Abstract
Rapid infection diagnosis is critical to improving patient treatment and outcome. Recent studies have shown microbial lipids to be sensitive and selective biomarkers for identifying bacterial and fungal species and antimicrobial resistance. Practical procedures for microbial lipid biomarker analysis will therefore improve patient outcomes and antimicrobial stewardship. However, current lipid extraction methods require significant hands-on time and are thus not suited for direct adoption as a clinical assay for microbial identification. Here, we have developed a method for lipid extraction directly on the surface of stainless-steel matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) plates, termed fast lipid analysis technique or FLAT, which facilitates the identification of bacterial and fungal species using a sub-60-minute workflow. Additionally, our method detects lipid A modifications in Gram-negative bacteria that are associated with antimicrobial resistance, including to colistin.
Subject terms: Infectious-disease diagnostics, Membrane lipids, Mass spectrometry, Microbiology techniques, Biochemical assays
Introduction
Rapid administration of appropriate antimicrobial therapy significantly improves patient survival1,2, making rapid microbial diagnostics a critical need. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS or simply MALDI) of proteins in the 2000 to 20,000 m/z range is the clinical standard for microbial identification (ID)3. MALDI protein ID offers a simple workflow, low cost-per-sample, and rapid time to result using colonies from solid growth medium. Current MALDI ID systems include MALDI Biotyper (Bruker Daltonics, Billerica, MA) and VITEK MS (bioMérieux, France). These mature platforms still have significant limitations. Identification at the species level can be unreliable for some common clinical species. For example, Shigella cannot be reliably distinguished from Escherichia coli. Finally, while researchers report MALDI-based approaches to antimicrobial resistance, MALDI-based clinical tests are not available or limited for antimicrobial resistance4–10. Consequently, antibiotic susceptibility testing typically requires sample culture in appropriate growth mediums to obtain pure cultures, followed by parallel subculture in serially-diluted antibiotics, potentially requiring days to weeks depending on the growth rate of the microbe. As multi-drug resistant bacteria and fungi continue to emerge, rapid determination of antimicrobial resistance is increasingly critical to antimicrobial stewardship11–14. Current early resistance tests such as PCR-based assays have made important contributions, but the expense of these tests limits them to specific applications13,15.
Leung et al. reported a novel MALDI-TOF MS method (BACLIB) using microorganism-specific lipids in the 1000–2400 m/z range as a species-specific “chemical barcode” primarily comprised of lipid A in Gram-negative bacteria and of cardiolipins and lipoteichoic acid in Gram-positive bacteria16. Similar to MALDI protein ID, they reported a lipid reference library with entries for over 50 species, including the clinically-relevant ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) and intrinsically colistin-resistant bacteria, such as Morganella morganii and Serratia marcescens16.
The BACLIB approach exploits the diverse yet consistent membrane lipids present in bacterial and fungal species. Gram-negative bacterial membranes contain lipid A, the lipid anchor of lipopolysaccharide (LPS), comprising a β-1′,6-linked diglucosamine backbone, a species-specific pattern of four to seven fatty acyl chains, and one or two terminal phosphate groups. Lipids extracted from Gram-positive bacterial membranes include cardiolipins and lipoteichoic acid (LTA), which also have species-specific patterns16. Fungal membranes are composed of phospholipids, glycosphingolipids, and sterols17.
Furthermore, antimicrobial resistance can be mediated via modification of membrane lipids. For example, addition of phosphoethanolamine, l-amino-4-arabinose (Ara4N), or galactosamine to the above-mentioned lipid A terminal phosphate groups reduces membrane permeability to polymyxins, such as colistin (polymyxin E)18,19. Membrane lipid modifications conferring antimicrobial resistance have been characterized by MALDI18–29. Subsequent to reporting the BACLIB method, Leung et al. showed the ability to determine colistin resistance MALDI patterns in the ESKAPE pathogens (Klebsiella pneumoniae, Acinetobacter baumannii) and those driven by the mcr-1 gene, a phosphoethanolamine transferase20,21,26,28,29. Thus, MALDI is an attractive platform for a rapid resistance assay and the approach of Leung et al. yields a single test combining multiplex microbial ID and resistance assays. However, the approach in Leung et al. involves centrifugation and labor-intensive washing steps and requires overnight lyophilization—impractical for a clinical test. Liang et al. describe a 1-h sodium acetate-based lipid extraction28, but the protocol also includes centrifugation and ethanol washing steps that are problematic for a clinical technique. Furniss et al. recently described a rapid lipid extraction using acetic acid8, but as with Liang et al., centrifugation and labor-intensive handling steps are required. Correa-Martínez et al. describe an antimicrobial susceptibility test on a MALDI plate: bacteria are incubated in microdroplets containing a range of concentrations of an antibiotic, then expression of resistance markers is measured via MALDI9,10, but at least 3 h incubation and several delicate pipetting manipulations are required.
To achieve the speed and limited hands-on time necessary for clinical use, we developed a new extraction method—FLAT (“fast lipid analysis technique”), that requires less than an hour of elapsed time and is highly parallelizable and amenable to automation. By directly extracting lipids on the heated surface of a stainless steel MALDI plate, FLAT minimizes handling steps and thus demands significantly less hands-on time than previous lipid analysis methods. Furthermore, FLAT can identify bacteria and fungi, while simultaneously detecting antimicrobial resistance signals that are not reported by current clinical MALDI ID systems, thus demonstrating the significant potential of FLAT in a clinical diagnostic laboratory setting.
Materials and methods
Microbial strains and culture
We applied the FLAT method to 149 replicates of 35 bacterial strains as follows: 20 unique clinical and laboratory adapted isolates of Gram-negative bacteria (E. coli, K. pneumoniae, A. baumannii, P. aeruginosa, M. morganii, and S. marcescens) including seven strains transformed with the mcr-1 plasmid26,30, and 15 unique clinical and laboratory adapted isolates of Gram-positive bacteria (S. aureus, B. cereus, and B. mycoides). In addition, we applied the FLAT method to four replicates of two laboratory adapted isolates of Candida auris. We compared FLAT results to results with lipid microextraction31 on 157 replicates of the same and similar strains of the same bacterial and fungal species. A complete list of strains and replicates is provided in Supplementary Table S1 online. Bacterial samples were cultured overnight at 37 °C on Difco LB agar plates (BD, Franklin Lakes NJ). Liquid bacterial samples were cultured overnight at 37 °C in Difco LB broth (BD, Franklin Lakes NJ) shaking at 180 RPM. C. auris samples were cultured overnight at 37 °C on Tryptic Soy Agar (MilliporeSigma, Burlington MA).
FLAT extraction of microbial lipids
Microbial colony smears or liquid samples were applied to a target location on a stainless steel MALDI plate. The target plate was incubated in a humidified chamber for 30 min at 110 °C. The MALDI plate was washed with deionized water from a squeeze bottle, allowed to air dry, then 1 μL of norharmane matrix solution was applied (10 mg/mL in 12:6:1, v/v/v chloroform/methanol/water) to each target location. Following the method of Leung et al.16, spectra were acquired from target locations in negative ion mode using a Microflex LRF MALDI-TOF MS (Bruker, Billerica MA) in reflectron mode with a limited mass range of 1000–2400 m/z. Typically, 300 laser shots were summed to acquire each mass spectrum.
Microextraction of microbial lipids
The lipid microextraction method used by Leung et al. was originally described by El Hamidi et al.16,27,31. Briefly, bacterial pellets were treated with an isobutyric acid/NaOH mixture, incubated 30–45 min at 100 °C, then centrifuged at 2000×g for 15 min to remove cell debris. Supernatants were transferred to clean tubes, diluted 1:1 with distilled water, and lyophilized overnight. The resulting dry pellets were washed twice in methanol, resuspended in 12:6:1, v/v/v chloroform/methanol/water, then 1 µL was dispensed onto a stainless steel MALDI plate. Matrix application and spectra acquisition conditions were otherwise identical to those used in the FLAT samples.
Computational analysis of mass spectra
Spectra were exported from the mass spectrometer as mzXML files and read with a Python program (source available below) using the mzXML reader in Pyteomics 4.3.232. Spectra were resampled onto a regular grid and background corrected with SNIP (Statistics-sensitive, Non-linear Iterative Peak-clipping)33. The similarity between two spectra is measured by cosine similarity: the cosine of the angle formed by the spectra treated as normalized vectors. In this way, a similarity between each spectrum and every other spectrum of interest was computed as a scalar number.
To measure the similarity between one group of spectra and another group, such as between spectra for one species and another species, the cosine similarities from each spectrum in one group to each spectrum in the other group were averaged, then the averages were averaged (if a spectrum appears in both groups, its self-similarity was omitted from the averages).
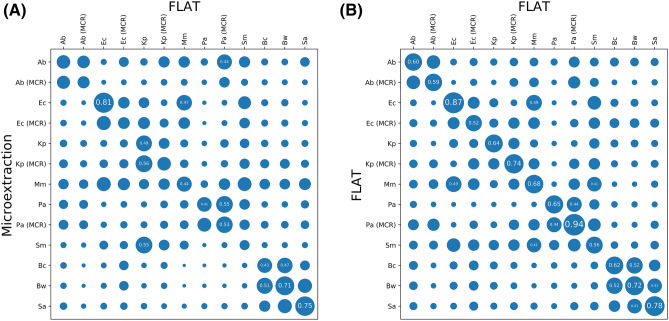
To evaluate two sets of spectra relative to species-specific signals, each set was sub-grouped by species, then the similarity was measured between each subgroup of one set and every subgroup of the other set. The result is a matrix of similarities, as is often used to compare the similarity of individual spectra, but here shows the average similarity of multiple spectra from the same species. Results are presented as bubblemaps in Fig. 4, with larger bubbles indicating greater similarity, and similarity values labeled numerically on a scale of 0.0–1.0 where similarity exceeds 0.4.
Figure 4.
FLAT spectra are distinctive by bacterial species and similar to equivalent Microextraction spectra. (A) A heatmap demonstrating that FLAT produces spectra consistent with microextraction. (B) A heatmap demonstrating FLAT species and mcr-1 status differentiation. In both cases, similarity between two groups of spectra was computed via the mean cosine similarity across all pairs of spectra, and is visualized as a circle with a proportionally scaled diameter. A total of 149 FLAT spectra and 148 microextraction spectra were used in this comparison, from strains and samples as described in Supplementary Tables S1 and S2 online. CR: mcr-1 transformed; Ab: Acinetobacter baumannii; Ec: Escherichia coli; Kp: Klebsiella pneumoniae Pa: Pseudomonas aeruginosa; Mm: Morganella morganii; Sm: Serratia marcescens; Bc: Bacillus cereus; Bw: Bacillus mycoides (previously known as Bacillus weihenstephanensis37); Sa: Staphylococcus aureus.
Results
The FLAT method for extracting microbial signature lipids
FLAT (Fig. 1) resembles current MALDI protein ID systems34–36 in that a microbial colony smear or liquid sample is directly applied to a target location on a MALDI plate. As described in “Materials and methods”, we applied FLAT to 149 replicates of 35 bacterial strains and four replicates of two unique C. auris strains. Total processing time, from the start of sample handling to the end of mass spectra acquisition, was less than 1 h in all cases. We extracted 157 replicates of the same and similar strains via lipid microextraction, as described in “Materials and methods”. MALDI spectra from these extractions were then analyzed and compared as follows.
Figure 1.
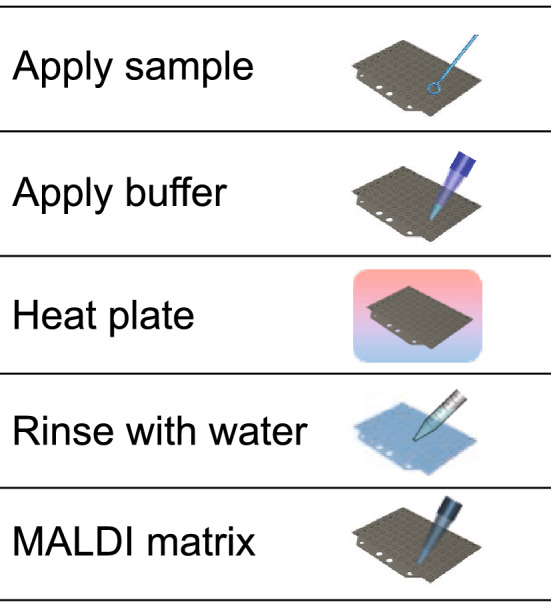
FLAT extraction workflow. A sample is applied to a stainless steel MALDI target plate, followed by FLAT extraction buffer (for Gram-negative species; 0.2 M anhydrous citric acid, 0.1 M trisodium citrate dihydrate) or no buffer (for Gram-positive and fungal species). The entire plate is incubated in a humidified chamber at 110 °C for 30 min and then rinsed thoroughly with water. MALDI matrix is applied to sample spots and lipid spectra are collected using MALDI-TOF MS.
Buffered and unbuffered FLAT extraction
Acidic conditions facilitate extraction of lipid A molecular species16,28,31, which are important for identifying Gram-negative bacteria16. However, acidic conditions may interfere with lipid extraction for some Gram-positive and fungal species. We therefore performed FLAT extraction both with and without a buffer on all samples. For each sample, two target spots were applied and treated as follows: on one target spot, 1 µL of buffer (0.2 M citric acid, 0.1 M trisodium citrate) was dispensed before extraction; the buffer was not applied to the second spot. Characteristic lipid A ions of Gram-negative bacteria were only observed in spectra from buffered spots. Characteristic lipid ions of Gram-positive bacteria and fungi were observed in spectra from both buffered and unbuffered spots. However, for Gram-positive bacteria and fungi, unbuffered spots consistently produced higher spectral quality than buffered spots. Consequently, in the following studies, spectra from buffered spots were used for Gram-negative bacteria (Figs. 2A,B and 4), while spectra from unbuffered spots were used for Gram-positive bacteria and fungi (Fig. 2C,D).
Figure 2.
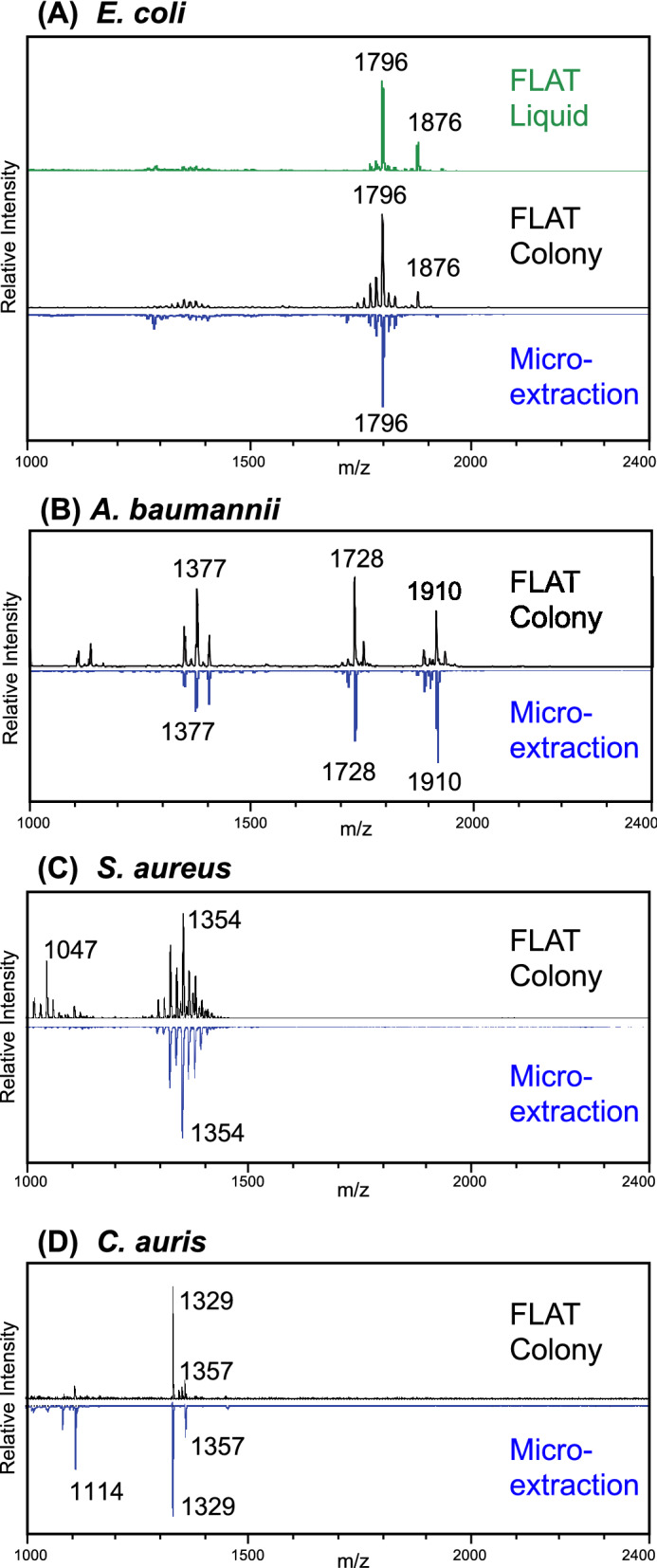
Representative FLAT spectra compared to microextractions spectra of the same strain. (A) Escherichia coli ATCC 25922 colony smear and liquid culture via FLAT is compared to microextraction. The characteristic 1796 m/z lipid A ion28 is observed in all spectra. Additionally, an ion at 1876 m/z is observed with FLAT, but not with microextraction. This is plausibly a lipid A ion, and may have been previously observed in E. coli28, but is not typically extracted via microextraction. Colony smears via FLAT are compared to microextraction in (B) through (D), with the same base peak shown for both methods for each species. (B) Acinetobacter baumannii SM1536. Previously reported ions16 are observed. (C) Staphylococcus aureus NRS484. The pattern of ions from 1300 to 1400 m/z is highly similar in both methods. An ion at 1047 m/z in FLAT may be present at very low intensity in microextraction. (D) Candida auris AR0384. Two ions at 1329 and 1357 m/z are present in both spectra. An ion at 1114 m/z in microextraction may be present at low intensity in FLAT.
Comparison of FLAT to lipid microextraction
We compared MALDI spectra produced by FLAT extraction to the spectra produced by lipid microextraction to determine if the methods extract similar lipid profiles. In lipid microextraction, methanol washing removes phospholipids in the relevant mass range, suppressing their signal. FLAT does not require depletion of phospholipids in the same way, so some differences may be expected between FLAT and lipid microextraction. Figure 2 shows representative FLAT and lipid microextraction method spectra, demonstrating that the two methods produce highly similar spectra with the same characteristic ions and giving confidence that FLAT can be applied to microbial and resistance ID as described. Supplementary Figure S1 compares FLAT and microextraction of E. coli over the mass range 800–2200 m/z, showing that FLAT extracts lipids below 1000 m/z. Supplementary Figure S2 shows a FLAT mass spectrum of E. coli from 600 to 800 m/z. However, phospholipids below 1000 m/z generally do not differentiate bacteria by species16.
Comparison of colony smears to liquid culture
We compared FLAT extraction of a liquid sample to FLAT extraction of a colony smear of the same strain. From overnight liquid culture of E. coli ATCC 25922, 1 µL was applied to a MALDI target spot and allowed to dry. A colony smear of the same E. coli strain was applied to a second target spot. The FLAT method with buffer was performed on the MALDI plate and spectra collected. Lipid microextraction was performed on the same E. coli strain. Figure 2A shows the resulting MALDI spectra, all of which have the characteristic E. coli lipid A ion at 1796 m/z as their principal ion.
Antimicrobial resistance detection
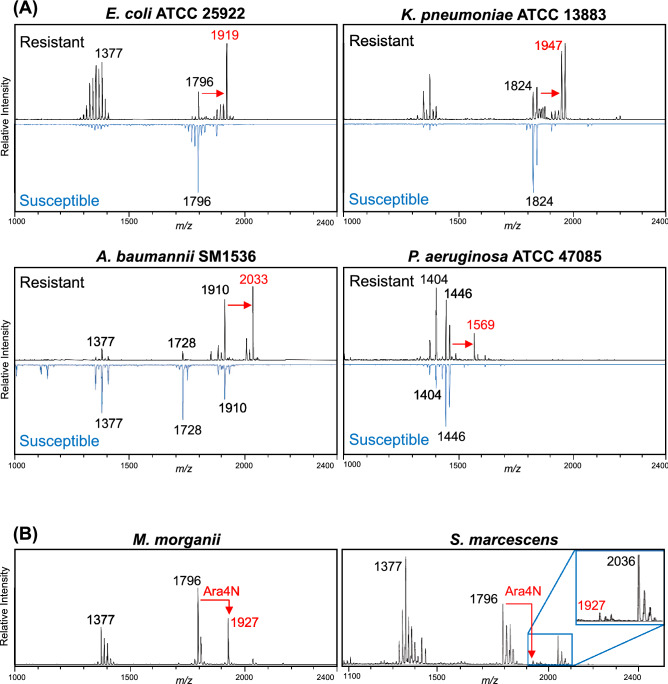
Lipid spectra are uniquely suited to identifying colistin resistance-associated ions, as previously described18,19,21–23,25,27–30. We evaluated FLAT as a rapid antimicrobial resistance assay, using bacterial strains that are either intrinsically colistin-resistant or have colistin resistance conferred through acquisition of a plasmid expressing the mcr-1 gene26,30. The mcr-1 plasmid encodes a phosphoethanolamine (PEtN) transferase and is responsible for the addition of a PEtN group to the terminal phosphate moieties of lipid A, thereby masking their negative charge and reducing membrane interactions through electrostatic binding to cationic antibiotics, such as colistin (polymyxin E). The addition of PEtN can be tracked with mass spectrometry via an increase in mass of the signature ion(s) present in the unmodified organism (Δm/z 123)27. To confirm the broad applicability of FLAT, we analyzed seven mcr-1-expressing strains from four different species (E. coli, K. pneumoniae, A. baumannii, and P. aeruginosa) and detected PEtN-modified lipid A in 100% of spectra (Fig. 3A, labeled in red). We also analyzed two bacterial species intrinsically resistant to colistin: M. morganii and S. marcescens. Lipid A from these bacterial backgrounds was modified with amino-containing sugars, including Ara4N, thereby conferring colistin resistance on these species in the absence of mcr-1 (Fig. 3B).
Figure 3.
Detection of antibiotic resistance. (A) FLAT was used on isolates expressing the gene mcr-1 from a plasmid leading to colistin resistance via phosphoethanolamine (PEtN) addition to lipid A (observed as Δm/z 123, shown in red). In resistant spectra, the intensity of the unmodified lipid A base peak is less than that of the modified ion, increasing the relative intensity of other ions in the spectra, especially ions between 1300 and 1400 m/z in E. coli and K. pneumonia. This effect is not clear in A. baumannii and P. aeruginosa, because in each case the intensity of the most prominent unmodified lipid A ion is similar to the intensity of the modified ion. (B) Intrinsically colistin-resistant bacteria Morganella morganii and Serratia marcescens were analyzed via FLAT for resistance-associated peaks. Lipid A modification with l-amino-4-arabinose (Ara4N) was observed (as Δm/z 131) in M. morganii spectra. Lipid A modification with Ara4N was observed (as Δm/z 131) in both species.
Computational comparison of FLAT and lipid microextraction
To compare FLAT and lipid microextraction semi-quantitatively, we evaluated spectra from 149 replicates of 35 strains of three Gram-positive and six Gram-negative bacterial species, including seven mcr-1 expressing strains and five strains intrinsically resistant to colistin. We compared these FLAT spectra to 148 unique lipid microextraction spectra from the same nine species. Generally, the same strains were used to generate both FLAT and lipid microextraction spectra, although additional strains were added for lipid microextraction to make a more rigorous comparison. Evaluated strains are summarized above and in Supplementary Tables S1 and S2 online.
FLAT and lipid microextraction spectra were compared by species and colistin resistance status using the averaged cosine similarity method described in “Materials and methods”, producing the two-dimensional bubble map in Fig. 4A. Larger circles represent more similar spectra. The prominent diagonal indicates that FLAT and lipid microextraction spectra are overall similar, with differences in the relative intensity of extracted lipids.
Differentiation of species and colistin resistance
We evaluated the ability of FLAT to differentiate species and colistin resistance status by evaluating the 149 FLAT spectra of three Gram-positive and six Gram-negative bacterial species described above. In this case, the FLAT spectra were compared to themselves, to specifically measure the degree to which FLAT produces differential signals by species and colistin resistance. Results are shown in Fig. 4B, where a much more prominent diagonal than in Fig. 4A is observed. In each case, the value on the diagonal is the most similar (largest) value in its column and row, indicating that FLAT produced distinct spectra for species, for colistin resistance status, and for each unique combination of species and colistin resistance status.
Discussion
FLAT rapidly extracts microbial lipid barcodes
We have shown by inspection and via average cosine similarity that, for a variety of microbial species, FLAT and lipid microextractions produce similar spectra at the species level and also that FLAT produces reliably distinct spectra for each species. FLAT thus shows obvious potential for automated species identification and antimicrobial resistance detection. In future work, we intend to demonstrate such automated identification, although not using cosine similarity as a starting point. FLAT readily detects modifications to microbial lipids, allowing detection of some but not all types of antimicrobial resistance. As with the 123 m/z difference due to phosphoethanolamine addition, specific lipid modifications conferring resistance can be detected as mass differences, even in species in which that specific resistance has not previously been observed.
Like lipid microextraction spectra, FLAT spectra provide a barcode that uniquely identifies microbial species. Unlike lipid microextraction and proposed alternatives, FLAT extraction can be accomplished in less than an hour without centrifugation. FLAT is thus a practical clinical method and amenable to straightforward automation, unlike lipid microextraction and alternatives.
When testing clinical samples, Gram status may be unknown, so a FLAT-based clinical assay can use two MALDI target spots per sample: one target spot with buffer is for Gram-negative bacteria while another spot without buffer is for Gram-positive bacteria and fungi. Samples would then be applied to both target spots. Normally, only one spot produces a high-quality spectrum and that spot would be used for identification. If both spots produce high-quality spectra, then either or both can be used for identification, requiring minimal additional processing time.
FLAT rapidly detects antimicrobial resistance
Detection of antimicrobial resistance is critical for providing effective patient treatment regimes. MALDI-based methods for antibiotic resistance have been proposed4–7, but to date have not resulted in a clinical diagnostic test. However, lipid spectra are uniquely suited to rapidly identify resistance-associated ions, as previously described, particularly in Gram-negative bacteria25,27,28. We evaluated FLAT as a rapid antimicrobial resistance assay, using bacterial strains that are either intrinsically colistin-resistant or have colistin resistance conferred by the mcr-1 plasmid. FLAT reliably detected both intrinsic colistin resistance and resistance conferred by mcr-1. In total, we demonstrate that flat extraction is capable of detecting antibiotic-resistance-associated structures in Gram-negative bacteria, thereby providing species-level identification and antibiotic susceptibility information in a single assay.
FLAT demonstrates potential as a clinical assay
Similar to lipid microextraction, FLAT extracts microbial lipid barcodes. Further, FLAT barcode spectra can be used in place of lipid microextraction spectra to identify bacteria and fungi and detect colistin resistance, using the BACLIB method. Ultimately, FLAT has important advantages to lipid microextraction and other alternatives for the BACLIB method, in that it takes less than an hour, uses clinic-friendly reagents, has low hands-on time, and does not require centrifugation. Therefore, FLAT is a potential clinical approach to the BACLIB microbial ID and resistance technique of Leung et al. FLAT thus offers important advantages to current clinical assays, in a clinic-friendly format.
Supplementary Information
Acknowledgements
The authors thank Dr. Alison Scott for her advice and assistance in preparing this manuscript. DRG and RKE thank the National Institutes of Health for funding from R01GM111066 and R01AI147314. DRG thanks the International Centre for Cancer Vaccine Science project carried out within the International Research Agendas program of the Foundation for Polish Science co-financed by the European Union under the European Regional Development Fund (MAB/2017/03) for support.
Author contributions
All authors made substantial contributions to experimental design, manuscript writing, and manuscript revision. C.E.C., L.M.L., F.M.G., S.R., and M.S. also performed mass spectrometry experiments. C.E.C., P.K., L.M.L., F.M.G., S.R., and M.S. performed cell culture and extraction procedures. M.S. performed computational comparison experiments.
Competing interests
EN, DRG, and RKE have a significant financial interest in Pataigin LLC, the company developing microbial diagnostic technology based on MS analysis of bacterial lipids. Aspects of the method described herein are covered by the following patents: United States patent 9,273,339 (inventors DRG and RKE), United States Patent 10,465,223 (inventors DRG and RKE), international patent application PCT/US2014/059853 (inventors DRG and RKE) and International patent application PCT/US2019/058213 (inventors MS and EN). All other authors declare no competing financial interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Matthew Sorensen and Courtney E. Chandler.
Contributor Information
Matthew Sorensen, Email: matthew@pataigin.com.
Robert K. Ernst, Email: Rkernst@umaryland.edu
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-78401-3.
References
- 1.Liu VX, et al. The timing of early antibiotics and hospital mortality in sepsis. Am. J. Respir. Crit. Care Med. 2017;196:856–863. doi: 10.1164/rccm.201609-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sterling SA, Miller WR, Pryor J, Puskarich MA, Jones AE. The impact of timing of antibiotics on outcomes in severe sepsis and septic shock: A systematic review and meta-analysis. Crit. Care Med. 2015;43:1907–1915. doi: 10.1097/CCM.0000000000001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vrioni G, Tsiamis C, Oikonomidis G, Theodoridou K, Kapsimali V, Tsakris A. MALDI-TOF mass spectrometry technology for detecting biomarkers of antimicrobial resistance: Current achievements and future perspectives. Ann. Transl. Med. 2018;6:240. doi: 10.21037/atm.2018.06.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparbier K, Schubert S, Weller U, Boogen C, Kostrzewa M. Matrix-assisted laser desorption ionization-time of flight mass spectrometry-based functional assay for rapid detection of resistance against β-lactam antibiotics. J. Clin. Microbiol. 2012;50:927–937. doi: 10.1128/JCM.05737-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung JS, et al. Rapid detection of antibiotic resistance based on mass spectrometry and stable isotopes. Eur J. Clin. Microbiol. Infect. Dis. 2014;33:949–955. doi: 10.1007/s10096-013-2031-5. [DOI] [PubMed] [Google Scholar]
- 6.Lange C, Schubert S, Jung J, Kostrzewa M, Sparbier K. Quantitative matrix-assisted laser desorption ionization-time of flight mass spectrometry for rapid resistance detection. J. Clin. Microbiol. 2014;52:4155–4162. doi: 10.1128/JCM.01872-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AWT, et al. Comprehensive evaluation of the MBT STAR-BL module for simultaneous bacterial identification and β-Lactamase-mediated resistance detection in gram-negative rods from cultured isolates and positive blood cultures. Front. Microbiol. 2018;9:334. doi: 10.3389/fmicb.2018.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furniss RCD, et al. Detection of colistin resistance in Escherichia coli by Use of the MALDI biotyper sirius mass spectrometry system. J. Clin. Microbiol. 2019;57:e01427–e1519. doi: 10.1128/JCM.01427-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correa-Martínez CL, Idelevich EA, Sparbier K, Kostrzewa M, Becker K. Rapid detection of extended-spectrum β-lactamases (ESBL) and AmpC β-lactamases in Enterobacterales: Development of a screening panel using the MALDI-TOF MS-based direct-on-target microdroplet growth assay. Front. Microbiol. 2019;10:13. doi: 10.3389/fmicb.2019.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correa-Martínez CL, Idelevich EA, Sparbier K, et al. Development of a MALDI-TOF MS-based screening panel for accelerated differential detection of carbapenemases in Enterobacterales using the direct-on-target microdroplet growth assay. Sci. Rep. 2020;10:4988. doi: 10.1038/s41598-020-61890-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Belkum A, et al. Developmental roadmap for antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2019;17:51–62. doi: 10.1038/s41579-018-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ventola CL. The antibiotic resistance crisis: Part 1: causes and threats. Pharm. Ther. 2015;40:277–283. [PMC free article] [PubMed] [Google Scholar]
- 13.Tsalik EL, Bonomo RA, Fowler VG., Jr New molecular diagnostic approaches to bacterial infections and antibacterial resistance. Annu. Rev. Med. 2018;69:379–394. doi: 10.1146/annurev-med-052716-030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Park KS, Karim AM, Lee CR, Lee SH. How to minimise antibiotic resistance. Lancet Infect. Dis. 2016;16:17–18. doi: 10.1016/S1473-3099(15)00467-3. [DOI] [PubMed] [Google Scholar]
- 15.Morel, C., et al. Ensuring Innovation in Diagnostics for Bacterial Infection: Implications For Policy. 55–56 (European Observatory on Health Systems and Policies, 2016). [PubMed]
- 16.Leung LM, et al. Identification of the ESKAPE pathogens by mass spectrometric analysis of microbial membrane glycolipids. Sci. Rep. 2017;7:6403. doi: 10.1038/s41598-017-04793-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lösel, D. M. Lipids in the structure and function of fungal membranes. In Biochemistry of Cell Walls and Membranes in Fungi (eds Kuhn, P. J. et al.) 119–133 (Springer, Berlin, 1990). ISBN: 978-3-642-74217-0D.
- 18.Gunn JS. Bacterial modification of LPS and resistance to antimicrobial peptides. J. Endotoxin Res. 2001;7:57–62. doi: 10.1177/09680519010070011001. [DOI] [PubMed] [Google Scholar]
- 19.Ernst RK, et al. Unique lipid a modifications in Pseudomonas aeruginosa isolated from the airways of patients with cystic fibrosis. J. Infect. Dis. 2007;196:1088–1092. doi: 10.1086/521367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mustapha MM, Li B, Pacey MP, Mettus RT, et al. Phylogenomics of colistin-susceptible and resistant XDR Acinetobacter baumannii. J. Antimicrob. Chemother. 2018;73:2952–2959. doi: 10.1093/jac/dky290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung LM, Cooper VS, Rasko DA, et al. Structural modification of LPS in colistin-resistant, KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2017;72:3035–3042. doi: 10.1093/jac/dkx234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qureshi ZA, Hittle LE, Ohara JA, et al. Colistin-resistant Acinetobacter baumannii: Beyond carbapenem resistance. Clin. Infect. Dis. 2015;60:1295–1303. doi: 10.1093/cid/civ048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelletier MR, Casella LG, Jones JW, et al. Unique structural modifications are present in the LPS from colistin-resistant strains ofAcinetobacter baumannii. Antimicrob. Agents. Chemother. 2013;57:4831–4840. doi: 10.1128/AAC.00865-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaffer SA, Harvey MD, Goodlett DR, Ernst RK. Structural heterogeneity and environmentally regulated remodeling of Francisella tularensis subspecies novicida lipid A characterized by tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2007;18:1080–1092. doi: 10.1016/j.jasms.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park YK, Lee JY, Ko KS. Transcriptomic analysis of colistin-susceptible and colistin-resistant isolates identifies genes associated with colistin resistance in Acinetobacter baumannii. Clin. Microbiol. Infect. 2015;8(765):e1–7. doi: 10.1016/j.cmi.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Liu YY, et al. Structural modification of lipopolysaccharide conferred by mcr-1 in Gram-negative ESKAPE pathogens. Antimicrob Agents Chemother. 2017;6:e00580–e617. doi: 10.1128/AAC.00580-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung LM, et al. Structural modification of LPS in colistin-resistant, KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2017;72:3035–3042. doi: 10.1093/jac/dkx234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang T, et al. Rapid microbial identification and antibiotic resistance detection by mass spectrometric analysis of membrane lipids. Anal. Chem. 2019;91:1286–1294. doi: 10.1021/acs.analchem.8b02611. [DOI] [PubMed] [Google Scholar]
- 29.Leung LM, et al. A prospective study of Acinetobacter baumannii complex isolates and colistin susceptibility monitoring by mass spectrometry of microbial membrane glycolipids. J. Clin Microbiol. 2019;57:e01100–e1118. doi: 10.1128/JCM.01100-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu YY, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 31.El Hamidi A, Tirsoaga A, Novikov A, Hussein A, Caroff M. Microextraction of bacterial lipid A: Easy and rapid method for mass spectrometric characterization. J. Lipid Res. 2005;46:1773–1778. doi: 10.1194/jlr.D500014-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Levitsky LI, Klein JA, Ivanov MV, Gorshkov MV. Pyteomics 4.0: Five years of development of a python proteomics framework. J. Proteome Res. 2019;18:709–714. doi: 10.1021/acs.jproteome.8b00717. [DOI] [PubMed] [Google Scholar]
- 33.Ryan CG, Clayton E, Griffin WL, Sie SH, Cousens DR. SNIP, A statistics-sensitive background treatment for the quantitative analysis of PIXE spectra in geoscience applications. Nucl. Instrum. Meth. B. 1988;34:396–402. doi: 10.1016/0168-583X(88)90063-8. [DOI] [Google Scholar]
- 34.Mellmann A, et al. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry in comparison to 16S rRNA gene sequencing for species identification of nonfermenting bacteria. J. Clin. Microbiol. 2008;46:1946–1954. doi: 10.1128/JCM.00157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elssner T, Kostrzewa M, Maier T, Kruppa G. Microorganism identification based on MALDI-TOF-MS fingerprints. In: Vaseashta A, Braman E, Susmann P, editors. NATO Science for Peace and Security Series A: Chemistry and Biology. Berlin: Springer; 2011. pp. 99–113. [Google Scholar]
- 36.Pence MA, Mc Elvaniatekippe E, Wallace MA, Burnham CAD. Comparison and optimization of two MALDI-TOF MS platforms for the identification of medically relevant yeast species. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1703–1712. doi: 10.1007/s10096-014-2115-x. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Lai Q, Shao Z. Genome analysis-based reclassification of Bacillus weihenstephanensis as a later heterotypic synonym of Bacillus mycoides. Int. J. Syst. Evol. Microbiol. 2018;68:106–112. doi: 10.1099/ijsem.0.002466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.