To the Editor: The syndrome of Covid-19 infection includes myalgias and elevated creatine kinase levels in at least a third of patients.1-3 Whether the elevation in creatine kinase level is caused by viral infection of muscle, toxic effects of cytokines, or another mechanism is unclear. There are few reports of muscle-biopsy findings in patients with Covid-19.4 We describe a patient with Covid-19 infection and myopathy who had a muscle-biopsy specimen showing evidence of virus-induced type I interferonopathy.
A 38-year-old man who had no recent history of illness or medication use presented with weakness, myalgias, and fever. He had generalized muscle weakness, which was more severe proximally than distally, and was unable to walk, abduct his shoulders, or flex his hips against gravity. Heliotrope rash, Gottron’s sign or papules, and nail-bed changes were absent. He had bibasilar infiltrates and a positive reverse-transcriptase–polymerase-chain-reaction assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA. The serum level of creatine kinase was 29,800 U per liter (reference range, 39 to 308), high-sensitivity troponin T 3157 ng per liter (reference range, 0 to 14), and C-reactive protein 55 mg per liter (reference value, <10), and myoglobinuria was not present. Findings on electrocardiography, echocardiography, and cardiac magnetic resonance imaging were normal. A description of the methods used for the immunohistochemical analysis of muscle is provided in the Supplementary Appendix, available with the full text of this letter at NEJM.org.
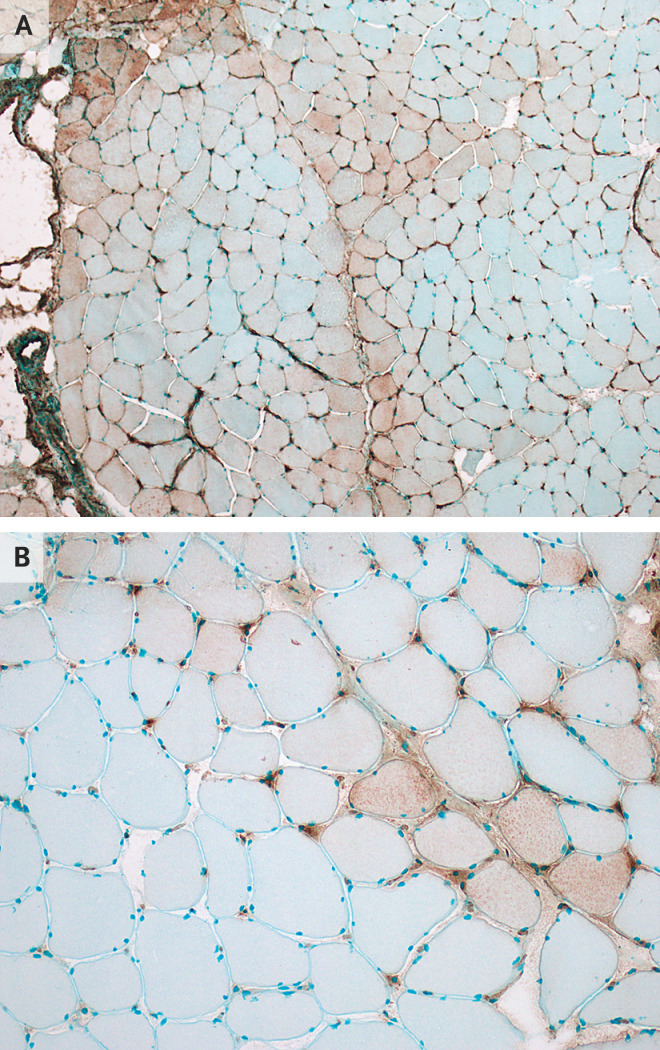
A biopsy specimen of his left deltoid muscle showed mild perivascular inflammation in a few vessels but no necrotic or regenerating fibers or perifascicular atrophy. Immunohistochemical analysis revealed abnormal expression of major-histocompatibility-complex class I antigen on sarcolemma and sarcoplasm and abnormal presence of myxovirus resistance protein A on muscle fibers and capillaries (Figure 1A and 1B). There was no membrane attack complex deposition on muscle fibers or capillaries. Immunohistochemical testing did not detect SARS-COV-2 in the muscle.
Figure 1. Immunohistochemical Analysis of a Biopsy Specimen from the Left Deltoid Muscle in a Patient with Covid-19.
Panel A shows abnormal expression of major-histocompatibility-complex (MHC) class I antigen on the sarcolemma and sarcoplasm of muscle fibers, with a predilection for perifascicular muscle fibers. Expression of MHC class I antigen on capillaries is normal. Panel B shows abnormal expression of myxovirus resistance protein A (MxA) on sarcolemma and sarcoplasm of muscle fibers, with a predilection for perifascicular muscle fibers, and on capillaries. MxA is not normally expressed on capillaries. Abnormal expression of MxA is seen with type I interferonopathies.
He was treated with intravenous remdesivir (single initial dose of 200 mg, followed by a dose of 100 mg daily for 4 days) and intravenous methylprednisolone (1 g daily for 3 days), followed by oral prednisone (60 mg daily). When discharged 14 days after the onset of illness, he was able to walk and the creatine kinase level was 5130 U per liter.
Myxovirus resistance protein A is a type I interferon-inducible protein expressed in response to viral infection, including to SARS-CoV-2.5 Deposition of this protein in muscle fibers and capillaries is an early feature of dermatomyositis, occurring before characteristic perifascicular atrophy. Our patient did not have clinical features of dermatomyositis or deposition of membrane attack complex on capillaries, another characteristic feature of dermatomyositis, which makes it unlikely that he had dermatomyositis independent of SARS-CoV-2. Increased expression of type I interferon in tissue up-regulates other proteins that are toxic to endothelial cells, muscle, and the lung. The findings in the muscle-biopsy specimen from this patient suggest that his SARS-CoV-2 myopathy may also have been due to type I interferonopathy.
Supplementary Appendix
Disclosure Forms
This letter was published on November 20, 2020, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Guidon AC, Amato AA. COVID-19 and neuromuscular disorders. Neurology 2020;94:959-969. [DOI] [PubMed] [Google Scholar]
- 2.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L-Q, Huang T, Wang Y-Q, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 2020;92:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang H, Charmchi Z, Seidman RJ, Anziska Y, Velayudhan V, Perk J. COVID-19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve 2020;62:E57-E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulia MS, O’Brien TP, Hou PC, Schuman A, Sambursky R. Multi-tiered screening and diagnosis strategy for COVID-19: a model for sustainable testing capacity in response to pandemic. Ann Med 2020;52:207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.