Abstract
The two branches of the autonomic nervous system (ANS) have been individually linked to changes in cognitive functioning: The parasympathetic nervous system (PNS) has been associated with healthy cognitive aging, whereas excessive sympathetic nervous system (SNS) activity has been linked to heightened cognitive decline. Despite these separate findings and despite the integrative nature of the ANS, little work has examined the two branches simultaneously to better understand their interactive effects on changes in cognitive functioning in midlife adults. We examined cognitive change in two waves of the Midlife in the United States (MIDUS) study cognitive project and indexed PNS and SNS activity from heart rate variability and epinephrine levels, respectively, from the MIDUS biomarker project (minimum n = 843, 57.9% female, mean age at first wave = 53.8 years). Our findings indicate that greater PNS responsivity (i.e., greater withdrawal and greater recovery) in response to cognitive challenge is associated with attenuated cognitive decline, but only among individuals with low SNS levels; at higher SNS levels, the effects of the PNS on cognitive decline are attenuated. These results suggest that future research targeting the ANS and cognitive aging should consider both ANS branch’s effects simultaneously.
1. Introduction
Cognitive function typically declines with advancing age (Hughes et al., 2018) and, although normative, cognitive decline is a costly consequence of aging (Hurd et al., 2013). Most research on age-related cognitive decline has focused on older populations and the pathology associated with the clinically-relevant loss of cognitive functioning (e.g., Alzheimer’s disease; Salthouse, 2010). But the early stages of the pathogenesis of cognitive decline are an important point at which interventions to prevent further decline in functionality may be most impactful. Understanding early changes in biological systems that may portend later cognitive decline are therefore crucial targets for early intervention to prevent later detrimental consequences of cognitive decline.
The autonomic nervous system (ANS) has been implicated directly in healthy cognitive functioning and indirectly in several physiological processes that relate to healthy cognitive functioning (Hugdahl, 1996; Thayer et al., 2009). Two foundational theoretical frameworks support this perspective. The neurovisceral integration model posits that an overlapping set of neural structures regulates both the parasympathetic nervous system (PNS) and cognitive functioning (Thayer & Lane, 2000). This neural architecture co-occurs in cortical-subcortical networks connected to the ventromedial prefrontal cortex (vmPFC), an area of the brain that demonstrates robust associations with so-called “top-down” control of cognition and executive function (Alvarez & Emory, 2006; Thayer, Ash, Fredrikson, Sollers, & Wager, 2012). In a second, complementary framework, polyvagal theory (Porges et al., 1995), higher-level brain structures are postulated to be intricately linked to ANS activity (and PNS activity specifically) in order to modulate energy usage in pursuit of avoiding threat and facilitating social functions. Further, polyvagal theory describes the PNS’s inhibitory relationship with the sympathetic nervous system (SNS) and other metabolic systems. Rapid inhibition and disinhibition of this “vagal brake” in response to challenge results in efficient mobilization of energy and an effective return to calm states, respectively (Porges, 2007). These theoretical frameworks thus suggest PNS activity may relate to or directly support cognitive functioning via neural and metabolic pathways.
Accordingly, increased indices of PNS activity, such as higher levels of high-frequency heart rate variability (HF HRV) – a measure of cardiac PNS functioning – has been associated with better performance on several measures of executive function (Thayer, Hansen, Saus-Rose, & Johnsen, 2009). HF HRV is also thought to be a trait-like predictor of individual differences in cognitive control, implicated in a variety of psychopathologies marked by deficits in self-regulation (Beauchaine & Thayer, 2015). Indeed, meta-analyses estimate a significant, small association of higher PNS activity with better “top-down” regulation of cognitive functioning and other behavioral and emotional processes, with some evidence that publication bias may inflate these associations (Holzman & Bridgett, 2017; Zahn et al., 2016). This pattern has been observed cross-sectionally and prospectively in mid-life adults (Frewen et al., 2013; Zeki Al Hazzouri et al., 2017). However, other research failed to find strong support for integrated neural control of both parasympathetic activity and cognitive functioning in the forebrain despite observing correlations between HF HRV and two cognitive tests (a test of working memory and interference control; Jennings et al., 2015).
Within longitudinal studies, the association of PNS activity and change in cognitive functioning is somewhat inconsistent as well: Higher resting PNS activity has been linked to attenuated declines in some aspects of cognitive functioning in older adults (Mahinrad et al., 2016); other work has not found associations between PNS activity and changes in cognitive functioning in mid-life adults (Britton et al., 2008).
The degree to which the PNS reacts to and recovers from a cognitive task has been related to cognitive functioning as well. Within polyvagal theory, a robust PNS withdrawal and subsequent recovery indicates a well-regulated mobilization of energy to meet the needs of a given challenge (Porges, 2001; 2007), such as a cognitive task. Faster recovery of PNS activity after a cognitive challenge has been associated with improved task-switching performance, a measure of executive functioning (Kimhy et al., 2013). In other work, greater PNS responsivity to cognitive challenge – i.e., a strong, parasympathetic withdrawal and/or a strong recovery, which has also been labeled “parasympathetic flexibility” (Miller et al., 2013) – has been linked to improved performance on broad tests of cognitive functioning Mathewson et al., 2010; Kassam et al., 2009; and Duschek et al., 2009.
Overall, indices of resting PNS activity and PNS responsivity have been associated with cognitive functioning, with mixed evidence suggestive that PNS activity may relate to longer-term changes in cognitive functioning. However, the PNS is only one half of the autonomic nervous system and works in tandem with the sympathetic nervous system (SNS) to regulate physiological arousal and allostasis (Karatsoreos & McEwen, 2011). The SNS is responsible for activation of the classic “fight or flight” response to stressors and, within the neurovisceral integration and polyvagal frameworks, is posited to be under the inhibitory control of the prefrontal cortex (Thayer & Lane, 2000; 2009; Porges, 2007). Thus, although the two branches do function independently, they work in concert to maintain proper physiological activation for an organism’s present needs (Porges, 2001). This interdependence between the two branches of the ANS may be best conceptualized as autonomic space (Berntson, Cacioppo, & Quiggley, 1993). Because of the potent effects of sympathetic activation on physiological arousal, inhibition of the SNS at rest is thought to allow the PNS to regulate energy expenditure on a moment-to-moment basis that results in better-regulated physiological activations to meet nonthreatening demands (Thayer & Lane, 2009).
Like the PNS, the SNS has been independently linked to cognitive functioning (Giuliano et al., 2018). Moderate levels of resting SNS activation may support physiological arousal necessary for cognitive functioning (Lovallo, 2011; Yerkes & Dodson, 1908). Other work has found that impaired sympathetic activity is associated with dementia among individuals with Parkinson’s Disease (Poewe, 2007) and that pharmacologically blocking sympathetic activity impairs episodic memory for emotional stimuli (van Stegeren et al., 1998). But over longer-term periods, chronic activation of the SNS can lead to a cascade of negative, downstream consequences on the neural and vascular anatomy that supports healthy cognitive aging (Rozanski & Kubzansky, 2005; McEwen, 1998). This work suggests that moderate levels of SNS activity may play a role in maintaining healthy cognitive functioning but may also lead to decrements in cognitive functioning at higher levels of activity.
Few studies have examined the PNS and SNS as interactive influences on cognitive functioning, despite burgeoning evidence of the ANS’ influence on cognitive functioning and the known interdependence of its two branches. In one study, phasic PNS fluctuations were associated with better working memory performance but resting SNS activity moderated this association in a sample of young mothers considered at-risk for chronic stress (Giuliano et al., 2017). Elevated PNS activity was associated with greater working memory capacity, but only when resting SNS activity was low; higher levels of resting SNS activity blocked the association between PNS fluctuations and working memory capacity. However, the interactive relationship of the PNS and SNS on long-term changes in cognitive function across the lifespan is unknown.
We examined the contribution of both branches of the ANS to changes in cognitive function across two waves of the MIDUS study (approximately nine years’ time). Based on prior evidence in younger samples (Giuliano et al., 2017), we hypothesized that the interaction of the PNS and SNS would be significantly related to changes in cognitive functioning, such that higher PNS responsivity would relate to attenuated decline in cognitive functioning when SNS activity was lower but not when SNS activity was higher.
2. Method
The Midlife in the United States (MIDUS) is a national longitudinal study of health and well-being that allows researchers to evaluate aging as an integrated bio-psychosocial process (Ryff & Kruger, 2019). Current data span thirty years in three collection waves, each wave separated by approximately ten years. Biomarker data from the second wave (MIDUS 2) and cognitive data from both the second and third wave (MIDUS 3) are used in the current analysis.
2.1. Biomarker subproject
The biomarker subproject of MIDUS 2 was conducted 2004–2006 on a sample of 1,255 respondents, all of whom completed an initial baseline survey. Comprehensive biological assessments were performed on this sample subset at three general clinical research centers (UCLA, University of Wisconsin, and Georgetown University).
2.1.1. Sympathetic nervous system.
As part of a broader examination of biopsychosocial health, 12-hour urinary samples were collected. SNS tone was primarily evaluated using 12-hour urine epinephrine (EPI) concentration, adjusted for creatinine levels1. EPI concentrations were submitted to natural-log transformation to correct a positive skew in the distribution. We followed-up our analyses on EPI with exploratory analyses that replicated our primary models but with EPI replaced by creatinine-corrected norepinephrine concentrations, another marker of SNS tone (Grassli & Esler, 1999).
2.1.2. Parasympathetic nervous system.
PNS control of heart rate is achieved through efferent traffic of the vagus nerve to the sinoatrial node. Vagus nerve traffic, or vagal tone, responds rapidly to stimuli and regulates beat-to-beat fluctuations in heart rate. Thus, in order to examine PNS activity, relative power of the high frequency component of heart rate variability (HF HRV) was derived from ECG recordings. Inter-beat intervals from the HR series from each five-minute epoch were interpolated into the frequency domain. The high-frequency component (0.15 – 0.4 Hz) of the waveform is thought to emanate solely from PNS control (Berntson et al., 1997). HF HRV values were natural-log transformed to correct a positive skew in the distribution.
2.1.2.1. Baseline PNS and dynamic PNS responses.
To index baseline PNS functioning, participants were seated for ten minutes while ECG was recorded continuously. Dynamic PNS responses were indexed from HRV recorded during two cognitive tasks presented during the Biomarker subproject. These tasks consisted of a math task and the Stroop color-naming task that were adaptively adjusted to maintain similar levels of difficulty for each participant2. Task order was randomized across participants. HR was recorded continuously during these tasks and during five-minute recovery periods that followed each task.
In line with prior work based on the MIDUS study sample (Kimhy et al., 2013; Crowley et al., 2016), we focused on PNS recovery scores, which were produced by subtracting the task HF HRV from the HF HRV of the epoch immediately following each task (i.e., the first recovery period for the first task and the second recovery period for the second task) and averaging the two differences. Larger negative values indicate an attenuated PNS recovery; larger positive values indicate heightened PNS recovery. Simple subtractive change scores across epochs are appropriate for indexing general responses across multiple tasks (Llabre et al., 1991).
We also examined reactivity scores, which we produced by subtracting the HF HRV value from the epoch immediately preceding each task (i.e., the baseline epoch for the first task and the recovery period that preceded the second task) from the task HF HRV and averaged the two differences. For this measure of reactivity, larger negative values indicate heightened PNS withdrawal (reactivity) in response to the cognitive tasks; larger positive values indicate reduced PNS withdrawal (reactivity) in response to the cognitive tasks.
2.1.2.2. Quadratic index of PNS responsivity.
As a follow-up to the individual PNS recovery and reactivity indices, we explored an integrative measure of PNS responsivity indexed by the quadratic pattern of the HF HRV response to cognitive challenge. For each of the cognitive challenges (i.e., math and Stroop tasks), a quadratic line was fit for each participant to the HF HRV value from the epoch that preceded the task, the task epoch, and the epoch that followed the task; the model also included a fixed effect for the linear line through these three epochs. The resulting quadratic estimates were normalized (Z-score) across the sample within each cognitive task. These normalized values were averaged into a single index of PNS responsivity – that is, the degree to which the PNS reacted to and recovered from the cognitive challenges (Miller et al., 2013). With this index, larger positive numbers are indicative of greater PNS responsivity.
2.1.3. Orthostatic (physical) challenge.
Orthostasis, or changing positions from sitting to standing, results in venous blood pooling as circulating blood volume shifts from the chest to the distensible venous capacitance system below the diaphragm (Nordkamp et al., 2013; Abhoud et al., 1976). To compensate for the resulting drop in blood pressure, a series of cardiopulmonary reflexes ultimately result in reduced parasympathetic activity and increased sympathetic activity. Hence, measuring reactivity to orthostasis provides an index of autonomic response to a purely physical challenge. Such cardiovascular reflexes may be less likely to be associated with central nervous system activity relevant to cognitive functioning (Porges, 2007). Comparing PNS responsivity in orthostatic and cognitive challenges allows for examination of the specificity of the interactive autonomic associations with change in cognitive functioning.
Participants were exposed to the orthostatic challenge following the cognitive tasks. Participants stood and, after a several minute calibration period, cardiovascular data were recorded for 5 minutes. We calculated an index of PNS reactivity to this orthostatic challenge by subtracting HF HRV in the last recovery epoch of the cognitive challenges from the HF HRV while standing (i.e., larger negative values indicate greater PNS withdrawal in response to physical challenge). There was no recovery period following orthostatic challenge.
2.2. Cognitive Subproject
The MIDUS 2 cognitive subproject was conducted 2004–2006 on a sample of 3,487 respondents, all of whom completed the initial baseline survey. The subsequent MIDUS 3 cognitive project was conducted approximately 10 years later on a sample of 2,693 respondents. The goal of the cognitive project was to determine how cognition is related overall to mental and physical health. During each wave of assessment, the Brief Test of Adult Cognition by Telephone (BTACT; Tun & Lachman, 2006; Kimhy et al., 2013) was administered to the participants. The BTACT includes subtests that measure episodic memory (immediate recall and delayed recall of a 15-word list), working memory span (backward digit span), verbal fluency (category fluency), inductive reasoning (number series completion), speed of processing (backward counting task), and reaction time (task-switching test).
Prior research on the MIDUS sample showed that: i) both word recall tests loaded onto an episodic memory factor and the rest of the subtests loaded onto an executive function factor (i.e., factor eigen values >1 in exploratory factor analysis); ii) the BTACT had good concurrent and discriminant validity; and iii) significant differences due to mode of testing (in-person or telephone) were not evident among a subset of participants (Lachman, Agrigoroaei, Tun, & Weaver, 2014). In other work, the BTACT has been shown to have good test-retest reliability across two (reliability coefficients range from r = .55 to .94) and four weeks (r = .52 to .85, except for category fluency, r = .28; Whitbourne, Neupert, & Lachman, 2008; Lachman et al., 2014). In the MIDUS 3 cognitive project, the same episodic and executive functioning factors were evident and were equivalent across occasions (i.e., tests of longitudinal measurement invariance fit a weak invariance model; Hughes et al., 2018). Our analyses focus on the summary BTACT score at each time point; see supplement for analyses of the individual episodic memory and executive functioning factors.
2.3. Analyses
2.3.1. Primary.
To examine the interactive effects of PNS and SNS on changes in cognitive functioning, multilevel models were specified that regressed cognitive functioning (i.e., BTACT score) on a three-way, cross-level interaction between a dummy code representing the MIDUS wave (MIDUS Wave 2 = 0; MIDUS Wave 3 = 1), HF HRV (baseline, recovery, or reactivity), and EPI concentration.
2.3.2. Secondary.
We followed the primary analyses with several secondary analyses: 1) We substituted the quadratic index of PNS responsivity for the individual measures of PNS recovery or reactivity; 2) we explored if age moderated the relationship between change in cognitive functioning and the interactive effects of PNS and SNS; 3) we repeated the primary models with creatinine-corrected norepinephrine levels (NE) replacing EPI levels as an indicator of SNS activity; 4) we re-ran the models using PNS reactivity to orthostatic challenge as the index of parasympathetic activity, which allows for the examination of the specificity of the PNS response to cognitive challenge compared to physical challenge; 5) due to collinearity between heart rate and HRV (de Geus et al., 2019), we re-ran models with baseline heart rate and heart rate reactivity and recovery replacing the HF HRV measures; 6) finally, we examined gender as a moderator due to known gender differences in cognitive decline in older adults (Lin et al., 2015).
2.3.3. Covariates.
All analyses co-varied participant gender (Male = 0; Female = 1), age at MIDUS 2 (centered at age = 50), and the number of years between completing the MIDUS 2 and MIDUS 3 cognitive sub-studies. As in prior work (Crowley et al., 2011; 2016), prescription and over-the-counter medications that could alter cardiovascular, autonomic, or cognitive functioning were counted for each participant and included as a continuous covariate (Table 1). Participants’ self-reported whether they had experienced any of a series of health conditions (Table 2); their answers of the following conditions were included as dummy-coded covariates in each model (“Yes” or “Borderline” = 1; “No” or “Unsure” = 0): Heart disease, high blood pressure, circulation problems, heart murmur, stroke, emphysema, and depression.
Table 1.
Usage frequencies of drug classes that may affect autonomic, cardiovascular, or cognitive functioning.
| Drug Class | AHFS Pharmacologic-Therapeutic classification # | Participants reporting usage (number of reported uses) | Percent of sample reporting usage |
|---|---|---|---|
| Parasympathomimetic (Cholinergic) Agents | 120400 | 2 | 0.23 |
| Sympathomimetic (Adrenergic) Agents | 121200 | 44 (52) | 5.06 |
| Sympatholytic (Adrenergic Blocking) Agents | 121600 | 4 | 0.46 |
| Autonomic Drugs, Miscellaneous | 129200 | 2 | 0.23 |
| a-Adrenergic Blocking Agents | 242000 | 14 | 1.61 |
| b-Adrenergic Blocking Agents | 242400 | 113 | 13.00 |
| Dihydropyridines | 242808 | 39 | 4.49 |
| Calcium-Channel Blocking Agents, Miscellaneous | 242892 | 18 | 2.07 |
| Angiotensin-Converting Enzyme Inhibitors | 243204 | 92 | 10.59 |
| Angiotensin II Receptor Antagonists | 243208 | 60 | 6.90 |
| Selective Serotonin Agonists | 283228 | 13 | 1.50 |
| Adrenals | 680400 | 39 (41) | 4.49 |
| Selective Serotonin- and Norepinephrine-reuptake Inhibitors | 28160416 | 21 | 2.42 |
| Selective-serotonin Reuptake Inhibitors | 28160420 | 77 | 8.86 |
| Serotonin Modulators | 28160424 | 9 | 1.04 |
| Tricyclics and Other Norepinephrine-reuptake Inhibitors | 28160428 | 16 | 1.84 |
| Antidepressants, Miscellaneous | 28160492 | 11 | 1.27 |
Table 2.
Frequencies of health conditions that may impact HF HRV or cognitive functioning.
| Condition | Participants reporting conditions | Percent of sample reporting condition |
|---|---|---|
| Heart Disease | 72 | 8.29 |
| High Blood Pressure | 291 | 33.49 |
| Circulation problems | 77 | 8.86 |
| Heart Murmur | 110 | 12.66 |
| TIA or Stroke | 27 | 3.11 |
| Emphysema/COPD | 22 | 2.53 |
| Depression | 199 | 22.90 |
Thus, the model to test Time × HF HRV × EPI for participant j at MIDUS wave i consisted of:
Level 1:
Level 2:
where ɣ0yMedicalConditionj represents the list of dummy-coded medical conditions for participant j. Secondary analyses added age as an additional cross-level moderator or replaced EPI concentration with creatinine-corrected NE concentration. A random intercept for Waveij per participants was included in every model.
All analyses were run in R (v3.5.1; R core team, 2018) using the lme4 package (Bates et al., 2015). Graphs were produced using the ggplot2 package (Wickham, 2009). All code is available on OSF (https://osf.io/yt49f/), and data for the MIDUS studies can be accessed via the Inter-university Consortium for Political and Social Research (https://www.icpsr.umich.edu/icpsrweb/ICPSR/series/203).
3. Results
3.1. Participants
Participants included in the current analysis (n = 944; 57.9% female; mean age at MIDUS 2 = 54.2 years, SD = 10.9, range = [34, 83]) completed MIDUS 2 and MIDUS 3 on average nine years apart (Mean = 9.34 years, SD = 0.83, range = [8, 12]). Of the 944 participants, n = 14 were missing urine epinephrine data, and n = 2 were missing both waves’ BTACT cognition scores and so were excluded from those analyses. An additional n = 73 were missing 1 BTACT score, but these participants were included in analyses as multilevel models can generally account for this degree of missingness. Participants who were missing baseline HF HRV (n = 63), HF HRV reactivity (n = 77), or HF HRV recovery data (n = 88) were excluded from those specific analyses. Our final sample size thus varied depending on the analysis being conducted (baseline HRV: n = 867; HRV reactivity: n = 854; HRV recovery: n = 843). In the largest of these analytic samples, 81% identified as European-American, 14% as African-American, 3% as multiracial, and 2% were of some other racial identity; 4% of the sample identified as Hispanic.
3.2. Preliminary Results
As reported elsewhere (Hughes et al., 2018), cognitive functioning as measured by the summary BTACT score generally declined in this subsample across the approximately 10 years from MIDUS 2 to MIDUS 3 (B = −0.184, 95% CI[−0.223, −0.145], p < .001); similar patterns are evident for episodic memory (B = −0.117, [−0.179, −0.056], p <.001) and executive functioning score (B = −0.326, [−0.363, −0.289], p <.001). See Table 3 for descriptive statistics of study variables.
Table 3.
Means (SD or range) of study variables split by of age groups1 and for the full sample
| Full Sample (n = 869): Means (SD or range) of study variables1 | |
|---|---|
| Age (years) | |
| MIDUS 2, Biomarker Project | 53.8 (10.9) |
| MIDUS 3, Cognitive Project | 63.1 (10.9) |
| BTACT Scores (standardized units) | |
| MIDUS 2 | 0.194 (0.928) |
| MIDUS 3 | 0.032 (0.667) |
| Parasympathetic Nervous System (high-frequency heart rate variability, natural-log ms2) | |
| Baseline | 4.91 (1.26) |
| Stroop | 4.32 (1.29) |
| Stroop Recovery | 4.90 (1.27) |
| Math | 4.74 (1.23) |
| Math Recovery | 4.91 (1.25) |
| Orthostatic Challenge | 4.08 (1.32) |
| Mean Cognitive Reactivity | −0.357 (0.579) |
| Mean Cognitive Recovery | 0.357 (0.624) |
| Orthostatic Challenge Reactivity | −0.856 (0.879) |
| Sympathetic Nervous System (creatinine-corrected, μg/g) | |
| Epinephrine | 0.144 (0.118) |
| Norepinephrine | 2.03 (1.59) |
Notes: Full sample in this table includes participants with epinephrine data available, at least one BTACT score, and HF HRV baseline, reactivity, or recovery data available.
3.3. Autonomic Predictors of Changes in Cognitive Functioning
3.3.1. Baseline ANS Functioning.
Baseline PNS activity was linked to marginal changes in BTACT scores from MIDUS 2 to MIDUS 3 (Wave × HF HRV: B = 0.030, [ 0.001, 0.061], p = .057), which seems to be driven by executive functioning (Wave × HF HRV: B = 0.035, [0.005, 0.061], p = .020; Table S1). These analyses suggest that higher vagal tone (i.e., greater HF HRV values) at rest related to attenuated declines in cognitive functioning. In contrast, baseline SNS activity was not associated with change in cognitive functioning scores (Wave × EPI: ps > .1; Table S2).
3.3.2. PNS responsivity to Cognitive Challenge.
PNS recovery from the cognitive tasks in the Biomarker project predicted change in BTACT scores (B = 0.071, [0.007, 0.135], p = .030; Table S3). Here again, higher vagal tone (as indexed by greater HF HRV recovery from the laboratory tasks) was associated with weaker declines in cognitive functioning. PNS reactivity to the cognitive tasks was not associated with changes in cognitive functioning (Wave × HF HRV: ps > .15).
3.4. Interactive effects of PNS and SNS on Change in Cognitive Functioning
3.4.1. Baseline PNS measure.
Baseline PNS and SNS activity were not found to interactively predict change in cognitive functioning (ps > .12).
3.4.2. PNS responsivity to Cognitive Challenge.
3.4.2.1. PNS Recovery.
SNS activity was found to moderate the association of dynamic PNS response with changes in cognitive functioning. Specifically, Wave × PNS recovery × SNS was associated with changes in BTACT scores (B = 0.176, [0.305, 0.048], p = .007). This association was reflected in executive functioning scores (B = −0.248, [−0.367, −0.130], p < 0.001) more so than episodic memory scores (Table S4). We explored this interaction by plotting point estimates of the slope linking MIDUS 2 to MIDUS 3 BTACT scores across the range of PNS recovery scores for individuals with low (−1SD) and high (+1SD) epinephrine concentrations. We subsequently calculated simple slopes for the change in BTACT scores from MIDUS 2 to MIDUS 3 at high and low PNS recovery and epinephrine levels. For individuals with lower SNS activity, PNS recovery was positively associated with changes in BTACT scores – i.e., stronger HF HRV recovery from the laboratory tasks predicted attenuated declines in cognitive functioning (estimate of MIDUS 2 to MIDUS 3 simple slope at +1SD HRV recovery: B = −0.104, [−0.188, −0.019]) and weaker HF HRV recovery predicted greater declines in cognitive functioning (−1SD HRV recovery: B = −0.321, [0.402, 0.240]; Figure 1). But at higher levels of SNS activity, BTACT changes were roughly equivalent at high (B = −0.168, [−0.248, −0.087]) and low PNS recovery scores (B = −0.137, [−0.218, −0.056]), suggesting the association between PNS recovery and BTACT changes was blocked.
Figure 1.
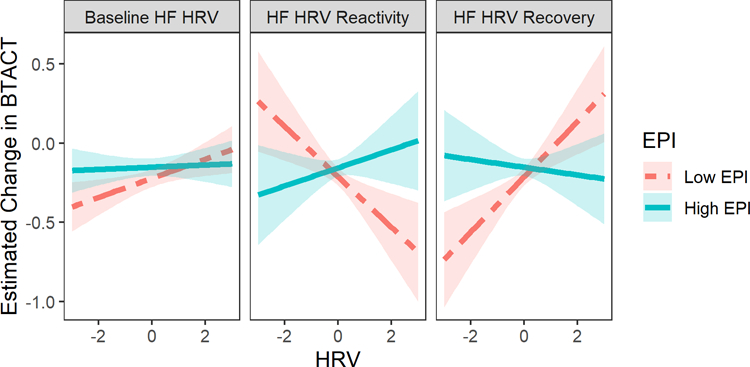
Estimated change in BTACT scores (MIDUS 3 minus MIDUS 2) at ±1SD EPI concentration across a range of HF HRV values. Point estimates of change in BTACT represent the slope connecting MIDUS 2 to MIDUS 3 derived from the multilevel model. Larger positive HRV values for baseline and recovery represent stronger vagal tone; larger negative HRV values for reactivity represent greater PNS vagal withdrawal.
3.4.2.2. PNS Reactivity.
An interaction between PNS reactivity and SNS levels on changes in BTACT score was also found (Wave × PNS reactivity × SNS: B = 0.192, [0.054, 0.330], p = .006), which was reflected in both executive functioning and episodic memory (Table S5). However, decomposing this interaction in a manner similar to the PNS recovery analysis revealed that the association of PNS reactivity with changes in BTACT score was seemingly in the opposite direction of the effect observed for PNS recovery. For individuals with low SNS activity, PNS reactivity correlated negatively with change in BTACT scores; greater PNS withdrawal (larger, negative PNS reactivity values) in response to the cognitive challenge predicted attenuated declines in cognitive functioning (−1SD PNS reactivity: B = −0.121, [0.203, 0.039]) and weaker PNS withdrawal was associated with greater declines (+1SD PNS reactivity: B = −0.305, [−0.385, −0.225]; Figure 1). But at higher SNS activity levels, BTACT changes were roughly equivalent for individuals with greater (B = −0.189, [0.271, 0.108]) and weaker PNS withdrawal (B = −0.123, [−0.204, −0.043]).
3.5. Secondary Analyses
3.5.1. Quadratic Index of Parasympathetic Responsivity.
Integrating across the results for the two indices of dynamic PNS activity suggests that PNS responsivity – greater withdrawal, greater recovery – is associated with improved cognitive functioning under conditions of low SNS activity, and that high SNS activity blocks this association. Here we examined PNS responsivity more formally by replacing reactivity or recovery with a quadratic index that encapsulates the reactivity and recovery measures. Unsurprisingly, this model revealed a similar pattern of results when PNS responsivity was more formally tested (Wave × PNS responsivity × SNS: B = −0.128, [−0.218, −0.037], p = .006; Table S6). For individuals with lower SNS activity, PNS responsivity was positively associated with changes in BTACT scores – i.e., greater responsivity predicted attenuated declines in cognitive functioning (B = −0.111, [−0.194, 0.028]) and weaker responsivity predicted stronger declines (B = −0.316, [−0.396, −0.236]) – but at higher levels of SNS activity, BTACT changes across MIDUS waves were roughly equivalent at high (B = −0.177, [−0.258, −0.096]) and low PNS responsivity scores (B = −0.129, [−0.209, −0.048]).
3.5.2. Interactions with Age.
We examined if the patterns observed were evident across the age range in the MIDUS study. Age did not robustly moderate the Wave × PNS recovery × SNS interaction on changes in BTACT scores (B = 0.011, [0.001, 0.023], p = .062); weaker effects were evident for baseline PNS (B = 0.004, [0.001, 0.009], p = .102) and PNS reactivity (B = −0.011, [−0.023, 0.002], p = .104; Table S7), though the direction of the effect was similar across all indices (see Figure S1). These exploratory results are therefore inconclusive due to their relative weakness and lack of consistency across PNS measures.
3.5.3. Other Indices of SNS Activity.
We repeated our analyses with creatinine-corrected norepinephrine (NE) concentrations replacing epinephrine. Models with NE generally replicate the effects seen with EPI. Specifically, NE moderates the association of PNS recovery and reactivity with changes in cognitive functioning (Table S8, Figure S2); this interaction was not evident for baseline PNS activity. The interactive effects were in the same direction as the EPI analyses. This suggests that the interaction between PNS and SNS on changes in cognitive functioning is not specific to one index of SNS activity.
3.5.4. Orthostatic Challenge.
We examined if the interaction between PNS reactivity to a physical challenge (orthostasis, or standing upright) and SNS activity predicted changes in cognitive functioning. Unlike the PNS responses to cognitive challenge, PNS reactivity to physical challenge did not interactively predict change in cognitive functioning with SNS (B = 0.030, [−0.053, 0.113], p = .484; Table S9). Thus, the prospective links between ANS activity and changes in cognitive function appear to be specific to dynamic responses to cognitive, and not physical challenges.
3.5.5. Examining HR instead of PNS Activity.
We examined if the HR × SNS interaction similarly predicted change in cognitive functioning. Replacing PNS activity with HR did not replicate the primary models (Baseline HR: B = 0.0002, [−0.007, 0.007], p = .943; HR reactivity: B = −0.012, [−0.031, 0.007], p = .214; HR recovery: B = 0.0135, [0.006, 0.0330], p = .173). This indicates the effect may be specific to PNS activity as indexed by HF HRV.
3.5.6. Gender Differences.
Finally, we explored gender differences in the PNS × SNS interaction. A main effect of gender was evident such that women demonstrated higher episodic memory and men demonstrated higher executive functioning (all ps < .002; see Tables S1–S5). Because of the opposing directions of these effects, no gender differences were evident for BTACT scores. Robust differences between men and women were not evident in the association of the PNS × SNS interaction with change in cognitive functioning (B = 0.055, [−0.321, 0.211], p = .686).
4. Discussion
Using a novel integrative model of the two branches of the ANS, our study demonstrates that changes in cognitive functioning are predicted by the interactive effects of the PNS and the SNS in midlife adults. Previous theorizing and literature examining the ANS has focused almost exclusively on linking PNS or SNS activity separately to outcome measurements (Thayer & Lane, 2000; Greaney et al., 2017). Following prior evidence that the PNS and SNS interactively predict working memory in a sample of young, at-risk mothers (Giuliano et al., 2017), here we show the interactive effect of the ANS extends to predicting broader changes in cognitive functioning across a nine-year period in a sample of middle-aged men and women. The best maintenance of cognitive function was predicted by a combination of stronger PNS responsivity to cognitive challenge (i.e., stronger withdrawal and stronger recovery) and lower SNS activity. These results appeared to be specific to responses to the cognitive challenge; PNS reactivity to orthostatic challenge did not predict changes in cognitive functioning.
The results in this study were most robustly observed when PNS responsivity, or the strength of reactivity and recovery, to a cognitive challenge were considered, rather than baseline PNS functioning. These results are in line with initial evidence of the interactive ANS effects on working memory, which were based on dynamic PNS responses to a cognitive task moderated by resting SNS activity (Giuliano et al., 2017). Dynamic PNS responses may impact cognitive function more so when SNS activity is low due to a wider range of variability available in autonomic space for the PNS to withdrawal, as opposed to when SNS activity is high and physiological activation is near ceiling, leaving less autonomic space for PNS withdrawal to further increase physiological arousal (Berntson, Cacciopo, & Quigley, 1993a).
The effects observed here – in which autonomic measurement occurred independent of the first wave of cognitive testing and nearly ten years before the second wave – suggest that PNS responsivity to cognitive challenges may be a trait-like indicator of the psychophysiological processes underlying cognitive functioning. These findings are also consistent with longitudinal work in young children showing that individual-level variance in PNS activity during laboratory visits is primarily accounted for by trait-level variance (Gatzke-Kopp & Ram, 2018). The present results extend prior research showing that trait-like measures of PNS and SNS activity make independent contributions to neural mechanisms of executive function (Giuliano et al., 2018).
The prospective effects on cognitive functioning observed here may also be explained by peripheral interactions between the ANS branches. In some prior work, greater PNS responsivity was associated with reduced carotid atherosclerosis in young to early-midlife adults (Heponiemi et al., 2007). The SNS is classically viewed as one part of the redundant systems that regulate vascular contractility (de Lucia et al., 2019). These peripheral physiological outcomes may each impact blood flow to the brain, which could subsequently impact cognitive functioning. Further, increasing the vasodilation capabilities of small arterioles and capillaries – for example, via aerobic exercise and concomitant increases in PNS activity and reductions in SNS activity (Hyodo et al., 2012; Mueller, 2007) – can increase the nutrient delivery to the brain and consequently increasing cognitive function (Ogoh et al., 2008).
Other work suggests PNS responsivity to a cognitive task may indicate a trait-like ability to orient to and cope with challenging tasks (Porges, 2001; Thayer et al., 2012) or to adaptively expend energy in the face of challenge (Duschek et al., 2009); each process may in turn optimize cognitive functioning. A comparison to PNS responsivity to orthostatic challenge – a purely physical task – further indicates the specificity of the results to cognitive challenge; however, the orthostatic challenge did not contain a recovery period, somewhat limiting this comparison. The present findings suggest these PNS responses are more beneficial when SNS activity is low; higher SNS activity, perhaps indexing higher chronic stress levels (Lucini et al., 2005; Cacioppo et al., 2000; Pike et al., 1997), may blunt these salubrious peripheral mechanisms.
4.1. Limitations
Guiding theoretical frameworks, the neurovisceral integration (Thayer & Lane, 2000) and polyvagal models (Porges, 1995), suggest direct links between PNS activity as indexed by HF HRV and central nervous system functioning that may underlie cognitive functioning (but cf. Jennings et al., 2015). Hence, longitudinal changes in autonomic functioning may co-occur with changes in cognitive functioning. In this analysis, we examined longitudinal changes in cognitive function, but biomarker data were only available from one time point (MIDUS 2). It is not clear if the associations observed with change in cognitive functioning would be evident at individual time points; indeed, interactive effects were not robust at the baseline cognitive measurement when accounting for longitudinal change in cognitive functioning. Future work should examine ANS biomarkers measured simultaneous to cognitive testing to determine if changes in ANS co-occur with changes in cognitive functioning. Future work may also attempt to modulate ANS functioning via behavioral or pharmacological intervention to examine putative mechanistic pathways.
The BTACT provides an effective way to examine broad cognitive health outcomes at scale but is not focal in terms of exact cognitive processes that may be impacted across time. We note in supplementary analyses that the episodic memory and executive functioning factors generally paralleled the primary results focused on the summary BTACT score. Future examinations of autonomic contributions to cognitive health would benefit from more specific tests of cognitive functioning like those found in prior work (e.g., visuospatial working memory; Giuliano et al., 2017).
This report relied on creatinine-corrected urinary epinephrine as an index of baseline measurements of SNS activity. This measure of SNS activity is the result of an array of downstream processes operating over the course of several hours; in comparison, HF HRV is considered fairly specific to the PNS and operates on the order of hundreds of milliseconds (Berntson, Cacioppo, & Quigley, 1993b; Thayer & Lane, 2000). Such differences in measurement specificity and systemic breadth may impede interpretation or useful translation of these results. Future work could focus on more direct indices of SNS activity, measured using techniques like microneurography (Greaney et al., 2017) or impedance cardiography (Goedhart et al., 2006), which may better match the timescale of the PNS measures and could be indexed concurrent to the cognitive tasks.
Finally, two demographic issues may limit interpretation. First, longitudinal studies consistently retain subjects that are healthier than the average person within the target population. In one examination of changes in cognitive functioning, attrition was modeled as a significant contributor to a sample’s decline in cognitive functioning (Sliwinski et al., 2003). This survivor bias also suggests that the present results may not generalize to more clinically-relevant populations, such as those who have or will develop mild cognitive impairment, Alzheimer’s disease, or other clinical outcomes. Second, racial and ethnic differences exist in cognitive decline in the US (Mehta & Yeo, 2017), but the MIDUS sample is fairly homogenous relative to US demographics. Future work should continue to investigate the interactive effects of the ANS on changes in cognitive functioning in diverse, pre-clinical populations who may be at risk for cognitive decline in order to explore the ANS as a modifiable therapeutic target.
4.2. Significance
The investigation of the biomarkers for cognitive decline may have clinical implications for assessing and treating cognitive decline in an aging population. Reduced resting SNS activity coupled with increased dynamic vagal tone during challenge is associated with maintenance of cognitive function. For clinicians, it may be beneficial to assess ANS function and determine if an intervention is necessary, especially since ANS activity is a modifiable factor in health and well-being. For example, exercise is linked to improved PNS activity (Hyodo et al., 2012), reduced sympathetic activity (Mueller, 2007), and improved cognitive functioning (Erickson & Kramer, 2009). Although speculative, future research should investigate the effects of interventions that alter the interactive effects of the PNS and SNS as a possible target for bolstering cognitive functioning across the lifespan.
Supplementary Material
Acknowledgements
Knight, Shank, and Clarke were partially supported by National Institute on Aging Grant T32 AG049676 to The Pennsylvania State University. Since 1995, the MIDUS study has been funded by the John D. and Catherine T. MacArthur Foundation Research Network, National Institute on Aging (P01-AG020166; U19-AG051426). Biomarker data collection was further supported by the NIH National Center for Advancing Translational Sciences (NCATS) Clinical and Translational Science Award (CTSA) program as follows: UL1TR001409 (Georgetown); UL1TR001881 (UCLA); 1UL1RR025011 (UW).
Footnotes
Conflict of Interest
All authors declare they have no conflict of interest.
Low-frequency heart rate variability (LF HRV) is available in the MIDUS project data as well and had been theorized to link to sympathetic activity. However, more recent work suggests LF HRV is not a reliable indicator of cardiovascular sympathetic activity (Heathers, 2011).
Because of the adaptive nature of these tasks, performance outcomes are not suitable indices of cognitive functioning.
References
- Alvarez JA, & Emory E (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychology review, 16(1), 17–42. 10.1007/s11065-006-9002-x [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Beauchaine TP, & Thayer JF (2015). Heart rate variability as a transdiagnostic biomarker of psychopathology. International Journal of Psychophysiology, 98(2), 338–350. 10.1016/j.ijpsycho.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1993a). Cardiac psychophysiology and autonomic space in humans: Empirical perspectives and conceptual implications. Psychological Bulletin, 114(2), 296–322. https://psycnet.apa.org/doi/10.1037/0033-2909.114.2.296 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, & Quigley KS (1993b). Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology, 30(2), 183–196. 10.1111/j.1469-8986.1993.tb01731.x [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, … & van der Molen MW (1997). Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. [DOI] [PubMed] [Google Scholar]
- Britton A, Singh-Manoux A, Hnatkova K, Malik M, Marmot MG, & Shipley M (2008). The association between heart rate variability and cognitive impairment in middle-aged men and women. Neuroepidemiology, 31(2), 115–121. 10.1159/000148257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Burleson MH, Poehlmann KM, Malarkey WB, Kiecolt-Glaser JK, Berntson GG, … & Glaser R (2000). Autonomic and neuroendocrine responses to mild psychological stressors: effects of chronic stress on older women. Annals of Behavioral Medicine, 22(2), 140–148. 10.1007/BF02895778 [DOI] [PubMed] [Google Scholar]
- Crowley OV, Kimhy D, McKinley PS, Burg MM, Schwartz JE, Lachman ME, … & Sloan RP (2016). Vagal recovery from cognitive challenge moderates age-related deficits in executive functioning. Research on Aging, 38(4), 504–525. https://psycnet.apa.org/doi/10.1177/0164027515593345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley OV, McKinley PS, Burg MM, Schwartz JE, Ryff CD, Weinstein M, … & Sloan RP (2011). The interactive effect of change in perceived stress and trait anxiety on vagal recovery from cognitive challenge. International Journal of Psychophysiology, 82(3), 225–232. 10.1016/j.ijpsycho.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus EJ, Gianaros PJ, Brindle RC, Jennings JR, & Berntson GG (2019). Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology, 56(2), e13287 https://dx.doi.org/10.1111%2Fpsyp.13287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucia C, Piedepalumbo M, Paolisso G, & Koch WJ (2019). Sympathetic nervous system in age-related cardiovascular dysfunction: Pathophysiology and therapeutic perspective. The international journal of biochemistry & cell biology, 108, 29–33. 10.1016/j.biocel.2019.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duschek S, Muckenthaler M, Werner N, & del Paso GAR (2009). Relationships between features of autonomic cardiovascular control and cognitive performance. Biological psychology, 81(2), 110–117. 10.1111/psyp.13287 [DOI] [PubMed] [Google Scholar]
- Erickson KI, & Kramer AF (2009). Aerobic exercise effects on cognitive and neural plasticity in older adults. British journal of sports medicine, 43(1), 22–24. 10.1136/bjsm.2008.052498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen J, Finucane C, Savva GM, Boyle G, Coen RF, & Kenny RA (2013). Cognitive function is associated with impaired heart rate variability in ageing adults: the Irish longitudinal study on ageing wave one results. Clinical Autonomic Research, 23(6), 313–323. 10.1007/s10286-013-0214-x [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp L, & Ram N (2018). Developmental dynamics of autonomic function in childhood. Psychophysiology, 55(11), e13218 10.1111/psyp.13218 [DOI] [PubMed] [Google Scholar]
- Giuliano RJ, Gatzke-Kopp LM, Roos LE, & Skowron EA (2017). Resting sympathetic arousal moderates the association between parasympathetic reactivity and working memory performance in adults reporting high levels of life stress. Psychophysiology, 54(8), 1195–1208. 10.1111/psyp.12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano RJ, Karns CM, Bell TA, Petersen S, Skowron EA, Neville HJ, & Pakulak E (2018). Parasympathetic and sympathetic activity are associated with individual differences in neural indices of selective attention in adults. Psychophysiology, 55(8), e13079 10.1111/psyp.13079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedhart AD, Kupper N, Willemsen G, Boomsma DI, & de Geus EJ (2006). Temporal stability of ambulatory stroke volume and cardiac output measured by impedance cardiography. Biological Psychology, 72(1), 110–117. 10.1016/j.biopsycho.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Grassi G, & Esler M (1999). How to assess sympathetic activity in humans. Journal of hypertension, 17(6), 719–734. 10.1097/00004872-199917060-00001 [DOI] [PubMed] [Google Scholar]
- Greaney JL, Kenney WL, & Alexander LM (2017). Sympathetic function during whole body cooling is altered in hypertensive adults. Journal of Applied Physiology, 123(6), 1617–1624. https://dx.doi.org/10.1152%2Fjapplphysiol.00613.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathers JAJ (2011). Sympathovagal balance from heart rate variability: An obituary. Experimental Physiology, 97(4), 556 10.1113/expphysiol.2011.063867 [DOI] [PubMed] [Google Scholar]
- Heponiemi T, Elovainio M, Pulkki L, Puttonen S, Raitakari O, & Keltikangas-Järvinen L (2007). Cardiac autonomic reactivity and recovery in predicting carotid atherosclerosis: the cardiovascular risk in young Finns study. Health Psychology, 26(1), 13–21. 10.1037/0278-6133.26.1.13 [DOI] [PubMed] [Google Scholar]
- Hinnant JB, & El-Sheikh M (2009). Children’s externalizing and internalizing symptoms over time: The role of individual differences in patterns of RSA responding. Journal of abnormal child psychology, 37(8), 1049–1061. https://psycnet.apa.org/doi/10.1007/s10802-009-9341-1 [DOI] [PubMed] [Google Scholar]
- Holzman JB, & Bridgett DJ (2017). Heart rate variability indices as bio-markers of top-down self-regulatory mechanisms: A meta-analytic review. Neuroscience and Biobehavioral Reviews, 74, 233–255. 10.1016/j.neubiorev.2016.12.032 [DOI] [PubMed] [Google Scholar]
- Hugdahl K (1996). Cognitive influences on human autonomic nervous system function. Current opinion in neurobiology, 6(2), 252–258. 10.1016/s0959-4388(96)80080-8 [DOI] [PubMed] [Google Scholar]
- Hughes ML, Agrigoroaei S, Jeon M, Bruzzese M, & Lachman ME (2018). Change in cognitive performance from midlife into old age: Findings from the Midlife in the United States (MIDUS) study. Journal of the International Neuropsychological Society, 24(8), 805–820. 10.1017/S1355617718000425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, & Langa KM (2013). Monetary costs of dementia in the United States. New England Journal of Medicine, 368(14), 1326–1334. 10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo K, Dan I, Suwabe K, Kyutoku Y, Yamada Y, Akahori M, … Soya H (2012). Acute moderate exercise enhances compensatory brain activation in older adults. Neurobiology of Aging, 33(11), 2621–2632. 10.1016/j.neurobiolaging.2011.12.022 [DOI] [PubMed] [Google Scholar]
- Jennings JR, Allen B, Gianaros PJ, Thayer JF, & Manuck SB (2015). Focusing neurovisceral integration: Cognition, heart rate variability, and cerebral blood flow. Psychophysiology, 52(2), 214–224. 10.1111/psyp.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, & McEwen BS (2011). Psychobiological allostasis: resistance, resilience and vulnerability. Trends in cognitive sciences, 15(12), 576–584. 10.1016/j.tics.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Kimhy D, Crowley OV, McKinley PS, Burg MM, Lachman ME, Tun PA, … Sloan RP (2013) The association of cardiac vagal control and executive functioning--findings from the MIDUS study. Journal of Psychiatric Research, 47(5), 625–635. 10.1016/j.jpsychires.2013.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S, Tun PA, & Weaver SL (2014). Monitoring cognitive functioning: Psychometric properties of the Brief Test of Adult Cognition by Telephone. Assessment, 21(4), 404–417. 10.1177/1073191113508807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM, & Alzheimer’s Disease Neuroimaging Initiative. (2015). Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimer’s & dementia: translational research & clinical interventions, 1(2), 103–110. 10.1016/j.trci.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Ironson GH, & Schneiderman N (1991). The reliability and specificity of delta versus residualized change as measures of cardiovascular reactivity to behavioral challenges. Psychophysiology, 28(6), 701–711. 10.1111/j.1469-8986.1991.tb01017.x [DOI] [PubMed] [Google Scholar]
- Lovallo WR (2011). Do low levels of stress reactivity signal poor states of health? Biological psychology, 86(2), 121–128. https://dx.doi.org/10.1016%2Fj.biopsycho.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucini D, Di Fede G, Parati G, & Pagani M (2005). Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension, 46(5), 1201–1206. [DOI] [PubMed] [Google Scholar]
- Mahinrad S, Jukema JW, van Heemst D, Macfarlane PW, Clark EN, de Craen AJ, & Sabayan B (2016). 10-Second heart rate variability and cognitive function in old age. Neurology, 86(12), 1120–1127. [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York academy of sciences, 840(1), 33–44. [DOI] [PubMed] [Google Scholar]
- Mehta KM, & Yeo GW (2017). Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimer’s & Dementia, 13(1), 72–83. 10.1016/j.jalz.2016.06.2360 [DOI] [PubMed] [Google Scholar]
- Miller JG, Chocol C, Nuselovici JN, Utendale WT, Simard M, & Hastings PD (2013). Children’s dynamic RSA change during anger and its relations with parenting temperament, and control of aggression. Biological Psychology, 92, 417–425. https://psycnet.apa.org/doi/10.1016/j.biopsycho.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PJ (2007). Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clinical and experimental pharmacology and physiology, 34(4), 377–384. 10.1111/j.1440-1681.2007.04590.x [DOI] [PubMed] [Google Scholar]
- Nordkamp LRO, van Dijk N, & Wieling W (2013). Orthostatic challenge tests: Active standing and head-up tilt In Electrical diseases of the heart (pp. 197–207). Springer, London: 10.1007/978-1-4471-4978-1_12 [DOI] [Google Scholar]
- Ogoh S, Hayashi N, Inagaki M, Ainslie PN, & Miyamoto T (2008). Interaction between the ventilatory and cerebrovascular responses to hypo-and hypercapnia at rest and during exercise. The Journal of Physiology, 586(17), 4327–4338. 10.1113/jphysiol.2008.157073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike JL, Smith TL, Hauger RL, Nicassio PM, Patterson TL, McClintick J, …& Irwin MR (1997). Chronic life stress alters sympathetic, neuroendocrine, and immune responsivity to an acute psychological stressor in humans. Psychosomatic medicine, 59(4), 447–457. 10.1097/00006842-199707000-00015 [DOI] [PubMed] [Google Scholar]
- Poewe W (2007). Dysautonomia and cognitive dysfunction in Parkinson’s disease. Movement disorders, 22(S17), S374–S378. 10.1002/mds.21681 [DOI] [PubMed] [Google Scholar]
- Porges SW (1995). Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology, 32(4), 301–318. [DOI] [PubMed] [Google Scholar]
- Porges SW (2001). The polyvagal theory: phylogenetic substrates of a social nervous system. International journal of psychophysiology, 42(2), 123–146. [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological psychology, 74(2), 116–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- Rozanski A, & Kubzansky LD (2005). Psychologic functioning and physical health: a paradigm of flexibility. Psychosomatic medicine, 67, S47–S53. 10.1097/01.psy.0000164253.69550.49 [DOI] [PubMed] [Google Scholar]
- Ryff CD & Kruger RF (2019). Approaching human health as an integrative challenge: Introduction and Overview In Ryff CD & Kruger RF (Eds.), The Oxford handbook of integrative health science (pp. 3–22). Oxford University Press. [Google Scholar]
- Salthouse TA (2010). Selective review of cognitive aging. Journal of the International neuropsychological Society, 16, 754–760. 10.1017/S1355617710000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski MJ, Hofer SM, Hall C, Buschke H, & Lipton RB (2003). Modeling memory decline in older adults: the importance of preclinical dementia, attrition, and chronological age. Psychology and aging, 18(4), 658 10.1037/0882-7974.18.4.658 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ III, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, & Johnsen BH (2009). Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine, 37(2), 141–153. 10.1007/s12160-009-9101-z [DOI] [PubMed] [Google Scholar]
- Thayer JF & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3): 201–16. 10.1016/s0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2009). Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, 33(2), 81–88. 10.1016/j.neubiorev.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Tun PA, & Lachman ME (2006). Telephone assessment of cognitive function in adulthood: The brief test of adult cognition by telephone. Age and Ageing, 35(6), 629–632. 10.1093/ageing/afl095 [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Everaerd W, Cahill L, McGaugh JL, & Gooren LJG (1998) Memory for emotional events: differential effects of centrally versus peripherally acting β-blocking agents. Psychopharmacology, 138(3), 305–310. 10.1007/s002130050675 [DOI] [PubMed] [Google Scholar]
- Whitbourne SB, Neupert SD, & Lachman ME (2008). Daily physical activity: Relation to everyday memory in adulthood. Journal of Applied Gerontology, 27, 331–349. 10.1177/0733464807312175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2009). ggplot2: elegant graphics for data analysis. Springer-Verlag; New York. [Google Scholar]
- Yerkes RM, & Dodson JD (1908). The relation of strength of stimulus to rapidity of habit-formation. Journal of comparative neurology and psychology, 18(5), 459–482. 10.1002/cne.920180503 [DOI] [Google Scholar]
- Zahn D, Adams J, Krohn J, Wenzel M, Mann CG, Gomille LK, Jacobi-Scherbening V, & Kubiak T (2016). Heart rate variability and self-control – A meta-analysis. Biological Psychology, 115, 9–26. 10.1016/j.biopsycho.2015.12.007 [DOI] [PubMed] [Google Scholar]
- Zeki Al Hazzouri A, Elfassy T, Carnethon MR, Lloyd-Jones DM, & Yaffe K (2017). Heart rate variability and cognitive function in middle-age adults: The coronary artery risk development in young adults. American journal of hypertension, 31(1), 27–34. 10.1093/ajh/hpx125 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


