Key Points
CAR T-cell therapy was safe and effective in a DLBCL patient with coexisting autoimmune neuropathy.
CD19 CAR T-cell therapy may control refractory autoantibodies and monoclonal gammopathies.
Introduction
Chimeric antigen receptor (CAR) T cells are genetically engineered T cells that attach to specifically targeted tumor-associated antigens and destroy the tumor cells through T-cell activation. CAR T cells have revolutionized the treatment of relapsed/refractory large B-cell lymphomas and other hematologic malignancies.1,2 Patients with a history of autoimmune disease were excluded from the landmark clinical trials that led to the approval of CAR T-cell therapy.3 Therefore, it is not known whether CAR T cells are safe to administer to patients with autoimmune conditions. Whether immune effector cell–associated neurotoxicity syndrome (ICANS), which complicates CAR T-cell therapy, could worsen preexisting neurologic conditions associated with lymphoma is also not established. Interestingly, preclinical murine studies have shown promising outcomes with CAR T-cell therapy in autoimmune conditions, including the eradication of systemic lupus erythematosus and autoimmune polyneuropathy.4,5
Multifocal motor neuropathy (MMN) is a rare, pure motor neuropathy characterized by asymmetric slowly progressive distal muscle weakness.6 MMN is a demyelinating and axonal disorder, with ∼40% to 50% of cases showing presence of immunoglobulin M (IgM) antibodies against the peripheral nerve myelin glycolipids GM1 and GD1b.7 These anti-GM1 autoantibodies associated with MMN have also been associated with IgM monoclonal gammopathy and B-cell lymphoma.8-11 Although MMN patients rapidly respond to IV immunoglobulin, the improvement is not sustained. Most patients require higher and more frequent doses of IV immunoglobulin over time, and if disease is refractory, second-line agents include rituximab and cyclophosphamide.12-16
Here, we describe the successful outcome with CAR T-cell therapy of a patient with diffuse large B-cell lymphoma (DLBCL) and an associated MMN that became refractory to IV immunoglobulin.
Case description
A 53-year-old man with a 10-year history of MMN controlled with monthly IV immunoglobulin presented with a 2-month history of pruritus, right axillary lymphadenopathy (LAD), and worsening lower-extremity weakness. His MMN at this time was progressing despite IV immunoglobulin, with ambulation limited to assistance with a wheelchair. On physical examination, he had extensive generalized muscle atrophy, with paresis of both upper and lower extremities, enlarged right axillary LAD, and palpable splenomegaly. Laboratory evaluation was notable for pancytopenia, with a leukocyte count of 3.1 × 109/L, hemoglobin of 10 g/dL, and platelet count of 104 × 109/L. Lactate dehydrogenase was elevated to 452 U/L. Serum protein electrophoresis revealed a biclonal M spike of 0.9 g/dL, IgG κ of 1633 mg/dL, and IgM κ of 551 mg/dL. Ganglioside antibody profile revealed antiganglioside antibodies, including anti-GM1 IgG/IgM of 149 and anti-GD1b IgG/IgM of 97. Computed tomography (CT) scan showed bulky right-sided axillary and subpectoral LAD with marked splenomegaly. Positron emission tomography (PET)/CT scan showed extensive fluorodeoxyglucose-avid adenopathy involving the bilateral cervical, bilateral axillary, and porta hepatis regions (maximum standardized uptake value range, 4-31).
A biopsy of the axillary lymph node confirmed the diagnosis of DLBCL. Immunohistochemical staining showed a population of atypical lymphocytes positive for CD20, CD5, CD23, BCL-2, BCL-6, PAX5, and MUM1 and negative for CD10, cyclin D1, SOX11, c-MYC, HHV8, EBV, and PD-L1. Ki-67 proliferation index was 70%. Immunoglobulin gene rearrangement studies revealed a clonal κ light chain rearrangement. Bone marrow aspiration and biopsy revealed 25% involvement of lymphoma, with multiple cytogenetic changes including extra copies of BCL-6 in 3q27, MYC in 8q24, and BCL-2 in 18q21 and isochromosome 17q leading to relative loss of 17p. Fluorescence in situ hybridization studies were negative for rearrangements involving IGH-BCL-2, BCL-6, and MYC genes. Because of the concurrent findings of stage IV DLBCL of the non–germinal center B-cell–like subtype and monoclonal gammopathy, MYD88 mutation testing was performed and was positive for L265P mutation, which is usually seen in Waldenström macroglobulinemia and IgM-expressing lymphoplasmacytic lymphoma. κ and λ light chain stains showed a nonspecific staining pattern and no distinct cytoplasmic light chain restriction.
Methods
Our patient proceeded to treatment with 6 cycles of dose-adjusted rituximab, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin. IV immunoglobulin for the MMN was withheld during chemotherapy. At the end of 6 cycles, he was in complete metabolic remission by PET/CT. The monoclonal gammopathy was no longer detected, but the ganglioside antibody profile remained positive for anti-GM1 IgG/IgM and anti-GD1b IgG/IgM (Figure 1). He was restarted on IV immunoglobulin every 3 weeks, with gradual improvement in his motor strength, such that he was able to walk without a walker and move his fingers.
Figure 1.
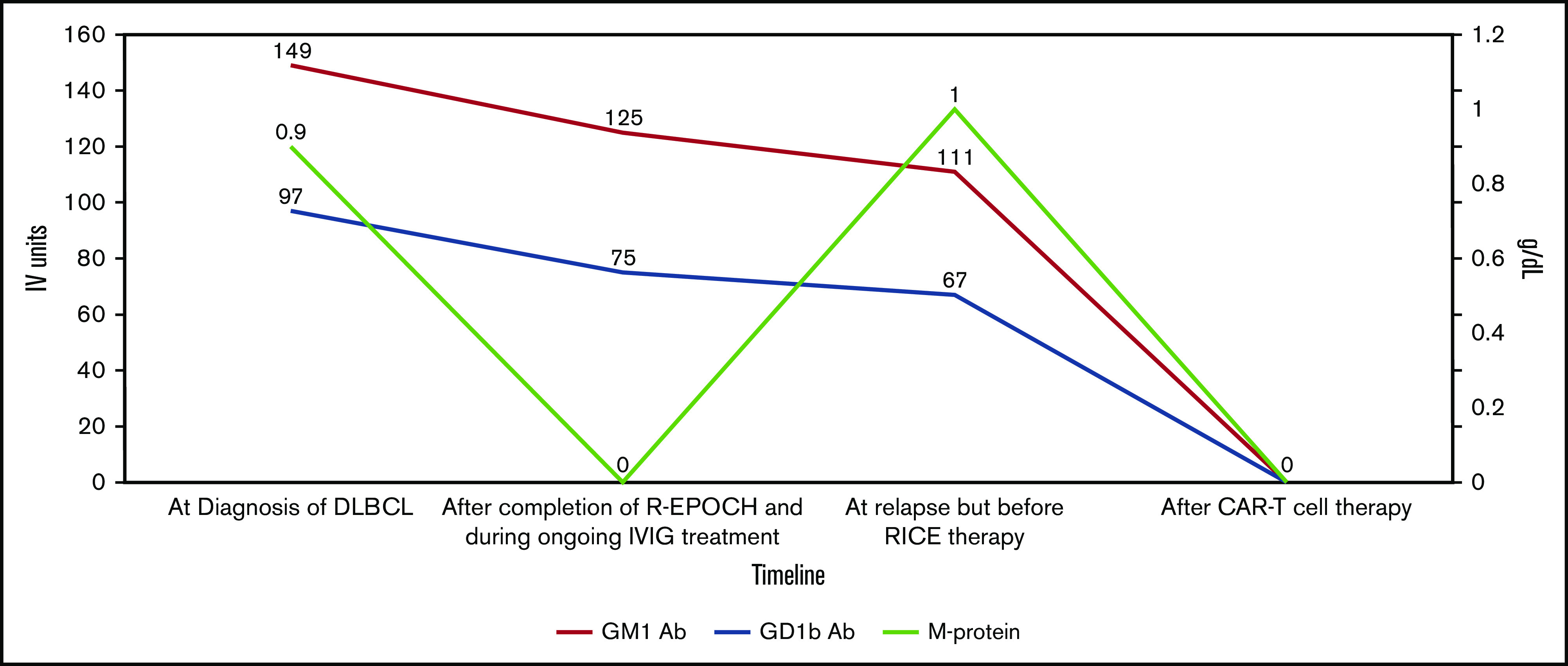
Ganglioside antibody profile and M protein levels during treatment course for DLBCL. Red line shows the levels of GM1 antibody, blue line shows levels of GD1B antibody, and light green line shows M protein levels. IVIG, IV immunoglobulin; R-EPOCH, rituximab plus etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; RICE, rituximab plus ifosfamide, carboplatin, and etoposide.
Six months later, a PET/CT scan showed new evidence of disease in the neck, chest, abdomen, and pelvis, as well as patchy involvement of bone marrow by biopsy. Relapsed DLBCL was confirmed on inguinal node biopsy. The IgM κ monoclonal gammopathy also reappeared before his systemic relapse was diagnosed and rapidly reached pretreatment levels. The patient at best had stable disease per Lugano 2014 criteria after 2 cycles of rituximab, ifosfamide, carboplatin, and etoposide, with persistent disease in the left axillary, inguinal, and portal/portocaval nodes (maximum standardized uptake value, 27.5). His strength continued to improve on IV immunoglobulin, and he was able to perform most of his activities of daily living, including ambulation with minimal assistance, although distal limb atrophy and weakness persisted.
He next proceeded to CAR T-cell therapy with axicabtagene ciloleucel (axi-cel). This decision was made accepting the possible risk that the treatment might worsen the MMN, because the patient had limited therapeutic options. Before CAR T-cell infusion, he received lymphodepleting chemotherapy with fludarabine and cyclophosphamide.
His CAR T-cell course was complicated by neutropenic fever on day 4 after axi-cel infusion (ie, day +4), which was treated with empiric antibiotics. He then developed grade 2 cytokine release syndrome (CRS), as evidenced by fever, hypotension, and tachycardia on day +6, which required intensive care unit admission for close monitoring; he was treated with 2 doses of tocilizumab. This was followed by grade 4 ICANS with progressively worsening altered mental status and generalized seizures, requiring intubation on day +8 and treatment with high-dose steroids and a third dose of tocilizumab. His initial steroid regimen was 1 g of IV methylprednisolone for 3 days followed by dexamethasone taper over 5 days once symptoms improved. An electroencephalogram performed on the day after ICANS development revealed moderate to severe slowing and intermittent rhythmic δ activity, representing a likely interictal pattern seen in encephalopathy, for which prophylaxis with levetiracetam was continued. After recovery from CRS and ICANS, he received 2 daily doses of 50 g of IV immunoglobulin for his MMN maintenance therapy. He was discharged home on day +16 after CAR T-cell infusion, with a biweekly regimen of IV immunoglobulin for the MMN.
His 30-day posttreatment PET/CT showed complete metabolic remission of his lymphoma, which was sustained on his 3- and 6-month posttreatment imaging. His muscle weakness continued to improve, and he was ambulating with a walker 2 months after and without any assistance 6 months after CAR T-cell therapy, with no decline in motor function before his IV immunoglobulin infusions. The monoclonal protein and the anti-GM1 and anti-GD1b antibodies became undetectable, and the patient continues to receive IV immunoglobulin (Figure 1).
Results and discussion
Although CAR T-cell therapy with the 2 US Food and Drug Administration–approved products axi-cel and tisagenleucel has revolutionized the treatment of refractory lymphoma, the treatment carries the risk of life-threatening adverse effects, which often require intensive care.17-19 Because of the concern that autoimmune conditions might worsen during the CRS provoked by extensive T-cell activation, patients with autoimmune conditions were excluded from the initial trials in lymphoma patients.20 Indeed, immunotherapy with checkpoint inhibitors, which also work by activating T cells, can exacerbate autoimmune conditions and cause new-onset autoimmune disorders.21-23 Another concern is that the severe neurologic toxicity seen in some patients after CAR T-cell infusion could exacerbate preexisting neurologic conditions.
With these concerns in mind, but with awareness of the rapid progression of the life-threatening lymphoma, our patient proceeded with CAR T-cell therapy; despite development of both severe CRS and ICANS, no immediate flare of motor weakness from MMN occurred during treatment. Although he was receiving IV immunoglobulin concurrently, it is speculated that the profound B-cell aplasia accompanying CAR T-cell infusion was instrumental in initiating a partial but continuing improvement in his motor weakness. The complete resolution of monoclonal gammopathy and anti-GM1 and anti-GD1b antibodies coincided with this improvement. This suggests a potential role of CAR T-cell therapy in removing autoreactive antibody-producing B-cell clones that are resistant to immunosuppression and chemoimmunotherapy. However, prospective clinical trials are necessary to evaluate the role of CAR T-cell therapy in the treatment of refractory autoimmune diseases and chemotherapy-resistant monoclonal gammopathies of clinical significance causing organ damage, which are predominantly mediated by IgM antibodies produced by CD-19+ B cells and plasmacytoid B cells.
To our knowledge, this is the first report of the successful and safe treatment of relapsed/refractory DLBCL and coexisting autoimmune MMN with monoclonal gammopathy with CD19-directed CAR T-cell therapy.
Acknowledgments
The authors thank the patient for his willingness to share his case with the scientific community and all those who participated in his care.
Footnotes
For data sharing requests, e-mail the corresponding author, Aaron P. Rapoport (arapopo@umm.edu).
Authorship
Contribution: A.P.R. and V.M. identified the patient case; K.S.J. collected the data and prepared the first manuscript draft and figures; I.S., N.G.H., V.M., and A.P.R. assisted in the writing of the manuscript; and all authors actively participated in reviewing and editing the manuscript.
Conflict--interest disclosure: The authors declare no competing financial interests.
Correspondence: Aaron P. Rapoport, University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center, 22 S. Greene St, Baltimore, MD 21201; e-mail: arapopo@umm.edu.
References
- 1.Anderson JK, Mehta A. A review of chimeric antigen receptor T-cells in lymphoma. Expert Rev Hematol. 2019;12(7):551-561. [DOI] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kansal R, Richardson N, Neeli I, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. 2019;11(482):eaav1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abraham PM, Quan SH, Dukala D, Soliven B. CD19 as a therapeutic target in a spontaneous autoimmune polyneuropathy. Clin Exp Immunol. 2014;175(2):181-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joint Task Force of the EFNS and the PNS European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. J Peripher Nerv Syst. 2010;15(4):295-301. [DOI] [PubMed] [Google Scholar]
- 7.Yeh WZ, Dyck PJ, van den Berg LH, Kiernan MC, Taylor BV. Multifocal motor neuropathy: controversies and priorities. J Neurol Neurosurg Psychiatry. 2020;91(2):140-148. [DOI] [PubMed] [Google Scholar]
- 8.Vlam L, Piepers S, Sutedja NA, et al. Association of IgM monoclonal gammopathy with progressive muscular atrophy and multifocal motor neuropathy: a case-control study. J Neurol. 2015;262(3):666-673. [DOI] [PubMed] [Google Scholar]
- 9.Fermand JP, Bridoux F, Dispenzieri A, et al. Monoclonal gammopathy of clinical significance: a novel concept with therapeutic implications. Blood. 2018;132(14):1478-1485. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi M, Mori K, Yamazaki S, Suda K, Sato N, Oshimi K. Multifocal motor neuropathy caused by a B-cell lymphoma producing a monoclonal IgM autoantibody against peripheral nerve myelin glycolipids GM1 and GD1b. Br J Haematol. 2003;123(4):600-605. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Moreno JM, Castilla JM, Garcia-Escudero A, Izquierdo G. Multifocal motor neuropathy with conduction blocks and prurigo nodularis. A paraneoplastic syndrome in a patient with non-Hodgkin B-cell lymphoma? [in Spanish]. Neurologia. 2004;19(4):220-224. [PubMed] [Google Scholar]
- 12.Nguyen TP, Chaudhry V. Multifocal motor neuropathy. Neurol India. 2011;59(5):700-706. [DOI] [PubMed] [Google Scholar]
- 13.Baumann A, Hess CW, Sturzenegger M. IVIg dose increase in multifocal motor neuropathy: a prospective six month follow-up. J Neurol. 2009;256(4):608-614. [DOI] [PubMed] [Google Scholar]
- 14.Terenghi F, Cappellari A, Bersano A, Carpo M, Barbieri S, Nobile-Orazio E. How long is IVIg effective in multifocal motor neuropathy? Neurology. 2004;62(4):666-668. [DOI] [PubMed] [Google Scholar]
- 15.Feldman EL, Bromberg MB, Albers JW, Pestronk A. Immunosuppressive treatment in multifocal motor neuropathy. Ann Neurol. 1991;30(3):397-401. [DOI] [PubMed] [Google Scholar]
- 16.Rüegg SJ, Fuhr P, Steck AJ. Rituximab stabilizes multifocal motor neuropathy increasingly less responsive to IVIg. Neurology. 2004;63(11):2178-2179. [DOI] [PubMed] [Google Scholar]
- 17.Feins S, Kong W, Williams EF, Milone MC, Fraietta JA. An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am J Hematol. 2019;94(S1):S3-S9. [DOI] [PubMed] [Google Scholar]
- 18.Chow VA, Shadman M, Gopal AK. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood. 2018;132(8):777-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia Borrega J, Gödel P, Rüger MA, et al. In the eye of the storm: immune-mediated toxicities associated with CAR-T cell therapy. HemaSphere. 2019;3(2):e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tocut M, Brenner R, Zandman-Goddard G. Autoimmune phenomena and disease in cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev. 2018;17(6):610-616. [DOI] [PubMed] [Google Scholar]
- 21.Inaba H, Ariyasu H, Takeshima K, Iwakura H, Akamizu T. Comprehensive research on thyroid diseases associated with autoimmunity: autoimmune thyroid diseases, thyroid diseases during immune-checkpoint inhibitors therapy, and immunoglobulin-G4-associated thyroid diseases. Endocr J. 2019;66(10):843-852. [DOI] [PubMed] [Google Scholar]
- 22.Orabona C, Mondanelli G, Puccetti P, Grohmann U. Immune checkpoint molecules, personalized immunotherapy, and autoimmune diabetes. Trends Mol Med. 2018;24(11):931-941. [DOI] [PubMed] [Google Scholar]
- 23.Cappelli LC, Shah AA, Bingham CO III. Immune-related adverse effects of cancer immunotherapy—implications for rheumatology. Rheum Dis Clin North Am. 2017;43(1):65-78. [DOI] [PMC free article] [PubMed] [Google Scholar]


