity of tendon: Fascicular and interfascicular tendon compartments have distinct proteomic composition. Sci Rep. 2016;6:20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Barrett AS, Wither MJ, Hill RC, Dzieciatkowska M, D’Alessandro A, Reisz JA, Hansen KC. Hydroxylamine chemical digestion for insoluble extracellular matrix characterization. J Proteome Res. 2017;16:4177–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Obermaier C, Griebel A, Westermeier R. Principles of protein labeling techniques In. Principles of protein labeling techniques. Proteomic Profiling: Springer; 2015. [Google Scholar]
- 167.Bagert JD, Xie YJ, Sweredoski MJ, Qi Y, Hess S, Schuman EM, Tirrell DA. Quantitative, time-resolved proteomic analysis by combining bioorthogonal noncanonical amino acid tagging and pulsed stable isotope labeling by amino acids in cell culture. Mol Cell Proteomics. 2014;13:1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McClatchy DB, Dong M-Q, Wu CC, Venable JD, Yates JR. 15N metabolic labeling of mammalian tissue with slow protein turnover. J Proteome Res. 2007;6:2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Saleh AM, Wilding KM, Calve S, Bundy BC, Kinzer-Ursem TL. Non-canonical amino acid labeling in proteomics and biotechnology. J Biol Eng. 2019;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT). Proc Natl Acad Sci U S A. 2006;103:9482–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dieterich DC, Hodas JJ, Gouzer G, Shadrin IY, Ngo JT, Triller A, Tirrell DA, Schuman EM. In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nat Neurosci. 2010;13:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Calve S, Witten AJ, Ocken AR, Kinzer-Ursem TL. Incorporation of non-canonical amino acids into the developing murine proteome. Sci Rep. 2016;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Saleh AM, Jacobson KR, Kinzer-Ursem TL, Calve S. Dynamics of Non-Canonical Amino Acid-Labeled Intra-and Extracellular Proteins in the Developing Mouse. Cell Mol Bioeng. 2019;12:495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dörrbaum AR, Kochen L, Langer JD, Schuman EM. Local and global influences on protein turnover in neurons and glia. Elife. 2018;7:e34202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.McLeod CM, Mauck RL. High fidelity visualization of cell-to-cell variation and temporal dynamics in nascent extracellular matrix formation. Sci Rep. 2016;6:38852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Loebel C, Mauck RL, Burdick JA. Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat Mater. 2019;18:883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ji W, Li L, Eniola-Adefeso O, Wang Y, Liu C, Feng C. Non-invasively visualizing cell–matrix interactions in two-photon excited supramolecular hydrogels. J Mater Chem B. 2017;5:7790–7795. [DOI] [PubMed] [Google Scholar]
- 178.Gupta SK, Mishra NC, Dhasmana A. Decellularization methods for scaffold fabrication In. Decellularization methods for scaffold fabrication. Decellularized Scaffolds and Organogenesis: Springer; 2017. [Google Scholar]
- 179.Mayorca-Guiliani AE, Willacy O, Madsen CD, Rafaeva M, Heumüller SE, Bock F, Sengle G, Koch M, Imhof T, Zaucke F. Decellularization and antibody staining of mouse tissues to map native extracellular matrix structures in 3D. Nat Protoc. 2019;14:3395–3425. [DOI] [PubMed] [Google Scholar]
- 180.Acuna A, Drakopoulos MA, Leng Y, Goergen CJ, Calve S. Three-dimensional visualization of extracellular matrix networks during murine development. Dev Biol. 2018;435:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Acuna A, Sofronici S, Goergen C, Calve S. In Situ Measurement of Native Extracellular Matrix Strain. Exp Mech. 2019;59:1307–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]