Abstract
Background:
Although adjuvant transcatheter arterial chemoembolization (TACE) has been used to prevent recurrence after surgery in patients with hepatocellular carcinoma (HCC), the survival benefits from adjuvant TACE remain controversial. We sought to systematically evaluate the data on the effectiveness of adjuvant TACE for HCC, as well as identify patient populations that might benefit from adjuvant TACE.
Methods:
The PubMed, Embase, Medline and Cochrane library were systematically searched for studies published before July 2019 that compared adjuvant TACE versus surgery alone for HCC. The study endpoints were overall survival (OS) and disease-free survival (DFS). Patients with large HCC (⩾5 cm), multinodular HCC, microvascular invasion (MVI), or portal vein tumor thrombosis (PVTT) were analyzed in subgroup analyses.
Results:
Twenty-four studies with 6977 patients were included in the analytic cohort. The pooled analysis demonstrated that adjuvant TACE was associated with a better OS and DFS [hazard ratio (HR): 0.67 and 0.67, both p < 0.01]. In subgroup analyses, pooled results revealed that adjuvant TACE was associated with an improved OS and DFS in patients with multinodular HCC (HR: 0.79 and 0.31, both p < 0.01), MVI (HR: 0.62 and 0.67, both p < 0.01), or PVTT (HR: 0.49 and 0.58, both p < 0.01), but not among patients with large HCC (⩾5 cm).
Conclusion:
Postoperative adjuvant TACE may be effective to improve OS and DFS in patients with multinodular HCC, or HCC with MVI or PVTT. Future randomized controlled trials are needed to better define the benefit of adjuvant TACE in subset patients with HCC.
Keywords: adjuvant therapy, disease-free survival, hepatocellular carcinoma, overall survival, transcatheter arterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related mortality worldwide.1 Surgical resection, liver transplantation and tumor ablation are the three curative modalities used to treat HCC.2 Surgical resection remains the primary curative treatment option due to limited donor availability for liver transplantation and technical limitations associated with tumor ablation. Even after curative surgery, the prognosis for patients with HCC is still, however, poor due to the high incidence of postoperative recurrence that is a major cause of subsequent mortality.3–5 Factors associated with HCC recurrence include large tumor size (⩾5 cm), multi-nodularity, microvascular vascular invasion (MVI), and the presence of portal vein tumor thrombosis (PVTT).6,7 Various adjuvant therapies have been used at several centers especially among patients with a high risk of HCC recurrence in an attempt to reduce the possibility of recurrence after surgery. Adjuvant therapeutic approaches have included interferon, sorafenib, immunotherapy, and systemic chemotherapy.8–14 None of these therapies have, however, been determined to be an effective regimen to international authoritative guidelines.15–17
Postoperative transcatheter arterial chemoembolization (TACE), which can be performed 1–2 months after curative resection of HCC, is also a proposed adjuvant therapy to prevent postoperative recurrence.18–21 Adjuvant TACE has been proposed to theoretically eliminate intrahepatic micro-metastases or residual tumor foci, thus preventing recurrence and improving patient survival after resection of HCC.18–21 The role of adjuvant TACE remains controversial, with several studies reporting no survival benefit or even a decrease in overall survival (OS) and disease-free survival (DFS).22,23 Specifically, postoperative TACE may impair hepatic and immunological functions and thus have an unintended adverse effect. The role of adjuvant TACE among patients after resection of HCC remains debated, with some investigators suggesting that adjuvant TACE may only benefit specific subsets of patients.24,25 A comprehensive review of current data on adjuvant TACE may provide an evidence-based approach to identifying which patients might benefit from adjuvant TACE after surgical resection of HCC.
As such, the aim of this study was to perform a systematic review and meta-analysis of pooled published results on long-term outcomes of HCC patients who underwent adjuvant TACE after surgery. In addition to assessing outcomes among patients who did versus did not receive adjuvant TACE, subgroup analyses were performed to identify specific cohorts of patients who might benefit the most from adjuvant TACE.
Methods
A systematic review and meta-analysis on existing published medical literature was conducted following the Cochrane Collaboration guidelines.26
Literature search strategy
The PubMed, Embase and Cochrane Library were searched for studies published before July 2019 using the following terms and search strategy to identify relevant studies: (“Hepatocellular Carcinoma [MeSH]” OR “Liver Cancer” OR “Hepatic Cancer” OR “Primary Hepatic Carcinoma” OR “PHC” OR “Hepatocellular Cancer” OR “HCC”) AND (“Chemoembolization [MeSH]” OR “Transarterial Chemoembolization” OR “Transcatheter Arterial Chemoembolization” OR “TACE” OR “Chemotherapy”) AND (“Hepatectomy [MeSH]” OR “Surgical [MeSH]” OR “Surgical Resection” OR “Surgery” OR “Hepatic Resection” OR “Liver Resection”). The references of the included studies, relevant reviews and meta-analysis were manually screened to identify other eligible studies. Only studies written in English, regardless of patient population, were included.
Eligibility criteria
The inclusion criteria for eligible studies were: (1) Studies that reported patients undergoing surgical resection for HCC; (2) Surgical resection with or without adjuvant TACE was compared; (3) Information on long-term survival was provided. Studies that met any one of the following criteria were excluded: (1) Studies on patients with recurrent or metastatic HCC; (2) Patients received any preoperative/neoadjuvant treatments; (3) No survival comparison of patients who did versus did not receive adjuvant TACE; (4) Replicated data reported by the same authors; (5) Abstracts, reviews, case reports, letters to the editor, and articles written in languages other than English.
Data extraction
Two reviewers (L.L. and C.L.) independently performed data extraction and a third author (T.Y.) cross-checked the data. Any disagreement was resolved through discussion. The data extracted included the surname of the first author, year of publication, study type, period of patient inclusion, number of patients, mean tumor size (cm), number of patients with Child–Pugh (A/B), number of patients with cirrhosis, number of patients with HBsAg (+), number of patients with multiple tumor numbers (⩾2), median OS (months), and median DFS (months). The hazard ratios (HRs) associated with the OS curves were extracted to assess prognosis. The methods for data extraction and calculation, especially the data in the Kaplan–Meier curves, were adopted from methods described in detail by Tierney et al.27 and Parmar et al.28 A calculation spreadsheet in Microsoft Excel was developed to obtain the observed minus expected events (O-E), the variance (V), the HR, the log (HR), and its standard error (SE) for each of the individual trials.
Quality assessment
Cochrane Handbook for Systematic Reviews of Interventions was used to assess the quality of the randomized controlled trials (RCTs) included in the meta-analysis. Sequence generation of randomization, allocation concealment, blinding of patients and personnel and blinding of outcome assessment were evaluated. The bias within and across the studies was further assessed based on the Risk of Bias in Non-randomised Studies - of Interventions (ROBINS-I)29 by the Cochrane Bias Methods Group (BMG). The Grading of Recommendations Assessment, Development and Evaluation (GRADE) System was used to assess the quality of the evidence and the strength of the recommendations.30
Data analysis
The Review Manager (RevMan, the Cochrane Collaboration, Oxford, UK) version 5.3 was used for data pooling. The primary endpoint of the meta-analysis was OS and DFS. The effect measures for OS and DFS were expressed as HR. If adjusted ratios are reported in some studies, such as using propensity score matching analysis, adjusted HRs are used in the analysis. The effect measures for survival rates (at 1-, 3- and 5-year) and DFS rates (at 1-, 2- and 3-year) were expressed as odds ratio (OR). The pooled HR, OR and 95% confidence interval (95% CI) associated with the various outcomes were calculated. Statistical method of Exp(O-E)/Var was adopted to calculate pooled HR and Mantel–Haenszel was adopted to calculate pooled OR. According to the updating Cochrane handbook, random-effects model was chosen as a priority for all analyses, and then the alternative test was performed as a sensitivity test. The results of data pooling in the meta-analysis were presented as “forest plots.” Generally, heterogeneity among studies was assessed using the I2 statistic and the chi-square (X2)-based Q test. A p < 0.1 or I2 >50 indicated significant heterogeneity.31 The 95% CI of the pooled ratio was provided for analysis of statistically significant, as well as the effect range estimate.
Results
Included studies
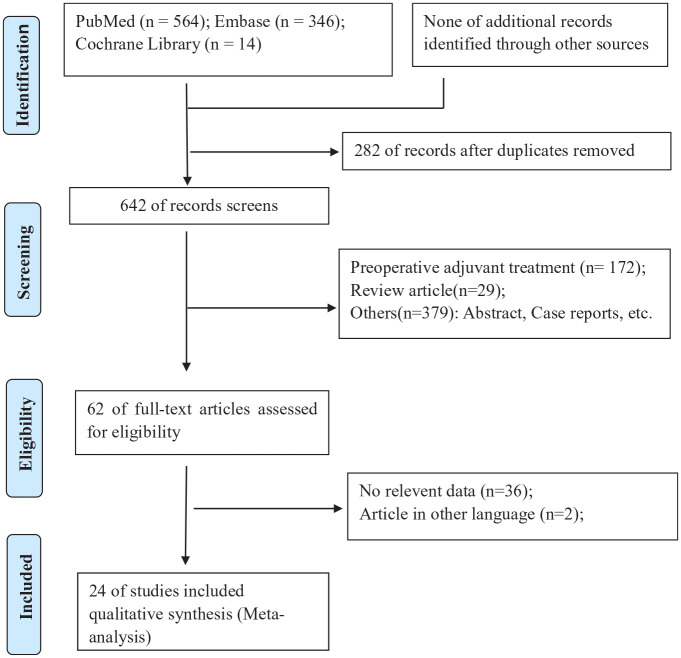
Through searches of PubMed (n = 564), Embase (n = 346) and Cochrane library (n = 14) databases, 924 articles were identified. Some 282 duplicate references were excluded. After abstract reviewing, 580 of the 642 original articles were eliminated for failure to meet the inclusion criteria. In addition, of the remaining 62 studies, 38 were excluded after reviewing the full text due to incomplete data or non-English language. Eventually, 24 studies [nine RCTs20,23,32–38 and 15 non-randomized controlled trials (NRCTs)18,19,21,22,39–49] were included in the systematic review. The search and screening processes of the medical literature review are summarized in Figure 1.
Figure 1.
PRISMA flow diagram showing selection of articles for review.
Quality assessment
Methodological quality was unknown risk of bias in four RCTs20,36–38 and high risk of bias in the remaining five RCTs23,32–35 (Supplement Table 1). Nine NRCTs18,21,22,39,42–44,48,49 were of relatively moderate risk of bias; six NRCTs19,40,41,45–47 were of relatively serious risk of bias (Supplement Table 2).
Baseline characteristics
Twenty-four studies including nine RCTs20,23,32–38 and 15 NRCTs18,19,21,22,39–49 including 6912 patients were published between 1994 and 2019. Among the entire cohort, 5627 (81%) patients were male. TACE was performed 1–2 months after curative resection of HCC by using lipiodol-based regimens, including the administration of an anticancer-in-oil emulsion followed by embolic agents. Mean tumor size was reported in 11 studies,18,19,21,23,36,37,40,41,44,46,48 involving 1948 (30%) patients with a median size of 7 cm (range 3–10). Some 743 (18%) patients with multiple tumors (⩾2) were reported in 13 studies18–23,34,35,37,42,44,46,49 involving 3980 patients. In addition, 3492 (74%) patients with HBsAg (+) were reported in 17 studies18–23,32,34–37,39,40,42,44,46,49 involving 4720 patients. The detailed baseline characteristics of the enrolled studies and patients are shown in Table 1.
Table 1.
Baseline characters of studies and patients.
| Name | Study | Period | Group | Number | Tumor size | Child (A/B) | Cirrhosis (%) | HBsAg (+ %) | Tumors (⩾2, %) | Median OS | Median DFS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Izumi et al.32 | RCT | 1987–1992 | S-TACE | 23 | NA | NA | NA | 6 (26) | NA | 49 | 28 |
| Surgery | 27 | NA | NA | NA | 2 (7) | NA | 41 | 19 | |||
| Li et al.33 | RCT | 1990–1993 | S-TACE | 70 | NA | NA | NA | NA | NA | NA | NA |
| Surgery | 70 | NA | NA | NA | NA | NA | NA | NA | |||
| Ewards et al.23 | RCT | 1991–1995 | S-TACE | 30 | 9 | NA | 17 (57) | 25 (83) | 11 (37) | NA | NA |
| Surgery | 36 | 10 | NA | 19 (53) | 31 (86) | 15 (42) | NA | NA | |||
| Li et al.34 | RCT | 1998–2001 | S-TACE | 39 | NA | 23/16 | NA | 32 (82) | 6 (15) | NA | NA |
| Surgery | 45 | NA | 22/23 | NA | 37 (82) | 9 20) | NA | NA | |||
| Li et al.35 | RCT | 1998–2001 | S-TACE | 35 | NA | 18/17 | NA | 29 (83) | 19 (54) | NA | NA |
| Surgery | 37 | NA | 15/22 | NA | 34 (92) | 17 (46) | NA | NA | |||
| Peng et al.36 | RCT | 1996–2004 | S-TACE | 51 | 9 | 44/7 | 42 (82) | 31 (61) | NA | 13 | NA |
| Surgery | 53 | 8 | 46/7 | 37 (70) | 40 (75) | NA | 9 | NA | |||
| Zhong et al.37 | RCT | 2001–2004 | S-TACE | 57 | 10 | 56/1 | NA | 53 (93) | 44 (77) | 23 | 6 |
| Surgery | 58 | 10 | 58/0 | NA | 52 (90) | 42 (72) | 14 | 4 | |||
| Wei et al.38 | RCT | 2009–2012 | S-TACE | 116 | NA | 116/0 | 50 (43) | NA | NA | 44 | 17 |
| Surgery | 118 | NA | 116/2 | 42 (36) | NA | NA | NA | NA | |||
| Wang et al.20 | RCT | 2011–2014 | S-TACE | 140 | NA | NA | 72 (51) | 29 (21) | 38 (27) | 22 | 26 |
| Surgery | 140 | NA | NA | 66 (47) | 39 (28) | 31 (22) | 9 | 24 | |||
| Tanaka et al.38 | NRCT | NA | S-TACE | 24 | NA | NA | 6 (25) | 6 (25) | 6 (25) | NA | NA |
| Surgery | 41 | NA | NA | 26 (63) | 9 (22) | 8 (20) | NA | NA | |||
| Ren et al.39 (1) | NRCT | 1995–1998 | S-TACE | 108 | NA | 106/2 | 84 (78) | 27 (25) | NA | NA | NA |
| Surgery | 190 | NA | 187/3 | 149 (78) | 47 (25) | NA | NA | NA | |||
| Ren et al.39 (2) | NRCT | 1995–1998 | S-TACE | 77 | NA | 77/0 | 71 (92) | 11 (14) | NA | NA | NA |
| Surgery | 174 | NA | 165/5 | 152 (87) | 43 (25) | NA | NA | NA | |||
| Xi et al.40 | NRCT | 1996–2001 | S-TACE | 145 | 7 | 145/0 | NA | 117 (81) | NA | NA | NA |
| Surgery | 576 | 7 | 560/16 | NA | 450 (78) | NA | NA | NA | |||
| Li et al.41 | NRCT | 2005–2010 | S-TACE | 35 | 6 | 34/1 | 32 (91) | NA | NA | NA | NA |
| Surgery | 41 | 7 | 39/2 | 36 (88) | NA | NA | NA | NA | |||
| Chen et al.42 | NRCT | 2001–2007 | S-TACE | 766 | NA | 754/12 | NA | 668 (86) | 120 (16) | NA | NA |
| Surgery | 1158 | NA | 1133/25 | NA | 1005 (87) | 128 (11) | NA | NA | |||
| Liu et al.43 (1) | NRCT | 1998–2006 | S-TACE | 112 | NA | NA | NA | NA | NA | NA | NA |
| Surgery | 138 | NA | NA | NA | NA | NA | NA | NA | |||
| Liu et al.43 (2) | NRCT | 1998–2006 | S-TACE | 66 | NA | NA | NA | NA | NA | NA | NA |
| Surgery | 112 | NA | NA | NA | NA | NA | NA | NA | |||
| Li et al.19 | NRCT | 2006–2009 | S-TACE | 26 | 5 | 12/14 | 17 (65) | 13 (50) | 4 (15) | 35 | NA |
| Surgery | 34 | 5 | 16/18 | 21 (62) | 17 (50) | 8 (24) | 15 | NA | |||
| Sun et al.18 | NRCT | 2004–2013 | S-TACE | 137 | 7 | 135/2 | 88 (64) | 121 (88) | 11 (8) | NA | NA |
| Surgery | 185 | 7 | 182/3 | 109 (59) | 163 (88) | 17 (9) | NA | NA | |||
| Jiang et al.44 | NRCT | 2007–2010 | S-TACE | 61 | 6 | NA | 51 (84) | 50 (82) | 17 (28) | 32 | NA |
| Surgery | 61 | 6 | NA | 51 (84) | 52 (85) | 15 (25) | 28 | NA | |||
| Liu et al.45 | NRCT | 2005–2013 | S-TACE | 162 | NA | NA | NA | NA | NA | 56 | 23 |
| Surgery | 205 | NA | NA | NA | NA | NA | 35 | 21 | |||
| Qi et al.22 | NRCT | 2012–2014 | S-TACE | 91 | NA | NA | 79 (87) | 77 (85) | 23 (25) | NA | NA |
| Surgery | 109 | NA | NA | 89 (82) | 96 (88) | 25 (23) | NA | NA | |||
| Bai et al.46 | NRCT | 2009–2010 | S-TACE | 31 | 12 | 31/1 | 28 (90) | 6 (19) | 6 (19) | 22 | 14 |
| Surgery | 51 | 10 | 47/4 | 47 (92) | 11 (22) | 9 (18) | 9 | 7 | |||
| Liu et al.47 | NRCT | 2010–2014 | S-TACE | 62 | NA | 59/3 | NA | NA | NA | NA | NA |
| Surgery | 55 | NA | 54/1 | NA | NA | NA | NA | NA | |||
| Tong et al.48 | NRCT | 2010–2014 | S-TACE | 83 | 4 | 81/2 | 35 (42) | NA | NA | 38 | NA |
| Surgery | 83 | 3 | 80/3 | 36 (43) | NA | NA | 31 | NA | |||
| Ye et al.49 (1) | NRCT | 2012–2015 | S-TACE | 72 | NA | 70/2 | 63 (88) | 66 (92) | 10 (14) | NA | NA |
| Surgery | 187 | NA | 180/7 | 156 (83) | 168 (90) | 32 (17) | NA | NA | |||
| Ye et al.49 (2) | NRCT | 2012–2015 | S-TACE | 86 | NA | 84/2 | 72 (84) | 72 (84) | 13 (15) | NA | 37 |
| Surgery | 174 | NA | 172/2 | 143 (82) | 156 (90) | 37 (21) | NA | 13 | |||
| Wang et al.21 | NRCT | 2004–2015 | S-TACE | 57 | 6 | 54/3 | 49 (86) | 47 (82) | 11 (19) | NA | NA |
| Surgery | 57 | 6 | 54/3 | 46 (81) | 51 (89) | 11 (19) | NA | NA |
Ren (1) and (2) was divided into two group by without or with risk factors for residual tumor; Liu H (1) and (2) was divided into two group by serum γ-glutamyl transpeptidase (GGT) ⩽80 U/L or GGT >80 U/L; Ye (1) and (2) was divided into two group by without or with microvascular invasion.
DFS, disease-free survival; NA, not available; NRCT, non-randomized controlled trial; OS, overall survival; RCT, randomized controlled trial; S-TACE, surgery followed by adjuvant transcatheter arterial chemoembolization.
Overall survival
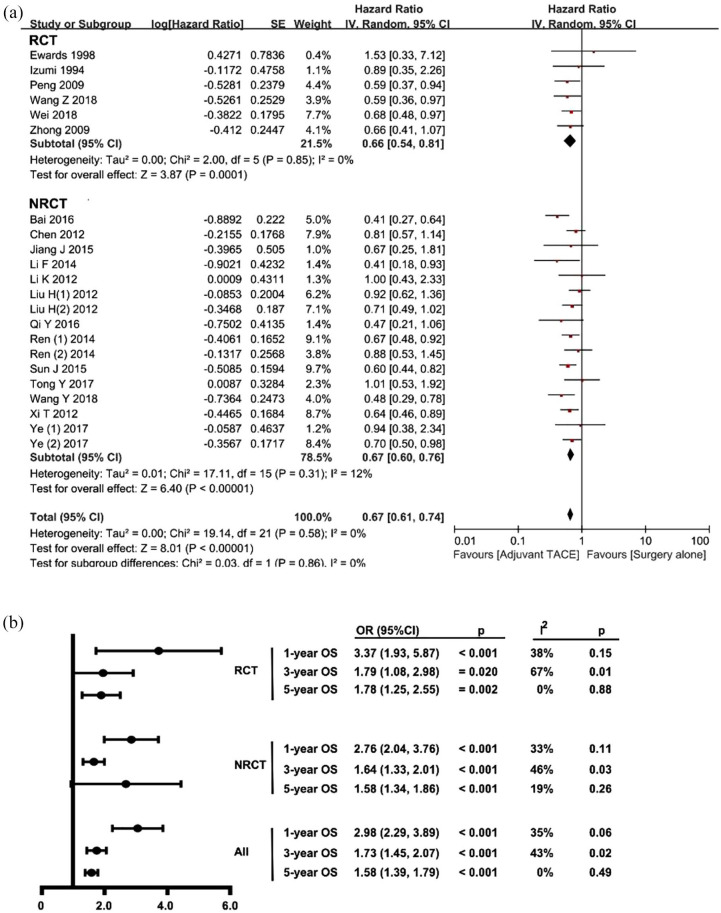
The OS was calculated based on the six RCTs20,32,33,36–38 and 15 NRCTs18,19,21,22,39–46,48,49 that incorporated 6573 patients (n = 2572, 39% for surgery followed by adjuvant TACE versus n = 4001, 61% for surgery alone). The pooled HR for OS among all studies was 0.67 (95% CI 0.60–0.76, p < 0.001; I2 = 0%, p = 0.58), which was in favor of the surgery followed by adjuvant TACE group (Figure 2A). No significant publication bias was noted in the funnel plot (Supplement Figure 1). Furthermore, the pooled analysis of all studies demonstrated that patients who underwent surgery followed by adjuvant TACE had better 1-, 3-, and 5-year survival versus patients who had surgery only (Figure 2B). Of note, the survival benefit was consistent among RCTs and NRCTs. While there was a heterogeneity at 3 years in RCT studies (I2 = 67%, p = 0.01), other studies demonstrated no significant heterogeneity. The pooled effect was estimated by using the random-effect model as demonstrated in a forest plot (Supplement Figure 2A–C). No significant publication bias was noted in the funnel plot (Supplement Figure 3A–C).
Figure 2.
Forest plots comparing the overall survival between surgery followed by adjuvant TACE and surgery alone.
NRCT, non-randomized controlled trial; RCT, randomized controlled trial; TACE, transcatheter arterial chemoembolization
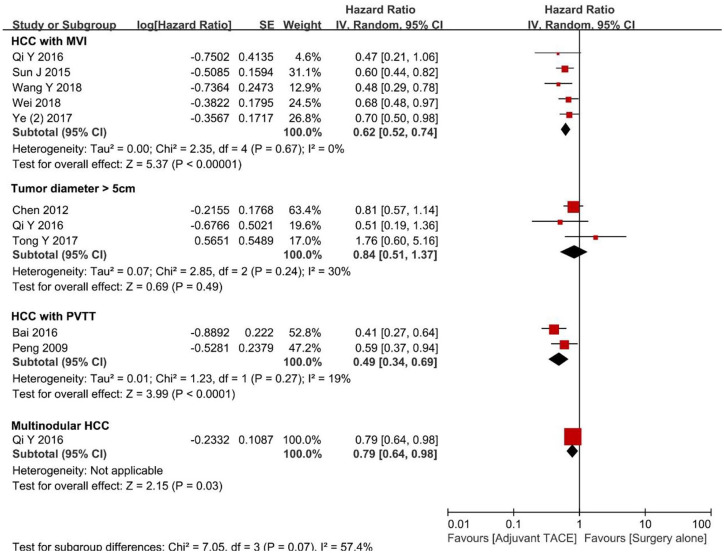
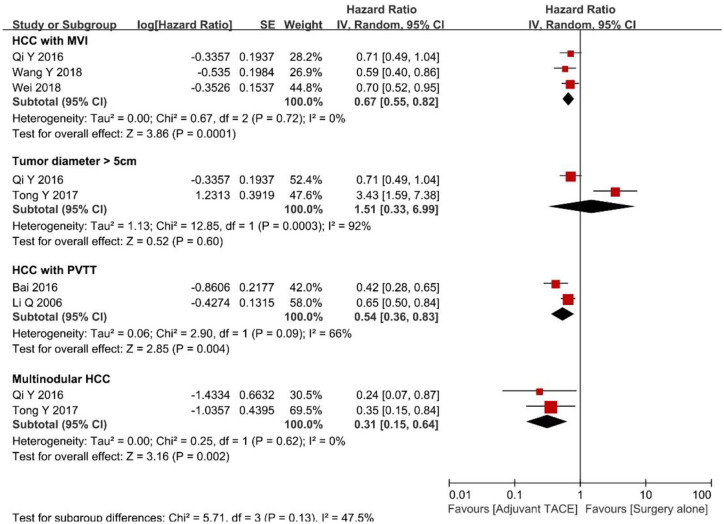
Potential differences in OS among patients who underwent surgery followed by adjuvant TACE versus patients who underwent surgery alone were further examined by stratifying patients according to several risk factors (Figure 3). Specifically, among patients with tumor diameter ⩾5 cm, the pooled HR demonstrated there were no difference among patients who underwent surgery followed by adjuvant TACE versus surgery alone (HR 0.84, 95% CI 0.51–1.37, p = 0.49; I2 = 30%, p = 0.24). In contrast, among patients with multinodular HCC (⩾2), as well as patients who had HCC with MVI or PVTT, the pooled HRs were in favor of surgery followed by adjuvant TACE (HR 0.79, 95% CI 0.64–0.98, p = 0.03; HR 0.62, 95% CI 0.52–0.74, p < 0.001; I2 = 0%, p = 0.67 and HR 0.49, 95% CI 0.34–0.69, p < 0.001; I2 = 19%, p = 0.27, respectively). No significant publication bias was noted in the funnel plot (Supplement Figure 3D).
Figure 3.
Forest plots comparing the overall survival stratified by different risk factors.
HCC, hepatocellular carcinoma; MVI, microvascular vascular invasion; PVTT, portal vein tumor thrombosis; TACE, transcatheter arterial chemoembolization
Disease-free survival
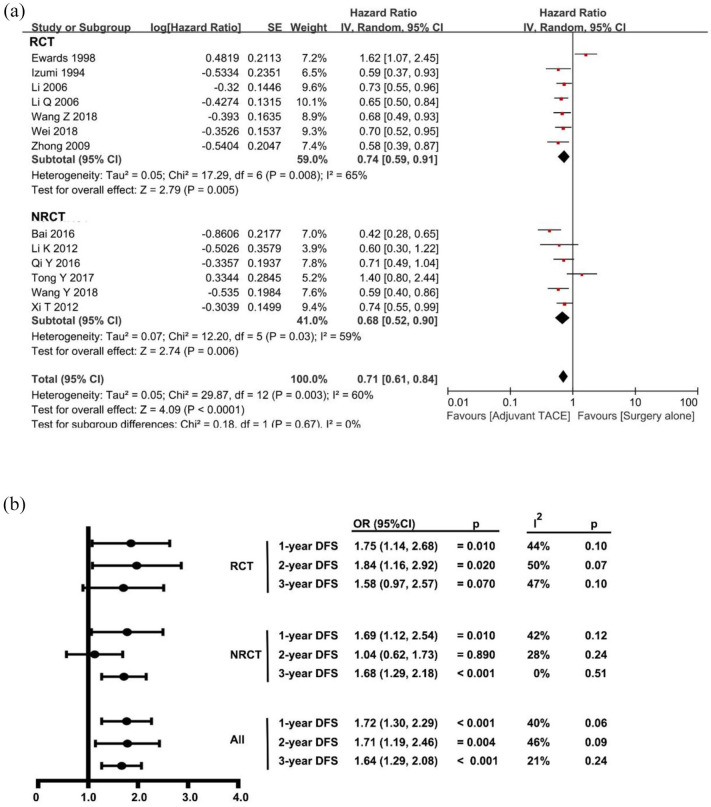
The pooled DFS was calculated based on the seven RCTs20,23,32,34,35,37,38 and six NRCTs21,22,40,41,46,48 that comprised 2260 patients (n = 882, 39% for surgery followed by adjuvant TACE versus n = 1378, 61% for surgery alone). The pooled HR of DFS for all studies was 0.71 (95% CI 0.61–0.84, p < 0.001; I2 = 60%, p = 0.003), which was in favor of adjuvant TACE after surgical resection. The potential reason for the heterogeneity may have been due to the inclusion of the study by Edward et al.;23 when this study was excluded, there were no significant heterogeneity (I2 = 18%, p = 0.27) (Figure 4A). No significant publication bias was demonstrated in the funnel plot (Supplement Figure 4). At the same time, the pooled analysis of all studies demonstrated that patients who underwent surgery followed by adjuvant TACE had a better 1-, 2-, and 3-year DFS than patients who had surgery only (Figure 4B). Though there were no statistical differences in 3-year DFS in the RCT subgroup and 2-year DFS in the NRCT subgroup, patients who underwent surgery followed by adjuvant TACE still had a better survival than patients who had surgery only. In addition, there was good consistency in the reported survival benefit in all included studies. The pooled effect was estimated by using the random-effect model as shown in forest plots (Supplement Figure 5A–C). No significant publication bias was noted in the funnel plot (Supplement Figure 6A–C).
Figure 4.
Forest plots comparing the disease-free survival between surgery followed by adjuvant TACE and surgery alone.
NRCT, non-randomized controlled trial; RCT, randomized controlled trial; TACE, transcatheter arterial chemoembolization
Potential differences in DFS among patients who underwent surgery followed by adjuvant TACE versus surgery alone were examined by stratifying patients according to several risk factors (Figure 5). For patients with tumor diameter ⩾5 cm, the pooled HR demonstrated no significant difference between surgery followed by adjuvant TACE versus surgery alone (HR 1.51, 95% CI 0.33–6.99, p = 0.60; I2 = 92%, p < 0.001). In contrast, among patients with multinodular HCC (⩾2), or HCC with MVI or PVTT, the pooled HRs were in favor of a survival benefit for patients undergoing surgery followed by adjuvant TACE (HR 0.31, 95% CI 0.15–0.64, p = 0.002; I2 = 0%, p = 0.62; HR 0.67, 95% CI 0.55–0.82, p < 0.001; I2 = 0%, p = 0.72 and HR 0.54, 95% CI 0.36–0.83, p = 0.004; I2 = 66%, p = 0.09, respectively). No significant publication bias was noted in the funnel plot (Supplement Figure 6D).
Figure 5.
Forest plots comparing the disease-free survival stratified by different risk factors.
HCC, hepatocellular carcinoma; MVI, microvascular vascular invasion; PVTT, portal vein tumor thrombosis
Sensitivity analysis
A sensitivity analysis was performed, in which one study at a time was removed and the others analyzed to estimate whether the results could have been affected markedly by a single study. Apart from 3-year in RCTs and 2-year in NRCTs for DFS (Figure 4B), the sensitivity analysis demonstrated the results were well stable.
Discussion
This meta-analysis aimed to evaluate the effectiveness of adjuvant TACE after surgery for HCC using the latest published data. In addition, we sought to identify patient populations who might benefit the most from this adjuvant therapy. To this end, 24 studies (nine RCTs and 15 NRCTs) comprising 6912 patients were included in the meta-analysis. Of note, adjuvant TACE following resection of HCC was noted to be associated with an improvement in both OS and DFS. In particular, certain subgroups of patients benefited the most from adjuvant TACE such as HCC patients with multinodular HCC, as well as patients with HCC who had MVI or PVTT. In contrast, there appeared not to be benefit of adjuvant TACE when assessing only tumor size (⩾5 cm) alone.
Adjuvant TACE has been most often used as a means to prevent postoperative recurrence in a few Eastern countries. The proposed mechanism of adjuvant TACE is the elimination of intrahepatic micro-metastases, residual small foci or dissociated cancer cells due to extrusion at the time of surgery.18,19,50 While some studies have reported no benefit of adjuvant TACE after HCC resection, other studies have demonstrated a survival benefit, especially among patients at a high recurrence risk. In 2010, Zhong et al. performed a meta-analysis of six RCTs involving 659 HCC patients to evaluate the efficacy of postoperative TACE.14 The authors reported that adjuvant TACE decreased the 1- and 3-year incidences of death among patients undergoing HCC resection. In 2014, Cheng et al. performed a meta-analysis of six RCTs to assess the beneficial effects of adjuvant TACE after HCC resection.51 These authors reported that adjuvant TACE offered potential benefits after curative HCC resection among patients who had a mean tumor size >5 cm, which was different than the results of the current study. In 2015, Qi et al. published a meta-analysis including 19 RCT and NRCT studies, which suggested that adjuvant TACE improved DFS among patients with HCC.52 In this study, however, the difference in OS was not statistically significant (HR = 0.85, 95% CI 0.72–1.00, p = 0.06), and the heterogeneity among studies was statistically significant (I2 = 70%, p < 0.001).
Compared with previous meta-analyses,14,51,52 the current review was much more extensive as it included 24 studies (nine RCTs and 15 NRCTs) comprising 6912 patients. In addition, the method of data extraction and calculation was more robust as it was adopted in detail from Tierney et al.27 and Parmar et al.28 Data included in the Kaplan–Meier curve analyses, in particular, were more extensive than previous analyses. Of note, the pooled HR in the current study demonstrated significant improvements in OS and DFS among patients who received adjuvant TACE. Importantly, there was no significant heterogeneity. In addition, the survival benefit of adjuvant TACE had a good consistency among RCTs and NRCTs. Pooled OR analyses at 1-, 3- and 5-year for OS, as well as DFS 1-, 2- and 3-year were consistent with previous results of pooled HR.
Another strength of the current study was the subset analyses we performed to identify patient populations who might benefit the most from adjuvant TACE. Specifically, patients who had adjuvant TACE versus surgery alone were examined relative to such risk factors as tumor size ⩾5 cm, multinodular HCC, MVI and PVTT. In subgroup analyses, the pooled results indicated that adjuvant TACE was associated with improved OS and DFS among patients with multinodular HCC, as well as HCC with MVI or PVTT. In contrast, there was no differential survival benefit to adjuvant TACE relative to tumor size of ⩾5 cm.
Several limitations should be considered when interpreting data from the current study. The usage of gelatin sponge and/or related material may difference in each patient, though TACE were performed 1–2 months after curative resection of HCC by using lipiodol-based regimens. Lack of standardized technique of intra-arterial therapy was the major limitation. Then, the consistency and representativeness of patients included was also suboptimal. This heterogeneity in the selection of patients may have led to selection bias. In addition, not all RCTs were high-quality studies and many NRCTs were predominantly retrospective in nature. As such there may be inherent selection bias from some of the studies. Moreover, many studies did not disclose some factors, such as median OS, DFS or the value of HR, which led to the pooling results of some factors only from a few articles. Finally, as all studies were performed in Asia, the results of this meta-analysis might not be applicable to patients in Western countries.
In conclusion, this systematic review and meta-analysis provides updated evidence to support adjuvant TACE as a possible treatment to improve the long-term oncological prognosis for patients undergoing curative resection for HCC, especially in those patients with multinodular HCC, MVI or PVTT. Future RCTs are still needed to better define the benefit of adjuvant TACE in subset patients with HCC.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_1756284820977693 for Survival benefits from adjuvant transcatheter arterial chemoembolization in patients undergoing liver resection for hepatocellular carcinoma: a systematic review and meta-analysis by Lei Liang, Chao Li, Yong-Kang Diao, Hang-Dong Jia, Hao Xing, Timothy M. Pawlik, Wan Yee Lau, Feng Shen, Dong-Sheng Huang, Cheng-Wu Zhang and Tian Yang in Therapeutic Advances in Gastroenterology
Footnotes
Author contributions: Lei Liang, Chao Li and Yong-Kang Diao contributed equally to this work. Lei Liang, Chao Li, Feng Shen, Dong-Sheng Huang, Cheng-Wu Zhang and Tian Yang conceived and designed the study. Lei Liang and Chao Li searched the literature and extracted the data. Lei Liang, Chao Li, Yong-Kang Diao, Hang-Dong Jia and Hao Xing wrote the manuscript. Feng Shen and Tian Yang proofread the manuscript. Timothy M. Pawlik, Wan Yee Lau and Dong-Sheng Huang made critical revision of the manuscript. Tian Yang obtained funding. All authors approved the final version of the manuscript. The authors declare no competing financial interests.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (No. 81672699 and 81972726, Dr Yang).
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Lei Liang  https://orcid.org/0000-0003-3294-2978
https://orcid.org/0000-0003-3294-2978
Hang-Dong Jia  https://orcid.org/0000-0001-7638-2605
https://orcid.org/0000-0001-7638-2605
Tian Yang  https://orcid.org/0000-0002-8333-3673
https://orcid.org/0000-0002-8333-3673
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lei Liang, Department of Hepatobiliary, Pancreatic and Minimal Invasive Surgery, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, Zhejiang, China; Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province, Hangzhou, China.
Chao Li, Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, The Second Military Medical University, Shanghai, China.
Yong-Kang Diao, Department of Hepatobiliary, Pancreatic and Minimal Invasive Surgery, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, Zhejiang, China; Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province, Hangzhou, China.
Hang-Dong Jia, Department of Hepatobiliary, Pancreatic and Minimal Invasive Surgery, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, Zhejiang, China; Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province, Hangzhou, China.
Hao Xing, Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, The Second Military Medical University, Shanghai, China.
Timothy M. Pawlik, Department of Surgery, Ohio State University, Wexner Medical Center, Columbus, OH, USA
Wan Yee Lau, Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, The Second Military Medical University, Shanghai, China Faculty of Medicine, the Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, N.T., Hong Kong SAR, China.
Feng Shen, Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, The Second Military Medical University, Shanghai, China.
Dong-Sheng Huang, School of Clinical Medicine, Hangzhou Medical College, Hangzhou, Zhejiang, China.
Cheng-Wu Zhang, Department of Hepatobiliary, Pancreatic and Minimal Invasive Surgery, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, No. 158, Shangtang Road, Hangzhou, Zhejiang, China.
Tian Yang, Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, The Second Military Medical University, No. 225, Changhai Road, Shanghai, 200438, China.
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65: 87–108. [DOI] [PubMed] [Google Scholar]
- 2. Vitale A, Peck-Radosavljevic M, Giannini EG, et al. Personalized treatment of patients with very early hepatocellular carcinoma. J Hepatol 2017; 66: 412–423. [DOI] [PubMed] [Google Scholar]
- 3. Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015; 261: 947–955. [DOI] [PubMed] [Google Scholar]
- 4. Cucchetti A, Piscaglia F, Cescon M, et al. Conditional survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Clin Cancer Res 2012; 18: 4397–4405. [DOI] [PubMed] [Google Scholar]
- 5. Tsai TJ, Chau GY, Lui WY, et al. Clinical significance of microscopic tumor venous invasion in patients with resectable hepatocellular carcinoma. Surgery 2000; 127: 603–608. [DOI] [PubMed] [Google Scholar]
- 6. Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003; 38: 200–207. [DOI] [PubMed] [Google Scholar]
- 7. Wu JC, Huang YH, Chau GY, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol 2009; 51: 890–897. [DOI] [PubMed] [Google Scholar]
- 8. Sun HC, Tang Z-Y, Wang L, et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol 2006; 132: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang S-N, Chuang S-C, Lee K-T. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: a pilot study. Hepatol Res 2014; 44: 523–531. [DOI] [PubMed] [Google Scholar]
- 10. Lee JH, Lee JH, Lim YS, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015; 148: 1383–1391e6. [DOI] [PubMed] [Google Scholar]
- 11. Kohno H, Nagasue N, Hayashi T, et al. Postoperative adjuvant chemotherapy after radical hepatic resection for hepatocellular carcinoma (HCC). Hepatogastroenterology 1996; 43: 1405–1409. [PubMed] [Google Scholar]
- 12. Liu CJ, Lee PH, Lin DY, et al. Heparanase inhibitor PI-88 as adjuvant therapy for hepatocellular carcinoma after curative resection: a randomized phase II trial for safety and optimal dosage. J Hepatol 2009; 50: 958–968. [DOI] [PubMed] [Google Scholar]
- 13. Chua TC, Saxena A, Chu F, et al. Hepatic resection with or without adjuvant iodine-131-lipiodol for hepatocellular carcinoma: a comparative analysis. Int J Clin Oncol 2011; 16: 125–132. [DOI] [PubMed] [Google Scholar]
- 14. Zhong J-H, Li H, Li L-Q, et al. Adjuvant therapy options following curative treatment of hepatocellular carcinoma: a systematic review of randomized trials. Eur J Surg Oncol 2012; 38: 286–295. [DOI] [PubMed] [Google Scholar]
- 15. Bruix J, Sherman M. and American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Verslype C, Rosmorduc O, Rougier P, et al. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23(Suppl. 7): vii41–vii48. [DOI] [PubMed] [Google Scholar]
- 17. European Association for the Study of the Liver and European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–943. [DOI] [PubMed] [Google Scholar]
- 18. Sun JJ, Wang K, Zhang CZ, et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann Surg Oncol 2016; 23: 1344–1351. [DOI] [PubMed] [Google Scholar]
- 19. Li F, Guo Z, Zhang Y, et al. Postoperative adjuvant arterial chemoembolization improves the survival of hepatitis B virus-related hepatocellular carcinoma: a retrospective control study. Ir J Med Sci 2015; 184: 753–759. [DOI] [PubMed] [Google Scholar]
- 20. Wang Z, Ren Z, Chen Y, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res 2018; 24: 2074–2081. [DOI] [PubMed] [Google Scholar]
- 21. Wang YY, Wang LJ, Xu D, et al. Postoperative adjuvant transcatheter arterial chemoembolization should be considered selectively in patients who have hepatocellular carcinoma with microvascular invasion. HPB (Oxford). Epub ahead of print 22 September 2018. DOI: 10.1016/j.hpb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 22. Qi Y-P, Zhong J-H, Liang Z-Y, et al. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma involving microvascular invasion. Am J Surg. Epub ahead of print 6 August 2018. DOI: 10.1016/j.amjsurg.2018.07.054. [DOI] [PubMed] [Google Scholar]
- 23. Lai EC, Lo CM, Fan ST, et al. Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg 1998; 133: 183–188. [DOI] [PubMed] [Google Scholar]
- 24. Zhong J-H, Li L-Q. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: a meta-analysis. Hepatol Res 2010; 40: 943–953. [DOI] [PubMed] [Google Scholar]
- 25. Zhong J-H, Ma L, Li L-Q. Postoperative therapy options for hepatocellular carcinoma. Scand J Gastroenterol 2014; 49: 649–661. [DOI] [PubMed] [Google Scholar]
- 26. Panic N, Leoncini E, de Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One 2013; 8: e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 29. Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Izumi R, Shimizu K, Iyobe T, et al. Postoperative adjuvant hepatic arterial infusion of lipiodol containing anticancer drugs in patients with hepatocellular carcinoma. Hepatology 1994; 20: 295–301. [PubMed] [Google Scholar]
- 33. Li JQ, Zhang YQ, Zhang WZ, et al. Randomized study of chemoembolization as an adjuvant therapy for primary liver carcinoma after hepatectomy. J Cancer Res Clin Oncol 1995; 121: 364–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Q, Wang J, Sun Y, et al. Postoperative transhepatic arterial chemoembolization and portal vein chemotherapy for patients with hepatocellular carcinoma: a randomized study with 131 cases. Dig Surg 2006; 23: 235–240. [DOI] [PubMed] [Google Scholar]
- 35. Li Q, Wang J, Sun Y, et al. Efficacy of postoperative transarterial chemoembolization and portal vein chemotherapy for patients with hepatocellular carcinoma complicated by portal vein tumor thrombosis–a randomized study. World J Surg 2006; 30: 2004–2011; discussion 2012–2013. [DOI] [PubMed] [Google Scholar]
- 36. Peng B-G, He Q, Li J-P, et al. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg 2009; 198: 313–318. [DOI] [PubMed] [Google Scholar]
- 37. Zhong C, Guo RP, Li JQ, et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol 2009; 135: 1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei W, Jian P-E, Li S-H, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun (Lond) 2018; 38: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ren Z-G, Lin Z-Y, Xia J-L, et al. Postoperative adjuvant arterial chemoembolization improves survival of hepatocellular carcinoma patients with risk factors for residual tumor: a retrospective control study. World J Gastroenterol 2004; 10: 2791–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xi T, Lai EC, Min AR, et al. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: a non-randomized comparative study. Hepatogastroenterology 2012; 59: 1198–1203. [DOI] [PubMed] [Google Scholar]
- 41. Li KW, Wen TF, Li X, et al. The effect of postoperative TACE on prognosis of HCC with microscopic venous invasion. Hepatogastroenterology 2012; 59: 1944–1946. [DOI] [PubMed] [Google Scholar]
- 42. Chen X, Zhang B, Yin X, et al. Lipiodolized transarterial chemoembolization in hepatocellular carcinoma patients after curative resection. J Cancer Res Clin Oncol 2013; 139: 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu H, Zhang A, Qian N, et al. Postoperative transarterial chemoembolization benefits patients with high γ-glutamyl transferase levels after curative hepatectomy for hepatocellular carcinoma: a survival stratification analysis. Tohoku J Exp Med 2012; 227: 269–280. [DOI] [PubMed] [Google Scholar]
- 44. Jiang JH, Guo Z, Lu HF, et al. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: propensity score analysis. World J Gastroenterol 2015; 21: 4627–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu W, Wang K, Bao Q, et al. Hepatic resection provided long-term survival for patients with intermediate and advanced-stage resectable hepatocellular carcinoma. World J Surg Oncol 2016; 14: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bai T, Chen J, Xie Z-B, et al. The efficacy and safety of postoperative adjuvant transarterial embolization and radiotherapy in hepatocellular carcinoma patients with portal vein tumor thrombus. OncoTargets Ther 2016; 9: 3841–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu C, Sun L, Xu J, et al. Clinical efficacy of postoperative adjuvant transcatheter arterial chemoembolization on hepatocellular carcinoma. World J Surg Oncol 2016; 14: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tong Y, Li Z, Liang Y, et al. Postoperative adjuvant TACE for patients of hepatocellular carcinoma in AJCC stage I: friend or foe? A propensity score analysis. Oncotarget 2017; 8: 26671–26678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ye JZ, Chen JZ, Li ZH, et al. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol 2017; 23: 7415–7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kubo S, Takemura S, Sakata C, et al. Adjuvant therapy after curative resection for hepatocellular carcinoma associated with hepatitis virus. Liver Cancer 2013; 2: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheng X, Sun P, Hu Q-G, et al. Transarterial (chemo)embolization for curative resection of hepatocellular carcinoma: a systematic review and meta-analyses. J Cancer Res Clin Oncol 2014; 140: 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Qi X, Liu L, Wang D, et al. Hepatic resection alone versus in combination with pre- and post-operative transarterial chemoembolization for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget 2015; 6: 36838–36859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_1756284820977693 for Survival benefits from adjuvant transcatheter arterial chemoembolization in patients undergoing liver resection for hepatocellular carcinoma: a systematic review and meta-analysis by Lei Liang, Chao Li, Yong-Kang Diao, Hang-Dong Jia, Hao Xing, Timothy M. Pawlik, Wan Yee Lau, Feng Shen, Dong-Sheng Huang, Cheng-Wu Zhang and Tian Yang in Therapeutic Advances in Gastroenterology