Abstract
Objectives:
Sleep duration and physical activity decline with age during adolescence. Earlier school schedules may contribute to these declines. The aim of this longitudinal study was to track changes in sleep and activity of Icelandic youth from primary to secondary school and compare students who enrolled in secondary schools with traditional and college-style schedules.
Methods:
We measured free-living sleep and activity with wrist actigraphy and body composition by dual-energy x-ray absorptiometry in 145 students at age 15 and age 17, when 58% attended schools with college-style scheduling. Differences from 15 to 17 and between students of different school structures were assessed with mixed-effect models.
Results:
Actigraphs were worn for 7.1±0.4 nights at 15 and 6.9±0.4 nights at 17. Overall, sleep duration decreased from 6.6±0.7 h/night to 6.2±0.7 h/night from age 15 to 17 (p<0.001). Students with traditional schedules reduced school-night sleep duration 26 min/night at follow-up (p<0.001), while sleep duration did not change for college-style students. All students went to bed later on school nights at follow-up, but only college-style students rose later. Sleep efficiency and awakenings did not differ by schedule-type. Neither sex changed body fat percentage, but average school-day activity decreased by 19% (p<0.001) on follow-up and did not differ by schedule-type.
Conclusions:
Over the two-year period, adolescents decreased weekly sleep duration and activity, but only those continuing traditional schedules reduced school-night sleep. This suggests greater individual control of school schedule may preserve sleep duration in this age group, which may be beneficial during the transition into adulthood.
Keywords: accelerometer, adolescents, physical activity, body composition, school schedule, sleep duration
INTRODUCTION
Sleep and physical activity are important for health and maturation in young people.1 Teenagers aged 14–17 years are recommended to sleep 8–10 hours each night2 with a consistent schedule3 and accumulate at least 60 minutes of vigorous activity 3 or more days each week.4 Cross-sectional studies have shown that insufficient sleep is associated with higher prevalence of obesity,5 lower academic achievement,6 decreased cognitive function,7 and increased symptoms of depression8. Laboratory-based sleep deprivation has also been shown to impair cognitive function,9 cause increased daytime sleepiness,10 and promote positive energy balance.11 Poor sleep quality is also associated with increased risk of obesity and diabetes,12 reduced academic performance,13 and increased symptoms of depression8 and anxiety,14 indicating that sleep quantity and quality are important during this critical period of development. Both cross-sectional and longitudinal data demonstrate that physical activity is inversely correlated with body fat percentage and depressive symptoms and positively correlated with cardiorespiratory fitness.15 During adolescence, sleep and activity are reported to decline markedly,16 and behaviors formed during this stage are known to continue into adulthood.17 Many factors are thought to contribute to these changes, including psychosocial factors, increased hours of homework,18 and greater extracurricular commitments.19 Biological development also likely contributes to changes in adolescent sleep, as older adolescents accumulate homeostatic sleep pressure more slowly20 are less sensitive to its effects21,22 than younger adolescents. Melatonin release is also delayed in older adolescents relative to younger counterparts, creating a propensity towards later bed- and rise times.23 However, early school start times often prohibit shifting rise times later, resulting in sleep curtailment for older adolescents.1,22 Most studies of sleep and activity in this age group have relied on reported measures, which generally have earlier bedtimes than objective measures24,25 and tend to over-report sleep duration26 and physical activity,11 while under-reporting nighttime awakenings.27 Studies employing concurrent objective measurements of sleep and activity for this critical period of development are sparse.
In a previous study, we objectively measured sleep and activity in a cohort of 15-year-old Icelandic adolescents.28 Sleep duration was found to be insufficient, particularly on school nights, when approximately 90% of participants spent less than the recommended 8–10 hours/night in bed. We postulated that this was due to late bedtimes (00:22 on average) combined with early school start times (08:10–08:20).28 The late bedtimes are likely caused by a variety of biological and societal factors, including the drive toward later bedtimes in older adolescents29 and an observed time-zone in Iceland that shifts sunsets 1.5 hour later than typically dictated by geography.30 Alarmed by these results, we conducted a follow-up study to investigate whether this trend continued two years later. Interestingly, after attending compulsory school with traditional schedules (approximately 08:00–15:00) from ages 6 through 16, Icelandic students apply to upper-secondary schools with either similar traditional schedules or college-style scheduling systems – where school start times can vary from day-to-day.31 Reasons for applying to schools of each type vary–some traditional schools are known for academic rigor and some college-style schools offer different academic concentrations. However, the college-style structure often gives students more opportunity to shape their school schedule. This transition presented a unique opportunity to test the hypothesis that adolescents enrolled in college-style systems have different changes in sleep and activity than those continuing traditional school schedules.
The aims of this study were to quantify changes in sleep and physical activity in Icelandic students as they transition from the last year of compulsory school at age 15 to the second year of upper-secondary school at age 17 and determine whether differences in school scheduling affect these changes. We hypothesized that sleep and physical activity would decline as reported in other adolescent populations, but that the greater control of class scheduling in the college-style system would curtail these declines.
METHODS
Participants
All students in second grade, aged seven to eight years, in six of the largest primary schools in Reykjavik, Iceland were invited to participate in a longitudinal cohort studying health, cardiovascular fitness, and physical activity initiated in 2006 (N = 320, 82% participated).32 At age 15, students from this cohort and all others who enrolled in these same schools were invited to participate (N = 411); 315 agreed (response rate 77%), and 281 had complete data for sleep and body mass index (BMI) and were reported on previously.28 Five of these participants had incomplete data for physical activity or body fat percentage, leaving 276 with complete data for all measurements (Figure 1). Two years later, 168 agreed to repeat the measurements and 145 had complete data, including 61 students (47 girls) attending secondary schools with traditional schedules and 84 (45 girls) attending secondary schools with college-style schedules. Non-participation was due to scheduling conflicts, lack of interest, or because subjects were unreachable for follow-up (n=108).
Figure 1.
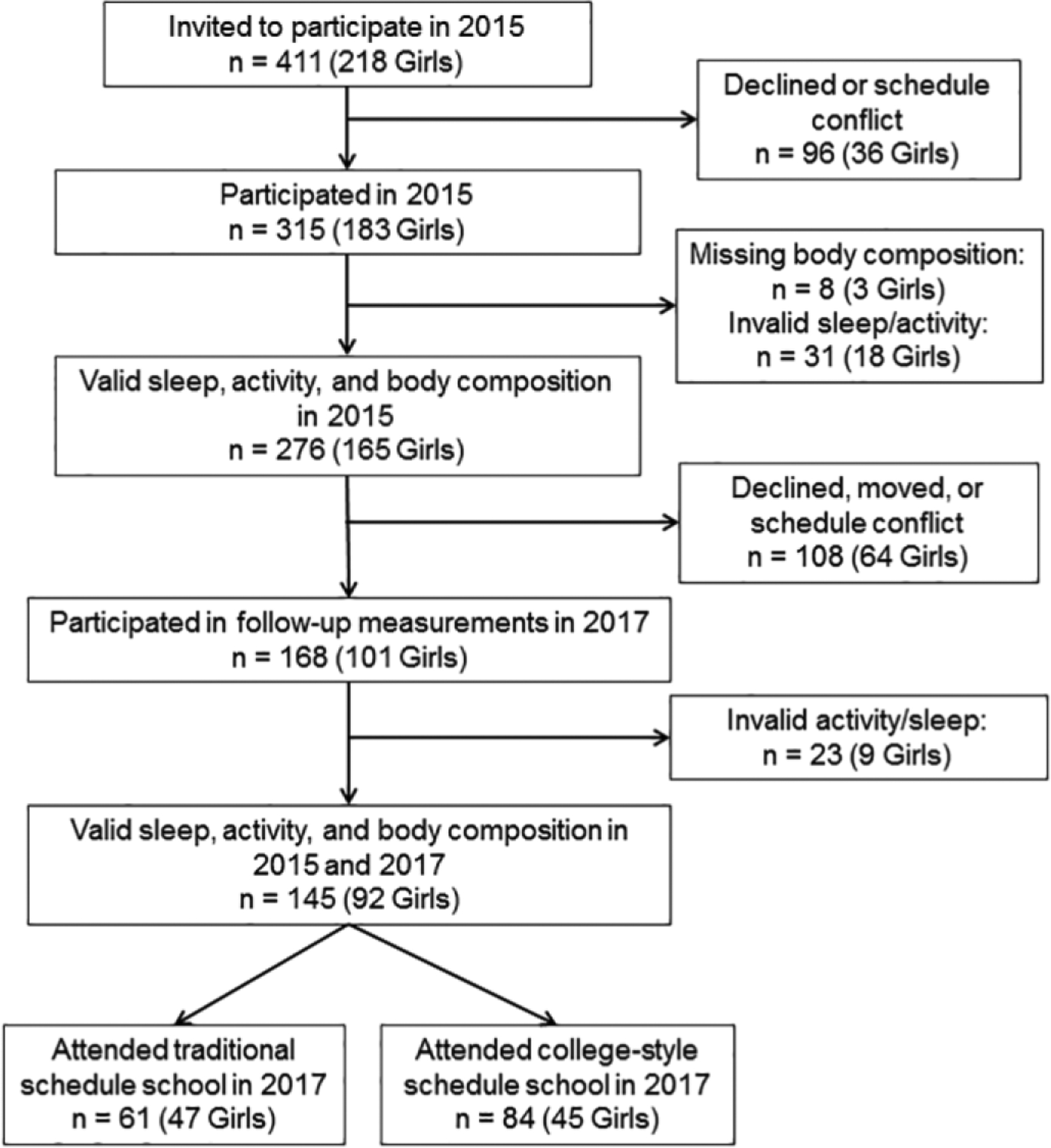
Flowchart describing study participation.
The National Bioethics Committee and the Icelandic Data Protection Authority approved the study (Study number: VSNb2015020013/13.07). Written informed consent was obtained from participants and guardians of underage participants. Full confidentiality was ensured, and the study was conducted in agreement with the guidance provided in the Declaration of Helsinki.
Sleep and Activity Measurements
Participants were instructed to wear an ActiGraph GT3X+ accelerometer (ActiGraph Inc., Pensacola, FL, USA) on their non-dominant wrist, continuously, for seven consecutive days. Due to holidays or schedule conflicts, some devices were collected after more than seven days (8–12 days) and some students (23 at age 15 and 31 at age 17) continued to wear the device for the extended period. Conversely, battery failures occurred in three cases resulting in shorter recordings of 5–6 days. Raw tri-axial accelerometer data was recorded at 80 samples/second and subsequently filtered and aggregated into one-minute activity counts with Actilife software (v6.13.0, ActiGraph, Pensacola, FL, USA). Periods of sixty or more consecutive minutes of zero counts in all axes were identified as non-wear28 by customized programs in Matlab (R2016B, Mathworks, Natick, MA, USA). Data was considered valid if the device was worn for ≥14 h on ≥3 school nights and ≥1 non-school night for sleep assessment33 and ≥ 4 days34 with at least 1 non-school day for activity analysis. Daily physical activity was represented by the tri-axial vector magnitude of each minute averaged from 12 midnight to 12 midnight the next day34 and normalized for wear-time.35
Concurrent with the week of actigraphy, participants were verbally instructed to complete a paper-based sleep diary each morning and evening. The diary was labeled for each day of the week and included time getting in bed, time of sleep onset, time of awakening, time leaving bed, and ratings for ease of falling asleep, daytime sleepiness, and daily mood. At age 15, 84% of all participants returned the sleep log, including 82% (N=119) of those with valid actigraphy and body composition data at both time points. At age 17, 80 % of all participants returned the sleep log, which also included 82% of those with valid actigraphy data at both time points. Those with valid actigraphy data who returned the sleep log completed 6.7±0.8 and 6.8±0.6 days on average while wearing the actigraph at age 15 and 17, respectively.
Bed- and rise-times were detected from the actigraphy recording in the Actilife software with an algorithm validated for adolescents36 and adjusted by two expert scorers based on visual inspection and participant sleep logs, when available.33 The mid-sleep time (midpoint between bed- and rise-times), time in bed, total sleep time, number of awakenings, minutes of wakefulness after sleep onset (WASO), and sleep efficiency (percentage of the period spent asleep) were computed for each sleep period.28,36 Averages of all valid school nights and non-school nights are presented. We quantified intra-individual variability of bedtime, rise-time, time in bed, and sleep duration using the standard deviation of all valid school nights. The longest nightly sleep period from 12 noon until 12 noon the next day was used in the analyses.
Body Composition Measurements
BMI was calculated from height and weight measured by trained personnel28 and converted to age- and sex-corrected z-score using the International Task Force for Obesity guideline.37 Body fat percentage was measured with dual energy X-ray absorptiometry (Lunar, GE Healthcare, Madison, WI, USA) which has been validated against direct imaging methods such as magnetic resonance imaging to detect longitudinal changes in adolescent body composition.38 Daily scans of a manufacturer-issued standard quality-assurance phantom for body composition measurement (IQA phantom, GE Healthcare) yielded a coefficient of variation of 0.19%.
Socioeconomic Status
Participant-reported parental educational attainment was used as a proxy for socioeconomic status.39 Students provided the educational attainment of both mother and father from the following options (presented in Icelandic): 1 = “elementary degree”, 2 = “secondary degree”, 3 = “trade school degree”, 4 = “university degree”, 5 = “other”, 6 = “do not know”, 7 = “do not want to answer”. For the current analysis, responses were recoded into a binary variable: 1 = “parent with a university degree” or 0 = “no parent with a university degree or did not answer”, as described previously.40
Sports Participation
Students self-reported participation in sports at age 15 and 17 by answering the question “Do you participate in sports?” with the options: 1 = “Yes”, 2 = “No”, 3 = “I did, but I don’t anymore” (presented in Icelandic). For the current analysis, responses were recoded into a binary variable: 1 = “participating in sports” or 0 = “not participating in sports”.
Icelandic Secondary School Scheduling
Upper-secondary schools in Iceland can adopt one of two course-scheduling systems.31 Class-based (traditional) schools offer a single daily course schedule to all students, which typically begins between 08:10–08:30 and finishes around 15:30, similar to compulsory schools. In unit-credit-based (college-style) schools, students can choose from several offerings of the same course occurring at various times. Students are only required to be present at school during their scheduled course times. Thus, daily schedules of unit-credit students are more individualized, like those of college students in many countries, and school start times can vary from 08:30 to 16:00.
National Examinations
To determine whether secondary school types may have differed in academic rigor, we compared student scores on standardized national examinations in Icelandic, English, and mathematics. The examinations are administered by the Icelandic Directorate of Education to all tenth-grade students (age 15) and used as part of the application criteria for secondary schools. Icelandic and English exams assess reading comprehension, writing, and grammar using multiple choice, short answer, and essay questions. The mathematics exam assesses knowledge of operations, geometry, and numerical understanding with multiple choice, word problems, and sentence completion. Scores are nationally normalized to a mean of 30, standard deviation of 10, and range from 0 to 60.41 We were unable to retrieve national exam information for 9 students with complete data for sleep, activity, and body composition, and 13 students were absent for testing in one or more subject area. Ultimately, test scores were available for 129 students (80 girls, 49 boys) in English, 129 students (82 girls, 47 boys) in Icelandic, and 126 (77 girls, 49 boys) in mathematics. No similar academic metrics could be included at age 17 since there are no analogous standardized national examinations after age 15 in the Icelandic education system.
Statistical Analyses
Chi-squared tests and unpaired T-tests showed no differences in the gender distribution, socioeconomic status, body composition, or activity of participants with and without complete follow-up data at age 15 (Table S1). Participants who completed follow-up had higher scores in English and mathematics, slightly less sleep time variability on school nights (−10 min), and slightly better sleep quality on non-school nights (2 fewer awakenings/night) than those who did not, but otherwise did not differ in sleep or activity at age 15 (Table S1).
Chi-squared tests were also used to assess differences in sex distribution and parental education between students attending schools of each schedule type at follow-up. Mixed-effect models with Tukey’s post-hoc tests were used for all other comparisons. Paired comparisons adjusted for parental education were used to assess changes in body composition from age 15 to 17 for boys and girls separately. Using unpaired analyses, we found few sex differences in parental education, sport participation, test scores, sleep, or activity at either age; compared to boys, girls had greater non-school day activity at 15,28 and earlier school night bedtimes at 17 (Table S2). Therefore, data from both sexes were combined. Paired analyses, adjusted for sex and parental education, were used to assess within subject differences from age 15 to 17 and from school days to non-school days at each age. Paired comparisons were again used to test for within subject difference from age 15 to 17 separately by school structure at follow-up, while unpaired analyses adjusted for sex and parental education were used to test for differences between school structures at each measurement. Linear regression was used to test for cross-sectional and longitudinal associations between body composition parameters and measures of sleep and physical activity. All regression models were adjusted for parental education. Age 17 cross-sectional models and all longitudinal models were additionally adjusted for age 17 school structure. All regression models involving body fat percentage were also adjusted for sex. All results are reported as mean ± standard deviation, unless otherwise noted. Bonferroni correction was used in all tests with multiple comparisons and corrected p-values < 0.05 were considered significant. Analyses were conducted using RStudio (v1.0.153 Boston, MA, USA) with R (v3.4.2, https://www.r-project.org/) and GraphPad Prism (v7, La Jolla, CA).
RESULTS
Changes in Body Composition from Age 15 to 17
Body composition measures are presented by sex in Table 1. Boys and girls were taller and heavier at age 17 than at age 15, but BMI z-scores and body fat percentage did not change. Boys were taller, heavier, and had lower body fat percentage than girls at both time points, but their BMIs did not differ. These results confirm expected growth patterns and known gender differences in this age group.32,33
Table 1.
Body composition of all participants at age 15 and 17.
| Boys (N=53) |
Girls (N=92) |
Boys Vs Girls |
||||||
|---|---|---|---|---|---|---|---|---|
| 15y old | 17y old | p (15 Vs 17) | 15y old | 17y old | p (15 Vs 17) | p (15y) | p (17y) | |
| Age, years | 15.8 ± 0.3 | 17.6 ± 0.3 | <0.001 | 15.9 ± 0.3 | 17.7 ± 0.3 | <0.001 | 0.09 | 0.33 |
| Height, cm | 178.8 ± 6.1 | 182.1 ± 5.9 | <0.001 | 167.3 ± 5.8 | 168. ± 5.8 | 0.001 | <0.001 | <0.001 |
| Weight, kg | 69.2 ± 12.5 | 74.3 ± 12.1 | <0.001 | 62.1 ± 10.3 | 65.5 ± 12.6 | <0.001 | <0.001 | <0.001 |
| Body mass index, kg/m2 | 21.6 ± 3.5 | 22.4 ± 3.3 | 0.003 | 22.2 ± 3.5 | 23.2 ± 4.3 | <0.001 | 1.00 | 1.00 |
| Body mass index, z-score | 0.53 ± 0.92 | 0.48 ± 0.92 | 1.00 | 0.52 ± 0.94 | 0.58 ± 1.03 | 0.832 | 1.00 | 1.00 |
| Body fat, % | 17.9 ± 8.0 | 18.0 ± 7.3 | 1.00 | 29.9 ± 6.8 | 30.9 ± 7.5 | 0.08 | <0.001 | <0.001 |
Results are means ± standard deviation. All statistical tests were controlled for multiple comparisons.
Changes in Sleep and Activity from Age 15 to 17
Over all measured nights, time in bed decreased from 7.5±0.7 h/night at age 15 to 7.1±0.8 h/night at age 17 and sleep duration decreased from 6.6±0.7 h/night to 6.2±0.8 h/night from age 15 to 17 (both p<0.001). On school nights, time in bed and total sleep time did not change from age 15 to 17, although students went to bed and rose later (both p<0.01) and increased intra-individual variability (i.e. within subject standard deviation) of time in bed and total sleep time at 17 (both p<0.001; Table 2). On non-school nights at age 17, students went to bed later (p<0.001) but did not change their rise time from age 15, resulting in shorter total sleep time (p<0.001; Table 2).
Table 2.
Sleep and physical activity for all participants on school days and non-school days at age 15 and 17.
| School Days |
Non-School Days |
School Vs Non- School |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 15y old | 17y old | p (15 Vs 17) | 15y old | 17y old | p (15 Vs 17) | p (15y) | p (17y) | ||
| Sleep | |||||||||
| Time in bed, h/day | 7.1 ± 0.8 | 6.8 ± 0.9 | 0.115 | 8.3 ± 1.4 | 7.8 ± 1.3 | <0.001 | <0.001 | <0.001 | |
| Total sleep time, h/day | 6.2 ± 0.7 | 6.0 ± 0.8 | 0.09 | 7.3 ± 1.3 | 6.9 ± 1.2 | <0.001 | <0.001 | <0.001 | |
| Bed time, clock time ± min | 00:20 ± 47.5 | 00:53 ± 62.0 | <0.001 | 01:36 ± 70.7 | 02:24 ± 84.1 | <0.001 | <0.001 | <0.001 | |
| Mid-sleep time, clock time ± min | 03:52 ± 36.1 | 04:20 ± 57.0 | <0.001 | 05:49 ± 64.0 | 06:19 ± 78.5 | <0.001 | <0.001 | <0.001 | |
| Rise time, clock time ± min | 07:25 ± 37.4 | 07:47 ± 61.5 | 0.008 | 10:04 ± 76.7 | 10:15 ± 89.7 | 0.5 | <0.001 | <0.001 | |
| WASO, min/night | 48.5 ± 21.5 | 48.1 ± 21.2 | 1.0 | 59.6 ± 28.0 | 52.8 ± 24.7 | 0.01 | <0.001 | 0.03 | |
| Awakenings, number/night | 18.2 ± 5.4 | 17.9 ± 5.6 | 1.0 | 21.9 ± 8.1 | 20.3 ± 7.3 | 0.03 | <0.000 | <0.001 | |
| Sleep efficiency, % | 88.4 ± 4.4 | 88.0 ± 4.8 | 1.0 | 88.0 ± 5.0 | 88.4 ± 4.9 | 1.0 | 0.9 | 0.6 | |
| Recorded Nights, N | 4.7 ± 0.6 | 5.3 ± 0.7 | <0.001 | 2.5 ± 0.6 | 2.0 ± 0.3 | <0.001 | <0.001 | <0.001 | |
| Valid nights, N | 4.6 ± 0.6 | 5.2 ± 0.8 | <0.001 | 2.5 ± 0.6 | 2.0 ± 0.3 | <0.001 | <0.001 | <0.001 | |
| Invalid nights, N | 0.07 ± 0.33 | 0.08 ± 0.31 | 1.0 | 0.02 ± 0.14 | 0.03 ± 0.16 | 1.0 | 0.3 | 0.3 | |
| Sleep Variability | |||||||||
| Time in bed variability, min | 51.6 ± 34.8 | 76.8 ± 47.5 | <0.001 | ||||||
| Sleep time variability, min | 47.3 ± 28.7 | 68.9 ± 43.5 | <0.001 | ||||||
| Bed time variability, min | 45.0 ± 33.3 | 48.7 ± 26.8 | 0.2 | ||||||
| Rise time variability, min | 38.8 ± 40.8 | 56.8 ± 43.4 | <0.001 | ||||||
| Physical Activity | |||||||||
| Activity, counts/wear min/day | 2207 ± 529 | 1787±382 | <0.001 | 1796 ± 556 | 1733 ± 575 | 0.6 | <0.001 | 0.6 | |
| Wear time, h/day | 23.9 ± 0.5 | 22.2 ± 1.1 | <0.001 | 23.9 ± 0.5 | 23.7 ± 1.0 | 0.5 | 1.0 | <0.001 | |
| Valid days, N | 3.7 ± 0.6 | 5.4 ± 1.0 | <0.001 | 2.5 ± 0.6 | 2.0 ± 0.3 | <0.001 | <0.001 | <0.001 |
Results are means ± standard deviation, WASO = minutes of wakening after sleep onset, Comparisons adjusted for sex, parental education, and multiple comparisons.
Sleep quality measures (i.e. nightly awakenings, WASO, and sleep efficiency) did not change from age 15 to 17 on school nights, but awakenings and WASO were both lower on non-school nights at age 17 (p<0.05, Table 2). Students went to bed later, rose later, slept longer, and had greater awakenings and WASO on non-school nights than school nights during both measurements (all p<0.001, Table 2), with the increase in WASO being directly proportional to the increase in sleep duration (~5.5 minutes of WASO/hour of sleep; p<0.001).
Average activity decreased by 13.1% over all days (2042±494 to 1773±393 counts/min of wear/day, p<0.001) and by 19.0% on school days (p<0.001) from 15 to 17 (Table 2). However, there was no change in non-school day activity from 15 to 17 (Table 2). At age 15 students were 18.5% more active on school days than non-school days (p<0.001); but two years later, activity on school days and non-school days was not different (Table 2).
Changes in Body Composition, Sleep, and Activity by School Schedule-Type
Participants attending both secondary school systems did not differ in parental education, sports participation (Table 3), BMI z-score, or activity at either age (Figure 2, Table S3 and S4). Combined data from both sexes showed that body fat increased from 25.6±8.4% to 26.9±8.6% in students that continued traditional school schedules (p=0.04) but did not change for students that switched to college-style schedules. However, there were no changes in body fat when boys and girls were analyzed separately (Figure 2, Table S3 and S4). At age 15, students who went on to attend traditional-schedule schools at age 17 scored higher than those who went on to attend college-style schools on national exams in Icelandic and mathematics (both p<0.001; Table 3).
Table 3.
Characteristics and sleep on school days and non-school days at age 15 and 17 of students attending traditional and college-style schedule schools at age 17.
| Traditional Schedule (N=61, 77% Female) | College-Style Schedule (N=84, 53.6% Female) | Traditional Vs College-style |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 15y old | 17y old | p (15 vs 17) | 17y old | 17y old | p (15 vs 17) | p (15y) | p (17y) | ||||
| Characteristics | |||||||||||
| Parent with university degree, N (%) | 47 (77%) | 56 (67%) | 0.24 | ||||||||
| Organized sports participation, N (%) | 48 (79%) | 31 (51%) | < 0.001 | 53 (63%) | 37 (44%) | 0.005 | 0.1 | 1.0 | |||
| National English exama,b,c | 35.76 ± 8.09 | 33.09 ± 9.19 | 0.14 | ||||||||
| National Icelandic exama,b,d | 38.69 ± 7.85 | 31.97 ± 8.22 | <0.001 | ||||||||
| National mathematics exama,b,e | 38.25 ± 7.67 | 31.63 ± 8.56 | <0.001 | ||||||||
| School Nights | |||||||||||
| Time in bed, h/day | 7.2 ± 0.7 | 6.7 ± 0.8 | <0.001 | 7.0 ± 0.8 | 6.9 ± 0.9 | 1.0 | 0.3 | 0.9 | |||
| Mid-sleep time, clock time ± min | 03:42 ± 31.8 | 03:54 ± 40.4 | 0.2 | 03:59 ± 37.6 | 04:38 ± 60.4 | <0.001 | 0.1 | <0.001 | |||
| WASO, min/night | 47.7 ± 18.7 | 45.0 ± 17.4 | 1.0 | 49.1 ± 23.4 | 50.3 ± 23.4 | 1.0 | 1.0 | 0.5 | |||
| Awakenings, number/night | 18.5 ± 4.7 | 17.2 ± 4.5 | 0.2 | 18.0 ± 5.8 | 18.4 ± 6.2 | 1.0 | 1.0 | 0.8 | |||
| Sleep efficiency, % | 88.7 ± 3.8 | 88.6 ± 4.3 | 1.0 | 88.1 ± 4.7 | 87.5 ± 5.2 | 0.8 | 1.0 | 0.6 | |||
| Time in bed variability, min | 47.4 ± 36.0 | 65.8 ± 48.9 | 0.04 | 54.7 ± 33.8 | 84.7 ± 45.1 | <0.001 | 0.9 | 0.02 | |||
| Sleep time variability, min | 44.8 ± 31.4 | 57.5 ± 42.4 | 0.2 | 49.2 ± .6.5 | 77.2 ± 42.7 | <0.001 | 1.0 | 0.002 | |||
| Bed time variability, min | 40.6 ± 32.5 | 41.8 ± 26.1 | 1.00 | 48.2 ± 33.6 | 53.8 ± 26.2 | 0.7 | 0.7 | 0.11 | |||
| Rise time variability, min | 27.7 ± 21.6 | 42.3 ± 37.5 | 0.2 | 46.8 ± 49.0 | 67.3 ± 44.6 | 0.003 | 0.02 | 0.001 | |||
| Recorded nights, N | 4.7 ± 0.6 | 5.3 ± 0.7 | <0.001 | 4.’ ± 0.6 | 5.3 ± 0.7 | <0.001 | 1.0 | 1.0 | |||
| Valid nights, N | 4.7 ± 0.6 | 5.2 ± 0.8 | <0.001 | 4.6 ± 0.6 | 5.2 ± 0.8 | <0.001 | 1.0 | 1.0 | |||
| Invalid nights, N | 0.03 ± 0.26 | 0.02 ± 0.15 | 0.6 | 0.08 ± 0.38 | 0.07 ± 0.26 | 0.5 | 1.0 | 1.0 | |||
| Non-school Nights | |||||||||||
| Time in bed, h/day | 8.4 ± 1.2 | 7.8 ± 1.2 | 0.053 | 8.3 ± 1.5 | 7.8 ± 1.4 | 0.01 | 1.0 | 1.0 | |||
| Mid-sleep time, clock time ± min | 05:31 ± 59.1 | 06:08 ± 70.6 | 0.003 | 06:03 ± 64.4 | 06:27 ± 83.3 | 0.04 | 0.04 | 0.6 | |||
| WASO, min | 58.87 ± 25.7 | 50.0 ± 20.1 | 0.1 | 60.1 ± 29.7 | 54.8 ± 27.5 | 0.5 | 1.0 | 1.0 | |||
| Awakenings, number/night | 22.2 ± 7.6 | 19.6 ± 6.4 | 0.1 | 21.8 ± 8.5 | 20.8 ± 8.0 | 1.0 | 1.0 | 1.0 | |||
| Sleep efficiency, % | 88.2 ± 4. 7 | 88.8 ± 4.6 | 1.0 | 87.9 ± 5.2 | 88.2 ± 5.1 | 1.0 | 1.0 | 1.0 | |||
| Recorded nights, N | 2. ± 0.6 | 2.0 ± 0.3 | <0.001 | 2.5 ± 0.6 | 2.0 ± 0.3 | <0.001 | 0.4 | 1.0 | |||
| Valid nights, N | 2.6 ± 0.7 | 2.0 ± 0.3 | <0.001 | 2.5 ± 0.6 | 2.0 ± 0.4 | <0.001 | 0.9 | 1.0 | |||
| Invalid nights, N | 0.02 ± 0.13 | 0.02 ± 0.13 | 1.0 | 0.02 ± 0.15 | 0.04 ± 0.19 | 1.0 | 0.9 | 1.0 | |||
Results are means ± standard deviation; All comparisons corrected for sex, parental education, and multiple comparisons; WASO = minutes of wakening after sleep onset;
Nationally normalized (mean = 30, standard deviation = 10, maximum score = 60); National test scores available for:
55 traditional schedule students (13 boys, 42 girls),
74 college-style schedule students (36 boys, 38 girls),
74 college-style schedule students (34 boys, 40 girls),
71 college-style schedule students (36 boys, 35 girls).
Figure 2.
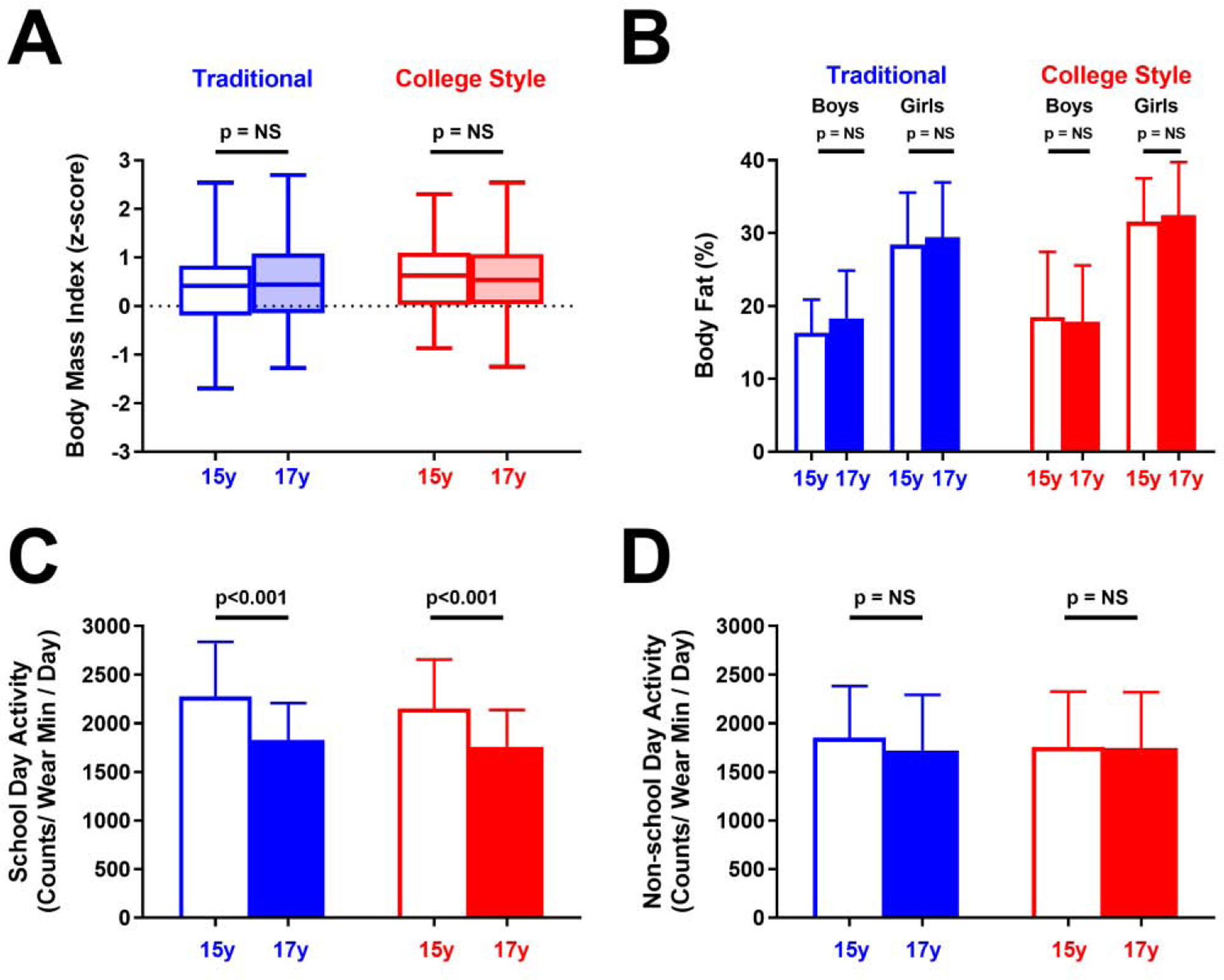
Body composition and physical activity by age 17 school-schedule type. (A, B) Traditional (blue) and college-style (red) students showed no change in body-mass index z-score (A) or body fat percentage (B) from age 15 (unfilled) to 17 (filled). (C, D) Traditional and college-style students showed similar school day reduction in activity (C) and no change in non-school day activity (D) from age 15 to 17. Box (median with first and third quartile) and whiskers (95% confidence interval) are used for BMI z-score in (A); all other results are means (boxes) with standard deviations (error bars). P-values indicate paired, within school-type comparisons between ages 15 and 17 adjusted for sex, parental education, and multiple comparisons.
Over all measured nights, time in bed decreased from age 15 to 17 from 7.61±0.66 to 7.02±0.73 h/night for traditional schedule students and from 7.45±0.72 to 7.13±0.79 h/night college-style students (both p≤0.001). Similarly, total sleep time over all nights declined from 6.73±0.59 to 6.22±0.77 h/night for traditional schedule students and from 6.54±0.69 to 6.25±0.74 h/night for college-style students (both p≤0.001). However, the magnitude of these reductions did not differ by schedule type. On school nights, students of both school types went to bed later at age 17 compared to age 15 (+25 minutes for traditional, +40 minutes for college-style, both p<0.01; Figure 3A), but those in college-style schools rose later at age 17 (p<0.001; Figure 3A). As a result, time in bed and total sleep time on school nights was reduced at age 17 for traditional schedule students but did not change for students with college-style schedules (Table 3, Figure 3B). On non-school nights, students of both school schedule types went to bed more than 40 minutes later (both p<0.001) but did not differ in rise time at age 15 and 17 (Figure 3C).Time in bed and sleep duration on non-school nights did not change for traditional schedule student between ages 15 and 17, but both were reduced for college-style students (both p<0.05) - perhaps indicating a reduction in catch-up sleep (Table 3 and Figure 3D).
Figure 3.
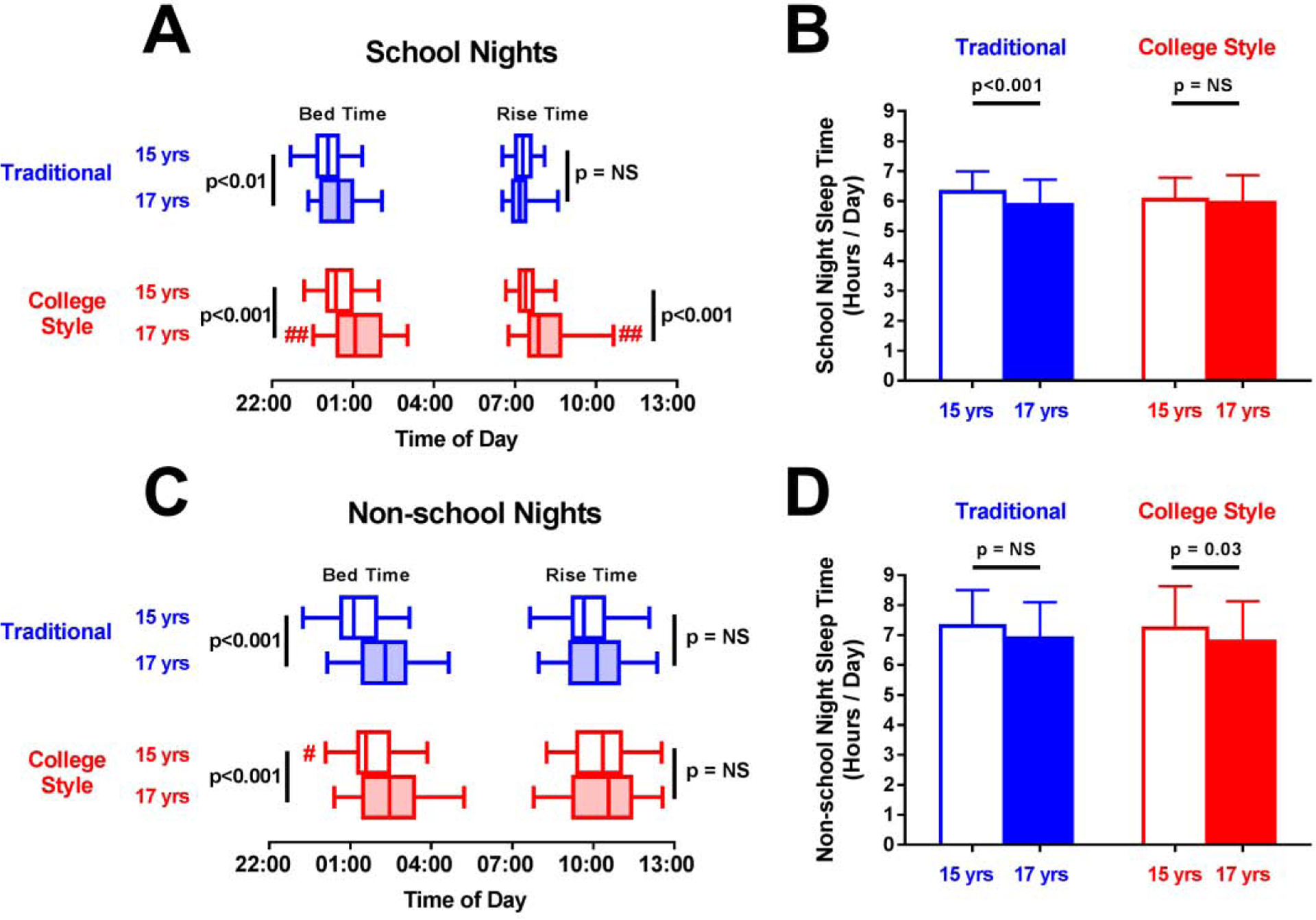
Sleep schedule and duration on school nights and non-school nights by age 17 school-schedule type. (A, B) On school nights, both traditional (blue) and college-style (red) students went to bed later at 17 (filled) versus 15 (unfilled), but college-style student rose later at age 17 (A). Thus, only traditional-school students reduced sleep duration at 17 (B). (C, D) On non-school-nights, both traditional and college-style students went to bed later and rose at the same time at age 17 versus 15 (C), but only college-style students reduced sleep duration (D). Box (median with first and third quartile) and whiskers (95% confidence interval) are used for bed- and rise-times; mean (boxes) with standard deviation (error bars) is used for sleep duration. P-values with black bars indicate paired, within school-type comparisons between ages 15 and 17. # (p<0.05) and ## (p<0.01) indicate significant differences between traditional and college-style students at age 15 or 17. All comparisons adjusted for sex, parental education, and multiple comparisons.
Sleep quality (awakenings, WASO, and sleep efficiency) did not differ by school schedule-type on either school nights or non-school nights at age 15 or 17 (Table 3). Students of both school types had increased variability of time spent in bed at age 17 compared to 15, but only college-style students also showed greater intra-individual variability in rise time and total sleep time on school nights (p≤0.02 vs. traditional schedule students; Table 3).
In sex-specific analyses, changes in sleep patterns from age 15 to 17 for girls from each schedule-type were similar to those of the entire group (Table S3). Changes in sleep for boys attending college-style schools were also largely similar to the entire group, with one notable exception - they did not increase variability in school day rise time at age 17 (Table S4). Conversely, the small number of boys attending traditional schools at age 17 in our sample (N=14) had few significant changes in sleep from age 15 (Table S4).
Regression Analysis of Body Composition Versus Sleep and Activity
Linear regression was used to explore potential cross-sectional and longitudinal associations between body composition and sleep and activity (Table S5). Mean sleep duration over all nights was negatively correlated with BMI z-score at age 15 (p=0.03), but this association did not persist at age 17 and change in sleep duration from age 15 to 17 was not associated with change in BMI z-score. There were no significant cross-sectional or longitudinal associations between sleep duration and body fat percentage or between sleep quality measures (e.g. sleep efficiency and WASO) and BMI or body fat percentage (Table S5).
After adjusting for sex and school schedule type, physical activity was negatively correlated to body fat percentage at age 17 (p=0.002), but not at age 15. Physical activity did not correlate with BMI z-score at either age, and longitudinal changes in activity did not correlate with changes in body fat or BMI z-score.
DISCUSSION
In this longitudinal study of Icelandic adolescents, we found that nightly sleep duration over the whole week decreased from age 15 to 17. However, on school nights, this reduction was only seen in students who continued a traditional schedule and not in those who switched to a college-style scheduling system. We also observed a profound reduction in school day activity at age 17, but it did not differ by school scheduling system.
Our previous study of 15-year-old Icelandic adolescents revealed that only 10.9% spent the recommended 8 h/night in bed on school nights, with an average sleep duration of 6.2 hours per school night,28 which is among the shortest reported in this age group.42,43 At age 17, school night sleep duration remained low, at 6.0 h/night, and the percentage of students getting the recommended amount of sleep on school nights dropped to 6.9%. These findings suggest a widespread and consistent pattern of short sleep, which is associated with problems in cognition,7 obesity,5 and poor academic achievement,6 and may lead to continued sleep problems and other deleterious health effects in adulthood.17
The shortened sleep duration from age 15 to 17 is in line with previous findings of a 14 min/year decline in sleep during adolescence.1,44 The sleep reduction observed here stems from a 33 min/night later bedtime which was not compensated by a similar shift in rise time. The later bedtime may be the result of a shift in circadian rhythm caused by delayed melatonin release29 and a slower accumulation of sleep pressure20 at this stage of puberty. Previous work has also demonstrated that older adolescents are less sensitive to sleep pressure than younger adolescents and adults21,22. Thus, despite experiencing the same deleterious effects of sleep deprivation, they do not notice it as readily and are less likely to alter their sleep schedule accordingly. Alternatively, changes in sleep timing and the reduction in sleep may be the result of external changes, such as increased media usage,40 reduced parental supervision, more recreational activities, work, and/or increased academic demands. While some studies using objective methods have reported comparably short adolescent sleep durations,42,45 the average bedtime of 01:19 at age 17 is notably later than the reported bedtimes of similarly aged groups from other countries.43,46 This may be due in part to a 1.5 hour mismatch between Iceland’s geographical position and its adopted time-zone of Greenwich Meantime.30
Students who attended schools with college-style course scheduling had later rise-times than those who continued in schools with traditional schedules resulting in better preservation of total sleep time from age 15 to 17. College-style students also reduced time in bed and total sleep time on non-school nights while traditional schedule students did not, perhaps indicating reduced need for catchup sleep amongst college-style student. These finding supports the idea that later school start times may be more suitable for the natural sleep patterns of older adolescents. Meta-analysis of previous cross-sectional and longitudinal studies has demonstrated that delaying school start times by 15–130 minutes is associated with longer sleep durations,47 which can lead to enhanced cognitive performance, improved attention levels, and greater academic success.1 Although students of the two school types did not differ in sleep quality measures such as sleep efficiency and WASO, college-style students had more intra-individual night-to-night variability in total sleep duration and rise-times on school nights than their counterparts with traditional schedules. The higher variability in sleep schedule may be due to greater day-to-day variation in class schedule amongst college-style students, specifically more varied school day start times. High nightly variability in sleep is known to negatively impact body composition,48 perceived health, and cognitive function. Thus, it is recommended that adolescents maintain a consistent sleep routine.3
Across the entire group, average activity declined by 13.1% over all measured days between age 15 and 17, explained mainly by a 19% reduction on school days. This substantial reduction in activity is in line with previous studies conducted in Iceland49 and elsewhere16,50 that suggest adolescent activity declines about 7% per year. These findings highlight the large impact school day activity has on overall activity in this age group.51 Further study is needed to determine if changes to physical education policy can mitigate the reduction in school day activity.
Changes in activity and BMI did not differ between students attending traditional and college-style schools. Similarly, sex-specific comparisons demonstrated no significant changes in body fat percentage for either school type. These results suggest that changes in activity and body composition are likely due to factors in addition to school schedule type. Cross-sectional regression demonstrated that sleep duration and physical activity were inversely correlated with measures of body composition, but the trends were not consistent at both ages and there were no longitudinal associations between body composition and sleep or activity. However, a longer duration between baseline and follow up is likely needed to detect whether changes in sleep and/or activity habits are associated with meaningful changes in body composition.
To our best knowledge, this is the first longitudinal study to objectively measure changes in sleep and physical activity in a cohort of youth (age 15 to 17) during a change in educational structure. Most previous studies of sleep in this age group have relied on self- or parent-report of typical time in bed or bed- and rise-times and have observed average sleep durations of 7 hours or more, with a few notable exceptions, mostly from Asian countries.52 Self-reported measures do not normally account for onset latency or brief awakenings during sleep53 and tend to over-report sleep time.26,53 In the current analysis, bedtimes and rise-times were detected in the actigraphy recordings and, thus, may be closer to sleep onset and awakening times rather than times of getting in and out of bed. For instance, over all nights for participants with concurrent actigraphy and sleep log information, actigraphy-measured bedtimes were closer to reported sleep onset times than reported time of entering bed. This methodological difference may partly explain the shorter sleep duration found in this study, although some studies of this age group with comparable objective measures have reported longer sleep durations.45 However, longitudinal trends in bedtime over all measured nights were consistent whether using actigraphy-measured bedtime (00:46 ± 47 min at 15y vs. 01:12 ± 59 min at 17y, p<0.001), reported sleep onset time (00:40 ± 50 min at 15y vs. 00:58 ± 58 min at 17y, p<0.001), or reported time of entering bed (00:07 ± 47 min at 15y vs. 00:22 ± 55 min at 17y, p<0.001), and all three measures were highly correlated (r>0.76 at both ages).
During this study, we focused our analysis on the primary nightly sleep period. Daytime naps were rare, with only 14 total naps identified in 8 different subjects.28 A recent study demonstrated approximately 62% of their teenage participants reported taking naps and both actigraphy-detected and self-reported naps were associated with shorter and more disrupted night-time sleep.54 However, although wrist actigraphy has high accuracy and sensitivity compared to polysomnography, its specificity is limited55, and there is currently no accepted criterion for scoring actigraphy-assessed naps54. We felt that the automated sleep detection algorithm may have been inadequate to detect naps and, although participants were instructed to log sleep periods, our sleep diary did not explicitly ask about napping. Thus, there were no validated automated methods or confirmatory logs to determine whether short periods of inactivity outside of the primary sleep period were naps.
We did not assess the prevalence of delayed sleep phase syndrome (DSPS) on school days following a weekend or holiday, when sleep schedules were likely shifted later. However, previous studies found a 2–3% prevalence of DSPS in adolescents.56,57 Thus, it is unlikely that the presence of DSPS significantly affected the results of our study.
Holiday periods and seasonal changes in daylength and weather can affect sleep and activity58. Due to schedule conflicts, age 15 measurements were collected from April to June and age 17 measurements from February to April. More holidays occurred during the age 15 data collection period, resulting in fewer school nights and more non-school nights for some students at age 15 than at age 17. Daylength and weather were also different during the age 15 and 17 measurement periods. However, there were few significant cross-sectional correlations between either sleep or activity and daylength: activity was positively correlated to daylength at age 15 and bedtime was inversely correlated to daylength at age 17. The differences in daylength from the age 15 measurement to the age 17 measurement were similar for students of the two school schedule types. Thus, it was unlikely that changes in daylength alone could explain difference in sleep duration or schedule between the two school types.
Assignment to traditional and college-style schools at age 16 was not experimentally controlled. Thus, we could not control the gender distribution between different secondary school schedule systems. Similarly, students did not report reasons for secondary school preferences, and we cannot rule out other potential causes for differences in sleep schedule and duration changes between the two school types. For instance, we did not directly measure academic workload or participation in all extra-curricular activities and, thus, cannot assess how these factors may have impacted sleep and activity. However, students attending traditional-schedule schools had higher average national exam test scores at age 15, supporting the notion that these schools may have more selective academic entrance requirements. Conversely, participation in organized sports did not differ across school type at either age, suggesting factors other than school type contribute to a decline in sports participation. Future studies with the opportunity to randomized school assignment should consider these factors in experimental design.
Due to the longitudinal design, we did not power the study to detect a predefined difference. However, data from this study is useful to design intervention studies in the field of sleep and health in adolescents. Sample size was relatively small, although it was representative of Reykjavík students in this age group during the study period (i.e., 1,355 15-year-olds in 2015 and 1,382 17-year-olds in 2017).59 Finally, the participants were Icelandic, generally healthy, and few were overweight or obese (14% at age 15 and 17% at age 17), potentially limiting generalizability.
CONCLUSIONS
From age 15 to 17, Icelandic adolescents reduced sleep duration, became less active, and shifted to a later bedtime. However, students attending schools with college-style systems with greater control of their school schedule showed less drastic change in sleep duration. These results suggest that school schedules can impact adolescent sleep patterns, but further follow-up is needed to determine if different schedules lead to any long-term differences in health or sleep behavior in adulthood.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Hans Haraldsson for additional statistical support, Cindy Clark of the NIH Library Writing Center and the University of Iceland Writing Center for manuscript editing assistance, study participants and staff of participating schools, and master’s students and researchers involved in data collection.
FUNDING
Primary funding was supplied by the Icelandic Centre for Research (RANNIS) (grant number: 152509-051). Additional support was provided by the Ministry of Education, Science and Culture; the Iceland Primary Health Care Research Fund, and doctoral grants from the University of Iceland Research Fund. KYC and RJB were funded by the National Institute of Diabetes and Digestive and Kidney Diseases intramural research program (Z01 DK071013 and Z01 DK071014).
ABBREVIATIONS:
- BMI
Body mass index
- WASO
Wake after sleep onset
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Owens J, Adolescent Sleep Working Group. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921–e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirshkowitz M, Whiton K, Albert SM, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. [DOI] [PubMed] [Google Scholar]
- 3.Gruber R, Carrey N, Weiss SK, et al. Position statement on pediatric sleep for psychiatrists. Can Child Adolesc Psychiatr Rev. 2014;23(3):174. [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Global recommendations on physical activity for health. World Health Organization, Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 5.Garaulet M, Ortega FB, Ruiz JR, et al. Short sleep duration is associated with increased obesity markers in European adolescents: effect of physical activity and dietary habits. The HELENA study International journal of obesity (2005). 2011;35(10):1308–1317. [DOI] [PubMed] [Google Scholar]
- 6.Hysing M, Harvey AG, Linton SJ, Askeland KG, Sivertsen B. Sleep and academic performance in later adolescence: results from a large population-based study. Journal of sleep research. 2016;25(3):318–324. [DOI] [PubMed] [Google Scholar]
- 7.Lo JC, Ong JL, Leong RL, Gooley JJ, Chee MW. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep. 2016;39(3):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sivertsen B, Harvey AG, Lundervold AJ, Hysing M. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16–18 years. Eur Child Adolesc Psychiatry. 2014;23(8):681–689. [DOI] [PubMed] [Google Scholar]
- 9.Carskadon MA, Harvey K, Dement WC. Sleep loss in young adolescents. Sleep. 1981;4(3):299–312. [DOI] [PubMed] [Google Scholar]
- 10.Fallone G, Owens JA, Deane J. Sleepiness in children and adolescents: clinical implications. Sleep Med Rev. 2002;6(4):287–306. [DOI] [PubMed] [Google Scholar]
- 11.Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110(14):5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nedeltcheva AV, Scheer FA. Metabolic effects of sleep disruption, links to obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2014;21(4):293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bogels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Med Rev. 2010;14(3):179–189. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, Su H, Zou Y, Chen J, Wu J, Chang W. Sleep quality of Chinese adolescents: distribution and its associated factors. J Paediatr Child Health. 2012;48(2):138–145. [DOI] [PubMed] [Google Scholar]
- 15.Hallal PC, Victora CG, Azevedo MR, Wells JC. Adolescent physical activity and health. Sports Med. 2006;36(12):1019–1030. [DOI] [PubMed] [Google Scholar]
- 16.Dumith SC, Gigante DP, Domingues MR, Kohl HW 3rd. Physical activity change during adolescence: a systematic review and a pooled analysis. International journal of epidemiology. 2011;40(3):685–698. [DOI] [PubMed] [Google Scholar]
- 17.Dregan A, Armstrong D. Adolescence sleep disturbances as predictors of adulthood sleep disturbances—a cohort study. J Adolesc Health. 2010;46(5):482–487. [DOI] [PubMed] [Google Scholar]
- 18.Adam EK, Snell EK, Pendry P. Sleep timing and quantity in ecological and family context: a nationally representative time-diary study. J Fam Psychol. 2007;21(1):4–19. [DOI] [PubMed] [Google Scholar]
- 19.Short MA, Gradisar M, Lack LC, et al. A cross-cultural comparison of sleep duration between US And Australian adolescents: the effect of school start time, parent-set bedtimes, and extracurricular load. Health Educ Behav. 2013;40(3):323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58(3):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28(11):1446–1454. [DOI] [PubMed] [Google Scholar]
- 22.Tarokh L, Saletin JM, Carskadon MA. Sleep in adolescence: Physiology, cognition and mental health. Neurosci Biobehav Rev. 2016;70:182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12(3):278–289. [DOI] [PubMed] [Google Scholar]
- 24.Brychta RJ, Rognvaldsdottir V, Guethmundsdottir SL, et al. Longitudinal Change in Adolescent Bedtimes Measured by Self-Report and Actigraphy. J Meas Phys Behav. 2019;2(4):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfson AR, Carskadon MA, Acebo C, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26(2):213–216. [DOI] [PubMed] [Google Scholar]
- 26.Short MA, Gradisar M, Lack LC, Wright H, Carskadon MA. The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Med. 2012;13(4):378–384. [DOI] [PubMed] [Google Scholar]
- 27.Werner H, Molinari L, Guyer C, Jenni OG. Agreement rates between actigraphy, diary, and questionnaire for children’s sleep patterns. Arch Pediatr Adolesc Med. 2008;162(4):350–358. [DOI] [PubMed] [Google Scholar]
- 28.Rognvaldsdottir V, Gudmundsdottir SL, Brychta RJ, et al. Sleep deficiency on school days in Icelandic youth, as assessed by wrist accelerometry. Sleep Med. 2017;33:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21(8):871–881. [DOI] [PubMed] [Google Scholar]
- 30.Thorleifsdottir B, Björnsson J, Benediktsdottir B, Gislason T, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53(1):529–537. [DOI] [PubMed] [Google Scholar]
- 31.Ragnarsdóttir G, Ásgeir Jóhannesson I. Curriculum, crisis and the work and well-being of Icelandic upper secondary school teachers. Education Inquiry. 2014;5(1):24045. [Google Scholar]
- 32.Magnusson KT, Hrafnkelsson H, Sigurgeirsson I, Johannsson E, Sveinsson T. Limited effects of a 2-year school-based physical activity intervention on body composition and cardiorespiratory fitness in 7-year-old children. Health Educ Res. 2012;27(3):484–494. [DOI] [PubMed] [Google Scholar]
- 33.Sadeh A The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15(4):259–267. [DOI] [PubMed] [Google Scholar]
- 34.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181. [DOI] [PubMed] [Google Scholar]
- 35.Ozemek C, Kirschner MM, Wilkerson BS, Byun W, Kaminsky LA. Intermonitor reliability of the GT3X+ accelerometer at hip, wrist and ankle sites during activities of daily living. Physiol Meas. 2014;35(2):129. [DOI] [PubMed] [Google Scholar]
- 36.Sadeh A, Sharkey M, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. [DOI] [PubMed] [Google Scholar]
- 37.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7(4):284–294. [DOI] [PubMed] [Google Scholar]
- 38.Bridge P, Pocock NA, Nguyen T, et al. Validation of longitudinal DXA changes in body composition from pre- to mid-adolescence using MRI as reference. J Clin Densitom. 2011;14(3):340–347. [DOI] [PubMed] [Google Scholar]
- 39.Marco CA, Wolfson AR, Sparling M, Azuaje A. Family socioeconomic status and sleep patterns of young adolescents. Behav Sleep Med. 2012;10(1):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hrafnkelsdottir S, Brychta R, Rognvaldsdottir V, et al. Less screen time and more frequent vigorous physical activity is associated with lower risk of reporting negative mental health symptoms among Icelandic adolescents. PLoS One. 2018;13(4):e0196286–e0196286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saevarsson ES, Gudmundsdottir SL, Kantomaa M, et al. Above average increases in body fat from 9 to 15 years of age had a negative impact on academic performance, independent of physical activity. Acta Paediatr. 2019;108(2):347–353. [DOI] [PubMed] [Google Scholar]
- 42.Matthews KA, Dahl RE, Owens JF, Lee L, Hall M. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep. 2012;35(10):1353–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hysing M, Pallesen S, Stormark KM, Lundervold AJ, Sivertsen B. Sleep patterns and insomnia among adolescents: a population‐based study. Journal of sleep research. 2013;22(5):549–556. [DOI] [PubMed] [Google Scholar]
- 44.Olds T, Blunden S, Petkov J, Forchino F. The relationships between sex, age, geography and time in bed in adolescents: a meta-analysis of data from 23 countries. Sleep Med Rev. 2010;14(6):371–378. [DOI] [PubMed] [Google Scholar]
- 45.Tonetti L, Fabbri M, Filardi M, Martoni M, Natale V. Effects of sleep timing, sleep quality and sleep duration on school achievement in adolescents. Sleep Med. 2015;16(8):936–940. [DOI] [PubMed] [Google Scholar]
- 46.Knutson KL, Lauderdale DS. Sociodemographic and behavioral predictors of bed time and wake time among US adolescents aged 15 to 17 years. J Pediatr. 2009;154(3):426–430. e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowers JM, Moyer A. Effects of school start time on students’ sleep duration, daytime sleepiness, and attendance: a meta-analysis. Sleep Health. 2017;3(6):423–431. [DOI] [PubMed] [Google Scholar]
- 48.Golley RK, Maher CA, Matricciani L, Olds T. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. International journal of obesity (2005). 2013;37(4):546. [DOI] [PubMed] [Google Scholar]
- 49.Gestsdottir S, Svansdottir E, Ommundsen Y, et al. Do aerobic fitness and self-reported fitness in adolescence differently predict body image in young adulthood? An eight year follow-up study. Ment Health Phys Act. 2016;10:40–47. [Google Scholar]
- 50.Collings PJ, Wijndaele K, Corder K, et al. Magnitude and determinants of change in objectively-measured physical activity, sedentary time and sleep duration from ages 15 to 17.5 y in UK adolescents: the ROOTS study. Int J Behav Nutr Phys Act. 2015;12(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marks J, Barnett LM, Strugnell C, Allender S. Changing from primary to secondary school highlights opportunities for school environment interventions aiming to increase physical activity and reduce sedentary behaviour: a longitudinal cohort study. Int J Behav Nutr Phys Act. 2015;12(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: A review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12(2):110–118. [DOI] [PubMed] [Google Scholar]
- 53.Arora T, Broglia E, Pushpakumar D, Lodhi T, Taheri S. An investigation into the strength of the association and agreement levels between subjective and objective sleep duration in adolescents. PLoS One. 2013;8(8):e72406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jakubowski KP, Hall MH, Lee L, Matthews KA. Temporal Relationships Between Napping and Nocturnal Sleep in Healthy Adolescents. Behav Sleep Med. 2017;15(4):257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson EO, Roth T, Schultz L, Breslau N. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117(2):e247–256. [DOI] [PubMed] [Google Scholar]
- 57.Sivertsen B, Pallesen S, Stormark KM, Boe T, Lundervold AJ, Hysing M. Delayed sleep phase syndrome in adolescents: prevalence and correlates in a large population based study. BMC Public Health. 2013;13:1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brychta RJ, Arnardottir NY, Johannsson E, et al. Influence of day length and physical activity on sleep patterns in older Icelandic men and women. J Clin Sleep Med. 2016;12(02):203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iceland S Population by sex and age 1841–2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


