Abstract
Objective
Hepatocellular carcinoma (HCC) is a common malignancy worldwide. Although the contradictory role of ADORA1 has been explored in certain types of cancers, its clinical significance and function in hepatocellular carcinoma cells are largely unknown.
Materials and Methods
The level of ADORA1 in HCC tissues and cells was evaluated by RT-PCR. The function of ADORA1 overexpression on HCC cell proliferation and invasion was assessed by MTS, transwell analysis, and colony formation assay. In addition, a mouse subcutaneous xenograft model was used to study in vivo effects. The efficacy of knockdown of ADORA1 sensitizes to chemotherapy was assessed by staining with Annexin V/propidium iodide followed with flow cytometry and nuclei fragmentation.
Results
In this study, ADORA1 was identified to be up-regulated in HCC tissues compared with adjacent normal tissue. High ADORA1 mRNA expression predicted poor survival in hepatocellular carcinoma patients. Ectopic expression of ADORA1 increased hepatocellular carcinoma cell proliferation and invasion. ADORA1 knockdown inhibited HCC cell growth and sensitized to chemotherapy. Furthermore, ADORA1 activated PI3K/AKT oncogenic signaling pathways. Treatment with PI3K inhibitor LY294002 blocked the effects of ADORA1 on tumor growth in either ADORA1-overexpressing or -deficiency cells. Finally, overexpression of ADORA1 stimulates HCC tumor growth in vivo. Treatment of ADORA1 antagonist oppositely suppressed HCC xenograft tumor growth.
Conclusion
ADORA1 serves as an important oncoprotein and a promoter of cell proliferation through PI3K/AKT signaling pathway in hepatocellular carcinoma.
Keywords: ADORA1, hepatocellular carcinoma, PI3K, AKT, tumor progression
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy worldwide and the second frequent cause of cancer-related death in Asia, particularly in China.1 Nearly 841,000 new cases are diagnosed every year, mortality reached to 782,000. To date, classical chemotherapy is the main treatment for HCC.2 Despite great improvement in therapeutic strategies past two decades, the overall survival of HCC patients is still unsatisfactory, with 30% of the 5-year survival rate.3 Therefore, it is of great urgency to clarify the molecular mechanisms underlying the progression in HCC and thus provide novel therapeutic modalities for cancer treatment.
Adenosine A1 receptor (ADORA1) is one of G-protein coupled receptor family members, including A1, A2A, A2B and A3 receptors.4 It binds with extracellular adenosine accumulating in the tumor microenvironment and subsequently activates downstream signaling cascades that contribute to diverse cancer progression, including renal cancer,5 colorectal cancer,6 and breast cancer.7 However, the role of ADORA1 between cancer types is mutually contradictory. ADORA1 is reported to overexpressed in renal cancer cells and presents an oncogene function to promote renal cancer cell progression and migration.5 Oppositely, up-regulation of ADORA1 by Metformin substantially induces the apoptosis and inhibits the proliferation of human colon cancer cells.6 Similar tumor suppressor role of ADORA1 is also found in breast cancer with ADORA1 antagonist.8
However, the expression pattern, tumor-related role and molecular mechanism of ADORA1 in HCC are currently unknown. In this work, we aimed to explore the biological effect and regulatory mechanisms of ADORA1 in HCC.
Materials and Methods
Patients and Tissue Samples
Tumor samples and adjacent normal tissue samples of 23 patients (with written informed consent) who were diagnosed as hepatocellular carcinoma from March 2010 to March 2012 at Guangxi Medical University Cancer Hospital were analyzed in the current study. The samples were snap-frozen in liquid nitrogen immediately after resection.
Cell Culture and Reagents
Three human HCC cell lines (HepG2, SMMC 7721 and BEL 7402) and normal human liver cells (HL-7702) were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (China). All cells were cultured in RPMI-1640 (DMEM) medium (Gibco, Life Technologies, California, USA) supplemented with 10% fetal bovine serum (Gibco) and penicillin (100UI/mL)/streptomycin (100 mg/mL) (Gibco), and were incubated in an incubator at 37°C with 5% CO2. 1, 3-dipropyl-8-cyclopentylxanthine (DPCPX) was purchased from Sigma-Aldrich (USA). DPCPX was dissolved in dimethyl sulfoxide (DMSO).
Cell Proliferation, Apoptosis and Colony Formation Assay
In cell proliferation assay, 1×104 cells/well were seeded into a 96-well plate. At different experimental end time point, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay was carried out using the MTS assay kit (Thermofisher, Shanghai, China) according to the manufacturer’s protocol.
In 5-Bromo-2′-deoxyuridine (BrdU) assay, the cells were cultured for three days according to designed experimental condition before labeling with (3 µg/mL) BrdU (Thermofisher) for 4 h. The analysis of BrdU was performed as previously described.9
In colony formation assay, 1 × 104 cells/well were seed in the 6-well plate after designed experimental treatment. After 2 weeks, cell colonies were fixed in 10% formalin and stained with crystal violet (0.1% w/v).
The apoptosis of cell was analyzed by Hoechst-33,258 (Thermofisher) nuclear staining. After treatment, the cells were stained with Hoechst-33,258 staining medium (0.1% Hoechst, 0.5% NP-40, 4% formaldehyde in PBS). At least 400 cells in each group were count, and the cells with fragmented nuclear were considered as apoptosis cells.
Cell Invasion
The 24-well BD BioCoat Matrigel Invasion Chambers from BD Bioscience were used to perform cell invasion assay. Briefly, 2–4 × 105 cells of each experimental group were seeded onto the upper wells with a thin layer of matrigel basement membrane matrix. After 2 days, the membranes where have migrated cells were stained with crystal violet (Fisher Scientific). Then the cells with crystal violet were counted under a light microscope (Zeiss Axio Observer) in five random fields (magnification; 40×). Each assay was performed in triplicate.
Real-Time PCR (RT-PCR)
The real-time PCR assay was performed as in previous studies.10 The concentration of total RNA was measured with Nanodrop 2000 micro-volume spectrophotometer (Thermo Scientific). Real-time PCR (RT-PCR) reactions were performed on an Mx3000P real-time PCR system (Stratagene USA). An SYBR Green master mix from ABI (ABI, USA) which operates on a 7500 Real-Time PCR System (ABI, USA) was employed to carry out real-time PCR. Expression levels of the genes to be analyzed were normalized to the expression of β-actin as a reference gene, while the 2−ΔΔCt method was employed to calculate the relative difference or fold change in gene expression. The primers used to amplify the gene were: 5′- CAAAGTGACAGTGGGTGTGG-3′ and 5′- GCCAGGTCCTTCACTGTCTC-3′ for ADORA1; 5′- TGACGTGGACATCCGCAAAG-3′ and 5′- CTG GAA GGT GGA CAG CGA GG-3′ for β-actin.
siRNA Knockdown
Lipofectamine 2000 (Invitrogen) was used to transfect siRNA into cells according to the manufacturer’s protocol. 200 pmoles of siRNA was used in each knockdown experiments. siRNA against ADORA1 and negative control were purchased from Genepharma (Shanghai, China).
Western Blotting
For Western blotting assay, the whole-cell lysates containing protease inhibitors and prepared in RIPA lysis buffer (150 mM NaCl, contains 10 mM Tris, pH7.3, 0.1% sodium dodecyl sulfate (SDS), 1% Triton X-100, 1% deoxycholate, and 5μM ethylenediaminetetraacetic acid) were used for Western blotting. Centrifugation of the lysates was done at 12,000r/min for 5 min at 4°C. The measurement of protein concentration was made using a BCA protein assay kit which is a product of Beyotime Biotechnology. SDS-PAGE (10%) was used to separate 30 μg of protein from each sample and then electrophoretically transmitted to a nitrocellulose membrane. The excess binding sites on the membrane were then saturated with a BSA blocking buffer (5% bovine serum albumin (BSA, Sigma-Aldrich, USA) in TBST buffer) and kept for overnight incubation with primary antibodies at 4°C. The blots were then set up to be incubated with an HRP secondary antibody for an hour at room temperature after which the FlourChemE system (Protein Simple) is used to visualize them according to the instruction elaborated by the manufacturer.
In vivo Xenograft Mouse Model
The animal experiments in the current study were approved by Guangxi Medical University Animal Care and Use Committee and followed by the guidance of Animal Welfare Regulations (USDA 1985; US Code, 42 USC § 289d). ADORA1 overexpression and vector HepG2 cell lines (1 × 106 cells) were injected subcutaneously into a 6-week-old BALB/c athymic nude mouse. (n=6 or each group). Similarly, SMMC 7721 cells (1 × 106 cells) were injected subcutaneously into the right flank of a 6-week-old BALB/c athymic nude mouse. Ten days after tumor inoculation, two groups of mice were treated with PBS (Control) or DPCPX (1 mg/kg) by IP injection (once/2 days for 15 days). (n=6 or each group). The tumor progress was monitored every 2 days and euthanized all mice at 20 days after injection. Tumor volumes were calculated (width2×length×0.5), and the final tumor weight was measured at the day when the animal was euthanized. Once xenograft tumors for the study of Mice were euthanized, and tumors were dissected for Western-blot analysis.
Statistical Analysis
Statistical significance is analyzed by Student’s t-test (paired) for bar graphs and growth curves with GraphPad Prism (v.5). The chi-square test was used to evaluate the associations between ADORA1 expression and clinicopathological factors in HCC. P < 0.05 is considered to statistical difference.
Results
ADORA1 is Up-Regulated in HCC Tissues and Cell Lines
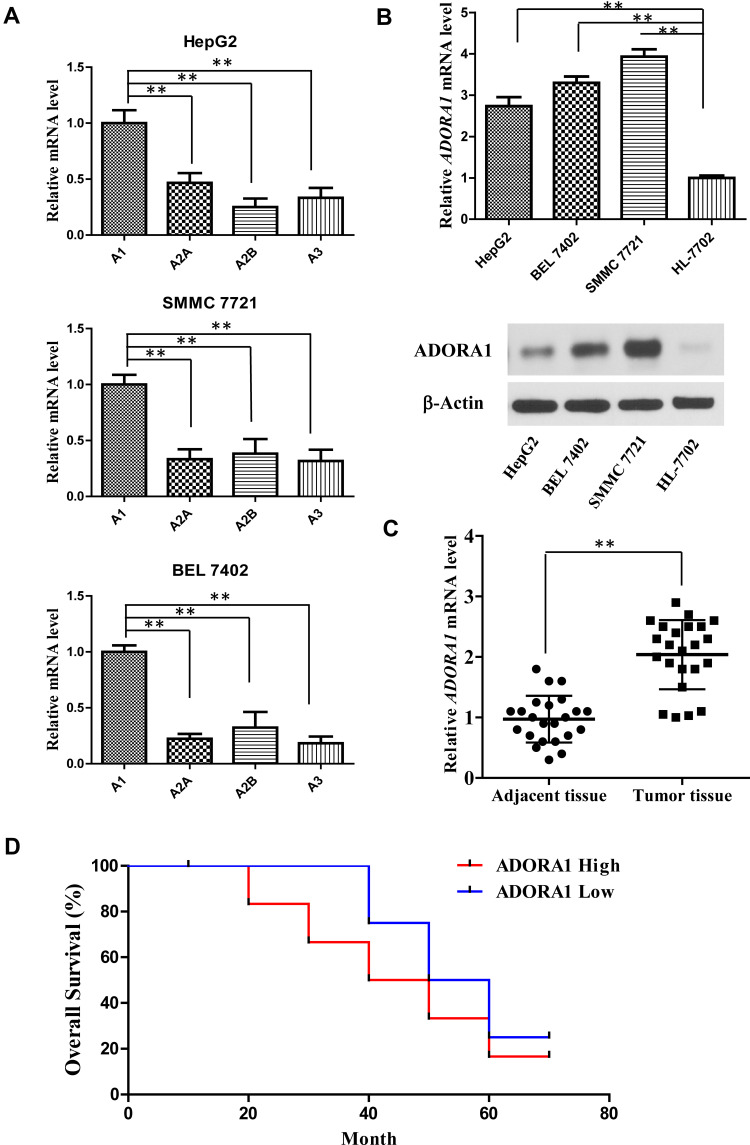
To explore the roles of ADORA1 in HCC progression, we first measured the expression levels of all four A1, A2A, A2B and A3 receptors in three HCC cell lines (HepG2, SMMC 7721 and BEL 7402). As shown in Figure 1A, the mRNA expression of ADORA1 was significantly higher than the other three family members in all the three cell lines. Second, ADORA1 expression was increased in all three HCC cell lines (HepG2, SMMC 7721 and BEL 7402) at both mRNA and protein level, compared with normal human liver cells (HL-7702) (Figure 1B). Third, the expression of ADORA1 was analyzed in tissue samples obtained from 23 pairs of human liver tumor and adjacent normal tissue (Table 1). As shown in Figure 1C, the mRNA expression of ADORA1 was higher in the tumor tissues compared with the adjacent normal mucosa tissues. Moreover, the data analysis with poor overall survival (OS) in the patients also suggested being correlated with high ADORA1 expression (Figure 1D). These results suggest that ADORA1 is up-regulated in HCC tissues and cell lines, a finding that might be relevant to HCC progression.
Figure 1.
ADORA1 is up-regulated in HCC tissues and cell lines. (A) The mRNA levels of all four A1, A2A, A2B and A3 receptors in three HCC cell lines (HepG2, SMMC 7721 and BEL 7402) were measured by RT-PCR. (B) The mRNA and protein level of ADORA1 in HCC cell lines (HepG2, SMMC 7721 and BEL 7402) and normal human liver cells (HL-7702) were measured by RT-PCR and Western-blotting. (C) The mRNA level of ADORA1 in 23 pairs of adjacent tissues and primary tumors in HCC patients were measured by RT-PCR. (D) The overall survival of HCC patients with high and low ADORA1 expression was analyzed by Kaplan–Meier estimator. **, p<0.01.
Table 1.
The Association Between ADORA1 Expression and Clinicopathological Features of HCC Patients
| Clinicopathological Features | ADORA1 Expression | P value | |
|---|---|---|---|
| Low | High | ||
| Age | 0.671 | ||
| <55 years | 5 | 6 | |
| ≥55 years | 6 | 6 | |
| Gender | 0.652 | ||
| Male | 3 | 5 | |
| Female | 7 | 8 | |
| Differentiation | 0.428 | ||
| Well and moderate | 4 | 5 | |
| Poor | 8 | 6 | |
| TNM stage | 0.051 | ||
| I–II | 4 | 6 | |
| III–IV | 3 | 10 | |
ADORA1 Promotes HCC Cell Proliferation and Invasion
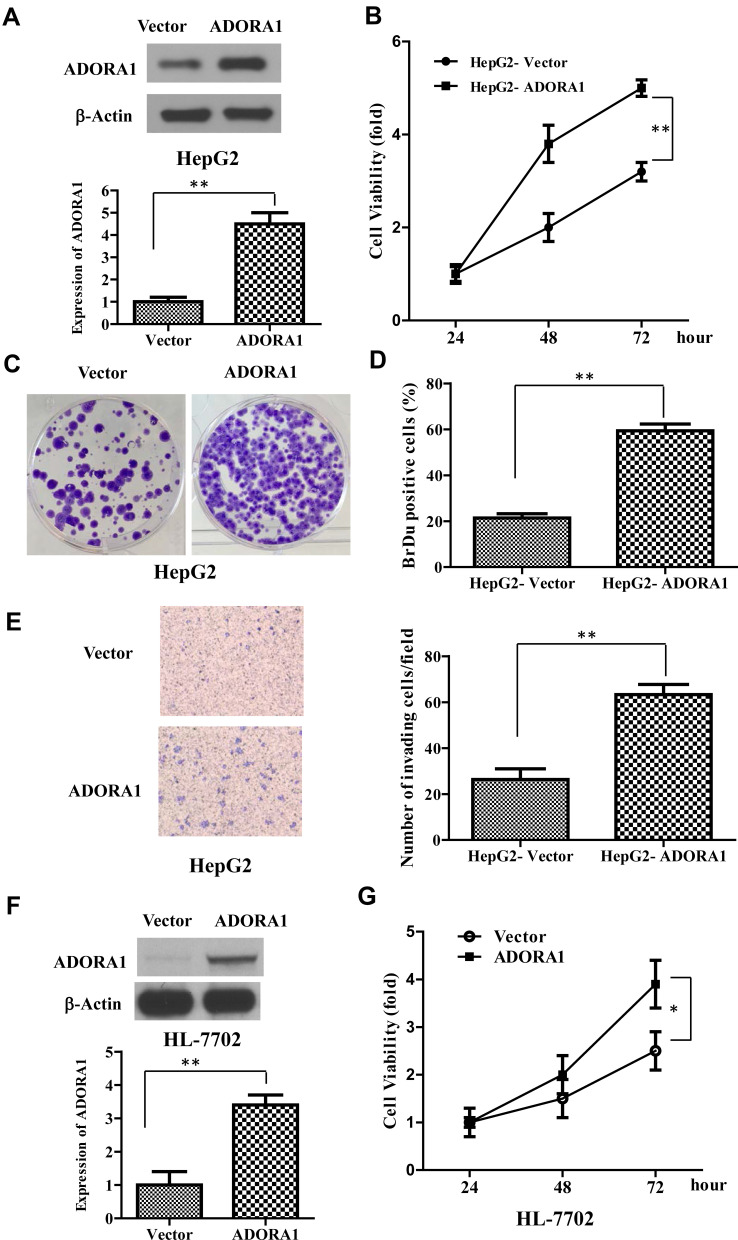
Since ADORA1 expression is relatively lower in HepG2 cells, we first established stable overexpressed ADORA1 HepG2 cells (HepG2-ADORA1) with pcDNA-3.1- ADORA1vector transfection and G418 screening (Figure 2A). The cell growth in HepG2-ADORA1 was significantly faster compared with HepG2 cells transfected with control pcDNA-3.1 vector (Figure 2B). The result from BrdU assay and colony formation assay further suggested that the proliferation of HCC cells was enhanced by ADORA1 overexpression (Figure 2C and D). Furthermore, ADORA1 overexpression resulted in enhanced invasion in transwell assays (Figure 2E). In normal human liver cells (HL-7702), overexpression of ADORA1 also enhanced cell growth efficacy (Figure 2F and G). These results suggest that ADORA1 stimulating the proliferation and invasion of HCC cells.
Figure 2.
ADORA1 promotes HCC cell proliferation and invasion. (A) The stable overexpressed ADORA1 HepG2 cells (HepG2- ADORA1) was established with pcDNA-3.1- ADORA1 vector transfection and G418 screening. Top, the overexpression of ADORA1 was confirmed with Western-blotting. Bottom, relative quantification of ADORA1. (B) The cell growth of HepG2-ADORA1 and HepG2-Vector cells was analyzed by MTS assay in the indicated time points. (C) Representative pictures of colony formation assay of HepG2-ADORA1 and HepG2-Vector cells. (D) Quantification of BrdU assay of HepG2-ADORA1 and HepG2-Vector cells. (E) Invasion of HepG2-ADORA1 and HepG2-Vector cells was analyzed by Tranwell assay. (F) the overexpression of ADORA1 in HL-7702 cells by transfection of pcDNA-3.1- ADORA1 vector. Top, the overexpression of ADORA1 was confirmed with Western-blotting. Bottom, relative quantification of ADORA1. (G) The cell growth of HL-7702-ADORA1 and HL-7702-Vector cells was analyzed by MTS assay in the indicated time points. Data are presented as mean ± SD from three independent experiments. *p<0.05; **p<0.01.
ADORA1 Knockdown Inhibits HCC Cell Growth and Sensitizes to Chemotherapy
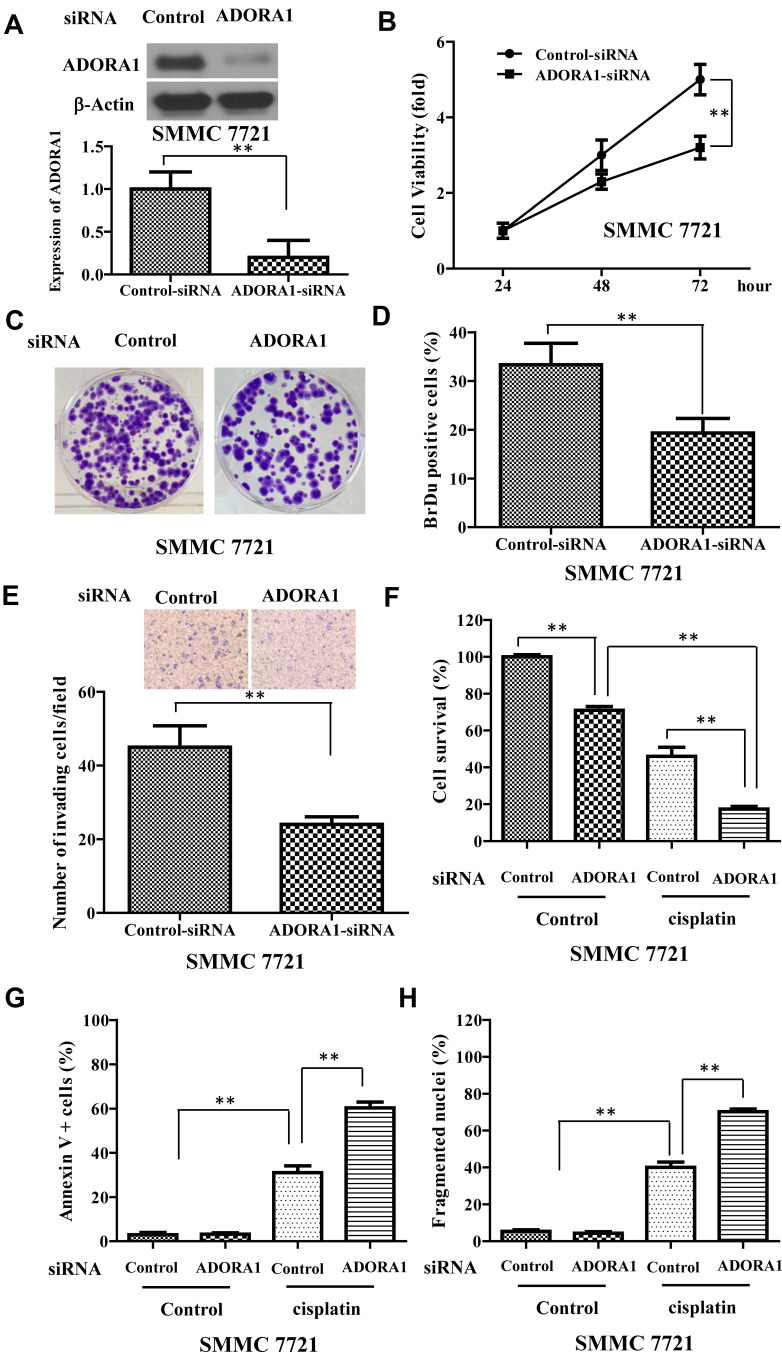
In order to confirm the oncogene function of ADORA1 in HCC progression, we knocked down ADORA1 with siRNA in SMMC 7721 cells, where the relatively highest expression level was observed among all the three HCC cell lines. As shown in Figure 3A, ADORA siRNA greatly slowed down the cell growth in SMMC 7721 cells, compared with control siRNA transfection (Figure 3B). BrdU assay and colony formation assay further confirmed the attenuated cell growth in SMMC 7721 cells transfected with ADORA siRNA (Figure 3C and D). In the meantime, the invasion of SMMC 7721 cells was also suppressed by ADORA knockdown (Figure 3E). Moreover, ADORA siRNA transfection significantly suppressed the cell survival and enhanced apoptosis with cisplatin treatment together, compared with single cisplatin treatment in SMMC 7721 cells (Figure 3F–H). Collectively, these results confirmed the potential oncogenic role of ADORA1 in HCC, also indicated that the promised cotreatment strategy by ADORA1 knockdown with common chemotherapy.
Figure 3.
ADORA1 knockdown inhibits HCC cell growth and sensitizes to chemotherapy. (A) The ADORA1 expression in SMMC 7721 with or without ADORA1 siRNA transfection was confirmed with Western-blotting. Bottom, relative quantification of ADORA1. (B) The cell growth of SMMC 7721 cells with or without ADORA1 siRNA transfection was analyzed by MTS assay in the indicated time points. (C) Representative pictures of colony formation assay of SMMC 7721 cells with or without ADORA1 siRNA transfection. (D) Quantification of BrdU assay of SMMC 7721 cells with or without ADORA1 siRNA transfection. (E) Invasion of SMMC 7721 cells with or without ADORA1 siRNA transfection was analyzed by Tranwell assay. (F) The cell growth of SMMC 7721 cells with or without ADORA1 siRNA transfection following with cisplatin treatment was analyzed by MTS assay. (G) The cell apoptosis of SMMC 7721 cells with or without ADORA1 siRNA transfection following with cisplatin treatment. Cells was stained with Annexin V/propidium iodide, and analyzed by flow cytometry. (H) The cell apoptosis was analyzed by nuclei fragmentation. Data are presented as mean ± SD from three independent experiments. **p<0.01.
Overexpression of ADORA1 Activates PI3K/AKT Signaling Pathway in HCC
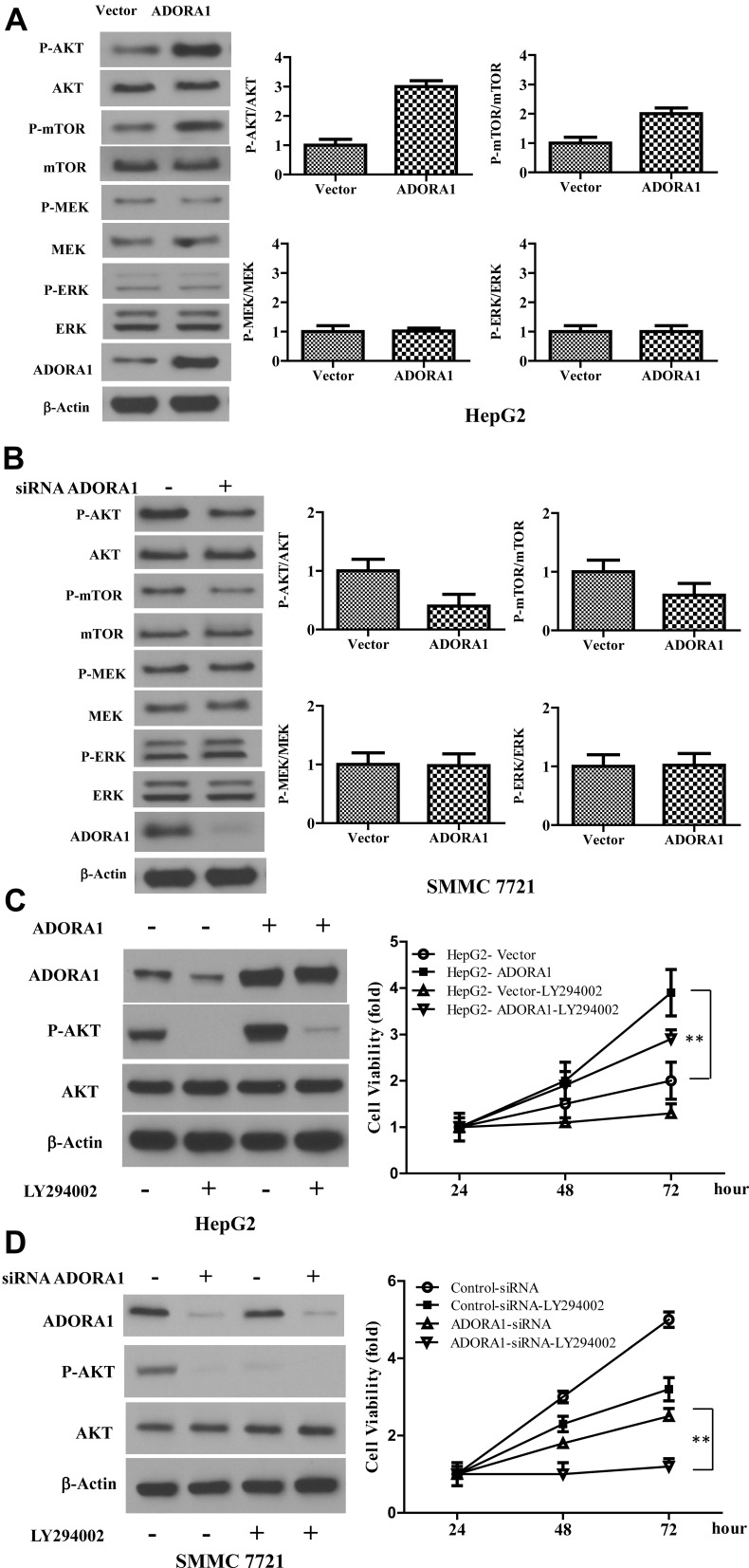
To explore the mechanism underlying the oncogenic role of ADORA1 in HCC, we analyzed classical cell survival signaling pathway, including PI3K/AKT and MAPK pathway. As shown in Figure 4A, phosphorylation of ERK and MEK remained unchanged in HepG2-ADORA1 and HepG2 cells transfected with control pcDNA-3.1 vector. The activation of several key components in PI3K/AKT signaling was significantly increased, including phosphorylation of AKT and mTOR. Oppositely, phosphorylation of AKT and mTOR was decreased after ADORA1 siRNA transfection in SMMC 7721 cells, compared with control siRNA transfection. MAPK pathway still was unchanged under the same experimental condition (Figure 4B). LY294002, PI3K inhibitor abrogated the activation of AKT and increased cell proliferation due to overexpression of ADORA1, but did not change ADORA1 expression (Figure 4C). In the meantime, knockdown of ADORA1 with siRNA inhibited activation of AKT and cell proliferation, LY294002 further slowed down the cell growth in SMMC 7721 cells (Figure 4D). These results suggest that ADORA1 stimulating the proliferation of HCC cells via activation of PI3K/AKT signaling pathway.
Figure 4.
Overexpression of ADORA1 activates PI3K/AKT signaling pathway in HCC. (A) The expression of indicated genes in HepG2-ADORA1 and HepG2-Vector cells were with Western-blotting. (B) The expression of indicated genes in SMMC 7721 cells with or without ADORA1 siRNA transfection were with Western-blotting. (C) HepG2-ADORA1 or HepG2-Vector cells was treatment with or without LY294002 treatment for 24 hours. Left, the expressions of indicated genes were analyzed by Western-blotting. Right, the cell growth was analyzed by MTS assay in the indicated time points. (D) SMMC 7721 cells was transfected with siRNA ADORA1 and then treated with LY294002. Left, the expressions of indicated genes were analyzed by Western-blotting. Right, the cell growth was analyzed by MTS assay in the indicated time points. Data are presented as mean ± SD from three independent experiments. **p<0.01.
Overexpression of ADORA1 Stimulates HCC Tumor Growth in vivo and DPCPX Suppressed HCC Xenograft Tumor Growth in vivo
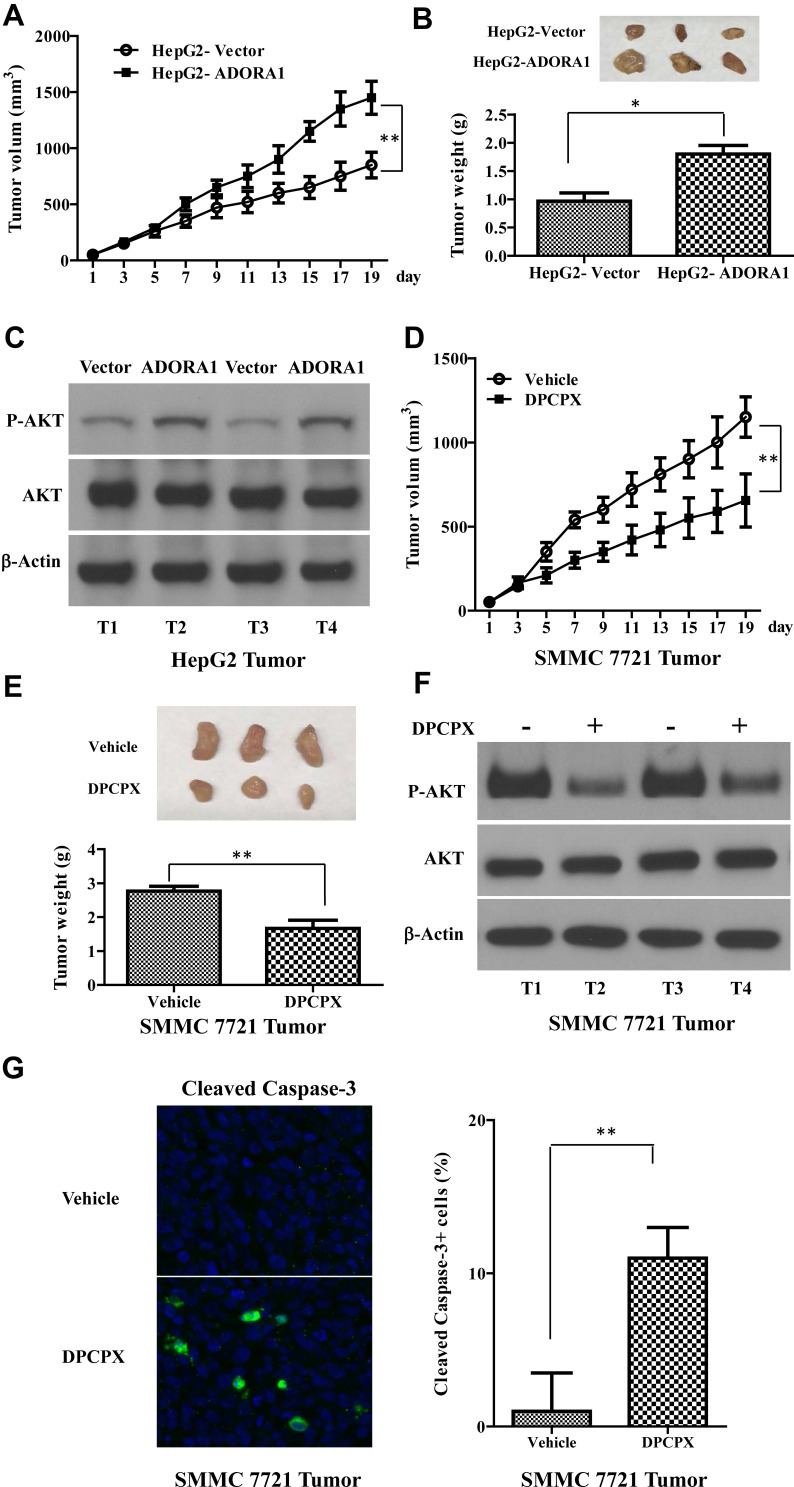
To determine the oncogenic role of ADORA1 in HCC in vivo, we first established xenograft mice model using HepG2-ADORA1 and HepG2-vector cell lines. As shown in Figure 5A and B, the tumor burden was distinctly greater in HepG2-ADORA1 group compared with vector group. The phosphorylation of AKT was also increased in HepG2-ADORA1 tumor compared with vector tumor (Figure 5C). Furthermore, 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), an ADORA1 antagonist, was found that significantly suppressed the tumor growth in SMMC 7721 xenograft mice (Figure 5D and E). The phosphorylation of AKT was shown decreased after DPCPX treatment (Figure 5F), but a significant reduction in activated caspase-3 (Figure 5G). These results indicate that ADORA1 stimulates HCC tumor growth in vivo.
Figure 5.
Overexpression of ADORA1 stimulates HCC tumor growth in vivo and DPCPX suppressed HCC xenograft tumor growth in vivo. (A) HepG2-ADORA1 and HepG2-Vector cells (1 × 106 cells) were injected into nude mice. The tumor curves show the time course of tumor volume. N=6 mice/group. (B) Top, the representative images of tumors. Bottom, the weight of xenograft tumor 20 days after inoculation. (C) The expression of indicated genes in HepG2-ADORA1 and HepG2-Vector tumors were with Western-blotting. (D) SMMC 7721 cells (1 × 106 cells) were injected into nude mouse. The tumor curves show the time course of tumor volume in treated with PBS (Control) or DPCPX. N=6 mice/group. (E) Top, the representative images of tumors. Bottom, the weight of xenograft tumor 20 days after inoculation. (F) The expression of indicated genes in SMMC 7721 tumors were with Western-blotting. (G) The representative images of cleaved Caspase-3staining. Data are the mean + SD of three tumors randomly chosen in each group. *p<0.05; **p<0.01.
Discussion
Adenosine, also named purine nucleoside base, is distributed throughout the entire body and participates in multiple physiological behaviors and diseases.11–13 Mostly, the extracellular adenosine activates downstream signaling cascades as a ligand that binds to adenosine receptors. Among the four direct G-protein coupled receptor family members of Adenosine, ADORA1 is the most sensitive to adenosine and has most extensive actions. Early studies elucidated the neuroprotective function of ADORA1 as an endogenous neuroprotective substance. The activation of ADORA1 on sensory nerve ending actually presents analgesic effect by suppressing adenylyl cyclase (AC) and cyclic adenosine monophosphate (cAMP) concentration.14 ADORA1 deficiency mice show severe anxiety behavior. ADORA1 antagonist also brings anxiety-causing side effect.15 More recently, the wider functions of ADORA1 were explored beyond neuroprotection, including pulmonary hypertension,16 cancers.6,8,9
In the current study, we for the first time revealed the up-regulated expression pattern of ADORA1 in HCC progression, as well as the correlation with poor clinical consequences. Consistent with previous studies, our result suggested that among the G-protein coupled receptor family members of Adenosine, ADORA1 seems to be the highly promising anti-cancer target due to its unique dysregulation pattern in diverse cancers, including colon cancer, astrocytoma and melanoma.17,18 Combined with the direct anti-tumor efficacy of ADORA1 antagonist DPCPX in HCC xenograft mice model, our finding supports the oncogenic role of ADORA1 in certain types of cancer progression and tumor-related function of ADORA1 is cancer classification dependent.
Although both of the pro- or anti-cancer cell proliferation was tight with ADORA1 expression, the underlying mechanism is still related with PI3K/AKT and MARK-ERK pathway, which are the two most important cell survival signaling.19 In previous study, ERK/JNK signaling was suggested to mediate the oncogenic role of ADORA1 in Renal cell carcinoma (RCC).5 By checking the activation of core genes in above two signaling, we excluded the possibility of MARK-ERK mediates overexpression of ADORA1 promotes HCC progression. Oppositely, phosphorylation of AKT and downstream activation of mTOR seem quite essential for pro-cancer progression function of ADORA1 in HCC from both in vitro and in vivo data of the current study. PI3K inhibitor not only suppressed the overexpression of ADORA1 induced cell proliferation, but also further inhibited cell proliferation in ADORA1 deficiency cells.
Besides cell proliferation-related oncogenic function of ADORA1, using ADORA1 antagonist also has been suggested to trigger cell apoptosis and help potential clinical outcome in RCC5 and melanoma.20 Although not to direct check if apoptosis happens after inhibition of ADORA1, we observed that ADORA1 knockdown sensitized the common chemotherapy drug in clinical treatment, by not only further attenuating cisplatin resulted in cell growth inhibition, but also enhancing cisplatin triggered cell apoptosis. More recently, ADORA1 has been shown to participate in the PD-1 based checkpoint anti-cancer strategy in non-small cell lung cancer (NSCLC).21 Given the complexity and etiology of viral hepatitis with HCC in China,22 plus the success exploration on anti–PD-1/PD-L1 blockade immunotherapy in treating Hepatitis B Virus infection-related advanced hepatocellular carcinoma,23 development of FDA-approved ADORA1 antagonists could light up a bright light in HCC, as well as cancer patient outcome, especially with current PD-1-based multiple immune therapies.24
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
Helsinki declaration and analogous ethical standards and guidelines were thoroughly consulted while designing the various aspects of the study. This study was reviewed and approved by the Ethics Committee of Guangxi Medical University Cancer Hospital.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Craig AJ, Von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2019:1–14. [DOI] [PubMed] [Google Scholar]
- 2.Balogh J, Victor D, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41. doi: 10.2147/JHC.S61146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greten TF, Wang XW, Korangy F. Current concepts of immune based treatments for patients with HCC: from basic science to novel treatment approaches. Gut. 2015;64(5):842–848. doi: 10.1136/gutjnl-2014-307990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haskó G, Linden J, Cronstein B, et al. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–770. doi: 10.1038/nrd2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Tong L, Chu X, et al. The adenosine A1 receptor antagonist DPCPX inhibits tumor progression via the ERK/JNK pathway in renal cell carcinoma. Cell Physiol Biochem. 2017;43(2):733–742. doi: 10.1159/000481557 [DOI] [PubMed] [Google Scholar]
- 6.Lan B, Zhang J, Zhang P, et al. Metformin suppresses CRC growth by inducing apoptosis via ADORA1. Front Biosci. 2017;22(2):248–257. doi: 10.2741/4484 [DOI] [PubMed] [Google Scholar]
- 7.Mirza A, Basso A, Black S, et al. RNA interference targeting of A1 receptor-overexpressing breast carcinoma cells leads to diminished rates of cell proliferation and induction of apoptosis. Cancer Biol Ther. 2005;4(12):1355–1360. doi: 10.4161/cbt.4.12.2196 [DOI] [PubMed] [Google Scholar]
- 8.Lin Z, Yin P, Reierstad S, et al. Adenosine A1 receptor, a target and regulator of estrogen receptorα action, mediates the proliferative effects of estradiol in breast cancer. Oncogene. 2010;29(8):1114–1122. doi: 10.1038/onc.2009.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Chen M-N, Du J, et al. Differential expression of adenosine P1 receptor ADORA1 and ADORA2A associated with glioma development and tumor-associated epilepsy. Neurochem Res. 2016;41(7):1774–1783. doi: 10.1007/s11064-016-1893-1 [DOI] [PubMed] [Google Scholar]
- 10.Marzec M, Kasprzycka M, Liu X, et al. Oncogenic tyrosine kinase NPM/ALK induces activation of the rapamycin-sensitive mTOR signaling pathway. Oncogene. 2007;26(38):5606–5614. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima Y, Kanno T, Nagaya T, et al. Adenosine deaminase inhibitor EHNA exhibits a potent anticancer effect against malignant pleural mesothelioma. Cell Physiol Biochem. 2015;35(1):51–60. doi: 10.1159/000369674 [DOI] [PubMed] [Google Scholar]
- 12.Liberman N, O’Brown ZK, Earl AS, et al. N6-adenosine methylation of ribosomal RNA affects lipid oxidation and stress resistance. Sci Adv. 2020;6(17):eaaz4370. doi: 10.1126/sciadv.aaz4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlachogiannis NI, Gatsiou A, Silvestris DA, et al. Increased adenosine-to-inosine RNA editing in rheumatoid arthritis. J Autoimmun. 2020;106:102329. doi: 10.1016/j.jaut.2019.102329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taiwo Y, Levine J. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44(1):131–135. doi: 10.1016/0306-4522(91)90255-M [DOI] [PubMed] [Google Scholar]
- 15.Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83–244. doi: 10.1016/S0301-0082(03)00087-X [DOI] [PubMed] [Google Scholar]
- 16.Valasarajan C, Laria JC, Laria NC, et al. Targeting ADORA1/PDE10A signalosome regulated cAMP microenvironment as a novel therapeutic approach for treating pulmonary hypertension In: C26. LET IT Bleed: Endothelial Injury and Angiogenesis in Pulmonary Hypertension. American Thoracic Society; 2019:A4392–A4392. [Google Scholar]
- 17.Sai K, Yang D, Yamamoto H, et al. A1 adenosine receptor signal and AMPK involving caspase-9/-3 activation are responsible for adenosine-induced RCR-1 astrocytoma cell death. Neurotoxicology. 2006;27(4):458–467. doi: 10.1016/j.neuro.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 18.Koszałka P, Gołuńska M, Urban A, et al. Specific activation of A3, A2A and A1 adenosine receptors in CD73-knockout mice affects B16F10 melanoma growth, neovascularization, angiogenesis and macrophage infiltration. PLoS One. 2016;11(3):e0151420. doi: 10.1371/journal.pone.0151420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012;40(1):139–146. doi: 10.1042/BST20110609 [DOI] [PubMed] [Google Scholar]
- 20.Dastjerdi MN, Valiani A, Mardani M, Ra MZ. Adenosine A1 receptor modifies P53 expression and apoptosis in breast cancer cell line Mcf-7. Bratisl Lek Listy. 2016;117(4):242–246. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Kuang X, Zhang Y, et al. ADORA1 inhibition promotes tumor immune evasion by regulating the ATF3-PD-L1 axis. Cancer Cell. 2020;37(3):324–339. doi: 10.1016/j.ccell.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 22.Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10(1):34. doi: 10.1038/nrgastro.2012.199 [DOI] [PubMed] [Google Scholar]
- 23.Li B, Yan C, Zhu J, et al. Anti–PD-1/PD-L1 blockade immunotherapy employed in treating hepatitis B virus infection–related advanced hepatocellular carcinoma: a literature review. Front Immunol. 2020;11:1037. doi: 10.3389/fimmu.2020.01037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boussiotis VA, Longo DL. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. 2016;375(18):1767–1778. doi: 10.1056/NEJMra1514296 [DOI] [PMC free article] [PubMed] [Google Scholar]