Abstract
Objective
The purpose of this study was to investigate the impact of obesity on disease activity and disease outcome in patients with inflammatory bowel disease.
Patients and methods
The impact of obesity on inflammatory bowel disease disease activity and outcome was retrospectively assessed in 3075 patients enrolled in the prospective nation-wide Swiss inflammatory bowel disease cohort between July 2006 and September 2018. Baseline characteristics, disease activity and disease course in 325 obese inflammatory bowel disease patients (body mass index ≥30 kg/m2) were compared to 1725 normal weight inflammatory bowel disease individuals (body mass index 18.5-24.9).
Results
Among 3075 patients in the prospective Swiss inflammatory bowel disease cohort, 325 patients (10.6%) were obese, namely, 194 Crohn’s disease patients, 131 ulcerative colitis, and inflammatory bowel disease-unclassified patients. Disease activity scores were elevated in obese Crohn’s disease (Crohn’s Disease Activity Index 33 vs 20, p = 0.001), but not ulcerative colitis patients. Obese Crohn’s disease, but not ulcerative colitis patients were less likely to be in remission based on a Crohn’s Disease Activity Index less than 100 and a calprotectin less than 100 ug/g. In a multivariate regression model, obesity was negatively associated with disease remission in Crohn’s disease (odds ratio 0.610, 95% confidence interval 0.402–0.926, p = 0.020), but not ulcerative colitis. Increased soft stool frequency was observed in both obese Crohn’s disease and ulcerative colitis patients. Adjusted Cox regression models revealed increased risk of complicated disease course in obese Crohn’s disease patients (hazard ratio 1.197, 95% confidence interval 1.046–1.370, p = 0.009). No association between obesity and disease progression, index treatment failure was seen neither in Crohn’s disease nor ulcerative colitis.
Conclusion
Obesity is associated with decreased rates of disease remission and increased risk of complicated disease course in Crohn’s disease over a six-year follow-up period. No effects were seen on disease progression and index treatment failure neither in Crohn’s disease nor ulcerative colitis.
Keywords: Inflammatory bowel disease, obesity, body mass index, natural history, Crohn’s disease, ulcerative colitis, disease activity, disease outcome, quality of life
Key Summary
What is known
Prevalence of obesity, defined as a body mass index (BMI) of ≥30 kg/m2, has dramatically increased over the past 25 years and reached epidemic proportions.
Obesity is common among patients with inflammatory bowel disease (IBD).
Data on the impact of a BMI of ≥30 kg/m2 on IBD disease outcome have been conflicting.
What is new
In our nationwide Swiss IBD cohort:
Obesity rate in IBD patients was 11%.
Obese Crohn’s disease (CD), but not ulcerative colitis (UC) patients showed increased disease activity scores compared to normal weight individuals.
Adjusted Cox regression models revealed increased risk of a complicated disease course in obese CD patients over a six-year follow-up period.
No association between obesity and disease progression, index treatment failure was seen neither in CD nor UC.
Introduction
Inflammatory bowel disease (IBD), with its two subtypes of Crohn's disease (CD) and ulcerative colitis (UC), is a chronic inflammatory disorder of the gastrointestinal tract that can result in malabsorption syndrome.1 However, in IBD clinical practice both overweight and obese patients are frequently encountered.2
Prevalence of being overweight, defined as a body mass index (BMI) of ≥25 kg/m2, and obesity (BMI ≥30 kg/m2) has dramatically increased over the past 25 years and reached epidemic proportions.3 It is estimated that 2.1 billion people suffer from overweight and 600 m suffer from obesity worldwide.4 Paralleling growing rates of obesity, prevalence and incidence of IBD have been increasing over the past years.5–7 In IBD, rates of obesity also appear to be on the rise.8,9 In the USA, it is currently estimated that 15–40% of IBD patients are obese.2 It is currently not entirely clear whether increasing obesity rates are causally linked with higher IBD rates. At least, premorbid obesity has been recently associated with the development of CD, but not UC.10 Obesity-associated dysbiosis might be a contributing factor.2 Obesity has further been demonstrated to increase the risk of autoimmune disorders, such as rheumatoid arthritis, psoriasis and psoriatic arthritis.11,12 In terms of mechanism, mesenteric fat has been shown to be an important source of C-reactive protein (CRP) triggering local inflammation and bacterial translocation in CD.13
Despite these mechanistic insights, data on the impact of obesity on disease activity and course in IBD patients have been conflicting.2 It is known from other autoimmune disorders, that obesity can negatively affect disease activity and result in worse outcomes.14,15 Obesity per se represents a chronic, low-grade inflammatory state and might perpetuate the release of pro-inflammatory cytokines.15 It has been further shown that obesity is associated with inadequate response to anti-tumour necrosis factor (anti-TNF) treatment in several immune-mediated diseases.16 However, in CD obesity has not been consistently associated with increased prevalence of IBD-related complications, while in UC a cohort of 202 patients did not reveal an association between obesity and disease severity.2,17,18 To date it is not known if weight reduction should be uniformly recommended in IBD practice, and whether a decrease of BMI has a positive impact on future disease course. Contrary to expectations, weight loss might even negatively affect IBD; in a case series from Mayo Clinic higher incidence rates of de novo IBD cases after bariatric surgery were observed, although it has yet to be determined whether this is a direct effect of weight loss or rather a consequence of changes in the microbiome post-surgically.19
In light of these uncertainties, we used prospectively collected data from the nation-wide Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS) to investigate the impact of obesity on disease activity and disease course in patients with IBD.
Methods
Study design
This study was a retrospective analysis of prospectively obtained data from the SIBDCS. Enrolment into the SIBDCS started in 2006 with patient recruitment from all regions across Switzerland. All patients had been previously diagnosed with IBD according to international guidelines.20,21 The SIBDCS is funded by the Swiss National Science Foundation (SNSF). Institutional review board (IRB) approval has been obtained from the local ethics committee of each participating centre (IRB approval number EK-1316, approved on 5 February 2007). All patients had provided written informed consent prior to inclusion into the SIBDCS.22
Study population and data collection
Inclusion criteria for the SIBDCS have been published elsewhere.22 Patients included in the SIBDCS undergo structured assessment of disease activity at baseline and during annual follow-up visits. Detailed questionnaires are completed by both the patient and the respective IBD physician. For the purpose of this study, we included normal weight and obese IBD patients. The following inclusion criteria were applied: (a) diagnosis of UC or CD; (b) enrolment into the SIBDCS between 2006–2018; (c) available data on weight and BMI at enrolment and during follow-up. Data were collected up to 6 September 2018. For the longitudinal analyses of the development of complications, we excluded patients with complications (see definitions) at baseline, and at least one annual follow-up visit was required.
Definitions and outcome measures
Normal weight was defined as a BMI of 18.5–24.9 kg/m2, and for the definition of obesity the threshold of ≥30 kg/m2 was used. The following parameters were assessed: (a) clinical disease activity measured by the Crohn's Disease Activity Index (CDAI) for CD23 and the modified Truelove Witts Activity Index (MTWAI) for UC;24,25 (b) disease location at diagnosis, enrolment and follow-up according to the Montreal classification (L1–L4 in CD and E1–E3 in UC);26 (c) disease behaviour in CD according to the Montreal classification (B1–B3);26 (d) quality of life measured by the IBDQ and the SF-36 questionnaire;27,28 (e) biological disease activity measured by CRP and faecal calprotectin; and (f) presence of extraintestinal manifestations (EIMs). The following EIMs were reported and analysed: arthritis/inflammatory arthralgia, pyoderma gangrenosum, erythema nodosum, aphthous stomatitis/oral ulcers, axial spondylarthropathy, uveitis/iritis and primary sclerosing cholangitis.
To assess whether or not obesity affects disease course, we looked at three different outcomes as follows:
Disease progression:
• CD: progression from inflammatory (B1) to penetrating/fibrostenotic phenotype (B2, B3).
• UC: more proximal extension (E1 to E2/E3, E2 to E3).
Disease complications:
• CD: development of fistula (perianal, non-perianal, any fistula), development of strictures (intestinal stenosis), surgery (intestinal resection, fistula and abscess surgery) and hospitalization.
• UC: surgery (intestinal resection) and hospitalization.
Index treatment failure:
• Need for new anti-TNF.
Statistical analyses
All statistical analyses were performed with the statistical package program STATA (version 13.1, College Station, Texas, USA). Data distribution was analysed using Normal-QQ-Plots. Quantitative data are presented as (a) mean and standard deviation (SD) for normally data; and (b) median and interquartile range (IQR) in the case of a non-normal distribution. Categorical data are summarised as percentage of the total group. Quantitative data were compared using Student's t-test for normally distributed data and using Wilcoxon rank-sum test for non-normally distributed data. Categorical data were compared using the chi-square test. For assessment of development of complications, Kaplan-Meier curves and Cox proportional hazard models were computed. Cox proportional hazard models were adjusted (multivariate) for baseline disease modifiers (sex, age at diagnosis, smoking at baseline and steroid treatment at baseline) to generate an adjusted hazard ratio (HR), the Bonferroni method was applied in order to avoid random significance generation where applicable. For the purpose of this study, a p-value of <0.05 was considered statistically significant.
Results
Patient characterization
Characteristics of the patients considered for the purposes of these analyses are shown in Table 1. Of the total 3075 patients enrolled in the SIBDCS (median BMI of 23.9 kg/m2, IQR 21.3–26.7, range 13.7–56.8), we identified 325 patients (10.6%) with an obese BMI of ≥30 kg/m2 (median BMI of 32.9 kg/m2, IQR 31.1–35.3, range 30.0–56.8). Of those 325 patients, 194 were diagnosed with CD (59.7%) and 131 with UC/IBD unclassified (IBD-U) (40.3%). Their median age at IBD diagnosis was 32 years (IQR 24–43) with a disease duration of 13 years (IQR 7–21). Eighty-four patients were current smokers (25.9%). Median follow-up within the IBD cohort was 6.0 years (IQR 3.6–9.0). Compared to normal weight patients, obese CD and UC patients were older at diagnosis and study enrolment (age at diagnosis 31 years vs 25 years, p < 0.001 for CD patients and 34 years vs 29 years, p < 0.001 for UC patients). Among obese UC patients, more male patients were observed when compared to normal weight individuals (60.3 vs 47.2%, p = 0.006). Obese CD patients were less likely to be treated with steroids (39.7 vs 48.6%, p = 0.023). There were no further differences between obese and normal weight patients with regards to disease duration, past and current anti-TNF and/or immunomodulatory treatment, surgical history and smoking status, neither in CD nor UC patients.
Table 1.
Patient demographics and disease characteristics in all patients and stratified by inflammatory bowel disease (IBD) subtype.
|
All patients |
Crohn’s disease |
Ulcerative colitis/IBD-U |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI category | Normal | Obese | p | Normal | Obese | p | Normal | Obese | p |
| Number of patients | 1725 | 325 | 977 | 194 | 748 | 131 | |||
| Gender | |||||||||
| Male | 778 (45.1) | 164 (50.5) | 425 (43.5) | 85 (43.8) | 353 (47.2) | 79 (60.3) | |||
| Female | 947 (54.9) | 161 (49.5) | 0.075 | 552 (56.5) | 109 (56.2) | 0.936 | 395 (52.8) | 52 (39.7) | 0.006 |
| Diagnosis | |||||||||
| CD | 977 (56.6) | 194 (59.7) | |||||||
| UC | 709 (41.1) | 123 (37.9) | |||||||
| IBD-U | 39 (2.3) | 8 (2.5) | 0.546 | ||||||
| Age at diagnosis | 26, 20–35 | 32, 24–43 | 25, 19–34 | 31, 23–42 | 29, 21–37 | 34, 26–43 | |||
| (median, IQR, range) | 1–82 | 10–78 | <0.001 | 1–81 | 10–78 | <0.001 | 7–82 | 12–73 | <0.001 |
| Age at enrolment | 37, 27–49 | 45, 36–56 | 37, 26–50 | 44, 35–56 | 38, 29–47 | 46, 36–55 | |||
| (median, IQR, range) | 17–85 | 18–85 | <0.001 | 17–85 | 18–65 | <0.001 | 17–84 | 18–81 | <0.001 |
| Disease duration | 12, 6–50 | 13, 7–21 | 12, 6–21 | 13, 7–22 | 11, 6–18 | 13, 7–20 | |||
| (median, IQR, range) | 0–59 | 0–49 | 0.079 | 0–57 | 0–47 | 0.405 | 0–59 | 0–49 | 0.102 |
| Previous treatment | |||||||||
| 5-ASA | 1274 (73.9) | 245 (75.4) | 0.564 | 570 (58.3) | 118 (60.8) | 0.521 | 704 (94.1) | 127 (97.0) | 0.294 |
| Steroids | 1447 (83.9) | 265 (81.5) | 0.296 | 843 (86.3) | 164 (84.5) | 0.522 | 604 (80.8) | 101 (77.1) | 0.334 |
| Immunomodulators | 1244 (72.2) | 249 (76.6) | 0.094 | 796 (81.5) | 162 (83.5) | 0.503 | 448 (59.9) | 87 (66.4) | 0.158 |
| CNI | 104 (6.0) | 17 (5.2) | 0.575 | 20 (2.1) | 4 (2.1) | 1.000 | 84 (11.2) | 13 (9.9) | 0.660 |
| Anti-TNF | 845 (49.0) | 169 (52.0) | 0.319 | 590 (60.4) | 123 (63.4) | 0.432 | 255 (34.1) | 46 (35.1) | 0.820 |
| Current treatment | |||||||||
| 5-ASA | 818 (47.4) | 154 (47.4) | 0.991 | 237 (24.3) | 52 (26.8) | 0.452 | 581 (77.7) | 102 (77.9) | 0.962 |
| Steroids | 828 (48.0) | 139 (42.8) | 0.083 | 475 (48.6) | 77 (39.7) | 0.023 | 353 (47.2) | 62 (47.3) | 0.977 |
| Immunomodulators | 872 (50.6) | 185 (56.9) | 0.035 | 552 (56.5) | 123 (63.4) | 0.076 | 320 (42.8) | 62 (47.3) | 0.333 |
| CNI | 39 (2.3) | 9 (2.8) | 0.578 | 3 (0.3) | 1 (0.5) | 0.516 | 36 (4.8) | 8 (6.1) | 0.531 |
| Anti-TNF | 717 (41.6) | 142 (43.7) | 0.476 | 519 (53.1) | 105 (54.1) | 0.798 | 198 (26.5) | 37 (28.2) | 0.672 |
| History of surgery | |||||||||
| No | 1139 (66.0) | 215 (66.2) | 486 (49.7) | 106 (54.6) | 665 (88.9) | 113 (86.3) | |||
| Yes | 586 (34.0) | 110 (33.8) | 0.965 | 491 (50.3) | 88 (45.4) | 0.213 | 83 (11.1) | 18 (13.7) | 0.381 |
| Smoking status | |||||||||
| Non-smoker | 1288 (74.7) | 239 (73.5) | 654 (66.9) | 129 (66.5) | 634 (84.8) | 110 (84.0) | |||
| Smoker | 428 (24.8) | 84 (25.9) | 320 (32.8) | 63 (32.5) | 108 (14.4) | 21 (16.0) | |||
| Unknown | 9 (0.5) | 2 (0.6) | 0.773 | 3 (0.3) | 2 (1.0) | 0.339 | 6 (0.8) | 0 (0.0) | 0.683 |
5-ASA: aminosalicylates; anti-TNF: anti-tumour necrosis factor; BI: body mass index; CD: Crohn’s disease; CNI: calcineurin inhibitor; IBD-U: IBD unclassified; IQR: interquartile range; UC: ulcerative colitis.
Disease activity
Disease behaviour was comparable between obese and normal weight CD patients. However, obese CD patients were less likely to have isolated ileal disease L1 (14.4 vs 24.3%, p = 0.003). Despite lower rates of stenosis (33 vs 43.1%, p = 0.009), obese CD patients had higher CDAI scores (median 33 vs 20, p = 0.001) and CRP levels (5 vs 3 mg/l, p < 0.001) compared to normal weight individuals. In fact, obese CD patients were less likely to be in remission based on a CDAI of < 100 (94.7% vs 97.8%, p = 0.017) and a faecal calprotectin of < 100 ug/g (25.6% vs 46.4%, p = 0.015), but not based on a faecal calprotectin of < 250 ug/g. Frequency of soft stools was also increased (8 vs 4 per week, p = 0.001). Obese CD patients reported higher prevalence of EIMs (63.4 vs 54.4%, p = 0.020), particularly arthritis/arthralgia (57.2 vs 46.2%; p = 0.005) and axial spondylarthropathy (9.8 vs 5.7%, p = 0.035. For details see Table 2. As the impact of obesity on disease activity at baseline might be confounded by numerous factors, particularly IBD medication, disease duration, smoking and age, we performed a multivariate logistic regression (dependent variable CDAI < 100). In this model, obesity remained significantly associated with disease activity. As indicated by an odds ratio (OR) < 1, a BMI of ≥30 kg/m2 was an independent negative predictor for presence of disease remission (OR 0.610, 95% CI 0.402–0.926, p = 0.020). For details see Supplementary Material Table 1.
Table 2.
Disease activity and quality of life in Crohn’s disease.
| BMI category | Normal (n = 977) | Obese (n = 194) | p |
|---|---|---|---|
| Fistulas | |||
| Perianal | 237 (24.3) | 50 (25.8) | 0.654 |
| Any | 323 (33.1) | 65 (33.5) | 0.904 |
| Abscess | |||
| No | 744 (76.2) | 148 (76.3) | |
| Yes | 233 (23.8) | 46 (23.7) | 0.967 |
| Intestinal stenosis | |||
| No | 556 (56.9) | 130 (67.0) | |
| Yes | 421 (43.1) | 64 (33.0) | 0.009 |
| Behaviour | |||
| B1 (inflammatory) | 364 (37.3) | 87 (44.9) | |
| B1p (B1 + perianal disease) | 144 (14.7) | 31 (16.0) | |
| B2 (stricturing) | 200 (20.5) | 28 (14.4) | |
| B2p (B2 + perianal disease) | 124 (12.7) | 20 (10.3) | |
| B3 (penetrating) | 72 (7.4) | 11 (5.7) | |
| B3p (B3 + perianal disease) | 73 (4.5) | 17 (8.8) | 0.195 |
| EIM | |||
| Arthritis | 451 (46.2) | 111 (57.2) | 0.005 |
| Uveitis/iritis | 104 (10.6) | 27 (13.9) | 0.187 |
| PG | 12 (1.2) | 2 (1.0) | 1.000 |
| EN | 67 (6.9) | 19 (9.8) | 0.174 |
| Oral ulcers | 141 (14.4) | 25 (12.9) | 0.573 |
| AS | 56 (5.7) | 19 (9.8) | 0.035 |
| PSC | 8 (0.8) | 1 (0.5) | 1.000 |
| Any | 531 (54.4) | 123 (63.4) | 0.020 |
| CDAI score | 20, 6–53 | 33, 6–69 | |
| (median, IQR, range) | 0–345 | 0–280 | 0.001 |
| Number of soft stools | 4, 0–14 | 8, 0–25 | |
| (median, IQR, range) | 0–140 | 0–100 | 0.001 |
| Disease location at diagnosis | |||
| L1 (ileal) | 237 (24.3) | 28 (14.4) | |
| L2 (colonic) | 195 (20.0) | 45 (23.2) | |
| L3 (ileo-colonic) | 449 (46.0) | 88 (45.4) | |
| L4 (upper GI only) | 10 (1.0) | 1 (0.5) | |
| Unknown/Unclear | 86 (8.8) | 32 (16.5) | 0.002 |
| CRP in mg/l | 3, 1–7 | 5, 3–12 | |
| (median, IQR, range) | 0–155 | 0–245 | <0.001 |
| Faecal calprotectin in ug/g | 105, 32–293 | 139, 61–296 | |
| (median, IQR, range) | 0–3500 | 15–1835 | 0.467 |
| IBDQ Bowel score | 59, 51–65 | 57, 49–63 | |
| (median, IQR, range) | 22–70 | 30–70 | 0.071 |
| IBDQ Systemic score | 26,21–30 | 23, 19–28 | |
| (median, IQR, range) | 7–35 | 9–35 | 0.001 |
| IBDQ Emotional score | 68, 57–75 | 66, 56–76 | |
| (median, IQR, range) | 14–84 | 28–84 | 0.922 |
| IBDQ Social score | 34, 28–35 | 31, 26–35 | |
| (median, IQR, range) | 6–35 | 7–35 | 0.005 |
| IBDQ Total score | 185, 159–202 | 177, 150–198 | |
| (median, IQR, range) | 66–224 | 78–223 | 0.091 |
| SF36 Physical score | 51, 44–56 | 47, 36–53 | |
| (median, IQR, range) | 16–72 | 13–67 | <0.001 |
| SF36 Mental score | 47, 38–54 | 48, 38–55 | |
| (median, IQR, range) | 10–66 | 18–66 | 0.285 |
| Medical resources consumption (in last 3 months) | |||
| Hospitalization | 71 (14.7) | 21 (21.0) | 0.114 |
| Outpatient visit | 164 (33.7) | 28 (28.3) | 0.297 |
| Work absence (in last 3 months) | |||
| No | 315 (86.5) | 45 (80.4) | |
| Yes | 49 (13.5) | 11 (19.6) | 0.218 |
AS: axial spondylarthropathy; BMI: body mass index; CDAI: Crohn’s Disease Activity Index; CRP: C-reactive protein; EIM: extraintestinal manifestation; EN: erythema nodosum; GI: gastro-intestinal; IQR: interquartile range; PG: pyoderma gangrenosum; PSC: primary sclerosing cholangitis.
No difference in terms of disease location and extent was seen between obese and normal weight UC patients. We observed differences in the number of bowel movements (p = 0.021) and CRP levels (4 vs 2 mg/l, p = 0.008) between obese and normal weight UC patients, but not in terms of clinical activity score (MTWAI), number of bloody stools, faecal calprotectin and presence of any EIMs (Table 3). Remission rates were not different between the two groups based on a MTWAI of < 3 (63.1 vs 63.4%, p = 0.944) and a faecal calprotectin of < 100 ug/g (48.1 vs 53.2%, p = 0.630). Using the same multivariate logistic regression model as in CD, obesity status did not predict disease remission (defined as MTWAI < 3) after correction for confounding factors (Supplementary Material Table 2).
Table 3.
Disease activity and quality of life in ulcerative colitis.
| BMI category | Normal (n = 748) | Obese (n = 131) | p |
|---|---|---|---|
| EIM | |||
| Arthritis | 250 (33.4) | 56 (42.8) | 0.039 |
| Uveitis/iritis | 46 (6.2) | 10 (7.6) | 0.521 |
| PG | 9 (1.2) | 3 (2.3) | 0.402 |
| EN | 29 (3.9) | 4 (3.1) | 0.806 |
| Oral ulcers | 47 (6.3) | 5 (3.8) | 0.270 |
| AS | 18 (2.4) | 6 (4.6) | 0.159 |
| PSC | 41 (5.5) | 1 (0.8) | 0.014 |
| Any | 325 (43.5) | 59 (45.0) | 0.735 |
| MTWAI score | 2, 0–4 | 2, 1–4 | |
| (median, IQR, range) | 0–17 | 0–16 | 0.136 |
| Total number of bowel movement/day | |||
| 1–2 | 438 (60.8) | 59 (45.7) | |
| 3–4 | 133 (18.5) | 35 (27.1) | |
| 5–6 | 75 (10.4) | 15 (11.6) | |
| 7–9 | 38 (5.3) | 9 (7.0) | |
| 10+ | 36 (5.0) | 11 (8.5) | 0.021 |
| Bloody stools | |||
| None | 514 (71.2) | 94 (72.9) | |
| Occasionally with BM | 142 (19.7) | 25 (19.4) | |
| >50% of BM | 38 (5.3) | 8 (6.2) | |
| With every BM | 28 (3.9) | 2 (1.6) | 0.627 |
| Disease location at diagnosis | |||
| Pancolitis | 273 (36.5) | 57 (43.5) | |
| Left-sided colitis | 234 (31.3) | 32 (24.4) | |
| Proctitis | 147 (19.7) | 28 (21.4) | |
| Unknown/unclear | 94 (12.6) | 14 (10.7) | 0.298 |
| CRP in mg/l | 2, 1–5 | 4, 1–6 | |
| (median, IQR, range) | 0–130 | 0–71 | 0.008 |
| Faecal calprotectin in ug/g | 83, 30–495 | 102, 58–260 | |
| (median, IQR, range) | 1–3450 | 9–1043 | 0.683 |
| IBDQ Bowel score | 61, 52–66 | 60, 53–65 | |
| (median, IQR, range) | 16–70 | 16–70 | 0.357 |
| IBDQ Systemic score | 27, 22–30 | 23, 19–29 | |
| (median, IQR, range) | 5–35 | 6–35 | <0.001 |
| IBDQ Emotional score | 70, 60–76 | 67, 59–75 | |
| (median, IQR, range) | 12–84 | 51–84 | 0.371 |
| IBDQ Social score | 34, 30–35 | 33, 28–35 | |
| (median, IQR, range) | 5–35 | 6–35 | 0.227 |
| IBDQ Total score | 190, 165–205 | 184, 162–201 | |
| (median, IQR, range) | 44–224 | 62–224 | 0.086 |
| SF36 Physical score | 53, 46–57 | 50, 39–56 | |
| (median, IQR, range) | 16–68 | 17–65 | 0.002 |
| SF36 Mental score | 49, 41–54 | 48, 39–54 | |
| (median, IQR, range) | 15–62 | 15–61 | 0.381 |
| Medical resources consumption (in last 3 months) | |||
| Hospitalization | 42 (10.3) | 9 (12.9) | 0.517 |
| Outpatient visit | 109 (26.6) | 23 (32.4) | 0.311 |
| Work absence (in last 3 months) | |||
| No | 304 (94.1) | 42 (91.3) | |
| Yes | 19 (5.9) | 4 (8.7) | 0.509 |
AS: axial spondylarthropathy; BM: bowel movement; BMI: body mass index; CRP: C–reactive protein; EIM: extraintestinal manifestation; EN: erythema nodosum; IQR: interquartile range; MTWAI: modified Truelove Witts Activity Index; PG: pyoderma gangrenosum; PSC: primary sclerosing cholangitis.
Quality of life
Quality of life was affected in obese CD and UC patients, although to a lesser extent in the latter group. IBDQ systemic and social scores (23 vs 26, p = 0.001, 31 vs 34, p = 0.005) as well as SF-36 physical score (47 vs 51, p < 0.001) were decreased in obese compared to normal weight CD patients. We observed no difference in terms of use of medical resources and absence from work in the past 3 months between obese and normal weight patients, neither in CD nor UC (Tables 2 and 3).
Disease outcome
To investigate the impact of obesity on disease course, we analysed the follow-up of all obese patients – stratified by IBD subtype – and compared them to normal weight individuals. There was no difference with regards to the length of the total follow-up between the two groups (6.0 vs 5.9 years).
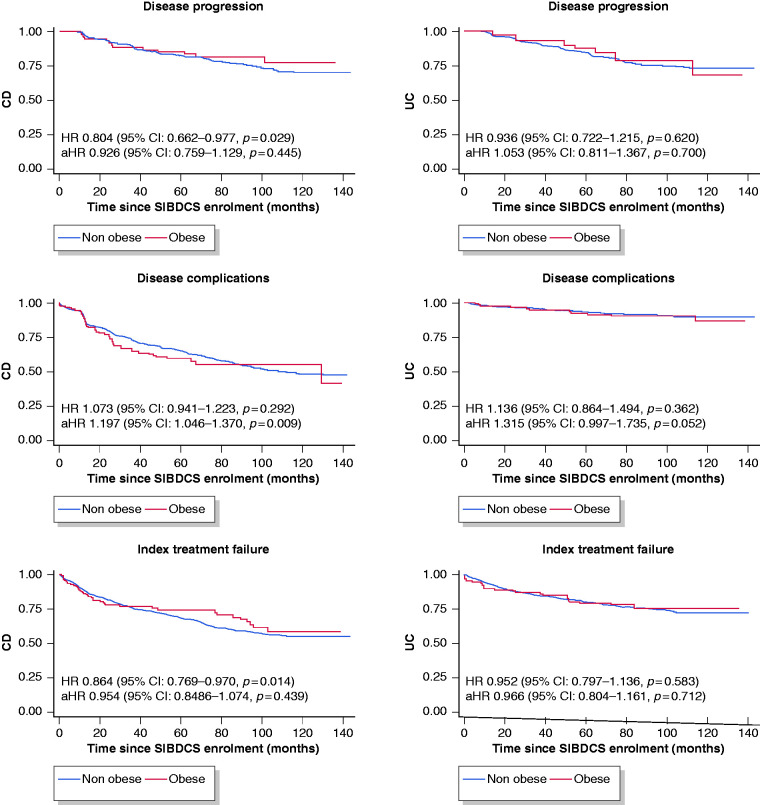
In CD, obesity was negatively associated with disease progression (HR 0.804, 95% CI 0.662–0.977, p = 0.029). However, in the adjusted model (corrected for sex, age at diagnosis, smoking at baseline and steroid treatment at baseline), there was no difference between obese and non-obese CD patients (HR 0.926 (95% CI: 0.759–1.129, p = 0.445). In the univariate model, there was no significant association between obesity and development of disease complications. However, when adjusting for above mentioned modifiers, obese patients were more likely to develop complicated disease in the follow-up period (HR 1.197, 95% CI 1.046–1.370, p = 0.009). While there was an association between obesity and index treatment failure in the univariate analysis (HR 0.864, 95% CI 0.769–0.970, p = 0.014), this association was no longer significant after adjusting for confounding factors (HR 0.954, 95% CI 0.848–1.076, p = 0.439) nor multivariate analysis.
In UC patients, we did not detect significant association of obesity with disease progression (HR 0.936, 95% CI 0.722–1.215, p = 0.620), disease complications (HR 1.136, 95% CI 0.864–1.494, p = 0.362) nor index treatment failure (HR 0.966, 95% CI 0.804–1.161, p = 0.712). However, in the adjusted model, obese patients showed a clear trend towards higher rates of complicated disease (HR 1.315, 95% CI 0.997–1.735, p = 0.052). For details see Figure 1 (Kaplan-Meier curves and unadjusted/adjusted HR).
Figure 1.
Kaplan-Meier curves and unadjusted/adjusted hazard ratios (aHRs) for disease progression, disease complications and index treatment failure in obese vs non-obese patients, stratified by inflammatory bowel disease (IBD) subtype. Normal body mass index (BMI) is reference (hazard ratio (HR) = 1). CD: Crohn’s disease; CI: confidence interval; SIBDCS: Swiss Inflammatory Bowel Disease Cohort Study; UC: ulcerative colitis.
Discussion
Obesity is common among patients with IBD. Data on the impact of a BMI of ≥30 kg/m2 on IBD disease outcome have been conflicting.2 We investigated the impact of obesity on IBD disease activity and disease outcome in a large Swiss cohort with a follow-up of 6 years. Our main findings are as follows: (a) obesity rate in patients enrolled into the SIBDCS was 11%; (b) obese CD, but not UC patients showed increased disease activity scores compared to normal weight individuals; (c) in a multivariate logistic regression model, obesity was negatively associated with disease remission in CD patients; and (d) obese CD patients were more likely to develop disease complications, while no association between obesity and disease progression or index treatment failure was seen, neither in CD nor UC.
Obesity has been associated with increased disease activity and worse clinical outcomes in other autoimmune diseases, such as rheumatoid arthritis and psoriatic arthritis, which might be attributed to obesity being considered a low chronic inflammatory state associated with the increased release of proinflammatory cytokines.14,15 In IBD, data have been less consistent. Some studies reported an association between obesity and penetrating CD as well as higher prevalence of colonic involvement, while others did not.17,18,29–31 In UC, a lower prevalence of pancolitis has been reported in obese patients in one study; as such, no clear association between obesity and UC activity has been established to date.2,18 A recent and comprehensive analysis of patient-reported data in more than 7000 IBD patients suggests increased risk of persistent disease activity or relapse in both CD and UC patients.32 Whilst we observed no differences with regards to disease behaviour (B1–B3 in CD) and extent (E1–E3 in UC) between obese and normal weight patients in our cohort, we found that obese CD, but not UC patients, showed increased disease activity scores compared to normal weight patients. The daily soft stool frequency was increased from a median of four times per day in normal weight patients to eight times per day in obese CD patients. In our estimation, this is a clinically meaningful difference. The finding of higher disease activity in obese CD patients is particularly noteworthy, as BMI is an integral component of the CDAI score, in which a high BMI actually lowers the score by a maximum of 10 points.23 If BMI was to be excluded, these differences would have been even more striking. Based on CDAI and faecal calprotectin ( < 100 ug/g), obese CD patients were less likely to be in disease remission compared to normal weight controls. Negative association between obesity and clinical remission remained significant in a multivariate logistic regression model. However, there was no difference between obese and non-obese patients with regards to remission rates when using higher faecal calprotectin cut-off values (such as 250 ug/g).33 Although we observed no differences in MTWAI between obese and normal weight UC patients, higher stool frequency in obese patients compared to normal weight UC patients could be interpreted as an indication for increased UC activity – if calprotectin and CRP data were not available. An association between soft stools and obesity has been previously shown in a non-IBD population.34 As endoscopic disease activity was not systematically collected and assessed in the Swiss IBD cohort, it is not possible to differentiate between mild ongoing disease activity and complete disease remission. Possible explanations for an increased stool frequency in the context of the latter might be higher rates of bile acid malabsorption, faster colonic transit or irritable bowel syndrome (as a result from dysbiosis).35–37 Taken together, the difference in disease activity between obese and non-obese IBD patients might not be as large as seen in other autoimmune diseases. Nonetheless, the significant impact of obesity on quality of life and increased stool frequency in both obese UC and CD patients should urge treating physicians to take weight into account when managing IBD patients.
So far, data on the impact of obesity on disease outcome in IBD have been conflicting.2 While some studies reported decreased rates of IBD surgery, hospitalizations and initiation of anti-TNF treatment, others demonstrated increased risk for surgery, severe hospitalizations and longer hospital stays in obese patients when compared to normal weight patients.2,29,38 In other studies, no differences in disease outcomes between obese and normal weight patients were observed.39,40 The recent and comprehensive analysis from Jain and colleagues revealed an independent and dose-dependent association between obesity and risk of disease relapse in CD and UC.32 In our study, we show that obese CD patients were indeed more likely to develop disease complications compared to non-obese patients. Although the p-value did not reach statistical significance, obese UC patients appear to have a worse outcome with regards to the development of complications, too. In contrast, we did not detect any association between obesity and disease progression or index treatment failure, neither in CD nor UC.
Our study has several strengths. The availability of a relatively large number of patients (>3000 patients within the cohort, 325 with an obese BMI) and a long follow-up period (6 years) make our cohort data more robust and our findings more reliable than those reported in previous studies. In addition, stringent inclusion and exclusion criteria combined with data collection in a prospective manner and close (annual) follow-up minimised the drawbacks of a retrospective data analysis. Furthermore, we analysed both patient- and physician-reported outcomes. Finally, our outcome analyses have been adjusted for several disease modifiers and therefore make the findings more reliable.
Nevertheless, our study has also some limitations. Due to the nature of the SIBDCS data collection, it was not possible to assess efficacy of anti-TNF treatment in obese patients compared to normal weight individuals. This type of analysis would be of particular interest given previous findings of rapid anti-TNF clearance and low trough levels in obese patients.2,41 Given that the SIBDCS is not a population-based study, obesity prevalence estimations were not possible, and patients with a more complicated disease course recruited at tertiary referral centres might be overrepresented. Thus, our findings cannot be applied one to one to a general IBD population. Index treatment failure was defined as need for new anti-TNF despite the availability of newer biologics. However, the latter have been introduced in Switzerland only very recently. A further limitation is the lack of endoscopic data. Finally – although data was prospectively collected – we report on a retrospective analysis.
In conclusion, in our study obesity was associated with increased disease activity and decreased quality of life, particularly in CD. We further observed – in a multivariate Cox proportional hazard model – a significant association between obesity and development of disease complications in CD. However, obesity does not appear to have an impact on disease progression or index treatment failure. Weight changes might be beneficial, but cannot be uniformly recommended based on the current data. Additional analyses of large population-based datasets are merited to further study the relationship between obesity and IBD disease course.
Acknowledgements
The authors would like to thank all patients and members of the SIBDCS group for their contribution to this work. Members of the SIBDCS group are: Karim Abdelrahman, Gentiana Ademi, Patrick Aepli, Amman Thomas, Claudia Anderegg, Anca-Teodora Antonino, Eva Archanioti, Eviano Arrigoni, Diana Bakker de Jong, Bruno Balsiger, Polat Bastürk, Peter Bauerfeind, Andrea Becocci, Dominique Belli, José M Bengoa, Luc Biedermann, Janek Binek, Mirjam Blattmann, Stephan Boehm, Tujana Boldanova, Jan Borovicka, Christian P Braegger, Stephan Brand, Lukas Brügger, Simon Brunner, Patrick Bühr, Bernard Burnand, Sabine Burk, Emanuel Burri, Sophie Buyse, Dahlia-Thao Cao, Ove Carstens, Dominique H Criblez, Sophie Cunningham, Fabrizia D’Angelo, Philippe de Saussure, Lukas Degen, Joakim Delarive, Christopher Doerig, Barbara Dora, Susan Drerup, Mara Egger, Ali El-Wafa, Matthias Engelmann, Jessica Ezri, Christian Felley, Markus Fliegner, Nicolas Fournier, Montserrat Fraga, Yannick Franc, Pascal Frei, Remus Frei, Michael Fried, Florian Froehlich, Raoul I Furlano, Luca Garzoni, Martin Geyer, Laurent Girard, Marc Girardin, Delphine Golay, Ignaz Good, Ulrike G Bigler, Beat Gysi, Johannes Haarer, Marcel Halama, Janine Haldemann, Pius Heer, Benjamin Heimgartner, Beat Helbling, Peter Hengstler, Denise Herzog, Cyrill Hess, Roxane Hessler, Klaas Heyland, Thomas Hinterleitner, Claudia Hirschi, Petr Hruz, Pascal Juillerat, Carolina Khalid-de Bakker, Stephan Kayser, Céline Keller, (Christina Knellwolf (-Grieger)), Christoph Knoblauch, Henrik Köhler, Rebekka Koller, Claudia Krieger(-Grübel), Patrizia Künzler, Rachel Kusche, Frank Serge Lehmann, Andrew Macpherson, Michel H Maillard, Michael Manz, Astrid Marot, Rémy Meier, Christa Meyenberger, Pamela Meyer, Pierre Michetti, Benjamin Misselwitz, Patrick Mosler, Christian Mottet, Christoph Müller, Beat Müllhaupt, Leilla Musso, Michaela Neagu, Cristina Nichita, Jan Niess, Andreas Nydegger, Nicole Obialo, Diana Ollo, Cassandra Oropesa, Ulrich Peter, Daniel Peternac, Laetitia Marie Petit, Valérie Pittet, Daniel Pohl, Marc Porzner, Claudia Preissler, Nadia Raschle, Ronald Rentsch, Alexandre Restellini, Sophie Restellini, Jean-Pierre Richterich, Frederic Ris, Branislav Risti, Marc A Ritz, Gerhard Rogler, Nina Röhrich, Jean-Benoît Rossel, Vanessa Rueger, Monica Rusticeanu, Markus Sagmeister, Gaby Saner, Bernhard Sauter, Mikael Sawatzki, Michael Scharl, Martin Schelling, Susanne Schibli, Hugo Schlauri, Dominique Schluckebier, Daniela Schmid, Sybille Schmid (-Uebelhart), Jean-François Schnegg, Alain Schoepfer, Vivianne Seematter, Frank Seibold, Mariam Seirafi, Gian-Marco Semadeni, Arne Senning, Christiane Sokollik, Joachim Sommer, Johannes Spalinger, Holger Spangenberger, Philippe Stadler, Peter Staub, Dominic Staudenmann, Volker Stenz, Michael Steuerwald, Alex Straumann, Bruno Strebel, Andreas Stulz, Michael Sulz, Aurora Tatu, Michela Tempia-Caliera, Joël Thorens, Kaspar Truninger, Radu Tutuian, Patrick Urfer, Stephan Vavricka, Francesco Viani, Jürg Vögtlin, Roland Von Känel, Dominique Vouillamoz, Rachel Vulliamy, Paul Wiesel, Reiner Wiest, Stefanie Wöhrle, Samuel Zamora, Silvan Zander, Tina Wylie, Jonas Zeitz and Dorothee Zimmermann.
Specific author contributions were as follows: study concept and design: TG, MBB, GR and SRV; acquisition and analysis of data: TG, FP and JBR; interpretation of data: TG, FP, MBB, LB, PS, MS, AMS, ES, GR and SRV; drafting of manuscript: TG, FP, MBB and SRV; critical revision of the manuscript for important intellectual content: JBR, LB, PS, MS, AMS, ES, AS and GR; supervision: TG and SRV. All authors approved the final version of the manuscript.
Footnotes
Declaration of conflicting interests: The author(s) declare that they have no conflict of interests to report.
Ethics approval: Institutional review board (IRB) approval has been obtained from the local ethics committee of each participating centre (IRB approval number EK-1316, approved on 5 February 2007).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research grants from the Swiss National Science Foundation to AMS (grant No. 32003B_160115/1), GR (grant No. 310030-120312), to SRV (grant No. 320000-114009/3 and 32473B_135694/1), to TG (grant No. P2ZHP3_168561) and to the Swiss IBD Cohort (grant no. 33CS30_148422).
Informed consent: Informed consent was approved prior to inclusion into the SIBDCS.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 2010; 28: 573–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh S, Dulai PS, Zarrinpar A, et al. Obesity in IBD: Epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol 2017; 14: 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384: 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54.e42; quiz e30. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan GG. The global burden of IBD: From 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015; 12: 720–727. [DOI] [PubMed] [Google Scholar]
- 7.Ng SC, Zeng Z, Niewiadomski O, et al. Early course of inflammatory bowel disease in a population-based inception cohort study From 8 countries in Asia and Australia. Gastroenterology 2016; 150: 86–95.e83; quiz e13–e84. [DOI] [PubMed] [Google Scholar]
- 8.Blain A, Cattan S, Beaugerie L, et al. Crohn's disease clinical course and severity in obese patients. Clin Nutr 2002; 21: 51–57. [DOI] [PubMed] [Google Scholar]
- 9.Moran GW, Dubeau MF, Kaplan GG, et al. The increasing weight of Crohn's disease subjects in clinical trials: A hypothesis-generating time-trend analysis. Inflamm Bowel Dis 2013; 19: 2949–2956. [DOI] [PubMed] [Google Scholar]
- 10.Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Measures of obesity and risk of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 2015; 21: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin B, Yang M, Fu H, et al. Body mass index and the risk of rheumatoid arthritis: A systematic review and dose-response meta-analysis. Arthritis Res Ther 2015; 17: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterry W, Strober BE, Menter A, et al. Obesity in psoriasis: The metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br J Dermatol 2007; 157: 649–655. [DOI] [PubMed] [Google Scholar]
- 13.Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, et al. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut 2012; 61: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iannone F, Lopalco G, Rigante D, et al. Impact of obesity on the clinical outcome of rheumatologic patients in biotherapy. Autoimmun Rev 2016; 15: 447–450. [DOI] [PubMed] [Google Scholar]
- 15.Versini M, Jeandel PY, Rosenthal E, et al. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun Rev 2014; 13: 981–1000. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Facciorusso A, Singh AG, et al. Obesity and response to anti-tumor necrosis factor-α agents in patients with select immune-mediated inflammatory diseases: A systematic review and meta-analysis. PLoS One 2018; 13: e0195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pringle PL, Stewart KO, Peloquin JM, et al. Body mass index, genetic susceptibility, and risk of complications among individuals with Crohn's disease. Inflamm Bowel Dis 2015; 21: 2304–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stabroth-Akil D, Leifeld L, Pfützer R, et al. The effect of body weight on the severity and clinical course of ulcerative colitis. Int J Colorectal Dis 2015; 30: 237–242. [DOI] [PubMed] [Google Scholar]
- 19.Braga Neto MB, Gregory M, Ramos GP, et al. De-novo inflammatory bowel disease after bariatric surgery: A large case series. J Crohns Colitis 2018; 12: 452–457. [DOI] [PubMed] [Google Scholar]
- 20.Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn's disease 2016: Part 1: Diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 21.Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017; 11: 649–670. [DOI] [PubMed] [Google Scholar]
- 22.Pittet V, Michetti P, Mueller C, et al. Cohort profile update: The Swiss Inflammatory Bowel Disease Cohort Study (SIBDCS). Int J Epidemiol 2019; 48: 385–386f. [DOI] [PubMed] [Google Scholar]
- 23.Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's Disease Activity Index. National Cooperative Crohn's Disease Study. Gastroenterology 1976; 70: 439–444. [PubMed] [Google Scholar]
- 24.Truelove SC, Witts LJ. Cortisone in ulcerative colitis: Preliminary report on a therapeutic trial. Br Med J 1954; 2: 375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Truelove SC, Witts LJ. Cortisone in ulcerative colitis: Final report on a therapeutic trial. Br Med J 1955; 2: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19: 5A–36A. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989; 96: 804–810. [PubMed] [Google Scholar]
- 28.Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 29.Flores A, Burstein E, Cipher DJ, et al. Obesity in inflammatory bowel disease: A marker of less severe disease. Dig Dis Sci 2015; 60: 2436–2445. [DOI] [PubMed] [Google Scholar]
- 30.Mendall MA, Gunasekera AV, John BJ, et al. Is obesity a risk factor for Crohn's disease? Dig Dis Sci 2011; 56: 837–844. [DOI] [PubMed] [Google Scholar]
- 31.Nic Suibhne T, Raftery TC, McMahon O, et al. High prevalence of overweight and obesity in adults with Crohn's disease: Associations with disease and lifestyle factors. J Crohns Colitis 2013; 7: e241–e248. [DOI] [PubMed] [Google Scholar]
- 32.Jain A, Nguyen NH, Proudfoot JA, et al. Impact of obesity on disease activity and Patient-Reported Outcomes Measurement Information System (PROMIS) in inflammatory bowel diseases. Am J Gastroenterol 2019; 114: 630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reenaers C, Bossuyt P, Hindryckx P, et al. Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United European Gastroenterol J 2018; 6: 1117–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballou S, Singh P, Rangan V, et al. Obesity is associated with significantly increased risk for diarrhoea after controlling for demographic, dietary and medical factors: A cross-sectional analysis of the 2009–2010 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2019; 50: 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadik R, Abrahamsson H, Ung KA, et al. Accelerated regional bowel transit and overweight shown in idiopathic bile acid malabsorption. Am J Gastroenterol 2004; 99: 711–718. [DOI] [PubMed] [Google Scholar]
- 36.Delgado-Aros S, Camilleri M, Garcia MA, et al. High body mass alters colonic sensory-motor function and transit in humans. Am J Physiol Gastrointest Liver Physiol 2008; 295: G382–G388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajilić-Stojanović M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011; 141: 1792–1801. [DOI] [PubMed] [Google Scholar]
- 38.Pavelock N, Masood U, Minchenberg S, et al. Effects of obesity on the course of inflammatory bowel disease. Proc (Bayl Univ Med Cent) 2019; 32: 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seminerio JL, Koutroubakis IE, Ramos-Rivers C, et al. Impact of obesity on the management and clinical course of patients with inflammatory bowel disease. Inflamm Bowel Dis 2015; 21: 2857–2863. [DOI] [PubMed] [Google Scholar]
- 40.Hass DJ, Brensinger CM, Lewis JD, et al. The impact of increased body mass index on the clinical course of Crohn's disease. Clin Gastroenterol Hepatol 2006; 4: 482–488. [DOI] [PubMed] [Google Scholar]
- 41.Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: A population pharmacokinetic study. Inflamm Bowel Dis 2014; 20: 2247–2259. [DOI] [PubMed] [Google Scholar]