Abstract
Background:
Frailty and decreased functional status are risk factors for adverse kidney transplant (KT) outcomes. Our objective was to examine the efficacy of an exercise intervention on frailty and decreased functional status in a cohort of patients with advanced chronic kidney disease (CKD).
Methods:
We conducted a prospective study involving 21 adults with ≥ stage 4 CKD who were 1) frail or pre-frail by Fried phenotype and/or 2) had lower extremity impairment [Short Physical Performance Battery score ≤ 10]. The intervention consisted of two supervised outpatient exercise sessions per week for 8 weeks.
Results:
Among our cohort, median participant age was 62 years (interquartile range, 53–67) and 85.7% had been evaluated for KT. Following the study, participants reported satisfaction with the intervention and multiple frailty parameters improved significantly, including fatigue, physical activity, walking time, and grip strength. Lower extremity impairment also improved (90.5% to 61.9%, p=0.03). No study-related adverse events occurred.
Conclusions:
Preliminary data from this study suggest that a supervised, outpatient exercise intervention is safe, acceptable, feasible, and associated with improved frailty parameters, and lower extremity function, in patients with advanced CKD. Further studies are needed to confirm these findings and determine whether this prehabilitation strategy improves KT outcomes.
Keywords: frailty, transplants, renal insufficiency, chronic
INTRODUCTION
The transplant community is facing a challenging combination of problems – kidney transplant (KT) candidates are becoming older and more medically complex while transplant waiting times continue to increase.1–3 These synergistic factors contribute to high rates of functional decline in KT candidates.1 Specifically, the combination of comorbidities, sarcopenia, and uremia-associated inflammation contribute to the development of frailty and decreased functional status in many patients.4–6 Frailty is commonly defined as a syndrome of multi-system physiologic dysfunction which leads to decreased ability to recover from adverse medical events.7 Decreased functional status refers to challenges performing activities of daily living. Although there is a lack of consensus regarding the optimal measure of frailty and functional status in KT patients, the two most commonly studied measures include: 1) the Fried frailty phenotype, a composite measure of wasting, exhaustion, physical activity, walking speed, and grip strength, and 2) the Short Physical Performance Battery (SPPB), a composite measure of lower extremity function.8,9 By the time KT candidates are transplanted, nearly one-quarter of KT candidates are frail by the Fried frailty phenotype, and approximately half have lower extremity (LE) impairment defined as SPPB scores ≤ 10.10,11 Pre-transplant frailty and LE impairment are strongly associated with a myriad of adverse outcomes before and after KT, including decreased rates of transplantation, waitlist mortality, delayed graft function, longer hospital length of stay, rehospitalizations, delirium, cognitive dysfunction, decreased quality of life, immunosuppression intolerance, and death.10,12–24
Although interventions have been shown to modify frailty in older, community-dwelling adults, anti-frailty interventions in KT candidates are lacking. In the current healthcare environment where transplant centers face numerous resource constraints, optimizing potentially modifiable risk factors-- such as frailty--to reduce adverse outcomes and maximize patient and graft survival is imperative.1 In fact, a recent Frailty Consensus Statement published by the American Society of Transplantation (AST) highlighted the urgent need for effective frailty interventions in transplant candidates.25 While interventions incorporating exercise improve frailty in the general population, anti-frailty interventions in KT candidates are understudied. Moreover, the preferred mode and duration of anti-frailty exercise interventions in KT candidates is unknown.26–28
We believe an ideal intervention for frail transplant candidates should be individualized, standardized, and widely available given that transplant candidates are often geographically dispersed from their transplant centers. The ideal intervention should also be supervised given that numerous studies involving non-transplant populations have shown that supervised interventions are more effective than unsupervised interventions.29–32 One intervention meeting these criteria is pulmonary rehabilitation (PR), a regimen of graduated aerobic, strength and flexibility training conducted in monitored clinical rehabilitation settings across the country.33,34 PR has been shown to improve frailty and LE function in patients with lung disease.35–39 Given that the exercise program utilized in PR is not specific to patients with lung disease, we hypothesized that PR would also improve frailty and LE function in patients with advanced chronic kidney disease (CKD), including KT candidates. The objective of this study was to examine the safety, acceptability, feasibility, and preliminary efficacy of PR on frailty, frailty parameters, LE function, body composition, and health-related quality of life (HRQOL) in patients with advanced CKD.
MATERIALS AND METHODS
Patient population.
The study was approved by the Mayo Clinic Institutional Review Board in accordance with the Helsinki Declaration of 1975 and was registered on ClinicalTrials.gov (NCT03535584). All patients provided written informed consent. We conducted a prospective study at Mayo Clinic, Rochester, Minnesota, USA between 7/2018 and 10/2019. Potentially eligible individuals with stage 4 or 5 CKD (age ≥ 18 years) who lived within 70 miles of our center were identified from our KT waiting list, dialysis units, CKD clinic, and/or during KT evaluations and approached to assess interest in study participation. Interested patients were screened for inclusion criteria: 1) frail or pre-frail by Fried frailty phenotype and/or 2) LE impairment (see Frailty testing below).8,9 Patients with moderate to severe active cardiopulmonary disease were excluded from the study. Moderate to severe cardiovascular disease was defined as a history of untreated myocardial ischemia, recent myocardial infarction with or without revascularization, heart transplant candidate, left ventricular assist device recipient, or known arrhythmia. Moderate to severe pulmonary disease was defined as known significant restrictive or obstructive lung disease by pulmonary function testing, lung transplant candidate, and/or need for oxygen supplementation. Participants received parking passes and remuneration.
Patient data.
Demographic information was obtained from participant self-report and abstracted from the electronic medical record. Self-reported functional status was obtained using the Karnofsky Performance Status scale.40 Estimated glomerular filtration rate (eGFR) in participants not on dialysis was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.41
Exercise intervention.
The exercise intervention consisted of 8 weeks of PR according to American Thoracic Society guidelines.34 Specifically, participants were asked to complete two exercise sessions per week for 8 weeks (16 total sessions) in the outpatient PR unit at our center under the supervision of a licensed respiratory therapist. Participants receiving in-center hemodialysis were preferentially scheduled on non-dialysis days. Each exercise session lasted ≤ 60 minutes; sessions lasting less than 60 minutes were terminated by participants. Exercise training was individualized and progressive and included three components: 1) endurance training, 2) strength training, and 3) flexibility training (see Table 1).
Table 1.
Description of exercise intervention
| Exercise component | Description |
|---|---|
| Endurance training |
|
| Strength training |
|
| Flexibility training |
|
Safety.
Study personnel collected vital signs before each exercise session and monitored the participants’ degree of dyspnea, oxygen saturation, and heart rate during exercise. Study protocol included blood pressure checks as needed during exercise (based on symptoms). Any adverse events experienced during the study were recorded.
Acceptability.
Upon study completion, participants were asked to answer the following questions (1 = strongly disagree to 5 = strongly agree): 1) I felt the exercise program was beneficial to my overall health, 2) I felt the exercise program was beneficial to my mental health, 3) I felt the exercise program was beneficial to my physical health, 4) I will continue to exercise regularly on my own after completing this program, 5) I feel more confident about exercising on my own after completing this program. Patients were also asked open-ended questions about their experience with the exercise intervention.
Feasibility.
Feasibility was assessed by the ability to accrue subjects and maintain participant involvement for the duration of the exercise intervention (8 weeks). Reasons for withdrawing from the study were recorded. We also examined the number of exercise sessions completed and information regarding each session (e.g., vitals, duration, treadmill speed, etc.).
Frailty testing.
Frailty testing was performed by trained study coordinators (K.T., R.W.) or physical therapists at baseline (prior to initiation of the exercise intervention), halfway through the study (after 4-weeks), and upon study completion (after 8-weeks). Frailty was defined in accordance with the Fried frailty phenotype: wasting, exhaustion , low physical activity, slow walking speed , and weakness .42 “Frail” was defined as ≥ 3 criteria, “pre-frail” as 1–2 criteria, and “non-frail” as none of the criteria. In addition, in order to detect potential response to the intervention, alternate measures of muscle mass were determined by electrical impedance using the InBody 770 body composition analyzer (InBody USA, Cerritos, CA), including the fat mass index, skeletal muscle index (SMI), and appendicular skeletal muscle mass index (ASMI) defined as fat mass, skeletal muscle mass, and appendicular skeletal muscle mass (sum of muscle mass in both arms and legs) divided by the square of height, respectively.
Short physical performance battery (SPPB).
LE function was measured using the SPPB which is a composite measure of balance, gait speed, and chair stands.43 Measurements were performed by trained study coordinators (K.T., R.W.) or physical therapists at baseline, after 4-weeks, and upon study completion as outlined above. During the SPPB, participants receive scores ranging from 0 (unable to perform) to 4 (no difficulty performing) for each of the three components. Component scores are then summed to provide a total SPPB score ranging from 0 to 12.43 For this study, LE impairment was defined as a total SPPB score ≤ 10 based on published literature showing scores ≤ 10 are associated with adverse outcomes and mortality in KT candidates.10,23,24
Other study measures.
HRQOL was measured using the Kidney Disease Quality of Life Short Form (KDQOL-SF), Version 1.3, which has been validated in KT recipients and includes both the Medical Outcomes Study Questionnaire Short Form 36 Health Survey (SF-36) and kidney disease-specific scales.44–46 In addition to the standard scales of the SF-36 which include the energy/fatigue scale, we also calculated the physical and mental component scores and a kidney disease-specific component summary score as previously described.21
Statistical analysis.
Categorical variables were summarized as counts and percentages with continuous variables summarized via medians and interquartile ranges (IQR). Pre- and post-intervention comparisons were made using Wilcoxon signed-rank test for continuous variables and McNemar’s test for categorical variables. Differences between groups were tested using Wilcoxon rank-sum and Fisher’s exact tests. The primary endpoint was frailty at study completion (8-weeks). Secondary outcomes included change in frailty parameters, body composition, SPPB scores, and HRQOL. In participants unable to complete the 8-week intervention, 4-week measures were used as censored data. For purposes of analysis, chair stand time was set as 60 seconds in participants unable to complete the test. P-values ≤0.05 were considered statistically significant. Analyses were conducted with JMP, version 14, SAS Institute, Inc.
RESULTS
Patient characteristics.
Of the 29 individuals screened, 27 met frailty or SPPB criteria for eligibility. Two patients were ineligible, because they were both non-frail and had a SPPB score > 10. Twenty-one participants completed at least eight sessions of PR and the 4-week follow-up testing and were therefore included in the final analysis per study design (Figure 1). The eight participants who withdrew prior to study completion did not differ from non-withdrawing participants in terms of age, BMI, race, sex, diabetes, dialysis dependence, frailty phenotype score, or SPPB score. Baseline characteristics of the study cohort are shown in Table 2. Overall, the median participant age was 62 years (range, 42–87; IQR, 53–67), 57.1% were male, 85.7% were white non-Hispanic, 66.7% were on dialysis, and 85.7% had been evaluated for KT. Three participants had not been evaluated for KT. Baseline testing revealed that 38.1% of participants were frail (meeting ≥ 3 Fried criteria), 42.9% were pre-frail, and 19.1% were non-frail. Median SPPB score was 9 (IQR, 7–10) and 90.5% of participants had a SPPB score ≤ 10. Overall, 28.6% of participants (n=6/21) were both frail and had a SPPB score ≤ 10 (Figure 2).
Figure 1.
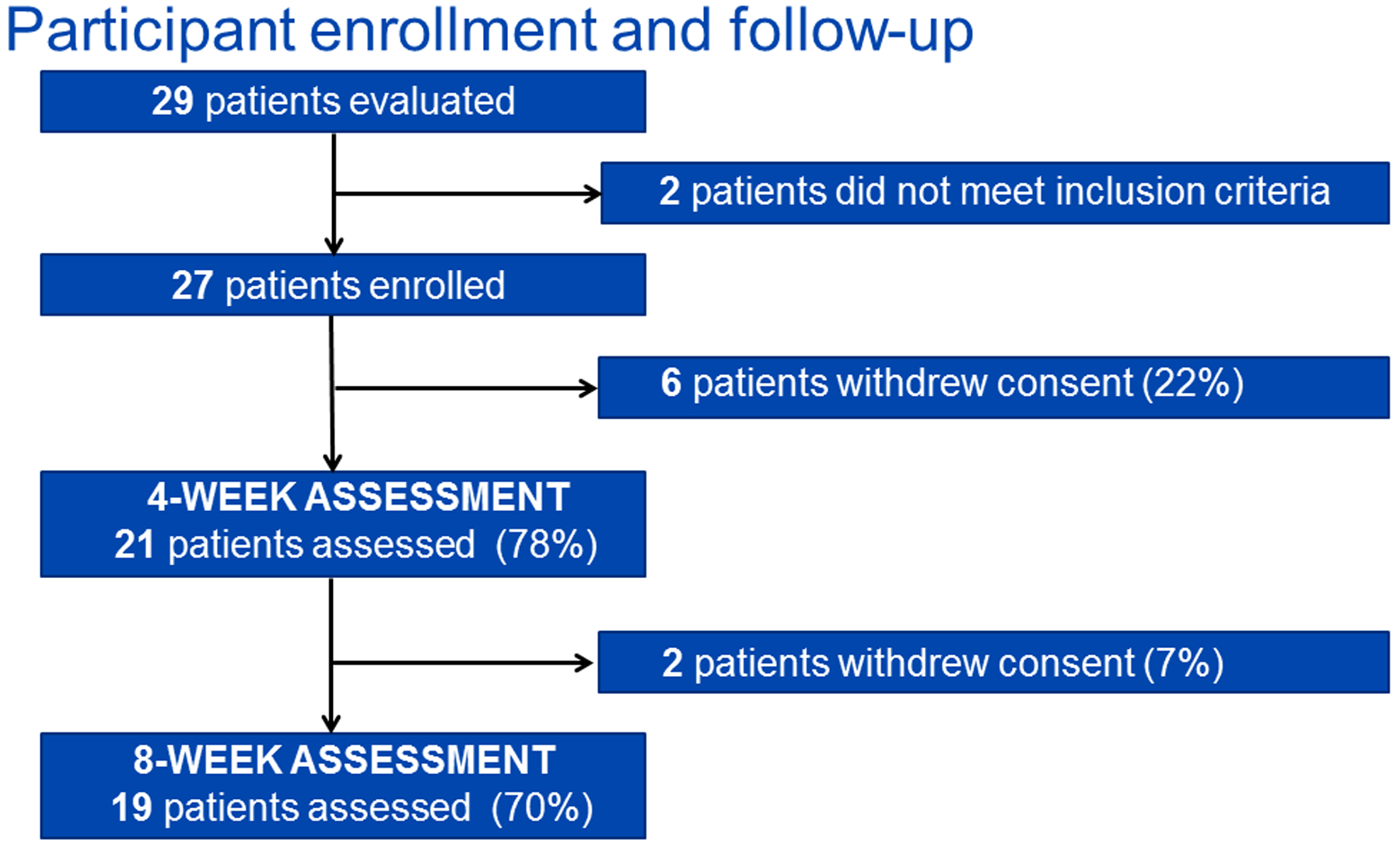
Enrollment and follow-up of study participants.
Table 2.
Baseline demographics
| Variable | Total (n=21)1 |
|---|---|
| Age (years) | 62 [53–67] |
| Male | 12 (57.1%) |
| Race/ethnicity | |
| White non-Hispanic | 18 (85.7%) |
| Black non-Hispanic | 1 (4.5%) |
| White Hispanic | 1 (4.5%) |
| Other | 1 (4.5%) |
| CKD Stage 4 | 6 (28.6%) |
| CKD Stage 5 | |
| Non-dialysis | 1 (4.8%) |
| Dialysis dependent | 14 (66.7%) |
| Dialysis modality (n=14) | |
| Hemodialysis | 11 (78.6%) |
| Peritoneal dialysis | 3 (21.4%) |
| Time on dialysis (years) (n=14) | 3.0 [0.7–7.0] |
| Cause of ESRD | |
| Glomerulonephritis | 1 (4.8%) |
| Polycystic disease | 3 (14.3%) |
| Diabetes | 8 (38.1%) |
| Hypertension | 3 (14.3%) |
| Other | 6 (28.6%) |
| History of prior kidney transplant, n (%) | 3 (14.3%) |
| Comorbidities | |
| Diabetes | 14 (66.7%) |
| Cardiovascular disease | 5 (23.8%) |
| Peripheral vascular disease | 8 (38.1%) |
| Rheumatoid or other arthritis | 4 (19.0%) |
| Lower extremity amputation | 0 (0.0%) |
| Hypertension | 19 (90.5%) |
| History of cancer | 6 (28.6%) |
| Chronic obstructive pulmonary disease | 5 (23.8%) |
| Smoking status | |
| Former smoker | 5 (23.8%) |
| Active smoker | 0 (0%) |
| Never smoked | 16 (76.2%) |
| BMI (kg/m2) | 30.5 [27.1–55.1] |
| Karnofsky score (baseline) | 80 [70–80] |
| Evaluated for kidney transplant | 18 (85.7%) |
Median [IQR]
Figure 2.
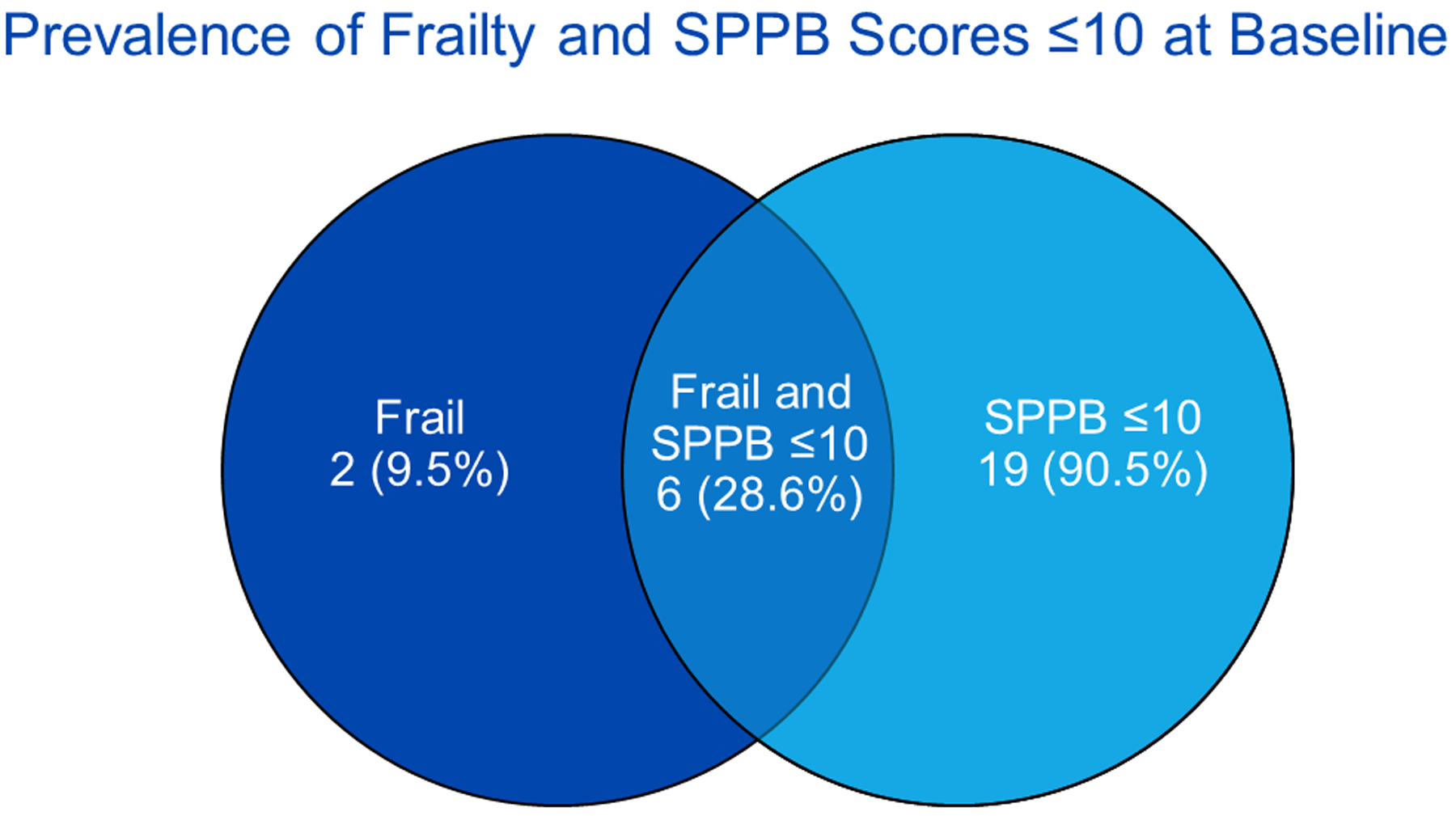
Prevalence of frailty and SPPB scores ≤ 10 at baseline (n=21). SPPB = Short Physical Performance Battery.
Safety, acceptability, and feasibility.
Reassuringly, none of the participants experienced any adverse events during the exercise sessions, and no study-related adverse events occurred. Assessment of the acceptability of the intervention was obtained in 95.2% of participants (n=20). Overall, 100% of respondents reported that the exercise intervention was beneficial to their overall health [median score 5 (IQR, 4–5)] and their physical health [median score 4 (IQR, 4–5)]. Furthermore, 90.0% of participants reported that the exercise intervention was beneficial to their mental health [median score 4 (IQR, 4–5)], 90.0% reported that they were planning to continue to exercise following the intervention [median score 4 (IQR, 4–5)], and 90.0% felt more confident about exercise following the intervention [median score 4 (IQR, 4–5)]. Participant comments following completion of the exercise intervention included “…I can breathe better…[I] have more stamina”; “when I started, I couldn’t walk very long…last day, I walked for over 70 minutes”; “I worked at my own pace…I liked the one on one support and positive encouragement”; “my leg muscles improved.”
Of the 27 accrued participants, 6 (22.2%) withdrew prior to completing at least 8 exercise sessions and 2 additional participants withdrew (7.4%) prior to completing all 16 exercise sessions. Reasons for withdrawal included participant health problems (n=5), lack of time (n=1), lack of transportation (n=1), and other (n=1). Of the 21 participants included in the final study cohort, 90.5% (n=19) completed all 16 PR sessions and 8-week follow-up testing. Median time between enrollment and completion of the exercise intervention was 8.7 weeks (IQR, 8.0–11.9).
Frailty.
Overall, the prevalence of frailty according to Fried frailty phenotype testing decreased following the exercise intervention, but this improvement was not significant (38.1% versus 23.8%, p=0.18) (Figure 3 and Table 3). Among the entire study cohort, median frailty score did not significantly improve [median change 0 (IQR, −1 to 0), p=0.13] by the end of the 8-week period (Table 3). However, the subgroup of participants who were both frail and had a SPPB score ≤ 10 at baseline experienced a significant improvement in median frailty score [median change −1 (IQR, −2.25 to −1) versus 0 (IQR, 0 to 0), p=0.001]. Analysis of individual frailty parameters among the entire cohort, revealed significantly improved physical activity, walking speed, and grip strength following the exercise intervention (Table 3). The prevalence of low physical activity decreased from 28.6% to 9.5% of participants (p=0.046) following the intervention. Furthermore, participant walking time improved by a median of 0.6 seconds (p=0.0002) and grip strength improved by a median of 2.0 kg (p=0.03) after PR. Although the proportion of participants endorsing exhaustion by CES-D (a dichotomous variable) did not change following the intervention, it significantly decreased in the subgroup of participants who were both frail and had a SPPB score ≤ 10 at baseline (33.3% versus 0.0%, p=0.0495). Fatigue as measured by the KDQOL did significantly improve among the entire cohort [median improvement 8.3 points (IQR, −5.0 to 33.8, p=0.0492].
Figure 3.
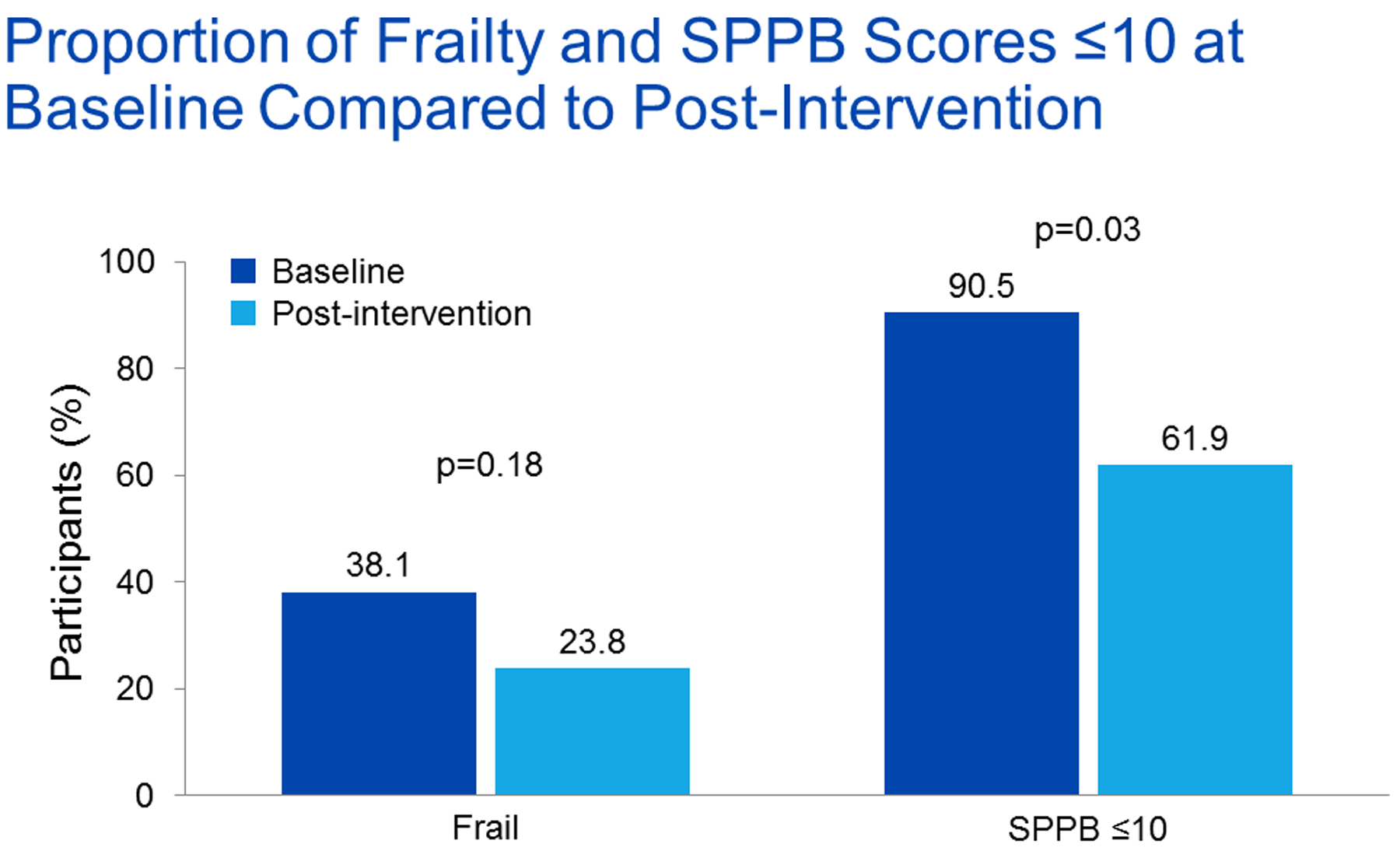
Proportion of frailty and SPPB scores ≤ 10 at baseline compared to post-intervention (n=21, McNemar’s test). SPPB = Short Physical Performance Battery.
Table 3.
Outcomes before and after exercise intervention
| Outcome | Baseline1 | Post-intervention1 | Median difference | p-value2 |
|---|---|---|---|---|
| Frail | 38.1% (n=8/21) | 23.8% (n=5/21) | 0.18 | |
| Frailty parameters | ||||
| SPPB score | 9 [7–10] | 10 [9–11.5] | 1 [0–2] | 0.0007 |
| SPPB | ||||
| Body composition | ||||
| Total minutes exercising on all equipment | 30 [20–41.3] | 40 [30–45] | 9 [−1.5 to 20.5] | 0.02 |
Median [IQR];
Wilcoxon signed rank test for continuous variables and McNemar’s test for categorical variables;
n=21 at baseline, n=20 post-intervention
As anticipated, wasting, as defined by the Fried frailty phenotype (self-reported unintentional weight loss of > 10 lbs in the prior year), did not improve (33.3% before versus 23.8% after PR, p=0.16). Among the entire study cohort, participants did not experience a change in body composition parameters and weight following the exercise intervention (Table 3). Furthermore, participants who were overweight or obese at baseline did not experience different changes in body composition parameters compared to participants who were not overweight or obese at baseline (data not shown). However, participants with baseline wasting experienced a decrease in BMI following the intervention [median change of −0.3 kg/m2 (IQR, −1.5 to −0.2)], while participants without baseline wasting did not [median change of 0.05 kg/m2 (IQR, −0.13 to 0.18), p=0.02]. This decrease in BMI appeared to reflect a loss of muscle mass rather than a loss of body fat. For example, participants with baseline wasting experienced a decrease in SMI of −0.1 kg/m2 (IQR −0.3 to 0) compared to a change of 0.1 kg/m2 (IQR, −0.02 to 0.3) in participants without baseline wasting (p=0.008). Similarly, participants with baseline wasting experienced a decrease in ASMI of −0.2 kg/m2 (IQR, −0.6 to −0.05) compared to a change of −0.04 kg/m2 (IQR, −0.2 to 0.2) in participants without baseline wasting (p=0.04). Participants with baseline wasting also experienced less improvement in chair stand time following the intervention [median improvement of 1.8 seconds (IQR, −4.2 to 0.8) versus median improvement of 6.3 seconds (IQR, −13.8 to −2.1), p=0.03].
SPPB.
Overall, the proportion of participants with a SPPB score ≤ 10 following the exercise intervention decreased significantly compared to baseline (90.5% to 61.9%, p=0.03) (Figure 2). Furthermore, the SPPB score significantly improved (Table 3). Of the SPPB component scores, both the balance test score and the chair stand test score significantly improved. Gait speed test time also significantly improved but not enough to improve the gait speed test score. Among our cohort, 57.1% (n=12) of participants experienced an improvement in gait speed ≥ 0.1 m/s, an increase consistent with clinically meaningful improvement and reduced mortality in older adults.47,48 Time to completion of the exercise intervention was no different in participants who experienced an improvement in SPPB score compared to those who did not [8.6 weeks (IQR, 8.0–10.3) versus 12.3 weeks (IQR, 7.9–13.6), p=0.16]. Other than the improvement in the energy/fatigue scale of the SF-36 mentioned above, the exercise intervention did not appear to be associated with improvement in HRQOL (data not shown). The time participants spent exercising per session increased by 9 minutes from a median of 30 minutes to median of 40 minutes (IQR, −2 to 21 minutes) following the exercise intervention (p=0.02) (Figure 4 and Table 3). This increase in time represented a 29.0% improvement compared to baseline.
Figure 4.
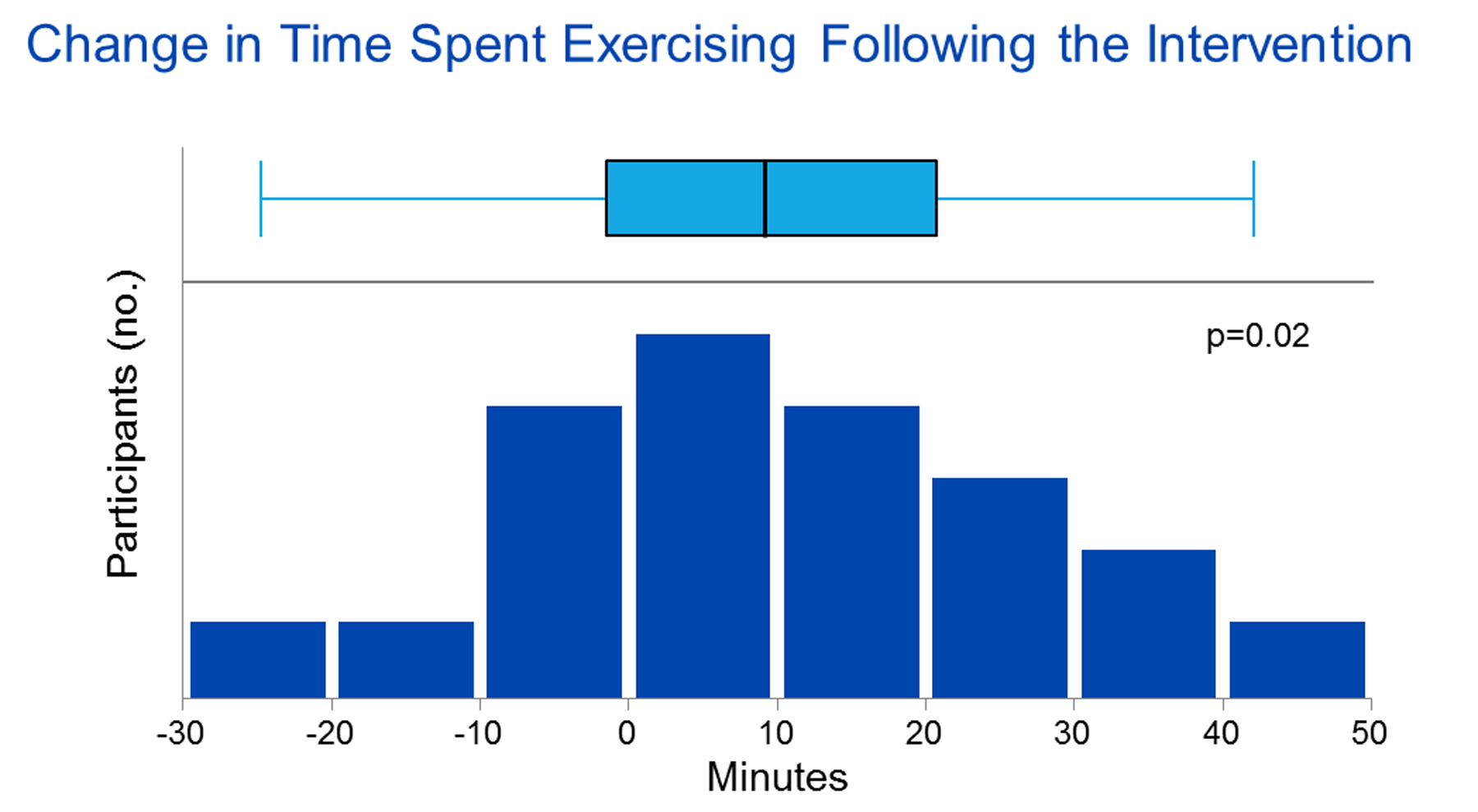
Box plot of interquartile range, range, and median change in time spent exercising following the intervention (n=21, Wilcoxon signed-rank).
DISCUSSION
Our study is the first to examine the effect of an 8-week, PR-based exercise intervention on frailty in KT candidates and individuals with stage 4–5 CKD. Overall, we found that PR was safe, well-received by participants, and feasible. The intervention was associated with promising improvements in measured frailty parameters, including fatigue, physical activity, walking speed, and grip strength. LE function also significantly improved, and participants improved their exercise time per session by nearly 30%.
Our study shows that PR-based exercise programs are safe, acceptable, and feasible in KT candidates and patients with advanced CKD. No study-related adverse events were observed. In terms of acceptability, we found that all respondents reported that the exercise intervention was beneficial to their overall health. The majority of respondents planned on continuing to exercise and reported feeling more confident about exercise following the intervention. In terms of feasibility, our withdrawal rate of 29.6% is comparable, if not better than, rates in other studies involving center-based exercise programs in patients with advanced CKD. For example, in a study by Chen at al. examining a 6-month intradialytic exercise intervention, 24.0% of participants were lost to follow-up or discontinued the intervention, while in a study by McAdams-Demarco et al. examining a 2-month exercise intervention involving weekly supervised exercise sessions, 58.3% of participants completed fewer than 4 exercise sessions.49,50
As outlined in the AST’s recent consensus statement, developing effective frailty interventions in solid organ transplant candidates is a priority for the transplant community.25 Although no studies have examined the effect of exercise on frailty in CKD patients, prior studies involving geriatric adults have demonstrated that frailty is indeed modifiable.27 For example, Cameron et al. found that a 12-month, multidisciplinary intervention incorporating nutrition, mental health, social engagement, and exercise components improved frailty prevalence, physical activity, walking speed, and grip strength in a cohort of older, community-dwelling adults (n=216).51 Likewise, Cesari et al. found that a 12-month intervention incorporating home- and center-based exercise improved frailty prevalence and physical activity among older, community dwelling adults (n=424).52 Although we did not demonstrate an overall improvement in frailty prevalence by Fried’s frailty phenotype among our entire study cohort, we believe this was likely because our study was underpowered. Furthermore, utilizing the Fried frailty phenotype as a benchmark in interventional studies is challenging given that it includes self-report measures subject to bias and numerous dichotomous outcomes. However, we did find that frailty scores significantly improved in participants who were both frail and had a SPPB score ≤ 10 at baseline suggesting that frailty is modifiable even in these especially vulnerable patients.
Our study demonstrates that PR improves fatigue and LE function in KT candidates and patients with advanced CKD. The ability of our intervention to improve fatigue is important given that fatigue is a highly prevalent and disabling symptom in CKD patients and associated with increased mortality.53–55 In terms of LE function, we found that the proportion of participants with a SPPB score ≤ 10 decreased following the intervention (90.5% to 61.9%, p=0.03). This finding is significant, because SPPB scores ≤ 10 have been associated with decreased listing for KT, death on the waiting list, decreased likelihood of transplantation, and increased post-KT mortality.10,16,24 We also found that PR was associated with improved balance and walking speed, with 57.0% of participants experiencing a clinically meaningful improvement. Patients with low balance scores have been shown to experience longer hospital lengths of stay and rehospitalizations following KT, whereas slow walking speed is associated with hospitalization and death in CKD patients.15,56,57 In contrast to our effect on SPPB scores, a recent study by Sheshadri al. found that a 3-month home-based exercise intervention involving pedometers and weekly telephone reminders was not associated with improvement in either fatigue or SPPB scores in a cohort of dialysis patients (n=60).58 This may be due to differences in the patient population, however.
Not surprisingly, no study to date, including ours, has demonstrated an effect of exercise on the Fried frailty parameter of wasting (unintentional weight loss of > 10 pounds over the past 12 months). While this parameter was originally included in the Fried criteria to reflect loss of lean body mass in older adults, it may not apply to individuals with CKD in whom weight loss often reflects changes in volume status or dietary restrictions.8 Furthermore, short-term exercise interventions cannot improve on what is by definition a 12-month parameter. Thus, in our study we examined the impact of PR on body composition as an alternative measure of muscle mass but found no impact of the intervention on this parameter. Prior studies examining the impact of exercise on body composition in CKD patients demonstrate conflicting results with some showing improvement in lean leg mass and SMI and others showing no effect.59–61 In our study, we found that patients with baseline wasting experienced greater loss of muscle mass following the intervention. This finding may reflect increased catabolism and malnutrition in this subgroup of patients who may benefit from multidisciplinary interventions which incorporate both exercise and nutritional supplementation.62
Researchers, patients, and providers agree that KT candidates would benefit from prehabilitation prior to transplant surgery given the strong association between frailty and adverse KT outcomes.1,25,50,63,64 In contrast to lengthier exercise interventions which could delay transplantation, 8-weeks of PR appears to be a viable option associated with much quicker improvement. These findings are consistent with other published studies of PR in patients with lung disease. For example, a large meta-analysis of randomized controlled trials performed in patients with chronic obstructive pulmonary disease showed that 8–12 weeks of PR improves fatigue and exercise capacity.65 Furthermore, 8-weeks of PR has been shown to improve frailty in patients with lung disease.39 In addition to its efficacy, PR is an attractive research intervention to trial in KT candidates because it is standardized33,34 and widely geographically available in part due to coverage established for patients with qualifying conditions under the Medicare Improvements for Patients and Providers Act.65,66 Thus if future studies confirm the impact of PR in frail KT candidates, a PR-based prehabilitation strategy may be easily disseminated and potentially reimbursable.
Limitations of our study include the small sample size, single-center design, and lack of a control group. Furthermore, our study cohort was predominantly Caucasian and English-speaking. Thus, findings from our study should be interpreted with caution and may not generalize to other transplant centers. Validation of our findings in a larger, multi-site study utilizing both randomization and blinding would be important prior to widespread implementation. Although, we did not demonstrate an improved KDQOL measure in this study, it will be important to follow-up in a larger cohort as such increased exercise capacity could improve pre-transplant quality of life. Also, future studies should assess whether improvement in physical activity is sustained after completion of a PR-based intervention and whether stage of CKD is related to improvement.
In conclusion, 8-weeks of PR appears to be safe, acceptable, feasible, and associated with significant improvement in fatigue, physical activity, walking speed, grip strength, SPPB scores, balance test scores, chair stand test scores, and exercise time in KT candidates and individuals with advanced CKD. Further studies involving large, multicenter cohorts are need to confirm these findings, assess sustainability of improvements, and examine ease of dissemination and implementation of the intervention. Future studies would benefit from examining the effect of multidisciplinary interventions combining nutritional supplementation with exercise on body composition. Finally, further studies are needed to determine whether improving frailty and LE function in KT candidates improves healthcare utilization and mortality.
ACKNOWLEDGEMENTS
We thank Dr. Roberto Benzo and his laboratory personnel for their assistance and use of the InBody 770 body composition analyzer.
FUNDING
Dr. Kennedy is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number HL 128859. Dr. Lorenz and Dr. Hickson are supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (Award Number DK 123313 and DK 109134, respectively). The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. This study was supported by the Mayo Clinic CTSA through grant number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). This study was also supported by the Mayo Clinic Robert and Billie Pirnie Endowment Award for Transplant Research.
ABBREVIATIONS
- KT
Kidney Transplant
- SPPB
Short Physical Performance Battery
- LE
Lower Extremity
- AST
American Society of Transplantation
- PR
Pulmonary Rehabilitation
- CKD
Chronic Kidney Disease
- HRQOL
Health-Related Quality of Life
- eGFR
Estimated Glomerular Filtration Rate
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- CES-D
Center for Epidemiologic Studies of Depression Scale
- BMI
Body Mass Index
- SMI
Skeletal Muscle Index
- ASMI
Appendicular Skeletal Muscle Index
- KDQOL-SF
Kidney Disease Quality of Life Short Form
- SF-36
Medical Outcomes Study Questionnaire Short Form 36 Health Survey
- IQR
Interquartile Range
Footnotes
DISCLOSURE
The authors declare no conflicts of interest.
Clinical Trial NCT03535584
REFERENCES
- 1.Cheng XS, Myers JN, Chertow GM, et al. Prehabilitation for kidney transplant candidates: Is it time? Clin Transplant. 2017;31(8). [DOI] [PubMed] [Google Scholar]
- 2.Hartmann EL, Wu C. The evolving challenge of evaluating older renal transplant candidates. Adv Chronic Kidney Dis. 2010;17(4):358–367. [DOI] [PubMed] [Google Scholar]
- 3.Tso PL. Access to renal transplantation for the elderly in the face of new allocation policy: a review of contemporary perspectives on “older” issues. Transplant Rev (Orlando). 2014;28(1):6–14. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr. 2017;68:135–142. [DOI] [PubMed] [Google Scholar]
- 5.Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol. 2013;24(3):337–351. [DOI] [PubMed] [Google Scholar]
- 6.Wu PY, Chao CT, Chan DC, Huang JW, Hung KY. Contributors, risk associates, and complications of frailty in patients with chronic kidney disease: a scoping review. Ther Adv Chronic Dis. 2019;10:2040622319880382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAdams-DeMarco MA, Chu NM, Segev DL. Frailty and Long-Term Post-Kidney Transplant Outcomes. Curr Transplant Rep. 2019;6(1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 10.Haugen CE, Agoons D, Chu NM, et al. Physical Impairment and Access to Kidney Transplantation. Transplantation. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez Fernandez M, Martinez Miguel P, Ying H, et al. Comorbidity, Frailty, and Waitlist Mortality among Kidney Transplant Candidates of All Ages. Am J Nephrol. 2019;49(2):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu NM, Gross AL, Shaffer AA, et al. Frailty and Changes in Cognitive Function after Kidney Transplantation. J Am Soc Nephrol. 2019;30(2):336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147(2):190–193. [DOI] [PubMed] [Google Scholar]
- 14.Haugen CE, Mountford A, Warsame F, et al. Incidence, Risk Factors, and Sequelae of Post-kidney Transplant Delirium. J Am Soc Nephrol. 2018;29(6):1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenz EC, Cheville AL, Amer H, et al. Relationship between pre-transplant physical function and outcomes after kidney transplant. Clin Transplant. 2017;31(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz EC, Cosio FG, Bernard SL, et al. The Relationship Between Frailty and Decreased Physical Performance With Death on the Kidney Transplant Waiting List. Prog Transplant. 2019;29(2):108–114. [DOI] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg. 2017;266(6):1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15(1):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13(8):2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, mycophenolate reduction, and graft loss in kidney transplant recipients. Transplantation. 2015;99(4):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and Postkidney Transplant Health-Related Quality of Life. Transplantation. 2018;102(2):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, Inflammatory Markers, and Waitlist Mortality Among Patients With End-stage Renal Disease in a Prospective Cohort Study. Transplantation. 2018;102(10):1740–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nastasi AJ, Bryant TS, Le JT, et al. Pre-kidney transplant lower extremity impairment and transplant length of stay: a time-to-discharge analysis of a prospective cohort study. BMC Geriatr. 2018;18(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nastasi AJ, McAdams-DeMarco MA, Schrack J, et al. Pre-Kidney Transplant Lower Extremity Impairment and Post-Kidney Transplant Mortality. Am J Transplant. 2018;18(1):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobashigawa J, Dadhania D, Bhorade S, et al. Report from the American Society of Transplantation on frailty in solid organ transplantation. Am J Transplant. 2019;19(4):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Negm AM, Kennedy CC, Thabane L, et al. Management of Frailty: A Systematic Review and Network Meta-analysis of Randomized Controlled Trials. J Am Med Dir Assoc. 2019;20(10):1190–1198. [DOI] [PubMed] [Google Scholar]
- 27.Puts MTE, Toubasi S, Andrew MK, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing. 2017;46(3):383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheshadri A, Johansen KL. Prehabilitation for the Frail Patient Approaching ESRD. Semin Nephrol. 2017;37(2):159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fokkenrood HJ, Bendermacher BL, Lauret GJ, Willigendael EM, Prins MH, Teijink JA. Supervised exercise therapy versus non-supervised exercise therapy for intermittent claudication. Cochrane Database Syst Rev. 2013(8):CD005263. [DOI] [PubMed] [Google Scholar]
- 30.Lacroix A, Kressig RW, Muehlbauer T, et al. Effects of a Supervised versus an Unsupervised Combined Balance and Strength Training Program on Balance and Muscle Power in Healthy Older Adults: A Randomized Controlled Trial. Gerontology. 2016;62(3):275–288. [DOI] [PubMed] [Google Scholar]
- 31.Stout NL, Baima J, Swisher AK, Winters-Stone KM, Welsh J. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature (2005–2017). PM R. 2017;9(9S2):S347–S384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson L, Ellis B, Leng GC. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2008(4):CD000990. [DOI] [PubMed] [Google Scholar]
- 33.Rochester CL, Vogiatzis I, Holland AE, et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am J Respir Crit Care Med. 2015;192(11):1373–1386. [DOI] [PubMed] [Google Scholar]
- 34.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. [DOI] [PubMed] [Google Scholar]
- 35.Attwell L, Vassallo M. Response to Pulmonary Rehabilitation in Older People with Physical Frailty, Sarcopenia and Chronic Lung Disease. Geriatrics (Basel). 2017;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy C, Novotny P, Stevens E, Kirsch J, Garrett J, LeBrasseur N, Benzo R Prospective Trial Using Pulmonary Rehabilitation to Treat Frailty. Am J Transplant. 2018;18(S4):276–277.28801953 [Google Scholar]
- 37.Kennedy CC, LeBrasseur N, Wise R, Sciurba F, Benzo R. Does pulmonary rehabilitation impact frailty? Am J Transplant. 2015(15 (Suppl 3)). [Google Scholar]
- 38.Larsson P, Borge CR, Nygren-Bonnier M, Lerdal A, Edvardsen A. An evaluation of the short physical performance battery following pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. BMC Res Notes. 2018;11(1):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maddocks M, Kon SS, Canavan JL, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71(11):988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karnofsky DA BJ. The clinical evaluation of chemotherapeutic agents in cancer. New York: Columbia University Press; 1949. [Google Scholar]
- 41.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 43.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. [DOI] [PubMed] [Google Scholar]
- 44.Barotfi S, Molnar MZ, Almasi C, et al. Validation of the Kidney Disease Quality of Life-Short Form questionnaire in kidney transplant patients. J Psychosom Res. 2006;60(5):495–504. [DOI] [PubMed] [Google Scholar]
- 45.Hays RD, Amin N, Leplege A, et al. Kidney Disease Quality of Life Short Form (KDQOL-SFtm), Version 1.2: A manual for use and scoring (French questionnaire, France) Santa Monica, CA: RAND; 1997. [Google Scholar]
- 46.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3(5):329–338. [DOI] [PubMed] [Google Scholar]
- 47.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55(11):1727–1734. [DOI] [PubMed] [Google Scholar]
- 48.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. [DOI] [PubMed] [Google Scholar]
- 49.Chen JL, Godfrey S, Ng TT, et al. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant. 2010;25(6):1936–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McAdams-DeMarco MA, Ying H, Van Pilsum Rasmussen S, et al. Prehabilitation prior to kidney transplantation: Results from a pilot study. Clin Transplant. 2019;33(1):e13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cameron ID, Fairhall N, Langron C, et al. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cesari M, Vellas B, Hsu FC, et al. A physical activity intervention to treat the frailty syndrome in older persons-results from the LIFE-P study. J Gerontol A Biol Sci Med Sci. 2015;70(2):216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansen KL. Exercise in the end-stage renal disease population. J Am Soc Nephrol. 2007;18(6):1845–1854. [DOI] [PubMed] [Google Scholar]
- 54.Picariello F, Norton S, Moss-Morris R, Macdougall IC, Chilcot J. Fatigue in Prevalent Haemodialysis Patients Predicts All-cause Mortality and Kidney Transplantation. Ann Behav Med. 2019;53(6):501–514. [DOI] [PubMed] [Google Scholar]
- 55.Weisbord SD, Fried LF, Arnold RM, et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16(8):2487–2494. [DOI] [PubMed] [Google Scholar]
- 56.Kutner NG, Zhang R, Huang Y, Painter P. Gait Speed and Mortality, Hospitalization, and Functional Status Change Among Hemodialysis Patients: A US Renal Data System Special Study. Am J Kidney Dis. 2015;66(2):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roshanravan B, Robinson-Cohen C, Patel KV, et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol. 2013;24(5):822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheshadri A, Kittiskulnam P, Lazar AA, Johansen KL. A Walking Intervention to Increase Weekly Steps in Dialysis Patients: A Pilot Randomized Controlled Trial. Am J Kidney Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baria F, Kamimura MA, Aoike DT, et al. Randomized controlled trial to evaluate the impact of aerobic exercise on visceral fat in overweight chronic kidney disease patients. Nephrol Dial Transplant. 2014;29(4):857–864. [DOI] [PubMed] [Google Scholar]
- 60.Lopes LCC, Mota JF, Prestes J, et al. Intradialytic Resistance Training Improves Functional Capacity and Lean Mass Gain in Individuals on Hemodialysis: A Randomized Pilot Trial. Arch Phys Med Rehabil. 2019;100(11):2151–2158. [DOI] [PubMed] [Google Scholar]
- 61.Muras-Szwedziak K, Masajtis-Zagajewska A, Pawlowicz E, Nowicki M. Effects of a Structured Physical Activity Program on Serum Adipokines and Markers of Inflammation and Volume Overload in Kidney Transplant Recipients. Ann Transplant. 2019;24:569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dedeyne L, Deschodt M, Verschueren S, Tournoy J, Gielen E. Effects of multi-domain interventions in (pre)frail elderly on frailty, functional, and cognitive status: a systematic review. Clin Interv Aging. 2017;12:873–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng XS, Lentine KL, Koraishy FM, Myers J, Tan JC. Implications of Frailty for Peritransplant Outcomes in Kidney Transplant Recipients. Curr Transplant Rep. 2019;6(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Pilsum Rasmussen S, Konel J, Warsame F, et al. Engaging clinicians and patients to assess and improve frailty measurement in adults with end stage renal disease. BMC Nephrol. 2018;19(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015(2):CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MLN Matters Number: MM6823. 2010. [12/7/2019]. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/MM6823.pdf.
- 67.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]


