Abstract
In 1990 the first fungal secondary metabolite biosynthetic gene was cloned in Aspergillus nidulans. Thirty years later, >30 biosynthetic gene clusters (BGCs) have been linked to specific natural products in this one fungal species. While impressive, over half of the BGCs in A. nidulans remain uncharacterized and their compounds structurally and functionally unknown. Here, we provide a comprehensive review of past advances that have enabled A. nidulans to rise to its current status as a natural product powerhouse focusing on the discovery and annotation of secondary metabolite clusters. From genome sequencing, heterologous expression, and metabolomics to CRISPR and epigenetic manipulations, we present a guided tour through the evolution of technologies developed and utilized in the last 30 years. These insights provide perspective to future efforts to fully unlock the biosynthetic potential of A. nidulans and, by extension, the potential of other filamentous fungi.
Keywords: historical perspective, biosynthesis, Aspergillus nidulans, natural products, genomics, bioinformatics, molecular biology
1. Introduction
The fungal kingdom encompasses an enormous variety of species that have secured ecological niches as symbionts, pathogens, and decomposers (Mohanta and Bae, 2015). Fundamental to thriving in these diverse roles is the production of secondary metabolites that enable fungi to defend against competitors, thwart host attack, communicate with neighboring microorganisms, and protect themselves from toxins (Keller, 2019). Indeed, fungi represent some of the most prolific producers of secondary metabolites which have been evolutionarily refined to interact with biological macromolecules (Keller, 2019; Keller et al., 2005). It is for these reasons that fungal secondary metabolites so often demonstrate marked human impacts, some life-saving, and others deadly. This pronounced functional diversity is paralleled by the distinct structural variation of fungal secondary metabolites. Fungi have evolved into adept synthetic chemists, utilizing enzymatic assembly lines to biosynthesize an impressive range of sterically complex molecules (Macheleidt et al., 2016). The ingenuity of secondary metabolite assembly in fungi has, even after decades of study, only just begun to be explored.
The genus Aspergillus is comprised of over 300 species with remarkable medicinal and commercial impact (Samson et al., 2014). Within this genus, A. nidulans has received widespread recognition as a model eukaryote. Recognized by Pontecorvo in the late 1940s as an excellent candidate for genetic experiments (Pontecorvo et al., 1953), A. nidulans has been the subject of thousands of research papers (>5000 publications in Pubmed), significantly advancing our understanding of cellular physiology and development, enzymology, chromatin structure, DNA repair, recombination, cell biology, primary metabolism and metabolic pathway regulation (Chemudupati et al., 2019; Dimou et al., 2019; Horio et al., 2019; Marcos et al., 2020; Németh et al., 2019). This organism is also the premier model for exploring fungal secondary metabolism. Like many filamentous fungi, A. nidulans contains dozens of biosynthetic gene clusters (BGCs), each hypothesized to yield distinct and diverse secondary metabolites. Of the ~70 predicted BGCs in this species, (Drott et al., 2020; Inglis et al., 2013), 31 core biosynthetic genes in A. nidulans have been linked to their secondary metabolite products (Romsdahl and Wang, 2019), with the majority reported within the past 15 years.
While impressive in comparison to other fungi, one can also ask, why is it that after nearly 75 years of study, over half of secondary metabolites in A. nidulans have yet to be identified? Also, how can we access these undiscovered compounds? The answers to these questions are complex and incompletely understood. However, we can glean insight into the future by looking into the past. Here, we offer a historical perspective and current understanding of secondary metabolite biosynthesis in A. nidulans and provide a guide for more systematic exploration of secondary metabolites for other fungi. Focusing on BGCs that have been linked to downstream products, our review covers technological advances in molecular biology, bioinformatics, and comparative genomics that have enabled identification of secondary metabolites and their biosynthetic genes, from the first linkage to the present day.
2. Timeline at a Glance
Compared to the nearly 75-year relationship between scientists and A. nidulans, the timeline of secondary metabolite BGC discovery is relatively short (Figure 1). Remarkably, the last 30 years have led to the discovery of over 50 downstream secondary metabolites that have been definitively linked core biosynthetic genes and associated tailoring enzymes, transporters, and/or regulatory elements (Table 1, Figure 2). Although a substantial number of these pathways have been characterized extensively, many others have only been partially elucidated. Other biosynthetic genes, discussed in brief later in the manuscript, have only been linked to intermediate products, and the final downstream metabolites remain unknown to this day.
Figure 1.
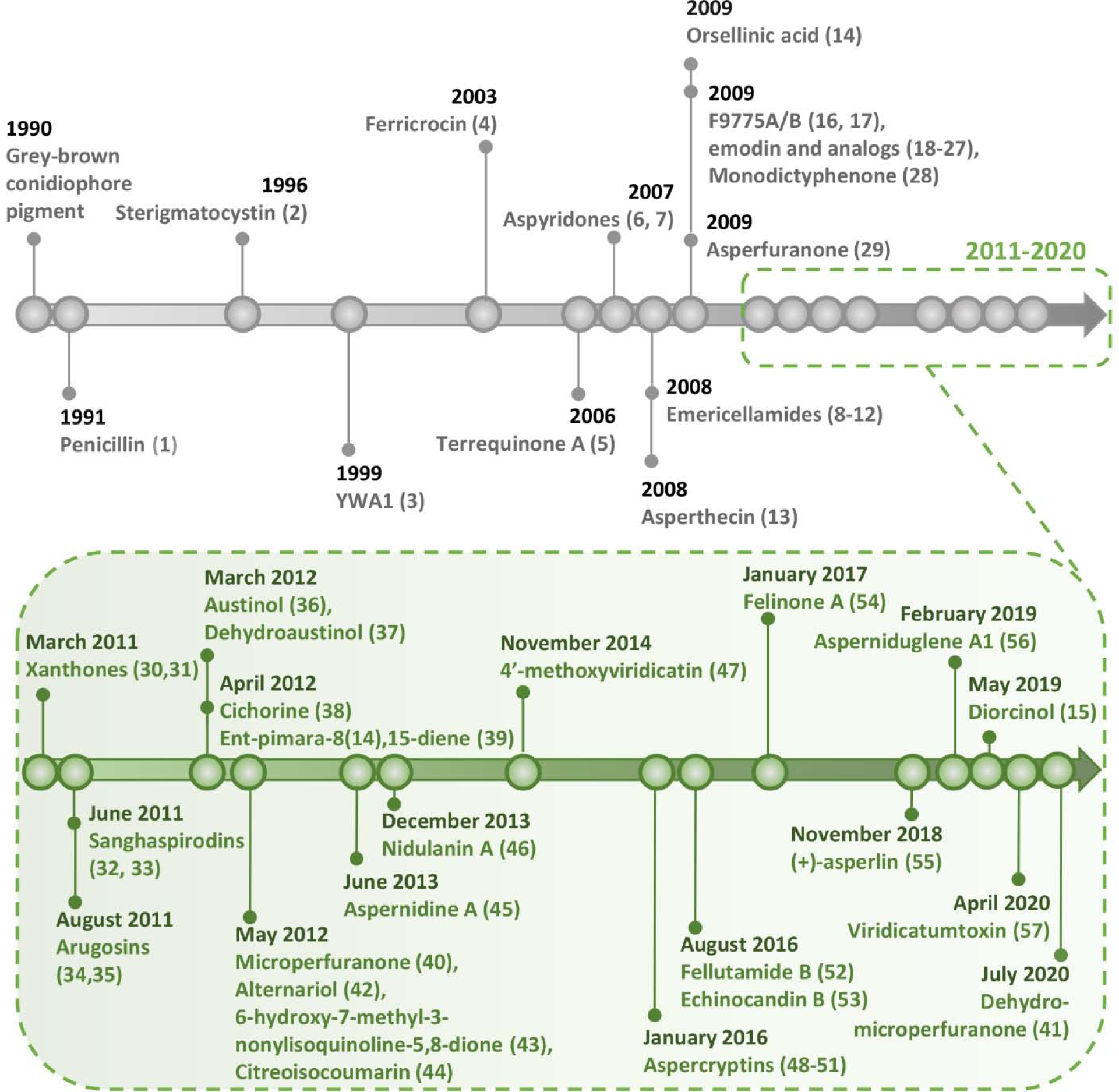
Timeline of BGC-metabolite linkage in Aspergillus nidulans. Numbers in parentheses correspond to structures outlined in Table 1 and Figure 2. Associated references are in Table 1.
Table 1.
A. nidulans compounds and their biosynthetic gene clusters. Gene cluster size is based on primary references and may represent incompletely annotated clusters.
| ID | Compound Name | Genes in Cluster | Backbone Gene Name | Backbone Gene Typea | References |
|---|---|---|---|---|---|
| - | grey-brown conidiophore pigment | 3 | ivoA (AN10576) | NRPS | (Birse and Clutterbuck, 1990; Sung et al., 2017) |
| 1 | penicillin | 3 | acvA (AN2621) | NRPS | (MacCabe et al., 1991) |
| 2 | sterigmatocystin | 25 | stcA (AN7825) | NR-PKS | (Brown et al., 1996b) |
| 3 | YWA1 | 2 | wA (AN8209) | NR-PKS | (Watanabe et al., 1999) |
| 4 | ferricrocin | 2 | sidC (AN0607) | NRPS | (Eisendle et al., 2003) |
| 5 | terrequinone A | 5 | tdiA (AN8513) | NRPS-like | (Bok et al., 2006; Bouhired et al., 2007) |
| 6,7 | aspyridones | 8 | apdA (AN8412) | hybrid | (Bergmann et al., 2007) |
| 8–12 | emericellamides | 4 | easA (AN2545) easB (AN2547) | NRPS HR-PKS |
(Chiang et al., 2008) |
| 13 | asperthecin | 3 | aptA (AN6000) | NR-PKS | (Szewczyk et al., 2008) |
| 14, 15 | orsellinic acid, diorcinol | 3 | orsA (AN7909) | NR-PKS | (Feng et al., 2019; Sanchez et al., 2010; Schroeckh et al., 2009) |
| 16,17 | F9775A/B | 3 | orsA (AN7909) | NR-PKS | (Bok et al., 2009; Sanchez et al., 2010) |
| 18–27 | emodin and analogs | 5 | mdpG (AN1050) | NR-PKS | (Bok et al., 2009) |
| 28 | monodictyphenone | 10 | mdpG (AN1050) | NR-PKS | (Bok et al., 2009) |
| 29 | asperfuranone | 7 | afoE (AN1034) afoG (AN1036) | NR-PKS HR-PKS |
(Chiang et al., 2009) |
| 30,31 | xanthones | 13 | mdpG (AN1050) | NR-PKS | (Sanchez et al., 2011) |
| 32,33 | sanghaspirodins | 2 | mdpG (AN1050) orsA (AN7909) | NR-PKS NR-PKS |
(Scherlach et al., 2011) |
| 34,35 | arugosins | 10 | mdpG (AN1050) | NR-PKS | (Nielsen et al., 2011) |
| 36 | austinol | 14 | ausA (AN8383) | NR-PKS | (Lo et al., 2012) |
| 37 | dehydroaustinol | 14 | ausA (AN8383) | NR-PKS | (Lo et al., 2012) |
| 38 | cichorine | 7 | cicF/pkbA (AN6448) | NR-PKS | (Ahuja et al., 2012; Sanchez et al., 2012) |
| 39 | ent-pimara-8(14),15-diene | 8 | AN1594 | TC | (Bromann et al., 2012) |
| 40,41 | microperfuranone, dehydromicroperfuranone | 2 | micA (AN3396) | NRPS-like | (Roux et al., 2020; Yeh et al., 2012) |
| 42 | alternariol | 1 | pkgA (AN6448) | NR-PKS | (Ahuja et al., 2012) |
| 43 | 6-hydroxy-7-methyl-3-nonylisoquinoline-5,8-dione | 3 | pkiA (AN3386) | NR-PKS | (Ahuja et al., 2012) |
| 44 | citreoisocoumarin | 2 | pkgA (AN6448) | NR-PKS | (Ahuja et al., 2012) |
| 45 | aspernidine A | 6 | pkfA (AN3230) | NR-PKS | (Yaegashi et al., 2013) |
| 46 | nidulanin A | 4 | nlsA (AN1242) | NRPS | (Andersen et al., 2013) |
| 47 | 4’-methoxyviridicatin | 14 | asqK (AN9226) | NRPS | (Ishikawa et al., 2014) |
| 48–51 | aspercryptins | 15 | pkbA (AN6448) atnA (AN7884) | NR-PKS NRPS |
(Chiang et al., 2016; Henke et al., 2016) |
| 52 | fellutamide B | 7 | inpA (AN3495) inpB (AN3496) | NRPS NRPS |
(Yeh et al., 2016) |
| 53 | echinocandin B | 12 | aniA | NRPS | (Hüttel et al., 2016) |
| 54 | felinone A | 2 | dbaI (AN7903) | NR-PKS | (Oakley et al., 2017) |
| 55 | (+)-asperlin | 10 | alnA (AN11191) | HR-PKS | (Grau et al., 2018) |
| 56 | asperniduglene A1 | 4 | sdgA (AN1784) | HR-PKS | (Lin et al., 2019a) |
| 57 | Viridicatumtoxinb | 13 | vrtA | NR-PKS | (Chooi et al., 2010; Drott et al., 2020) |
NRPS = nonribosomal peptide synthetase, HR-PKS = highly reducing polyketide synthase, NR-PKS = non-reducing polyketide synthase, TC = terpene cyclase
gene designations are based on those from Penicillium aethiopicum (Chooi et al., 2010).
Figure 2.
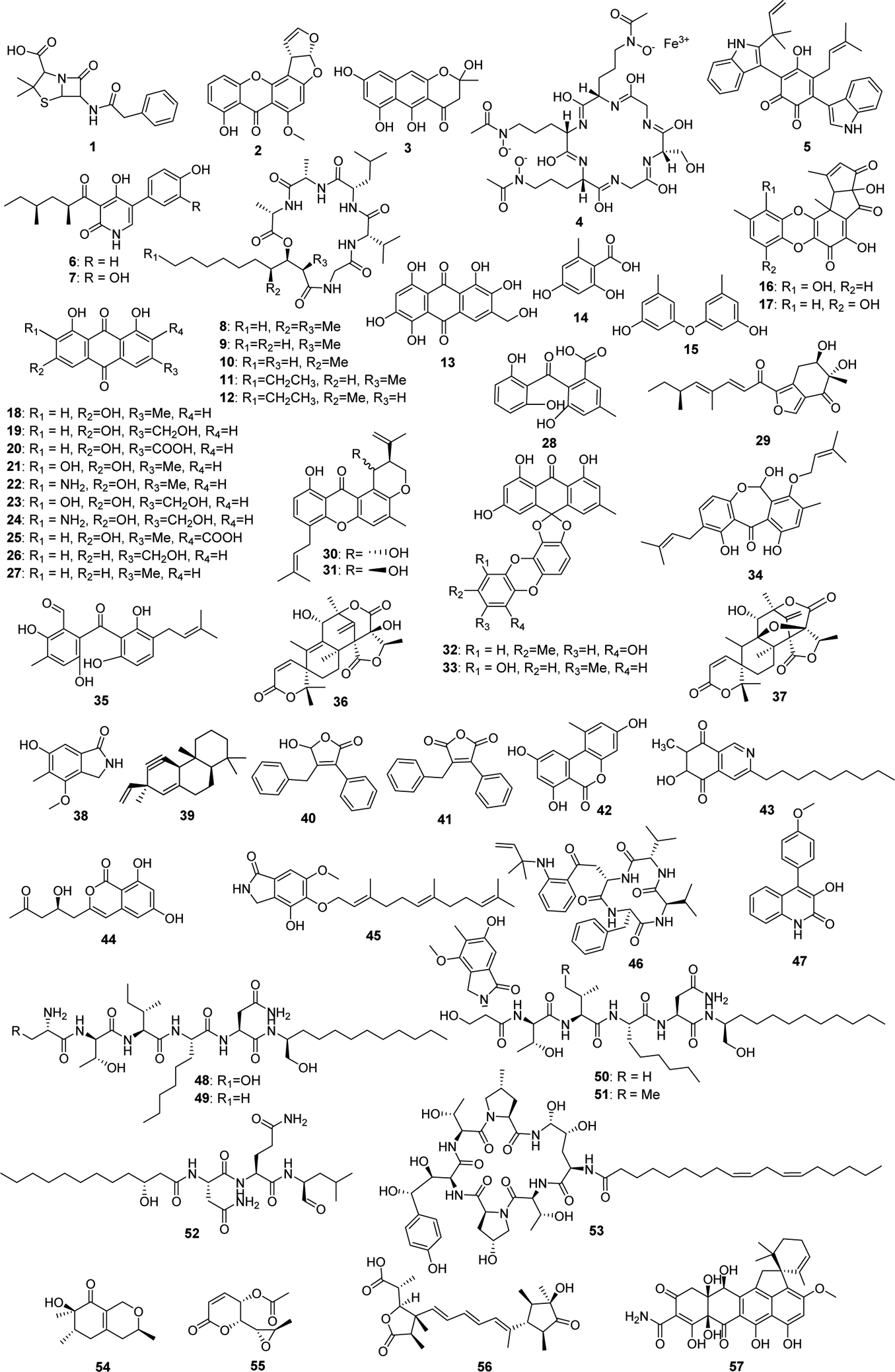
Structures of A. nidulans downstream secondary metabolites. Numbers correspond to compounds listed in timeline in Figure 1 and Table 1.
2.1. Decade 1 (1990–1999): Mutant Hunts and the Pre-Genomic Era
The first decade of exploration of A. nidulans secondary metabolism saw the discovery of only four biosynthetic pathways (Figure 1). These pathways were responsible for formation of the grey-brown conidiophore pigment (Birse and Clutterbuck, 1990; Sung et al., 2017), the life-saving antibiotic penicillin (Litzka et al., 1996; MacCabe et al., 1991; Spröte et al., 2008), the important mycotoxin sterigmatocystin (Brown et al., 1996a; Brown et al., 1996b; Kelkar et al., 1996; Kelkar et al., 1997; Keller et al., 1995; Keller et al., 2000), and the green spore pigment precursor YWA1 (Watanabe et al., 1999). These early discoveries were facilitated by development of a transformation system where gene targeting strategies using plasmids containing selectable markers, often marker genes to restore auxotrophic strains to prototrophy (e.g. pyrG encoding orotidine 5’-monophosphate decarboxylase restoring uracil/uridine prototrophy to a pyrG89 mutant) (Oakley et al., 1987), were used to replace the gene of interest. Although enormously useful, these early strategies suffered from the time-consuming nature of experiments such as the need to screen large numbers of transformants to identify the correct mutant (due to the propensity of the plasmid to enter into non-homologous regions of the genome). Nonetheless, these strategies laid an important foundation for our understanding of enzymatic assembly and regulation and served as the inspiration for the catalyzing technologies of the next decade. Undoubtedly, without the discovery of these gene clusters, particularly the penicillin and sterigmatocystin gene clusters, many of today’s “gold-standard” technologies would not exist.
2.2. Decade 2 (2000–2009): Accelerated Discovery in the Genomic Age
The turn of the millennium also marked a turning point for A. nidulans secondary metabolite research. While the first few years were slow, seeing only the discovery of the ferricrocin gene cluster using technologies established in the previous decade (Eisendle et al., 2003), the second half of the decade marked the birth of several groundbreaking advances catalyzing a wave of accelerated discoveries. Fusion PCR, developed broadly in 2002 (Kuwayama et al., 2002) and applied to A. nidulans a few years later (Nayak et al., 2006; Szewczyk et al., 2006; Yang et al., 2004; Yu et al., 2004; Zarrin et al., 2005), allowed for amplification of DNA (typically flanking regions of the gene to be deleted or overexpressed) without the need for a restriction enzyme or ligation step.
The power of genome mining - made available in 2005 by publication of the A. nidulans genome (Galagan et al., 2005) - combined with the use of fusion PCR facilitated rapid discovery of metabolites from 2006–2009, nearly tripling the number of BGC characterizations and multiplying the number of linked downstream metabolites seven-fold (Figure 1) (Bergmann et al., 2007; Bok et al., 2009; Bok et al., 2006; Bouhired et al., 2007; Chiang et al., 2009; Chiang et al., 2008; Schroeckh et al., 2009; Szewczyk et al., 2008). What’s more, this decade marked the discovery of the laeA global regulator of secondary metabolism (Bok and Keller, 2004), early exploration into the impact of chromatin packaging on gene expression (Bok et al., 2009), and the manipulation of culture conditions of metabolite discovery (Schroeckh et al., 2009). With these technologies, researchers now had the tools they needed to access the “low hanging fruit” of the A. nidulans secondary metabolome.
2.3. Decade 3 (2010–2019): Innovative Strategies Awaken Silent Clusters
From 2010–2019, the number of characterized biosynthetic pathways in A. nidulans more than doubled as a result of continuous and creative efforts by the scientific community, successfully identifying an additional 27 downstream metabolites originating from 15 core biosynthetic genes (Figures 1–2). Although it seemed as though scientists were “bridging the gap” between characterized and uncharacterized clusters, the invention of algorithmic genome mining tools such as SMURF (Khaldi et al., 2010) and antiSMASH (Medema et al., 2011) revealed that the biosynthetic capacity of fungi was even greater than previously acknowledged. Increasingly during exploration, researchers encountered the ongoing challenge of “silent” gene clusters that could not be activated under standard laboratory conditions. Indeed, large scale backbone gene deletion studies led to notable metabolite differences in only a fraction of mutants (Ahuja et al., 2012; Nielsen et al., 2011; Sanchez et al., 2012; Sanchez et al., 2011), indicating that the majority of the deleted genes were not expressed under normal conditions.
Having depleted much of the “low-hanging fruit,” researchers shifted to developing clever methods to activate silent clusters, including continued manipulation of culture conditions (Andersen et al., 2013), induction of pathway-specific regulators (Bromann et al., 2012; Lin et al., 2019a; Sung et al., 2017; Yeh et al., 2016; Yeh et al., 2012), management of the chromatin landscape (Ahuja et al., 2012; Chiang et al., 2010; Henke et al., 2016), confrontations with other microbes (Fischer et al., 2018; Nützmann et al., 2011), and the creation of “genetic dereplication” strains with altered secondary metabolite and gene expression profiles (Chiang et al., 2016). These approaches were often paired with gene expression data to identify induced changes and link metabolites to their corresponding gene clusters. Increasing utilization of gene expression data also enabled the discovery of several “split” biosynthetic pathways in which metabolites were formed by biosynthetic genes that were clustered into two or more groups (Andersen et al., 2013; Lo et al., 2012; Sanchez et al., 2011; Scherlach et al., 2011).
3. An Evolution of Technologies
In the last thirty years, technological advances in molecular biology, bioinformatics, and comparative genomics have led to the rapid advancement in our understanding of A. nidulans secondary metabolite biosynthesis. A depiction of some of the diverse chemical entities discovered during this time and the important technologies contributing to these discoveries, can be seen in Figure 3.
Figure 3.

An overview of technological advances and strategies for identifying secondary metabolites and their gene clusters from Aspergillus nidulans.
3.1. Strain Manipulation Methods: Then and Now
From 1990–2003, researchers primarily relied on conventional cloning technologies to target genes for functional analysis (Miller et al., 1985). These traditional cloning techniques utilize restriction endonucleases to prepare DNA fragments for cloning and a self-replicating plasmid, which are typically propagated in a bacterial organism such as Escherichia coli. The insert can then be spliced into the vector plasmid using DNA ligase to form a new vector which is used to transform the fungal organism under study (Miller et al., 1985). Although hugely important for insights into biosynthetic pathways in A. nidulans, these techniques were quite tedious and time-consuming, and discovery efforts were greatly improved by the introduction of fusion PCR as mentioned above (Kuwayama et al., 2002). Fusion PCR remains a popular technique to modify expression of natural product genes in A. nidulans, as noted by the numerous papers published using this method since 2006.
3.2. Sequencing of the A. nidulans Genome: Possibilities Awaken
Arguably the single most important contribution to the field of A. nidulans secondary metabolite biosynthesis is that of the A. nidulans FGSC A4 reference genome, published with 13-fold coverage for the first time in 2005 (Galagan et al., 2005). This contribution was by no means an individual effort, instead involving input from the Broad Institute of MIT and Harvard, The Institute for Genomic Research (TIGR), and global academic participation of universities from five continents around the globe (Galagan et al., 2005). Community participation in the A. nidulans genome did not stop there, as continued data curation has remained necessary to this day. Indeed, the creation of numerous community databases, continually refined, has enabled researchers to annotate clusters, identify errors in existing datasets, and find gene clusters that had been missed in previous analyses (Yeh et al., 2012). Notable databases include the Aspergillus genome database (AspGD, although now not maintained) (Arnaud et al., 2010), the Central Aspergillus Data Repository (CADRE) (Mabey Gilsenan et al., 2012), the Joint Genome Institute Fungal Genomics Program (JGI) (Grigoriev et al., 2011), and FungiDB (Basenko et al., 2018; Stajich et al., 2012). Such efforts have been facilitated by the production of gene cluster detection algorithms such as SMURF (Khaldi et al., 2010) and antiSMASH (Blin et al., 2019) enabling automated annotation and interpretation of gene functions in a high-throughput fashion.
The availability of the A. nidulans genome made possible an incredible range of discoveries. For example, genomic data enabled identification of the nkuA gene, a critical player in DNA repair by enabling nonhomologous end joining. Upon deletion of nkuA, however, researchers saw a large decrease in the frequency of nonhomologous integration events, with a 90% success rate for correct gene targeting (Nayak et al., 2006).
With these tools in hand, large scale manipulations of the entire A. nidulans genome were now possible, resulting in several ambitious large-scale deletion studies. In 2008, the emericellamide biosynthetic pathway was discovered following the deletion of 6 randomly selected non-ribosomal peptide synthase (NRPS) backbone genes (Chiang et al., 2008). High-performance liquid chromatography (HPLC) experiments revealed that one of the NRPS deletion mutants showed a loss of production of several metabolites which were isolated and shown to be the antibiotic compound emericellamide A, as well as four new analogs, emericellamides C-F. Additional genes surrounding the backbone NRPS were deleted identifying a polyketide synthase (PKS), an acyltransferase, and an AMP-dependent CoA-ligase that were also essential for emericellamide biosynthesis (Chiang et al., 2008).
A few years later, researchers deleted 10 non-reducing polyketide synthase (NR-PKS) genes using genome sequencing data in an attempt to link these uncharacterized backbone genes to their biosynthetic products (Sanchez et al., 2011). This genome-based deletion analysis revealed the biosynthetic backbone gene, mpdG, for the production of prenyl xanthones including shamixanthone, emericellin, variecoxanthone A, and epishamixanthone. Again, targeted deletions were utilized to determine the boundaries of the cluster, identifying 10 genes required for biosynthesis (Sanchez et al., 2011). In another study, large-scale PKS deletions were completed and the resulting strains were grown on a variety of media types, revealing that the xanthone PKS mpdG is also required for the biosynthesis of arugosins A and H (Nielsen et al., 2011). Such strategies could also be used for targeted discovery of specific metabolite biosynthetic pathways. For example, using the logic that the aromatic group present in cichorine was produced by a NRPKS backbone, gene deletions targeting NR-PKS backbone enzymes were completed and mutants evaluated for a loss of cichorine production (Sanchez et al., 2012). Deletions of upstream and downstream genes from the identified PKS identified a regulator, a transporter, and four tailoring genes involved in cichorine biosynthesis.
One particularly eye-opening discovery made possible by the availability of the A. nidulans genome is that numerous biosynthetic pathways are “split” into more than one cluster (Andersen et al., 2013; Lo et al., 2012; Sanchez et al., 2011; Scherlach et al., 2011). For example, the prenyltransferase enzyme required to prenylate shamixanthone and its analogs is not located near the PKS backbone gene. Using homology searches, researchers identified several putative prenyltransferases in the genome and identified one that led to the elimination of xanthone biosynthesis. Interestingly, an oxidoreductase adjacent to this prenyltransferase was also found to be essential for converting the precursor emericellin into downstream products (Sanchez et al., 2011). Likewise, the prenyltranserase required for austinol and dehydroaustinol biosynthesis is separated from the PKS gene cluster, and was only found using homology-based genome mining searches (Lo et al., 2012). Subsequent deletions revealed a pathway involving at least 14 genes separated into two distinct gene clusters.
In addition to homology searches, researchers have discovered split pathways through the utilization of gene expression data (Andersen et al., 2013; Scherlach et al., 2011). For example, the sanghaspirodins are produced through a convergence of two separate polyketide pathways. Using full genome microarray and qRT-PCR to monitor gene expression, researchers were able to identify distinct genomic regions that were activated by growth conditions in which the sanghaspirodins were produced (Scherlach et al., 2011). In a particularly elegant study, Andersen et al. designed a gene expression compendium by combining DNA expression array for A. nidulans grown under a variety of growth conditions with genomic data. Using this platform, they accurately predicted the extent of 13/16 known BGCs, and produced nearly accurate predictions for the remaining three. Even more impressively, the group utilized a data clustering approach to identify cross-chemistry between split clusters (Andersen et al., 2013). Not only did they validate this method by reproducing the gene cluster predictions for known split pathways (Lo et al., 2012; Sanchez et al., 2012), they also identified a new split cluster, responsible for the formation of nidulanin A.
Although most of the genome mining efforts have been focused on enzymatic genes, some BGCs contain non-enzymatic genes that protect the producing organism from toxic effects of the biosynthesized metabolite (Gilchrist et al., 2018; Keller, 2015). Based on this knowledge, researchers prioritized an uncharacterized gene cluster from the A. nidulans genome that contained a proteasome subunit with no obvious role in natural product synthesis (Yeh et al., 2016). Induced expression of this cluster revealed the product to be fellutamide B, a proteasome inhibitor. Indeed, by deleting the putative proteasome subunit inpE and activating expression of the rest of the inp cluster, researchers definitively illustrate that the proteasome subunit is required for A. nidulans’ resistance to internally synthesized fellutamide B (Yeh et al., 2016).
Perhaps the greatest insight gained through genome mining efforts was that the biosynthetic potential of A. nidulans, and the fungal kingdom as a whole, is considerably larger than previously realized. This realization prompted researchers to develop methods to manipulate pathway regulation in an effort to activate “silent” clusters and identify their metabolites.
3.3. Giving A Voice to the Voiceless: Understanding and Manipulating Pathway Regulation
The A. nidulans genome made apparent the fact that the vast majority of BGCs remain silent under standard laboratory growth conditions. To overcome this bottleneck and to identify the potential virulence factors, toxins, and druggable molecules encoded by these cryptic clusters, new strategies were developed to access this untapped biosynthetic potential. These strategies involve a combination of genetic and chemical manipulations of fungi, and have provided considerable insight into the various genetic and environmental cues that govern gene expression in A. nidulans.
3.3.1. The Impact of Culture Conditions
Even before the A. nidulans genome was published in 2005, scientists had begun to explore the impact of culture conditions on metabolite production in microorganisms. Indeed, the “One Strain Many Compounds”, or “OSMAC” approach was coined in 2002, in which systematic alteration in culture conditions can be used to increase both the yield and the number of secondary metabolites from a single microorganism (Bode et al., 2002). Not until several years later was this approach was successfully used to induce formation of silent metabolites in A. nidulans, however. In 2010, the orsellinic acid/F9775 gene cluster was expanded using the OSMAC approach (Sanchez et al., 2010). Additionally, two isoindole alkaloids, aspernidines A and B, were discovered using the OSMAC approach (Scherlach et al., 2010). It was not until three years later, however, that the biosynthetic pathway for these molecules was discovered (Yaegashi et al., 2013). The OSMAC approach has also been essential for elucidating the roles of biosynthetic enzymes in particular pathways. For example, it was utilized to link arugosins to the PKS mpdG, which also plays a role in the biosynthesis of emodin and xanthones (Kelkar et al., 1996; Nielsen et al., 2011; Sanchez et al., 2011), as well as to identify several intermediate compounds in the cichorine biosynthetic pathway (Sanchez et al., 2012). The biosynthetic pathways of the sanghaspirodins (Scherlach et al., 2011) and nidulanin A (Andersen et al., 2013) were also identified, at least in part, by using clever culture conditions inspired by the OSMAC approach.
3.3.2. Microbial Interactions
One reason that many organisms fail to produce secondary metabolites in laboratory culture is because they lack the complex, mixed-microbial community that they interact with in natural settings. Although relatively underexplored in A. nidulans secondary metabolism, the impact of interspecies associations on A. nidulans biosynthesis was explored and utilized to activate orsellinic acid production (Schroeckh et al., 2009). By growing A. nidulans in the presence of a collection of actinomycetes occupying the same environmental niche, researchers were able to discover of the orsellinic acid pathway. Interestingly, induction of the pathway only occurred when direct contact between the fungus and bacterium was made possible, and not when fungi were treated with bacterial supernatant, suggesting that direct contact was required for metabolite generation (Schroeckh et al., 2009). This method has proven lucrative in identifying new fungal secondary metabolites in other species and provides support for the hypothesis that natural products are the chemical coinage in cross-species, cross-Kingdom interactions (Németh et al., 2019; Netzker et al., 2018; Stroe et al., 2020).
3.3.3. Pathway-specific Regulation of Secondary Metabolism
One of the earliest genetic strategies for activating a cryptic gene locus was successfully utilized for the discovery of aspyridones A and B (Bergmann et al., 2007). Through genome mining, researchers found a hybrid PKS-NRPS backbone gene, and recognized that it was silent given the lack of PKS-NRPS hybrid molecules in the fungal extracts under study. Using homology searches, researchers identified a putative activator gene in the PKS-NRPS gene cluster, which they placed under the control of the inducible alcohol dehydrogenase (alcA) promoter. This led to the production of inducible mutant strains, which produced the aspyridones and provided proof of concept for this important strategy (Bergmann et al., 2007). Indeed, this strategy was later used to identify the asperfuranone biosynthetic pathway (Chiang et al., 2009), and to refine the monodictyphenone pathway (Chiang et al., 2010). A similar strategy was utilized to overexpress a Zn(II)2Cys6-type transcription factor, which served as a pathway-specific activator for the diterpene gene cluster encoding ent-pimara-8(14),15-diene (Bromann et al., 2012).
In 2012, Ahuja et al. attempted to replace the promoters of all putative transcription factors in 17 uncharacterized natural product gene clusters in A. nidulans (Ahuja et al., 2012). Interestingly, despite previous success (Bergmann et al., 2007; Chiang et al., 2010; Chiang et al., 2009), these transformations generally produced no or minimal increases in natural product biosynthesis. With this in mind, the group took this strategy a step further, replacing not just the promoters of putative regulators, but the promoters of all unknown natural product genes with NR-PKS backbones with the regulatable alcA promoter (Ahuja et al., 2012). These transformations were largely successful, enabling determination of at least one product from all eight NR-PKS backbones. It is worth noting, however, that while a handful of these products represent end-products of the biosynthetic pathway, including alternariol and citreoisocoumarin, the majority of identified metabolites likely represent intermediates and stable precursors rather than end metabolite (Ahuja et al., 2012). This approach has remained a popular strategy, leading to the subsequent discoveries of the microperfuranone (Yeh et al., 2012), grey-brown conidiophore pigment (Sung et al., 2017), and asperniduglene A1 (Lin et al., 2019a) biosynthetic pathways.
Another strategy was developed in 2018 to manipulate specific biosynthetic pathways, paramount for the discovery of the (+)-asperlin biosynthetic pathway (Grau et al., 2018). After transcription factor promoter replacement yielded no biosynthetic products and replacement of promoters in SM backbone genes yielded only an intermediate compound, researchers instead developed a synthetic transcription factor that was not subject to inhibition by post-translational modification (Grau et al., 2018). By choosing the afoA transcription factor, known to function in late stationary growth where secondary metabolites can be produced at very high levels, and fusing it with the DNA-binding domain of the transcription factor associated with the silent cluster under study, researchers were able to activate production of the antibiotic (+)-asperlin and identify the boundaries of the biosynthetic cluster (Grau et al., 2018).
3.3.4. Global Regulation of Secondary Metabolism
In addition to gaining insight into pathway-specific regulation, researchers have made great strides in understanding global regulation of secondary metabolism in A. nidulans. The first, and incredibly impactful, discovery was that of the positive global regulator of secondary metabolism, LaeA (Bok and Keller, 2004). The authors noted that the identification of global regulators of fungal biosynthesis would enable facile and comprehensive manipulation of secondary metabolite production. To identify global regulators, authors evaluated sterigmatocystin production through a mutagenesis screen, targeting mutants that lost the ability to produce sterigmatocystin. Complementation and subsequent sequencing of one of these mutants revealed the ORF laeA, unique to filamentous fungi. Deletion of laeA blocked expression of numerous known and unknown metabolites, including sterigmatocystin and penicillin, while overexpression increased production of these metabolites (Bok and Keller, 2004). LaeA was thereafter found be a member of the important Velvet complex (whose core members are LaeA, VeA and VelB), required for secondary metabolism and light regulated development in all filamentous fungi examined to date (Bayram et al., 2008; Kopke et al., 2013; López-Berges et al., 2013; Rahnama et al., 2019; Saha et al., 2020; Schumacher et al., 2015). The discovery of laeA has had major impacts in the field of fungal secondary metabolism, and the original manuscript describing its discovery has been cited more than 800 times (Bok and Keller, 2004).
Two years later, researchers utilized the recently published A. nidulans genome in combination with laeA manipulation as a genome mining tool to identify fungal BGCs and their boundaries (Bok et al., 2006). In this study, deletion of laeA enabled identification of clear BGC signatures in DNA expression profiles in which genes belonging to the same cluster were suppressed in laeA mutants. Researchers used these signatures to look for ORFs in genetic regions that were suppressed in deletion mutants (Bok et al., 2006). This strategy led to the discovery of terrequinone A, along with putative genes involved in its biosynthesis. The biosynthetic pathway boundaries were confirmed a year later using targeted deletions of predicted genes (Bouhired et al., 2007). Indeed, prior to the discovery of terrequinone A, the discovery of metabolites preceded the identification of their biosynthetic genes. Using laeA, however, the directionality could be reversed, from gene to metabolite, for the first time.
It was not until 10 years later, in 2017, that another master regulator, mcrA, was discovered for Aspergillus secondary metabolism (Oakley et al., 2017). Prior to this work, the majority research done on fungal secondary metabolism was targeted towards positive regulators of secondary metabolism, such as the global regulator laeA. Authors reasoned that the identification of negative master regulators would enable upregulation of cryptic gene clusters, a problem that has become increasingly relevant in recent years (Oakley et al., 2017). Using a genetic screen aimed to identify mutations that induced transcription of the NRPS backbone involved in nidulanin A biosynthesis, researchers identified a negative regulator of nidulanin A biosynthesis, mcrA. Deletion of this gene stimulated production even in strains that lacked laeA. Using the mcrA deletion mutant, researchers determined that A. nidulans could produce the antibiotic felinone A, and were able to propose a biosynthetic pathway for this compound (Oakley et al., 2017). This was followed by a discovery of a LaeA-like methyltransferase, LlmG, that when overexpressed induced the production of metabolites from at least 7 known secondary metabolite pathways (Grau et al., 2019). Previous research which had identified but not characterized LlmG, had instead shown that LlmF actually interfered with sterigmatocystin biosynthesis through a cellular inhibition of the LaeA partner, VeA, from entry into the nucleus (Palmer et al., 2013). Overall, this supports an as yet unknown mechanism of LaeA-like methyltransferase influence on global regulation of fungal natural products.
3.3.5. Manipulating the Chromatin Landscape
In eukaryotic organisms such as fungi, DNA is packaged into a dynamic nucleoprotein complex called chromatin in which fundamental genetic processes such as gene transcription occur (Ruiz-Velasco and Zaugg, 2017). Chromatin organization can render DNA more or less accessible to proteins that read sequences, and as such, chromatin architecture represents the fundamental level at which gene expression is controlled. This control is wielded through diverse chemical modifications of histones and DNA, which can fold or unfold chromatin, ultimately hiding or exposing key regulatory elements required for gene expression. Sumoylation of core histones, in which small ubiquitin like modifiers are added post-translationally, for example, is correlated with transcriptional suppression, and sumoylation at other targets, such as transcription factors, can promote activity of other chromatin modifying enzymes that decrease gene expression as well (Szewczyk et al., 2008). In 2008, researchers found that deletion of sumO, a gene encoding a small ubiquitin-like sumoylation protein in A. nidulans, dramatically increased the production of the asperthecin (Szewczyk et al., 2008). The overproduction of this metabolite enabled researchers to complete targeted deletions to monitor asperthecin production and to identify the biosynthetic pathway for this compound.
Histone 3 lysine 4 methylation is often associated with gene silencing, and researchers used this knowledge to identify CclA, an ortholog of Bre2 in Saccharomyces cerevisiae involved in H3K4 di and tri-methylation. Deletion of this gene activated the expression of a number of secondary metabolite clusters, including the monodictyphenone/emodin pathway and the F9775 A and B pathway (Bok et al., 2009). Years later, a study was completed using a mutant of A. nidulans with downregulated levels of the histone deacetylase RpdA (Henke et al., 2016). When mass spectral signals were compared with the wild-type fungus, many metabolites appeared to be upregulated in the RpdA mutant strain. One of these metabolites was the known metabolite aspercryptin B1, whose biosynthesis had been elucidated earlier that same year (Chiang et al., 2016). Using MS/MS fragmentation patterns, researchers were able to identify several new aspercryptins using the RpdA mutant and use this information to refine the previously reported biosynthetic pathway (Henke et al., 2016). The most recent addition of chromatin modifying proteins to elucidate Aspergillus natural products includes the reader protein SntB (Horio et al., 2019; Nødvig et al., 2018; Pfannenstiel et al., 2018).
3.3.6. Production of “Genetic Dereplication” Strains
As more and more biosynthetic pathways are characterized, researchers have been able to utilize this knowledge to gain insight into additional biosynthetic pathways. Indeed, a large number of secondary metabolite genes were discovered by using sterigmatocystin deletion mutants. By preventing production of this major polyketide product, it is easier to detect metabolites of lower abundance. Furthermore, in some cases, the liberation of the malonyl-CoA required for sterigmatocystin biosynthesis enables upregulation of metabolites that also rely on the incorporation of this precursor. This strategy was paramount for the elucidation of the asperfuranone (Chiang et al., 2009), F9775 A/B, monodictyphenone/emodin (Bok et al., 2009), xanthone (Sanchez et al., 2011), and microperfuranone (Yeh et al., 2012) biosynthetic pathways.
Many of these associated discoveries were used to take this strategy a step further in 2016, where Chiang et al. developed a “genetic dereplication” strain lacking clusters for sterigmatocysin, emericellamides, asperfuranone, monodictyphenone, terrequinone, F9775 A/B, asperthecin, and austinol/dehydroaustinol. In this strain, one large MS detectable peak was identified and purified, and found to be aspercryptin. Given the size of aspercryptin, researchers were able to identify an NRPS containing 6 adenylation domains from the genome as a candidate for biosynthesis. It and adjacent genes were deleted, confirming the biosynthetic players in aspercryptin production (Chiang et al., 2016).
3.3.7. CRISPR Technology
The CRISPR-Cas9 genome editing technology reached the Aspergillus world in 2015 where, as a visual test, a guide RNA was employed to mutate A. nidulans yA (encoding a laccase for spore pigmentation) resulting in yellow pigmented colonies (Nødvig et al., 2015). Since then several studies have further improved this technology to allow for a more versatile system to edit Aspergillus genomes. This included the use of a nkuA deficient strain, targeting of more than one allele, marker free targeting and use of AMAI autonomously replicating sequence for episomal based regulation (Nødvig et al., 2018; Roux et al., 2020; Vanegas et al., 2019). Cpf1-mediated CRISPR experiments have also shown promise for efficient gene editing in Aspergilli (Vanegas et al., 2019). A recent and exciting development is the CRISPRa technology, (CRISPR-mediated transcriptional activation) in which an enzymatically deficient Cas12a (Cpf1) or Cas9-, incapable of cutting DNA but still capable of targeting a specific DNA sequence, fuses to transcriptional activation domains, upregulating gene expression, and allowing for activation of cryptic secondary metabolites. As a proof of concept, Roux et al. recently utilized CRISPRa to coactivate both the nonribosomal peptide synthetase-like (NRPS-like) gene micA and the P450 micB in A. nidulans, resulting in the discovery of the BGC product as dehydromicroperfuranone (Roux et al., 2020).
3.4. Heterologous Expression: Enabling Discoveries from Diverse Organisms
The established genetic system of A. nidulans has been successfully utilized for decades to uncover the vast biosynthetic potential contained within its genome. This advanced genetic system, in addition to enabling discovery of natively produced secondary metabolites, has seen great success as a heterologous expression system to study secondary metabolite production in phylogenetically diverse fungal and even botanical organisms (Table 2, Figure 4). Fungal systems like A. nidulans are particularly well suited to expressing large secondary metabolism genes containing introns from other fungi (Chiang et al., 2013; Yaegashi et al., 2014). Researchers can take advantage of well-established genetic manipulation approaches in A. nidulans to produce mutants with clean backgrounds, nutritional markers, inducible promoters, and pigment deficiencies and apply them to study fungal systems without an established genetic background (de Reus et al., 2019; Yin et al., 2013; Zhang et al., 2017). In addition to enabling discovery of new metabolites and their secondary metabolite pathways, heterologous expression systems can also be utilized to improve yields of important natural products (Maiya et al., 2009) and to gain insight into the dynamics that follow a horizontal gene transfer event (de Reus et al., 2019). While Table 2 and Figure 4 illustrate a large number of manuscripts expressing heterologous compounds in A. nidulans, this is not an absolute number, and we apologize to any authors whose work we did not capture in this review.
Table 2.
Compounds derived from heterologously expressed genes in Aspergillus nidulans. ID numbers are associated with compound structures in Figure 4.
| ID | Compound Name | Parent Organism | Reference |
|---|---|---|---|
| 58 | tryprostatin B | Aspergillus fumigatus | (Maiya et al., 2009) |
| 59 | DHMP | Penicillium brevicompactum | (Hansen et al., 2012) |
| 60 | asperfuranone | Aspergillus terreus | (Chiang et al., 2013) |
| 61 | geodin | Aspergillus terreus | (Nielsen et al., 2013) |
| 62 | chanoclavine-I | Aspergillus fumigatus | (Ryan et al., 2013) |
| 63 | neosartoricin B | Trichophyton tonsurans | (Yin et al., 2013) |
| 64 | penicillin K | Penicillium chrysogenum | (Unkles et al., 2014) |
| 65 | terezine D | Aspergillus terreus | (Bok et al., 2015) |
| 66 | Sch210972 | Chaetomium globosum | (Sato et al., 2015) |
| 67 | myceliothermophin A/Ea | Myceliophthora thermophile | (Li et al., 2016) |
| 68 | ent-kaurene | Fusarium fujikuroi | (Bromann et al., 2016) |
| 69 | gamma-terpinene | Citrus unshiu | (Bromann et al., 2016) |
| 70 | subglutinol C/Db | Metarhizum robertsii ARSEF 23 | (Kato et al., 2016) |
| 71 | citreoviridin | Aspergillus terreus var. aureus | (Lin et al., 2016) |
| 72 | valactamide A | Aspergillus terreus | (Clevenger et al., 2017) |
| 73 | flaviolin | Pestalotiopsis fici | (Zhang et al., 2017) |
| 74 | scylatone | Pestalotiopsis fici | (Zhang et al., 2017) |
| 75 | zaragozic acid A | Curvularia lunata | (Liu et al., 2017) |
| 76 | asperphenamate | Penicillium brevicompactum | (Li et al., 2018b) |
| 77 | aurovertinsc | Calcarisporium arbuscula | (Ma et al., 2018) |
| 78 | benzomalvin A | Aspergillus terreus | (Clevenger et al., 2018) |
| 79 | acu-dioxomorpholine | Aspergillus aculeatus | (Robey et al., 2018) |
| 80 | alternapyronesd | Parastagonospora nodorum | (Li et al., 2018a) |
| 81 | β-carotene | Fusarium fujikuroi | (Wiemann et al., 2018) |
| 82 | viriditoxin | Paecilomyces variotii | (Hu et al., 2019a) |
| 83 | elsinochrome A | Parastagonospora nodorum | (Hu et al., 2019b) |
| 84 | trichoxide | Trichoderma virens | (Liu et al., 2019) |
| 85 | stemphyloxin II | Parastagonospora nodorum | (Li et al., 2019b) |
| 86 | bikaverin | Fusarium fujikuroi | (de Reus et al., 2019) |
| 87, 88 | burnettramic acid A/B | Aspergillus burnettii | (Li et al., 2019a) |
| 89 | ilicicolin H/Je | Neonectria sp. DH2 | (Lin et al., 2019b) |
| 90 | fumihopaside A | Aspergillus fumigatus | (Ma et al., 2019) |
| 91 | mycophenolic acid | Penicillium griseofulvum | (Chen et al., 2019) |
| 92 | phomacin D/Ef | Parastagonospora nodorum | (Li et al., 2020b) |
| 93 | nanangelenin A | Aspergillus nanangensis | (Li et al., 2020a) |
| 94 | terreazepine | Aspergillus terreus | (Caesar et al., 2020) |
| 95 | laetiporic acidsg | Laetiporus sulphureus | (Seibold et al., 2020) |
| 96 | 6,8-dihydroxy-3-methylisocoumarin | Penicillium crustosum | (Xiang et al., 2020) |
| 97 | terrestric acid | Penicillium crustosum | (Fan et al., 2020) |
| 98 | flavoglaucin | Aspergillus ruber | (Nies et al., 2020) |
| 99 | beauveriolide I/IIIh | Cordyceps militaris | (Wang et al., 2020) |
| 100 | thermolidesi | Talaromyces thermophilus | (Zhang et al., 2020) |
myceliothermophin A shown as example structure;
subglutinol C shown as example structure;
aurovertin B shown as example structure;
alternapyrone A shown as example structure;
ilicicolin H shown as example structure;
phomacin D shown as example structure;
laetiporic acid A shown as example structure;
beauveriolide I shown as example structure;
thermolide C shown as example structure
Figure 4.
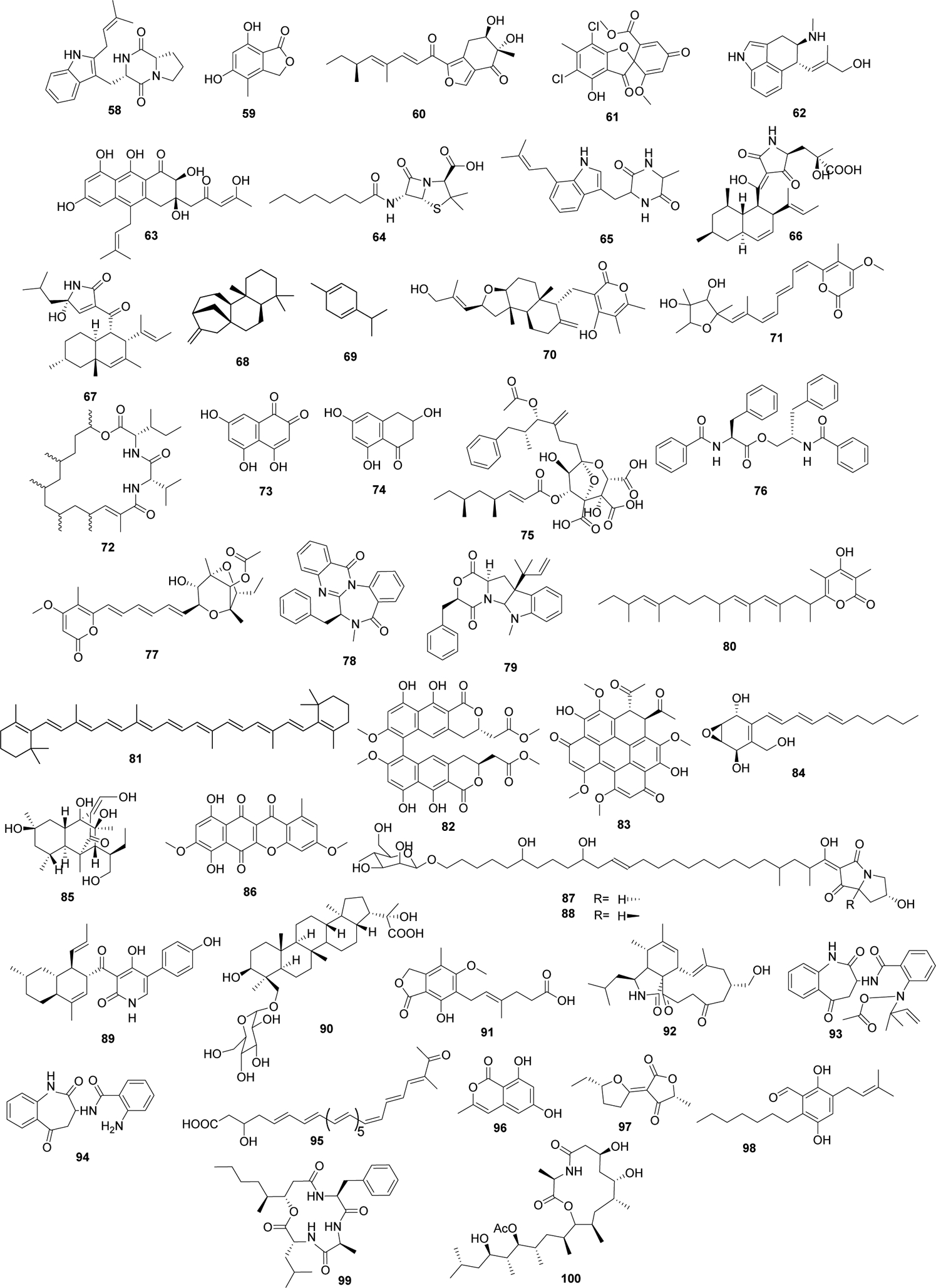
Structures of compounds derived from heterologously expressed genes in A. nidulans. Compound numbers correspond to those listed in Table 2.
A variety of strategies have been developed to successfully exploit A. nidulans as a heterologous host. Traditional approaches, such as those used for the discovery of neosartoricin B, typically involve the over-expression of each gene cluster of interest individually utilizing selection markers (Yin et al., 2013). Although this approach has been widely successful, it is quite laborious and limited to small gene clusters (Wiemann et al., 2018). To overcome this limitation, other strategies have been developed enabling single- or multi-step transfer of large gene clusters into the host (Chiang et al., 2013; Nielsen et al., 2013). For example transferred genes can be placed under the control of the alcA promoter using fusion PCR prior to A. nidulans transformation (Chiang et al., 2013; Lin et al., 2016). This approach can be adapted to incorporate large gene clusters using a marker recycling strategy in which genes containing selectable markers are introduced iteratively into A. nidulans (Chiang et al., 2013). Nielsen et al. developed another approach in which amplified fragments are combined into large fragments (~15 kb) prior to insertion into the transformation vector using USER cloning (Nielsen et al., 2013). For especially large pathways, such as that involved in geodin biosynthesis, additional fragments can be incorporated using a marker recycling strategy.
More recently, the co-inducible nitrate expression system, or CoIN, was developed utilizing one-step yeast recombinational cloning (Wiemann et al., 2018). Although yeast recombinational cloning theoretically enables assembly of PCR fragments into extremely large vectors, it is often subject to uncontrolled recombination events between identical genetic regions. To overcome this, Weimann et al. produced an expression system in which heterologous gene clusters can be co-induced using nitrate-inducible sterigmatocystin promoters, and successfully utilized the approach to express β-carotene in A. nidulans (Wiemann et al., 2018).
Over the last 5 years, scientists have begun using the AMA1 autonomous fungal replicating element (Aleksenko and Clutterbuck, 1997) as a tool for heterologous expression in A. nidulans. The utilization of this element enables incorporation of large BGCs into a heterologous host, which is difficult to achieve through chromosomal integration approaches (Clevenger et al., 2017; Hu et al., 2019b; Liu et al., 2019). In A. nidulans, AMA1 was first used for the identification of the Sch21072 biosynthetic pathway (Sato et al., 2015). In 2019, a multiple marker AMA1 vector set was developed by Hu et al., which enabled combinatorial testing of multiple biosynthetic genes to identify pathway intermediates of the perylenequinone elsinochrome A, allowing a more comprehensive characterization of this biosynthetic pathway (Hu et al., 2019b).
The AMA1 element has also been essential in an untargeted method utilizing ‘Fungal Artificial Chromosomes’ (FACs) to systematically annotate the biosynthetic capacity of fungi (Bok et al., 2015; Clevenger et al., 2017). FAC vector backbones are large Aspergillus/E. coli shuttle vectors containing randomly sheared genomic DNA, accessory genes, and regulatory elements from a fungal parent, as well as the AMA1 element that are capable of self-propagation in both A. nidulans and E. coli. While the first FAC study aimed to demonstrate that FACs function as expression vectors by producing a precursor from the known metabolite astechrome pathway (Bok et al., 2015), subsequent applications of the approach have incorporated a metabolite scoring system capable of differentiating heterologously expressed metabolites from those originating from the host organism. This approach has been used to discover novel secondary metabolites and their biosynthetic pathways including valactamide A (Clevenger et al., 2017) and terreazepine(Caesar et al., 2020), as well as to elucidate the biosynthesis of known metabolites including the benzomalvins (Clevenger et al., 2018) and acu-dioxomorpholines (Robey et al., 2018).
4. Where do we go from here?
4.1. The Lingering Challenge of the Silent Majority
It is clear that while we have made significant strides in completing the characterization of A. nidulans secondary metabolite pathways, there remain several challenges impeding this goal. The largest hurdle that must still be overcome is that of cryptic biosynthetic pathways. Undoubtedly, the abundance of such pathways in fungal genomes provides an unparalleled opportunity to discover new molecules with bioactive properties, and the strategies that have been developed to activate gene clusters have laid a strong foundation for future discoveries. Nonetheless, successful activation of the untapped potential of A. nidulans, and the fungal kingdom as a whole, will require not only the tools discussed above but comprehensive understanding of the myriad factors that regulate gene expression and translation, bioinformatic and phylogenetic tools to assist in prediction of BGC ‘viability’ and, we suggest, the largely ignored area of the role of cell biology on secondary metabolite synthesis.
4.2. “Strained” Information and Limitations of the A4 Reference Genome
The A4 reference genome of A. nidulans has been a powerhouse of information enabling the discovery of dozens of important secondary metabolites from this organism (Galagan et al., 2005). In recent years, scientists have begun to uncover a remarkable degree of within-species diversity, in which different isolates of the same organism illustrate markedly different phenotypes (Bastos et al., 2020; Drott et al., 2020; Horn and Dorner, 1999; Susca et al., 2016). We take note of pioneering work that examined of 36 BGC in 66 strains of the fungus A. fumigatus (Lind et al., 2017). This study identified a diversity of BGC polymorphisms that provided a blueprint for drivers of BGC macroevolutionary patterns including point mutations, recombination, and genomic deletion and insertion events as well as horizontal gene transfer from distant fungi. Two informative take home messages for future BGC mining strategies were that (i) due to mutational decay, not all BGCs will be active in all strains of a species and (ii) different isolates of the same species can possess different and active BGCs.
This latter point has is exemplified in A. nidulans. For example, A. nidulans NRRL 8112 was found to produce echinocandin B (Figure 1) by Eli Lilly as early as 1976 (Higgens and Michel, 1977), but the gene cluster encoding its production remained elusive. When genetic sequences between this strain and the A4 reference strain where compared, researchers found the echinocandin BGC present in A. nidulans NRRL 8112 but absent in the A4 reference strain (Hüttel et al., 2016).
More recently Drott et al. compared BGCs of two A. nidulans clinical isolates to the A4 reference strain (Drott et al., 2020). Using antiSMASH version 5.0 (Blin et al., 2019) and manual curation, this group identified a total of 75 BGCs from clinical isolate MO80069 and 74 BGCs from strain SP260548, compared to the 72 BGCs found in the A4 reference genome. Interestingly, only 67 of the 72 identified gene clusters were identified by previous annotations (Inglis et al., 2013), suggesting that the number of secondary metabolite clusters, even in the workhorse strain, may be greater than previously realized. Most notably, six clusters were not found in the A4 reference strain, and were either found in one or both clinical isolates, including the viridicatumtoxin cluster previously unknown in A. nidulans (Figure 1). HPLC-MS metabolite profiles of the three isolates also showed considerable differences, with the MO80069 and SP260548 strains showing two and four unique metabolites respectively. Future work will be required to identify these metabolites and determine whether or not they correspond to the unique clusters found within these organisms, or if they are products of yet uncharacterized clusters that are also found in the A4 reference genome. It is likely that continued sampling of more isolates will reveal new BGCs and secondary metabolite products and will also provide insight into the overall within-species variation of A. nidulans. This prospect is particularly exciting, as it offers a unique avenue to evaluate differences in BGC content and regulation of gene expression in phenotypically different strains of the same organism.
4.3. What can cell biology tell us?
Although yeasts are used as heterologous tools for secondary metabolite synthesis, they contain only a few known endogenous BGCs (Krause et al., 2018), and it is clear that filamentous fungi are the champions of natural product synthesis in Kingdom Fungi. Experimental data support a critical role for these metabolites in life style niches of the producing fungi, be it structural support, protection from abiotic or biotic assaults or signaling with other organisms (Keller, 2019) and, additionally, the shape and mode of growth of these complex fungi suggest a developmental involvement in secondary metabolism. Because many natural products and their precursors are toxic, even to the producing fungus, filamentous fungi have evolved transport pathways to shuttle and specialized vesicles to hold both enzymes and precursors of BGCs. Studies remain sparse on this topic, but the data indicate that transport and secretion pathways are critical for full expression of secondary metabolites. This is shown by a thorough characterization of temporal and spatial movement of penicillin biosynthetic enzymes in A. nidulans and Pencillium chrysogenum (Martín, 2020), of ‘toxisomes’ involved in aflatoxin biosynthesis in A. parasiticus and in terpene synthesis in Fusarium graminearum (Boenisch et al., 2019; Flynn et al., 2019; Linz et al., 2012), vesicles and enzyme trafficking needed for fumiquinazoline production in A. fumigatus (Lim et al., 2014) and subcellular compartmentalization and movement of melanin enzymes in a non-conventional secretory pathway in A. fumigatus (Upadhyay et al., 2016). Filamentous fungi maintain a balance of endo- and exocytosis at their growing tip, a region associated with secretion of secondary metabolites. Interestingly, a recent bioinformatic approach in A. nidulans looking for proteins containing motifs indicative of endocytic recycling, identified not only expected cytoskeleton related proteins but also at least one secondary metabolite protein, PkiC a fatty acid synthase required for cichorine synthesis (Commer et al., 2020).
5. Concluding Thoughts and Future Perspectives
Over the last 30 years, research into A. nidulans secondary metabolites has driven discoveries and synergistic development of technologies that now converge to increase our collective pace in the years ahead. Indeed, every major discovery made in the last 30 years was only possible through the continued collaboration and effort of the increasingly interdisciplinary field. Through the combination of bioinformatics, genome mining, fusion PCR, CRISPR technology, expression profiling, and culture manipulation, researchers have become increasingly effective in discovering and activating stubbornly silent clusters and tapping into the biosynthetic dark matter that remains, even in an organism as thoroughly studied as A. nidulans. Given the dynamic and interactive factors, both genetic and environmental, that regulate BGC activity and evolution, it is unsurprising that interdisciplinary strategies are required to induce expression of silent genes, to determine their expression into functional proteins, and to harness all the cellular machinery that enables facile secondary metabolite synthesis. The growing community in this arena can now “shine a light” into the darkness and accelerate our insights into the numerous interacting factors that influence biosynthesis of natural products to identify new fungal metabolites and improve the human condition.
Acknowledgements
A sincere thank you to Nicholas Raffa for his constructive criticism of the manuscript. The authors would also like to acknowledge financial support from the National Institutes of Health (grant F32GM132679 to L.K.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest
References
- Ahuja M, et al. , 2012. Illuminating the diversity of aromatic polyketide synthases in Aspergillus nidulans. J Am Chem Soc. 134, 8212–8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksenko A, Clutterbuck AJ, 1997. Autonomous plasmid replication in Aspergillus nidulans: AMA1 and MATE elements. Fungal Genet Biol. 21, 373–87. [DOI] [PubMed] [Google Scholar]
- Andersen MR, et al. , 2013. Accurate prediction of secondary metabolite gene clusters in filamentous fungi. Proc Natl Acad Sci. 110, E99–E107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud MB, et al. , 2010. The Aspergillus Genome Database, a curated comparative genomics resource for gene, protein and sequence information for the Aspergillus research community. Nucleic Acids Res. 38, D420–D427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basenko EY, et al. , 2018. FungiDB: An Integrated Bioinformatic Resource for Fungi and Oomycetes. J Fungi (Basel, Switzerland). 4, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos RW, et al. , 2020. Functional Characterization of Clinical Isolates of the Opportunistic Fungal Pathogen Aspergillus nidulans. mSphere. 5, e00153–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayram Ö, et al. , 2008. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science. 320, 1504–1506. [DOI] [PubMed] [Google Scholar]
- Bergmann S, et al. , 2007. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat Chem Biol. 3, 213–217. [DOI] [PubMed] [Google Scholar]
- Birse CE, Clutterbuck AJ, 1990. N-Acetyl-6-hydroxytryptophan oxidase, a developmentally controlled phenol oxidase from Aspergillus nidulans. Microbiology. 136, 1725–1730. [DOI] [PubMed] [Google Scholar]
- Blin K, et al. , 2019. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47, W81–W87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode HB, et al. , 2002. Big effects from small changes: possible ways to explore nature’s chemical diversity. Chembiochem. 3, 619–27. [DOI] [PubMed] [Google Scholar]
- Boenisch MJ, et al. , 2019. Nanoscale enrichment of the cytosolic enzyme trichodiene synthase near reorganized endoplasmic reticulum in Fusarium graminearum. Fungal Genet Biol. 124, 73–77. [DOI] [PubMed] [Google Scholar]
- Bok JW, et al. , 2009. Chromatin-level regulation of biosynthetic gene clusters. Nat Chem Biol. 5, 462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok JW, et al. , 2006. Genomic mining for Aspergillus natural products. Chem Biol. 13, 31–37. [DOI] [PubMed] [Google Scholar]
- Bok JW, Keller NP, 2004. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot Cell. 3, 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok JW, et al. , 2015. Fungal artificial chromosomes for mining of the fungal secondary metabolome. BMC Genomics. 16, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhired S, et al. , 2007. Accurate prediction of the Aspergillus nidulans terrequinone gene cluster boundaries using the transcriptional regulator LaeA. Fungal Genet Biol. 44, 1134–1145. [DOI] [PubMed] [Google Scholar]
- Bromann K, et al. , 2016. Engineering Aspergillus nidulans for heterologous ent-kaurene and gamma-terpinene production. Appl Microbiol Biotechnol. 100, 6345–6359. [DOI] [PubMed] [Google Scholar]
- Bromann K, et al. , 2012. Identification and characterization of a novel diterpene gene cluster in Aspergillus nidulans. PloS One. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D, et al. , 1996a. Aspergillus has distinct fatty acid synthases for primary and secondary metabolism. Proc Natl Acad Sci. 93, 14873–14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D, et al. , 1996b. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc Natl Acad Sci. 93, 1418–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar LK, et al. , 2020. Heterologous expression of the unusual terreazepine biosynthetic gene cluster reveals a promising approach for identifying new chemical scaffolds. mBio. 11, e01691–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemudupati M, et al. , 2019. The mode of mitosis is dramatically modified by deletion of a single nuclear pore complex gene in Aspergillus nidulans. Fungal Genet Biol. 130, 72–81. [DOI] [PubMed] [Google Scholar]
- Chen X, et al. , 2019. Immunosuppressant mycophenolic acid biosynthesis employs a new globin-like enzyme for prenyl side chain cleavage. Acta Pharmaceutica Sinica B. 9, 1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y-M, et al. , 2013. An efficient system for heterologous expression of secondary metabolite genes in Aspergillus nidulans. J Am Chem Soc. 135, 7720–7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y-M, et al. , 2010. Genetic characterization of the monodictyphenone gene cluster in Aspergillus nidulans. Appl Environ Microbiol. 76, 2067–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y-M, et al. , 2009. A gene cluster containing two fungal polyketide synthases encodes the biosynthetic pathway for a polyketide, asperfuranone, in Aspergillus nidulans. J Am Chem Soc. 131, 2965–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y-M, et al. , 2008. Molecular genetic mining of the Aspergillus secondary metabolome: discovery of the emericellamide biosynthetic pathway. Chem Biol. 15, 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang YM, et al. , 2016. Development of genetic dereplication strains in Aspergillus nidulans results in the discovery of aspercryptin. Angew Chem Int. 55, 1662–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chooi Y-H, et al. , 2010. Identification of the viridicatumtoxin and griseofulvin gene clusters from Penicillium aethiopicum. Chem Biol. 17, 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger KD, et al. , 2017. A scalable platform to identify fungal secondary metabolites and their gene clusters. Nat Chem Biol. 13, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger KD, et al. , 2018. Interrogation of benzomalvin biosynthesis using fungal artificial chromosomes with metabolomic scoring (FAC-MS): discovery of a benzodiazepine synthase activity. Biochemistry. 57, 3237–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commer B, et al. , 2020. Localization of NPFxD motif-containing proteins in Aspergillus nidulans. Fungal Genet Biol. 103412. [DOI] [PubMed] [Google Scholar]
- de Reus E, et al. , 2019. Metabolic and regulatory insights from the experimental horizontal gene transfer of the aurofusarin and bikaverin gene clusters to Aspergillus nidulans. Mol Microbiol. 112, 1684–1700. [DOI] [PubMed] [Google Scholar]
- Dimou S, et al. , 2019. The peroxisomal SspA protein is redundant for purine utilization but essential for peroxisome localization in septal pores in Aspergillus nidulans. Fungal Genet Biol. 132, 103259. [DOI] [PubMed] [Google Scholar]
- Drott M, et al. , 2020. Diversity of secondary metabolism in Aspergillus nidulans clinical isolates. Msphere. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisendle M, et al. , 2003. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding l-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol Microbiol. 49, 359–375. [DOI] [PubMed] [Google Scholar]
- Fan J, et al. , 2020. Formation of Terrestric Acid in Penicillium crustosum Requires Redox-Assisted Decarboxylation and Stereoisomerization. Org Lett. 22, 88–92. [DOI] [PubMed] [Google Scholar]
- Feng C, et al. , 2019. Biosynthesis of Diphenyl Ethers in Fungi. Org Lett. 21, 3114–3118. [DOI] [PubMed] [Google Scholar]
- Fischer J, et al. , 2018. Chromatin mapping identifies BasR, a key regulator of bacteria-triggered production of fungal secondary metabolites. Elife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn CM, et al. , 2019. Expression of the Fusarium graminearum terpenome and involvement of the endoplasmic reticulum-derived toxisome. Fungal Genet Biol. 124, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan JE, et al. , 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature. 438, 1105–1115. [DOI] [PubMed] [Google Scholar]
- Gilchrist CLM, et al. , 2018. Panning for gold in mould: can we increase the odds for fungal genome mining? Org Biomol Chem. 16, 1620–1626. [DOI] [PubMed] [Google Scholar]
- Grau MF, et al. , 2018. Hybrid transcription factor engineering activates the silent secondary metabolite gene cluster for (+)-asperlin in Aspergillus nidulans. ACS Chem Biol. 13, 3193–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau MF, et al. , 2019. Overexpression of an LaeA-like Methyltransferase Upregulates Secondary Metabolite Production in Aspergillus nidulans. ACS Chem Biol. 14, 1643–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev IV, et al. , 2011. Fueling the future with fungal genomics. Mycology. 2, 192–209. [Google Scholar]
- Hansen BG, et al. , 2012. Involvement of a natural fusion of a cytochrome P450 and a hydrolase in mycophenolic acid biosynthesis. Appl Environ Microbiol. 78, 4908–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke MT, et al. , 2016. New aspercryptins, lipopeptide natural products, revealed by HDAC inhibition in Aspergillus nidulans. ACS Chem Biol. 11, 2117–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgens CE, Michel KH, Antibiotic A-22082 and process for production thereof. Google Patents, 1977. [Google Scholar]
- Horio T, et al. , 2019. SUMOlock reveals a more complete Aspergillus nidulans SUMOylome. Fungal Genet Biol. 127, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn B, Dorner J, 1999. Regional differences in production of aflatoxin B1 and cyclopiazonic acid by soil isolates of Aspergillus flavus along a transect within the United States. Appl Environ Microbiol. 65, 1444–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, et al. , 2019a. Fungal Dirigent Protein Controls the Stereoselectivity of Multicopper Oxidase-Catalyzed Phenol Coupling in Viriditoxin Biosynthesis. J Am Chem Soc. 141, 8068–8072. [DOI] [PubMed] [Google Scholar]
- Hu J, et al. , 2019b. Heterologous biosynthesis of elsinochrome A sheds light on the formation of the photosensitive perylenequinone system. Chem Sci. 10, 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttel W, et al. , 2016. Echinocandin B biosynthesis: a biosynthetic cluster from Aspergillus nidulans NRRL 8112 and reassembly of the subclusters Ecd and Hty from Aspergillus pachycristatus NRRL 11440 reveals a single coherent gene cluster. BMC Genomics. 17, 570–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis DO, et al. , 2013. Comprehensive annotation of secondary metabolite biosynthetic genes and gene clusters of Aspergillus nidulans, A. fumigatus, A. niger and A. oryzae. BMC Microbiol. 13, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa N, et al. , 2014. Non-Heme Dioxygenase Catalyzes Atypical Oxidations of 6, 7‐Bicyclic Systems To Form the 6, 6-Quinolone Core of Viridicatin Type Fungal Alkaloids. Angew Chem Int. 53, 12880–12884. [DOI] [PubMed] [Google Scholar]
- Kato H, et al. , 2016. New natural products isolated from Metarhizium robertsii ARSEF 23 by chemical screening and identification of the gene cluster through engineered biosynthesis in Aspergillus nidulans A1145. J Antibiot. 69, 561–566. [DOI] [PubMed] [Google Scholar]
- Kelkar HS, et al. , 1996. Aspergillus nidulans stcP encodes an O-methyltransferase that is required for sterigmatocystin biosynthesis. Appl Environ Microbiol 62, 4296–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelkar HS, et al. , 1997. Aspergillus nidulans stcL encodes a putative cytochrome P-450 monooxygenase required for bisfuran desaturation during aflatoxin/sterigmatocystin biosynthesis. J Biol Chem. 272, 1589–1594. [DOI] [PubMed] [Google Scholar]
- Keller NP, 2015. Translating biosynthetic gene clusters into fungal armor and weaponry. Nat Chem Biol. 11, 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller NP, 2019. Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol. 17, 167–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller NP, et al. , 1995. StcS, a putative P-450 monooxygenase, is required for the conversion of versicolorin A to sterigmatocystin in Aspergillus nidulans. Appl Environ Microbiol. 61, 3628–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller NP, et al. , 2005. Fungal secondary metabolism — from biochemistry to genomics. Nat Rev Microbiol. 3, 937–947. [DOI] [PubMed] [Google Scholar]
- Keller NP, et al. , 2000. Requirement of monooxygenase-mediated steps for sterigmatocystin biosynthesis by Aspergillus nidulans. Appl Environ Microbiol. 66, 359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaldi N, et al. , 2010. SMURF: genomic mapping of fungal secondary metabolite clusters. Fungal Genet Biol. 47, 736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopke K, et al. , 2013. Members of the Penicillium chrysogenum velvet complex play functionally opposing roles in the regulation of penicillin biosynthesis and conidiation. Eukaryot Cell. 12, 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause DJ, et al. , 2018. Functional and evolutionary characterization of a secondary metabolite gene cluster in budding yeasts. Proc Natl Acad Sci. 115, 11030–11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwayama H, et al. , 2002. PCR-mediated generation of a gene disruption construct without the use of DNA ligase and plasmid vectors. Nucleic Acids Res. 30, e2–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. , 2019a. Discovery and Heterologous biosynthesis of the burnettramic acids: rare PKS-NRPS-derived bolaamphiphilic pyrrolizidinediones from an Australian fungus, Aspergillus burnettii. Org Lett. 21, 1287–1291. [DOI] [PubMed] [Google Scholar]
- Li H, et al. , 2020a. Biosynthesis of a New Benzazepine Alkaloid Nanangelenin A from Aspergillus nanangensis Involves an Unusual l-Kynurenine-Incorporating NRPS Catalyzing Regioselective Lactamization. J Am Chem Soc. 142, 7145–7152. [DOI] [PubMed] [Google Scholar]
- Li H, et al. , 2019b. Biosynthesis of a Tricyclo[6.2.2.0(2,7)]dodecane System by a Berberine Bridge Enzyme-Like Aldolase. Chemistry. 25, 15062–15066. [DOI] [PubMed] [Google Scholar]
- Li H, et al. , 2018a. Chemical Ecogenomics-Guided Discovery of Phytotoxic α-Pyrones from the Fungal Wheat Pathogen Parastagonospora nodorum. Org Lett. 20, 6148–6152. [DOI] [PubMed] [Google Scholar]
- Li H, et al. , 2020b. Genomics-Driven Discovery of Phytotoxic Cytochalasans Involved in the Virulence of the Wheat Pathogen Parastagonospora nodorum. ACS Chem Biol. 15, 226–233. [DOI] [PubMed] [Google Scholar]
- Li L, et al. , 2016. Biochemical Characterization of a Eukaryotic Decalin-Forming Diels–Alderase. J Am Chem Soc. 138, 15837–15840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, et al. , 2018b. Asperphenamate biosynthesis reveals a novel two-module NRPS system to synthesize amino acid esters in fungi. Chem Sci. 9, 2589–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim FY, et al. , 2014. Co ordination between BrlA regulation and secretion of the oxidoreductase FmqD directs selective accumulation of fumiquinazoline C to conidial tissues in Aspergillus fumigatus. Cell Microbiol. 16, 1267–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T-S, et al. , 2016. Biosynthetic pathway of the reduced polyketide product citreoviridin in Aspergillus terreus var. aureus revealed by heterologous expression in Aspergillus nidulans. Org Lett. 18, 1366–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TS, et al. , 2019a. Discovery and Elucidation of the Biosynthesis of Aspernidgulenes: Novel Polyenes from Aspergillus nidulans by Using Serial Promoter Replacement. ChemBioChem. 20, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, et al. , 2019b. Heterologous Expression of Ilicicolin H Biosynthetic Gene Cluster and Production of a New Potent Antifungal Reagent, Ilicicolin J. Molecules. 24, 2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind AL, et al. , 2017. Drivers of genetic diversity in secondary metabolic gene clusters within a fungal species. PLoS biology. 15, e2003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz JE, et al. , 2012. Proteomic and biochemical evidence support a role for transport vesicles and endosomes in stress response and secondary metabolism in Aspergillus parasiticus. J Proteome Res. 11, 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzka O, et al. , 1996. The Aspergillus nidulans penicillin-biosynthesis gene aat (penDE) is controlled by a CCAAT-containing DNA element. Eur J Biochem. 238, 675–682. [DOI] [PubMed] [Google Scholar]
- Liu L, et al. , 2019. Fungal Highly Reducing Polyketide Synthases Biosynthesize Salicylaldehydes That Are Precursors to Epoxycyclohexenol Natural Products. J Am Chem Soc. 141, 19538–19541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, et al. , 2017. Identification and Heterologous Production of a Benzoyl-Primed Tricarboxylic Acid Polyketide Intermediate from the Zaragozic Acid A Biosynthetic Pathway. Org Lett. 19, 3560–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo H-C, et al. , 2012. Two separate gene clusters encode the biosynthetic pathway for the meroterpenoids austinol and dehydroaustinol in Aspergillus nidulans. J Am Chem Soc. 134, 4709–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Berges MS, et al. , 2013. The velvet complex governs mycotoxin production and virulence of Fusarium oxysporum on plant and mammalian hosts. Mol Microbiol. 87, 49–65. [DOI] [PubMed] [Google Scholar]
- Ma K, et al. , 2019. Characterization and Biosynthesis of a Rare Fungal Hopane-Type Triterpenoid Glycoside Involved in the Antistress Property of Aspergillus fumigatus. Org Lett. 21, 3252–3256. [DOI] [PubMed] [Google Scholar]
- Ma Z, et al. , 2018. Rational design for heterologous production of aurovertin-type compounds in Aspergillus nidulans. Appl Microbiol Biotechnol. 102, 297–304. [DOI] [PubMed] [Google Scholar]
- Mabey Gilsenan J, et al. , 2012. CADRE: the Central Aspergillus Data REpository 2012. Nucleic Acids Res. 40, D660–D666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCabe A, et al. , 1991. Delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. Molecular characterization of the acvA gene encoding the first enzyme of the penicillin biosynthetic pathway. J Biol Chem. 266, 12646–12654. [PubMed] [Google Scholar]
- Macheleidt J, et al. , 2016. Regulation and role of fungal secondary metabolites. Annu Review Genet. 50, 371–392. [DOI] [PubMed] [Google Scholar]
- Maiya S, et al. , 2009. Improved tryprostatin B production by heterologous gene expression in Aspergillus nidulans. Fungal Genet Biol. 46, 436–440. [DOI] [PubMed] [Google Scholar]
- Marcos AT, et al. , 2020. Nitric oxide homeostasis is required for light-dependent regulation of conidiation in Aspergillus. Fungal Genet Biol. 137, 103337. [DOI] [PubMed] [Google Scholar]
- Martín JF, 2020. Transport systems, intracellular traffic of intermediates and secretion of β-lactam antibiotics in fungi. Fungal Biol Biotechnol. 7, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema MH, et al. , 2011. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 39, W339–W346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BL, et al. , 1985. Direct and indirect gene replacements in Aspergillus nidulans. Mol Cell Biol. 5, 1714–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanta TK, Bae H, 2015. The diversity of fungal genome. Biol Proced Online. 17, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak T, et al. , 2006. A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics. 172, 1557–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Németh Z, et al. , 2019. l-Arabinose induces d-galactose catabolism via the Leloir pathway in Aspergillus nidulans. Fungal Genet Biol. 123, 53–59. [DOI] [PubMed] [Google Scholar]
- Netzker T, et al. , 2018. Microbial interactions trigger the production of antibiotics. Curr Op Microbiol. 45, 117–123. [DOI] [PubMed] [Google Scholar]
- Nielsen ML, et al. , 2011. A genome-wide polyketide synthase deletion library uncovers novel genetic links to polyketides and meroterpenoids in Aspergillus nidulans. FEMS Microbiol Lett. 321, 157–166. [DOI] [PubMed] [Google Scholar]
- Nielsen MT, et al. , 2013. Heterologous reconstitution of the intact geodin gene cluster in Aspergillus nidulans through a simple and versatile PCR based approach. PloS One. 8, e72871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies J, et al. , 2020. Biosynthesis of the Prenylated Salicylaldehyde Flavoglaucin Requires Temporary Reduction to Salicyl Alcohol for Decoration before Reoxidation to the Final Product. Organic Lett. 22, 2256–2260. [DOI] [PubMed] [Google Scholar]
- Nødvig CS, et al. , 2018. Efficient oligo nucleotide mediated CRISPR-Cas9 gene editing in Aspergilli. Fungal Genet and Biol. 115, 78–89. [DOI] [PubMed] [Google Scholar]
- Nødvig CS, et al. , 2015. A CRISPR-Cas9 system for genetic engineering of filamentous fungi. PloS One. 10, e0133085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nützmann H-W, et al. , 2011. Bacteria-induced natural product formation in the fungus Aspergillus nidulans requires Saga/Ada-mediated histone acetylation. Proc Natl Acad Sci. 108, 14282–14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B, et al. , 1987. Cloning, mapping and molecular analysis of the pyrG (orotidine-5’-phosphate decarboxylase) gene of Aspergillus nidulans. Gene. 61 3, 385–99. [DOI] [PubMed] [Google Scholar]
- Oakley CE, et al. , 2017. Discovery of McrA, a master regulator of Aspergillus secondary metabolism. Mol Microbiol. 103, 347–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JM, et al. , 2013. Secondary metabolism and development is mediated by LlmF control of VeA subcellular localization in Aspergillus nidulans. PLoS Genet. 9, e1003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannenstiel BT, et al. , 2018. The epigenetic reader SntB regulates secondary metabolism, development and global histone modifications in Aspergillus flavus. Fungal Genet Biol. 120, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontecorvo G, et al. , The Genetics of Aspergillus nidulans In: Demerec M, (Ed.), Advances in Genetics. Academic Press, 1953, pp. 141–238. [DOI] [PubMed] [Google Scholar]
- Rahnama M, et al. , 2019. The LaeA orthologue in Epichloë festucae is required for symbiotic interaction with Lolium perenne. Fungal Genet Biol. 129, 74–85. [DOI] [PubMed] [Google Scholar]
- Robey MT, et al. , 2018. Identification of the first diketomorpholine biosynthetic pathway using FAC-MS technology. ACS Chem Biol. 13, 1142–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romsdahl J, Wang CCC, 2019. Recent advances in the genome mining of Aspergillus secondary metabolites (covering 2012–2018). MedChemComm. 10, 840–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, et al. , 2020. CRISPR-mediated activation of biosynthetic gene clusters for bioactive molecule discovery in filamentous fungi. ACS Synth Biol. [DOI] [PubMed] [Google Scholar]
- Ruiz-Velasco M, Zaugg JB, 2017. Structure meets function: how chromatin organisation conveys functionality. Curr Op Syst Biol. 1, 129–136. [Google Scholar]
- Ryan KL, et al. , 2013. Partial reconstruction of the ergot alkaloid pathway by heterologous gene expression in Aspergillus nidulans. Toxins. 5, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha P, et al. , 2020. MoLAEA Regulates Secondary Metabolism in Magnaporthe oryzae. Msphere. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RA, et al. , 2014. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol. 78, 141–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JF, et al. , 2010. Molecular genetic analysis of the orsellinic acid/F9775 gene cluster of Aspergillus nidulans. Mol Biosyst. 6, 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JF, et al. , 2012. Identification and molecular genetic analysis of the cichorine gene cluster in Aspergillus nidulans. MedChemComm. 3, 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JF, et al. , 2011. Genome-based deletion analysis reveals the prenyl xanthone biosynthesis pathway in Aspergillus nidulans. J Am Chem Soc. 133, 4010–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, et al. , 2015. Involvement of Lipocalin-like CghA in Decalin-Forming Stereoselective Intramolecular [4+2] Cycloaddition. ChemBioChem. 16, 2294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherlach K, et al. , 2011. Two induced fungal polyketide pathways converge into antiproliferative spiroanthrones. ChemBioChem. 12, 1836–1839. [DOI] [PubMed] [Google Scholar]
- Scherlach K, et al. , 2010. Aspernidine A and B, prenylated isoindolinone alkaloids from the model fungus Aspergillus nidulans. J Antibiot. 63, 375–377. [DOI] [PubMed] [Google Scholar]
- Schroeckh V, et al. , 2009. Intimate bacterial–fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc Natl Acad Sci. 106, 14558–14563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J, et al. , 2015. The VELVET Complex in the Gray Mold Fungus Botrytis cinerea: Impact of BcLAE1 on Differentiation, Secondary Metabolism, and Virulence. Mol Plant Microbe Interact. 28, 659–74. [DOI] [PubMed] [Google Scholar]
- Seibold PS, et al. , 2020. The Laetiporus polyketide synthase LpaA produces a series of antifungal polyenes. J Antibiot. 73, 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spröte P, et al. , 2008. Identification of the novel penicillin biosynthesis gene aatB of Aspergillus nidulans and its putative evolutionary relationship to this fungal secondary metabolism gene cluster. Mol Microbiol. 70, 445–461. [DOI] [PubMed] [Google Scholar]
- Stajich JE, et al. , 2012. FungiDB: an integrated functional genomics database for fungi. Nucleic Acids Res. 40, D675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroe MC, et al. , 2020. Targeted induction of a silent fungal gene cluster encoding the bacteria-specific germination inhibitor fumigermin. Elife. 9, e52541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung CT, et al. , 2017. Overexpression of a three-gene conidial pigment biosynthetic pathway in Aspergillus nidulans reveals the first NRPS known to acetylate tryptophan. Fungal Genet Biol. 101, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susca A, et al. , 2016. Variation in fumonisin and ochratoxin production associated with differences in biosynthetic gene content in Aspergillus niger and A. welwitschiae isolates from multiple crop and geographic origins. Front Microbiol. 7, 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk E, et al. , 2008. Identification and characterization of the asperthecin gene cluster of Aspergillus nidulans. Appl Environ Microbiol. 74, 7607–7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk E, et al. , 2006. Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protocols. 1, 3111–3120. [DOI] [PubMed] [Google Scholar]
- Unkles SE, et al. , 2014. Synthetic biology tools for bioprospecting of natural products in eukaryotes. Chem Biol. 21, 502–508. [DOI] [PubMed] [Google Scholar]
- Upadhyay S, et al. , 2016. Subcellular compartmentalization and trafficking of the biosynthetic machinery for fungal melanin. Cell Rep. 14, 2511–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas KG, et al. , 2019. Cpf1 enables fast and efficient genome editing in Aspergilli. Fungal Biol Biotechnol. 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. , 2020. Genome mining and biosynthesis of the Acyl-CoA:cholesterol acyltransferase inhibitor beauveriolide I and III in Cordyceps militaris. J Biotechnol. 309, 85–91. [DOI] [PubMed] [Google Scholar]
- Watanabe A, et al. , 1999. Re-identification of Aspergillus nidulans wA gene to code for a polyketide synthase of naphthopyrone. Tetrahedron Lett. 40, 91–94. [Google Scholar]
- Wiemann P, et al. , 2018. CoIN: co-inducible nitrate expression system for secondary metabolites in Aspergillus nidulans. Fungal Biol Biotechnol. 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang P, et al. , 2020. Isocoumarin formation by heterologous gene expression and modification by host enzymes. Org Biomol Chem. 18, 4946–4948. [DOI] [PubMed] [Google Scholar]
- Yaegashi J, et al. , 2014. Recent advances in genome mining of secondary metabolite biosynthetic gene clusters and the development of heterologous expression systems in Aspergillus nidulans. J Ind Microbiol Biotechnol. 41, 433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaegashi J, et al. , 2013. Molecular genetic characterization of the biosynthesis cluster of a prenylated isoindolinone alkaloid aspernidine A in Aspergillus nidulans. Org Lett. 15, 2862–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, et al. , 2004. Rapid production of gene replacement constructs and generation of a green fluorescent protein-tagged centromeric marker in Aspergillus nidulans. Eukaryot Cell. 3, 1359–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H-H, et al. , 2016. Resistance gene-guided genome mining: serial promoter exchanges in Aspergillus nidulans reveal the biosynthetic pathway for fellutamide B, a proteasome inhibitor. ACS Chem Biol. 11, 2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H-H, et al. , 2012. Molecular genetic analysis reveals that a nonribosomal peptide synthetase-like (NRPS-like) gene in Aspergillus nidulans is responsible for microperfuranone biosynthesis. Appl Microbiol Biotechnol. 96, 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W-B, et al. , 2013. Discovery of cryptic polyketide metabolites from dermatophytes using heterologous expression in Aspergillus nidulans. ACS Synth Biol. 2, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J-H, et al. , 2004. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet Biol. 41, 973–981. [DOI] [PubMed] [Google Scholar]
- Zarrin M, et al. , 2005. A rapid method for promoter exchange in Aspergillus nidulans using recombinant PCR. Fungal Genet Biol. 42, 1–8. [DOI] [PubMed] [Google Scholar]
- Zhang J-M, et al. , 2020. Heterologous and Engineered Biosynthesis of Nematocidal Polyketide–Nonribosomal Peptide Hybrid Macrolactone from Extreme Thermophilic Fungi. J Am Chem Soc. 142, 1957–1965. [DOI] [PubMed] [Google Scholar]
- Zhang P, et al. , 2017. A cryptic pigment biosynthetic pathway uncovered by heterologous expression is essential for conidial development in Pestalotiopsis fici. Mol Microbiol. 105, 469–483. [DOI] [PubMed] [Google Scholar]


