Abstract
Background
Intracranial metastatic disease (IMD) is a serious and known complication of human epidermal growth factor receptor 2 (HER2)-positive breast cancer. The role of targeted therapy for patients with HER2-positive breast cancer and IMD remains unclear. In this study, we sought to evaluate the effect of HER2-targeted therapy on IMD from HER2-positive breast cancer.
Methods
We searched MEDLINE, EMBASE, CENTRAL, and gray literature sources for interventional and observational studies reporting survival, response, and safety outcomes for patients with IMD receiving HER2-targeted therapy. We pooled outcomes through meta-analysis and examined confounder effects through forest plot stratification and meta-regression. Evidence quality was evaluated using GRADE (PROSPERO CRD42020161209).
Results
A total of 97 studies (37 interventional and 60 observational) were included. HER2-targeted therapy was associated with prolonged overall survival (hazard ratio [HR] 0.47; 95% confidence interval [CI], 0.39–0.56) without significantly prolonged progression-free survival (HR 0.52; 95% CI, 0.27–1.02) versus non-targeted therapy; the intracranial objective response rate was 19% (95% CI, 12–27%), intracranial disease control rate 62% (95% CI, 55–69%), intracranial complete response rate 0% (95% CI, 0–0.01%), and grade 3+ adverse event rate 26% (95% CI, 11–45%). Risk of bias was high in 40% (39/97) of studies.
Conclusion
These findings support a potential role for systemic HER2-targeted therapy in the treatment of patients with IMD from HER2-positive metastatic breast cancer.
Keywords: brain metastases, breast cancer, HER2/neu, molecular targeted therapy
Key Points.
We performed a meta-analysis of survival, response, and safety outcomes for 7157 patients from 97 studies.
HER2-targeted therapy was associated with prolonged overall survival for patients with IMD from HER2-positive breast cancer.
Our results support a potential role for systemic HER2-targeted therapy for this patient population.
Importance of the Study.
We reviewed the literature and meta-analyzed outcomes for HER2-targeted therapy in patients with HER2-positive breast cancer and IMD. HER2-targeted therapy was associated with prolonged overall survival, notable response proportions, and an adverse event rate that may depend on drug structure. These findings support a potential role for HER2-targeted therapy in the treatment of IMD from HER2-positive metastatic breast cancer. Future trials should include patients with IMD to determine optimal treatment combinations and sequences and illuminate the role of novel therapies that may have efficacy in the central nervous system.
Intracranial metastatic disease (IMD) is one of the most feared complications of breast cancer, the most common cancer in women and the second most frequent cause of IMD, accounting for 15–20% of all brain metastases.1–3 Expression of the human epidermal growth factor receptor 2 (HER2) is associated with an increased risk of IMD (odds ratio 2.7; 95% confidence interval [CI], 2–3.7) compared to other breast cancer subtypes.4,5 Up to 50% of women with HER2-positive breast cancer develop IMD over their lifetime.6–11 Furthermore, the incidence of IMD in women with HER2-positive breast cancer is increasing due to advances in detection and improved systemic disease control.12 Diagnosis with IMD has significant implications for prognosis: the median survival for patients with HER2-positive metastatic breast cancer is 26.3–30 months with IMD versus 42.5–47.9 months without brain involvement.11,13–15 Furthermore, diagnosis with IMD may result in reduced quality of life because of neurological deficit, as well as a “loss of hope and a fear of loss of self.” 3,16
Treatment for IMD in patients with HER2-positive breast cancer has historically been limited to surgical resection and radiotherapy; the role for chemotherapy has generally been disappointing.17–20 The intracranial efficacy of chemotherapy is thought to be limited by cell-intrinsic resistance and poor penetration of drugs across the blood–brain barrier.16,20
The finding of prolonged survival with HER2 inhibition in women with HER2-positive metastatic breast cancer21–25 and the increased permeability of novel HER2 inhibitors into the brain26 have led to an interest in HER2-targeted therapy as a treatment of IMD from HER2-positive metastatic disease.16,27 Guidelines from the National Comprehensive Cancer Network,18 Congress of Neurological Surgeons,17 and European Association of Neuro-Oncology19 reflect the paucity of evidence to support or condemn the use of HER2-targeted therapy for IMD.
Although prior systemic reviews have been conducted, these studies do not speak to HER2 targeting agents developed since trastuzumab and lapatinib, and one is not restricted to patients with HER2-positive disease.28,29 Our understanding of outcomes among patients with HER2-positive breast cancer brain metastases who receive HER2-targeted therapy thus remains limited. To address this limitation, we conducted this systematic review and meta-analysis to update the literature on the effects of HER2-targeted therapy on survival, response, and safety outcomes in patients with HER2-positive breast cancer and IMD.
Methods
Eligibility Criteria
Included studies reported outcomes for patients with IMD from HER2-positive breast cancer who received post-IMD HER2-targeted therapy. Details are available in Supplementary Methods.
Search Strategy
On January 27, 2020, we searched multiple databases and gray literature sources. The full search strategy is available in Supplementary Methods and Supplementary Tables S1–S3).
Study Selection
Retrieved records underwent title-and-abstract review then full-text review. Independent reviewers (A.W.E. and F.G.) screened the studies in duplicate using the eligibility criteria (Supplementary Tables S4 and S5). Reasons for exclusion at full-text review were recorded. Disagreements were resolved by discussion. Cohen’s κ statistic was calculated for both steps.
Data Extraction
Two independent reviewers (A.W.E. and F.G.) extracted all study outcomes and characteristics in duplicate. Disagreements were resolved through discussion. Only data specific to patients with IMD from HER2-positive breast cancer were extracted.
Synthesis of Results
Principal summary measures were hazard ratios (HRs) for survival outcomes and proportions for response and safety outcomes. We estimated summary effect sizes through meta-analyses with random effects models using the inverse variance method. Tests for heterogeneity included I2, τ2, and Q statistics. Analysis was performed using the statistical programming language R (version 3.6.1, R Core Team, 2019)30 and the R packages robvis31 and meta.32
Additional Analyses
We conducted subgroup and sensitivity analyses and meta-regression to estimate subgroup effect sizes, assess robustness, and investigate confounders (Supplementary Methods).
Risk of Bias
We assessed risk of bias in randomized controlled trials (RCTs) using the Cochrane Risk of Bias (RoB 2) tool,33 cohort studies using the Newcastle–Ottawa Scale,34 and the one non-randomized controlled trial (NRCT) using the ROBINS-I tool.35 Independent reviewers (A.W.E. and F.G.) assessed risk in duplicate and resolved discrepancies through discussion. We assessed evidence quality using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework,36 and publication bias through Egger’s test and funnel plot inspection.
Results
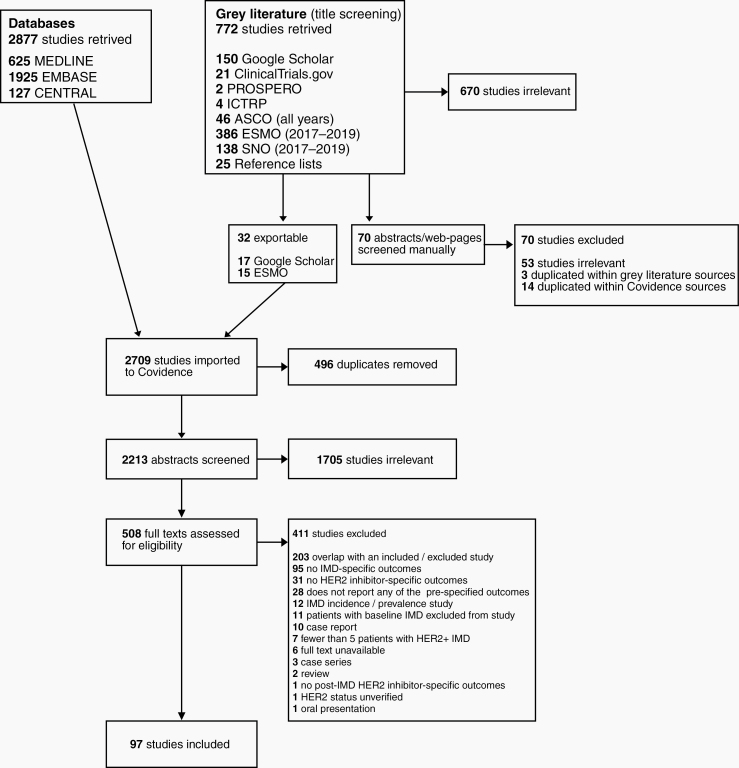
The literature search yielded 3449 records, from which we included 97 studies and 7157 patients (Figure 1).11,37–132 The 97 included studies were 4 RCTs, 1 NRCT, 32 single-arm interventional trials, 1 prospective cohort study, and 59 retrospectives cohort studies. Thirty-six of the 41 comparative studies compared HER2-targeted therapy to a non-targeted therapy, and 5 compared different HER2-targeted therapies to one another. Median follow-up ranged from 6.25 to 26 months (Supplementary Table S6). Pharmaceutical industry funding was disclosed by 49% (48/97) of studies (Supplementary Table S7). Trial characteristics are listed in Table 1.
Figure 1.
PRISMA flow diagram. Search queries were conducted in PubMed, EMBASE, CENTRAL, and gray literature source from their inception to January 27, 2020 for studies reporting survival, response, and safety outcomes for patients with IMD from HER2-positive breast cancer who received HER2-targeted therapy. Cohen’s κ statistic for inter-rater reliability at title-and-abstract (0.71) and full-text screening stages (0.67) indicated substantial agreement between reviewers.
Table 1.
Characteristics of Included Studies
| Author | Year | Publication Type | Study Design | Therapy | Therapy (n) | Comparator | Comparator (n) |
|---|---|---|---|---|---|---|---|
| Chan, A. et al.37 | 2019 | Abstr. | RCT | AC-TH or TCH | 64 | AC-T | 37 |
| Krop, I. et al.38 | 2015 | Art. | RCT | T-DM1 | 45 | Lapatinib + capecitabine | 50 |
| Murthy, R. et al.39 | 2019 | Art. | RCT | Tucatinib + trastuzumab + capecitabine | 198 | Placebo + trastuzumab + capecitabine | 93 |
| Takano, T. et al.40 | 2018 | Art. | RCT | Trastuzumab + capecitabine | 6 | Lapatinib + capecitabine | 7 |
| Bian, L. et al.41 | 2013 | Art. | NRCT | Trastuzumab + capecitabine | 4 | Lapatinib + capecitabine | 12 |
| Brufsky, A. et al.11 | 2011 | Art. | Pro. Coh. | Trastuzumab | 258 | No trastuzumab | 119 |
| Bartsch, R. et al.42 | 2011 | Art. | Ret. Coh. | Trastuzumab ± lapatinib | 43 | No HER2-targeted therapy | 37 |
| Bartsch, R. et al.43 | 2007 | Art. | Ret. Coh. | Trastuzumab | 17 | No trastuzumab | 36 |
| Braccini, A. et al.44 | 2013 | Art. | Ret. Coh. | Trastuzumab and/or lapatinib | 89 | No HER2-targeted therapy | 20 |
| Chen, J. et al.45 | 2014 | Abstr. | Ret. Coh. | HER2-targeted therapy | 24 | No HER2-targeted therapy | 36 |
| Church, D. et al.46 | 2008 | Art. | Ret. Coh. | Trastuzumab | 18 | No trastuzumab | 8 |
| Gomes, D. et al.47 | 2015 | Abstr. | Ret. Coh. | Trastuzumab and/or lapatinib | NR | No HER2-targeted therapy | NR |
| Gori, S. et al.48 | 2019 | Art. | Ret. Coh. | Trastuzumab and/or lapatinib | 102 | No HER2-targeted therapy | 52 |
| Griguolo, G. et al.49 | 2018 | Art. | Ret. Coh. | Pertuzumab, trastuzumab, T-DM1, and/or lapatinib | 22 | No HER2-targeted therapy | 10 |
| Hayashi, N. et al.50 | 2015 | Art. | Ret. Coh. | Trastuzumab and/or lapatinib | 283 | No HER2-targeted therapy | 149 |
| Hulsbergen, A. et al.51 | 2020 | Art. | Ret. Coh. | Trastuzumab and/or lapatinib | 8 | No HER2-targeted therapy | 7 |
| Kaplan, M. et al.52 | 2013 | Art. | Ret. Coh. | Lapatinib + capecitabine | 46 | Trastuzumab-based therapy | 65 |
| Kaplan, M. et al.53 | 2015 | Art. | Ret. Coh. | Trastuzumab ± lapatinib | 20 | No HER2-targeted therapy | 30 |
| Karam, I. et al.54 | 2011 | Art. | Ret. Coh. | Trastuzumab + RT | 130 | RT | 46 |
| Kim, J. et al.55 | 2019 | Art. | Ret. Coh. | Lapatinib + SRS | 43 | SRS | 41 |
| Le Scodan, R. et al.56 | 2011 | Art. | Ret. Coh. | Trastuzumab | 32 | No trastuzumab | 20 |
| Metro, G. et al.57 | 2011 | Art. | Ret. Coh. | Lapatinib + capecitabine | 30 | Trastuzumab-based therapy | 23 |
| Metro, G. et al.58 | 2007 | Art. | Ret. Coh. | Trastuzumab | 10 | No trastuzumab | 10 |
| Miller, J. et al.59 | 2017 | Art. | Ret. Coh. | Trastuzumab or lapatinib or pertuzumab or T-DM1 | 82 | No HER2-targeted therapy | 17 |
| Morikawa, A. et al.60 | 2018 | Art. | Ret. Coh. | Trastuzumab and/or lapatinib | 80 | No HER2-targeted therapy | 20 |
| Mounsey, L. et al.61 | 2018 | Art. | Ret. Coh. | Trastuzumab, lapatinib, T-DM1, and/or pertuzumab | 76 | No HER2-targeted therapy | 47 |
| Mueller, V. et al.62 | 2016 | Abstr. | Ret. Coh. | Trastuzumab or lapatinib or T-DM1 or Trastuzumab + pertuzumab | 155 | No HER2-targeted therapy | 317 |
| Niwinska, A. et al.63 | 2013 | Abstr. | Ret. Coh. | Trastuzumab or lapatinib | NR | No HER2-targeted therapy | NR |
| Niwinska, A. et al.64 | 2010 | Art. | Ret. Coh. | Trastuzumab and/or lapatinib | 105 | No HER2-targeted therapy | 118 |
| Okita, Y. et al.65 | 2013 | Art. | Ret. Coh. | Trastuzumab | 12 | No trastuzumab | 15 |
| Ou, D. et al.66 | 2019 | Art. | Ret. Coh. | HER2-targeted therapy | 22 | No HER2-targeted therapy | 17 |
| Park, I. et al.67 | 2009 | Art. | Ret. Coh. | Trastuzumab | 29 | No trastuzumab | 49 |
| Park, Y. et al.68 | 2009 | Art. | Ret. Coh. | Trastuzumab | 40 | No trastuzumab | 37 |
| Parsai, S. et al.69 | 2019 | Art. | Ret. Coh. | Lapatinib + SRS | 50 | SRS | 76 |
| Tarhan, M. et al.70 | 2013 | Art. | Ret. Coh. | Trastuzumab and/or lapatinib | 21 | No HER2-targeted therapy | 15 |
| Witzel, I. et al.71 | 2011 | Art. | Ret. Coh. | Trastuzumab | NR | No trastuzumab | NR |
| Yap, Y. et al.72 | 2012 | Art. | Ret. Coh. | Trastuzumab and/or lapatinib | 115 | No HER2-targeted therapy | 165 |
| Yomo, S. et al.73 | 2013 | Art. | Ret. Coh. | Lapatinib + SRS | 24 | SRS | 16 |
| Zhang, C. et al.74 | 2016 | Art. | Ret. Coh. | Trastuzumab | 33 | No trastuzumab | 35 |
| Zhang, Q. et al.75 | 2016 | Art. | Ret. Coh. | Trastuzumab and/or lapatinib | 24 | No HER2-targeted therapy | 36 |
| Zhukova, L. et al.76 | 2018 | Abstr. | Ret. Coh. | Trastuzumab ± lapatinib | NR | No HER2-targeted therapy | NR |
| Bhargava, P. et al.77 | 2019 | Abstr. | Ret. Coh. | Lapatinib and/or trastuzumab or T-DM1 or trastuzumab (intrathecal) | 102 | — | NA |
| Bartsch, R. et al.78 | 2009 | Art. | Ret. Coh. | Trastuzumab | 40 | — | NA |
| Bidard, F. et al.79 | 2009 | Art. | Ret. Coh. | Trastuzumab ± lapatinib | 6 | — | NA |
| Fabi, A. et al.80 | 2018 | Art. | Ret. Coh. | T-DM1 | 87 | — | NA |
| Figura, N. et al.81 | 2019 | Art. | Ret. Coh. | Trastuzumab (intrathecal) | 18 | — | NA |
| Gamucci, T. et al.82 | 2019 | Art. | Ret. Coh. | Pertuzumab + trastuzumab + taxanes | 21 | — | NA |
| Gavila, J. et al.83 | 2019 | Art. | Ret. Coh. | Trastuzumab + lapatinib | 38 | — | NA |
| Gori, S. et al.84 | 2012 | Art. | Ret. Coh. | Trastuzumab | 16 | — | NA |
| Grell, P. et al.85 | 2012 | Abstr. | Ret. Coh. | Lapatinib | 31 | — | NA |
| Hardy-Werbin, M. et al.86 | 2019 | Art. | Ret. Coh. | T-DM1 | 5 | — | NA |
| Huang, C. et al.87 | 2010 | Abstr. | Ret. Coh. | Lapatinib + capecitabine | 52 | — | NA |
| Jackisch, C. et al.88 | 2014 | Art. | Ret. Coh. | Trastuzumab | 90 | — | NA |
| Jacot, W. et al.89 | 2016 | Art. | Ret. Coh. | T-DM1 | 39 | — | NA |
| Mailliez, A. et al.90 | 2016 | Abstr. | Ret. Coh. | T-DM1 | 14 | — | NA |
| Martin Huertas, R. et al.91 | 2019 | Abstr. | Ret. Coh. | T-DM1 | 8 | — | NA |
| McCabe Y. et al.92 | 2016 | Abstr. | Ret. Coh. | T-DM1 | 23 | — | NA |
| Metro, G. et al.93 | 2010 | Abstr. | Ret. Coh. | Trastuzumab + chemotherapy or ET | 10 | — | NA |
| Michel, L. et al.94 | 2015 | Art. | Ret. Coh. | T-DM1 | 6 | — | NA |
| Montagna, E. et al.95 | 2009 | Art. | Ret. Coh. | Trastuzumab | 36 | — | NA |
| Okines, A. et al.96 | 2018 | Art. | Ret. Coh. | T-DM1 | 16 | — | NA |
| Riahi, H. et al.97 | 2010 | Abstr. | Ret. Coh. | Trastuzumab + WBRT | 31 | — | NA |
| Rossi, M. et al.98 | 2016 | Art. | Ret. Coh. | Trastuzumab | 40 | — | NA |
| Vasista, A. et al.99 | 2019 | Art. | Ret. Coh. | Trastuzumab | 29 | — | NA |
| Vici, P. et al.100 | 2017 | Art. | Ret. Coh. | T-DM1 | 61 | — | NA |
| Bachelot, T. et al.101 | 2011 | Art. | Sing. Int. | Lapatinib + capecitabine | 45 | — | NA |
| Bartsch, R. et al.102 | 2008 | Art. | Sing. Int. | Trastuzumab + gemcitabine | 5 | — | NA |
| Bonneau, C. et al.103 | 2018 | Art. | Sing. Int. | Trastuzumab (intrathecal) | 16 | — | NA |
| Borges, V. et al.104 | 2018 | Art. | Sing. Int. | Tucatinib + T-DM1 | 30 | — | NA |
| Christodoulou, C. et al.105 | 2017 | Art. | Sing. Int. | Lapatinib + WBRT | 12 | — | NA |
| de Azambuja, E. et al.106 | 2013 | Art. | Sing. Int. | Lapatinib + temozolomide | 16 | — | NA |
| Falchook, G. et al.107 | 2013 | Art. | Sing. Int. | Trastuzumab + lapatinib + bevacizumab | 10 | — | NA |
| Freedman, R. et al.108 | 2019 | Art. | Sing. Int. | Neratinib | 40 | — | NA |
| Giotta, F. et al.109 | 2010 | Art. | Sing. Int. | Lapatinib + capecitabine | 14 | — | NA |
| Gutierrez, M. et al.110 | 2015 | Abstr. | Sing. Int. | Trastuzumab (intrathecal) | 19 | — | NA |
| Hurvitz, S. et al.111 | 2018 | Art. | Sing. Int. | Lapatinib + everolimus + capecitabine | 19 | — | NA |
| Leone, J. et al.112 | 2019 | Art. | Sing. Int. | Trastuzumab + cabozantinib | 21 | — | NA |
| Lin, N. et al.113 | 2009 | Art. | Sing. Int. | Lapatinib | 242 | — | NA |
| Lin, N. et al.114 | 2016 | Abstr. | Sing. Int. | Pertuzumab + trastuzumab | 40 | — | NA |
| Lin, N. et al.115 | 2008 | Art. | Sing. Int. | Lapatinib | 39 | — | NA |
| Lin, N. et al.116 | 2013 | Art. | Sing. Int. | Lapatinib + WBRT + trastuzumab | 35 | — | NA |
| Lin, N. et al.117 | 2011 | Art. | Sing. Int. | Lapatinib + capecitabine or topotecan | 22 | — | NA |
| MacPherson, I. et al.118 | 2019 | Art. | Sing. Int. | Trastuzumab + epertinib or capecitabine | 5 | — | NA |
| Metzger, O. et al.119 | 2017 | Abstr. | Sing. Int. | Tucatinib + trastuzumab | 41 | — | NA |
| Montemurro, F. et al.120 | 2017 | Abstr. | Sing. Int. | T-DM1 | 399 | — | NA |
| Morikawa, A. et al.121 | 2019 | Art. | Sing. Int. | Lapatinib + capecitabine | 11 | — | NA |
| Murthy, R. et al.122 | 2018 | Art. | Sing. Int. | Tucatinib ± capecitabine ± trastuzumab | 29 | — | NA |
| Naskhletashvili, D. et al.123 | 2010 | Abstr. | Sing. Int. | Trastuzumab + capecitabine | 5 | — | NA |
| Niwinska, A. et al.124 | 2010 | Art. | Sing. Int. | Trastuzumab + chemotherapy | 52 | — | NA |
| Pistilli, B. et al.125 | 2018 | Art. | Sing. Int. | Trastuzumab + buparlisib + capecitabine | 9 | — | NA |
| Ro, J. et al.126 | 2012 | Art. | Sing. Int. | Lapatinib + capecitabine | 58 | — | NA |
| Shawky, H. et al.127 | 2014 | Art. | Sing. Int. | Lapatinib + capecitabine | 21 | — | NA |
| Sutherland, S. et al.128 | 2010 | Art. | Sing. Int. | Lapatinib + capecitabine | 34 | — | NA |
| Toi, M. et al.129 | 2009 | Art. | Sing. Int. | Lapatinib | 10 | — | NA |
| Van Swearingen, A. et al.130 | 2018 | Art. | Sing. Int. | Trastuzumab + everolimus + vinorelbine | 32 | — | NA |
| Yardley, D. et al.131 | 2015 | Art. | Sing. Int. | T-DM1 | 26 | — | NA |
| Yardley, D. et al.132 | 2018 | Art. | Sing. Int. | Lapatinib + cabazitaxel | 11 | — | NA |
Art., article; Abstr., abstract; RCT, randomized controlled trial; NRCT, non-randomized controlled trial; Pro. Coh., prospective cohort study; Ret. Coh., retrospective cohort study; Sing. Int., single-arm interventional trial; AC-TH, doxorubicin + cyclophosphamide then trastuzumab + paclitaxel; TCH, paclitaxel + cyclophosphamide + trastuzumab; AC-T, doxorubicin + cyclophosphamide then paclitaxel; T-DM1, trastuzumab emtansine; RT, radiotherapy; SRS, stereotactic radiosurgery; —, none; NA, not applicable.
Overall Survival
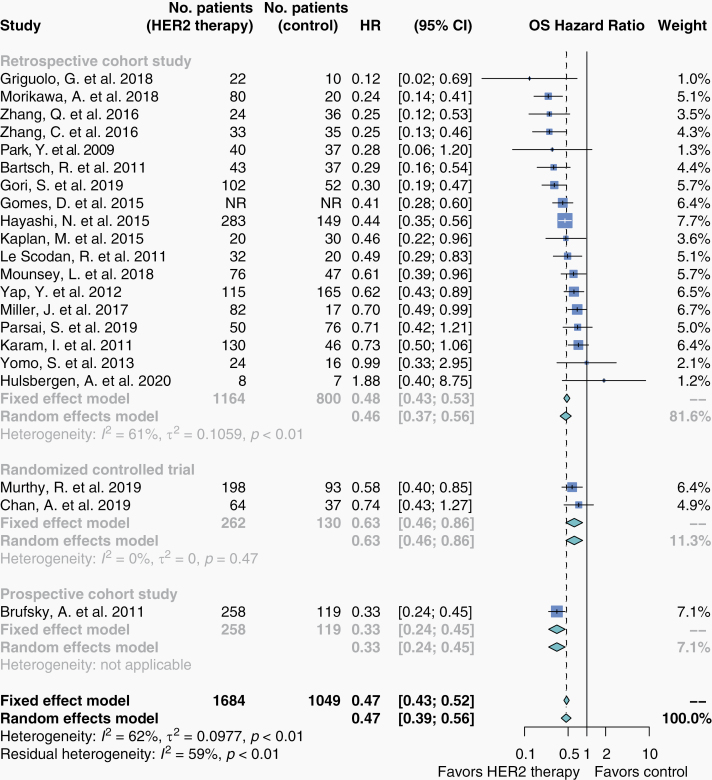
A meta-analysis of the 21 studies reporting overall survival (OS) HR comparing HER2-targeted therapy to non-targeted therapy showed HER2-targeted therapy was associated with prolonged OS (HR 0.47; 95% CI, 0.39–0.56; n = 3059; Figure 2). Summary estimates for individual agents for OS and all other outcomes are presented in Supplementary Table S8. Seventy-two studies reported OS in formats ineligible for meta-analysis (Supplementary Table S9).
Figure 2.
Overall survival in patients who received HER2-targeted therapy versus non-targeted therapy. Hazard ratios for overall survival were extracted from eligible studies and pooled in a meta-analysis. Studies here are stratified by study design. The size of each box represents the weight of each study in the meta-analysis. The vertical solid line represents the point of equivalence between HER2-targeted therapy and comparators. The vertical dashed and dotted lines represent the points of summary for fixed and random effects models, respectively, and the diamonds represent 95% CI. Analyses were performed with the R programming language30 and the R package meta.32
Progression-Free Survival
A meta-analysis of 4 studies showed that HER2-targeted therapy was not associated with prolonged progression-free survival (PFS; HR 0.52; 95% CI, 0.27–1.02; n = 475; Supplementary Figure S1). Twenty-nine studies reported PFS in formats ineligible for meta-analysis (Supplementary Table S10). Additional outcomes related to disease progression were reported in formats ineligible for meta-analysis: intracranial progression-free survival (iPFS), intracranial time to progression (iTTP), time to progression (TTP), and intracranial duration of response (iDoR) (Supplementary Tables S11–S14). Benefit with HER2-targeted therapy was seen in both studies reporting comparative iPFS (Supplementary Table S11) and in 3 of 4 studies reporting comparative iTTP (Supplementary Table S12). Comparative estimates for TTP and iDoR were not reported (Supplementary Tables S13 and S14).
Intracranial Objective Response Rate
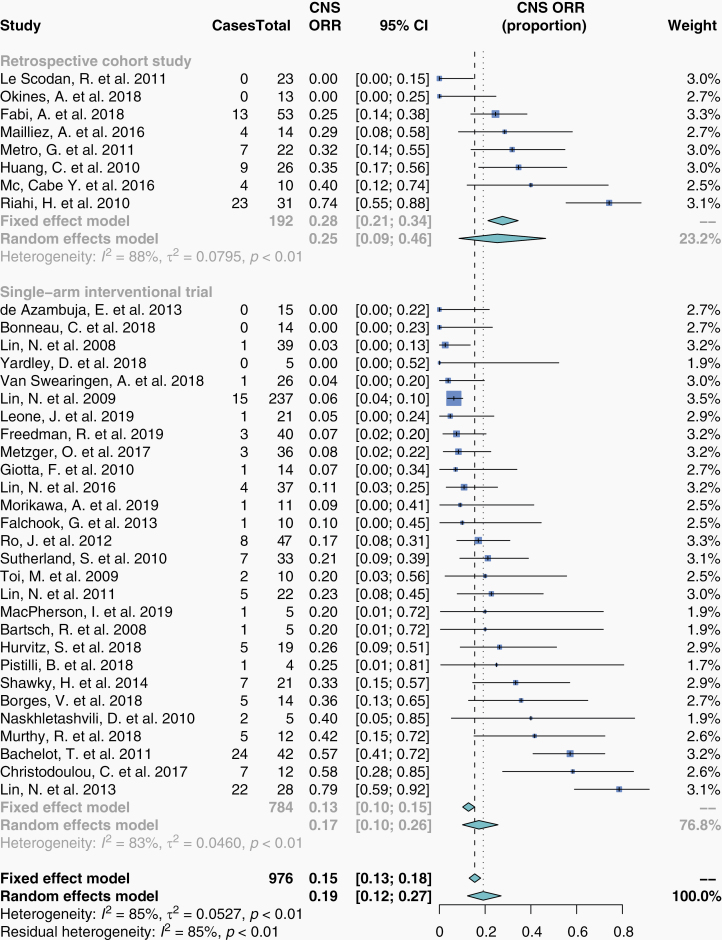
We performed a meta-analysis for intracranial objective response rate (iORR) proportions from 36 studies. These were 28 single-arm interventional trials and 8 retrospective cohort studies. The summary estimate for iORR as a proportion was 19% (95% CI, 12–27%; n = 976; Figure 3).
Figure 3.
Intracranial objective response rate in patients who received HER2-targeted therapy. Proportions for iORR were extracted from eligible studies and pooled in a meta-analysis. Studies here are stratified by study design. The size of each box represents the weight of each study in the meta-analysis. The vertical dashed and dotted lines represent the points of summary for fixed and random effects models, respectively, and the diamonds represent 95% CI. Analyses were performed with the R programming language30 and the R package meta.32
Intracranial Disease Control Rate
We performed a meta-analysis for intracranial disease control rate (iDCR) proportions from 33 studies. These were 1 NRCT, 25 single-arm interventional trials, and 7 retrospective cohort studies. The summary estimate for iDCR as a proportion was 62% (95% CI, 54–69%; n = 922; Supplementary Figure S2). Stratification by HER2-targeted agent and by publication before versus after 2018 produced distinct subgroup estimates and resolved some heterogeneity (Supplementary Figures S3 and S4).
Intracranial Complete Response Rate
We then performed a meta-analysis on intracranial complete response rate (iCRR) proportions from 30 studies. These were 25 single-arm interventional trials and 5 retrospective cohort studies. The summary estimate for iCRR as a proportion was 0% (95% CI, 0–1%; n = 891; Supplementary Figure S5).
Safety
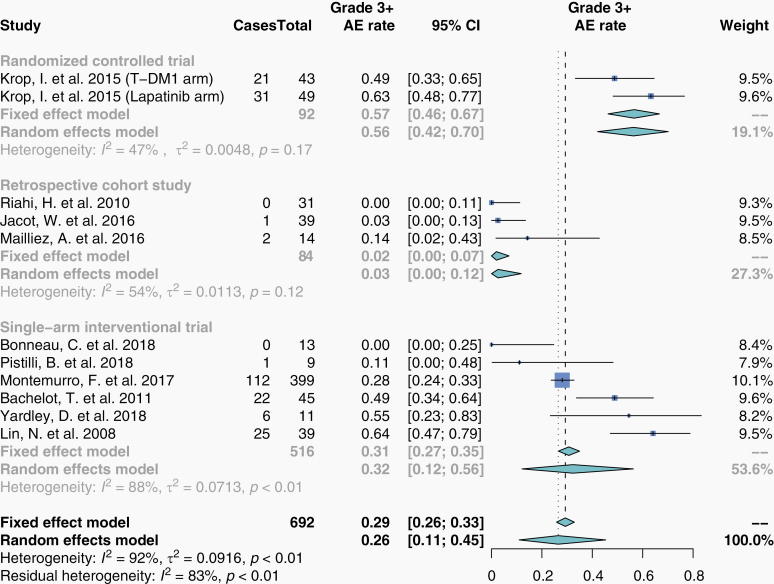
Studies reported CTCAE grade 3+ adverse events as either a number of total events (15 studies; Supplementary Table S15) or as a number of patients who experienced events (10 studies; Figure 4). Summary estimate for grade 3+ adverse event rate from studies reporting patient numbers was 26% (95% CI, 11–45%; Figure 4). Stratification by drug structure (monoclonal antibody vs small-molecule inhibitor) produced distinct subgroup estimates and resolved some heterogeneity (Supplementary Figure S6). Only one study reported a central nervous system (CNS)-specific serious adverse event rate, which was 8% (30/399) in patients receiving T-DM1.120
Figure 4.
Grade 3+ CTCAE adverse event rate in patients who received HER2-targeted therapy. Proportions for grade 3+ CTCAE adverse event rate were extracted from eligible studies and pooled in a meta-analysis. Studies here are stratified by study design. The size of each box represents the weight of each study in the meta-analysis. The vertical dashed and dotted lines represent the points of summary for fixed and random effects models, respectively, and the diamonds represent 95% CI. Analyses were performed with the R programming language30 and the R package meta.32
Additional Analyses
Sensitivity analyses
Sensitivity analyses did not produce significantly different summary estimates (Figures 2–4, Supplementary Figures S1, S2, and S5). Of note, omission of one study37 produced a significant summary estimate for PFS (HR 0.41; 95% CI, 0.30–0.56; n = 374).
Meta-regression
Meta-regression for OS, iORR, iDCR, and iCRR did not show association between selected characteristics and summary estimates (Supplementary Table S16). Two coefficients in the model for grade 3+ adverse event rate were significant: drug structure (small-molecule inhibitor vs monoclonal antibody, β = 0.33, P = .02) and study design (retrospective cohort study vs RCT, β = −0.47, P = .01).
Risk of Bias
Risk of bias varied among the included studies (Supplementary Figures S7–S11). Egger’s test and visual inspection of funnel plots suggested asymmetry due to publication bias in the summary estimates for iDCR (P = .01, Supplementary Figure S12) and iCRR (P = .02, Supplementary Figure S13) and were undetected for other summary estimates (Supplementary Figures S14–S17).
GRADE
Evidence certainty level differed between outcomes and study designs (Table 2).
Table 2.
GRADE Summary of Findings
| HER2-Targeted Therapy Compared To Control For Patients With Intracranial Metastatic Disease From HER2-Positive Breast Cancer | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Certainty Assessment | Summary of Findings | ||||||||||
| Participants (studies) follow-up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias or effect size | Overall certainty of evidence | Study event rates (%) | Relative effect (95% CI) | Anticipated absolute effects | ||
| With control | With HER2- targeted therapy | Risk with control | Risk difference with HER2-targeted therapy | ||||||||
| Overall survival (OS), RCTs | |||||||||||
| 392 (2 RCTs), follow-up NR | Not seriousa | Not seriousb | Not serious | Not serious | None | ⨁⨁⨁⨁ HIGH | 130 participants | 262 participants | HR 0.63 (0.46– 0.86) [OS] | All patients | |
| 50 per 100 | 15 fewer per 100 (from 23 fewer to 5 fewer) | ||||||||||
| OS, observational studies | |||||||||||
| 2341 (19 observational studies), follow-up range 0.23–53 months | Seriousc | Not seriousd | Not serious | Not serious | Strong associatione | ⨁⨁◯◯ LOW | 919 participants | 1422 participants | HR 0.45 (0.37– 0.54) [OS] | All patients | |
| 50 per 100 | 23 fewer per 100 (from 27 fewer to 19 fewer) | ||||||||||
| Progression-free survival, RCTs | |||||||||||
| 392 (2 RCTs), follow-up NR | Not serious | Seriousf | Not serious | Seriousg | None | ⨁⨁◯◯ LOW | 130 participants | 262 participants | HR 0.74 (0.29–1.90) [Progression- free survival] | All patients | |
| 50 per 100 | 10 fewer per 100 (from 32 fewer to 23 more) | ||||||||||
| Progression-free survival, observational studies | |||||||||||
| 83 (2 observational studies), follow-up range 1–39 months | Serioush | Not seriousb | Not serious | Not serious | Strong associationi | ⨁⨁◯◯ LOW | 42 participants | 41 participants | HR 0.32 (0.19 to 0.55) [Progression- free survival] | All patients | |
| 50 per 100 | 30 fewer per 100 (from 38 fewer to 18 fewer) | ||||||||||
CI, confidence interval; HR, hazard ratio.
aLow for both studies (RoB 2).
b I-squared 0%, tau-squared 0.
c68% (13/19) studies Agency for Health Research and Quality (AHRQ) “poor.”
d I-squared 63%, tau-squared 0.104.
eHR 0.45.
f I-squared 89%, tau-squared 0.417.
g95% CI, 0.29–1.90.
h50% (1/2) studies AHRQ “poor.”
iHR 0.32.
Discussion
In our meta-analysis, HER2-targeted therapy was associated with prolonged OS (HR 0.47; 95% CI, 0.39–0.56) in patients with HER2-positive breast cancer and IMD, with an iORR of 22% (95% CI, 14–30%), an iDCR of 62% (95% CI, 55–69%), an iCRR of 0% (95% CI, 0–0.01%), and a grade 3+ adverse event rate of 26% (95% CI, 11–45%). HER2-targeted therapy did not have a statistically significant effect on PFS (HR 0.52; 95% CI, 0.27–1.02).
The lack of prolonged PFS with HER2-targeted therapy may be an artifact of multiple data limitations. First, only 4 of 29 eligible studies included PFS data amenable to pooling. Second, the RECIST 1.1 criteria used to evaluate PFS do not distinguish between systemic and intracranial progression. Hence, a patient experiencing CNS benefit may be taken off therapy due to systemic progression. Third, this estimate was produced through pooling studies with different designs and treatments; this variety may both account for this result and reduce its credibility. Prolonged iPFS and iTTP were reported with HER2-targeted therapy versus non-targeted therapy by 2 and 3 studies, respectively (Supplementary Tables S11 and S12).
Subgroup analysis suggested that estimates for individual HER2-targeted agents were similar (Supplementary Table S8). Stratification of grade 3+ adverse event rate by drug structure suggested that antibody-based therapies were associated with lower rates of grade 3+ adverse events compared to small-molecule inhibitors (Supplementary Figure S6). This could be the result of greater pharmacokinetic distribution of small-molecule inhibitors compared to antibodies,133,134 an inherent difference in toxicity between classes or a spurious product of multiple comparisons.
Sensitivity analyses showed that our results were robust. Meta-regression revealed significant coefficients for study design and drug structure in modeling grade 3+ adverse event rate, although this analysis was underpowered due to the small ratio between the number of studies (k = 11) and model variables (n = 3).
Risk of bias varied in our study. Seventy-five percent (24/32) of single-arm interventional studies did not report central or blinded outcome measurement. Fifty-six percent (20/36) of comparative cohort studies either did not control or did not report control of confounders between study arms (Supplementary Figure S7). Most cohort studies did not report adequate follow-up (62%, 37/60) or follow-up completeness (82%, 49/60); Supplementary Figure S9).
Our results were consistent with previous reviews of trastuzumab and lapatinib for IMD from HER2-positive breast cancer.28,29 Reviews of other HER2-targeted therapies are lacking.
Since the execution of our literature search, the HER2CLIMB, CLEOPATRA, EMILIA, and KAMILLA trials have reported intracranial antitumor activity with the addition of tucatinib to trastuzumab and capecitabine, pertuzumab to trastuzumab plus docetaxel, T-DM1 versus lapatinib plus capecitabine, and T-DM1, respectively.38,135–137
Progress in the field of breast cancer brain metastases is still limited by infrequent evaluation of CNS-specific endpoints. This is reflected in the paucity of comparative intracranial results in our study: Of 36 studies comparing HER2-targeted therapy to a non-targeted comparator, none reported iORR, iDoR, iTTR, and iBCLS for both experimental and control arms, while only 1 trial reported iCRR, 2 reported iDCR and iPFS, and 4 evaluated iTTP. To obtain high-quality data regarding the efficacy of systemic therapy for the treatment of breast cancer patients with IMD, intracranial outcomes need to be collected prospectively in relevant RCTs. More liberal inclusion of patients with IMD should also be considered in the design of future clinical trials.138–140
Limitations
Our study had several limitations. First, patients with IMD from HER2-positive breast cancer were a subgroup in many of the included studies and therefore, outcomes for these patients were often few and secondary. Second, heterogeneity was substantial or considerable in most of our summary estimates. This was expected as our study employed broad inclusion criteria. To resolve heterogeneity, our subgroup analyses and meta-regression identified important factors for several outcomes, although these may be false positives from multiple comparisons. Third, many outcomes were reported in formats that precluded meta-analysis. PFS, for example, was reported as an HR comparing HER2-targeted therapy to non-targeted therapy by only 4 of 29 studies reporting PFS. A more accurate approximation of effects could be achieved with increased reporting of meta-analyzable endpoints. Fourth, several outcomes key to clarifying the role of HER2-targeted therapy in the management of IMD were under-reported, such as comparative intracranial response and safety outcomes, and CNS-specific clinical features and mortality.
Conclusions
Our study reviewed the literature and meta-analyzed outcomes for HER2-targeted therapy in patients with HER2-positive breast cancer and IMD. We find that HER2-targeted therapy is associated with prolonged OS, notable response proportions, and an adverse event rate that may depend on drug structure. Our findings support a role for HER2-targeted therapy in the treatment of IMD from HER2-positive metastatic breast cancer. Future trials for HER2-positive metastatic breast cancer should include patients with IMD to determine optimal treatment combinations and sequences, and further illuminate the role of novel therapies that may have efficacy in the CNS.
Supplementary Material
Acknowledgments
We thank Kaitlin Fuller, MLIS for consultation in creating the protocol and search strategies. We thank Alex Koziarz, MSc for review of our search strategy according to the PRESS checklist.141
Preliminary data from this study were presented in virtual poster format at the SNO Conference on Brain Metastases, Aug 14, 2020.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement. K.J.J. is a consultant and/or speaker for Apobiologix, Amgen, Esai, Genomic Health Inc., Novartis, Purdue Pharma, Pfizer, Roche.
Authorship statement. Project design: A.W.E. and S.D.; literature search and screening: A.W.E. and F.G.; data extraction: A.W.E. and F.G.; statistical analysis: A.W.E. and F.G.; manuscript draft and editing: A.W.E., F.G., S.H., K.J.J., A.S., M.A., and S.D.; project supervision: S.D.
References
- 1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. [DOI] [PubMed] [Google Scholar]
- 2. Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer. 1996;78(8):1781–1788. [PubMed] [Google Scholar]
- 3. Mayer M. A patient perspective on brain metastases in breast cancer. Clin Cancer Res. 2007; 13(6):1623–1624. [DOI] [PubMed] [Google Scholar]
- 4. Graesslin O, Abdulkarim BS, Coutant C, et al. . Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol. 2010;28(12):2032–2037. [DOI] [PubMed] [Google Scholar]
- 5. Martin AM, Cagney DN, Catalano PJ, et al. . Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017;3(8):1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bendell JC, Domchek SM, Burstein HJ, et al. . Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. [DOI] [PubMed] [Google Scholar]
- 7. Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27(31):5278–5286. [DOI] [PubMed] [Google Scholar]
- 8. Olson EM, Najita JS, Sohl J, et al. . Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22(4): 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pestalozzi BC, Holmes E, de Azambuja E, et al. . CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013;14(3):244–248. [DOI] [PubMed] [Google Scholar]
- 10. Brosnan EM, Anders CK. Understanding patterns of brain metastasis in breast cancer and designing rational therapeutic strategies. Ann Transl Med. 2018;6(9):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brufsky AM, Mayer M, Rugo HS, et al. . Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. [DOI] [PubMed] [Google Scholar]
- 12. Gil-Gil MJ, Martinez-Garcia M, Sierra A, et al. . Breast cancer brain metastases: a review of the literature and a current multidisciplinary management guideline. Clin Transl Oncol. 2014;16(5):436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gobbini E, Ezzalfani M, Dieras V, et al. . Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer. 2018;96:17–24. [DOI] [PubMed] [Google Scholar]
- 14. Jung SY, Rosenzweig M, Sereika SM, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. 2012;23(1):103–112. [DOI] [PubMed] [Google Scholar]
- 15. Hurvitz SA, O’Shaughnessy J, Mason G, et al. . Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERs. Clin Cancer Res. 2019;25(8):2433–2441. [DOI] [PubMed] [Google Scholar]
- 16. Achrol AS, Rennert RC, Anders C, et al. . Brain metastases. Nat Rev Dis Primers. 2019;5(1):5. [DOI] [PubMed] [Google Scholar]
- 17. Elder JB, Nahed BV, Linskey ME, Olson JJ. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of emerging and investigational therapies for the treatment of adults with metastatic brain tumors. Neurosurgery. 2019;84(3):E201–E203. [DOI] [PubMed] [Google Scholar]
- 18. Nabors LB, Portnow J, Ahluwalia M, et al. . NCCN Clinical Practice Guidelines in Oncology – Central Nervous System Cancers. Version 2.2020 2020. www.nccn.org. Accessed July 6, 2020.
- 19. Soffietti R, Abacioglu U, Baumert B, et al. . Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol. 2017;19(2):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8(6):344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Slamon DJ, Leyland-Jones B, Shak S, et al. . Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. [DOI] [PubMed] [Google Scholar]
- 22. Geyer CE, Forster J, Lindquist D, et al. . Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. [DOI] [PubMed] [Google Scholar]
- 23. Awada A, Colomer R, Inoue K, et al. . Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016;2(12):1557–1564. [DOI] [PubMed] [Google Scholar]
- 24. Verma S, Miles D, Gianni L, et al. ; EMILIA Study Group Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swain SM, Miles D, Kim SB, et al. . Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. [DOI] [PubMed] [Google Scholar]
- 26. Duchnowska R, Loibl S, Jassem J. Tyrosine kinase inhibitors for brain metastases in HER2-positive breast cancer. Cancer Treat Rev. 2018;67:71–77. [DOI] [PubMed] [Google Scholar]
- 27. Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17(5):279–299. [DOI] [PubMed] [Google Scholar]
- 28. Larsen PB, Kümler I, Nielsen DL. A systematic review of trastuzumab and lapatinib in the treatment of women with brain metastases from HER2-positive breast cancer. Cancer Treat Rev. 2013;39(7):720–727. [DOI] [PubMed] [Google Scholar]
- 29. Petrelli F, Ghidini M, Lonati V, et al. . The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: a systematic review and pooled analysis. Eur J Cancer. 2017;84:141–148. [DOI] [PubMed] [Google Scholar]
- 30. R Core Team. R Foundation for Statistical Computing. R: A language and environment for statistical computing 2019.
- 31. McGuinness LA. robvis: an R package and web application for visualising risk-of-bias assessments 2019. https://github.com/mcguinlu/robvis. Accessed July 2, 2020.
- 32. Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–45. [Google Scholar]
- 33. Sterne JAC, Savović J, Page MJ, et al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 34. Wells G, Shea B, O’Connell D, et al. . The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 9, 2019.
- 35. Sterne JA, Hernán MA, Reeves BC, et al. . ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan A, Spera G, MacHado A, et al. . Central nervous system as first site of relapse in patients with HER2 positive early breast cancer treated in the BCIRG-006 trial. Cancer Res. 2019; Conference:2018 San Antonio Breast Cancer Symposium. United States. 2079 (2014 Suppl 2011). [Google Scholar]
- 38. Krop IE, Lin NU, Blackwell K, et al. . Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murthy RK, Loi S, Okines A, et al. . Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. [DOI] [PubMed] [Google Scholar]
- 40. Takano T, Tsurutani J, Takahashi M, et al. . A randomized phase II trial of trastuzumab plus capecitabine versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer previously treated with trastuzumab and taxanes: WJOG6110B/ELTOP. Breast. 2018;40:67–75. [DOI] [PubMed] [Google Scholar]
- 41. Bian L, Wang T, Zhang S, Jiang Z. Trastuzumab plus capecitabine vs. lapatinib plus capecitabine in patients with trastuzumab resistance and taxane-pretreated metastatic breast cancer. Tumour Biol. 2013;34(5):3153–3158. [DOI] [PubMed] [Google Scholar]
- 42. Bartsch R, Berghoff A, Pluschnig U, et al. . Impact of systemic anti-HER2 treatment on overall survival in patients with brain metastases from HER2-overexpressing breast cancer. Eur J Cancer. 2011; European Multidisciplinary Cancer Congress. Stockholm, Sweden. Conference Publication: 47(Suppl. 1) (p S348). [Google Scholar]
- 43. Bartsch R, Rottenfusser A, Wenzel C, et al. . Trastuzumab prolongs overall survival in patients with brain metastases from Her2 positive breast cancer. J Neurooncol. 2007;85(3):311–317. [DOI] [PubMed] [Google Scholar]
- 44. Braccini AL, Azria D, Thezenas S, Romieu G, Ferrero JM, Jacot W. Prognostic factors of brain metastases from breast cancer: impact of targeted therapies. Breast. 2013;22(5):993–998. [DOI] [PubMed] [Google Scholar]
- 45. Chen J, Zhang Q, Yu X, Zhang Z, Guo X. Outcome of brain metastases from HER 2-positive breast cancer: Difference in survival benefit from anti-her 2 treatment after WBRT with regard to prior targeted therapy. Int J Radiat Oncol Biol Phys. 2014; Conference:56th Annual Meeting of the American Society for Radiation Oncology, ASTRO 2014. San Francisco, CA United States. Conference Publication; 90(1s):S247. [Google Scholar]
- 46. Church DN, Modgil R, Guglani S, et al. . Extended survival in women with brain metastases from HER2 overexpressing breast cancer. Am J Clin Oncol. 2008;31(3):250–254. [DOI] [PubMed] [Google Scholar]
- 47. Gomes DBD, Deeba RE, Suki D, et al. . Impact of systemic therapy on the outcomes of patients with metastatic breast cancer to brain: MD Anderson Cancer Center (MDACC) experience 1999–2012. J Clin Oncol. 2015;33(Suppl. 15):1046. [Google Scholar]
- 48. Gori S, Puglisi F, Moroso S, et al. . The HERBA study: a retrospective multi-institutional Italian study on patients with brain metastases from HER2-positive breast cancer. Clin Breast Cancer. 2019;19(4):e501–e510. [DOI] [PubMed] [Google Scholar]
- 49. Griguolo G, Pouderoux S, Dieci MV, et al. . Clinicopathological and treatment-associated prognostic factors in patients with breast cancer leptomeningeal metastases in relation to tumor biology. Oncologist. 2018;23(11):1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hayashi N, Niikura N, Masuda N, et al. . Prognostic factors of HER2-positive breast cancer patients who develop brain metastasis: a multicenter retrospective analysis. Breast Cancer Res Treat. 2015;149(1):277–284. [DOI] [PubMed] [Google Scholar]
- 51. Hulsbergen AFC, Cho LD, Mammi M, et al. . Systemic therapy following craniotomy in patients with a solitary breast cancer brain metastasis. Breast Cancer Res Treat. 2020;180(1):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaplan MA, Isikdogan A, Koca D, et al. . Clinical outcomes in patients who received lapatinib plus capecitabine combination therapy for HER2-positive breast cancer with brain metastasis and a comparison of survival with those who received trastuzumab-based therapy: a study by the Anatolian Society of Medical Oncology. Breast Cancer. 2014;21(6):677–683. [DOI] [PubMed] [Google Scholar]
- 53. Kaplan MA, Ertugrul H, Firat U, et al. . Brain metastases in HER2-positive metastatic breast cancer patients who received chemotherapy with or without trastuzumab. Breast Cancer. 2015;22(5):503–509. [DOI] [PubMed] [Google Scholar]
- 54. Karam I, Nichol A, Woods R, Tyldesley S. Population-based outcomes after whole brain radiotherapy and re-irradiation in patients with metastatic breast cancer in the trastuzumab era. Radiat Oncol. 2011;6:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim JM, Miller JA, Kotecha R, et al. . Stereotactic radiosurgery with concurrent HER2-directed therapy is associated with improved objective response for breast cancer brain metastasis. Neuro Oncol. 2019;21(5):659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Le Scodan R, Jouanneau L, Massard C, et al. . Brain metastases from breast cancer: prognostic significance of HER-2 overexpression, effect of trastuzumab and cause of death. BMC Cancer. 2011;11:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Metro G, Foglietta J, Russillo M, et al. . Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2011;22(3):625–630. [DOI] [PubMed] [Google Scholar]
- 58. Metro G, Sperduti I, Russillo M, Milella M, Cognetti F, Fabi A. Clinical utility of continuing trastuzumab beyond brain progression in HER-2 positive metastatic breast cancer. Oncologist. 2007;12(12):1467–1469; author reply 1469–1471. [DOI] [PubMed] [Google Scholar]
- 59. Miller JA, Kotecha R, Ahluwalia MS, et al. . Overall survival and the response to radiotherapy among molecular subtypes of breast cancer brain metastases treated with targeted therapies. Cancer. 2017;123(12):2283–2293. [DOI] [PubMed] [Google Scholar]
- 60. Morikawa A, Wang R, Patil S, et al. . Characteristics and prognostic factors for patients with HER2-overexpressing breast cancer and brain metastases in the era of HER2-targeted therapy: an argument for earlier detection. Clin Breast Cancer. 2018;18(5):353–361. [DOI] [PubMed] [Google Scholar]
- 61. Mounsey LA, Deal AM, Keith KC, et al. . Changing natural history of HER2-positive breast cancer metastatic to the brain in the era of new targeted therapies. Clin Breast Cancer. 2018;18(1):29–37. [DOI] [PubMed] [Google Scholar]
- 62. Mueller V, Loibl S, Laakmann E, et al. . Brain Metastases in Breast Cancer Network Germany (BMBC, GBG 79): treatment patterns and clinical outcome of more than 1000 patients with brain metastases from breast cancer. J Clin Oncol. 2016;34(Suppl. 15):2070. [Google Scholar]
- 63. Niwinska A, Murawska M. Good results of intensive systemic treatment of patients with HER2-positive breast cancer with brain metastases. Breast. 2013;22(Suppl. 23):S40. [Google Scholar]
- 64. Niwińska A, Murawska M, Pogoda K. Breast cancer subtypes and response to systemic treatment after whole-brain radiotherapy in patients with brain metastases. Cancer. 2010;116(18):4238–4247. [DOI] [PubMed] [Google Scholar]
- 65. Okita Y, Narita Y, Suzuki T, et al. . Extended trastuzumab therapy improves the survival of HER2-positive breast cancer patients following surgery and radiotherapy for brain metastases. Mol Clin Oncol. 2013;1(6):995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ou D, Cao L, Xu C, Kirova Y, Chen JY. Upfront brain radiotherapy may improve survival for unfavorable prognostic breast cancer brain metastasis patients with Breast-GPA 0-2.0. Breast J. 2019;25(6): 1134–1142. [DOI] [PubMed] [Google Scholar]
- 67. Park IH, Ro J, Lee KS, Nam BH, Kwon Y, Shin KH. Trastuzumab treatment beyond brain progression in HER2-positive metastatic breast cancer. Ann Oncol. 2009;20(1):56–62. [DOI] [PubMed] [Google Scholar]
- 68. Park YH, Park MJ, Ji SH, et al. . Trastuzumab treatment improves brain metastasis outcomes through control and durable prolongation of systemic extracranial disease in HER2-overexpressing breast cancer patients. Br J Cancer. 2009;100(6):894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Parsai S, Miller JA, Juloori A, et al. . Stereotactic radiosurgery with concurrent lapatinib is associated with improved local control for HER2-positive breast cancer brain metastases. J Neurosurg. 2019;132(2):503–511. [DOI] [PubMed] [Google Scholar]
- 70. Tarhan MO, Demir L, Somali I, et al. . The clinicopathological evaluation of the breast cancer patients with brain metastases: predictors of survival. Clin Exp Metastasis. 2013;30(2):201–213. [DOI] [PubMed] [Google Scholar]
- 71. Witzel I, Kantelhardt EJ, Milde-Langosch K, et al. . Management of patients with brain metastases receiving trastuzumab treatment for metastatic breast cancer. Onkologie. 2011;34(6):304–308. [DOI] [PubMed] [Google Scholar]
- 72. Yap YS, Cornelio GH, Devi BC, et al. . Brain metastases in Asian HER2-positive breast cancer patients: anti-HER2 treatments and their impact on survival. Br J Cancer. 2012;107(7):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yomo S, Hayashi M, Cho N. Impacts of HER2-overexpression and molecular targeting therapy on the efficacy of stereotactic radiosurgery for brain metastases from breast cancer. J Neurooncol. 2013;112(2):199–207. [DOI] [PubMed] [Google Scholar]
- 74. Zhang C, Wang L, Wang L, Cui S. A retrospective study on the efficacy of trastuzumab in HER2-positive and tamoxifen-refractory breast cancer with brain metastasis. Biodrugs. 2016;30(1):33–40. [DOI] [PubMed] [Google Scholar]
- 75. Zhang Q, Chen J, Yu X, et al. . Survival benefit of anti-HER2 therapy after whole-brain radiotherapy in HER2-positive breast cancer patients with brain metastasis. Breast Cancer. 2016;23(5):732–739. [DOI] [PubMed] [Google Scholar]
- 76. Zhukova LG, Lubennikova E, Ganshina I, et al. . Clinical outcome of Russian HER2-positive breast cancer patients with brain metastases: retrospective review. J Clin Oncol. 2018;36(Suppl. 15):e13025. [Google Scholar]
- 77. Bhargava PG, Shenoy R, Rathnasamy N, et al. . Clinical profile and outcome of HER2 positive breast cancer patients with brain metastases treated with HER2 targeted therapy: real-world experience. Ann Oncol. 2019;30(Suppl. 5):127. [Google Scholar]
- 78. Bartsch R, De Vries C, Pluschnig U, et al. . Predicting for activity of second-line trastuzumab-based therapy in her2-positive advanced breast cancer. BMC Cancer. 2009;9:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bidard FC, Guilhaume MN, Gauthier H, Cottu PH, Diéras V, Pierga JY. Meningeal carcinomatosis in HER2-overexpressing breast cancers. J Neurooncol. 2009;93(2):287–288. [DOI] [PubMed] [Google Scholar]
- 80. Fabi A, Alesini D, Valle E, et al. . T-DM1 and brain metastases: clinical outcome in HER2-positive metastatic breast cancer. Breast. 2018;41:137–143. [DOI] [PubMed] [Google Scholar]
- 81. Figura NB, Rizk VT, Mohammadi H, et al. . Clinical outcomes of breast leptomeningeal disease treated with intrathecal trastuzumab, intrathecal chemotherapy, or whole brain radiation therapy. Breast Cancer Res Treat. 2019;175(3):781–788. [DOI] [PubMed] [Google Scholar]
- 82. Gamucci T, Pizzuti L, Natoli C, et al. . A multicenter REtrospective observational study of first-line treatment with PERtuzumab, trastuzumab and taxanes for advanced HER2 positive breast cancer patients. RePer Study. Cancer Biol Ther. 2019;20(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gavila J, De La Haba J, Bermejo B, et al. . A retrospective, multicenter study of the efficacy of lapatinib plus trastuzumab in HER2-positive metastatic breast cancer patients previously treated with trastuzumab, lapatinib, or both: the Trastyvere study. Clin Transl Oncol. 2019;15:15. [DOI] [PubMed] [Google Scholar]
- 84. Gori S, Montemurro F, Spazzapan S, et al. . Retreatment with trastuzumab-based therapy after disease progression following lapatinib in HER2-positive metastatic breast cancer. Ann Oncol. 2012;23(6):1436–1441. [DOI] [PubMed] [Google Scholar]
- 85. Grell P, Kandrnal V, Zbynek B, Vyzula R. Lapatinib efficacy according to metastatic sites in trastuzumab pretreated patients with HER2-positive metastatic breast cancer: an analysis form IntERB registry in the Czech Republic. J Clin Oncol. 2012;30(Suppl. 15):e11072. [Google Scholar]
- 86. Hardy-Werbin M, Quiroga V, Cirauqui B, et al. . Real-world data on T-DM1 efficacy—results of a single-center retrospective study of HER2-positive breast cancer patients. Sci Rep. 2019;9(1):12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Huang C, Chen S, Liu M, et al. . Taiwanese patients with breast cancer with brain metastasis (BM) enrolled in the Lapatinib Expanded Access Program (LEAP). J Clin Oncol. 2010;28(Suppl. 15):1111. [Google Scholar]
- 88. Jackisch C, Schoenegg W, Reichert D, et al. . Trastuzumab in advanced breast cancer - a decade of experience in Germany. BMC Cancer. 2014;14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jacot W, Pons E, Frenel JS, et al. . Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res Treat. 2016;157(2):307–318. [DOI] [PubMed] [Google Scholar]
- 90. Mailliez A, Girard E, Boulanger T, Giraud C, Bonneterre J, Le Rhun E. Response to ado-trastuzumab emtansine according to RANO criteria in central nervous system metastases of HER2 positive breast cancers. J Clin Oncol. 2016;34(Suppl. 15):605. [Google Scholar]
- 91. Martin Huertas R, Lopez Miranda E, Corral De la Fuente E, et al. . Efficacy of trastuzumab-emtansine (T-DM1) in HER2-positive breast cancer (BC) with brain metastases (BM): A single institution experience. Ann Oncol. 2019;30(Suppl. 3):iii60–iii60. [Google Scholar]
- 92. McCabe CY, Wardley AM, Armstrong AC, Howell SJ. T-DM1 to induce response in central nervous system (CNS) metastases from Her2 +ve metastatic breast cancer (MBC). J Clin Oncol. 2016;34(Suppl. 15):582–582. [Google Scholar]
- 93. Metro G, Fabi A, Foglietta J, et al. . Trastuzumab-based therapy after disease progression following lapatinib and capecitabine in HER2-positive (HER21) metastatic breast cancer (MBC). Ann Oncol. 2010;21(Suppl. 8):viii100–viii101. [DOI] [PubMed] [Google Scholar]
- 94. Michel LL, Bermejo JL, Gondos A, Marmé F, Schneeweiss A. T-DM1 as a new treatment option for patients with metastatic HER2-positive breast cancer in clinical practice. Anticancer Res. 2015;35(9):5085–5090. [PubMed] [Google Scholar]
- 95. Montagna E, Cancello G, D’Agostino D, et al. . Central nervous system metastases in a cohort of metastatic breast cancer patients treated with trastuzumab. Cancer Chemother Pharmacol. 2009;63(2):275–280. [DOI] [PubMed] [Google Scholar]
- 96. Okines A, Irfan T, Khabra K, et al. . Development and responses of brain metastases during treatment with trastuzumab emtansine (T-DM1) for HER2 positive advanced breast cancer: a single institution experience. Breast J. 2018;24(3):253–259. [DOI] [PubMed] [Google Scholar]
- 97. Riahi Idrissi H, Chargari C, Pierga J, et al. . Whole brain radiotherapy with concurrent trastuzumab for treatment of brain metastases in breast cancer patients. Int J Radiat Oncol Biol Phys. 2010;78(Suppl. 3):S238–S238. [DOI] [PubMed] [Google Scholar]
- 98. Rossi M, Carioli G, Bonifazi M, et al. . Trastuzumab for HER2+ metastatic breast cancer in clinical practice: cardiotoxicity and overall survival. Eur J Cancer. 2016;52:41–49. [DOI] [PubMed] [Google Scholar]
- 99. Vasista A, Ryan L, Naher S, et al. . Survival and cardiac toxicity in patients with HER2-positive, metastatic breast cancer treated with trastuzumab in routine clinical practice. Asia Pacific J Clin Oncol. 2019;28:28. [DOI] [PubMed] [Google Scholar]
- 100. Vici P, Pizzuti L, Michelotti A, et al. . A retrospective multicentric observational study of trastuzumab emtansine in HER2 positive metastatic breast cancer: a real-world experience. Oncotarget. 2017;8(34):56921–56931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bachelot T, Romieu G, Campone M, et al. . Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. [DOI] [PubMed] [Google Scholar]
- 102. Bartsch R, Wenzel C, Gampenrieder SP, et al. . Trastuzumab and gemcitabine as salvage therapy in heavily pre-treated patients with metastatic breast cancer. Cancer Chemother Pharmacol. 2008;62(5):903–910. [DOI] [PubMed] [Google Scholar]
- 103. Bonneau C, Paintaud G, Trédan O, et al. . Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer. 2018;95:75–84. [DOI] [PubMed] [Google Scholar]
- 104. Borges VF, Ferrario C, Aucoin N, et al. . Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. JAMA Oncol. 2018;4(9):1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Christodoulou C, Kalogera-Fountzila A, Karavasilis V, et al. . Lapatinib with whole brain radiotherapy in patients with brain metastases from breast and non-small cell lung cancer: a phase II study of the Hellenic Cooperative Oncology Group (HeCOG). J Neurooncol. 2017;134(2):443–451. [DOI] [PubMed] [Google Scholar]
- 106. de Azambuja E, Zardavas D, Lemort M, et al. . Phase I trial combining temozolomide plus lapatinib for the treatment of brain metastases in patients with HER2-positive metastatic breast cancer: the LAPTEM trial. Ann Oncol. 2013;24(12):2985–2989. [DOI] [PubMed] [Google Scholar]
- 107. Falchook GS, Moulder SL, Wheler JJ, Jiang Y, Bastida CC, Kurzrock R. Dual HER2 inhibition in combination with anti-VEGF treatment is active in heavily pretreated HER2-positive breast cancer. Ann Oncol. 2013;24(12):3004–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Freedman RA, Gelman RS, Anders CK, et al. ; Translational Breast Cancer Research Consortium TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Giotta F, Latorre A, Oliva S, et al. . Lapatinib as salvage therapy in metastatic breast cancer: results from an expanded access program. Ann Oncol. 2010;21(Suppl. 4):iv63–iv64. [Google Scholar]
- 110. Gutierrez M, Fourme EM, Le Rhun E, et al. . Final results of the phase I “HIT” study: a multicenter phase I-II study evaluating trastuzumab administered by intrathecal injection for leptomeningeal meningitis of HER2+ metastatic breast cancer (MBC). Cancer Res. 2015;75(Suppl. 9):P5-19–17. [Google Scholar]
- 111. Hurvitz S, Singh R, Adams B, et al. . Phase Ib/II single-arm trial evaluating the combination of everolimus, lapatinib and capecitabine for the treatment of HER2-positive breast cancer with brain metastases (TRIO-US B-09). Ther Adv Med Oncol. 2018;10:1758835918807339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Leone JP, Duda DG, Hu J, et al. . A phase II study of cabozantinib alone or in combination with trastuzumab in breast cancer patients with brain metastases. Breast Cancer Res Treat. 2019; 20:20. [DOI] [PubMed] [Google Scholar]
- 113. Lin NU, Diéras V, Paul D, et al. . Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. [DOI] [PubMed] [Google Scholar]
- 114. Lin NU, Kumthekar P, Sahebjam S, et al. . Pertuzumab (P) plus high-dose trastuzumab (H) for the treatment of central nervous system (CNS) progression after radiotherapy (RT) in patients (pts) with HER2-positive metastatic breast cancer (MBC): primary efficacy analysis from the phase II PATRICIA study. Cancer Res. 2020;80(Suppl. 4):P1-18-03. [Google Scholar]
- 115. Lin NU, Carey LA, Liu MC, et al. . Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26(12):1993–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lin NU, Freedman RA, Ramakrishna N, et al. . A phase I study of lapatinib with whole brain radiotherapy in patients with Human Epidermal Growth Factor Receptor 2 (HER2)-positive breast cancer brain metastases. Breast Cancer Res Treat. 2013;142(2):405–414. [DOI] [PubMed] [Google Scholar]
- 117. Lin NU, Eierman W, Greil R, et al. . Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol. 2011;105(3):613–620. [DOI] [PubMed] [Google Scholar]
- 118. Macpherson IR, Spiliopoulou P, Rafii S, et al. . A phase I/II study of epertinib plus trastuzumab with or without chemotherapy in patients with HER2-positive metastatic breast cancer. Breast Cancer Res. 2019;22(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Metzger O, Barry W, Krop I, et al. . Phase I dose-escalation trial of ONT-380 in combination with trastuzumab in patients (pts) with HER2 breast cancer brain metastases. Cancer Res. 2017;77(Suppl. 4):P1-12-04. [Google Scholar]
- 120. Montemurro F, Ellis P, Delaloge S, et al. . Safety and efficacy of trastuzumab emtansine (T-DM1) in 399 patients with central nervous system metastases: Exploratory subgroup analysis from the KAMILLA study. Cancer Res. 2017;77(Suppl. 4):P1-12-10. [Google Scholar]
- 121. Morikawa A, de Stanchina E, Pentsova E, et al. . Phase I study of intermittent high-dose lapatinib alternating with capecitabine for HER2-positive breast cancer patients with central nervous system metastases. Clin Cancer Res. 2019;25(13):3784–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Murthy R, Borges VF, Conlin A, et al. . Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19(7):880–888. [DOI] [PubMed] [Google Scholar]
- 123. Naskhletashvili DR, Gorbounova V, Bychkov MB, et al. . Capecitabine-based therapy for patients with brain metastases from breast cancer. Ann Oncol. 2010;21(Suppl. 38) (pp viii118–viii119). [Google Scholar]
- 124. Niwińska A, Tacikowska M, Murawska M. The effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death. Int J Radiat Oncol Biol Phys. 2010;77(4):1134–1139. [DOI] [PubMed] [Google Scholar]
- 125. Pistilli B, Pluard T, Urruticoechea A, et al. . Phase II study of buparlisib (BKM120) and trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer resistant to trastuzumab-based therapy. Breast Cancer Res Treat. 2018;168(2):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ro J, Park S, Kim SB, et al. . Clinical outcomes of HER2-positive metastatic breast cancer patients with brain metastasis treated with lapatinib and capecitabine: an open-label expanded access study in Korea. BMC Cancer. 2012;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shawky H, Tawfik H. All-oral combination of lapatinib and capecitabine in patients with brain metastases from HER2-positive breast cancer—a phase II study. J Egypt Natl Cancer Inst. 2014;26(4): 187–194. [DOI] [PubMed] [Google Scholar]
- 128. Sutherland S, Ashley S, Miles D, et al. . Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases—the UK experience. Br J Cancer. 2010;102(6):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Toi M, Iwata H, Fujiwara Y, et al. . Lapatinib monotherapy in patients with relapsed, advanced, or metastatic breast cancer: efficacy, safety, and biomarker results from Japanese patients phase II studies. Br J Cancer. 2009;101(10):1676–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Van Swearingen AED, Siegel MB, Deal AM, et al. . LCCC 1025: a phase II study of everolimus, trastuzumab, and vinorelbine to treat progressive HER2-positive breast cancer brain metastases. Breast Cancer Res Treat. 2018;171(3):637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yardley DA, Krop IE, LoRusso PM, et al. . Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer previously treated with chemotherapy and 2 or more HER2-targeted agents: results from the T-PAS expanded access study. Cancer J. 2015;21(5):357–364. [DOI] [PubMed] [Google Scholar]
- 132. Yardley DA, Hart LL, Ward PJ, et al. . Cabazitaxel plus Lapatinib as therapy for HER2+ metastatic breast cancer with intracranial metastases: results of a dose-finding study. Clin Breast Cancer. 2018;18(5):e781–e787. [DOI] [PubMed] [Google Scholar]
- 133. Levêque D, Gigou L, Bergerat JP. Clinical pharmacology of trastuzumab. Curr Clin Pharmacol. 2008;3(1):51–55. [DOI] [PubMed] [Google Scholar]
- 134. Medina PJ, Goodin S. Lapatinib: a dual inhibitor of human epidermal growth factor receptor tyrosine kinases. Clin Ther. 2008;30(8):1426–1447. [DOI] [PubMed] [Google Scholar]
- 135. Lin NU, Borges V, Anders C, et al. . Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020:JCO2000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Swain SM, Baselga J, Miles D, et al. . Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25(6):1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Montemurro F, Delaloge S, Barrios CH, et al. . Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial☆. Ann Oncol. 2020;31(10):1350–1358. [DOI] [PubMed] [Google Scholar]
- 138. Lin NU, Prowell T, Tan AR, et al. . Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol. 2017;35(33):3760–3773. [DOI] [PubMed] [Google Scholar]
- 139. Camidge DR, Lee EQ, Lin NU, et al. . Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018;19(1):e20–e32. [DOI] [PubMed] [Google Scholar]
- 140. US Department of Health and Human Services, Food and Drug Administration. Cancer Clinical Trial Eligibility Criteria: Brain Metastases Guidance for Industry. July 2020. https://www.fda.gov/media/121317/download. Accessed July 13, 2020.
- 141. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.