Abstract
Visceral myopathy with abnormal intestinal and bladder peristalsis includes a clinical spectrum with megacystis microcolon intestinal hypoperistalsis syndrome and chronic intestinal pseudo-obstruction. The vast majority of cases are caused by dominant variants in ACTG2; however, the overall genetic architecture of visceral myopathy has not been well-characterized. We ascertained 53 families, with visceral myopathy based on megacystis, functional bladder/gastrointestinal obstruction or microcolon. A combination of targeted ACTG2 sequencing and exome sequencing was used. We report a molecular diagnostic rate of 64% (34/53), of which 97% (33/34) is attributed to ACTG2. Strikingly, missense mutations in five conserved arginine residues involving CpG dinucleotides, accounted for 49% (26/53) of disease in the cohort. As a group, the ACTG2- negative cases had a more favorable clinical outcome and more restricted disease. Within the ACTG2-positive group, poor outcomes (characterized by total parenteral nutrition dependence, death or transplantation) were invariably due to one of the arginine missense alleles. Analysis of specific residues suggests a severity spectrum of p.ARG178 > p.R257 > p.R40 along with other less frequently reported sites p.R63 and p.R211. These results provide genotype-phenotype correlation for ACTG2-related disease and demonstrate the importance of arginine missense changes in visceral myopathy.
Keywords: ACTG2, dysmotility, megacystis-microcolon intestinal hypoperistalsis, smooth muscle, visceral myopathy
INTRODUCTION
Visceral myopathy (MIM: 155310) is a rare disorder of smooth muscle dysfunction with phenotypes ranging from massively distended bladder requiring catheterization with functional intestinal dysmotility causing severe feeding intolerance to a milder presentation with a predominant involvement of the gastrointestinal system manifesting as intermittent abdominal distention and functional intestinal obstruction. Clinicians have characterized patients within this broad phenotypic spectrum as megacystis-microcolon intestinal hypoperistalsis syndrome (MMIHS), chronic intestinal pseudo-obstruction (CIPO), or hollow visceral myopathy. The rarity of the disease and the overlap between the different phenotypes have made it difficult to accurately determine the incidence and prevalence (Downes, Cheruvu, Karunaratne, De Giorgio, & Farmer, 2018). A nationwide epidemiologic survey in Japan reported CIPO prevalence of 1.0 and 0.8 cases per 100,000 in males and females, respectively, with an incidence of 0.21 and 0.24 cases per 100,000 (Iida, Ohkubo, Inamori, Nakajima, & Sato, 2013). In the United States, it has previously been estimated that approximately 100 infants with CIPO are born every year (Di Lorenzo, 1999).
A number of genes have been identified underlying these phenotypes with the majority of molecularly-diagnosed cases caused by monoallelic variants in ACTG2 resulting in dominant alleles, with biallelic variants in additional smooth muscle genes underlying some recessive cases
The genetic basis of visceral myopathy remained elusive for many years. Though some studies implicated myopathy in MMIHS/CIPO (Puri, Lake, Gorman, O’Donnell, & Nixon, 1983; Rolle, O’Briain, Pearl, & Puri, 2002), others suggested abnormal innervation (Kapur, 2003), GI hormonal imbalance (Hammar et al., 2012; Taguchi et al., 1989) and perturbation of the cells of Cajal, the intrinsic intestinal pacemaker (Piotrowska et al., 2003) as the underlying mechanism. Evidence from mouse models indicated that impairment of cholinergic neurotransmission, which initiates the process leading to smooth muscle contraction, results in dilated bladder, and mydriasis (Lev-Lehman, Bercovich, Xu, Stockton, & Beaudet, 2001).
We and others identified ACTG2 as the first single gene for the disease traits of visceral myopathy (Lehtonen et al., 2012), and MMIHS/CIPO (Thorson et al., 2014; Wangler et al., 2014). Subsequent reports, supported by functional data from mouse models, established the important role of ACTG2 in these disorders (Halim et al., 2016; Holla, Bock, Busk, & Isfoss, 2014; Klar et al., 2015; Lu et al., 2016; Matera et al., 2016; Milunsky, Baldwin, et al., 2017; Milunsky, Lazier, et al., 2017; Moreno et al., 2016; Tuzovic et al., 2015; Whittington, Poole, Dutta, & Munn, 2017). ACTG2 encodes a muscle actin isoform predominantly expressed in the intestinal smooth muscle (Miwa et al., 1991; Szucsik & Lessard, 1995) which, together with myosin, comprises the apparatus responsible for muscle contraction and relaxation.
Although ACTG2 emerged as the first major causative gene, additional genes have been found to play a role in the pathogenesis. ACTA2 also encodes a smooth muscle actin gene; variation in it causes multisystemic smooth muscle dysfunction syndrome (MIM #613834). This Mendelian condition includes bladder hypotonicity and abnormal intestinal peristalsis; however, in those cases there is also significant involvement of vascular and ciliary smooth muscle leading to vascular aneurysms and mydriasis.
Autosomal recessive forms of MMIHS are caused by biallelic loss-of-function variants in other proteins involved in actin-myosin interactions: MYH11 (myosin heavy chain) (Gauthier et al., 2015); MYLK (myosin light chain kinase) (Halim, Brosens, et al., 2017); LMOD1 (leiomodin 1, an actin-binding protein expressed primarily in vascular and visceral smooth muscle) (Halim, Wilson, et al., 2017) and MYL9 (regulatory myosin light chain) (Moreno et al., 2018). Other genes that are implicated in intestinal hypoperistalsis, but usually with other distinguishing phenotypic features include genes causing mitochondrial disorders (TYMP and POLG); EDNRB3, EDN3 and SOX10 associated with Waardenburg syndrome with Hirschsprung disease; SGOL1, RAD21, FLNA2 and L1CAM.
These studies have highlighted smooth muscle structural proteins and pathways related to smooth muscle function and provided mechanistic insight from case series and reports. Such novel gene discoveries provide further molecular insights into disease pathology, nevertheless, heterozygous variants in ACTG2 remain the most common finding on molecular testing (Ravenscroft et al., 2018). On the basis of data from a small number of cases, some genotype-phenotype correlations have been proposed. In one series, three patients with missense mutations affecting Arg178 were found to have microcolon, while seven patients with Arginine substitutions at another site (Arg38, Arg148, Arg178) were not (Matera et al., 2016). In another cohort of 11 subjects, the observation of severe phenotypes was made for individuals with mutations affecting Arg178 (Halim et al., 2016). However, these observations were not tested statistically due to the small number of cases. In addition, the overall likelihood of finding specific molecular diagnoses for these disorders has not been well defined as most reported cases come from a subset of molecularly diagnosed cases. We, therefore, studied 53 unrelated families with visceral myopathy and report an allelic series. The clinical findings, molecular diagnostic rates, and genotype-phenotype correlations in this cohort provide new insights into the genetic architecture of visceral myopathy.
METHODS
All patients were recruited to the Baylor College of Medicine (BCM) Visceral Myopathy Cohort. All subjects provided informed consent for the study under IRB protocols (H-7777 or H-29697) at BCM. Patients were recruited to the study during two different time intervals; the first from 1999 to 2001, and then from 2013 to the present. Patients with clinical findings of prenatal or postnatal megacystis, microcolon, intestinal hypomotility across a range of ages, intestinal pseudo-obstruction, prune belly syndrome or dependence on total parenteral nutrition (TPN) due to intestinal functional obstruction were ascertained and referred or recruited. Subjects were excluded if they had documented spina bifida, diagnosed Hirschsprung disease or a known secondary cause for neurogenic bladder. We have previously described 15 probands from this cohort in the process of uncovering ACTG2 as the causative gene (Wangler et al., 2014). In another family in the cohort, homozygous variants in MYLK were later identified (Halim, Brosens, et al., 2017). DNA samples from these and additional probands and family members were isolated and stored for further studies.
Targeted ACTG2 Sequencing
We designed a set of primers that cover all the exons and intron-exon boundaries of the ACTG2 gene (Table S1). We utilized these for Targeted ACTG2 Sanger sequencing and for confirmation of results of research exome or external clinical exome and segregation studies in available family members.
Exome Sequencing
Genomic DNA (1 μg) was fragmented by sonication. Illumina paired-end libraries from genomic DNA samples were constructed (Lupski et al., 2013). Pre-capture libraries were pooled and hybridized in solution to the BCM-HGSC CORE exome capture design (Bainbridge et al., 2011) (52Mb; NimbleGen). Captured DNA fragments were sequenced on an Illumina HiSeq 2000 platform, producing 9–10 gigabase-pairs (Gb) of sequence for each personal genome and achieving an average of 95% of the targeted exome bases covered to an average depth of 20x or greater, with mean coverage of target bases of over 100x. Raw sequence reads were mapped and aligned to the GRCh37 (hg19) human genome reference assembly using the HGSC Mercury analysis pipeline (http://www.tinyurl.com/HGSC-Mercury/) (Reid et al., 2014) or the Baylor Genetics analysis pipeline (Liu et al., 2019).
Clinical Data Analysis
Study subjects recruited after 2013 were assessed by clinicians or genetic counselor co-authors by direct history, physical exam and pedigree analysis. In some cases, subjects had been previously referred from centers around the world before 2013 in which case medical information was gathered from communication with health care providers or review of medical records after authorization for release of medical information was provided by the families. In addition, we asked families direct questions to verify information, and determine physicians and hospitals for specific health history events. We also utilized a clinical questionnaire completed by the patients or their families to collect a standard set of data including demographic information and clinical symptoms. This was not possible for all the subjects such that 40 of 53 cases have complete clinical information.
Meta-Analysis
We performed a review of the literature for cohorts and case reports of ACTG2-related visceral myopathy/MMIHS/CIPO. For each of the unrelated probands, we documented the ACTG2 variant, presence or absence of megacystis, microcolon, any GI involvement, any genitourinary (GU) involvement, and the outcome. Outcomes were arbitrarily divided into poor (defined as dependence on total parenteral nutrition, visceral transplant or death in early childhood) versus more favorable outcome (all other cases).
RESULTS
Clinical characteristics of the BCM visceral myopathy cohort
The BCM cohort includes 53 probands with detailed clinical assessment available for 40 cases and their families. The study population consisted of 66% female and 34% male probands consistent with previous studies suggesting a female preponderance (Tables 1 and S2) (Gosemann & Puri, 2011). Mean maternal age in the cohort was 31 years (range 22–40) and mean paternal age was 32 years (range 20–44).
Table 1 –
BCM Visceral Myopathy Cohort overview
| All N/Total (%) | All ACTG2 N/Total (%) | ACTG2 Negative N/Total (%) | |
|---|---|---|---|
| Demographics | |||
| Number of Probands | 53 | 33 | 20 |
| Sex | |||
| Male | 16/40 (40) | 13/28 (46.4) | 3/12 (25) |
| Female | 24/40 (60) | 15/28 (53.6) | 9/12 (75) |
| Clinical Features | |||
| GU Features | |||
| Megacystis | 28/36 (77.8) | 24/27 (89) | 4/9 (44.4) |
| Fetal Bladder Procedure | 4/35 (11.4) | 3/27 (11.1) | 1/8 (12.5) |
| Catheterization or Vesicostomy | 27/34 (79.4) | 24/26 (92.3) | 3/8 (37.5) |
| GI Features | |||
| Microcolon | 18/34 (52.9) | 16/26 (61.5) | 2/8 (25) |
| Bilious Emesis | 15/32 (46.8) | 13/24 (54.2) | 2/8 (25) |
| TPN Dependence | |||
| Total | 11/36 (30.5) | 9/27 (33.3) | 2/9 (22) |
| Partial | 17/36 (47.2) | 16/27 (59.2) | 1/9 (11) |
| Minimal | 8/36 (22.2) | 2/27 (7.4) | 6/9 (67) |
| Abdominal Surgery | 19/36 (52.7) | 15/26 (57.7) | 4/10 (40) |
| GI + GU involvement | 33/38 (86.8) | 28/28 (100) | 5/10 (50) |
Note: N indicates number of cases; Total represents cases with complete clinical information.
Abbreviations: BCM, Baylor College of Medicine; Cath, bladder catheterization; GI, gastrointestinal; GU, genitourinary, TPN, total parenteral nutrition.
We observed prenatal or postnatal megacystis in 77.8% with bladder catheterization requirement in 79.4% of our cohort, and a fetal bladder diversion surgery having been undertaken during pregnancy in four subjects (11.4%), two of whom were recruited in the first interval of ascertainment and two in the more recent recruitment (Table 1 and Figure S1a–c). Microcolon was identified in 53% of the subjects and 52.8% of the subjects underwent abdominal surgery in the first weeks of life with bilious emesis noted in the first days of life in 43.7% (Table 1 and Figure S1d–f). The overwhelming majority of the cohort required TPN or partial parenteral nutrition at some point in life, we noted that 30% of the subjects were totally dependent on TPN for nutrition, whereas an additional 47.2% required partial or temporary parenteral nutrition (Table 1 and Figure S1g). Taken together, the subjects in our cohort represented a medically complex population of individuals with high rates of surgical, bladder and nutritional intervention with similar rates as previous clinical meta-analyses of MMIHS (Gosemann & Puri, 2011).
As a group, patients in the cohort had symptoms affecting both GI and GU systems such as megacystis and TPN dependence; however, there were five notable subjects (13.1%) with disease involvement apparently restricted to either the GI or the GU system (Figure S1h).
We categorized a number of outcomes given the high rates of complications or medical procedures in the cohort. Subjects received care in different hospitals in different countries where medical practices could vary making surgical outcomes challenging to characterize. Patients with visceral myopathy can also have a number of complications related to previous abdominal surgery, infection of central lines, liver disease from long-term TPN, and hospital-related infections. We arbitrarily divided the cohort into dichotomous clinical groups defined on the basis of “poor outcomes” versus more “favorable outcomes” (Figure S1i).
Molecular Diagnostic Rates
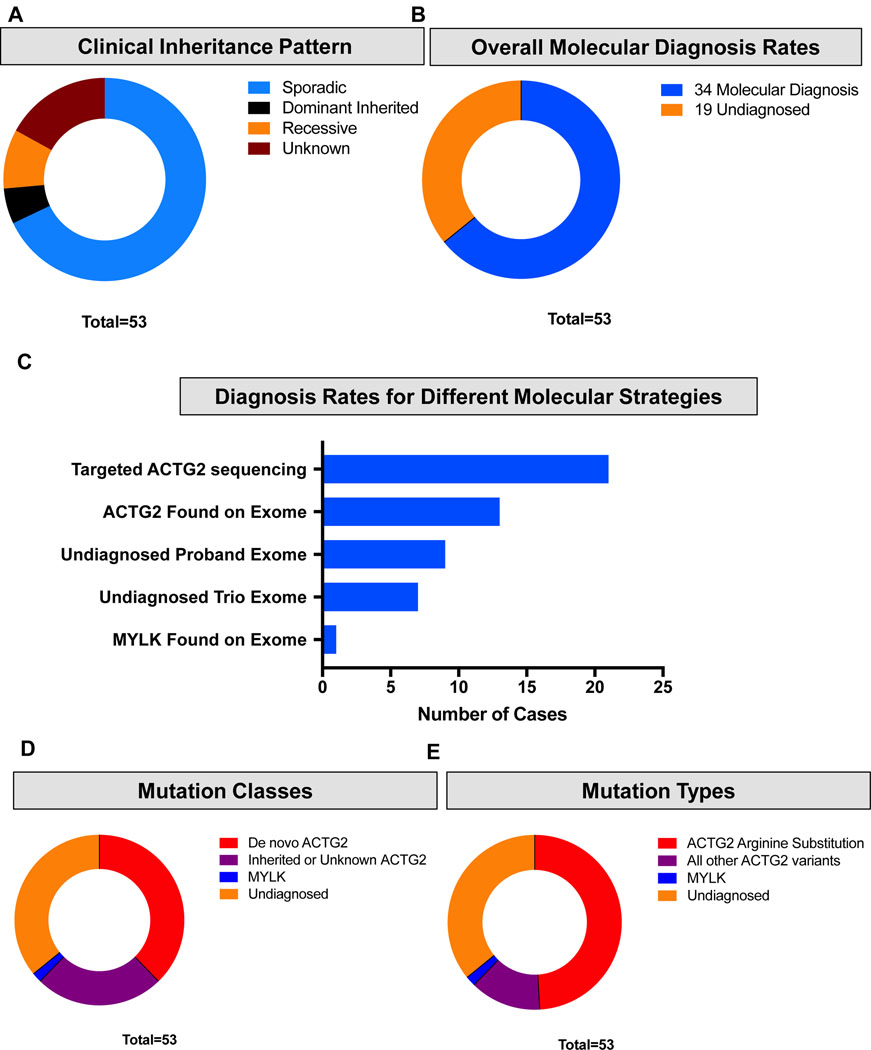
The inheritances pattern of MMIHS varies depending on genetic cause with ACTG2 cases presenting as sporadic or a dominant trait (Wangler et al., 2014), but familial cases with consanguinity point to the recessive loci (Nakamura, O’Donnell, & Puri, 2019). In the present cohort, five families exhibited an apparently-recessive pattern of inheritance where affected siblings had apparently-unaffected parents (Fam10, Fam19, Fam33, Fam36 and Fam44); consanguinity was reported in three of these cases (3/53, 5.6%). In four families the pedigree suggested a dominantly inherited disease (affected child with affected parent; Fam13, Fam34, Fam53 and Fam54). In the majority of cases (38/53, 71.7%) the disease was apparently sporadic (Figure 1a and Table S2).
Figure 1 –
Molecular characterization of the cohort. (a) Apparent patterns of inheritance in the Baylor College of Medicine (BCM) Visceral Myopathy Cohort. Blue represents sporadic cases in the family. Brown represents families with multiple affected members over at least two generations suggesting a dominant inheritance pattern. Orange represents families with affected siblings born to asymptomatic parents suggesting a recessive pattern of inheritance. Red represents unknown pattern of inheritance. (b) Overall molecular diagnosis rates in the BCM Visceral Myopathy Cohort. Blue represents cases with a molecular diagnosis. Orange represents cases without a molecular diagnosis. (c) Number of cases diagnosed by different molecular strategies (exome versus targeted testing). (d) Inheritance patterns observed in the cohort. Red represents de novo ACTG2 cases. Purple represents ACTG2 cases with unknown pattern of inheritance. Blue represents MYLK (autosomal recessive). Orange represents cases lacking a molecular diagnosis. (e) Variant types in the BCM cohort. Red represents missense variants in ACTG2 affecting Arginine residues. Purple represents all other ACTG2 variants. Blue represents variants in MYLK. Orange represents undiagnosed cases.
Upon molecular analysis, individuals in our cohort received a molecular diagnosis of a pathogenic mutation in 64.1% (34/53) of cases (Figure 1b and Table 1). Novel variants have been submitted to Clinvar. Overall, 20 subjects were diagnosed by targeted research testing of ACTG2 using Sanger sequencing or targeted next-generation sequencing, and another 14 subjects were found to have ACTG2 variants by exome sequencing for a total of 33 ACTG2-positive cases (Figure 1c). In one family with the apparent recessive disease we previously identified a pathogenic homozygous MYLK variant (Halim et al., 2016)
Of the cases that received a molecular diagnosis, 20 were due to de novo ACTG2 variants, consistent with the observation of the cohort that the majority of the cases appear sporadically, and suggesting an estimate of 37.7% (20/53) of cases of visceral myopathy are due to de novo ACTG2 events (Figure 1d). We noted that 26/53 (49%) of the cases, both de novo and inherited had ACTG2 arginine missense substitutions (Figure 1e). For 19 families a molecular pathogenic diagnosis has not been identified to date, including 17 sporadic cases and two pairs of siblings. Seven of these families had undergone trio-exome sequencing.
Clinical Features of the ACTG2 positive versus negative cases
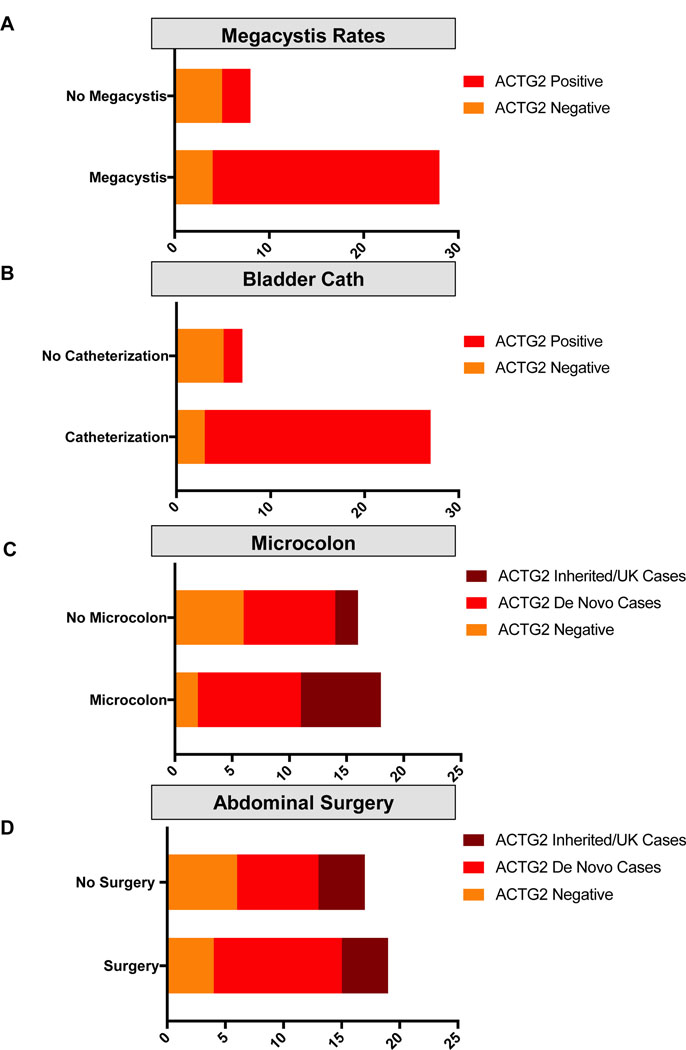
In comparing the cases in our cohort, testing positive for an ACTG2 pathogenic variant to those without, we noted clinical differences. Of the 28 individuals in our cohort with confirmed megacystis, 24 were ACTG2-positive (85.7%), whereas of the eight known to not have megacystis, only three of eight were positive for ACTG2 (37.5%, Figure 2a). Similarly, 92.3% (24/26) of the ACTG2-positive cases required bladder catheterization, compared to only 37.5% (3/8) of the ACTG2- negative cases (Fisher’s Exact test; p = .0035; Figure 2b). Microcolon was identified in 61.5% (16/26) of the ACTG2-positive cases, while only 25% (2/8) had microcolon in the ACTG2- negative cases (Figure 2c). Finally, abdominal surgery in the first weeks of life was performed in 57.7% (15/26) of the ACTG2-positive cases compared to 40% (4/10) in the ACTG2-negative cases (Figure 2d). Taken together the ACTG2- positive and negative cases comprised somewhat clinically distinct groups, particularly in regard to the GU features.
Figure 2 –
Clinical features in the ACTG2-positive versus negative cases. (a,b) Genitourinary features (megacystis [a] and bladder decompression/catheterization [b]). Red represents cases found to harbor ACTG2 pathogenic variants. Orange represents cases without pathogenic variants in ACTG2. (c,d) Gastrointestinal features (microcolon [c] and abdominal surgery in the first weeks of life [d]). Brown represents cases with inherited ACTG2 variants or variants with unknown inheritance pattern. Red represents cases found to harbor de novo ACTG2 pathogenic variants. Orange represents cases without pathogenic variants in ACTG2.
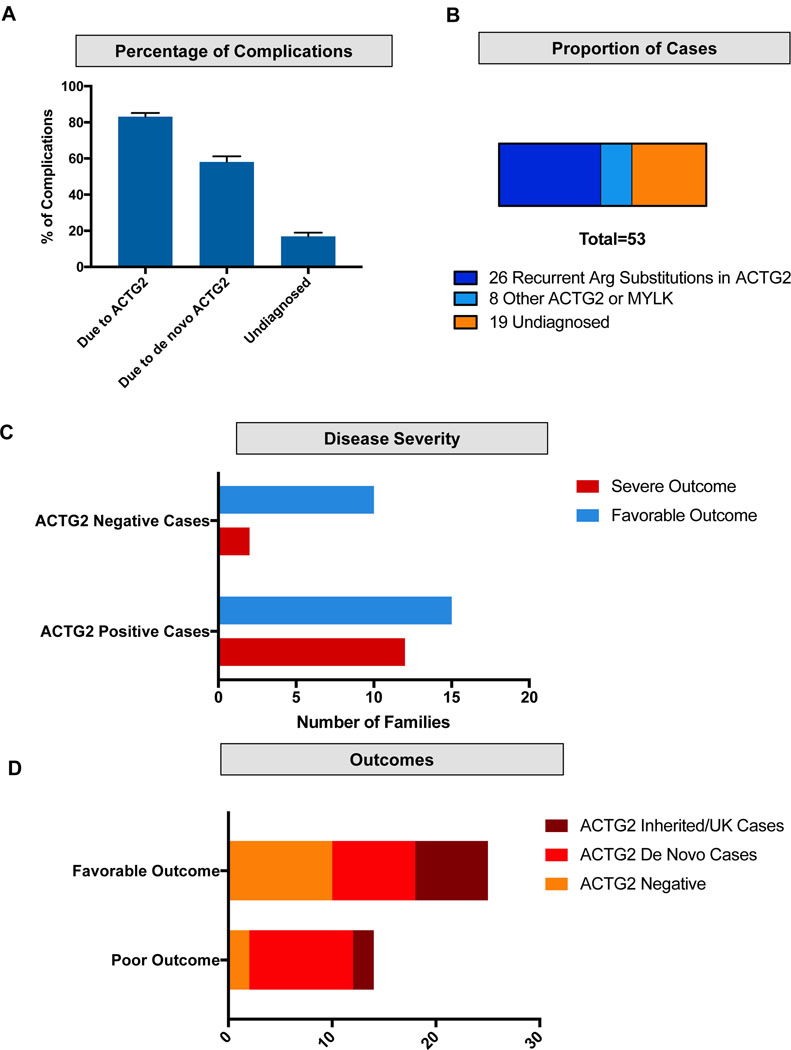
We also noted that for each disease feature or complication classically defined in MMIHS (e.g., megacystis and microcolon), between 75 and 90% could be attributed to ACTG2 (Figure 3a). In addition, we noted that missense substitutions in arginine residues accounted for 49% of all the cases in our cohort (Figure 3b). Notably, there was a large difference in outcomes between the ACTG2-positive and - negative groups (Figure 3c,d). Testing positive for ACTG2 in our cohort led to a 44.4% chance (12/27) of a patient having a poor outcome and severe disease, compared to a 16.7% chance (2/12) of a poor outcome in those testing negative, although the results were not statistically significant (Fisher’s Exact test; p = .1509). In aggregate, these data suggest stringent clinical selection of cases of severe visceral myopathy could predict high rates of positive ACTG2 testing (Figure 3c). Our findings demonstrate that the vast majority of cases with megacystis, microcolon, newborn abdominal surgery, bladder catheterization, and poor long-term prognosis belong to an ACTG2-positive group.
Figure 3 –
Burden of disease attributed to ACTG2. (a) Proportion of disease complications (including megacystis, fetal bladder procedure, bilious emesis, abdominal surgery in the first weeks of life, microcolon, abnormal gastrointestinal motility study and need for bladder catheterization) that is attributed to all ACTG2 cases, ACTG2 de novo cases and ACTG2 negative cases. (b) Proportion of cases with arginine variants (blue), all other molecularly solved cases (light blue) and cases lacking a molecular diagnosis (orange) in the Baylor College of Medicine cohort. (c) Overall outcomes in the cohort. Poor outcome is defined as death in early childhood, dependence on total parenteral nutrition, or cases undergoing visceral transplant. Favorable outcome is defined as lacking these features. Brown represents proportion attributed to ACTG2 inherited cases or of unknown inheritance. Red represents ACTG2 de novo cases. Orange represents ACTG2 negative cases. (d) Disease severity in the ACTG2-positive versus ACTG2-negative. Red represents poor outcomes defined as above. Blue represents more favorable outcomes.
Overall, the undiagnosed group was more clinically heterogeneous than the ACTG2-positive group. One case had prune belly syndrome with no GI involvement (Fam38-1). These cases also had a number of additional features reported including multiple congenital anomalies (Fam31-1), developmental delays and myoclonic jerks (Fam40-1), symptoms resembling postural orthostatic tachycardia syndrome and recurrent pancreatitis (Fam45-1), spina bifida occulta (Fam48-1) and multiple café-au-lait macules in 2 siblings (Fam33-1 and Fam33-4).
Recurrent arginine missense mutations in ACTG2
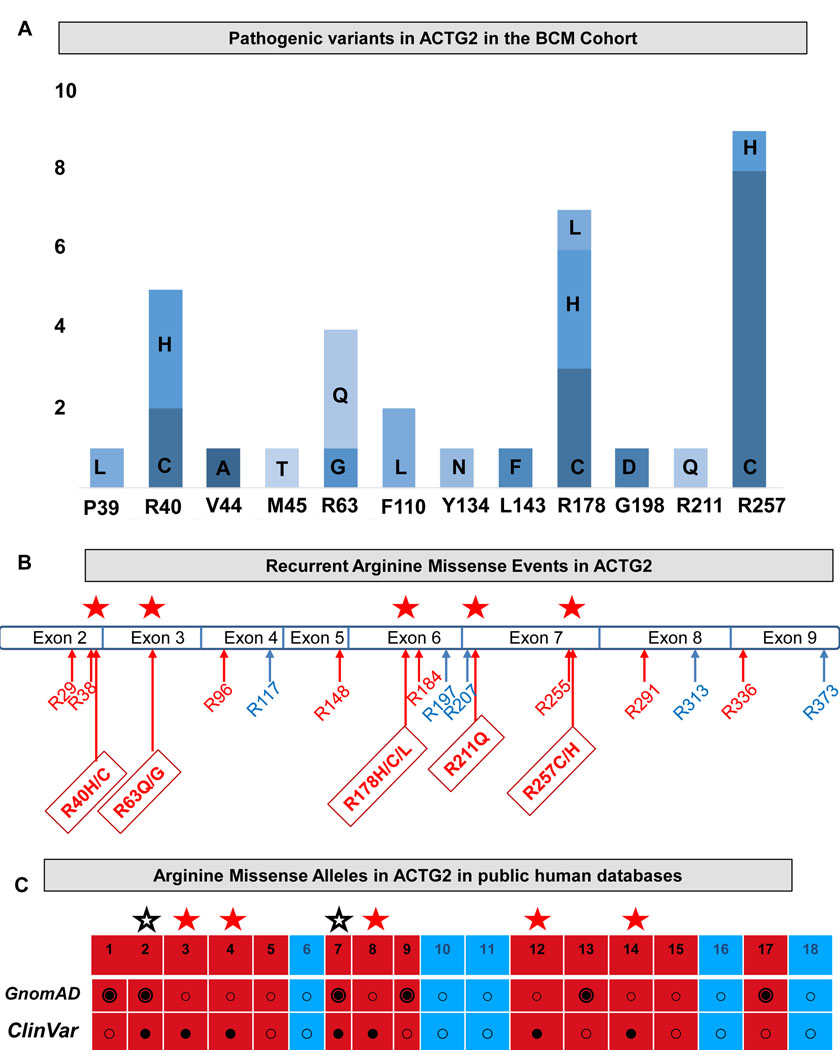
The pathogenic variants in our cohort were all coding missense substitutions. These included 17 unique variants, affecting 12 unique amino acid positions of the ACTG2 protein (Figure 4a and Table S3). The ACTG2 gene encodes a 376 amino acid actin protein which is nearly identical at the amino acid level to the five other paralogs in the human genome (ACTG1, ACTC1, ACTA1, ACTA2 and ACTB) (Figure S2). All of the actin proteins share 18 identical arginine residues distributed across the protein. In ACTG2, 13 of the 18 arginine residues are encoded by a “CGX” (CGG, CGC, CGA and CGU) codon with a CpG dinucleotide at that site (Figure 4b, sites in red). We observed five recurrent arginine sites in which multiple recurrent arginine missense substitutions were identified (red stars). These sites accounted for 78.7% of the ACTG2-positive cases and nearly 50% of the cases in our entire cohort.
Figure 4 –
ACTG2 variants. (a) ACTG2 variants in the Baylor College of Medicine cohort. Bars represent number of probands with a variant at each position shown (e.g., P39 indicates proline at position 39 or p.Pro39). The amino acid change is indicated on the graph. (b) Arginine residues shown on the ACTG2 exon structure. Red labels indicate sites encoded by a “CGX” codon (CpG dinucleotides). Blue labels indicate all other Arginine residues. Red stars indicate a recurrently-mutated site. Positions of the arginine residues are labeled (e.g., R40H/C indicates p.Arg40His and p.Arg40Cys). (c) Arginine missense variants in public human databases (GnomAD and Clinvar). Black filled double circles in gnomAD indicate presence of the allele at Minor allele frequency (MAF) > 0 in the database. Black dots represent pathogenic or likely pathogenic variants in ClinVar. Red boxes represent positions encoded by CpG dinucleotides. Blue boxes represent all other arginine variants. Red stars indicate recurrent pathogenic variants in our cohort, all present in ClinVar and absent from GnomAD. Black stars indicate Arginine missense alleles that are present in both ClinVar as pathogenic or likely pathogenic variants in ClinVar which are also present at MAF > 0 in GnomAD.
Next, we examined public databases including gnomAD and ClinVar (Harrison et al., 2016; Lek et al., 2016) and we catalogued which of these and the other arginine sites have been noted to have missense substitutions. We found that the five recurrent sites were all absent in gnomAD, but present in ClinVar. We also noted that p.Arg38 and p.Arg148 are additional arginine sites encoded by a CGX codon where subsitutions that are noted as pathogenic in ClinVar occur that are clearly causative for MMIHS from previous publications (Holla et al., 2014; Lehtonen et al., 2012; Matera et al., 2016; Ravenscroft et al., 2018), but interestingly missense variants at these two sites are also present in gnomAD (Figure 4c): p.Arg38Cys, p.Arg148Cys and p.Arg148His were each present in a heterozygous state with a minor allele frequency of 1/251,154 (0.000004). Of note, we observed a total of ten stopgain ACTG2 alleles (six unique). Of these, four were an arginine nonsense mutation p.Arg211Ter with a minor allele frequency of 0.000017 in gnomAD. These findings of loss-of-function alleles in gnomAD but missing from clinical cohorts, are in line with our previous analysis that suggested that haploinsufficiency of ACTG2 is unlikely; indeed we had not observed these variants in our cohort (Wangler et al 2014).
Our data pointed to a central and prevalent role for the ACTG2 locus in this disease phenotype, and to sporadic cases due to de novo events and less commonly dominant inherited disease. We examined the paternal ages in our cohort (Figure S3a) and observed a modest effect in which the average paternal age at time of birth in the families with a de novo ACTG2 mutation was 34 versus 29 years in all other cases. This difference was statistically significant (Fisher’s Exact test; p=0.0432).
Since the earliest efforts to apply genomics to MMIHS, consanguinity has been observed in some cases. Despite, the data on ACTG2 and de novo events, it was recently suggested that based on the rates of consanguinity that MMIHS should be considered primarily an autosomal recessive disorder (Nakamura et al., 2019). We, therefore, explored the apparent recessive cases in our cohort. Parental consanguinity was reported in three families in the cohort with affected siblings (Fam10, Fam33 and Fam36), of which one case was found to harbor a homozygous variant in MYLK (Fam36) (Halim, Brosens, et al., 2017). Interestingly, one other nonconsanguineous case that appeared recessive with two affected siblings, one of whom underwent intestinal transplant, was ultimately found to be due to the ACTG2 p.Arg178Cys variant (Figure S3b). Significant intrafamilial variability was observed accounting for this observation as the siblings inherited a pathogenic variant in ACTG2 from their mother who had been undiagnosed. Notably, she had prolonged labor due to uterine atony, a feature also reported in another family (Fam51) in the cohort as well as other cases in the literature (Klar et al., 2015; Sipponen, Karikoski, Nuutinen, Markkola, & Kaitila, 2009). In an additional family with multiple affected members (Fam34) symptoms ranged from chronic constipation with or without urinary tract infections to severely affected individuals requiring catheterizations and abdominal surgeries (Figure S4a). Ages at diagnosis ranged from the prenatal period to adulthood in this family. Given the wide range of intrafamilial variability, apparent recessive cases with affected siblings still does not exclude a dominant model with incomplete penetrance in the parents, and ACTG2 testing can still be useful. We also postulated that parental mosaicism for ACTG2 could account for these observations but we did not observe mosaicism in our parental samples.
We also had previously identified a variant in ACTG2 which appeared to affect an alternative transcript (NC_000002.11 (NM_001615.3):c.255+210C>A encoding p.Phe110Leu on transcript Uc010fex.1). This variant was found in Fam19 in two affected siblings (Figure S4b). We then found this variant in another family Fam18, in which the proband and an unaffected sibling were positive for this variant. The Fam18-1 proband was also positive for a de novo ACTG2 variant (chr2:74141963: NM_001615:c.770G>A: p.Arg257His). These data suggest the previously reported p.Phe110Leu allele may be a benign variant. However, an impact as a modifier or incompletely penetrant allele could not be ruled out (Figure S4b).
Additional clinical features of ACTG2-positive group
The majority of individuals testing positive for ACTG2 exhibited the classic MMIHS phenotype including a combination of symptoms suggesting bladder and intestinal dysmotility. Two cases (Fam55-1 and Fam57-1) presented initially with neurogenic bladder alone during the first months of life but later developed significant GI dysmotility: Fam55-1 developed severe abdominal distension with Clostridium difficile infection at age 1 year and had an ileostomy performed. Fam57-1 presented with more severe bladder symptoms and no apparent GI dysmotility at birth, but by age four months she developed massive gastric distension requiring decompression by a gastric tube and was started on 80% parenteral nutrition. Prune belly was also seen in ACTG2-positive cases (Fam9, Fam16). In adult patients, features including cholecystitis, cholelithiasis, biliary sludge, gastric and colonic polyps, gastritis, hepatitis, nephrolithiasis and a simple renal cyst were reported. Developmental delays were noted in two cases: one subject with a p.Arg40His variant had mild initial gross motor delays (Fam49-1). Another subject with a p.Arg178Cys variant was born prematurely at 34 weeks. She had microcephaly, cortical thumbs, mild hypertonia, and mild global developmental delays (Fam44-4). Chromosomal microarray analysis was nondiagnostic. Mydriasis was not noted in the ACTG2-positive subjects.
ACTG2 variants and genotype-phenotype correlations
Because we observed a high proportion of arginine missense substitutions in ACTG2 in our cohort, we sought to study whether these alleles were associated with differences in clinical outcomes. We reasoned, because these particular CGX codons were sites of recurrent mutation that predicting clinical outcomes from these alleles would be particularly useful as they are highly likely to continue to be observed in new cases. Indeed, it has previously been suggested that specific arginine missense variants such as those affecting the arginine residue at position 178 (Arg178) may have a higher rate of microcolon or early death (Halim et al., 2016; Matera et al., 2016). In our cohort, all the probands with variants affecting Arg178 were TPN-dependent and at least one of them had undergone a multiorgan transplant including large and small intestine, spleen, pancreas, part of the esophagus, left kidney, and liver.
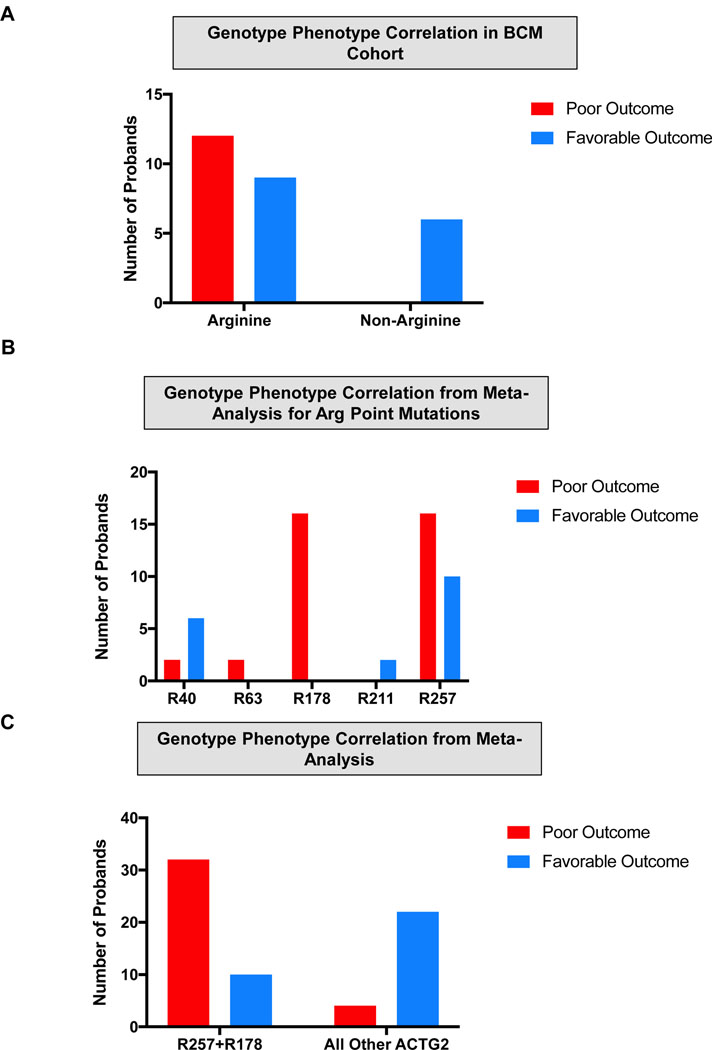
We performed a genotype-phenotype correlation in which we compared the outcomes in the 33 patients in our cohort with confirmed ACTG2 missense substitutions. We divided the ACTG2-positive group into arginine substitution and non-arginine substitutions and correlated to the previously characterized poor or favorable outcome characterization based on transplant, TPN or disease-related mortality. We found that all the poor outcomes in the ACTG2-positive group came from individuals with arginine substitutions (Figure 5a). Those with an arginine substitution in our cohort had a 57.1% risk of death in childhood, TPN dependence and/or transplantation, whereas we found no individuals with non-arginine substitutions suffering these outcomes (Fisher’s Exact test; p=0.0149). These results demonstrate a seemingly robust genotype-phenotype correlation in which the most severely affected cases invariably have arginine substitutions in ACTG2.
Figure 5 –
Genotype-phenotype correlations for ACTG2 Arginine missense variants. Positions of the arginine residues are labeled (e.g., R40 indicates arginine at position 40 or p.Arg40). (a,b) Outcomes of arginine versus non-arginine missense variants in the Baylor College of Medicine (BCM) cohort (a) and meta-analysis incorporating cases from the BCM cohort and cases from the literature (b). Red bars represent poor outcome defined as death in early childhood, total parenteral nutrition dependence and patient undergoing visceral transplant for intestinal dysmotility. Blue bars represent cases lacking these features. (c) Outcomes of specific Arginine missense variants. Red bars represent poor outcome defined as above. Blue bars represent more favorable outcomes lacking these features.
Next, we examined whether our cohort compared with previously reported cases. We collected information from previously published ACTG2 cases and attempted the same genotype-phenotype analysis incorporating previously reported cases from the literature (Table S4). Our results were similar to those from the BCM cohort alone. We found that the non-arginine substitutions reported were not in association with death, transplant or TPN dependence. Interestingly, the arginine substitutions incorporating our cohort and the literature had a 63.8% (37/58) chance of poor outcome, an estimate very consistent with that from our cohort alone. These differences were statistically significant (Fisher’s Exact test; p<0.0001).
We show that the arginine substitutions, in general, are associated with more severe outcomes, but previous studies have suggested specific effects of specific mutations such as p.Arg178 (Halim et al., 2016; Ravenscroft et al., 2018). We also compared the outcome measure across each of the five recurrent arginine missense mutations (Figure 5b). Interestingly, we observed that all 17 individuals with missense alleles affecting p.Arg178 had a poor outcome compared to 16/26 with a missense mutations affecting position p.Arg257 (Fisher’s Exact test; p=0.01; Figure 5c). For missense substitutions affecting residues at positions 40, 63 and 211, there were too few cases (two each) to assess. We did observe that cases affecting an arginine residue at position 40 (p.Arg40) trended toward a more favorable outcome (6/8). We also examined the identification of microcolon, and found a striking difference between individuals with p.Arg178 variants versus p.Arg257. Out of 20 individuals with p.Arg178, 19 were found to have microcolon, whereas microcolon was reported in only five out of 20 with p.Arg257 (Fisher’s Exact test; p<0.0001; Figure S5). In summary, visceral myopathy represents a spectrum of severity and there is a robust genotype-phenotype correlation, ACTG2-positive cases are more likely to be severe cases than ACTG2-negative ones, arginine missense substitutions are more severe than non-arginine missense alleles. Finally, the recurrent arginine alleles impacting p.Arg178 are most severe as a group, followed by p.Arg257, then p.Arg40 with insufficient numbers to conclude on other arginine substitutions. These data demonstrate that these arginine missense substitutions are the primary determinants of the amount and severity of disease in our cohort.
DISCUSSION
In this study, we utilize a large cohort of 53 families with MMIHS to demonstrate that a set of recurrent missense substitutions affecting arginine residues account for the majority of cases. We apply a combination of targeted gene sequencing and exome sequencing to show that ACTG2 testing has a high diagnostic yield in this clinical population. This is particularly true in more severe cases of MMIHS. In addition, we provide evidence for a genotype-phenotype correlation within ACTG2-positive cases and show that the recurrent missense substitutions affecting arginine residues are responsible for cases of MMIHS with poor outcomes. Our results have both diagnostic significance for clinical molecular studies of patients with MMIHS, as well as prognostic and genetic counseling implications.
One of the key insights of our study is the central role that ACTG2 mutations and particularly arginine missense substitutions play in this spectrum of disease. Previously, evidence from some cohorts had also pointed to this predominant role for this locus in MMIHS (Ravenscroft et al., 2018), however, others have suggested that MMIHS is mostly an autosomal recessive disorder and that consanguinity is a major genetic risk factor (Nakamura et al., 2019), and indeed a number of recessive loci have been described (Gauthier et al., 2015; Halim, Brosens, et al., 2017; Halim, Wilson, et al., 2017; Moreno et al., 2018). Our cohort included four families with affected siblings and three with consanguinity, but interestingly, one of these cases was ultimately explained by dominant inheritance of an ACTG2 variant with incomplete penetrance in the mother. We did perform exome sequencing on 17 of the 19 undiagnosed cases in our cohort aimed at identifying additional recessive loci, of which one family was found to have a homozygous MYLK variant (Halim, Brosens, et al., 2017). However, in our cohort these appear to be quite rare, and it was striking to observe more cases in the cohort that affect one of the five recurrent arginine substitutions (26/53), and ACTG2 in general (33/53), than all those that remain undiagnosed (19/53).
We also observed a wide range of phenotypic severity in our ACTG2- positive cases, ranging from the most severe to older individuals with lifetime abdominal discomfort but no firm diagnosis. Our data, therefore, suggest ACTG2 testing should be considered in a much wider range of clinical conditions affecting GI or GU function. Though exome sequencing would seem most advantageous given the ability to detect all the known MMIHS loci, ACTG2 targeted testing could be a reasonable alternative in some clinical settings, and nearly half of the cases can be diagnosed by sequencing five specific CpG dinucleotides within ACTG2.
These observations also provide a greater context for understanding the recurrent arginine missense substitutions in ACTG2. In our larger cohort we saw an obvious impact on severity of disease for cases affecting p.Arg178 and p.Arg257 with even greater severity in the former group. Of the 18 encoded arginine residue within the ACTG2 gene, 13 are at a CpG dinucleotide site. Of these, six are found to be sites of missense substitution within gnomAD database. Interestingly, two of these six sites are listed in ClinVar as pathogenic variants, p.Arg38 and p.Arg148. One possible interpretation is that these sites are associated with less severe disease. Indeed in our cohort, we observed wide interfamilial variability for a p.Arg40His variant. With our larger cohort and examining other published cases, we were able to suggest a severity spectrum for these specific sites with p.Arg178 and p.Arg257 accounting for most of the severe cases and p.Arg40 with more mixed severity. With the information from gnomAD, an allelic series for severity spectrum for these specific alleles could be proposed with p.Arg178>p.Arg257>p.Arg40>p.Arg38 and p.Arg148. Whether p.Arg63 and p.Arg211 also fall into this less severe category will await further studies.
Regardless of the rates of complications within each group, patients with any severity of visceral myopathy face difficult medical challenges and the absence of transplantation or lifetime TPN dependence does not reduce the tremendous impact that visceral myopathy can have on quality of life, severe medical complications or costly and complex medical care. Some individuals in our cohort did undergo transplant, but then had good outcomes after their intervention, therefore our data cannot provide an overall prognosis. Instead we used TPN dependence or transplantation as a proxy for gastrointestinal severity. Although those with a negative ACTG2 sequencing result seem less severe as a group according to these measures, a number of severe cases are identified in this cohort. Likewise, while ACTG2-positive cases are more likely to have a severe phenotype, individual cases within this group can be very mildly impacted. Our results are limited to our own cohort and should not be interpreted as predictive for other cases, particularly with negative ACTG2 results as other genetic factors could impact these cases. Conversely, while nearly all the most severe disease occurred in individuals with arginine substitutions, a number of individuals with arginine substitutions can have milder phenotypes. Whether additional modifier loci could impact severity remains to be determined. In addition, other variants in ACTG2 itself could also impact differences in severity. For example, we observed an p.Phe110Leu variant affecting an alternate transcript (Uc010fex.1) in two siblings with comparatively mild GI disease. We then observed this same variant in another family, in which the proband had a de novo R257 allele. Whether this allele could secondarily impact severity remains to be determined.
Our study had some inherent limitations. Our ascertainment, as for any rare disease cohort is susceptible to selection bias as we primarily relied on referral to BCM from co-authors or external researchers or clinicians. With clinical ACTG2 testing now available, our study represented families interested in genetic research and seen at academic centers which likely selected for more severe cases. We also have recruited individuals over two periods of time, from 1999–2001 and then from 2013 to present. Subjects were referred for fetal megacystis, microcolon, functional gastrointestinal or functional bladder obstruction. In addition, the management of these patients could vary with surgical and medical interventions that could complicate the picture of severity. Other groups have suggested that very specific clinical designations (such as CIPO with megacystis) need to be applied (Matera et al., 2016) and we did not categorize our cases according to these clinical designations for analysis purposes. Though this might have some advantages, in examining our cohort, we observed subjects with the same mutation who can be labeled with different clinical designations such as MMIHS, CIPO and hollow visceral myopathy by different providers, and in the absence of specific clinical diagnostic criteria, we chose to recruit subjects with a range of phenotypes and then analyze according to clinical features. Though this ascertainment issue is inherent to our study design, it primarily led to the inclusion of a number of cases that did not meet strict criteria for MMIHS or CIPO and who often ultimately had non-diagnostic molecular studies. This bias would therefore lead us to underestimate the impact of ACTG2 in MMIHS/CIPO, which actually strengthens our conclusion that ACTG2, and not recessive loci as others have proposed (Nakamura et al., 2019), is the primary genetic factor in MMIHS. Indeed, in a highly clinically selected cohort it is possible that the rate of ACTG2-positive cases could be even higher than 60%. Despite having access to 53 families with this rare condition, our numbers still did not allow sufficient power to compare most of the specific mutations and additional recruitment will be needed to further study this. Nonetheless, we propose a severity spectrum based on our observations in which ACTG2-positive cases are more likely to be severe than ACTG2-negative, arginine missense substitutions are more common and more severe within the ACTG2-positive group and we propose an arginine severity spectrum (p.Arg178 > p.Arg63 > p.Arg257 > p.Arg40 > p.Arg211, p.Arg38 and p.Arg148; Table 2).
Table 2.
Genotype-phenotype correlation for visceral myopathy
| Group | Inheritance | Phenotypes | Outcomes Observed |
|---|---|---|---|
| ACTG2:p.Arg178 | De novo or dominant inherited | MMIHS-Most severe neonatal presentation with microcolon | High rates of mortality and transplantation |
| ACTG2:p.Arg257 | De novo or dominant inherited | MMIHS or CIPO. Typically Severe neonatal presentation usually without microcolon and more variability | High rates of mortality and transplantation |
| ACTG2:p.Arg40 | De novo or dominant inherited | MMIHS or CIPO. Severity range and more moderate or mild cases than groups above | Moderate to high rates of mortality and transplantation |
| ACTG2:Other Arg | De novo or dominant inherited | MMIHS or CIPO. Severity range and more moderate or mild cases than groups above | Moderate rates of mortality and transplantation |
| Non-Arg ACTG2 | De novo or dominant inherited | MMIHS or CIPO. Severity range and more moderate or mild cases than groups above | Moderate to high rates of mortality and transplantation |
| ACTG2-negative | Sporadic or ultra-rare recessive | Less severe as a group than ACTG2 positive, but some severe cases | Low rates of mortality and transplantation |
Abbreviations: CIPO, chronic intestinal pseudo-obstruction; MMIHS, megacystis-microcolon intestinal hypoperistalsis syndrome
In conclusion, visceral myopathy represents a spectrum of clinical severity due to smooth muscle myopathy. We provide an effort to overlay this clinical severity spectrum with a molecular classification. In the future, providing classes such as “ACTG2-positive” versus “ACTG2-negative” and “p.Arg178” versus “p.Arg40” can help apply molecular methods to aid in the classification of a variable set of phenotypes. In addition, testing the ACTG2 gene by exome or targeted testing is clearly a promising approach to providing better genetic counseling and diagnosis to patients with symptoms ranging from newborns with microcolon to adults with longstanding GI discomfort.
Supplementary Material
Figure S3 - (a) A family with an apparently-recessive pattern of inheritance. An ACTG2 pathogenic variant (p.Arg178Cys) was identified in two affected maternal half-siblings (shaded blue) with features of MMIHS. The variant was found to be inherited from their mother who did not report symptoms of intestinal or bladder dysmotility but did have uterine atony. (b) Paternal ages in the cohort. Cases with de novo ACTG2 variants had a modestly higher mean paternal age (34 versus 29 years) which was statistically significant.
Figure S1 - Clinical features observed in the BCM cohort. (a-f) The prevalence of megacystis, need for catheterization, bladder decompression procedure in the fetal period, microcolon, abdominal surgery in the first weeks of life and neonatal bilious emesis. Orange represents the proportion with the designated feature. Blue represents the proportion without the designated feature. (g) Parenteral nutrition. Red represents proportion of total parenteral nutrition (TPN) dependence. Orange represents partial or temporary parenteral nutrition. Blue represents minimal or no requirement for parenteral nutrition. (h) Involvement of both gastrointestinal (GI) and genitourinary (GU) systems versus symptoms restricted to either one of the systems. (i) Outcomes observed in the cohort. Orange represents poor outcomes defined as death in early childhood, dependence on total parenteral nutrition or patient undergoing visceral transplant for intestinal dysmotility. Blue represents proportion of cases without these outcomes.
Figure S2 - Amino Acid Sequence alignment of the actin genes.
Figure S5 - Comparison of the p.Arg257 and the p.Arg178, the two most frequent sites of pathogenic ACTG2 variants. Data was pooled from the BCM Visceral Myopathy Cohort and meta-analysis of cases from the literature. A dramatic difference is observed for the rate of microcolon.
Figure S4 - (a) Pedigree of Fam34. Fam34 is a multi-generation pedigree with multiple affected family members. The p.Arg40His variant testing was positive for individuals with a red star. The CIPO or MMIHS phenotypes were observed (light blue and blue respectively). Complications of MMIHS are indicated in the legend. (b) Segregation of a p.Phe110Leu variant which is non-coding in most transcripts of the ACTG2 gene. This variant is observed in two affected individuals in Fam19. It is also observed in one affected and one unaffected individual in Fam18. Notably, a de novo pathogenic p.Arg257His variant is also seen in Fam18-1 suggesting the p.Phe110Leu variant is potentially benign, although a modifying effect of this variant cannot be ruled out.
Acknowledgements
We acknowledge Pradnya Bhadane for organizing the obtained medical records. This study was funded in part by the National Human Genome Research Institute (NHGRI)/National Heart Lung and Blood Institute (NHLBI) grant UM1 HG006542 to the Baylor Hopkins Center for Mendelian Genomics and the National Institute of Neurological Disorders and Stroke (NINDS) grant R35 NS105078-01 to J. R. L. J.E.P. was supported by NHGRI (K08 HG008986). B.C. is a senior clinical investigator of the Research Foundation, Flanders.
Funding information:
National Human Genome Research Institute (NHGRI)/National Heart Lung and Blood Institute (NHLBI), Grant/Award Number: UM1 HG006542; National Institute of Neurological Disorders and Stroke (NINDS), Grant/Award Number: R35 NS105078-01; NHGRI, Grant/Award Number: K08 HG008986
Footnotes
Conflict of Interests
Baylor College of Medicine and Miraca Holdings Inc. have formed a joint venture with shared ownership and governance of Baylor Genetics (BG), formerly the Baylor Miraca Genetics Laboratories, which performs chromosomal microarray analysis and clinical exome sequencing. J.R.L. serves on the Scientific Advisory Board of BG. J.R.L. has stock ownership in 23 and Me, is a paid consultant for Regeneron Pharmaceuticals, and is a coinventor on multiple US and European patents related to molecular diagnostics for inherited neuropathies, eye diseases, and bacterial genomic fingerprinting.
Data Availability
All the data that support the findings of this study are available upon request form the corresponding author. The data beyond that included in the manuscript and supplemental files are not publically available due to privacy concerns.
References
- Bainbridge MN, Wang M, Wu Y, Newsham I, Muzny DM, Jefferies JL, & Gibbs RA (2011). Targeted enrichment beyond the consensus coding DNA sequence exome reveals exons with higher variant densities. Genome Biol, 12(7), R68 10.1186/gb-2011-12-7-r68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo C. (1999). Pseudo-obstruction: current approaches. Gastroenterology, 116(4), 980–987. doi: 10.1016/s0016-5085(99)70082-x [DOI] [PubMed] [Google Scholar]
- Downes TJ, Cheruvu MS, Karunaratne TB, De Giorgio R, & Farmer AD (2018). Pathophysiology, Diagnosis, and Management of Chronic Intestinal Pseudo-Obstruction. J Clin Gastroenterol, 52(6), 477–489. doi: 10.1097/MCG.0000000000001047 [DOI] [PubMed] [Google Scholar]
- Gauthier J, Ouled Amar Bencheikh B, Hamdan FF, Harrison SM, Baker LA, Couture F, . . . Soucy JF (2015). A homozygous loss-of-function variant in MYH11 in a case with megacystis-microcolon-intestinal hypoperistalsis syndrome. Eur J Hum Genet, 23(9), 1266–1268. doi: 10.1038/ejhg.2014.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosemann JH, & Puri P. (2011). Megacystis microcolon intestinal hypoperistalsis syndrome: systematic review of outcome. Pediatr Surg Int, 27(10), 1041–1046. doi: 10.1007/s00383-011-2954-9 [DOI] [PubMed] [Google Scholar]
- Halim D, Brosens E, Muller F, Wangler MF, Beaudet AL, Lupski JR, . . . Alves MM (2017). Loss-of-Function Variants in MYLK Cause Recessive Megacystis Microcolon Intestinal Hypoperistalsis Syndrome. Am J Hum Genet, 101(1), 123–129. doi: 10.1016/j.ajhg.2017.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim D, Hofstra RM, Signorile L, Verdijk RM, van der Werf CS, Sribudiani Y, . . . Alves MM (2016). ACTG2 variants impair actin polymerization in sporadic Megacystis Microcolon Intestinal Hypoperistalsis Syndrome. Hum Mol Genet, 25(3), 571–583. doi: 10.1093/hmg/ddv497 [DOI] [PubMed] [Google Scholar]
- Halim D, Wilson MP, Oliver D, Brosens E, Verheij JB, Han Y, . . . Miano JM (2017). Loss of LMOD1 impairs smooth muscle cytocontractility and causes megacystis microcolon intestinal hypoperistalsis syndrome in humans and mice. Proc Natl Acad Sci U S A, 114(13), E2739-E2747. doi: 10.1073/pnas.1620507114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar O, Ohlsson B, Veress B, Alm R, Fredrikson GN, & Montgomery A. (2012). Depletion of enteric gonadotropin-releasing hormone is found in a few patients suffering from severe gastrointestinal dysmotility. Scand J Gastroenterol, 47(10), 1165–1173. doi: 10.3109/00365521.2012.706826 [DOI] [PubMed] [Google Scholar]
- Harrison SM, Riggs ER, Maglott DR, Lee JM, Azzariti DR, Niehaus A, . . . Rehm HL. (2016). Using ClinVar as a Resource to Support Variant Interpretation. Curr Protoc Hum Genet, 89, 8 16 11–18 16 23. doi: 10.1002/0471142905.hg0816s89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holla OL, Bock G, Busk OL, & Isfoss BL (2014). Familial visceral myopathy diagnosed by exome sequencing of a patient with chronic intestinal pseudo-obstruction. Endoscopy, 46(6), 533–537. doi: 10.1055/s-0034-1365142 [DOI] [PubMed] [Google Scholar]
- Iida H, Ohkubo H, Inamori M, Nakajima A, & Sato H. (2013). Epidemiology and clinical experience of chronic intestinal pseudo-obstruction in Japan: a nationwide epidemiologic survey. J Epidemiol, 23(4), 288–294. doi: 10.2188/jea.je20120173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur RP (2003). Neuronal dysplasia: a controversial pathological correlate of intestinal pseudo-obstruction. Am J Med Genet A, 122A(4), 287–293. doi: 10.1002/ajmg.a.20470 [DOI] [PubMed] [Google Scholar]
- Klar J, Raykova D, Gustafson E, Tothova I, Ameur A, Wanders A, & Dahl N. (2015). Phenotypic expansion of visceral myopathy associated with ACTG2 tandem base substitution. Eur J Hum Genet, 23(12), 1679–1683. doi: 10.1038/ejhg.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen HJ, Sipponen T, Tojkander S, Karikoski R, Jarvinen H, Laing NG, . . . Tuupanen S. (2012). Segregation of a missense variant in enteric smooth muscle actin gamma-2 with autosomal dominant familial visceral myopathy. Gastroenterology, 143(6), 1482–1491 e1483. doi: 10.1053/j.gastro.2012.08.045 [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, . . . Exome Aggregation C. (2016). Analysis of protein-coding genetic variation in 60,706 humans. Nature, 536(7616), 285–291. doi: 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Lehman E, Bercovich D, Xu W, Stockton DW, & Beaudet AL (2001). Characterization of the human beta4 nAChR gene and polymorphisms in CHRNA3 and CHRNB4. J Hum Genet, 46(7), 362–366. doi: 10.1007/PL00010921 [DOI] [PubMed] [Google Scholar]
- Liu P, Meng L, Normand EA, Xia F, Song X, Ghazi A, . . . Yang Y. (2019). Reanalysis of Clinical Exome Sequencing Data. N Engl J Med, 380(25), 2478–2480. doi: 10.1056/NEJMc1812033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Xiao Y, Huang J, Tao Y, Yan W, Lu L, . . . Cai W. (2016). Mutation in Actin gamma-2 Responsible for Megacystis Microcolon Intestinal Hypoperistalsis Syndrome in 4 Chinese Patients. J Pediatr Gastroenterol Nutr, 63(6), 624–626. doi: 10.1097/MPG.0000000000001204 [DOI] [PubMed] [Google Scholar]
- Lupski JR, Gonzaga-Jauregui C, Yang Y, Bainbridge MN, Jhangiani S, Buhay CJ, . . . Gibbs RA (2013). Exome sequencing resolves apparent incidental findings and reveals further complexity of SH3TC2 variant alleles causing Charcot-Marie-Tooth neuropathy. Genome Med, 5(6), 57. doi:gm461 [pii] 10.1186/gm461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera I, Rusmini M, Guo Y, Lerone M, Li J, Zhang J, . . . Ceccherini I. (2016). Variants of the ACTG2 gene correlate with degree of severity and presence of megacystis in chronic intestinal pseudo-obstruction. Eur J Hum Genet, 24(8), 1211–1215. doi: 10.1038/ejhg.2015.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milunsky A, Baldwin C, Zhang X, Primack D, Curnow A, & Milunsky J. (2017). Diagnosis of Chronic Intestinal Pseudo-obstruction and Megacystis by Sequencing the ACTG2 Gene. J Pediatr Gastroenterol Nutr, 65(4), 384–387. doi: 10.1097/MPG.0000000000001608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milunsky A, Lazier J, Baldwin C, Young C, Primack D, & Milunsky JM (2017). Prenatal diagnosis of chronic intestinal pseudo-obstruction and paternal somatic mosaicism for the ACTG2 pathogenic variant. Prenat Diagn, 37(12), 1254–1256. doi: 10.1002/pd.5171 [DOI] [PubMed] [Google Scholar]
- Miwa T, Manabe Y, Kurokawa K, Kamada S, Kanda N, Bruns G, . . . Kakunaga T. (1991). Structure, chromosome location, and expression of the human smooth muscle (enteric type) gamma-actin gene: evolution of six human actin genes. Mol Cell Biol, 11(6), 3296–3306. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1710027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno CA, Metze K, Lomazi EA, Bertola DR, Barbosa RH, Cosentino V, . . . Cavalcanti DP (2016). Visceral myopathy: Clinical and molecular survey of a cohort of seven new patients and state of the art of overlapping phenotypes. Am J Med Genet A, 170(11), 2965–2974. doi: 10.1002/ajmg.a.37857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno CA, Sobreira N, Pugh E, Zhang P, Steel G, Torres FR, & Cavalcanti DP (2018). Homozygous deletion in MYL9 expands the molecular basis of megacystis-microcolon-intestinal hypoperistalsis syndrome. Eur J Hum Genet, 26(5), 669–675. doi: 10.1038/s41431-017-0055-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, O’Donnell AM, & Puri P. (2019). Consanguinity and its relevance for the incidence of megacystis microcolon intestinal hypoperistalsis syndrome (MMIHS): systematic review. Pediatr Surg Int, 35(2), 175–180. doi: 10.1007/s00383-018-4390-6 [DOI] [PubMed] [Google Scholar]
- Piotrowska AP, Rolle U, Chertin B, De Caluwe D, Bianchi A, & Puri P. (2003). Alterations in smooth muscle contractile and cytoskeleton proteins and interstitial cells of Cajal in megacystis microcolon intestinal hypoperistalsis syndrome. J Pediatr Surg, 38(5), 749–755. doi: 10.1016/jpsu.2003.50159 [DOI] [PubMed] [Google Scholar]
- Puri P, Lake BD, Gorman F, O’Donnell B, & Nixon HH (1983). Megacystis-microcolon-intestinal hypoperistalsis syndrome: a visceral myopathy. J Pediatr Surg, 18(1), 64–69. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/6834228 [DOI] [PubMed] [Google Scholar]
- Ravenscroft G, Pannell S, O’Grady G, Ong R, Ee HC, Faiz F, . . . Laing NG (2018). Variants in ACTG2 underlie a substantial number of Australasian patients with primary chronic intestinal pseudo-obstruction. Neurogastroenterol Motil, 30(9), e13371. doi: 10.1111/nmo.13371 [DOI] [PubMed] [Google Scholar]
- Reid JG, Carroll A, Veeraraghavan N, Dahdouli M, Sundquist A, English A, . . . Boerwinkle E. (2014). Launching genomics into the cloud: deployment of Mercury, a next generation sequence analysis pipeline. BMC Bioinformatics, 15, 30. doi: 10.1186/1471-2105-15-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolle U, O’Briain S, Pearl RH, & Puri P. (2002). Megacystis-microcolon-intestinal hypoperistalsis syndrome: evidence of intestinal myopathy. Pediatr Surg Int, 18(1), 2–5. doi: 10.1007/s003830200001 [DOI] [PubMed] [Google Scholar]
- Sipponen T, Karikoski R, Nuutinen H, Markkola A, & Kaitila I. (2009). Three-generation familial visceral myopathy with alpha-actin-positive inclusion bodies in intestinal smooth muscle. J Clin Gastroenterol, 43(5), 437–443. doi: 10.1097/MCG.0b013e31817d3f84 [DOI] [PubMed] [Google Scholar]
- Szucsik JC, & Lessard JL (1995). Cloning and sequence analysis of the mouse smooth muscle gamma-enteric actin gene. Genomics, 28(2), 154–162. doi: 10.1006/geno.1995.1126 [DOI] [PubMed] [Google Scholar]
- Taguchi T, Ikeda K, Shono T, Goto S, Kubota M, Kawana T, . . . Toyohara T. (1989). Autonomic innervation of the intestine from a baby with megacystis microcolon intestinal hypoperistalsis syndrome: I. Immunohistochemical study. J Pediatr Surg, 24(12), 1264–1266. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2593057 [DOI] [PubMed] [Google Scholar]
- Thorson W, Diaz-Horta O, Foster J 2nd, Spiliopoulos M, Quintero R, Farooq A, . . . Tekin M. (2014). De novo ACTG2 mutations cause congenital distended bladder, microcolon, and intestinal hypoperistalsis. Hum Genet, 133(6), 737–742. doi: 10.1007/s00439-013-1406-0 [DOI] [PubMed] [Google Scholar]
- Tuzovic L, Tang S, Miller RS, Rohena L, Shahmirzadi L, Gonzalez K, . . . Anyane-Yeboa K. (2015). New Insights into the Genetics of Fetal Megacystis: ACTG2 Mutations, Encoding gamma-2 Smooth Muscle Actin in Megacystis Microcolon Intestinal Hypoperistalsis Syndrome (Berdon Syndrome). Fetal Diagn Ther, 38(4), 296–306. doi: 10.1159/000381638 [DOI] [PubMed] [Google Scholar]
- Wangler MF, Gonzaga-Jauregui C, Gambin T, Penney S, Moss T, Chopra A, . . . Beaudet A. (2014). Heterozygous de novo and inherited mutations in the smooth muscle actin (ACTG2) gene underlie megacystis-microcolon-intestinal hypoperistalsis syndrome. PLoS Genet, 10(3), e1004258. doi: 10.1371/journal.pgen.1004258 PGENETICS-D-13-03436 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington JR, Poole AT, Dutta EH, & Munn MB (2017). A Novel Mutation in ACTG2 Gene in Mother with Chronic Intestinal Pseudoobstruction and Fetus with Megacystis Microcolon Intestinal Hypoperistalsis Syndrome. Case Rep Genet, 2017, 9146507. doi: 10.1155/2017/9146507 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S3 - (a) A family with an apparently-recessive pattern of inheritance. An ACTG2 pathogenic variant (p.Arg178Cys) was identified in two affected maternal half-siblings (shaded blue) with features of MMIHS. The variant was found to be inherited from their mother who did not report symptoms of intestinal or bladder dysmotility but did have uterine atony. (b) Paternal ages in the cohort. Cases with de novo ACTG2 variants had a modestly higher mean paternal age (34 versus 29 years) which was statistically significant.
Figure S1 - Clinical features observed in the BCM cohort. (a-f) The prevalence of megacystis, need for catheterization, bladder decompression procedure in the fetal period, microcolon, abdominal surgery in the first weeks of life and neonatal bilious emesis. Orange represents the proportion with the designated feature. Blue represents the proportion without the designated feature. (g) Parenteral nutrition. Red represents proportion of total parenteral nutrition (TPN) dependence. Orange represents partial or temporary parenteral nutrition. Blue represents minimal or no requirement for parenteral nutrition. (h) Involvement of both gastrointestinal (GI) and genitourinary (GU) systems versus symptoms restricted to either one of the systems. (i) Outcomes observed in the cohort. Orange represents poor outcomes defined as death in early childhood, dependence on total parenteral nutrition or patient undergoing visceral transplant for intestinal dysmotility. Blue represents proportion of cases without these outcomes.
Figure S2 - Amino Acid Sequence alignment of the actin genes.
Figure S5 - Comparison of the p.Arg257 and the p.Arg178, the two most frequent sites of pathogenic ACTG2 variants. Data was pooled from the BCM Visceral Myopathy Cohort and meta-analysis of cases from the literature. A dramatic difference is observed for the rate of microcolon.
Figure S4 - (a) Pedigree of Fam34. Fam34 is a multi-generation pedigree with multiple affected family members. The p.Arg40His variant testing was positive for individuals with a red star. The CIPO or MMIHS phenotypes were observed (light blue and blue respectively). Complications of MMIHS are indicated in the legend. (b) Segregation of a p.Phe110Leu variant which is non-coding in most transcripts of the ACTG2 gene. This variant is observed in two affected individuals in Fam19. It is also observed in one affected and one unaffected individual in Fam18. Notably, a de novo pathogenic p.Arg257His variant is also seen in Fam18-1 suggesting the p.Phe110Leu variant is potentially benign, although a modifying effect of this variant cannot be ruled out.