Abstract
In the present study we examined the inactivation of SARS-CoV-2 by synthetic conjugated polymers and oligomers developed in our laboratories as antimicrobials for bacteria, fungi and non-enveloped viruses. The results show highly effective light induced inactivation with several of these oligomers and polymers including irradiation with near-UV and visible light. In the best case, one oligomer induced a 5-log reduction in pfu/mL within 10 min. In general, the oligomers are more active than the polymers; however, the polymers are active with longer wavelength visible irradiation. Although not studied quantitatively, the results show that in the presence of the agents at concentrations similar to those used in the light studies, there is essentially no dark inactivation of the virus. Since three of the five materials/compounds examined are quaternary ammonium derivatives, this study indicates conventional quaternary ammonium antimicrobials may not be active against SARS-CoV-2. Our results suggest several applications involving the incorporation of these materials in wipes, sprays, masks and clothing and other Personal Protection Equipment (PPE) that can be useful in preventing infections and the spreading of this deadly virus and future outbreaks from similar viruses.
Keywords: Coronavirus inactivation, conjugated polyelectrolytes, SARS-Cov-2 virus, COVID-19, photodynamic inactivation, antimicrobial materials
Graphical Abstract
INTRODUCTION
It is hard to overstate the devastation brought to the world by SARS-CoV-2 through the highly contagious Covid-19 disease. In contrast, of the six previously encountered human coronaviruses, four are common and remain active but pose only minor threats to world health.1 Three recent (since 2000) coronaviruses originally infected animals but have jumped to humans and pose existential threats to human health. SARS coronavirus (SARS-CoV) emerged in 2002 and caused Severe Acute Respiratory Syndrome (SARS). SARS-CoV emerged in an outbreak in February 2003 in Asia and rapidly spread to two dozen countries in Asia, South America, North America and Europe before being contained.2–7 According to the World Health Organization (WHO), there were 8,098 cases worldwide and of those 774 died.8 In the US there were 8 verified cases and no deaths and there have been no cases of SARS subsequent to 2003. Middle East Respiratory Syndrome (MERS) emerged in Saudi Arabia in 2012 and has since spread to several countries, including the US. MERS causes severe respiratory symptoms, including death. There have been two nonfatal confirmed cases in the US in May 2014. MERS is a threat to travelers to the Middle East and travel advisories are posted on the CDC Website. In contrast to the SARS and MERS, Covid-19 has spread to all inhabited parts of the world, excluding Antarctica. As of November 8, 2020, there are 50.1 million cases and 1.25 million deaths worldwide, and of those 10 million cases and 238,000 deaths have occurred in the US.8
The major attempt at controlling the spread of Covid-19 includes several efforts to produce vaccines. Although promising candidates are already in human clinical trials, it is likely that unforeseen problems may delay the availability of a safe and effective vaccine and its widespread deployment. Several diagnostic tests for of SARS-CoV-2 have been developed; however, uneven test distribution and delays in obtaining results have hindered knowledge of viral spread and hampered the ability to develop an effective response. Several conventional disinfectants are active against SARS-CoV-2 and the most commonly used of these are bleach, hydrogen peroxide, and alcohol solutions.9 Recent reports describe surface coatings that are active.10 While many disinfectants are effective for use as cleaning solutions and wipes, the volatility and/or corrosivity of the “active agents” limit prolonged sterilization of surfaces or objects by these reagents.
Here we present an alternative approach to long lasting disinfection: cationic phenylene ethynylene polymers and oligomers (conjugated electrolytes).11–17 Over the past decade we and others have demonstrated the effectiveness of these compounds and derivative materials against bacteria, fungi, biofilms, and viruses.18–28 The antimicrobial activity of these compounds includes both light-activated and dark-active pathways. Encouragingly, these materials exhibit relatively low toxicity against mammalian cells or the human skin.29–31 Depending upon the selection of materials and properties such as solubility and stability, it is possible to control the lifetime of these materials in coatings, with photodegradation as the main route of inactivation.32 The most common byproducts of photodegradation, due to oxidative cleavage, are aldehydes and carboxylic acids that are unlikely to damage the environment.32 In addition, these materials can also bind selectively with certain proteins or protein fragments and with light-irradiation, the conjugated electrolytes may photosensitize the oxidation of proteins at the specific binding sites.33 It is envisioned that the antipathogen polymers and oligomers reported here as active vs. SARS-CoV-2 could be used in a variety of formats, for example as the active agent in disinfecting solutions or wipes, impregnated or surface grafted to textiles and/or as long-lasting surface coatings.34–36
The present paper reports a pilot study of four phenylene ethynylene materials and compounds and one polythiophene as inactivators of SARS-CoV-2 in aqueous suspensions. The five materials were chosen as representatives of a much larger group of polymeric and oligomeric conjugated electrolytes that have been previously shown to be highly active against microbes.12, 13, 18, 20, 37–39 In this study, samples of each material in solution were incubated with aqueous suspensions of SARS-CoV-2. The samples were incubated in the dark and under UV-visible light irradiation in a photoreactor. After incubation for increasing amounts of time, the samples were analyzed for virus activity. As detailed below, it was found that all five of the tested materials were effective against SARS-CoV-2, but with effectiveness that differed significantly among the tested group.
RESULTS
Oligomer and Polymer Structures and Rationale for Selection
The structures of the five materials tested are shown in Figure 1. Three are oligomeric phenylene ethynylenes (OPEs, compounds 1, 2, and 3) and two are cationic conjugated polymers (poly-4 and poly-5).
Figure 1.
Structures of oligomers (1-3) and polymers (poly-4, and poly-5) used in this study.
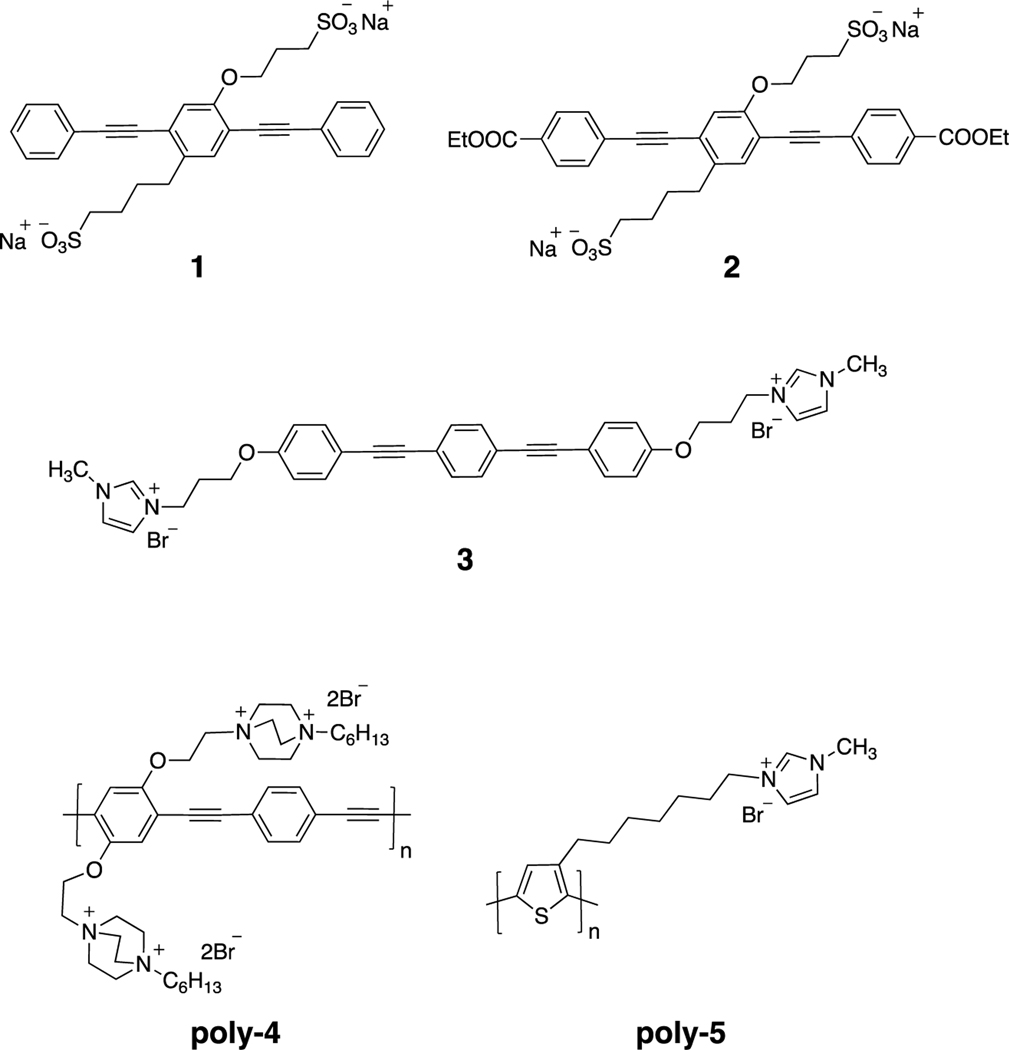
The synthesis of 3 is reported in Supplemental Material and the syntheses of all other materials have been reported in the literature.37, 40–42 As shown in Figure 2, all of the materials absorb in the near-UV and/or visible regions of the spectrum. The final concentration of materials chosen for this study was 10 μg/mL in solutions that contain an active compound and SARS-CoV-2. This concentration was selected to give moderate absorption throughout the sample and yet minimizing a potential “inner filter” effect.43, 44 The fact that the poly-4 absorbs much more strongly at this concentration suggests the possibility that an “inner filter” effect could diminish its light-activated viral inactivation activity.
Figure 2.
Absorption spectra of the five phenylene ethynylene materials at 10 μg/mL in water. Spectra were recorded on a Lambda 35 U/Vis spectrometer (PerkinElmer, Waltham, MA) in quartz cuvettes (PerkinElmer, Waltham, MA).
Our previous studies testing the antiviral activities of oligomeric and polymeric phenylene ethynylene materials were performed with the non-enveloped RNA bacteriophages MS2 and T4.20 In contrast, SARS-CoV-2 is an enveloped virus containing several proteins that shield its internal RNA. Recent reports suggest that the virus has an acidic coat and might be reactive towards negatively charged reagents.45 We hypothesize that the viral envelope might have both positively and negatively charged regions that could interact with charged compounds such as OPEs and PPEs. Thus, the selection of the test compounds for the pilot project was in-part based on charge: oligomers 1 and 2 are negatively charged. Moreover, oligomer 2 has been shown to associate strongly with several proteins46–48 and in fact, induces protein oxidation upon irradiation.33 The cationic polymer poly-4 has become our workhorse compound for antimicrobial work.39 Cationic oligomer 3 has not been studied extensively but was selected for investigation due to our findings that phenylene ethynylene polymers, such as poly-5 containing pendant imidazolium groups are unusually reactive in light induced bacterial killing.22, 38
Photoactive Oligomers Potently inhibit SARS-CoV-2
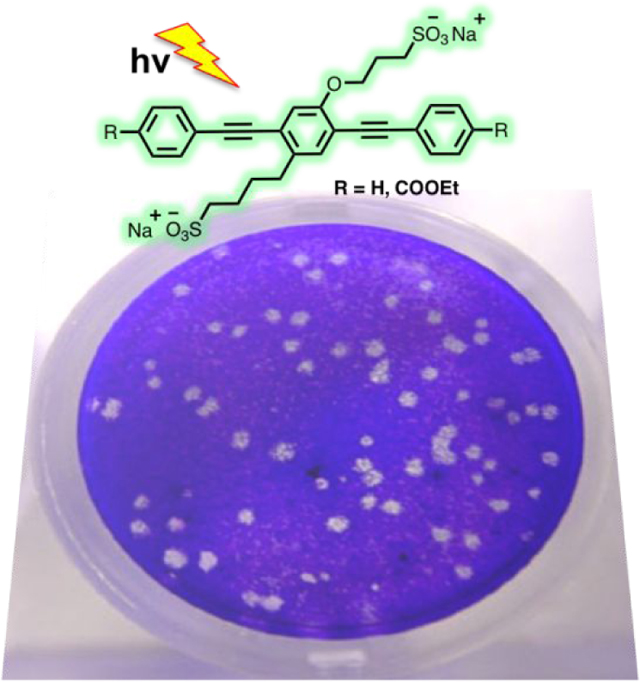
SARS-CoV-2 efficiently infects and causes cytopathic effects in Vero E6 cells.49 Vero E6 cells have an impaired type I interferon response and are therefore highly susceptible to virus infection and cell death.50, 51 Vero cells stained with crystal violet are commonly used to define infectious particle concentrations as expressed in plaque forming units (pfu) in viral stocks.52 A representative sample with a low pfu concentration is shown in Figure 3.
Figure 3.
Crystal violet stain of a Vero E6 cell monolayer 3 days post SARS-CoV-2 infection. A viral plaque, which appears as white circular features in the image, begins when a virus infects a cell within the cell monolayer. The virus infected cell subsequently lyses and spreads the infection to adjacent cells where the infection-to-lysis cycle is repeated. The infected cell area creates a plaque, an area of dead cells surrounded by uninfected, live cells, which can be seen by adding a crystal violet solution that colors the cytoplasm of healthy cells.
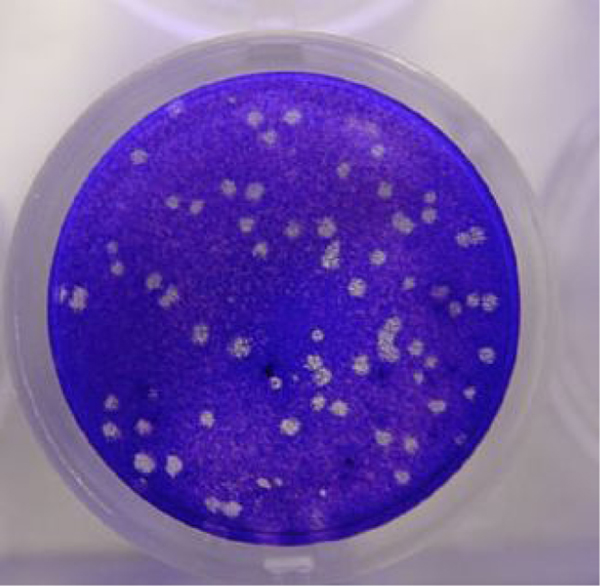
We first tested the near UV light (300–400 nm)-activated inhibitory effect of oligomers 1, 2 and 3 against SARS-CoV-2 infection on Vero cells.53,54 Figure 4 shows the reduction in pfu when SARS-CoV-2 is mixed in cell culture media with the oligomers and incubated with near UV light irradiation. Compounds 1 and 3 demonstrated immediate activity and ablated any viral plaque formation in as fast as 15–20 minutes of irradiation for oligomer 1 and 10 minutes irradiation for oligomer 3 in these conditions (Figure 4). While not as striking, oligomer 2 also showed a clear ability to decrease plaque formation by 30 minutes incubation and irradiation (Figure 4). Importantly, consistent with previous findings, these oligomers demonstrated no cytopathic effects when added to Vero cell monolayers in the absence of virus (data not shown).22, 31, 37, 55 In addition, the effect of the compounds alone in the absence of light for viral inhibition was investigated. Significant cytopathic effect of the virus on the Vero cells was observed following incubation for 60 min in dark conditions in the presence of compounds. Although quantitative data were not collected, we conclude that light activation is required for antiviral activity.
Figure 4.
Antiviral activity of photoactive oligomers against SARS-CoV-2 with near UV light irradiation for the indicated time periods. A) Oligomer 1; B) Oligomer 2; C) Oligomer 3; D) Control (no oligomer). For each sample 10 μg/mL of oligomer was incubated with 2.6 × 105 pfu/mL SARS-CoV-2 and irradiated. Viral titer was quantified by plaque assay on Vero E6 cells. Values shown are the average titer from at least three independent experiments (±SEM). Asterisks (*) denotes statistically significant differences between starting titer (inoculum) and samples incubated with compound and in light. Statistical analysis performed with one-way ANOVA analysis, * denotes p adjusted < 0.05.
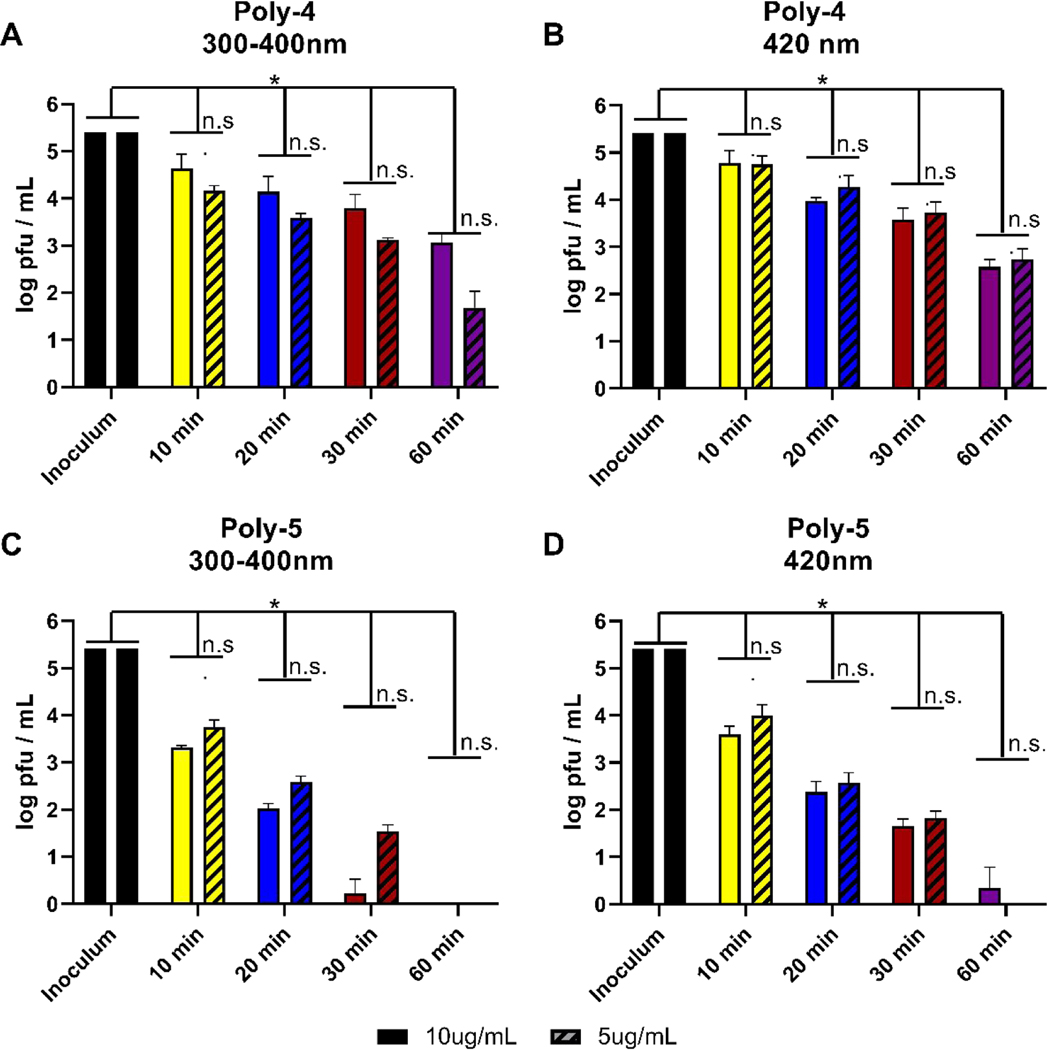
We then expanded our investigations to include photoactive polymers poly-4 and poly-5 which, unlike the oligomers, absorb in the visible region as well as the near-UV (Figure 2), making them likely to be active at these wavelengths as well. As shown in Figure 5A, when incubated with SARS-CoV-2 at a concentration of 10 μg/mL, poly-4 reduced plaque formation by almost 1.5 logs after 20 minutes of exposure to near UV light. While statistically significant when compared to inoculum titer, this reduction is minimally greater than that of the control with no compound added when exposed to either near UV or visible light (Figure 4D). In contrast, poly-5 is capable of effectively eliminating viral plaque formation in as little as 30 minutes incubation in near UV light and reducing viral titers by nearly 5 logs with 60 minutes of irradiation in the visible light (Figure 5B). Because of the potential “inner filter” effect from poly-4 and poly-5 during light incubation, we tested their ability to inhibit SARS-CoV-2 plaque formation at a lower concentration of 5 μg/mL under the same conditions. This lower concentration of either polymer demonstrated statistically insignificant differences in viral titer compared to the inhibition observed using 10 μg/mL (Figure 5). Similar to the oligomer results and previously reported data,22, 31, 37 we observed neither cytopathic effects of the polymers alone on Vero cells nor reduction in viral titer by plaque assay when polymers were incubated with the virus in the dark (data not shown). We therefore conclude that the “inner filter” effect is not interfering with the antiviral activity of poy-4 and poly-5 in these conditions. Further, poly-5 demonstrated effective inhibition of SARS-CoV-2 in both near UV and visible ranges of light, making it a strong candidate for further study and potential use to combat viral environmental contamination.
Figure 5.
Antiviral activity of photoactive polymers against SARS-CoV-2. Poly-4 (A, B) or poly-5 (C, D) was incubated with 2.6 × 105 pfu/mL SARS-CoV-2 with near UV light or visible light irradiation for the noted times. Viral titer was quantified by plaque assay on Vero E6 cells. Values plotted are the average titer calculated from at least three independent experiments (±SEM). Asterisks (*) denotes statistically significant differences between starting titer (inoculum) and samples incubated with compound and in light. Statistical analysis performed with two-way ANOVA analysis, * denotes p adjusted < 0.05.
DISCUSSION
The most important and significant result of this study is that all five materials tested show antiviral activity against SARS-CoV-2 under irradiation with light absorbed by the specific material. The three oligomers are active when irradiated with near-UV light and the two polymers are active under both visible and near-UV irradiation. Both anionic (1 and 2) and cationic (3) oligomers are active; this is reasonable for non-covalent binding of the oligomers to protein components, likely the viral spike proteins, of SARS-CoV-2. We have shown in a previous simulation study that the binding of phenylene ethynylene-based oligomers to the MS2 virus capsid protein assembly is mediated by van der Waals interactions between the hydrophobic OPE backbone and the capsid protein and strong electrostatic interactions between the OPE charged side chains and charged residues on the capsid surface.56 A combination of hydrophobic and electrostatic interactions between the oligomers and polymers and the SARS-CoV-2 spike protein likely contribute to the compounds’ antiviral activities in this study. Of the 4 structural protein components, the membrane protein M is a likely target for both oligomers and polymers to bind and be in close proximity to interior RNA.3 As shown in Figure 4D, when suspensions of SARS-CoV-2 are irradiated with near UV or visible light without oligomers or polymers present, there is some inactivation of the virus, especially upon prolonged irradiation with near-UV. However, it is clear that most of the inactivation of the virus under irradiation is due to excitation of the oligomers and polymers.
None of the five materials studied thus far exhibit antiviral activity in the dark. This is important but not surprising since SARS-CoV-2 is an enveloped virus and in the dark the interactions between oligomers and polymers and membrane proteins are not expected to be strong enough to denature the protein or break covalent bonds. However, it seems to be the case that binding or “docking” brings the oligomers and polymers sufficiently close to the virus such that the reactive oxygen species (ROS) generated at the binding sites on the virus can penetrate the virus and cause damages to the membrane coat protein M, RNA and/or inner proteins. Quite likely, the smaller oligomers may penetrate into the interior of the virus perhaps even binding to the N-protein that is directly connected to the virus RNA.3 Since we have shown in other studies that both negatively and positively charged OPEs can bind with misfolded proteins or protein fragments,46–48 binding of the polymers and oligomers used in this study with viral proteins and subsequent generation of singlet oxygen by irradiation close to the RNA seems a likely path for virus inactivation.20 The observation that the cationic quaternary ammonium poly-4 is inactive in the dark is a harbinger that classic quaternary ammonium surfactants may not be active against the virus.
As mentioned previously and detailed in Figure 4, anionic oligomer 1 and cationic oligomer 3 show rapid and effective inactivation of SARS-CoV-2 under irradiation with near UV light with essentially complete inactivation in 10–15 min, while anionic oligomer 2 shows much slower inactivation under the same conditions. While oligomer 2 has been shown in other studies to bind strongly to misfolded proteins and fragments,46–48 it seems reasonable as 2 is more hydrophilic than 1 or 3 and better solubilized in water but less able to penetrate into the interior of the virus. The curious effect that oligomer 2 is less active than 1 may also be associated with the fact that the ester groups have been shown to give rise to rapid excited state quenching of the oligomer in water. This quenching may reduce the ability of the compound to sensitize reactive oxygen species.57, 58
For the two polymers used in this study, irradiation with either near UV or visible light results in inactivation of SARS-CoV-2 as shown in Figure 5. Poly-5 shows rapid and efficient inhibition of the virus with essentially complete inactivation in 20–30 min under either near-UV or visible irradiation at levels of both 5 and 10 μg/mL. Poly-4 is significantly less reactive than Poly-5. At this point it is premature to speculate on the cause of the differences. An attractive feature of both polymeric systems examined is that they are reactive under visible irradiation and potentially can be “passive” antiviral agents under ambient irradiation conditions.
It is worth considering the practical applications of these materials. We can envision that these materials can be used in the prevention of Covid-19 and other virus-based diseases as well as for future virus threats. In previous studies we have shown that similar materials prepared in our laboratories can be incorporated into antimicrobial surface coatings. For example, textiles where compound are covalently attached via electrospinning or non-covalently incorporated by adsorption have exhibited broad spectrum antimicrobial properties.11, 36 Other studies have shown that these materials are not likely to harm the environment from their degradation byproducts and also not harmful to human skin or to several other types of mammalian cells.29, 30 It seems likely that these materials, used as pure components or mixtures in wipes, sprays, personal protective equipment (PPE) items, clothing for athletes, warfare fighters, and paints and hard surface coatings, can provide lasting disinfection of surfaces in rooms, vehicles, outdoor and indoor spaces.
CONCLUSIONS
In this study, we have tested five representative conjugated oligomers and polymers from an array of phenylene ethynylene-based cationic and anionic conjugated materials against SARS-CoV-2, the virus that causes the Covid-19 disease. Light activation of the materials at the wavelengths where they absorb (i.e., for 1 – 3 near UV and for poly-4 and poly-5 both near-UV and visible) gives rise to moderate to very strong inactivation of the virus. The antiviral activities of the compounds are likely due to binding of the compounds to viral proteins that brings the compounds in close proximity to the virus, followed by light-activated singlet oxygen and ROS generation that ultimately damage and inactivate the virus. An important result of these studies is the finding that there is essentially no dark inactivation of the virus. Since three of the five materials/compounds examined are quaternary ammonium derivatives, and show strong antimicrobial activity vs bacteria and non-enveloped viruses, this study indicates conventional quaternary ammonium antimicrobials may not be active against SARS-CoV-2. Our findings open the way towards a host of applications that may mitigate disease infections from SARS-CoV-2 as well as current and future virus threats to human health.
MATERIALS AND METHODS
Cells and viruses
Vero E6 cells (ATCC, CRL-1586) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated FBS, 1% pen/strep, 2mM L-glut, 1% non-essential amino acids, 1% HEPES. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, Isolate USA-WA1/2020) was acquired from BEI Resources (NR-52281) and propagated in Vero E6 cells for 3 days. Infectious virus was isolated by harvesting cellular supernatant and spinning at 1000 rpm for 10 minutes to remove cellular debris and stored at −80°C. SARS-CoV-2 infectious particles were quantified in media by standard plaque assay.52 Briefly, Vero E6 cells were treated with serial dilutions of SARS-CoV-2 in DMEM (4% FBS) and incubated at 37°C for 1 hr. An overlay of 2.4% cellulose (colloidal microcrystalline, Sigma #435244) and 2x DMEM (5% FBS) was added to each well and incubated for 3 days at 37°C and 5% CO2. On day 3, cells were fixed with 4% paraformaldehyde and stained with crystal violet to visualize and count plaques.
Oligomer and polymer incubations with SARS-CoV-2
Individual compounds were diluted to 10 μg/mL or 5 μg/mL in DMEM culture media (4% FBS). SARS-CoV-2 was added to the media solution containing a compound at a final concentration of 2.6×105 pfu/mL. Virus incubated in media was supplemented with a volume of RNA/DNAse-free water equal to that used for the compound dilutions. Samples were then incubated at room temperature inside a Luzchem light chamber and exposed to either no light, bulbs emitting 420 nm light, or bulbs emitting 300–400 nm light for the specified time intervals (see Supporting Information for spectral distributions and irradiance). The concentration of infectious virus present in the samples was then quantified by plaque assay.
Statistical Analysis
Results were analyzed and visualized with GraphPad Prism software. Statistical analyses to determine reduction in pfu following incubation with oligomers 1–3 and untreated controls utilized a one-way ANOVA analysis with a Dunnett’s multiple comparison test correction. Results comparing the effects of multiple polymer concentrations in various light exposures were analyzed with a two-way ANOVA and Sidak’s multiple comparison test correction.
Supplementary Material
Acknowledgement
AMK was supported by NIH grant 1K22AI141680–01A1. KSS acknowledges the Welch Foundation for support (Grant No. AX-0045–20110629).
Footnotes
Supporting Information
Synthesis and characterization of oligomer 2, spectral distribution of light sources used for near-UV and visible irradiation of samples. This material is available free via the internet at http://pubs.acs.org.
Conflict of Interest
KSS and DGW have interest in a start up company, BioSafe, LLC that has licensed patents related to the materials that are the focus of this work.
References
- 1.National Center for Immunization and Respiratory Diseases (Ncird), Division of Viral Diseases Updated February 15, 2020. https://www.cdc.gov/ncird/index.html.
- 2.Zhou P; Yang X-L; Wang X-G; Hu B; Zhang L; Zhang W; Si H-R; Zhu Y; Li B; Huang C-L; Chen H-D; Chen JC; Luo Y; Guo H; Jiang R-D; Liu M-Q; Chen Y; Shen X-R; Wang X; Zheng X-S; Zhao K; Chen Q-J; Deng F; Liu L-L; Yan B; Zhan F-X; Wang Y-Y; Xiao G-F; Shi Z-L, A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astuti I; Ysrafi, Severe Acute Respiratory Syndrome Coronavirus 2 (Sars-Cov-2): An Overview of Viral Structure and Host Response. Diabetes Metab Syndr. 2020, 14, 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q; Guan X; Wu P; Wang X; Zhou L; Tong Y; Ruiqi Ren R; Leung KSM; Lau EHY; Wong JY; Xing X; Xiang N; Wu Y; Li C; Chen Q; Li D; Liu T; Zhao J; Liu M; Tu W; Chen C; Jin L; Yang R; Wang Q; Zhou S; Wang R; Liu H; Luo Y; Liu Y; Shao G; Li H; Tao Z; Yang Y; Deng Z; Liu B; Ma Z; Zhang Y; Shi G; Lam TTY; Wu JT; Gao GF; Cowling BJ; Yang B; Leung GM; Feng Z, Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shereen MA; Khan S; Kazmi A; Bashir N; Siddique R, Covid-19 Infection: Origin, Transmission, and Characteristics of Human Coronaviruses. J. Adv. Res. 2020, 24, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo Y-R; Cao Q-D; Hong Z-S; Tan Y-Y; Chen S-D; Jin H-J; Tan K-S; Wang D-Y; Yan Y, The Origin, Transmission and Clinical Therapies on Coronavirus Disease 2019 (Covid-19) Outbreak – an Update on the Status. Mil. Med. Res. 2020, 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu F; Zhao S; Yu B; Chen Y-M; Wang W; Song Z-G; Hu Y; Tao Z-W; Tian J-H; Pei Y-Y; Yuan M-L; Zhang Y-L; Dai F-H; Liu Y; Wang Q-M; Zheng J-J; Xu L; Holmes EC; Zhang Y-Z, A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coronavirus Disease (Covid-19) Weekly Epidemiological Update and Weekly Operational Update; World Health Organization (WHO): 2020. [Google Scholar]
- 9.EPA Listed Disinfectants for SARS-CoV-2 https://www.epa.gov/pesticide-registration/listn-disinfectants-coronavirus-covid-19 (accessed November 8, 2020).
- 10.Behzadinasab S; Chin A; Hosseini M; Poon L; Ducker WA, A Surface Coating That Rapidly Inactivates Sars-Cov-2. ACS Appl. Mater. Interfaces 2020, 12 (31), 34723–34727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chemburu S; Corbitt T; Ista L; Ji E; Fulghum J; Lopez G; Ogawa K; Schanze KS; Whitten D, Light-Induced Biocidal Action of Conjugated Polyelectrolytes Supported on Colloids. Langmuir 2008, 24, 11053–11062. [DOI] [PubMed] [Google Scholar]
- 12.Corbitt TS; Sommer JR; Chemburu S; Ogawa K; Ista LK; Lopez GP; Whitten DG; Kirk S Schanze KS, Conjugated Polyelectrolyte Capsules: Light-Activated Antimicrobial Micro “Roach Motels”. ACS Appl. Mater. Interfaces 2009, 1, 4852. [DOI] [PubMed] [Google Scholar]
- 13.Corbitt TC; Ding L; Ji E; Ista LK; Ogawa K; Lopez GP; Schanze KS; Whitten DG, Light and Dark Biocidal Activity of Cationic Poly(Arylene Ethylene) Conjugated Polyelectrolytes. Photochem. Photobiol. Sci. 2009, 8, 998–1005. [DOI] [PubMed] [Google Scholar]
- 14.Ding L; Chi EY; Chemburu S; Ji E; Schanze KS; Lopez GP; Whitten DG, Insight into the Mechanism of Antimicrobial Poly(Phenylene Ethynylene) Polyelectrolytes: Interactions with Photphatidylglycerol Lipid Membranes. Langmuir 2009, 25, 13742–13751. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y; Corbitt TS; Jett SD; Tang Y; Schanze KS; Chi EY; Whitten DG, Direct Visualization of Bactericidal Action of Cationic Conjugated Polymers and Oligomers. Langmuir 2012, 28, 65–70. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y; Jett SD; Crum J; Schanze KS; Chi EY; Whitten DG, Understanding the Dark and Light-Enhanced Bactericidal Action of Cationic Conjugated Polyelectrolytes and Oligomers. Langmuir 2013, 29, 781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y; Schanze KS; Chi EY; Whitten DG, When Worlds Collide: Interactions at the Interface between Biological Systems and Synthetic Cationic Polyelectrolytes and Oligomers. Langmuir 2013, 29, 10635–10647. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y; Chi EY; Natvig DO; Schanze KS; Whitten DG, Antimicrobial Activity of Cationic Conjugated Polyelectrolytes and Oligomers against Saccharaomyces Cerevisiae Vegetative Cells and Ascospores. ACS Appl. Mater. Interfaces 2013, 5, 45594561. [DOI] [PubMed] [Google Scholar]
- 19.Dascier D; Ji E; Parthasarathy A; Schanze KS; Whitten DG, Efficacy of End-Only Functionalized oligo(Arylene-Ethynylene)s in Killing Bacterial Biofilms. Langmuir 2012, 28, 11286–11290. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y; Canady T; Zhou Z; Tang Y; Price D; Bear D; Chi E; Schanze KS; Whitten DG, Cationic Phenylene Ethynylene Polymers and Oligomers Exhibit Efficient Antiviral Activity. ACS Appl. Mater. Interfaces 2011, 3, 2209–2214. [DOI] [PubMed] [Google Scholar]
- 21.Pappas HC; Sylejmani R; Graus MS; Donabedian PL; Whitten DG; Neumann AK, Antifungal Properties of Cationic Phenylene Ethynylenes and Their Impact on Beta-Glucan Exposure. Antimicrob. Agents Chemother. 2016, 60, 4519–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagadesan P; Yu Z; Barboza-Ramos I; Lara HH; Vazquez-Munoz R; López-Ribot JL; Schanze KS, Light-Activated Antifungal Properties of Imidazolium-Functionalized Cationic Conjugated Polymers. Chem. Mater. 2020, 32 (14), 6186–6196. [Google Scholar]
- 23.Feng GX; Mai CK; Zhan RY; Bazan GC; Liu B, Narrow Band Gap Conjugated Polyelectrolytes for Photothermal Killing of Bacteria. J. Mater. Chem. B 2015, 3 (37), 7340–7346. [DOI] [PubMed] [Google Scholar]
- 24.Wang B; Feng GX; Seifrid M; Wang M; Liu B; Bazan GC, Antibacterial Narrow-Band-Gap Conjugated Oligoelectrolytes with High Photothermal Conversion Efficiency. Angew. Chem.-Int. Edit. 2017, 56 (50), 16063–16066. [DOI] [PubMed] [Google Scholar]
- 25.He P; Lv FT; Liu LB; Wang S, Cationic Conjugated Polymers for Detection and Inactivation of Pathogens. Science China-Chemistry 2017, 60 (12), 1567–1574. [Google Scholar]
- 26.Wang B; Wang M; Mikhailovsky A; Wang S; Bazan GC, A Membrane-Intercalating Conjugated Oligoelectrolyte with High-Efficiency Photodynamic Antimicrobial Activity. Angew. Chem.-Int. Edit. 2017, 56 (18), 5031–5034. [DOI] [PubMed] [Google Scholar]
- 27.Xu QL; He P; Wang JW; Chen H; Lv FT; Liu LB; Wang S; Yoon J, Antimicrobial Activity of a Conjugated Polymer with Cationic Backbone. Dyes Pigm. 2019, 160, 519–523. [Google Scholar]
- 28.Zhou Z; Ergene C; Lee JY; Shirley DJ; Carone BR; Caputo GA; Palermo EF, Sequence and Dispersity Are Determinants of Photodynamic Antibacterial Activity Exerted by Peptidomimetic Oligo(Thiophene)s. ACS Appl. Mater. Interfaces 2019, 11 (2), 1896–1906. [DOI] [PubMed] [Google Scholar]
- 29.Wilde KN; Whitten DG; Canavan HE, In Vitro Cytotoxicity of Antimicrobial Conjugated Electrolytes: Interactions with Mammalian Cells. ACS Appl. Mater. Interfaces 2013, 5, 9305–9311. [DOI] [PubMed] [Google Scholar]
- 30.Wilde KN; Nguyen PA; Whitten DG; Canavan HE, Skin Irritation Testing of Antimicrobial Conjugated Electrolytes. Biointerphases 2017, 12, 02C403. [DOI] [PubMed] [Google Scholar]
- 31.Wang S; Jagadesan P; Sun H; Hu R; Li Z; Huang Y; Liu L; Wang S; Younus M; Schanze KS, Fluorescence Imaging of Mammalian Cells with Cationic Conjugated Polyelectrolytes. ChemPhotoChem 2020, DOI: 10.1002/cptc.202000192. [DOI] [Google Scholar]
- 32.Hill EH; Goswami S; Evans DG; Schanze KS; Whitten DG, Photochemistry of a Model Cationic p-Phenylene Ethynylene in Water. J. Phys. Chem. Lett. 2012, 3, 1363–1368. [DOI] [PubMed] [Google Scholar]
- 33.Fanni AM; Monge FA; Hammond JJ; Martinez JS; Whitten DG; Chi EY, Controlled Photosensitizing Activity of Oligomeric p-Phenylene Ethynylenes on Amyloid-Β Fibrils. Biophys. J. 2019, 116 (3, Supplement 1), 275a. [Google Scholar]
- 34.Ogawa K; Chemburu S; Lopez GP; Whitten DG; Schanze KS, Conjugated Polyelectrolyte-Grafted Silica Microspheres. Langmuir 2007, 23 (8), 4541–4548. [DOI] [PubMed] [Google Scholar]
- 35.Chemburu S; Corbitt TS; Ista LK; Ji E; Fulghum J; Lopez GP; Ogawa K; Schanze KS; Whitten DG, Light-Induced Biocidal Action of Conjugated Polyelectrolytes Supported on Colloids. Langmuir 2008, 24 (19), 11053–11062. [DOI] [PubMed] [Google Scholar]
- 36.Ista LK; Dascier D; Ji E; Parthasarathy A; Corbitt TS; Schanze KS; Whitten DG, Conjugated-Polyelectrolyte-Grafted Cotton Fibers Act as “Micro Flypaper” for the Removal and Destruction of Bacteria. ACS Appl. Mater. Interfaces 2011, 3 (8), 2932–2937. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y; Pappas HC; Zhang L; Wang S; Cai R; Tan W; Wang S; Whitten DG; Schanze KS, Selective Imaging and Inactivation of Bacteria over Mammalian Cells by Imidazolium-Substituted Polythiophene. Chem. Mater. 2017, 29, 6389–6395. [Google Scholar]
- 38.Parthasarathy A; Pappas HC; Hill EH; Huang Y; Whitten DG; Schanze KS, Conjugated Polyelectrolytes with Imidazolium Solubilizing Groups. Properties and Application to Photodynamic Inactivation of Bacteria. ACS Appl. Mater. Interfaces 2015, 7 (51), 28027–28034. [DOI] [PubMed] [Google Scholar]
- 39.Scheberl A; Khalil ML; Maghsoodi F; Strach EW; Yang J; Chi EY; Schanze KS; Reimhult E; Whitten DG, Quantitative Determination of Dark and Light-Activated Antimicrobial Activity of Poly(Phenylene Ethynylene), Polythiophene, and Oligo(Phenylene Ethynylene) Electrolytes. ACS Appl. Mater. Interfaces 2020, 12 (19), 21322–21329. [DOI] [PubMed] [Google Scholar]
- 40.Tang Y; Hill EH; Zhou Z; Evans DG; Schanze KS; Whitten DG, Synthesis, Self-Assembly, and Photophysical Properties of Cationic Oligo(p-Phenyleneethynylene)s. Langmuir 2011, 27 (8), 4945–4955. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Z; Corbitt TS; Parthasarathy A; Tang Y; Ista LK; Schanze KS; Whitten DG, “End-Only” Functionalized Oligo(Phenylene Ethynylene)S: Synthesis, Photophysical and Biocidal Activity. J. Phys. Chem. Lett. 2010, 1 (21), 3207–3212. [Google Scholar]
- 42.Zhao XY; Pinto MR; Hardison LM; Mwaura J; Muller J; Jiang H; Witker D; Kleiman VD; Reynolds JR; Schanze KS, Variable Band Gap Poly(Arylene Ethynylene) Conjugated Polyelectrolytes. Macromolecules 2006, 39 (19), 6355–6366. [Google Scholar]
- 43.Kubista M; Sjoback R; Eriksson S; Albinsson B, Experimental Correction for the Inner-Filter Effect in Fluorescence Spectra. Analyst 1994, 119 (3), 417–419. [Google Scholar]
- 44.Rodriguez HB; Roman ES, Effect of Concentration on the Photophysics of Dyes in Light-Scattering Materials. Photochem. Photobiol. 2013, 89 (6), 1273–1282. [DOI] [PubMed] [Google Scholar]
- 45.Das UN, Can Bioactive Lipids Inactivate Coronavirus (Covid-19)? Archives of Medical Research 2020, 51 (3), 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fanni AM; Monge FA; Lin C-Y; Thapa A; Bhaskar K; Whitten DG; Chi EY, High Selectivity and Sensitivity of Oligomeric P-Phenylene Ethynylenes for Detecting Fibrillar and Prefibrillar Amyloid Protein Aggregates. ACS Chem. Neurosci. 2019, 10 (3), 1813–1825. [DOI] [PubMed] [Google Scholar]
- 47.Donabedian PL; Evanoff M; Monge FA; Whitten DG; Chi EY, Substituent, Charge, and Size Effects on the Fluorogenic Performance of Amyloid Ligands: A Small-Library Screening Study. ACS Omega 2017, 2 (7), 3192–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donabedian PL; Pham TK; Whitten DG; Chi EY, Oligo(P-Phenyleneethynylene) Electrolytes: A Novel Molecular Scaffold for Optical Tracking of Amyloids. ACS Chem. Neurosci. 2015, 6, 1526–1535. [DOI] [PubMed] [Google Scholar]
- 49.Kim J-M; Chung Y-S; Jo HJ; Lee N-J; Kim MS; Woo SH; Park S; Kim JW; Kim HM; Han M-G, Identification of Coronavirus Isolated from a Patient in Korea with Covid-19. Osong Public Health Res Perspect 2020, 11 (1), 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ammerman NC; Beier-Sexton M; Azad AF, Growth and Maintenance of Vero Cell Lines. Current Protocols in Microbiology 2008, 11 (1), A.4E.1-A.4E.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desmyter J; Melnick JL; Rawls WE, Defectiveness of Interferon Production and of Rubella Virus Interference in a Line of African Green Monkey Kidney Cells (Vero). J. Virol. 1968, 2, 955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendoza EJ; Manguiat K; Wood H; Drebot M, Two Detailed Plaque Assay Protocols for the Quantification of Infectious Sars-Cov-2. Current Protocols in Microbiology 2020, 57 (1), cpmc105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The spectral distributions of the near-UV and visible light sources used are provided in the Supporting Information. The integrated near-UV irradiance that is provided by the source (6.7 mW-cm−2) is approximately 3 times greater than the integrated near-UV irradiance in 1sun solar light, see ref. 54.
- 54.Solar Spectrum https://en.wikipedia.org/wiki/Air_mass_(solar_energy)#/media/File:Solar_Spectrum.png (accessed November 8, 2020).
- 55.In vivo studies in which a patch coated with poly-4 was placed under a flat of mice skin and examined over a period of one month showed no damage to the skin or accumulation of the poly-4 in vital organs (data not shown).
- 56.Martin TD; Hill EH; Whitten DG; Chi EY; Evans DG, Oligomeric Conjugated Polyelectrolytes Display Site-Preferential Binding to an Ms2 Viral Capsid. Langmuir 2016, 32 (47), 12542–12551. [DOI] [PubMed] [Google Scholar]
- 57.Donabedian PL; Creyer MN; Monge FA; Schanze KS; Chi EY; Whitten DG, Detergent-Induced Self-Assembly and Controllable Photosensitizer Activity of Diester Phenylene Ethynylenes. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (28), 7278–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hill EH; Evans DG; Whitten DG, The Influence of Structured Interfacial Water on the Photoluminescence of Carboxyester-Terminated Oligo-p-Phenylene Ethynylenes. J. Phys. Org. Chem. 2014, 27 (4), 252–257. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.