Abstract
Objective
To determine the association between levels of low density lipoprotein cholesterol (LDL-C) and all cause mortality, and the concentration of LDL-C associated with the lowest risk of all cause mortality in the general population.
Design
Prospective cohort study.
Setting
Denmark; the Copenhagen General Population Study recruited in 2003-15 with a median follow-up of 9.4 years.
Participants
Individuals randomly selected from the national Danish Civil Registration System.
Main outcome measures
Baseline levels of LDL-C associated with risk of mortality were evaluated on a continuous scale (restricted cubic splines) and by a priori defined centile categories with Cox proportional hazards regression models. Main outcome was all cause mortality. Secondary outcomes were cause specific mortality (cardiovascular, cancer, and other mortality).
Results
Among 108 243 individuals aged 20-100, 11 376 (10.5%) died during the study, at a median age of 81. The association between levels of LDL-C and the risk of all cause mortality was U shaped, with low and high levels associated with an increased risk of all cause mortality. Compared with individuals with concentrations of LDL-C of 3.4-3.9 mmol/L (132-154 mg/dL; 61st-80th centiles), the multivariable adjusted hazard ratio for all cause mortality was 1.25 (95% confidence interval 1.15 to 1.36) for individuals with LDL-C concentrations of less than 1.8 mmol/L (<70 mg/dL; 1st-5th centiles) and 1.15 (1.05 to 1.27) for LDL-C concentrations of more than 4.8 mmol/L (>189 mg/dL; 96th-100th centiles). The concentration of LDL-C associated with the lowest risk of all cause mortality was 3.6 mmol/L (140 mg/dL) in the overall population and in individuals not receiving lipid lowering treatment, compared with 2.3 mmol/L (89 mg/dL) in individuals receiving lipid lowering treatment. Similar results were seen in men and women, across age groups, and for cancer and other mortality, but not for cardiovascular mortality. Any increase in LDL-C levels was associated with an increased risk of myocardial infarction.
Conclusions
In the general population, low and high levels of LDL-C were associated with an increased risk of all cause mortality, and the lowest risk of all cause mortality was found at an LDL-C concentration of 3.6 mmol/L (140 mg/dL).
Introduction
Low density lipoprotein cholesterol (LDL-C) is a well established causal risk factor for the development of atherosclerosis and cardiovascular disease.1 High levels of LDL-C consistently predict a risk of future atherosclerotic cardiovascular events in a variety of populations throughout the world. Also, many randomised controlled trials of treatment with lipid lowering agents have clearly shown that lowering LDL-C levels reduces the risk of atherosclerotic cardiovascular events in the future.1 2 3 4
Because lowering levels of LDL-C reduces cardiovascular disease outcomes, the general perception is that high levels of LDL-C are associated with an increased risk of mortality but low levels are not. Studies on the association between LDL-C levels and the risk of all cause mortality, however, have provided conflicting results, with some studies showing a counterintuitive inverse association (lower mortality with increasing levels of LDL-C) 5 6 7 and some showing no association.8 9 10 Most of these studies were conducted in individuals aged 65 and older, and in historical population based cohorts. Also, a recent study in young Koreans not taking lipid lowering drugs showed a U shaped relation between levels of LDL-C and mortality.11 Studies on the association between levels of LDL-C and cardiovascular mortality found different results, with some studies showing a positive association only8 12 and some showing a U shaped association.11 Thus the association between LDL-C levels and the risk of all cause and cause specific mortality in the general population is unclear. Also, the concentration of LDL-C where the risk of mortality is lowest is not defined.
In this study, we determined the association between levels of LDL-C and the risk of all cause and cause specific mortality. Also, we identified the LDL-C level associated with the lowest mortality in individuals in the contemporary ongoing Copenhagen General Population Study.
Methods
Study population
The study included individuals of Danish descent from the Copenhagen General Population Study, an ongoing cohort study with the first round of examinations in participants recruited in 2003-15. Invited individuals were aged 20-100 and randomly selected from the national Danish Civil Registration System, reflecting the Danish general population (43% participation rate). All participants completed a self-administered questionnaire, including questions on lifestyle factors and medical treatment, underwent a physical examination, and gave blood samples for biochemical measurements.
Endpoints
The number of deaths from any cause was obtained from the Danish Civil Registration System, a complete register of all residents in Denmark since 1968 without losses to follow-up. The cause of death from January 1977 onwards was retrieved from the national Danish Causes of Death Registry, based on the codes of the International Classification of Diseases, seventh, eighth, and 10th revisions (ICD-7, ICD-8, and ICD-10), and classified as cardiovascular, cancer, or other mortality. If one of the first three ranked causes of death had a cardiovascular diagnosis (ICD-10 codes I00-I90), death was categorised as cardiovascular mortality. The remaining deaths were classified as cancer mortality if one of the first three ranked causes of death had a cancer diagnosis (ICD-10 codes C00-C96), and as other mortality if death was not classified as cardiovascular or cancer mortality.
Information on diagnoses of non-fatal and fatal myocardial infarction (ICD-8 code 410 and ICD-10 codes I21-I22) was obtained from the national Danish Patient Registry, a registry with information on all hospital contacts in Denmark from January 1977 onwards (outpatients and emergency wards from 1995), and the national Danish Causes of Death Registry (ICD-9 was never used in Denmark). Information on diagnoses of non-fatal and fatal cancer (ICD-7 codes 140-205 and ICD-10 codes C00-D09, excluding common skin cancers) was obtained from the national Danish Cancer Registry and the national Danish Causes of Death Registry.
Laboratory analyses
All blood samples were collected in the non-fasting state.13 Concentrations of LDL-C were calculated with the Friedewald equation as:
Total cholesterol − high density lipoprotein cholesterol – triglycerides/2.2 in mmol/L (total cholesterol − high density lipoprotein cholesterol – triglycerides/5 in mg/dL) when triglyceride concentrations were less than 4 mmol/L (352 mg/dL), and were measured directly (Konelab) when triglyceride concentrations were 4 mmol/L or more (≥352 mg/dL). Concentrations of total cholesterol, high density lipoprotein cholesterol, triglycerides, and direct LDL-C were measured by standard hospital assays (Roche and Konelab).
Covariates
Statistical analyses were adjusted for a priori defined covariates (that is, for well known risk factors for mortality).14 Sex and age were derived from the Civil Registration Number. Blood pressure was measured at the physical examination. In the questionnaire, participants reported on their smoking status and cumulative number of pack years, lipid lowering treatment, and diabetes. Diagnoses of diabetes, cardiovascular disease, cancer, or chronic obstructive pulmonary disease before entry into the study were obtained from the national Danish Patient Registry. Individuals with diabetes were identified as those having a registered diagnosis in the national Danish Patient Registry, a non-fasting plasma glucose concentration of more than 11 mmol/L (198 mg/dL), treatment with antidiabetic drugs, or self-reported diabetes from the questionnaire.
Statistical analyses
Only participants with an LDL-C measurement at baseline were included in the study; 847 individuals were excluded because of missing LDL-C measurements. Data on potential confounders were more than 99% complete. The remaining missing values were imputed by multivariable chained imputation with fully conditional specification15; imputed and reported results were similar.
Associations between levels of LDL-C and the risk of all cause mortality, cause specific mortality, myocardial infarction, and cancer were estimated by Cox proportional hazards regression models with 95% confidence intervals, with age as the underlying time scale (participants enter the risk set at baseline age and exit at censoring/event age=age adjustment) and left truncation (delayed entry at study examination). Follow-up started on the day of examination and ended at the first occurrence of death, myocardial infarction, cancer, emigration, or in December 2018. Individuals with a previous myocardial infarction or cancer were excluded when myocardial infarction or cancer was the endpoint. Multivariable adjusted statistical analyses were adjusted for age (as time scale), sex, current smoking, cumulative number of pack years, systolic blood pressure, lipid lowering treatment, diabetes, cardiovascular disease, cancer, and chronic obstructive pulmonary disease at baseline.
The associations between levels of LDL-C and all endpoints were evaluated on a continuous scale with restricted cubic spline curves based on Cox proportional hazards models. To balance best fit and overfitting in the main splines for mortality, myocardial infarction, and cancer, the number of knots, between three and seven, was chosen as the lowest value for the Akaike information criterion, but if within two of each other for different knots, the lowest number of knots was chosen. The same number of knots from the main splines was also applied in splines for stratified analyses to allow direct comparison of overall and stratified analyses, including test of interaction. Interaction of levels of LDL-C with covariates for stratification on all cause mortality was examined by including two factor interaction terms in the Cox proportional regression model. The concentration of LDL-C associated with the lowest risk of mortality was the concentration with the lowest hazard ratio on the spline curve. The association between levels of LDL-C on a continuous scale and all cause mortality was also evaluated with fractional polynomials based on the Cox proportional hazards models. Furthermore, the associations between seven predefined LDL-C categories and all cause mortality were examined: five equally distributed categories of LDL-C were defined by the 20th, 40th, 60th, and 80th centiles, and to evaluate the highest and lowest levels of LDL-C, two additional categories were defined by the 5th and 95th centiles. The reference category for these analyses was the LDL-C level associated with the lowest risk of all cause mortality.
Hazard ratios and 95% confidence intervals for categories of LDL-C are presented with and without regression dilution bias; restricted cubic splines and the results in the main paper are reported without this correction. Correction for regression dilution bias was done with a non-parametric method to correct for underestimation caused by random measurements and long term fluctuations.16 With LDL-C measurements from 9604 individuals with no atherosclerotic cardiovascular disease and who were not treated with lipid lowering agents participating in the 2003-15 examination and in the follow-up examination about 10 years later, a regression dilution ratio of 0.64 was determined for LDL-C (this ratio was used for the overall analyses for all individuals, regardless of follow-up time for the individual person—that is, the regression coefficients were multiplied by 1/0.64). Spline curves were not adjusted for regression dilution bias as we are not aware of a method to do this calculation.
In sensitivity analyses, pretreatment levels of LDL-C were estimated in individuals receiving lipid lowering treatment as baseline LDL-C measurements multiplied by 1.43 for individuals with no concurrent diagnoses of ischaemic heart disease or stroke, and by 1.67 for individuals with known ischaemic heart disease or stroke, corresponding to a 30% and 40% reduction, respectively.17 All statistical analyses were performed in Stata/SE 15.1.
Patient and public involvement
No patients were involved directly in the design of the study, recruitment, or conduct of the study because our cohort consisted of normal individuals from the population at large (not patients) and because our study was planned in the year 2000 when direct patient involvement was not used in Denmark.
Results
The study included 108 243 individuals with 1 002 361 person years of follow-up (median follow-up 9.4 years, range 0-15 years). We found 11 376 (10.5%) deaths during follow-up, with a median age of 81 (range 26-106) at the time of death. Table 1 shows the baseline characteristics by LDL-C centile categories.
Table 1.
Baseline characteristics of 108 243 individuals in the Copenhagen General Population Study
| Centile (mmol/L, mg/dL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1st-5th (<1.8, <70) | 6th-20th (1.8-2.3, 70-92) | 21st-40th (2.4-2.8, 93-112) | 41st-60th (2.9-3.3, 113-131) | 61st-80th (3.4-3.9, 132-154) | 81st-95th (4.0-4.8, 155-189) | 96th-100th (>4.8, >189) | All | |
| No of individuals | 6412 (6) | 15 681 (14) | 21 289 (20) | 22 207 (21) | 21 892 (20) | 15 999 (15) | 4763 (4) | 108 243 |
| Women | 3202 (50) | 9068 (58) | 11 973 (56) | 12 385 (56) | 11 710 (53) | 8537 (53) | 2699 (57) | 59 574 (55) |
| Age (years) | 62 (47-72) | 56 (45-68) | 56 (46-67) | 58 (48-67) | 59 (50-67) | 60 (51-67) | 60 (52-67) | 58 (48-67) |
| Smoker | 961 (15) | 2441 (16) | 3291 (15) | 3674 (17) | 3872 (18) | 3203 (20) | 1107 (23) | 18 549 (17) |
| Pack years, ever smokers | 20 (8-38) | 15 (5-30) | 14 (5-29) | 15 (6-30) | 16 (7-30) | 18 (7-31) | 19 (8-32) | 16 (6-30) |
| Systolic blood pressure (mm Hg) | 137 (124-151) | 136 (122-151) | 137 (124-152) | 140 (126-154) | 141 (128-156) | 144 (130-159) | 145 (132-160) | 140 (126-155) |
| Lipid lowering treatment | 3030 (47) | 4166 (27) | 2891 (14) | 1584 (7) | 849 (4) | 373 (2) | 132 (3) | 13 025 (12) |
| Diabetes | 1249 (19) | 1218 (8) | 822 (4) | 563 (3) | 401 (2) | 269 (2) | 83 (2) | 4605 (4) |
| Atherosclerotic cardiovascular disease | 1806 (28) | 2262 (14) | 1756 (8) | 1413 (6) | 1200 (5) | 817 (5) | 223 (5) | 9477 (9) |
| Cancer | 557 (9) | 1093 (7) | 1393 (7) | 1474 (7) | 1508 (7) | 1081 (7) | 327 (7) | 7433 (7) |
| Chronic obstructive pulmonary disease | 1218 (19) | 2517 (16) | 3224 (15) | 3324 (15) | 3246 (15) | 2331 (15) | 666 (14) | 16 526 (15) |
| LDL-C (mmol/L) | 1.6 (1.4-1.7) | 2.2 (2.0-2.3) | 2.7 (2.6-2.8) | 3.2 (3.1-3.3) | 3.7 (3.6-3.9) | 4.4 (4.2-4.6) | 5.3 (5.1-5.7) | 3.2 (2.6-3.8) |
| LDL-C (mg/dL) | 62 (54-66) | 85 (77-89) | 104 (101-108) | 124 (119-128) | 143 (139-150) | 170 (162-178) | 205 (197-219) | 124 (101-147) |
Values are median (interquartile range) or number (%).
LDL-C=low density lipoprotein cholesterol.
LDL-C and all cause mortality
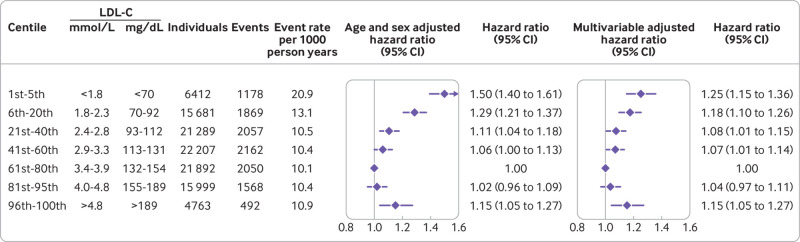
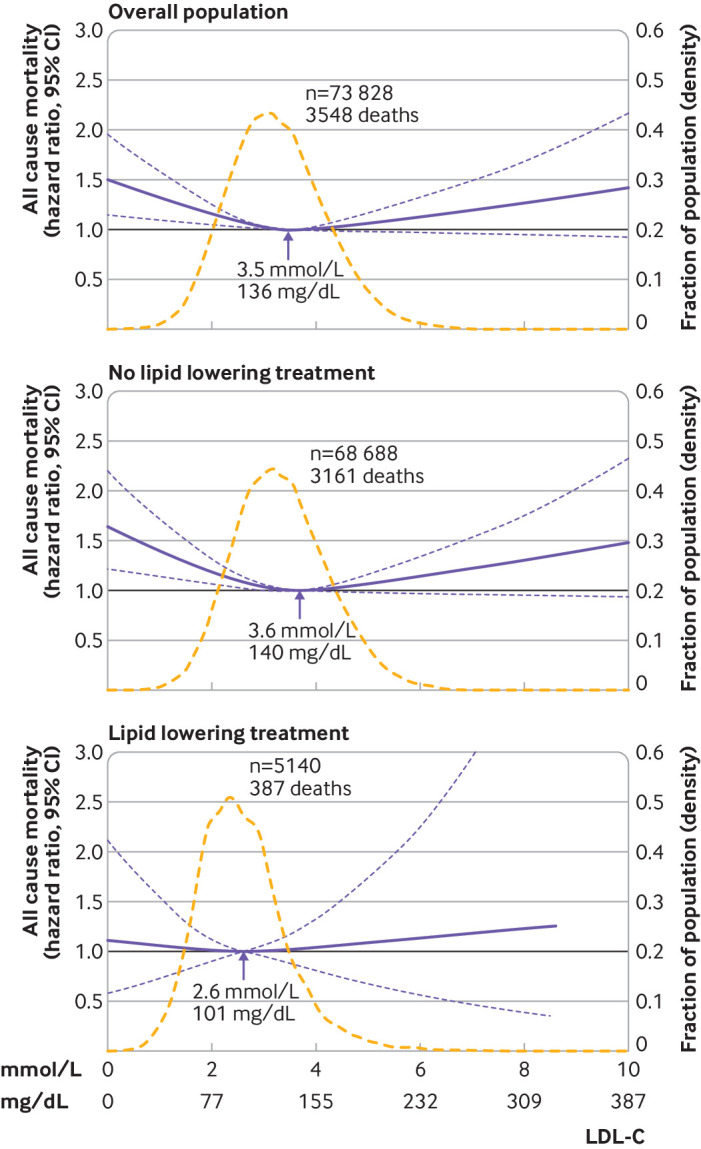
The association between levels of LDL-C on a continuous scale and risk of all cause mortality was U shaped; low and high levels of LDL-C were associated with an increased risk of all cause mortality (fig 1). This association was also found in those not receiving lipid lowering treatment. For individuals receiving lipid lowering treatment, however, the 95% confidence interval included the hazard ratio of 1.0 for any level of LDL-C (P value for interaction between LDL-C levels and lipid lowering treatment on all cause mortality was <0.001) (fig 1, eFig 1). Compared with individuals with concentrations of LDL-C of 3.4-3.9 mmol/L (132-154 mg/dL; 61st-80th centiles), the multivariable adjusted hazard ratio for all cause mortality was 1.25 (95% confidence interval 1.15 to 1.36) for individuals with concentrations of LDL-C less than 1.8 mmol/L (<70 mg/dL; 1st-5th centiles) and 1.15 (1.05 to 1.27) for individuals with concentrations of LDL-C greater than 4.8 mmol/L (>189 mg/dL; 96th-100th centiles) (fig 2).
Fig 1.
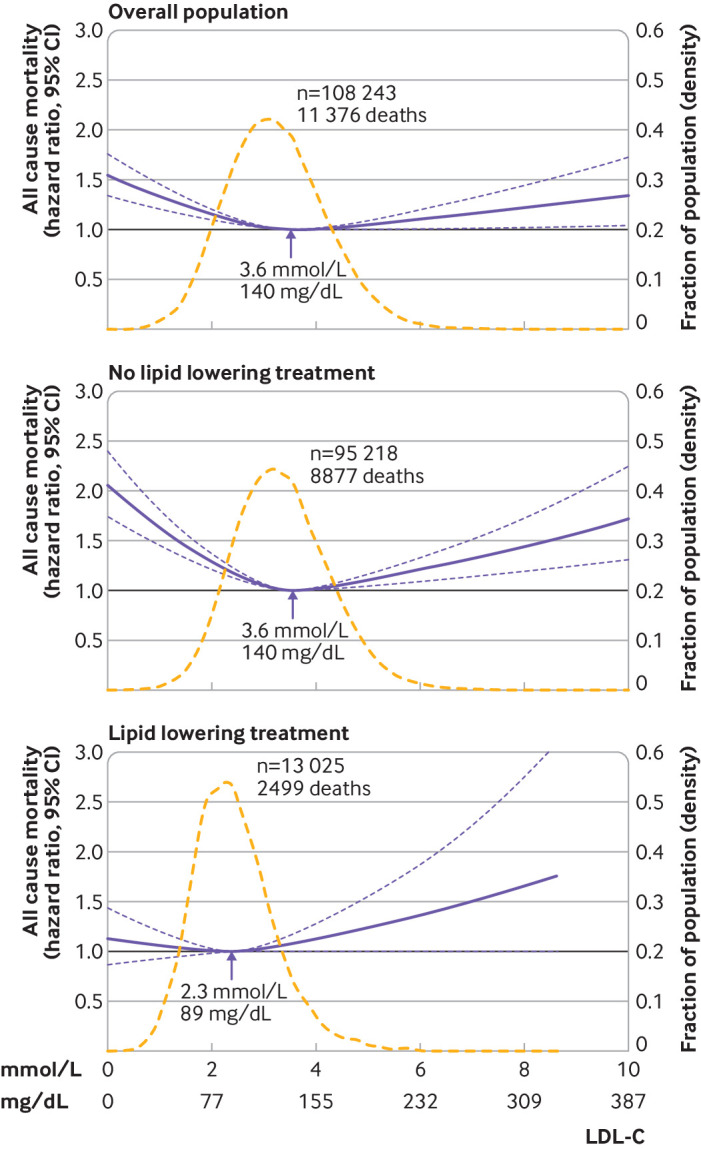
Multivariable adjusted hazard ratios for all cause mortality according to levels of low density lipoprotein cholesterol (LDL-C) on a continuous scale. Solid blue lines are multivariable adjusted hazard ratios, with dashed blue lines showing 95% confidence intervals derived from restricted cubic spline regressions with three knots. Reference lines for no association are indicated by the solid bold lines at a hazard ratio of 1.0. Dashed yellow curves show the fraction of the population with different levels of LDL-C. Arrows indicate the concentration of LDL-C with the lowest risk of all cause mortality. Analyses were adjusted for age, sex, current smoking, cumulative number of pack years, systolic blood pressure, lipid lowering treatment, diabetes, cardiovascular disease, cancer, and chronic obstructive pulmonary disease at baseline. Based on individuals from the Copenhagen General Population Study followed for a mean 9.4 years
Fig 2.
Hazard ratios for all cause mortality according to categories of levels of low density lipoprotein cholesterol (LDL-C), sex and age adjusted, and multivariable adjusted. Multivariable adjusted analyses were adjusted for age, sex, current smoking, cumulative number of pack years, systolic blood pressure, lipid lowering treatment, diabetes, cardiovascular disease, cancer, and chronic obstructive pulmonary disease at baseline. Based on individuals from the Copenhagen General Population Study followed for a mean 9.4 years
An increased risk of all cause mortality at low levels of LDL-C were seen in men and women (eFigs 2-3). Also, the association was most pronounced in individuals aged 65 or younger (eFig 4). For categories of age, the P value for interaction between low levels of LDL-C and age on all cause mortality was <0.001 (eFig 5).
LDL-C level with the lowest risk of all cause mortality
The concentration of LDL-C associated with the lowest risk of all cause mortality in multivariable adjusted analyses was 3.6 mmol/L (140 mg/dL) in the overall population and in individuals not receiving lipid lowering treatment, compared with 2.3 mmol/L (89 mg/dL) in individuals receiving lipid lowering treatment (fig 1). Similar levels were seen in men and women and across age groups, except for men and women receiving lipid lowering treatment where the lowest risk of all cause mortality was at a concentrations of LDL-C of 2.7 mmol/L (105 mg/dL) and 1.9 (74 mg/dL), respectively (eFig 2, eFig 4).
LDL-C and cause specific mortality
In the overall population, the 95% confidence interval included the hazard ratio of 1.0 at any concentration of LDL-C for cardiovascular mortality whereas low levels of LDL-C were associated with an increased risk of cancer and other mortality (fig 3, eFig 6). In individuals not receiving lipid lowering treatment, the associations with cardiovascular, cancer, and other mortality were U shaped (eFig 6). In individuals receiving lipid lowering treatment, low levels of LDL-C were associated with increased cancer mortality but otherwise the 95% confidence interval included the hazard ratio of 1.0 at any concentration of LDL-C for cardiovascular, cancer, and other mortality (eFig 6). Also, the P value for interaction between levels of LDL-C and lipid lowering treatment was <0.001 for cardiovascular and other mortality, and 0.04 for cancer mortality.
Fig 3.
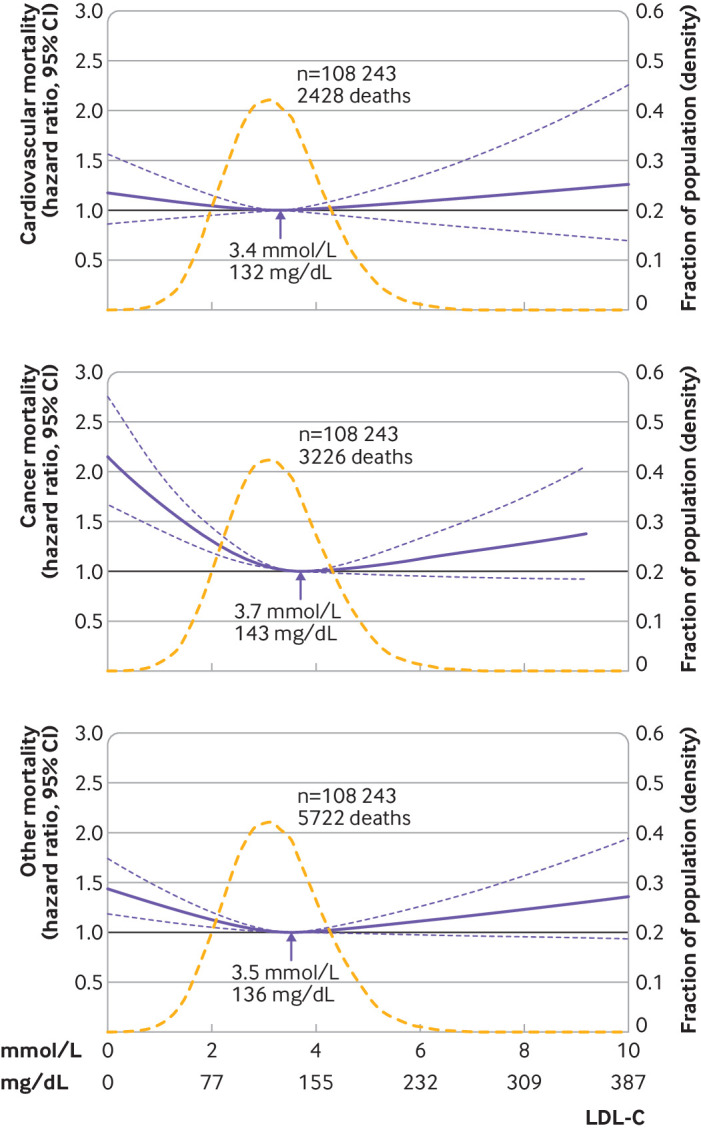
Multivariable adjusted hazard ratios for cause specific mortality according to levels of low density lipoprotein cholesterol (LDL-C) on a continuous scale in the overall population. Solid blue lines are multivariable adjusted hazard ratios, with dashed blues lines showing 95% confidence intervals derived from restricted cubic spline regressions with three knots. Reference lines for no association are indicated by solid bold lines at a hazard ratio of 1.0. Dashed yellow curves show fraction of population with different levels of LDL-C. Arrows indicate the concentration of LDL-C with the lowest risk of mortality. Analyses were adjusted for age, sex, current smoking, cumulative number of pack years, systolic blood pressure, lipid lowering treatment, diabetes, cardiovascular disease, cancer, and chronic obstructive pulmonary disease at baseline. Based on individuals from the Copenhagen General Population Study followed for a mean 9.4 years
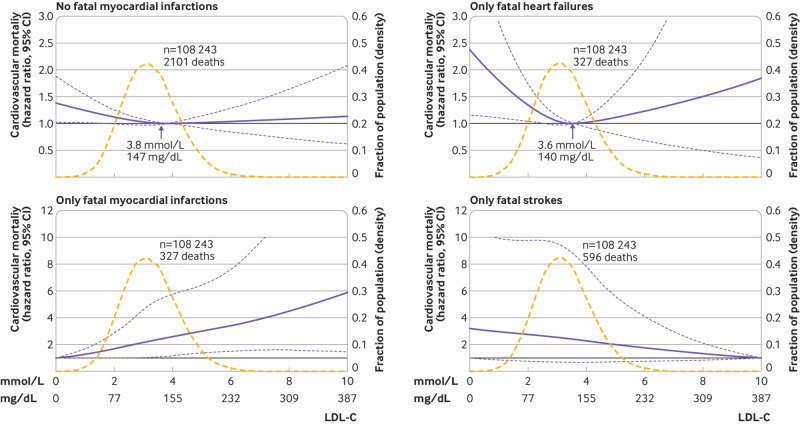
Analysing cardiovascular mortality by ICD-10 codes showed that 13% of individuals died from myocardial infarction, 13% from heart failure, and 25% from any stroke (eTable 1). For cardiovascular mortality not including fatal myocardial infarction, the results were similar to overall cardiovascular mortality (fig 4). Any increase in LDL-C levels was associated with an increased risk of fatal myocardial infarction, although low levels of LDL-C were associated with an increased risk of fatal heart failure but the 95% confidence interval was wide (fig 4). For any fatal stroke, the 95% confidence interval included a hazard ratio of 1.0 at any concentration of LDL-C (fig 4).
Fig 4.
Multivariable adjusted hazard ratios for different cardiovascular mortality endpoints according to levels of low density lipoprotein cholesterol (LDL-C) on a continuous scale. Solid blue lines are multivariable adjusted hazard ratios, with dashed blue lines showing 95% confidence intervals derived from restricted cubic spline regressions with three knots. Reference lines for no association are indicated by solid bold lines at a hazard ratio of 1.0. Dashed yellow curves show fraction of population with different levels of LDL-C. Arrows indicate the concentration of LDL-C with the lowest risk of mortality. Analyses were adjusted for age, sex, current smoking, cumulative number of pack years, systolic blood pressure, lipid lowering treatment, diabetes, cardiovascular disease, cancer, and chronic obstructive pulmonary disease at baseline. Based on individuals from the Copenhagen General Population Study followed for a mean 9.4 years
LDL-C and myocardial infarction
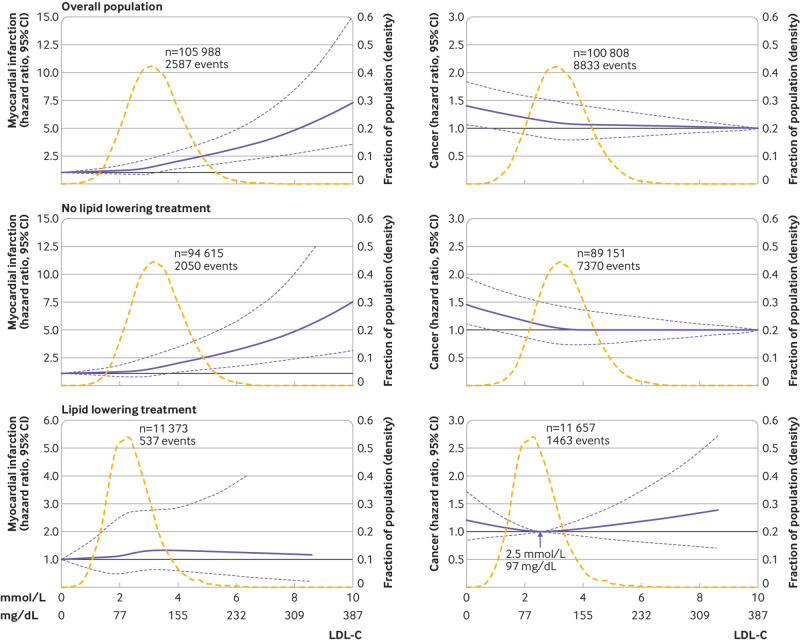
Any increase in LDL-C levels was associated with an increased risk of myocardial infarction in the overall cohort and in individuals not receiving lipid lowering treatment, although the 95% confidence interval included a hazard ratio of 1.0 at any concentration of LDL-C in individuals receiving lipid lowering treatment (P value for interaction between LDL-C levels and lipid lowering treatment on risk of myocardial infarction was 0.04) (fig 5).
Fig 5.
Multivariable adjusted hazard ratios for myocardial infarction and cancer according to levels of low density lipoprotein cholesterol (LDL-C) on a continuous scale. Solid blue lines are multivariable adjusted hazard ratios, with dashed blue lines showing 95% confidence intervals derived from restricted cubic spline regressions, with four knots for myocardial infarction and three knots for cancer. Reference lines for no association are indicated by solid bold lines at a hazard ratio of 1.0. Dashed yellow curves show fraction of population with different levels of LDL-C. Arrow indicates the concentration of LDL-C with the lowest risk of cancer. Analyses were adjusted for age, sex, current smoking, cumulative number of pack years, systolic blood pressure, lipid lowering treatment, diabetes, cardiovascular disease, cancer, and chronic obstructive pulmonary disease at baseline. Based on individuals from the Copenhagen General Population Study followed for a mean 9.4 years
LDL-C and cancer
Very low levels of LDL-C were associated with an increased risk of cancer in the overall population and in individuals not receiving lipid lowering treatment, although the 95% confidence interval included a hazard ratio of 1.0 at any concentration of LDL-C in individuals receiving lipid lowering treatment (P value for interaction between LDL-C levels and lipid lowering treatment on risk of cancer was 0.02) (fig 5).
Sensitivity analyses
The U shaped association between LDL-C levels on a continuous scale and all cause mortality was similar when a statistical method other than restricted cubic splines was used: with fractional polynomials, the concentration of LDL-C associated with the lowest risk of all cause mortality was 4.1 mmol/L (159 mg/dL) in the overall population, 4.0 mmol/L (155 mg/dL) in individuals not receiving lipid lowering treatment, and 2.1 mmol/L (82 mg/dL) in individuals receiving lipid lowering treatment (eFig 7 versus fig 1).
To assess whether the positive association between low levels of LDL-C and an increased risk of all cause mortality could be explained by reverse causation as a result of severe disease, we excluded individuals with less than five years of follow-up (start of follow-up began five years after the baseline examination) and individuals with atherosclerotic cardiovascular disease, cancer, and chronic obstructive pulmonary disease at the start of the study. We found that the results were similar to the main analyses although the association was slightly reduced (fig 6, and eFigs 8-10 versus fig 1). Starting follow-up five years after the baseline examination excluded individuals dying within five years of baseline and individuals with less than five years of follow-up. Excluding only those dying within five years of the baseline examination gave similar results. Also, we found similar results when restricting analyses to individuals aged 40-70 and with no chronic diseases at baseline.
Fig 6.
Multivariable adjusted hazard ratios for all cause mortality according to levels of low density lipoprotein cholesterol (LDL-C) on a continuous scale with the start of follow-up at year 5 and after exclusion of individuals with known atherosclerotic cardiovascular disease, cancer, and chronic obstructive pulmonary disease at baseline. Solid blue lines are multivariable adjusted hazard ratios, with dashed blue lines showing 95% confidence intervals derived from restricted cubic spline regressions with three knots. Reference lines for no association are indicated by solid bold lines at a hazard ratio of 1.0. Dashed yellow curves show fraction of population with different levels of LDL-C. Arrows indicate the concentration of LDL-C with the lowest risk of all cause mortality. Analyses were adjusted for age, sex, current smoking, cumulative number of pack years, systolic blood pressure, lipid lowering treatment, diabetes, cardiovascular disease, cancer, and chronic obstructive pulmonary disease at baseline. Based on individuals from the Copenhagen General Population Study followed for a mean 9.4 years
Estimated pretreatment levels of LDL-C were examined to evaluate whether low levels of LDL-C caused by lipid lowering treatment, representing a high risk group with higher pretreatment levels, could explain the association between low levels of LDL-C and mortality. The results from these analyses gave similar results for all cause, cancer, and other mortality at low levels of LDL-C whereas the hazard ratios were nominally higher at high levels of LDL-C (eFig 11 versus fig 1, and fig 3). Low and high levels of LDL-C were associated with an increased risk of cardiovascular mortality. To assess the magnitude of effect size underestimation caused by random measurements and long term fluctuations, the hazard ratios and 95% confidence intervals for the association between LDL-C categories and all cause mortality were corrected for regression dilution bias (eFig 12 versus fig 2).
Discussion
In this study of 108 243 individuals from a contemporary ongoing general population cohort, we found a U shaped association between levels of LDL-C and the risk of all cause mortality, with low and high levels associated with an increased risk. The concentration of LDL-C with the lowest risk of all cause mortality was 3.6 mmol/L (140 mg/dL), well above the generally considered optimal concentration. These new results are likely to have implications for the interpretation of levels of LDL-C in clinical practice. As expected, the risk of myocardial infarction increased with any increase in the level of LDL-C.
Possible explanations for our findings
The association between low levels of LDL-C and an increased risk of all cause mortality could be explained by reverse causation. Debilitation and illness have been hypothesised to cause a decrease in levels of cholesterol18 19 and, in this study, comorbidities were more frequent in individuals with the lowest levels of LDL-C. Also, consistent with the theory that low levels of LDL-C are an indirect marker of severe disease, the association between low levels of LDL-C and the risk of all cause mortality was strongest in the age and sex adjusted model, and substantially reduced when adjusting for baseline comorbidities. An association remained after this adjustment, however, and after excluding individuals with less than five years of follow-up and known cardiovascular disease, cancer, and chronic obstructive pulmonary disease at baseline. Whether the remaining association, despite extensive comorbidity adjustment, can be attributed to residual confounding in terms of alternative mechanisms is unclear. The more pronounced association in individuals aged 65 or younger could point to an epiphenomenon where a pathophysiological abnormality, possibly genetic, causes an increased risk of mortality and decreased levels of LDL-C in parallel.
The U shaped association between levels of LDL-C and mortality might be similar to the obesity paradox, which is largely explained by methodological issues, including reverse causation.20 In contrast with the obesity paradox, however, the U shaped association between levels of LDL-C and mortality in our study remained similar when analyses were restricted to healthy individuals aged 40-70 with no chronic diseases. This finding indicates that the obesity paradox and the U shaped association between levels of LDL-C and mortality are caused by different mechanisms.
Previous studies
Most studies investigating the relation between levels of LDL-C and the risk of all cause mortality have found no association 8 9 10 or an inverse association.5 6 7 Our study showed that the inverse association can be explained by the increased risk of all cause mortality associated with low levels of LDL-C rather than representing an actual decreased risk at high levels of LDL-C. Also, a recent study in young Koreans not taking lipid lowering drugs showed an association between low levels of LDL-C and an increased risk of all cause mortality, cardiovascular mortality, and cancer mortality,11 similar to our results in the group of individuals not receiving lipid lowering treatment.
No previous study has examined the concentration of LDL-C associated with the lowest risk of all cause mortality in a general population cohort. One study in people aged 65 and over reported the lowest all cause risk of mortality at a concentration of LDL-C of 4.9 mmol/L (190 mg/dL) for women and 3.8 mmol/L (147 mg/dL) for men.21 In our study, we consistently found the lowest risk of all cause mortality at concentrations of LDL-C of 3.6-3.7 mmol/L (140-143 mg/dL) for men and women and across the age groups (≤65 or >65).
Previous studies on the association between total cholesterol and risk of mortality showed a reversed J shaped or U shaped association, with the highest risk of all cause, cancer, and other mortality found at the lowest levels of total cholesterol, although positive, inverse, and no association with cardiovascular mortality have been reported.18 22 23 Also, we have recently found a similar U shaped association between levels of high density lipoprotein cholesterol and risk of all cause mortality.24
Lipid lowering treatment
The relatively low number of individuals receiving lipid lowering treatment in Denmark has been confirmed in previous studies.25 26 In our study, in individuals receiving lipid lowering treatment, the association between low levels of LDL-C and an increased risk of all cause, cancer, and other mortality was weaker than for individuals not receiving lipid lowering treatment. Any increase in levels of LDL-C, however, was associated with an increased risk of cardiovascular mortality but the 95% confidence intervals were wider and included a hazard ratio of 1.0 for all cause, cardiovascular, and other mortality at any concentration of LDL-C. This finding indirectly indicates a non-causal association and suggests that the reduction in levels of LDL-C caused by lipid lowering treatment does not explain the increased risk of mortality at low levels of LDL-C but rather low LDL-C levels is a predictor for mortality. Hence it would be incorrect to use our data as an argument against the use of lipid lowering treatment in the prevention of atherosclerotic cardiovascular disease and mortality. A recent meta-analysis of studies in individuals at high risk of atherosclerotic cardiovascular disease showed that more intensive lowering of levels of LDL-C was associated with a greater reduction in the risk of all cause and cardiovascular mortality.4 The remaining association between low levels of LDL-C and cancer mortality together with the association between very low levels of LDL-C and an increased risk of cancer (fatal and non-fatal) supports the hypothesis of a decrease in LDL-C levels because of debilitation and illness.
Clinical importance
Our results could be important for understanding what is a “normal and healthy” level of LDL-C in the general population (that is, when the focus is not limited to myocardial infarction and atherosclerotic cardiovascular disease). The finding of the lowest risk of all cause mortality at a concentration of LDL-C of 3.6 mmol/L (140 mg/dL) implies that in individuals with an otherwise low risk of atherosclerotic cardiovascular disease, an LDL-C level of around this value is not necessarily hazardous in itself. Any increase in LDL-C, however, was associated with an increased risk of myocardial infarction and death from myocardial infarction. Together, these results indicate the importance of assessing the absolute risk of atherosclerotic cardiovascular disease in deciding when to use lipid lowering treatment,27 28 rather than starting treatment based solely on a moderate increase in levels of LDL-C.
Strengths and limitations
The strengths of our study include, firstly, the size of the cohort in terms of the large number of individuals recruited, with no individuals lost to follow-up. Secondly, information on cause of death for every individual was obtained from Danish registries. Thirdly, we adjusted for several confounders with an effect on mortality risk.14 Fourthly, the strong positive association between any increase in levels of LDL-C and an increased risk of myocardial infarction supports the validity of this study.
A limitation of our study is that it included only white individuals living in a Western country, which could limit the applicability of our results to other ethnicities; however, we are not aware of data to suggest that our results are not applicable to other ethnicities living in countries with a similar standard of living and healthcare system to Denmark. A recent study in young Koreans of supposedly comparable affluence to people in Denmark showed similar results to our study.11 In less affluent and less developed countries, levels of LDL-C associated with the lowest mortality could differ from our results. We only had information on lipid lowering treatment at baseline and cannot rule out that the results might have been influenced by individuals starting or stopping treatment with lipid lowering agents during follow-up. We could not adjust for weight loss, which has been associated with decreases in LDL-C levels, as this information was not available in our cohort. Some results were corrected for regression dilution bias to visualise the possible underestimation of the effect estimates; however, the main figures show unadjusted results and the true values are likely to lie somewhere between the corrected and uncorrected values. Finally, we could not deal with the question of causality because the design of the study was observational. This question could theoretically be looked at in mendelian randomisation analyses, modelling non-linear and U shaped relations.29 30 31 Modelling of U shaped associations in mendelian randomisation analyses, however, requires high statistical power and numerous genetic instruments explaining a large fraction of the variation in plasma concentrations of LDL-C. Such genetic data with sufficient statistical power were not available in our cohort. Nevertheless, future studies with more statistical power than our study could provide further insight into the potential causal nature of the association of levels of LDL-C with mortality with non-linear mendelian randomisation analyses.
Conclusions
Low and high levels of LDL-C were associated with an increased risk of all cause mortality in individuals in the general population. Similar results were seen for cancer and other mortality whereas no association was found for cardiovascular mortality overall. Also, individuals in the general population with a concentration of LDL-C of 3.6 mmol/L (140 mg/dL) live the longest. This finding, if confirmed in more studies, will have important clinical and public health implications.
What is already known on this topic
Conflicting results have been reported on the association between levels of LDL-C and all cause mortality
Most previous studies were conducted in individuals aged over 65 in historical populations
What this study adds
Low and high levels of LDL-C were associated with an increased risk of all cause mortality in the general population
The lowest risk of all cause mortality was found at a concentration of LDL-C of 3.6 mmol/L (140 mg/dL)
Acknowledgments
We thank staff and participants of the Copenhagen General Population Study for their contributions.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: All authors contributed to the study design, acquisition, analyses, and interpretation of the data. CDLJ drafted the initial manuscript and AL, MBM, and BGN critically revised the manuscript for important intellectual content. Final approval of the version to be published was given by all authors. BGN is the guarantor and he had full access to all the data in the study, takes responsibility for the work and conduct of the study, and controlled the decision to publish. The corresponding author attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was supported by Herlev and Gentofte Hospital’s Research Fund and the Department of Clinical Biochemistry, Herlev and Gentofte Hospital, Copenhagen University Hospital, Denmark. The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from Herlev and Gentofte Hospital’s Research Fund and the Department of Clinical Biochemistry, Herlev and Gentofte Hospital, Copenhagen University Hospital, Denmark for the submitted work; BGN declares commercial ties with AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Amarin, Novartis, Novo Nordisk, Silence Therapeutics, and Esperion; the remaining coauthors have no competing interests.
Ethical approval: The study was approved by Herlev and Gentofte Hospital, the ethics committee of the Capital Region of Denmark (H-KF-01-144/01), and the Danish Data Protection Agency. Written informed consent was given by each participant.
Data sharing: Additional data are available from the corresponding author on reasonable request.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Results will, after scientific publication, be disseminated to the public in general.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459-72. 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78. 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 3. Baigent C, Blackwell L, Emberson J, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670-81. 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA 2018;319:1566-79. 10.1001/jama.2018.2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schupf N, Costa R, Luchsinger J, Tang MX, Lee JH, Mayeux R. Relationship between plasma lipids and all-cause mortality in nondemented elderly. J Am Geriatr Soc 2005;53:219-26. 10.1111/j.1532-5415.2005.53106.x [DOI] [PubMed] [Google Scholar]
- 6. Akerblom JL, Costa R, Luchsinger JA, et al. Relation of plasma lipids to all-cause mortality in Caucasian, African-American and Hispanic elders. Age Ageing 2008;37:207-13. 10.1093/ageing/afn017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bathum L, Depont Christensen R, Engers Pedersen L, Lyngsie Pedersen P, Larsen J, Nexøe J. Association of lipoprotein levels with mortality in subjects aged 50+ without previous diabetes or cardiovascular disease: a population-based register study. Scand J Prim Health Care 2013;31:172-80. 10.3109/02813432.2013.824157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kronmal RA, Cain KC, Ye Z, Omenn GS. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med 1993;153:1065-73. 10.1001/archinte.1993.00410090025004 [DOI] [PubMed] [Google Scholar]
- 9. Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA 1998;279:585-92. 10.1001/jama.279.8.585 [DOI] [PubMed] [Google Scholar]
- 10. Psaty BM, Anderson M, Kronmal RA, et al. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality: the Cardiovascular Health Study. J Am Geriatr Soc 2004;52:1639-47. 10.1111/j.1532-5415.2004.52455.x [DOI] [PubMed] [Google Scholar]
- 11. Sung KC, Huh JH, Ryu S, et al. Low levels of low-density lipoprotein cholesterol and mortality outcomes in non-statin users. J Clin Med 2019;8:1571. 10.3390/jcm8101571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abdullah SM, Defina LF, Leonard D, et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease. Circulation 2018;138:2315-25. 10.1161/CIRCULATIONAHA.118.034273 [DOI] [PubMed] [Google Scholar]
- 13. Nordestgaard BG, Langsted A, Mora S, et al. European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) joint consensus initiative Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J 2016;37:1944-58. 10.1093/eurheartj/ehw152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Organization WH. Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization, 2009. [Google Scholar]
- 15. White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 16. Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999;150:341-53. 10.1093/oxfordjournals.aje.a010013 [DOI] [PubMed] [Google Scholar]
- 17. Jones PH, Davidson MH, Stein EA, et al. STELLAR Study Group Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol 2003;92:152-60. 10.1016/S0002-9149(03)00530-7 [DOI] [PubMed] [Google Scholar]
- 18. Jacobs D, Blackburn H, Higgins M, et al. Report of the Conference on Low Blood Cholesterol: mortality associations. Circulation 1992;86:1046-60. 10.1161/01.CIR.86.3.1046 [DOI] [PubMed] [Google Scholar]
- 19. Ranieri P, Rozzini R, Franzoni S, Barbisoni P, Trabucchi M. Serum cholesterol levels as a measure of frailty in elderly patients. Exp Aging Res 1998;24:169-79. 10.1080/036107398244300 [DOI] [PubMed] [Google Scholar]
- 20. Greenberg JA. Correcting biases in estimates of mortality attributable to obesity. Obesity (Silver Spring) 2006;14:2071-9. 10.1038/oby.2006.242 [DOI] [PubMed] [Google Scholar]
- 21. Tikhonoff V, Casiglia E, Mazza A, et al. Low-density lipoprotein cholesterol and mortality in older people. J Am Geriatr Soc 2005;53:2159-64. 10.1111/j.1532-5415.2005.00492.x [DOI] [PubMed] [Google Scholar]
- 22. Petersen LK, Christensen K, Kragstrup J. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing 2010;39:674-80. 10.1093/ageing/afq129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeong SM, Choi S, Kim K, et al. Association of change in total cholesterol level with mortality: a population-based study [correction in: PLoS One 2019;14:e0215934]. PLoS One 2018;13:e0196030. 10.1371/journal.pone.0196030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J 2017;38:2478-86. 10.1093/eurheartj/ehx163 [DOI] [PubMed] [Google Scholar]
- 25. Siggaard-Andersen N, Freiberg JJ, Nordestgaard BG. Only a fraction of patients with ischaemic diseases or diabetes are treated to recommended target values for plasma lipids. Dan Med J 2012;59:A4470. [PubMed] [Google Scholar]
- 26. Langsted A, Freiberg JJ, Nordestgaard BG. Extent of undertreatment and overtreatment with cholesterol-lowering therapy according to European guidelines in 92,348 Danes without ischemic cardiovascular disease and diabetes in 2004-2014. Atherosclerosis 2017;257:9-15. 10.1016/j.atherosclerosis.2016.11.025 [DOI] [PubMed] [Google Scholar]
- 27. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mach F, Baigent C, Catapano AL, et al. ESC Scientific Document Group 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111-88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 29. Burgess S, Davies NM, Thompson SG, EPIC-InterAct Consortium Instrumental variable analysis with a nonlinear exposure-outcome relationship. Epidemiology 2014;25:877-85. 10.1097/EDE.0000000000000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Staley JR, Burgess S. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to Mendelian randomization. Genet Epidemiol 2017;41:341-52. 10.1002/gepi.22041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun YQ, Burgess S, Staley JR, et al. Body mass index and all cause mortality in HUNT and UK Biobank studies: linear and non-linear mendelian randomisation analyses. BMJ 2019;364:l1042. 10.1136/bmj.l1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material