Highlights
-
•
We report one case of human rabies with a long incubation period in Wuhan, China.
-
•
In Wuhan, many free-roaming dogs are not only dangerous to domestic dogs and livestock, they are also a threat to humans.
-
•
Epidemiology of rabies with emphasis on its potential to spread in urban-rural fringe in Wuhan has been discussed.
-
•
The elimination of RABV from free-roaming domestic dogs will drive a considerable reduction in human disease.
-
•
We showed that the efficient collaboration was important between the hospital and a professional laboratory for rabies diagnosis.
Keywords: Glycoprotein gene sequences, Human rabies, Rabies virus
Abstract
Rabies remains endemic in China and continues to pose a major threat to public health with a nearly 100 % case fatality rate in humans. We confirmed a case of human rabies in Wuhan, in May 2018. The patient had got a dog bite wound 3 years before symptoms of confusion, hydrophobia, and photophobia onset. On May 14, our laboratory confirmed that the patient was infected with a rabies virus that circulates in dogs in China and died on May 24, two weeks later after admission. Complete glycoprotein gene sequences determined for this isolate indicated the source of a RABV infection was dog-related RABV variants.
Introduction
Rabies virus(RABV) belongs to the genus Lyssavirus of Rhabdoviridae that infect a variety of mammals, including humans causing fatal viral encephalitis [1]. Human infection usually results from a bite, scratch, or lick from an infected animal [2]. Dogs are the main source of human rabies deaths, contributing up to 99% of all rabies transmissions to humans [2]. The incubation period for rabies usually varies from 1 to 3 months, but may also vary from less than a week to greater than a year depending on the age, proximity to the brain, severity of exposure, the rabies viral load, the species of the animal, and the variant of the virus [3,4].
According to the World Health Organization (WHO) estimates, globally, rabies causes approximately 59,000 human deaths every year, 95 % of which occur in the developing countries of Asia and Africa [2]. To date, 16 different lyssavirus species have been established and two more are awaiting further assessment [5]. RABV is commonly subdivided into six phylogenetic clades, namely the Africa 2, Africa 3, Arctic-related, Asian, Cosmopolitan, and Indian subcontinent clades [6]. The current Chinese RABV strains can also be clustered into six major and geographically distinct phylogenetic subclades [7,8].
As the largest developing country, China is a high-risk region, and the number of human rabies cases is second only to that in India [8]. Despite the existence of effective rabies vaccines for dogs, dog-transmitted human rabies persists and has reemerged in China. The latest human rabies epidemic wave in China began in 1997 (222 cases), reaching its peak in 2007 (3,303 cases) and then rapidly declining following the implementation of comprehensive control measures throughout the country [9]. A total of 290 human rabies cases were reported in 22 provinces in China in 2019, a decrease of 43.80 % (226/516) compared with 2017.
Recently, one RABV infections was diagnosed in Wuhan. In this study, our laboratory confirmed that the patient was infected with a rabies virus. Therefore, complete G gene sequences determined for this isolate from human patient to analyse their potential genetic relationship with other Chinese isolates.
The study
On May 6, 2018, a 63-year-old woman, lived in Huangpi, Wuhan, sought treatment nearby clinic because of persistent itching of the skin on my right lower arm, but it did not relieve. Four days later, on 10 May, she went to the Emergency Department (ED) of Wuhan Golden yingtan Hospital with fever, chest tightness, waist pain, gas kick, dyspnea, hydrophobia, and photophobia, consistent with rabies virus infection. She had no past history of pre-/post-exposure prophylaxis(PrEP/PEP) vaccination. Hospital staff members questioned family about the patient history for the possibility of exposure to a rabid animal bite or scratc, and the husband and son of the patient reported that she had been bitten on her left ankle by a stray dog 3 years before symptom onset, suffered from hemorrhage. After the bite, the village doctor only applied some traditional Chinese medicine to stop bleeding, however, did not wash the wound thoroughly in that site and not immediately resort to hospital treatment such as the wound disinfection, local infiltration of rabies immunoglobulin and administration of rabies vaccines. She was neurologically evaluated and diagnosed with an encephalitic syndrome. A presumptive diagnosis of suspected rabies was made on the first day of admission. Her blood pressure measured was 127/74 mmHg, temperature 36.3 °C, while her pulse and respiratory rates were 80 beats per minute and 18 breaths per minute. Laboratory results were notable for abnormal blood coagulation status of the patients, partial phromboplastin time (APTT) level of 18.9 s (reference range, 24−36 s), prothrombin time (PT) level of 11.2 s (reference range, 12–16 s), antithrombin (At III) level of 126 % (reference range, 103.2%~113.8%) and DDimer level of 2.79 μg/mL (reference range, <0.2 μg/mL).
The same day, she was transferred to the intensive care unit (ICU). Based on clinical symptoms, the primary treatment for human cases was intensive supportive care treatment and the use of an anti-excitatory strategy that included sedative and analgesic medicine, with ventilator-assisted ventilation and continuous renal replacement therapy. Because rabies PEP was ineffective for treatment of rabies after the onset of symptoms, PEP was not administered.
Beacuse the hospital lacked the capacity to perform basic tests that could dectet the viral antigens to help support the diagnosis of rabies virus in the patient, on 14 May, CSF, serum and saliva specimens were collected and submitted to Rabies Diagnosis Center of Wuhan Institute of Biologic Products,Co.,Ltd for rabies testing. No neutralizing antibodies to rabies virus were detected in serum or CSF with a modified rapid fluorescent focus inhibition test (RFFIT) [10] using the calibrated WHO international standard immunoglobulin adjusted to 2 international units (IU) and a naive bovine serum as PC(positive control)and NC (negative control), respectively. On May 14, rabies was confirmed by the detection of rabies virus RNA by reverse transcription polymerase–chain reaction (RT-PCR) in saliva. Intracranial inoculations of suckling mice with a 20 % suspension of three saliva samples showed typical rabies symptoms within 14 days. The diagnosis was again confirmed again by the detection of rabies virus RNA by RT-PCR and for detection of rabies virus antigen with double antibody sandwich ELISA in brain of suckling mice.
Despite treatment with sedative and analgesic medicine with ventilator-assisted ventilation, the patient’s condition progressively worsened. On May 24, the family decided to withdraw advanced medical support, and the patient died shortly thereafter. Case history obtained through family member, revealed that the patient was bitten by a stray dog on her left ankle 3 years ago and the dog was clubbed to death with sticks on the same day, a common measure in Wuhan where animal rabies is enzootic. According to her son and husband sharing information, she had no scratches or bites from domestic or wild animals before the onset of the disease, except that she was bitten by dogs three years ago. No tests were carried out on the dog to confirm rabies. The patient did not receive appropriate wound treatment, including fushing and disinfection of the wound site and had not initiated any rabies PEP vaccination schedule at the medical facility. While after 3 years, she developed the first symptoms.
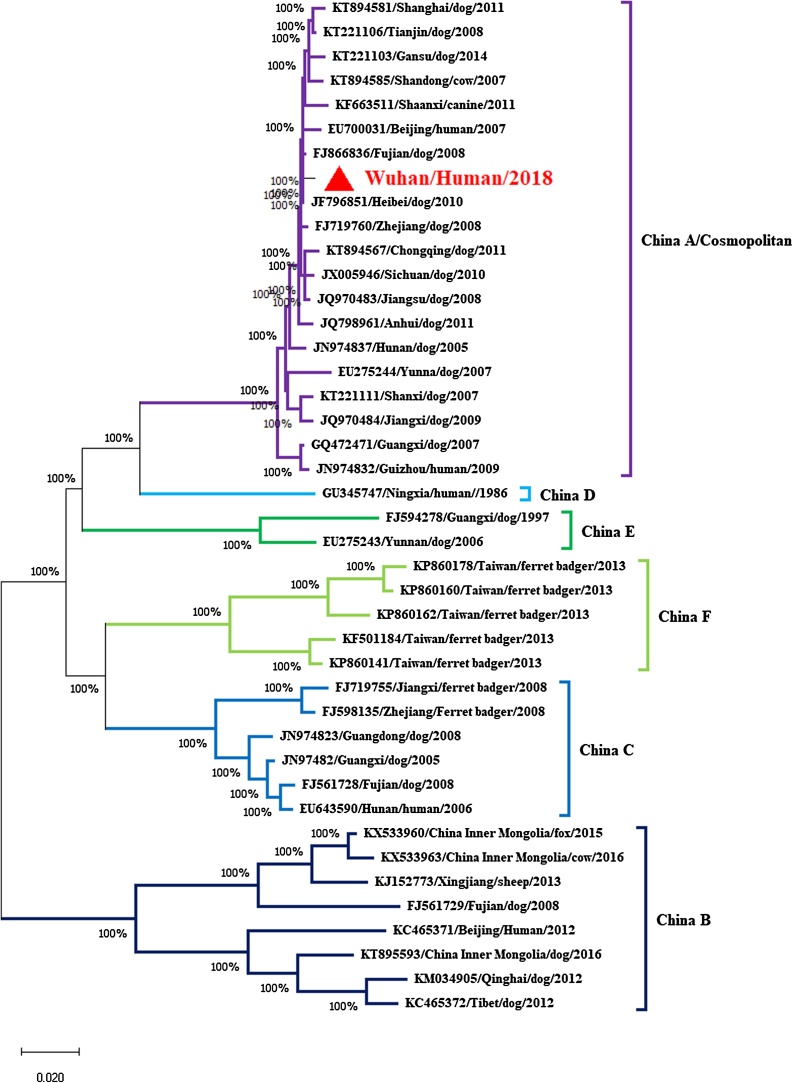
The complete G gene was amplified as two overlapping fragments, G1 and G2, using two pairs of primers in combination, which were adapter primers M13FM221/M13RG781 and M13RMSL2/M13FL2 [11]. Phylogenetic and molecular evolutionary analyses were conducted using the MEGA X software and the neighborjoining (NJ) algorithm with 1000 bootstrap replications [12]. Sequencing and analyzing of the glycoprotein gene of rabies virus isolates from human case indicated that the causal virus was a lyssavirus of the rabies virus species belonging to China-lineage A (Fig. 1), which was included in a branch representing the cosmopolitan lineage [7,13].
Fig. 1.
Phylogenetic analysis of representative Chinese RABV G gene sequences. The tree was constructed by a NJ algorithm using MEGAX software. Bootstrap values are shown at the branch nodes. China RABV lineages A–F are indicated.
Conclusion
Dog-associated RABVs are the main causative agents of human rabies case in the inner Chinese provinces [14]. Here, we describe the human rabies case from Wuhan in 2018. We show that the human RABV strains cluster with RABV sequences previously identified in Wuhan of Hubei province, which was belong to cosmopolitan dog RABV. Our report demonstrates that RABV is still present in Wuhan, where free-roaming dog populations act as the principal reservoir. This shows the need for further epidemiological research and control measures on the management of dog populations. Since dog bites are the main source of human infections, mass dog vaccination campaigns and responsible dog ownership represent the most effective course of action to reduce rabies incidence [2].
The handling of the case described is an example of efficient collaboration between the hospital and a professional laboratory, Rabies Diagnosis Center of Wuhan Institute of Biologic Products,Co.,Ltd. There was constant and rapid exchange of information between these entities to confirm the case and to identify the exposed individuals. This case highlights the importance of prompt rabies diagnosis to minimize health care–associated exposures.
Ethical approval
Ethical Approval was given by Wuhan Golden yingtan Hospital.
Author’s statement
All authors have seen and approved this version of the manuscript, and it is not submitted for publication anywhere.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
This work has received funding from National "Major New Drug Creation" Science and Technology Major Project (2015ZX09102021) and 2017 Hubei Province Technology Innovation Project (Major Project).
References
- 1.Delmas O., Holmes E.C., Talbi C., Larrous F., Dacheux L., Bouchier C. Genomic diversity and evolution of the lyssaviruses. PLoS One. 2008;3(4):e2057. doi: 10.1371/journal.pone.0002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization W.H. 2018. WHO expert consultation on rabies. Third report. WHO technical report series (1012) 1-139, back cover. [Google Scholar]
- 3.Apanga P.A., Awoonor-Williams J.K., Acheampong M., Adam M.A. A presumptive case of human rabies: a rare survived case in rural ghana. Front Public Health. 2016;4:256. doi: 10.3389/fpubh.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller T., Freuling C.M. Chapter 6 - rabies in terrestrial animals. In: Fooks A.R., Jackson A.C., editors. Rabies. fourth edition. Academic Press; Boston: 2020. pp. 195–230. [Google Scholar]
- 5.Rupprecht C.E., Kuzmin I.V., Yale G., Nagarajan T., Meslin F.X. Priorities in applied research to ensure programmatic success in the global elimination of canine rabies. Vaccine. 2019;37(Suppl. 1):A77–A84. doi: 10.1016/j.vaccine.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Oude M.B., Farag E., GeurtsvanKessel C., Schapendonk C., van der Linden A., Kohl R. First molecular analysis of rabies virus in Qatar and clinical cases imported into Qatar, a case report. Int J Infect Dis. 2020;96:323–326. doi: 10.1016/j.ijid.2020.04.070. [DOI] [PubMed] [Google Scholar]
- 7.Meng S., Xu G., Wu X., Lei Y., Yan J., Nadin-Davis S.A. Transmission dynamics of rabies in China over the last 40 years: 1969-2009. J Clin Virol. 2010;49(1):47–52. doi: 10.1016/j.jcv.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Wang L., Wu X., Bao J., Song C., Du J. Phylodynamic and transmission pattern of rabies virus in China and its neighboring countries. Arch Virol. 2019;164(8):2119–2129. doi: 10.1007/s00705-019-04297-8. [DOI] [PubMed] [Google Scholar]
- 9.Tu C., Feng Y., Wang Y. Animal rabies in the People’s Republic of China. Rev Sci Tech. 2018;37(2):519–528. doi: 10.20506/rst.37.2.2820. [DOI] [PubMed] [Google Scholar]
- 10.Smith J., Yager P.A., Baer G.M. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus-neutralizing antibody. Lab Tech Rabies. 1996:181–191. [Google Scholar]
- 11.Meng S., Lv W., Wu J., Wang J., Xu G., Yan J. Comparison of three strategies for sequencing rabies viral G gene. J Appl Virol. 2013:2. [Google Scholar]
- 12.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng S.L., Yan J.X., Xu G.L., Nadin-Davis S.A., Ming P.G., Liu S.Y. A molecular epidemiological study targeting the glycoprotein gene of rabies virus isolates from China. Virus Res. 2007;124(1–2):125–138. doi: 10.1016/j.virusres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Wang L., Tang Q., Liang G. Rabies and rabies virus in wildlife in mainland China, 1990-2013. Int J Infect Dis. 2014;25:122–129. doi: 10.1016/j.ijid.2014.04.016. [DOI] [PubMed] [Google Scholar]