Abstract
The PKA-inhibitor (PKI) family members PKIα, PKIβ and PKIγ bind with high affinity to PKA and block its kinase activity, modulating the extent and duration of PKA-mediated signaling events. While PKA is a well-known regulator of physiological and oncogenic events, the role of PKI proteins in these pathways has remained elusive. Here, by measuring activation of the MAPK pathway downstream of GPCR-Gαs-cAMP signaling, we show that the expression levels of PKI proteins can alter the balance of activation of two major cAMP targets: PKA and EPAC. Our results indicate that PKA maintains repressive control over MAPK signaling as well as a negative feedback on cAMP concentration. Overexpression of PKI and its subsequent repression of PKA dysregulates these signaling pathways, resulting in increased intracellular cAMP and enhanced activation of EPAC and MAPK. We also find that amplifications of PKIA are common in prostate cancer and are associated with reduced progression free survival. Depletion of PKIA in prostate cancer cells leads to reduced migration, increased sensitivity to anoikis and reduced tumor growth. By altering PKA activity PKI can act as a molecular switch, driving GPCR-Gαs-cAMP signaling towards activation of EPAC-RAP1 and MAPK, ultimately modulating tumor growth.
Keywords: cAMP, GPCR, EPAC, PKI, PKA
INTRODUCTION
Genomic alterations in heterotrimeric G proteins and G protein coupled receptors (GPCRs) that result in the dysregulation of their expression and activity are observed in numerous human malignancies (1, 2). Particularly interesting is the case of the cAMP activating heterotrimeric G protein Gαs, which can have both tumor promoting and suppressive functions. Activating mutations in Gαs are common in human cancer (1) and GNAS (the gene coding for Gαs) is considered a cancer driver gene in endocrine tumors (2–4). On the other hand, inactivation of Gαs can promote tumor growth by regulating cell differentiation in somatic stem cell compartments, like the skin and neural progenitors (5–7).
This disparity may arise due to Gαs activating two major signaling branches downstream of cAMP: PKA (protein kinase A) and EPACs (exchange proteins directly activated by cAMP). A diversity of downstream crosstalk between these pathways can determine specific signaling outcomes in different cell lineages (8, 9).
The tumor suppressive roles of Gαs have been attributed to its activation of PKA. Loss of PKA activity results in the activation of hedgehog signaling and tumor formation in the skin (5). PKA has also been shown to regulate mesenchymal-to-epithelial transition and to induce differentiation of tumor initiating cells in breast cancer (10). PKA can block mitogenic signaling pathways through inhibition of MAPK, AKT and mTOR (9, 11–14) and can block the YAP1 oncogene through activation of LATS kinases (5, 15, 16). All this evidence points towards a specific role of PKA driving tumor suppression downstream of Gαs and cAMP and indicates that additional proteins might participate in the modulation of the signaling outputs of Gαs-cAMP.
Among the proteins that can alter the balance of cAMP signaling is the PKA-inhibitor (PKI) protein family. PKIs specifically block the kinase activity of PKA by binding to the free catalytic subunits, preventing the subsequent phosphorylation of substrates (17, 18). The PKI family is composed of three different genes, PKIA, PKIB and PKIG, which code for the corresponding PKIα, PKIβ and PKIγ proteins (19). PKIs bear a pseudo-substrate sequence that binds to PKA with varying affinities (PKIα showing highest affinity) and a nuclear export signal that regulates their intracellular localization (19–22). PKI knockout studies in mice have yielded minor responses (23, 24), possibly due to compensatory upregulation of PKA regulatory subunits such as RIα (24). However, overexpression has revealed more profound phenotypic consequences in animal studies. Regulation of cAMP signaling is important in cardiac homeostasis, with PKIα expression imparting a cardioprotective function to β-adrenergic signaling via enhancing MAPK signaling (25). Expression of the PKA inhibitory domain of PKIα in the basal cells in the skin of mice leads to rapid expansion of epidermal basal cells and the onset of basal cell carcinoma (5). Thus, by blocking PKA activity, PKI proteins could have a potential role in regulating the output of GPCR-cAMP activation, modulating physiological and pathological functions of PKA and Gαs.
In this study we characterize the differential effect of PKI protein expression levels on GPCR-cAMP signaling using as a readout the activation of ERK downstream of cAMP. Our results indicate that PKI proteins can act as a molecular switch, driving GPCR-Gαs-cAMP signaling away from PKA and towards activation of EPACs resulting in enhanced MAPK signaling. We also demonstrate that PKI overexpression leads to sustained increases in cAMP levels, which might explain the overactivation of EPAC proteins as a result of PKA inhibition. Finally, through the utilization of publicly available cancer genomic datasets and established prostate cancer cell lines, we demonstrate a role for PKI in enhancing prostate cancer aggressiveness both in vitro and in vivo. This activity of PKI proteins can have multiple implications in our understanding of the physiological and pathological processes mediated by GPCRs, Gαs and cAMP.
MATERIALS AND METHODS
DNA constructs
Constructs for PKI1−24, inPKI1−24 and GNASR201C were described previously (5). GFP- PKIα, PKIβ and PKIγ proteins were cloned using gBlocks® Gene Fragment (Integrated DNA Technologies, Coralville, IA, USA) coding for the full-length sequences of human PKIs cloned downstream of GFP. pGL4.29[luc2P/CRE/Hygro] (CRE-Luc) and pGL4.33[luc2P/SRE/Hygro] (SRE-Luc) were purchased from Promega (Madison, WI, USA). Flag-RAP1b and Flag-RAP1b S179AS180A were a gift from Philip Stork (Addgene plasmids 118325 and 118326) (26). pCS6-RAP1GAP was purchased from Transomic Technologies (Huntsville, AL, USA) [pCS6(BC054490)]. The pGloSensor™−22F cAMP Plasmid (E2103) was purchased from Promega (Madison, WI, USA).
Cell culture and transfections
All cells were cultured at 37°C in the presence of 5% CO2. HEK293 cell were obtained from AddexBio (San Diego, CA, USA) and cultured in DMEM (Sigma-Aldrich Inc, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS) (Sigma-Aldrich Inc, St. Louis, MO, USA) and antibiotic/antimycotic solution (Sigma-Aldrich Inc, St. Louis, MO, USA). RWPE-1 (ATCC CRL-11609) were obtained from ATCC (Manassass, VA, USA) and maintained in Keratinocyte Serum Free Medium (K-SFM) (Thermo Fisher Scientific, Waltham, MA, USA). LNCaP (C0019003) was obtained from AddexBio and maintained in RPMI-1640 (Corning [Corning, NY, USA] 10–040-CV) + 10% FBS. VCaP (AddexBio, C0019001) was maintained in DMEM + 10% FBS, supplemented with 10% conditioned media. DU145 (ATCC HTB-81) were maintained in EMEM (ATCC, 30–2003) + 10% FBS. And PC3 (ATCC CRL-1435) were maintained in RPMI-1640 + 10% FBS. For overexpression experiments, cells were transfected one day after plating. DNA constructs were transfected at a concentration of 158 ng/cm2 using Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer instructions. For stimulation experiments, cells were incubated for a minimum of 24 hours after transfection, then serum starved overnight (serum free DMEM) prior to stimulation for the times indicated in the figures. Forskolin (FSK) and 3-isobutyl-1-methylxanthine (IBMX) were added in combination at concentrations of 10 μM and 100 μM respectively for times indicated in the figures, or 6 hours prior to the CRE-Luc assays. Di-methyl-Prostaglandin E2 (mPGE2) was added at a concentration of 5 μM for times indicated on figures, and 6 hours prior to CRE-Luc and SRE-Luc assays. The EPAC inhibitor ESI-09 was added at a concentration of 10 μM, 3 hours prior to stimulation in respective experiments. DMSO served as control (Con) treatment for stimulation experiments. For siRNA experiments, cells were transfected with the corresponding siRNAs one day after plating and were treated/harvested 48hs after transfection. The siRNAs obtained from Dharmacon/Horizon (Lafayette, CO, USA) were the following: NonTargeting (control) siGENOME Non-Targeting siRNA Pool #2, catalog no. D-001206–14-20, PKIα siGENOME Human PKIA (5569) - SMARTpool, catalog no. M-012321–00-0005, PKIβ siGENOME Human PKIB (5570) - SMARTpool, catalog no. M-008224–01-0005, PKIγ siGENOME Human PKIG (11142) - SMARTpool, catalog no. M-017218–02-0005, EPAC1 siGENOME Human RAPGEF3 (10411) - SMARTpool, catalog no. M-007676–01-0005, EPAC2 siGENOME Human RAPGEF4 (11069) - SMARTpool, catalog no. M-009511–01-0005. siRNAs were transfected at a concentration of 5.2 pmol/cm2 individually or 2.1 pmol/cm2 each siRNA in combination using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. To establish stable shRNA expressing cells, lentivirus particles were generated containing SMARTvector Inducible shRNA vectors (Dharmacon) expressing: shControl (Non-targeting shRNA hEF1a-TurboRFP; Cat#: VSC11541; Clone ID: SVC17020101), shPKIA (Human PKIA shRNA hEF1a-TurboRFP; Cat#: V3SH11252–228265267; Clone ID: V3IHSHER_8202917), or shPKIB (Human PKIB shRNA hEF1a-TurboRFP; Cat#: V3SH11252–228570517; Clone ID: V3IHSHER_8508167). Lentiviruses were produced using Lenti-X HEK293-T cells (Takara Bio [Mountain View, CA, USA], 632180) using TransIT-293 Transfection Reagent (Mirus Bio [Madison, WI, USA], MIR 2700). Supernatant containing lentivirus particles was concentrated using Lenti-X Concentrator (Takara Bio, 631232). Cells were selected with 5 μg/mL puromycin dihydrochloride (Gibco [Gaithersburg, MD, USA], A1113803) until a resistant population was obtained, and maintained with 1 μg/mL puromycin. Expression of shRNA was stimulated by 1 μg/mL doxycycline hydrochloride (Sigma Aldrich, D3447) at least 48 hours prior to experiments. Anoikis experiments were performed by seeding DU145 cells into ultra-low adhesion plates (Corning, 3471) in low serum (1%) media for 24 hours. For transwell migration, DU145-shPKI cells were seeded into the top chamber of a 8 um pore transwell insert (24-well format, Sigma Aldrich, CLS3422) in serum free medium (DMEM w/o FBS). The lower chamber was treated with normal serum media. Cells were allowed to migrate for 24 hours, fixed and stained with crystal violet (Sigma Aldrich, C6158) solution with 3% paraformaldehyde. Remaining cells in the inner membrane were removed with a cotton swab and the bottom membrane was fully imaged using a Keyence (Itasca, IL, USA) BZ-X700 microscope (20X) with automatic stage and focus. Stained cells were marked and counted using ImageJ software.
Gene Expression Analysis and Quantitative PCR
RNA was isolated and processed using RNeasy Plus Mini Kit (Qiagen, Germantown, MD, USA) according to manufacturer’s instructions. Cells were lysed using the Precellys lysing kit (Bertin Corp., Rockville, MD, USA). mRNA expression profiling was performed in the CCR-Sequencing Facility at the NIH. cDNA was synthesized from one microgram of RNA using the SensiFAST cDNA Synthesis Kit (Bioline, Memphis, TN, USA), which was used as template for quantitative polymerase chain reaction (qRT–PCR) analysis using the SensiFAST SYBR Hi-ROX Kit (Bioline, Memphis, TN, USA). Samples were analyzed using a 7900HT Fast Real-Time PCR System. Oligonucleotides used for amplification were (Gene, Forward sequence 5’→3’, Reverse sequence 5’→3’): RPLP0, 5’-TGTCTGCTCCCACAATGAAAC-3’, 5’-TCGTCTTTAAACCCTGCGTG-3’; PKIA, 5’-CTTCGTTGTGCATCTTCTTCAC-3’, 5’-GGAAGAACAGGTAGAAGAAATGC-3’; PKIB, 5’-AGAGTTCAGCTGCCACAGAC-3’, 5’-CAGCACTCTTGATAGATTATGAGCC-3’; PKIG, 5’-TTCCCTCCACCTGTCCTT-3’, 5’-CCTACTCGGACTTCATCTCCT-3’; EPAC1 (RAPGEF3), 5’-CATTGAGCCCCAGAGATGTG-3’, 5’-TCCGTGAGAGAGGTGATGG-3’; EPAC2 (RAPGEF4), 5’-CATCTTGGTCATGTTCTTTAAGTCTG-3’, 5’GAGCTGCCTCTATCGTCTTAC-3’.
Immunoblot Analysis and Immunofluorescence
Western blot assays were performed as described previously (27, 28) and repeated at least 2 independent times. Antibodies used were: anti-GAPDH (Cell Signaling [Danvers, MA, USA], catalog no. 5174; 1:5000), anti-phospho-ERK (Cell Signaling [Danvers, MA, USA], catalog no. 4370; 1:1000), anti-ERK (Cell Signaling [Danvers, MA, USA], catalog no. 4695; 1:2000), anti-phospho-AKT (T308) (Cell Signaling [Danvers, MA, USA], catalog no. 13038; 1:1000), anti-phospho-AKT (S473) (Cell Signaling [Danvers, MA, USA], catalog no. 4060; 1:1000), anti-AKT (Cell Signaling [Danvers, MA, USA], catalog no. 4691; 1:2000), anti-phospho-CREB (Cell Signaling [Danvers, MA, USA], catalog no. 9198; 1:1000), anti-CREB (Cell Signaling [Danvers, MA, USA], catalog no. 9197; 1:2000), anti-phospho-PKA substrates (Cell Signaling [Danvers, MA, USA], catalog no. 9624; 1:1000), anti-PKA catalytic subunit (Santa Cruz Biotechnology [Dallas, TX, USA], catalog no. sc-28315; 1:500), anti-GFP (Cell Signaling [Danvers, MA, USA], catalog no. 2956; 1:2000), anti-phospho-Src (Y416) (Cell Signaling [Danvers, MA, USA], catalog no. 2101; 1:1000), anti-phospho-Src (S17) (Cell Signaling [Danvers, MA, USA], catalog no. 12432; 1:1000), anti-Src (Cell Signaling [Danvers, MA, USA], catalog no. 2109; 1:1000), anti-phospho-c-Raf (S259) (Cell Signaling [Danvers, MA, USA], catalog no. 9421; 1:500), anti-phospho-B-Raf (S445) (Cell Signaling [Danvers, MA, USA], catalog no. 2696; 1:500), anti-phospho-MEK 1/2 (S217/S221) (Cell Signaling [Danvers, MA, USA], catalog no. 9154; 1:1000), anti-phospho-MEK 1 (S298) (Cell Signaling [Danvers, MA, USA], catalog no. 9128; 1:1000), anti-MEK1/2 (Cell Signaling [Danvers, MA, USA], catalog no. 8727; 1:2000), anti-RAP1 (Cell Biolabs [San Diego, CA, USA] [RAP1-pulldown kit supplied]; 1:500), anti-glu-glu-Tag (Biolegend [San Diego, CA, USA], catalog no. 901801; 1:500), anti-RAP1GAP (Santa Cruz Biotechnology [Dallas, TX, USA], catalog no. sc-166586; 1:1000), anti-RAP1a/RAP1b (RAP1a/b) (Cell Signaling [Danvers, MA, USA], catalog no. 4938; 1:1000), anti-FLAG-tag (Cell Signaling [Danvers, MA, USA], catalog no. 8146; 1:2000), anti-Cleaved PARP (Cell Signaling [Danvers, MA, USA], catalog no. 5625; 1:1000), anti-Cleaved Caspase 3 (Cell Signaling [Danvers, MA, USA], catalog no. 9661; 1:250), HRP-anti-Mouse-IgG (secondary) (Invitrogen [Carlsbad, CA, USA] catalog no. A28177; 1:5000), HRP-anti-Rabbit-IgG (secondary) (Thermo Scientific [Waltham, MA, USA], catalog no. 31462; 1:5000), HRP-anti-Goat-IgG (secondary) (Life Technologies [Carlsbad, CA, USA], catalog no. A15999; 1:5000). IF was performed as previously described (29). Nuclei were stained with Hoechst 33342 (Invitrogen, Carlsbad, CA, USA). Images were obtained using a Leica SP8 confocal microscope with LASX software (equipped with either a high-contrast Plan-Apochromat 20x oil CS2 objective at 1.30 NA; Leica Microsystems [Buffalo Grove, IL, USA]).
CRE-/SRE-Luciferase Assays
Luciferase assays were performed as described previously (5). Briefly, cells in 24 well plates were co-transfected with SRE-Luc or CRE-Luc (105 ng / cm2) plus the indicated DNA constructs (99 ng / cm2), and a Renilla Luciferase Vector (21 ng / cm2) (Promega, Madison, WI, USA). After 24 hours, cells were serum starved overnight and then luciferase activity was measured using a Dual-Glo Luciferase Assay Kit (Promega, Madison, WI, USA) and a Microtiter plate luminometer (SpectraMax iD3, Molecular Devices LLC [San Jose, CA, USA]). Firefly luciferase activity was normalized to Renilla luciferase in every sample.
RAP1-GTP pulldown assay
Active GTP-bound RAP1 (RAP1-GTP) was isolated using the Rap1 Activation Assay Kit (Cell Biolabs, San Diego, CA, USA) according to the manufacturer protocol. Cells were lysed in kit-supplied lysis buffer supplemented with protease inhibitors (cOmplete ULTRA Tablets [Indianapolis, IN, USA]) and phosphatase inhibitor cocktail (phosSTOP [Indianapolis, IN, USA]) and lysed by gently passing 3–4 times through a 26–1/2 gauge needle. Pulldown and input samples were probed for RAP1 by SDS-PAGE (as described previously).
GloSensor cAMP assay
HEK293 cells were seeded into 96-well cell culture plates (Corning [Corning, NY, USA], 3610). One day after plating, 50 ng pGloSensor-22F cAMP plasmid DNA was co-transfected with 50 ng either inPKI1−24 or PKI1−24 plasmid DNA per well. For GNASR201C experiments, cells were transfected with 50 ng pGloSensor and 30 ng PKI plasmid DNA + 30 ng GNASR201C or empty vector. 48 hours after transfection, cell media was changed to CO2 independent media (Gibco [Gaithersburg, MD, USA], 18045088) supplemented with 10% FBS and 2% GloSensor cAMP Reagent stock solution. Plates were equilibrated at room temperature protected from light for 2 hours prior to reading luminescence. Luminescence kinetics were measured using a Microtiter plate reader (SpectraMax iD3, Molecular Devices LLC [San Jose, CA, USA]), set to read luminescence approximately every 30 seconds for 70 minutes. Luminescence was recorded for at least 5 minutes initially to obtain baseline luminescence and then cells were stimulated with 1 μM FSK or 5 μM mPGE2; DMSO served as a negative vehicle control. Assays with FSK were performed in the absence of PDE inhibitor (IBMX) due to the reporter becoming locked in an active state and saturating luminescence, masking changes to cAMP abundance. GNASR201C cAMP levels were measured in cells with the corresponding transfection without any stimulation.
RNA-sequencing
The sequencing quality of the ~43–60 million reads per sample was assessed using FastQC (version 0.11.5), Preseq (version 2.0.3), Picard tools (version 1.119), and RSeQC (version 2.6.4). Reads were trimmed using Cutadapt (version 1.14) to remove sequencing adapters, prior to mapping to the human reference genome, hg19, using STAR (version 2.5.2b) in a two-pass mode. Expression levels were quantified using RSEM version 1.3.0 with GENCODE annotation version 19. DESeq2 (version 1.20.0) was used to assess differential expression between conditions. Significant differentially expressed genes were defined as having a false-discovery rate corrected-p value < 0.05 and a fold-change ≥ 2.
Subcutaneous xenografts
Animal studies were carried out according to National Institutes of Health–Intramural Animal Care and Use Committee approved protocols, in compliance with the Guide for the Care and Use of Laboratory Animals. DU145 cells stably expressing inducible shControl or shPKIA constructs were pre-treated 48 hours with 1 μg/mL doxycycline and 2×106 cells were injected subcutaneously into the flanks of 10 athymic nude mice (NU/J) (The Jackson Laboratory [Bar Harbor, ME, USA], Stock No: 002019) (shControl-DU145: N=5; shPKIA-DU145: N=5). Animals were maintained on chow containing 200 mg doxycycline/kg (Bio-Serv [Flemington, NJ, USA], S3888). After 8 weeks, all animals were euthanized, and tumors were excised and measured. Volume was calculated via the formula: V = 0.5*(L*l2) (with L = longest diameter, l = shortest diameter).
Genomic alteration in PKI genes
Data from The Cancer Genome Atlas (TCGA) was analyzed for genomic alterations in PKI genes using the cBio cancer genomics portal (cBioPortal) (30, 31) with the terms PKIA, PKIB and PKIG. All datasets were from TCGA PanCancer Atlas and filtered to include datasets with at least 150 samples. The cancer types displayed correspond to: Prostate: Prostate Adenocarcinoma; Liver: Liver Hepatocellular Carcinoma; Breast: Breast Invasive Carcinoma; Lung adeno.: Lung Adenocarcinoma; Pancreas: Pancreatic Adenocarcinoma; Bladder: Bladder Urothelial Carcinoma; Uterine: Uterine Corpus Endometrial Carcinoma; Ovarian: Ovarian Serous Cystadenocarcinoma; Colorectal: Colorectal Adenocarcinoma; Stomach: Stomach Adenocarcinoma; Melanoma: Skin Cutaneous Melanoma; Esophagus: Esophageal Adenocarcinoma; Sarcoma; H&N: Head and Neck Squamous Cell Carcinoma; Lung squ.: Lung Squamous Cell Carcinoma; PCPG: Pheochromocytoma and Paraganglioma; Cervical: Cervical Squamous Cell Carcinoma; ccRCC: Kidney Renal Clear Cell Carcinoma; LGG: Brain Lower Grade Glioma; GBM: Glioblastoma Multiforme; AML: Acute Myeloid Leukemia; pRCC: Kidney Renal Papillary Cell Carcinoma; Thyroid: Thyroid Carcinoma. Expression values correspond to RNA-sequencing data from TCGA (log RNA seq v2 RSEM). Mutation refers to missense mutation.
Statistical analysis
Statistical analyses, variation estimation and validation of test assumptions were carried out using the Prism 7 statistical analysis program (GraphPad Software, San Diego, CA, USA). Grouped analyses were performed with two-way ANOVA with post-hoc multiple comparisons tests, which are described in figure legends. Significance is designated by asterisks in the figure panels. Asterisks denote statistical significance (non-significant or ns, P > 0.05; *P < 0.05; **P < 0.01; *** P < 0.001; and ****P < 0.0001).
Data availability
RNAseq primary and processed data generated in this manuscript have been deposited in the GEO database under accession code GSE133091. All other data is contained within the manuscript. Request for additional data should be made to the corresponding author (RI-B) at ramiro.iglesias-bartolome@nih.gov.
RESULTS
Inhibition of PKA by PKI potentiates the activation of ERK downstream of cAMP
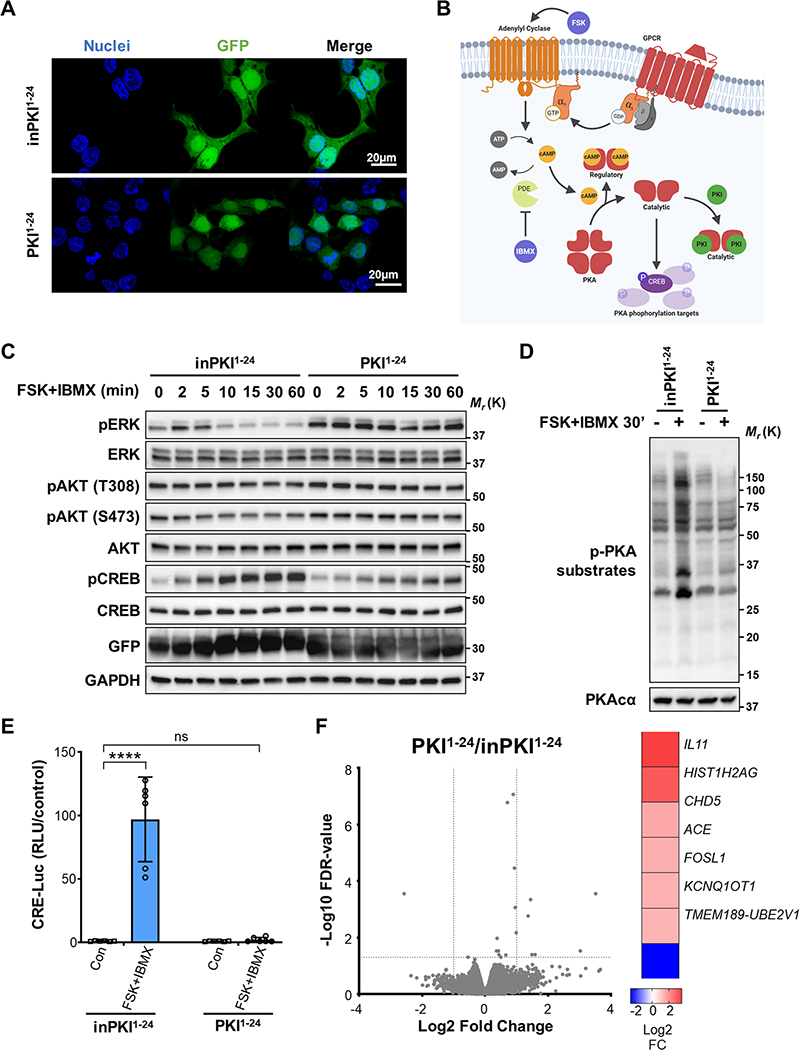
To analyze whether the expression levels of PKI proteins can affect the differential activation of pathways downstream of cAMP, we utilized constructs in which GFP is fused to the isolated PKA binding domain of PKIα (PKI1−24) or a mutated form of PKI1−24 that cannot bind and block PKA as a control (inactive PKI or inPKI1−24) (5) (Fig. 1A). Production of cAMP was induced in HEK293 cells using a combination of 10 μM forskolin (FSK), an activator of adenylyl cyclases (ACs), and 100 μM 3-isobutyl-1-methylxanthine (IBMX), a phosphodiesterase (PDE) inhibitor (depicted in Fig. 1B). Control cells (inPKI1−24) stimulated with FSK and IBMX showed a rapid but transient induction of ERK phosphorylation (pERK) at 2 and 5 min, followed by a consistent, potent reduction in the levels of pERK by 30 min after stimulation (Fig. 1C). This inhibition of pERK was accompanied by an increase in the phosphorylation of CREB (pCREB), indicating activation of PKA (Fig. 1C). Interestingly, cells transduced with active PKI1−24 exhibited increased ERK phosphorylation at baseline, which remained elevated relative to controls over time (Fig. 1C). We also observed an increase in the activation of AKT, although the effect of PKI1−24 on AKT phosphorylation was not as pronounced as the effect on pERK (Fig. 1C). At 30 minutes following stimulation in control cells (the time point that pERK was most reduced), PKA activity was substantially increased as shown by PKA-phosphorylated substrates (Fig. 1D). This response was abrogated with expression of PKI1−24 (Fig. 1D). Consistent with changes to PKA activation, CREB-mediated transcription was significantly increased 6 hours following stimulation in control cells as measured by a CREB-activated luciferase reporter (CRE-Luc), which was eliminated by active PKI1−24 (Fig. 1E).
Figure 1: PKI potentiates the activation of ERK downstream of cAMP.
A. Immunofluorescence (IF) image showing cellular localization of GFP-tagged PKI1−24 peptides (green). Cell nuclei are stained with Hoescht 33342 (blue). B. Illustration showing PKA signaling mediated by cAMP and inhibited by PKI after stimulation with FSK, an adenylyl cyclase (AC) activator, and IBMX, a phosphodiesterase (PDE) inhibitor. C. Western blot time course of the phosphorylated target proteins in cells transduced with control inPKI1−24 or active PKI1–24. Cells were stimulated with FSK and IBMX for the times indicated. D. Western blot image of PKA-phosphorylated substrates following FSK and IBMX stimulation for 30 min in cells transduced with inPKI1−24 or PKI1–24. E. CRE-Luc reporter assay showing PKA activation of CREB with (+) or without (−) FSK and IBMX stimulation for 6 hrs in cells transduced with control inPKI1−24 or active PKI1–24. Results shown are normalized means ± SD, N = 6, and analyzed with two-way ANOVA with Sidak’s post-hoc multiple comparisons test. F. RNA-seq analysis of gene expression differences in HEK293 cells transfected with PKI1−24 compared with cells expressing inPKI1−24; N = 3. Volcano plot shows gene expression changes expressed as Log2 fold change and -Log10 of false discovery rate (FDR)-corrected p values, dotted lines indicate fold change 2 and a p value of 0.05 in the x and y axes respectively. Significantly differentially expressed genes following PKI1−24 expression are indicated in the heat map (right) with their corresponding Log2 fold change. For all panels: ****, p<0.0001; ns, not-significant p>0.05.
Our results show that inhibition of PKA activity by PKI can affect ERK phosphorylation levels, although it is not clear if this effect is mediated directly through signaling cascades or due to transcriptional effects of PKA on regulators of MAPK signaling. To test these possibilities, we performed RNA sequencing (RNAseq) of unstimulated cells expressing PKI1−24 or inPKI1−24 as a control. We did not observe any significant differences in the mRNA level of phosphatases or kinase members of the MAPK family or related genes following PKI1−24 expression (Fig. 1F), indicating that the direct modulation of PKA signaling by PKI alters the phosphorylation state of ERK downstream of cAMP.
We next tested the effect of reduced expression of endogenous PKIs on ERK activation. Quantitative polymerase chain reaction with reverse transcription (RT-qPCR) analysis for PKIs mRNA showed that HEK293 cells express PKIα, PKIβ and PKIγ mRNAs, albeit at different levels (Fig. 2A). Transduction of cells with pooled-small interference RNA (siRNA) targeting each of the PKI messengers alone or in combination led to a significant decrease in the expression levels of individual PKI mRNAs (Fig. 2B). At 30 minutes after FSK and IBMX stimulation, depletion of endogenous PKIα and PKIβ, but not PKIγ corresponded to only a mild increase in overall cAMP-mediated PKA activity as shown by PKA-phosphorylated substrates (Fig. 2C). However, knockdown of PKIs did reduce the basal levels of ERK phosphorylation and increased CREB phosphorylation, which was potentiated by activation of cAMP (Fig. 2D). Strikingly, depletion of PKIs had a more profound effect on PKA-mediated transcriptional activity as shown by the significantly increased CRE-Luc response compared with cells transduced with control siRNA (Fig. 2E).
Figure 2: Endogenous PKI proteins modulate ERK phosphorylation.
A. RT-qPCR analysis of endogenous PKI mRNAs relative to PKIA expression. Results shown are normalized means ± SD, N = 3. B. RT-qPCR of PKI mRNAs in cells transfected with siRNA for individual PKIA, PKIB, and PKIG, or in combination (siPKIABG). Expression relative to non-targeting control siRNA (siCon) transfected cells. Results shown are normalized means ± SD, N = 3, and analyzed with two-way ANOVA with Tukey’s post-hoc multiple comparisons test. C. Western blot panel of PKA-phosphorylated substrates after each siRNA transfection in cells with (+) or without (−) FSK and IBMX for 30 min. D. Western blot panel of indicated targets after depletion of endogenous PKIs and stimulated with (+) or without (−) FSK and IBMX for 30 min. E. CRE-Luc assay in cells treated with DMSO (Con) or FSK+IBMX for 6 hrs in control and PKI depleted cells. Results shown are normalized means ± SD, N = 5, and analyzed with two-way ANOVA with Tukey’s post-hoc multiple comparisons test. F. IF image showing localization of GFP-tagged PKIs. G. Western blot of PKA-phosphorylated substrates in cells transduced with or without PKIs and stimulated with (+) or without (−) FSK and IBMX for 30 min. H. Western blot panel showing changes to cAMP-mediated phosphorylation of target proteins in cells expressing full-length PKIs and stimulated (+) or not (−) with FSK and IBMX for 30 min. I. CRE-Luc assay showing alterations to PKA-mediated transcriptional activity in cells transduced with full-length PKIs or GFP as a control, then treated with DMSO (Con) or FSK+IBMX for 6 hrs. Results shown are normalized means ± SD, N = 6, and analyzed with two-way ANOVA with Tukey’s post-hoc multiple comparisons test. For all panels: **, p<0.01; ***, p<0.001; ****, p<0.0001; ns, not-significant p>0.05.
To further corroborate that PKIs can regulate ERK activation, we constructed GFP fusion proteins for full length human PKIα, PKIβ and PKIγ (Fig. 2F). Overexpression of full-length PKI proteins reduced PKA activity as demonstrated by the levels of PKA-phosphorylated substrates (Fig. 2G). Transduction with full-length PKIs partially blocked the inhibitory effect of cAMP on ERK phosphorylation and reduced CREB phosphorylation (Fig. 2H). Expression of PKIs also abrogated PKA-CREB transcriptional activity as shown by the CRE-Luc assay (Fig. 2I). Taken together, our results indicate that cAMP induces a PKA-dependent inhibition of ERK phosphorylation that can be modulated by altering the expression levels of PKI proteins.
PKI proteins can act as a molecular switch, increasing activation of EPAC and ERK while reducing PKA activity
cAMP production in cells leads to the activation of two different sets of proteins, PKA and EPAC (32, 33). EPAC1/2 are guanine nucleotide exchange factors (GEFs) for the RAS family members RAP1 and RAP2 (13, 32–34). Since EPAC-mediated activation of RAP1 can lead to an increase in MAPK signaling and ERK phosphorylation (9, 11, 13, 35–38), we hypothesized that the increased activation of ERK downstream of cAMP in the presence of PKI could be due to a disbalance in the activities of EPAC and PKA.
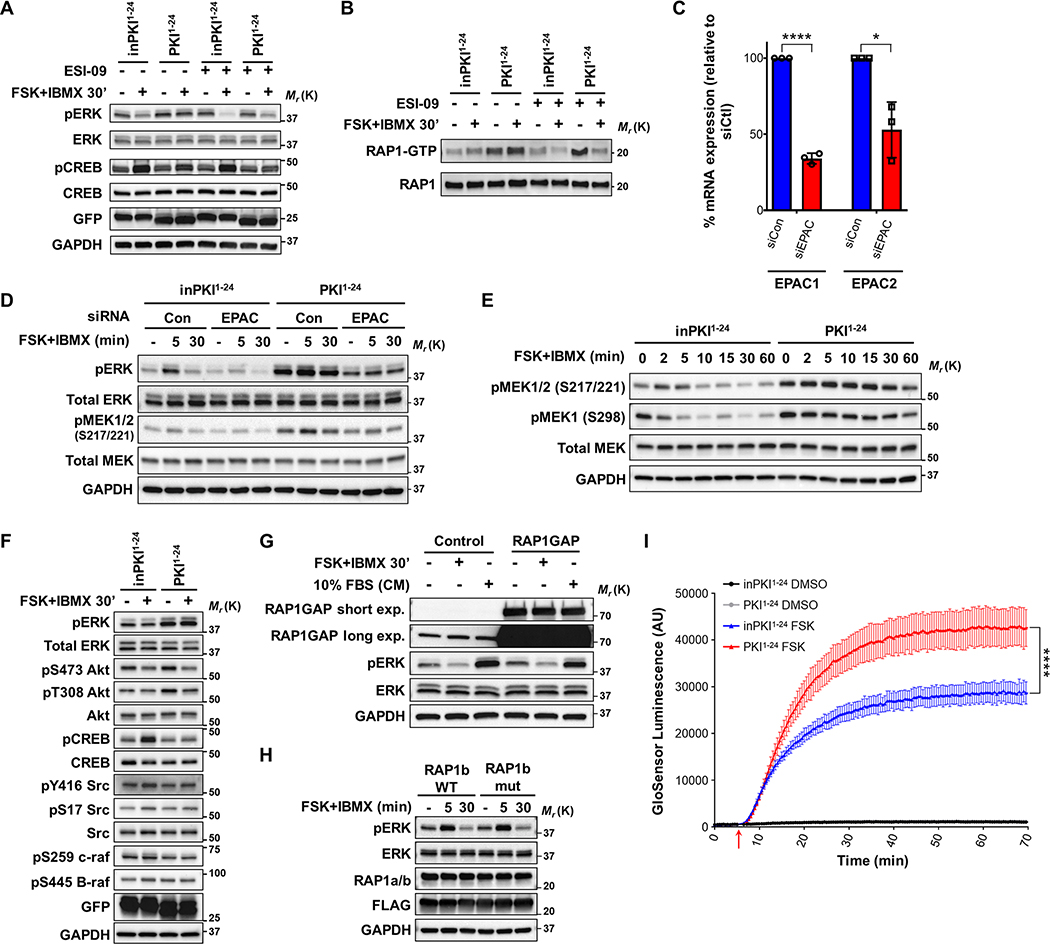
To analyze the effect of EPAC we took advantage of the EPAC inhibitor ESI-09 (39, 40). Treatment with ESI-09 resulted in lower levels of pERK following FSK+IBMX treatment in both control cells and in cells expressing PKI1−24 (Fig. 3A), indicating that the protective effect of PKI on pERK might be partially mediated by EPAC. Indeed, analysis of the activation of the downstream EPAC target RAP1 showed that PKI1−24 potentiated the basal and cAMP-induced levels of the active GTP-bound RAP1 (RAP1-GTP), which is blocked by ESI-09 treatment (Fig. 3B).
Figure 3: PKI proteins enhance cAMP production, increasing activation of EPAC and RAP1.
A. Western blot panel of phosphorylated target proteins in cells stimulated (+) or not (–) with FSK and IBMX in the presence (+) or absence (–) of the EPAC inhibitor ESI-09. B. GTP-bound (active) RAP-1 pulldown assay in cells treated as in panel A. C. RT-qPCR of EPAC mRNAs in cells transfected with siRNA targeting RAPGEF3 and RAPGEF4 (EPAC1 and EPAC2) mRNA in combination (siEPAC). Expression measured relative to non-targeting control siRNA (siCon) transfected cells. Results shown as normalized means ± SD, N = 3 and analyzed using unpaired Two-tailed t-tests between respective siCon and siEPAC treated samples. D. Western blot analysis of cells expressing either inPKI1−24 or PKI1−24 treated with siCon or siEPAC and unstimulated (–) or treated with FSK+IBMX for 5 or 30 minutes. E. Western blot time course of MEK phosphorylation after FSK and IBMX stimulation for the indicated times in cells expressing inPKI1−24 or PKI1–24. F. Western blot panel of the indicated phosphorylated proteins involved in MAPK signaling after FSK and IBMX stimulation for 30 min in cells expressing inPKI1−24 or PKI1–24. G. Western blot analysis of ERK phosphorylation in cells overexpressing RAP1GAP or empty vector (Control) either unstimulated (–), treated with FSK+IBMX for 30 minutes (FSK+IBMX; +), or maintained in complete medium [10% FBS (CM); +]. The RAP1GAP blot was exposed for a short period (short exp.) to detect overexpressed RAP1GAP, and a long exposure (long exp.) to detect endogenous RAP1GAP. H: Western blot analysis of ERK phosphorylation in cells overexpressing either wild-type FLAG-tagged RAP1b (RAP1b WT) or non-PKA-phosphorylatable FLAG-tagged RAP1b mutant protein (RAP1b mut). Cells were untreated (–) or stimulated with FSK+IBMX for 5 or 30 minutes. I. GloSensor cAMP luminescence kinetics assay in cells expressing either inPKI1−24 or PKI1–24. Cells were treated with vehicle control (DMSO) or FSK at the timepoint indicated by the red arrow. Plotted data points represent mean luminescence ± SD at each timepoint, N=6; plots of FSK stimulated cells were analyzed using the ‘plateau followed by one phase association’ non-linear regression method, with an extra sum-of-squares F test comparison of the final plateau between inPKI1−24 and PKI1–24. For all panels: *, p<0.05; ****, p<0.0001.
We then tested the effects of reduced EPAC expression on ERK phosphorylation. Knockdown of EPAC1/2 by pooled siRNA reduced basal ERK phosphorylation and diminished the short term (5 min) cAMP-induced activation of pERK (Fig. 3C and D). While PKI1−24 expression increases overall ERK phosphorylation, depletion of EPAC1/2 partially abrogated this effect (Fig. 3D). These results indicate that overexpression of PKI leads to the concomitant blockage of PKA and increased activity of EPAC, resulting in increased levels of RAP1-GTP and pERK.
RAP1 has been shown to increase B-RAF activation of MEK and consequently ERK phosphorylation (35, 41, 42). It is known that RAP1 can activate MAPK downstream through two major pathways, first via complexing with B-RAF leading to activation of MEK1/2 at serine 217/221 (S217/221) (43, 44) or via Rac1-PAK activation which can activate MEK1 at serine 298 (S298) (45, 46). Activation of cAMP by FSK+IBMX in control cells led to a transient increase in the phosphorylation of MEK at the B-RAF-dependent residues S217/221, followed by a decrease below the unstimulated level (Fig. 3E). Overexpression of PKI1−24 increased the basal levels of pMEK and prevented its inactivation by cAMP (Fig. 3E). We also observed an overall increase on PAK-dependent MEK phosphorylation at S298 (47) in cells expressing PKI1−24 compared to controls (Fig. 3E). The alteration to MEK phosphorylation was also observed to be dependent on EPACs, with reduced pMEK S217/221 in cells treated with EPAC1/2 siRNA compared to control siRNA treated cells (Fig. 3D). Analyses of Src, B-RAF and C-RAF phosphorylation showed no differences in cells treated with FSK+IBMX and transduced with either control inPKI1−24 or active PKI1−24 (Fig. 3F), although RAP1 activation of B-RAF is not mediated by phosphorylation but by protein-protein interactions (42, 44).
Our data suggests that the effect of PKI on ERK phosphorylation is partially dependent on upregulation of EPAC-RAP1 activity. Since PKI directly binds and inhibits PKA catalytic subunits, it is unclear how PKI expression can alter activation of EPAC and RAP1. It has previously been shown that PKA can downregulate RAP1, either through reduced GTP loading on RAP1 by PKA-mediated activation of RAP1GAP (48) or by direct PKA phosphorylation of RAP1 (26, 44, 49). To determine if the alterations to MAPK activity are due to differential RAP1 interaction with RAP1GAP, we overexpressed RAP1GAP and treated cells with either FSK+IBMX or 10% fetal bovine serum (FBS) in the media. Stimulation of cAMP in cells with RAP1GAP overexpression did not show substantial changes in pERK compared to control cells (Fig. 3G). We also did not observe differences in ERK activation when we utilized a RAP1b mutant protein (RAP1b mut) containing alanine substitutions at two PKA phosphorylation sites (S179A, S180A) (26) (Fig. 3H). Together, these results suggest that the cAMP mediated changes in pERK are not due to direct PKA regulation on RAP1 activity.
An additional possibility for the crosstalk between EPAC and PKA activities is that PKA can directly modulate the levels of cAMP. PKA can phosphorylate and inactivate ACs (50, 51) and can increase the activity of PDEs (52), reducing the levels of cAMP. To measure changes in cAMP abundance, we utilized a luminescent biosensor for real-time monitoring of intracellular cAMP (GloSensor 22F cAMP) (53, 54). Cells expressing PKI1−24 stimulated with FSK yielded significantly increased and sustained levels of cAMP compared to inPKI1−24 transduced cells (Fig. 3I). These results suggest that PKI overexpression disrupts the negative feedback of PKA on cAMP concentration, leading to increased cAMP which could explain the observed overactivation of EPAC-RAP1 by PKI.
PKI potentiates cAMP production and enhances ERK activation downstream of Gαs and GPCR signaling
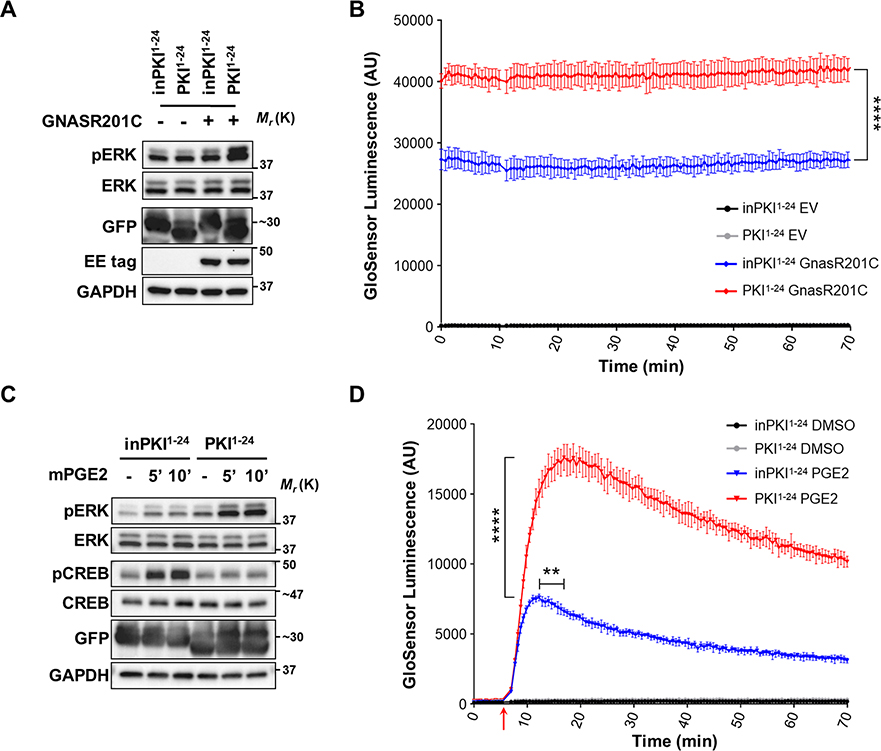
Our results indicate that overexpression of PKI can act as a molecular switch, modifying the activation levels of the two main branches downstream of cAMP, PKA and EPAC, leading to increased ERK activity. We next explored whether this mechanism was relevant when cAMP production was triggered upstream of adenylyl cyclases by activation of Gαs and GPCR signaling. To specifically test Gαs-cAMP signaling we used an oncogenic mutant form of Gαs (GNASR201C) which is constitutively active due to a defect on GTP hydrolysis (1). We found that GNASR201C protein was able to increase the basal levels of pERK and that this increase was further potentiated by the presence of PKI1−24 (Fig. 4A). PKI1−24 overexpression also led to a significant and sustained increase in cAMP abundance in cells with GNASR201C overexpression (Fig. 4B).
Figure 4: PKI proteins enhance ERK activation downstream of Gαs and GPCR signaling.
A. Western blot panel of phosphorylated proteins in HEK293 cells expressing (+) or not (–) a constitutively active mutant form of Gαs (GNASR201C) in combination with active PKI1−24 or control inPKI1–24. B. GloSensor cAMP luminescence assay of inPKI1−24 or PKI1−24 expressing cells with either empty vector (EV) or GNASR201C overexpression. Plotted data points represent the mean luminescence ± SD at each timepoint, N=4, and analyzed using an unpaired Two-tailed t-test between the inPKI1−24 and PKI1−24 + GNASR201C cells. C. Western blot analysis of phosphorylated proteins in HEK293 cells expressing inPKI1−24 or PKI1−24, stimulated for the indicated time with di-methyl-PGE2 (mPGE2). D. GloSensor cAMP luminescence assay of inPKI1−24 or PKI1−24 expressing cells treated either with vehicle control (DMSO) or mPGE2 at the timepoint indicated by the red arrow. Plotted data points represent the mean luminescence ± SD at each timepoint, N=5; statistics for mPGE2 treated cells were analyzed using an unpaired Two-tailed t-test comparing the mean maximum luminescence, and separately with the Mann-Whitney test to compare the mean time to maximal luminescence between inPKI1−24 and PKI1−24 cells. For all panels: **, p<0.01; ****, p<0.0001.
We next tested how PKIs may modulate signaling downstream of endogenous Gαs-coupled GPCRs by using prostaglandin E2 (PGE2) (55). PGE2 signals by binding to Gαs-coupled prostaglandin E receptors. PGE2 stimulation led to increased ERK activation that was potentiated by expression of PKI1−24 (Fig. 4C). GloSensor assays showed that PKI1−24 also results in significantly increased cAMP production following GPCR activation with PGE2, as well as a significant delay in the time until cAMP levels began to decline (Fig. 4D). Therefore, we conclude that PKI overexpression can modulate the response of cells to multiple signals that converge on the cAMP pathway.
PKI shifts transcriptional responses downstream of GPCR signaling
While protein phosphorylation changes due to cAMP induction are usually rapid and transient, they can lead to complex alterations in cell fate through the activation and inhibition of transcriptional networks. We took advantage of two sets of reporters to analyze the effect of PKI expression on gene regulatory networks by using the downstream reporters CRE-Luc and SRE-Luc, that measure downstream transcriptional activation by PKA and ERK respectively (56) (Fig. 5A). Cells transduced with these reporters and either PKI1−24 or full-length PKI proteins were stimulated with PGE2. In both cases, we observed that overexpression of PKIs was able to block PGE2-induced activation of CREB transcription (Fig. 5B and C), and at the same time potentiate the activation of downstream ERK transcriptional pathways (Fig. 5D and E). Altogether, these results support the hypothesis that PKIs act as molecular switches downstream of Gαs-coupled GPCRs.
Figure 5: PKI proteins switch transcriptional responses downstream of PGE2 signaling.
A. Graphical representation of CRE-Luc and SRE-Luc reporter assays to test transcriptional activation downstream of PKA or ERK respectively. Dotted lines indicate indirect regulation of the target. B-E: Luciferase assays in cells transfected with the indicated constructs and treated with DMSO (Con) or mPGE2 for 6 hours. B. CRE-Luc reporter assay of cells expressing inPKI1−24 or PKI1−24; Mean ± SD, N = 6, and analyzed with two-way ANOVA with Tukey’s post-hoc multiple comparisons test. C. CRE-Luc reporter assay of cells expressing GFP or full-length GFP-PKIs; Mean ± SD, N = 6, and analyzed with two-way ANOVA with Tukey’s post-hoc multiple comparisons test. D. SRE-Luc reporter assay of cells expressing inPKI1−24 or PKI1−24; Mean ± SD, N = 6, and analyzed with two-way ANOVA with Tukey’s post-hoc multiple comparisons test. E. SRE-Luc reporter assay of cells expressing GFP or full-length GFP-PKIs; Mean ± SD, N = 8, and analyzed with two-way ANOVA with Tukey’s post-hoc multiple comparisons test. For all panels: *, p<0.05; **, p<0.001; ***, p<0.001; ****, p<0.0001; ns, not-significant p>0.05.
PKIα expression is associated with aggressive prostate cancer phenotypes
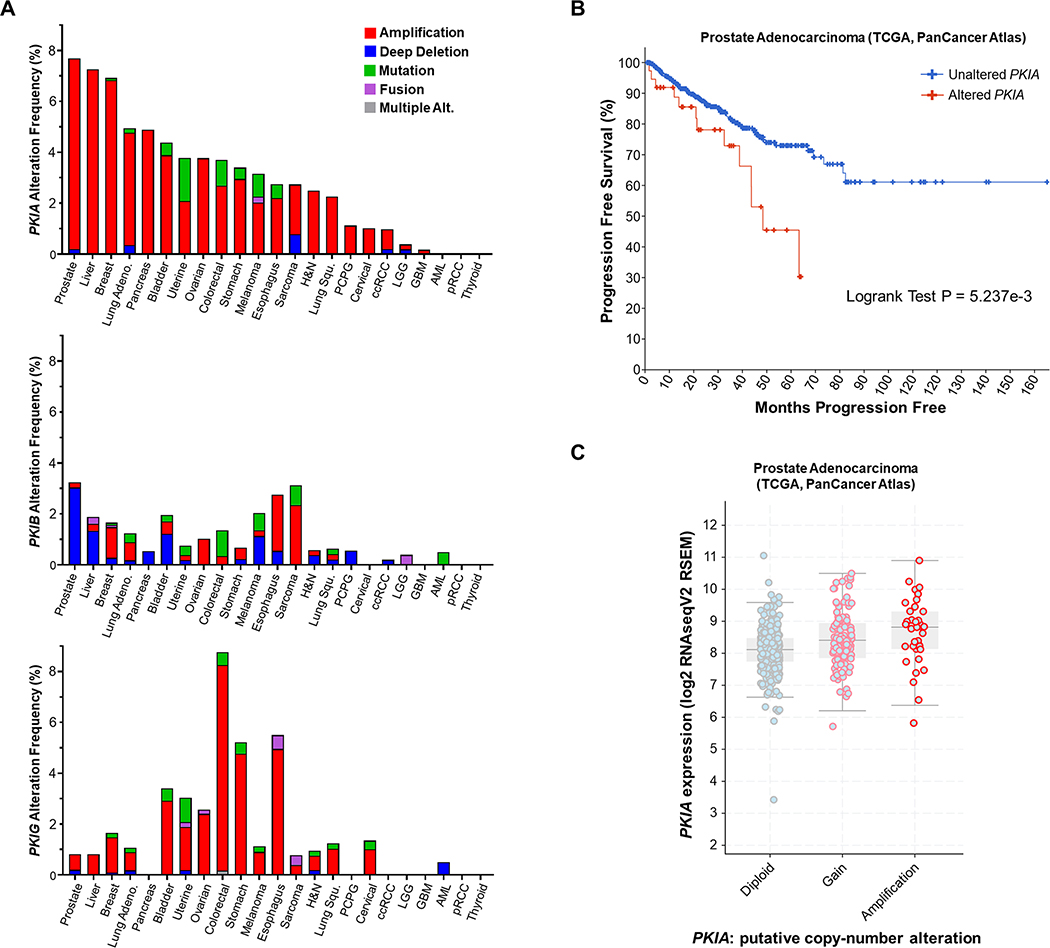
Multiple alterations in genes coding for heterotrimeric G proteins and GPCRs are observed in human malignancies (1, 2) but the presence of genomic alterations in PKIs has not been studied. To characterize potential alteration in PKI genes that could indicate a role for this protein family in pathological conditions in humans, we utilized data from The Cancer Genome Atlas (TCGA) and analyzed genomic alterations in PKI genes using the cBio cancer genomics portal (cBioPortal) (30, 31). Studies were filtered to include the TCGA PanCancer Atlas collection. The gene coding for PKIα (PKIA) showed the highest overall levels of gene amplifications across numerous cancer types, particularly in prostate, liver, breast, and lung cancers, among others (Fig. 6A). Interestingly, PKIA only showed minor alterations in glioma (LGG), glioblastoma (GBM) and no alteration in thyroid cancer (Fig. 6A). Since prostate adenocarcinoma exhibited the greatest frequency of PKIA gene amplifications, we further investigated the potential role for PKIA in prostate cancer progression. We found that there was a significant reduction in the time to disease progression in the group with PKIA alterations compared to unaltered cases (Fig. 6B). Furthermore, PKIA mRNA expression was increased consistently with PKIA gene amplification (Fig. 6C).
Fig. 6: PKI genomic alterations are frequent in cancer and PKIA amplification is associated with aggressive prostate cancer.
A. TCGA PanCancer Atlas analysis of PKIA, PKIB, and PKIG gene alteration frequencies in human cancers. Cancer type (X-axis) is ordered by most common PKIA gene alteration frequency. B. cBioPortal analysis of the TCGA prostate adenocarcinoma PanCancer Atlas dataset for progression-free survival (months) between patients with PKIA gene alterations vs. those with unaltered PKIA. Significance (LogRank test and exact P value) calculated in cBioPortal. C. cBioPortal Log2 RNAseq analysis of PKIA mRNA expression vs. copy-number alteration of samples in the TCGA PanCancer Atlas prostate adenocarcinoma dataset.
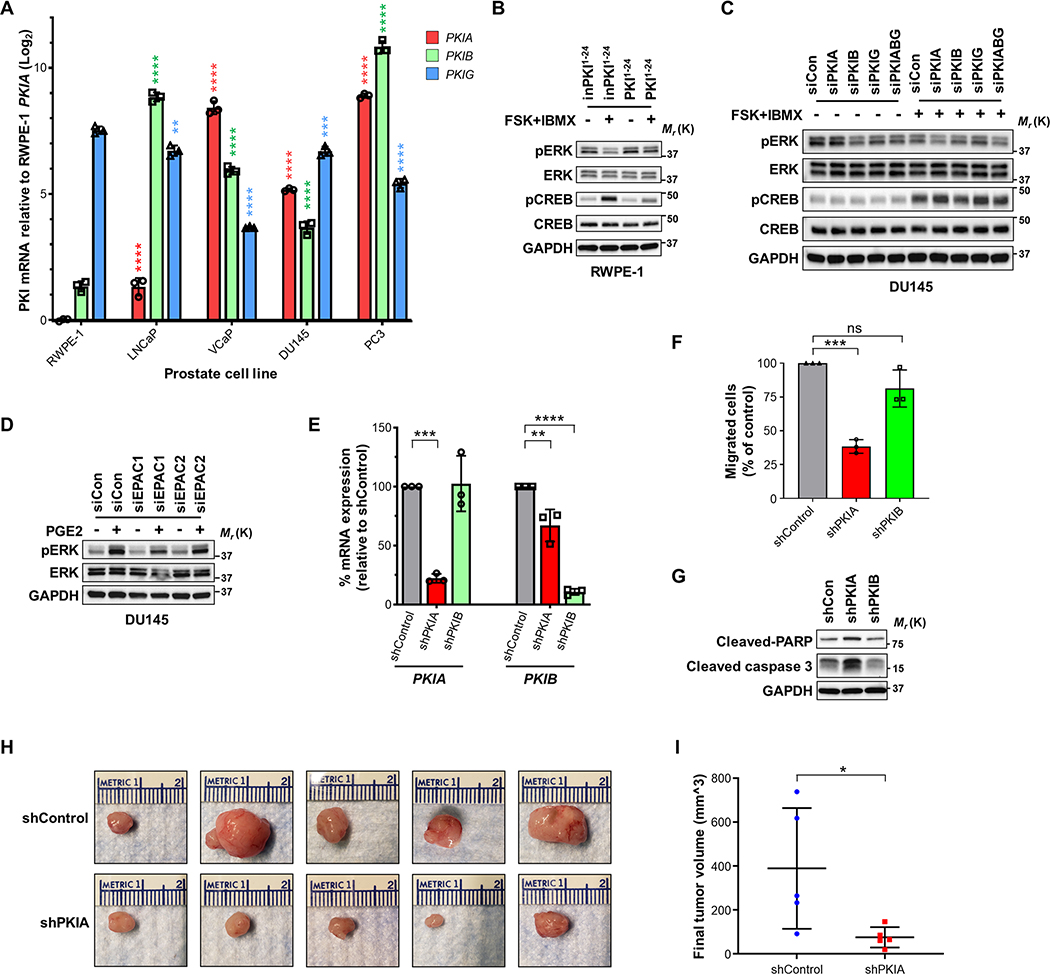
We next measured PKI expression levels in the established prostate cancer cell lines LNCaP, VCaP, DU145, and PC3, compared to an immortalized normal prostate epithelial line, RWPE-1. PKIA mRNA was significantly elevated in all prostate cancer lines relative to RWPE-1, with even higher expression in the more aggressive castration-resistant prostate cancer (CRPC) cell lines VCaP, DU145, and PC3 (Fig. 7A). To determine if PKIs can alter cAMP signaling in prostate cells, PKI levels were modulated by overexpression in normal RWPE-1 (PKI1−24) or depletion in tumorigenic DU145 cells (siPKIs). Overexpression of PKI1−24 in RWPE-1 cells treated with FSK+IBMX resulted in a similar ERK activation profile as observed in HEK293 cells; FSK+IBMX stimulation reduced pERK in the inPKI1−24 expressing cells, which was prevented by PKI1−24 expression (Fig. 7B). Conversely, in DU145 cells, siRNA depletion of PKIA or the combination of siPKIs resulted in reduced pERK levels following FSK+IBMX stimulation (Fig. 7C). It was also observed that PGE2 stimulation in DU145 cells resulted in increased pERK that was partially reduced by EPAC1 knockdown, indicating a potential role of EPAC in MAPK regulation in these cells (Fig. 7D).
Fig. 7: PKIα supports aggressive prostate cancer phenotypes in vitro and in vivo.
A. RT-q-PCR analysis of PKI mRNA between non-transformed prostate epithelial cell line, RWPE-1, and prostate adenocarcinoma cell lines LNCaP, VCaP, DU145, and PC3. Log2 mRNA expression relative to PKIA in RWPE-1 cells; Mean ± SD, N = 3, and analyzed with two-way ANOVA with Tukey’s post-hoc multiple comparisons test. B. Western blot image of phosphorylated target proteins following FSK and IBMX stimulation for 30 min in RWPE-1 cells transduced with inPKI1−24 or PKI1–24. C. Western blot panel of indicated targets DU145 cells after depletion of endogenous PKIs and stimulated with (+) or without (–) FSK and IBMX for 30 min. D. Western blot analysis of DU145 cells treated with siCon, siEPAC1 or siEPAC2 and unstimulated (–) or treated (+) with PGE2 for 5 minutes. E. RT-qPCR of PKIA and PKIB mRNAs in DU145 cells stably transduced with doxycycline-inducible shRNA targeting PKIA and PKIB (shPKIA and shPKIB) after 48 hours of 1 μg/mL doxycycline stimulation. Expression measured relative to non-targeting control shRNA (shControl) transduced cells. Results shown as normalized means ± SD, N = 3 and analyzed individually (for each mRNA target) using one-way ANOVA with Dunnett’s post-hoc multiple comparisons test. F. Transwell migration (24 hours) of DU145 cells expressing shPKIA or shPKIB compared to shControl from serum-free media towards full serum media. Results shown as normalized means ± SD, N = 3 and analyzed using one-way ANOVA with Dunnett’s post-hoc multiple comparisons test. G. Western blot image of apoptosis markers for anoikis analysis of shPKIA or shPKIB expressing DU145 cells compared to shControl cells following 24-hour culture in anchorage-free conditions in low serum (1% FBS) media. H. Images of final xenograft tumors from subcutaneously injected shControl and shPKIA DU145 cells; N = 5 animals injected for each shRNA. I. Final tumor volumes measured from xenograft (panel H). Plotted showing individual tumor volume scatter plot with Mean ± SD, N = 5 each, and analyzed with the unpaired Mann-Whitney U test.
To determine if PKIα is involved in prostate cancer growth and metastasis, DU145 cells were stably transduced with lentiviral doxycycline-inducible shRNA constructs targeting PKIA or PKIB and compared to control shRNA (shControl) (Fig. 7E). Transwell migration assays indicated that shPKIA but not shPKIB significantly reduced migration of DU145 cells compared to shControl (Fig. 7F). Sensitivity to anoikis as a measure of anchorage-independent metastatic survival (57) was also measured. Depletion of PKIA resulted in increased sensitivity to anoikis in DU145 cells, as shown by induction of the apoptotic markers cleaved PARP and cleaved caspase 3 (Fig. 7G). To analyze the role of PKIα in vivo, DU145 cells expressing shPKIA or shControl were subcutaneously xenografted into athymic nude mice. Final tumor volumes showed that shPKIA tumors were significantly smaller compared to shControl (Fig. 7H and 7I). Altogether, our data reveals that PKIα is involved in driving more aggressive prostate cancer phenotypes both in vitro and in vivo.
DISCUSSION
In this study, we demonstrate that expression levels of PKI proteins can alter the activation of the GPCR-cAMP effectors PKA and EPAC, modulating the state of MAPK signaling (Fig. 8). Our results show that PKI proteins can act as molecular switches that divert GPCR-cAMP signaling towards increased EPAC activity. While PKIs and PKA do not directly regulate EPAC, its overactivation could be due to the disruption of PKA feedback loops on the production and degradation of cAMP (50–52). PKI inhibition of PKA results in increased and sustained cAMP concentrations that, in turn, can intensify the activation of EPAC and RAP1. Since RAP1 has been shown to increase MEK and ERK phosphorylation (35, 41, 42), the activation of MAPK mediated by PKI is probably due in part to RAP1. In line with this, both B-RAF and PAK dependent MEK activation sites are phosphorylated at a higher level with PKI overexpression. Additional regulatory loops exist that might expand the effect of PKI crosstalk into the MAPK pathway. For example, it is known that PKA antagonizes RAS signaling by phosphorylating C-Raf (58), which might contribute to the observed reduction in MEK and ERK phosphorylation in cells treated with FSK.
Figure 8: PKI acts as a molecular switch downstream of GPCR-Gαs signaling.
Simplified model depicting the findings of our study. PKI expression levels can alter the activation state of PKA, leading to the disruption of PKA modulation of cAMP levels and MAPK. In turn, increased levels of cAMP can lead to activation of EPAC and RAP1, which can lead to increase MEK and ERK phosphorylation. Dotted lines indicate indirect regulation of the target.
Our mechanistic studies of PKI were largely based on overexpression of the PKA-binding domain of PKIα, although knockdown experiments and expression of full-length PKI proteins showed similar trends. The limited effect of experiments with endogenous PKI proteins in HEK293 cells could be due to the numerous feedback mechanisms that regulate cAMP and PKA levels. For example, PKIα knockout mice show a decrease in PKA activity rather than the expected increase due to upregulation of the expression of regulatory subunits of PKA (23). These feedback loops might also help explain the lack of an additive effect of knocking down all endogenous PKI proteins and indicate that cells keep a very tight control on the activity levels of PKA catalytic subunits. The use of FSK and IBMX to stimulate and maintain cAMP levels in cells allowed us to demonstrate that PKI proteins directly modulate cAMP-PKA-EPAC signaling rather than additional GPCR-dependent pathways that feed into MAPK signaling. FSK and IBMX bypass the traditional GPCR-G-protein axis, so they are a powerful tool to directly study modulation of cAMP without the confounding activity of other pathways activated by GPCRs. However, we were able to confirm that our results are also relevant for endogenous activation of cAMP by GPCRs by using PGE2.
While PKI peptides have been extensively used to study PKA function, our study highlights the importance of understanding the biological consequences of altered PKI protein expression on downstream cAMP signaling. PKI protein levels regulate glucose homeostasis (59), cardiomyocyte hypertrophy (60), neuronal potentiation (61) and morphine antinociceptive tolerance (19). Further evidence also suggests PKI proteins may dysregulate signaling programs and cell fate decisions important in cancer. The recently described roles of PKA in tumor suppression (5, 6) and mesenchymal-to-epithelial transition (10) suggest that PKIs could be unique regulatory components for these pathways. Indeed, overexpression of the PKA-binding domain of PKIα is sufficient to induce rapid tumor formation in the skin by blocking PKA-induced cell differentiation (5). Here, we show that numerous genomic alterations are present in PKI genes across a diverse set of human cancers, particularly genomic amplifications in PKIA. In prostate cancer patients, amplification of PKIA was associated with increased PKIα expression and reduced progression-free survival. Prostate cancer cell lines showed significantly increased PKI mRNA levels, although it is not clear what genetic drivers regulate this increased expression. Depletion of PKIA mRNA in the CRPC cell line DU145 resulted in reduced metastatic phenotypes in vitro (reduced migration and increased sensitivity to anoikis) and reduced tumor growth potential in vivo. Supporting our results for a role of PKIs in prostate cancer, it has been shown that PKIβ can affect growth and invasiveness in prostate cancer cells (62), although we did not observe any effects mediated by PKIβ in DU145 cells.
In our study we focused on PKI modulation of ERK downstream of GPCR-Gαs-cAMP signaling. Consistent with our findings in HEK293 cells, PKI and EPAC expression levels can modulate MAPK signaling in prostate cell lines. While this might indicate that the effects of PKI in prostate cancer could be mediated by regulation of MAPKs, PKIs could affect other pathways. For example, in the skin, PKI overexpression leads to the activation of oncogenic YAP1 and GLI signaling (5). In addition, we show that PKI expression levels can alter downstream events mediated by PGE2. PGE2 is an important component of the tumor microenvironment, having a predominant role in tumor growth (55), indicating that PKIs could impact prostaglandin-receptor signaling in cells. Finally, it has been shown that PKI proteins can bind all PKA catalytic subunits (63), even those with activating mutations (64), suggesting that PKIs could have regulatory roles in both oncogenic and tumor suppressive functions of PKA.
Overall, our data suggests that by differentially regulating the output of GPCR signaling, the levels of expression of PKI proteins can modulate physiological and pathological roles of Gαs, PKA and Gαs-coupled GPCRs. Additional research is needed to fully unravel the impact of PKIs in the complex cAMP-activated signaling networks regulating tumor growth.
ACKNOWLEDGMENTS
We thank the members of the CCR Sequencing Facility at the Frederick National Laboratory for Cancer Research for their help during sample preparation, sequencing and data processing. This work used the computational resources of the NIH High-Performance Computing Biowulf Cluster. Illustrations were created with BioRender.com. The results shown here are in part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (ZIA BC 011764 and ZIA BC 011763).
Non-standard abbreviations
- AC
Adenylyl cyclase
- cAMP
Cyclic adenosine monophosphate
- CREB
cAMP-response element binding protein
- CRPC
Castration-resistant prostate cancer
- EPAC
Exchange protein directly activated by cAMP
- ERK/MAPK
Extracellular-signal-regulated kinase / Mitogen-activated protein kinase
- FSK
Forskolin
- GAP
GTPase activating protein
- GPCR
G-protein coupled receptor
- IBMX
3-isobutyl-1-methylxanthine
- PDE
Phosphodiesterase
- PGE2
Prostaglandin E2
- PKA
Protein kinase A
- PKI
PKA inhibitor protein
- RAP
Ras-related protein
Footnotes
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
REFERENCES
- 1.O’Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, and Gutkind JS (2013) The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nature reviews. Cancer 13, 412–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, Colaprico A, Wendl MC, Kim J, Reardon B, Ng PK, Jeong KJ, Cao S, Wang Z, Gao J, Gao Q, Wang F, Liu EM, Mularoni L, Rubio-Perez C, Nagarajan N, Cortes-Ciriano I, Zhou DC, Liang WW, Hess JM, Yellapantula VD, Tamborero D, Gonzalez-Perez A, Suphavilai C, Ko JY, Khurana E, Park PJ, Van Allen EM, Liang H, Group MCW, Cancer Genome Atlas Research, N., Lawrence MS, Godzik A, Lopez-Bigas N, Stuart J, Wheeler D, Getz G, Chen K, Lazar AJ, Mills GB, Karchin R, and Ding L (2018) Comprehensive Characterization of Cancer Driver Genes and Mutations. Cell 173, 371–385 e318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, and Spiegel AM (1991) Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. The New England journal of medicine 325, 1688–1695 [DOI] [PubMed] [Google Scholar]
- 4.Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, and Vallar L (1989) GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 340, 692–696 [DOI] [PubMed] [Google Scholar]
- 5.Iglesias-Bartolome R, Torres D, Marone R, Feng X, Martin D, Simaan M, Chen M, Weinstein LS, Taylor SS, Molinolo AA, and Gutkind JS (2015) Inactivation of a Galpha(s)-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol 17, 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He X, Zhang L, Chen Y, Remke M, Shih D, Lu F, Wang H, Deng Y, Yu Y, Xia Y, Wu X, Ramaswamy V, Hu T, Wang F, Zhou W, Burns DK, Kim SH, Kool M, Pfister SM, Weinstein LS, Pomeroy SL, Gilbertson RJ, Rubin JB, Hou Y, Wechsler-Reya R, Taylor MD, and Lu QR (2014) The G protein alpha subunit Galphas is a tumor suppressor in Sonic hedgehog-driven medulloblastoma. Nature medicine 20, 1035–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kool M, Jones DT, Jager N, Northcott PA, Pugh TJ, Hovestadt V, Piro RM, Esparza LA, Markant SL, Remke M, Milde T, Bourdeaut F, Ryzhova M, Sturm D, Pfaff E, Stark S, Hutter S, Seker-Cin H, Johann P, Bender S, Schmidt C, Rausch T, Shih D, Reimand J, Sieber L, Wittmann A, Linke L, Witt H, Weber UD, Zapatka M, Konig R, Beroukhim R, Bergthold G, van Sluis P, Volckmann R, Koster J, Versteeg R, Schmidt S, Wolf S, Lawerenz C, Bartholomae CC, von Kalle C, Unterberg A, Herold-Mende C, Hofer S, Kulozik AE, von Deimling A, Scheurlen W, Felsberg J, Reifenberger G, Hasselblatt M, Crawford JR, Grant GA, Jabado N, Perry A, Cowdrey C, Croul S, Zadeh G, Korbel JO, Doz F, Delattre O, Bader GD, McCabe MG, Collins VP, Kieran MW, Cho YJ, Pomeroy SL, Witt O, Brors B, Taylor MD, Schuller U, Korshunov A, Eils R, Wechsler-Reya RJ, Lichter P, Pfister SM, and Project IPT (2014) Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer cell 25, 393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng X, Ji Z, Tsalkova T, and Mei F (2008) Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 40, 651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stork PJ, and Schmitt JM (2002) Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol 12, 258–266 [DOI] [PubMed] [Google Scholar]
- 10.Pattabiraman DR, Bierie B, Kober KI, Thiru P, Krall JA, Zill C, Reinhardt F, Tam WL, and Weinberg RA (2016) Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science 351, aad3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozengurt E (2007) Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol 213, 589–602 [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith ZG, and Dhanasekaran DN (2007) G protein regulation of MAPK networks. Oncogene 26, 3122–3142 [DOI] [PubMed] [Google Scholar]
- 13.Schmidt M, Dekker FJ, and Maarsingh H (2013) Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol Rev 65, 670–709 [DOI] [PubMed] [Google Scholar]
- 14.Jewell JL, Fu V, Hong AW, Yu FX, Meng D, Melick CH, Wang H, Lam WM, Yuan HX, Taylor SS, and Guan KL (2019) GPCR signaling inhibits mTORC1 via PKA phosphorylation of Raptor. Elife 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim M, Kim M, Lee S, Kuninaka S, Saya H, Lee H, Lee S, and Lim DS (2013) cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. The EMBO journal 32, 1543–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, and Guan KL (2013) Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes & development 27, 1223–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashby CD, and Walsh DA (1972) Characterization of the interaction of a protein inhibitor with adenosine 3’,5’-monophosphate-dependent protein kinases. I. Interaction with the catalytic subunit of the protein kinase. J Biol Chem 247, 6637–6642 [PubMed] [Google Scholar]
- 18.Ashby CD, and Walsh DA (1973) Characterization of the interaction of a protein inhibitor with adenosine 3’,5’-monophosphate-dependent protein kinases. II. Mechanism of action with the holoenzyme. J Biol Chem 248, 1255–1261 [PubMed] [Google Scholar]
- 19.Dalton GD, and Dewey WL (2006) Protein kinase inhibitor peptide (PKI): a family of endogenous neuropeptides that modulate neuronal cAMP-dependent protein kinase function. Neuropeptides 40, 23–34 [DOI] [PubMed] [Google Scholar]
- 20.Taylor SS, Buechler JA, and Yonemoto W (1990) cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem 59, 971–1005 [DOI] [PubMed] [Google Scholar]
- 21.Wen W, Harootunian AT, Adams SR, Feramisco J, Tsien RY, Meinkoth JL, and Taylor SS (1994) Heat-stable inhibitors of cAMP-dependent protein kinase carry a nuclear export signal. J Biol Chem 269, 32214–32220 [PubMed] [Google Scholar]
- 22.Wen W, Meinkoth JL, Tsien RY, and Taylor SS (1995) Identification of a signal for rapid export of proteins from the nucleus. Cell 82, 463–473 [DOI] [PubMed] [Google Scholar]
- 23.Gangolli EA, Belyamani M, Muchinsky S, Narula A, Burton KA, McKnight GS, Uhler MD, and Idzerda RL (2000) Deficient gene expression in protein kinase inhibitor alpha Null mutant mice. Mol Cell Biol 20, 3442–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belyamani M, Gangolli EA, and Idzerda RL (2001) Reproductive function in protein kinase inhibitor-deficient mice. Mol Cell Biol 21, 3959–3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Szeto C, Gao E, Tang M, Jin J, Fu Q, Makarewich C, Ai X, Li Y, Tang A, Wang J, Gao H, Wang F, Ge XJ, Kunapuli SP, Zhou L, Zeng C, Xiang KY, and Chen X (2013) Cardiotoxic and cardioprotective features of chronic beta-adrenergic signaling. Circ Res 112, 498–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi M, Dillon TJ, Liu C, Kariya Y, Wang Z, and Stork PJS (2013) PKA-dependent phosphorylation of Rap1 regulates its membrane localization and cell migration. [DOI] [PMC free article] [PubMed]
- 27.Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, and Gutkind JS (2012) mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell stem cell 11, 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaque JP, Dorsam RT, Feng X, Iglesias-Bartolome R, Forsthoefel DJ, Chen Q, Debant A, Seeger MA, Ksander BR, Teramoto H, and Gutkind JS (2013) A genome-wide RNAi screen reveals a Trio-regulated Rho GTPase circuitry transducing mitogenic signals initiated by G protein-coupled receptors. Molecular cell 49, 94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iglesias-Bartolome R, Uchiyama A, Molinolo AA, Abusleme L, Brooks SR, Callejas-Valera JL, Edwards D, Doci C, Asselin-Labat M-L, Onaitis MW, Moutsopoulos NM, Silvio Gutkind J, and Morasso MI (2018) Transcriptional signature primes human oral mucosa for rapid wound healing. Science Translational Medicine 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, and Schultz N (2012) The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2, 401–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, and Schultz N (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6, pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, and Bos JL (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396, 474–477 [DOI] [PubMed] [Google Scholar]
- 33.Gloerich M, and Bos JL (2010) Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50, 355–375 [DOI] [PubMed] [Google Scholar]
- 34.Bos JL (2018) From Ras to Rap and Back, a Journey of 35 Years. Cold Spring Harb Perspect Med 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan Y, and Huang XY (1998) Analysis of the Gs/mitogen-activated protein kinase pathway in mutant S49 cells. J Biol Chem 273, 14533–14537 [DOI] [PubMed] [Google Scholar]
- 36.Keiper M, Stope MB, Szatkowski D, Bohm A, Tysack K, Vom Dorp F, Saur O, Oude Weernink PA, Evellin S, Jakobs KH, and Schmidt M (2004) Epac- and Ca2+ -controlled activation of Ras and extracellular signal-regulated kinases by Gs-coupled receptors. J Biol Chem 279, 46497–46508 [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Dillon TJ, Pokala V, Mishra S, Labudda K, Hunter B, and Stork PJ (2006) Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol Cell Biol 26, 2130–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelinas JN, Banko JL, Peters MM, Klann E, Weeber EJ, and Nguyen PV (2008) Activation of exchange protein activated by cyclic-AMP enhances long-lasting synaptic potentiation in the hippocampus. Learn Mem 15, 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsalkova T, Mei FC, Li S, Chepurny OG, Leech CA, Liu T, Holz GG, Woods VL Jr., and Cheng X (2012) Isoform-specific antagonists of exchange proteins directly activated by cAMP. Proc Natl Acad Sci U S A 109, 18613–18618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Y, Chen H, Boulton S, Mei F, Ye N, Melacini G, Zhou J, and Cheng X (2015) Biochemical and pharmacological characterizations of ESI-09 based EPAC inhibitors: defining the ESI-09 “therapeutic window”. Sci Rep 5, 9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vossler MR, Yao H, York RD, Pan MG, Rim CS, and Stork PJ (1997) cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89, 73–82 [DOI] [PubMed] [Google Scholar]
- 42.Qiu W, Zhuang S, von Lintig FC, Boss GR, and Pilz RB (2000) Cell type-specific regulation of B-Raf kinase by cAMP and 14–3-3 proteins. J Biol Chem 275, 31921–31929 [DOI] [PubMed] [Google Scholar]
- 43.Matallanas D, Birtwistle M, Romano D, Zebisch A, Rauch J, von Kriegsheim A, and Kolch W (2011) Raf family kinases: old dogs have learned new tricks. Genes Cancer 2, 232–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi M, Li Y, Dillon TJ, and Stork PJ (2017) Phosphorylation of Rap1 by cAMP-dependent Protein Kinase (PKA) Creates a Binding Site for KSR to Sustain ERK Activation by cAMP. J Biol Chem 292, 1449–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, and Cobb MH (1997) Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. The EMBO journal 16, 6426–6438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, and Birukov KG (2008) Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol 215, 715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eblen ST, Slack JK, Weber MJ, and Catling AD (2002) Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol 22, 6023–6033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAvoy T, Zhou MM, Greengard P, and Nairn AC (2009) Phosphorylation of Rap1GAP, a striatally enriched protein, by protein kinase A controls Rap1 activity and dendritic spine morphology. Proc Natl Acad Sci U S A 106, 3531–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lou L, Urbani J, Ribeiro-Neto F, and Altschuler DL (2002) cAMP inhibition of Akt is mediated by activated and phosphorylated Rap1b. J Biol Chem 277, 32799–32806 [DOI] [PubMed] [Google Scholar]
- 50.Iwami G, Kawabe J, Ebina T, Cannon PJ, Homcy CJ, and Ishikawa Y (1995) Regulation of adenylyl cyclase by protein kinase A. J Biol Chem 270, 12481–12484 [DOI] [PubMed] [Google Scholar]
- 51.Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, Wong W, Hoshi N, Langeberg LK, Cooper DM, Dessauer CW, and Scott JD (2006) Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Molecular cell 23, 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Omori K, and Kotera J (2007) Overview of PDEs and their regulation. Circ Res 100, 309–327 [DOI] [PubMed] [Google Scholar]
- 53.Binkowski BF, Fan F, and Wood KV (2011) Luminescent biosensors for real-time monitoring of intracellular cAMP. Methods Mol Biol 756, 263–271 [DOI] [PubMed] [Google Scholar]
- 54.Fan F, Binkowski BF, Butler BL, Stecha PF, Lewis MK, and Wood KV (2008) Novel genetically encoded biosensors using firefly luciferase. ACS Chem Biol 3, 346–351 [DOI] [PubMed] [Google Scholar]
- 55.Wang D, and Dubois RN (2010) Eicosanoids and cancer. Nature reviews. Cancer 10, 181–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng Z, Garvin D, Paguio A, Stecha P, Wood K, and Fan F (2010) Luciferase Reporter Assay System for Deciphering GPCR Pathways. Curr Chem Genomics 4, 84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guadamillas MC, Cerezo A, and Del Pozo MA (2011) Overcoming anoikis--pathways to anchorage-independent growth in cancer. J Cell Sci 124, 3189–3197 [DOI] [PubMed] [Google Scholar]
- 58.Dumaz N, and Marais R (2003) Protein kinase A blocks Raf-1 activity by stimulating 14–3-3 binding and blocking Raf-1 interaction with Ras. J Biol Chem 278, 29819–29823 [DOI] [PubMed] [Google Scholar]
- 59.Blanchet E, Van de Velde S, Matsumura S, Hao E, LeLay J, Kaestner K, and Montminy M (2015) Feedback inhibition of CREB signaling promotes beta cell dysfunction in insulin resistance. Cell Rep 10, 1149–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sotomayor-Flores C, Rivera-Mejias P, Vasquez-Trincado C, Lopez-Crisosto C, Morales PE, Pennanen C, Polakovicova I, Aliaga-Tobar V, Garcia L, Roa JC, Rothermel BA, Maracaja-Coutinho V, Ho-Xuan H, Meister G, Chiong M, Ocaranza MP, Corvalan AH, Parra V, and Lavandero S (2020) Angiotensin-(1–9) prevents cardiomyocyte hypertrophy by controlling mitochondrial dynamics via miR-129–3p/PKIA pathway. Cell Death Differ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Lecea L, Criado JR, Rivera S, Wen W, Soriano E, Henriksen SJ, Taylor SS, Gall CM, and Sutcliffe JG (1998) Endogenous protein kinase A inhibitor (PKIalpha) modulates synaptic activity. J Neurosci Res 53, 269–278 [DOI] [PubMed] [Google Scholar]
- 62.Chung S, Furihata M, Tamura K, Uemura M, Daigo Y, Nasu Y, Miki T, Shuin T, Fujioka T, Nakamura Y, and Nakagawa H (2009) Overexpressing PKIB in prostate cancer promotes its aggressiveness by linking between PKA and Akt pathways. Oncogene 28, 2849–2859 [DOI] [PubMed] [Google Scholar]
- 63.Manschwetus JT, Bendzunas GN, Limaye AJ, Knape MJ, Herberg FW, and Kennedy EJ (2019) A Stapled Peptide Mimic of the Pseudosubstrate Inhibitor PKI Inhibits Protein Kinase A. Molecules 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SR, Sang L, and Yue DT (2016) Uncovering Aberrant Mutant PKA Function with Flow Cytometric FRET. Cell Rep 14, 3019–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RNAseq primary and processed data generated in this manuscript have been deposited in the GEO database under accession code GSE133091. All other data is contained within the manuscript. Request for additional data should be made to the corresponding author (RI-B) at ramiro.iglesias-bartolome@nih.gov.