Abstract
Aquatic heavy metal pollution is a growing concern. To facilitate heavy metal monitoring in water, we developed transgenic Daphnia that are highly sensitive to heavy metals and respond to them rapidly. Metallothionein A, which was a metal response gene, and its promoter region was obtained from Daphnia magna. A chimeric gene fusing the promoter region with a green fluorescent protein (GFP) gene was integrated into D. magna using the TALEN technique and transgenic Daphnia named D. magna MetalloG were produced. When D. magna MetalloG was exposed to heavy metal solutions for 1 h, GFP expression was induced only in their midgut and hepatopancreas. The lowest concentrations of heavy metals that activated GFP expression were 1.2 µM Zn2+, 130 nM Cu2+, and 70 nM Cd2+. Heavy metal exposure for 24 h could lower the thresholds even further. D. magna MetalloG facilitates aqueous heavy metal detection and might enhance water quality monitoring.
Subject terms: Environmental chemistry, Assay systems
Introduction
Excessive amounts of heavy metals are hazardous to human and ecological health. Heavy metals are discharged from industrial and natural sources. They are major aquatic toxicants, so the concern around heavy metal pollution in water is increasing in recent years. The maximum allowable metal concentrations in environmental and drinking water are strictly regulated in many countries. For regulation of aquatic heavy metal, many monitoring methods have been developed1, including instrumental analyses such as flame atomic absorption spectrometry, electrothermal atomic absorption spectrometry, inductively coupled plasma optical emission spectrometry, and inductively coupled plasma mass spectrometry2. Nevertheless, these techniques are expensive and time-consuming; therefore, simple, rapid, and efficient methods are desired for continuous heavy metal monitoring (REF).
Biomonitoring is one of the simplest ways to detect toxicants, i.e., evaluating toxicity based on biological responses. Many organisms such as algae, zooplankton, insects, and fish have been used as biomonitors for aquatic pollutants in the past3. In addition to these conventional species, frogs has also been studied as a target species for heavy metals4. However, advances in genetic engineering have changed the classical endpoints, because gene expression change can be used as one of the endpoints. Alterations in gene expression occur before phenotypic changes appear, with the latter utilized as classical endpoints. Recently, the use of toxicogenomics based on transcription profiles has emerged5. It detects and explains global transcription changes induced by toxicants and it is recognized as a sensitive method; however, it is time-consuming and costly. It is informative to evaluate the expression of target genes that can be activated by toxicants. Moreover, the application of green fluorescent protein (GFP) in transgenic animals facilitates detection of gene expression changes caused by environmental changes or the presence of toxicants. In these transgenic animals, GFP is designed to be induced by the promoter of an activated gene. To monitor water quality, transgenic zebrafish and medaka have been developed6 to detect specific pollutants including metals7, aromatic carbons8, and estrogens9.
Gene expression changes in response to heavy metals have been extensively investigated. Metallothionein is one of the genes activated by heavy metal exposure10. It is a small, highly conserved protein found in organisms ranging from bacteria to humans11, and is rich in cysteine residues, the thiol groups of which bind metals. Metallothionein is, therefore, be an excellent biomarker for metal exposure12. Transgenic fish carrying the red fluorescent protein gene DsRed2 under the control of the metallothionein gene have been constructed13. Metallothionein gene Ia1 obtained from green mussels was fused to DsRed2 and introduced into zebrafish. Transgenic zebrafish responded to 4.4 μM Cd, 7.9 μM Cu, and 306 μM Zn. The use of the metallothionein gene was also reported for Caenorhabditis elegans14, whose lowest observed effect concentrations were 5 μM for Cd, 10 μM for Cu, and 250 μM for Zn. The use of transgenic animals for metal monitoring is simple; however, the detection limit is still very high.
Daphnia magna is a small freshwater crustacean that is used as an aquatic pollution bioindicator and model organism in ecotoxicology. As a rule, the adverse effects of aquatic pollutants have been evaluated using standard protocols such as OECD test guidelines 202 and 211 for acute and chronic toxicity testing, respectively. Daphnia are generally more sensitive to environmental changes than fish, and therefore it is expected that transgenic Daphnia could be used as an indicator of heavy metals. It has been reported that the metallothionein gene of D. pulex is activated by exposures to Cd11 and other heavy metals15. Therefore, the daphnid metallothionein gene might serve as a sensitive biomarker even in Daphnia. Recently, we successfully applied genome editing to Daphnia16–19 as well as conventional transgenic methods15. In the present study, we developed D. magna as a transgenic biomonitoring animal. Using the genome editing technique, we selected the metallothionein gene as a biomarker and visualized changes in its expression by linking it to GFP, thereby generating germ line transmitted transgenic Daphnia.
Materials and methods
Metal analysis
Test solution samples for chemical analysis were prepared in a plastic cup, separate from each test. The samples were collected at the start and the end of the exposure period to determine dissolved heavy metal concentrations. The collected samples were diluted using ultrapure water and nitric acid (final concentration of 0.13 M), spiked with an internal standard (Ga and In, the final concentration of 2 μg/L and 10 μg/L), and then analyzed using ICP-MS (Agilent 8800, Agilent Technologies, Inc.).
Daphnia culture
D. magna (NIES clone) were obtained from the National Institute for Environmental Studies (Tsukuba, Japan). Cultures with a density of 16 individuals/L were maintained at 23 ± 1 °C under a 14 h light/10 h dark photoperiod in 5 L of Aachener Daphnien Medium (ADaM)20. A 0.08 mL aliquot of a 7 × 109 cells/mL Chlorella suspension was added daily to each culture21.
Heavy metal exposure
For mRNA expression analysis of MT genes, neonate Daphnia less than 24 h old were used, which corresponded to < 96 h after oviposition because it takes 72 h for the neonate to swim out from the brood chamber. For GFP fluorescence analysis, juvenile D. magna MetalloG Daphnia (7 d post-oviposition) was used. Five each of neonate or juvenile daphniids were placed in 250 ml of ADaM medium and exposed to various metal concentrations over a 24-h or 48 h period. They were incubated at 23 ± 1 °C under a 14 h light/10 h dark photoperiod. Stock solutions of ZnCl2, CuSO4, and CdCl2 (Nacalai Tesque; Kyoto, Japan) were added to make up the final metal concentrations. Nominal concentrations were 0.59 μM (80 μg/l), 2.9 μM (400 μg/l), and 14.7 μM (2000 μg/l) for the ZnCl2 exposure, 64 nM (10.2 μg/l) and 320 nM (51 μg/l) for CuSO4 exposure. There was no feeding during exposure to heavy metals. Exposures were performed independently in triplicate.
Quantification of mRNA
Total RNA was extracted from five daphniids using Sepasol-RNA I solution (Nacalai Tesque; Kyoto, Japan) according to the manufacturer’s protocol. Around 0.5 μg of total RNA was generally obtained. Concentrations of RNA were determined by A260 measured by Nanodrop. One-fifth of the total RNA was used in cDNA synthesis with 250 ng of random primers (Invitrogen, Carlsbad, CA, USA) and 200U of SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). Quantitative polymerase chain reaction (qPCR) was performed with SYBR GreenER qPCR Supermix Universal (Invitrogen, Carlsbad, CA, USA) using the Mx3005P real-time (RT)-PCR system (Agilent Technologies, Santa Clara, CA, USA). PCR amplifications were performed with 200 nM primers in triplicate using the following conditions: 2 min at 50 °C and 10 min at 95 °C, followed by a total of 40 two-temperature cycles (15 s at 95 °C and 1 min at 60 °C) in a volume of 20 μl. Metallothionein gene coding sequences were obtained from the GenBank database (A: KF561474.1, B: KF561476.1, and C: KF561475.1) and the primers for the qPCR were designed. The primers for the metallothionein gene quantification were Metallo-A forward: CTGTTGCCAAAACAATTGCTCA; Metallo-A reverse: CTCCAGTGGCACAAATGCAAG; Metallo-B forward: TGCATTCACCACCAGTAGCG; Metallo-B reverse: GCCAAGGTAAATGCTCTCGTGTG; Metallo-C forward: GTGCCCTCGTTGTCAAGGTG; and Metallo-C reverse: ATTGGTTCCACACGTGCAGT. The primers used to amplify L32 were the same as described previously22, whose expression was used as a control. Generally, cDNA corresponding to 30 ng of total RNA was used and reaction was performed by using Ex-Taq (Takara Bio Inc., Siga, Japan). Metallothionein gene expressions were normalized to the ribosomal protein L32 level. For the estimation of the gene expression levels, 2−ΔΔCτ method was used (REF:Livac). On completion of the assay, a dissociation stage was carried out for melting curve analysis.
To determine tissue-specific mRNA expression, ten individuals were used for one exposure, and they were soaked in RNA later (Thermo Fisher Scientific, Yokohama, Japan) after the exposure. They were dissected under a stereomicroscope and midgut and hepatopancreas were separated from other tissues. Total RNAs were prepared from the midgut and hepatopancreas and other tissues as described above. Both RNAs were used for qPCR using the same primers and conditions as above.
To evaluate the copy number of the mRNA, ten-fold dilution series of each plasmid DNA containing the target sequence prepared to make the standard curve.
Generation of transgenic Daphnia
To make a reporter gene that can respond to heavy metal exposure, the promoter region of MT-A was fused to the GFP gene. The MT-A gene containing the promoter region and coding sequence was amplified from purified genomic DNA by PCR. Daphnia genomic DNA was prepared as described preciously17. The primer sequences were TCTCAGTCCAGGTTTGGTTAGGA and TGTCTTAGTTGGGTGCCATTCTC. The amplified DNA fragment was cloned into a pCR-Blunt II-TOPO vector (Thermo Fisher Scientific, Yokohama, Japan), generating pCR-MT-A. After the confirmation of the sequence, to obtain the DNA fragment containing MT-A promoter region and its vector, MT-A open reading frame and the putative three prime untranslated regions (3′-UTR) were removed by PCR using primers GCCCTTGCTCACCATGTTGATTGAAGATTTGGAATAGTGT and TTGTCCAAACTCATCAAGGGCGAATTCCAGCAC. The GFP coding sequence and polyA signal sequence was obtained from pAcGFP-C1 (Clontech Laboratories, Inc., CA, USA) using primers ATGGTGAGCAAGGGCGCCGA and GATGAGTTTGGACAAACCACAACTAGAATGCAGTG. By fusing these two DNA fragments using In-fusion HD Cloning Kit (Clontech Laboratories, Inc., CA, USA), GFP coding sequence and SV40 poly A signal sequence were inserted under the control of MT-A, which was designated as pCR-MTApro-GFP. As a NHEJ targeting site of TALEN, eyeless-targeting recognition sites17 was used because the site was successfully used in the previous study (REF). To fuse the eyeless site with pCR-MTApro-GFP, the MT-A promoter region with GFP DNA fragment was introduced to the pCS-ey2-4XJHRE-H2B-GFP-ey1, which is the plasmid containing eyeless site and used in the previous study (REF: Nakanishi T., Kato Y., Matsuura T., Watanabe H., (2016) TALEN-mediated knock-in) using the In-fusion Cloning Kit using the primers AAACCTCCACTGAGACTCCGACTGGCGTAATAGCGA and GCGGCCGTTACTAGTCGGTACCCAGCTTTTGTTCC. The chimeric gene was amplified by PCR using primers TCTCAGTGGAGGTTTGGTTAGGA and ACTAGTAACGGCCGCCAGTG. TALEN target sequences, CCGGCGAGAATTCTCGGTCG and ACAACAACACCTTCGGACG were located in exon 6 of eyeless gene. The ey1 TALEN mRNAs targeting these sites were synthesized as described previously18 (Supplementary Fig. S1).
Microinjection was performed as described previously17,23. Eggs were obtained from two- to three-week-old Daphnia directly after ovulation. The eggs were placed in ice-cold M4 medium24 containing 80 mM sucrose. The ey1 TALEN mRNA (500 ng/μl) and GFP reporter plasmids (50 ng/μl) were mixed with the injection marker Alexa Fluor 568 dye (Life Technologies Inc., Grand Island, NY, USA) at a final concentration of 0.01 µM. The microinjection was performed on ice and the injected eggs were incubated in a 96-well chamber at 23 °C until hatching.
The transgenic line was screened by the expression of GFP in offspring (Supplementary Fig. S1). GFP expression was observed under a Leica M165C fluorescence stereoscopic microscope (Leica Microsystems Heidelberg GmbH, Mannheim, Germany) fitted with a 480-nm excitation filter and a 510-nm barrier filter (GFP2 filter set). To confirm the transgene, inverse PCR was performed. Approximately 2 μg of genomic DNA was prepared from the transgenic animals, digested with EcoR I, and circularized by ligation using T4 DNA ligase at 4 °C for 24 h. The PCR product using primers derived from the vector sequence (GGGAGAAAGGCGGACAGGTATC and GGTAGCTCTTGATCCGGCAAAC) was sequenced to clarify the junction sequence (Supplementary Fig. S2) after cloning into a pBlunt II-TOPO vector using an In-fusion HD cloning kit (Takara Bio Inc., Siga, Japan).
Imaging of D. magna MetalloG
GFP expression was observed under a Leica M165C fluorescence stereoscopic microscope (Leica Microsystems Heidelberg GmbH, Mannheim, Germany) fitted with a 480-nm excitation filter and a 510-nm barrier filter (GFP2 filter set). Light field and fluorescent images were recorded with a color digital camera (Leica DC500; Leica Microsystems Heidelberg GmbH, Mannheim, Germany) mounted on the microscope. GFP expression was quantified based on the protocol described previously25.
To observe endogenous GFP expression of D. magna MetalloG, eggs were isolated from brood chamber of D. magna MetalloG just after ovulation and placed in 96 well plate containing ADaM medium.
Results and discussion
Heavy metal analysis
In order to confirm actual concentrations of heavy metals used for the exposure, each medium was sampled and heavy metals was measured by ICP-MS. ADaM medium, which was used as a control, contained certain amount of Zn (0.1–0.15 µM) in addition to trace amount of Mn, Cu, Cd and Pb (Supplementary Table S1), which were derived from sea salt used for ADaM preparation. ADaM also contains 1.45 µM Se. As mentioned above, these metals may induce GFP expression in a control medium as a background. Estimated concentrations of heavy metals by ICP-MS were generally close to nominal concentrations (Supplementary Table S2).
Metallothionein gene expression changes with heavy metal exposure
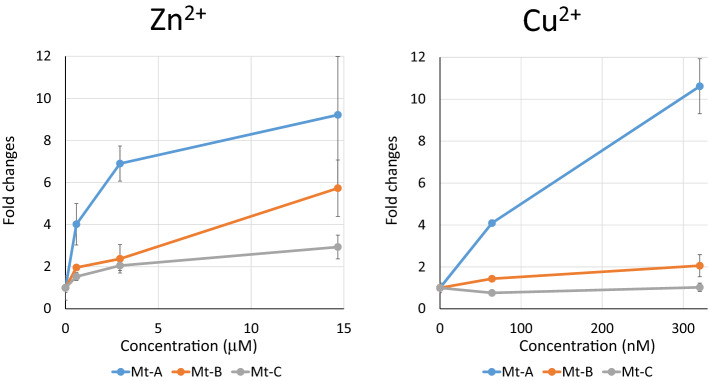
By searching the daphnia genome database (http://arthropods.eugenes.org/EvidentialGene/daphnia/), we identified three MT genes named MT-A, MT-B and MT-C. To select a metallothionein gene that could be effectively and strongly activated by heavy metals, quantitative PCR analysis was performed after exposing daphniids to CuSO4. Daphniids were subjected to 64 nM and 320 nM Cu2+ for 24 h and the mRNA expression levels of their MT genes were evaluated by q-PCR. MT-A showed the highest response to Cu2+ whereas MT-B was only slightly but not significantly activated. MT-C did not respond to Cu2+ (Fig. 1). Daphniids were also exposed to different concentrations of ZnCl2 for 24 h and the expression levels of MT-A, MT-B, and MT-C were examined. Only MT-A showed > 6× activation in 3 µM and 15 µM Zn2+ exposures, and the induction levels were almost the same for both concentrations. MT-B was activated only at the highest Zn2+ concentration (15 µM ); however, MT-C was not significantly induced even at that dose (Fig. 1).
Figure 1.
MT gene expression changes in response to heavy metal exposure. Indicated concentrations of Zn2+ and Cu2+ were exposed to neonate Daphnia (< 24 h) for 24 h. Five daphniids were used in 250 mL exposure and total RNA was extracted from the five daphniids. Expression levels of each MT gene were estimated by qPCR, normalized to the L32 level, and divided by the control (unexposed) expression level. The exposure was repeated three times. Bar = SE, *p < 0.05, +p ≤ 0.1.
MT-A alone showed the highest response to heavy metals. In the present study, MT-B showed weak activation and MT-C hardly respond at all to heavy metals. Our findings are comparable to those of a previous study12. The promoter regions of MT-A and MT-B have three metal response element (MRE) motifs and one MRE motif26,27, respectively. The presence of MRE in the promoter regions of Daphnia MT genes was also mentioned in another study15. Therefore, MRE might contribute to metal response and its occurrence in triplicate in MT-A might explain the fact that this metallothionein showed the strongest response to heavy metal exposures.
Transgenic Daphnia
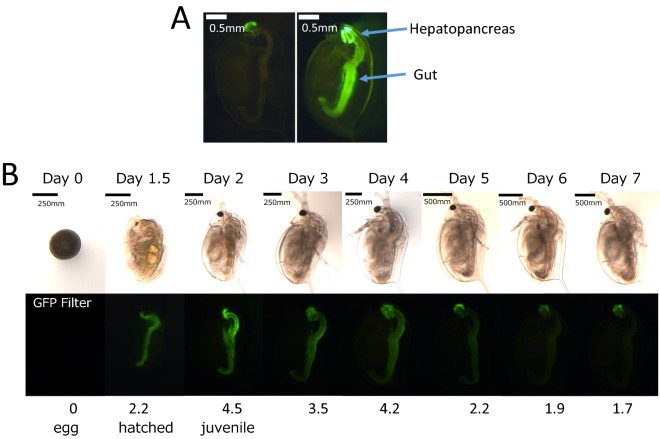
We obtained the promoter region and 5′-UTR of MT-A to drive the GFP gene since this metallothionein displayed the strongest response to ZnCl2. We obtained 1675 bp upstream sequence from the first ATG of MT-A and used as the MT-A promoter because coding sequence of another gene (Chitinase) was located further upstream region. We included a recognition sequence of eyeless-targeting TALEN named ey1 TALEN as previously constructed18 into the donor DNA (Supplementary Fig. S1), because we previously reported that Daphnia magna eyeless (Dma-ey, ortholog of mammalian pax6) gene can be used as a target site for recombination. This plasmid was knocked-in via TALEN-mediated non-homologous end-joining repair as described previously18. We injected 374 eggs and isolated one transgenic line designated as D. magna MetalloG (Fig. 2A, Supplementary Fig. S1). Transmission of the GFP reporter gene was confirmed by PCR using the progeny of the line and the integration site was sequenced. The transgenic Daphnia is suppsed to have 6 kb (3 kb of reporter gene and 3 kb of its vector) DNA fragment as a transgene. The junction sequence is shown in the Supporting Information (Supplementary Fig. S2).
Figure 2.
Heavy metal response image of D. magna MetalloG and endogenous GFP expression of D. magna MetalloG. (A) D. magna MetalloG daphnia at 7 days old exposed to 30 μM of ZnCl2 for 48 h (right panel) and control D. magna MetalloG (unexposed) Daphnia (left panel) at the same age. (B) Eggs were obtained from D. magna MetalloG brood chamber after ovulation (assigned as Day 0) and cultured in ADaM. Pictures were taken for one week using the same parameters throughout. Bar = 250 µm. Relative fluorescence intensities were calculated by the method of Törner et al. (23) and indicated under the figures.
Endogenous expression of GFP
D. magna MetalloG was cultured in ADaM and endogenous GFP expression was observed. During embryogenesis, GFP fluorescence could not be detected (data not shown). After hatching, GFP fluorescence was detected in the midgut and hepatopancreas (Fig. 2B day 1.5). The GFP fluorescence intensity in these organs was highest at day 2, then gradually decreased. At days 6 and 7, fluorescence in the midgut almost disappeared, and weak GFP expression could only be detected in the hepatopancreas (Fig. 2B). When the metal-free medium was used (M4 medium24 without any heavy metals), GFP expression could not be detected (data not shown). This result suggests that GFP expression observed at day 2 in ADaM medium was induced by the presence of metals contained in the ADaM medium itself (Supplementary Table S1). To develop a higher S/N ratio of D. magna MetalloG, we attempted to use the metal-free medium, however the D. magna MetalloG could not survive in the medium, suggesting the importance of MT-A expression or presence of heavy metals during early stages.
Tissue specific expression of MT-A and GFP
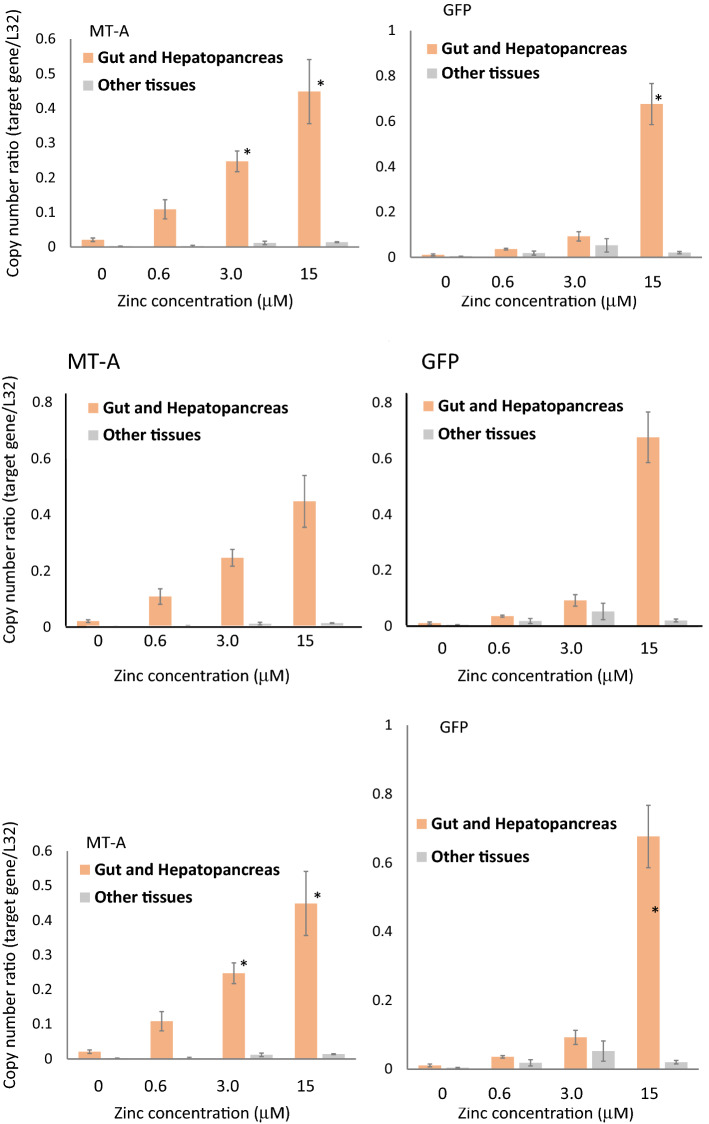
To compare the expression patterns of endogenous MT-A and GFP, D. magna MetalloG were dissected and their midgut and hepatopancreas and other tissues were separated after exposure to various concentrations of Zn2+. As shown in Fig. 3, the expression of both MT-A mRNA and GFP mRNA was exclusively observed in midgut and hepatopancreas and there was little expression in other tissues. They increased to a similar extent in a dose-dependent manner in the midgut and hepatopancreas. Therefore, the MT-A promoter used to drive GFP reflects endogenous MT-A expression well.
Figure 3.
Tissue-specific MT-A and GFP expressions. Neonatal Daphnia (< 24 h) were exposed to different concentrations of Zn2+ for 24 h. Midgut and hepatopancreas of D. magna MetalloG were dissected and mRNA was purified. mRNA was also purified from the remaining tissue. The mRNA expression levels of MT-A and GFP were estimated by qPCR. Relative amounts of mRNA to that of the ribosomal L32 protein gene were estimated by ΔΔCτ method and indicated in Y-axis. The quantification was performed three times. Bar = SE.
Although in situ hybridization is necessary to precisely compare the specific expression of endogenous MT-A and GFP transgene, our comparison of mRNA expression levels in the midgut and in other D. magna MetalloGtissues supports the fact that GFP expression mimics that of MT-A. This suggests that the promoter used in the present study contained both metal response element and DNA elements essential for tissue-specific expression in the hepatopancreas and midgut. Since these tissues are continuously exposed to the external environment, it is reasonable that the MT gene is expressed to protect cells from heavy metals existing in the external environment. It is notable that MT-A mRNA and GFP mRNA expression were comparable to the expression level of ribosomal L32 protein mRNA (Supplementary Table S3). As ribosomal proteins are ones of proteins highly expressed in cells, this result suggests the presence of abundant MT mRNA and probably MT protein in these tissues. When mRNA was prepared, a nearly tenfold induction of MT-A mRNA was observed (Fig. 1) and a greater than 20-fold induction was observed in the isolated tissues. This higher induction of mRNA facilitates the induction and detection of GFP. Heavy metal accumulation in the hepatopancreas has been reported in crayfish28 and heavy metal MT induction in the same organ was indicated in crabs29, which suggests that the hepatopancreas sequesters and detoxifies heavy metals in crustaceans. GFP expression of the hepatopancreas in D. magna MetalloG suggests that hepatopancreas function is widely conserved in crustacean including Daphnia and tissue functions in this organism.
GFP expression by single heavy metal exposure
Based on endogenous GFP expression, seven-day-old Daphnia was used in heavy metal exposure tests when the background GFP levels were at a minimum (Fig. 2B day 7). When D. magna MetalloG was exposed to Zn2+ for 1 h, the GFP expression in the hepatopancreas increased. GFP expression was significantly enhanced at Zn2+ concentrations 1.2 µM (Fig. 4) and GFP intensity increased with Zn2+ concentration. These results were consistent with those of the MT-A gene activation test (Fig. 1).
Figure 4.
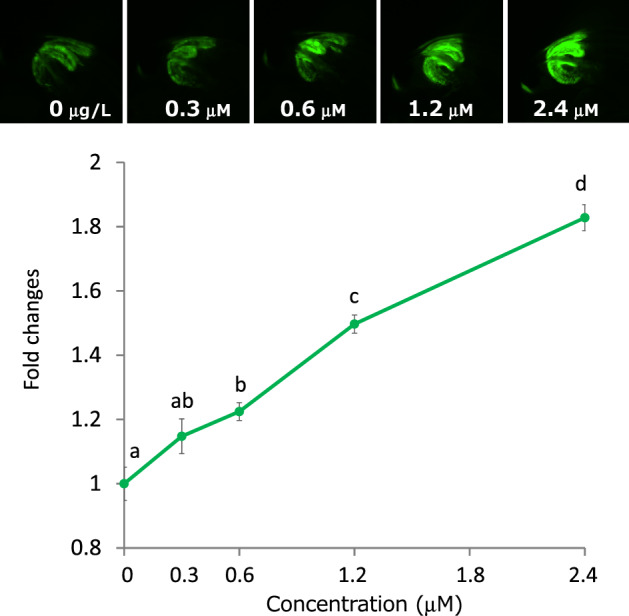
GFP expression changes after ZnCl2 exposure for 1 h. Top: representative images of hepatopancreas exposed to ZnCl2 for 1 h. Bottom: GFP expressions were quantified and fold changes vs. 0 mg/L fluorescence intensity were calculated. Five daphniids were exposed in 10 mL of ADaM for 1 h. Fold changes vs. unexposed control were plotted. N = 5; **p < 0.01 (t-test).
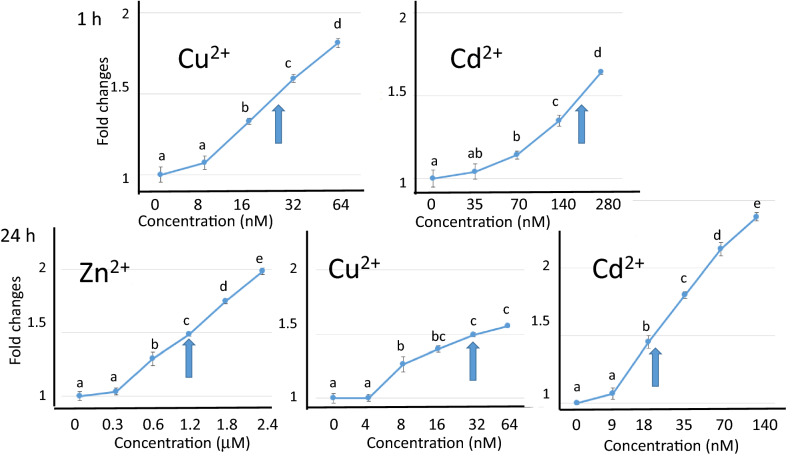
The responses of D. magna MetalloG to Cu2+ and Cd2+ were also examined after 1 h exposure. As shown in Fig. 5, GFP expression was enhanced in a dose-dependent manner in the presence of either Cu2+ or Cd2+. The minimum concentrations of Cu2+ and Cd2+ that significantly enhanced GFP expression were 130 nM and 70 nM, respectively (Fig. 5, Supplementary Table S4).
Figure 5.
Dose depenent GFP expression changes after heavy metal exposure for 1 h or 24 h. Five daphniids were exposed in 10 mL of ADaM containing indicated metal ions for 1 h or 24 h. GFP expression was quantified and normalized to the control (0 µg/L) expression level. Numbers on x-axis indicate concentrations of each heavy metal (µM for Zn2+ and nM for Cu2+ and Cd2+). Y-axis indicates fold changes. Arrows indicate ECIR1.5 values. Top: 1 h exposure, Bottom: 24 h exposure. N = 5; ***p < 0.001; **p < 0.01; *p < 0.05 (t-test).
To determine whether longer exposures to heavy metals reduced detection levels, we examined GFP expression changes after 24 h. GFP expression increased with metal exposure time (Fig. 5). Significant enhancements of GFP expression were observed with 0.6 µM ZnCl2, 67 nM Cu2+, and 18 nM of Cd2+. These values did not change even after 48 h exposure (Supplementary Table S4) and longer (Supplementary Fig. S3). These values were much lower than EC50 values of conventional chronic toxicity test for 24 h. When EC50 values were determined using wild-type, they were as follows: Zn2+: 180 µM, Cu2+: 0.43 µM, and Cd2+: 1.7 µM (Supplementary Table S4). Therefore, the GFP response in transgenic Daphnia might prove useful in the detection of heavy metals.
GFP expression by multiple heavy metals exposure
To examine whether D. magna MetalloG responded to multiple metal exposure, D. magna MetalloG was exposed to 0.9 µM of Zn2+, 20 nM of Cu, or 110 nM of Cd2+, and a combination of these metals. As shown in Fig. 6, when Cd2+ was combined with other metals, GFP fluorescence was significantly induced at 1 h. GFP fluorescence was the highest when the three metals were mixed and exposed. Although there was no significance in the exposure of Zn2+ and Cu2+, GFP was also induced. These results indicated that GFP fluorescence could be induced additively when multiple heavy metals were exposed. After 24 h exposure, each metal induced GFP expression and additive effect of the mixture of metals could be prominently observed. Only one dose of the three metals were applied in the present study, additional experiments using various concentrations of metals would further clarify the GFP induction profile. Measured concentrations of the medium are indicated in Supplementary Table S5, which shows measured concentrations were close to nominal concentrations.
Figure 6.
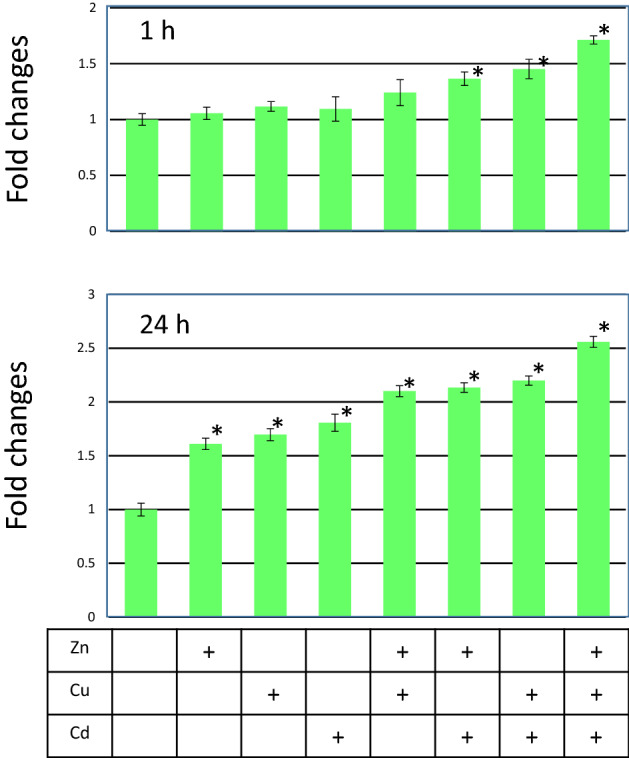
GFP expression by multiple heavy metals exposure. Seven day old D. magna MetalloG was exposed to 0.9 µM of Zn2+, 20 nM of Cu2+, or 110 nM of Cd2+, and a combination of these metals. After 1 h and 24 h exposure, GFP expression was quantified, These values were divided by that of control (0 µg/L) expression level and fold changes were calculated. Metal ions used for the exposure are indicated in the boxes (+presence of ion indicated on the left column (Y-axis indicates fold changes. *p < 0.05 (t-test) Measured concentrations are indicated in Supplementary Table S5.
Advantages of D. magna MetalloG
The use of GFP has been proposed previously6 and applied as a reporter gene in other species. To detect heavy metals in the water, heavy metal monitoring medaka have been reported. The GFP in the transgenic medaka is driven by Hsp70, which responds to 110 nM Cd2+ after 24 h exposure30. D. magna MetalloG can respond to 18 nM Cd2+ after 24 h; therefore, the Daphnia biosensor is more sensitive than the medaka. In addition, Hsp70 can respond not only to metals but to other stimuli including heat; therefore, the expression of GFP does not necessarily indicate the presence of heavy metals. Conversely, the metallothionein gene responds to particular metals and could, therefore, be useful for the classification of toxicants. Recently, transgenic zebrafish containing the GFP gene under the metallothionein promoter have been developed; however, the transgenic zabrafish only responded to high metal concentrations (Cd2+: 0.5 ppm (4.5 µM), Zn2+: 20 ppm (306 µM);, and Cu2+: 0.3 ppm (4.7 µM))31. While the promoter used for the detection of heavy metal was MT, sensitivity to metals was more than hundred times higher in Daphnia. To detect heavy metals in soil, a transgenic nematode was also established32. In the transgenic nematode, the lowest observed effect concentration to Cd2+ was reported as 5 µM (0.9 ppm, 900 µg/L). These results indicate that Daphnia is a suitable species for the detection of heavy metals in much lower concentrations, which might be reasonable because Daphnia is known to be more sensitive to heavy metals than fish. Therefore, D. magna MetalloG is the most sensitive and rapid GFP-expressing biosensor ever developed.
Compared to zebrafish and medaka, Daphnia has application advantages. Daphnia is an invertebrate; therefore, there are no legal ethical issues with this genus during experimental studies. As the regulation of animal use is becoming stricter, the application of invertebrates is preferable to vertebrates. In addition, Daphnia can mature in one week and start reproduction, which facilitates propagation and use. Based on genome editing techniques in Daphnia developed in our laboratory, this is the first reported study of a transgenic strain utilized as a biosensor strain. Our technique provides several types of biosensors using Daphnia in the near future.
Perspective of the use of D. magna MetalloG
D. magna MetalloG still has problems that need to be improved. One is endogenous expression and the other is tissue specific expression. GFP expression was detected from Daphnia’s exposure to the ADaM, which contained trace amounts of Mn, Rb, Sr, Co, Se, and other metals (Supplementary Table S1). To eliminate these heavy metal ions, we examined if metal free synthetic medium could be used for the test. When the synthetic water was used (M4 medium without any metal ions), the endogenous GFP expression was significantly reduced; however, the daphniids died. By reducing the amounts of metals in medium to minimum levels, it might be possible to decrease the lower limit of sensitivity. Exclusive GFP expression might not be desirable as a biosensor because observation of Daphnia with high magnification is required. If the Daphnia expresses GFP in their entire body, it becomes much easier to detect the expression, possibly without using a microscope. Therefore, the rational design of the promoter by eliminating the DNA element responsible for tissue-specificity and, adding multiple MER might help further development of Daphnia as a biosensor.
The detection limits to these heavy metals of D. magna MetalloGnking Water Regulations, USEPA33 Inorganic Maximum Contaminant Level Goal of Copper and Cadmium were assigned as 1.3 mg/L and 0.005 mg/L, respectively. Secondary Maximal Contaminant Level of Zinc was assigned as 5 mg/L in Secondary Drinking Water Standards, USEPA34. Thus, D. magna MetalloG might be useful for detecting heavy metals near regulatory levels.
In conclusion, we developed a genome-edited Daphnia that can express GFP that responds to heavy metals. The sensitivity of GFP expression is much higher than that of conventional acute toxicity testing. In addition, genome-edited Daphnia can detect heavy metals much faster (1 h) than the conventional method (24–48 h). Daphnia is particularly sensitive to aquatic chemical stresses. By introducing a sensitive reporter gene into a susceptible organism, a highly accurate bioassay could be created. The introduction of other reporter genes responsive to other toxicants can build genome-edited Daphnia that facilitate water quality monitoring and contaminant identification.
Supplementary information
Author contributions
T.A. made the transgenic animal and performed the main experiments. Q.D.N., K.T., A.O., T.M. took pictures and made figures. N.T. did exposure and evaluation. H.Y., H.W. measured metal concentration. T.M. and Y.K. wrote the main manuscript. H.W. supervised the experiments and finalized the manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-78572-z.
References
- 1.Elzwayie A, Afan HA, Allawi MF, El-Shafie A. Heavy metal monitoring, analysis and prediction in lakes and rivers: state of the art. Environ. Sci. Pollut. Res. 2017;24:12104–12117. doi: 10.1007/s11356-017-8715-0. [DOI] [PubMed] [Google Scholar]
- 2.Winefordner JD, et al. Comparing several atomic spectrometric methods to the super stars: special emphasis on laser induced breakdown spectrometry, LIBS, a future super star. J. Anal. At. Spectrom. 2004;19:1061. doi: 10.1039/b400355c. [DOI] [Google Scholar]
- 3.Zhou Q, Zhang J, Fu J, Shi J, Jiang G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta. 2008;606:135–150. doi: 10.1016/j.aca.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Prokić MD, et al. Antioxidative responses of the tissues of two wild populations of Pelophylax kl esculentus frogs to heavy metal pollution. Ecotoxicol. Environ. Saf. 2016;128:21–29. doi: 10.1016/j.ecoenv.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Iguchi T, Watanabe H, Katsu Y. Toxicogenomics and ecotoxicogenomics for studying endocrine disruption and basic biology. Gen. Comp. Endocrinol. 2007;153:25–29. doi: 10.1016/j.ygcen.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Gong Z, Ju B, Wan H. Green fluorescent protein (GFP) transgenic fish and their applications. Genetica. 2001;111:213–225. doi: 10.1023/A:1013796810782. [DOI] [PubMed] [Google Scholar]
- 7.Blechinger SR, Warren JT, Kuwada JY, Krone PH. Developmental toxicology of cadmium in living embryos of a stable transgenic zebrafish line. Environ. Health Perspect. 2002;110:1041–1046. doi: 10.1289/ehp.021101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng GHB, Gong Z. GFP transgenic medaka (Oryzias latipes) under the Inducible cyp1a promoter provide a sensitive and convenient biological indicator for the presence of TCDD and other persistent organic chemicals. PLoS ONE. 2013;8:e64334. doi: 10.1371/journal.pone.0064334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng Z, Shan T, Tong Y, Lam SH, Gong Z. Development of estrogen-responsive transgenic medaka for environmental monitoring of endocrine disrupters. Environ. Sci. Technol. 2005;39:9001–9008. doi: 10.1021/es050728l. [DOI] [PubMed] [Google Scholar]
- 10.Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu. Rev. Pharmacol. Toxicol. 1999;39:267–294. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Reyero N, et al. Biomarker discovery and transcriptomic responses in Daphnia magna exposed to munitions constituents. Environ. Sci. Technol. 2009;43:4188–4193. doi: 10.1021/es803702a. [DOI] [PubMed] [Google Scholar]
- 12.Poynton HC, Varshavsky JR, Chang B, Cavigiolio G, Chan S, Holman PS, Loguinov AV, Bauer DJ, Komachi K, Theil EC. Daphnia magna ecotoxicogenomics providesmechanistic insights into metal toxicity. Environ. Sci. Technol. 2007;80:1044–1050. doi: 10.1021/es0615573. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Yan Y, Wang J, Wu W, Xu L. Generation of mt:egfp transgenic zebrafish biosensor for the detection of aquatic zinc and cadmium. Environ. Toxicol. Chem. 2016;35:2066–2073. doi: 10.1002/etc.3362. [DOI] [PubMed] [Google Scholar]
- 14.Ma H, Glenn TC, Jagoe CH, Jones KL, Williams PL. A transgenic strain of the nematode caenorhabditis elegans as a biomonitor for heavy metal contamination. Environ. Toxicol. Chem. 2009;28:1311. doi: 10.1897/08-496.1. [DOI] [PubMed] [Google Scholar]
- 15.Asselman J, et al. Functional characterization of four metallothionein genes in Daphnia pulex exposed to environmental stressors. Aquat. Toxicol. 2012;110–111:54–65. doi: 10.1016/j.aquatox.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakanishi T, Kato Y, Matsuura T, Watanabe H. CRISPR/Cas-mediated targeted mutagenesis in Daphnia magna. PLoS ONE. 2014;9:e98363. doi: 10.1371/journal.pone.0098363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naitou A, Kato Y, Nakanishi T, Matsuura T, Watanabe H. Heterodimeric TALENs induce targeted heritable mutations in the crustacean Daphnia magna. Biol. Open. 2015;4:364–369. doi: 10.1242/bio.20149738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakanishi T, Kato Y, Matsuura T, Watanabe H. TALEN-mediated homologous recombination in Daphnia magna. Sci. Rep. 2015;5:18312. doi: 10.1038/srep18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato Y, Matsuura T, Watanabe H. Genomic integration and Germline transmission of plasmid injected into Crustacean Daphnia magna Eggs. PLoS ONE. 2012;7:e45318. doi: 10.1371/journal.pone.0045318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klüttgen B, Dülmer U, Engels M, Ratte HT. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 1994;28:743–746. doi: 10.1016/0043-1354(94)90157-0. [DOI] [Google Scholar]
- 21.Kato Y, et al. Molecular cloning and sexually dimorphic expression of DM-domain genes in Daphnia magna. Genomics. 2008;91:94–101. doi: 10.1016/j.ygeno.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Kato Y, Kobayashi K, Watanabe H, Iguchi T. Environmental sex determination in the branchiopod crustacean Daphnia magna: deep conservation of a Doublesex gene in the sex-determining pathway. PLoS Genet. 2011;7:e1001345. doi: 10.1371/journal.pgen.1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato Y, et al. Development of an RNA interference method in the cladoceran crustacean Daphnia magna. Dev. Genes Evol. 2011;220:337–345. doi: 10.1007/s00427-011-0353-9. [DOI] [PubMed] [Google Scholar]
- 24.Elendt BP, Bias WR. Trace nutrient deficiency in Daphnia magna cultured in standard medium for toxicity testing Effects of the optimization of culture conditions on life history parameters of D. magna. Water Res. 1990;24:1157–1167. doi: 10.1016/0043-1354(90)90180-E. [DOI] [Google Scholar]
- 25.Törner K, Nakanishi T, Matsuura T, Kato Y, Watanabe H. Optimization of mRNA design for protein expression in the crustacean Daphnia magna. Mol. Genet. Genom. 2014;289:707–715. doi: 10.1007/s00438-014-0830-8. [DOI] [PubMed] [Google Scholar]
- 26.Stuart GW, Searle PF, Palmiter RD. Identification of multiple metal regulatory elements in mouse metallothionein-I promoter by assaying synthetic sequences. Nature. 1985;317:828–831. doi: 10.1038/317828a0. [DOI] [PubMed] [Google Scholar]
- 27.Westin G, Schaffner W. A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. EMBO J. 1988;7:3763–3770. doi: 10.1002/j.1460-2075.1988.tb03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kouba A, Buřič M, Kozák P. Bioaccumulation and effects of heavy metals in crayfish: A review. Water. Air. Soil Pollut. 2010;211:5–16. doi: 10.1007/s11270-009-0273-8. [DOI] [Google Scholar]
- 29.Olafson RW, Kearns A, Sim RG. Heavy metal induction of metallothionein synthesis in the hepatopancreas of the crab Scylla serrata. Comp. Biochem. Physiol. Part B Comp. Biochem. 1979;62:417–424. doi: 10.1016/0305-0491(79)90112-3. [DOI] [Google Scholar]
- 30.Ng GHB, Xu H, Pi N, Kelly BC, Gong Z. Differential GFP Expression Patterns Induced by Different Heavy Metals in Tg(hsp70:gfp) Transgenic Medaka (Oryzias latipes) Mar. Biotechnol. 2015;17:317–327. doi: 10.1007/s10126-015-9620-5. [DOI] [PubMed] [Google Scholar]
- 31.Pawar N, Gireesh-Babu P, Sivasubbu S, Chaudhari A. Transgenic zebrafish biosensor for the detection of cadmium and zinc toxicity. Curr. Sci. 2016;111:1697–1701. doi: 10.18520/cs/v111/i10/1697-1701. [DOI] [Google Scholar]
- 32.Aschner M, Martinez-Finley EJ. Revelations from the nematode caenorhabditis elegans on the complex interplay of metal toxicological mechanisms. J. Toxicol. 2011 doi: 10.1155/2011/895236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Primary Drinking Water Regulations. https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#.
- 34.Secondary Drinking Water Standards: Guidance for Nuisance Chemicals. https://www.epa.gov/dwstandardsregulations/secondary-drinking-water-standards-guidance-nuisance-chemicals.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.