Abstract
Alcohol use disorder (AUD) is characterized by dysfunction in motivational, mood‐stress regulation, and sleep systems that interact in complex ways to heighten the risk of relapse during abstinence. Emerging data suggest that excessive and chronic alcohol use disrupts sleep homeostasis and, in abstinence, subjects with AUD are known to experience insomnia that may persist for weeks to years, which we propose to refer to as insomnia associated with alcohol cessation (IAAC). The purpose of this review is to provide an update of pharmacological approaches to therapy including compounds in development, to raise awareness of the prevalence of and unmet need in IAAC and highlight differences in treatment consideration for IAAC as compared to insomnia disorder. We performed a search of select electronic databases to identify studies of pharmacological agents used to treat sleep disturbances in abstinent or treatment‐seeking patients with alcohol use disorder. The search, conducted in June 2019 and updated in December 2019, yielded 1,188 abstracts after duplicates were removed, of which 36 full‐text articles were assessed for eligibility. Eighteen studies were included, 15 randomized controlled trials and three open‐label studies. Several classes of medications including antidepressants, anticonvulsants, and antipsychotics have been evaluated for their effectiveness in treating sleep disturbances in abstinent or treatment‐seeking patients with AUD. None of these medications are approved by the FDA for the treatment of IAAC, and the currently available evidence for these agents is limited. Randomized, controlled clinical trials are warranted to evaluate the efficacy and safety of medications in the treatment of IAAC.
Keywords: alcohol cessation, alcohol use disorder, alcohol withdrawal, Insomnia, pharmacological treatment, treatment
Emerging data suggest that excessive and chronic alcohol use disrupts sleep homeostasis and, in abstinence, subjects with alcohol use disorder (AUD) are known to experience insomnia that may persist for weeks to years, which we propose to refer to as insomnia associated with alcohol cessation (IAAC). The purpose of this review is to provide an update of pharmacological approaches to therapy including compounds in development, to raise awareness of the prevalence of and unmet need in IAAC and highlight differences in treatment consideration for IAAC as compared to insomnia disorder. Several classes of medications including antidepressants, anticonvulsants, and antipsychotics have been evaluated for their effectiveness in treating sleep disturbances in abstinent or treatment‐seeking patients with AUD yet none of these medications are approved by the FDA for the treatment of IAAC and the currently available evidence for these agents is limited.
1. INTRODUCTION
The acute and chronic use of alcohol has been shown to cause disturbances in sleep patterns in humans. In patients with alcohol use disorder (AUD) in recovery, insomnia is characterized using polysomnography (PSG) as increased sleep‐onset latency, sleep fragmentation, rapid eye movement (REM) sleep percentage, and reductions in sleep efficiency, the percentage of slow‐wave sleep (SWS), and REM latency. 1 , 2 Insomnia commonly occurs in the acute withdrawal phase (1 to 2 weeks) and early recovery phase (2 to 8 weeks after detoxification). In the acute withdrawal phase, insomnia symptoms are variable and can improve over the detoxification period, 3 yet may continue into the sustained recovery phase (3 or more months after the detoxification phase). During the sustained recovery phase, sleep‐related disturbances can continue for up to 3 years. 4 , 5 , 6
From a clinical and pathophysiological perspective, it is currently controversial if insomnia in patients with AUD is distinct from insomnia disorder. This point is highlighted in the differing criteria within the two major insomnia nosology systems. The Diagnostic and Statistical Manual of Mental Disorders, 5th edition, separates substance/medication‐induced sleep disorder (including that from alcohol) from insomnia disorder, the former being characterized as a prominent and severe disturbance in sleep causing clinically significant distress or impairment that developed during or soon after the substance intoxication or after withdrawal from exposure. 7 The International Classification of Sleep Disorders, 3rd edition, in a departure from its previous version, no longer promotes the concept that insomnia arises as a secondary form of sleep disturbance related to an underlying primary disorder such as substance use disorder, providing a single diagnosis of chronic insomnia disorder for all patients who have persistent and frequent insomnia. 8 However, when evaluating treatment options, and in particular pharmacological options, there is a clear need for differing considerations for patients with AUD in recovery with insomnia versus the general insomnia population. In patients with AUD in recovery, reducing the risk of alcohol relapse and concerns of abuse and addiction are of the utmost importance when evaluating treatment for insomnia. Therefore, we propose to refer to this disorder as insomnia associated with alcohol cessation (IAAC).
1.1. Prevalence of IAAC
Sleep complaints are common among patients with AUD during both acute and sustained abstinence. 5 During the acute withdrawal phase, estimates of sleep disturbances based on surveys of treatment‐seeking alcohol‐dependent patients ranged from 57% to 92%. 9 , 10 , 11 Additionally, Brower and Perron, 2010 12 analyzed data from the 2001 to 2002 National Epidemiologic Survey on Alcohol and Related Conditions (N = 43 093 adults) and determined in participants with a lifetime diagnosis of alcohol dependence and acute alcohol withdrawal, the prevalence of self‐reported acute alcohol withdrawal‐related insomnia was 32%, and acute alcohol withdrawal‐related sleep disturbance was 50% in this population. 12
Sleep‐related disturbances including insomnia may improve as detoxification progresses yet remain high during recovery and have been demonstrated to persist for up to 3 years. 6 The prevalence of self‐reported insomnia symptoms in a sample of 164 patients undergoing alcohol detoxification in an inpatient clinical research setting was 90% upon admission (Day 2) and 51% by Day 28. 13 Additionally, in a prospective analysis of the Pittsburgh Sleep Quality Index (PSQI) administered to 119 alcohol‐dependent patients who met the inclusion criteria in a 1‐month residential treatment program, 69% of patients reported sleep disturbances upon admission, and 49% reported sleep disturbances upon discharge. 14 Furthermore, in a naturalistic, longitudinal outcomes study, alcohol‐dependent patients were interviewed using the Sleep Problems Questionnaire, Time‐Line Follow‐Back Interview, and Brief Symptom Inventory. The interview results showed that 47% (n = 125) of 267 patients reported insomnia at baseline. Among all patients followed in the study, 62% (64/103) of those who had insomnia at baseline continued to have insomnia at 6 months. 15 In a recent study of 77 patients with alcohol dependence electively admitted to a hospital for detoxification, 70.1% had PSQI scores greater than five at admission which fell to 59.7% at discharge. 3 From the above analyses, it is clear that the incidence of insomnia associated with alcohol cessation both during and after detoxification is high.
1.2. Effects of alcohol on sleep: progression to IAAC
Acutely, alcohol has several effects related to neurotransmitters that are involved in the control of normal sleep, including an increase in mesolimbic dopaminergic activity, alteration of serotonin receptor function, the facilitation of gamma‐aminobutyric acid (GABA) inhibition, the inhibition of glutamate excitation, an increase in endogenous opioid synthesis and release, and the promotion of adenosine signaling 16 , 17 ; with chronic intake, these effects become more exaggerated. As a result, the control of sleep and wake is altered in AUD.
Patients with AUD who are actively drinking commonly have an inability to initiate and maintain sleep and a disruption in the organization of sleep stages. These patients have increases in sleep‐onset latency, REM sleep latency, and SWS, as well as a reduction and fragmentation in total sleep time and REM sleep suppression. 1 , 2 , 17 , 18
With alcohol cessation in patients with AUD, a full complement of sleep dysfunction may emerge, including REM and non‐REM (NREM) deficiencies, which are associated with an increased risk of alcohol relapse, and disruptions in sleep homeostasis, which, in preclinical sleep deprivation studies, have been associated with impairment of adenosine regulatory mechanisms. 1 , 19 , 20 , 21 Patients with AUD in the early recovery phase often experience increased sleep fragmentation, increased awakenings, especially in the second half of the night, frequent sleep‐stage transitions, and body movements. 4 , 17 Compared with healthy controls, patients in early recovery have shown polysomnogram abnormalities, including reduced sleep efficiency, total sleep time, and SWS. Patients in early recovery also show shortened NREM to REM cycles, an increase in sleep‐onset latency, an increase in REM sleep percentage, and reduced REM latency. 1 , 2 , 22 An increase in sleep‐onset latency, a reduction in the percentage of SWS and REM latency, and a reduction in sleep efficiency are all predictors of alcohol relapse. 20
1.3. Underlying neurobiology of IAAC
Many sleep abnormalities including insomnia may improve with time for many patients after continued abstinence from alcohol. 23 However, chronic alcohol use in some patients may cause permanent neurologic and functional alterations to the sleep centers of the brain, leading to persistent symptoms that require independent treatment. 4 , 5 Researchers have developed several theories based on available data to explain the neurobiological mechanisms underlying the persistent sleep‐wake disturbances experienced by patients with AUD, even after months to years of abstinence from alcohol. 1 , 24 Using studies conducted in animals, Thakkar et al 21 theorized that adenosine and the wake‐promoting cholinergic neurons of the basal forebrain are important mediators of sleep homeostasis. Based on the results in preclinical studies, these researchers suggested that alcohol alters sleep homeostasis by down‐regulation of the adenosine system and dysregulation of the cholinergic neurons in the basal forebrain. 21
As yet, no studies have directly compared and contrasted the neuropathophysiology of insomnia disorder with that of IAAC. It is, therefore, controversial as to whether the neurobiological underpinnings of insomnia disorder and IAAC differ. It is also currently unclear why, in some individuals, IAAC resolves over time with continued abstinence while it persists in others. Furthermore, pre‐existing insomnia prior to alcohol abuse further complicates the separation of these populations for study.
The sensitivity and responsiveness of the sleep homeostat can be assessed by challenges such as assessing response to auditory stimulation during sleep or recovery after sleep deprivation. Subjects with AUD relative to age‐matched controls show a lower incidence and amplitude of evoked EEG K‐complexes to tones presented during sleep. 25 In the Michigan Longitudinal Study, response to reduced sleep time, produced by a three‐hour phase delay, was compared between healthy controls and abstinent AUD patients. In the control group, after recovery from reduced sleep, controls had enhanced slow‐wave sleep and slow‐wave activity (rebound). However, rebound was not present in abstinent AUD patients; that is, they do not recover. 26 , 27 , 28 Further research in this area is needed to advance understanding of the interaction between alcohol use disorder, abstinence, and insomnia.
1.4. Correlations with relapse
Many researchers have concluded that untreated sleep disturbances can contribute to the risk of alcohol relapse after a period of abstinence and that disturbed sleep is an important predictor of relapse. 4 , 29 Subjective and objective indicators of sleep disturbances that have been evaluated in alcohol‐dependent patients after the acute abstinence period, such as difficulty in falling asleep, decreased total sleep time and decreased sleep efficiency, predict the likelihood of relapse during longer periods of abstinence. 1 , 4 , 21 , 30 , 31
In an early polysomnography study, 32 found 10 of 28 subjects with AUD had relapsed at three months of abstinence. Those relapsing had reduced REM latency, increased REM %, and in a replication cohort, the 6 of 17 who relapsed had a shortened REM latency and greater REM %. 20 extended this work showing that persistently abnormal recordings of eye movement density in REM sleep and REM latency in primary alcohol‐dependent inpatients at 14 months were associated with alcohol relapse. Other relapse predictors in this study were an increase in sleep‐onset latency, a reduction in the percentage of SWS, and a reduction in sleep efficiency. Similarly, 33 found 36 of 74 AUD patients relapsed at five months and that individuals who relapsed showed an increased sleep latency, reduced REM latency, and reduced NREM stage 4%. Researchers have also determined that persistent insomnia and sleep fragmentation after five months of abstinence predicted relapse over 12‐14 months of sustained abstinence. 2 , 20 One study also suggests subjective impressions of sleep, sleep‐onset latency, and wakefulness after sleep onset may be better predictors of relapse than PSG measures. 34 Lastly, a recent study established a link between craving in treatment‐seeking patients with alcohol dependence and insomnia as measured using the Short Sleep Index. 31 Although insomnia and sleep‐stage disturbance have been associated with craving and risk of relapse in alcohol‐dependent patients, treatment of sleep disturbances for the prevention of relapse has not been adequately studied 31 , 35 and would be helpful in definitively establishing causality.
1.5. Treatment of IAAC
Insomnia is an easily recognizable symptom in patients with alcohol use disorder in recovery, and if addressed, may improve treatment outcomes for this patient population. 2 , 4 Comprehensive management of insomnia in this population involves a wide array of treatments and interventions to reduce symptoms and increase functionality.
Nonpharmacological interventions used to treat IAAC in patients with AUD include sleep‐hygiene education, cognitive‐behavioral therapy (CBT) for insomnia, relaxation therapy, and bright‐light therapy. 36 While a thorough review of these treatment modalities is outside the scope of the current review, these options are an important component of the armamentarium of the healthcare provider and demand mention. Three controlled studies 37 , 38 , 39 and one uncontrolled study 40 evaluated the efficacy of CBT in treating insomnia in abstinent patients with AUD. One controlled study evaluated the efficacy of progressive muscle relaxation, 41 and one uncontrolled study evaluated bright‐light therapy 42 in treating insomnia in abstinent patients with AUD. All these interventions demonstrated efficacy in reducing self‐reported symptoms of insomnia. However, studies that utilize PSG as a primary outcome are needed. Additionally, it is currently unclear if nonpharmacological treatments improve drinking outcomes such as relapse which highlights the need for further randomized controlled studies. 36 , 38 , 43
While physicians may be reluctant to utilize medication in this group, there may be legitimate reasons to do so. In concert with nonpharmacological approaches, many patients with IAAC may benefit from consideration of pharmacological interventions, especially if sleep disturbances are accompanied by daytime dysfunction or psychological distress and persist beyond four weeks of alcohol cessation. 4 , 36 In a 2000 national survey of addiction‐medicine physicians, only 22% of physicians (n = 311) offered pharmacological treatment to more than half of patients in early recovery from AUD who had sleep problems. 44 This highlights a key difference in treatment considerations from insomnia disorder, where pharmacological treatments are more prevalent.
Specific treatment goals for IAAC have not been thoroughly described in the published literature or clinical practice guidelines. Additionally, clinical practice guidelines regarding the treatment of IAAC are insufficient. However, the treatment goals for IAAC, in general, are to improve sleep, increase the ability to function during the day, and reduce the risk of alcohol relapse. 35 This article aims to review current and potential future pharmacological treatment options in patients with IAAC. Therefore, we identified and reviewed studies that evaluated the efficacy of pharmacological agents in the treatment of sleep disturbances in patients with alcohol use disorder who were abstaining from alcohol or were treatment‐seeking.
2. METHODS
We performed a search of the published literature in MEDLINE (Ovid and PubMed), EMBASE, and PsycINFO through June of 2019 by searching abstracts and titles using the following terms: alcoholism, “alcohol depen*,” “alcohol withdrawal,” or “alcohol use disorder” in conjunction with insomnia or “sleep*.” The literature search was updated in December 2019 which did not result in the inclusion of any additional studies.
2.1. Eligibility criteria and study selection
Studies that prospectively evaluated drug effects on insomnia‐related sleep parameters and sampled subjects with alcohol use disorder who were treatment‐seeking or abstinent at study entry were included. Review articles and meta‐analyses were excluded yet reviewed to ensure all eligible published literature was captured; case reports were also excluded. We restricted results to those written in the English language and original, full‐text published studies from the year 1990 and after. All resulting titles and abstracts were reviewed, and the full text was reviewed for any publication that was potentially relevant.
Additionally, we attempted to identify novel molecules in development that may be useful in the treatment of insomnia associated with alcohol cessation. We searched four databases, Cortellis for Competitive Intelligence (Clarivate Analytics), Pharmaprojects (Informa PLC), GlobalData (GlobalData PLC), and ClinicalTrials.gov through July of 2019 to identify any drugs in development targeting insomnia in the United States. We believe these four databases, taken together, provide a comprehensive record of drug products in development.
3. RESULTS
The search of the published literature yielded 1,188 abstracts after duplicates were removed (Figure 1). Of these, 997 were excluded as they did not evaluate a pharmacological agent. Of the remaining 190 abstracts screened, 154 did not meet inclusion criteria and 36 full‐text articles were assessed for eligibility. Of these, 15 did not assess insomnia or sleep parameters and in three articles, the subjects were not treatment‐seeking or abstinent at study entry. Eighteen studies were included, 15 randomized controlled trials and three open‐label studies.
FIGURE 1.
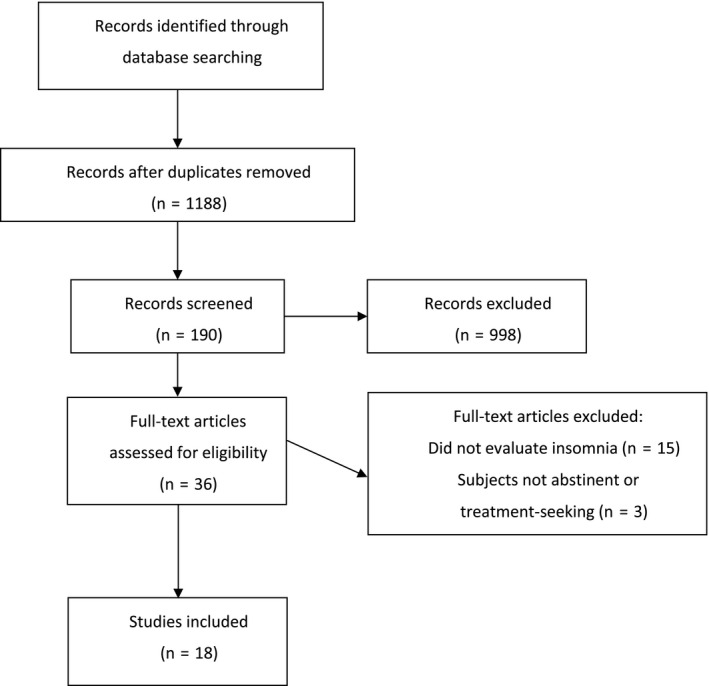
Flow diagram of literature search
Several classes of medications have been evaluated for their effectiveness in treating sleep disturbances in abstinent or treatment‐seeking patients with AUD (eg, antidepressants, anticonvulsants, melatonin agonists, and antipsychotics) and are described here and summarized in Table 1.
TABLE 1.
Studies that evaluated the effect of pharmacological treatment on sleep outcomes in alcohol use cessation or treatment
| Citation | Medication | RCT | n | Daily dose | Duration of treatment | Sleep outcome measured | Results | Effect on alcohol use |
|---|---|---|---|---|---|---|---|---|
| Antidepressants | ||||||||
| Le Bon (2003) | Trazodone | Yes | 18 | 50‐200 mg | 4 wk | PSG | Sleep efficiency increase when calculated after sleep onset | NA |
| Friedmann (2008) | Trazodone | Yes | 173 | 50‐150 mg | 12 wk | PSQI | Improved sleep quality during administration (mean change from baseline −3.02; 95% CI −3.38 to −2.67) | Reduction in abstinent days compared to placebo |
| Anticonvulsants | ||||||||
| Malcolm (2002) | Carbamazepine/ lorazepam | Yes | 136 | 200‐800 mg/2‐8 mg | 5 days | Sleep Quality VAS | Significant effect of drug group on sleep quality that favored carbamazepine (adjusted mean 62.1 vs 51.2; P = .0186) | NA |
| Malcolm (2007) | Gabapentin/ Lorazepam | Yes | 75 | 400‐1200/4‐6 mg | 4 days | BDI and ESS | Those with multiple previous withdrawals reported reduced sleep disturbances and sleepiness in gabapentin compared to lorazepam group | No difference |
| Brower (2008) | Gabapentin | Yes | 21 | 1500 mg | 6 wk | SPQ and PSG | No effect | Significantly delayed the onset to heavy drinking |
| Trevisan (2008) | Gabapentin | Yes | 57 | 1200 mg | 4 wk | PSQI | No effect | No effect |
| Anton (2009) | Gabapentin/flumazenil (IV) | Yes | 60 | Up to 1200 mg/2mg | 39 days | ISI and ESS |
Decreased insomnia symptoms in those with low withdrawal symptoms on gabapentin vs placebo (P = .015) Lower daytime sleepiness in gabapentin group vs placebo (P < .004) |
Decreased alcohol use in patients with high withdrawal symptoms |
| Anton (2011) | Gabapentin/Naltrexone | Yes | 146 | 1200 mg/50 mg | 6 wk | ISI | Gabapentin/Naltrexone group reported better sleep compared to placebo or naltrexone‐only group (P = .02 and P = .03, respectively) | Gabapentin/ Naltrexone group had improvements in drinking outcomes over naltrexone alone |
| Mason (2014) | Gabapentin | Yes | 150 | 900 or 1800 mg | 12 wk | PSQI | Significant improvement in sleep compared to placebo (P < .001) | Significantly improved rates of abstinence and no heavy drinking compared to placebo |
| Karam‐Hage (2003) | Gabapentin/ Trazodone | No | 50 | 300‐1800 mg/50‐300 mg | Up to 6 wk | SPQ | Both groups improved significantly, however, gabapentin group improved more than the trazodone group from baseline (8.8 vs 6.1, P = .023) | NA |
| Melatonin Agonists | ||||||||
| Brower (2011a) | Ramelteon | No | 5 | 8 mg | 4 wk | ISI | Significant improvement insomnia score from baseline (mean 17.6 to 9.8; Cohen's d = 1.31) | NA |
| Grosshans (2014) | Agomelatine | No | 9 | 25‐50 mg | 6 wk | PSQI | Significant improvement in insomnia score from baseline (mean 13.1 to 7.8, P < .01) | NA |
| Antipsychotics | ||||||||
| Litten (2012) | Quetiapine XR | Yes | 218 | Up to 400 mg | 12 wk | PSQI | Significant improvement in sleep compared to placebo (P = .009) | No effect |
| Chakravorty (2014) | Quetiapine XR | Yes | 20 | Up to 400 mg | 8 wk | PSG | No effect on sleep efficiency yet a reduction in wake after sleep‐onset time from baseline (P = .03) | NA |
| Other Agents | ||||||||
| Staner (2006) | Acamprosate | Yes | 24 | 1998 mg | 3 wk | PSG | Decreased wake time after sleep onset and increased stage 3 and REM sleep latency compared to placebo (P < .05) | NA |
| Perney (2012) | Acamprosate | Yes | 592 | 2000 or 3000 mg | 24 wk | SSI | Percentage of mean change over baseline was significantly improved with compared with placebo (P = .001) | NA |
| Irwin (2009) | Etanercept | Yes | 18 | 25 mg | Single dose, crossover | PSG | Significant decrease in amount and percentage of REM sleep compared to placebo | NA |
| Petrakis (2016) | Prazosin | Yes | 96 | 16 mg | 13 wk | PSQI | No effect | No effect |
Abbreviations: BDI, Beck Depression Inventory, Item 16; ESS, Epworth Sleepiness Scale; ISI, Insomnia Severity Index; IV, intravenous; n, number of evaluated subjects; NA, not applicable, not investigated; PSG, polysomnography; PSQI, Pittsburgh Sleep Quality Index; RCT, randomized clinical trial; SPQ, Sleep Problems Questionnaire; SSI, Short Sleep Index; VAS, visual analog scale; XR, extended‐release.
3.1. Sedative antidepressants
3.1.1. Trazodone
Trazodone 50‐200 mg/day has been shown to improve sleep in two randomized, double‐blind, placebo‐controlled studies 45 , 46 , and while it is not approved by the FDA for the treatment of insomnia, it is the most frequently prescribed medication for the treatment of IAAC. 36 , 44 However, one of these studies showed evidence of an increased risk for alcohol relapse with trazodone. 45
Le Bon et al 46 evaluated the efficacy of trazodone (50‐200 mg/day) administered before bedtime in increasing sleep efficiency in 18 patients (16 completers; trazodone, n = 8; placebo, n = 8) with AUD who had completed alcohol detoxification. Trazodone significantly improved sleep efficiency after sleep onset and reduced wake time after sleep onset compared with the placebo group (for which there was no benefit) on the first night of treatment and after four weeks of treatment in abstinent patients. Non‐REM sleep was also significantly improved after the first night of treatment with trazodone. 46 Friedman et al 45 evaluated the efficacy of trazodone (50‐150 mg) administered at bedtime for 12 weeks in 173 alcohol‐dependent inpatients (who were discharged 3 to 5 days after detoxification). Some primary outcomes in the study were the proportion of days abstinent and the proportion of drinks per drinking day, and sleep quality was a secondary outcome. Although trazodone improved sleep quality at 12 weeks compared with baseline as measured by the PSQI (mean PSQI change = −3.02; 95% confidence interval [CI]: −3.38 to −2.67), sleep quality worsened in the trazodone group at 6 months with no significant differences noted compared with placebo. Furthermore, patients in the trazodone group had comparable or worse drinking outcomes versus placebo during and after treatment. At six months, placebo‐treated patients had an increased percentage of abstinent days and fewer drinks per drinking day. At 6 months, 9% of trazodone‐treated patients and 14% of placebo‐treated patients had continuous abstinence. 45
3.2. Anticonvulsants
3.2.1. Carbamazepine
One double‐blind active‐comparator trial randomized 136 patients with mild to moderate alcohol withdrawal to receive carbamazepine or lorazepam for five days on a fixed‐dose tapering schedule. When adjusted for time since last drink, visual analog measures of sleep quality indicated a statistically significant main effect of medication on sleep that favored carbamazepine over lorazepam (adjusted mean = 62.1 vs 51.2 for lorazepam; P = .0186). 47
3.2.2. Gabapentin
Six randomized, double‐blind, placebo‐controlled or active‐comparator studies have evaluated the efficacy of gabapentin 400‐1800 mg/day in the treatment of individuals with IAAC. 48 , 49 , 50 , 51 , 52 , 53 Of the six studies, three showed significant improvements in sleep parameters, 50 , 51 , 53 and four of the six studies showed a positive effect on drinking outcomes. 48 , 50 , 51 , 53
Malcolm et al, 52 in a double‐blind, randomized controlled clinical study, evaluated the efficacy of gabapentin compared to lorazepam in improving sleep disturbances and daytime sleepiness in outpatients being treated for mild to moderate alcohol withdrawal. The patients received a 4‐day fixed‐dose taper of gabapentin (range = 400‐1200 mg) or lorazepam (range = 4 mg [two doses of 2 mg] to 6 mg [three doses of 2 mg]) (n = 75) with an 8‐day follow‐up period (n = 68). In the early treatment period, self‐reports of insomnia were lower while daytime sleepiness was higher with lorazepam than with gabapentin. In the follow‐up period, patients previously taking lorazepam reported increased insomnia and daytime sleepiness while patients on gabapentin reported continued improvement in sleep measures. Gabapentin and lorazepam were similar in efficacy related to self‐reports of sleep and sleepiness in patients with limited previous alcohol withdrawals. However, in patients with multiple previous alcohol withdrawals, gabapentin significantly reduced sleep disturbances and sleepiness compared with lorazepam. 52 Brower et al 48 evaluated the efficacy of gabapentin 1500 mg administered at bedtime for six weeks in the treatment of insomnia and prevention of relapse in 21 patients (gabapentin, n = 10; placebo, n = 11) with AUD whose insomnia persisted for at least one week of abstinence with no withdrawal symptoms. There were no significant differences between the gabapentin and placebo groups in subjective sleep reports and polysomnography; however, there was a nonsignificant trend for rebound insomnia with gabapentin. Gabapentin significantly delayed the onset of relapse at six weeks and 12 weeks. For the 14 patients who completed the study, there was a significant relationship between improvement in sleep and good drinking outcomes during the first six weeks. 48 Trevisan et al 49 evaluated the efficacy of gabapentin 1200 mg/day; valproic acid 1500 mg/day; and placebo in the treatment of acute alcohol withdrawal over five days and the prevention of relapse and symptoms of prolonged alcohol withdrawal (including psychiatric distress and sleep disturbances) over four weeks in 57 male veterans in an alcohol detoxification program. The three treatments had similar effects on drinking outcomes, relapse rates, and improved sleep. 49 Anton et al 50 evaluated the efficacy of the combination of flumazenil 2 mg administered intravenously for two consecutive days and gabapentin (up to 1200 mg nightly) administered orally for 39 days on drinking outcomes and sleep parameters in 60 alcohol‐dependent patients who were required to be abstinent at least 72 hours before randomization. Upon randomization, 44 patients had low alcohol withdrawal symptoms, and 16 patients had high alcohol withdrawal symptoms. Patients with high alcohol withdrawal symptoms had better drinking outcomes after the combination of medications. Patients with lower withdrawal scores had better sleep with the combination of medications, as measured by the Insomnia Severity Index, and patients with high alcohol withdrawal symptoms had better sleep with placebo. There was no relationship between treatment effect on sleep and drinking outcomes. 50
In a separate study, Anton et al 51 compared the efficacies of gabapentin titrated to 1200 mg/day (for 6 weeks) plus naltrexone titrated to 50 mg/day (for 16 weeks; n = 50), naltrexone 50 mg/day alone (for 16 weeks; n = 50), or placebo (n = 50) in the treatment of alcohol dependence in patients who were able to maintain abstinence for 4 days before randomization. Of the 150 randomized patients, four patients did not have drinking data available and, therefore, were not included in the analysis. For the first six weeks, the gabapentin‐naltrexone group had a longer time to relapse, a lower percentage of heavy drinking days, and fewer drinks per drinking day than the group taking naltrexone alone. The gabapentin‐naltrexone group had significantly better sleep than the naltrexone or placebo groups. Poor sleep quality was significantly related to relapse in the naltrexone group, but not in the gabapentin‐naltrexone group. 51 Mason et al 53 evaluated the efficacy of gabapentin 900 mg/day and 1800 mg/day on measures including the rates of relapse and insomnia in 150 alcohol‐dependent patients for 12 weeks who were abstinent from alcohol at least three days before randomization. Gabapentin significantly improved rates of both abstinence and no heavy drinking, as well as significantly reduced the PSQI total score compared with placebo in a linear dose‐related manner. 53
Additionally, one open‐label study was conducted to evaluate gabapentin in patients experiencing insomnia after alcohol cessation. Karam‐Hage and Brower 54 evaluated the efficacy of gabapentin (n = 34; range = 300‐1800 mg) compared with trazodone (n = 16; range = 50‐300 mg) at bedtime in alcohol‐dependent outpatients with persisting insomnia despite 4 or more weeks of abstinence at baseline and up to 6 weeks in an open pilot study. Patients in the gabapentin group reported significant improvement in total SPQ scores compared with patients in the trazodone group (P = .023). At follow‐up, patients in the gabapentin group reported less initial insomnia and “feeling tired and worn out” compared with patients in the trazodone group. 54
Gabapentin has some inherent advantages in clinical practice because it is not metabolized by the liver, does not lower the seizure threshold, and does not require frequent blood tests. However, gabapentin has been associated with abuse liability 55 and may increase suicidal thoughts and behavior, which may require careful risk assessment in the AUD patient population due to the high comorbidity with mood disorder in AUD. 36 , 48 , 49 , 53 , 56
3.3. Melatonin agonists
3.3.1. Ramelteon and agomelatine
A small, open‐label, 4‐week study evaluated the efficacy of ramelteon 8 mg/night in treating insomnia in five patients with AUD and primary or alcohol‐induced insomnia. Ramelteon improved Insomnia Severity Index scores from a mean (standard deviation [SD]) of 17.6 (3.6) to 9.8 (7.6), increased total sleep time from 5.0 (1.0) to 6.3 (1.6) hours, and decreased sleep‐onset latency from 50.3 (20.9) to 23.5 (21.4) minutes. 57
Subjective sleep quality was improved in a small, open‐label, 6‐week study that evaluated the efficacy of agomelatine 25‐50 mg/day in treating insomnia in 9 abstinent patients with AUD. Treatment with agomelatine significantly decreased the PSQI total score from a mean (SD) of 13.1 (1.7) to 7.8 (1.7). 58 Agomelatine has some limitations in clinical practice because of its potential for causing hepatotoxicity, and it is not approved by the US Food and Drug Administration (FDA) for use in the United States. 36 , 58
3.4. Antipsychotics
3.4.1. Quetiapine
Two randomized, double‐blind, placebo‐controlled studies demonstrated that quetiapine up to 400 mg/day significantly improved sleep parameters compared with those of placebo in patients with AUD.
In an 8‐week study of 20 patients (quetiapine XR, n = 10; placebo, n = 10) with AUD in early recovery who complained of sleep‐related disturbance, patients treated with quetiapine XR experienced significantly greater improvement in wake after sleep onset, as measured by polysomnography, and initial (although not sustained) improvements in insomnia symptoms, as measured by the Insomnia Severity Index. The effect of quetiapine on drinking outcomes was not evaluated. No significant differences in PSQI scores were noted between treatment groups. 59 In a 12‐week study of 224 very heavy drinking patients with AUD (218 completers), sleep quality (as measured by the PSQI) improved with both groups but was greater with quetiapine (least‐squares [LS] mean, 4.1, 95% CI: 3.6‐4.6) as compared with placebo (LS mean, 5.1, 95% CI: 4.6‐5.6). Quetiapine was not effective in improving the percentage of days of heavy drinking. 60
Only modest improvements in sleep scores were noted, and these improvements were not sustained upon follow‐up in the 8‐week study. 59 Also, adverse effects (increased weight, increased triglyceride levels, dizziness, dry mouth, dyspepsia, increased appetite, sedation, and daytime somnolence) were more common with quetiapine than with placebo. 60 Another point of consideration for the AUD population is that there is a concern for misuse and abuse of quetiapine based on case reports in the published medical literature. 36 , 61 , 62
3.5. Other medications
3.5.1. Acamprosate
Acamprosate is approved by the FDA for the maintenance of abstinence from alcohol in patients with alcohol dependence who are abstinent at treatment initiation. 63 Two randomized, double‐blind, placebo‐controlled studies showed significant improvements in sleep parameters in patients with AUD during alcohol abstinence.
In a 3‐week study (acamprosate, n = 12; placebo, n = 12), polysomnography data showed that acamprosate 666 mg administered three times daily (initiated 8 days before alcohol withdrawal and continued during the 15 days following alcohol withdrawal) significantly reduced wake time after sleep onset (11.5 ± 12.1 vs 13.9 ± 13.0) and increased Stage 3 sleep (6.2 ± 2.5 vs 4.4 ± 2.2) percent and REM latency (82.1 ± 23.5 vs 62.6 ± 34.9) minutes compared with placebo after two weeks of abstinence (all treatment effects, P < .05). 64 In a post hoc analysis of a 24‐week study (n = 592; 292 completers), acamprosate 2000 or 3000 mg/day significantly reduced the percentage of alcohol‐dependent patients who experienced subjective sleep disturbance symptoms at six months compared with placebo (from 40.2% at baseline for all patients to 19.5% with acamprosate and 26.1% with placebo; P = .04). The percentage of mean change over baseline in the Short Sleep Index score was significantly improved with acamprosate (−39.1%) compared with placebo (−2.6%) (P = .001) 65 .
3.5.2. Prazosin
Prazosin showed some promise in patients with post‐traumatic stress disorder‐without comorbid AUD 66 , 67 however, in a randomized, double‐blind, placebo‐controlled, 13‐week study in 96 veterans with post‐traumatic stress disorder and AUD (abstinent for two days before randomization), prazosin 16 mg/day did not significantly improve sleep parameters (including PSQI) or alcohol use compared with placebo. 68
3.5.3. Etanercept
Etanercept is a tumor necrosis factor‐alpha (TNF‐α) blocker indicated for the treatment of a variety of inflammatory diseases, such as rheumatoid arthritis and plaque psoriasis. 69 Circulating levels of TNF‐α have been correlated with increases in REM sleep. 70 In a randomized, double‐blind, placebo‐controlled, crossover, single‐dose study in 21 (18 completers) abstinent alcohol‐dependent male patients, etanercept 25 mg significantly reduced the amount and percentage of REM sleep compared with placebo. The reduction in REM sleep reported with etanercept approached those values found in control subjects. 70
These 19 studies evaluated patients at differing time points during abstinence, yet they did not uniformly measure drinking outcomes, a key limitation in the study designs. Additionally, most of these studies did not establish the temporal relationship between the subjects’ sleep disturbances and alcohol use disorder. Furthermore, none of these medications are approved by the FDA for the treatment of insomnia in patients with AUD or IAAC and the currently available evidence for these agents is limited.
3.6. Pharmacological treatments for insomnia disorder currently in development
Considering therapeutic targets in sleep research, our search identified eight novel compounds currently being studied for use in the United States. Three of these target the orexin system, a new pharmacological target for insomnia disorder, and recognized as a major addition to treatment armamentarium. The first dual orexin antagonist (DORA) approved by the FDA was Merck's suvorexant, which blocks both orexin‐1 and orexin‐2 receptors. A key differentiator for this class is the reduction of wakefulness, rather than increasing sleep drive. 71 DORAs that are being developed include lemborexant and daridorexant (previously referred to as nemorexant). A follow‐on approach is to target only the orexin‐2 receptors, which proponents argue will lead to an improved risk‐benefit ratio. 72 One such molecule in development is seltorexant, which recently reported beneficial sleep onset and maintenance effects in a Phase 2 study of subjects with insomnia disorder. 72 Importantly the orexin antagonists, dual and selective, are currently targeted toward insomnia disorder rather than IAAC. We do note that suvorexant is currently listed on ClinicalTrials.gov as being studied in patients with comorbid sleep disorder and alcohol dependence (NCT03897062); however, preclinical work with suvorexant demonstrated that in the period following alcohol withdrawal orexin antagonism had sleep‐promoting effect yet may exacerbate some aspects of sleep and EEG pathology, 73 leading the authors to suggest that the approach may not be best suited to this vulnerable population.
Other compounds in development for insomnia disorder target gamma‐aminobutyric acid receptors (eg, EVT‐201 and SAGE‐217), 74 , 75 which may not be appropriate for IAAC as previously discussed. Piromelatine, a melatonin MT1/2/3 and serotonin 5‐HT‐1A/1D receptors agonist, is in development for sleep disorders and Alzheimer's disease. 76 Lumateperone is a serotonin 2a receptor antagonist which also modulates dopaminergic and glutaminergic systems from Intra‐Cellular Therapies, Inc; it is currently being developed for schizophrenia and is also being studied in Phase 2 trials for “sleep disorders associated with neuropsychiatric disorders”. 77 , 78 Also, Imbrium Therapeutics has advanced a novel, potentially first‐in‐class small molecule nociceptin/orphanin‐FQ receptor agonist to a randomized, double‐blind, placebo‐controlled, multi‐center, parallel‐group Phase 2 study (NCT04035200) to evaluate the compound's safety, efficacy, and tolerability in adults with moderate or severe AUD who are experiencing IAAC. Prior studies with this molecule in patients with insomnia disorder demonstrated increases in sleep efficiency over placebo, and reduction of sleep fragmentation throughout the night. Importantly, this treatment is minimally metabolized by the liver, increasing its suitability for use in patients with a history of significant alcohol use who are more likely to have impaired liver function. 79
4. DISCUSSION AND DIRECTIONS FOR FUTURE RESEARCH
The prevalence of insomnia associated with alcohol cessation in patients with AUD is high; however, limited prevalence data describe the persistence of sleep‐related disorders for patients with AUD who are in a sustained abstinence period. 4 , 12 Patients with IAAC have been shown to experience increased sleep‐onset latency, sleep fragmentation, and REM sleep percentage, as well as reductions in sleep efficiency, the percentage of SWS, and REM latency. 1 , 2 If these sleep disturbances go untreated, they are likely to contribute to lower quality of life and increase the risk of relapse in this patient population. 4 , 29 Further research is needed to provide prevalence data for IAAC and to fully characterize the physiology of sleep in patients with AUD in early and sustained recovery. 4
Data from sleep studies suggest a progression of disturbance in sleep continuity to IAAC. 1 , 2 , 4 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 80 While currently controversial, emerging data are directed toward understanding the physiological differences between IAAC and insomnia disorder. 30 , 81 What is abundantly clear is that when employing treatment options, these two populations need to be considered differently.
Although sedative‐hypnotic agents (eg, benzodiazepine receptor agonists, including Z drugs) are considered first‐line treatment for insomnia, these agents have abuse liability and risk for overdose when combined with alcohol, both of which are significant concerns in the alcohol‐dependent population. 36 Furthermore, the safety and efficacy of these medications have not been evaluated in controlled clinical trials for the management of insomnia in patients with AUD or IAAC. Additionally, clinicians are hesitant to prescribe sleep‐inducing agents such as benzodiazepines because of concerns regarding abuse, overdose, and risk of alcohol relapse in this patient population. 44
Currently available pharmacological interventions have shown some benefit in improving insomnia associated with alcohol cessation in patients with AUD; however, they have not been extensively studied in this patient population, are not approved by FDA for such use, and there are limitations to their use due to misuse and abuse potential, unfavorable safety profile, or risk of relapse. 35 , 36 The ideal pharmacological treatment for IAAC would have the following minimal attributes: efficacious with a low potential for abuse, low risk for adverse effects involving the respiratory system and favorable safety profile in hepatically compromised individuals. 35 , 36 In addition, pharmacological treatment should ideally reduce the risk of relapse and should be compatible with comorbid medical and psychiatric disorders often present in this patient population as well as with nonpharmacological treatment approaches. Most important, the efficacy and safety of any treatment should be supported by a substantial body of research consisting of controlled clinical trials with a methodological focus on the AUD population in early and sustained recovery. 4 , 36
Other methodological considerations for future studies in this population should include the following: firstly, inclusion criteria with currently accepted definitions of insomnia (and excluding those with other sleep disorders), secondly the impact of insomnia‐focused interventions on drinking outcomes, thirdly the role of medical and psychiatric comorbidities in predicting outcomes for insomnia treatments, and fourthly the efficacy of pharmacological and nonpharmacological monotherapy versus combination therapy. 4 , 35 , 36
Looking toward future possible novel treatment options, we are aware of eight novel compounds in development for the treatment of insomnia in the United States, one of which specifically targeting IAAC. With an increasing awareness of the importance of IAAC and a move toward studying therapeutic approaches for niche sleep indications, we are hopeful that efficacious and safe treatment options for this vulnerable patient population appropriate for use in early and sustained recovery will become available.
CONFLICT OF INTEREST
TAR has received compensated consultation support from Purdue Pharma LP for this work. JA and JT are former employees of Purdue Pharma LP GW is a current employee of Imbrium Therapeutics, a subsidiary of Purdue Pharma LP This work was funded by Purdue Pharma LP
AUTHOR CONTRIBUTIONS
Timothy A. Roehrs, PhD, Jessica Auciello, PharmD, MPH, Jack Tseng, PhD and Garth Whiteside, PhD have read and approved the final version of the manuscript for submission to Neuropsychopharmacology Reports and agree to be accountable for all aspects of the work; each has made a substantial contribution to the conception, design, gathering, analysis and/or interpretation of data and a substantial contribution to the writing and intellectual content of the article; and acknowledge that they have exercised due care in ensuring the integrity of the work. As such, all authors meet criteria for authorship as defined by Neuropsychopharmacology Reports.
Roehrs TA, Auciello J, Tseng J, Whiteside G. Current and potential pharmacological treatment options for insomnia in patients with alcohol use disorder in recovery. Neuropsychopharmacol Rep. 2020;40:211–223. 10.1002/npr2.12117
Funding information
All funding was provided by Purdue Pharma LP
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Roehrs T, Roth T. Medication and substance abuse In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 6th ed Philadelphia, PA: Elsevier, 2017; p. 1380–9. [Google Scholar]
- 2. Kolla BP, Bostwick JM. Insomnia: the neglected component of alcohol recovery. J Addict Res Ther. 2011;2(1).e2 [Google Scholar]
- 3. Neu P, Sofin Y, Danker‐Hopfe H. The effect of detoxification on sleep: how does sleep quality change during. Qualified detoxification treatment?. J Addict. 2018;2018:9492453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arnedt JT, Conroy DA, Brower KJ. Treatment options for sleep disturbances during alcohol recovery. J Addict Dis. 2007;26(4):41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hyde M, Roehrs T, Roth T. Alcohol, alcoholism, and sleep In: Lee‐Chiong TL, editor. Sleep medicine essentials. Hoboken, NJ: Wiley‐Blackwell, 2009; p. 257–60. [Google Scholar]
- 6. Chakravorty S, Chaudhary NS, Brower KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40(11):2271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Psychiatric Association . Substance/medication‐induced sleep disorder. The diagnostic and statistical manual of mental disorders. 5th ed Washington, DC; 2013. [Google Scholar]
- 8. American Academy of Sleep Medicine . International classification of sleep disorders. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 9. Caetano R, Clark CL, Greenfield TK. Prevalence, trends, and incidence of alcohol withdrawal symptoms: analysis of general population and clinical samples. Alcohol Health Res World. 1998;22(1):73–9. [PMC free article] [PubMed] [Google Scholar]
- 10. Escobar‐Cordoba F, Avila‐Cadavid JD, Cote‐Menendez M. Complaints of insomnia in hospitalized alcoholics. Rev Brasil Psiquiatria. 2009;31(3):261–4. [DOI] [PubMed] [Google Scholar]
- 11. Steinig J, Foraita R, Happe S, Heinze M. Perception of sleep and dreams in alcohol‐dependent patients during detoxication and abstinence. Alcohol Alcohol. 2011;46(2):143–7. [DOI] [PubMed] [Google Scholar]
- 12. Brower KJ, Perron BE. Prevalence and correlates of withdrawal‐related insomnia among adults with alcohol dependence: results from a national survey. Am J Addict. 2010;19(3):238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wallen GR, Brooks AT, Whiting B, Clark R, Krumlauf MC, Yang LI, et al. The prevalence of sleep disturbance in alcoholics admitted for treatment: a target for chronic disease management. Fam Commun Health. 2014;37(4):288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kolla BP, Schneekloth T, Biernacka J, Mansukhani M, Geske J, Karpyak V, et al. The course of sleep disturbances in early alcohol recovery: an observational cohort study. Am J Addict. 2014;23(1):21–6. [DOI] [PubMed] [Google Scholar]
- 15. Brower KJ, Krentzman A, Robinson EA. Persistent insomnia, abstinence, and moderate drinking in alcohol‐dependent individuals. Am J Addict. 2011;20(5):435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lovinger DM. Serotonin's role in alcohol's effects on the brain. Alcohol Health Res World. 1997;21(2):114–20. [PMC free article] [PubMed] [Google Scholar]
- 17. Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5(4):287–97. [DOI] [PubMed] [Google Scholar]
- 18. Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health. 2001;25(2):110–25. [PMC free article] [PubMed] [Google Scholar]
- 19. Clasadonte J, McIver SR, Schmitt LI, Halassa MM, Haydon PG. Chronic sleep restriction disrupts sleep homeostasis and behavioral sensitivity to alcohol by reducing the extracellular accumulation of adenosine. J Neurosci. 2014;34(5):1879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcohol Clin Exp Res. 1998;22(8):1796–802. [PubMed] [Google Scholar]
- 21. Thakkar MM, Sharma R, Sahota P. Alcohol disrupts sleep homeostasis. Alcohol. 2015;49(4):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia AN, Salloum IM. Polysomnographic sleep disturbances in nicotine, caffeine, alcohol, cocaine, opioid, and cannabis use: a focused review. Am J Addict. 2015;24(7):590–8. [DOI] [PubMed] [Google Scholar]
- 23. Perney P, Lehert P. Insomnia in alcohol‐dependent patients: prevalence, risk factors and acamprosate effect: an individual patient data meta‐analysis. Alcohol Alcohol. 2018;53(5):611–8. [DOI] [PubMed] [Google Scholar]
- 24. Colrain IM, Nicholas CL, Baker FC. Alcohol and the sleeping brain In: Sullivan EV, Pfefferbaum A, editors. Handbook of clinical neurology. 125. 3rd series ed. Waltham, MA: Elsevier BV, 2014; p. 415–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Colrain IM, Crowley KE, Nicholas CL, Padilla M, Baker FC. The impact of alcoholism on sleep evoked Delta frequency responses. Biol Psychiatry. 2009;66(2):177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armitage R, Hoffmann R, Conroy DA, Arnedt JT, Brower KJ. Effects of a 3‐hour sleep delay on sleep homeostasis in alcohol dependent adults. Sleep. 2012;35(2):273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brower KJ, Hoffmann R, Conroy DA, Arnedt JT, Armitage R. Sleep homeostasis in alcohol‐dependent, depressed and healthy control men. Eur Arch Psychiatry Clin Neurosci. 2011;261(8):559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry. 2002;51(8):632–41. [DOI] [PubMed] [Google Scholar]
- 29. Roehrs T, Roth T. Sleep, alcohol, and quality of life In: Verster JC, Pandi‐Perumal SR, Streiner DL, editors. Sleep and quality of life in medical illnesses. Totowa, NJ: Humana Press, 2008; p. 333–9. [Google Scholar]
- 30. Roehrs TA, Roth T. Sleep disturbance in substance use disorders. Psychiatr Clin North Am. 2015;38(4):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He S, Brooks AT, Kampman KM, Chakravorty S. The relationship between alcohol craving and insomnia symptoms in alcohol‐dependent individuals. Alcohol Alcohol. 2019;54(3):287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gillin JC, Smith TL, Irwin M, Butters N, Demodena A, Schuckit M. Increased pressure for rapid eye movement sleep at time of hospital admission predicts relapse in nondepressed patients with primary alcoholism at 3‐month follow‐up. Arch Gen Psychiatry. 1994;51(3):189–97. [DOI] [PubMed] [Google Scholar]
- 33. Brower KJ, Aldrich MS, Hall JM. Polysomnographic and subjective sleep predictors of alcoholic relapse. Alcohol Clin Exp Res. 1998;22(8):1864–71. [PubMed] [Google Scholar]
- 34. Conroy DA, Todd Arnedt J, Brower KJ, Strobbe S, Consens F, Hoffmann R, et al. Perception of sleep in recovering alcohol‐dependent patients with insomnia: relationship with future drinking. Alcohol Clin Exp Res. 2006;30(12):1992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brower KJ. Assessment and treatment of insomnia in adult patients with alcohol use disorders. Alcohol. 2015;49(4):417–27. [DOI] [PubMed] [Google Scholar]
- 36. Schubert JR, Arnedt JT. Management of insomnia in patients with alcohol use disorder. Curr Sleep Med Rep. 2017;3(2):38–47. [Google Scholar]
- 37. Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive‐behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. 2011;49(4):227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chakravorty S, Morales KH, Arnedt JT, Perlis ML, Oslin DW, Findley JC, et al. Cognitive behavioral therapy for insomnia in alcohol‐dependent veterans: a randomized, controlled pilot study. Alcohol Clin Exp Res. 2019;43(6):1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Currie SR, Clark S, Hodgins DC, El‐Guebaly N. Randomized controlled trial of brief cognitive‐behavioural interventions for insomnia in recovering alcoholics. Addiction. 2004;99(9):1121–32. [DOI] [PubMed] [Google Scholar]
- 40. Zhabenko O, Zhabenko N, Conroy DA, et al. An open uncontrolled pilot trial of online cognitive‐behavioral therapy for insomnia for Ukrainian alcohol‐dependent patients In: Andrade ALM, De Micheli D, editors. Innovations in the treatment of substance addiction. Cham, Switzerland: Springer International Publishing, 2016; p. 165–81. [Google Scholar]
- 41. Greeff AP, Conradie WS. Use of progressive relaxation training for chronic alcoholics with insomnia. Psychol Rep. 1998;82(2):407–12. [DOI] [PubMed] [Google Scholar]
- 42. Schmitz M, Frey R, Pichler P, Röpke H, Anderer P, Saletu B, et al. Sleep quality during alcohol withdrawal with bright light therapy. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(6):965–77. [DOI] [PubMed] [Google Scholar]
- 43. Miller MB, Donahue ML, Carey KB, Scott‐Sheldon LAJ. Insomnia treatment in the context of alcohol use disorder: a systematic review and meta‐analysis. Drug Alcohol Depend. 2017;181:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Friedmann PD, Herman DS, Freedman S, Lemon SC, Ramsey S, Stein MD. Treatment of sleep disturbance in alcohol recovery: a national survey of addiction medicine physicians. J Addict Dis. 2003;22(2):91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Friedmann PD, Rose JS, Swift R, Stout RL, Millman RP, Stein MD. Trazodone for sleep disturbance after alcohol detoxification: a double‐blind, placebo‐controlled trial. Alcohol Clin Exp Res. 2008;32(9):1652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Le Bon O, Murphy JR, Staner L, Hoffmann G, Kormoss N, Kentos M, et al. Double‐blind, placebo‐controlled study of the efficacy of trazodone in alcohol post‐withdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol. 2003;23(4):377–83. [DOI] [PubMed] [Google Scholar]
- 47. Malcolm R, Myrick H, Roberts J, Wang W, Anton RF. The differential effects of medication on mood, sleep disturbance, and work ability in outpatient alcohol detoxification. Am J Addict. 2002;11:141–50. [DOI] [PubMed] [Google Scholar]
- 48. Brower KJ, Myra Kim H, Strobbe S, Karam‐Hage MA, Consens F, Zucker RA. A randomized double‐blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Trevisan LA, Ralevski E, Keegan K, Oville A, Vuppalapati D, Gonzalez G, et al. Alcohol detoxification and relapse prevention using valproic acid versus gabapentin in alcohol‐dependent patients. Addict Disord Treat. 2008;7(3):119–28. [Google Scholar]
- 50. Anton RF, Myrick H, Baros AM, Latham PK, Randall PK, Wright TM, et al. Efficacy of a combination of flumazenil and gabapentin in the treatment of alcohol dependence: relationship to alcohol withdrawal symptoms. J Clin Psychopharmacol. 2009;29(4):334–42. [DOI] [PubMed] [Google Scholar]
- 51. Anton RF, Myrick H, Wright TM, Latham PK, Baros AM, Waid LR, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Malcolm R, Myrick LH, Veatch LM, Boyle E, Randall PK. Self‐reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24–32. [PubMed] [Google Scholar]
- 53. Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karam‐Hage M, Brower KJ. Open pilot study of gabapentin versus trazodone to treat insomnia in alcoholic outpatients. Psychiatry Clin Neurosci. 2003;57(5):542–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith RV, Havens JR, Walsh SL. Gabapentin misuse, abuse and diversion: a systematic review. Addiction. 2016;111(7):1160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pfizer Inc . Neurontin® [package insert]. New York, NY: 2017. [Google Scholar]
- 57. Brower KJ, Conroy DA, Kurth ME, Anderson BJ, Stein MD. Ramelteon and improved insomnia in alcohol‐dependent patients: a case series. J Clin Sleep Med. 2011;7(3):274–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grosshans M, Mutschler J, Luderer M, Mann K, Kiefer F. Agomelatine is effective in reducing insomnia in abstinent alcohol‐dependent patients. Clin Neuropharmacol. 2014;37(1):6–8. [DOI] [PubMed] [Google Scholar]
- 59. Chakravorty S, Hanlon AL, Kuna ST, Ross RJ, Kampman KM, Witte LM, et al. The effects of quetiapine on sleep in recovering alcohol‐dependent subjects: a pilot study. J Clin Psychopharmacol. 2014;34(3):350–4. [DOI] [PubMed] [Google Scholar]
- 60. Litten RZ, Fertig JB, Falk DE, Ryan ML, Mattson ME, Collins JF, et al. A double‐blind, placebo‐controlled trial to assess the efficacy of quetiapine fumarate XR in very heavy‐drinking alcohol‐dependent patients. Alcohol Clin Exp Res. 2012;36(3):406–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim S, Lee G, Kim E, Jung H, Chang J. Quetiapine misuse and abuse: is it an atypical paradigm of drug seeking behavior? J Res Pharm Pract. 2017;6(1):12–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sansone RA, Sansone LA. Is seroquel developing an illicit reputation for misuse/abuse? Psychiatry. 2010;7(1):13–6. [PMC free article] [PubMed] [Google Scholar]
- 63. Forest Pharmaceuticals Inc . Campral® [package insert]. St. Louis, MO: 2012. [Google Scholar]
- 64. Luc S, Peter B, Thierry D, Isabelle G, Muriel M, Frederic L, et al. Effects of acamprosate on sleep during alcohol withdrawal: A double‐blind placebo‐controlled polysomnographic study in alcohol‐dependent subjects. Alcohol Clin Exp Res. 2006;30(9):1492–9. [DOI] [PubMed] [Google Scholar]
- 65. Perney P, Lehert P, Mason BJ. Sleep disturbance in alcoholism: proposal of a simple measurement, and results from a 24‐week randomized controlled study of alcohol‐dependent patients assessing acamprosate efficacy. Alcohol Alcohol. 2012;47(2):133–9. [DOI] [PubMed] [Google Scholar]
- 66. Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, et al. Prazosin effects on stress‐ and cue‐induced craving and stress response in alcohol‐dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012;36(2):351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Simpson TL, Malte CA, Dietel B, Tell D, Pocock I, Lyons R, et al. A pilot trial of prazosin, an alpha‐1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2015;39(5):808–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Petrakis IL, Desai N, Gueorguieva R, Arias A, O'Brien E, Jane JS, et al. Prazosin for veterans with post‐traumatic stress disorder and comorbid alcohol dependence: a clinical trial. Alcohol Clin Exp Res. 2016;40(1):178–86. [DOI] [PubMed] [Google Scholar]
- 69. Immunex Corporation . Enbrel® [package insert]. Thousand Oaks, CA; 2017. [Google Scholar]
- 70. Irwin MR, Olmstead R, Valladares EM, Breen EC, Ehlers CL. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol Psychiatry. 2009;66(2):191–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Merck Sharp & Dohme Corp . Belsomra [package insert]. Whitehouse Station, NJ: 2018. [Google Scholar]
- 72. De Boer P, Drevets WC, Rofael H, van der Ark P, Kent JM, Kezic I, et al. A randomized Phase 2 study to evaluate the orexin‐2 receptor antagonist seltorexant in individuals with insomnia without psychiatric comorbidity. J Psychopharmacol. 2018;32(6):668–77. [DOI] [PubMed] [Google Scholar]
- 73. Sanchez‐Alavez M, Benedict J, Wills DN, Ehlers CL. Effect of suvorexant on event‐related oscillations and EEG sleep in rats exposed to chronic intermittent ethanol vapor and protracted withdrawal. Sleep. 2019;42(4):zsz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hoffmann E, Nomikos GG, Kaul I, et al. SAGE‐217, A novel GABAA receptor positive allosteric modulator: clinical pharmacology and tolerability in randomized phase I dose‐finding studies. Clin Pharmacokinet [Internet]. 2019. July 29 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Walsh JK, Thacker S, Knowles LJ, Tasker T, Hunneyball IM. The partial positive allosteric GABA(A) receptor modulator EVT 201 is efficacious and safe in the treatment of adult primary insomnia patients. Sleep Med. 2009;10(8):859–64. [DOI] [PubMed] [Google Scholar]
- 76. She M, Hu X, Su Z, Zhang C, Yang S, Ding L, et al. Piromelatine, a novel melatonin receptor agonist, stabilizes metabolic profiles and ameliorates insulin resistance in chronic sleep restricted rats. Eur J Pharmacol. 2014;727:60–5. [DOI] [PubMed] [Google Scholar]
- 77. Kumar B, Kuhad A, Kuhad A. Lumateperone: a new treatment approach for neuropsychiatric disorders. Drugs Today. 2018;54(12):713–9. [DOI] [PubMed] [Google Scholar]
- 78. Spiegelhalder K, Regen W, Nanovska S, Baglioni C, Riemann D. Comorbid sleep disorders in neuropsychiatric disorders across the life cycle. Curr Psychiatry Rep. 2013;15(6):364. [DOI] [PubMed] [Google Scholar]
- 79. Imbrium Therapeutics . Imbrium Therapeutics advances insomnia pipeline through initiation of a Phase 2 study of potential first‐in‐class molecule for insomnia associated with alcohol cessation. Stamford, CT: 2019. Available from https://www.imbriumthera.com/news/imbrium-therapeutics-advances-insomnia-pipeline-through-initiation-of-a-phase-2-study-of-potential-first-in-class-molecule-for-insomnia-associated-with-alcohol-cessation/ [Google Scholar]
- 80. Roehrs T, Papineau K, Rosenthal L, Roth T. Ethanol as a hypnotic in insomniacs: self administration and effects on sleep and mood. Neuropsychopharmacology. 1999;20(3):279–86. [DOI] [PubMed] [Google Scholar]
- 81. Roehrs T, Gumenyuk V, Drake C, Roth T. Physiological correlates of insomnia. Curr Topics Behav Neurosci. 2014;21:277–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


