Abstract
Prenatal exposure to per- and polyfluoroalkyl substances (PFAS), a ubiquitous class of chemicals, is associated with adverse outcomes such as pre-eclampsia, low infant birth weight, and later-life adiposity. The objectives of this study were to examine PFAS levels in the placenta and identify sociodemographic risk factors in a high-risk pregnancy cohort (n = 122) in Chapel Hill, North Carolina. Of concern, PFOS, PFHxS, PFHpS, and PFUnA were detected above the reporting limit in 99, 75, 55, and 49% of placentas, respectively. Maternal race/ethnicity was associated with significant differences in PFUnA levels. While the data from this high-risk cohort did not provide evidence for an association with hypertensive disorders of pregnancy, fetal growth, or gestational age, the prevalence of detectable PFAS in the placenta suggests a need to biomonitor for exposure to PFAS during pregnancy. Future research should investigate factors underlying the differences in PFAS levels in association with a mother’s race/ethnicity, as well as potential effects on pregnancy and child health.
Graphical Abstract
1. INTRODUCTION
Per- and polyfluoroalkyl acids (PFAS) are a diverse chemical family of synthetic organic structures used for a variety of applications in both the United States and worldwide due to their water- and grease-resistant properties.1 PFAS are found in fabric surface treatment, pesticides, fire-fighting foam, food packaging, and a variety of other sources, reaching humans via air, contaminated water, food, and breast milk.2–8 PFAS consist of alkyl chains in which hydrogen atoms have been replaced by fluorine. These fluorine–hydrogen bonds are exceptionally strong, making PFAS resistant to degradation and highly persistent in the environment and humans.9 Due to their persistence and pervasive use, PFAS have been detected in human blood worldwide.10,11 Between 1999 and 2012, a number of PFAS were found at detectable levels in blood in over 98% of the U.S. population.12,13 Concern is growing regarding widespread exposure to PFAS, and a number of PFAS are currently listed as Contaminants of Emerging Concern (CECs) by the U.S. Environmental Protection Agency.14
PFAS exposure in pregnant women has been associated with an increased risk of fertility problems, pre-eclampsia, and miscarriage.15–21 In utero PFAS exposure has been associated with an array of negative effects in children: low infant birth weight,22–27 congenital cerebral palsy in boys,28 dysregulated pubertal onset later in life,29 and greater adiposity.30 Animal models have also shown deleterious effects of exposure to PFAS, including altered pubertal onset, reduced postnatal survival, and disturbed lactation in rodents exposed in utero.31–34 While a number of studies have focused on pregnancy as a time window of exposure to PFAS by assessing maternal blood or serum, few have focused on the placenta that is critical for understanding the effect of PFAS during pregnancy.35,36 As the transducer of the maternal to fetal environment during pregnancy, the placenta is important for adequate transport of nutrients, removal of waste, and secretion of hormones. Studies have shown that PFAS can transfer between maternal and fetal circulation via the placenta.37–40 Additionally, the placenta:maternal serum ratios of PFAS have been found to increase with gestational age, suggestive of the bioaccumulation of PFAS in the placenta over time.41 Despite growing concern over the effects of PFAS on pregnancy, fetal development, and their ability to cross the placenta, only three studies to date, all of which studied women outside of the United States, have detailed PFAS levels in the placenta.40–42 This study aimed to address this research gap in understanding the levels of PFAS in the placentas of a U.S.-based cohort and identify risk factors associated with high exposure.
In addition, the majority of studies on PFAS exposure focus on two perfluoroalkyl acids, mainly perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA);43 however, over 3000 PFAS are estimated to be in production.1 While some of these chemicals, including PFOS and PFOA, have been discontinued by major manufacturers in the United States, their persistence in the environment and continued use internationally make them a continued toxicological concern.44 Moreover, hexafluoropropylene oxide dimer acid (HFPO-DA, ammonium salt with trade name: GenX), a modern replacement for PFOA, has recently contaminated drinking water from the Cape Fear River in the Wilmington area, NC, warranting immediate analysis of the prevalence of PFAS in NC populations.45
In the present study, we set out to characterize the PFAS level in the placenta and understand risk factors associated with high PFAS exposure in a high-risk pregnancy cohort in the United States. To carry out this research, we utilized specimens from a cohort of pregnant women at risk for preterm birth in North Carolina. The primary goals of this work were to (i) establish baseline data on 22 different PFAS levels in the placenta, (ii) identify sociodemographic risk factors associated with increased placental PFAS levels in this cohort, and (iii) investigate whether PFAS placental levels change with gestational age at delivery, fetal growth, or hypertensive disorders of pregnancy (preeclampsia and gestational hypertension).
2. MATERIALS AND METHODS
2.1. Study Population.
This cross-sectional study was derived from the UNC Preterm Birth Biobank (PTB) Study.46 The original purpose of this overarching study was to establish a database and tissue bank to evaluate clinical and biochemical markers of preterm birth. Participants were identified and recruited through UNC-CH obstetrics and gynecology clinics and inpatient obstetric units at the North Carolina Women’s Hospital from 2015 to 2018. Women at high risk for spontaneous preterm birth due to (a) a prior spontaneous preterm birth, (b) short mid-trimester transvaginal cervical length (<25 mm, assessed between 16.0 and 23.9 weeks’ gestation) in the current pregnancy, (c) twin or triplet gestation, or (d) antepartum admission due to threatened preterm labor following cervical change and/or cervical dilation in the setting of at least six symptomatic uterine contractions per hour were included in the UNC PTB Biobank. Women carrying fetuses with major structural anomalies or aneuploidy were excluded. Pregnancies were dated by the last menstrual period (if available) and ultrasound using standard American College of Obstetricians and Gynecologists criteria.47 All pregnancy management decisions were made at the discretion of the primary obstetric provider. This study was approved by the Institutional Review Board of the University of North Carolina, Chapel Hill (IRB# 14–2855), and all women provided written, informed consent.
Baseline clinical and demographic information (including maternal educational attainment, race and ethnicity, marital status, and prepregnancy body mass index (kg/m2)) were collected from participants through structured interviews and supplemented by a review of electronic medical records. In addition, the antenatal course, delivery indications, and pregnancy outcomes (including infant gender, birth weight, gestational age at delivery) were obtained from the electronic medical record following delivery.
After delivery, three full-thickness placental biopsies were obtained from the fetal side, 2 cm from the placental cord insertion site using a standard 3 mm punch biopsy tool, and frozen at −80 °C within 24 h of delivery until analysis. Care was taken to avoid biopsies of placental sites with obvious gross abnormalities. Two hundred and seventy-one women were enrolled in the UNC PTB Biobank study: 2 had a first-trimester miscarriage, 13 were lost to follow-up or had missing data on gestational age at delivery, resulting in 256 viable placentas, of which 122 had enough remaining placental sample to be used in the present analysis.
2.2. Placenta PFAS Quantification.
Extraction and quantification by liquid chromatography–mass spectrometry (LC–MS/MS) method established utilized in this study were previously established in Bangma et al. 2018 and included 15 total PFAS.48 In the current study, the method was updated to include a total of 22 PFAS as follows: perfluorobutyric acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), perfluorooctanic acid (PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUdA), perfluorododecanoic acid (PFDoA), perfluorotridecanoic acid (PFTrDA), perfluorotetradecanoic acid (PFTeDA), perfluorohexadecanoic acid (PFHxDA), perfluorobutanesulfonic acid (PFBS), perfluoropentanesulfonic acid (PFPeS), perfluorohexanesulfonic acid (PFHxS), perfluoroheptanesulfonic acid (PFHpS), perfluorooctane sulfonic acid (PFOS), perfluorononanesulfonic acid (PFNS), perfluorodecanesulfonic acid (PFDS), perfluorododecancesulfonic acid (PFDoS), 1H,1H,2H,2H-perfluorooctane sulfonic acid (6:2 FTS), and 2,3,3,3-tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy)-propanoic acid (HFPO-DA also known as GenX). In-depth information detailing the Bangma et al. 2018 method as it was utilized for placenta PFAS quantification can be found in the Supporting Information (SI).
2.3. Statistical Analysis.
PFHpS, PFHxS, PFOS, and PFUnA were identified as having approximately 50% or more of samples over the reporting limit (RL). Using an established gravimetric method, the reporting limit (RL) is calculated in two ways: (1) using the lowest detectable calibrant divided by the mass of the extracted sample or (2) taking the average amount of compound detected in the blanks divided by the mass of the extracted sample.49,50 To be conservative, from the two methods of calculating the RL, the RL that was the highest for that PFAS and sample was designated as the respective RL for PFAS in that sample (Supporting Information File S1). The concentrations determined include isomers for applicable PFAS. For statistical analysis, all samples below the RL were set to half the RL detection limit for that sample and PFAS.51 This allowed for the preservation of sample size for the PFAS included in the statistical analysis. Spearman rank correlations between concentrations of each of the PFAS were calculated to evaluate patterns of co-occurrence.
For this analysis, the race was categorized as non-hispanic white, hispanic white, non-hispanic black, or other. Education was trichotomized into less than high school, completed high school, or further education beyond high school. Marital status was dichotomized as married or not married. Delivery gestational age was maintained as a continuous variable for regression analyses and was also trichotomized into very preterm (delivery before 340/7 weeks of gestation), preterm (delivery between 340/7 and 366/7 weeks of gestation), and term (delivery ≥ 370/7 weeks of gestation) for a categorical variable. Insurance was trichotomized into private insurance, Medicaid/Medicare insurance, or self-pay. Maternal age was trichotomized into below 20 years old at delivery (<20 years), between 20 and 35 years of age at delivery (≥20 to <35 years), and over 35 years and older at delivery (≥35 years). Prepregnancy BMI was categorized into underweight (BMI <18.5 kg/m2), normal weight (≥18.5–24.9 kg/m2), overweight (≥25.0–29.9 kg/m2), and obese (≥30.0 kg/m2) categories. Gestational hypertension was defined as new-onset hypertension, with at least two blood pressure readings ≥ 140/90 at least 6 h apart at or after 20 weeks’ gestation. Pre-eclampsia was defined as hypertension accompanied by new-onset proteinuria (urine protein:creatine ratio ≥0.30 or 24 h urine protein ≥ 300 mg/dL) and/or laboratory abnormalities (platelet count <100 per microliter, serum aspartate transaminase, or aminotransferase twice normal or ≥70 U/L) and/or severe headache or visual disturbance. Women with chronic hypertension were considered to have super-imposed pre-eclampsia if they had an exacerbation of their hypertension in addition to one of the above laboratory abnormalities or physical symptoms. Because the American College of Obstetricians and Gynecologists recognize that preeclampsia can develop in the absence of proteinuria, and the management of gestational hypertension and pre-eclampsia is similar, these diagnoses were combined into one “hypertensive disorders of pregnancy” variable, dichotomized as yes or no.47 For assessing the association with fetal growth, small for gestational age (SGA), appropriate for gestational age (AGA), and large for gestational age (LGA) newborns were identified using gender-specific fetal growth curves from a nationally representative, racially diverse reference population. Small for gestational age was defined as birth weight in grams ≤10th percentile and large for gestational age was defined as birth weight in grams ≥ 90th percentile of this reference population.52 Those born <23 weeks’ gestation were considered as having missing data for fetal growth category because there is no nationally representative data on birth weight distributions below this gestational age from the reference used. Smoking status was not included in analyses as only 9 mothers reported active smoking during pregnancy and 112 mothers reported nonsmoking status.
Distributions of each PFAS were evaluated for normality via Shapiro–Wilks tests of both original and log-transformed data. All PFAS evaluated were determined to have non-normal distributions in both original and log-transformed forms; thus, to examine differences across categorical variables, non-parametric tests were employed to test the equality of medians. Wilcoxon tests were used to evaluate differences between dichotomous sociodemographic variables and hypertensive disorders of pregnancy, and Kruskal–Wallis tests were used to evaluate differences between sociodemographic variables with more than two groups and fetal growth categories. The p-values of these tests are reported with significance defined as p < 0.05.
To evaluate whether PFAS levels varied by gestational age, both univariate and multivariate linear regression models were performed to generate crude and adjusted estimates of change in PFAS levels by gestational age. For the multivariate linear regression, control variables were identified as having significant or borderline significant differences in medians across groups from the sociodemographic risk factor analysis described above. To assess temporal changes in PFAS concentrations of the placenta, univariate linear regression models were used to evaluate the association between delivery date and PFAS concentration. For this model, births in 2018 were excluded due to a low sample size (n = 2 births in 2018). All analyses were conducted in SAS 9.4 software.
3. RESULTS
3.1. Study Population Characteristics.
This cohort of 122 women included 42 (37.5%) non-hispanic white, 30 (26.8%) hispanic white, 37 (33.0%) non-hispanic black, and 3 (2.7%) women who self-reported other for race/ethnicity (Table 1). The majority (63.1%) of women were between 20 and 34 years old. Just over half (53.9%) had private medical insurance, with 38.5% using Medicaid/Medicare insurance. In this high-risk group, the mean gestational age at delivery was 33.9 weeks. A total of 44 women (36.9%) delivered very preterm (less than 34 weeks), 12 women (9.8%) delivered preterm (between 34 and 37 weeks), and 65 women (53.3%) delivered term (37 weeks or over). Fourteen (11.5%) women had pregnancies complicated by hypertensive disorders. The mean birth weight was 2,375 g (range 159–4300 g). In comparison to the National Vital Statistics data from 2016 and 2017 representatives of all births in the United States, the present cohort differs slightly with regard to racial demographics (U.S. population, 2017: 51.68% white mothers, 14.54% to non-hispanic black, 23.3% hispanic) but is similar with regard to the percentage of insurance types (U.S. population, 2017: 49.1% private insurance, 43% Medicaid).53,54 As expected, given the high-risk characteristics of the cohort, the mean gestational age at delivery (prevalence of preterm births) and mean birth weight differed from the general U.S. population.53,54
Table 1.
Study Population Characteristics (N = 122) of Women with Placental Samples Analyzed for PFAS Levels (Chapel Hill, NC)
| range | mean (SD) | N | (%)a | missing values (%)b | |
|---|---|---|---|---|---|
| race and hispanic ethnicity | 10 (8.2) | ||||
| white, non-hispanic | 42 | (37.5) | |||
| white, hispanic | 30 | (26.8) | |||
| black, non-hispanic | 37 | (33.0) | |||
| other | 3 | (2.7) | |||
| maternal smoking | 1 (<1) | ||||
| yes | 9 | (7.4) | |||
| no | 112 | (92.6) | |||
| child’s gender | 1 (<1) | ||||
| female | 54 | (44.6) | |||
| male | 67 | (55.4) | |||
| maternal prepregnancy BMI (kg/m2) | 17–56 | 30.9 (8.82) | 0 | ||
| <18.5 | 6 | (4.9) | |||
| 18.5 ≤ 25 | 24 | (19.7) | |||
| 25 ≤ 30 | 30 | (24.6) | |||
| 30+ | 62 | (50.8) | |||
| maternal age | 20–44 | 31.0 (6.51) | 1 (<1) | ||
| below 20 years old (<20) | 0 | (0) | |||
| between 20 and 25 years old (≥20 to <35) | 77 | (63.1) | |||
| over 35 years old (≥35) | 44 | (36.1) | |||
| maternal medical insurance | 18 (14.8) | ||||
| private insurance | 56 | (53.9) | |||
| Medicare/Medicaid | 40 | (38.5) | |||
| self-pay | 8 | (7.7) | |||
| maternal education | 20 (16.4) | ||||
| less than high school | 13 | (12.8) | |||
| completed high school | 42 | (41.2) | |||
| more than high school | 34 | (33.3) | |||
| declined | 13 | (12.8) | |||
| marital status | 8 (6.6) | ||||
| married | 81 | (71.0) | |||
| not married | 33 | (29.0) | |||
| gestational age (weeks) | 18–41 | 33.9 (5.55) | 1 (<1) | ||
| preterm delivery | |||||
| very preterm (<34 weeks) | 44 | (36.4) | |||
| preterm (34 < weeks <37) | 12 | (9.9) | |||
| term (≥37 weeks) | 65 | (53.7) | |||
| hypertensive complications of pregnancy | 1 (<1) | ||||
| no hypertensive complications | 107 | (88.4) | |||
| pre-eclampsia and/or gestational hypertension | 14 | (11.6) | |||
| birth weight (grams) | 159–4300 | 2375 (1085) | 5 (4.1) | ||
| small for gestational age (SGA)/large for gestational age (LGA) | 9 (7.4) | ||||
| SGA | 9 | 8.0 | |||
| AGA | 92 | 81.4 | |||
| LGA | 12 | 10.6 |
Percent of nonmissing observations.
Percent of all observations.
3.2. PFAS Levels in the Study Population.
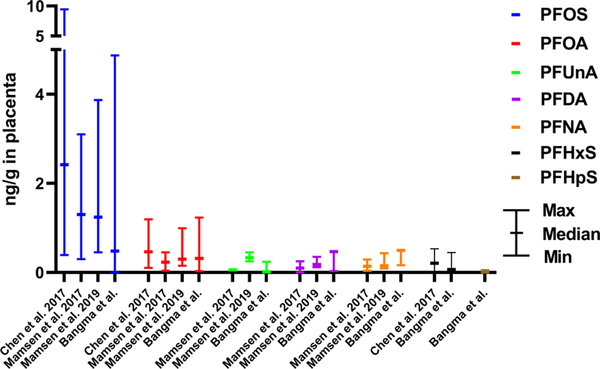
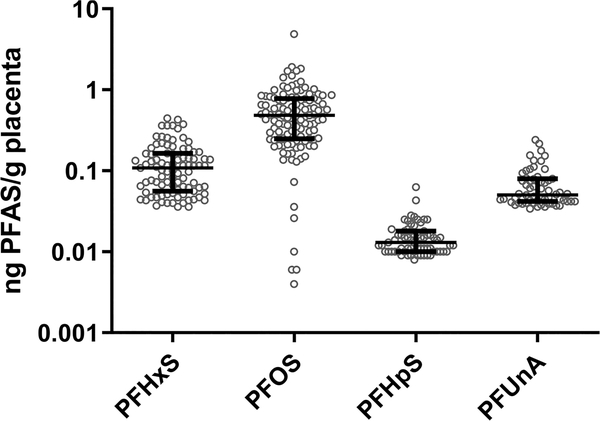
Of the 22 PFAS examined in the placenta, 11 PFAS were detected in at least one placenta sample (n = 122) (Table 2). Compared to three other non-U.S.-based studies of placenta PFAS levels, these data are relatively consistent, with PFOS and PFOA found at the maximum values (Figure 1). PFHxS, PFHpS, PFOS, and PFUnA were detected over the RL in approximately 50% or more of samples. As a significant lack of detection would skew results, for all subsequent analyses, only these four PFAS are considered and values below the RL were imputed as half the RL. The highest levels detected were of PFOS, followed by PFHxS, PFUnA, and then PFHpS (Figure 2). PFOS was detected at a median of 0.480 ng/g and a maximum of 4.87 ng/g. Comparatively, PFHxS had a median of 0.067 ng/g and a maximum of 0.446 ng/g and PFUnA had a median value under the RL and a maximum of 0.240 ng/g. PFHpS was found to have a median concentration of 0.009 ng/g and a maximum of 0.063 ng/g. Correlation between the levels of PFHxS, PFHpS, PFOS, and PFUnA was common (Supporting Information Figure S1 and Table S1). Specifically, all pairings other than PFUnA and PFHxS were significantly positively correlated. The most significant correlation was PFHpS and PFHxS (Spearman correlation coefficient = 0.532, p-value = 2.907 × 10−10).
Table 2.
| PFPeS | PFHxSc | PFHpSc | PFOSc | PFHxA | PFOA | PFNA | PFDA | PFUnAc | PFTriA | PFTA | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| chain length | 5 | 6 | 7 | 8 | 6 | 8 | 9 | 10 | 11 | 13 | 14 |
| n | 122 | 122 | 122 | 122 | 122 | 122 | 122 | 122 | 122 | 122 | 122 |
| % >RL | 31 | 75 | 55 | 99 | 1.6 | 27 | 21 | 39 | 49 | 29 | 7 |
| maximum (ng/g) | 0.035 | 0.446 | 0.063 | 4.87 | 5.87 | 1.23 | 0.494 | 0.465 | 0.240 | 0.336 | 0.111 |
| minimum (ng/g) | <0.005 | <0.033 | <0.008 | <0.001 | <1.32 | <0.290 | <0.148 | <0.030 | <0.033 | <0.050 | <0.049 |
| median (ng/g) | <0.005 | 0.067 | 0.009 | 0.480 | <1.45 | <0.315 | <0.163 | <0.031 | <0.031 | <0.057 | <0.054 |
PFAS with approximately 50% or more of samples above the reporting limit (RL) were included in the statistical analysis.
Values below the RL are listed in this table as “<RL”.
Included in the statistical analysis.
Figure 1.
Maximum, minimum, and median (means are indicated when medians were not reported in the literature) PFAS levels measured by ng/g wet weight in the human placenta as reported in the literature for Chen et al. 2017 (n = 32), Mamsen et al. 2019 (n = 78), Mamsen et al. 2017 (n = 34, means reported), and placentas included in the current study, Bangma et al. (n = 122). Frequently reported PFAS, including PFOS, PFOA, PFNA, and PFDA, were included in the graph as well as any additional PFAS detected in ~50% of the placenta in the current study, including PFUnA, PFHxS, and PFHpS.
Figure 2.
Whisker plots of the distribution of PFAS of the four PFAS included in statistical analysis: PFHpS, PFHxS, PFOS, and PFUnA. Circles represent individual PFAS values, and bars represent median and interquartile ranges.
The median and interquartile ranges of PFAS in the total cohort and stratified by select sociodemographic characteristics were calculated (Table 3). Median and interquartile ranges for the remaining sociodemographic characteristics can be found in the Supporting Information Table S2. Additionally, p-value results of Wilcoxon or Kruskal–Wallis test for difference in median levels are displayed. Two comparisons were statistically significant (p < 0.05). PFUnA levels in the placenta were significantly different according to race/ethnicity status (p = 0.0002). One comparison was approaching the statistical significance: difference in PFHpS levels in the placenta in mothers of different ages (p = 0.0936).
Table 3.
Comparisons of Medians, Interquartile Range (IQR), and Maximum and Minimum Values of PFAS Levels (ng/g) by Selected Demographic Variablesa,b,c
| PFHxS |
PFOS |
PFHpS |
PFUnA |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | median | IQR | min | max | p-value | median | IQR | min | max | p-value | median | IQR | min | max | p-value | median | IQR | min | max | p-value | |
| 122 | 0.067 | 0.115 | 0.017 | 0.446 | 0.480 | 0.525 | 0.001 | 4.87 | 0.009 | 0.010 | 0.004 | 0.0630 | 0.027 | 0.032 | 0.002 | 0.240 | |||||
| race and hispanic ethnicity | 0.16 | 0.16 | 0.37 | <0.01 | |||||||||||||||||
| white non-hispanic | 42 | 0.117 | 0.130 | 0.017 | 0.446 | 0.454 | 0.500 | 0.006 | 1.42 | 0.010 | 0.012 | 0.004 | 0.028 | 0.019 | 0.020 | 0.016 | 0.100 | ||||
| white hispanic | 30 | 0.069 | 0.102 | 0.017 | 0.427 | 0.626 | 0.552 | 0.129 | 4.87 | 0.009 | 0.008 | 0.005 | 0.063 | 0.047 | 0.056 | 0.018 | 0.240 | ||||
| black non-hispanic | 37 | 0.052 | 0.079 | 0.017 | 0.362 | 0.432 | 0.407 | 0.001 | 1.71 | 0.006 | 0.010 | 0.004 | 0.025 | 0.040 | 0.042 | 0.017 | 0.217 | ||||
| other | 3 | 0.047 | 0.165 | 0.043 | 0.208 | 0.552 | 0.386 | 0.495 | 0.881 | 0.013 | 0.003 | 0.011 | 0.014 | 0.081 | 0.111 | 0.045 | 0.156 | ||||
| maternal age | 0.82 | 0.35 | 0.09 | 0.21 | |||||||||||||||||
| <20 | 1 | 0.064 | 0 | 0.064 | 0.064 | 0.166 | 0 | 0.166 | 0.166 | 0.004 | 0 | 0.004 | 0.004 | 0.017 | 0 | 0.017 | 0.017 | ||||
| 20 ≤ 35 | 77 | 0.067 | 0.102 | 0.017 | 0.446 | 0.484 | 0.486 | 0.001 | 1.91 | 0.009 | 0.009 | 0.004 | 0.043 | 0.034 | 0.028 | 0.002 | 0.134 | ||||
| 35+ | 44 | 0.068 | 0.116 | 0.017 | 0.427 | 0.485 | 0.569 | 0.004 | 4.87 | 0.011 | 0.011 | 0.004 | 0.063 | 0.022 | 0.057 | 0.016 | 0.240 | ||||
| missing | 0 | ||||||||||||||||||||
P-value of the test of difference in medians is listed. The table lists tests that were found to be statistically significant or trending toward statistical significance; for complete results of all comparisons conducted, see the Supporting Information Table S2.
P-values obtained from the Wilcoxon test for sociodemographic variables of two groups (child’s gender and marital status) and from Kruskal–Wallis test for all other sociodemographic variables, which have more than two groups.
Values below the RL have been imputed as half the RL.
3.3. PFAS Levels and Race/Ethnicity.
Significant differences in PFUnA levels were observed by maternal race and ethnicity. The three women of other race/ethnicities had the highest maximum and median values: 0.156 and 0.081 ng/g, respectively. This was followed by hispanic white, non-hispanic white, and non-hispanic black women, according to their median value. No other comparisons of PFAS levels by race/ethnicity were significant, and the order from the highest to lowest median values was not consistent across the different PFAS.
3.4. PFAS Levels and Maternal Age.
Differences by maternal age in PFHpS were trending toward significance (p = 0.09). Older mothers were observed to have higher levels of PFHpS than younger mothers. The median PFHpS in mothers under 20 years old was 0.004 ng/g, mothers between 20 and 35 years old had a median level of 0.009 ng/g, and mothers over the age of 35 had a median level of 0.011 ng/g.
3.5. PFAS Levels and Hypertensive Complications of Pregnancy.
No significant differences in PFHxS, PFHpS, or PFOS by hypertensive disorders of pregnancy were found (Supporting Information Table S3). A borderline significant difference was observed of PFUnA levels by hypertensive complications of pregnancy status (p = 0.09). In women with hypertensive complications of pregnancy, the median PFUnA level was 0.044 ng/g, compared to 0.020 ng/g in women without hypertensive complications of pregnancy. All median values were higher in placentas of mothers with hypertensive complications of pregnancy. Associations were examined in a sex-specific manner (e.g., stratified by fetal sex), and no significant differences were observed (data not shown).
3.6. PFAS Levels and Fetal Growth.
Fetal growth was categorized into small for gestational age (SGA), appropriate for gestational age (AGA), and large for gestational (LGA). Of the cohorts, 8.0% was SGA and 10.6% was LGA. No significant differences in median placental levels of PFOS, PFHxS, PFHpS, nor PFUnA were seen by fetal growth categories (Supporting Information Table S4). No consistent patterns were seen for PFAS levels across fetal growth categories. Associations were examined in a sex-specific manner (e.g., stratified by fetal sex), and no significant differences were observed (data not shown).
3.7. PFAS Levels and Gestational Age.
Gestational ages in this cohort ranged from 18 to 41 weeks. Both crude and adjusted linear regression models regressing PFAS level onto gestational age showed no significant association (Supporting Information Table S5A,B). β values representing the predicted change in the PFAS level in μg/g with each additional week of gestation were positive for PFHxS and PFHpS (increase in the placental PFAS level with an increase in gestational age) and negative for PFOS and PFUnA (a decrease in the placental PFAS level with an increase in gestational age) in both the crude and adjusted models.
For the remaining seven PFAS detected below 50% RL, associations between gestational age and detection of PFAS above the RL were investigated utilizing unadjusted binomial regression models. PFAS levels were dichotomized into two categories (detected above RL or detected below RL). PFOA was significantly more likely (p = 0.04) to be detected above RL in later gestational ages compared to that in earlier gestation age placentas (Supporting Information Table S6). Associations were examined in a sex-specific manner (e.g., stratified by fetal sex), and no significant differences were observed (data not shown).
3.8. PFAS Levels Over Time (Year of Delivery).
Dates of birth in this cohort ranged from 04/12/2015 to 01/12/2018. As only two participants had births in 2018, for the analysis of the effect of year of delivery, these two observations were removed. PFOS, PFHxS, and PFUnA were all found to decrease in concentration in placental tissues over time (Supporting Information Table S7). However, only PFHxS significantly decreased over time, with a predicted change with each passing year of −0.024 ng/g (p-value = 0.04). PFHpS showed an increase in levels with each subsequent year; however, this association was not significant.
4. DISCUSSION
PFAS exposure during pregnancy has been associated with health risks for both the mother and the fetus.15,16,20,22,26,27,30,55 Despite growing concern over the effects of prenatal exposure to PFAS, and the understanding that PFAS can cross the maternal fetal barrier via the placenta, a limited number of studies have evaluated PFAS levels in the placenta.37–40 Investigating the placenta separately from maternal serum can lend additional information as to the extent to which PFAS accumulate in the placenta. This is particularly important because it cannot be assumed that serum concentrations are representative of PFAS accumulation in specific critical organs. For example, studies have observed certain PFAS in higher levels in brain and lung compartments despite low rates of detection in serum.56 The present study provides novel information as it is among the first to quantify and analyze placental levels of PFAS in a U.S. cohort. Overall, there were three major findings. First, many of the placental samples had levels of specific PFAS chemicals similar to international studies. Of concern, PFOS, PFHxS, PFHpS, and PFUnA were detected above the reporting limit in 99, 75, 55, and 49% of placentas, respectively. Second, placental PFAS levels varied by race/ethnicity for PFUnA. Finally, associations between the levels of PFAS in the placenta in the study cohort and gestational age, hypertensive disorders of pregnancy, and fetal growth were performed but did not reach statistical significance. While placental PFAS were not associated with pregnancy or child health outcomes in this high-risk cohort, the prevalence of their detection in the placenta suggests a need to biomonitor during pregnancy.
Of the four PFAS detected above RL in approximately 50% or more of samples, PFOS was found in the highest concentration. This finding is consistent with the current literature. Previously published studies on PFAS in the placenta are limited to three studies: one in China and two in Denmark.40–42 Similar to the results from the present study, these studies found PFOS and PFOA as having the highest and second highest median ng/g wet weights, respectively, compared to other PFAS. Moreover, in the analysis of PFAS in serum measured by the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2016, PFOS was consistently found in the greatest concentration compared to other PFAS.57 Notably, of the four PFAS detected above RL in the present study, only PFOS and PFHxS are currently tracked by NHANES; therefore, comparison to the general US population for exposure to PFHpS and PFUnA is not possible at this time. In NHANES, the geometric means in the serum of PFOS and PFHxS (2015–2016) are 4.72 and 1.18 μg/L, respectively.57 Despite PFOS being phased out of production in the United States since 2002,57,58 the presence of PFOS in the placenta in this study highlights the persistence of PFAS and signifies the importance of continued PFAS monitoring and reduction efforts.
In the present study, maternal race and ethnicity were associated with significant differences in PFUnA levels, with those who self-reported their race/ethnicity as other than non-hispanic white, hispanic white, and non-hispanic black having the greatest measured median wet weight in ng/g of PFUnA, while non-hispanic black women had the lowest. PFAS concentrations have been found to vary by race/ethnicity in nonpregnancy cohorts; however, consistent patterns have not been established and different PFAS appear to demonstrate varied distributions by race and ethnicity.59–61 Research concerning associations between race/ethnicity and PFAS levels in pregnant women is limited. To our knowledge, the only studies to investigate race/ethnicity and PFAS in pregnant U.S. women are the Health Outcomes and Measures of the Environment (HOME) Study and Project Viva. The HOME study observed significantly different serum PFAS concentrations in pregnant mothers based on maternal race.36,62,63 In addition, both Project Viva and the HOME Study found that maternal race can be a predictor of PFAS levels in children, with children born to black mothers showing lower PFAS levels than children born to white mothers.62–64 Additional research on the relationship between PFAS levels and maternal race and ethnicity is warranted to discern environmental exposure disparities, sources, and pathways.
Finally, this study evaluated changes in PFAS with gestational age at birth and pre-eclampsia or gestational hypertension. Previously, Mamsen and colleagues investigated placentas in the first through third trimester and identified five PFAS, including PFOS, PFOA, PFDA, PFNA, and PFUnA, that increased as gestational age increased.41,42 In the present study, none of the PFAS displayed a significant association between PFAS levels and gestational age. The differences between the Mamsen studies and the current study could be due to several factors. First, this high-risk cohort is enriched with placentas from preterm deliveries compared to the larger U.S. population. As a result, the placentas are likely physiologically distinct from term placentas, which may influence PFAS accumulation.65,66 Second, the current study investigated placentas from mid-second to third trimester pregnancies (18–41 weeks’ gestation), while Mamsen et al. investigated placentas from the first to third trimesters. Third, our study cohort placentas were obtained from live births, while the Mamsen studies investigated PFAS in the placenta and fetal organs from intrauterine fetal demise (IUFD). In addition, in the present study, we observed no associations between pre-eclampsia or gestational hypertension and placental PFAS. Previous literature is divided on the subject of PFAS and pre-eclampsia with one study reporting no association,17 while other studies report associations for at least one PFAS, including PFOS, PFOA, PFNA, and PFBS.15,16,19,20 It is important to note that placental studies are limited to cross-sectional design and therefore hindered by the potential for reverse causality.67,68
While this study is among the first to examine PFAS placental levels in a U.S. cohort, it is not without limitations. Concentrations of PFAS quantified in the placenta are usually lower than matching fetal cord serum and maternal serum.40 This limitation often reduces the number of PFAS measured above the reporting limit and can impact the ability of researchers to identify associations between PFAS and potential risk factors; for example, PFUnA, in our study, was detected above the RL in 49% of placentas. Future studies will investigate PFAS in matching maternal serum from this cohort. Finally, the cohort used in this study comprises women at high risk for spontaneous preterm birth in North Carolina. As mentioned, the exposure levels and associations detected in this cohort are likely not generalizable to the broader U.S. population of pregnant women due to the geographical and obstetric characteristics of this cohort.69 Specifically, pathologies of the placenta may influence bioaccumulation of PFAS, and thus, reverse causality cannot be excluded with a cross-sectional study. Future research should investigate the associations between PFAS accumulation in the placenta and pregnancy complications in a more generalizable cohort.
For the 122 placentas investigated, similar PFOS and PFOA levels were observed in this U.S.-based cohort as compared to cohorts in China and Denmark. Maternal race/ethnicity was associated with significant differences in PFUnA levels. No significant associations between PFAS and fetal growth, gestational age, or hypertensive disorders of pregnancy were observed in the current study. Overall, this study expands on the limited data describing PFAS levels in the placenta and further highlights the need for biomonitoring of PFAS during pregnancy among women of child-bearing age.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded in part by the NIH (T32-ES007018, R01-MD011609, and K24-ES031131) and the NC PFAST network.
Footnotes
Certain commercial equipment or instruments are identified in the paper to adequately specify the experimental procedures. Such identification does not imply recommendations or endorsement by the NIST nor does it imply that the equipment or instruments are the best available for the purpose. The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.9b07102.
Additional materials and methods; cohort comparison to the National Vital Statistics Birth Data 2016 and 2017; all calculated RLs (File S1, XLSX); associations between each of PFAS levels measured in the placenta (Figure S1); Spearman correlation coefficients of the correlations between PFAS measured in the placenta (Table S1); comparisons of medians of PFAS levels by selected demographic variables (Table S2); comparisons of medians, IQR, and maximum and minimum values of PFAS levels by hypertensive disorders of pregnancy (Table S3); comparisons of medians, IQR, and maximum and minimum values of PFAS levels by fetal growth (Table S4); β values, standard error, and associated p-values of crude linear regressions of PFAS level onto gestational age; β values, standard error, and associated p-values of adjusted, multivariate linear regressions of PFAS level onto gestational age (Table S5); crude binomial regression models between gestational age and PFAS detection above RL for the seven PFAS detected below RL in approximately 50% or more of samples (Table S6); β values, standard error, and associated p-values of crude linear regressions of PFAS level onto delivery date (Table S7); and NIST Standard Reference Material 1947 CoA compared to PFAS values obtained in this study (Table S8) (PDF)
Contributor Information
Jacqueline Bangma, Department of Environmental Sciences and Engineering, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27516, United States.
Lauren A. Eaves, Department of Environmental Sciences and Engineering, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27516, United States.
Kirsi Oldenburg, Department of Environmental Sciences and Engineering, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27516, United States.
Jessica L. Reiner, Chemical Sciences Division, Hollings Marine Laboratory, National Institute of Standards and Technology, Charleston, South Carolina 29412, United States
Tracy Manuck, Department of Obstetrics and Gynecology, Division of Maternal Fetal Medicine, University of North Carolina-Chapel Hill, Chapel Hill, North Carolina 27599, United States; Institute for Environmental Health Solutions, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States.
Rebecca C. Fry, Department of Environmental Sciences and Engineering, Gillings School of Global Public Health, Institute for Environmental Health Solutions, Gillings School of Global Public Health, and Curriculum in Toxicology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27516, United States; .
REFERENCES
- (1).KEMI. Occurrence and Use of Highly Fluorinated Substances and Alternatives; KEMI, Swedish Chemicals Agency Stockholm; : Sweden, 2015. [Google Scholar]
- (2).Eriksson U; Kärrman A World-wide indoor exposure to polyfluoroalkyl phosphate esters (PAPs) and other PFASs in household dust. Environ. Sci. Technol. 2015, 49, 14503–14511. [DOI] [PubMed] [Google Scholar]
- (3).Hu XC; Andrews DQ; Lindstrom AB; Bruton TA; Schaider LA; Grandjean P; Lohmann R; Carignan CC; Blum A; Balan SA; Higgins CP; Sunderland EM Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, No. 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hopkins ZR; Sun M; DeWitt JC; Knappe DR Recently Detected Drinking Water Contaminants: GenX and Other Per-and Polyfluoroalkyl Ether Acids. J. - Am. Water Works Assoc. 2018, No. 107. [Google Scholar]
- (5).Haug LS; Thomsen C; Brantsæter AL; Kvalem HE; Haugen M; Becher G; Alexander J; Meltzer HM; Knutsen HK Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ. Int. 2010, 36, 772–778. [DOI] [PubMed] [Google Scholar]
- (6).Tittlemier SA; Pepper K; Seymour C; Moisey J; Bronson R; Cao X-L; Dabeka RW Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J. Agric. Food Chem. 2007, 55, 3203–3210. [DOI] [PubMed] [Google Scholar]
- (7).Fromme H; Mosch C; Morovitz M; Alba-Alejandre I; Boehmer S; Kiranoglu M; Faber F; Hannibal I; Genzel-Boroviczeny O; Koletzko B; Volkel W Pre- and postnatal exposure to perfluorinated compounds (PFCs). Environ. Sci. Technol. 2010, 44, 7123–7129. [DOI] [PubMed] [Google Scholar]
- (8).Haug L; Huber S; Becher G; Thomsen C Characterisation of human exposure pathways to perfluorinated compounds–comparing exposure estimates with biomarkers of exposure. Environ. Int. 2011, 37, 687–693. [DOI] [PubMed] [Google Scholar]
- (9).Key B; Howell R; Criddle C Fluorinated Organics in the Biosphere. Environ. Sci. Technol. 1997, 31, No. 2445. [Google Scholar]
- (10).Fromme H; Tittlemier S; Volkel W; Wilhelm M; Twardelle D Perfluorinated compounds–exposure assessment for the general population in Western countries. Int. J. Hyg. Environ. Health 2009, 212, 239–270. [DOI] [PubMed] [Google Scholar]
- (11).Kannan K; Corsolini S; Falandysz J; Fillmann G; Kumar K; Loganathan B; Mohd M; Olivero J; Van Wouwe N; Yang J; ALdoust K Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol. 2004, 38, 4489–4495. [DOI] [PubMed] [Google Scholar]
- (12).US EPA. Drinking Water Advisory for Perfluorooctane Sulfonate (PFOS); United States Environmental Protection Agency: Washington, DC, 2016; pp 1–88. [Google Scholar]
- (13).Calafat AM; Wong LY; Kuklenyik Z; Reidy JA; Needham LL Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Emerging Contaminants and Federal Facility Contaminants of Concern. U.S. Environmental Protection Agency, 2019. https://www.epa.gov/fedfac/emerging-contaminants-and-federal-facilitycontaminants-concern.
- (15).Huang R; Chen Q; Zhang L; Luo K; Chen L; Zhao S; Feng L; Zhang J Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and the risk of hypertensive disorders of pregnancy. Environ. Health 2019, 18, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wikström S; Lindh CH; Shu H; Bornehag C-G Early pregnancy serum levels of perfluoroalkyl substances and risk of preeclampsia in Swedish women. Sci. Rep. 2019, 9, No. 2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Starling AP; Engel SM; Richardson DB; Baird DD; Haug LS; Stuebe AM; Klungsøyr K; Harmon Q; Becher G; Thomsen C; Sabaredzovic A; Eggesbø M; Hoppin JA; Travlos GS; Wilson RE; Trogstad LI; Magnus P; MP L Perfluoroalkyl substances during pregnancy and validated preeclampsia among nulliparous women in the Norwegian Mother and Child Cohort Study. Am. J. Epidemiol. 2014, 179, 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Vestergaard S; Nielsen F; Andersson A-M; Hjøllund NH; Grandjean P; Andersen HR; Jensen TK Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Hum. Reprod. 2012, 27, 873–880. [DOI] [PubMed] [Google Scholar]
- (19).Savitz DA; Stein CR; Bartell SM; Elston B; Gong J; Shin HM; Wellenius GA Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology 2012, 23, 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Stein CR; Savitz DA; Dougan M Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am. J. Epidemiol. 2009, 170, 837–846. [DOI] [PubMed] [Google Scholar]
- (21).Jensen TK; Andersen LB; Kyhl HB; Nielsen F; Christesen HT; Grandjean P Association between Perfluorinated Compound Exposure and Miscarriage in Danish Pregnant Women. PLoS One 2015, 10, No. e0123496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Arbuckle TE; Kubwabo C; Walker M; Davis K; Lalonde K; Kosarac I; Wen SW; Arnold DL Umbilical cord blood levels of perfluoroalkyl acids and polybrominated flame retardants. Int. J. Hyg. Environ. Health 2013, 216, 184–194. [DOI] [PubMed] [Google Scholar]
- (23).Fei C; McLaughlin J; Tarone R; J, O. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ. Health Perspect. 2007, 115, 1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lauritzen H; Larose T; Øien T; Sandanger T; Odland J; van de Bor M; Jacobsen G Maternal serum levels of perfluoroalkyl substances and organochlorines and indices of fetal growth: a Scandinavian case–cohort study. Pediatr. Res. 2017, 81, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Maisonet M; Terrell M; McGeehin M; Christensen K; Holmes A; Calafat A; Marcus M Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ. Health Perspect. 2012, 120, 1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Apelberg BJ; Witter FR; Herbstman JB; Calafat AM; Halden RU; Needham LL; Goldman LR Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ. Health Perspect. 2007, 115, 1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Fei C; McLaughlin JK; Tarone RE; et al. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ. Health Perspect. 2007, 115, 1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Liew Z; Ritz B; Bonefeld-Jorgensen EC; Henriksen TB; Nohr EA; Bech BH; Fei C; Bossi R; von Ehrenstein OS; Streja E; Uldall P; Olsen J Prenatal exposure to perfluoroalkyl substances and the risk of congenital cerebral palsy in children. Am. J. Epidemiol. 2014, 180, 574–581. [DOI] [PubMed] [Google Scholar]
- (29).Ernst A; Brix N; Lauridsen L; Olsen J; Parner E; Liew Z; Olsen L; Ramlau-Hansen C Exposure to Perfluoroalkyl Substances during Fetal Life and Pubertal Development in Boys and Girls from the Danish National Birth Cohort. Environ. Health Perspect. 2019, 127, No. 017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Braun J; Chen A; Romano M; Calafat A; Webster G; Yolton K; Lanphear B Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity 2015, 24, 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Lau C; Thibodeaux JR; Hanson RG; Narotsky MG; Rogers JM; Lindstrom AB; Strynar MJ Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci. 2006, 90, 510–518. [DOI] [PubMed] [Google Scholar]
- (32).Lau C; Butenhoff JL; Rogers JM The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol. Appl. Pharmcol. 2004, 198, 231–241. [DOI] [PubMed] [Google Scholar]
- (33).Lau C; Thibodeaux JR; Hanson RG; Rogers JM; Grey BE; Stanton ME; Butenhoff JL; Stevenson LA Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol. Sci. 2003, 74, 382–392. [DOI] [PubMed] [Google Scholar]
- (34).Tucker DK; Macon MB; Strynar MJ; Dagnino S; Andersen E; Fenton SE The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod. Toxicol. 2015, 54, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lauritzen H; Larose T; Øien T; Odland J; van de Bor M; Jacobsen G; Sandanger T Factors Associated with Maternal Serum Levels of Perfluoroalkyl Substances and Organochlorines: A Descriptive Study of Parous Women in Norway and Sweden. PLoS One 2016, 11, No. e0166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Sagiv SK; Rifas-Shiman SL; Webster TF; Mora AM; Harris MH; Calafat AM; Ye X; Gillman MW; Oken E Sociodemographic and Perinatal Predictors of Early Pregnancy Per- and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ. Sci. Technol. 2015, 49, 11849–11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Glynn A; Berger U; Bignert A; Ullah S; Aune M; Lignell S; Darnerud PO Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ. Sci. Technol. 2012, 46, 9071–9079. [DOI] [PubMed] [Google Scholar]
- (38).Porpora MG; Lucchini R; Abballe A; Ingelido AM; Valentini S; Fuggetta E; Cardi V; Ticino A; Marra V; Fulgenzi AR Placental transfer of persistent organic pollutants: a preliminary study on mother-newborn pairs. Int. J. Environ. Res. Public Health 2013, 10, 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Gao K; Zhuang T; Liu X; Fu J; Zhang J; Fu J; Wang L; Zhang A; Liang Y; Song M; Jiang G Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFASs) and Association between the Placental Transfer Efficiencies and Dissociation Constant of Serum Proteins-PFAS Complexes. Environ. Sci. Technol. 2019, No. 6529. [DOI] [PubMed] [Google Scholar]
- (40).Chen F; Yin S; Kelly BC; Liu W Chlorinated polyfluoroalkyl ether sulfonic acids in matched maternal, cord, and placenta samples: a study of transplacental transfer. Environ. Sci. Technol. 2017, 51, 6387–6394. [DOI] [PubMed] [Google Scholar]
- (41).Mamsen LS; Björvang RD; Mucs D; Vinnars M-T; Papadogiannakis N; Lindh CH; Andersen CY; Damdimopoulou P Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies. Environ. Int. 2019, 124, 482–492. [DOI] [PubMed] [Google Scholar]
- (42).Mamsen LS; Jönsson BA; Lindh CH; Olesen RH; Larsen A; Ernst E; Kelsey TW; Andersen CY Concentration of perfluorinated compounds and cotinine in human foetal organs, placenta, and maternal plasma. Sci. Total Environ. 2017, 596, 97–105. [DOI] [PubMed] [Google Scholar]
- (43).Monroy R; Morrison K; Teo K; Atkinson S; Kubwabo C; Stewart B; Foster WG Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples. Environ. Res. 2008, 108, 56–62. [DOI] [PubMed] [Google Scholar]
- (44).Calafat AM; Kuklenyik Z; Reidy JA; Caudill SP; Tully JS; Needham LL Serum concentrations of 11 polyfluoroalkyl compounds in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Sci. Technol. 2007, 41, 2237–2242. [DOI] [PubMed] [Google Scholar]
- (45).Sun M; Arevalo E; Strynar M; Lindstrom A; Richardson M; Kearns B; Pickett A; Smith C; Knappe DR Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett. 2016, 3, 415–419. [Google Scholar]
- (46).Manuck TA; Smeester L; Martin EM; Tomlinson MS; Smith C; Varner MW; Fry RC Epigenetic Regulation of the Nitric Oxide Pathway, 17-α Hydroxyprogesterone Caproate, and Recurrent Preterm Birth. Am. J. Perinatol. 2018, 35, 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).ACOG. Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Am. J. Obstet. Gynecol. 2019, 133, e1–e25. [DOI] [PubMed] [Google Scholar]
- (48).Bangma JT; Reiner JL; Lowers RH; Cantu TM; Scott J; Korte JE; Scheidt DM; McDonough C; Tucker J; Back B Perfluorinated alkyl acids and fecundity assessment in striped mullet (Mugil cephalus) at Merritt Island national wildlife refuge. Sci. Total Environ. 2018, 619, 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Reiner JL; O’Connell SG; Moors AJ; Kucklick JR; Becker PR; Keller JM Spatial and temporal trends of perfluorinated compounds in beluga whales (Delphinapterus leucas) from Alaska. Environ. Sci. Technol. 2011, 45, 8129–8136. [DOI] [PubMed] [Google Scholar]
- (50).Kurtz AE; Reiner JL; West KL; Jensen BA Perfluorinated Alkyl Acids in Hawaiian Cetaceans and Potential Biomarkers of Effect: Peroxisome Proliferator-Activated Receptor Alpha and Cytochrome P450 4A. Environ. Sci. Technol. 2019, 53, No. 2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Keller JM; Kannan K; Taniyasu S; Yamashita N; Day RD; Arendt MD; Segars AL; Kucklick JR Perfluorinated Compounds in the Plasma of Loggerhead and Kemp’s Ridley Sea Turtles from the Southeastern Coast of the United States. Environ. Sci. Technol. 2005, 39, 9101–9108. [DOI] [PubMed] [Google Scholar]
- (52).Olsen IE; Groveman SA; Lawson ML; Clark RH; Zemel BS New intrauterine growth curves based on United States data. Pediatrics 2010, 125, e214–24. [DOI] [PubMed] [Google Scholar]
- (53).Hamilton BE; Osterman MJ; Driscoll AK; Rossen LM Births: Provisional Data for 2017; National Vital Statistics Report, 2018; pp 14–22. [PubMed] [Google Scholar]
- (54).Martin JA; Hamilton BE; Osterman MJK; Driscoll AK; Drake P Births: Final Data for 2016; National Vital Statistics Report, 2018; pp 1–55. [PubMed] [Google Scholar]
- (55).Sagiv SK; Rifas-Shiman SL; Fleisch AF; Webster TF; Calafat AM; Ye X; Gillman MW; Oken E Early-Pregnancy Plasma Concentrations of Perfluoroalkyl Substances and Birth Outcomes in Project Viva: Confounded by Pregnancy Hemodynamics? Am. J. Epidemiol. 2018, 187, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Pérez F; Nadal M; Navarro-Ortega A; Fàbrega F; Doming JL; Barceló D; Farré M Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [DOI] [PubMed] [Google Scholar]
- (57).Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals; National Center for Environmental Health (U.S.) Division of Laboratory Sciences: Atlanta, Georgia, 2019; pp 1–866. [Google Scholar]
- (58).US EPA. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS); U.S. Environmental Protection Agency Office of Water, 2016; pp 1–88. [Google Scholar]
- (59).Christensen K; Raymond M; Blackowicz M; Liu Y; Thompson B; Anderson H; Turyk M Perfluoroalkyl substances and fish consumption. Environ. Res. 2017, 154, 145–151. [DOI] [PubMed] [Google Scholar]
- (60).Park S; Peng Q; Ding N; Mukherjee B; Harlow S Determinants of per- and polyfluoroalkyl substances (PFAS) in midlife women: Evidence of racial/ethnic and geographic differences in PFAS exposure. Environ. Res. 2019, 175, 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Barton KE; Starling AP; Higgins CP; McDonough CA; Calafat AM; Adgate JL Sociodemographic and behavioral determinants of serum concentrations of per-and polyfluoroalkyl substances in a community highly exposed to aqueous film-forming foam contaminants in drinking water. Int. J. Hyg. Environ. Health 2020, 223, 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Kato K; Wong L; Chen A; Dunbar C; Webster G; Lanphear B; Calafat A Changes in Serum Concentrations of Maternal Poly- and Perfluoroalkyl Substances over the Course of Pregnancy and Predictors of Exposure in a Multiethnic Cohort of Cincinnati, Ohio Pregnant Women during 2003–2006. Environ. Sci. Technol. 2014, 48, 9600–9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Kingsley S; Eliot M; Kelsey K; Calafat A; Ehrlich S; Lanphear B; Chen A; Braun J Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environ. Res. 2018, 165, 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Harris M; Rifas-Shiman S; Calafat A; Ye X; Mora A; Webster T; Oken E; Sagiv S Predictors of Per- and Polyfluoroalkyl Substance (PFAS) Plasma Concentrations in 6–10 Year Old American Children. Environ. Sci. Technol. 2017, 51, 5193–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Doğan K; Salihoglu O; Sever N; Tombul T; Sari E; Yasar L Do placental histopathologic characteristics differ with gestational ages in preterm and term deliveries? Fetal Pediatr. Pathol. 2015, 34, 365–374. [DOI] [PubMed] [Google Scholar]
- (66).Paquette AG; Brockway HM; Price ND; Muglia LJ Comparative transcriptomic analysis of human placentae at term and preterm delivery. Biol. Reprod. 2018, 98, 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Savitz DA; Wellenius GA Invited commentary: exposure biomarkers indicate more than just exposure. Am. J. Epidemiol. 2018, 187, 803–805. [DOI] [PubMed] [Google Scholar]
- (68).Dhingra R; Winquist A; Darrow LA; Klein M; Steenland K A study of reverse causation: Examining the associations of perfluorooctanoic acid serum levels with two outcomes. Environ. Health Perspect. 2017, 125, 416–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Kleinbaum DG; Morgenstern H; Kupper LL Selection bias in epidemiologic studies. Am. J. Epidemiol. 1981, 113, 452–463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.