Supplemental Digital Content is available in the text.
Keywords: biochemical adherence assessment, hypertension, meta-analysis, trough concentration
Abstract
Biochemical drug screening by liquid chromatography-tandem mass spectrometry in plasma is an accurate method for the quantification of plasma concentrations of antihypertensive medications in patients with hypertension. Trough concentrations could possibly be used as drug-specific cutoff values in the biochemical assessment of (non-)adherence. We performed a literature review and meta-analysis of pharmacokinetic studies to determine plasma trough concentrations of amlodipine, hydrochlorothiazide, and valsartan. PubMed was searched for pharmacokinetic studies up to September 2020. Eligible studies reported steady-state mean trough concentration and their variance. Pooled trough concentrations were estimated using a three-level random effects meta-analytic model. Moderator analyses were performed to explore sources of heterogeneity. One thousand three hundred eighteen potentially relevant articles were identified of which 45 were eligible for inclusion. The pooled mean trough concentration was 9.2 ng/mL (95% CI, 7.5–10.8) for amlodipine, 41.0 ng/mL (95% CI, 17.4–64.7) for hydrochlorothiazide, and 352.9 ng/mL (95% CI, 243.5–462.3) for valsartan. Substantial heterogeneity was present for all 3 pooled estimates. Moderator analyses identified dosage as a significant moderator for the pooled trough concentration of amlodipine (β1=0.9; P<0.05), mean age, and mean body weight for the mean trough concentration of hydrochlorothiazide (β1=2.2, P<0.05, respectively, β1=−4.0, P<0.05) and no significant moderators for valsartan. Plasma trough concentrations of amlodipine, hydrochlorothiazide, and valsartan, measured with liquid chromatography-tandem mass spectrometry, are highly heterogeneous over the different studies. Use of the pooled trough concentration as a cutoff in the biochemical assessment of adherence can result in inaccurate diagnosis of (non-)adherence, which may seriously harm the patient-physician relationship, and is therefore not recommended.
Blood pressure (BP) control is generally low in hypertensive populations with prevalence rates ranging from 10% to 44%.1,2 Medication nonadherence is a known behavioral contributor to poor BP control and is associated with an increased risk of cardiovascular disease, hospitalization, and increased health care costs.3,4 Moreover, nonadherent uncontrolled patients are at greater risk of being exposed to unnecessary and costly diagnostic tests for assessment of secondary causes of hypertension and invasive device-based therapies.5 Identification of nonadherence to antihypertensive drug treatment is therefore of major importance. The European Society for Patient Adherence, Compliance, and Persistence defines medication adherence as the process by which the patients take their medications as prescribed. Nonadherence can occur in 3 different phases of adherence: initiation (patient do not initiate treatment), implementation (actual dosing does not correspond to the prescribed dosing regimen because of delays, omits or extra doses), or persistence (discontinuation of treatment).6
Biochemical drug screening in plasma or urine by liquid chromatography tandem mass spectrometry is an objective method for medication adherence assessment.7 This method can be used to assess adherence in all 3 phases of adherence, allowing simultaneous and sensitive detection of different antihypertensive drugs and their metabolites and also creating the opportunity to link medication exposure to BP when blood sampling is accompanied by BP measurement.8
Biochemical drug screening is most often performed qualitatively with the purpose to detect the presence or absence of antihypertensive drugs or metabolites using the limit of detection (LOD), the lowest amount of a drug in a sample which can be detected. Also used is lower limit of quantitation (LLOQ), the lowest amount of a drug in a sample, which can be quantitatively determined with a certain accuracy, precision, and reproducibility.9 Patients will be classified as adherent to treatment when the drug or a metabolite is present at a concentration of at least its LLOQ or LOD and conversely is classified as nonadherent to treatment when the concentration of the drug or metabolite is less than its LLOQ or LOD. Overall, approaches based on the LLOQ or the LOD are qualitative screening methods, which can detect only complete nonadherence at one point in time. More erratic or irregular adherence behavior may not be detected. Moreover, qualitative liquid chromatography-tandem mass spectrometry is not able to identify white coat adherence, defined as an increase in adherence to treatment regimens before a clinical appointment. Finally, the LLOQ and LOD highly depend on the sensitivity of the analytical assay and not on the therapeutic range of the drug.10 Ongoing improvements of the analytical assay, resulting in lower detection limits, will therefore increase the risk of misclassification of partially nonadherent patients.
The biochemical assessment of adherence may be improved by quantitative analysis, evaluating measured drug concentrations, especially in the implementation and persistence phase of adherence. A possible way to perform quantitative biochemical drug screening is to compare the measured plasma drug concentration (Cx) with the trough concentration (Cmin), the minimum plasma concentration at steady state, assuming that adherent patients will at least have a plasma drug concentration above this limit. To implement this method in clinical practice, a reliable trough concentration per antihypertensive drug should be identified.
Therefore, the aim of this study was to perform a literature review and meta-analysis of pharmacokinetic studies to determine plasma population trough concentrations of 3 frequently prescribed antihypertensive drugs with different pharmacokinetic properties and from different antihypertensive drug classes; amlodipine, hydrochlorothiazide, and valsartan.
Methods
The data that support the findings of this study are available from the senior author upon reasonable request. Requests to access the dataset may be sent to W. Spiering at w.spiering@umcutrecht.nl.
Literature Search
We conducted a literature search via PubMed (including articles up to Sept 1, 2020) for studies describing the pharmacokinetics of amlodipine, hydrochlorothiazide, and valsartan. These antihypertensive drugs were selected because they belong to the most widely used classes of antihypertensive drugs, are the preferred 3-drug class combination if BP is not controlled by a 2-drug single-pill combination according to the 2018 European Society of Cardiology (ESC)/European Society of Hypertension (ESH) Guidelines,11 and possess different pharmacokinetic properties (eg, bioavailability, Tmax, volume of distribution and elimination). PubMed was searched for each drug separately with terms for the generic drug name and pharmacokinetics (Table S1 in the Data Supplement). All articles were screened for relevant title/abstracts using predefined in- and exclusion criteria. After title and abstract screening, full texts of the remaining articles were independently screened by 2 authors (E.H. Groenland and M.E.A.M. van Kleef). In addition, reference lists of all eligible articles were hand-searched for additional eligible studies.
Inclusion and Exclusion Criteria
We included prospective cohort and pharmacokinetic intervention studies that reported the steady state plasma trough concentration and their variance (SD or SE) in healthy subjects or patients with hypertension. We excluded single-dose studies because the trough concentrations in these studies are unlikely to match with the trough concentrations in patients chronically treated with antihypertensives, since steady-state concentration is not reached after a single dose. Moreover, we excluded studies that did not provide a measure of variability (or data to calculate the variability) for the mean trough concentration. Because of limited sensitivity of analytical methods other than liquid or gas chromatography, we excluded studies that applied such methods. Last, case reports, case series, narrative reviews, and articles in languages other than English, German, or Dutch were excluded.
Data Extraction
Data extraction was carried out by one researcher (E.H. Groenland) and verified by another (M.E.A.M. van Kleef). Extracted information included general study information such as journal, author, year of publication, study region, study design, and number of patients enrolled. Furthermore, we extracted information about drug dose, dosing frequency, treatment period, and analytical method. When available, age, sex, and body weight were extracted. Specifically, we extracted data on the plasma trough concentration of each drug (mean, SD or SE). Where measures were available only in graphical format, the software Digitizelt version 2.3.2 (Digitizelt, Braunschweig, Germany)12 was used, when possible, to extract the data. Discrepancies observed between the data extracted were resolved through discussion, and when discrepancies could not be resolved, a third reviewer K.C.M. van der Elst was consulted.
Data Analyses
To provide an overall estimate of the mean trough concentration per antihypertensive drug, mean trough concentrations from individual studies were pooled. Since most of the studies provided multiple mean trough concentrations (ie, with and without co-medication, multiple dosages), we applied a three-level random effects meta-analytic model, taking dependency between the mean trough concentrations into account.13 Moreover, moderator analyses were performed to explore sources of heterogeneity.14 A detailed description of the used methodology is provided in Methods in the Data Supplement. Results were graphically presented in forest plots. All analyses were performed using the statistical software package R version 3.5.1. and the metafor package.15 For all analyses, a P value of <0.05 was considered statistically significant.
Results
Literature Search and Review Process
Figure 1 shows the flow diagram for the study inclusion. The search generated a total of 1318 potentially relevant studies; 492 for amlodipine, 535 for hydrochlorothiazide, and 291 for valsartan. On the basis of the title and abstracts, we identified 335 possibly relevant articles. After full text screening, 44 studies met the eligibility criteria. Additionally, 1 study was identified from the reference lists.16 This additional study was not indexed with a term related to pharmacokinetics and therefore not included in our search results.
Figure 1.
Flowchart showing the process of study selection. HPLC indicates high-performance liquid chromatography; and LC-MS, liquid chromatography mass spectrometry.
Description of the Included Studies
Table S2 reports the characteristics and key findings from the included studies. Most studies were open-label trials set up to evaluate the pharmacokinetics of the antihypertensive drug alone or in relation to other drugs. Males were overrepresented in most study populations; 22 studies consisted of at least 80% males of which 13 studies contained only men. Of the 45 included studies, 37 studies evaluated the interaction of the antihypertensive drug with other, mostly cardiovascular, medication. Thirty-eight studies reported multiple mean trough concentration obtained from measurements in different populations, measurements after different drug dosages, or measurements after combination with other drugs. Therefore, 93 mean trough concentrations were included in the meta-analysis.
Amlodipine
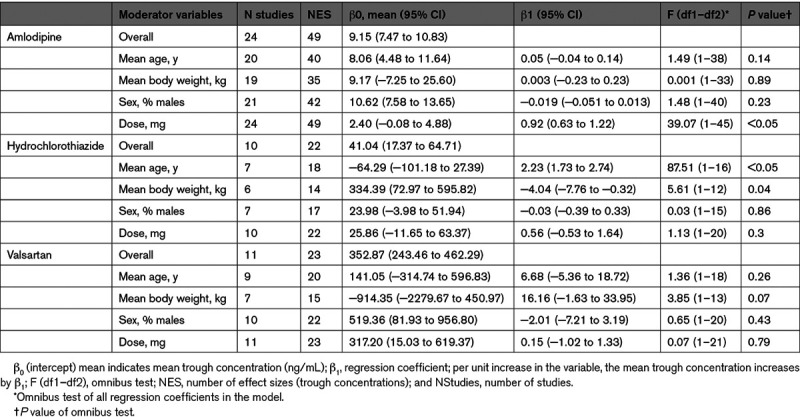
Data on the trough concentration and variance of amlodipine was available in 24 studies.16–39 These 24 studies reported a total of 49 trough concentrations. The pooled mean trough concentration for amlodipine was 9.2 ng/mL (95% CI, 7.5–10.80; Figure 2A). We found significant variability of trough concentrations within studies (at level 2; likelihood ratio test [LRT], 476.4; P<0.05) as well as between studies (at level 3; LRT, 29.3; P<0.05). Of the total variance, 14% and 85% were distributed at levels 2 and 3, respectively, and 0.8% was the percentage of sampling variance that was calculated using the formula of Cheung.13 Moderator analyses showed a significant moderating effect for dose. The mean trough concentration increased as studies applied a higher dose (β1=0.9; per mg increase in dose the mean trough concentration increases by 0.9 ng/mL, P<0.05; Table).
Figure 2.

Forest plots trough concentrations. Forest plots trough concentration amlodipine (A), hydrochlorothiazide (B), and valsartan (C). Forest plots ordered by the height of the mean trough concentration. The diamonds indicate the pooled estimate for the mean trough concentration from the meta-analysis, based on the multilevel random-effect model. A–D, The different trough concentrations derived from the same study, see Table S1 in the Data Supplement for further specification.
Table.
Results of Moderator Analyses
Hydrochlorothiazide
Data on the trough concentration and variance of hydrochlorothiazide were available in 10 studies.18,39–47 These 10 studies reported a total of 22 trough concentrations. The pooled mean trough concentration for hydrochlorothiazide was 41.0 ng/mL (95% CI, 17.4–64.7; Figure 2B). We found significant variability of trough concentrations within studies (at level 2; LRT, 98.7; P<0.05) as well as between studies (at level 3; LRT, 4.43, P=0.05). Of the total variance, 45% and 55% were distributed at levels 2 and 3, respectively, and 0.2% was the percentage of sampling variance that was calculated using the formula of Cheung.13 Moderator analyses showed a significant moderating effect for mean age and mean body weight. The pooled mean trough concentration increased as the mean age increased (β1=2.2, P<0.05) and decreased as the mean body weight increased (β1=−4.0, P<0.05). A multiple-moderators model, including these 2 covariates, indicated that only mean age had a unique moderating effect on the mean trough concentration (β1=2.1, P<0.05; Table).
Valsartan
Data on the trough concentration of valsartan was available in 11 studies.18,24–26,36,39,48–52 These 11 studies reported a total of 23 trough concentrations. The pooled mean trough concentration for valsartan was 352.9 ng/mL (95% CI, 243.5–462.3; Figure 2C). The variability within studies (at level 2) was not significant (LRT, 2.7; P=0.1). However, we did find significant variability of trough concentrations between studies (at level 3; LRT, 12.8; P=0.05). Of the total variance, 7% and 88% were distributed at levels 2 and 3, respectively, and 5% was the percentage of sampling variance that was calculated using the formula of Cheung.13 Moderator analyses showed no significant moderating effect for the preselected study characteristics (Table).
Discussion
The present study was designed to formulate a pooled trough concentration for amlodipine, hydrochlorothiazide, and valsartan; 3 frequently prescribed antihypertensive drugs from different classes with different pharmacokinetic properties, with the aim to use these values in the quantitative biochemical assessment of medication adherence (implementation and persistence phase) in patients with uncontrolled hypertension. Our meta-analysis resulted in a pooled trough concentration of 9.3 ng/mL (95% CI, 7.6–11.0) for amlodipine, 41.0 ng/mL (95% CI, 17.4–64.7) for hydrochlorothiazide, and 352.9 ng/mL (95% CI, 243.5–462.3) for valsartan. However, substantial heterogeneity within- and between studies was present, which could only partly be explained by differences in dose in case of amlodipine and differences in mean age for hydrochlorothiazide.
The substantial heterogeneity within- and between studies in the present meta-analysis indicates large between-individual variability in trough concentrations. This substantial variability in pharmacokinetic parameters of antihypertensive drugs corresponds to results from previous studies investigating the variability in plasma concentrations of BP-lowering drugs.53,54 The large variability in trough concentrations is most likely explained by differences in drug-, dose-, and patient characteristics, including adherence behavior. In this meta-analysis, univariate moderator analysis revealed drug dosage as a significant moderator for the pooled mean plasma trough concentration of amlodipine with a value of 0.92 ng/mL per mg increase in dosage. This observation is in line with a previous study investigating the influence of dosage on the plasma concentration of amlodipine.54 Although not expected, dosage was not a significant moderator on the pooled mean trough concentrations of hydrochlorothiazide and valsartan. One of the reasons could be the limited amount of studies investigating different dosages for these antihypertensive drugs. The pooled mean trough concentration of hydrochlorothiazide significantly decreased with increasing mean body weight (β1=−4.0, P=0.04), which is probably because of a higher volume of distribution in patients with increased body weight. Furthermore, the pooled mean trough concentration of hydrochlorothiazide increased with increase in mean age (β1=2.2, P<0.05). This was in accordance with earlier findings that showed a reduced renal clearance of hydrochlorothiazide with increasing age, resulting in higher plasma concentrations.55 The lack of an effect of age on the trough concentration of amlodipine and valsartan in the current study is probably because of a limited number of studies including older people. Certainly, there are many other factors that can influence the mean plasma concentrations of these 3 antihypertensive drugs (eg, renal- and hepatic function, interacting comedications, and the degree of adherence). However, because of the limited availability of these data in the included studies, we were not able to evaluate the influence of these factors.
The large variation in plasma trough concentrations, as demonstrated in the present study, discourages the use of the pooled trough concentration as a reliable cutoff in the biochemical assessment of adherence. Few alternatives for quantitative drug screening have previously been proposed.56,57 In 2018, the concept of indexed plasma drug concentrations for drug adherence screening in hypertensive patients was proposed.56 This concept involves comparison of the measured plasma drug concentrations (Cx) of antihypertensive drugs with the expected Cmax (Cx/Cmax) for each drug and dose using published reference values. When these indexed plasma concentrations are used, different drugs and doses can be compared on the same relative scale. Moreover, plasma half-lives of the tested drugs, timing of the drug intake, and timing of blood sampling may be used to define a particular Cx/Cmax value as a common threshold for same-day drug use. However, the choice of an appropriate Cmax from published data is nevertheless a crucial prerequisite for the application of this method. Just like the retrieved trough concentrations in our meta-analysis, the Cmax is also highly variable and should therefore not be used as a reliable threshold.
Use of published therapeutic reference ranges as a cutoff for adherence is further discouraged by findings from a recent German study that reported serum concentrations of antihypertensive drugs below the literature-based reference ranges despite supervised intake of these drugs.57 To overcome this limitation, a novel method which is based on the dose-related concentration was introduced. This method compares the measured concentration of an antihypertensive drug with trough drug concentrations calculated individually for each patient. Although the cutoff values in that study were also based on parameters from pharmacokinetic studies conducted in selected study populations, which do not entirely reflect the variability in the population, it was shown that all patients attending the nephrology ward had measured drug concentrations above the lower limit of the dose-related concentration, after supervised antihypertensive drug intake. Therefore, their approach might be a promising method for future quantitative drug screening.
Strengths of our meta-analysis include the application of a 3-level random effects model for the meta-analysis of the trough concentration from the individual studies. By using this model, we were able to pool multiple trough concentrations derived from the same study since this model takes dependency between mean trough concentrations into account. An important advantage of the 3-level approach is that all the relevant information produced in primary studies can be preserved and maximum statistical power can be achieved. In addition, we performed moderator analysis, which gave us the opportunity to explore within-study and between-study variance.
Several limitations of the present meta-analysis need to be taken into account. First, by limiting our search to the PubMed database, we could have missed some relevant studies. However, this limited search already resulted in highly variable trough concentrations. Therefore, extension of the search to other databases will probably not change our main finding of substantial within- and between- study heterogeneity. Moreover, reference check of the included studies yielded only one additional reference, indicating that the amount of missed studies is limited. Second, since data about clinical characteristics from the individual studies was sparse, we were restricted in the possibilities to perform moderator analyses. Consequently, there may be a true moderating effect of several study and/or patient characteristics, which we were unable to detect in the present study. Also, the limited amount of studies did not allow us to construct a multiple-moderator model to explore the presence of multicollinearity.20 Third, as most of the included studies were performed in young to middle aged, healthy, and mainly male individuals it is questionable whether these results could be translated to patients with uncontrolled hypertension. These patients are characterized by a higher age and generally suffer from multiple comorbidities (eg, renal insufficiency), which is often an indication for additional, possibly interacting, drug treatment, which may in theory further increase the between-individual pharmacokinetic variability.58 The pooled trough concentrations and the amount of heterogeneity reported in this study are therefore likely to be underestimated.
Perspectives
Then, how are we supposed to apply our findings into clinical practice? The goal of biochemical adherence assessment is to accurately distinguish between adherent and nonadherent patients and to use this information in a shared decision-making approach to ultimately improve drug adherence and BP control. To implement quantitative screening in daily clinical practice, reliable cutoff values are required. As illustrated in our meta-analysis, trough concentrations of the 3 different antihypertensive drugs are highly variable, which means that a drug concentration below the trough concentration could also be the result of a deviation from typical pharmacokinetics. Therefore, trough concentrations are not suitable as cutoff values for the quantitative biochemical assessment of drug adherence as this increases the risk of misclassification of adherent patients as nonadherent. Performing biochemical assessment in urine instead of plasma will even further increase the risk of misclassification as some antihypertensive drugs are extensively metabolized or have a low urinary excretion. Addressing of nonadherence in patients that are adherent could seriously harm the patient-physician relationship, might work counterproductive in adherence management, and should therefore be prevented. Hence, as long as a reliable cutoff value for quantitative drug screening is lacking a conservative approach is preferred and biochemical assessment of adherence should be performed qualitatively.
In conclusion, the plasma trough concentrations of amlodipine, hydrochlorothiazide, and valsartan are highly heterogeneous. Use of the pooled trough concentrations, retrieved by our meta-analysis, as a cutoff for the biochemical assessment of adherence in clinical practice is therefore not recommended. Before implementation of a quantitative drug screening into clinical practice, drug-, dose-, and patient-specific lower limits based on individual patient data from pharmacokinetic studies are needed, to take into account factors that influence drug exposure.
Acknowledgments
We are grateful to Dr E.M. van Maarseveen for his keen interest and consistent encouragement during this work.
Sources of Funding
None.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BP
- blood pressure
- LLOQ
- lower limit of quantification
- LOD
- level of detection
- LRT
- likelihood ratio test
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.120.16061.
For Sources of Funding and Disclosures, see page 92.
Contributor Information
Eline H. Groenland, Email: e.h.groenland@umcutrecht.nl.
Monique E.A.M. van Kleef, Email: m.e.a.vankleef@umcutrecht.nl.
Michiel L. Bots, Email: m.l.bots@umcutrecht.nl.
Frank L.J. Visseren, Email: f.l.j.visseren@umcutrecht.nl.
Novelty and Significance
What Is New?
The biochemical assessment of drug adherence may be improved by performing a quantitative analysis, evaluating measured drug concentrations. For use in clinical practice, reliable cutoff values are needed to decide whether a patient is (non-)adherent. A potential cutoff value could be the trough concentration.
This is the first study that performed a meta-analysis on the trough concentrations of 3 frequently prescribed antihypertensive drugs; amlodipine, hydrochlorothiazide, and valsartan.
What Is Relevant?
The pooled mean trough concentrations of the 3 investigated antihypertensive drugs suffer from substantial heterogeneity indicating large between-individual variability in trough concentrations.
Use of the pooled mean plasma trough concentrations, retrieved by our meta-analysis, as a cutoff for the biochemical assessment of drug adherence in clinical practice is therefore not recommended.
Summary
Biochemical drug screening in plasma or urine by liquid chromatography tandem mass spectrometry is an objective method for medication adherence assessment. Quantitative plasma concentrations could possibly improve biochemical assessment of adherence to antihypertensive drugs. However, use of our pooled trough concentrations is not recommended because of the large between-individual differences. Therefore, a concentration below this concentration is not sufficient to establish nonadherence.
References
- 1.Fang J, Alderman MH, Keenan NL, Ayala C, Croft JB. Hypertension control at physicians’ offices in the United States. Am J Hypertens. 2008;21:136–142. doi: 10.1038/ajh.2007.35 [DOI] [PubMed] [Google Scholar]
- 2.Pereira M, Lunet N, Azevedo A, Barros H. Differences in prevalence, awareness, treatment and control of hypertension between developing and developed countries. J Hypertens. 2009;27:963–975. doi: 10.1097/hjh.0b013e3283282f65 [DOI] [PubMed] [Google Scholar]
- 3.Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, Borghi C, Brignoli O, Caputi AP, Cricelli C, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598–1605. doi: 10.1161/CIRCULATIONAHA.108.830299 [DOI] [PubMed] [Google Scholar]
- 4.Will JC, Zhang Z, Ritchey MD, Loustalot F. Medication adherence and incident preventable hospitalizations for hypertension. Am J Prev Med. 2016;50:489–499. doi: 10.1016/j.amepre.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 5.Rimoldi SF, Scherrer U, Messerli FH. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245–1254. doi: 10.1093/eurheartj/eht534 [DOI] [PubMed] [Google Scholar]
- 6.De Geest S, Zullig LL, Dunbar-Jacob J, Helmy R, Hughes DA, Wilson IB, Vrijens B. ESPACOMP Medication adherence reporting guideline (EMERGE). Ann Intern Med. 2018;169:30–35. doi: 10.7326/M18-0543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:217047 doi: 10.1155/2015/217047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Punt AM, Stienstra NA, van Kleef MEA, Lafeber M, Spiering W, Blankestijn PJ, Bots ML, van Maarseveen EM. Screening of cardiovascular agents in plasma with LC-MS/MS: a valuable tool for objective drug adherence assessment. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1121:103–110. doi: 10.1016/j.jchromb.2019.05.013 [DOI] [PubMed] [Google Scholar]
- 9.Burnier M, Wuerzner G, Struijker-Boudier H, Urquhart J. Measuring, analyzing, and managing drug adherence in resistant hypertension. Hypertens (Dallas, Tex 1979). 2013;62:218–225. doi: 10.1161/HYPERTENSIONAHA.113.00687 [DOI] [PubMed] [Google Scholar]
- 10.Hamdidouche I, Jullien V, Boutouyrie P, Billaud E, Azizi M, Laurent S. Drug adherence in hypertension: from methodological issues to cardiovascular outcomes. J Hypertens. 2017;35:1133–1144. doi: 10.1097/HJH.0000000000001299 [DOI] [PubMed] [Google Scholar]
- 11.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 12.Bormann I. Digitizer Software—Digitize a Scanned Graph or Chart Into (x, y) Data. 2016 https://www.digitizeit.de/
- 13.Cheung MWL. A guide to conducting a meta-analysis with non-independent effect sizes. Neuropsychol Rev. 2019;29:387–396. doi: 10.1007/s11065-019-09415-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assink M, Wibbelink CJM. Fitting three-level meta-analytic models in R: a step-by-step tutorial. Quant Methods Psychol. 2016;12:154–174 [Google Scholar]
- 15.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48 [Google Scholar]
- 16.Webster J, Robb OJ, Jeffers TA, Scott AK, Petrie JC, Towler HM. Once daily amlodipine in the treatment of mild to moderate hypertension. Br J Clin Pharmacol. 1987;24:713–719. doi: 10.1111/j.1365-2125.1987.tb03236.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abernethy DR. An overview of the pharmacokinetics and pharmacodynamics of amlodipine in elderly persons with systemic hypertension. Am J Cardiol. 1994;73:10A–17A. doi: 10.1016/0002-9149(94)90269-0 [DOI] [PubMed] [Google Scholar]
- 18.Bhad P, Ayalasomayajula S, Karan R, Leon S, Riviere GJ, Sunkara G, Jarugula V. Evaluation of pharmacokinetic interactions between amlodipine, valsartan, and hydrochlorothiazide in patients with hypertension. J Clin Pharmacol. 2011;51:933–942. doi: 10.1177/0091270010376963 [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Hu P, Jiang J, Liu T, Zhong W, Liu H, Zhao Q. Pharmacokinetic and pharmacodynamic profiles of a fixed-dose combination of olmesartan medoxomil and amlodipine in healthy Chinese males and females. Clin Drug Investig. 2012;32:783–790. doi: 10.1007/s40261-012-0026-0 [DOI] [PubMed] [Google Scholar]
- 20.Faulkner JK, McGibney D, Chasseaud LF, Perry JL, Taylor IW. The pharmacokinetics of amlodipine in healthy volunteers after single intravenous and oral doses and after 14 repeated oral doses given once daily. Br J Clin Pharmacol. 1986;22:21–25. doi: 10.1111/j.1365-2125.1986.tb02874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillen M, Yang C, Wilson D, Valdez S, Lee C, Kerr B, Shen Z. Evaluation of pharmacokinetic interactions between lesinurad, a new selective urate reabsorption inhibitor, and CYP enzyme substrates sildenafil, amlodipine, tolbutamide, and repaglinide. Clin Pharmacol drug Dev. 2017;6:363–376. doi: 10.1002/cpdd.324 [DOI] [PubMed] [Google Scholar]
- 22.Glesby MJ, Aberg JA, Kendall MA, Fichtenbaum CJ, Hafner R, Hall S, Grosskopf N, Zolopa AR, Gerber JG; Adult AIDS Clinical Trials Group A5159 Protocol Team. Pharmacokinetic interactions between indinavir plus ritonavir and calcium channel blockers. Clin Pharmacol Ther. 2005;78:143–153. doi: 10.1016/j.clpt.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 23.Guo C, Pei QI, Tan H, Huang Z, Yuan H, Yang G. Effects of genetic factors on the pharmacokinetics and pharmacodynamics of amlodipine in primary hypertensive patients. Biomed Rep. 2015;3:195–200. doi: 10.3892/br.2014.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He YL, Ligueros-Saylan M, Sunkara G, Sabo R, Zhao C, Wang Y, Campestrini J, Pommier F, Dole K, Marion A, et al. Vildagliptin, a novel dipeptidyl peptidase IV inhibitor, has no pharmacokinetic interactions with the antihypertensive agents amlodipine, valsartan, and ramipril in healthy subjects. J Clin Pharmacol. 2008;48:85–95. doi: 10.1177/0091270007307880 [DOI] [PubMed] [Google Scholar]
- 25.Hsiao HL, Langenickel TH, Greeley M, Roberts J, Zhou W, Pal P, Rebello S, Rajman I, Sunkara G. Pharmacokinetic drug-drug interaction assessment between LCZ696, an angiotensin receptor neprilysin inhibitor, and hydrochlorothiazide, amlodipine, or carvedilol. Clin Pharmacol Drug Dev. 2015;4:407–417. doi: 10.1002/cpdd.183 [DOI] [PubMed] [Google Scholar]
- 26.Jung JA, Lee SY, Kim JR, Ko JW, Jang SB, Nam SY, Huh W. A pharmacokinetic and pharmacodynamic drug interaction between rosuvastatin and valsartan in healthy subjects. Drug Des Devel Ther. 2015;9:745–752. doi: 10.2147/DDDT.S76942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim CO, Sil Oh E, Kim C, Park MS. Pharmacokinetic interaction between amlodipine and lobeglitazone, a novel peroxisome proliferator-activated receptor-gamma agonist, in healthy subjects. Clin Ther. 2015;37:1999, 2006–e1. doi: 10.1016/j.clinthera.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 28.Kim JR, Kim S, Huh W, Ko JW. No pharmacokinetic interactions between candesartan and amlodipine following multiple oral administrations in healthy subjects. Drug Des Devel Ther. 2018;12:2475, 2483 doi: 10.2147/DDDT.S172568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuramoto K, Ichikawa S, Hirai A, Kanada S, Nakachi T, Ogihara T. Azelnidipine and amlodipine: a comparison of their pharmacokinetics and effects on ambulatory blood pressure. Hypertens Res. 2003;26:201–208. doi: 10.1291/hypres.26.201 [DOI] [PubMed] [Google Scholar]
- 30.Leenen FH, Coletta E. Pharmacokinetic and antihypertensive profile of amlodipine and felodipine-ER in younger versus older patients with hypertension. J Cardiovasc Pharmacol. 2010;56:669–675. doi: 10.1097/FJC.0b013e3181fc45bb [DOI] [PubMed] [Google Scholar]
- 31.Noh YH, Lim HS, Kim MJ, Kim YH, Choi HY, Sung HR, Jin SJ, Lim J, Bae KS. Pharmacokinetic interaction of telmisartan with s-amlodipine: an open-label, two-period crossover study in healthy Korean male volunteers. Clin Ther. 2012;34:1625–1635. doi: 10.1016/j.clinthera.2012.05.010 [DOI] [PubMed] [Google Scholar]
- 32.Park JW, Kim KA, Il Kim Y, Park JY. Pharmacokinetic and haemodynamic interactions between amlodipine and losartan in human beings. Basic Clin Pharmacol Toxicol. 2019;125:345–352. doi: 10.1111/bcpt.13244 [DOI] [PubMed] [Google Scholar]
- 33.Prasad PP, Stypinski D, Vyas KH, Gonasun L. Lack of interaction between modified-release fluvastatin and amlodipine in healthy subjects. Clin Drug Investig. 2004;24:323–331. doi: 10.2165/00044011-200424060-00002 [DOI] [PubMed] [Google Scholar]
- 34.Preston RA, Chung M, Gaffney M, Alonso A, Baltodano NM, Epstein M. Comparative pharmacokinetics and pharmacodynamics of amlodipine in hypertensive patients with and without type II diabetes mellitus. J Clin Pharmacol. 2001;41:1215–1224. doi: 10.1177/00912700122012760 [DOI] [PubMed] [Google Scholar]
- 35.Rohatagi S, Lee J, Shenouda M, Haworth S, Bathala MS, Allison M, Rubets I, Heyrman R, Noveck R, Salazar DE. Pharmacokinetics of amlodipine and olmesartan after administration of amlodipine besylate and olmesartan medoxomil in separate dosage forms and as a fixed-dose combination. J Clin Pharmacol. 2008;48:1309–1322. doi: 10.1177/0091270008322176 [DOI] [PubMed] [Google Scholar]
- 36.Seong SJ, Ohk B, Kang WY, Gwon MR, Kim BK, Cho S, Yang DH, Lee HW, Yoon YR. Pharmacokinetic drug interactions between amlodipine, valsartan, and rosuvastatin in healthy volunteers. Adv Ther. 2019;36:1642–1656. doi: 10.1007/s12325-019-00976-9 [DOI] [PubMed] [Google Scholar]
- 37.Son M, Guk J, Kim Y, Woo Chae D, Heo YA, Soh D, Park K. Pharmacokinetic interaction between rosuvastatin, telmisartan, and amlodipine in healthy male Korean subjects: a randomized, open-label, multiple-dose, 2-period crossover study. Clin Ther. 2016;38:1845–1857. doi: 10.1016/j.clinthera.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 38.Stangier J, Su CA, Roth W. Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients. J Int Med Res. 2000;28:149–167. doi: 10.1177/147323000002800401 [DOI] [PubMed] [Google Scholar]
- 39.Vaidyanathan S, Valencia J, Kemp C, Zhao C, Yeh CM, Bizot MN, Denouel J, Dieterich HA, Dole WP. Lack of pharmacokinetic interactions of aliskiren, a novel direct renin inhibitor for the treatment of hypertension, with the antihypertensives amlodipine, valsartan, hydrochlorothiazide (HCTZ) and ramipril in healthy volunteers. Int J Clin Pract. 2006;60:1343–1356. doi: 10.1111/j.1742-1241.2006.01164.x [DOI] [PubMed] [Google Scholar]
- 40.Beermann B, Groschinsky-grind M. Antihypertensive effect of various doses of hydrochlorothiazide and its relation to the plasma level of the drug. Eur J Clin Pharmacol. 1978;13:195–201. doi: 10.1007/bf00609982 [DOI] [PubMed] [Google Scholar]
- 41.Giudicelli JF, Richer C, Mattei A. Pharmacokinetics and biological effects of captopril and hydrochlorothiazide after acute and chronic administration either alone or in combination in hypertensive patients. Br J Clin Pharmacol. 1987;23suppl 151S–63S. doi: 10.1111/j.1365-2125.1987.tb03122.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ismail Z, Triggs EJ, Smithurst BA, Parke W. The pharmacokinetics of amiloride-hydrochlorothiazide combination in the young and elderly. Eur J Clin Pharmacol. 1989;37:167–171. doi: 10.1007/BF00558226 [DOI] [PubMed] [Google Scholar]
- 43.Kher A, Fillastre JP, Fourtillan JB, Lefebvre MA, Ingrand I. Pharmacokinetics of a fixed combination of sotalol and hydrochlorothiazide in hypertensive patients with moderate renal insufficiency. Eur J Clin Pharmacol. 1984;27:361–362. doi: 10.1007/BF00542176 [DOI] [PubMed] [Google Scholar]
- 44.Lindamood C, Ortiz S, Shaw A, Rackley R, Gorski JC. Effects of commonly administered agents and genetics on nebivolol pharmacokinetics: drug-drug interaction studies. J Clin Pharmacol. 2011;51:575–585. doi: 10.1177/0091270010370846 [DOI] [PubMed] [Google Scholar]
- 45.Sabanathan K, Castleden CM, Adam HK, Ryan J, Fitzsimons TJ. A comparative study of the pharmacokinetics and pharmacodynamics of atenolol, hydrochlorothiazide and amiloride in normal young and elderly subjects and elderly hypertensive patients. Eur J Clin Pharmacol. 1987;32:53–60. doi: 10.1007/BF00609957 [DOI] [PubMed] [Google Scholar]
- 46.McCrea JB, Lo MW, Tomasko L, Lin CC, Hsieh JY, Capra NL, Goldberg MR. Absence of a pharmacokinetic interaction between losartan and hydrochlorothiazide. J Clin Pharmacol. 1995;35:1200–1206. doi: 10.1002/j.1552-4604.1995.tb04047.x [DOI] [PubMed] [Google Scholar]
- 47.Weir SJ, Dimmitt DC, Lanman RC, Morrill MB, Geising DH. Steady-state pharmacokinetics of diltiazem and hydrochlorothiazide administered alone and in combination. Biopharm Drug Dispos. 1998;19:365–371. doi: 10.1002/(sici)1099-081x(199809)19:6<365::aid-bdd112>3.0.co;2-r [DOI] [PubMed] [Google Scholar]
- 48.Ayalasomayajula S, Langenickel T, Pal P, Boggarapu S, Sunkara G. Clinical pharmacokinetics of sacubitril/valsartan (LCZ696): a novel angiotensin receptor-neprilysin inhibitor. Clin Pharmacokinet. 2017;56:1461–1478. doi: 10.1007/s40262-017-0543-3 [DOI] [PubMed] [Google Scholar]
- 49.Ayalasomayajula S, Schuehly U, Pal P, Chen F, Zhou W, Sunkara G, Langenickel TH. Effect of the angiotensin receptor-neprilysin inhibitor sacubitril/valsartan on the pharmacokinetics and pharmacodynamics of a single dose of furosemide. Br J Clin Pharmacol. 2018;84:926–936. doi: 10.1111/bcp.13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen CL, Desai-Krieger D, Ortiz S, Kerolous M, Wright HM, Ghahramani P. A single-center, open-label, 3-way crossover trial to determine the pharmacokinetic and pharmacodynamic interaction between nebivolol and valsartan in healthy volunteers at steady state. Am J Ther. 2015;22:e130–e140. doi: 10.1097/MJT.0000000000000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad PP, Yeh CM, Gurrieri P, Glazer R, McLeod J. Pharmacokinetics of multiple doses of valsartan in patients with heart failure. J Cardiovasc Pharmacol. 2002;40:801–807. doi: 10.1097/00005344-200211000-00018 [DOI] [PubMed] [Google Scholar]
- 52.Sunkara G, Reynolds CV, Pommier F, Humbert H, Yeh C, Prasad P. Evaluation of a pharmacokinetic interaction between valsartan and simvastatin in healthy subjects. Curr Med Res Opin. 2007;23:631–640. doi: 10.1185/030079906X167471 [DOI] [PubMed] [Google Scholar]
- 53.Ågesen FN, Weeke PE, Tfelt-Hansen P, Tfelt-Hansen J; for ESCAPE-NET. Pharmacokinetic variability of beta-adrenergic blocking agents used in cardiology. Pharmacol Res Perspect. 2019;7:e00496 doi: 10.1002/prp2.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peeters LEJ, Feyz L, Boersma E, Daemen J, van Gelder T, Koch BCP, Versmissen J. Clinical applicability of monitoring antihypertensive drug levels in blood. Hypertens (Dallas, Tex 1979). 2020;76:80–86. doi: 10.1161/HYPERTENSIONAHA.120.15038 [DOI] [PubMed] [Google Scholar]
- 55.Williams RL, Mordenti J, Upton RA, Lin ET, Gee WL, Blume CD, Benet LZ. Effects of formulation and food on the absorption of hydrochlorothiazide and triamterene or amiloride from combination diuretic products. Pharm Res. 1987;4:348–352. doi: 10.1023/a:1016409606936 [DOI] [PubMed] [Google Scholar]
- 56.Sandbaumhuter FA, Haschke M, Vogt B, Bohlender JM. Indexed plasma drug concentrations for drug adherence screening in hypertensive patients. Ann Cardiol Angeiol (Paris). 2018;67:119–126. doi: 10.1016/j.ancard.2018.04.020 [DOI] [PubMed] [Google Scholar]
- 57.Ritscher S, Hoyer M, Wunder C, Obermuller N, Toennes SW. Evaluation of the dose-related con centration approach in therapeutic drug monitoring of diuretics and beta-blockers - drug classes with low adherence in antihypertensive therapy. Sci Rep. 2019;9:15652 doi: 10.1038/s41598-019-52164-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peeters LEJ, Kester MP, Feyz L, Van Den Bemt PMLA, Koch BCP, Van Gelder T, Versmissen J. Pharmacokinetic and pharmacodynamic considerations in the treatment of the elderly patient with hypertension. Expert Opin Drug Metab Toxicol. 2019;15:287–297. doi: 10.1080/17425255.2019.1588249 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


