Abstract
BACKGROUND AND AIMS:
Per- and polyfluoroalkyl substances (PFAS) are widespread and persistent pollutants that have been shown to have hepatotoxic effects in animal models. However, human evidence is scarce. We evaluated how prenatal exposure to PFAS associates with established serum biomarkers of liver injury and alterations in serum metabolome in children.
APPROACH AND RESULTS:
We used data from 1,105 mothers and their children (median age, 8.2 years; interquartile range, 6.6–9.1) from the European Human Early-Life Exposome cohort (consisting of six existing population-based birth cohorts in France, Greece, Lithuania, Norway, Spain, and the United Kingdom). We measured concentrations of perfluorooctane sulfonate, perfluorooctanoate, perfluorononanoate, perfluorohexane sulfonate, and perfluoroundecanoate in maternal blood. We assessed concentrations of alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyltransferase in child serum. Using Bayesian kernel machine regression, we found that higher exposure to PFAS during pregnancy was associated with higher liver enzyme levels in children. We also measured child serum metabolomics through a targeted assay and found significant perturbations in amino acid and glycerophospholipid metabolism associated with prenatal PFAS. A latent variable analysis identified a profile of children at high risk of liver injury (odds ratio, 1.56; 95% confidence interval, 1.21–1.92) that was characterized by high prenatal exposure to PFAS and increased serum levels of branched-chain amino acids (valine, leucine, and isoleucine), aromatic amino acids (tryptophan and phenylalanine), and glycerophospholipids (phosphatidylcholine [PC] aa C36:1 and Lyso-PC a C18:1).
CONCLUSIONS:
Developmental exposure to PFAS can contribute to pediatric liver injury.
Nonalcoholic fatty liver disease (NAFLD) is increasingly diagnosed at younger ages, currently affecting 8%−12% of the general pediatric population in the United States and Europe.(1) In large population studies, the prevalence of elevated serum levels of alanine aminotransferase (ALT), a biomarker of liver injury, has almost tripled among U.S. adolescents.(2) If current trends continue, NAFLD prevalence is estimated to increase by 20% over the next 10 years,(3) resulting in increased morbidity from cardiovascular disease, chronic kidney disease, and type 2 diabetes.(4) Hence, there is an urgent need to identify modifiable risk factors for liver injury and NAFLD that can be targeted for more-efficient prevention strategies.
Recently, it has been postulated that environmental chemicals with endocrine disrupting activity, commonly known as endocrine disrupting chemicals (EDCs), have the ability to promote metabolic changes that can result in fatty liver—a hypothesis referred to as the “Metabolism Disrupting Chemical” hypothesis.(5) In line with this, per- and polyfluoroalkyl substances (PFAS), a class of EDCs, concentrate considerably within the liver(6,7) and may affect liver function.(8) PFAS are chemically and thermally stable synthetic compounds widely used in various industrial applications and consumer products, including fire-fighting foams, nonstick coatings, water- and stain-repellent textiles, and food packaging.(9) These substances have long half-lives in human tissues (estimated at up to 7 years, depending on the PFAS), and recent human biomonitoring studies show widespread exposure, with the most highly detected PFAS being perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), perfluorononanoate (PFNA), and perfluorohexane sulfonate (PFHxS).(10,11)
Animal studies show that PFAS exposure causes elevated liver enzymes, liver enlargement, and hepatic steatosis,(12,13) and that this PFAS-induced hepatotoxicity can start in utero.(14,15) Indeed, the “Developmental Origins of Health and Disease” paradigm highlights the importance of pollutant exposures during early development in eliciting metabolic changes and increased disease risk, even after the exposure has occurred.(16) Nevertheless, in humans, evidence on the effects of PFAS on liver injury is limited to cross-sectional studies in adults(17–20) and only a few studies in children.(21,22) Furthermore, most of these studies examined effects of each PFAS compound separately; however, humans are simultaneously exposed to several PFAS that may have additive or interactive effects.(23)
Dysregulation of lipid and amino acid metabolism has been linked to liver injury and NAFLD pathogenesis(24); however, mechanisms underlying the effects of prenatal PFAS exposure on child liver injury remain unclear. Experimental studies have shown that PFAS can increase oxidative stress while disrupting nuclear factor erythroid 2–related factor 2–regulated hepatic antioxidant defenses, exert immunotoxic effects, and induce hepatocyte caspase 3–mediated apoptosis.(25–28) Moreover, they have been shown to affect the activity of peroxisome proliferator-activated receptor-alpha and -gamma and hepatocyte nuclear factor 4-alpha, which play an important role in transcriptional regulation of lipid and amino acid metabolism.(29,30) In a cross-sectional study of children with biopsy-proved NAFLD, we demonstrated that concurrent exposure to PFAS was associated with increased risk for nonalcoholic steatohepatitis (NASH) and higher plasma levels of phosphoethanolamine, tyrosine, phenylalanine, aspartate, and creatine.(22) Here, in a well-characterized multicenter pregnancy cohort of 1,105 mothers and their children who were followed up to the age of 6–10 years, we studied how prenatal exposure to PFAS associates with liver enzymes and alterations in serum metabolome in childhood. We hypothesized that increased prenatal plasma PFAS concentrations would be associated with increased risk of liver injury and alterations in key metabolic pathways implicated in NAFLD pathophysiology.
Participants and Methods
STUDY SUBJECTS
This study was part of the Human Early-Life Exposome project (HELIX) project,(31) a collaboration across six established and ongoing longitudinal, population-based birth-cohort studies in Europe: the BiB (Born in Bradford) study in the United Kingdom, the EDEN (Étude des Déterminants pré et postnatals du développement et de la santé de l’Enfant) study in France, the INMA (INfancia y Medio Ambiente) cohort in Spain, the KANC (Kaunas cohort) in Lithuania, the MoBa (Norwegian Mother, Father and Child Cohort Study),(32) and the RHEA Mother Child Cohort study in Crete, Greece. Across these cohorts, a subcohort of 1,301 mothers and their singleton children (~200 children in each cohort) was followed in 2014–2015 for a clinical examination, a computer-assisted interview with the mother, and the collection of additional biological samples. Data collection was standardized across cohorts and performed by trained staff. The full HELIX protocol and database are described elsewhere.(31)
Our study population consisted of 1,105 (85%) mothers and their children (median age, 8.2 years; interquartile range, 6.6–9.1) from the HELIX subcohort, included based on availability of information on PFAS exposure during pregnancy and on liver enzyme levels and serum metabolomic profiling in childhood. All participating families provided written informed consent. Approval for the HELIX project was obtained from the local ethical committees at each site. Additionally, the current study was approved by the University of Southern California Institutional Review Board.
PLASMA PFAS CONCENTRATIONS IN PREGNANCY
Maternal blood samples were collected in mid-pregnancy for the INMA (mean gestational age [SD], 13.7 [2.0] weeks), MoBa (18.7 [0.9] weeks), and RHEA cohorts (14.1 [3.7] weeks) and in late pregnancy for the BiB (26.6 [1.4] weeks), EDEN (26.1 [1.2] weeks), and KANC cohorts (39.4 [1.3] weeks). Measurement of PFAS was performed at the Department of Environmental Exposure and Epidemiology at the Norwegian Institute of Public Health (NIPH) in plasma or serum samples for BiB, in serum samples for EDEN and RHEA, in whole-blood samples for KANC, and in plasma samples for MoBa. For INMA, PFAS were measured in plasma samples at the Institute for Occupational Medicine, RWTH Aachen University in Germany. Concentrations of PFOS, PFOA, PFNA, PFHxS, and perfluoroundecanoate (PFUnDA) were determined using column-switching liquid chromatography (LC) coupled to a triple-quadrupole mass spectrometer in serum or plasma and online solid-phase extraction and ultra-high-performance LC coupled with tandem mass spectrometry (MS) in whole blood.(33) A ratio of 1:1 was assumed for serum and plasma, whereas 1:2 ratios were assumed for whole-blood versus serum/plasma.(33) The limit of detection (LOD) was 0.02 μg/L for samples assessed at NIPH, whereas for samples assessed at RWTH Aachen University, LODs were 0.05 μg/L for PFNA and 0.1 μg/L for the other PFAS. We used all available PFAS measures and applied quantile regression imputation of left-censored data (QRILC) to obtain singly imputed values for samples with concentration below LOD (ranging from 0.3% to 4.7% across PFAS). QRILC imputes missing elements using random draws from a truncated distribution estimated by a quantile regression and has been shown to have high imputation accuracy.(34) Details about quality assurance and quality control have been reported(33) and are briefly described in Supporting Text S1.
BLOOD SAMPLE COLLECTION IN HELIX CHILDREN
Children provided blood samples during the subcohort follow-up visit at the end of the clinical examination after a median (5th, 95th percentile) fasting time of 3.3 (2.2, 5.9) hours. Blood samples were collected and processed according to identical predefined standardized protocols across all six cohorts.
LIVER ENZYME LEVELS IN CHILDHOOD
We assessed levels of ALT, aspartate aminotransferase (AST), and gamma-glutamyltransferase (GGT) in child serum at the Biochemistry Laboratory of the Clínica Universidad de Navarra using homogenous enzymatic colorimetric methods on a Colorimetry Cobas 8000 analyzer, according to the manufacturer’s instructions (Roche Diagnostics GmbH, Mannheim, Germany). All coefficients of variation (CVs) were <3%.
METABOLITE PROFILING IN CHILDHOOD
Child metabolite levels were quantified in serum at Imperial College of London (London, UK), using the targeted metabolomics AbsoluteIDQ p180 Kit (Biocrates Life Sciences AG, Innsbruck, Austria). The kit allows the targeted analysis of 188 metabolites of different classes, including amino acids, biogenic amines, acylcarnitines, glycerophospholipids, sphingolipids, and sum of hexoses, thus covering a wide range of analytes and metabolic pathways in one targeted assay. Of the total 188 metabolites, 42 were analyzed quantitatively by LC-electrospray ionization (ESI)-MS/MS with the use of external calibration standards at seven different concentrations and isotope-labeled internal standards for most analytes. The other 146 metabolites were analyzed by flow injection analysis-ESI-MS/MS using a 1-point internal standard calibration with 14 representative internal standards. We excluded 11 serum metabolites having a CV >30% and >30% of the data below LOD, thus leaving 177 metabolites to be used for further analysis. Median CV across these metabolites was 11.9%. Details about the assessment of serum metabolites in HELIX children have been published.(35)
STATISTICAL ANALYSES
We defined liver injury risk as having any liver enzyme concentration above the 90th percentile for the study population (ALT, ≥22.7 IU/L; AST, ≥41.4 IU/L; or GGT, ≥17.1 IU/L). Maternal concentrations of PFAS were right-skewed and log2-transformed to improve model fit. Spearman’s correlation coefficients were computed to assess pair-wise correlations between individual PFAS compounds.
We examined the association of prenatal PFAS mixture exposure with liver injury risk using Bayesian kernel machine regression (BKMR), a nonparametric flexible modeling approach that can accommodate for both correlation and nonlinearity when evaluating the PFAS exposure response. Details about the BKMR models used in our study can be found in Supporting Text S2. Information on covariates was obtained through interviews, self-administered questionnaires, ad-hoc measurements, or medical records. We identified potential confounders and predictors of the outcomes of interest based on previous knowledge and a directed acyclic graph approach (Supporting Fig. S1). We included the following covariates in the models: cohort of inclusion, maternal age (in years), maternal education level (low, middle, or high), maternal prepregnancy body mass index (BMI; in kg/m2), child ethnicity (white, other), child age (in years), and child sex.
We performed several sensitivity analyses in the PFAS mixture-response association. First, we made further adjustment for child plasma levels of PFAS. Second, we additionally adjusted for child weight status (normal weight vs. overweight or obese) based on the age- and sex-specific BMI cutoffs proposed by the World Health Organization.(36) Third, we adjusted for gestational weight gain, available food indicators of maternal diet quality (consumption of fish, fruits, and vegetables; in times per week), child sedentary behavior (minutes per week), and food indicators of child diet quality (fish intake, total fruit and vegetable intakes, and total sugar-sweetened beverage consumption; in times per week). Fourth, we repeated analysis while excluding one cohort at a time to assess whether a specific cohort had a marked influence on the overall mixture effect. Fifth, we repeated analysis after stratifying by sex, given that metabolic effects in children of prenatal PFAS exposure have been previously suggested to differ by sex.(37) Sixth, we conducted stratified analyses by mid- and late-pregnancy period of PFAS assessment to assess the extent to which maternal physiological differences between these periods can affect the results.
Serum concentrations of metabolites in childhood were right-skewed and log10-transformed to improve normality. We then applied xMWAS, an automated framework for data-driven integration and differential network analysis,(38) to identify metabolites that were differentially associated with PFAS between children at high and low risk for liver injury. Details about this approach are provided in Supporting Text S3. In each group of children, we used xMWAS to first select the metabolites with the largest association to PFAS and then generate a global association network. We then compared the eigenvector centrality of each metabolite between the high- and low-risk networks to identify those with differences in their network topology. This can gauge the relative importance of a metabolite in discriminating biological processes between the two risk groups.(39,40) If a metabolite was not present in one of the networks, its eigenvector centrality was considered 0. Metabolites with an absolute value of difference in their eigenvector centrality >0.2 were considered to have differential contribution to, and thus disparate importance in, the high-risk group as compared to low risk.(39) Upon identification of the differentiating metabolites in the high- versus low-risk group, we annotated them to their Kyoto Encyclopedia of Genes and Genomes (KEGG) and Chemical Entities of Biological Interest identifiers and performed pathway overpresentation analysis, using ConsensusPathDB,(41) to characterize dysregulated metabolic pathways that are both associated with PFAS exposure and risk for liver injury. KEGG pathways with more than two annotated metabolites and a P value < 0.05 based on the hypergeometric test were kept for further evaluation.
Last, to provide insight into the joint contribution of prenatal PFAS and the metabolites to liver injury risk, we performed an integrated latent variable analysis to estimate latent unknown clusters(42) of children associated with increased susceptibility to liver injury by incorporating information on their prenatal PFAS mixture exposure and the metabolites identified from the network analysis. Details about this method are provided in Supporting Text S4.
All analyses were done using R software (version 3.5.3; R Foundation for Statistical Computing, Vienna, Austria). We used the software packages bkmr for BKMR analysis, xMWAS for network analysis, and LUCIDus for the integrated latent variable analysis.
Results
STUDY POPULATION CHARACTERISTICS
No differences in liver enzyme levels were observed between boys and girls. A total of 253 children (22.8%) were classified as being at risk for liver injury. The percentage of children at risk for liver injury was highest in the Greek cohort (31.6%) and lowest in the Lithuanian cohort (4.4%; Table 1). Children at risk for liver injury were more likely to be nonwhites, overweight or obese, and born from mothers with low educational status and higher BMI prepregnancy.
TABLE 1.
Sociodemographic Characteristics of the Study population overall and by Child liver Injury Risk
| Liver Injury Risk* |
||||
|---|---|---|---|---|
| All (N = 1,105) | Low (n = 852) | High (n = 253) | P for Difference† | |
| Cohort of inclusion | <0.001 | |||
| BiB, UK | 95 (8.6) | 40 (4.7) | 55 (21.7) | |
| EDEN, France | 196 (17.7) | 154 (18.1) | 42 (16.6) | |
| INMA, Spain | 212 (19.2) | 164 (19.3) | 48 (19) | |
| KANC, Lithuania | 166 (15) | 155 (18.2) | 11 (4.4) | |
| MoBa, Norway | 271 (24.5) | 254 (29.8) | 17 (6.7) | |
| RHEA, Greece | 165 (14.9) | 85 (10) | 80 (31.6) | |
| Maternal characteristics | ||||
| Age at birth (years) | 31.0 (4.7) | 31.1 (4.7) | 30.0 (4.8) | 0.93 |
| Prepregnancy BMI (kg/m2) | 24.5 (4.8) | 24.1 (4.4) | 25.8 (5.7) | <0.001 |
| Normal weight (<25 kg/m2) | 703 (63.6) | 564 (66.2) | 139 (54.9) | |
| Overweight (≥25–<30 kg/m2) | 262 (23.7) | 198 (23.2) | 64 (25.3) | |
| Obesity (≥30 kg/m2) | 140 (12.7) | 90 (10.6) | 50 (19.8) | |
| Maternal smoking in pregnancy | ||||
| No | 948 (85.8) | 738 (86.6) | 210 (83.0) | 0.15 |
| Yes | 157 (14.2) | 114 (13.4) | 43 (17.0) | |
| Education | <0.001 | |||
| Low | 124 (11.2) | 83 (9.7) | 41 (16.2) | |
| Medium | 382 (34.6) | 277 (32.5) | 105 (41.5) | |
| High | 599 (54.2) | 492 (57.8) | 107 (42.3) | |
| Parity | 0.11 | |||
| Nulliparous | 509 (46.0) | 398 (46.7) | 112 (44.3) | |
| Multiparous | 596 (54.0) | 454 (53.3) | 141 (55.7) | |
| Child characteristics | <0.001 | |||
| Age (years) | 8.2 (1.6) | 8.3 (1.6) | 7.9 (1.7) | |
| Sex | 0.45 | |||
| Male | 597 (54) | 455 (53.4) | 142 (56.1) | |
| Female | 508 (46) | 397 (46.6) | 111 (43.9) | |
| Race/ethnicity | <0.001 | |||
| White | 1,031 (93.3) | 817 (95.9) | 214 (84.6) | |
| Other | 74 (6.7) | 35 (4.1) | 39 (15.4) | |
| BMI (kg/m2) | 17 (2.6) | 16.8 (2.2) | 17.9 (3.5) | <0.001 |
| Weight status‡ | <0.001 | |||
| Normal weight | 878 (79.5) | 712 (83.6) | 166 (65.6) | |
| Overweight | 161 (14.6) | 112 (13.2) | 49 (19.4) | |
| Obesity | 66 (6.0) | 28 (3.3) | 38 (15.0) | |
| Liver enzyme concentrations | ||||
| ALT (IU/L) | 15.7 (6.2) | 13.8 (3.6) | 22.2 (8.5) | <0.001 |
| AST (IU/L) | 30.8 (9.3) | 28.2 (5.1) | 39.7 (13.6) | <0.001 |
| GGT (IU/L) | 12.7 (3.6) | 11.7 (2.2) | 16.2 (5.1) | <0.001 |
Data are expressed as mean (SD) or n (%).
Liver injury risk was defined as any liver enzyme level above the 90th percentile.
P value for difference between the low- and high-liver-injury risk groups was calculated using the chi-square test for categorical characteristics and the Mann-Whitney U test for continuous ones.
Weight status was defined according to the World Health Organization BMI cutoffs.
Maternal blood PFAS concentrations of the study population are displayed in Supporting Table S1. Median (25th, 75th percentile) concentrations of PFOA, PFOS, PFHxS, PFNA, and PFUnDA in pregnancy were 2.38 (1.45, 3.45), 6.74 (4.43, 10.35), 0.59 (0.34, 0.93), 0.72 (0.47, 1.11), and 0.20 (0.13, 0.30) ng/mL, respectively. Pair-wise correlations between the PFAS compounds revealed moderate-to-high correlations, with PFOS-PFHxS and PFOA-PFHxS correlations being the highest (Spearman’s rho = 0.70 and 0.65, respectively; Supporting Table S1).
PRENATAL PFAS EXPOSURE AND LIVER INJURY RISK IN CHILDREN
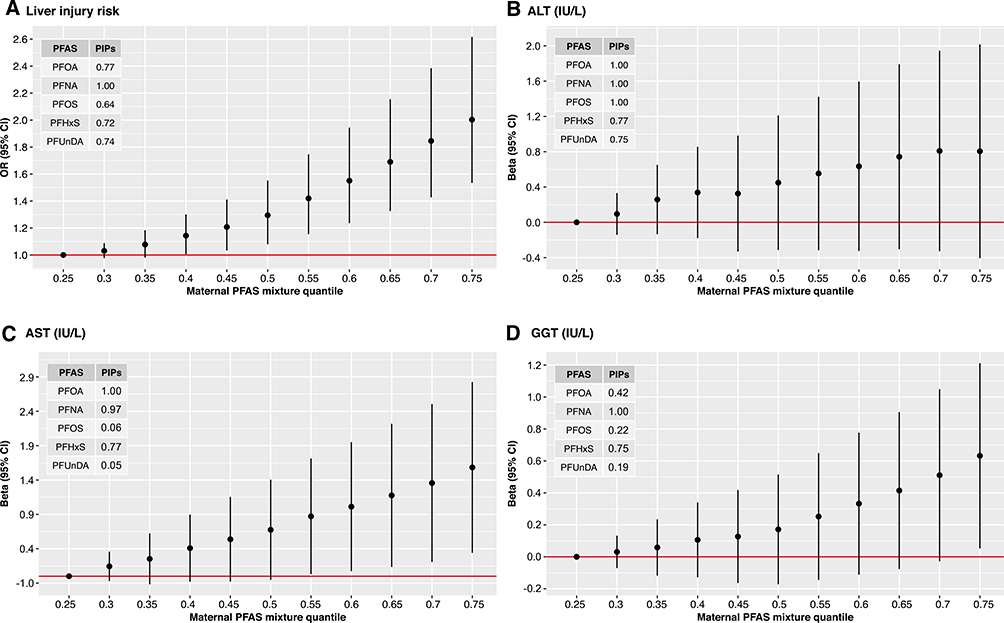
Higher prenatal exposure to PFAS mixture was associated with increased risk for liver injury during childhood (Fig. 1). In Fig. 1A, we display the estimated change in the risk as maternal concentrations of all five PFASs were simultaneously fixed at the same percentile (over a range between 0.3 and 0.75, with 0.05% increments) and compared to when those PFAS concentrations were each at their 25th percentile. PFNA and PFOA had the greatest contribution to the mixture effect, as suggested by their posterior inclusion probabilities in the mixture-response function for liver injury risk (Fig. 1A). Children who were highly exposed to prenatal PFAS mixture (75th percentile) had 2-fold higher risk for liver injury (odds ratio [OR], 2.0; 95% confidence interval [CI], 1.53–2.61) compared to children with low levels of exposure (25th percentile). Similar positive associations with the prenatal PFAS mixture exposure were found when we analyzed the three liver enzymes as separate outcomes (Fig. 1B–D). When exploring the effect of each of the separate PFAS while holding the remaining PFAS within the mixture constant, increased risks were also observed for PFNA and PFOA (Supporting Fig. S2A). In further analyses examining synergism between PFAS within the mixture, we observed that the effects of PFHxS, PFOA, PFOS, and PFUnDA were more pronounced at higher levels of PFNA (Supporting Fig. S2B).
FIG. 1.
Joint effect of prenatal PFAS mixture on liver injury risk and individual liver enzyme levels in children. Effect estimates were calculated by BKMR models adjusted for cohort, maternal age, education level, and prepregnancy BMI, child ethnicity, age, and sex. Graphs A, B, C and D depict the mixture response function for liver injury risk, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyltransferase (GGT), respectively. Graphs show the difference in the effect estimates when all exposures are at a particular quantile compared to when all are at the 25th quantile. Circle represents effect estimates, black vertical lines represent 95% CIs, and red horizontal lines represent the null. Tables within the graphs depict the posterior inclusion probabilities (PIPs) of each PFAS in the mixture-response function for each outcome. PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; PFUnDA, perfluoroundecanoate.
Correlations between maternal and child blood PFAS were low to moderate, with the highest correlations being observed for maternal PFOS with child PFHxS and PFOS (Spearman’s rho = 0.53 and 0.50, respectively). Effect estimates for the prenatal PFAS mixture on liver injury risk did not materially change when we adjusted for child PFAS levels (Supporting Fig. S3A). Effect estimates also did not materially change when we took into account weight status in childhood (Supporting Fig. S3B). Additional adjustment for gestational weight gain, maternal diet-quality indicators, and child lifestyle risk factors for NAFLD, including sedentary behavior and diet-quality indicators, did not change considerably the results (Supporting Fig. S3C). Significant associations of prenatal PFAS mixture exposure with increased liver injury risk were also observed when we repeated the analysis excluding one cohort at a time, as well as when we excluded the two cohorts contributing most to the cases of increased liver enzymes (Supporting Fig. S3D). In stratified analysis by sex, we found that the effect of prenatal PFAS mixture was greater in girls than in boys, although effect estimates were in the same direction and had overlapping CIs (Supporting Fig. S3E). When we stratified by trimester of pregnancy for PFAS assessment, we observed that effect estimates were slightly stronger when maternal PFAS concentrations were assessed in mid-pregnancy (first to second trimester) compared to late pregnancy, but estimates had the same direction and overlapping CIs (Supporting Fig. S3F).
PRENATAL PFAS EXPOSURE AND THE CHILD SERUM METABOLOME
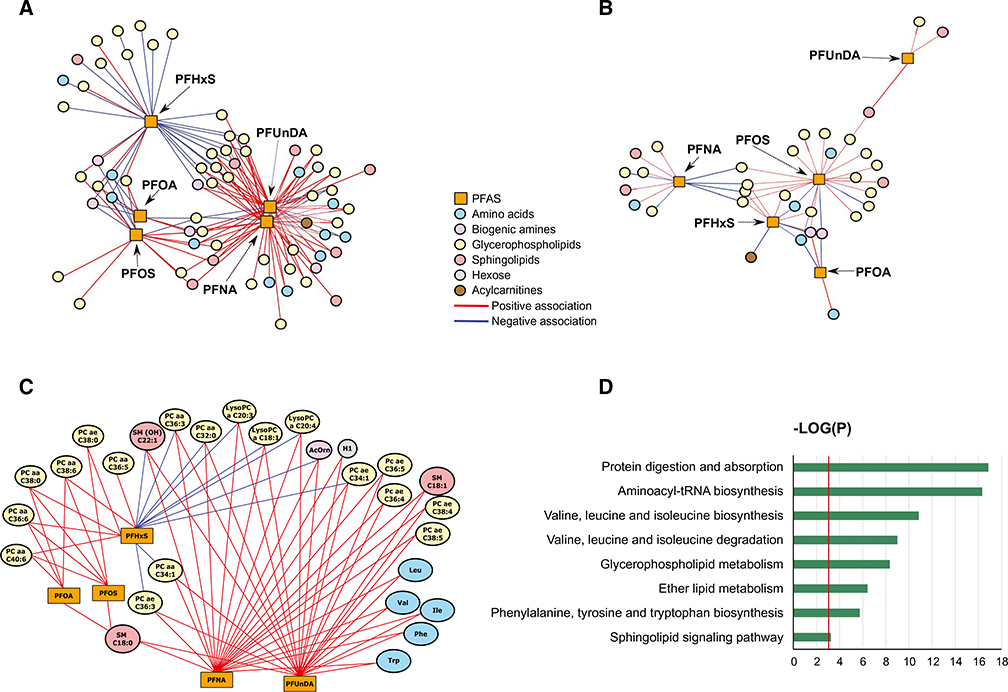
We observed that the network of children at high risk for liver injury involved 66 metabolites that were associated with PFAS (Fig. 2A; Supporting Table S2). In comparison, the network of low risk involved 34 metabolites (Fig. 2B; Supporting Table S3). A total of 29 metabolites had the greatest change in eigenvector centrality across the two networks (|delta eigenvector centrality|≥0.2). These metabolites included five amino acids, one biogenic amine, 19 glycerophospholipids, three sphingomyelins, and one hexose and exhibited both positive and negative associations with maternal serum PFAS in children at high risk for liver injury (Fig. 2C).
FIG. 2.
Integrated differential network analysis of maternal blood PFAS during pregnancy and child serum metabolites. (A) Network structure of PFAS and metabolites in children at high liver injury risk. (B) Network structure of PFAS and metabolites in children at low liver injury risk. (C) Network structure of PFAS and metabolites with differential contribution to the high- versus low-risk network (|delta eigenvector centrality| ≥0.2) in children at high liver injury risk. Pair-wise association scores between PFAS and metabolites were estimated using sparse partial least squares regression. Graphs A, B, and C depict significant PFAS-metabolite associations at P < 0.05. (D) Overpresentation analysis of metabolites with differential contribution to the high- versus low-risk network. Blue bars indicate −log(P) based on the hypergeometric test. Red vertical line indicates the P value threshold of 0.05.
Pathway analysis of these metabolites showed that the most dysregulated pathways were related to protein and amino acid metabolism (Fig. 2D). Specifically, six pathways, including protein digestion and absorption, aminoacyl-tRNA (transfer RNA) biosynthesis, valine, leucine, and isoleucine biosynthesis, valine, leucine, and isoleucine degradation, and phenylamine, tyrosine, and tryptophan biosynthesis, were over-represented in the network of high liver injury risk. PFAS-associated metabolic perturbations were also observed for some lipid metabolism pathways, including glycerophospholipid metabolism, ether lipid metabolism, and sphingolipid signaling pathway.
IDENTIFICATION OF CHILDREN AT RISK FOR LIVER INJURY BASED ON PRENATAL PFAS EXPOSURE AND SERUM METABOLOME
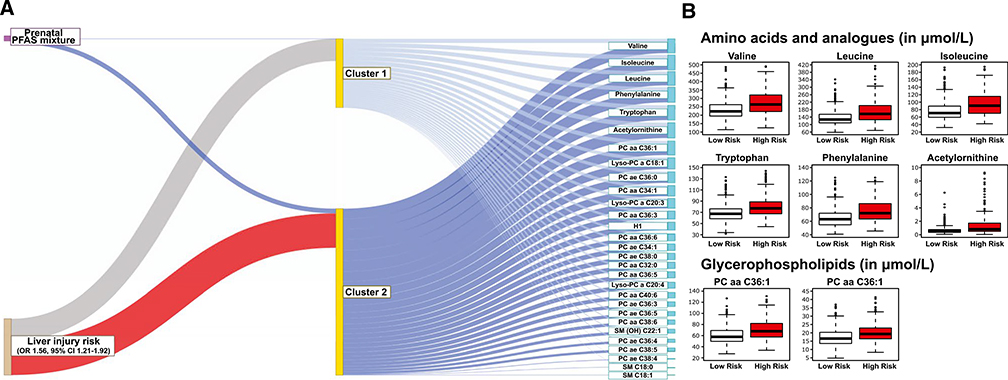
The integrated latent variable analysis estimated two subgroups of children (Fig. 3A). The high-risk subgroup had 56% increased risk for liver injury (OR, 1.56; 95% CI, 1.21–1.92) compared to the low-risk subgroup. This group was characterized by increased prenatal PFAS levels and increased child serum levels of branched-chain amino acids (BCAAs; valine, leucine, and isoleucine), the aromatic amino acids (AAAs) tryptophan and phenylalanine, biogenic amine acetylornithine, and glycerophospholipids phosphatidylcholine (PC) aa C36:1 and Lyso-PC a C18:1 (Fig. 3B; Supporting Table S4).
FIG. 3.
Integrated latent variable analysis on prenatal PFAS-mixture exposure and child serum metabolome. (A) Identification of a subgroup of children at risk of liver injury. The thick blue line connecting PFAS-mixture exposure to Cluster 2 indicate positive association (OR for 75th vs. 25th percentile, 2.16; 95% CI, 1.84, 2.53), compared to Cluster 1 (reference). The blue lines connecting the clusters to metabolites indicate positive associations. The thick red line connecting Cluster 2 and liver injury risk shows that children in the Cluster 2 had a higher risk for liver injury (OR, 1.56; 95% CI, 1.21–1.92) compared to those in Cluster 1. (B) Distribution of selected amino acid and glycerophospholipid metabolites in children with high probability of inclusion to Cluster 2 (P (Highrisk|PFASmixture, Metabolites)≥0.5) compared to those with low probability of inclusion. Median μmol/L values (25th-75th percentile) for children with low versus high inclusion probability were 223.0 (195.0, 265.0) versus 264.0 (220.0, 320.3) for valine, 127.0 (109.0, 155.0) versus 157.0 (126.0, 201.5) for leucine, 70.8 (59.94, 89.4) versus 90.6 (70.2, 115.3) for isoleucine, 67.4 (58.6, 76.3) versus 77.1 (67.5, 88.7) for tryptophan, 63.1 (54.8, 72.0) versus 72.1 (63.4, 85.9) for phenylalanine, 0.52 (0.35, 0.77) versus 0.81 (0.50, 1.71) for acetylornithine, 57.8 (49.7, 69.2) versus 67.8 (57.5, 81.7) for PC aa C36:1, and 16.6 (13.6, 20.3) versus 19.3 (16.4, 22.9) for Lyso-PC a C18:1.
Discussion
In this well-characterized multicenter cohort of European mothers and their children, we demonstrate that maternal exposure to PFAS mixture during pregnancy is associated with increased liver injury risk in childhood. We also integrated maternal PFAS blood concentrations with targeted serum metabolomic profiling in children and show that prenatal PFAS exposure is associated with alterations in key amino acids and lipid pathways characterizing liver injury risk. Given that the prevalence of NAFLD in children is rapidly increasing(1) and that PFAS can efficiently cross the placenta barrier and deposit to fetal tissues,(6) these results have potential implications for public health and prevention policy.
PFAS have been in production for over 55 years, resulting in ubiquitous exposure levels. Maternal blood concentrations of PFAS in our study population were lower than those reported in the U.S. National Health and Nutrition Examination Survey (NHANES) female population over the period 1999–2010, when the recruitment of participating mothers in our study occurred, and slightly higher than recent U.S. levels (collection period 2011–2016).(11) The in utero or early-life period is a critical developmental period of high vulnerability to PFAS. In line with our findings, animal studies have shown that prenatal exposure to PFOA and PFOS causes hepatocellular injury and fatty liver in the offspring.(14,15) Previous human cross-sectional studies have also shown positive associations of plasma or serum concentration of PFAS, including PFOS, PFOA, and PFNA, with liver enzymes levels in U.S adolescents and adults in NHANES,(19,43) adult populations from Sweden,(20) and U.S communities drinking PFAS-contaminated water.(17,18) In a previous U.S. study involving children with physician-diagnosed NAFLD, we showed that plasma PFOS and PFHxS levels were associated with increased risk for NASH.(22) The only pregnancy cohort study conducted previously found that maternal serum PFAS concentrations were not associated with ALT levels in U.S children.(21) A major strength of the present study is that the complicated interplay of PFAS exposure was treated in the analysis as a mixture, compared with previous studies that studied them only as single independent compounds. This approach is appropriate, given that in the real world, PFAS occur together as contaminant mixtures in soil, water, and consumer products, are highly correlated, and, as observed in our study, they may exert synergistic hepatotoxic effects.
We found a slightly stronger association of prenatal PFAS mixture in girls than in boys, findings consistent with previous research showing that females are more susceptible than males to PFAS hepatotoxicity.(44) The liver is a sexually dimorphic organ, given that it expresses both androgen and estrogen receptors and is responsive to sex steroids. Experimental studies have shown that PFAS interfere with sex steroid pathways by disturbing expression of genes involved in sex steroid biosynthesis and activity of sex steroid receptors,(30,45) which, in turn, could confer sex differences in PFAS hepatotoxicity.(46)
The exact mechanisms through which PFAS can affect liver injury and NAFLD remain unclear. Among children with NAFLD, we previously showed that plasma PFAS were associated with alterations in several amino acid pathways (e.g., valine, leucine and isoleucine degradation, and tyrosine metabolism) and glycerophospholipid metabolism.(22) Our study confirms these results, showing that not only childhood exposure, but also prenatal exposure to PFAS are associated with alterations in amino acid (e.g., valine, leucine, and isoleucine degradation and biosynthesis; phenylalanine, tyrosine, and tryptophan biosynthesis) and lipid (e.g., glycerophospholipid) metabolism. These are key metabolic pathways that have been well linked to liver injury and NAFLD pathogenesis in both human and animal studies.(24) The integrated latent analysis identified a high-risk profile of children characterized by high prenatal PFAS exposure and increased child serum concentrations of BCAAs, AAAs, and glycerophospholipids. These results strengthen an emerging body of evidence from animal(47) and human studies,(22,48) showing that PFAS exposure is associated with alterations in BCAA, AAA, and glycerophospholipid levels. Circulating levels of BCAAs and AAAs have been reported to be elevated in children with NAFLD.(49,50) BCAAs and AAAs are associated with hepatic insulin resistance, a key pathophysiological feature of NAFLD, possibly as a result of impaired hepatic tricarboxylic acid cycle metabolism and mitochondrial function.(51,52) PFAS disrupt hepatic lipid metabolism by interacting with peroxisome proliferator-activated receptors and other receptors attributable to their structural similarities with fatty acids.(8) Moreover, abnormal glycerophospholipid levels can induce hepatic lipotoxicity and inflammation, the hallmark of NAFLD, through endoplasmic reticulum stress activation and inflammatory extracellular vesicle release.(53) Consequently, altered glycerophospholipid metabolism has been reported in both blood and liver tissue samples from NAFLD patients.(50,54)
Strengths of our study include the multicentric, prospective design involving mother-child pairs of six countries spanning north to south in Europe, with comprehensive assessment of PFAS exposure in a critical developmental time period (pregnancy) and detailed information regarding covariates and predictors of the outcome during follow-up. When we adjusted for childhood exposure to PFAS, results did not change, underlying the importance of the pregnancy as a key time period of exposure for the development of liver injury. The integrated analyses of chemical exposures and metabolomics, with the goal of identifying latent variables representing distinct groups of children at risk for liver injury, have the potential to offer a personalized paradigm with potential clinical application to the identification of pediatric populations at risk for liver injury.
Although large in scale, our study has a number of limitations. As in any observational study, residual confounding cannot be ruled out, and it might have biased the observed associations. However, our results remained similar following adjustment for a large variety of social and lifestyle factors and are in accordance with previous experimental and human evidence on PFAS hepatotoxicity; this argues against residual confounding as the sole explanation for our results. We characterized liver injury based on serum liver enzymes levels given that the current diagnostic gold-standard liver biopsy for NAFLD has well-known limitations of high cost, risk, and ethical restrictions in large population studies, such as HELIX. However, we do not expect any misclassification in the outcome to be informed by prenatal PFAS status. Although we used a longitudinal study design, metabolomic biomarkers in children were assessed at the same time point as liver enzyme levels, thus limiting the ability to disentangle any mediating effect of the observed PFAS-associated metabolic perturbations on liver injury. We acquired the serum metabolomics data using a standardized, targeted LC-MS/MS assay, which exhibits high sensitivity and specificity of the quantification, provides explicit metabolite identification, and has high interlaboratory reproducibility.(55) Although this analytical method has been widely used in large-scale epidemiology studies, thereby facilitating future comparisons, it is limited in the number of metabolites identified and provided partial coverage of the metabolome. Supplementing the current study with untargeted metabolomic approaches, such as high-resolution MS, in the future would help expand metabolite coverage and capture more pathways potentially relevant to PFAS hepatotoxicity.
In conclusion, our results suggest that in utero exposure to PFAS can contribute to liver injury in childhood and that alterations in BCAAs, AAAs, and glycerophospholipids may jointly characterize this association. The study addresses a critical gap in our current understanding of the etiology of the NAFLD epidemic in youth and has potential to lead to avenues for fatty liver disease prevention and treatment starting early in life.
Supplementary Material
Acknowledgment:
We acknowledge the input of the entire HELIX consortium. We are grateful to all the participating families in the six cohorts (BiB, EDEN, INMA, KANC, MoBa, and RHEA cohorts), that took part in this study. We are equally grateful to all the fieldworkers for their dedication and efficiency in this study. A full roster of the INMA and RHEA study investigators can be found at http://www.proyectoinma.org/en/inma-project/inma-project-researchers/ and http://www.rhea.gr/en/about-rhea/the-rheateam/,respectively. The Born in Bradford study is only possible because of the enthusiasm and commitment of the participating children and parents. We are grateful to all the participants, health professionals, and researchers who have made Born in Bradford happen. We are also grateful to all the participating families in Norway who take part in the ongoing MoBa cohort study. We are grateful to all the participants, health professionals, and researchers who have participated in the Kaunas KANC cohort. We thank all the children and families participating in the EDEN-HELIX mother-child cohort. We are grateful to Joane Quentin, Lise Giorgis-Allemand, and Rémy Slama (EDEN study group) for their work on the HELIX project. We thank Sonia Brishoual, Angelique Serre, and Michele Grosdenier (Poitiers Biobank, CRB BB-0033-00068, Poitiers, France) for biological sample management and Prof. Frederic Millot (principal investigator), Elodie Migault, Manuela Boue, and Sandy Bertin (Clinical Investigation Center, Inserm CIC1402, CHU de Poitiers, Poitiers, France) for planning and investigational actions. We are also grateful to Veronique Ferrand-Rigalleau, Celine Leger, and Noella Gorry (CHU de Poitiers, Poitiers, France) for administrative assistance. We also acknowledge the commitment of the members of the EDEN Mother-Child Cohort Study Group: I. Annesi-Maesano, J.Y. Bernard, J. Botton, M.A. Charles, P. Dargent-Molina, B. de Lauzon-Guillain, P. Ducimetière, M. de Agostini, B. Foliguet, A. Forhan, X. Fritel, A. Germa, V. Goua, R. Hankard, M. Kaminski, B. Larroque, N. Lelong, J. Lepeule, G. Magnin, L. Marchand, C. Nabet, F. Pierre, M.J. Saurel-Cubizolles, M. Schweitzer, and O. Thiebaugeorges.
The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 308333—the HELIX project. INMA data collections were supported by grants from the Instituto de Salud Carlos III, CIBERESP, and the Generalitat de Catalunya-CIRIT. KANC was funded by the grant of the Lithuanian Agency for Science Innovation and Technology (6-04-2014_31V-66). For a full list of funding that supported the EDEN cohort, see the publication: Heude B et al. Cohort Profile: The EDEN mother-child cohort on the prenatal and early postnatal determinants of child health and development. Int J Epidemiol 2016;45:353-363. The Norwegian Mother, Father and Child Cohort Study (MoBa) is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research. The Rhea project was financially supported by European projects and the Greek Ministry of Health (Program of Prevention of Obesity and Neurodevelopmental Disorders in Preschool Children, in Heraklion district, Crete, Greece: 2011–2014; ‘Rhea Plus’: Primary Prevention Program of Environmental Risk Factors for Reproductive Health, and Child Health: 2012–2015). The KANC cohort was financially supported by the Lithuanian Agency for Science Innovation and Technology on September 13, 2015, No. 31V-77. Dr. Maribel Casas received funding from Instituto de Salud Carlos III (Ministry of Economy and Competitiveness; MS16/00128). This work was supported by National Institute of Environmental Health Sciences (NIEHS): R21ES029681 (Chatzi, Conti, McConnell, Stratakis, and Vos), R01ES030691 (Chatzi, Conti, and McConnell), R01ES029944 (Chatzi, Conti, and Valvi), R01ES030364 (Chatzi, Conti, and McConnell), R01ES028903 (Chatzi, McConnell, and Valvi), F32ES029828 (Jin), P30ES007048 (Chatzi, Conti, McConnell, and Stratakis), and R01ES030691 (Chatzi, Conti, and McConnell). Additional funding from the National Institutes of Health (NIH) supported Dr. Conti (P01CA196569, R01CA140561, and R01ES016813) and Dr. Stratakis (P30DK048522). The CRG/UPF Proteomics Unit is part of the Spanish Infrastructure for Omics Technologies (ICTS OmicsTech), and it is a member of the ProteoRed PRB3 consortium, which is supported by grant PT17/0019 of the PE I+D+i 2013–2016 from the Instituto de Salud Carlos III (ISCIII) and ERDF. We acknowledge support from the Spanish Ministry of Science, Innovation and Universities, “Centro de Excelencia Severo Ochoa 2013–2017”, SEV-2012-0208, and “Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement de la Generalitat de Catalunya” (2017SGR595).
Abbreviations:
- AAA
aromatic amino acid
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BCAA
branched-chain amino acid
- BKMR
Bayesian kernel machine regression
- BMI
body mass index
- CI
confidence interval
- CV
coefficient of variation
- GGT
gamma-glutamyltransferase
- LC
liquid chromatography
- LOD
limit of detection
- MS
mass spectrometry
- NAFLD
nonalcoholic fatty liver disease
- OR
odds ratio
- PC
phosphatidylcholine
- PFAS
per- and polyfluoroalkyl substances
- PFHxS
perfluorohexane sulfonate
- PFNA
perfluorononanoate
- PFOA
perfluorooctanoate
- PFOS
perfluorooctane sulfonate
- PFUnDA
perfluoroundecanoate
Footnotes
Potential conflict of interest: Nothing to report.
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.31483/suppinfo.
REFERENCES
- 1 ).Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One 2015;10:e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.) Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr 2013;162:496–500.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3 ).Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4 ).Stefan N, Haring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2018. August 30 10.1016/S2213-8587(18)30154-2. [DOI] [PubMed] [Google Scholar]
- 5 ).Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 2017;68:3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6 ).Mamsen lS, Bjorvang RD, Mucs D, Vinnars MT, Papadogiannakis N, Lindh CH, et al. Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ Int 2019;124:482–492. [DOI] [PubMed] [Google Scholar]
- 7 ).Perez F, Nadal M, Navarro-Ortega A, Fabrega F, Domingo JL, Barcelo D, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 2013;59:354–362. [DOI] [PubMed] [Google Scholar]
- 8 ).Wahlang B, Jin J, Beier JI, Hardesty JE, Daly EF, Schnegelberger RD, et al. Mechanisms of environmental contributions to fatty liver disease. Curr Environ Health Rep 2019;6:80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9 ).Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol 2011;45:7954–7961. [DOI] [PubMed] [Google Scholar]
- 10 ).Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol 2004;38:4489–4495. [DOI] [PubMed] [Google Scholar]
- 11 ).CDC. Fourth National Report on Human Exposure to Environmental Chemicals, updated tables, January 2019. Atlanta, GA: CDC. [Google Scholar]
- 12 ).Hui Z, Li R, Chen L. The impact of exposure to environmental contaminant on hepatocellular lipid metabolism. Gene 2017;622:67–71. [DOI] [PubMed] [Google Scholar]
- 13 ).Das KP, Wood CR, Lin MT, Starkov AA, Lau C, Wallace KB, et al. Perfluoroalkyl acids-induced liver steatosis: effects on genes controlling lipid homeostasis. Toxicology 2017;378:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14 ).lv Z, li g, Li Y, Ying C, Chen J, Chen T, et al. Glucose and lipid homeostasis in adult rat is impaired by early-life exposure to perfluorooctane sulfonate. Environ Toxicol 2013;28:532–542. [DOI] [PubMed] [Google Scholar]
- 15 ).Quist EM, Filgo AJ, Cummings CA, Kissling GE, Hoenerhoff MJ, Fenton SE. Hepatic mitochondrial alteration in CD-1 mice associated with prenatal exposures to low doses of perfluorooctanoic acid (PFOA). Toxicol Pathol 2015;43:546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16 ).Wesolowski SR, Kasmi KC, Jonscher KR, Friedman JE. Developmental origins of NAFLD: a womb with a clue. Nat Rev Gastroenterol Hepatol 2017;14:81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17 ).Darrow LA, Groth AC, Winquist A, Shin HM, Bartell SM, Steenland K. Modeled perfluorooctanoic acid (PFOA) exposure and liver function in a Mid-Ohio valley community. Environ Health Perspect 2016;124:1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18 ).Gallo V, Leonardi G, Genser B, Lopez-Espinosa MJ, Frisbee SJ, Karlsson L, et al. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect 2012;120:655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19 ).Gleason JA, Post GB, Fagliano JA. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007–2010. Environ Res 2015;136:8–14. [DOI] [PubMed] [Google Scholar]
- 20 ).Salihovic S, Stubleski J, Karrman A, Larsson A, Fall T, Lind L, et al. Changes in markers of liver function in relation to changes in perfluoroalkyl substances—a longitudinal study. Environ Int 2018;117:196–203. [DOI] [PubMed] [Google Scholar]
- 21 ).Mora AM, Fleisch AF, Rifas-Shiman SL, Woo Baidal JA, Pardo L, Webster TF, et al. Early life exposure to per- and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environ Int 2018;111:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22 ).Jin R, McConnell R, Catherine C, Xu S, Walker DI, Stratakis N, et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in children: an untargeted metabolomics approach. Environ Int 2020;134:105220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 ).Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. Environmental pollutants and child health—a review of recent concerns. Int J Hyg Environ Health 2016;219:331–342. [DOI] [PubMed] [Google Scholar]
- 24 ).Sookoian S, Pirola CJ. NAFLD. Metabolic make-up of NASH: from fat and sugar to amino acids. Nat Rev Gastroenterol Hepatol 2014;11:205–207. [DOI] [PubMed] [Google Scholar]
- 25 ).Kim HS, Jun Kwack S, Sik Han E, Seok Kang T, Hee Kim S, Young HS. Induction of apoptosis and CYP4A1 expression in Sprague-Dawley rats exposed to low doses of perfluorooctane sulfonate. J Toxicol Sci 2011;36:201–210. [DOI] [PubMed] [Google Scholar]
- 26 ).Wan C, Han R, Liu L, Zhang F, Li F, Xiang M, et al. Role of miR-155 in fluorooctane sulfonate-induced oxidative hepatic damage via the Nrf2-dependent pathway. Toxicol Appl Pharmacol 2016;295:85–93. [DOI] [PubMed] [Google Scholar]
- 27 ).Zhang l, Krishnan p, Ehresman DJ, Smith PB, Dutta M, Bagley BD, et al. Editor’s highlight: perfluorooctane sulfonate-choline ion pair formation: a potential mechanism modulating hepatic steatosis and oxidative stress in mice. Toxicol Sci 2016;153:186–197. [DOI] [PubMed] [Google Scholar]
- 28 ).Corsini E, Luebke RW, Germolec DR, DeWitt JC. Perfluorinated compounds: emerging POPs with potential immunotoxicity. Toxicol Lett 2014;230:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29 ).Tan X, Xie G, Sun X, Li Q, Zhong W, Qiao P, et al. High fat diet feeding exaggerates perfluorooctanoic acid-induced liver injury in mice via modulating multiple metabolic pathways. PLoS One 2013;8:e61409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30 ).Buhrke T, Kruger E, Pevny S, Rossler M, Bitter K, Lampen A. Perfluorooctanoic acid (PFOA) affects distinct molecular signalling pathways in human primary hepatocytes. Toxicology 2015;333:53–62. [DOI] [PubMed] [Google Scholar]
- 31 ).Maitre L, de Bont J, Casas M, Robinson O, Aasvang GM, Agier L, et al. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open 2018;8:e021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32 ).Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, et al. Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 2016;45:382–388. [DOI] [PubMed] [Google Scholar]
- 33 ).Haug LS, Sakhi AK, Cequier E, Casas M, Maitre L, Basagana X, et al. In-utero and childhood chemical exposome in six European mother-child cohorts. Environ Int 2018;121:751–763. [DOI] [PubMed] [Google Scholar]
- 34 ).Wei R, Wang J, Su M, Jia E, Chen S, Chen T, et al. Missing value imputation approach for mass spectrometry-based metabolomics data. Sci Rep 2018;8:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35 ).Lau CE, Siskos AP, Maitre L, Robinson O, Athersuch TJ, Want EJ, et al. Determinants of the urinary and serum metabolome in children from six European populations. BMC Med 2018;16:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36 ).de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37 ).Shelly C, Grandjean P, Oulhote Y, Plomgaard P, Frikke-Schmidt R, Nielsen F, et al. Early life exposures to perfluoroalkyl substances in relation to adipokine hormone levels at birth and during childhood. J Clin Endocrinol Metab 2019;104:5338–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38 ).Uppal K, Ma C, Go YM, Jones DP, Wren J. xMWAS: a data-driven integration and differential network analysis tool. Bioinformatics 2018;34:701–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39 ).Lichtblau Y, Zimmermann K, Haldemann B, Lenze D, Hummel M, Leser U. Comparative assessment of differential network analysis methods. Brief Bioinform 2017;18:837–850. [DOI] [PubMed] [Google Scholar]
- 40 ).Dablander F, Hinne M. Node centrality measures are a poor substitute for causal inference. Sci Rep 2019;9:6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41 ).Kamburov A, Pentchev K, Galicka H, Wierling C, Lehrach H, Herwig R. ConsensusPathDB: toward a more complete picture of cell biology. Nucleic Acids Res 2011;39:D712–D717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42 ).Peng C, Wang J, Asante I, Louie S, Jin R, Chatzi L, et al. A Latent Unknown Clustering Integrating Multi-Omics Data (LUCID) with Phenotypic Traits. Bioinformatics 2020;36:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43 ).lin Cy, lin ly, Chiang CK, Wang WJ, Su YN, Hung KY, et al. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in US adults. Am J Gastroenterol 2010;105:1354–1363. [DOI] [PubMed] [Google Scholar]
- 44 ).Attanasio R Sex differences in the association between perfluoroalkyl acids and liver function in US adolescents: analyses of NHANES 2013–2016. Environ Pollut 2019;254:113061. [DOI] [PubMed] [Google Scholar]
- 45 ).Kjeldsen LS, Bonefeld-Jorgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int 2013;20:8031–8044. [DOI] [PubMed] [Google Scholar]
- 46 ).Grossmann M, Wierman ME, Angus P, Handelsman DJ. Reproductive endocrinology of nonalcoholic fatty liver disease. Endocr Rev 2019;40:417–446. [DOI] [PubMed] [Google Scholar]
- 47 ).Yu N, Wei S, Li M, Yang J, Li K, Jin L, et al. Effects of perfluorooctanoic acid on metabolic profiles in brain and liver of mouse revealed by a high-throughput targeted metabolomics approach. Sci Rep 2016;6:23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48 ).Alderete TL, Jin R, Walker DI, Valvi D, Chen Z, Jones DP, et al. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: a proof-of-concept analysis. Environ Int 2019;126:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49 ).Khusial RD, Cioffi Ce, Caltharp SA, Krasinskas AM, Alazraki A, Knight-Scott J, et al. Development of a plasma screening panel for pediatric nonalcoholic fatty liver disease using metabolomics. Hepatol Commun 2019;3:1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50 ).Jin R, Banton S, Tran VT, Konomi JV, Li S, Jones DP, et al. Amino acid metabolism is altered in adolescents with nonalcoholic fatty liver disease—an untargeted, high resolution metabolomics study. J Pediatr 2016;172:14–19.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51 ).Sunny NE, Kalavalapalli S, Bril F, Garrett TJ, Nautiyal M, Mathew JT, et al. Cross-talk between branched-chain amino acids and hepatic mitochondria is compromised in nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab 2015;309:E311–E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52 ).Gaggini M, Carli F, Rosso C, Buzzigoli E, Marietti M, Della Latta V, et al. Altered amino acid concentrations in NAFLD: impact of obesity and insulin resistance. Hepatology 2018;67:145–158. [DOI] [PubMed] [Google Scholar]
- 53 ).Musso G, Cassader M, Paschetta E, Gambino R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology 2018;155: 282–302.e8. [DOI] [PubMed] [Google Scholar]
- 54 ).Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 2007;46:1081–1090. [DOI] [PubMed] [Google Scholar]
- 55 ).Siskos AP, Jain P, Romisch-Margl W, Bennett M, Achaintre D, Asad Y, et al. Interlaboratory reproducibility of a targeted metabolomics platform for analysis of human serum and plasma. Anal Chem 2017;89:656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.