Abstract
Objective.
Tinnitus is a condition that causes distress and impairment across cognitive, functional, and psychiatric spectra. In the psychiatric realm, tinnitus has long been associated with depression. To better characterize the co-occurrence of depression and tinnitus, we performed a systematic review of the prevalence of depression among patients with tinnitus.
Data Sources.
We comprehensively examined original studies reporting the prevalence of depression in adult populations with tinnitus, as indexed in the PubMed and Web of Science databases and published from January 2006 to August 2016.
Review Methods.
All identified articles were reviewed independently by 2 researchers, with a third reviewer for adjudication. Included studies were evaluated for threats to validity across 3 domains—representativeness, response rate, and ascertainment of outcome—on a 4-point modified Newcastle-Ottawa Quality Assessment Scale.
Results.
Twenty-eight studies were included, representing 15 countries and 9979 patients with tinnitus. Among the included studies, the median prevalence of depression was 33%, with an interquartile range of 19% to 49% and an overall range of 6% to 84%. Studies were high quality overall, with a mean score of 3.3 (SD = 0.76), and 89% utilized a validated tool to ascertain depression.
Conclusions.
We conducted one of the largest contemporary comprehensive reviews, which suggests a 33% prevalence of depression among patients with tinnitus. Our review reaffirms that a substantial proportion of patients with tinnitus have depression, and we recommend that all who treat tinnitus should screen and treat their patients for depression, if present.
Keywords: tinnitus, depression, quality assessment, systematic review
Tinnitus is the perception of sound in the absence of an external auditory stimulus.1 It is very common; a recent study conducted in the United States reported that tinnitus affects 9.6% of the adult population.2 Population-based studies of other nations demonstrated similar prevalence estimates ranging from 4.6% to 30%.3–6 Tinnitus can manifest differently in regard to laterality, loudness, and type of sound. In turn, tinnitus can have varying impacts on the quality of life, from mild annoyance to moderate functional impairment and, in extreme cases, suicide.7
Available therapies for tinnitus have limited success and are primarily focused on mitigating the impact of the sound on patients’ lives. Success rates of these tools are largely dependent on the patient’s perception of severity.8 As such, characterizing and quantifying the level of distress and impairment across different cognitive, functional, and psychiatric spectra within tinnitus experiencers has long been an active and necessary area of study. A number of studies demonstrated an association between tinnitus and a variety of psychological and psychiatric disorders, most commonly depression.9,10
The prevailing theory behind the relationship between tinnitus and depression is that tinnitus triggers depression in depression-prone individuals.11 Another theory is that the relationship is bidirectional, with a cyclical process unfolding in which psychological processes contribute to worsening awareness and severity of tinnitus.11 Tinnitus is also a known side effect of a number of antidepressant agents. Although the exact mechanism of the relationship between tinnitus and depression remains a point of debate—and the reported prevalence of depression and tinnitus has varied considerably—there is a broad evidence base demonstrating the association between depression and tinnitus.9,10 In this study, we aimed to systematically and comprehensively review the current evidence on the prevalence of depression among patients with tinnitus to quantify the extent of depressive distress in this population.
Methods
This review adhered to the guidelines outlined by the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses).12 This includes a 27-item checklist and 4-phase flow diagram designed to ensure standard undertaking and reporting of systematic reviews and meta-analyses.
Eligible studies were original studies that reported the prevalence of depression diagnoses or depressive symptoms sensu lato among adult patients (>18 years) with tinnitus. Studies were included regardless of clinical setting, provided that tinnitus was a prominent symptom (eg, vestibular schwannoma) or was the primary complaint. We excluded studies that were not original full-length research articles, such as poster abstracts, review articles, meta-analyses, and commentaries. Case reports and studies in which there were < 5 patients were also excluded. In addition, articles deemed to be unrelated and excluded were as follows: animal studies, studies that did not describe the prevalence of depression in tinnitus, and studies that reported a prevalence but excluded patients on the basis of a psychiatric condition. Last, we excluded foreign language studies that would require full-text review and did not have a suitable translation.
Search Strategy
We performed a comprehensive literature search of articles on tinnitus and depression that were indexed in PubMed and Web of Science and published from January 2006 to August 2016 (10 full calendar years from onset of study).
For the PubMed database, we employed keywords from the Medical Subject Headings (MeSH) terms with plain text terms to develop the following advanced search strategy: (tinnitus[MeSH] OR tinnitus) AND (“Depressive Disorder”[MeSH] OR “Depression”[MeSH] or depress*) AND “adult”[MeSH Terms]. For the Web of Science database, we employed the following search strategy: TS (topic) = (tinnitus AND depress*). For this search, the following indices were utilized: SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, BKCI-S, BKCI-SSH, ESCI, CCR-EXPANDED, IC.
Screening
All articles identified during the search were independently reviewed by 2 researchers (J.W.S., M.A.) for inclusion on the basis of the inclusion and exclusion criteria. This was completed in 2 stages: first by abstract and then, if meeting criteria, by full-text review. Inconsistencies regarding inclusion of a given study were adjudicated by a third reviewer (K.M.). Foreign language studies were evaluated at the abstract and full-text levels as long as there was a suitable English translation. As the last step of the screening process, studies that were included after full-text review were evaluated for any potential overlap in study base. In the case that there was overlap, we excluded studies that were subpopulations of other studies. The studies resultant of this final screen advanced to quality review and data extraction.
Quality Review and Data Extraction
Our systematic review aimed to capture a measure of prevalence and thus dealt only with cross-sectional data, even if the original study design was a different type. We therefore elected to apply a modified version of the Newcastle-Ottawa Quality Assessment Scale adapted for cross-sectional studies to assess bias (see supplemental material, available in the online version of the article).13 We assessed bias in each of the following domains on an overall scale of 0–4: representativeness of the sample, response, and ascertainment of the outcome. Studies that were deemed to be at high risk of bias included (1) those that employed criteria that selected for a narrow subset of the target population of patients with tinnitus (eg, selecting for or excluding a population that had a specific comorbidity), (2) those that did not have a satisfactory response rate (<50%) or did not report a response rate, and (3) those that used a nonvalidated measure for ascertainment of depression (eg, self-report).
Quality scores were tabulated independently by 2 researchers for each included study. Any discrepancies regarding quality review were discussed, and a consensus was established. This process was also conducted for all other data that were extracted: year of study, instrument, study design, country, and prevalence of depression (by severity, if available). These data were examined and summarized with descriptive statistics, including means, medians, and interquartile ranges.
Results
Study Characteristics
Our database search identified 590 unique abstracts. Of these, 202 advanced to full-text review, of which 28 were ultimately included in the systematic review (Figure 1).7,8,11,14–38 The included studies were published between 2007 and 2016 and represented 9979 patients with tinnitus from 15 countries (5 from Brazil, 5 from the United States, and 3 from Germany; 2 each from Italy, Belgium, and Sweden; 1 each from Nigeria, Netherlands, Turkey, Egypt, Switzerland, Japan, Finland, Korea, and Australia). Of the included studies that reported it, the mean age ranged from 36 to 68 years, with the median being 49.2. Of the included studies that reported sex, the percentage male ranged from12.5% to 77.1%, with the median being 51%. A full summary of the characteristics of the included studies is provided in Table I.
Figure 1.
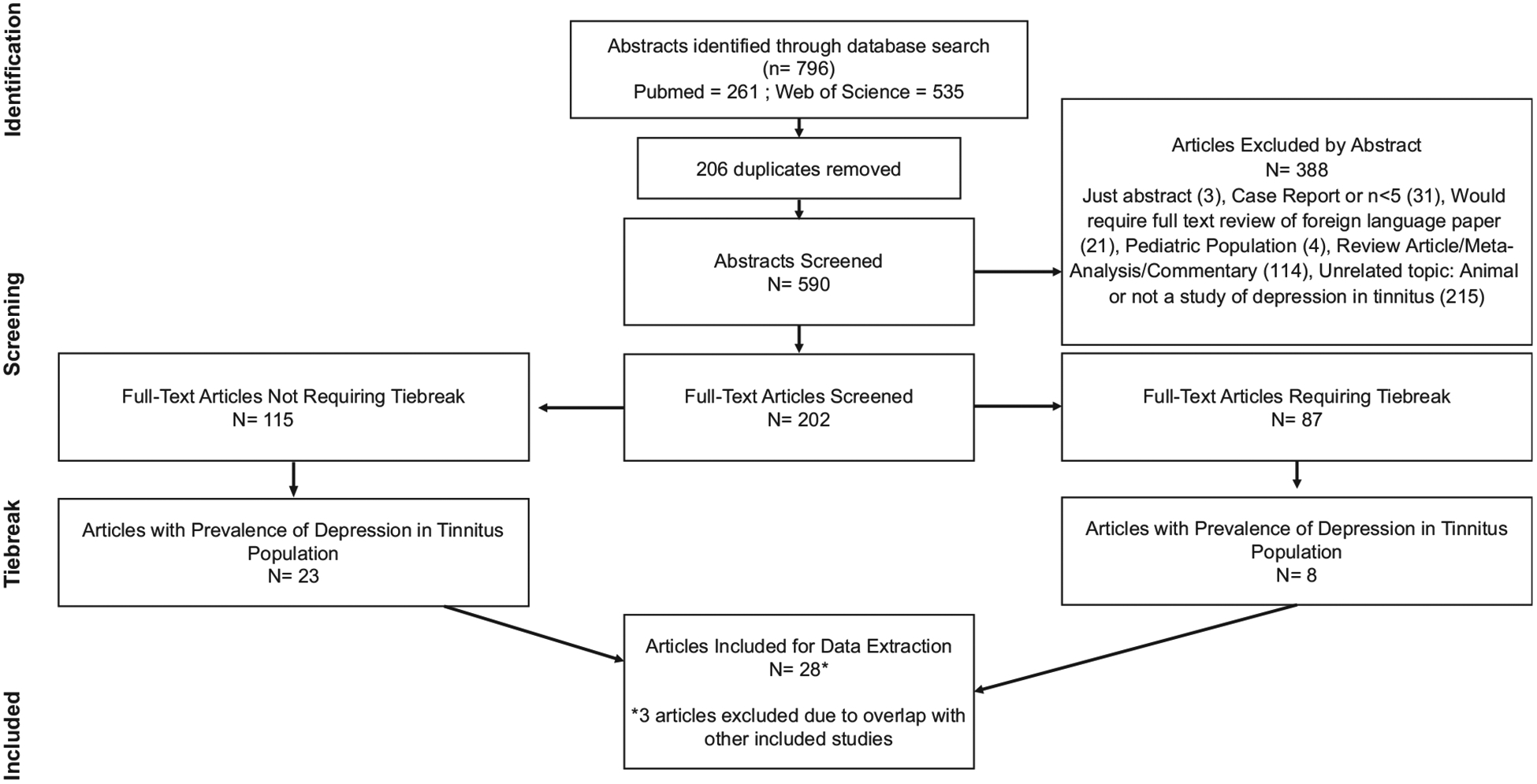
Flowchart of the article review process to arrive at the final articles included for data extraction and analysis: abstract identification, abstract screening, and full-text screening by tiebreak status.
Table I.
Studies Included in the Review.
| Study | Year | Country | Tinnitus Population Studied | Instrument | Patients with Tinnitus, n | Men, % | Mean Age or Range, y | Depression Prevalence, % | Quality Score |
|---|---|---|---|---|---|---|---|---|---|
| Adoga7 | 2008 | Nigeria | All tinnitus patients who presented to ENT clinic. | HADS | 92 | 45.7 | 36.0 | 17.4 | 4 |
| Andersson14 | 2009 | Sweden | Cochlear implant recipients who reported tinnitus | HADS | 107 | 38.7 | 54.4 | 5.6 | 3 |
| Barros Suzuki15 | 2016 | Brazil | Patients with tinnitus complaints for > 1 y without improvement with drug therapies and no tinnitus treatment for at least 3 mo | HADS | 10 | 50 | 41–78 | 10.0 | 3 |
| Bartels16 | 2008 | Netherlands | Consecutive patients with chronic tinnitus seen at ENT clinic | HADS ≥8 | 265 | 69.8 | 55.4 | 49.1 | 4 |
| Belli8 | 2008 | Turkey | Patients with tinnitus symptoms for at least 1 mo with no drug therapies for 3 mo prior to the study | SCI for DSM-III | 85 | 45.6 | 38.4 | 5.6 | 4 |
| Carlsson17 | 2015 | Sweden | Patients with severe to profound hearing impairment who reported tinnitus | HADS ≥8 | 704 | 51 | 68.0 | 24.6 | 3 |
| Crocetti18 | 2009 | Italy | Patients with idiopathic tinnitus for at least 6 mo (exclusion criteria: Meniere’s disease and chronic pain) | BDI | 108 | 66.7 | 48.0 | 13.0 | 4 |
| Das19 | 2012 | USA | Patients with nonpulsatile tinnitus of at least 6 mo (exclusion criteria: active diagnosis of any acute or chronic brain-related neurologic condition) | PHQ-9 ≥6 | 37 | 54 | 50.0 | 24.3 | 2 |
| Fernandes20 | 2013 | Brazil | Patients with orofacial pain and self-reported tinnitus | RDC/TMD | 129 | 17.9 | 37.7 | 68.2 | 3 |
| Folmer21 | 2008 | USA | Consecutive patients with chronic tinnitus presenting to ENT clinic for initial appointment | aBDI | 196 | 68.4 | 52.1 | 49.0 | 4 |
| Gomaa22 | 2014 | Egypt | Patients with subjective tinnitus associated with hearing loss presenting to ENT clinic | DASS | 100 | 40 | 20–60 | 84 | 4 |
| Granjeiro23 | 2013 | Brazil | Patients with tinnitus and normal hearing (exclusion criteria: drug treatment for tinnitus in past 6 mo; tinnitus <6 mo; history of acoustic trauma, vascular disease, ear surgery, vestibular disorders, head trauma, and neurologic diseases; recent intake of ototoxic drugs and chemo-/radiotherapy). | BDI | 68 | 45.6 | 36.8 | 66.2 | 3 |
| Hilgenberg24 | 2012 | Brazil | Patients with tinnitus receiving regular dental care (exclusion criteria: Ménière’s disease, history of noise exposure or ear surgery or infections; frequent use of headphones; abuse of ototoxic medication or substances, including fluoxetine and alcohol; and systemic diseases [eg, diabetes, hypertension] beyond control) | RDC/TMD | 100 | 16 | 39.2 | 70.0 | 3 |
| Jacques25 | 2013 | Belgium | Patients with chronic tinnitus consulting an audiophonology center. | Clinical diagnosis of MDD (exact criteria unclear) | 80 | — | — | 28.8 | 2 |
| Langguth26 | 2007 | Germany | Consecutive patients presenting to tinnitus clinic for tinnitus as their primary complaint | BDI ≥10 | 72 | 69.4 | 49.3 | 55.6 | 3 |
| Meyer27 | 2014 | Switzerland | Chronic tinnitus patients volunteering for EEG study | BDI | 24 | 54.2 | 39.8 | 50.0 | 4 |
| Michikawa28 | 2010 | Japan | Patients who self-reported tinnitus in the past year in a community-based cross-sectional study | Self-report questiona | 243 | 44.2 | >65 | 33.7 | 2 |
| Milhomem Rocha29 | 2015 | Brazil | Concomitant tinnitus and musical hallucinations | SCID | 16 | 12.5 | 61.4 | 68.8 | 3 |
| Nondahl30 | 2011 | USA | Patients who self-reported tinnitus in the past year from the Beaver Dam Offspring Study, an epidemiologic cohort study of aging | CES-D ≥ 16 | 345 | 51.3 | 50.8 | 24.6 | 4 |
| Ooms11 | 2011 | Belgium | Consecutive tinnitus patients seen at ENT clinic | BDI-II | 136 | 64.7 | 49.1 | 33.1 | 4 |
| Robinson31 | 2008 | USA | Patients with self-reported tinnitus distress volunteering for cognitive-behavior therapy trial (exclusion criterion: inability to participate in group) | HRSD | 65 | 52 | 55 | 24.6 | 4 |
| Salonen32 | 2007 | Finland | Subjects who responded to survey on hearing loss and reported tinnitus with or without annoyance | aBDI | 343 | 41.2 | 70–85 | 19.0 | 3 |
| Seo33 | 2016 | Korea | Subjects who were part of KNHANES and reported experiencing tinnitus in the past year | Self-report questionb | 3949 | — | 48.3 | 18.2 | 2 |
| Shargorodsky34 | 2010 | USA | Subjects who were part of NHANES and reported experiencing tinnitus in the past year. | WHO WMH-CIDI | 2265 | — | 20–39 | 7.9 | 4 |
| Sireci35 | 2012 | Italy | Patients with tinnitus referred to audiology clinic | SCL 90-R | 191 | 58.1 | 48.6 | 33.0 | 4 |
| Trevis36 | 2016 | Australia | Patients who self-identified as experiencing constant tinnitus for at least 3 mo | BDI-II | 26 | 58 | 40.3 | 42.3 | 4 |
| Vtillmann37 | 2013 | Germany | Patients with chronic tinnitus responding to mailed questionnaires | HADS≥8 | 118 | 77.1 | 55.6 | 41.5 | 3 |
| Zirke38 | 2013 | Germany | Patients with tinnitus for at least 3 mo admitted to tinnitus center | CIDI | 100 | 45 | 49.6 | 37.0 | 2 |
Abbreviations: aBDI, abbreviated Beck Depression Inventory; (0–4, no or minimal depression; 5–7, mild depression; 8–15, moderate depression; and ≥16, severe depression); BDI, Beck Depression Inventory; CES-D, Center for Epidemiologic Studies Depression Scale; CIDI, Composite International Diagnostic Interview (evaluated per criteria of the International Classification of Diseases, Tenth Revision and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition); DASS, Depression, Anxiety and Stress Scale; DSM-III, Diagnostic and Statistical Manual of Mental Disorders, Third Edition; EEG, electroencephalogram; ENT, ear, nose, and throat; HADS, Hospital Anxiety and Depression Scale (unless otherwise stated, >10); HRSD, Hamilton Rating Scale for Depression; KNHANES, Korea National Health and Nutrition Examination Survey; MDD, major depressive disorder; NHANES, National Health and Nutrition Examination Survey; PHQ-9, Patient Health Questionnaire-9; RDC/TMD, Research Diagnostic Criteria for Temporomandibular Disorders; SCI, Structured Clinical Interview; SCL 90-R, Symptom Checklist-90 R; WHO WMH-CIDI, World Health Organization World Mental Health Composite International Diagnostic Interview.
Depression was evaluated with the question “Do you feel sad, depressed, or miserable?”
Depression was evaluated with the question “Have you experienced a depressed mood for 2 or more continuous weeks during the previous year?”
Quality Assessment
Of the included studies, quality scores ranged from 2 to 4, with a mean score of 3.3 (SD = 0.76).
With respect to the representativeness of the sample domain, the majority earned a point for being truly or somewhat representative; 46% were rated truly representative (studies that captured all patients in a given period or random sampling); and 25% were rated somewhat representative of the target population (studies that used nonrandom sampling, including those that recruited volunteers in person or via mailed questionnaires). The remaining 29% of studies were rated as utilizing a selected group and did not earn a point. These studies featured substantial inclusion criteria (eg, treatment-resistant tinnitus, severe to profound hearing impairment, concomitant musical hallucinations) or exclusion criteria (eg, chronic disease comorbidity, systemic disease beyond control, Ménière’s disease).
With respect to the response domain, 75% of the included studies were awarded a point for achieving comparability between respondents and nonrespondents. These included studies that comprised all patients (avoiding the issue of nonrespondents completely) or studies that were based on nationally representative cohorts (eg, Korea National Health and Nutrition Examination Survey, National Health and Nutrition Examination Survey). The remaining 25% did not achieve a satisfactory response rate (<50% respondents) or, more commonly, did not sufficiently comment on nonrespondent characteristics.
With respect to the ascertainment domain, the majority of studies (89%) earned 2 points for employing a validated measure of depression. The most common validated measures used for evaluating depression were variants of the Beck Depression Index (BDI, BDI-II, abbreviated BDI: 25% of studies) and the Hospital Anxiety and Depression Scale (21% of studies). Other validated measurement tools employed included the Patient Health Questionnaire-9, the Symptom Checklist-90 R, the Hamilton Rating Scale for Depression, and structured interview techniques coupled with criteria from the Diagnostic and Statistical Manual of Mental Disorders. Of the 3 studies not earning a point in this domain, 2 employed self-report measures of depression in the form of 1 question on experiencing depressed mood, and 1 utilized a clinical diagnosis of depression, although it did not provide sufficient description of how the diagnosis was ascertained.
Findings
The median prevalence of depression was 33%, with an interquartile range of 19% to 49%. The overall range was 6% to 84%. Five studies comprising 547 patients provided a breakdown of severe depression; 23% of the total patients scored as severely depressed. Notably, 2 of the groups among the highest prevalence of depression had a significant concomitant symptom: orofacial pain (68%) and musical hallucinations (69%). However, a similar prevalence of depression was also identified in studies without such concomitant conditions.
Discussion
There are multiple reviews on the association of tinnitus and depression, but this review is the first to systematically review the data on prevalence of depression among patients with tinnitus. In our review, we provide a strong pool of evidence further establishing a substantial burden of depression among patients with tinnitus, with a median prevalence of 33% among our 28 studies and nearly 10,000 patients. This is considerably greater than estimates in comparable general populations. For instance, data from the National Health and Nutritional Examination Survey showed that 8.1% of American adults experienced depressive symptoms in a given 2-week period from 2013 to 2016.39 Furthermore, the studies that provided a breakdown of depression showed a disproportional number of patients with tinnitus experiencing severe depression.
This review suggests that the burden of depression is comparable to that of other medical conditions, such as poststroke (33%) and myocardial infarction (31%) states.40,41 Screening for depression among patients with stroke or myocardial infarction is routine, due to a known association with worse clinical outcomes. The impact of depression and its treatment on tinnitus outcomes is not yet fully elucidated and warrants further study outside the scope of this review. However, given the similar burden and the possible cyclical relationship between depression and tinnitus, our results suggest that there may be value in similarly screening and treating depression in cases of tinnitus.
To achieve the highest-quality data, we employed an all-inclusive search query to ensure that our results were applicable to the general population, regardless of clinical setting or cultural differences. Our vast search on PubMed and Web of Science sought a large group of heterogenous data, thus allowing our findings to be as applicable to the general population as possible. Based on studies from 15 countries during the most recent decade, our data set is one of the largest contemporary analyses to evaluate the prevalence of depression among those affected by tinnitus.9,42–44 This includes studies written in different languages, albeit with suitable translations into English. Only a minority of screened studies were excluded due to absence of a suitable translation (n = 21).
A major critique of similar large-scale analyses is the lack of validation of the data quality.42,43 A comparable large study by Trevis et al reported a quality score of 64%.10 We verified the quality of our data utilizing a modified Newcastle-Ottawa Quality Assessment Scale, achieving a mean score of 3.3 out of 4, indicating that included studies were consistently of high quality with respect to representativeness, response, and use of validated measures of depression. Excluded studies commonly had narrow inclusion criteria, low response rates, and nonvalidated measurements of depression.
A second critique of comparable studies involves the “positive result” bias that occurs with patients presenting to ear, nose, and throat clinics. In theory, these patients are more likely to seek help than the general population, often with worse tinnitus symptoms and a higher likelihood of suffering from concomitant depression. However, a 2010 study by Krog et al focused solely on the general population and found a weak link between tinnitus and depression.45 To compromise on these polarizing perspectives, our data employed studies including populations of “help seekers” and “non-help seekers” to maximize generalizability. While we sought to include the highest-quality data possible, we chose to avoid excluding several patient populations, namely those with preexisting psychosomatic conditions. For example, a recent study by Pinto et al chose to exclude those with known psychosis, personality and somatoform disorders, otitis, outer ear diseases, Ménière’s disease, and somatosensory tinnitus and those with tinnitus due to metabolic derangements.9 Studies such as this offer select value to a homogenous population yet fail to identify the true prevalence of the largely heterogenous population experiencing tinnitus.
While utilizing a variety of study types to achieve this goal, we acknowledge that a limitation of any systematic review lies within the heterogeneous nature of the data. In our work, this was particularly evident in the variety of tools used to diagnose depression. Although the majority (89%) employed validated measures with various tools, there was heterogeneity in the scores needed to define “depression.” Nonetheless, numerous studies found a high degree of convergent validity among assessment measures.46–48 For example, a recent study commissioned by the National Health Services (Scotland) examining depression severity in the primary care setting found a high concordance in classification across the Hospital Anxiety and Depression Scale, Patient Health Questionnaire-9, and BDI.49 Additionally, although we quantified an important association between tinnitus and depression, there were limited data on the temporality of onset of tinnitus versus depression. As such, we are limited in our ability to comment on the underpinnings of the association.
Acknowledging the inherent limitations of systematic reviews and meta-analyses, our review still holds much value. It is the most robust contemporary review to quantify a long-studied association of depression and tinnitus from a cohort of high-quality studies, and it establishes depression to be far more prevalent in the tinnitus population than the general population, similar to other highly morbid diseases, such as myocardial infarction and stroke. Our results suggest that the routine screening and treatment of depression among patients with tinnitus warrant strong consideration and further investigation.
Supplementary Material
Funding source:
None.
Sponsorships: None.
Footnotes
Competing interests: Matthew R. Amans, Covidien—consultant, Stryker Neurovascular—consultant.
Supplemental Material
Additional supporting information is available in the online version of the article.
References
- 1.Esmaili AA, Renton J. A review of tinnitus. Aust J Gen Pract. 2018;4:205–208. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142:959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jalessi M, Farhadi M, Asghari A, et al. Tinnitus: an epidemiologic study in Iranian population. Acta Med Iran. 2013;51:886–891. [PubMed] [Google Scholar]
- 4.Park RJ, Moon JD. Prevalence and risk factors of tinnitus: the Korean National Health and Nutrition Examination Survey 2010–2011, a cross-sectional study. Clin Otolaryngol. 2014;39: 89–94. [DOI] [PubMed] [Google Scholar]
- 5.Khedr EM, Ahmed MA, Shawky OA, Mohamed ES, El Attar GS, Mohammad KA. Epidemiological study of chronic tinnitus in Assiut, Egypt. Neuroepidemiology. 2010;35:45–52. [DOI] [PubMed] [Google Scholar]
- 6.Sindhusake D, Mitchell P, Newall P, Golding M, Rochtchina E, Rubin G. Prevalence and characteristics of tinnitus in older adults: the Blue Mountains Hearing Study. Int J Audiol. 2003; 42:289–294. [DOI] [PubMed] [Google Scholar]
- 7.Adoga AA, Adoga AS, Obindo JT. Tinnitus and the prevalence of co-morbid psychological stress. Niger J Med. 2008; 17:95–97. [DOI] [PubMed] [Google Scholar]
- 8.Belli S, Belli H, Bahcebasi T, Ozcetin A, Alpay E, Ertem U. Assessment of psychopathological aspects and psychiatric comorbidities in patients affected by tinnitus. Eur Arch Otorhinolaryngol. 2008;265:279–285. [DOI] [PubMed] [Google Scholar]
- 9.Pinto PCL, Marcelos CM, Mezzasalma MA, Osterne FJV, de Melo Tavares de Lima MA, Nardi AE. Tinnitus and its association with psychiatric disorders: systematic review. J Laryngol Otol. 2014;128:660–664. [DOI] [PubMed] [Google Scholar]
- 10.Trevis KJ, McLachlan NM, Wilson SJ. A systematic review and meta-analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62–86. [DOI] [PubMed] [Google Scholar]
- 11.Ooms E, Meganck R, Vanheule S, Vinck B, Watelet J-B, Dhooge I. Tinnitus severity and the relation to depressive symptoms: a critical study. Otolaryngol Head Neck Surg. 2011;145:276–281. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG: PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 13.Herzog R,Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson G, Freijd A, Baguley DM, Idrizbegovic E. Tinnitus distress, anxiety, depression, and hearing problems among cochlear implant patients with tinnitus. J Am Acad Audiol. 2009;20:315–319. [DOI] [PubMed] [Google Scholar]
- 15.Barros Suzuki FA de, Suzuki FA, Yonamine FK, Onishi ET, Penido NO. Effectiveness of sound therapy in patients with tinnitus resistant to previous treatments: importance of adjustments. Braz J Otorhinolaryngol. 2016;82:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartels H, Middel BL, van der Laan BF, Staal MJ, Albers FW. The additive effect of co-occurring anxiety and depression on health status, quality of life and coping strategies in help-seeking tinnitus sufferers. Ear Hear. 2008;29:947–956. [DOI] [PubMed] [Google Scholar]
- 17.Carlsson PI, Hjaldahl J, Magnuson A, et al. Severe to profound hearing impairment: quality of life, psychosocial consequences and audiological rehabilitation. Disabil Rehabil. 2015;37: 1849–1856. [DOI] [PubMed] [Google Scholar]
- 18.Crocetti A, Forti S, Ambrosetti U, Bo LD. Questionnaires to evaluate anxiety and depressive levels in tinnitus patients. Otolaryngol Head Neck Surg. 2009;140:403–405. [DOI] [PubMed] [Google Scholar]
- 19.Das SK, Wineland A, Kallogjeri D, Piccirillo JF. Cognitive speed as an objective measure of tinnitus. Laryngoscope. 2012;122:2533–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandes G, Gonçalves DA de G, de Siqueira JTT de, Camparis CM. Painful temporomandibular disorders, self reported tinnitus, and depression are highly associated. Arq Neuropsiquiatr. 2013;71:943–947. [DOI] [PubMed] [Google Scholar]
- 21.Folmer RL, Griest SE, Martin WH. Obsessive-compulsiveness in a population of tinnitus patients. Int Tinnitus J. 2008;14: 127–130. [PubMed] [Google Scholar]
- 22.Gomaa MA, Elmagd MH, Elbadry MM, Kader RM. Depression, Anxiety and Stress Scale in patients with tinnitus and hearing loss. Eur Arch Otorhinolaryngol. 2014;271:2177–2184. [DOI] [PubMed] [Google Scholar]
- 23.Granjeiro RC, Kehrle HM, de Oliveira TS, Sampaio AL, de Oliveira CA. Is the degree of discomfort caused by tinnitus in normal-hearing individuals correlated with psychiatric disorders? Otolaryngol Head Neck Surg. 2013;148:658–663. [DOI] [PubMed] [Google Scholar]
- 24.Hilgenberg PB, Saldanha AD, Cunha CO, Rubo JH, Conti PC. Temporomandibular disorders, otologic symptoms and depression levels in tinnitus patients. J Oral Rehabil. 2012;39:239–244. [DOI] [PubMed] [Google Scholar]
- 25.Jacques D, Nozeret Y, Zdanowicz N, Reynaert C, Garin P, Gilain C. Tinnitus and psychiatric comorbidities in liaison psychiatry analysis of three years in an audiophonology centre. Psychiatr Danub. 2013;25(suppl 2):S102–S104. [PubMed] [Google Scholar]
- 26.Langguth B, Kleinjung T, Fischer B, Hajak G, Eichhammer P, Sand PG. Tinnitus severity, depression, and the big five personality traits. Prog Brain Res. 2007;166:221–225. [DOI] [PubMed] [Google Scholar]
- 27.Meyer M, Luethi MS, Neff P, Langer N, Büchi S. Disentangling tinnitus distress and tinnitus presence by means of EEG power analysis. Neural Plast. 2014;2014:468546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michikawa T, Nishiwaki Y, Kikuchi Y, et al. Prevalence and factors associated with tinnitus: a community-based study of Japanese elders. J Epidemiol. 2010;20:271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milhomem Rocha SC, Kii MA, Pereira CB, Borelli DT, Forlenza O, Sanchez TG. Multidisciplinary assessment of patients with musical hallucinations, tinnitus and hearing loss. Psychopathology. 2015;48:251–255. [DOI] [PubMed] [Google Scholar]
- 30.Nondahl DM, Cruickshanks KJ, Huang GH, et al. Tinnitus and its risk factors in the Beaver Dam Offspring Study. Int J Audiol. 2011;50:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson SK, Viirre ES, Bailey KA, et al. A randomized controlled trial of cognitive-behavior therapy for tinnitus. Int Tinnitus J. 2008;14:119–126. [PubMed] [Google Scholar]
- 32.Salonen J, Johansson R, Joukamaa M. Alexithymia, depression and tinnitus in elderly people. Gen Hosp Psychiatry. 2007;29: 431–435. [DOI] [PubMed] [Google Scholar]
- 33.Seo JH, Kang JM, Hwang SH, Han KD, Joo YH. Relationship between tinnitus and suicidal behaviour in Korean men and women: a cross-sectional study. Clin Otolaryngol. 2016;41: 222–227. [DOI] [PubMed] [Google Scholar]
- 34.Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010; 123:711–718. [DOI] [PubMed] [Google Scholar]
- 35.Sireci F, Ballacchino A, Agrifoglio M, Ferrara S, Mucia M, Salvago P. Psychopathologic diseases in patients with tinnitus: a case-control of an outpatient cohort. Acta Medica Mediterranea. 2012;28:167–170. [Google Scholar]
- 36.Trevis KJ, McLachlan NM, Wilson SJ. Psychological mediators of chronic tinnitus: the critical role of depression. J Affect Disord. 2016;204:234–240. [DOI] [PubMed] [Google Scholar]
- 37.Vollmann M, Scharloo M, Langguth B, Kalkouskaya N, Salewski C. Illness representations as mediators of the relationship between dispositional optimism and depression in patients with chronic tinnitus: a cross-sectional study. Psychol Health. 2013;29:81–93. [DOI] [PubMed] [Google Scholar]
- 38.Zirke N, Seydel C, Arsoy D, et al. Analysis of mental disorders in tinnitus patients performed with Composite International Diagnostic Interview. Qual Life Res. 2013;22:2095–2104. [DOI] [PubMed] [Google Scholar]
- 39.Brody DJ, Pratt LA, Hughes JP. Prevalence of depression among adults aged 20 and over: United States, 2013–2016. NCHS Data Brief. 2018;(303):1–8. [PubMed] [Google Scholar]
- 40.Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:1330–1340. [DOI] [PubMed] [Google Scholar]
- 41.Thombs BD, Bass EB, Ford DE, et al. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med. 2006;21:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durai M, Searchfield G. Anxiety and depression, personality traits relevant to tinnitus: a scoping review. Int J Audiol. 2016; 55:605–615. [DOI] [PubMed] [Google Scholar]
- 43.Geocze L, Mucci S, et al. Systematic review on the evidences of an association between tinnitus and depression. Braz J Otorhinolaryngol. 2013;79:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hébert S, Canlon B, Hasson D, et al. Tinnitus severity is reduced with reduction of depressive mood—a prospective population study in Sweden. PLoS One. 2012;7(5):e37733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krog N, Engdahl B, Tambs K. The association between tinnitus and mental health in a general population sample: results from the HUNT Study. J Psychosom Res. 2010;69:289–298. [DOI] [PubMed] [Google Scholar]
- 46.Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S454–S466. [DOI] [PubMed] [Google Scholar]
- 47.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J Psychosom Res. 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 48.Kung S, Alarcon RD, Williams MD, Poppe KA, Jo Moore M, Frye MA. Comparing the Beck Depression Inventory-II (BDI-II) and Patient Health Questionnaire (PHQ-9) depression measures in an integrated mood disorders practice. J Affect Disord. 2013;145:341–343. [DOI] [PubMed] [Google Scholar]
- 49.Cameron I, Cardy A, Crawford J. Assessing the validity of the PHQ-9, HADS, BDI-II and QIDS-SR16 in measuring of depression in a UK sample of primary care patients with a diagnosis of depression. Edinburgh, Scotland: Healthcare Improvement Scotland; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


