Abstract
Background:
The coronavirus disease 2019 (COVID-19) pandemic is responsible for millions of infections worldwide, and a substantial number of these patients will be admitted to the intensive care unit (ICU). Our objective was to describe the characteristics, outcomes and management of critically ill patients with COVID-19 pneumonia at a single designated pandemic centre in Montréal, Canada.
Methods:
A descriptive analysis was performed on consecutive critically ill patients with COVID-19 pneumonia admitted to the ICU at the Jewish General Hospital, a designated pandemic centre in Montréal, between Mar. 5 and May 21, 2020. Complete follow-up data corresponding to death or discharge from hospital health records were included to Aug. 4, 2020. We summarized baseline characteristics, management and outcomes, including mortality.
Results:
A total of 106 patients were included in this study. Twenty-one patients (19.8%) died during their hospital stay, and the ICU mortality was 17.0% (18/106); all patients were discharged home or died, except for 4 patients (2 awaiting a rehabilitation bed and 2 awaiting long-term care). Twelve of 65 patients (18.5%) requiring mechanical ventilation died. Prone positioning was used in 29 patients (27.4%), including in 10 patients who were spontaneously breathing; no patient was placed on extracorporeal membrane oxygenation. High-flow nasal cannula was used in 51 patients (48.1%). Acute kidney injury was the most common complication, seen in 20 patients (18.9%), and 12 patients (11.3%) required renal replacement therapy. A total of 53 patients (50.0%) received corticosteroids.
Interpretation:
Our cohort of critically ill patients with COVID-19 had lower mortality than that previously described in other jurisdictions. These findings may help guide critical care decision-making in similar health care systems in further COVID-19 surges.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in December 2019. As of Nov. 13, 2020, millions of infections have occurred, and COVID-19 has claimed more than 1.3 million lives, with an overall mortality rate of about 3% and mortality of up to 60% in the critically ill.1–6 Limited information is available to describe the outcomes of patients admitted to intensive care units (ICUs) in Canada.
The Jewish General Hospital was the first designated pandemic centre for adults in Montréal, Canada, the epicentre of COVID-19 in Canada during the first wave of the pandemic.7 The first positive case of SARS-CoV-2 infection in the province of Quebec was diagnosed on Feb. 27, 2020. The Jewish General Hospital admitted Quebec’s first patient with COVID-19 to the ICU on Mar. 5. As of May 27, 2020, the hospital had admitted 17% of all ICU patients with COVID-19 in the city.8
Severe COVID-19 pneumonia is associated with progressive dyspnea, increasing hypoxemia and acute respiratory distress syndrome (ARDS), often occurring after 7–10 days of symptom onset, and requires ICU admission for ongoing respiratory support.9 Management of severe COVID-19 pneumonia is similar to that of other types of viral pneumonia causing respiratory failure and includes standard lung protective mechanical ventilation, prone positioning and consideration for neuromuscular blockade, inhaled nitric oxide and extracorporeal membrane oxygenation (ECMO).4
Our objective was to describe the characteristics, outcomes and management of critically ill patients with COVID-19 pneumonia in the ICU of a designated pandemic centre.
Methods
Design and participants
A retrospective chart review was performed on all consecutive patients with SARS-CoV-2 infection, as determined by a positive polymerase chain reaction (PCR) test, who were admitted to the ICU with severe or critical COVID-19 pneumonia, between Mar. 5 and May 21, and who had complete follow-up data available to Aug. 4, 2020. Severe COVID-19 pneumonia is defined as adults with clinical signs of pneumonia with a respiratory rate greater than 30 breaths/min or an oxygen saturation measurement of less than 90% on room air. Critical disease is associated with ARDS or sepsis.10
We had no strict criteria for ICU admission, and admission decisions were left at the discretion of the treating intensivist. Consultation with intensivists from the emergency department or COVID-19 wards was encouraged when patients required more than 5 L/min of oxygen or were showing rapid clinical deterioration. Patients who tested positive for SARS-CoV-2 infection, and were asymptomatic for COVID-19 pneumonia and admitted for other medicosurgical reasons were excluded from the study.
Setting
This study was conducted at the Jewish General Hospital in Montréal, a 637-bed, university-affiliated, tertiary care adult hospital. The hospital was designated by the provincial government as the initial receiving centre for all adult patients with COVID-19 in Montréal during the first wave of pandemic admissions. The Jewish General Hospital ICU is a mixed medical–surgical unit with a maximum baseline capacity of 24 patients.
During the following 3 months, many changes occurred in our institution and in the province. All elective surgeries were cancelled at our hospital on Mar. 16, 2020, and by the second week of March, the Quebec government declared a provincewide state of emergency. Quarantine and social distancing measures were initiated, generalized closures were implemented and, by the third week of March, only essential services were kept open.
To respond to the pandemic, a plan was implemented to increase the number of available ICU beds at the Jewish General Hospital from 24 to 50. Because of the surge of patients in our ICU, on Mar. 21, the province expanded the list of designated hospitals in the Montréal region that could admit patients with COVID-19.
Program processes and interventions
Staffing in the Jewish General Hospital ICU consisted of attending physicians with critical care training, critical care residents and other trainees. As the ICU census expanded, by Mar. 28, 2020, critical care and anesthesia attending physicians provided in-house coverage 24 hours per day. Resident physician, ICU nursing and respiratory therapy capacity was increased by redeployment from other areas of the hospital, which allowed nurse-to-patient ratios to be maintained at a maximum of 1:1.2. All patients were cared for in single rooms, and all health care workers in the unit had access to personal protective equipment.
From Mar. 5 to Apr. 24, 2020, our initial COVID-19 institutional guideline, established by infectious diseases specialists, recommended that all patients with severe COVID-19 pneumonia be prescribed azithromycin 500 mg daily for 5 days and hydroxychloroquine 200 mg taken orally 3 times per day for 10 days. All ICU patients received treatment with standard weight-based thromboprophylaxis with low-molecular-weight heparin or, if contraindicated, unfractionated heparin. However, intermediate dosing (e.g., dalteparin 7500 units by subcutaneous injection daily) was permitted at the discretion of the treating physician. Thromboembolic events were diagnosed using computed tomography pulmonary angiography or venous duplex ultrasonography. These examinations were performed as clinically indicated at the discretion of the treating physician.
Management of mechanical ventilation was at the discretion of the treating physician. In our ICU, pressure-regulated volume control was the default mode used for most patients with ARDS, with a ventilation goal of 6 mL/kg predicted body weight. Fraction of inspired oxygen (Fio2) titration for intubated patients was supported using either the ALVEOLI low or high positive end-expiratory pressure (PEEP) table, depending on the patient’s oxygen needs and response to initial PEEP titration.11
Prone positioning was generally initiated when the Fio2 requirement was sustained above 65%. Prone positioning was also used in nonintubated patients receiving high-flow nasal cannula (HFNC) at the discretion of the treating intensivist to minimize the required Fio2. Neuromuscular blockade was used when required to maintain patient–ventilator synchrony and plateau pressures less than 30 cm H2O, after maximizing sedation and opioids. The use of noninvasive mask ventilation was not approved for use in patients with COVID-19 during the study period, and our institution does not provide HFNC outside of the ICU.
Variables and outcomes
Collected variables included demographic information, comorbidities (including Charlson Comorbidity Index score),12 initial vital signs, laboratory results and COVID-19-specific symptoms. Scores measuring severity of illness, including an Acute Physiology And Chronic Health Evaluation II (APACHE II) score13 and a modified Sequential Organ Failure Assessment (SOFA) score, were recorded.14 Data on interventions, including medical therapies, oxygenation methods and ventilation strategies, were collected for all patients (Appendix 1, available at www.cmajopen.ca/content/8/4/E788/suppl/DC1). Clinical outcomes, including length of stay, morbidity and mortality, were also determined. ICU mortality was defined as a death occurring during the ICU stay. Hospital mortality was defined as death occurring anytime during the ICU or subsequent ward stay. Morbidities recorded included secondary infections, thromboembolic events, renal failure and tracheostomies.
The local ICU daily census of ICU patients with and without COVID-19 was recorded. The ICU census of COVID-19 patients in the province of Quebec was also recorded.
Data sources and extraction
All clinical data were obtained from the hospital’s electronic medical records. Data extraction was performed by 2 reviewers (S.D. and S.S.Y.) independently and in duplicate using a standardized form. Disagreement between the reviewers was resolved through a consensus process. If the 2 reviewers could not agree, the final decision went to a third reviewer (J.L.). To ensure the interrater reliability, an independent reviewer (J.L.) randomly examined 20% of all study patient files.
Census data were collected locally from our hospital’s information technology department and provincially from the website of Quebec’s ministry of health and social services (Ministère de la Santé et des Services sociaux).8
Statistical analysis
Baseline characteristics were summarized by counts and percentages or medians and interquartile ranges (IQRs), as appropriate. All analyses were performed using SAS version 9.4 and STATA version 15.
Ethics approval
This study was approved by the local research ethics board of Centre intégré universitaire en santé et services sociaux (CIUSSS) West-Central Montreal, Jewish General Hospital.
Results
During the study period, we admitted 106 patients to the ICU with COVID-19 pneumonia, out of 461 (23.0%) hospital admissions for COVID-19 in our institution. At the time of the analysis, only 4 patients (3.8%) remained in hospital, with 2 patients awaiting a rehabilitation bed and 2 awaiting a long-term care bed. A total of 76 patients (71.7%) were admitted directly from the emergency department. Four patients who tested positive for SARS-CoV-2 infection and were admitted for other medical or surgical reasons were excluded from our analysis.
Table 1 presents the demographic characteristics and baseline clinical and laboratory characteristics of the patients. Median age was 66 (IQR 54–74) years, and 64 (60.4%) of the patients were male. Eight patients (7.6%) were not candidates for intubation because of advanced directives. The median Charlson Comorbidity Index score was 3 (IQR 1–4), the median SOFA score on admission was 5 (IQR 3–8) and the median APACHE II score was 15 (IQR 11–20).
Table 1:
Baseline demographic and clinical characteristics of patients admitted to the intensive care unit with coronavirus disease 2019 pneumonia
| Characteristic | No. (%) of patients* n = 106 |
|---|---|
| Age, yr, median (IQR) | 66 (54–74) |
| Sex, male | 64 (60.4) |
| Body mass index, median (IQR), n = 76 | 29.4 (25.3–33.8) |
| Pregnant | 3 (2.8) |
| “No intubation” advanced directive | 8 (7.6) |
| Nursing home resident | 11 (10.4) |
| Charlson Comorbidity Index score, median (IQR) | 3 (1–4) |
| SOFA score,† median (IQR) | 5 (3–8) |
| APACHE II score, median (IQR) | 15 (11–20) |
| Pao2/Fio2,‡ median (IQR), n = 63 | 133 (95–174) |
| Comorbidities | |
| Hypertension | 55 (51.9) |
| Diabetes mellitus | 30 (28.3) |
| Coronary artery disease | 15 (14.2) |
| Obesity, n = 89 | 41 (46.1) |
| Chronic kidney disease | 10 (9.4) |
| Chronic obstructive lung disease | 8 (7.6) |
| Asthma | 10 (9.4) |
| Malignancy | 10 (9.4) |
| Immunocompromised§ | 12 (11.3) |
Note: APACHE II = Acute Physiology And Chronic Health Evaluation II, Fio2 = fraction of inspired oxygen, IQR = interquartile range, Pao2 = partial pressure of oxygen, SOFA = Sequential Organ Failure Assessment.
Unless stated otherwise.
SOFA score excluding the neurologic component; worst SOFA score calculated after 24 h of admission.
Worst Pao2/FiO2 calculated 24 h postintubation.
Diagnosis of HIV or treatment with chronic corticosteroid, chemotherapy or biologic medication.
Interventions
Sixty-five patients (61.3%) required endotracheal intubation, with 24 patients (22.6%) requiring neuromuscular blockade. HFNC was used for 51 patients (48.1%), including its use as initial respiratory support in 56.9% (29/51); and 39.2% (20/51) receiving HFNC did not require intubation. A total of 29 patients (27.4%) underwent prone positioning; 13 of these patients (44.8%) were spontaneously breathing. Three of 13 spontaneously breathing patients who underwent prone positioning (23.1%) were subsequently intubated. No patient required ECMO.
The most frequently prescribed medical therapies were azithromycin (94, 88.7%), hydroxychloroquine (75, 70.8%), broad-spectrum antibiotics (71, 67.0%) and corticosteroids (53, 50.0%). The most common corticosteroid used was dexamethasone (33/53, 62.3%) (Table 2).
Table 2:
Critical care management of patients admitted to the intensive care unit with coronavirus disease 2019 pneumonia
| Intervention | No. (%) of patients* n = 106 |
|---|---|
| Respiratory management | |
| Invasive mechanical ventilation | 65 (61.3) |
| High PEEP† | 22 (33.9) |
| PEEP at 24 h postintubation, median (IQR), cm H2O, n = 63 | 10 (8–12) |
| Prone positioning | 29 (27.4) |
| Intubated | 19 (65.5) |
| Not intubated | 10 (34.5) |
| HFNC | 51 (48.1) |
| Initial respiratory support‡ | 29 (56.9) |
| Postextubation support | 24 (47.1) |
| Medication | |
| Neuromuscular blockade | 24 (22.6) |
| Corticosteroids | 53 (50.0) |
| Symptom onset to receipt of corticosteroids, d, median (IQR) | 13 (10–17) |
| Azithromycin | 94 (88.7) |
| Hydroxychloroquine | 75 (70.8) |
| Tocilizumab | 11 (10.4) |
| Oseltamivir | 8 (7.5) |
| Lopinavir–ritonavir | 6 (5.7) |
Note: HFNC = high-flow nasal cannula, IQR = interquartile range, PEEP = positive end-expiratory pressure.
Unless stated otherwise.
High PEEP defined as PEEP > 15 cm H2O during mechanical ventilation.
Two patients had HFNC for both indications.
Outcomes
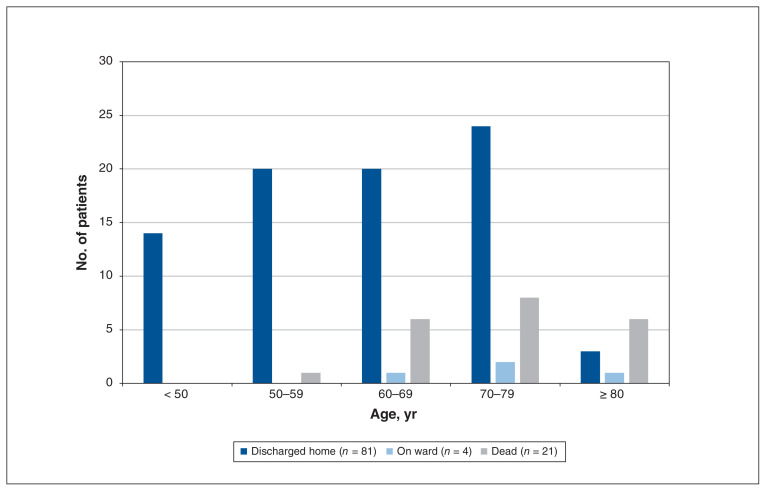
Our ICU mortality was 17.0% (18/106) and hospital mortality was 19.8% (21/106). Hospital mortality fell to 13.3% (13/98) when patients with an advanced directive order for no intubation were excluded. No patient younger than 50 years died; 3 patients (2.8%) died on the medical ward (Figure 1).
Figure 1:
Disposition of 106 patients admitted to the intensive care unit with coronavirus disease 2019 pneumonia, by age.
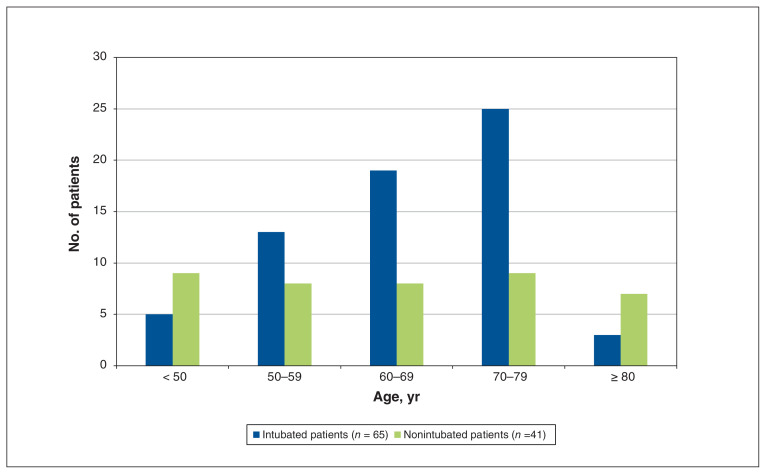
ICU mortality in patients requiring mechanical ventilation was 18.5% (12/65), with most ventilated patients being older than 60 years (Figure 2). The median duration of ventilation was 12 (IQR 8–18) days, with 41.5% (27/65) of patients requiring mechanical ventilation for more than 14 days. The median ICU and hospital length of stay were 10 (IQR 4–17) and 19 (IQR 10–31) days, respectively.
Figure 2:
Intubation status of 106 patients admitted to the intensive care unit with coronavirus disease 2019 pneumonia, by age.
The most common complication was acute kidney injury (20/106, 18.9%), and thromboembolic events occurred in 15.1% (16/106) of patients (Table 3).
Table 3:
Outcomes and complications in critically ill patients with coronavirus disease 2019 pneumonia
| Variable | No. (%) of patients* n = 106 |
|---|---|
| Outcomes | |
| Hospital mortality | 21 (19.8) |
| ICU mortality | 18 (17.0) |
| ICU mortality in mechanically ventilated patients, n = 65 | 12 (18.5) |
| Currently on medical ward† | 4 (3.8) |
| Duration of mechanical ventilation, d, median (IQR) | 12 (8–18) |
| ICU length of stay, d, median (IQR) | 10 (4–17) |
| Hospital length of stay, d, median (IQR) | 19 (10–31) |
| Tracheostomy | 7 (6.6) |
| Complications | |
| ICU-acquired infection‡ | 22 (20.8) |
| Atrial fibrillation | 24 (22.6) |
| Acute kidney injury§ | 20 (18.9) |
| Renal replacement therapy | 12 (11.3) |
| Thrombotic events¶ | 16 (15.1) |
| Pulmonary embolism or deep vein thrombosis | 12 (11.3) |
| Ischemic stroke | 7 (6.6) |
| Peripheral arterial thrombosis | 1 (0.9) |
Note: ICU = intensive care unit, IQR = interquartile range.
Unless stated otherwise.
Two patients awaiting rehabilitation and 2 patients awaiting long-term care.
Positive culture results with pathogenic organisms.
Greater than 2 times baseline creatinine.
Thromboembolic event confirmed by imaging; 2 patients had > 1 thrombotic events.
Proportion of provincial ICU load
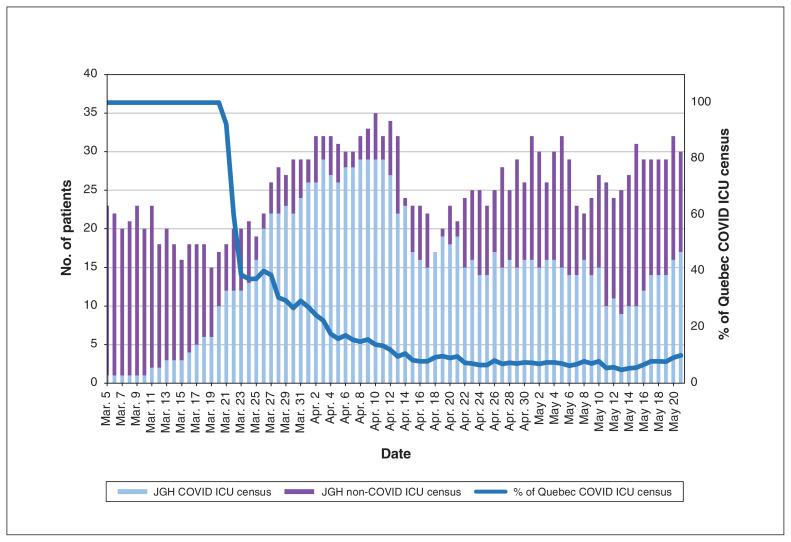
The Jewish General Hospital ICU admitted most of the province’s critically ill patients with COVID-19 pneumonia in the first few weeks of the pandemic. This proportion decreased to eventually stabilize at 10% of all provincial ICU admissions for COVID-19 pneumonia, as other ICUs in Montréal were activated by the provincial pandemic protocol (Figure 3).
Figure 3:
Daily coronavirus disease 2019 (COVID-19) census. Note: ICU = intensive care unit, JGH = Jewish General Hospital.
Interpretation
In our descriptive analysis of critically ill patients with COVID-19, we found an ICU mortality of 17.0%. Despite comparable baseline characteristics, our cohort had a substantially lower mortality rate than those from China, Europe and the United States.3,5,15,16 Although the overall mortality rate is similar to that from another Canadian study (in which 24% of patients were still admitted to hospital), in our cohort, no patients remained in ICU at the time of analysis, providing robust data of mortality rates.17
Our management practices evolved as we gained clinical experience and new data emerged in the literature. We avoided the use of HFNC on patients with COVID-19 early in the pandemic because of the theoretical risk of increased aerosolization and the initial paucity of data on how SARS-CoV-2 is transmitted.18,19 Although it was permitted to use HFNC based on the original protocol, most clinicians were reluctant to do so during the first month owing to the theoretical risk of increased aerosolization and the initial paucity of data on how SARS-CoV-2 is transmitted. However, as literature emerged showing a minimal associated risk to health care workers, a substantial proportion of patients were treated with HFNC.20 All patients receiving HFNC were treated in negative-pressure rooms with standard personal protective equipment and KN95 masks. Importantly, only 3 health care workers in the ICU tested positive for SARS-CoV-2 infection during the study period, all of whom had an alternative higher risk exposure outside of the ICU (Stephanie Petizian, ICU nursing manager, Jewish General Hospital, Montréal: personal communication, 2020). As we began using HFNC more often, we noted that a large proportion of the patients receiving HFNC never required intubation, which is consistent with the current literature on respiratory failure in patients without COVID-19.21
A large proportion of our patients received corticosteroids in the context of cytokine release syndrome or severe ARDS. Corticosteroids were given late (median 13 d) after the start of symptoms, and most patients were prescribed a regimen of dexamethasone as described by Villar and colleagues.22 Corticosteroid use for coronavirus-associated respiratory failure and cytokine release syndrome has been controversial; however, a short course of corticosteroids given after the peak of viral shedding may be warranted, as the risk of worsening infection and outcome appears low.23–28 Indeed, the results of the recently published Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial show that low-dose dexamethasone improves mortality in severe COVID-19 and is now considered standard of care.29 It remains possible that our frequent treatment with corticosteroids and tocilizumab was responsible for the shorter duration of mechanical ventilation that we observed as compared with that observed by Mitra and colleagues.17
At the outset, our local infectious diseases guideline recommended hydroxychloroquine for all patients based on preliminary reports of efficacy;30 however, this was subsequently discontinued on Apr. 24, 2020, after the emergence of data showing lack of benefit and ongoing questions of safety.31,32 Although no patient died from a malignant arrhythmia, it remains possible patients may have experienced other adverse effects, including delirium or drug–drug interactions.
With use of HFNC, standard lung-protective ventilation, prone positioning and corticosteroids, there were no patients who met local criteria for ECMO. Although it remains possible that some patients could have benefited from early ECMO use, current reports suggest a very high mortality associated with the use of ECMO in COVID-19.33–35 Moreover, rational use of such a resource-intensive therapy is critical in the context of a pandemic.33–35
During the study period, although local capacity was at times under stress, ICU resources were never completely overwhelmed, which may have contributed to our relatively low ICU mortality. Redeployment of nursing and respiratory therapists from other areas of the hospital to increase ICU capacity was critical. Another possible contributor to the lower mortality observed in both our study and that of another Canadian cohort is the publicly funded Canadian health system, which strives to minimize financial and other socioeconomic barriers to accessing health services.17 Reports from the US show high rates of death in marginalized populations and, in particular, Black Americans, who have increased barriers to health care.36,37
Limitations
This study has several important limitations. Given the observational nature of our study, any causal link between our interventions and the observed outcomes is speculative. The small sample precludes any ability to link specific therapies or changes in practice and mortality. Thromboembolic events occurred in an important proportion of patients in our descriptive analysis; however, individual dosing of thromboprophylaxis was not available for analysis, and patients were not systematically screened for thromboembolic events, thereby limiting our interpretation. It is also a single-centre study in a tertiary care academic hospital in Canada, and the results obtained may not be generalizable to other centres.
Conclusion
The ICU mortality of 17% in our cohort of critically ill patients with COVID-19 at a Canadian tertiary care centre appears to be lower than that reported in other countries. These results may help clinicians and health care administrators with decisions surrounding critical care management of COVID-19. Further studies are urgently needed to help understand the appropriate use of mechanical ventilation strategies, and the role of HFNC, prone positioning, and antiviral, antithrombotic or other novel therapies in COVID-19 that may have contributed to our findings.
Supplementary Material
Acknowledgements
The authors acknowledge the dedicated work of the entire ICU team, including nurses, respiratory therapists, medical residents, orderlies, pharmacy staff, housekeeping staff and clerical staff, for their tireless and devoted work during this unprecedented medical event. The authors also acknowledge assistance from the Department of Anesthesia and the Division of Infectious Diseases at the Jewish General Hospital as well as the hospital and provincial health care leadership.
Footnotes
Competing interests: Paul Warshawsky reports personal fees from Gilead Sciences Canada, outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Stephen Yang, Jed Lipes, Sandra Dial, Blair Schwartz and Dev Jayaraman contributed to the conception, design, data analysis and drafting of the manuscript. Denny Laporta, Evan Wong, Craig Baldry, Paul Warshawsky, Patricia McMillan, David Hornstein and Michel de Marchie contributed to revising the manuscript and providing insight into the interpretation of results. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work. Stephen Yang and Jed Lipes are co–first authors.
Data sharing: A deidentified data set is held on a secure server at Jewish General Hospital, and data requests may be granted provided there is an appropriate data-sharing agreement.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/8/4/E788/suppl/DC1.
References
- 1.Baud D, Qi X, Nielsen-Saines K, et al. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20:773. doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–70. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–81. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murthy S, Gomersall CD, Fowler RA. Care for critically ill patients with COVID-19. JAMA. 2020;323:1499–500. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVID-19 coronavirus pandemic. Worldometer. [accessed 2020 Nov. 13]. Available: www.worldometers.info/coronavirus.
- 7.Coronavirus disease (COVID-19): outbreak update. Ottawa: Public Health Agency of Canada; [accessed 2020 Sept. 13]. updated 2020 Oct. 20. Available: www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection.html#a1. [Google Scholar]
- 8.Coronavirus (COVID-19). Ministère de la Santé et des Services sociaux. [accessed 2020 Sept. 13]. updated 2020 Oct. 23. Available: https://msss.gouv.qc.ca/professionnels/maladies-infectieuses/coronavirus-2019-ncov.
- 9.Wang D, Yin Y, Hu C, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care. 2020;24:188. doi: 10.1186/s13054-020-02895-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical management of COVID-19 interim guidance. Geneva: World Health Organization; 2020. [accessed 2020 Sept. 13]. Available: www.who.int/publications/i/item/clinical-management-of-covid-19. [Google Scholar]
- 11.Brower RG, Lanken PN, MacIntyre N, et al. National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 14.Lambden S, Laterre PF, Levy MM, et al. The SOFA score — development, utility and challenges of accurate assessment in clinical trials. Crit Care. 2019;23:374. doi: 10.1186/s13054-019-2663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA. 2020;323:1612–4. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auld SC, Caridi-Scheible M, Blum JM, et al. Emory COVID-19 Quality and Clinical Research Collaborative. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019. Crit Care Med. 2020;48:e799–804. doi: 10.1097/CCM.0000000000004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitra AR, Fergusson NA, Lloyd-Smith E, et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: a case series. CMAJ. 2020;192:E694–701. doi: 10.1503/cmaj.200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loh N-HW, Tan Y, Taculod J, et al. The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can J Anaesth. 2020;67:893–4. doi: 10.1007/s12630-020-01634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran K, Cimon K, Severn M, et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Yang B, Li Q, et al. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–77. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45:563–72. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 22.Villar J, Ferrando C, Martínez D, et al. dexamethasone in ARDS network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–76. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 23.Gomersall CD. Pro/con clinical debate: steroids are a key component in the treatment of SARS. Pro: Yes, steroids are a key component of the treatment regimen for SARS. Crit Care. 2004;8:105–7. doi: 10.1186/cc2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee N, Chan KCA, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–9. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B, Li M, Zhou Z, et al. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villar J, Confalonieri M, Pastores SM, et al. Rationale for prolonged corticosteroid treatment in the acute respiratory distress syndrome caused by coronavirus disease 2019. Crit Care Explor. 2020;2:e0111. doi: 10.1097/CCE.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang SS, Lipes J. Corticosteroids for critically ill COVID-19 patients with cytokine release syndrome: a limited case series. Can J Anaesth. 2020;67:1462–4. doi: 10.1007/s12630-020-01700-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye Z, Wang Y, Colunga-Lozano LE, et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020;192:E756–67. doi: 10.1503/cmaj.200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19 — preliminary report. N Engl J Med. 2020 Jul 17; doi: 10.1056/NEJMoa2021436. [Epub ahead of print] [DOI] [Google Scholar]
- 30.Gautret P, Lagier J-C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juurlink DN. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020;192:E450–3. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahévas M, Tran V-T, Roumier M, et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henry BM, Lippi G. Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): pooled analysis of early reports. J Crit Care. 2020;58:27–8. doi: 10.1016/j.jcrc.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parhar KKS, Lequier L, Blackwood J, et al. Optimizing provision of extracorporeal life support during the COVID-19 pandemic: practical considerations for Canadian jurisdictions. CMAJ. 2020;192:E372–4. doi: 10.1503/cmaj.200448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X, Cai S, Luo Y, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019 — induced acute respiratory distress syndrome: a multicenter descriptive study. Crit Care Med. 2020;48:1289–95. doi: 10.1097/CCM.0000000000004447. [DOI] [PubMed] [Google Scholar]
- 36.Ferdinand KC, Nasser SA. African-American COVID-19 mortality: a sentinel event. J Am Coll Cardiol. 2020;75:2746–8. doi: 10.1016/j.jacc.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okonkwo NE, Aguwa UT, Jang M, et al. COVID-19 and the US response: accelerating health inequities. BMJ Evid Based Med. 2020 Jun 3; doi: 10.1136/bmjebm-2020-111426. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.