Abstract
Chronic myeloid leukemia (CML), caused by constitutively active BCR-ABL1 fusion tyrosine kinase, has served as a paradigm for successful application of molecularly targeted cancer therapy. The development of the tyrosine kinase inhibitor (TKI) imatinib allows patients with CML to experience near-normal life expectancy. Specific point mutations that decrease drug binding affinity can produce TKI resistance, and second-and third-generation TKIs largely mitigate this problem. Some patients develop TKI resistance without known resistance mutations, with significant heterogeneity in the underlying mechanism, but this is relatively uncommon, with the majority of patients with chronic phase CML achieving long-term disease control. In contrast, responses to TKI treatment are short lived in advanced phases of the disease or in BCR-ABL1-positive acute lymphoblastic leukemia, with relapse driven by both BCR-ABL1 kinase-dependent and -independent mechanisms. Additionally, the frontline CML treatment with second-generation TKIs produces deeper molecular responses, driving disease burden below the detection limit for a greater number of patients. For patients with deep molecular responses, up to half have been able to discontinue therapy. Current efforts are focused on identifying therapeutic strategies to drive deeper molecular responses, enabling more patients to attempt TKI discontinuation.
Introduction
The development of ABL1 tyrosine kinase inhibitors (TKI) for the treatment of chronic myeloid leukemia (CML) is an outstanding example of the translation of basic science discoveries to clinical reality. It has also been an example of the translational research cycle—performing basic science studies as part of clinical trials to understand resistance mechanisms that can then be translated into improved therapies and outcomes. This work has transformed CML from a routinely fatal leukemia to one in which the lifespan of patients with CML approaches that of the general population (Gunnarsson et al., 2016).
CML
CML is characterized by a massive expansive of predominately mature myeloid lineage cells. Most patients with CML (~85%–90%) are diagnosed in chronic phase. In the pre-TKI era, patients would progress to accelerated-phase disease (defined by increasing blast count) within 3–4 years (Faderl et al., 1999). Transformation to an acute leukemia (blast crisis) would soon follow, with approximately 70% having myeloid features while 30% developed lymphoid blast crisis (Rosenthal et al., 1977). Although phenotypically resembling acute leukemia, response rates were exceedingly low to conventional intensive chemotherapy with a median survival of 4 months (Iacoboni et al., 1986). Before the introduction of TKIs, the mainstays of therapy were interferon alpha (IFN-α) and stem cell transplantation. Responses to IFN-α were rarely durable and treatment was associated with substantial toxicity. In younger individuals with histocompatibility leukocyte antigen-matched siblings, allogenic stem transplantation offered a 5-year survival rate of 60% (Horowitz et al., 1996); however, this treatment was accompanied by significant morbidity and mortality and was only available to a minority of patients.
Molecular Pathogenesis of CML
In 1959, Peter Nowell and David Hungerford described a specific chromosomal abnormality in the blood of patients with CML, naming it after their home city, the Philadelphia (Ph) chromosome (Nowell and Hungerford, 1960). Fourteen years later, Janet Rowley established that a reciprocal translocation between chromosomes 9 and 22 produced the Ph chromosome (Rowley, 1973). Subsequent work showed that this translocation event resulted in a fusion event between the Abelson murine leukemia viral oncogene homolog 1 (ABL1) on chromosome 9 and the breakpoint cluster region (BCR) gene on chromosome 22 (Groffen et al., 1984; Lugo et al., 1990). Unlike other myeloid malignancies, BCR-ABL1 is frequently the sole genetic abnormality detected in newly diagnosed CML patients. When detected, most co-occurring mutations are of unclear significance, although mutations in specific genes may predict inferior treatment responses (Branford et al., 2019). Thus, CML is relatively unique in that it is defined by the presence of a single driver oncogene, the BCR-ABL1 fusion.
BCR-ABL1 Fusions and Signaling Mechanisms
Although ubiquitously referred to as the Ph chromosome and BCR-ABL1, there are several breakpoints in BCR that produce distinct BCR-ABL1 isoforms. In patients with CML, the most common BCR-ABL1 isoform results from the fusion of BCR exon 13 or 14 (e13/e14) with ABL1 exon 2 (a2), producing a fusion gene referred to as e13a2 (b2a2) or e14a2 (b3a2) and a protein that has an apparent molecular weight of 210 kDa (p210). A rare subset of patients have an isoform that results from a fusion between BCR exon 19 and ABL1 exon 2, producing a larger protein (p230 BCR-ABL1) that is typically associated with a more indolent disease course (Pane et al., 1996).
After the discovery of the Ph chromosome, it was demonstrated that between 20% and 30% of patients with acute lymphoblastic leukemia (ALL) are also BCR-ABL1-positive (Ph+ ALL). These patients are typically older, have an increased risk of disease involving the central nervous system, and have a poorer prognosis as compared with BCR-ABL1-negative patients (Moorman et al., 2010). In patients with Ph+ ALL, two-thirds will express a shorter p190 isoform resulting from fusion of exon 1 of BCR (e1) to the same portion of ABL1 (e1a2) (Chissoe et al., 1995). This same isoform in also expressed in a rare subset of patients with CML. However, approximately one-third of Ph+ ALL patients will express one of the longer p210 BCR-ABL1 isoforms.
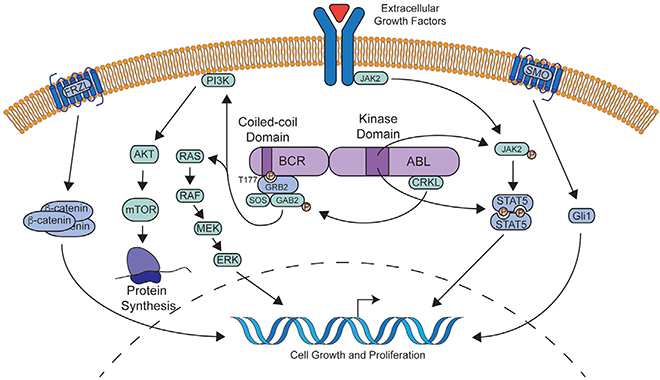
The fusion event between BCR and the ABL1 kinase leads to multiple oncogenic consequences. The coiled-coil domain of the BCR N terminus facilitates dimerization and constitutive autophosphorylation of the ABL1 tyrosine kinase domain, resulting in subsequent phosphorylation of numerous substrates, including GRB2/GAB2, CRKL, JAK/STAT family members, MAPK, and PI3K/AKT pathways (Brehme et al., 2009; Dorsey et al., 2002; Gallipoli et al., 2014; Goss et al., 2006; Hantschel et al., 2012; He et al., 2002; Hemmeryckx et al., 2002; Million and Van Etten, 2000; Pendergast et al., 1993; Reckel et al., 2017; Samanta et al., 2006; Sattler et al., 2002; Senechal et al., 1996; Seo et al., 2010; Xie et al., 2001). The signaling pathways downstream of BCR-ABL1 are summarized in Figure 1. Despite this complexity, all downstream pathways appear dependent on the tyrosine kinase activity of BCR-ABL1, which is crucial to the clinical efficacy of BCR-ABL1 TKIs.
Figure 1. Molecular Pathway Activation Downstream of BCR-ABL1.
BCR-ABL1 dimerizes leading to autophosphorylation at tyrosine 177 of BCR. This serves as a docking point for the GRB2/GAB2/SOS complex which activates multiple signaling pathways, including PI3K/AKT and MAPK. Autophosphorylation of key residues in the BCR-ABL1 kinase domain also in turn activate the JAK/STAT pathway likely via activation of JAK2 and direct phosphorylation of STAT5. In the setting of BCR-ABL1 TKI resistance, extracellular growth factors can act via the JAK/STAT pathway to sustain cell growth. Leukemia stem cells may uniquely depend on WNT/β-catenin and SHH/SMO signaling for survival in the face of BCR-ABL1 kinase inhibition.
Development of Targeted Therapies for BCR-ABL1-Positive Leukemia
The essential role of BCR-ABL1 kinase activity for oncogenic transformation provided the rationale for targeting this activity therapeutically. Scientists at Ciba-Geigy (now Novartis) developed a series of compounds with inhibitory activity toward tyrosine kinases. Screening experiments using this library identified lead compounds with activity against the ABL1 kinase. Optimization to improve oral bioavailability resulted in the development of imatinib (STI-571; Gleevec). Imatinib potently inhibits ABL1 tyrosine kinase activity in an ATP-competitive manner with relative specificity, but also demonstrated inhibitory activity against the tyrosine kinase activity of KIT and platelet-derived growth factor receptor (Druker et al., 1996). Treatment of cells transformed by BCR-ABL1 resulted in a dose-dependent inhibition of proliferation and induction of apoptosis. These results were confirmed in primary CML and Ph+ ALL cells as well as in committed progenitors from patients with CML. Importantly, imatinib exhibited little impact on normal hematopoietic colony formation at concentrations of up to 1 μM. Subsequent studies demonstrated that imatinib treatment potently inhibited the growth of BCR-ABL1-positive murine xenografts (le Coutre et al., 1999). These preclinical studies paved the way for clinical trials in patients with BCR-ABL1-positive leukemia.
Phase 1 trials with imatinib began in 1998 in patients with CML who were resistant to or intolerant of IFN-α (Druker et al., 2001a). Remarkably, 53/54 patients treated with at least 300 mg per day, demonstrated a complete hematologic response (CHR), with 13% of patients achieving clearance of the Ph chromosome from their marrows as assessed by conventional cytogenetics (complete cytogenetic response [CCR]). Phase 2 studies demonstrated a CCR rate of 41% with a progression-free survival rate of 89% after 18 months of treatment (Kantarjian et al., 2002). The landmark phase 3 IRIS trial compared IFN-α + cytarabine with imatinib monotherapy in newly diagnosed, previously untreated patients with chronic-phase CML and established imatinib as the standard-of-care for patients with CML. Although only 8.5% of patients treated with IFN-α + cytarabine achieved a CCR, imatinib treatment produced a 73.8% CCR rate (Kantarjian et al., 2002). Long-term follow-up of IRIS trial participants revealed a 10-year survival rate of 83.3% with infrequent serious side effects (Hochhaus et al., 2017a). Clinical trials were expanded to Ph+ ALL and blast crisis CML, where more modest CHR rates of 20% and 11% were observed, respectively (Druker et al., 2001a). A follow-up phase 2 trial in blast crisis CML revealed a sustained hematologic response in 31% of patients, with a median duration of response of 10 weeks (Sawyers et al., 2002). Similar results were seen in a phase 2 trial of Ph+ ALL, where a CHR was achieved in 19% of patients with an estimated progression-free survival of 2.2 months (Ottmann et al., 2002).
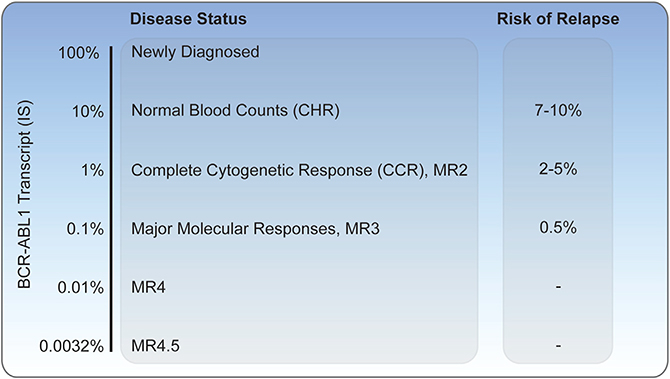
While normalization of blood counts (CHR) and clearance of the Ph chromosome from >20 bone marrow metaphases (CCR) were previously adequate measures for the evaluation of treatment effect, the majority of patients treated with imatinib achieve a CCR. Therefore, more sensitive monitoring approaches were needed to assess disease response. Quantitative reverse transcriptase PCR evaluation of peripheral blood BCR-ABL1 transcript levels enables the detection of low levels of disease with >3 logs more sensitivity than conventional cytogenetics (Hughes et al., 2003). The development of an international scale (IS) allowed standardization of transcript levels between laboratories. An IS value of 100% corresponds to the median level of transcripts among a large international cohort of newly diagnosed CML patients, with CHR and CCR achieved at IS values of 10% and 1%, respectively (Figure 2). In the IRIS trial, individuals who achieved a >3 log reduction in BCR-ABL1 transcript levels had a negligible risk of progression during the following 12 months, with a BCR-ABL1 PCR level of 0.1% being designated a major molecular response (MMR). Long-term follow-up has demonstrated that patients who achieve MMR have minimal to no risk of disease progression to accelerated or blast crisis at 5 years. Furthermore, no patient who achieved MMR by 18 months on therapy with imatinib died of CML with 10 years of follow-up, although some patients died of other causes.
Figure 2. Monitoring of Chronic-Phase CML on BCR-ABL1 TKI Therapy.
BCR-ABL1 transcript levels as measured by reverse-transcriptase PCR and their corresponding disease status. Risk of relapse per year numbers are derived from the original IRIS trial and subsequent European LeukemiaNet guidelines (Hughes et al., 2003; Marin et al., 2008).
Mechanisms of Resistance
In the IRIS study, approximately 17% of patients developed resistance to imatinib with 5 years of follow-up. As noted, most of the patients with advanced-phase disease rapidly became resistant to therapy. One of the critical observations in clinical isolates from patients at the time of resistance was that BCR-ABL1 tyrosine kinase activity was frequently restored as assessed by levels of phosphorylation of the adapter protein CRKL (Gorre et al., 2001). This indicated that imatinib was no longer able to inhibit its target.
Subsequent studies have shown that both BCR-ABL1 kinase-dependent and -independent mechanisms of resistance occur, with the breakdown of percentages differing between patients with primary resistance (failure to achieve an optimal response) and relapse after achieving a response. In the former, BCR-ABL1 kinase-independent mechanisms predominate, while in the latter at least 60% will have kinase-dependent resistance with BCR-ABL1 kinase domain mutations being the predominant mechanism. Of note, given the rapid emergence of resistant clones with kinase domain mutations in advanced-phase disease and a peak of relapse in chronic-phase patients with kinase domain mutations in the second to third year on therapy, it has been hypothesized that resistant clones may be present at the time of initiation of therapy and selected for on therapy as opposed to being induced by therapy (Iqbal et al., 2013; Schmitt et al., 2018). An additional insight that stemmed from the identification of BCR-ABL1 kinase domain mutations in the majority of imatinib-resistant patients has been validation of the concept that the clinical efficacy of the drug is linked to on-target inhibition of BCR-ABL1 kinase activity, rather than due to inhibition of secondary, off-targets. This paradigm, akin to experience with resistance to antibiotics and with human immunodeficiency virus-directed anti-retroviral therapy, has held true for a variety of activated kinase targets in other cancers, including EGFR and ROS1 in non-small-cell lung cancer (Awad et al., 2013; Kobayashi et al., 2009), FLT3 in AML (Smith et al., 2012), and KIT in gastrointestinal stromal tumor (Heinrich et al., 2006).
BCR-ABL1 KINASE DOMAIN MUTATIONS AS A MECHANISM OF RESISTANCE
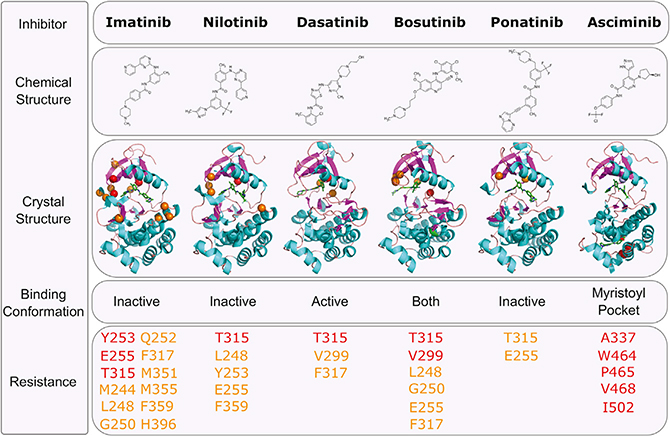
Crystallographic studies of imatinib in complex with the kinase domain of ABL1 revealed that the drug binds to a catalytically inactive conformation of the enzyme. In this state (type II), the DFG motif of the kinase is displaced outward and the activation loop remains in a substrate-mimicking closed form, precluding ATP from accessing its binding site (Nagar et al., 2002; Schindler et al., 2000). These studies also identified several critical residues within the ATP-binding site that form direct hydrogen bonds or productive van der Waals interactions with imatinib. Importantly, sequencing of the BCR-ABL1 kinase domain in CML patients with imatinib resistance has since revealed a spectrum of variants in or near the imatinib binding site (Hughes et al., 2006). These mutations largely center around the phosphate-binding loop (P loop; positions M244, G250, Q252, Y253, and E255), gatekeeper residue (T315 and F317), SH2 contact and C-lobe (M351, F359), and the activation loop (H396). Many of these variants have been studied in greater detail and have been shown to compromise imatinib binding through steric clash, elimination of direct contacts, and/or favoring an active conformation of the ABL1 kinase domain.
The subsequent development and approval of multiple second- and third-generation ABL1 TKIs has largely addressed the BCR-ABL1 point mutant resistance vulnerabilities of imatinib. Nilotinib was originally developed based on the imatinib scaffold, binding to the same pocket but with significantly higher affinity (Weisberg et al., 2005). This increased potency allows for efficacy against some imatinib-resistant point mutations, yet the similar network of interacting residues preserves some common resistance liabilities, such as mutations of Y253, E255, T315, and F359. By contrast, the dual SRC/ABL1 inhibitor dasatinib, which was developed contemporaneously with nilotinib, was found to bind at the ATP site in an active conformation of the ABL1 kinase domain (type I), demonstrating even greater potency against the kinase compared with either imatinib or nilotinib (Shah et al., 2004). As a function of dasatinib’s markedly different chemical structure and binding mode, BCR-ABL1 point mutations associated with resistance to this TKI center on variants of positions V299, T315, and F317. A second SRC/ABL1 inhibitor, bosutinib, which binds similarly to dasatinib, but with slightly reduced potency, exhibits similar vulnerabilities with the addition of variants at G250 and E255 (Golas et al., 2003; Redaelli et al., 2009).
Although all of the approved inhibitors above demonstrate partially overlapping resistance profiles, mutations of T315 (the most problematic of which is a change to isoleucine) remain a common gap in coverage. Often referred to as the “gatekeeper mutation,” due to its position at the entrance to the ATP-binding site, the T315I mutant produces resistance to imatinib, dasatinib, nilotinib, and bosutinib. Ponatinib, a highly potent ABL1 inhibitor, was specifically developed to retain efficacy against the T315I mutation. Its rigid structure and extensive network of productive contacts result in high-affinity binding to ABL1 and efficacy against all kinase domain point mutations, including T315I (O’Hare et al., 2009). Despite this, evidence of a dose-dependent increase in the risk of vascular occlusive events on ponatinib, likely the result of its broad kinase target profile beyond ABL1 which includes KDR, has limited its potential for a broader indication in CML (Massaro et al., 2018). An effort to retain the ability to target the T315I mutation but avoid potential off-target toxicities led to the development of asciminib (ABL001), the first allosteric ABL1 kinase inhibitor to reach clinical evaluation. Unlike each of the other approved TKIs for CML, asciminib uniquely binds to the myristoylation pocket of BCR-ABL1 (Wylie et al., 2017), which in c-ABL1 is critical in the regulation of the kinase and maintaining an auto-inhibited state (Hantschel et al., 2003). Preclinical studies have shown asciminib to be a potent and highly selective inhibitor of ABL1 with activity against many BCR-ABL1 point mutations, including T315I. In a phase 1 clinical trial of heavily pre-treated chronic-phase (and several accelerated-phase) CML patients, asciminib treatment resulted in CHR and CCR in 92% and 54% of patients, respectively (Hughes et al., 2019). MMR was achieved in 48% of patients by 12 months, including 57% of patients with intolerance or resistance to ponatinib. Although longer follow-up will be required to determine the spectrum of potential resistance vulnerabilities for this drug, available preclinical and clinical data to date suggest resistance mutations in and around the myristoylation pocket are predicted to be uniquely problematic for asciminib (Eide et al., 2019; Wylie et al., 2017).
The expanding arsenal of clinically available ABL1 TKIs over the past 15 years (Figure 3) has enabled the largely effective management of BCR-ABL1 point mutation-mediated resistance in CML. However, sequential TKI treatment in patients with resistance has been shown to have the potential to select for compound mutations (two mutations in the same BCR-ABL1 molecule) that confer resistance to many or all available TKIs (Khorashad et al., 2008; O’Hare et al., 2009; Shah et al., 2007; Stagno et al., 2009; Zabriskie et al., 2014). For example, while phase 2 evaluation of ponatinib showed impressive responses in highly refractory patients with a variety of BCR-ABL1 point mutations, among patients who discontinued therapy ~25% had evidence of a BCR-ABL1 compound mutation, more commonly in CML blast crisis and Ph+ ALL patients than those with chronic-phase disease (Cortes et al., 2013; Deininger et al., 2016). Notably, while single-agent TKI treatment of these mutants is ineffective, combined treatment with ATP site TKIs (particularly ponatinib) and asciminib has been proposed as a strategy for this type of resistance (Eide et al., 2019).
Figure 3. BCR-ABL1 Tyrosine Kinase Inhibitors and Resistance Mechanisms.
Chemical structures and published X-ray crystallographic structures of ABL1 complexed with kinase inhibitors are shown. Residues at which mutations are associated with strong resistance to a given TKI are indicated in red, while those associated with lesser degrees of resistance are listed in orange. Both T315 and E255 mutations do lead to an increase in the IC50 for ponatinib; however, they do not typically lead to clinical resistance in isolation, but do as a compound mutation. The structure of ABL1 complexed with asciminib shows nilotinib in the ATP-binding site for reference. T315I is indicated in purple for visual reference (Cowan-Jacob et al., 2007; Levinson and Boxer, 2012; O’Hare et al., 2009; Tokarski et al., 2006; Weisberg et al., 2005; Wylie et al., 2017).
Although BCR-ABL1 kinase domain mutations have traditionally been detected via Sanger sequencing, recent work using next-generation sequencing has revealed an increased sensitivity for low-level variants. In patients with suboptimal responses to therapy, 30%–40% will harbor a low-level resistance mutation, which will invariably be selected for unless therapy is changed (Soverini et al., 2020). These results demonstrate the clinical utility of more sensitive BCR-ABL1 kinase domain mutation screening in the setting of suboptimal treatment responses and argue for the incorporation of this approach in clinical algorithms.
BCR-ABL1 Kinase Domain Mutation-Independent Resistance Mechanisms
Although BCR-ABL1 kinase domain mutations are identified in many individuals with CML who relapse on TKI treatment, resistance can develop in others without apparent kinase domain mutations. There is also evidence that TKI resistance can develop through the reactivation of the signaling pathways downstream of BCR-ABL1, including MAPK, PI3K, SRC, and JAK/STAT despite effective BCR-ABL1 inhibition (Burchert et al., 2005; Donato et al., 2003; Gioia et al., 2011; Packer et al., 2011; Warsch et al., 2011; Zhou et al., 2008). In addition, many patients with CML will achieve some measure of disease control on BCR-ABL1 TKI therapy, but fail to achieve optimal responses and similarly harbor no explanatory kinase domain mutations. In some cases, such individuals can respond to an alternate TKI, suggesting that their disease remains dependent on BCR-ABL1 kinase activity and/or that additional non-kinase domain mutation-mediated resistance mechanisms are overcome by the off-target profile of the new therapy.
In solid tumors treated with targeted therapy, resistance frequently develops through lineage plasticity mechanisms, with transcriptional and epigenetic changes driving survival in the face of treatment (recently reviewed in Boumahdi and de Sauvage, 2020). Crucial to plasticity-mediated resistance is the development of a pool of slow-cycling cells with relative therapeutic resistance. BCR-ABL1 kinase domain mutation-independent resistance may be an analogous state in CML. Furthermore, the residual disease in patients failing to achieve a deep molecular response and in those that relapse after attempted treatment discontinuation may resist the effects of BCR-ABL1 inhibition via similar mechanisms. However, in contrast with solid tumors, individuals with a deep molecular response to BCR-ABL1-directed therapy rarely relapse while on therapy, a finding that may be related to the relative lack of genomic complexity in chronic-phase CML.
BCR-ABL1 kinase domain mutation-independent resistance is also associated with the presence of mutations in the epigenetic regulators ASXL1, DNMT3A, IDH1, and SETBP1 (Kim et al., 2017). Indeed, the presence of such mutations at the time of diagnosis is associated with an increased risk of poor treatment outcome (Branford et al., 2018) Furthermore, such mutations are also associated with progression to blast crisis (Giotopoulos et al., 2015; Grossmann et al., 2011). Precisely how these mutations drive TKI resistance in CML is unclear, although studies in AML provide evidence that such mutations facilitate some degree of differentiation arrest in leukemic blasts which may result in decreased TKI sensitivity.
Microenvironmental factors also play a role in driving TKI resistance. Stromal-derived cytokines promote STAT3 phosphorylation in primary CML cells, and BCR-ABL1-independent activation of STAT3 is seen in TKI-resistant primary CML cells (Strom et al., 2009; Traer et al., 2012; Wang et al., 2007; Weisberg et al., 2008). Bone marrow-derived placental growth factor levels are increased in CML and promote BCR-ABL1-independent CML cell proliferation (Schmidt et al., 2011). Fibroblast growth factor 2 released from the bone marrow stroma also drives imatinib resistance through activation of downstream MAPK signaling (Traer et al., 2014). Therefore, it appears that CML cells remain hypersensitive to certain microenvironmental-derived factors that may enable them to survive in the face of TKI therapy. Whether this property is the result of residual BCR-ABL1 kinase activity incompletely repressed by TKI therapy, kinase-independent functions of BCR-ABL1, or additional genomic alterations acquired during the process of malignant transformation remains an important avenue for further investigation.
Ph+ ALL and Advanced CML
The treatment of Ph+ ALL and advanced-phase CML remains a clinical challenge. In Ph+ ALL and lymphoid blast crisis CML, single-agent TKI therapy rarely produces deep or durable responses and relapse is inevitable (Druker et al., 2001b; Jones et al., 2008). Therefore, targeted therapy is typically added to conventional intensive chemotherapy. In pediatric Ph+ ALL, the combination of imatinib plus conventional chemotherapy produces markedly improved responses, with a disease-free survival rate of 70% at 5 years. Importantly, chemotherapy plus TKI showed an equivalent 5-year disease-free survival rate to conventional chemotherapy followed by allogenic hematopoietic stem cell transplantation (Schultz et al., 2014).
In myeloid blast crisis, intensive chemotherapy is largely ineffective and treatment with a second-generation TKI is typically used with the goal of inducing a second remission and proceeding to allogenic transplantation (Hehlmann, 2012). Driven by a stereotypic set of cytogenetics aberrations and point mutations, blast crisis is associated with decreased response rates to TKI therapy as well as a markedly increased rates of resistance (Johansson et al., 2002). TKI resistance is frequently driven by the acquisition of BCR-ABL1 point mutations and mutations in epigenetic regulators (Kantarjian et al., 2006). This increased genomic instability is thought to be driven by generation of reactive oxygen species, leading to DNA damage and increased mutation frequency (Koptyra et al., 2006). These findings argue that once disease has acquired the genomic instability to progress to the acute-phase, multi-agent therapy will be required to achieve long-term disease control.
Comparison of Responses and Resistance to TKIs in Chronic-Phase CML
At present, five inhibitors of BCR-ABL1 have been granted FDA approval for the treatment of chronic-phase CML (Figure 3; Table 1). Since the initial approval of imatinib, numerous studies have investigated strategies for attaining deeper molecular responses. High-dose imatinib (600–800 mg daily) drives more rapid molecular responses and achieves a higher rate of MMR at 1 year, yet does not alter the rate of progression-free survival at 5 years (Gafter-Gvili et al., 2011; Hehlmann et al., 2017). Dasatinib, nilotinib, and bosutinib have all been compared with imatinib as frontline therapy for chronic-phase CML (Kantarjian et al., 2010; Cortes et al., 2016, 2018a; Maiti et al., 2016; Wang et al., 2015; Hochhaus et al., 2016; Saglio et al., 2010; Hughes et al., 2019). In each case, superior molecular responses are observed at 1 year in comparison with imatinib, yet none of these drugs produce clinically significant alterations in long-term progression-free survival. Although ponatinib demonstrated marked improvements in early molecular responses compared with imatinib in previously untreated chronic-phase CML patients (Lipton et al., 2014, 2016), this trial was stopped early due to the increase risk of arteriovascular-occulsive events observed with ponatinib. For all agents, responses improve with time, with a greater number of patients achieving a deep molecular response after 5 years of therapy. Indeed the difference between second-generation TKIs and standard-dose imatinib narrows with long-term follow-up (Hochhaus et al., 2016; Maiti et al., 2016). The rate of achieving MR4.5 (defined as BCR-ABL1 transcripts <0.0032% IS) with standard-dose imatinib varies between studies, making cross-trial comparisons challenging. However, high-dose imatinib, dasatinib, and nilotinib all show a 10%–20% improvement in the MR4.5 rate in comparison with standard-dose imatinib at 5 years (Gafter-Gvili et al., 2011; Hehlmann et al., 2017; Rossari et al., 2018). The precise mechanism underlying the rate of deep molecular response is unclear. It is likely that drug potency plays a significant role, as imatinib demonstrates the highest IC50 of all clinically available BCR-ABL1 TKIs, and imatinib dose-escalation drives more rapid molecular responses. Evidence also suggests that pharmacokinetic differences in drug absorption and bioavailability may play a significant role, with some proposing that time of target inhibition is an important driver of disease response, requiring higher TKI dosing in some patients to durably inhibit CML cell growth (Josephs et al., 2013). However, a clear relationship between plasma half-life and disease response is not readily apparent. Dasatinib demonstrates a markedly lower half-life than imatinib, but twice-daily dasatinib drives similar clinical responses to once-daily dosing (Shah et al., 2008a), suggesting potency of inhibition perhaps outweighs time of target inhibition as a clinically important variable (Shah et al., 2008b; Snead et al., 2009). Complicating this interpretation, however, is the finding that while dasatinib is rapidly cleared from blood it has a relatively slow rate of dissociation from BCR-ABL1 and is retained intracellularly, arguing that it may display a longer time on-target than would be suggested by its half-life (Kumar and Lowery, 2017; O’Hare et al., 2013; Willemsen-Seegers et al., 2017). Thus, the development of high potency BCR-ABL1 TKIs with increased half-life remains a possible means of driving improved disease responses. As TKI discontinuation (discussed below) becomes an increasingly important clinical goal, strategies aimed at driving deeper molecular responses are of increased importance. Furthermore, diagnostic methods to identify those patients who are unlikely to achieve a satisfactory molecular response would be useful for the identification of patients who may benefit from up-front combination therapy.
Table 1.
Comparison of Potency and Response to BCR-ABL1 TKIs
| Drug | BCR-ABL1 Cellular IC50 (nM) | Plasma Half-Life (h) | Frontline Treatment at 1 Year | Frontline Treatment at 5 Years | |||
|---|---|---|---|---|---|---|---|
| Rate of CCR (%) | Rate of MMR (%) | Rate of MR4.5 (%) | Rate of MR4.5 (%) | PFS (%) | |||
| Imatinib 400 mg | 100–500 | 18 | 55–66 | 22–38 | 1–5 | 30–35 | 86–91 |
| High-dose Imatinib | 65 | 45–55 | 9 | 58a | 89 | ||
| Dasatinib | 0.8–1.8 | 3–5 | 77 | 46 | 5 | 42 | 85 |
| Nilotinib | 10–25 | 17 | 80 | 44 | 11 | 54 | 96 |
| Bosutinib | 42 | 32–39 | 77 | 47 | 8.1 | NA | NA |
| Ponatinib | 0.5 | 22 | 100b | 80b | 60b | NA | NA |
| Asciminib | 0.25 | 8 | 48b | NA | NA | NA | NA |
NA, data not yet available.
Data summarized from the following references: IC50 values (Rossari et al., 2018); half-life (Abbas et al., 2012; Cortes et al., 2012; Hazarika et al., 2008; Peng et al., 2004; Reckel et al., 2017); dasatinib versus imatinib (Cortes et al., 2016; Kantarjian et al., 2010); nilotinib versus imatinib (Hochhaus et al., 2016; Saglio et al., 2010); bosutinib versus imatinib (Cortes et al., 2018a); ponatinib versus imatinib (Lipton et al., 2014, 2016); high-dose versus standard-dose imatinib (Gafter-Gvili et al., 2011; Hehlmann et al., 2017).
MR4.5 rate for standard dose imatinib in CML IV study was 49%.
Only ten patients on ponatinib in the EPIC trial reached 1 year.
Residual Disease and TKI Discontinuation
Despite the marked change in the natural history of CML, significant challenges remain. Although side effects of therapy are generally mild, the majority of patients face many years to a lifetime of treatment. Thus, side effects from imatinib, such as fatigue, rashes, fluid retention, bone pain, and chronic diarrhea can be quite detrimental to quality of life (Deininger et al., 2003; Efficace et al., 2013). Other TKIs are associated with pleural effusions, increased risk of cardiovascular disease, QTc prolongation, and pancreatitis (Cortes et al., 2018b; Hazarika et al., 2008; Khoury et al., 2009; Porkka et al., 2010). Given the chronic nature of therapy, compliance is also a significant issue. Approximately 15% of patients monitored for medication adherence by a microelectronic device took less than 80% of the prescribed doses of imatinib (Ibrahim et al., 2011; Marin et al., 2010). Importantly, a strong correlation between adherence and response was noted, and none of the patients with adherence less than 80% achieved MMR.
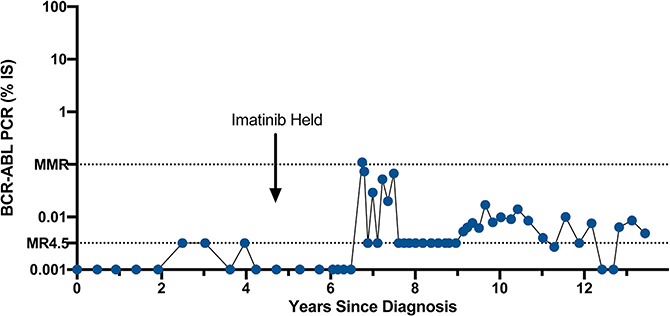
In individuals with CML who achieve deep molecular remissions, residual BCR-ABL1-positive cells can be identified in the long-term hematopoietic stem cell pool (Eisterer et al., 2005). Indeed, individuals with a negative BCR-ABL1 PCR may harbor as many as 6.5 × 106 residual leukemia cells (Sender et al., 2016). Despite this potentially large number of persistent leukemia cells, clinical trials were initiated to see if it would be possible to discontinue therapy. Starting with the STIM-Pilot/STIM1 trial, it was determined that some individuals who maintain undetectable BCR-ABL1 transcripts (as measured by an assay with at least a 4.5 log sensitivity) can safely discontinue imatinib therapy (Mahon et al., 2010; Rousselot et al., 2007). Multiple other trials with imatinib and second-generation TKIs have confirmed these initial results and found that approximately 50% of patients are able to discontinue therapy with long-term follow-up over 7 years in some studies (Hochhaus et al., 2017b; Imagawa et al., 2015; Mahon et al., 2018; Rea et al., 2017; Ross et al., 2013; Saussele et al., 2018; Takahashi et al., 2018). For patients who achieve a treatment-free remission (TFR), 50% will maintain undetectable levels of BCR-ABL1 transcripts, while 50% will develop low-level positive BCR-ABL1 transcripts yet maintain an MMR for at least several years. An example of such a patient is shown in Figure 4. This patient was off imatinib for 8 years and demonstrated a BCR-ABL1 PCR that fluctuated between undetectable and 0.1%. For the 50% of patients with recurrent disease (defined as loss of MMR), nearly all patients are able to regain their response with resumption of TKI treatment, although there have been rare reports of disease progression. Somewhat surprisingly, after another 2 years of maintaining undetectable BCR-ABL1 transcript levels, approximately 40% of these individuals will be able to successfully discontinue TKI treatment (Legros et al., 2017). This suggests that CML stem cells are continuously cleared by TKI therapy at a low rate or exist in a quiescent, insensitive state and stochastically pass through a transient-sensitive state. Interestingly, it is likely that most individuals in a TFR continue to harbor low-level residual disease when measured using ultrasensitive methodology (Bocchia et al., 2018; Cui et al., 2018). Therefore, it may be that once the CML stem cell number is reduced below a certain threshold, many individuals will experience long-term remission, either owing to the quiescent nature of the CML stem cell itself or immune-mediated disease control. An understanding of the biology of CML stem cells has become a major focus area as the clearance of residual disease would enable more patients to successfully discontinue therapy.
Figure 4. BCR-ABL1 Transcript Levels in a CML Patient in Long-Term Treatment-free Remission.
Example of an individual who underwent imatinib discontinuation in 2011 and has maintained a TFR since that time with fluctuating BCR-ABL1 PCR levels but maintenance of an MMR.
Studies to date suggest that residual BCR-ABL1-positive CML stem cells are relatively insensitive to BCR-ABL1 inhibition by TKIs (Corbin et al., 2011). Numerous signaling pathways are differentially activated in these cells as compared with BCR-ABL1-negative stem cells. CML stem cells are dependent on WNT/β-catenin signaling for survival in the face of BCR-ABL1 inhibition (Gregory et al., 2010). Combined inhibition of BCR-ABL1 and β-catenin synergistically induces apoptosis in CML blasts and progenitors, suggesting the possibility that dual therapy could eliminate CML stem cells (Zhou et al., 2017). Additional studies have implicated the Hedgehog pathway as a potential therapeutic target in CML stem cells. Hedgehog signaling is activated in CML stem cells and persists upon BCR-ABL1 inhibition, and pharmacologic inhibition of Hedgehog signaling prevents the expansion of CML stem cells (Dierks et al., 2008; Zhao et al., 2009). Unfortunately, early trials of Hedgehog inhibitors in combination with ABL1 TKIs were discontinued due to toxicity.
The JAK/STAT pathway has been implicated as a crucial downstream target of BCR-ABL1. Single-cell RNA sequencing of CML stem cells revealed a quiescent population with transcriptional evidence of both JAK/STAT and TNF pathway activation as compared with normal stem cells (Giustacchini et al., 2017). Consistent with this, combination therapy with nilotinib and the JAK/STAT inhibitor, ruxolitinib, decreased CML stem cell number in murine xenografts while sparing normal stem cells (Gallipoli et al., 2014). Epigenetic regulators have also been implicated in the persistence of CML stem cells in the face of TKI therapy. Combined inhibition of BCR-ABL1 and the epigenetic regulators EZH2 or SIRT1 resulted in synergistic elimination of CML stem cells (Li et al., 2012; Scott et al., 2016). Combined inhibition of BCR-ABL1 and the anti-apoptotic regulator BCL2 also markedly reduced CML stem cell numbers in murine models. Numerous other pathways have been implicated as critical to the maintenance of the leukemia stem cell, including the PI3K pathway, PML, PP2A, HIF, and autophagy pathway (Airiau et al., 2013; Bellodi et al., 2009; Cheloni et al., 2017; Chen et al., 2014; Ito et al., 2008; Lai et al., 2018). Collectively, these studies demonstrate differential activation of signaling pathways in CML and normal stem cells offering possible targets for combination therapy.
As a result of these preclinical studies, multiple clinical trials are investigating therapeutic combinations that may further reduce residual disease burden, allowing more patients to successfully achieve deep molecular responses and to discontinue therapy. Three trials are specifically investigating the combination of second-generation TKIs with ruxolitinib to promote deeper molecular responses or aid in a second attempt at TKI discontinuation (NCT02689440, NCT01702064, NCT03610971). In addition, there has been renewed interest in IFN-α in combination with BCR-ABL1 TKIs driven by the notion that IFN may augment immune responses leading to reduction in residual disease (NCT01933906, NCT02001818). Finally, the combination of dasatinib and the BCL2 inhibitor venetoclax is also under early-stage clinical investigation (NCT02689440). Dual BCR-ABL1 inhibition is an additional strategy that has the potential to drive deeper molecular responses, allowing treatment discontinuation in a larger number of patients. There is preclinical evidence that dual BCR-ABL1 inhibition with imatinib and dasatinib prevents the development of resistance to either agent (Burgess et al., 2005). Dual BCR-ABL1 inhibition with the allosteric inhibitor asciminib and ATP-binding site inhibitors is particularly appealing given that these two classes of drug bind to distinct domains of ABL1 and have differing mechanisms of resistance (Eide et al., 2019; Wylie et al., 2017). Collectively, these early-phase studies offer the possibility of producing deeper disease response in a larger number of patients, potentially allowing for increased numbers of patients to be eligible for TKI discontinuation.
Conclusion
The advent of BCR-ABL1-targeted therapy has markedly changed the natural history of CML, rendering a once-fatal disease into a manageable condition. Resistance is relatively uncommon and occurs through BCR-ABL1 kinase-dependent and -independent mechanisms. The development of multiple BCR-ABL1 TKIs has rendered on-target resistance a largely treatable condition, although the T315I mutation and compound mutations remain a clinical challenge. Patients with advanced disease (i.e., blast crisis or Ph+ ALL) remain an additional area of challenge. This scenario is globally quite similar to the experience with targeted therapy in the vast majority of other malignancies. Although impressive responses can be obtained, these are short-lived with the inevitable development of resistance. For such patients, combination therapy will likely be necessary to obtain longer-term responses.
Despite these advances, chronic TKI therapy is associated with a decreased quality of life and increased financial burden, underscoring discontinuation of therapy as an important clinical goal for many patients. An improved biological understanding of why BCR-ABL1-positive cells persist in some individuals on therapy may enable the development of strategies that allow more patients to discontinue therapy. The persistence of residual disease during targeted therapy is not unique to CML, and likely occurs in the vast majority of malignancies. Therefore, lessons learned regarding residual disease in CML may offer clues to how residual disease may be eradicated more broadly.
The relative success of targeted therapy in CML is likely due to the fact that chronic-phase disease becomes clinically evident early in the disease course. This allows for early treatment and cytoreduction, reducing the risk of genetic evolution. Therefore, to extend the success seen with CML to other forms of cancer, it is imperative to develop strategies that detect cancers when they are less genetically advanced, facilitating treatment earlier in the course of disease.
ACKNOWLEDGMENTS
Funding provided by KL2TR002370-03 to T.P.B., Howard Hughes Medical Institute and R01 CA065823-24 to B.J.D.
Footnotes
DECLARATION OF INTERESTS
B.J.D. potential competing interests—SAB: Aileron Therapeutics, Therapy Architects (ALLCRON), Cepheid, Vivid Biosciences, Celgene, RUNX1 Research Program, EnLiven Therapeutics, Gilead Sciences (inactive), Monojul (inactive); SAB & Stock: Aptose Biosciences, Blueprint Medicines, Iterion Therapeutics, Third Coast Therapeutics, GRAIL (SAB inactive); Scientific Founder: MolecularMD (inactive, acquired by ICON); Board of Directors & Stock: Amgen; Board of Directors: Burroughs Wellcome Fund, CureOne; Joint Steering Committee: Beat AML LLS; Founder: VB Therapeutics; Clinical Trial Funding: Novartis, Bristol-Myers Squibb, Pfizer; Royalties from Patent 6958335 (Novartis exclusive license) and OHSU and Dana-Farber Cancer Institute (one Merck exclusive license). The remaining authors have no competing interests to declare.
REFERENCES
- Abbas R, Hug BA, Leister C, Gaaloul ME, Chalon S, and Sonnichsen D (2012). A phase I ascending single-dose study of the safety, tolerability, and pharmacokinetics of bosutinib (SKI-606) in healthy adult subjects. Cancer Chemother. Pharmacol 69, 221–227. [DOI] [PubMed] [Google Scholar]
- Airiau K, Mahon F-X, Josselin M, Jeanneteau M, and Belloc F (2013). PI3K/mTOR pathway inhibitors sensitize chronic myeloid leukemia stem cells to nilotinib and restore the response of progenitors to nilotinib in the presence of stem cell factor. Cell Death Dis 4, e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad MM, Katayama R, McTigue M, Liu W, Deng Y-L, Brooun A, Friboulet L, Huang D, Falk MD, Timofeevski S, et al. (2013). Acquired resistance to crizotinib from a mutation in CD74–ROS1. N. Engl. J. Med 368, 2395–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M, Galavotti S, Young KW, Selmi T, Yacobi R, et al. (2009). Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J. Clin. Invest 119, 1109–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchia M, Sicuranza A, Abruzzese E, Iurlo A, Sirianni S, Gozzini A, Galimberti S, Aprile L, Martino B, Pregno P, et al. (2018). Residual peripheral blood CD26+ leukemic stem cells in chronic myeloid leukemia patients during TKI therapy and during treatment-free remission. Front. Oncol 8, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumahdi S, and de Sauvage FJ (2020). The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov 19, 39–56. [DOI] [PubMed] [Google Scholar]
- Branford S, Wang P, Yeung DT, Thomson D, Purins A, Wadham C, Shahrin NH, Marum JE, Nataren N, Parker WT, et al. (2018). Integrative genomic analysis reveals cancer-associated mutations at diagnosis of CML in patients with high-risk disease. Blood 132, 948–961. [DOI] [PubMed] [Google Scholar]
- Branford S, Kim DDH, Apperley JF, Eide CA, Mustjoki S, Ong ST, Nteliopoulos G, Ernst T, Chuah C, Gambacorti-Passerini C, et al. (2019). Laying the foundation for genomically-based risk assessment in chronic myeloid leukemia. Leukemia 33, 1835–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehme M, Hantschel O, Colinge J, Kaupe I, Planyavsky M, Köcher T, Mechtler K, Bennett KL, and Superti-Furga G (2009). Charting the molecular network of the drug target Bcr-Abl. Proc. Natl. Acad. Sci. U S A. 106, 7414–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchert A, Wang Y, Cai D, von Bubnoff N, Paschka P, Müller-Brüssel-bach S, Ottmann OG, Duyster J, Hochhaus A, and Neubauer A (2005). Compensatory PI3-kinase/Akt/mTor activation regulates imatinib resistance development. Leukemia 19, 1774–1782. [DOI] [PubMed] [Google Scholar]
- Burgess MR, Skaggs BJ, Shah NP, Lee FY, and Sawyers CL (2005). Comparative analysis of two clinically active BCR-ABL kinase inhibitors reveals the role of conformation-specific binding in resistance. Proc. Natl. Acad. Sci. U S A 102, 3395–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheloni G, Tanturli M, Tusa I, Ho DeSouza N, Shan Y, Gozzini A, Mazurier F, Rovida E, Li S, and Dello Sbarba P (2017). Targeting chronic myeloid leukemia stem cells with the hypoxia-inducible factor inhibitor acriflavine. Blood 130, 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Peng C, Abraham SA, Shan Y, Guo Z, Desouza N, Cheloni G, Li D, Holyoake TL, and Li S (2014). Arachidonate 15-lipoxygenase is required for chronic myeloid leukemia stem cell survival. J. Clin. Invest 124, 3847–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Kantarjian H, Shah NP, Bixby D, Mauro MJ, Flinn I, O’Hare T, Hu S, Narasimhan NI, Rivera VM, et al. (2012). Ponatinib in refractory Philadelphia chromosome-positive leukemias. New Engl. J. Med 367, 2075–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Kim D-W, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, Nicolini FE, Apperley JF, Khoury HJ, Talpaz M, et al. (2013). A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. New Engl. J. Med 369, 1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, Shah NP, Chuah C, Casanova L, Bradley-Garelik B, et al. (2016). Final 5-year study results of DASISION: the Dasatinib versus Imatinib Study in Treatment-Naíve Chronic Myeloid Leukemia Patients trial. J. Clin. Oncol 34, 2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim D-W, Dyagil I, Glushko N, Milojkovic D, le Coutre P, et al. (2018a). Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J. Clin. Oncol. 36, 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chissoe SL, Bodenteich A, Wang Y-F, Wang Y-P, Buriaton SW, Cran D, Clifbtree J, Freeman A, Iyer K, Jian L, et al. (1995). Sequence and analysis of the human ABL gene, the BCR gene, and regions involved in the Philadelphia chromosomal translocation. Genomics 27, 67–82. [DOI] [PubMed] [Google Scholar]
- Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, and Druker BJ (2011). Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J. Clin. Invest 121, 396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes JE, Apperley JF, DeAngelo DJ, Deininger MW, Kota VK, Rousselot P, and Gambacorti-Passerini C (2018b). Management of adverse events associated with bosutinib treatment of chronic-phase chronic myeloid leukemia: expert panel review. J. Hematol. Oncol 11, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Coutre P, Mologni L, Cleris L, Marchesi E, Buchdunger E, Giardini R, Formelli F, and Gambacorti-Passerini C (1999). In vivo eradication of human BCR/ABL-positive leukemia cells with an ABL kinase inhibitor. J. Natl. Cancer Inst 91, 163–168. [DOI] [PubMed] [Google Scholar]
- Cowan-Jacob SW, Fendrich G, Floersheimer A, Furet P, Liebetanz J, Rummel G, Rheinberger P, Centeleghe M, Fabbro D, and Manley PW (2007). Structural biology contributions to the discovery of drugs to treat chronic myelogenous leukaemia. Acta Crystallogr. D Biol. Crystallogr 63, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Zhu Z, Liu S, Li Q, Meng L, Cheng H, Zhong Z, Li W, You Y, Zhu X, et al. (2018). Monitoring of leukemia stem cells in chronic myeloid leukemia patients. Leuk. Lymphoma 59, 2264–2266. [DOI] [PubMed] [Google Scholar]
- Deininger MW, Hodgson JG, Shah NP, Cortes JE, Kim D-W, Nicolini FE, Talpaz M, Baccarani M, Müller MC, Li J, et al. (2016). Compound mutations in BCR-ABL1 are not major drivers of primary or secondary resistance to ponatinib in CP-CML patients. Blood 127, 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger MWN, O’Brien SG, Ford JM, and Druker BJ (2003). Practical management of patients with chronic myeloid leukemia receiving imatinib. J. Clin. Oncol 21, 1637–1647. [DOI] [PubMed] [Google Scholar]
- Dierks C, Beigi R, Guo G-R, Zirlik K, Stegert MR, Manley P, Trussell C, Schmitt-Graeff A, Landwerlin K, Veelken H, et al. (2008). Expansion of Bcr-Abl-positive leukemic stem cells is dependent on hedgehog pathway activation. Cancer Cell 14, 238–249. [DOI] [PubMed] [Google Scholar]
- Donato NJ, Wu JY, Stapley J, Gallick G, Lin H, Arlinghaus R, and Talpaz M (2003). BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood 101, 690–698. [DOI] [PubMed] [Google Scholar]
- Dorsey JF, Cunnick JM, Mane SM, and Wu J (2002). Regulation of the Erk2-Elk1 signaling pathway and megakaryocytic differentiation of Bcr-Abl(+) K562 leukemic cells by Gab2. Blood 99, 1388–1397. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, and Lydon NB (1996). Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 2, 561–566. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, et al. (2001a). Efficacy and safety of a specific inhibitor of the Bcr-Abl tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med 344, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, and Talpaz M (2001b). Activity of a specific inhibitor of the Bcr-Abl tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N. Engl. J. Med 344, 1038–1042. [DOI] [PubMed] [Google Scholar]
- Efficace F, Baccarani M, Breccia M, Cottone F, Alimena G, Deliliers GL, Baratè C, Specchia G, Di Lorenzo R, Luciano L, et al. (2013). Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia 27, 1511–1519. [DOI] [PubMed] [Google Scholar]
- Eide CA, Zabriskie MS, Savage Stevens SL, Antelope O, Vellore NA, Than H, Schultz AR, Clair P, Bowler AD, Pomicter AD, et al. (2019). Combining the allosteric inhibitor asciminib with ponatinib suppresses emergence of and restores efficacy against highly resistant BCR-ABL1 mutants. Cancer Cell 36, 431–443.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisterer W, Jiang X, Christ O, Glimm H, Lee KH, Pang E, Lambie K, Shaw G, Holyoake TL, Petzer AL, et al. (2005). Different subsets of primary chronic myeloid leukemia stem cells engraft immunodeficient mice and produce a model of the human disease. Leukemia 19, 435–441. [DOI] [PubMed] [Google Scholar]
- Faderl S, Talpaz M, Estrov Z, and Kantarjian HM (1999). Chronic Myelogenous Leukemia: Biology and Therapy. Ann. Intern. Med 131, 207. [DOI] [PubMed] [Google Scholar]
- Gafter-Gvili A, Leader A, Gurion R, Vidal L, Ram R, Shacham-Abulafia A, Ben-Bassat I, Lishner M, Shpilberg O, and Raanani P (2011). High-dose imatinib for newly diagnosed chronic phase chronic myeloid leukemia patients—systematic review and meta-analysis. Am. J. Hematol. 86, 657–662. [DOI] [PubMed] [Google Scholar]
- Gallipoli P, Cook A, Rhodes S, Hopcroft L, Wheadon H, Whetton AD, Jørgensen HG, Bhatia R, and Holyoake TL (2014). JAK2/STAT5 inhibition by nilotinib with ruxolitinib contributes to the elimination of CML CD34+ cells in vitro and in vivo. Blood 124, 1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia R, Leroy C, Drullion C, Lagarde V, Etienne G, Dulucq S, Lippert E, Roche S, Mahon F-X, and Pasquet J-M (2011). Quantitative phosphoproteomics revealed interplay between Syk and Lyn in the resistance to nilotinib in chronic myeloid leukemia cells. Blood 118, 2211–2221. [DOI] [PubMed] [Google Scholar]
- Giotopoulos G, van der Weyden L, Osaki H, Rust AG, Gallipoli P, Meduri E, Horton SJ, Chan W-I, Foster D, Prinjha RK, et al. (2015). A novel mouse model identifies cooperating mutations and therapeutic targets critical for chronic myeloid leukemia progression. J. Exp. Med 212, 1551–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustacchini A, Thongjuea S, Barkas N, Woll PS, Povinelli BJ, Booth CAG, Sopp P, Norfo R, Rodriguez-Meira A, Ashley N, et al. (2017). Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat. Med 23, 692–702. [DOI] [PubMed] [Google Scholar]
- Golas JM, Arndt K, Etienne C, Lucas J, Nardin D, Gibbons J, Frost P, Ye F, Boschelli DH, and Boschelli F (2003). SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res 63, 375–381. [PubMed] [Google Scholar]
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, and Sawyers CL (2001). Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293, 876–880. [DOI] [PubMed] [Google Scholar]
- Goss VL, Lee KA, Moritz A, Nardone J, Spek EJ, MacNeill J, Rush J, Comb MJ, and Polakiewicz RD (2006). A common phosphotyrosine signature for the Bcr-Abl kinase. Blood 107, 4888–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MA, Phang TL, Neviani P, Alvarez-Calderon F, Eide CA, O’Hare T, Zaberezhnyy V, Williams RT, Druker BJ, Perrotti D, et al. (2010). Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl. Cancer Cell 18, 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen J, Stephenson J, Heisterkamp N, de Klein A, Bartram C, and Grosveld G (1984). Philadelphia chromosomal breakpoints are clustered within a limited region, Bcr, on chromosome 22. Cell 36, 93–99. [DOI] [PubMed] [Google Scholar]
- Grossmann V, Kohlmann A, Zenger M, Schindela S, Eder C, Weissmann S, Schnittger S, Kern W, Müller MC, Hochhaus A, et al. (2011). A deep-sequencing study of chronic myeloid leukemia patients in blast crisis (BC-CML) detects mutations in 76.9% of cases. Leukemia 25, 557–560. [DOI] [PubMed] [Google Scholar]
- Gunnarsson N, Sandin F, Höglund M, Stenke L, Björkholm M, Lambe M, Olsson-Strömberg U, Richter J, and Själander A (2016). Population-based assessment of chronic myeloid leukemia in Sweden: striking increase in survival and prevalence. Eur. J. Haematol 97, 387–392. [DOI] [PubMed] [Google Scholar]
- Hantschel O, Nagar B, Guettler S, Kretzschmar J, Dorey K, Kuriyan J, and Superti-Furga G (2003). A myristoyl/phosphotyrosine switch regulates c-Abl. Cell 112, 845–857. [DOI] [PubMed] [Google Scholar]
- Hantschel O, Warsch W, Eckelhart E, Kaupe I, Grebien F, Wagner K-U, Superti-Furga G, and Sexl V (2012). BCR-ABL uncouples canonical JAK2-STAT5 signaling in chronic myeloid leukemia. Nat. Chem. Biol 8, 285–293. [DOI] [PubMed] [Google Scholar]
- Hazarika M, Jiang X, Liu Q, Lee S-L, Ramchandani R, Garnett C, Orr MS, Sridhara R, Booth B, Leighton JK, et al. (2008). Tasigna for chronic and accelerated phase Philadelphia chromosome-positive chronic myelogenous leukemia resistant to or intolerant of imatinib. Clin. Cancer Res 14, 5325–5331. [DOI] [PubMed] [Google Scholar]
- He Y, Wertheim JA, Xu L, Miller JP, Karnell FG, Choi JK, Ren R, and Pear WS (2002). The coiled-coil domain and Tyr177 of Bcr are required to induce a murine chronic myelogenous leukemia-like disease by Bcr/abl. Blood 99, 2957–2968. [DOI] [PubMed] [Google Scholar]
- Hehlmann R (2012). How I treat CML blast crisis. Blood 120, 737–747. [DOI] [PubMed] [Google Scholar]
- Hehlmann R, Lauseker M, Saußele S, Pfirrmann M, Krause S, Kolb HJ, Neubauer A, Hossfeld DK, Nerl C, Gratwohl A, et al. (2017). Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia 31, 2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, Eisenberg BL, von Mehren M, Fletcher CDM, Sandau K, et al. (2006). Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J. Clin. Oncol 24, 4764–4774. [DOI] [PubMed] [Google Scholar]
- Hemmeryckx B, Reichert A, Watanabe M, Kaartinen V, de Jong R, Pattengale PK, Groffen J, and Heisterkamp N (2002). BCR/ABL P190 transgenic mice develop leukemia in the absence of Crkl. Oncogene 21, 3225–3231. [DOI] [PubMed] [Google Scholar]
- Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim D-W, Issaragrisil S, le Coutre PD, Etienne G, Dorlhiac-Llacer PE, Clark RE, et al. (2016). Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 30, 1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, et al. (2017a). Long-term outcomes of imatinib treatment for chronic myeloid leukemia. New Engl. J. Med 376, 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochhaus A, Masszi T, Giles FJ, Radich JP, Ross DM, Gómez Casares MT, Hellmann A, Stentoft J, Conneally E, García-Gutiérrez V, et al. (2017b). Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia 31, 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz MM, Rowlings PA, and Passweg JR (1996). Allogeneic bone marrow transplantation for CML: a report from the International Bone Marrow Transplant Registry. Bone Marrow Transplant 17 (Suppl 3), S5–S6. [PubMed] [Google Scholar]
- Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, Baccarani M, Cortes J, Cross NCP, Druker BJ, et al. (2006). Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 108, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, Gathmann I, Bolton AE, van Hoomissen IC, Goldman JM, et al. (2003). Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N. Engl. J. Med 349, 1423–1432. [DOI] [PubMed] [Google Scholar]
- Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, Breccia M, Goh Y-T, Talpaz M, Hochhaus A, et al. (2019). Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. New Engl. J. Med 381, 2315–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni SJ, Plunkett W, Kantarjian HM, Estey E, Keating MJ, McCredie KB, and Freireich EJ (1986). High-dose cytosine arabinoside: treatment and cellular pharmacology of chronic myelogenous leukemia blast crisis. J. Clin. Oncol 4, 1079–1088. [DOI] [PubMed] [Google Scholar]
- Ibrahim AR, Eliasson L, Apperley JF, Milojkovic D, Bua M, Szydlo R, Mahon F-X, Kozlowski K, Paliompeis C, Foroni L, et al. (2011). Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood 117, 3733–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa J, Tanaka H, Okada M, Nakamae H, Hino M, Murai K, Ishida Y, Kumagai T, Sato S, Ohashi K, et al. (2015). Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol. 2, e528–e535. [DOI] [PubMed] [Google Scholar]
- Iqbal Z, Aleem A, Iqbal M, Naqvi MI, Gill A, Taj AS, Qayyum A, ur-Rehman N, Khalid AM, Shah IH, et al. (2013). Sensitive detection of pre-existing BCR-ABL kinase domain mutations in CD34+ cells of newly diagnosed chronic-phase chronic myeloid leukemia patients is associated with imatinib resistance: implications in the post-imatinib era. PLoS One 8, e55717. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, Rosenblatt J, Avigan DE, Teruya-Feldstein J, and Pandolfi PP (2008). PML targeting eradicates quiescent leukaemia-initiating cells. Nature 453, 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Fioretos T, and Mitelman F (2002). Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 107, 76–94. [DOI] [PubMed] [Google Scholar]
- Jones D, Thomas D, Yin CC, O’Brien S, Cortes JE, Jabbour E, Breeden M, Giles FJ, Zhao W, and Kantarjian HM (2008). Kinase domain point mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia emerge after therapy with BCR-ABL kinase inhibitors. Cancer 113, 985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs DH, Fisher DS, Spicer J, and Flanagan RJ (2013). Clinical pharmacokinetics of tyrosine kinase inhibitors: implications for therapeutic drug monitoring. Ther. Drug Monit 35, 562–587. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, Niederwieser D, Resta D, Capdeville R, Zoellner U, et al. (2002). Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med 346, 645–652. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, et al. (2006). Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. New Engl. J. Med 354, 2542–2551. [DOI] [PubMed] [Google Scholar]
- Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R, et al. (2010). Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. New Engl. J. Med 362, 2260–2270. [DOI] [PubMed] [Google Scholar]
- Khorashad JS, Milojkovic D, Mehta P, Anand M, Ghorashian S, Reid AG, De Melo V, Babb A, de Lavallade H, Olavarria E, et al. (2008). In vivo kinetics of kinase domain mutations in CML patients treated with dasatinib after failing imatinib. Blood 111, 2378–2381. [DOI] [PubMed] [Google Scholar]
- Khoury HJ, Guilhot F, Hughes TP, Kim D-W, and Cortes JE (2009). Dasatinib treatment for Philadelphia chromosome-positive leukemias: practical considerations. Cancer 115, 1381–1394. [DOI] [PubMed] [Google Scholar]
- Kim T, Tyndel MS, Zhang Z, Ahn J, Choi S, Szardenings M, Lipton JH, Kim H-J, and Kim Dong Hwan D (2017). Exome sequencing reveals DNMT3A and ASXL1 variants associate with progression of chronic myeloid leukemia after tyrosine kinase inhibitor therapy. Leukemia Res 59, 142–148. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, and Halmos B (2009). EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med 352, 786–792. [DOI] [PubMed] [Google Scholar]
- Koptyra M, Falinski R, Nowicki MO, Stoklosa T, Majsterek I, Nieborowska-Skorska M, Blasiak J, and Skorski T (2006). BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood 108, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, and Lowery RG (2017). A high-throughput method for measuring drug residence time using the transcreener ADP assay. SLAS Discov. 22, 915–922. [DOI] [PubMed] [Google Scholar]
- Lai D, Chen M, Su J, Liu X, Rothe K, Hu K, Forrest DL, Eaves CJ, Morin GB, and Jiang X (2018). PP2A inhibition sensitizes cancer stem cells to ABL tyrosine kinase inhibitors in BCR-ABL+ human leukemia. Sci. Transl Med 10, 10.1126/scitranslmed.aan8735. [DOI] [PubMed] [Google Scholar]
- Legros L, Nicolini FE, Etienne G, Rousselot P, Rea D, Giraudier S, Guerci-Bresler A, Huguet F, Gardembas M, Escoffre M, et al. (2017). Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer 123, 4403–4410. [DOI] [PubMed] [Google Scholar]
- Levinson NM, and Boxer SG (2012). Structural and spectroscopic analysis of the kinase inhibitor bosutinib and an isomer of bosutinib binding to the Abl tyrosine kinase domain. PLoS One 7, e29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wang L, Li L, Wang Z, Ho Y, McDonald T, Holyoake TL, Chen W, and Bhatia R (2012). Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell 21, 266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Lustgarten S, Trede NS, Rivera VM, Clackson T, Haluska FG, et al. (2014). EPIC: a phase III trial of ponatinib (PON) versus imatinib (IM) in patients (pts) with newly diagnosed CP-CML. J. Clin. Oncol 32, 7023. [Google Scholar]
- Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Assouline S, Etienne G, Nicolini FE, Coutre P.le, Clark RE, et al. (2016). Ponatinib versus imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol 17, 612–621. [DOI] [PubMed] [Google Scholar]
- Lugo T, Pendergast A, Muller A, and Witte. (1990). Tyrosine kinase activity and transformation potency of Bcr-Abl oncogene products. Science 247, 1079–1082. [DOI] [PubMed] [Google Scholar]
- Mahon F-X, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, et al. (2010). Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 11, 1029–1035. [DOI] [PubMed] [Google Scholar]
- Mahon F-X, Boquimpani C, Kim D-W, Benyamini N, Clementino NCD, Shuvaev V, Ailawadhi S, Lipton JH, Turkina AG, De Paz R, et al. (2018). Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single-group, phase 2, open-label study. Ann. Intern. Med 168, 461–470. [DOI] [PubMed] [Google Scholar]
- Maiti A, Kantarjian HM, Patel K, Borthakur G, Ravandi F, Verstovsek S, Ferrajoli A, Estrov Z, Kadia TM, Skinner JA, et al. (2016). Long term follow-up of frontline dasatinib in patients (pts) with early chronic phase chronic myeloid leukemia (CML-CP). J. Clin. Oncol 34, e18542. [Google Scholar]
- Marin D, Milojkovic D, Olavarria E, Khorashad JS, de Lavallade H, Reid AG, Foroni L, Rezvani K, Bua M, Dazzi F, et al. (2008). European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood 112, 4437–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin D, Bazeos A, Mahon F-X, Eliasson L, Milojkovic D, Bua M, Apperley JF, Szydlo R, Desai R, Kozlowski K, et al. (2010). Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J. Clin. Oncol 28, 2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro F, Molica M, and Breccia M (2018). Ponatinib: a review of efficacy and safety. Curr. Cancer Drug Targets 18, 847–856. [DOI] [PubMed] [Google Scholar]
- Million RP, and Van Etten RA (2000). The Grb2 binding site is required for the induction of chronic myeloid leukemia-like disease in mice by the Bcr/Abl tyrosine kinase. Blood 96, 664–670. [PubMed] [Google Scholar]
- Moorman AV, Chilton L, Wilkinson J, Ensor HM, Bown N, and Proctor SJ (2010). A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood 115, 206–214. [DOI] [PubMed] [Google Scholar]
- Nagar B, Bornmann WG, Pellicena P, Schindler T, Veach DR, Miller WT, Clarkson B, and Kuriyan J (2002). Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571). Cancer Res 62, 4236–4243. [PubMed] [Google Scholar]
- Nowell P, and Hungerford D (1960). A minute chromosome in human chronic granulocytic leukemia. Science 142, 1497. [DOI] [PubMed] [Google Scholar]
- O’Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, Adrian LT, Zhou T, Huang W-S, Xu Q, et al. (2009). AP24534, a panBCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 16, 401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare T, Eide CA, Agarwal A, Adrian LT, Zabriskie MS, MacKenzie RJ, LaTocha DH, Johnson KJ, You H, Luo J, et al. (2013). Threshold levels of ABL tyrosine kinase inhibitors retained in chronic myeloid leukemia cells determine their commitment to apoptosis. Cancer Res 73, 3356–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottmann OG, Druker BJ, Sawyers CL, Goldman JM, Reiffers J, Silver RT, Tura S, Fischer T, Deininger MW, Schiffer CA, et al. (2002). A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood 100, 1965–1971. [DOI] [PubMed] [Google Scholar]
- Packer LM, Rana S, Hayward R, O’Hare T, Eide CA, Rebocho A, Heidorn S, Zabriskie MS, Niculescu-Duvaz I, Druker BJ, et al. (2011). Nilotinib and MEK inhibitors induce synthetic lethality through paradoxical activation of RAF in drug-resistant chronic myeloid leukemia. Cancer Cell 20, 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pane F, Frigeri F, Sindona M, Luciano L, Ferrara F, Cimino R, Meloni G, Saglio G, Salvatore F, and Rotoli B (1996). Neutrophilic-chronic myeloid leukemia: a distinct disease with a specific molecular marker (BCR/ABL with C3/A2 junction). Blood 88, 2410–2414. [PubMed] [Google Scholar]
- Pendergast AM, Quilliam LA, Cripe LD, Bassing CH, Dai Z, Li N, Batzer A, Rabun KM, Der CJ, Schlessinger J, et al. (1993). BCR-ABLinduced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell 75, 175–185. [PubMed] [Google Scholar]
- Peng B, Hayes M, Resta D, Racine-Poon A, Druker BJ, Talpaz M, Sawyers CL, Rosamilia M, Ford J, Lloyd P, et al. (2004). Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J. Clin. Oncol 22, 935–942. [DOI] [PubMed] [Google Scholar]
- Porkka K, Khoury HJ, Paquette RL, Matloub Y, Sinha R, and Cortes JE (2010). Dasatinib 100 mg once daily minimizes the occurrence of pleural effusion in patients with chronic myeloid leukemia in chronic phase and efficacy is unaffected in patients who develop pleural effusion. Cancer 116, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea D, Nicolini FE, Tulliez M, Guilhot F, Guilhot J, Guerci-Bresler A, Gardembas M, Coiteux V, Guillerm G, Legros L, et al. (2017). Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood 129, 846–854. [DOI] [PubMed] [Google Scholar]
- Reckel S, Hamelin R, Georgeon S, Armand F, Jolliet Q, Chiappe D, Moniatte M, and Hantschel O (2017). Differential signaling networks of Bcr-Abl p210 and p190 kinases in leukemia cells defined by functional proteomics. Leukemia 31, 1502–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redaelli S, Piazza R, Rostagno R, Magistroni V, Perini P, Marega M, Gambacorti-Passerini C, and Boschelli F (2009). Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J. Clin. Oncol 27, 469–471. [DOI] [PubMed] [Google Scholar]
- Rosenthal S, Canellos GP, Whang-Peng J, and Gralnick HR (1977). Blast crisis of chronic granulocytic leukemia: morphologic variants and therapeutic implications. Am. J. Med 63, 542–547. [DOI] [PubMed] [Google Scholar]
- Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, Dang P, Goyne JM, Slader C, Filshie RJ, et al. (2013). Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood 122, 515–522. [DOI] [PubMed] [Google Scholar]
- Rossari F, Minutolo F, and Orciuolo E (2018). Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J. Hematol. Oncol 11, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, Blanchet O, Marit G, Gluckman E, Reiffers J, et al. (2007). Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood 109, 58–60. [DOI] [PubMed] [Google Scholar]
- Rowley JD (1973). A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 243, 290–293. [DOI] [PubMed] [Google Scholar]
- Saglio G, Kim D-W, Issaragrisil S, le Coutre P, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A, Hughes TP, et al. (2010). Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. New Engl. J. Med 362, 2251–2259. [DOI] [PubMed] [Google Scholar]
- Samanta AK, Lin H, Sun T, Kantarjian H, and Arlinghaus RB (2006). Janus kinase 2: a critical target in chronic myelogenous leukemia. Cancer Res 66, 6468–6472. [DOI] [PubMed] [Google Scholar]
- Sattler M, Mohi MG, Pride YB, Quinnan LR, Malouf NA, Podar K, Gesbert F, Iwasaki H, Li S, Van Etten RA, et al. (2002). Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell 1, 479–492. [DOI] [PubMed] [Google Scholar]
- Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, Janssen JJWM, Mayer J, Koskenvesa P, Panayiotidis P, et al. (2018). Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 19, 747–757. [DOI] [PubMed] [Google Scholar]
- Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, Schiffer CA, Talpaz M, Guilhot F, Deininger MWN, et al. (2002). Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Presented in part at the 43rd Annual Meeting of the American Society of Hematology, Orlando, FL, December 11, 2001. Blood 99, 3530–3539. [DOI] [PubMed] [Google Scholar]
- Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, and Kuriyan J (2000). Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science 289, 1938–1942. [DOI] [PubMed] [Google Scholar]
- Schmidt T, Masouleh BK, Loges S, Cauwenberghs S, Fraisl P, Maes C, Jonckx B, De Keersmaecker K, Kleppe M, Tjwa M, et al. (2011). Loss or inhibition of stromal-derived PlGF prolongs survival of mice with imatinib-resistant Bcr-Abl1+ leukemia. Cancer Cell 19, 740–753. [DOI] [PubMed] [Google Scholar]
- Schmitt MW, Pritchard JR, Leighow SM, Aminov BI, Beppu L, Kim DS, Hodgson JG, Rivera VM, Loeb LA, and Radich JP (2018). Single-molecule sequencing reveals patterns of preexisting drug resistance that suggest treatment strategies in Philadelphia-positive leukemias. Clin. Cancer Res 24, 5321–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz KR, Carroll A, Heerema NA, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M, Zheng HW, Davies SM, et al. (2014). Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: children’s Oncology Group study AALL0031. Leukemia 28, 1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MT, Korfi K, Saffrey P, Hopcroft LEM, Kinstrie R, Pellicano F, Guenther C, Gallipoli P, Cruz M, Dunn K, et al. (2016). Epigenetic reprogramming sensitizes CML stem cells to combined EZH2 and tyrosine kinase inhibition. Cancer Discov 6, 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, and Milo R (2016). Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14, e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senechal K, Halpern J, and Sawyers CL (1996). The CRKL adaptor protein transforms fibroblasts and functions in transformation by the BCR-ABL oncogene. J. Biol. Chem 271, 23255–23261. [DOI] [PubMed] [Google Scholar]
- Seo J-H, Wood LJ, Agarwal A, O’Hare T, Elsea CR, Griswold IJ, Deininger MWN, Imamoto A, and Druker BJ (2010). A specific need for CRKL in p210BCR-ABL-induced transformation of mouse hematopoietic progenitors. Cancer Res 70, 7325–7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NP, Tran C, Lee FY, Chen P, Norris D, and Sawyers CL (2004). Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305, 399–401. [DOI] [PubMed] [Google Scholar]
- Shah NP, Skaggs BJ, Branford S, Hughes TP, Nicoll JM, Paquette RL, and Sawyers CL (2007). Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. J. Clin. Invest 117, 2562–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NP, Kantarjian HM, Kim D-W, Réa D, Dorlhiac-Llacer PE, Milone JH, Vela-Ojeda J, Silver RT, Khoury HJ, Charbonnier A, et al. (2008a). Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J. Clin. Oncol 26, 3204–3212. [DOI] [PubMed] [Google Scholar]
- Shah NP, Kasap C, Weier C, Balbas M, Nicoll JM, Bleickardt E, Nicaise C, and Sawyers CL (2008b). Transient potent BCR-ABL inhibition is sufficient to commit chronic myeloid leukemia cells irreversibly to apoptosis. Cancer Cell 14, 485–493. [DOI] [PubMed] [Google Scholar]
- Smith CC, Wang Q, Chin C-S, Salerno S, Damon LE, Levis MJ, Perl AE, Travers KJ, Wang S, Hunt JP, et al. (2012). Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature 485, 260–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead JL, O’Hare T, Adrian LT, Eide CA, Lange T, Druker BJ, and Deininger MW (2009). Acute dasatinib exposure commits Bcr-Abl-dependent cells to apoptosis. Blood 114, 3459–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soverini S, Bavaro L, De Benedittis C, Martelli M, Iurlo A, Orofino N, Sica S, Sorà F, Lunghi F, Ciceri F, et al. (2020). Prospective assessment of NGS-detectable mutations in CML patients with nonoptimal response: the NEXT-in-CML study. Blood 135, 534–541. [DOI] [PubMed] [Google Scholar]
- Stagno F, Vigneri P, Del Fabro V, Stella S, Massimino M, Berretta S, Messina A, and Di Raimondo F (2009). Imatinib dose escalation to achieve molecular responses in patients with chronic myeloid leukemia in late chronic phase. Leuk. Res 33, e17. [DOI] [PubMed] [Google Scholar]
- Strom SS, Yamamura Y, Kantarijian HM, and Cortes-Franco JE (2009). Obesity, weight gain, and risk of chronic myeloid leukemia. Cancer Epidemiol. Biomarkers Prev 18, 1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Tauchi T, Kitamura K, Miyamura K, Saburi Y, Hatta Y, Miyata Y, Kobayashi S, Usuki K, Matsumura I, et al. (2018). Deeper molecular response is a predictive factor for treatment-free remission after imatinib discontinuation in patients with chronic phase chronic myeloid leukemia: the JALSG-STIM213 study. Int. J. Hematol 107, 185–193. [DOI] [PubMed] [Google Scholar]
- Tokarski JS, Newitt JA, Chang CYJ, Cheng JD, Wittekind M, Kiefer SE, Kish K, Lee FYF, Borzillerri R, Lombardo LJ, et al. (2006). The structure of Dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res 66, 5790–5797. [DOI] [PubMed] [Google Scholar]
- Traer E, MacKenzie R, Snead J, Agarwal A, Eiring AM, O’Hare T, Druker BJ, and Deininger MW (2012). Blockade of JAK2-mediated extrinsic survival signals restores sensitivity of CML cells to ABL inhibitors. Leukemia 26, 1140–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traer E, Javidi-Sharifi N, Agarwal A, Dunlap J, English I, Martinez J, Tyner JW, Wong M, and Druker BJ (2014). Ponatinib overcomes FGF2-mediated resistance in CML patients without kinase domain mutations. Blood 123, 1516–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shen Z-X, Saglio G, Jin J, Huang H, Hu Y, Du X, Li J, Meng F, Zhu H, et al. (2015). Phase 3 study of nilotinib vs imatinib in Chinese patients with newly diagnosed chronic myeloid leukemia in chronic phase: ENESTchina. Blood 125, 2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cai D, Brendel C, Barett C, Erben P, Manley PW, Hochhaus A, Neubauer A, and Burchert A (2007). Adaptive secretion of granulocytemacrophage colony-stimulating factor (GM-CSF) mediates imatinib and nilotinib resistance in BCR/ABL+ progenitors via JAK-2/STAT-5 pathway activation. Blood 109, 2147–2155. [DOI] [PubMed] [Google Scholar]
- Warsch W, Kollmann K, Eckelhart E, Fajmann S, Cerny-Reiterer S, Hölbl A, Gleixner KV, Dworzak M, Mayerhofer M, Hoermann G, et al. (2011). High STAT5 levels mediate imatinib resistance and indicate disease progression in chronic myeloid leukemia. Blood 117, 3409–3420. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Manley PW, Breitenstein W, Brüggen J, Cowan-Jacob SW, Ray A, Huntly B, Fabbro D, Fendrich G, Hall-Meyers E, et al. (2005). Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 7, 129–141. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Wright RD, McMillin DW, Mitsiades C, Ray A, Barrett R, Adamia S, Stone R, Galinsky I, Kung AL, et al. (2008). Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Mol. Cancer Ther 7, 1121–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen-Seegers N, Uitdehaag JCM, Prinsen MBW, de Vetter JRF, de Man J, Sawa M, Kawase Y, Buijsman RC, and Zaman GJR (2017). Compound selectivity and target residence time of kinase inhibitors studied with surface plasmon resonance. J. Mol. Biol 429, 574–586. [DOI] [PubMed] [Google Scholar]
- Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, Marzinzik AL, Pelle X, Donovan J, Zhu W, et al. (2017). The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature 543, 733–737. [DOI] [PubMed] [Google Scholar]
- Xie S, Wang Y, Liu J, Sun T, Wilson MB, Smithgall TE, and Arlinghaus RB (2001). Involvement of Jak2 tyrosine phosphorylation in Bcr-Abl transformation. Oncogene 20, 6188–6195. [DOI] [PubMed] [Google Scholar]
- Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA, Estrada J, Nicolini FE, Khoury HJ, Larson RA, Konopleva M, Cortes JE, et al. (2014). BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell 26, 428–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al. (2009). Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 458, 776–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Mak PY, Mu H, Mak DH, Zeng Z, Cortes J, Liu Q, Andreeff M, and Carter BZ (2017). Combined inhibition of b-catenin and Bcr-Abl synergistically targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo. Leukemia 31, 2065–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou LL, Zhao Y, Ringrose A, DeGeer D, Kennah E, Lin AE-J, Sheng G, Li X-J, Turhan A, and Jiang X (2008). AHI-1 interacts with BCR-ABL and modulates BCR-ABL transforming activity and imatinib response of CML stem/progenitor cells. J. Exp. Med 205, 2657. [DOI] [PMC free article] [PubMed] [Google Scholar]