Abstract
Physicians who treat patients with schizophrenia frequently encounter complex clinical situations not fully addressed by published treatment guidelines. Some of these situations lead to antipsychotic polypharmacy, often prescribed when clinical and social obstacles prevent access to clozapine and patients have had suboptimal responses to nonclozapine monotherapy. We offer our perspective on the place of antipsychotic polypharmacy in the current treatment guidelines for patients with schizophrenia. We summarize data on the prevalence of antipsychotic polypharmacy and describe common clinical situations in which this practice is encountered, along with the pharmacological underpinnings of this practice. We briefly review evidence on common risks of antipsychotic polypharmacy and describe the limited evidence for the possible benefits of such practice. Moreover, we take a look at alternative antipsychotic augmentation strategies that address all domains of psychosis.
Keywords: Antipsychotics, Schizophrenia
Schizophrenia affects more than 23 million people worldwide (1), many of whom do not respond adequately to their antipsychotic treatment regimen. Clinicians may thus recommend a combination of antipsychotic medications, along with other psychotropic drugs, in an effort to maximize treatment response. “Antipsychotic polypharmacy” is broadly defined as simultaneous prescribing of more than one antipsychotic (2). For practical applications, Ganguly et al. (3) defined antipsychotic polypharmacy as two or more antipsychotics prescribed concurrently with at least 14 days overlap, whereas more than 31 days overlap was defined as an “episode of polypharmacy.” Kreyenbuhl et al. (4) argued that only persisting, long-term combinations of antipsychotics (i.e., more than 30 or 60 days) should be defined as actual polypharmacy because short-term combinations may represent a transition during a switch in antipsychotics. Regardless of duration, the prescription of two or more antipsychotics is mentioned with caution in current practice guidelines. Here, we examine the current empirical evidence for the use of more than one antipsychotic medication in the treatment of schizophrenia. We explore current treatment guidelines and the prevalence of antipsychotic polypharmacy before discussing the risks and benefits of using antipsychotic combinations. Moreover, we briefly review combining antipsychotics with other classes of psychotropic medication and nonpharmacologic treatment interventions for schizophrenia.
Current State of Affairs in Antipsychotic Combinations
Use of Antipsychotic Combinations Among Patients With Schizophrenia as Reflected in Treatment Guidelines
Before examining polypharmacy among patients with schizophrenia, we reviewed practice recommendations from across the world. We reviewed guidelines from North America (United States), Asia (India), Europe, and Australia. In Figure 1 (5–11), we consolidate these treatment algorithms, showing which step is recommended in which guidelines. For the United States, we reviewed the 2004 and the 2019 editions of American Psychiatric Association (APA) Practice Guidelines for the Treatment of Patients With Schizophrenia (5, 11), the Texas Medication Algorithm Project (TMAP) antipsychotic algorithm for schizophrenia (7), and the 2017–2018 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults (6). The 2004 APA guideline and the Florida best practice guidelines do not recommend antipsychotic polypharmacy at any point. They instead focus on using adjunct medications to target particular residual symptoms, such as antidepressants for depressive symptoms (5, 6). The TMAP recommends clozapine and a nonclozapine antipsychotic combination after clozapine partial or nonresponse, noting that randomized controlled trials (RCTs) have shown inconsistent results for this practice. In the case of nonresponse, the TMAP recommends returning to another monotherapy trial before continuing with two antipsychotics or other combination treatments (7). The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines for treatment and management of psychosis in adults do not recommend combined antipsychotic use except in the case of short-term cross-titration. The NICE guidelines emphasize the assessment of psychosocial needs, comorbid illness, and medication compliance when evaluating medication ineffectiveness. They also mention the augmentation of clozapine with a secondary antipsychotic, but only after an extensive assessment of the reasons for the ineffectiveness of clozapine monotherapy (8). The Indian clinical practice guidelines note that augmentation of clozapine with a second antipsychotic may be considered after an inadequate response to clozapine alone. This same guideline notes that this practice is often considered in the management of agitation, but only for as short a duration as possible (9). The Royal Australian and New Zealand College of Psychiatrists (RANZCP) clinical guidelines note that after the failure of two antipsychotic trials, one may consider polypharmacy with careful monitoring. Moreover, the RANZCP guidelines recommend regular review to determine whether the regimen can be simplified (10).
Figure 1.
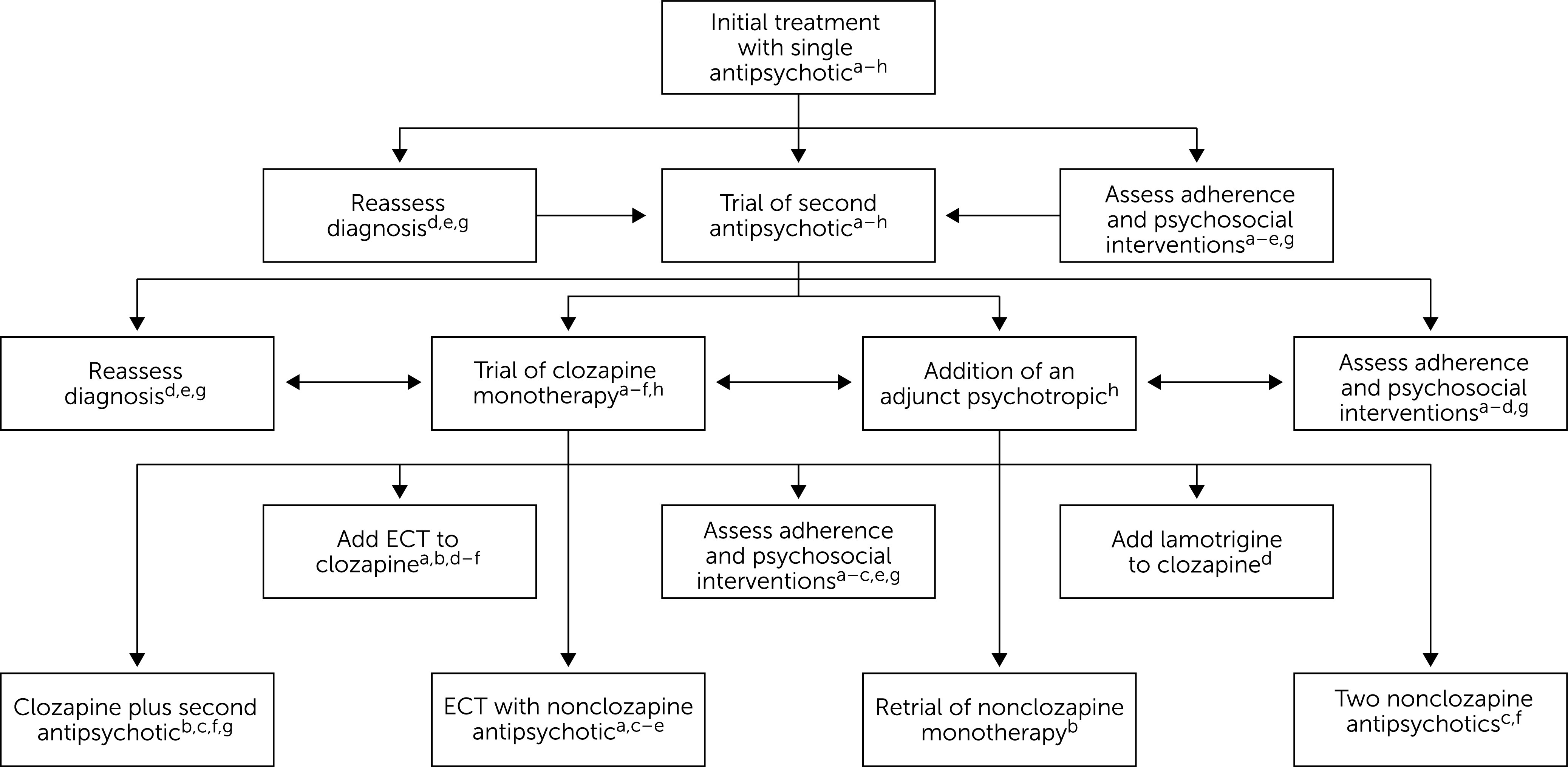
Consolidated treatment algorithm for patients with schizophrenia, based on worldwide guidelines
aAmerican Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia (5). bTexas Medication Algorithm Project (TMAP) schizophrenia treatment guidelines (7), first application. cTMAP schizophrenia treatment guidelines, second application after failure of initial application (7). d2017–2018 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults (6). eClinical Practice Guidelines for Management of Schizophrenia (9). fRoyal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines for the Management of Schizophrenia and Related Disorders (10). gPsychosis and Schizophrenia in Adults: Prevention and Management (National Institute for Health and Care Excellence guidelines) (8). hAmerican Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia 2019 (11). ECT, electroconvulsive therapy.
The 2019 APA practice guideline shifts to a more inclusive use of multiple psychotropics and antipsychotics as needed. The guideline explicitly recommends the augmentation of antipsychotic monotherapy with other psychotropics such as antidepressants for residual negative or depressive symptoms before a trial of clozapine monotherapy. It does highlight clozapine’s efficacy among patients who experience aggression and suicidality and advises that clozapine should not be delayed by excessive trials of augmentation treatment. This guideline also notes the beneficial role of antipsychotic combinations, if used cautiously, based on the evidence discussed later in this article (11).
Taken together, guidelines that recommend consideration of antipsychotic polypharmacy do so for patients who could be considered to have treatment-resistant schizophrenia. “A person with treatment-resistant schizophrenia” is currently defined as a patient who did not have a response to previous trials of one to three antipsychotics; did not have a response to a prospective trial of another antipsychotic (different from the one to which the patient previously had no response), and has had a prospective treatment response evaluation with a standardized instrument (e.g., Brief Psychiatric Rating Scale or Positive and Negative Syndrome Scale) (12). Overall, the guidelines indicate that the patient should have had no resolution of symptoms with as many as three antipsychotics if clozapine was not one of the two previous antipsychotics tried (see Figure 1).
What Is the Prevalence of Antipsychotic Polypharmacy?
No consensus exists on how frequently antipsychotic combinations are prescribed. Ganguly et al. (3) studied concomitant prescriptions of more than two antipsychotics among 31,435 Medicaid recipients with schizophrenia from California and Georgia in 1998–2000. Of Medicaid recipients with schizophrenia, 40% received antipsychotic polypharmacy for longer than 14 days (mean duration=149 days), and 23% received long-term (>60 days) polypharmacy (mean duration=236 days). The episodes involving clozapine were longer (mean=301 days) than those involving other antipsychotics (mean=225 days).
Kreyenbuhl et al. (4) found that among 61,257 patients with schizophrenia and schizoaffective disorder treated with antipsychotics recorded in the Department of Veterans Affairs National Psychosis Registry, the prevalence of combination antipsychotics was 20.0% for 30 days of combination treatment, 13.1% for more than 60 days, and 9.5% for more than 90 days. Of the 5,826 patients who remained on antipsychotics for longer than 90 days, 97.8% were treated with two antipsychotics, 1.3% were treated with three antipsychotics, and 0.02% were treated with four antipsychotics. Among patients who were prescribed long-term antipsychotic combinations, 4,319 (74.1%) were taking first- and second-generation antipsychotics, 1,058 (18.2%) were taking two second-generation antipsychotics, and 374 (6.4%) were taking two first-generation antipsychotics (4).
Tiihonen et al. (13), studying a cohort of inpatients in Finland, found that of 8,719 patients with first-episode schizophrenia followed for a median of 14 years, 41.6% were exposed to antipsychotic polypharmacy for more than 90 days, whereas among a general cohort of 62,250 inpatients with schizophrenia followed for the same duration, 57.5% received polypharmacy. Relevant studies on the frequency of antipsychotic combinations among large cohorts of patients with schizophrenia are described in Table 1 (3, 4, 13–16).
TABLE 1.
Frequency of antipsychotic combinations
| Study/country | N and populationa | Antipsychotic polypharmacy (prescribing ≥2 antipsychotics at the same time) as defined in each study | Prevalence of antipsychotic polypharmacy | Duration of follow-up |
|---|---|---|---|---|
| Leslie and Rosenheck (14), United States | N=34,925 VA outpatients with schizophrenia taking oral antipsychotics | Prescribed antipsychotics for one week June–Sept. 1999 | 6.8% (2,383) received prescriptions for combination antipsychotics | June–Sept 1999 |
| Jaffe and Levine (15), United States | N=7,130 inpatients in NY state psychiatric hospital system receiving antipsychotics | Polypharmacy episode ≥28 days | 37.3% (2,791) patients were on combination antipsychotics, of whom 0.12% (342) received three or more antipsychotics; mean duration of polypharmacy=97 days | 1999 |
| Ganguly et al. (3), United States | N=31,435 Medicaid recipients in California and Georgia, ≥16 years old | Study inclusion criteria: ≥14 days of antipsychotics combination; polypharmacy episode ≥31 days; long-term polypharmacy ≥ 60 days | Overall polypharmacy 40% (N=12,549); long-term polypharmacy 23% (N=7,222); mean duration of long-term polypharmacy=236 days | 1998–2000 |
| Humberstone et al (16), New Zealand | N=3,178 outpatients with schizophrenia and bipolar disorder on antipsychotics | Cross-sectional prescribing practice, March 2000 | Polypharmacy among 16.4% (N=521) of the total sample; 254 on oral antipsychotic only; 267 on oral + long-acting injectable antipsychotic | March 2000 |
| Kreyenbuhl et al. (4), United States | N=61,257 patients in VA National Psychosis Registry | ≥30 days polypharmacy | 20.0% (12,234) for ≥30 days | 2000 |
| ≥ 60 days polypharmacy | 13.1% (N=8,050) for ≥60 days | |||
| ≥ 90 days: long-term polypharmacy | 9.5% (N=5,826) for ≥90 days | |||
| Tiihonen et al. (13), Finland | N=8,719 persons with first-episode schizophrenia and N=62,250 inpatients with schizophrenia from Hospital Discharge Register, National Prescription Register, and National Death Register in Finland | >90 days polypharmacy | 41.6% (N=3,627) of patients with first-episode schizophrenia were on antipsychotic polypharmacy for >90 days; 57.5% of all inpatients with schizophrenia (N=62,250), were on polypharmacy when followed for the same duration of time | 1996–2015 (median follow-up=14 years) |
VA, Department of Veterans Affairs.
The variability in the frequency of antipsychotic combinations reported in the literature is in part because of the lack of consensus in defining an episode of antipsychotic polypharmacy. Many authors define polypharmacy as an episode of antipsychotics prescribed in combination for longer than 90 days, and shorter periods of polypharmacy are attributable to switching antipsychotics and short-term treatment of prominent symptoms in a certain domain of psychosis (17).
Antipsychotic Combinations: Friend or Foe?
Does Combining Antipsychotic Drugs Make Pharmacological Sense?
Although there is evidence of increased risk of adverse effects and a consistent association with inpatient admission, the practice of combining antipsychotics is broadly used and appears to be effective for a subgroup of patients. Correll et al. (18) performed a meta-analysis of patients with schizophrenia (N=1,216) on antipsychotic polypharmacy (mean±SD duration of 12±11.3 weeks) from 19 studies (double-blind, single-blind, open, and unclear blinding) performed in China, the United States, Japan, Israel, Turkey, Canada, Germany, and the United Kingdom. Both monotherapy and combination therapy arms included patients receiving both first- and second-generation antipsychotics, with the combination therapy including either oral or long-acting injectable drugs. The most commonly prescribed drugs were clozapine, chlorpromazine, risperidone, and sulpiride. Although the studies had a variety of outcomes, combination therapy was more effective than monotherapy, and more patients dropped out of the monotherapy groups than the combination antipsychotic groups. The combination therapy was more effective when treatment lasted longer than 10 weeks, when one of the antipsychotics was clozapine, and when treatment was initiated simultaneously with both antipsychotics, rather than when a second antipsychotic was added because of a lack of response. In regard to antipsychotic classes, combinations of a first- and second-generation antipsychotic were significantly more effective than monotherapy with either class. It is notable that in the studies conducted in China, clozapine was frequently prescribed in combination with other antipsychotics, and the combinations were initiated at the start of the trial rather than adding the second antipsychotic because of a lack of response to monotherapy.
Essock et al. (19) studied the effectiveness of switching from an antipsychotic combination to monotherapy versus remaining on the combination regimen among 127 patients with schizophrenia and schizoaffective disorder followed for six months. Patients who were randomized to monotherapy discontinued treatment (i.e., switched to another monotherapy agent or returned to an antipsychotic combination) significantly sooner than those who remained on an antipsychotic combination. However, the 69% of study participants who switched to another monotherapy lost weight (0.5 body mass index units on average), and their symptoms and number of hospitalizations were not significantly different from those of the combination group, whereas the patients on combination antipsychotics gained weight.
Foster et al. (20) compared the long-term (30-month) outcomes in time to relapse and clinical measures among patients who were initially on combination antipsychotics (N=50), on long-acting injectable antipsychotic monotherapy (N=20), or on oral antipsychotic monotherapy (N=206), after randomization to either long-acting injectable risperidone or a second-generation oral antipsychotic. The patients in the oral antipsychotic group had significantly fewer hospitalizations than those on combination antipsychotics at baseline (p=0.009). At 30-month follow-up, 68% of patients who were initially on combination antipsychotics had relapsed, whereas only 53% of patients who were initially on a long-acting injectable antipsychotic and 52% of those who were initially on an oral antipsychotic had relapsed. Although a chi-square test (χ2=3.85, df=2, p=0.146) showed no significant difference in the relapse rate among groups, the log-rank test showed a significant difference among the groups in time to first relapse (χ2=6.81, p=0.033), with a significantly longer time to relapse among the oral antipsychotic group (mean=562.8 days) than among the combination antipsychotic group (mean=409.5, p=0.011). In this study, patients who were switched from an antipsychotic combination to monotherapy appeared more vulnerable to relapse.
Next, we present case examples that illustrate the common clinical situations leading clinicians to use an antipsychotic combination and comment on the evidence supporting this practice.
Case example 1: optimizing treatment for a psychosis domain not fully addressed by the initial drug.
Ms. X is a 37-year-old Hispanic woman with a history of schizophrenia with significant thought and behavior disorganization and multiple past inpatient admissions for bizarre behavior (e.g., trying to run through a store window, walking into traffic) motivated by command-type auditory hallucinations. Ms. X is not using birth control, has no known medical conditions, does not use drugs, and drinks alcohol only occasionally. When evaluated by a psychiatrist for a second opinion, she is pleasant and appears cooperative, but she is unable to offer a coherent history because of her loose thought process and bizarre, poorly formed delusions. Family reports that although Ms. X is able to take a shower, dress herself, and do some household chores, she has to be redirected to follow a daily routine. She was partially stabilized on 500 mg/day quetiapine and 50 mg/day sertraline without any notable weight gain or obvious metabolic adverse effect. However, she reports severe constipation from the current medication. She has not had an inpatient admission in the past six months. However, she remains disorganized and incoherent at times, with odd delusions. She cannot drive and is unable to conduct any social engagements without the assistance of her parents.
The consulting psychiatrist suggests clozapine and explains the agranulocytosis monitoring requirements and other possible side effects: seizures, orthostatic hypotension, cardiomyopathy, increased risk of cardiovascular events, and—highly relevant to this case—constipation. The patient and family decide not to pursue clozapine because of the perceived burden of blood draw. They are also reluctant to switch antipsychotics because they perceive that the quetiapine successfully reduced Ms. X’s admissions. The patient and her family choose to add paliperidone to quetiapine to address her thought disorder and hallucinations. The patient also looks forward to eventually taking a lower dose of quetiapine, which may limit its only perceived side effect of chronic constipation.
This case potentially illustrates Kapur et al.’s (21) “kiss and run” hypothesis of quetiapine’s psychopharmacological action. Quetiapine is thought to have a low, apparently subtherapeutic, D2 receptor occupancy 12 hours after administration. However, at four to six hours postadministration, the D2 receptor occupancy measured with positron emission tomography is high, leading to the hypothesis that quetiapine dissociates from receptors faster than other antipsychotics (21). Adding a low-dose antipsychotic with consistently high D2 occupancy may be of utility in this case by addressing two domains of psychosis not fully addressed by the initial drug, namely the delusions and disorganized speech and behavior that continue to limit the patient’s function. In addition, paliperidone has minimal affinity for the muscarinic receptors, whose blockade is implicated in the side effects of constipation from antipsychotic medication (22), thus maximizing treatment of Ms. X’s symptoms without exacerbation of the adverse effects she is currently experiencing. Table 2 (19, 22–24) shows the receptor affinity of commonly used antipsychotics. The greater the number of plus signs, the stronger the affinity at that receptor. Antipsychotic combinations may also aim to optimize nondopaminergic (e.g., serotonergic, glutamatergic, and adrenergic) receptor occupancy to alleviate positive and negative symptoms (13). Adding a second antipsychotic can also address the affective domain of psychosis (depression or mania), insomnia, or aggression toward self or others.
TABLE 2.
Antipsychotics and affinity for neurotransmitter receptors clinically relevant in the treatment of schizophrenia
| Receptora | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Generation and antipsychotic | D1 | D2 | D3 | 5-HT2B | 5-HT2A | 5-HT1A | M1 | H1 | alpha1 |
| Second generation | |||||||||
| Clozapine | + | + | + | +++ | ++ | + | +++ | +++ | +++ |
| Olanzapine | ++ | ++ | ++ | ++ | +++ | ++ | +++ | ++ | |
| Quetiapine | + | + | + | ++ | ++ | + | ++ | +++ | +++ |
| Asenapine | +++ | +++ | +++ | +++ | ++++ | ++ | + | +++ | +++ |
| Zotepine | ++ | +++ | ++ | +++ | + | +++ | +++ | ||
| Risperidone | + | +++ | +++ | ++ | ++++ | + | ++ | +++ | |
| Paliperidone | ++ | +++ | +++ | ++ | +++ | + | ++ | +++ | |
| Ziprasidone | + | +++ | +++ | ++ | ++++ | ++ | ++ | ++ | |
| Iloperidone | + | +++ | ++ | +++ | ++ | ++ | ++++ | ||
| Lurasidone | +++ | +++ | +++ | ++ | |||||
| Aripiprazole | +++ | +++ | ++++ | ++ | +++ | ++ | ++ | ||
| Brexpiprazole | + | ++++ | ++ | ++ | ++++ | ++++ | ++ | ++ | |
| Cariprazine | ++++ | ++++ | ++++ | ++ | +++ | ++ | + | ||
| Sulpiride | ++ | ++ | |||||||
| First generation | |||||||||
| Chlorpromazine | ++++ | ++++ | ++++ | ++++ | ++++ | ||||
| Thioridazine | ++++ | ++++ | ++++ | ++++ | ++++ | ||||
| Perphenazine | ++++ | ++++ | + | +++ | ++ | ||||
| Trifluoperazine | ++++ | +++ | + | ++ | ++ | ||||
| Fluphenazine | ++++ | ++ | + | ++ | + | ||||
| Thiothixene | ++++ | + | + | +++ | ++ | ||||
| Haloperidol | ++++ | ++ | + | + | + | ||||
| Loxapine | +++ | ++++ | ++ | ++++ | +++ | ||||
Current evidence shows that antipsychotic therapeutic action is more likely to occur after at least 60% occupancy of D2 receptors is achieved; however, at 80% occupancy, the movement-associated adverse effects of antipsychotics are thought to begin (25). When using multiple antipsychotics, the likelihood of surpassing the therapeutic window is higher and motor-adverse effects may occur, including extrapyramidal symptoms, tardive dyskinesia, and akathisia. Motor side effects of antipsychotic treatment are largely attributed to dopamine blockade, with contributions from the antipsychotic effect of other neurotransmitters, for example, D2 blockade in the nigrostriatal pathway leading to Parkinsonism; dopamine blockade accompanied by acetylcholine, gamma‑aminobutyric acid, norepinephrine, serotonin, and neuropeptides in akathisia; and dopamine blockade and an increase in acute cholinergic activity in acute dystonia (26–28). Because the motor side effects of antipsychotics are rarely quantified in practice, they cannot be easily identified in the electronic medical record. In the absence of prospective studies addressing the motor side effects of antipsychotic combinations versus monotherapy, researchers have studied treatment with anticholinergic drugs prescribed concomitantly with antipsychotic combinations to generate data on the frequency of motor side effects. Generally, combinations that included first- and second-generation or two second-generation antipsychotics led to increased anticholinergic use, possibly reflecting increased motor side effects (28). It can be argued that anticholinergic medications may be prescribed prophylactically and therefore do not reflect the presence of motor side effects. However, in either case, the addition of another medication with its own side effect profile can be considered to be a negative outcome for the patient (26). Carnahan et al. (26) noted that the low propensity of extrapyramidal side effects found with second-generation antipsychotic monotherapy does not persist when second-generation antipsychotic drugs are combined. Neuroleptic malignant syndrome, which is thought to occur as a result of a marked and sudden reduction in central dopaminergic activity resulting from D2 receptor blockade within the nigrostriatal, hypothalamic, mesolimbic, and mesocortical pathways, has been found to be associated with antipsychotic combinations, although this association has primarily been noted in case reports (23). Currently, tardive dyskinesia and akathisia have not been clearly associated with combination antipsychotics (28).
Dopamine blockade also can lead to a lack of inhibition of prolactin release. This in turn leads to hyperprolactinemia and in some cases galactorrhea, amenorrhea, sexual dysfunction, osteoporosis, and even breast cancer (24). It appears that adding an antipsychotic drug with high D2 blockage potential to a drug with low D2 propensity increases the risk of hyperprolactinemia (25). The apparent exception is the addition of aripiprazole, which has been shown to decrease prolactin levels and is a possible treatment for antipsychotic-induced hyperprolactinemia (24, 29).
Case example 2: perceived difficulties in access and exposure to clozapine.
Mr. Y is a 56-year-old nonsmoking man with schizoaffective disorder who has had multiple depressed psychotic episodes, each leading to two- to three-week-long hospitalizations for paranoia and hostile behavior. He was discharged from his most recent hospitalization on 30 mg/day olanzapine and 40 mg/day fluoxetine. Despite good medication adherence, he was still depressed and at times had passive suicidal thoughts, paranoia, and auditory hallucinations with derogatory content. Eventually, he was switched from fluoxetine to amitriptyline, and his depressive symptoms resolved. He continued to have auditory hallucinations that interfered with his ability to perform activities of daily living and attend his peer-support program. He repeatedly refused clozapine monotherapy in inpatient and outpatient settings because of concerns about blood draw requirements and frequent trips to the pharmacy, even though his case manager offered to arrange home medication delivery. Eventually, Mr. Y’s outpatient psychiatrist offered to add fluphenazine to his regimen to address the hallucinations after a thorough discussion of possible motor side effects. The patient agreed to take fluphenazine and had remission of the hallucinations with 5 mg/day, while his fasting lipids, serum glucose, waist circumference, weight, and onset of involuntary movements were monitored. The patient became able to work 16 hours/week and consistently kept all his clinic appointments. However, he developed involuntary movements of his arms and trunk that, although manageable, did not resolve with further treatment.
Why not consider clozapine?
Patients in both case examples 1 and 2 would have been, according to existing guidelines, good candidates for clozapine. Among eligible patients, clozapine is infrequently prescribed (between 15% in the United States and 54% in the United Kingdom) despite guidelines recommending it after nonresponse to two other antipsychotics (see Figure 1) (30). Reluctance to use clozapine perpetuates the low frequency of clozapine prescriptions and thus prescribers’ and trainees’ limited exposure to patients who remit or recover on clozapine (31). Moreover, the mandatory monitoring of white blood and absolute neutrophil counts compounds prescribers’ reluctance to initiate the drug. This reluctance is further increased by the presence of other potentially serious adverse effects: orthostatic hypotension, bradycardia, syncope, seizure, myocarditis and cardiomyopathy, and increased mortality in elderly patients with dementia-related psychosis. Recent evidence has also suggested that clozapine may cause increased mortality risk among patients with pneumonia (32). Despite these concerns, the effectiveness of clozapine among patients with schizophrenia who are treatment resistant or who experience suicidal ideation remains superior (33, 34). In their 2019 meta-analysis on clozapine and long-term mortality risk, Vermuelen et al. (33) also found that continuous clozapine use has the lowest all-cause mortality risk of any antipsychotic.
Clozapine is a highly effective antipsychotic used for patients with treatment-resistant schizophrenia. However, 40%−60% of patients who meet this criterion (13) will have an incomplete response (35) to clozapine monotherapy. Clozapine in combination with a second antipsychotic currently has the best, although limited, evidence in terms of antipsychotic polypharmacy. Clozapine is commonly used in combination with amisulpride, haloperidol, and sulpiride and is increasingly used with aripiprazole as the augmenting antipsychotic to maximize D2 receptor blockade.
The combination of clozapine and risperidone is possibly the most well studied of the clozapine combination strategies. RCTs comparing clozapine and risperidone with clozapine monotherapy are equivocal (35, 36). Some studies have indicated that combination therapy is more efficacious than clozapine monotherapy for positive and negative symptoms and disorganized thoughts, some have indicated that it is less effective, and some have shown no difference. These mixed findings may indicate that specific populations benefit from the combination therapy, but the criteria to identify those populations have not yet been found.
Sulpiride, amisulpride, and levosulpiride are atypical antipsychotics in the benzamide class that are not approved in the United States, Canada, or Australia but are used in other countries. Clozapine with sulpiride has been reported to improve positive and negative symptoms (37). Sulpiride and risperidone have been found to be equally efficacious in combination with clozapine. In their 2017 review of clozapine combinations for the treatment of schizophrenia, Barber et al. (37) found that amisulpride combined with clozapine is reported to be more efficacious than quetiapine combined with clozapine and noted improvement in the global assessment of function, clinical global impression, and depression, but not psychosis.
The combination of clozapine and aripiprazole shows promise as a way to ameliorate some of the side effects of clozapine. Jeon and Kim (36), in their evaluation of the concerns with antipsychotic polypharmacy and metabolic syndrome (i.e., the state of hyperlipidemia, obesity, hypertension, and glucose intolerance), found two RCTs in which body weight and thus body mass index improved and cholesterol levels decreased, although psychosis did not improve. Jeon and Kim (36) also found open-label trials in which patients showed improved metabolic markers and decreased somnolence. Aripiprazole and clozapine combinations have also been shown to have fewer side effects than clozapine and haloperidol combinations but are similarly efficacious (37). Tiihonen et al. (13) explored the association of antipsychotic polypharmacy versus monotherapy with risk of rehospitalization among persons with schizophrenia and found that the lowest risk was observed with the combination of clozapine and aripiprazole (7%−14% lower than any antipsychotic monotherapy). They also found that patients treated with clozapine and aripiprazole had a better outcome in terms of both psychiatric hospital readmission and all-cause hospitalization than those treated with any other monotherapy or combination of antipsychotics.
Case example 3: minimizing side effects of the initial antipsychotic drug while achieving response or remission.
A 23-year-old man with schizophrenia, cocaine use disorder, and medication nonadherence was admitted for crisis stabilization five times in 12 months for active psychosis, a suicide attempt by an overdose of ibuprofen, and threats to harm others. At the last admission, he was grossly psychotic and threatened to harm his case manager. The patient did not improve after one week of inpatient care on 30 mg/day olanzapine. The inpatient unit team applied for the patient’s transfer to long-term care, and it took two additional weeks for a bed to be secured for him. His new inpatient treatment team, after reviewing the records and history obtained from the patient and his family, proposed starting treatment with a long-acting injectable antipsychotic, potentially olanzapine, because the patient had eventually showed improvement with an oral formulation. However, the patient adamantly refused to take a monthly injection, stating that he did not like needles. The patient also declined clozapine given the need for a weekly blood draw. After approximately four weeks of hospitalization, the patient became calmer and less paranoid, started attending groups, and frequently played basketball in the recreation area. However, he maintained active hallucinations with derogatory content that bothered him, particularly at night. The patient had a family history of diabetes and gained seven pounds on olanzapine. Given his partial response to high-dose olanzapine, recent weight gain, and family history, the psychiatrist recommended adding 5 mg/day aripiprazole, with the intent of addressing the remaining hallucinations and delusions and diminishing the metabolic side effects. After another two weeks of inpatient care, the patient stabilized on olanzapine (decreased to 20 mg/day) and 5 mg/day aripiprazole and was discharged in the care of an assertive community treatment (ACT) team. Subsequently, the patient attended Narcotics Anonymous and the local psychosocial clubhouse program. He played basketball with friend three times a week and lost five pounds. He inquired about scholarship opportunities at the local community college. Given the patient’s marked improvement, the ACT team psychiatrist and patient alike were reluctant to change the patient's medication, although it involved a combination of antipsychotics.
As reflected in this case example, if weight gain or metabolic disturbance is present but psychotic symptoms persist, clinicians may choose to add another antipsychotic with higher D2 receptor affinity and address the persisting symptoms while lowering the dose of the antipsychotic with higher liability of metabolic syndrome. Long-term use of antipsychotics among patients with schizophrenia increases the risk for metabolic syndrome (2, 38). The relationship between antipsychotic drug combinations and metabolic syndrome is complex and likely dependent on multifactorial issues such as multiple receptors, failed glucose homeostasis, and lifestyle factors. The mechanisms for the metabolic side effects of atypical antipsychotics are multiple. Coccurello and Moles (39) found that antipsychotics with the greatest weight gain liability share a high affinity for serotonin (5-HT2A, 5-HT2C, 5-HT6, and 5-HT7), muscarinic (M1, M2, M3, and M5), histamine (H1), adrenergic (alpha1 and alpha2 but also beta3), and dopamine (D1 and D2-like) receptors. Ijaz et al. (2) found insufficient data for the potential harm of antipsychotic polypharmacy, including metabolic syndrome, noting a potential protective effect of antipsychotic combinations that included aripiprazole for dyslipidemia and glucose metabolism compared with other combinations and monotherapy. The finding of a protective role of aripiprazole calls for further investigation. Data are emerging about the effectiveness of aripiprazole added to decrease the metabolic burden of clozapine (34).
The treatment team in case example 3 also had the option to completely cross-titrate a second antipsychotic (in this case, aripiprazole). However, improvement and stabilization while cross-titrating two antipsychotic drugs may result in the patient being kept on both antipsychotics to prevent illness relapse, which could lead to long-term polypharmacy. Although each of the patients in the case examples presented could ideally benefit from taking only one antipsychotic, the clinical and practical considerations in each case make antipsychotic polypharmacy necessary to stabilize the patients and allow them to progress toward recovery.
Other Considerations
Are all dimensions of psychosis being treated?
Among patients with residual symptoms despite a partial response to a single antipsychotic, distress could be caused by symptoms that belong to other domains of psychosis, requiring treatment with an alternative psychotropic agent. Here, we briefly discuss the augmentation of antipsychotics with various other classes of medications.
Antidepressants.
In their systematic review and meta-analysis, Helfer et al. (40) noted that depressive symptoms can occur in all phases of schizophrenia—prodromal, acute, and postpsychotic—and lead to poor outcome, increased relapse, and a higher suicide rate. The prevalence of depression in schizophrenia has been reported to be around 40% (41). On their own, depressive symptoms represent a domain of schizophrenia that can be difficult to disentangle clinically from the negative symptoms with which they overlap significantly in symptomatology, genetics, neurobiology, and neurocognition. Thus, among patients with schizophrenia, treating depressive symptoms is imperative. Helfer et al.’s systematic review of 82 trials suggests that antidepressants added to antipsychotics are efficacious in treating negative and depressive symptoms among persons with schizophrenia, albeit with small effect sizes (40). They also found that antidepressants were safe, with no difference between persons receiving antidepressant-antipsychotic combinations and respective control groups in dropout rates, exacerbation of psychosis, or adverse effects. Helfer et al. also found that selective serotonin reuptake inhibitors as a class—and fluvoxamine and citalopram in particular—showed a particularly beneficial effect for depressive and negative symptoms, and mirtazapine has promise particularly in the treatment of negative symptoms (40). An investigation of the use of other adjunctive psychotropics versus antipsychotic polypharmacy found that adjunctive antidepressant use reduced emergency department visits and hospitalization, even among patients with comorbid substance use disorder (42). In addition, the use of other adjunctive psychotropics versus antipsychotic polypharmacy led to a lower risk of diabetes mellitus and reduced mortality (42). Tiihonen et al. (43), in their examination of psychotropic use among persons with schizophrenia, also found that the concurrent use of antidepressants and antipsychotics was associated with decreased all-cause mortality and decreased suicide deaths when compared with concurrent use of benzodiazepines and antipsychotics.
Mood stabilizers.
A meta-analysis found that divalproex augmentation of antipsychotics among persons with schizophrenia and schizoaffective disorder significantly decreased psychiatric symptoms compared with treatment with antipsychotic monotherapy (44). However, in a subgroup analysis, the significant benefit on psychiatric symptoms was found to persist only in open trials, not in RCTs, and only in studies that lasted four weeks or less (44). Lithium augmentation of antipsychotic drugs has been evaluated in relatively small studies, yielding low-quality evidence. Although patients who received lithium augmentation had significantly better clinical response than patients treated only with antipsychotics, this benefit was not maintained after participants with schizoaffective disorder were excluded or when studies that were not double-blinded were excluded (45, 46). Stroup et al. (42) reported that there was no evidence for the efficacy of mood stabilizers in the treatment of schizophrenia. Moreover, they found that gabapentin was associated with high mortality and noted that it is associated with dizziness, somnolence, increased suicidal behavior, and suicide. As such, they recommended caution in its use. No other mood stabilizer used with this sample (e.g., lithium, divalproex, carbamazepine, lamotrigine) seemed to be associated with higher mortality.
Benzodiazepines.
Reviews by Tiihonen et al. (43) and Stroup et al. (42) found that combinations of antipsychotics and benzodiazepines were associated with higher mortality, postulated to be caused by the use of benzodiazepines at a higher than the daily defined dose. This, in turn, may cause benzodiazepine withdrawal, which could lead to increased mortality. They also postulated that discontinuation of long-term benzodiazepine use may lead to increased anxiety and suicidal behavior. Stroup et al. (42) found that, in addition to their association with the higher rate of mortality in the United States, benzodiazepines were associated with a higher number of emergency department visits and hospitalizations. Stroup et al. (42) found no benefit in the use of adjunct benzodiazepines when compared with the use of adjunctive antipsychotics, antidepressants, or mood stabilizers.
Neuromodulation.
Electroconvulsive therapy (ECT) is one of the oldest and most effective treatments in psychiatry. ECT was originally delivered to patients with schizophrenia and was the most popular treatment for acute psychosis until the introduction of chlorpromazine in 1952 (47). The use of ECT in the Western world later shifted to patients with unipolar and bipolar disorder, although it is still frequently used to treat patients schizophrenia in developing countries (48). ECT can be an effective treatment for acute phases of schizophrenia in patients with catatonia and those with marked positive symptoms or affective symptoms (47, 49). In their recent review of ECT and schizophrenia, Sanghani et al. (47) found growing evidence for the use of ECT as an augmentation strategy for antipsychotics, including evidence that the combination of clozapine and ECT is particularly effective among patients with treatment-resistant schizophrenia or those who do not respond to clozapine (48–50).
A less invasive option is the use of transcranial magnetic stimulation (TMS). A recent meta-analysis supported an effect of low-frequency repetitive TMS (rTMS) applied over the left temporo-parietal region on refractory auditory hallucinations and positive symptoms (51). There was no evidence supporting the use of other rTMS modalities, such as prefrontal or right-side temporo-parietal rTMS, in treatment for auditory hallucinations and positive symptoms. Treatment of negative and cognitive symptoms with this modality did not provide consistent results (51, 52).
Psychosocial intervention.
Each of the current schizophrenia treatment guidelines mentions the importance of psychosocial intervention in treatment response, particularly providing the patient with enough support beyond direct medication management, as illustrated in Figure 1. Interventions such as ACT, cognitive-behavioral therapy, cognitive remediation, family psychoeducation, illness self-management training, social skills training, supported employment, and art therapy have been shown to significantly assist in improving outcomes for those with severe mental illness. Chien et al. (53) found evidence for the efficacy of psychosocial intervention in relieving psychotic symptoms and improving function. However, studies and clinical implementation have been hindered by limited access to and inconsistent applications of psychosocial interventions.
Personalized medicine.
Given the risk of pharmacokinetic and pharmacodynamic drug interactions with polypharmacy, it would be beneficial to have an understanding of how individual patients metabolize drugs before combination therapy is pursued. This becomes increasingly relevant as patients age and may be prescribed nonpsychotropic medication that affects the liver enzymes involved in drug metabolism and overall treatment response. Personalized medicine appeared to hold significant promise in psychiatry in the early 2000s. Research has been translated into practice primarily by understanding cytochrome (CYP) P450 metabolism and thus the side effects of psychiatric drugs, whereas research on candidate genes with potential in predicting drug response has not yet yielded clinical outcomes. CYP450 genes code for liver enzymes contributing to phase 1 of the metabolism of antipsychotics, among many psychiatric drugs (54). Recently, Arranz et al. (55) demonstrated that in antipsychotic treatment among patients who receive pharmacogenetic testing and demonstrate CYP functional variants, careful dosing adjustments lead to a significant improvement in global (p=0.02), psychic (p=0.05), and other side effects (p=0.01) measured with a validated scale. CYP2D6 metabolizes chlorpromazine, haloperidol, perphenazine, and thioridazine, as well as risperidone, paliperidone, iloperidone, and aripiprazole. CYP1A2 metabolizes chlorpromazine, perphenazine, thioridazine, thiothixene, loxapine, and trifluoperazine, as well as clozapine and olanzapine. CYP3A4 metabolizes haloperidol, pimozide, lurasidone, aripiprazole, ziprasidone, and quetiapine. These enzymes are important in the metabolism of antipsychotic drugs, and it is thus important to consider the possibility of drug interactions when two or more antipsychotics are combined (56).
These enzymes are highly polymorphic, and their polymorphisms are thought to lead to distinct phenotypes, which can in turn influence drug response, particularly adverse effects, although factors such as age, ethnicity, drug interactions, active drug metabolites, and medical comorbidities can also influence testing results (57). Combining medications that are metabolized by the same CYP450 enzyme may be problematic, particularly when the enzyme is nonfunctional. Moreover, the Food and Drug Administration–approved prescription information for aripiprazole and risperidone contains guidance on adjusting dosing on the basis of CYP2D6 metabolism. Finally, the literature has recommended “CYP2D6 and CYP2C19 genotyping, preferably combined with therapeutic drug monitoring, for the four second-generation antipsychotics that are dependent on CYP2D6 for their metabolism (aripiprazole, brexpiprazole, iloperidone, and risperidone), clozapine, and atomoxetine” (58–60). Clinically, listening carefully to patients’ report of side effects occurring from low doses of an antipsychotic or, conversely, report of individual ineffective trials of large-dose antipsychotics, can offer clues to a possible CYP450 polymorphism (57).
Conclusions
To conclude, as summarized in Table 3, there is much interest in but limited evidence on concomitant treatment with multiple psychotropics and, in particular, with antipsychotic combinations among patients with schizophrenia. Currently, antipsychotic polypharmacy is used in various clinical situations. Some examples are optimizing treatment for a domain of psychosis not addressed by the initial drug (e.g., although the initial drug may cover thought disorganization or hallucinations, the second antipsychotic may address affective symptoms, insomnia, or aggression); minimizing the side effects of initial antipsychotic for a patient with a partial response (e.g., if metabolic disturbance is present, the second antipsychotic can address the persisting symptoms while allowing a lower dose of the first antipsychotic); perceived difficulties in access and exposure to clozapine; and when improvement during a cross-titration of two antipsychotic drugs may result in keeping the patient on both antipsychotics to prevent destabilization of the illness.
TABLE 3.
Practical aspects of treatment with antipsychotic combinations
| Evidence on antipsychotic combinations’ effectiveness is limited | Managing antipsychotic combinations | Switching back to monotherapy |
|---|---|---|
| Patients maintained on a combination antipsychotics longer than 10 weeks (18–20) | Watch for drug interactions, metabolic syndrome, motor side effects, hyperprolactinemia | (Re)consider clozapine; resolve barriers preventing access |
| Antipsychotic combinations including clozapine (18) | Work closely with primary care to monitor patient | Consider the risk of symptom relapse (19, 20) |
| Treatment simultaneously initiated with 2 antipsychotics (13, 18) | Monitor all domains of illness | |
| Patients with refractory schizophrenia and no practical access to clozapine | Anticipate medication discontinuation (19) |
Concerns remain about the use of antipsychotic combinations. Dissemination and adoption of emerging evidence about the potential benefit of antidepressants and other treatment modalities with lower side effect burden, such as psychosocial interventions and brain stimulation techniques, is still limited. With few exceptions (e.g., emerging data on aripiprazole combinations), patients face a heightened risk of side effects from combination antipsychotics compared with monotherapy. These risks include metabolic syndrome, motor side effects, and prolactinemia, which may ultimately lead to increased morbidity and mortality among this population. The primary reason for death among this population remains cardiac disease. Given the risk of increased medical complications, patients may need to be on multiple nonpsychotropic medications, which can in turn exacerbate the risks of polypharmacy
Thus, when maintaining a patient on antipsychotic, psychiatrists should bear in mind important considerations. Does the patient have refractory schizophrenia, or is the patient on a combination of antipsychotics for another reason? If the schizophrenia is not refractory, is it safe to return to monotherapy, and how can this be done? If the schizophrenia is refractory, can obstacles to the use of clozapine possibly be overcome by using social support, case management, or home visits for a blood draw? If a patient is maintained on an antipsychotic combination, the safest way to monitor for the risks of metabolic syndrome, motor side effects, and hyperprolactinemia could be to proactively integrate patient care with family medicine and specialists to monitor for and treat possible side effects. Moreover, psychiatrists need to consider the patient’s choice of treatment and psychosocial environment and involve the patient’s family, significant others, and caretakers in the choice of treatment.
Footnotes
The authors report no financial relationships with commercial interests.
References
- 1.Schizophrenia. Geneva, World Health Association, 2018. www.who.int/news-room/fact-sheets/detail/schizophrenia. Accessed Jul 10, 2019
- 2.Ijaz S, Bolea B, Davies S, et al. : Antipsychotic polypharmacy and metabolic syndrome in schizophrenia: a review of systematic reviews. BMC Psychiatry 2018; 18:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganguly R, Kotzan JA, Miller LS, et al. : Prevalence, trends, and factors associated with antipsychotic polypharmacy among Medicaid-eligible schizophrenia patients, 1998-2000. J Clin Psychiatry 2004; 65:1377–1388 [DOI] [PubMed] [Google Scholar]
- 4.Kreyenbuhl J, Valenstein M, McCarthy JF, et al. : Long-term combination antipsychotic treatment in VA patients with schizophrenia. Schizophr Res 2006; 84:90–99 [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. Arlington, VA, Guideline Writing Group , 2004 [Google Scholar]
- 6.2017–2018 Florida Best Practice Psychotherapeutic Medication Guidelines for Adults . Tampa,University of South Florida, Florida Medicaid Drug Therapy Management Program for Behavioral Health, 2018 [DOI] [PubMed]
- 7.Moore TA, Buchanan RW, Buckley PF, et al. : The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry 2007; 68:1751–1762 [DOI] [PubMed] [Google Scholar]
- 8.Psychosis and schizophrenia in adults: prevention and management. London, National Institute for Health and Care Excellence, 2014. www.nice.org.uk/guidance/cg178/chapter/1-Recommendations. Accessed Sep 30, 2019 [PubMed]
- 9.Grover S, Chakrabarti S, Kulhara P, et al. : Clinical practice guidelines for management of schizophrenia. Indian J Psychiatry 2017; 59(Suppl 1):S19–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galletly C, Castle D, Dark F, et al. : Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry 2016; 50:410–472 [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. Washington, DC, Guideline Writing Group , 2019 [Google Scholar]
- 12.Elkis H, Buckley PF: Treatment-resistant schizophrenia. Psychiatr Clin North Am 2016; 39:239–265 [DOI] [PubMed] [Google Scholar]
- 13.Tiihonen J, Taipale H, Mehtälä J, et al. : Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry 2019; 76:499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leslie DL, Rosenheck RA: Use of pharmacy data to assess quality of pharmacotherapy for schizophrenia in a national health care system: individual and facility predictors. Med Care 2001; 39:923–933 [DOI] [PubMed] [Google Scholar]
- 15.Jaffe AB, Levine J: Antipsychotic medication coprescribing in a large state hospital system. Pharmacoepidemiol Drug Saf 2003; 12:41–48 [DOI] [PubMed] [Google Scholar]
- 16.Humberstone V, Wheeler A, Lambert T: An audit of outpatient antipsychotic usage in the three health sectors of Auckland, New Zealand. Aust N Z J Psychiatry 2004; 38:240–245 [DOI] [PubMed] [Google Scholar]
- 17.Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA, American Psychiatric Publishing, 2013 [Google Scholar]
- 18.Correll CU, Rummel-Kluge C, Corves C, et al. : Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull 2009; 35:443–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Essock SM, Schooler NR, Stroup TS, et al. : Schizophrenia Trials Network: effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry 2011; 168:702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster A, Buckley P, Lauriello J, et al. : Combination antipsychotic therapies: an analysis from a longitudinal pragmatic trial. J Clin Psychopharmacol 2017; 37:595–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapur S, Zipursky R, Jones C, et al. : A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry 2000; 57:553–559 [DOI] [PubMed] [Google Scholar]
- 22.Stahl SM: Essential Psychopharmacology Online. Cambridge, UK, Cambridge University Press, 2018. https://stahlonline.cambridge.org/essential_4th.jsf
- 23.Langan J, Martin D, Shajahan P, et al. : Antipsychotic dose escalation as a trigger for neuroleptic malignant syndrome (NMS): literature review and case series report. BMC Psychiatry 2012; 12:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Tang Y, Wang C: Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One 2013; 8:e70179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCutcheon RA, Reis Marques T, Howes OD: Schizophrenia—an overview. JAMA Psychiatry 2019; 77:1–10 [DOI] [PubMed] [Google Scholar]
- 26.Carnahan RM, Lund BC, Perry PJ, et al. : Increased risk of extrapyramidal side-effect treatment associated with atypical antipsychotic polytherapy. Acta Psychiatr Scand 2006; 113:135–141 [DOI] [PubMed] [Google Scholar]
- 27.Rupniak NM, Jenner P, Marsden CD: Acute dystonia induced by neuroleptic drugs. Psychopharmacology (Berl) 1986; 88:403–419 [DOI] [PubMed] [Google Scholar]
- 28.Gallego JA, Nielsen J, De Hert M, et al. : Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf 2012; 11:527–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peuskens J, Pani L, Detraux J, et al. : The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs 2014; 28:421–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kar N, Barreto S, Chandavarkar R. Clozapine monitoring in clinical practice: beyond the mandatory requirement. Clin Psychopharmacol Neurosci 2016;14(4):323-329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen J, Dahm M, Lublin H, et al. : Psychiatrists’ attitude towards and knowledge of clozapine treatment. J Psychopharmacol 2010; 24:965–971 [DOI] [PubMed] [Google Scholar]
- 32.De Leon J, Sanz EJ, De Las Cuevas C: Data from the World Health Organization’s Pharmacovigilance Database supports the prominent role of pneumonia in mortality associated with clozapine adverse drug reactions. Schizophr Bull 2020; 46:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermeulen JM, van Rooijen G, van de Kerkhof MPJ, et al. : Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1-12.5 years. Schizophr Bull 2019; 45:315–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes TR, Paton C: Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs 2011; 25:383–399 [DOI] [PubMed] [Google Scholar]
- 35.Buckley PF, Gaughran F: Treatment-Refractory Schizophrenia: A Clinical Conundrum, 2014 ed. Berlin, Heidelberg: Springer; 2014 [Google Scholar]
- 36.Jeon SW, Kim YK: Unresolved issues for utilization of atypical antipsychotics in schizophrenia: antipsychotic polypharmacy and metabolic syndrome. Int J Mol Sci 2017; 18:2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barber S, Olotu U, Corsi M, et al. Clozapine combined with different antipsychotic drugs for treatment‐resistant schizophrenia. Cochrane Database of Systematic Reviews. 3:CD006324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rojo LE, Gaspar PA, Silva H, et al. : Metabolic syndrome and obesity among users of second generation antipsychotics: a global challenge for modern psychopharmacology. Pharmacol Res 2015; 101:74–85 [DOI] [PubMed] [Google Scholar]
- 39.Coccurello R, Moles A: Potential mechanisms of atypical antipsychotic-induced metabolic derangement: clues for understanding obesity and novel drug design. Pharmacol Ther 2010; 127:210–251 [DOI] [PubMed] [Google Scholar]
- 40.Helfer B, Samara MT, Huhn M, et al. : Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am J Psychiatry 2016; 173:876–886 [DOI] [PubMed] [Google Scholar]
- 41.Upthegrove R, Marwaha S, Birchwood M: Depression and schizophrenia: cause, consequence, or trans-diagnostic issue? Schizophr Bull 2017; 43:240–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stroup TS, Gerhard T, Crystal S, et al. : Comparative effectiveness of adjunctive psychotropic medications in patients with schizophrenia. JAMA Psychiatry 2019; 76:508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tiihonen J, Suokas JT, Suvisaari JM, et al. : Polypharmacy with antipsychotics, antidepressants, or benzodiazepines and mortality in schizophrenia. Arch Gen Psychiatry 2012; 69:476–483 [DOI] [PubMed] [Google Scholar]
- 44.Tseng PT, Chen YW, Chung W, et al. : Significant effect of valproate augmentation therapy in patients with schizophrenia: a meta-analysis study. Medicine (Baltimore) 2016; 95:e2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Citrome L: Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother 2009; 9:55–71 [DOI] [PubMed] [Google Scholar]
- 46.Leucht S, Helfer B, Dold M, et al. : Lithium for schizophrenia. Cochrane Database Syst Rev 2015; 10:CD003834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanghani SN, Petrides G, Kellner CH: Electroconvulsive therapy (ECT) in schizophrenia: a review of recent literature. Curr Opin Psychiatry 2018; 31:213–222 [DOI] [PubMed] [Google Scholar]
- 48.Grover S, Hazari N, Kate N: Combined use of clozapine and ECT: a review. Acta Neuropsychiatr 2015; 27:131–142 [DOI] [PubMed] [Google Scholar]
- 49.Sadock BJ, Sadock VA, Ruiz P: Electroconvulsive therapy; in Kaplan and Sadock’s Synopsis of Psychiatry, 11th ed. Edited by Pataki C, Sussman N. Philadelphia, Wolters Kluwer, 2014 [Google Scholar]
- 50.Kho KH, Blansjaar BA, de Vries S, et al. : Electroconvulsive therapy for the treatment of clozapine nonresponders suffering from schizophrenia—an open label study. Eur Arch Psychiatry Clin Neurosci 2004; 254:372–379 [DOI] [PubMed] [Google Scholar]
- 51.Guo Q, Li C, Wang J: Updated review on the clinical use of repetitive transcranial magnetic stimulation in psychiatric disorders. Neurosci Bull 2017; 33:747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenquist PB, Ahmed AO, McCall WV: Therapeutic brain stimulation in treatment-resistant schizophrenia; in Treatment-Refractory Schizophrenia: A Clinical Conundrum. Edited by Buckley PF, Gaughran F. New York, Springer, 2014 [Google Scholar]
- 53.Chien WT, Leung SF, Yeung FK, et al. : Current approaches to treatments for schizophrenia spectrum disorders, part II: psychosocial interventions and patient-focused perspectives in psychiatric care. Neuropsychiatr Dis Treat 2013; 9:1463–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fleeman N, Dundar Y, Dickson R, et al. : Cytochrome P450 testing for prescribing antipsychotics in adults with schizophrenia: systematic review and meta-analyses. Pharmacogenomics J 2011; 11:1–14 [DOI] [PubMed] [Google Scholar]
- 55.Arranz MJ, Gonzalez-Rodriguez A, Perez-Blanco J, et al. : A pharmacogenetic intervention for the improvement of the safety profile of antipsychotic treatments. Transl Psychiatry 2019; 9:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pouget JG, Shams TA, Tiwari AK, et al. : Pharmacogenetics and outcome with antipsychotic drugs. Dialogues Clin Neurosci 2014; 16:555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Foster A, Wang Z, Usman M, et al. : Pharmacogenetics of antipsychotic adverse effects: case studies and a literature review for clinicians. Neuropsychiatr Dis Treat 2007; 3:965–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chopra N, Ruan C, McCollum B, et al. : High doses of drugs extensively metabolized by CYP3A4 were needed to reach therapeutic concentrations in two patients taking inducers. Rev Colomb Psiquiatr 2020; 49:84–95 [DOI] [PubMed] [Google Scholar]
- 59.Swen JJ, van der Straaten T, Wessels JAM, et al. : Feasibility of pharmacy-initiated pharmacogenetic screening for CYP2D6 and CYP2C19. Eur J Clin Pharmacol 2012; 68:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spina E, Hiemke C, de Leon J: Assessing drug-drug interactions through therapeutic drug monitoring when administering oral second-generation antipsychotics. Expert Opin Drug Metab Toxicol 2016; 12:407–422 [DOI] [PubMed] [Google Scholar]


