Abstract
Topical hormone therapy with natural or synthetic ligands of nuclear hormone receptors such as glucocorticoids, vitamin D analogues and retinoids has a long and highly successful tradition in dermatology. Yet the dermatological potential of thyroid hormone receptor (TR) agonists has been widely ignored, despite abundant clinical, cell and molecular biology, mouse in vivo, and human skin and hair follicle organ culture data documenting a role of TR-mediated signalling in skin physiology and pathology. Here, we review this evidence, with emphasis on wound healing and hair growth, and specifically highlight the therapeutic potential of repurposing topical L-thyroxine (T4) for selected applications in future dermatological therapy. We underscore the known systemic safety and efficacy profile of T4 in clinical medicine, and the well-documented impact of thyroid hormones on, for example, human epidermal and hair follicle physiology, hair follicle epithelial stem cells and pigmentation, keratin expression, mitochondrial energy metabolism and wound healing. On this background, we argue that short-term topical T4 treatment deserves careful further preclinical and clinical exploration for repurposing as a low-cost, effective and widely available dermatotherapeutic, namely in the management of skin ulcers and telogen effluvium, and that its predictable adverse effects are well-manageable.
Keywords: bFGF, hair follicle, hormonal therapy, human skin, mitochondria, pigmentation, RXR, thyroid, TR, TRE, triiodothyronine, wound healing
1 ∣. INTRODUCTION
Dermatologists have used natural or synthetic agonists of glucocorticoid, vitamin D or retinoid nuclear hormone receptors, both systemically and topically, for decades in the management of skin diseases. In fact, the introduction of glucocorticoids, calcitriols and retinoids into the clinic each constituted landmark progress in skin therapies, and the range of dermatological indications for each of these distinct classes of chemicals rapidly expanded after they became widely accepted in clinical practice.1,2 Given the success of these agents, it is surprising that a fourth class of potent nuclear hormone receptor agonists has not been developed as part of the therapeutic arsenal of dermatology: endogenous and synthetic thyroid hormone receptor (TR) ligands.3
This “Cinderella status” of these peptide hormones among endocrinological dermatotherapy is even more surprising in view of the fact that clinicians have appreciated for decades how powerfully even slight variations in the thyroid hormone (TH) serum concentration impact the function of human skin and its appendages (Table 1, see below).4-7 Indeed, the cutaneous impact of the thyroid gland was recognized long before THs had even been isolated.8 Moreover, systemic therapy with L-thyroxine (T4) is among the most important and frequently prescribed medications in all of medicine.9 T4 is also highly affordable, very stable, and has an exceptionally well-characterized toxicological profile, which is backed up by decades of clinical experience.10 Finally, a host of clinical, cell biology, rodent in vivo, and human skin and hair follicle (HF) organ culture data have long documented a central role of TR-mediated signalling in skin physiology and additionally as a mediator of skin pathology (see below). However, none of this has led to the systematic exploration of T4 as a novel therapeutic in clinical dermatology.
TABLE 1.
| Sign | Thyroid dysfunction |
|---|---|
| Dryness | Mostly hypothyroidism |
| Hyperhidrosis | Hyperthyroidism |
| Dry and/or brittle hair | Hypothyroidism |
| Hair loss | Hypo- or hyperthyroidism |
| Yellow skin tint | Hypothyroidism |
| Pruritus | Hypo- or hyperthyroidism |
| Impaired wound healing | Hypothyroidism |
| Frontal fibrosing alopecia | More frequently associated with thyroid disorders |
| Effluvium | Hypo- and hyperthyroidism |
| Alopecia areata | Hyperthyroidism |
| Brittle nails | Hypo- and hyperthyroidism |
| Palmoplantar erythema | Hyperthyroidism |
| Hyperpigmentation | Hyperthyroidism |
| Vitiligo | Graves’ disease |
| Myxedema | Hypothyroidism |
Therefore, this Viewpoint essay synthesizes translationally relevant evidence to support mainly this straightforward message: mainstream dermatological research should embrace TR signalling as a promising target for pharmacological intervention in future skin therapies. Specifically, we argue that topical application of T4, with likely has much greater safety than systemic T4 and reaches high ligand concentrations directly in the target organ, deserves organized and focused exploration for repurposing in dermatology. We also discuss questions regarding methodological, toxicological, regulatory and product development hurdles that need to be overcome for repurposing T4 as a topical therapeutic in dermatology.
While other natural or synthetic TH analogues/thyromimetics such as triiodothyroacetic acid (TRIAC) and eprotirome also have potential dermatological application,11-14 we focus on the topical use of T4 itself, since the pharmacology and toxicology of this natural, FDA-approved TH have been well characterized over decades. Moreover, T4’s low cost and high penetrability into human skin15 facilitates the potential repurposing of T4 for the management of selected skin disorders.
Using imagery of the fairy tale popularized by the Brothers Grimm,16,17 dermatologists, endocrinologists and skin biologists have have long held in hand, but not used Cinderella's slipper, even though the identity of its enchanting owner known for decades (Kendall reported the isolation of T4 in 1915,18 and its impact on human skin has been known for as long as most readers of this essay can remember).5 We therefore propose that it is about time to invite this Cinderella among hormones that shape human skin physiology to the dance floor of dermatological therapy so as to systematically probe how she performs.
2 ∣. THYROID HORMONE (TH) BIOLOGY
THs are produced exclusively in the thyroid gland under the control of a complex neuroendocrine regulatory system, the hypothalamic–pituitary–thyroid (HPT) axis, which controls the development, growth and metabolism of vertebrate organisms,19,20 including the physiology of human skin.21,22 Stimulation of the pituitary gland by hypothalamus-derived thyrotropin-releasing hormone (TRH) induces pituitary thyrotropin (TSH) secretion. TSH then stimulates the thyroid gland to synthesize and secrete T4 and triiodothyronine (T3), which then negatively regulate TRH and TSH secretion.23
The overall potency of THs as endocrine growth regulators is perhaps most impressively documented in one of the most striking tissue remodelling events of vertebrate biology, metamorphosis (eg tail regression in frog tadpoles), which is strictly TH-regulated.24-26 If one searches for a comparably complex phenomenon in mammalian organisms that is also profoundly regulated by THs, a prominent example is the control of craniofacial development by THs.27
This complexity and diversity of TH functions is better understood if one calls to mind that THs induce a plethora of gene expression changes that utilize both genomic and non-genomic pathways.28-31
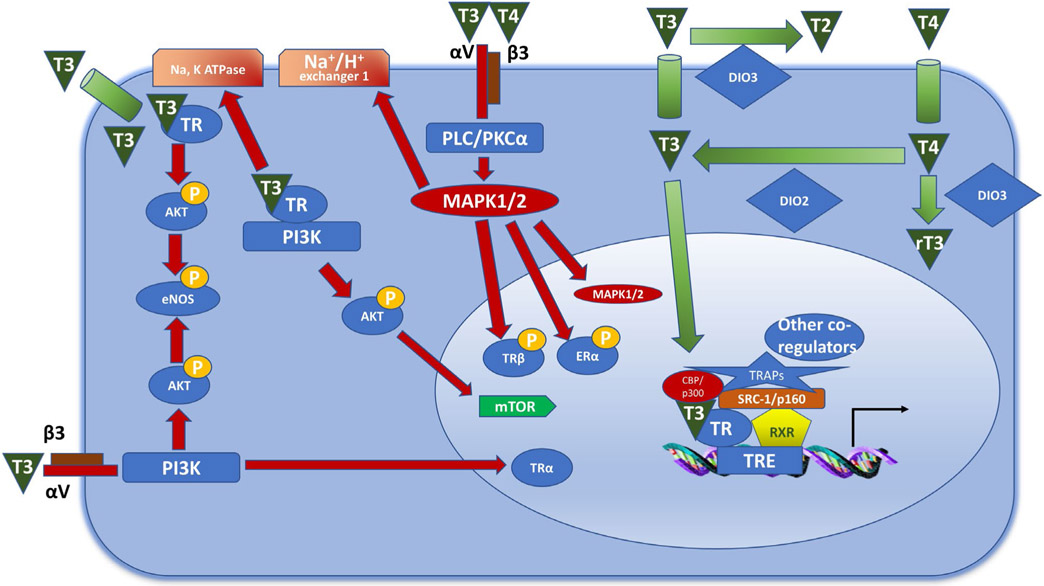
The classical genomic pathway requires intracellular binding of THs to the nuclear TH receptors c-erb Aα and Aβ (TRα/β); in humans, TRα1 seems to be the functionally most relevant product of the coding gene (THRA).32 In the nucleus, the TH-TR complex then preferentially binds to the promoter regions of genes that contain a defined TH response element (TRE) (details, Figure 1).33-35 Although, like all nuclear receptors, TR can sometimes regulate transcription as a homodimer, it more commonly interacts with retinoid X receptor (RXR), and the TR-RXR heterodimer is, arguably, one of the most transcriptionally active TR forms.36 The intricacy of TRE sequence interactions with the receptor complex can further determine the transcriptional output (activation or inhibition). Added levels of complexity of these interactions originate from additional protein interactions with the receptor complex, namely with co-activators or co-repressors (Fig. 1).7,37-40
FIGURE 1.
The genomic and non-genomic effects of thyroid hormones. The genomic effects of thyroid hormones are mediated by TRs. TR can act on the DNA as a homodimer or as a heterodimer with RXR, which is the more stable form. The heterodimer than localizes to thyroid response elements (TREs) that are in the promoter regions of the target genes. Following the recruitment of transcriptional co-activators (CBP/p300, SRC-1/p160, TRAPs), transcription is initiated. The non-genomic effects can be mediated by activation of plasma membrane proteins or via TRs located in the cytoplasm. Integrin αV/β3 can bind T3 and T4, leading to activation of the MAPK1/2 pathway following stimulation of PLC/PKCα. This results in trafficking of ERα and TRβ to the nucleus, and in increased activity of the sodium proton exchanger. Binding of T3 to integrin αV/β3 can also result in activation of the PI3K pathway, resulting in TRα trafficking to the nucleus. Binding of T3 to TRβ in the cytoplasm leads to Akt phosphorylation, which results in mTOR and eNOS activation. DIO, deiodinase; eNOS, endothelial nitric oxide synthase; ER, estrogen receptor; RXR, retinoid X receptor; TR, thyroid receptor; TRE, thyroid response element. Adapted from Davis et al,29 Chi et al50 and Jabbar et al51
In addition, there is a plethora of additional effects that are not mediated by direct TRE-TR binding such as TH-mediated miRNA regulation,41 DNA methylation,42 metabolic effects of TH,43 and post-translational modifications of T344 to name but a few.45 Given that T3 shows a considerably higher affinity to cognate nuclear receptors than T4, circulating T4 is thought to largely act after intracellular deiodination into T3 in peripheral organs, including human HFs.46-48 However, there is increasing appreciation that T4 may also exert direct, T3 conversion-independent effects, again including in human HFs.48,49 Such effects can be exerted in a TR-nuclear independent, non-genomic manner by partnering with different cytosolic mediators (see Figure 1 for more details).29,50,51 The plasma membrane transport of THs is facilitated and regulated by specialized, energy-dependent transporter systems,52 while TH metabolism and thereby local TH activity in a given tissue is enzymatically controlled via differentially expressed deiodinases.53-56 All of these elements are present and active in human skin (see below).
The cell- and tissue-level biological effects of THs are greatly complicated by intimate interactions of TR-mediated signalling with vitamin D receptor (VDR), glucocorticoid receptor (GR) and retinoid receptor-controlled pathways, for example by modulating the proliferation-inhibitory and differentiation-stimulatory response of epidermal keratinocytes to retinoids.57 As an example, the nuclear transcription factor coded for by the hairless gene, a key regulator of HF development and cycling that also exerts tumor suppression activity,58-60 acts as an important TR repressor, presumably by recruiting histone deacetylases.61 On the other hand, TR-mediated signalling suppresses HR expression in the CNS,62 but it is as yet unclear whether this also occurs in human skin. TH thus has heterogeneous, cell-context dependent effects as inducer of either cell proliferation or differentiation, dependent on signalling context and cell type.
3 ∣. CLINICAL POINTERS TO THYROID HORMONES AS MAJOR MODULATORS OF HUMAN SKIN FUNCTION
Both hypothyroid and hyperthyroid states, leading to abnormally low and excessively high TH serum levels, have long been known to induce clinically visible changes in human skin and hair phenotype (Table 1).5,63-67 In hyperthyroidism, skin changes include erythema, palmoplantar hyperhidrosis, acropathy and infiltrative dermopathy;68 Graves' disease may be associated with generalized pruritus, chronic urticaria, vitiligo and diffuse skin hyperpigmentation.69 In hypothyroidism, the skin typically is cool and dry with a pasty appearance and a boggy feel as the result of diffuse, excessive storage of mucopolysaccharides (myxoedema).70 The epidermis of hypothyroid patients typically is thin and hyperkeratotic.57 Interestingly, hypothyroid patients may also display a clinically asymptomatic small-fibre sensory neuropathy and reduced intraepidermal nerve fibre density.71 These clinical pointers already highlight that human skin physiology is impacted by THs on multiple levels.
Likely due, at least in part, to their exceptionally high metabolic and energetic demands, growing (anagen) human scalp HFs are extremely sensitive to even minor variations in TH serum level as a result of direct T3 and T4 effects on this skin appendage.48 Clinically, this presents as alopecia that may develop due to prolonged telogen effluvium, along with dry, brittle and dull hair shafts, in patients with hypothyroidism.5,72 Hyperthyroid states can also induce telogen effluvium, together with thinned hair shaft diameter and brittle, greasy hair,64,73 even though THs stimulate hair matrix proliferation.72 Hair shafts of patients with hyperthyroidism also show substantially reduced tensile strength.74 Although alopecia areata has been reported to be more prevalent in patients with hyperthyroidism, other studies have questioned this association.75,76 Yet, patients with frontal fibrosing alopecia reportedly have more often associated thyroid disorders than is generally seen in the European population.77 Early greying has been claimed to be related to autoimmune thyroid disease, and both hypo- and hyperthyroidism,78 whereas re-pigmentation of previously grey/white hair has been reported in some patients under systemic T4 therapy.79
4 ∣. CUTANEOUS TH BIOLOGY
That human skin and its appendages profoundly respond to changes in TH serum levels is indisputed. The diversity of the cutaneous effects of THs is mirrored in the fact that both TRα and TRß are widely expressed in human skin,80-83 including in the HF,48 and numerous resident cell populations in human skin express genes whose promoters carry a TRE (eg keratinocytes, adipocytes).84,85 Deiodination of T4 into T3 also occurs prominently in rodent and human skin, including in human scalp HFs.46-48 Though human keratinocytes express both TRs,81,86 the complexity of the signalling pathways that THs utilize (Figure 1), and the “promiscuous liaisons” of TR-, VDR- and retinoid receptor-mediated signalling,87 renders it extremely challenging to dissect the exact mechanisms by which THs exert their effects in intact human skin or HFs ex vivo or in vivo.
Gene expression profiling of T3-treated human keratinocytes in vitro has shown suppression of the expression of genes that are important for the remodelling by, attachment to and production of extracellular matrix, including integrin β4, plectin, collagen XVII, MMP1, MMP3 and MMP14.88-90 THs also change keratin expression in epidermal and HF keratinocytes, including keratin genes important for normal epidermal function, such as K5, K6, K16 and K17.25,38,39,48,70,91-95 Such regulation is mediated through binding of THs to TRE in keratin gene promoter regions.70 However, these regions are shared binding sites for glucocorticoid and retinoid receptors, thus providing complex interactive platforms for hormonal crosstalk. The TRE in keratin genes mediates binding of four monomers of GR and both homodimers and heterodimers of TR, RAR and RXR.38,39,91,94,96
An example for such possible complexity is the fact that while several keratin genes are known to have negative control elements in their promoter that are activated by TH at the cellular level (eg K5, K6, K14),39 the same keratin genes are up-regulated by TH administration in vivo in mice and ex vivo in human HFs.93 For example, K6 mRNA and protein expression is up-regulated in human HFs ex vivo, while K15 promoter activity and transcription are also induced by T3 in human HF epithelial stem cells ex vivo and in vitro.48,92 Possibly, this could reflect the simultaneous impact of intracutaneously generated retinoids, cortisol and/or vitamin D3 metabolites.97 Given that keratin genes can be also regulated by TSH, one is tempted to speculate that these seemingly contradictory effects of TH on keratin expression might also be indirectly mediated, for example by T3/T4-induced reduction in the amount of intracutaneously produced TSH.63,70,98
Interestingly, the deletion of TRs in TRα1/TRβ knockout mice results in substantially altered miRNAs levels, including the down-regulation of miR-21, miR-31, miR-34 and miR-203. This further suggests that intracutaneous TR-mediated signalling results in longer-term ripple effects, some of which are miRNA-mediated, which far exceed those induced by changes in the expression of TRE+ genes, and can induce skin defects by altering hair cycling, wound healing and stem cell function.41 This concept is also supported by the finding that THs differentially regulate peripheral clock activity in human skin, namely the expression level of BMAL and PERIOD1 in human scalp HFs.99 This is important if one considers that ca. 10% of mammalian genes are thought to be regulated by changes in clock gene activity,100 which impacts human skin and HF physiology profoundly.99,101,102
The majority of mechanistic studies that have dissected nuclear receptor binding utilized a reductionist in vitro approach, such as purified proteins, specific DNA binding sites and transient transfection in cell lines. However, the impact of hormone regulation on overall tissue physiology reflects the concurrence of multiple, simultaneous, interacting signals88,92,103 and is the net result of both genomic and non-genomic effects.104-106 This underscores the need for tissue-level studies to decipher the physiologically relevant functional effects of TH signalling in human skin.
Given the sweeping functional repercussions of direct and indirect TR-mediated signalling for mammalian skin homoeostasis and function, it is not surprising that intracutaneous TH metabolism underlies tight regulation by deiodinases, for example via the intraepidermal activity of the TH-inactivating type 3 deiodinase, which may function as an important coordinator of keratinocyte proliferation and differentiation in murine epidermis.53,54
Unfortunately, with the notable exception of keratinocytes and their progenitor cells,86 the impact of TH on most other resident cell populations of human skin is unknown or remains very incompletely explored. However, the observation that hyperthyroidism can be associated with skin hyperpigmentation (Table 1) and that both T3 and T4 significantly stimulate melanogenesis in human scalp HFs48 strongly suggests that the recognized key roles of TH in the regulation of pigment cell development, patterning and melanogenesis in lower vertebrate skin,107-109 translate to human skin. Moreover, skin phenotype analyses in TRα1 and TRß double-knockout mice suggest that TR signalling also down-modulates skin inflammation via AP-1, NF-kB- and STAT3-dependent suppression of selected cytokines and chemokines.86
There is evidence to support a critical role of TH in regulating epidermal differentiationand in establishing the epidermal barrier during development.110 For example, ex vivo studies with skin explants from fetal rats have shown that TH accelerated development of the stratum corneum and epidermal barrier formation.111,112 THs are important in maintaining the delicate balance between proliferation and differentiation of keratinocytes, and this is evident both in normal conditions such as epidermal regeneration during wound healing,93 and in pathological conditions such as skin cancer, by crosstalking with the Sonic hedgehog pathway.113 Also, the TH analogue, TRIAC, induces epidermal thickening in mice and stimulates epidermal keratinocyte proliferation, presumably via cyclin D1 activation resulting in quicker entrance into the S phase.14
These effects can be mediated either directly via the TRE in selected keratin promoters or indirectly, and it is plausible to ask whether such effects can be harnessed to treat inherited keratin disorders.70,114-117
5 ∣. DOES HUMAN SKIN DISPLAY A FUNCTIONAL PERIPHERAL EQUIVALENT OF THE CENTRAL HYPOTHALAMIC–PITUITARY–THYROID AXIS (HPT)?
Even though this is not widely appreciated among dermatologists and skin biologists, human skin is a major, “non-classical” site of expression and production of several neurohormones that regulate the synthesis and secretion of other key hormones, including cortisol and THs.6,97,114,118 For example, the existence of a fully functional peripheral equivalent of the hypothalamic–pituitary–adrenal axis (HPA: CRH→ACTH→cortisol) in human skin, that its existence was already proposed in 1996,119 along with cortisol production and negative feedback regulation, was first detected by examining human scalp HFs,120 while regulated intracutaneous cortisol production was subsequently also demonstrated in human epidermis.121-127
Before these HPA axis-related studies, Slominski et al had already reported transcript or protein expression for several key members of the HPT axis (HPT: TRH→TSH→T3/T4) in human skin and some of its constituent cell populations.128 For example, cultured human dermal and HF fibroblasts transcribe TRH,128 whereas TSH mRNA and protein are found in normal human epidermis98 and TSH receptor (TSHR) transcripts and protein in human skin and HF mesenchyme.63,129 Both TRH and its receptor are expressed in the epithelium of normal human scalp HFs.130,131
In the current context, it may be most important that intraepidermal TSH mRNA and protein expression are up-regulated by TRH and down-regulated by THs in organ-cultured human skin.98 Thus, intraepidermal TSH expression is regulated by the classical endocrine controls that define the central HPT axis, suggesting that at least some elements of the central HPT axis, with the notable exception of TH production itself, are established in human skin.21,22,98 Topical administration of T4 is likely to functionally impact on this partial, intracutaneous HPT axis equivalent. An additional “twist” to this complex plot is that a naturally occurring metabolite and by-product of cholesterol synthesis, farnesyl pyrophosphate, serves as a bona fide hormone that has high affinity to ligand binding pockets of multiple nuclear receptors including TR.132,133 This raises the intriguing possibility that, by regulating cholesterol synthesis,134 one can also modify the overall effects of hormone action,135 including that of THs.
6 ∣. THYROID HORMONES AS KEY REGULATORS OF HUMAN HAIR FOLLICLE FUNCTION AND STEM CELL BIOLOGY: PRECLINICAL EVIDENCE
Clinical observations have long documented the exceptional sensitivity of human scalp HFs to insufficient or excessive TH stimulation (Table 1). Together with the fact that human HFs express both TRs, with TRß1 likely being the predominant and functionally most relevant isoform48,80,81 and transcribe relevant deiodinase genes,48 it is suggested that exploration of TH effects on these human skin appendages will generate important pointers to potential dermatological applications of topical T4. That epithelially targeted depletion of type 3 deiodinase in mice induces hair cycle abnormalities53 further supports the importance of TH metabolism for HF function in vivo. TH may also sensitize the HF response to other hormones besides glucocorticoids, calcitriols and retinoids,87 such as growth hormone.136 This could be relevant in the context that both retinoids137 and growth hormone138 negatively regulate the growth of human scalp HFs ex vivo.
If one directly stimulates microdissected, organ-cultured human scalp HFs with T4, this promotes proliferation and inhibits the apoptosis of hair matrix keratinocytes, along with a prolongation of the hair growth phase (anagen).48 This is associated with a down-regulation of the intrafollicular expression of TGF-β2, the key catagen-promoting growth factor and of K14, while K6 in the outer root sheath of the HF is up-regulated; hair shaft production ex vivo is not significantly altered. Moreover, T4 strongly stimulates melanin synthesis in the HF pigmentary unit of human scalp HFs in a hair cycle-independent manner48—the first evidence that T4 regulates melanocyte functions within an intact human (mini-)organ.
THs are also important regulators of human HF epithelial stem cell (eHFSC) functions in the bulge: namely physiologically relevant concentrations of T3 and T4 enhance K15 promoter activity and expression in human eHFSCs in situ and in vitro,139 just as had been shown in earlier epithelial cell culture work.92 Interestingly, these THs also upregulate expression of the immunoinhibitory “no danger” cell surface molecule, CD200, on human eHFSC. Eventually, however, T3 and T4 inhibit clonal growth of these eHFSCs and induce both their differentiation and apoptosis after they have been isolated from the bulge and cultured in vitro.139
Human eHFSCs also express type 2 deiodinase 2, suggesting that they can convert T4 into T3 and actively regulate TH metabolism.139,140 Later in vivo studies in TR knockout mice confirmed that eHFSC underlies endocrine regulation by THs and suggested that TR-mediated signalling may promote emigration of bulge stem cells from their niche.141 Interestingly, mRNA and protein expression of TR3, an orphan member of the nuclear hormone receptor superfamily, is enriched in the bulge of human telogen HFs.142
Hormones can also affect human skin by regulating circadian rhythmicity, either centrally or peripherally. For example, melatonin is a potent regulator of the central and peripheral clock,143 and these effects can lead to changes in the skin by modulating, for example, mitochondria function.143 Therefore, it is important to note that THs are also important chronobiologically active hormones that regulate central clock activity,144,145 influence circadian clock oscillations,146,183 are essential for seasonal rhythms in mammals and show diurnal expression patterns.147,148 However, T4 also impacts on peripheral clock activity of human scalp HFs: T4 reduces intrafollicular BMAL1 and PER1 expression after 24 hours of scalp HF organ culture, while after 6 days, transcript and/or protein levels of all core clock and clock target genes (BMAL1, PER1, clock, CRY1, CRY2) are up-regulated.99 This effect of T4 on intrafollicular clock activity is likely to functionally impact on human HF biology, since the down-regulation of intrafollicular core clock gene expression prolongs anagen/inhibits catagen development102 and stimulates HF pigmentation.99 Since BMAL1 and PER1 are also expressed in the human HF bulge, the niche for eHFSCs,99 some of the stem cell effects of T4 might be indirectly mediated via TH-regulation of peripheral clock activity in human eHFSCs.
7 ∣. THYROID HORMONES AND WOUND HEALING
There is multiple level evidence that TH should promote wound healing (WH) in human skin, ranging from clinical data from patients with hypothyroidism to various experimental in vivo, ex vivo and in vitro models7,93,149-151 (see also below, “Skin ulcer management”). It is assumed that hypothyroidism is associated with delayed WH,152 and T4 reportedly is necessary for the healing of radiotherapy-induced neck fistula in hypothyroid patients.153,154 However, definitive clinical evidence on this is as yet missing, and there are reports that a hypothyroid state does not affect WH in pigs155 and even in humans.156
Nevertheless, a host of in vivo data from hypothyroid rats and mice as well as ex vivo evidence from experimentally wounded human skin demonstrate a key role for THs in WH.93,151,157-159 Mice treated with systemic (intra-peritoneal) T3 achieved a WH rate that was twice the rate of TH-deficient mice,93 and oral T3 also led to improved quality of wounds and increased the healing rate in rats.157,159 The same WH promoting effect was achieved in mice treated with topical T3 and in rats treated with the combination of Aloe vera, TH and silver sulfadiazine.93,158 WH was also accelerated in Guinea pigs with topical T3 administration, mainly by enhanced wound contraction.150 The TH effects on WH are very likely mediated by TRs, as mice lacking both TRα1 and TRβ showed impairment in WH, with delayed re-epithelialization that was mediated by a defect in keratinocyte proliferation.160 Finally, mice that selectively lack oncofetal protein type 3 deiodinase, the TH-inactivating enzyme in the epidermis, show delayed WH.53
The WH process requires coordination of multiple cellular functions and multiple cell types to properly execute this programme.161 Thus, finding a single molecule that can target multiple cell types and cellular processes with sufficient clinical efficacy represents a major challenge. T4 may be one such molecule: as our synthesis above has shown, T4 targets multiple cellular processes and cell types that contribute to WH, including epithelialization, extracellular matrix remodelling, angiogenesis and inflammation, to name but a few.162,163 TH can enhance keratinocyte proliferation in vitro,65 a process that is accompanied by increased expression of the “wound healing keratins,” such as K6, K16 and K17, in cultured human keratinocytes.93
T4 also has a pro-angiogenic effect that is mediated by alpha v beta 3 integrin receptors and dependent on MAPK pathway activation, as was demonstrated in the three-dimensional human microvascular endothelial sprouting model.164 Similar pro-angiogenic effects for T4 have also been observed in the chick chorioallantoic membrane model, and these effects were dependent on fibroblast growth factor 2.165 These angiogenesis-stimulating effects may translate to experimentally wounded human skin ex vivo.151 Finally, TH can modify TGFb signalling and attenuate fibrosis,166 while hypothyroid patients have asymptomatic small-fibre sensory neuropathy, which impairs WH.71 Thus, while the final verdict of T4 effects on human WH is still out, the vast majority of currently available indications suggests that this may well be the “bride” for whom the “princess” of translational WH research have long been looking.
8 ∣. POTENTIAL FUTURE USES OF TOPICAL L-THYROXINE IN DERMATOLOGICAL THERAPY
This re-exploration of the skin research literature on THs makes it abundantly clear that these multipotent peptide hormones rival the impact of endogenous steroid hormones in their importance for mammalian skin physiology, whose impact on human skin function they also modulate. But, based on what we have delineated above, which practical applications for topical T4 in clinical dermatology currently appear most promising and deserve to be explored with preference for the repurposing of T4? Figure 2 summarizes the answer, and salient details are provided below.
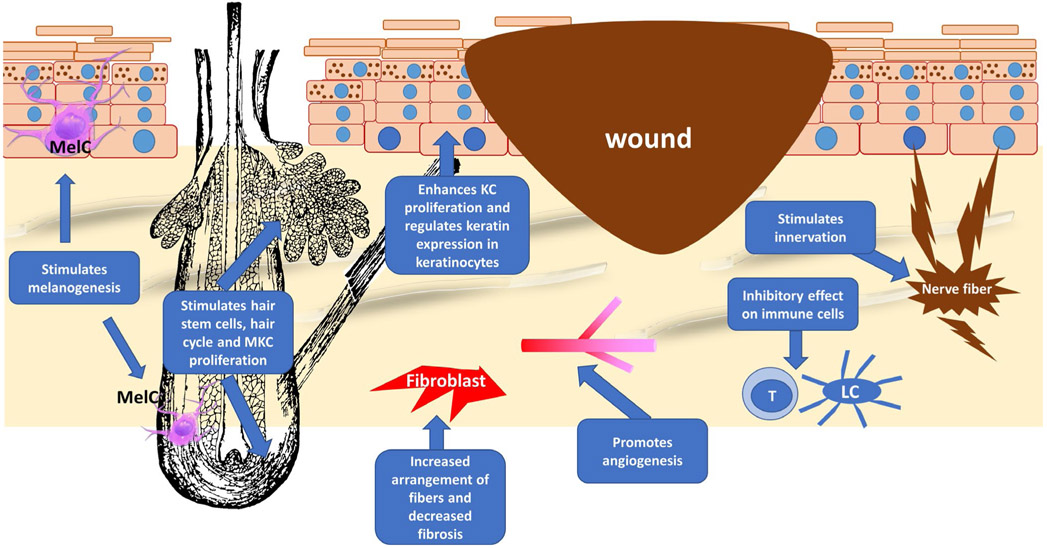
FIGURE 2.
The different aspects by which T4 can enhance wound healing. T4 can modulate the function of several components of the skin, which are important to the wound healing process, including the hair follicle, the production of extracellular fibres by the fibroblasts, angiogenesis, immune cells (eg T cells, Langerhans cells [LH]), epidermal keratinocytes (KCs) (keratin expression, proliferation and differentiation) and the peripheral nervous system. All these components can be a target for the thyroid hormone. Hair follicle image for this figure was obtained from https://www.publicdomainpictures.net and melanocyteimages from Wikipedia (https://en.wikipedia.org). MKC, matrix keratinocytes
8.1 ∣. Skin ulcer management
The relentlessly expanding “ulcer epidemic” is one of the most significant healthcare challenges faced by medical systems around the globe.167,168 Given hypothyroidism-associated WH abnormalities,7,160 direct evidence that topical T3 and T4 promotes acute WH in mice in vivo 93 and that T4 promotes epidermal repair and possibly even angiogenesis in wounded human skin ex vivo,151 skin ulcer management is a logical and urgent first-line candidate dermatological indication for topical T4.
That THs can counteract excessive fibrosis in mice in vivo166 would be a welcome added benefit under pathological WH conditions (eg hypertrophic scars, keloid disease, ulcer-associated skin fibrosis), if this applies to human skin. Also, given that epithelial–mesenchymal transition (EMT) is a physiological event during normal WH,169 the recently documented EMT-promoting effects of THs170 might be temporarily harnessed for promoting skin ulcer healing, at least during a defined window of the WH process. The striking boosting effects of THs on mitochondrial function and energy metabolism in human epidermis ex vivo171 should also facilitate WH under the severely hypoxic conditions that characterize most skin ulcers.172-174
We therefore advocate to rigorously explore whether topical T4 accelerates the speed and quality of skin ulcer healing. We explicitly do not envision this as a monotherapy, but as adjunct therapy to current standard of care for the major types of skin ulcers, that is diabetic foot ulcers, chronic venous insufficiency and peripheral arterial occlusion disease-associated ulcers.7
This novel hormonal ulcer treatment concept is strongly supported by the observation that T4 even accelerates re-epithelialization and potentially also angiogenesis in wounded human skin organ-cultured under highly pathological WH conditions that reproduce hypoxia, oxidative damage, hypoinsulinaemia, hypothyroidism and hyperglycaemia.175
There is mounting evidence that mitochondrial dysfunction may contribute to overall impairment of WH in patients. These effects may originate from the overall contribution of ageing, cellular senescence and other co-morbidities associated with patients with chronic ulcers.176 For example, mitochondrial oxidative phosphorylation was found only in diabetic patients with neuropathy regardless of the presence or absence of peripheral arterial disease and was associated with their inflammatory state.177 One of the better studied aspects of mitochondrial contributions to chronic ulcers relates to oxidative stress, including multiple approaches to mitigate it. It is well established that ROS production is increased in diabetic foot ulcers, due to a dysregulated mitochondrial electron transport chain,178 which in turn amplifies ROS production and advanced glycation end products,176,179-181 which promote the chronic inflammatory state of WH and prevent its progression to healing.182
However, successful WH is a highly complex, stringently choreographed sequence of events, whose dysregulation impairs WH and switches it into a chronic, non-healing state.183,184 Attempts to therapeutically target this choreography to promote ulcer healing may be beneficial, but can also cause problems or have no effect. For example, hyperbaric oxygen therapy (HBOT) shows a wide range of effects from clinical benefit to some patients to no improvements in others.185 Nrf2 (the master transcription factor which regulates oxidative damage responses in human skin and HFs)186-188 that protects from excessive ROS as well as SDF-1a levels is associated with clinically beneficial HBOT outcomes, suggesting that Nrf2 and SDF-1 levels may serve as biomarkers for identifying those ulcer patients who may benefit from HBOT therapy.189 Similar biomarkers may help to identify ulcer patients who promise optimal treatment outcomes after topical T4 therapy.
These challenges notwithstanding, one can hardly conceive of a more attractive new candidate ulcer therapeutic than topical T4, as it ticks all the right boxes: targeting of multiple WH-relevant cell populations and processes at once, immediate availability for use in humans at low cost and in practically every healthcare system worldwide, combined with likely limited and optimally characterized systemic toxicity.
8.2 ∣. Skin ageing
Declining mitochondrial function and energy metabolism are key characteristics of ageing tissues, and the restoration of mitochondrial bioenergetics has therefore been advocated as an effective approach for the treatment of numerous ageing-associated pathologies.190-196 Therefore, it is important to note that T3 and T4 increase mitochondrial activity and even biogenesis in human skin and HFs ex vivo, and this without evidence for the induction of excessive mitochondrial ROS production by THs.171,197 Specifically, T3 and T4 increase gene and protein expression of mitochondrial-encoded subunit 1 of cytochrome c oxidase (MTCO1), a subunit of respiratory chain complex IV, mitochondrial transcription factor A (TFAM) and porin.197 They also stimulate intrafollicular complex I/IV activity and mitochondrial biogenesis. T3 increases follicular heat production as an indicator for mitochondrial energy metabolism, whereas T3 and T4 both enhance ATP production in cultured human HF keratinocytes.197 Importantly, T3 and T4 actually reduce ROS formation and increase transcription of key ROS scavenging enzymes (catalase, superoxide dismutase 2) in HF keratinocytes.197
Concomitant with these (expected) boostering effects of TH on mitochondrial energy metabolism,171,197 T3 improves key skin ageing read-out parameters: it increases sirtuin-1, fibrillin-1, proliferator-activated receptor-gamma 1-alpha (PGC1α), collagen I and III transcription, while T4 decreases expression of the key molecular ageing indicators, cyclin-dependent kinase inhibitor 2A (p16ink4) and mammalian target of rapamycin (mTORC1/2), in organ-cultured human skin. Moreover, TH treatment enhances the deposition and improves the spatial arrangement of intradermal fibrillin-rich microfibrils and collagen III bundles ex vivo.171
Taken together, this strongly suggests that short-term application of THs exerts anti-ageing properties in human skin. Since these observations were made ex vivo, it remains to be explored whether T4 really exerts clinically relevant anti-ageing effects also after topical application to intact human skin in vivo, and at which point continuous TR stimulation and boostering of mitochondrial activity might promote undesired excessive ROS production and thus ageing-accelerating oxidative damage. One plausible approach to circumvent this theoretical problem would be to repetitively administer topical T4 only relatively short-term (eg 1-3 days), with several days of treatment-free intervals interspaced.
8.3 ∣. Skin atrophy
Steroid-induced skin atrophy has long been one of the most vexing problems of both topical and systemic glucocorticoid therapy in dermatology,198 to which eHFSCs are particularly sensitive, at least in mice.199 In view of the effects of TH in human skin in vivo139 and the epidermal effects of T4 described above,151,171 it is conceivable that topical T4 might be able to counteract the atrophogenic effects of glucocorticoids in human skin. Indeed, a first proof-of-principle clinical study suggests that TR stimulation with topically applied agonists may reverse glucocorticoid-induced skin atrophy in human patients in vivo.13
8.4 ∣. Telogen effluvium
THs modulate hair growth in rats, sheep, mice and humans (Table 1) in vivo.66,200 TH-dependent signalling can prolong the anagen phase of hair growth, change the expression of selected keratins, induce hair matrix KC proliferation and delay the beginning of apoptosis-driven HF involution (catagen) also in human scalp HFs.21,48 While THs do not significantly modulate new hair shaft formation ex vivo,48 they significantly stimulate mitochondrial energy metabolism in human scalp HFs.48,197 Furthermore, the synthetic TR agonist, KB2115 (eprotirome), which shows higher affinity for TR-β than for TR-α,201 also prolongs anagen in human scalp HFs ex vivo, accompanied by increased proliferation of matrix keratinocytes and decreased intrafollicular protein expression of the key catagen promoter, transforming growth factor-β2.11
Thus, it is reasonable to assume that topical T4 may be able to counteract the premature catagen induction that underlies various causes of telogen effluvium, including that associated with male pattern androgenetic alopecia and female pattern hair loss.202,203
8.5 ∣. Cicatricial alopecia: lichen planopilaris and frontal fibrosing alopecia
Lymphocytic cicatricial alopecias like lichen planopilaris and frontal fibrosing alopecia represent prototypic human eHFSC diseases.204 Given that TH stimulates K15 mRNA and protein expression by human eHFSCs,139 short-term topical T4 may also deserve consideration as an adjuvant therapy in the management of these alopecias. However, one critical question in this context is whether T4 therapy could eventually deplete the HF’s eSC niches by promoting emigration from the bulge, apoptosis and/or differentiation of K15+ eSCS upon long-term T4 administration.205-207 To exclude this possibility will require very careful preclinical scrutiny in vivo, for example in human scalp skin xenotransplants on SCID/beige mice.
8.6 ∣. Hair greying
Melanin production in the skin is closely regulated by different hormones and hormone-like neurotransmitters, such as L-dihydroxyphenylalanine, which bears structural resemblance to THs.208,209 T4 is also a strong stimulator of melanin production, and the fact that T4 potently stimulates melanin production in the human HF pigmentary unit ex vivo48 also encourages one to explore whether topical T4 offers an effective and safe remedy to halt or even reverse hair greying. This would be most valuable during the initial, melanocyte stem cell-independent phase of greying in human scalp HFs, where hair pigmentation can probably still be re-stimulated for several years,21 before progressive defects and depletion of the bulge melanocyte stem cell niche render greying irreversible.210
Interestingly, the key neuroendocrine HPT axis regulator of TH production and secretion in the thyroid gland, TRH, is also produced in human scalp HF themselves.130 It is remarkable that TRH itself strongly stimulates human HF pigmentation and activates the specialized melanocytes of the HF pigmentary unit, likely via upregulating MITF, while it does not stimulate melanogenesis in epidermal melanocytes.131 It is therefore conceivable that the co-administration of TRH or TRH-mimetic agonistic peptides with topical T4 could augment anti-hair greying effects of the latter.
8.7 ∣. Keratinization disorders
Keratinization disorders result from mutations in one of the keratin genes, resulting in inherited skin or hair disorders, based on the expression pattern of the mutated keratin.70,115,116 Since keratins form intermediate filaments by forming a heterodimer pair of keratins, it has been postulated that this keratin-modulating effect can be harnessed to upregulate the expression of non-mutated keratin genes or keratins that can compensate for the mutated, functionally defective keratin gene product and/or downregulate the mutated keratins.70,115,116 This may suffice to counteract, at least in part, some of the functional defects imparted by the mutated keratin. For example, the stimulatory effect of TH on K15 might be beneficial in patients with K14 mutations associated with epidermolysis bullosa simplex, since K15 can substitute for the mutated K14 in forming keratin pairs with K5.92 Better understanding of the effects of TH on K6, K16 and K17 might also provide a way to compensate for the mutated genes in pachyonychia congenita.
9 ∣. POTENTIAL TOXICITY AND CARCINOGENIC RISKS ASSOCIATED WITH TOPICAL L-THYROXINE THERAPY
THs rank among the most potent hormones in vertebrate biology, and the toxicity of chronically increased serum levels of T3 and/ or T4,211 independent of whether they are medication-induced or result from excessive TH secretion by the thyroid gland, is well-documented (Table 2). The high expression and activity level of deiodinases in epidermis and endothelial cells53,54 would be expected to reduce the risk of both, local overstimulation of TRs and high levels of systemic absorption of topically applied T4. Moreover, temporary topical T4 administration through the intact epidermal barrier in a relatively small skin area may not lead to a measurable increase in serum T3/T4 levels.
TABLE 2.
| Organ | Signs | Symptoms |
|---|---|---|
| Skin | Sweating; warm and moist skin; pretibial myxoedema; palmar erythema; onycholysis | Increased sweating; intolerance to heat |
| Hair | Hair loss and hair thinning | |
| Reproductive system | Men: Decreased libido; gynaecomastia Women: Reduced fertility and oligomenorrhea; spider angioma |
|
| Central nervous system | Hyperactivity; periodic paralysis | Anxiety; reduced concentration; nervousness; fatigue; hyperactivity; emotional lability; confusion; coma |
| Peripheral nervous system | Hyperreflexia | |
| Thyroid | Goitre; bruit | Swelling of the neck; tenderness |
| Cardiovascular system | High output failure; atrial arrhythmia; tachycardia; systolic hypertension; hyperdynamic precordium; | Shortness of breath; palpitations; chest pain |
| Muscles | Wasting; fine tremor | Tremor; weakness |
| Eyes (as part of Graves’ disease) | Periorbital oedema; ophthalmoplegia; eyelid lag and retraction; conjunctival injection; stare; proptosis; chemosis | Grittiness; soreness; diplopia |
| Gastrointestinal system | Weight loss; thin; cachectic; abdominal tenderness | Weight loss; increased appetite; hyperdefecation; diarrhoea; nausea; vomiting |
However, direct administration of T4 to skin ulcers and their surrounding skin with its impaired epidermal barrier function,212 in theory, may raise systemic TH levels to an extent that risks the induction of the well-appreciated adverse effects of excessive oral T4 administration or hyperthyroidism212 (Table 2). Since this could off-set any therapeutically desired effects of topical T4, especially in patients with cardiovascular disease and/or hyperthyroidism, the correlation of defined doses of topically applied T4 over time with resulting changes in serum T4,T3 and bTSH levels must be rigorously established, comparing the absorption through intact, non-inflamed adult human skin with inflamed, barrier-impaired skin and skin ulcers.
Since T4 downregulates the intraepidermal transcription and protein expression of bTSH in organ-cultured human skin,98 it is also conceivable that this T4-induced alteration in the neuroendocrine status of human epidermis might result in adverse skin effects, even though the functional role of bTSH in skin physiology98 remains largely unexplored. Moreover, given the central role of VDR and RXR signalling in the control of human keratinocyte proliferation and differentiation and overall skin physiology on the one hand, and the recognized impact of TR-mediated signalling on VDR and retinoid receptor activity/pathways on the other,87 it will also be important to search for deleterious effects of longer-term topical T4 application exerted via these indirect mechanisms of action.
While the systemic toxicity of topically administered T4 can easily be monitored and prevented by regular routine TH and bTSH serum measurements, the documented role of THs in carcinogenesis requires more careful consideration and scrutiny. Very recent epidemiological data support the long-suspected carcinogenic effect of long-term systemic T4 therapy: the latter was found to be associated with a significantly increased rate of several different types of cancer in Swedish patients, especially among women.213 Together with the body of work published by Dentice and collaborators that has highlighted the involvement of TH and their deiodinases in carcinoma-associated EMT, squamous cell carcinoma (SCC) and basal cell carcinoma,55,170,214,215 this strongly argues against long-term topical T4 therapy. Instead, it would appear well-advised to limit any form of topical T4 therapy in dermatology to maximally a couple of weeks, or perhaps ideally, to a pulse therapy regimen with sufficiently long treatment-free intervals so as to evade or minimize the risk of carcinogenicity.
10 ∣. HURDLES TO REPURPOSING L-THYROXINE AS TOPICALLY APPLIED DRUG IN DERMATOLOGY
A key regulatory hurdle in repurposing any drug approved for systemic application for topical administration is that its safety and toxicology as well as that of the chosen vehicle have to be rigorously re-evaluated and documented.216,217 This is associated with major drug development costs. At the same time, patent protection of any topical T4 application in dermatology is made very challenging by the wealth of “prior art” in this field (see above). Given this, from an industry perspective, a hurdle to T4 repurposing in dermatology is securing intellectual property perhaps by way of a novel delivery system or specific vehicle, judged to be patentable or perhaps as a drug–device combination. However, this does not prevent physicians to use T4 preparations off label for selected patients, provided sufficient safety.
For all of the candidate dermatological applications of topical T4 discussed above, development of a suitable vehicle that delivers an optimal intracutaneous T4 concentration without risking major systemic T4 toxicity is a methodological key challenge before clinical testing can begin. Obviously, the choice of vehicle will differ greatly, depending on whether topical T4 application via an intact epidermal barrier (where a critical role for inner ring deiodination in transcutaneous T4 penetration is recognized)218 or direct administration to the base and/or periphery of a bacterially superinfected skin ulcer is desired. Luckily, some suitable candidate vehicles have already been explored.15,219
11 ∣. CONCLUSIONS
We hope to have developed a compelling case here for exploring (short-term) topical therapy with T4 for a number of dermatological conditions. Given that very long-term therapy would be required for hair greying, skin ageing and keratinization disorders, which would enhance the risk of systemic toxicity and possibly even carcinogenicity, perhaps the most immediately persuasive dermatological applications for topical T4 are as an adjunct therapy in skin ulcer management and telogen effluvium, and for the prevention/restoration of glucocorticoid-induced skin atrophy. In these indications, short-term therapy (days to weeks) should suffice, and the simple monitoring of systemic TH levels under therapy should provide sufficient safeguarding against systemic adverse effects of topical T4.
Since, for the reasons delineated above, a major push from industry to invite this “Cinderella” among skin-related hormones to the dance floor of dermatological therapy is not expected any time soon, dermatologists themselves may have to play the role of the prince who accomplishes this, using individually formulated topical T4 prescriptions.
ACKNOWLEDGEMENTS
Writing of this essay was made possible by start-up funds of the Department of Dermatology, University of Miami, and by he NIHR Manchester Biomedical Research Centre to R.P, and our work is funded by Dr Phillip Frost Department of Dermatology and Cutaneous Surgery, NR015649 (MTC), AR060562 (MTC), AR073614 (MTC and RSK), DK119085 (RSK and MTC).
Funding information
Writing of this essay was made possible through start-up funds from the Department of Dermatology, University of Miami, to RP.
Footnotes
CONFLICT OF INTEREST
None declared. For the record, RP is founder & CEO of Monasterium Laboratory, Münster, Germany.
REFERENCES
- [1].Griffiths C, Barker J, Bleiker T, Chalmers RJG, Creamer D, Rook GA, Rook's Textbook of Dermatology, 9th ed., John Wiley & Sons Inc, Chichester, West Sussex: 2016. [Google Scholar]
- [2].Lebwohl MG, Heymann WR, Berth-Jones J, Coulson I, Treatment of Skin Disease: Comprehensive Therapeutic Strategies, 5th ed., Elsevier, Philadelphia, PA: 2017. [Google Scholar]
- [3].Litwack G, Thyroid Hormone, 1st ed., Academic Press, San Diego, CA: 2018. [Google Scholar]
- [4].Chaker L, Bianco AC, Jonklaas J, Peeters RP, Lancet 2017, 390, 1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Freinkel RK, Freinkel N, Arch. Dermatol 1972, 106, 349. [PubMed] [Google Scholar]
- [6].Paus R, in Rook’s Textbook of Dermatology (Eds: Griffiths CEM, Barker J, Bleiker T, Chalmers R, Creamer D), Vol 4, 9th ed., Wiley-Blackwell, Oxford: 2016. [Google Scholar]
- [7].Safer JD, Curr. Opin. Endocrinol. Diabetes Obes 2012, 19, 388. [DOI] [PubMed] [Google Scholar]
- [8].Horsley V, Br. Med. J 1885, 1, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sea M, Williams Textbook of Endocrinology, 14th ed., Elsevier, Philadelphia, PA: 2019. [Google Scholar]
- [10].Brunton L, Knollman B, Hilal-Dandan R, Goodman and Gilman's The Pharmacological Basis of Therapeutics, 13th ed., McGrawHill, New York, NY: 2017. [Google Scholar]
- [11].Olah A, Gherardini J, Bertolini M, Chéret J, Ponce L, Kloepper J, Bíró T, Soeberdt M, Abels C, Paus R, J. Invest. Dermatol 2016, 136, 1711. [DOI] [PubMed] [Google Scholar]
- [12].Yazdanparast P, Carlsson B, Oikarinen A, Risteli J, Faergemann J, Thyroid 2004, 14, 345. [DOI] [PubMed] [Google Scholar]
- [13].Yazdanparast P, Carlsson B, Oikarinen A, Risteli J, Lavin T, Faergemann J, Thyroid 2006, 16, 1157. [DOI] [PubMed] [Google Scholar]
- [14].Zhang B, Zhang A, Zhou X, Webb P, He W, Xia X, Int. J. Immunopathol. Pharmacol 2012, 25, 859. [DOI] [PubMed] [Google Scholar]
- [15].Padula C, Pappani A, Santi P, Int. J. Pharm 2008, 349, 161. [DOI] [PubMed] [Google Scholar]
- [16].Carruthers A, Cinderella - And Other Girls Who Lost Their Slippers (Origins of Fairy Tales), Pook Press, Alcester, Warwickshire, UK: 2015. [Google Scholar]
- [17].Grimm J, Grimm W Kinder- und Hausmärchen, Realschulbuchhandlung, Berlin: 1812. [Google Scholar]
- [18].Kendall EC, J Am Med Assoc 1915, 64, 2042. [Google Scholar]
- [19].Bassett JH, Williams GR, Bone 2008, 43, 418. [DOI] [PubMed] [Google Scholar]
- [20].Costa-e-Sousa RH, Hollenberg AN, Endocrinology 2012, 153, 4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paus R, J. Invest. Dermatol 2010, 130, 7. [DOI] [PubMed] [Google Scholar]
- [22].Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD, Adv. Anat. Embryol. Cell Biol 2012, 212:v, vii, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zoeller RT, Tan SW, Tyl RW, Crit. Rev. Toxicol 2007, 37, 11. [DOI] [PubMed] [Google Scholar]
- [24].Laurent I, Tang S, Astère M, Wang KR, Deng S, Xiao L, Li QF, Endocrine 2018, 61, 28. [DOI] [PubMed] [Google Scholar]
- [25].Mathisen PM, Miller L, Mol. Cell Biol 1989, 9, 1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nakai Y, Nakajima K, Yaoita Y, Zoolog. Sci 2017, 34, 414. [DOI] [PubMed] [Google Scholar]
- [27].Leitch VD, Bassett JHD, Williams GR, Nat. Rev. Endocrinol 2020, 16, 147. [DOI] [PubMed] [Google Scholar]
- [28].Chen CY, Tsai MM, Chi HC, Lin KH, Biochim. Biophys. Acta 2013, 1834, 2271. [DOI] [PubMed] [Google Scholar]
- [29].Davis PJ, Goglia F, Leonard JL, Nat. Rev. Endocrinol 2016, 12, 111. [DOI] [PubMed] [Google Scholar]
- [30].Iordanidou A, Hadzopoulou-Cladaras M, Lazou A, Mol. Cell Biochem 2010, 340, 291. [DOI] [PubMed] [Google Scholar]
- [31].Senese R, Cioffi F, de Lange P, Goglia F, Lanni A, J. Endocrinol 2014, 221, R1. [DOI] [PubMed] [Google Scholar]
- [32].Moran C, Agostini M, Visser WE, Schoenmakers E, Schoenmakers N, Offiah AC, Poole K, Rajanayagam O, Lyons G, Halsall D, Gurnell M, Chrysis D, Efthymiadou A, Buchanan C, Aylwin S, Chatterjee KK, Lancet Diabetes Endocrinol. 2014, 2, 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tata JR, Biochim. Biophys. Acta 2013, 1830, 3860. [DOI] [PubMed] [Google Scholar]
- [34].Verga Falzacappa C, Patriarca V, Bucci B, Mangialardo C, Michienzi S, Moriggi G, Stigliano A, Brunetti E, Toscano V, Misiti S, J. Cell Biochem 2009, 106, 835. [DOI] [PubMed] [Google Scholar]
- [35].Weitzel JM, Iwen KA, Mol. Cell Endocrinol 2011, 342, 1. [DOI] [PubMed] [Google Scholar]
- [36].Cheng SY, Leonard JL, Davis PJ, Endocr Rev. 2010, 31, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mourouzis I, Lavecchia AM, Xinaris C, J. Mol. Evol 2020, 88, 88. [DOI] [PubMed] [Google Scholar]
- [38].Jho SH, Vouthounis C, Lee B, Stojadinovic O, Im MJ, Brem H, Merchant A, Chau K, Tomic-Canic M, J. Invest. Dermatol 2005, 124, 1034. [DOI] [PubMed] [Google Scholar]
- [39].Jho SH, Radoja N, Im MJ, Tomic-Canic M, J. Biol. Chem 2001, 276, 45914. [DOI] [PubMed] [Google Scholar]
- [40].Mahajan MA, Das S, Zhu H, Tomic-Canic M, Samuels HH, Mol. Cell Biol 2004, 24, 4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ruiz-Llorente L, Contreras-Jurado C, Martinez-Fernandez M, Paramio JM, Aranda A, Thyroid 2018, 28, 921. [DOI] [PubMed] [Google Scholar]
- [42].Kyono Y, Subramani A, Ramadoss P, Hollenberg AN, Bonett RM, Denver RJ, Endocrinology 2016, 157, 3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gunin AG, Golubtsova NN, Kravtsova OA, Subbotkin AS, Subbotkina NO, Filippov FN, Bull. Exp. Biol. Med 2019, 166, 797. [DOI] [PubMed] [Google Scholar]
- [44].Liu YY, Brent GA, Methods Mol. Biol 2018, 1801, 47. [DOI] [PubMed] [Google Scholar]
- [45].Singh BK, Sinha RA, Yen PM, Int. J. Mol. Sci 2018, 19, 3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kaplan MM, Pan CY, Gordon PR, Lee JK, Gilchrest BA, J. Clin. Endocrinol. Metab 1988, 66, 815. [DOI] [PubMed] [Google Scholar]
- [47].Safer JD, Persons K, Holick MF, Thyroid 2009, 19, 181. [DOI] [PubMed] [Google Scholar]
- [48].van Beek N, Bodó E, Kromminga A, Gáspár E, Meyer K, Zmijewski MA, Slominski A, Wenzel BE, Paus R, J. Clin. Endocrinol. Metab 2008, 93, 4381. [DOI] [PubMed] [Google Scholar]
- [49].Maher SK, Wojnarowicz P, Ichu T-A, Veldhoen N, Lu L, Lesperance M, Propper CR, Helbing CC, Comp. Biochem. Physiol. Part D Genomics Proteomics 2016, 18, 44. [DOI] [PubMed] [Google Scholar]
- [50].Chi HC, Chen CY, Tsai MM, Tsai CY, Lin K-H, Biomed. Res. Int 2013, 2013, 601361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S, Nat. Rev. Cardiol 2017, 14, 39. [DOI] [PubMed] [Google Scholar]
- [52].Hennemann G, Docter R, Friesema EC, de Jong M, Krenning EP, Visser TJ, Endocr. Rev 2001, 22, 451. [DOI] [PubMed] [Google Scholar]
- [53].Mancino G, Sibilio A, Luongo C, Di Cicco E, Miro C, Cicatiello AG, Nappi A, Sagliocchi S, Ambrosio R, De Stefano MA, Di Girolamo D, Porcelli T, Murolo M, Saracino F, Perruolo G, Formisano P, Stornaiuolo M, Dentice M, Thyroid 2020, 30, 1066. [DOI] [PubMed] [Google Scholar]
- [54].Huang MP, Rodgers KA, O'Mara R, Mehta M, Abuzahra HS, Tannenbaum AD, Persons K, Holick MF, Safer JD, Thyroid 2011, 21, 1263. [DOI] [PubMed] [Google Scholar]
- [55].Miro C, Ambrosio R, De Stefano MA, Di Girolamo D, Di Cicco E, Cicatiello AG, Mancino G, Porcelli T, Raia M, Del Vecchio L, Salvatore D, Dentice M, Thyroid 2017, 27, 567. [DOI] [PubMed] [Google Scholar]
- [56].Sabatino L, Lubrano V, Balzan S, Kusmic C, Del Turco S, Iervasi G, Mol. Cell Biochem 2015, 399, 87. [DOI] [PubMed] [Google Scholar]
- [57].Garcia-Serrano L, Gomez-Ferreria MA, Contreras-Jurado C, Segrelles C, Paramio JM, Aranda A, PLoS One 2011, 6, e23825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Panteleyev AA, Botchkareva NV, Sundberg JP, Christiano AM, Paus R, Am. J. Pathol 1999, 155, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Panteleyev AA, Paus R, Ahmad W, Sundberg JP, Christiano AM, Exp. Dermatol 1998, 7, 249. [DOI] [PubMed] [Google Scholar]
- [60].Panteleyev AA, Paus R, Christiano AM, Am. J. Pathol 2000, 157, 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Maatough A, Whitfield GK, Brook L, Hsieh D, Palade P, Hsieh JC, J. Cell Biochem 2018, 119, 69. [DOI] [PubMed] [Google Scholar]
- [62].Hsieh JC, Estess RC, Kaneko I, Whitfield GK, Jurutka PW, Haussler MR, J. Endocrinol 2014, 220, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bodó E, Kromminga A, Bíró T, Borbíró I, Gáspár E, Zmijewski MA, van Beek N, Langbein L, Slominski AT, Paus R, J. Invest. Dermatol 2009, 129, 1126. [DOI] [PubMed] [Google Scholar]
- [64].Messenger AG, Br J Dermatol. 2000, 142, 633. [DOI] [PubMed] [Google Scholar]
- [65].Safer JD, Crawford TM, Fraser LM, Hoa M, Ray S, Chen TC, Persons K, Holick MF, Thyroid 2003, 13, 159. [DOI] [PubMed] [Google Scholar]
- [66].Safer JD, Fraser LM, Ray S, Holick MF, Thyroid 2001, 11, 717. [DOI] [PubMed] [Google Scholar]
- [67].Tsujio M, Yoshioka K, Satoh M, Watahiki Y, Mutoh K, Vet. Pathol 2008, 45, 505. [DOI] [PubMed] [Google Scholar]
- [68].Leonhardt JM, Heymann WR, Dermatol. Clin 2002, 20, 473. [DOI] [PubMed] [Google Scholar]
- [69].Artantas S, Gul U, Kilic A, Guler S, Eur. J. Intern. Med 2009, 20, 158. [DOI] [PubMed] [Google Scholar]
- [70].Ramot Y, Paus R, Tiede S, Zlotogorski A, BioEssays 2009, 31, 389. [DOI] [PubMed] [Google Scholar]
- [71].Magri F, Buonocore M, Oliviero A, Rotondi M, Gatti A, Accornero S, Camera A, Chiovato L, Eur. J. Endocrinol 2010, 163, 279. [DOI] [PubMed] [Google Scholar]
- [72].Schell H, Kiesewetter F, Seidel C, von Hintzenstern J, Dermatologica. 1991, 182, 23. [DOI] [PubMed] [Google Scholar]
- [73].Fistarol SK, Praxis (Bern 1994) 2002, 91, 1019. [DOI] [PubMed] [Google Scholar]
- [74].Stüttgen G, Funktionelle Dermatologie, Springer, Berlin: 1974. [Google Scholar]
- [75].Kinoshita-Ise M, Martinez-Cabriales SA, Alhusayen R, J. Dermatol. 2019, 46, 702. [DOI] [PubMed] [Google Scholar]
- [76].Lee S, Lee YB, Kim BJ, Lee WS, J. Am. Acad. Dermatol 2019, 80, 1410. [DOI] [PubMed] [Google Scholar]
- [77].Kanti V, Constantinou A, Reygagne P, Vogt A, Kottner J, Blume-Peytavi U, J. Eur. Acad. Dermatol. Venereol 2019, 33, 1976. [DOI] [PubMed] [Google Scholar]
- [78].Lerner AB, Am. J. Med 1971, 51, 141. [DOI] [PubMed] [Google Scholar]
- [79].Redondo P, Guzman M, Marquina M, Pretel M, Aguado L, Lloret P, Gorrochategui A, Actas Dermosifiliogr 2007, 98, 603. [PubMed] [Google Scholar]
- [80].Ahsan MK, Urano Y, Kato S, Oura H, Arase S, J. Med. Invest 1998, 44, 179. [PubMed] [Google Scholar]
- [81].Billoni N, Buan B, Gautier B, Gaillard O, Mahe YF, Bernard BA, Br. J. Dermatol 2000, 142, 645. [DOI] [PubMed] [Google Scholar]
- [82].Torma H, Karlsson T, Michaelsson G, Rollman O, Vahlquist A, Acta Derm. Venereol 2000, 80, 4. [DOI] [PubMed] [Google Scholar]
- [83].Torma H, Rollman O, Vahlquist A, Acta Derm. Venereol 1993, 73, 102. [DOI] [PubMed] [Google Scholar]
- [84].Vollberg TM Sr, Nervi C, George MD, Fujimoto W, Krust A, Jetten AM, Mol. Endocrinol 1992, 6, 667. [DOI] [PubMed] [Google Scholar]
- [85].Fonseca-Alaniz MH, Takada J, Alonso-Vale MI, Lima FB, J. Pediatr. 2007, 83(5 Suppl), S192. [DOI] [PubMed] [Google Scholar]
- [86].Contreras-Jurado C, Garcia-Serrano L, Gomez-Ferreria M, Costa C, Paramio JM, Aranda A, J. Biol. Chem 2011, 286, 24079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM, Nature 1992, 355, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tomic-Canic M, Stojadinovic O, Lee B, Walsh R, Blumenberg M, Clin. Transl. Sci 2008, 1, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Moeller LC, Dumitrescu AM, Refetoff S, Mol. Endocrinol 2005, 19, 2955. [DOI] [PubMed] [Google Scholar]
- [90].Pouyani T, Sadaka BH, Papp S, Schaffer L, In Vitro Cell Dev. Biol. Anim 2013, 49, 178. [DOI] [PubMed] [Google Scholar]
- [91].Radoja N, Diaz DV, Minars TJ, Freedberg IM, Blumenberg M, Tomic-Canic M, J. Invest. Dermatol 1997, 109, 566. [DOI] [PubMed] [Google Scholar]
- [92].Radoja N, Stojadinovic O, Waseem A, Tomic-Canic M, Milisavljevic V, Teebor S, Blumenberg M, Mol. Cell Biol 2004, 24, 3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Safer JD, Crawford TM, Holick MF, Endocrinology 2004, 145, 2357. [DOI] [PubMed] [Google Scholar]
- [94].Tomic-Canic M, Day D, Samuels HH, Freedberg IM, Blumenberg M, J. Biol. Chem 1996, 271, 1416. [DOI] [PubMed] [Google Scholar]
- [95].Tomic-Canic M, Sunjevaric I, Freedberg IM, Blumenberg M, J. Invest. Dermatol 1992, 99, 842. [DOI] [PubMed] [Google Scholar]
- [96].Lee B, Vouthounis C, Stojadinovic O, Brem H, Im M, Tomic-Canic M, J. Mol. Biol 2005, 345, 1083. [DOI] [PubMed] [Google Scholar]
- [97].Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R, Endocrinology 2018, 159, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Bodó E, Kany B, Gáspár E, Knüver J, Kromminga A, Ramot Y, Bíró T, Tiede S, van Beek N, Poeggeler B, Meyer KC, Wenzel BE, Paus R, Endocrinology 2010, 151, 1633. [DOI] [PubMed] [Google Scholar]
- [99].Hardman JA, Haslam IS, Farjo N, Farjo B, Paus R, PLoS One 2015, 10, e0121878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Forger DB, Biological, Clocks, Rhythms, and Oscillations: The Theory of Biological Timekeeping , Kindle ed., MIT Press, New York, NY: 2017. [PubMed] [Google Scholar]
- [101].Al-Nuaimi Y, Baier G, Watson RE, Chuong CM, Paus R, Exp. Dermatol 2010, 19, 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Al-Nuaimi Y, Hardman JA, Bíró T, Haslam IS, Philpott MP, Tóth BI, Farjo N, Farjo B, Baier G, Watson REB, Grimaldi B, Kloepper JE, Paus R, J. Invest. Dermatol 2014, 134, 610. [DOI] [PubMed] [Google Scholar]
- [103].Tomic-Canic M, Freedberg IM, Blumenberg M, Exp. Cell Res 1996, 224, 96. [DOI] [PubMed] [Google Scholar]
- [104].Jozic I, Vukelic S, Stojadinovic O, Liang L, Ramirez HA, Pastar I, Canic MT, J. Invest. Dermatol 2017, 137, 1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Stojadinovic O, Lee B, Vouthounis C, Vukelic S, Pastar I, Blumenberg M, Brem H, Tomic-Canic M, J. Biol. Chem 2007, 282, 4021. [DOI] [PubMed] [Google Scholar]
- [106].Stojadinovic O, Sawaya A, Pastar I, Tomic-Canic M, PLoS One 2013, 8, e63453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Guillot R, Muriach B, Rocha A, Rotllant J, Kelsh RN, Cerda-Reverter JM, PLoS One 2016, 11, e0166152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].McMenamin SK, Bain EJ, McCann AE, Patterson LB, Eom DS, Waller ZP, Hamill JC, Kuhlman JA, Eisen JS, Parichy DM, Science 2014, 345, 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wright MR, Lerner AB, Nature 1960, 185, 169. [DOI] [PubMed] [Google Scholar]
- [110].Antonini D, Sibilio A, Dentice M, Missero C, Front. Endocrinol 2013, 4, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Hanley K, Rassner U, Elias PM, Williams ML, Feingold KR, J. Invest. Dermatol 1996, 106, 404. [DOI] [PubMed] [Google Scholar]
- [112].Komuves LG, Hanley K, Jiang Y, Elias PM, Williams ML, Feingold KR, J. Invest. Dermatol 1998, 111, 429. [DOI] [PubMed] [Google Scholar]
- [113].Dentice M, Luongo C, Huang S, Ambrosio R, Elefante A, Mirebeau-Prunier D, Zavacki AM, Fenzi G, Grachtchouk M, Hutchin M, Dlugosz AA, Bianco AC, Missero C, Larsen PR, Salvatore D, Proc. Natl Acad. Sci. USA 2007, 104, 14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Paus R, Langan EA, Vidali S, Ramot Y, Andersen B, Trends Mol. Med 2014, 20, 559. [DOI] [PubMed] [Google Scholar]
- [115].Ramot Y, Olah A, Paus R, Br. J. Dermatol 2018, 178, 1469. [DOI] [PubMed] [Google Scholar]
- [116].Ramot Y, Paus R, BioEssays 2014, 36, 672. [DOI] [PubMed] [Google Scholar]
- [117].Ramot Y, Zhang G, Bíró T, Lisztes E, Funk W, Ingber A, Langbein L, Paus R, J. Dermatol. Sci 2011, 64, 67. [DOI] [PubMed] [Google Scholar]
- [118].Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR, Drug Discov. Today Dis. Mech 2008, 5, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Slominski A, Mihm MC, Int. J. Dermatol 1996, 35, 849. [DOI] [PubMed] [Google Scholar]
- [120].Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R, FASEB J. 2005, 19, 1332. [DOI] [PubMed] [Google Scholar]
- [121].Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M, J. Biol. Chem 2011, 286, 10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J, Endocr. Rev 2013, 34, 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Slominski RM, Tuckey RC, Manna PR, Jetten AM, Postlethwaite A, Raman C, Slominski AT, Genes Immun. 2020, 21, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Slominski A, Zjawiony J, Wortsman J, Semak I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, Tuckey RC, Eur. J. Biochem 2004, 271, 4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J, Am. J. Physiol. Endocrinol. Metab 2005, 288, E701. [DOI] [PubMed] [Google Scholar]
- [126].Slominski A, Zbytek B, Semak I, Sweatman T, Wortsman J, J. Neuroimmunol 2005, 162, 97. [DOI] [PubMed] [Google Scholar]
- [127].Skobowiat C, Dowdy JC, Sayre RM, Tuckey RC, Slominski A, Am. J. Physiol. Endocrinol. Metab 2011, 301, E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Slominski A, Pisarchik A, Wortsman J, Kohn L, Ain KB, Venkataraman GM, Chung JH, Giuliani C, Thornton M, Slugocki G, Tobin DJ, J. Invest. Dermatol 2002, 119, 1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Cianfarani F, Baldini E, Cavalli A, Marchioni E, Lembo L, Teson M, Persechino S, Zambruno G, Ulisse S, Odorisio T, D'Armiento M, J. Invest. Dermatol 2010, 130, 93. [DOI] [PubMed] [Google Scholar]
- [130].Gáspár E, Hardenbicker C, Bodó E, Wenzel B, Ramot Y, Funk W, Kromminga A, Paus R, FASEB J. 2010, 24, 393. [DOI] [PubMed] [Google Scholar]
- [131].Gáspár E, Nguyen-Thi KT, Hardenbicker C, Tiede S, Plate C, Bodó E, Knuever J, Funk W, Bíró T, Paus R, J. Invest. Dermatol 2011, 131, 2368. [DOI] [PubMed] [Google Scholar]
- [132].Das S, Schapira M, Tomic-Canic M, Goyanka R, Cardozo T, Samuels HH, Mol. Endocrinol 2007, 21, 2672. [DOI] [PubMed] [Google Scholar]
- [133].Vukelic S, Stojadinovic O, Pastar I, Vouthounis C, Krzyzanowska A, Das S, Samuels HH, Tomic-Canic M, J. Biol. Chem 2010, 285, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Palmer MA, Blakeborough L, Harries M, Haslam IS, Exp. Dermatol. 2020, 29, 299. [DOI] [PubMed] [Google Scholar]
- [135].Pastar I, Stojadinovic O, Sawaya AP, Stone RC, Lindley LE, Ojeh N, Vukelic S, Samuels HH, Tomic-Canic M, J. Cell Physiol 2016, 231, 2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ocaranza P, Lammoglia JJ, Iniguez G, Roman R, Cassorla F, Growth Horm. IGF Res 2014, 24, 42. [DOI] [PubMed] [Google Scholar]
- [137].Foitzik K, Spexard T, Nakamura M, Halsner U, Paus R, J. Invest. Dermatol 2005, 124, 1119. [DOI] [PubMed] [Google Scholar]
- [138].Alam M, Below DA, Cheret J, Langan EA, Bertolini M, Jimenez F, Paus R, J. Invest. Dermatol 2019, 139, 1593. [DOI] [PubMed] [Google Scholar]
- [139].Tiede S, Bohm K, Meier N, Funk W, Paus R, Eur. J. Cell Biol 2010, 89, 769. [DOI] [PubMed] [Google Scholar]
- [140].Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Brady JN, Udey MC, Vogel JC, J. Clin. Invest 2006, 116, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Contreras-Jurado C, Lorz C, Garcia-Serrano L, Paramio JM, Aranda A, Mol. Biol. Cell 2015, 26, 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Xie L, Yang R, Liu S, Lyle S, Cotsarelis G, Xiang L, Zhang L, Li B, Wan M, Xu X, Lab. Invest 2016, 96, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Slominski AT, Hardeland R, Zmijewski MA, Slominski RM, Reiter RJ, Paus R, J. Invest. Dermatol 2018, 138, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Kalsbeek A, la Fleur S, Fliers E, Circadian control of glucose metabolism. Mol. Metab 2014, 3, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Yoshimura T, Front. Neuroendocrinol 2013, 34, 157. [DOI] [PubMed] [Google Scholar]
- [146].Beasley LJ, Nelson RJ, Experientia 1982, 38, 870. [DOI] [PubMed] [Google Scholar]
- [147].Dardente H, J. Neuroendocrinol 2012, 24, 249. [DOI] [PubMed] [Google Scholar]
- [148].Dardente H, Hazlerigg DG, Ebling FJ, Front. Endocrinol 2014, 5, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Erdogan M, Ilhan YS, Akkus MA, Caboğlu SA, Ozercan I, Ilhan N, Yaman M, Acta Chir. Belg 1999, 99, 72. [PubMed] [Google Scholar]
- [150].Kassem R, Liberty Z, Babaev M, Trau H, Cohen O, Clin. Exp. Dermatol 2012, 37, 850. [DOI] [PubMed] [Google Scholar]
- [151].Zhang GY, Langan EA, Meier NT, Funk W, Siemers F, Paus R, PLoS One 2019, 14, e0212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Firouzi A, Fadaei Fathabadi F, Norozian M, Amini A, Abdollahifar MA, Noruzian M, J. Lasers Med. Sci 2018, 9, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Alexander MV, Zajtchuk JT, Henderson RL, Arch. Otolaryngol 1982, 108, 289. [DOI] [PubMed] [Google Scholar]
- [154].Talmi YP, Finkelstein Y, Zohar Y, Ann. Otol. Rhinol. Laryngol 1989, 98(4 Pt 1), 267. [DOI] [PubMed] [Google Scholar]
- [155].Cannon CR, Laryngoscope 1994, 104(S66), 1. [DOI] [PubMed] [Google Scholar]
- [156].Ladenson PW, Levin AA, Ridgway EC, Daniels GH, Am. J. Med 1984, 77, 261. [DOI] [PubMed] [Google Scholar]
- [157].Mehregan AH, Zamick P, J. Cutan. Pathol 1974, 1, 113. [DOI] [PubMed] [Google Scholar]
- [158].Tarameshloo M, Norouzian M, Zarein-Dolab S, Dadpay M, Gazor R, Lab. Anim. Res 2012, 28, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Zamick P, Mehregan AH, Plast Reconstr. Surg 1973, 51, 71. [DOI] [PubMed] [Google Scholar]
- [160].Contreras-Jurado C, Garcia-Serrano L, Martinez-Fernandez M, Ruiz-Llorente L, Paramio JM, Aranda A, PLoS One 2014, 9, e108137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Pastar I, Wong LL, Egger AN, Tomic-Canic M, Exp. Dermatol 2018, 27, 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hyter S, Indra AK, FEBS Lett. 2013, 587, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Schmuth M, Moosbrugger-Martinz V, Blunder S, Dubrac S, Biochim. Biophys. Acta 2014, 1841, 463. [DOI] [PubMed] [Google Scholar]
- [164].Mousa SA, Davis FB, Mohamed S, Davis PJ, Feng X, Int. Angiol 2006, 25, 407. [PubMed] [Google Scholar]
- [165].Davis FB, Mousa SA, O'Connor L, Mohamed S, Lin HY, Cao HJ, Davis PJ, Circ. Res 2004, 94, 1500. [DOI] [PubMed] [Google Scholar]
- [166].Alonso-Merino E, Martin Orozco R, Ruiz-Llorente L, Martínez-Iglesias OA, Velasco-Martín JP, Montero-Pedrazuela A, Fanjul-Rodríguez L, Contreras-Jurado C, Regadera J, Aranda A, Proc. Natl Acad. Sci. USA 2016, 113, E3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Kirsner RS, Am. J. Manag. Care 2018, 24(14 Spec No), SP607. [PubMed] [Google Scholar]
- [168].Singer AJ, Tassiopoulos A, Kirsner RS, N. Engl. J. Med 2018, 378, 302. [DOI] [PubMed] [Google Scholar]
- [169].Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, Tomic-Canic M, Cell Tissue Res. 2016, 365, 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Miro C, Di Cicco E, Ambrosio R, Mancino G, Di Girolamo D, Cicatiello AG, Sagliocchi S, Nappi A, De Stefano MA, Luongo C, Antonini D, Visconte F, Varricchio S, Ilardi G, Del Vecchio L, Staibano S, Boelen A, Blanpain C, Missero C, Salvatore D, Dentice M, Nat. Commun 2019, 10, 5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Vidali S, Chéret J, Giesen M, Haeger S, Alam M, Watson REB, Langton AK, Klinger M, Knuever J, Funk W, Kofler B, Paus R, J. Invest. Dermatol 2016, 136, 2003. [DOI] [PubMed] [Google Scholar]
- [172].Darwin E, Tomic-Canic M, Curr. Dermatol. Rep 2018, 7, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Tomic-Canic M, DiPietro LA, J. Invest. Dermatol 2019, 139, 997. [DOI] [PubMed] [Google Scholar]
- [174].Tomic-Canic M, Wong LL, Smola H, J. Wound Care 2018, 27, 646. [DOI] [PubMed] [Google Scholar]
- [175].Post H, Hundt JE, Zhang G, Depping R, Rose C, Langan EA, Paus R, Arch. Dermatol. Res 2020, 10.1007/s00403-020-02092-z in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Cano Sanchez M, Lancel S, Boulanger E, Neviere R, Antioxidants 2018, 7, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Tecilazich F, Dinh T, Lyons TE, Guest J, Villafuerte RA, Sampanis C, Gnardellis C, Zuo CS, Veves A, J. Vasc. Surg 2013, 57, 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Shah MS, Brownlee M, Circ. Res 2016, 118, 1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Peppa M, Stavroulakis P, Raptis SA, Wound Repair Regen. 2009, 17, 461. [DOI] [PubMed] [Google Scholar]
- [180].Schramm A, Matusik P, Osmenda G, Guzik TJ, Vascul. Pharmacol 2012, 56, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Zorov DB, Juhaszova M, Sollott SJ, Physiol. Rev 2014, 94, 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Artlett CM, J. Pathol 2013, 229, 157. [DOI] [PubMed] [Google Scholar]
- [183].Eming SA, Tomic-Canic M, Exp. Dermatol 2017, 26, 97. [DOI] [PubMed] [Google Scholar]
- [184].Eming SA, Martin P, Tomic-Canic M, Sci. Transl. Med 2014, 6, 265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Lam G, Fontaine R, Ross FL, Chiu ES, Adv. Skin Wound Care 2017, 30, 181. [DOI] [PubMed] [Google Scholar]
- [186].Haslam IS, Jadkauskaite L, Szabó IL, Staege S, Hesebeck-Brinckmann J, Jenkins G, Bhogal RK, Lim F-L, Farjo N, Farjo B, Bíró T, Schäfer M, Paus R, J. Invest. Dermatol 2017, 137, 295. [DOI] [PubMed] [Google Scholar]
- [187].Jian Z, Li K, Song PU, Zhu G, Zhu L, Cui T, Liu B, Tang L, Wang X, Wang G, Gao T, Li C, J. Invest. Dermatol 2014, 134, 2221. [DOI] [PubMed] [Google Scholar]
- [188].Ron-Doitch S, Soroka Y, Frusic-Zlotkin M, Barasch D, Steinberg D, Kohen R, Exp. Dermatol 2020, 10.1111/exd.14103 [DOI] [PubMed] [Google Scholar]
- [189].Dhamodharan U Karan A, Sireesh D, Vaishnavi A, Somasundar A, Rajesh K, Ramkumar KM, Free Radic. Biol. Med 2019, 138, 53. [DOI] [PubMed] [Google Scholar]
- [190].Fang EF, Scheibye-Knudsen M, Chua KF, Mattson MP, Croteau DL, Bohr VA, Nat. Rev. Mol. Cell Biol 2016, 17, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Poulose N, Raju R, Biochim. Biophys. Acta 2015, 1852, 2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Szeto HH, Br. J. Pharmacol 2014, 171, 2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Wallace DC, Philos. Trans. R Soc. Lond. B Biol. Sci 2013, 368, 20120267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Wallace DC, Fan W, Procaccio V, Annu. Rev. Pathol 2010, 5, 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Bellei B, Picardo M, Ageing Res. Rev 2020, 57, 100981. [DOI] [PubMed] [Google Scholar]
- [196].Flood KS, Houston NA, Savage KT, Kimball AB, Clin. Dermatol 2019, 37, 312. [DOI] [PubMed] [Google Scholar]
- [197].Vidali S, Knuever J, Lerchner J, Giesen M, Bíró T, Klinger M, Kofler B, Funk W, Poeggeler B, Paus R, J. Invest. Dermatol 2014, 134, 33. [DOI] [PubMed] [Google Scholar]
- [198].Schoepe S, Schacke H, May E, Asadullah K, Exp. Dermatol 2006, 15, 406. [DOI] [PubMed] [Google Scholar]
- [199].Chebotaev DV, Yemelyanov AY, Lavker RM, Budunova IV, J. Invest. Dermatol 2007, 127, 2749. [DOI] [PubMed] [Google Scholar]
- [200].Hale PA, Ebling FJ, J. Exp. Zool 1975, 191, 49. [DOI] [PubMed] [Google Scholar]
- [201].Shiohara H, Nakamura T, Kikuchi N, Ozawa T, Nagano R, Matsuzawa A, Ohnota H, Miyamoto T, Ichikawa K, Hashizume K, Bioorg. Med. Chem 2012, 20, 3622. [DOI] [PubMed] [Google Scholar]
- [202].Harries M, Tosti A, Bergfeld W, Blume-Peytavi U, Shapiro J, Lutz G, Messenger A, Sinclair R, Paus R, J. Eur. Acad. Dermatol. Venereol 2016, 30, 667. [DOI] [PubMed] [Google Scholar]
- [203].Rebora A, Clin. Cosmet. Investig. Dermatol 2019, 12, 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Harries MJ, Jimenez F, Izeta A, Hardman J, Panicker SP, Poblet E, Paus R, Trends Mol. Med 2018, 24, 435. [DOI] [PubMed] [Google Scholar]
- [205].Cicatiello AG, Ambrosio R, Dentice M, Mol. Cell Endocrinol 2017, 459, 84. [DOI] [PubMed] [Google Scholar]
- [206].Catalano V, Dentice M, Ambrosio R, Luongo C, Carollo R, Benfante A, Todaro M, Stassi G, Salvatore D, Cancer Res. 2016, 76, 1237. [DOI] [PubMed] [Google Scholar]
- [207].Ghorbani A, Naderi-Meshkin H, Curr. Stem Cell Res. Ther 2016, 11, 19. [DOI] [PubMed] [Google Scholar]
- [208].Slominski A, Tobin DJ, Shibahara S, Wortsman J, Physiol Rev. 2004, 84, 1155. [DOI] [PubMed] [Google Scholar]
- [209].Slominski A, Zmijewski MA, Pawelek J, L Pigment Cell Melanoma Res. 2012, 25, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Nishimura EK, Pigment Cell Melanoma Res. 2011, 24, 401. [DOI] [PubMed] [Google Scholar]
- [211].Sharma A, Stan MN, Mayo Clin. Proc 2019, 94, 1048. [DOI] [PubMed] [Google Scholar]
- [212].Roy S, Elgharably H, Sinha M, Ganesh K, Chaney S, Mann E, Miller C, Khanna S, Bergdall VK, Powell HM, Cook CH, Gordillo GM, Wozniak DJ, Sen CK, J. Pathol 2014, 233, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Wandell P, Carlsson AC, Li X, Sundquist J, Sundquist K, Cancer Epidemiol. 2020, 66, 101707. [DOI] [PubMed] [Google Scholar]
- [214].Di Girolamo D, Ambrosio R, De Stefano MA, Mancino G, Porcelli T, Luongo C, Di Cicco E, Scalia G, Del Vecchio L, Colao A, Dlugosz AA, Missero C, Salvatore D, Dentice M, J. Clin. Invest 2016, 126, 2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Nappi A, Di Cicco E, Miro C, Cicatiello AG, Sagliocchi S, Mancino G, Ambrosio R, Luongo C, Di Girolamo D, De Stefano MA, Porcelli T, Stornaiuolo M, Dentice M, Cancers 2020, 12, 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Abels C, Soeberdt M, Exp. Dermatol 2019, 28, 1002. [DOI] [PubMed] [Google Scholar]
- [217].Florian P, Flechsenhar KR, Bartnik E, Ding-Pfennigdorff D, Herrmann M, Bryce PJ, Nestle FO, Exp. Dermatol 2020, 29, 4. [DOI] [PubMed] [Google Scholar]
- [218].Santini F, Vitti P, Chiovato L, Ceccarini G, Macchia M, Montanelli L, Gatti G, Rosellini V, Mammoli C, Martino E, Chopra IJ, Safer JD, Braverman LE, Pinchera A, J. Clin. Endocrinol. Metab 2003, 88, 2825. [DOI] [PubMed] [Google Scholar]
- [219].Padula C, Nicoli S, Santi P, Int. J. Pharm 2009, 372, 12. [DOI] [PubMed] [Google Scholar]
- [220].Franklyn JA, Boelaert K, Lancet 2012, 379, 1155. [DOI] [PubMed] [Google Scholar]
- [221].Nayak B, Burman K, Endocrinol. Metab. Clin. North Am 2006, 35, 663. [DOI] [PubMed] [Google Scholar]
- [222].Devereaux D, Tewelde SZ, Emerg. Med. Clin. North Am 2014, 32, 277. [DOI] [PubMed] [Google Scholar]
- [223].Vaidya B, Pearce SH, BMJ 2014, 349, g5128. [DOI] [PubMed] [Google Scholar]