Abstract
Recently, a novel shape memory polymer foam based on the photopolymerization of poly(ε-caprolactone) diacrylate (PCLDA) has been developed. These PCLDA foams enter a temporary softened state when briefly treated with warm saline (Tsaline > Tm of PCLDA), allowing them to conform to irregular bone defect “boundaries” prior to shape setting. When coated with a mechanically stable polydopamine (PD) layer, these PCLDA foams have previously been demonstrated to induce hydroxyapatite deposition. In the present study, the osteoinductivity of these “self-fitting” PD-coated PCLDA (PD–PCLDA) materials was evaluated relative to uncoated PCLDA (U-PCLDA) controls using bone marrow-derived human mesenchymal stem cells (h-MSCs). When cultured in the absence of osteogenic media supplements, PD–PCLDA scaffolds expressed similar levels of Runx2, alkaline phosphatase, and osteopontin protein as U-PCLDA scaffolds cultured in the presence of osteogenic media supplements. In addition, PD–PCLDA scaffolds cultured without osteogenic supplements did not significantly promote undesired lineage progression (e.g., adipogenesis or chondrogenesis) of h-MSCs. Cumulatively, these data indicate that PD–PCLDA materials display increased osteoinductivity relative to U-PCLDA substrates. Future studies will examine tethered osteogenic factors or peptides toward augmenting the osteoinductive properties of the PD–PCLDA foams.
Keywords: bone tissue engineering, PCL foam, polydopamine coating, osteogenesis, mesenchymal stem cells
Graphical Abstract

1. INTRODUCTION
Autografts that are currently used to treat critically sized bone defects suffer from significant drawbacks such as limited availability, donor site morbidity, and often prolonged hospitalization or rehabilitation periods.1–4 Moreover, such grafts cannot be easily shaped to fit within an irregular bone defect,5 which in turn can increase the risk of graft resorption.6–9 In contrast, an engineered graft, fabricated by conditioning patient-derived cells within biomaterial scaffolds, that overcomes these limitations presents an attractive alternative for the treatment of bone defects.9,10 The successful development of such a graft hinges upon the appropriate design and functionalization of the biomaterial scaffold: in addition to being amenable for shaping in situ, the scaffold should support the growth and osteoblastic differentiation of progenitor cells and promote osseointegration upon implantation.11–13 The aim of the present study is to evaluate the potential of a polydopamine-coated shape memory polymer (SMP) scaffold based on poly(ε-caprolactone) diacrylate (PCLDA) toward promoting osteoblastic differentiation of human mesenchymal stem cells (h-MSCs).
A range of scaffold materials and fabrication methods have been evaluated for the repair of critically sized bone defects.10,14,15 When noninjectable materials are used, mold-based fabrication, postfabrication shaping, and computer-aided solid free-form fabrication techniques are often employed to ensure that the resulting scaffolds precisely match defect geometry.16,17 While this processing allows for the intimate contact between the scaffold and adjacent bone tissue that is necessary for healing, these fabrication methods are time- and cost-intensive. Thus, in situ curing of synthetic bone substitutes (e.g., putties and cements prepared with and without ceramic or glass ceramic fillers) has been explored.18–26 Unfortunately, these putties and cements often suffer from major limitations such as extreme exothermic cure (up to ~90 °C), slow setting times, brittle mechanical properties (leading to fracture during surgical contouring and postsurgery), and low pore interconnectivity and lack of degradation (leading to poor healing).18–26 Hydrogels have also been studied but are generally mechanically weak and often exhibit variable local curing times, leading to poor adhesion along defect boundaries.27 In addition, the cytotoxicity of hydrogel reagents (e.g., monomers, cross-linkers, and catalysts) may be problematic during in situ curing.27,28
In contrast, the PCLDA SMP scaffolds selected for examination herein are capable of “self-fitting” into irregular defects. Zhang et al. previously reported the fabrication of highly porous PCLDA-based SMP foams via the photopolymerization of PCLDA macromers in the presence of a fused salt template followed by salt-leaching and annealing.29–31 The resulting 3D scaffolds are nonbrittle, display interconnected pore structures with greater than 70% total porosity, and have compressive moduli exceeding 4 MPa.29 Thus, PCLDA foams possess appropriate structural and mechanical properties for use in a number of bone defect applications.32–34 Importantly, these PCLDA foams also display shape memory behavior and have previously been demonstrated to enter a temporary softened state when treated with 60 °C warm saline (Tsaline > Tm of the selected PCL).29 This softened state permits their manipulation and conformability along irregular bone defect “boundaries” prior to shape setting, and the high shape fixity of the PCLDA SMP foams allows retention of the fitted shape following cooling.29
To improve their osteoconductivity, PCLDA SMP foams have recently been prepared with a mussel-inspired polydopamine (PD) coating.29 PD coatings prepared under basic conditions in the presence of oxygen have previously been shown to stimulate hydroxyapatite (HAp) deposition as well as enhance a variety of osteoblast cell responses, including adhesion, spreading, proliferation, and differentiation.35–40 For PCLDA foams, PD coatings were used in place of conventional bioactive ceramic or glass fillers (e.g., calcium phosphates and sulfates, tricalcium phosphates, and HAp) to avoid compromising the softened-state ductility of the PCLDA SMP foams necessary for their “self-fitting” behavior. Indeed, Zhang et al. demonstrated that the pores of PCLDA foams could be uniformly coated with PD without compromising the softened state malleability and shape memory properties of the foams.29 Furthermore, the PD coating remained stably attached to the foam surface following the heating and shape modulation associated with defect-filling, and PD-coated foams stimulated HAp deposition when immersed in simulated body fluid.29
The primary goal of this study is to evaluate the capacity of PD-coated PCLDA (PD–PCLDA) substrates to promote osteoblastic differentiation of h-MSCs for potential application in bone defect repair. Briefly, photo-cross-linkable PCLDA was processed into 2D films or 3D foams. Samples were further treated with a dopamine hydrochloride solution to result in a PD coating on the substrate surfaces. An initial cell study was performed in the presence of osteogenic supplements to confirm that the PD–PCLDA films have a positive effect on h-MSC osteogenesis relative to uncoated PCLDA (U-PCLDA) films. We then examined the potential of PD–PCLDA foams to promote h-MSC osteogenesis in the absence of osteogenic supplements. In this second study, h-MSC expression of osteoblastic genes and/or proteins was evaluated following 2 weeks of culture within U-PCLDA and PD–PCLDA foams. Concurrently, the expression of undesired adipogenic and chondrogenic genes/proteins was also evaluated to assess the specificity of the observed osteogenic response.
2. MATERIALS AND METHODS
2.1. Polymer Synthesis
PCL diol (Mn ~ 10 000 g/mol; Sigma-Aldrich) was functionalized with terminal acrylate groups to result in PCLDA, as described in a previous report.30 1H NMR spectroscopy was performed to confirm the acrylation of terminal–OH groups and the integrity of the molecular weight of PCL. Concurrently, the cell adhesion peptide NH2-Arg-Gly-Asp-Ser-COOH (RGDS; American Peptide) was conjugated to 3.4 kDa acryloyl-PEG-succinimidyl valerate (Laysan Bio) at a 1:1 molar ratio to form acryloyl-PEG-RGDS (ACRL-PEG-RGDS). ACRL-PEG-RGDS was purified by dialysis against double deionized (ddI) water, lyophilized, and stored at −80 °C until further use.
2.2. Preparation and Characterization of PCLDA Substrates
2.2.1. Fabrication of PCLDA 2D Films and 3D Foams
PCLDA films were prepared by combining a solution containing 0.15 g/mL of PCLDA and 1 mM ACRL-PEG-RGDS. Photoinitiator solution consisting of 10 wt % 2,2-dimethoxy-2-phenylacetophenone (DMAP; Sigma-Aldrich) in N-vinylpyrrolidone (NVP; Sigma-Aldrich) was then added at 15 vol %. The resulting mixture was pipetted into silicone molds (45 mm × 22 mm × 1.8 mm; McMaster Carr) sandwiched between two glass slides. The sandwich assembly was exposed to ultraviolet light (6 mW/cm2, 365 nm) for 3 min, following which the upper glass slide and the silicone mold were removed. The edges of the solvent-swollen film were secured to the remaining slide with binder clips and air-dried overnight. The resulting film was then soaked in 1:1 water/ethanol mixture for 24 h and dried in vacuo for 4 h.
Porous PCLDA 3D foams were prepared according to a previously described protocol.29 Briefly, ddI water was added at 7.5 wt % to a 3 mL glass vial (I.D. = 12.9 mm) containing 1.8 g of granulated NaCl particles (459 ± 69 μm; Sigma-Aldrich). The wetted salt crystals were mechanically stirred, centrifuged at 4000 rpm for 15 min, and air-dried overnight to result in a fused-salt template. Subsequently, a solution containing 0.15 g/mL of PCLDA and 1 mM ACRL-PEG-RGDS was prepared in dichloromethane (Sigma-Aldrich) and combined with 15 vol % photoinitiator solution (10 wt % DMAP in NVP). Six hundred microliters of this solution was added to the fused salt template, and the sealed vial was then centrifuged at 2500 rpm for 10 min to aid macromer infiltration into the salt template. The vial was then exposed to UV light for 3 min and air-dried overnight. The 3D foam was removed from the vial and leached using a 1:1 water/ethanol mixture for 4 days, with daily exchange of the water/ethanol mixture, followed by air-drying overnight.
2.2.2. Polydopamine Coating Application and Assessment
Following successful fabrication, PCLDA films and 3D foams were coated with a layer of PD. Briefly, 3D foams were degassed using a syringe to ensure solution contact with the foam pores. The foams and films were then submerged for 16 h in a 2 mg/mL solution of dopamine hydrochloride (Sigma-Aldrich) in 10 mM Tris buffer, pH 8.5, with oxygenation provided by constant stirring at 150 rpm. The dopamine hydrochloride solution undergoes oxidative self-polymerization under these conditions, as evidenced visually by the brown coloration of the treated surfaces.41 The films and foams were then extensively rinsed with ddI water and dried in vacuo for 24 h. Thereafter, both uncoated (U-PCLDA) and coated (PD–PCLDA) films and foams were heat-treated at 85 °C for 1 h, followed by cooling to room temperature. PCLDA substrates coated with PD in this manner have previously been demonstrated to be capable of HAp deposition following immersion in simulated body fluid.29 Finally, the films were cut into 1 cm diameter discs, while the final dimensions of the 3D foams were 6 mm (diameter) × 3 mm (height).
The presence and uniformity of the resulting PD coatings have been confirmed in a prior report29 and were assessed herein by stereomicroscopy evaluation of the foam surface and cross-sections. Furthermore, the capacity of the PD-coatings to withstand the shape modulation associated with defect fitting was also evaluated by stereomicroscopy-based inspection of the coating following fitting within an irregular defect. The color of the films and foams was closely monitored for uniformity throughout the study to ensure that the PD coating remained undamaged during scaffold processing and subsequent culture.
2.2.3. Foam Bulk Compression Testing
The compressive moduli of the U-PCLDA and PD-PCLDA foams were evaluated using an EnduraTec ELF-3200 mechanical testing system equipped with a 50 lbf load cell. Following application of a 0.5 N preload, foams were compressed to 30% of their initial thickness at a constant strain rate of 1 mm/min. The average compressive modulus of the coated versus uncoated foams was then calculated from the slopes of the resulting stress–strain curves.
2.4. Cell Culture
Cryopreserved bone marrow derived h-MSCs (Lonza, Inc.) were thawed and expanded in MesenPRO RS medium (Life Technologies) supplemented with 10% MSC-qualified fetal bovine serum (FBS; Life Technologies) in a 37 °C-5% CO2 jacketed incubator. For each study, h-MSCs were harvested for use at passage 6. In the first set of experiments, the ability of the PD–PCLDA films to promote osteoblastic differentiation of h-MSCs in the presence of osteogenic supplements was investigated. In the second set of experiments, the capacity of the PD–PCLDA 3D foams to promote h-MSC osteogenesis in the absence of osteogenic supplements was examined.
2.4.1. Osteoblastic Differentiation of h-MSCs on PCLDA Films in the Presence of Osteogenic Supplements
U-PCLDA (n = 5) and PD–PCLDA (n = 7) films were sterilized using ethylene oxide (Andersen Sterilizers Inc.) and then secured within separate wells of a 24-well culture plate using Teflon ring inserts. h-MSCs were seeded on the films at ≈2500 cells/film and cultured for 2 weeks in osteogenic medium, which was exchanged every 2 days. This osteogenic medium (OM) consisted of Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies) supplemented with 10% MSC-qualified FBS (Life Technologies), 50 μg/mL L-ascorbic acid 2-phosphate (Sigma), 0.1 μM dexamethasone (Sigma), 10 mM β-glycerophosphate (Sigma), and 1% antibiotic/antimycotic solution (10,000 IU/mL penicillin, 10,000 μg/mL streptomycin, and 25 μg/mL amphotericin; Life Technologies). A portion of the h-MSC population seeded onto the films was harvested to serve as a day 0 control.
2.4.2. Osteoblastic Differentiation of h-MSCs within PCLDA 3D Foams in the Absence of Osteogenic Supplements
U-PCLDA (n = 5) and PD–PCLDA (n = 5) were placed within separate wells of a 12well culture plate and sterilized using ethylene oxide. After sterilization, the foams were transferred to a 96-well plate for the duration of culture. Fifty microliters of an h-MSC cell suspension (3.5 × 106 cells/mL) was pipetted onto the upper surface of each foam sample and incubated for 15 min. Thereafter, the foams were flipped within each well, and 50 μL of cell suspension was likewise seeded onto the other surface and incubated for 15 min. This process was then repeated for a total of 200 μL of applied cell suspension per sample. Following cell seeding, sample surfaces were rinsed with phosphate buffered saline (PBS) thrice in order to remove nonadherent cells, and the foams were subsequently transferred to a new 96-well plate. The U-PCLDA foams and the PD–PCLDA foams were then cultured for 2 weeks in growth medium (GM) without osteogenic supplements (DMEM supplemented with 10% MSC-qualified FBS and 1% antibiotic/antimycotic solution). The cell culture medium was exchanged every 2 days.
To assess the degree of osteogenic differentiation observed within GM-cultured foams, a day 0 time point as well as a positive osteogenic control were included. For day 0 samples, a portion of the h-MSC population seeded onto the foams was harvested for subsequent analyses. For the osteogenic positive control, a set of U-PCLDA 3D foams (n = 4) was cultured in the presence of OM for 2 weeks, with cell culture medium exchanged every 2 days. To evaluate the degree of nonspecific differentiation within the GM-cultured foams, positive adipogenic and chondrogenic culture controls were also included. For the adipogenic control, h-MSCs were cultured in 2D in the presence of h-MSC Adipogenic Differentiation Medium (Lonza, Inc.). For the chondrogenic control, h-MSCs were seeded at 1 × 106 cells/mL within a 6.0 kDa 10% PEGDA hydrogel to enforce a rounded cell morphology and were cultured for 2 weeks in chondrogenic medium (GM containing 10 ng/mL TGF-β3).42–44
2.5. Construct Imaging
At 72 h of culture, a set of foams were harvested for confocal imaging of cell attachment and spreading within the scaffolds. Following rinsing with PBS, the foams were fixed in formalin and subsequently stained with rhodamine phalloidin (Life Technologies). The cell-laden foams were then imaged using confocal microscopy (Zeiss LSM 510 META).
2.6. Construct Homogenization and mRNA Extraction for End-Point Analyses
After 2 weeks of culture, mRNA was extracted using a modified version of an extraction protocol described previously.45 PCLDA films were rinsed briefly with PBS, after which they were exposed to 330 μL of the lysis buffer provided with the Dynabeads mRNA Direct kit (Life Technologies) for 10 min at room temperature. Similarly, foam samples were briefly rinsed with PBS, transferred to 1.7 mL RNase-free microcentrifuge tubes containing 300 μL of lysis buffer, homogenized using plastic RNase-free pestles (Kimble Chase), and then incubated at room temperature for 10 min. Thereafter, the sample extracts were centrifuged for 5 min at 10000 rpm, and the polyA-mRNA in the supernatant was extracted using 20 μL of Dynabeads oligo(dT) magnetic beads. The remaining supernatant was stored at −80 °C for the evaluation of DNA, calcium, and protein. The mRNA-laden Dynabeads were rinsed and transferred to the tube containing 100 μL of 10 mM Tris-HCl buffer. Bound polyA-mRNA was then released into this buffer by heating the beads to 80 °C for 2 min. The resulting mRNA solution was stored at −80 °C until further analysis. DNA levels in the sample homogenates were assessed using the PicoGreen kit (Life Technologies), performed according to the manufacturer’s instructions.
2.6.1. Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR) for the Evaluation of Gene Expression
qRT-PCR was performed on each sample using a StepOne Real-Time PCR system (Life Technologies) and the SuperScript III Platinum One-Step qRT-PCR kit according to the manufacturer’s instructions. Validated qRT-PCR primers for human genes related to osteogenesis, chondrogenesis, and adipogenesis were purchased from Origene and Qiagen (Table S1). Approximately 3 ng of polyA-mRNA was combined with 5 μL of 1 mM primer in a total reaction volume of 25 μL. Gene amplification over 40 cycles was monitored by measuring the change in SYBR Green fluorescence, with ROX dye serving as a passive reference. A threshold fluorescence value in the exponential phase of amplification was identified using StepOne software v2.0, and a threshold cycle (Ct) was determined for each sample. Gene expression was normalized to a reference gene (β-actin), and the ΔΔCt method was used for the estimation of relative gene expression.46 For the 2D film studies, the U-PCLDA films served as the reference control, whereas the U-PCLDA foams cultured in osteogenic media served as the reference control for the 3D foam studies. Melting temperature analysis was performed for each reaction to verify the appropriate amplification product.
2.6.2. Calcium Quantification
Total calcium in 10 μL aliquots of the sample homogenates was quantified using the calcium CPC liquid color kit (Stanbio Laboratory) and then normalized by the measured end point sample DNA. To separate calcium deposited by h-MSCs from the mineralization intrinsically stimulated by the PCLDA scaffolds, coated and uncoated PCLDA foam and film controls were also analyzed for total calcium. These PCLDA foam and film controls contained no cells but otherwise experienced the same culture and extraction conditions as the cellularized samples.
2.6.3. Western Blot Analysis for Protein Expression
Immunoblots were performed to semiquantitatively compare extracellular matrix (ECM) protein and transcription factor levels between the different experimental groups. Sample homogenate volumes representing 30 ng of DNA for films, 300 ng of DNA for foams, 500 ng of DNA for the 2D adipogenic controls, and 2000 ng of DNA for the chondrogenic controls were then loaded into separate wells of sodium dodecyl sulfate polyacrylamide gels and separated using an applied electric field.
Following protein separation and transfer onto a nitrocellulose membrane, the membranes were blocked with a solution of 5% bovine serum albumin (BSA) in TBST/NaN3 (25 mM Tris-HCl, pH 7.5, 137 mM NaCl, 0.1% Tween 20, and 0.05% NaN3) for 1 h at room temperature. Primary antibodies against Runx2 (Clone M-70), tissue nonspecific alkaline phosphatase (TNAP; Clone TRA 2–54), osteopontin (OPN; Clone Akm2A1), Sox9 (Clone E-9), collagen II(α1) (Col II; Clone M2139), aggrecan (ACAN; Clone D-20), PPARγ (Clone I-18), adipsin (Clone H-55), and adipocyte-fatty acid binding protein (AFABP; Clone 3F4) were obtained from Santa Cruz Biotechnology, and a primary antibody against β-actin (AB8226) was obtained from Abcam. Osteocalcin (OCN) was not able to be analyzed by Western Blot due to its low molecular weight (MW ≈ 6 kDa) relative to the other proteins analyzed, which made it challenging to detect under the transfer conditions needed for the remaining proteins. Each primary antibody was then diluted in a solution of 5% BSA in TBST/NaN3 and applied overnight at 4 °C with constant shaking.
Bound primary antibody was detected by the application of an appropriate alkaline phosphatase (AP)-conjugated or horseradish peroxidase (HRP)-conjugated secondary antibody (Jackson Immunochemicals) for 1 h at room temperature. This was followed by the application of Novex AP chemiluminescent substrate (Life Technologies) or Luminol chemiluminescent reagent (Santa Cruz Biotechnology). Chemiluminescence was detected using a ChemiDoc XRS+ System equipped with ImageLab Software (Bio-Rad Laboratories), with exposure time controlled to avoid signal saturation. Band integrated optical density for each protein target was quantified using Adobe Photoshop CS2 (version 9.0) and normalized to β-actin.
2.8. Statistical Analyses
All data are reported as the mean ± standard error of the mean. Sample means were compared using ANOVA (SPSS version 23.0) followed by a Tukey posthoc test, with a p-value <0.05 considered statistically significant.
3. RESULTS
3.1. Osteoblastic Differentiation of h-MSCs on PCLDA Films in the Presence of Osteogenic Supplements
In the preliminary study, the capacity of PD–PCLDA films to promote osteoblastic differentiation of h-MSCs was investigated. Because this experiment was conducted in the presence of osteogenic supplements, only bone-related markers were evaluated at the study end point. Specifically, h-MSC expression of the osteogenic transcription factor Runx2, the bone-related enzyme TNAP, and bone ECM proteins OPN and OCN on PD–PCLDA films was compared to that on U-PCLDA films and to day 0.
h-MSCs cultured on PD–PCLDA films displayed a significant increase in the expression of Runx2 mRNA (2.4-fold; p = 0.045) and OPN mRNA (1.8-fold; p = 0.023) relative to U-PCLDA films (Figure 1A). However, OCN gene expression could not be distinguished between the two substrates, and TNAP mRNA expression levels were reduced on PD–PCLDA films relative to U-PCLDA films (1.9-fold; p < 0.001). In addition, levels of OPN and OCN mRNA were not significantly different on either substrate from day 0 levels, and the expression of Runx2 mRNA on both the U-PCLDA and PD–PCLDA films was significantly lower than day 0 (p < 0.046). Thus, mixed relationships in osteogenic marker mRNA expression between the U-PCLDA and PD–PCLDA films and between the films and day 0 existed, which were challenging to interpret without additional data.
Figure 1.
(A) Osteogenic gene expression, (B) osteogenic protein expression, and (C) deposition by h-MSCs cultured on U-PCLDA and PD–PCLDA films for 2 weeks in the presence of osteogenic medium (OM) containing L-ascorbic acid-2-phosphate, β-glycerophosphate, and dexamethasone. Results were normalized to the U-PCLDA construct values. *, indicates a significant difference relative to day 0, p < 0.05. #, indicates a significant difference relative to U-PCLDA; p < 0.05. Note osteocalcin (OCN) was only examined at the mRNA level due to its low molecular weight, which prevented its analysis by Western blot using 8% or 10% SDS–PAGE gels and transfer times used herein.
To further evaluate the gene expression data, we therefore incorporated conventional 2D osteogenic controls in which h-MSCs were cultured in osteogenic medium (OM) for 2 weeks. Associated h-MSC gene expression was then analyzed at 1, 3, 7, 10, and 14 days of culture (Figure S1). TNAP mRNA expression peaked at day 7 and then declined, while Runx2 and OPN mRNA expression peaked at around day 10 and then declined. Thus, the lower Runx2 gene expression on both the films relative to day 0 may actually indicate later-stage osteogenesis on these substrates relative to day 0 rather than reduced osteogenesis. Similarly, the reduced TNAP mRNA levels associated with PD–PCLDA films relative to U-PCLDA films may actually be indicative of more advanced osteogenesis on the PD–PCLDA films relative to the U-PCLDA films.
To clarify the meaning of the gene expression data, more definitive protein level analyses were conducted. Representative images of the resulting Western blots are given in Figure S2. At the protein level, substantial increases in Runx2, TNAP, and OPN were observed on the PD–PCLDA films relative to day 0 as well as relative to the U-PCLDA films (Figure 1B). In particular, h-MSCs cultured on the PD–PCLDA films exhibited a significant increase in Runx2 relative to U-PCLDA films (5.6-fold; p < 0.001) and relative to day 0 (8.3-fold; p < 0.001). TNAP and OPN protein levels on the PD–PCLDA films were also 2.4-fold (p = 0.005) and 5.2-fold (p = 0.010) greater, respectively, than on U-PCLDA films and over 65-fold greater than those on day 0 (p < 0.003; Figure 1B). The PD-coated substrates also appeared to support increased cell-mediated matrix mineralization (Figure 1C), with h-MSCs cultured on PD–PCLDA films depositing approximately 1.5-fold more calcium than cells cultured on uncoated films, although this difference was not statistically significant (p = 0.14). Combined, these data indicate that PD–PCLDA substrates are able to induce enhanced osteoblastic differentiation of h-MSCs compared to U-PCLDA substrates in the presence of osteogenic supplements.
3.2. Osteoblastic Differentiation of h-MSCs within PCLDA 3D Foams in the Absence of Osteogenic Supplements
Following the preliminary study with PCLDA films, a second set of experiments was conducted using PCLDA 3D foams to evaluate whether these PD-coated substrates would retain their capacity to promote h-MSC osteogenesis in the absence of osteogenic supplements.
3.2.1. Foam Characterization
Prior to the onset of the 3D cell studies, coated and uncoated PCLDA foams were characterized with respect to the uniformity and mechanical stability of the PD coating as well as with respect to foam mechanical properties. The PD coating method applied herein has previously been confirmed to result in a uniform, nanoscalethick PD layer on PCLDA SMP substrates.29 The presence and homogeneity of this nanoscale layer was assessed in the present work by stereomicroscopy evaluation of the foam surface and cross-sections. Representative images of the U-PCLDA and PD–PCLDA foams are shown in Figure 2A. Note the relatively uniform brown coloration of the pore walls of the PD-coated scaffolds, indicating the presence of the PD-coating (Figure 2A,B).41 As we have previously observed,29 the PD–PCLDA and U-PCLDA foams showed an interconnected pore structure with an overall porosity of ≈72%, and the PD-coating did not appear to alter the pore structure of the scaffolds (Figure 2B). In terms of foam mechanical properties, both U-PCLDA and PD–PCLDA foams displayed similar stress–strain responses to compression testing (Figure 2C). Furthermore, the average elastic modulus of coated foams (5.4 ± 0.1 MPa) could not be statistically distinguished from that of the uncoated foams (5.0 ± 0.3 MPa).
Figure 2.
(A) Representative image of U-PCLDA and PD–PCLDA foams. The U-PCLDA foam is white in coloration, whereas the PD–PCLDA foam displays a characteristic brown coloration. (B) Representative stereomicroscopy image of a PD–PCLDA foam section. (C) Representative stress–strain curves for U-PCLDA and PD–PCLDA foams under bulk compression.
To demonstrate the capacity of the PD–PCLDA foams to be shaped to a model defect without disruption or delamination of the PD-coating, foams were treated with 60 °C warm saline, pressed into a model defect, allowed to cool, and subsequently removed for stereomicroscopy evaluation (Figure 3).29 The PD-coating (evidenced by brown coloration) remained in contact with the PCLDA pore walls following the heating and shape modulation associated with defect fitting (Figure 3). Previous studies have also confirmed that the present PD–PCLDA foams retain the shape memory properties (shape retention and shape fixity) of their U-PCLDA counterparts.29
Figure 3.
(A) Macroscopic image of an uncompressed PD–PCLDA foam next to a model defect and (B) a representative stereomicroscopy image of the associated foam structure. (C) Macroscopic image of the same foam after being fitted and locked into the shape of the model defect and (D) a representative stereomicroscopy image of the associated foam structure. The scale bar in C applies to both A and C and equals 6 mm. The scale bar in D applied to both B and D and equals 150 μm.
3.2.2. PCLDA Foam Culture and Assessment of the Strength and Specificity of Osteogenic Differentiation in the Absence of Osteogenic Supplements
To assess the capacity of PD–PCLDA substrates to promote h-MSC osteogenesis in the absence of osteogenic supplements, U-PCLDA foams and PD–PCLDA foams were cultured in growth medium (GM) for a period of 2 weeks. The resulting constructs were termed U-PCLDA-GM and PD–PCLDA-GM, and associated h-MSC gene and protein expression was then compared to positive U-PCLDA controls cultured in OM (termed U-PCLDA-OM). In addition, the extent of undesired h-MSC differentiation was assessed relative to adipogenic and chondrogenic positive controls.
3.2.3. Osteogenic Differentiation
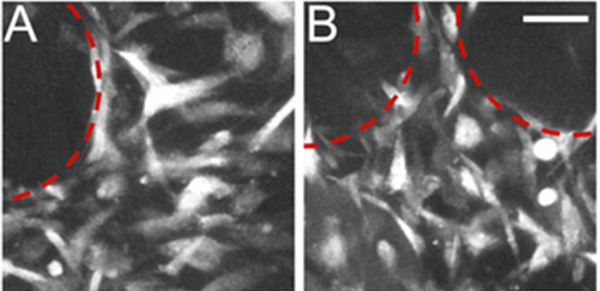
Figure 4 displays representative confocal images of h-MSC adhesion on U-PCLDA and PD–PCLDA foams, demonstrating that both scaffolds were able to support robust cell adhesion. Following 2 weeks of culture within the 3D foams, h-MSC gene expression of bone markers Runx2, TNAP, OPN, and OCN was investigated. At the mRNA level, the expression of Runx2, OPN, and OCN each appeared to be increased in the U-PCLDA-GM and PD–PCLDA-GM constructs relative to day 0, although these increases were generally only statistically significant for the U-PCLDA-GM constructs (Figure 5A). Similarly, the mRNA expression levels for Runx2, OPN, and OCN in the U-PCLDA-OM foams were statistically indistinguishable from day 0 levels, with TNAP being the only marker for which U-PCLDA-OM foam gene expression was significantly greater than day 0 levels (p < 0.001). These results would appear to indicate that osteogenesis was highest in the U-PCLDA-GM constructs, even beyond the level associated with foams cultured in OM. Despite this, calcium deposition within the U-PCLDA-OM foams was over 2-fold greater than that in the U-PCLDA-GM and the PD–PCLDA-GM foams (p < 0.001; Figure 5), indicating that stimulation with OM supplements was substantively increasing osteogenesis.
Figure 4.
Representative confocal microscopy images of rhodamine–phalloidin stained h-MSCs within (A) U-PCLDA-GM foams and (B) PD–PCLDA-GM foams. Red-dashed lines encircle pores within the foams. The scale bar in B applies to A and B and equals 50 μm.
Figure 5.
Expression of bone-related (A) mRNA, (B) proteins, and (C) calcium by h-MSCs cultured within U-PCLDA-GM, PD–PCLDA-GM, and U-PCLDA-OM constructs. Results were normalized to U-PCLDA-OM construct values. *, indicates a significant difference relative to day 0, p < 0.05. #, indicates a significant difference relative to U-PCLDA-GM, p < 0.05. $, indicates significant difference when compared with PD–PCLDA-GM, p < 0.05. Note that osteocalcin (OCN) was only examined at the mRNA level due to its low molecular weight, which prevented its analysis by Western blot using 8% or 10% SDS–PAGE gels and the transfer times used herein.
We hypothesized that the apparent discrepancy between the qRT-PCR and the calcium data was due to temporal shifts in h-MSC gene expression following osteogenic induction (Figure S1). To further evaluate this possibility, more definitive protein level analyses were conducted. Representative Western blot images are shown in Figure S3. At the protein level, U-PCLDA-GM, PD–PCLDA-GM, and U-PCLDA-OM each appeared to express significantly increased levels of TNAP and OPN relative to day 0. In particular, h-MSCs within U-PCLDA-OM positive controls produced 18.9-fold greater TNAP (p = 0.05), and 233-fold greater OPN (p < 0.001) compared to day 0 (Figure 5B). These protein level results were consistent with the calcium deposition measures, both of which indicated that the OM-treated U-PCLDA controls stimulated osteogenesis, in apparent contrast to the qRT-PCR data. Furthermore, the PD–PCLDA-GM foams were associated with significant increases in the protein-level production of Runx2 (3.8-fold; p = 0.015) and OPN (2-fold; p = 0.029) compared to U-PCLDA-GM foams (Figure 5B), and each osteogenic protein investigated was produced at statistically similar levels in the PD–PCLDA-GM constructs relative to the U-PCLDA-OM positive controls.
Combined, these protein level data indicate that the applied PD-coatings were able to stimulate h-MSC osteogenesis to a similar degree as osteogenic media. That said, h-MSC calcium deposition on the PD–PCLDA foams was significantly less than that on U-PCLDA-OM foams (p < 0.001; Figure 5C). This suggests that full osteoblastic maturation was not achieved on the PD–PCLDA-GM constructs relative to U-PCLDA-OM positive controls.
3.2.4. Undesired Adipogenic Differentiation
As h-MSCs can potentially differentiate into adipocytes and chondrocytes as well as into osteoblasts when cultured in the absence of osteogenic supplements, the expression of markers associated with these undesired lineages was also assessed at the mRNA and protein levels. For adipogenesis, the transcription factor PPARγ, the midterm adipogenic marker adipsin, and the late-term adipogenic marker AFABP were examined. At the mRNA level, U-PCLDA-GM, PD–PCLDA-GM, and U-PCLDA-OM constructs each displayed significantly increased PPARγ (p < 0.028) expression relative to day 0 controls (Figure 6A). However, adipsin mRNA levels for the U-PCLDA-GM PD–PCLDA-GM constructs could not be statistically distinguished from day 0. In addition, AFABP mRNA levels for the U-PCLDA-GM and the PD-PCLDA-FM foams were significantly lower than day 0 levels (p < 0.050). Contrary to expectation, the PD–PCLDA-GM and U-PCLDA-GM constructs were also associated with significantly lower levels of PPARγ (p < 0.017), adipsin (p < 0.002), and AFABP (p < 0.010) mRNA than the U-PCLDA-OM osteogenic controls. These data appeared to indicate that, although the U-PCLDA-GM and PD–PCLDA-GM constructs stimulated adipogenesis to some degree, the strength of this adipogenic response was markedly less that than that associated with an osteogenic control, which is generally considered nonadipogenic.
Figure 6.
Expression of adipogenic (A) mRNA and (B) proteins by h-MSCs cultured within U-PCLDA-GM, PD–PCLDA-GM, and U-PCLDA-OM constructs. Results were normalized to U-PCLDA-OM construct values. *, indicates a significant difference relative to day 0, p < 0.05. #, indicates a significant difference relative to U-PCLDA-GM, p < 0.05. $, indicates significant difference when compared with PD–PCLDA-GM, p < 0.05. For protein level analyses, h-MSCs cultured in adipogenic differentiation medium were included as an adipogenic control.
To further evaluate these qRT-PCR results, protein level analyses were conducted, and an adipogenic positive control was incorporated. Representative Western blot images are provided in Figure S4. In contrast to the gene expression data, no statistically significant differences were observed at the protein level for PPARγ, adipsin, or AFABP for the U-PCLDA-OM osteogenic controls relative to day 0 (Figure 6B). In addition, the U-PCLDA-GM, PD–PCLDA-GM, and U-PCLDA-OM constructs were associated with 35- to 45-fold lower levels of adipsin and AFABP than those of the adipogenic positive control. That said, h-MSCs seeded on the PD–PCLDA-GM foams produced PPARγ protein levels that were significantly greater than those of the U-PCLDA-GM foams (3.6-fold; p = 0.001) and which appeared to be greater than that associated with the adipogenic positive control (Figure 6B). Given that PPARγ is not specific to adipogenesis,47,48 the higher levels of PPARγ associated with the PD–PCLDA-GM foams relative to U-PCLDA-GM foams are difficult to interpret. However, these results may indicate that the PD-PCLDA-GM foams are in the early stages of an adipogenic response. Longer-term culture studies may therefore be warranted to more fully evaluate adipogenesis on PD–PCLDA foams.
3.2.5. Undesired Chondrogenic Differentiation
In addition to adipogenic markers, chondrogenesis was evaluated. Specifically, the chondrogenic transcription factor Sox9 and cartilage ECM proteins Col II and ACAN were investigated. At the mRNA level, U-PCLDA-GM constructs displayed significantly increased Sox9 (p < 0.001) and ACAN (p < 0.001) gene expression relative to day 0 controls (Figure 7A). Col II mRNA levels also appeared to be higher on U-PCLDA-GM foams relative to day 0. In contrast, PD–PCLDA-GM foams and U-PCLDA-OM constructs exhibited levels of Sox9, Col II, and ACAN mRNA that were statistically indistinguishable from day 0, although Col II expression on the PD–PCLDA-GM foams appeared to be greater than Col II expression in the U-PCLDA-OM foams. These results suggest that PD–PCLDA-GM foams supported reduced chondrogenesis relative to U-PCLDA-GM constructs and that chondrogenesis on the PD–PCLDA-GM foams was not substantially advanced from day 0.
Figure 7.
Expression of chondrogenic (A) mRNA and (B) proteins by h-MSCs cultured within U-PCLDA-GM, PD–PCLDA-GM, and U-PCLDA-OM constructs. Results were normalized to U-PCLDA-OM construct values. *, indicates a significant difference relative to day 0, p < 0.05. #, indicates a significant difference relative to U-PCLDA-GM, p < 0.05. $, indicates significant difference when compared with PD–PCLDA-GM, p < 0.05. For protein level analyses, a chondrogenic positive control was included.
To clarify these qRT-PCR data, more definitive protein level assessments were also conducted, and a chondrogenic positive control was incorporated. Representative Western blot images are provided in Figure S5. At the protein level, the expression of Sox9 and Col II in the U-PCLDA-GM, PD–PCLDA-GM, and U-PCLDA-OM foams could not be distinguished among the constructs (Figure 7B), and Sox9 levels in each treatment group were similar to the chondrogenic positive control. Although ACAN protein was not detected in the U-PCLDA-GM and PD–PCLDA-GM constructs, positive ACAN staining was present in the U-PCLDA-OM osteogenic controls and in the chondrogenic positive control. In addition, the U-PCLDA-GM, PD–PCLDA-GM, and U-PCLDA-OM constructs displayed approximately 60-fold lower levels of Col II than the chondrogenic positive control. These data indicate that PD–PCLDA-GM constructs supported chondrogenesis to a degree similar to that of the PD–PCLDA-OM osteogenic controls, which can be considered nonchondrogenic.
4. DISCUSSION
In the present study, PCLDA SMP substrates were functionalized with PD coatings with the goal of enhancing osteoinductivity without disrupting the ability of these materials to be compressed and fitted to irregularly shaped bone defects. Toward this end, h-MSCs were cultured on PD-coated PCLDA films in the presence of osteogenic supplements (L-ascorbic acid 2-phosphate, β-glycerophosphate, and dexamethasone), and cell phenotype was assessed by examining the expression of osteogenic mRNA and proteins as well as the degree of calcium deposition after 2 weeks of culture. The 2-fold motivation for this preliminary study was to confirm that the PD coatings enhanced h-MSC osteoblastic differentiation in the presence of osteogenic supplements and also to allow comparison with previous studies, most of which have been conducted in the presence of supplements.
h-MSCs cultured on PD–PCLDA films in the presence of OM showed significant elevation in the expression of Runx2, TNAP, and OPN proteins compared to that on the U-PCLDA films. Although β-glycerolphosphate and dexamethasone are well-known inducers of osteoblastic differentiation,49 our preliminary study conducted with PD-coated 2D films demonstrated that the coatings promoted osteogenic differentiation over and above the inherent differentiation induced by these supplements. The present results are also consistent with previous reports for stem cells cultured on PD-coated materials in the presence of osteogenic additives.50–52 Specifically, Rim et al. reported enhanced osteogenic differentiation of h-MSCs cultured on PD-coated electrospun substrates compared to uncoated controls,50 while Ge et al.51 and Lee et al.52 described similar results with mouse adipose-derived stem cells and periodontal ligament-derived stem cells, respectively.
Since our long-term goal is to use highly porous PD–PCLDA scaffolds for bone defect repair, a second follow-up study was conducted using PCLDA which had been processed into foams with shape memory properties.29 In these studies, osteogenic supplements were withdrawn to determine if the resulting PD–PCLDA foams were intrinsically capable of promoting h-MSC osteogenesis. PD–PCLDA-GM constructs displayed significantly higher protein-level expression of Runx2 and OPN than U-PCLDA-GM foams and similar Runx2 and OPN levels as those of osteogenic positive controls. Furthermore, the PD–PCLDA foams did not promote h-MSC lineage progression toward undesired adipocytic and chondrocytic phenotypes relative to chondrogenic and adipogenic positive controls. Indeed, the degree of adipogenic and chondrogenic response on the PD–PCLDA-GM foams was generally similar to U-PCLDA foams cultured in osteogenic medium, which is considered to be nonadipogenic and nonchondrogenic.
Despite the protein level results indicating increased osteogenesis on PD–PCLDA foams, full osteoblastic maturation as indicated by increased mineralization was not achieved on the PD–PCLDA foams when cultured in the absence of osteogenic supplements. Thus, although the present data indicate that PD–PCLDA substrates support an enhanced osteogenic response relative to U-PCLDA substrates, future studies will examine the incorporation of additional osteoinductive additives (such as BMP2)53–57 in order to stimulate greater osteoblastic maturation on PD–PCLDA foams. In addition, future studies will need to be conducted for longer culture periods to more fully confirm the specificity of h-MSC osteogenic differentiation within PD–PCLDA foams cultured in the absence of osteogenic supplements.
An additional limitation of the present studies is that equivalent bioavailability of the incorporated RGD adhesion peptide, which was included to ensure cell adhesion on the uncoated PCLDA substrates, was not confirmed between the U-PLCDA and PD–PCLDA substrates. Given that the PD coating was applied following RGD incorporation, it is possible that availability of the RGD peptide was substantially reduced on the PD–PCLDA substrates relative to the U-PCLDA substrates. Thus, some of the differences observed between the U-PCLDA and PD–PCLDA films and foams may be due to differential RGD availability. That said, RGD has been generally associated with increased osteogenic responses in in vitro culture.58 Therefore, it would be anticipated that a potential reduction in RGD bioavailability on the PD–PCLDA constructs relative to U-PCLDA constructs would be a disadvantage to the PD–PCLDA osteogenic response.
The mechanisms underlying the osteoinductivity of PD-coatings have not been clearly established in the literature, although previous studies have provided valuable insights. PD-coatings as prepared herein are known to bind Ca2+ ions and promote HAp formation when soaked in simulated body fluids.35,36,59 Indeed, Zhang et al. showed that the present PD–PCLDA foams were coated with mineral crystallites upon soaking in a simulated body fluid for 2 weeks, while uncoated PCLDA foams demonstrated no such deposits.29 Although alternative mechanisms have been proposed,52 the formation of such an apatite layer may support osteoblastic differentiation either through the release of ions or via the adsorption of osteoinductive proteins.60 Future studies may include examination of the internal cellular signaling underlying the osteoinductive properties of PD-coatings.
5. CONCLUSIONS
This study demonstrated that PD-coated PCLDA films promoted increased h-MSC osteogenic protein expression compared to that of uncoated PCLDA films when cultured in the presence of osteogenic supplements. PD–PCLDA 3D foams continued to induce significantly higher expression of bone-related proteins by h-MSCs relative to that of uncoated foams in the absence of osteogenic media supplements. Importantly, the PD-coated PCLDA foams also did not generally enhance h-MSC expression of adipogenic and chondrogenic proteins relative to U-PCLDA osteogenic controls, indicating that the differentiation stimulated by the PD-coatings may be specific to the osteogenic lineage. In summary, the results from this study indicate that PD-coated PCLDA SMP foams intrinsically promote osteoblastic differentiation of h-MSCs. Such foams could potentially serve as “self-fitting” scaffolds which can recruit progenitor cells and promote osteoinduction when used to treat critically sized bone defects.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Institutes of Health (R03 EB015202) for providing financial support to conduct this research.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomaterials.5b00445.
qRT-PCR primer sequences, temporal gene expression and alizarin red staining from 2D h-MSCs treated with osteogenic media, and representative Western blot images (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Elsalanty ME; Genecov DG Bone grafts in craniofacial surgery. Craniomaxillofacial trauma & reconstruction 2009, 2 (3), 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kinoshita Y; Maeda H Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications. Sci. World J. 2013, 2013, 863157–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Banwart JC; Asher MA; Hassanein RS Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine 1995, 20 (9), 1055–60. [DOI] [PubMed] [Google Scholar]
- (4).Cowley SP; Anderson LD Hernias through donor sites for iliac-bone grafts. J. Bone Jt. Surg., Am. Vol 1983, 65 (7), 1023–5. [PubMed] [Google Scholar]
- (5).Eufinger H; Wehmoller M; Machtens E; Heuser L; Harders A; Kruse D Reconstruction of Craniofacial Bone Defects with Individual Alloplastic Implants Based on Cad/Cam-Manipulated Ct-Data. J. Cranio Maxill Surg 1995, 23 (3), 175–181. [DOI] [PubMed] [Google Scholar]
- (6).Phillips JH; Rahn BA Fixation Effects on Membranous and Endochondral Onlay Bone-Graft Resorption. Plast Reconstr Surg 1988, 82 (5), 872–877. [DOI] [PubMed] [Google Scholar]
- (7).Neovius E; Engstrand T Craniofacial reconstruction with bone and biomaterials: Review over the last 11 years. J. Plast Reconstr Aes 2010, 63 (10), 1615–1623. [DOI] [PubMed] [Google Scholar]
- (8).Enneking WF; Eady JL; Burchardt H Autogenous cortical bone grafts in the reconstruction of segmental skeletal defects. J. Bone Jt. Surg., Am. Vol 1980, 62 (7), 1039–58. [PubMed] [Google Scholar]
- (9).Mistry AS; Mikos AG Tissue engineering strategies for bone regeneration. Adv. Biochem. Eng./Biotechnol. 2005, 94, 1–22. [DOI] [PubMed] [Google Scholar]
- (10).Calori GM; Mazza E; Colombo M; Ripamonti C The use of bone-graft substitutes in large bone defects: any specific needs? Injury 2011, 42 (Suppl 2), S56–63. [DOI] [PubMed] [Google Scholar]
- (11).Blokhuis TJ; Arts JJC. Bioactive and osteoinductive bone graft substitutes: Definitions, facts and myths. Injury 2011, 42, S26–S29. [DOI] [PubMed] [Google Scholar]
- (12).Albrektsson T; Johansson C Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Yu XH; Wang LP; Xia ZM; Chen L; Jiang X; Rowe D; Wei M Modulation of host osseointegration during bone regeneration by controlling exogenous stem cell differentiation using a material approach. Biomater. Sci. 2014, 2 (2), 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Burg KJ; Porter S; Kellam JF Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21 (23), 2347–59. [DOI] [PubMed] [Google Scholar]
- (15).Parikh SN Bone graft substitutes: past, present, future. J. Postgrad. Med 2002, 48 (2), 142–8. [PubMed] [Google Scholar]
- (16).Sachlos E; Czernuszka J Making tissue engineering scaffolds work. Review on the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. Eur. Cell Mater. 2003, 5, 29–40. [DOI] [PubMed] [Google Scholar]
- (17).Hollister SJ Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [DOI] [PubMed] [Google Scholar]
- (18).Hou Q; De Bank PA; Shakesheff KM Injectable scaffolds for tissue regeneration. J. Mater. Chem. 2004, 14, 1915–1923. [Google Scholar]
- (19).Migliaresi C; Motta A; DiBenedetto AT Injectable Scaffolds for Bone and Cartilage Regeneration In Engineering of Functional Skeletal Tissues; Bronner F, Farach-Carson MC, Mikos AG, Eds.; Springer-Verlag: London, 2007; Vol. 3, pp 95–109. [Google Scholar]
- (20).Gage E; Langevin C-J; Papay F Biomaterials in Craniofacial Surgery In Plastic and Reconstructive Surgery; Siemionow MZ, Eisenmann-Klein M, Eds.; Springer-Verlag: London, 2010; pp 125–135. [Google Scholar]
- (21).Van-der-Stok J; Van-Lieshout EMM; El-Massoudi Y; Van-Kralingen GH; Patka P Bone substitutes in the Netherlands - A systematic review. Acta Biomater. 2011, 7, 739–750. [DOI] [PubMed] [Google Scholar]
- (22).Low KL; Tan SH; Zein SHS; Roether JA; Mourino V; Boccaccini AR Calcium phosphate-based composites as injectable bone substitute materials. J. Biomed. Mater. Res., Part B 2010, 94B, 273–286. [DOI] [PubMed] [Google Scholar]
- (23).Slaughter BV; Khurshid SK; Fisher OZ; Khademhosseini A; Peppas NA Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Spierings PTJ. Properties of Bone Cement: Testing and Performance of Bone Cements In The Well-Cemented Total Hip Arthroplasty; Breusch S, Malchau H, Eds.; Springer: Germany, 2005; pp 67–78. [Google Scholar]
- (25).Serbetci K; Korkusuz F; Hasirci N Thermal and mechanical properties of hydroxyapatite impregnated acrylic bone cements. Polym. Test. 2004, 23, 145–155. [Google Scholar]
- (26).Giannoudis PV; Dinopoulos H; Tsiridis E Bone substitutes: An update. Injury 2005, 36, S20–S27. [DOI] [PubMed] [Google Scholar]
- (27).Williams CG; Elisseeff JH Injectable Systems for Cartilage Tissue Engineering In Scaffolding in Tissue Engineering; Ma PX, Elisseeff J, Eds.; CRC Press: Boca Raton, FL, 2006; pp 169–188. [Google Scholar]
- (28).Shin H; Temenoff JS; Mikos AG In vitro cytotoxicity of unsaturated oligo[poly(ethylene glycol) fumarate] macromers and their cross-linked hydrogels. Biomacromolecules 2003, 4, 552–560. [DOI] [PubMed] [Google Scholar]
- (29).Zhang D; George OJ; Petersen KM; Jimenez-Vergara AC; Hahn MS; Grunlan MA A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater. 2014, 10 (11), 4597–605. [DOI] [PubMed] [Google Scholar]
- (30).Zhang D; Petersen KM; Grunlan MA Inorganic-organic shape memory polymer (SMP) foams with highly tunable properties. ACS Appl. Mater. Interfaces 2013, 5 (1), 186–91. [DOI] [PubMed] [Google Scholar]
- (31).Zhang D; Burkes WL; Schoener CA; Grunlan MA Porous inorganic-organic shape memory polymers. Polymer 2012, 53 (14), 2935–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Karageorgiou V; Kaplan D Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26 (27), 5474–91. [DOI] [PubMed] [Google Scholar]
- (33).Murugan R; Ramakrishna S Development of nanocomposites for bone grafting. Compos. Sci. Technol. 2005, 65 (15), 2385–2406. [Google Scholar]
- (34).Moore WR; Graves SE; Bain GI Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71 (6), 354–61. [PubMed] [Google Scholar]
- (35).Zhou YZ; Cao Y; Liu W; Chu CH; Li QL Polydopamine-Induced Tooth Remineralization. ACS Appl. Mater. Interfaces 2012, 4 (12), 6901–6910. [DOI] [PubMed] [Google Scholar]
- (36).Yan PH; Wang JQ; Wang L; Liu B; Lei ZQ; Yang SR The in vitro biomineralization and cytocompatibility of polydopamine coated carbon nanotubes. Appl. Surf. Sci. 2011, 257 (11), 4849–4855. [Google Scholar]
- (37).Ku SH; Lee JS; Park CB Spatial Control of Cell Adhesion and Patterning through Mussel-Inspired Surface Modification by Polydopamine. Langmuir 2010, 26 (19), 15104–15108. [DOI] [PubMed] [Google Scholar]
- (38).Liu YT; Lee TM; Lui TS Enhanced osteoblastic cell response on zirconia by bio-inspired surface modification. Colloids Surf., B 2013, 106, 37–45. [DOI] [PubMed] [Google Scholar]
- (39).Shi X; Ostrovidov S; Shu Y; Liang X; Nakajima K; Wu H; Khademhosseini A Microfluidic generation of polydopamine gradients on hydrophobic surfaces. Langmuir 2014, 30 (3), 832–8. [DOI] [PubMed] [Google Scholar]
- (40).Xu MC; Zhang YF; Zhai D; Chang J; Wu CT Mussel-inspired bioactive ceramics with improved bioactivity, cell proliferation, differentiation and bone-related gene expression of MC3T3 cells. Biomater. Sci 2013, 1 (9), 933–941. [DOI] [PubMed] [Google Scholar]
- (41).Ku SH; Ryu J; Hong SK; Lee H; Park CB General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31 (9), 2535–2541. [DOI] [PubMed] [Google Scholar]
- (42).Gupta MS; Cooper ES; Nicoll SB Transforming growth factor-beta 3 stimulates cartilage matrix elaboration by human marrow-derived stromal cells encapsulated in photocrosslinked carboxymethylcellulose hydrogels: potential for nucleus pulposus replacement. Tissue Eng., Part A 2011, 17 (23–24), 2903–10. [DOI] [PubMed] [Google Scholar]
- (43).McCall JD; Luoma JE; Anseth KS Covalently tethered transforming growth factor beta in PEG hydrogels promotes chondrogenic differentiation of encapsulated human mesenchymal stem cells. Drug Delivery Transl. Res. 2012, 2 (5), 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Williams CG; Kim TK; Taboas A; Malik A; Manson P; Elisseeff J In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003, 9 (4), 679–88. [DOI] [PubMed] [Google Scholar]
- (45).Munoz-Pinto DJ; Jimenez-Vergara AC; Gharat TP; Hahn MS Characterization of sequential collagen-poly(ethylene glycol) diacrylate interpenetrating networks and initial assessment of their potential for vascular tissue engineering. Biomaterials 2015, 40 (0), 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Livak KJ; Schmittgen TD Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25 (4), 402–8. [DOI] [PubMed] [Google Scholar]
- (47).Yu WH; Li FG; Chen XY; Li JT; Wu YH; Huang LH; Wang Z; Li P; Wang T; Lahn BT; Xiang AP PPARgamma suppression inhibits adipogenesis but does not promote osteogenesis of human mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2012, 44 (2), 377–84. [DOI] [PubMed] [Google Scholar]
- (48).Kawai M; Rosen CJ PPARgamma: a circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 2010, 6 (11), 629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Bruder SP; Jaiswal N; Ricalton NS; Mosca JD; Kraus KH; Kadiyala S Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin. Orthop. Relat. Res. 1998, 355S, S247–56. [DOI] [PubMed] [Google Scholar]
- (50).Rim NG; Kim SJ; Shin YM; Jun I; Lim DW; Park JH; Shin H Mussel-inspired surface modification of poly(L-lactide) electrospun fibers for modulation of osteogenic differentiation of human mesenchymal stem cells. Colloids Surf., B 2012, 91, 189–197. [DOI] [PubMed] [Google Scholar]
- (51).Ge LP; Li QT; Huang Y; Yang SQ; Ouyang J; Bu SS; Zhong W; Liu ZH; Xing MMQ Polydopamine-coated paper-stack nanofibrous membranes enhancing adipose stem cells’ adhesion and osteogenic differentiation. J. Mater. Chem. B 2014, 2 (40), 6917–6923. [DOI] [PubMed] [Google Scholar]
- (52).Lee JS; Yi JK; An SY; Heo JS Increased Osteogenic Differentiation of Periodontal Ligament Stem Cells on Polydopamine Film Occurs via Activation of Integrin and PI3K Signaling Pathways. Cell. Physiol. Biochem. 2014, 34 (5), 1824–34. [DOI] [PubMed] [Google Scholar]
- (53).Chien CY; Liu TY; Kuo WH; Wang MJ; Tsai WB Dopamine-assisted immobilization of hydroxyapatite nanoparticles and RGD peptides to improve the osteoconductivity of titanium. J. Biomed. Mater. Res., Part A 2013, 101A (3), 740–747. [DOI] [PubMed] [Google Scholar]
- (54).Wu CT; Fan W; Chang J; Xiao Y Mussel-inspired porous SiO2 scaffolds with improved mineralization and cytocompatibility for drug delivery and bone tissue engineering. J. Mater. Chem. 2011, 21 (45), 18300–18307. [Google Scholar]
- (55).Ko E; Yang K; Shin J; Cho SW Polydopamine-Assisted Osteoinductive Peptide Immobilization of Polymer Scaffolds for Enhanced Bone Regeneration by Human Adipose-Derived Stem Cells. Biomacromolecules 2013, 14 (9), 3202–3213. [DOI] [PubMed] [Google Scholar]
- (56).Chien CY; Tsai WB Poly(dopamine)-Assisted Immobilization of Arg-Gly-Asp Peptides, Hydroxyapatite, and Bone Morphogenic Protein-2 on Titanium to Improve the Osteogenesis of Bone Marrow Stem Cells. ACS Appl. Mater. Interfaces 2013, 5 (15), 6975–6983. [DOI] [PubMed] [Google Scholar]
- (57).Lai M; Cai KY; Zhao L; Chen XY; Hou YH; Yang ZX Surface Functionalization of TiO2 Nanotubes with Bone Morphogenetic Protein 2 and Its Synergistic Effect on the Differentiation of Mesenchymal Stem Cells. Biomacromolecules 2011, 12 (4), 1097–1105. [DOI] [PubMed] [Google Scholar]
- (58).Yang F; Williams CG; Wang DA; Lee H; Manson PN; Elisseeff J The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 2005, 26 (30), 5991–8. [DOI] [PubMed] [Google Scholar]
- (59).Ryu J; Ku SH; Lee H; Park CB Mussel-Inspired Polydopamine Coating as a Universal Route to Hydroxyapatite Crystallization. Adv. Funct. Mater. 2010, 20 (13), 2132–2139. [Google Scholar]
- (60).Samavedi S; Whittington AR; Goldstein AS Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9 (9), 8037–8045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.