Abstract
OBJECTIVES
This study aims to understand the complex factors affecting heart transplant survival and to determine the importance of possible sex-specific risk factors.
BACKGROUND
Heart transplant allocation is primarily focused on preventing waitlist mortality. To prevent organ wastage, future allocation must balance risk of waitlist mortality with post-transplantation mortality. However, more information regarding risk factors after heart transplantation is needed.
METHODS
We included all adults (30,606) in the Scientific Registry of Transplant Recipients database who underwent isolated heart transplantation from January 1, 2004, to July 1, 2018. Mortality (8,278 deaths) was verified with the complete Social Security Death Index with a median follow-up of 3.9 years. Temporal decomposition was used to identify phases of survival and phase-specific risk factors. The random survival forests method was used to determine importance of mortality risk factors and their interactions.
RESULTS
We identified 3 phases of mortality risk: early post-transplantation, constant, and late. Sex was not a significant risk factor. There were several interactions predicting early mortality such as pretransplantation mechanical ventilation with presence of end-organ function (bilirubin, renal function) and interactions predicting later mortality such as diabetes and older age (donor and recipient). More complex interactions predicting early-, mid-, and late-mortality existed and were identified with machine learning (i.e., elevated bilirubin, mechanical ventilation, and dialysis).
CONCLUSIONS
Post-heart transplant mortality risk is complex and dynamic, changing with time and events. Sex is not an important mortality risk factor. To prevent organ wastage, end-organ dysfunction should be resolved before transplantation as much as possible. (J Am Coll Cardiol HF 2020;■: ■-■) © 2020 by the American College of Cardiology Foundation.
Keywords: heart transplantation, mechanical circulatory support, mortality, outcome assessment, sex
The new heart transplant allocation system provides broader sharing of donors and more granular distinction of medical urgency with an increase from 3 to 6 tiers (1). Although distribution of organs is now fairer with lower waitlist mortality, a recent analysis showed worse post-transplantation survival (78% survival 6 months) compared with 1-year survival of 91% and 3-year survival 84% under the previous allocation system (2,3). To reduce post-transplantation mortality, the factors affecting post-transplantation survival must be better understood. Although many variables associated with post-transplantation survival have been identified (4–6), the relationships among these variables (interactions) has not been explored. Interactions among risk factors are likely complex and may be sex-specific given our finding of more than 20 sex interactions predicting heart transplant waitlist mortality (7). In addition, post-transplantation survival is known to be non-proportional, making conventional statistical methods such as Cox proportional hazards regression and multivariable logistic regression inadequate for assessing post-transplantation mortality among heart transplant recipients. We used both conventional statistical methods (non-proportional hazards parametric model) and machine-learning analyses (random survival forests) to better understand the relationships among variables associated with post-transplantation survival to determine if prognostic risk factors are sex specific.
METHODS
POPULATION.
All adults in the Scientific Registry of Transplant Recipients (SRTR) who underwent heart transplantation in the United States between January 1, 2004, and July 1, 2018, were included in this analysis. We excluded recipients of multi-organ transplants (n = 1,527) and recipients <18 years of age at transplant (n = 5,434) because allocation criteria are different for multi-organ and pediatric candidates (8). The final cohort had 30,606 adult heart transplants.
DATABASE.
The SRTR includes data on all donors, wait-listed candidates, and transplant recipients in the United States submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration of the U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. Human error in data entry is minimized by error checks at time of data entry and internal verification of outliers. The study was approved by the Cleveland Clinic Institutional Review Board and informed consent was waived because data was de-identified by SRTR before their transmission to the investigators.
ENDPOINTS.
The primary endpoint was all-cause mortality assessed as time from heart transplantation to death or end of follow-up. SRTR mortality data were verified with the complete Social Security Death Master File made available through a waiver. During a median follow-up of 3.9 years, 50% of patients were followed beyond 4.1 years, 25% beyond 7.9 years, and 10% beyond 11 years.
DATA.
Baseline characteristics for women and men were reported at time of heart transplantation (Supplemental Table 1). Continuous variables are expressed as medians, accompanied by 25th and 75th (Q1, Q3) percentiles. Categorical variables are expressed as number of patients and frequency. All variables had a low level of missingness (<10%) except for HLA mismatch (10%), recipient pulmonary capillary wedge pressure (11%), recipient hepatitis B virus core antibody (11%), recipient median house income (13%), and recipient Epstein-Barr virus serology (15%). Missing data were imputed using multiple imputation by chained equations for parametric survival models (9) and missForest for the random forest analysis (10).
ANALYTICAL STRATEGY.
Two complimentary time-related analyses were performed, each with different strengths in meeting aims of this study. Both were directed at identifying risk factors for death after transplantation that may change across follow-up time based on risk factor strength and importance, and both methods were used in different ways to identify interactions among variables. The first method used a completely parametric non-proportional hazards model that addressed these risk factor changes by temporal decomposition (11). We used this model to explore interactions with sex. The second method was a completely non-parametric non-proportional hazards random survival forests (RSF) model that addressed changing importance of risk factors in predicting mortality at particular times following transplantation (12–14). We used this model to explore all evidences of interactions among variables with no presumptions.
Temporal decomposition method.
Three hazard components that, added together, constituted the overall instantaneous risk of mortality (hazard function) were designated as: 1) early hazard phase describing procedural risk; 2) constant hazard phase describing underlying risk; and 3) late-accelerated hazard phase. Each hazard phase was simultaneously modulated by risk factors.
In the focus on sex interactions, 2 parsimonious models were constructed by machine learning variable selection (15) using variables identified in Supplemental Table 1. One model was for females and another for males (Supplemental Methods). All variables appearing in either model were incorporated into an overall model plus their pairwise interaction with sex.
Non-parametric machine learning model.
RandomForestSRC software (14) was used to identify important predictors of mortality using 92 variables (Supplemental Table 1, Supplemental Methods). A total of 500 survival trees were grown under log-rank splitting. For each tree, 10 independent variables were selected at random as candidates to split a tree node.
Data-driven interaction detection used quantile regression (Supplemental Methods). Because of non-proportional hazards, interactions were identified at 4 preset times: 90 days, 1 year, 5 years, and 10 years. We investigated for each variable all possible interactions, and report the top 15 highly predictive interactions using the method of holdout variable importance (16).
RESULTS
RECIPIENT CHARACTERISTICS BY SEX.
The cohort consisted of 7,778 women and 22,828 men, with the majority of patients being white, older than 50 years of age, blood type A or O, privately insured, and transplanted urgently with a dilated cardiomyopathy (Table 1, Supplemental Table 2). Inotropes were more frequently used in women and mechanical circulatory support more frequently in men. Women were slightly younger than men, had a higher frequency of black patients, dilated cardiomyopathy, and Medicaid insurance, smaller body surface area, less history of prior cardiac surgery, and slightly worse renal function when compared to men. There were minimal sex differences in recipient hemodynamics, ischemic time, and donor age. Most donor deaths were from head trauma; hearts from donors dying from hypoxia or stroke were more frequently transplanted into female recipients. Recipient-donor predicted total heart mass differences were notable for oversizing hearts in transplanted women and under-sizing them in men.
Table 1.
Baseline Characteristics of Adult Heart Transplantations From January 1, 2004, to July 1, 2018 Total (N ¼ 30,606) Female Recipient (n ¼ 7,778) Male
| Total (N = 30,606) | Female Recipient (n = 7,778) | Male Recipient (n = 22,828) | |
|---|---|---|---|
| Age (yrs) | 56 (46, 62) | 53 (40, 60) | 56 (47, 63) |
| Race | |||
| White | 20,601 (67) | 4,777 (61) | 15,824 (69) |
| Black | 6,225 (20) | 2,049 (26) | 4,176 (18) |
| Hispanic | 2,456 (8) | 631 (8) | 1,825 (8) |
| Asian | 975 (3) | 214 (3) | 761 (3) |
| Other | 349 (1) | 107 (1) | 242 (1) |
| BMI (kg/m2) | 27 (24, 30) | 26 (22, 30) | 27 (24, 31) |
| Insurance at OHT | |||
| Private | 15,459 (51) | 4,020 (52) | 11,439 (50) |
| Medicare | 10,241 (34) | 2,324 (30) | 7,917 (35) |
| Medicaid | 3,707 (12) | 1,185 (15) | 2,522 (11) |
| Other | 1,140 (4) | 227 (3) | 913 (4) |
| ABO blood type | |||
| A | 12,469 (41) | 3,066 (39) | 9,403 (41) |
| B | 4,499 (15) | 1,150 (15) | 3,349 (15) |
| O | 11,942 (39) | 3,158 (41) | 8,784 (38) |
| AB | 1,696 (5.5) | 404 (5.2) | 1,292 (5.7) |
| Diagnosis | |||
| Dilated | 15,334 (50) | 4,890 (63) | 10,444 (46) |
| Ischemic | 11,680 (38) | 1,535 (20) | 10,145 (45) |
| Congenital | 1,014 (3.3) | 393 (5.1) | 621 (2.7) |
| Hypertrophic | 747 (2.4) | 338 (4.4) | 409 (1.8) |
| Restrictive | 948 (3.1) | 333 (4.3) | 615 (2.7) |
| Valvular | 524 (1.7) | 165 (2.1) | 359 (1.6) |
| Other | 311 (1.0) | 109 (1.4) | 202 (0.89) |
| Diabetes mellitus | 8,314 (27) | 1,723 (22) | 6,591 (29) |
| Dialysis | 1,154 (4) | 259 (3) | 895 (4) |
| Prior surgery (non-OHT) | 7,403 (25) | 1,475 (20) | 5,928 (27) |
| Infection with intravenous antibiotics | 3,135 (10) | 719 (10) | 2,416 (11) |
| Ventilator at OHT | 520 (1.7) | 149 (1.9) | 371 (1.6) |
| Inotrope at OHT | 11,917 (39) | 3,346 (43) | 8,571 (38) |
| VAD at OHT | |||
| LVAD | 10,407 (34) | 1,945 (25) | 8,462 (37) |
| RVAD ±LVAD | 913 (3.0) | 234 (3.0) | 679 (3.0) |
| MCS unspecified | 708 (2.3) | 158 (2.0) | 550 (2.4) |
| TAH | 343 (1.1) | 44 (0.57) | 299 (1.3) |
| ECMO | 227 (0.74) | 77 (0.99) | 150 (0.66) |
| IABP | 1,851 (6.0) | 441 (5.7) | 1,410 (6.2) |
| Mean PAP (mm Hg) | 27 (20, 34) | 26 (20, 33) | 27 (20, 35) |
| PCWP (mm Hg) | 18(12, 24) | 17(11, 23) | 18(12, 25) |
| Cardiac index (l/min/m2) | 2.2 (1.8, 2.6) | 2.2 (1.7, 2.6) | 2.2 (1.8, 2.7) |
| eGFR ml/min/1.73 m2 | 66 (49, 85) | 64 (47, 85) | 66 (50, 85) |
| Total bilirubin mg/dl | 0.80 (0.50,1.20) | 0.70 (0.40,1.10) | 0.80 (0.50,1.20) |
| Donor age (yrs) | 30 (22, 41) | 30 (21, 42) | 30 (22, 40) |
| Donor BMI (kg/m2) | 26 (23, 30) | 25 (22, 29) | 27 (24, 30) |
| Donor female | 8,900 (29) | 4,465 (57) | 4,435 (19) |
| Donor diabetes | 992 (3.3) | 282 (3.6) | 710 (3.1) |
| Donor hypertension | 4,400 (14) | 1,123 (15) | 3,277 (14) |
| Donor cause of death | |||
| Anoxia | 6,932 (23) | 2,009 (26) | 4,923 (22) |
| CVA | 6,237 (20) | 1,941 (25) | 4,296 (19) |
| Head trauma | 16,594 (54) | 3,593 (46) | 13,001 (57) |
| CNS tumor | 208 (0.68) | 54 (0.69) | 154 (0.67) |
| Other | 629 (2.1) | 177 (2.3) | 452 (2.0) |
| Ischemic time (h) | 3.2 (2.4, 3.8) | 3.2 (2.4, 3.8) | 3.2 (2.4, 3.8) |
| Predicted total heart mass (pHM*) | −0.86 (−13.0, 8.7) | −14 (−30.0, −0.92) | 2.0 (−7.9, 10.8) |
Values are n (%) or median (Q1, Q3), unless otherwise stated. *Predicted total heart mass (pHM) = [(pHM(recipient) - pHM(donor))/(pHM(recipient))]*100.
BMI = body mass index; CNS = central nervous system; CVA = cerebrovascular accident; ECMO = extracorporeal membrane oxygenation; eGFR = estimated glomerular filtration rate; IABP = intra-aortic balloon pump; LVAD = left ventricular assist device; MCS = mechanical circulatory support; OHT = orthotopic heart transplantation; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; RVAD = right ventricular assist device; TAH = total artificial heart; VAD = ventricular assist device
OVERALL SURVIVAL.
Overall, 8,278 patients died (2,020 females and 6,258 males) during the study period from January 1, 2004, to July 1, 2018 (Figure 1). One-month unadjusted survival for the cohort was 96% (96% women, 96% men), 1-year survival 89% (89% women, 90% men), and 12-year survival 50% (53% women, 50% men). Temporal decomposition analysis identified 3 hazard phases for mortality post-transplantation: early-, constant-, and late-phase (Central Illustration). In the early phase, risk of death was greatest during the first week post-heart transplantation and declined over 1 month with no significant sex-differences. The constant phase was nearly 4% mortality per year with no significant sex-differences. In the late phase, risk of death increased with time with women having better survival than men (Central Illustration).
FIGURE 1. Sex Specific Heart Transplantation Survival.
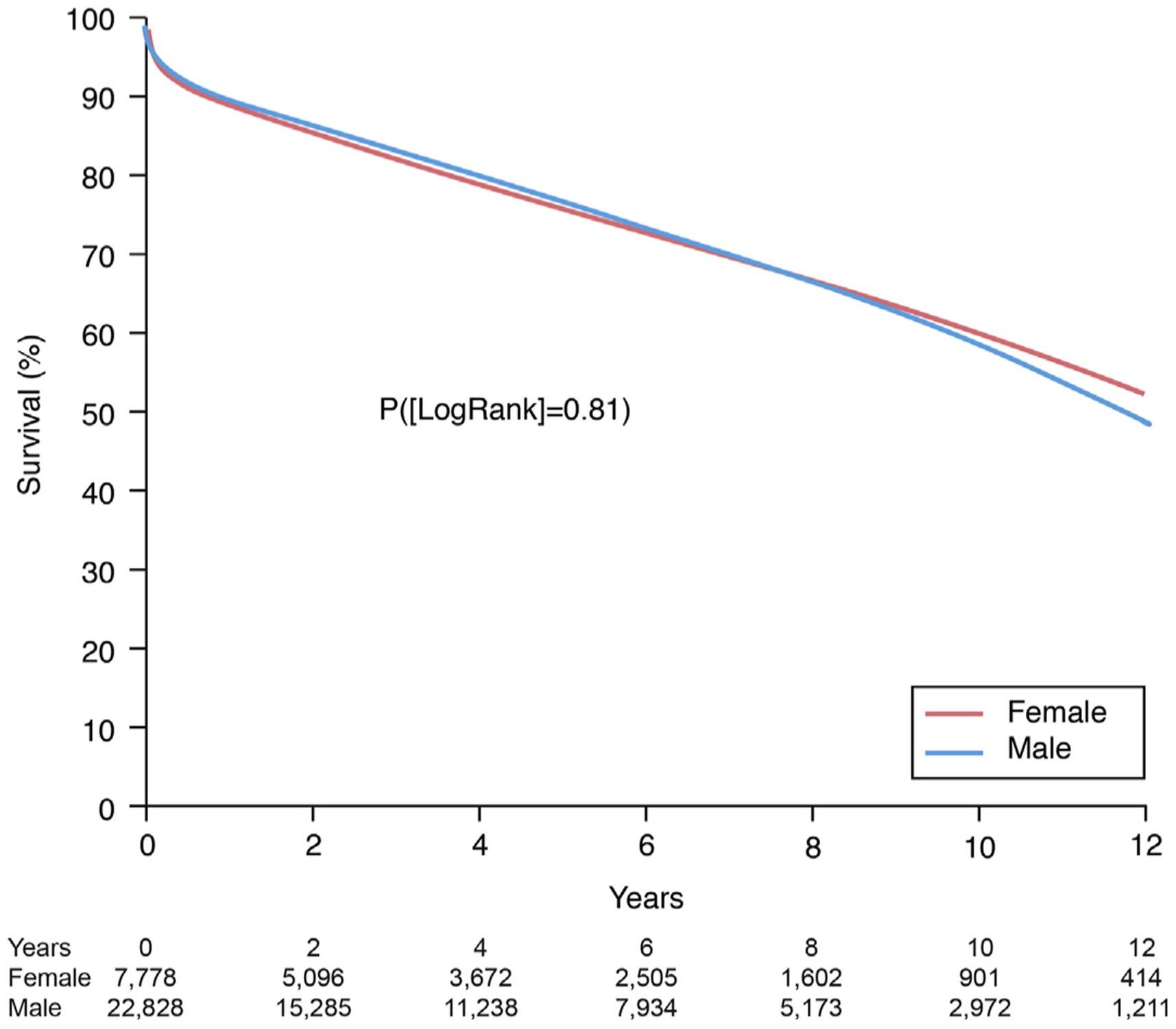
Survival curves were generated parametrically in a multiphase model for patients who underwent heart transplant from January 1, 2004, to June 30, 2018.
CENTRAL ILLUSTRATION.
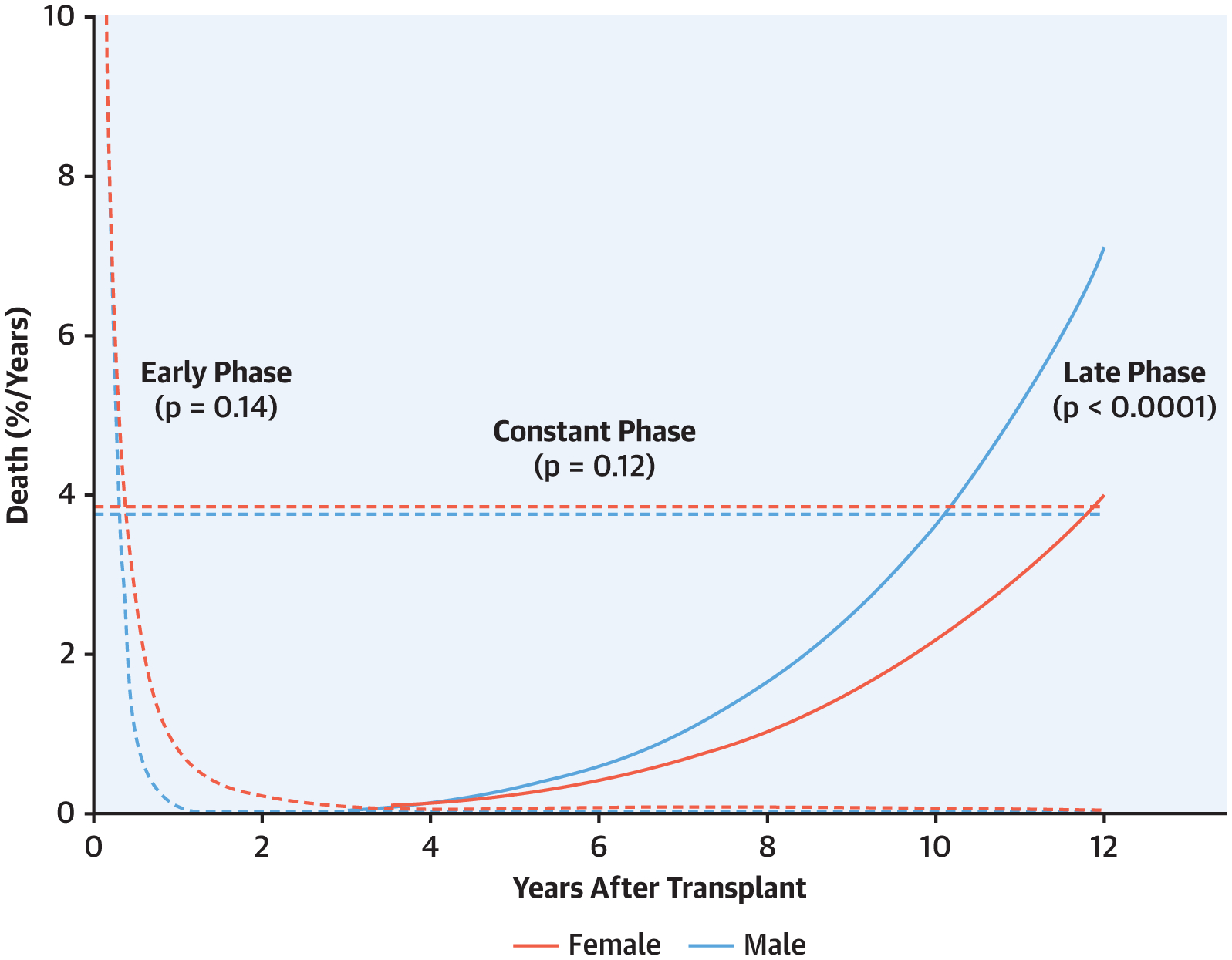
4% mortality per year and was mainly associated with non-modifiable factors such as age, race, and socioeconomic factors difficult to change. Finally, a late phase (solid lines) was notable for sex differences in long-term survival mainly due to diabetes mellitus, obesity, age and potential transplantation complications.
RISK FACTORS FOR POST-TRANSPLANTATION MORTALITY. Parametric modeling.
The most significant risk factors for mortality varied for each hazard phase (Table 2). For the early phase, these included renal function, hepatic function, congenital heart disease, extracorporeal membrane oxygenation, durable mechanical circulatory support before 2008, and mechanical ventilation at time of transplantation. For the constant phase, the most significant associations included recipient age, recipient black race, ischemic cardiomyopathy, and Medicaid insurance. For the late phase, the most significant associations were recipient age, larger recipient body surface area, and history of diabetes.
TABLE 2.
Overall: Multivariable Analysis on Risk Factors for Death
| Coefficient ± SE | p Value | Reliability (%)* | |
|---|---|---|---|
| Early phase | |||
| Older age† | 0.39 ± 0.05 | <0.0001 | 100 |
| Higher BMI‡ | 0.39 ± 0.06 | <0.0001 | 100 |
| Congenital disease | 1.06 ± 0.10 | <0.0001 | 100 |
| Bilirubin | 100 | ||
| Higher bilirubin§ | 0.56 ± 0.06 | <0.0001 | |
| Lower bilirubin∥ | 0.17 ± 0.04 | 0.001 | |
| Prior cardiac surgery | 0.26 ± 0.05 | 0.0005 | 98 |
| ECMO | 0.86 ± 0.14 | <0.0001 | 99 |
| Ventilation | 0.82 ± 0.11 | <0.0001 | 100 |
| LVAD/RVAD before 2008 | 0.59 ± 0.10 | <0.0001 | 99 |
| Longer ischemic time¶ | 0.02 ± 0.002 | <0.0001 | 100 |
| Multiple heart transplants | 0.31 ± 0.12 | 0.012 | 69 |
| GFR/dialysis interaction | |||
| Dialysis | −1.90 ± 0.28 | <0.0001 | 100 |
| GFR# | 0.04 ± 0.006 | <0.0001 | 100 |
| GFR** | −0.65 ± 0.07 | <0.0001 | 100 |
| Older donor†† | 0.10 ± 0.02 | 0.0001 | 99 |
| Constant phase | |||
| Young age‡‡ | 0.54 ± 0.05 | <0.0001 | 59 |
| Race black | 0.51 ± 0.04 | <0.0001 | 100 |
| Ischemic cardiomyopathy | 0.44 ± 0.04 | <0.0001 | 100 |
| Infection requiring intravenous drugs | 0.25 ± 0.06 | <0.0001 | 97 |
| Medicaid | 0.33 ± 0.05 | <0.0001 | 58 |
| Older donor | 0.01 ± 0.001 | <0.0001 | 100 |
| Late phase | |||
| Older age§§ | 1.45 ± 0.12 | <0.0001 | 100 |
| Larger BSA∥∥ | 0.10 ± 0.03 | 0.0004 | 63 |
| Diabetes | 0.70 ± 0.07 | <0.0001 | 98 |
Reliability: number of occurrences out of 1,000 bootstrap models.
Exp(age/50), exponential transformation.
(BMI/25)2, squared transformation.
(LOG[Bilirubin]), logarithmic transformation.
([1/Bilirubin]), inverse transformation.
(Ischemic time)2, squared transformation.
(GFR/65)2, squared transformation.
(LOG[GFR]), logarithmic transformation.
Exp(donor age/35), exponential transformation.
(50/age), inverse transformation.
(age/50)2, squared transformation.
(BSA)2, squared transformation.
BSA = body surface area; GFR = glomerular filtration rate: other abbreviations as in Table 1.
Non-parametric modeling.
Table 3 shows variables of importance for predicting 90-day, 1-year, 5-year, and 10-year survival identified by RSF. Variables such as age (donor and recipient), recipient body mass index, history of dilated cardiomyopathy, renal function, total bilirubin, and ischemic time were highly predictive of survival across all intervals. Other variables were predictive of survival for certain intervals such as height (donor and recipient), predicted heart mass, year of transplantation, mechanical ventilation and extracorporeal membrane oxygenation (ECMO) affecting early survival (survival ≤1 year) whereas race (especially black), private insurance, diabetes, and history of ischemic cardiomyopathy importantly affected long-term survival. Infection at time of transplantation requiring intravenous antibiotics was an important risk factor for 1- and 5-year survival.
TABLE 3.
Random Survival Forests Identification of Top 15 Risk Factors for Mortality
| 90 Days |
1 Year |
5 Years |
10 Years |
|
|---|---|---|---|---|
| Recipient | ||||
| Age | + | + | + | + |
| Height | + | + | ||
| Weight | + | + | + | |
| BMI | + | + | + | + |
| BSA | + | |||
| Predicted heart mass difference | + | |||
| White race | + | |||
| Black race | + | + | ||
| Medicare insurance | ||||
| Private insurance | + | + | + | |
| Congenital heart disease | ||||
| Dilated cardiomyopathy | + | + | + | + |
| Ischemic cardiomyopathy | + | |||
| Infection with intravenous antibiotics | + | + | ||
| Diabetes mellitus | + | + | ||
| eGFR (non-dialysis) | + | + | + | + |
| Dialysis | + | + | + | + |
| Total bilirubin | + | + | + | + |
| Mechanical ventilation | + | + | + | |
| ECMO | + | + | ||
| Donor | ||||
| Age | + | + | + | + |
| Donor height | + | + | ||
| Transplant | ||||
| Year | + | + | + | |
| Ischemic time | + | + | + | + |
Sex-specific risk factors.
Sex was not a significant risk factor for mortality (Table 2). Upon formal investigation for sex interactions, few were found to be significant for post-transplantation mortality (Figure 2, Supplemental Table 3). Sex interactions were found in the early-hazard phase of mortality with bilirubin, in the constant-hazard phase with larger body mass index and Medicaid, and in the late-hazard phase with larger body mass index and donor death due to brain tumor.
FIGURE 2. Heart Transplantation Risk Factors With Sex Interactions.
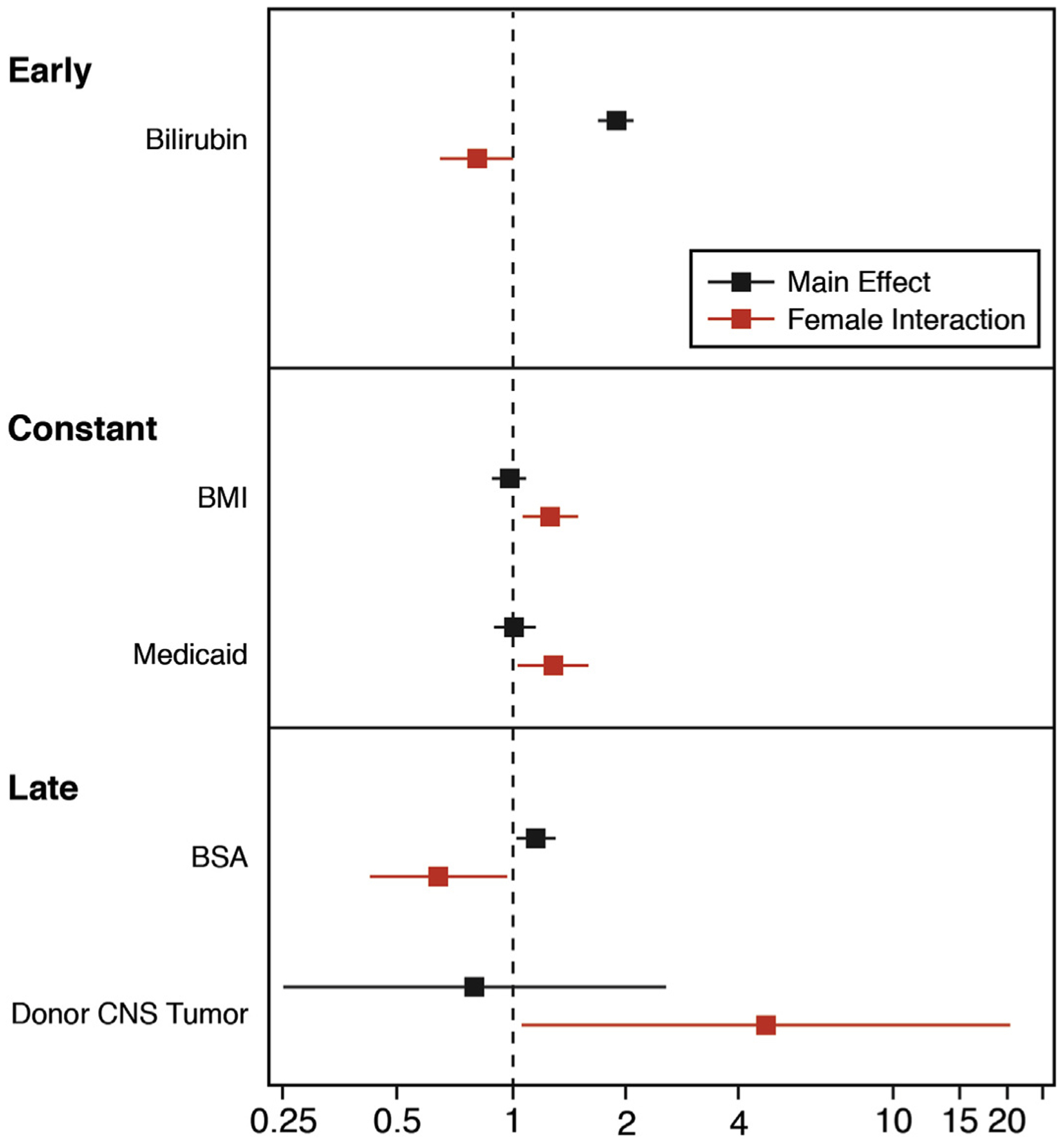
The cohort included all patients who underwent heart transplant from January 1, 2004 to June 30, 2018. Variables significant for either men or women were included in a final model and only those with sex interaction were included in the figure. The main effect and interaction are shown for early, constant, and late risk for post-transplant survival. The dashed vertical line is showing a hazard ratio of 1 (no effect). Sex interactions are identified in red. BMI = body mass index; BSA = body surface area; CNS = central nervous system.
Most important interactions.
At 90 days post-transplantation (Figure 3), the most important factors affecting survival were the presence of mechanical ventilation while undergoing dialysis, mechanical ventilation in the presence of abnormal total bilirubin, and abnormal total bilirubin when combined with abnormal renal function. For 1-year survival, the most important interactions were between ischemic time and total bilirubin, and ischemic time and estimated glomerular filtration rate. Mechanical ventilation and ECMO were also important factors. For 5-year survival, the most important interaction was between total bilirubin and recipient age. For 10-year survival, the most important interactions were between donor age and recipient age, total bilirubin and recipient age, and donor age and total bilirubin.
FIGURE 3. Interactions Among Variables of Importance Predicting Heart Transplantation Survival.
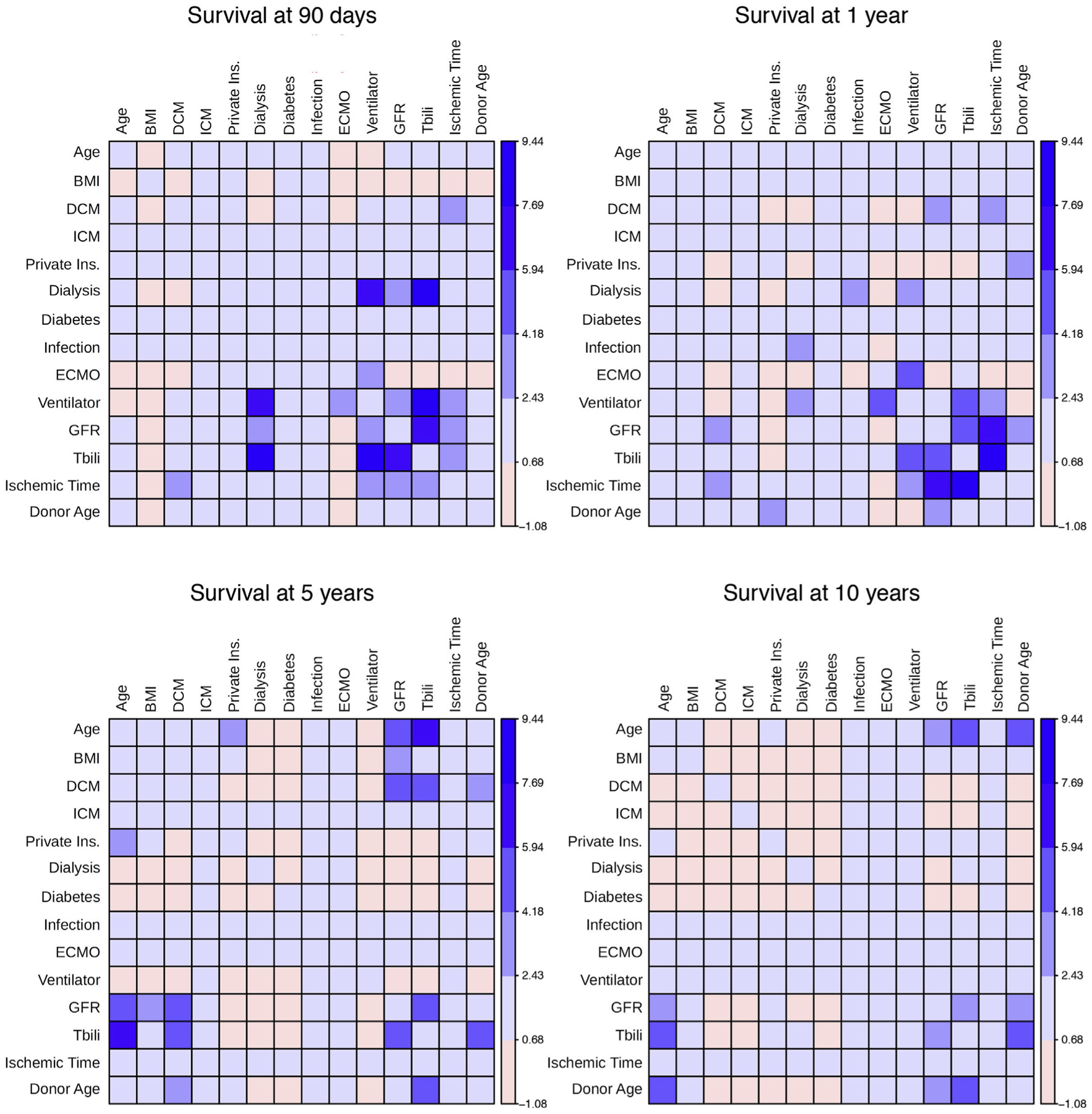
Heat map of interactions among variables of importance predicting survival at 90 days, 1 year, 5 years, and 10 years were identified with least significant interactions colored pink and most significant interactions colored dark blue. BMI = body mass index; DCM = dilated cardiomyopathy; ECMO = extracorporeal membrane oxygenation; GFR = glomerular filtration rate; ICM = ischemic cardiomyopathy; Ins. = insurance; Tbili = total bilirubin; other abbreviations as in Figure 2.
Figure 4 is a 3-dimensional plot showing the interaction between donor age and total bilirubin to predict 90-day, 1-year, 5-year, and 10-year survival. Donor age was a stronger predictor at 10 years compared to 90 days. Total bilirubin was a more constant predictor over time with its greatest predictive value early relative to late post-transplantation. The higher the bilirubin, the worse the survival. In Supplemental Figure 1, the relationship between bilirubin, dialysis, and mechanical ventilation was complex. Survival was worse with dialysis or mechanical ventilation at time of transplantation and elevated total bilirubin further increased this risk and was important even among those patients who were not on dialysis or receiving mechanical ventilation.
FIGURE 4.
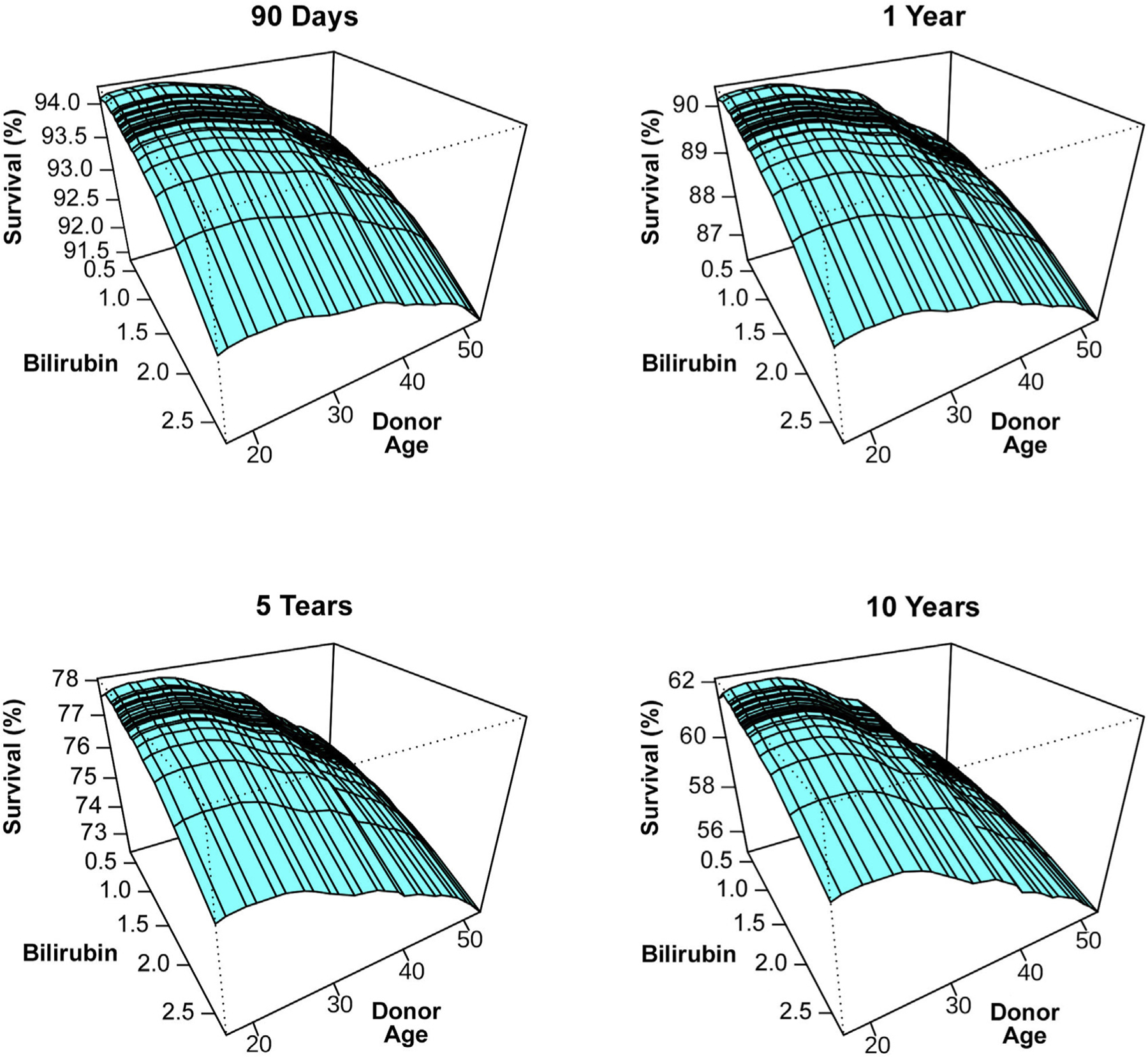
Predicted Heart Transplantation Survival According to Donor Age and Bilirubin the Interaction Between Donor Age and Total Bilirubin to Predict 90-Day, 1-Year, 5-Year, and 10-Year Heart Transplantation Survival Are Shown in 3-Dimensional Plots
DISCUSSION
PRINCIPAL FINDINGS.
Our study is an in-depth analysis of post-transplantation survival showing the complex relationships among risk factors, which vary over time. There were 4 principal findings. First, we identified 3 phases of survival: early post-operative, constant, and late-phase. Second, the factors predicting post-transplantation mortality varied in each phase, making it imperative that future efforts to create an allocation score focus on whether the goal is short-term or long-term survival. Third, despite sex being a strong predictor of waitlist mortality before heart transplantation (7,17–20), sex was not among the top variables predicting early postoperative, constant, or late survival. Fourth, machine learning rapidly identified variables of importance and complex interactions, whereas conventional statistical methods such as parametric modeling identified the different hazard phases of mortality but was time-consuming, limited by assumptions and identified only simple interactions (i.e., 2-way interactions). Therefore, both statistical methods were important and provided complementary information to better understand post-transplantation mortality.
Phases of post-transplantation survival.
The 3 phases of mortality post-transplantation have not been previously described. In fact, the published data is limited to analyses for heart transplantation focused on either early versus late mortality (4,21,22) or analyses at specific time points such as the annual publication of the International Society for Heart and Lung Transplantation (ISHLT) Registry with multivariable analyses on 1-, 5-,10-, and 20-year mortality (23), and the OPTN/SRTR report focused on 1-, 3-, and 5-year survival in the United States (24). Studies focusing on different time points have noted time-dependent risk factors for mortality. For instance, Nilsson et al. (5) noted different hazard ratios for risk prediction at 1, 5, and 10 years post-transplantation using the ISHLT database. This fact is critical to understanding the results of different studies and to the future success of a heart transplant allocation score that will balance waitlist mortality with post-transplantation survival.
Role of patient sex in survival.
Sex is a strong predictor of heart transplant waitlist mortality with many identified sex interactions (7,25). However, in our analysis and in a recent publication (26), sex was neither a significant nor important variable associated with predicting post-transplantation mortality despite women living longer than men. Furthermore, there were few sex interactions identified by either of 2 methods used and those identified were not highly predictive of survival. We did discover that sex differences in mortality was driven by sex differences in the late unadjusted hazard phase, yet other risk factors better predicted survival than sex when analyzing mortality by conventional parametric and machine learning non-parametric methods. Although we did not identify significant sex differences in post-transplantation survival, it is important to mention possible selection bias given the fact that women are less likely to survive than men on the transplant waiting list.
Risk factors predictive of post-transplantation mortality.
The majority of risk factors predicting mortality in our analysis were limited to recipient factors which have been previously described but not known to vary in strength across time or to have any interactions that change with time. One of the first studies to use both donor and recipient characteristics to predict graft loss within the first year of transplantation was performed by Hong et al (4). They analyzed 11,703 patients transplanted in the United States from 2001 to 2007 and found mostly recipient characteristics influencing early death including age, prior cardiac surgery, etiology (congenital and amyloidosis), diabetes complicated by stroke, renal function, serum bilirubin, mechanical ventilation, mechanical circulatory support, and hospitalization at time of transplantation. Donor risk factors included donor-heart ischemic time, heart transplant sex-mismatch, donor hepatitis C status, donor with insulin-dependent diabetes, and donor age. Their survival model, known as the risk stratification score, proceeded the time of effective hepatitis C therapy and routine use of continuous-flow left ventricular assist devices. Trivedi et al. (21) assessed 17,131 adult patients in the OPTN/United Network for Organ Sharing (UNOS) database transplanted between 2005 and 2013 to identify factors affecting long-term survival. High recipient risk factors affecting long-term mortality were age >65 years, body mass index >30 kg/m2, mean pulmonary artery pressure >30 mm Hg, total bilirubin >1.5 mg/dl, serum creatinine >1.5 mg/dl, prior heart transplant, prior cancer, mechanical ventilation, and use of non-continuous-flow left ventricular assist devices. There were few donor risk factors affecting long-term survival with the most essential being age >50 years, diabetes mellitus, and mismatch of donor/recipient sex. Risk of death for all patients receiving transplants increased with presence of multiple risk factors and ischemic time >4 h. Recipient risk factors were more predictive than donor and best outcome for a high-risk recipient was if donor was low risk. Finally, Nilsson et al. (5) recently published the International Heart Transplant Survival Algorithm (IHTSA) to assess heart transplant long-term survival. They included 56,625 adult patients in the ISHLT registry who underwent transplantation from 1994 to 2010. Their analysis identified 32 recipient risk variables and 11 donor risk factors with time-dependent hazards (1-, 5-, and 10-year survival post-transplantation). The most important variables predictive of mortality in their analysis were recipient age, type of heart disease, history of prior transplantation, and mechanical ventilation before transplantation. Donor factors included age, cause of death, sex, and diabetes.
Weiss et al (22) published another model called the Index for Mortality Prediction After Cardiac Transplantation (IMPACT) predicting 1-year mortality post-transplantation derived from 21,378 patients in the United States transplanted between 1997 and 2008 (Table 4). Their model used only recipient characteristics and found similar risk factors but also identified race (black patients had higher mortality risk than white patients), sex (women had higher risk than men), dialysis (one of the strongest risk factors for mortality), and infection requiring intravenous antibiotics at time of transplantation. Although ventricular assist devices were deemed high risk for mortality, the risk was much lower than ECMO. When IHTSA was compared to the IMPACT model, IHTSA better predicted 1-year mortality (27). IHTSA used a flexible, nonlinear artificial neural network to predict survival that could handle complex interactions unlike the other survival models that used conventional statistical methods (multivariable logistic regression and multivariable Cox-proportional hazards models) and assumed a linear relationship of continuous variables to outcome.
TABLE 4.
Comparison of Mortality Risk Predictors for Heart Transplantation
| RSS | IMPACT | Trivedi et al. (21) | IHTSA | |
|---|---|---|---|---|
| Mortality prediction | Early | Early | Late | Late |
| Recipient risk factors | ||||
| Age | + | + | + | + |
| Sex | + | + | ||
| Race | + | |||
| Height | + | |||
| Weight | + | |||
| Body mass index | + | |||
| Prior cardiac surgery | + | + | ||
| Type of heart disease | + | + | + | + |
| Diabetes mellitus | + | |||
| Diabetes mellitus with stroke | + | |||
| Hypertension | + | |||
| Prior cancer | + | |||
| Anti-arrhythmic drugs | + | |||
| Intravenous antibiotics | + | + | ||
| Prior PRBC transfusion | + | |||
| PRA >10% | + | |||
| ABO blood type | + | |||
| Bilirubin | + | + | + | + |
| Renal function | + | + | + | |
| Dialysis | + | |||
| Mean PAP | + | |||
| Systolic PAP | + | |||
| PVR | ||||
| ECMO | + | + | + | |
| VAD | + | + | + | + |
| TAH | + | |||
| IABP | + | |||
| Mechanical ventilation | + | + | + | + |
| Hospitalized at time of transplant | + | + | ||
| Donor risk factors | ||||
| Age | + | + | + | + |
| Sex | + | |||
| Weight | + | |||
| ABO blood type | + | |||
| Diabetes mellitus | + | + | ||
| Hepatitis C | + | |||
| Head trauma death | + | |||
| CVA death | + | |||
| Other | ||||
| Sex mismatch donor/recipient | + | + | ||
| Weight mismatch donor/recipient | + | |||
| Height mismatch donor/recipient | + | |||
| HLADR, 2 mismatch | + | |||
| Ischemic time | + | + | + | + |
| Transplant era | + |
IHTSA = International Heart Transplant Survival Algorithm; IMPACT = Index for Mortality Prediction After Cardiac Transplantation; PRA = panel reactive antibody, PRBC = packed red blood cell, PVR = pulmonary vascular resistance, RSS = risk stratification score; other abbreviations as in table 1
Interaction of variables in predicting post-transplantation survival.
Interactions were easily identified by RSF and their importance varied with follow-up time. To the best of our knowledge, these interactions have not been previously described. Among the most important was the relationship between hepatic and renal dysfunction. These variables reflected severity of illness with end-organ dysfunction at time of transplantation. Mechanical ventilation had an additive risk for early mortality. In contrast, age of donor and recipient at time of transplantation affected survival in the later phase. Based on our analysis, we believe these interactions should be considered in allocation decisions. For instance, long ischemic times may be better tolerated in the early postoperative period among those who have no significant end-organ dysfunction. Furthermore, to prevent organ wastage, transplantation should occur either before the development of end-organ dysfunction or after stabilizing the patient and improving organ function. We also envision that the many interactions discovered in our analysis will later be used to create an allocation score. Future allocation efforts must balance fair distribution of organs with utility.
STUDY LIMITATIONS.
The major limitation of our analysis was use of a large national heart transplant database restricted by accuracy of data entry, variables collected, and lack of data regarding organ recovery or failure necessary to create continuously updated mortality estimates (28). Although data entry is always limited by human error, the SRTR database minimizes this risk by edit checks, validation of data at time of entry, and internal verification when there are outliers. Data quality is further improved at every transplant center by UNOS and the Centers for Medicare and Medicaid Services (CMS) audit checks are performed routinely every few years (29). The data did not include known prognostic risk factors such as natriuretic peptides, serum sodium, heart rate, and blood pressure, although these are usually available and collected at individual centers (30–33). Our analysis is also retrospective, limited to a prospective database that does not include all confounders, and did not focus on the thresholds of values for end-organ dysfunction that still may result in safe transplantation. These factors will be necessary in the future to quantify transplant benefit or the balance between fairness of distribution with utility among critically ill patients. In addition, there are scant amount of variables that account for neighborhood risk factors that likely affect survival. Finally, the risk factors defining the late phase of mortality were likely related to transplantation complications such as malignancy, allograft vasculopathy, and renal failure, which are time-varying covariates that may not correlate with risk factors at time of transplantation. This important fact is why it is necessary to distinguish the different temporal phases of mortality and better understand their importance to improve clinical care and decision-making.
CONCLUSIONS
Recognizing the recipient and donor characteristics that are associated specifically with increased post-transplantation mortality has possible clinical implications for avoiding organ wastage. Chief among these is end-organ dysfunction (hepatic, renal, and pulmonary). We hypothesize that the timing of transplantation from ECMO should take into account patient stability and improvement/normalization of end-organ function as a strategy to reduce early post-transplantation mortality. If those goals cannot be met, transition to durable mechanical circulatory support is warranted.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
This is an indepth analysis of heart transplantation survival notable for the discovery of 3 phases of survival and many novel interactions predicting mortality. This study is relevant given recent concerns regarding possible worse post-transplantation survival with the new heart allocation system.
TRANSLATIONAL OUTLOOK:
Better pairing of donor and recipient can be achieved with better understanding of risk factors. This study is relevant given need to follow outcomes with the new heart allocation system.
Acknowledgments
Supported in part by grants HL141892 and GM125072 from the National Institutes of Health (NIH) and support from the Cystic Fibrosis Foundation. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
ABBREVIATIONS AND ACRONYMS
- ECMO
extracorporeal membrane oxygenation
- IHTSA
International Heart Transplant Survival Algorithm
- IMPACT
Index for Mortality Prediction After Cardiac Transplantation
- ISHLT
International Society for Heart and Lung Transplantation
- OPTN
Organ Procurement and Transplantation Network
- RSF
random survival forests
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For supplemental Methods, figures, and tables, please see the online version of this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Heart Failure author instructions page.
REFERENCES
- 1.Organ Procurement and Transplantation Network: Policies. Available at: http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_9.pdf. Accessed December 4, 2019. [Google Scholar]
- 2.Cogswell R, John R, Estep J, et al. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transplant 2020;39:1–4. [DOI] [PubMed] [Google Scholar]
- 3.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2017 annual data report: heart. Am J Transplant 2019;19 suppl 2:323–403. [DOI] [PubMed] [Google Scholar]
- 4.Hong KN, Iribarne A, Worku B, et al. Who is the high-risk recipient? Predicting mortality after heart transplant using pretransplant donor and recipient risk factors. Ann Thorac Surg 2011;92: 520–7. disc 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson J, Ohlsson M, Hoglund P, Ekmehag B, Koul B, Andersson B. The International Heart Transplant Survival Algorithm (IHTSA): a new model to improve organ sharing and survival. PLoS One 2015;10:e0118644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss ES, Allen JG, Kilic A, et al. Development of a quantitative donor risk index to predict short-term mortality in orthotopic heart transplantation. J Heart Lung Transplant 2012;31:266–73. [DOI] [PubMed] [Google Scholar]
- 7.Hsich EM, Blackstone EH, Thuita L, et al. Sex differences in mortality based on United Network for Organ Sharing status while awaiting heart transplantation. Circ Heart Fail 2017;10:e003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organ Procurement and Transplantation Network: Allocation of Hearts and Heart-Lungs. Available at: https://optn.transplant.hrsa.gov/governance/policies/. Accessed August 22, 2019. [Google Scholar]
- 9.Rubin DB. Multiple Imputations for Nonresponse in Surveys. New York, New York: John Wiley and Sons, Inc., 1987. [Google Scholar]
- 10.Tang F, Ishwaran H. Random forest missing data algorithms. Stat Anal Data Min 2017;10: 363–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackstone EH, Naftel DC, Turner ME. The decomposition of time - varying hazard into phases, each incorporating a separate stream of concomitant information. J Am Stat Assoc 1986; 81:615–24. [Google Scholar]
- 12.Ishwaran H, Kogalur UB, Blackstone EH, Lauer MS. Random survival forests. Ann Appl Stat 2008:841–60. [Google Scholar]
- 13.Ishwaran H Variable importance in binary regression trees and forests. Electron J Stat 2007; 1:519–37. [Google Scholar]
- 14.Ishwaran H, Kogalur UB. RandomForestSRC: Random Forest for Survival R, and Classification (RF-SRC), 2019. R package version 2.9.1. Available at: https://cran.r-project.org/package=randomForestSRC. Accessed December 4, 2019. [Google Scholar]
- 15.Rajeswaran J, Blackstone EH. Identifying risk factors: Challenges of separating signal from noise. J Thorac Cardiov Sur 2017;153:1136–8. [DOI] [PubMed] [Google Scholar]
- 16.Lu M, Ishwaran H. A prediction-based alternative to P values in regression models. J Thorac Cardiov Sur 2018;155:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsich EM, Starling RC, Blackstone EH, et al. Does the UNOS heart transplant allocation system favor men over women? J Am Coll Cardiol HF 2014;2:347–55. [DOI] [PubMed] [Google Scholar]
- 18.Morris AA, Cole RT, Laskar SR, et al. Improved outcomes for women on the heart transplant wait list in the modern era. J Card Fail 2015;21:555–60. [DOI] [PubMed] [Google Scholar]
- 19.Weidner G, Zahn D, Mendell NR, et al. Patients’ sex and emotional support as predictors of death and clinical deterioration in the waiting for a new heart study: results from the 1-year follow-up. Prog Transplant 2011;21:106–14. [DOI] [PubMed] [Google Scholar]
- 20.Hsich EM. Matching the market for heart transplantation. Circ Heart Fail 2016;9:e002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trivedi JR, Cheng A, Ising M, Lenneman A, Birks E, Slaughter MS. Heart transplant survival based on recipient and donor risk scoring: a UNOS database analysis. ASAIO J 2016;62:297–301. [DOI] [PubMed] [Google Scholar]
- 22.Weiss ES, Allen JG, Arnaoutakis GJ, et al. Creation of a quantitative recipient risk Index for Mortality Prediction After Cardiac Transplantation (IMPACT). Ann Thorac Surg 2011;92:914–21. disc 921–2. [DOI] [PubMed] [Google Scholar]
- 23.ISHLT Heart Transplant Data. Available at: https://ishltregistries.org/registries/slides.asp. Accessed August 13, 2019.
- 24.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2016 annual data report: heart. Am J Transplant 2018;18 suppl 1:291–362. [DOI] [PubMed] [Google Scholar]
- 25.Hsich EM, Thuita L, McNamara DM, et al. Variables of importance in the Scientific Registry of Transplant Recipients database predictive of heart transplant waitlist mortality. Am J Transplant 2019;19:2067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moayedi Y, Fan CPS, Cherikh WS, et al. Survival outcomes after heart transplantation: does recipient sex matter? Circ Heart Fail 2019; 12:e006218. [DOI] [PubMed] [Google Scholar]
- 27.Medved D, Ohlsson M, Hoglund P, Andersson B, Nugues P, Nilsson J. Improving prediction of heart transplantation outcome using deep learning techniques. Sci Rep 2018;8:3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blackstone EH, Rajeswaran J, Cruz VB, et al. Continuously updated estimation of heart transplant waitlist mortality. J Am Coll Cardiol 2018;72: 650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leppke S, Leighton T, Zaun D, et al. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando) 2013; 27:50–6. [DOI] [PubMed] [Google Scholar]
- 30.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 31.Fonarow GC, Adams KF Jr., Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005;293:572–80. [DOI] [PubMed] [Google Scholar]
- 32.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006;113:1424–33. [DOI] [PubMed] [Google Scholar]
- 33.Hsich EM, Grau-Sepulveda MV, Hernandez AF, et al. Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am Heart J 2012; 163:430–7, 437 e1–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


