Abstract
Background:
Undetected Alzheimer’s disease and stroke neuropathology is believed to account for a large proportion of decline in cognitive performance that is attributed to normal aging. This study examined the amount of variance in age-related cognitive change that is accounted for by Alzheimer’s disease and stroke using a novel pattern-recognition protocol.
Method:
Secondary analyses of data collected for the Health & Retirement Study (N=17,579) were used to objectively characterize patterns of cognitive decline associated with Alzheimer’s disease and stroke. The rate of decline in episodic memory and orientation was the outcome of interest, while algorithms indicative of Alzheimer’s disease and stroke pathology were the predictors of interest.
Results:
The average age of the sample was 67.54±10.45 years old at baseline and completed, on average, 14.20±3.56 years of follow-up. After adjusting for demographics, Alzheimer’s disease and stroke accounted for approximately half of age-associated decline in cognition (51.07–55.6% for orientation and episodic memory respectively) and explained variance attributed to random slopes in longitudinal multilevel models.
Discussion:
The results of this study suggested that approximately half of cognitive decline usually attributed to normal aging may be due to Alzheimer’s disease and stroke.
Keywords: neuroepidemiology, cerebrovascular disease, pattern recognition, adaptive diagnostics
Alzheimer’s disease and related dementias are extremely common and are estimated to affect 14 million people in the United States [1], and was recorded as the underlying cause of death in ≥110,000 deaths in 2018 [2]. More than 99% of all dementias fall into two main types: Alzheimer’s disease, which accounts for approximately 60–80% of all cases, and vascular dementia, primarily associated with major stroke in addition to other cerebrovascular causes and risk factors. Both Alzheimer’s disease and vascular dementia are known to impair cognitive performance [3]. However, dementia due to Alzheimer’s disease and cerebrovascular disease are both often preceded by substantial losses in cognitive functioning that can take years to accumulate sufficiently to cause clinical impairment.
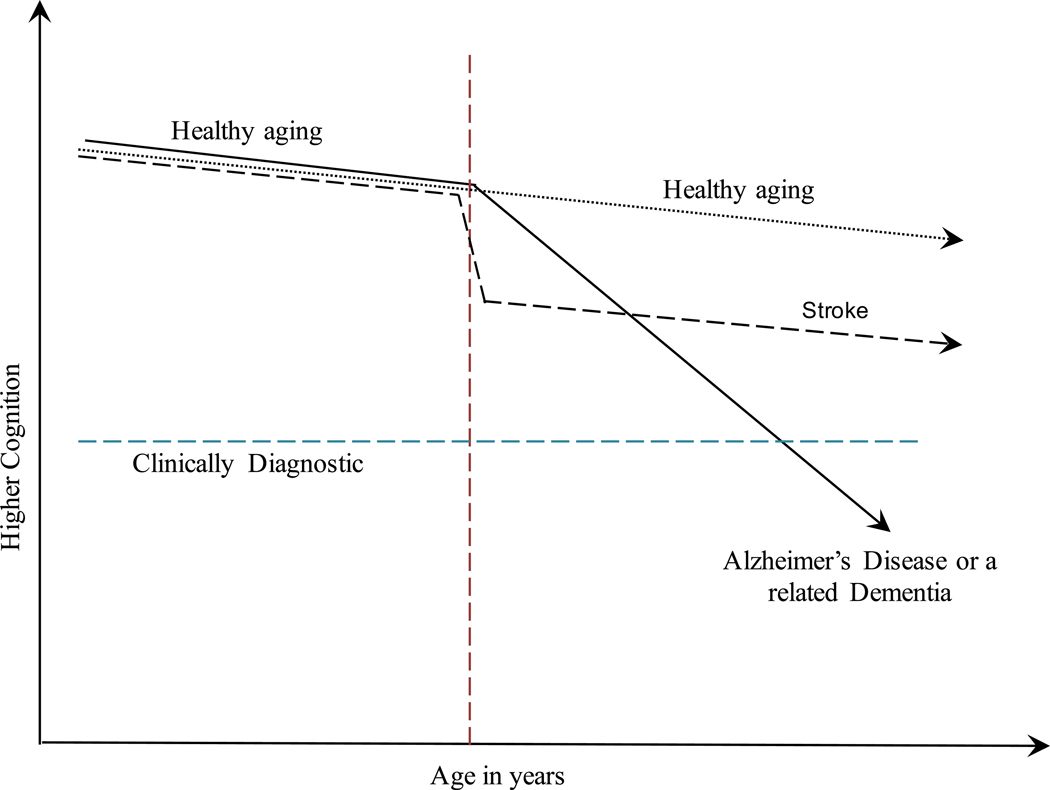
Recent work has suggested that approximately half (41–52%) of age-related cognitive decline observed in older adult populations may be attributable to the presence of age-related pathologies including Alzheimer’s disease and stroke [4, 5]. However, efforts thus far have been limited because disease diagnosis and identification of causes and rates of progression rely heavily on subjective reports from individuals and/or informants to characterize disease progress. Yet, clinical diagnoses for Alzheimer’s disease and stroke have long been differentiated based on the rate at which cognitive declines occur [6]. However, to date these diagnoses have relied almost exclusively on subjective individual or informant reports about rapidity of symptom onset to help differential diagnosis of Alzheimer’s disease and cerebrovascular disease or stroke [7]. However, whereas normal aging is conceptualized as a linear decline in cognitive performance throughout adulthood [8], cognitive decline evident in Alzheimer’s disease (as represented graphically in Figure 1) is characterized by decades [9] of progressive accelerated losses in cognitive functioning when compared to normal aging [10], and decline in stroke is often clinically characterized by a rapid loss in functioning which cause losses over a very short period of time before eventually stabilizing [11].
Figure 1.
Differential pathological characterization based on aging pattern in episodic memory: Vascular decline occurs quickly but stabilizes while Alzheimer’s-related decline represents slow but progressive declines in cognitive function with time.
This study relies on recent advances in pattern-recognition algorithms to clinically differentiate normal aging from Alzheimer’s disease [12] and ischemic events (i.e., major and silent “stroke”) [13]. The goal of this study was to determine the extent to which Alzheimer’s disease and stroke-like events identified using pattern recognition algorithms helped to explain associations between aging and cognitive decline in episodic memory and orientation in a national sample of older U.S. residents.
Methods
We utilized data from the Health and Retirement Study (HRS), a large representative sample of older adults in the United States, which collects cognitive data biennially starting in 1992 (response rate 81.6%). The HRS is open to enrollment at subsequent waves, and data are publicly available online (http://hrsonline.isr.umich.edu) [14]. Data from waves 3–12 were used in this analysis due to different parameters for tasks of interest in waves 1 & 2 compared to those used in waves 3 and beyond. Due to the intense analytic requirements for differential diagnostic routines, respondents without at least four valid observations were excluded. The analytic sample included 17,579 respondents who were followed up 7.9 times, on average, over the course of 14.20 (SD=3.56) years.
Measures
Episodic memory is a critical measure of cognitive functioning that is both sensitive to cognitive aging, AD [15], and, in its acute phase, stroke [16]. To measure episodic memory, respondents were first provided with a list of ten unrelated words and asked to immediately recall as many words as they could remember from the initial list in any order that they wished. Participants reported each recalled item aloud to the interviewer, and each correctly recalled word was worth one point. After completing a distractor task that lasted 10–15 minutes, respondents were again asked to correctly recall as many words from the initial list as possible. Recalled items were reported aloud to the interviewer with each correctly recalled item scoring a single point irrespective of the order in which the items were recalled. The verbal episodic memory index included the summation of both immediate and delayed tests (possible scores ranging from 0–20). The resulting index was normally distributed (mean = 10.83, SD = 3.44, skew = −0.05, kurtosis = 2.94).
Orientation is a common measure of dementia risk including both Alzheimer’s disease and vascular dementia. In the HRS, orientation was measured by testing participants on knowledge of common everyday facts, such as the day of the week or the current president’s name, and capacity to complete simple financial numeracy tasks. Confusion in these domains is believed to indicate disorientation in everyday life consistent with dementia, including Alzheimer’s disease and vascular dementia. Measures also included determining similarities between common items, identification of pictures of common items, and correctly being able to count and subtract numbers in reverse. A factor analysis clarified that these measures formed a single-factor solution (ξ=0.81; weights were 0.32 (backwards counting), 0.50 (subtract sevens), and 0.48 (orientation). In the HRS, orientation (with possible scores ranging from 0–15) is measured among individuals aged 65 and older using seven questions including the day of the week, the date including day, month, and year (e.g., “April 4th, 2018”); numeracy as measured using the ability to subtract seven from a random number and the ability to count backwards from another number; object naming for one man-made object and another natural object; and the names of the current president and vice president of the United States.
Alzheimer’s and stroke pathological declines
Under normal aging, which is often defined as the decline that is evident among individuals who are not exhibiting signs of cognitively impairing disease [8], cognitive decline occurs linearly at a steady rate that may differ between individuals. These methods have been described in depth elsewhere [17, 18]; briefly, the pattern recognition model sorts through the longitudinal data seeking a pattern defined a priori to match clinical expectations of Alzheimer’s disease, stroke, or normal aging. As such, a linear rate of decline was used as the null assumption whereas a pattern of piecewise-linear accelerated declines was identified for Alzheimer’s disease while a pattern of significant stepwise decline was identified for stroke (following Figure 1). To objectively detect Alzheimer’s-like and/or stroke-like patterns of decline, a pattern recognition protocol was applied to individual-level trajectories of episodic memory to determine which pattern maximized the log-likelihood; model fit statistics were retained. These trajectories were used both to characterize individuals as having either Alzheimer’s or stroke and also used to identify the timing of onset for Alzheimer’s and/or stroke pathology onset. Akaike’s information criterion (AIC) was used to compare linear models of healthy aging to pathological models for each individual, and then to differentiate between stroke and Alzheimer’s disease cognitive pathology. In cases where both Alzheimer’s disease and stroke pathology were identified, a mixed (both were identified and fit improved with both) or indeterminate (both were identified but neither disease was primary) category was also recorded for descriptive purposes. For the purposes of contextualizing these results, rates of decline were compared to each other and were compared with the average level of decline necessary to cause clinical diagnosis of amnestic mild cognitive impairment as characterized by a loss of 1.5 standard deviations in episodic memory. Subgroup analyses examined the mixed category to examine rapidity of rates of decline in this group. Models incorporated respondent’s age in years, sex, and race/ethnicity.
Statistical Analyses
Means, standard deviations, and percentages were used to describe the sample. Prevalence of disease outcomes in the sample were estimated using percentages and 95% confidence intervals (95% C.I.). For descriptive purposes, age-adjusted incidence rates (aIR) were also reported alongside accompanying 95% confidence intervals.
Longitudinal multilevel modeling is a common method used to mark the rate of cognitive decline [19]. In our longitudinal multilevel models, random intercepts were utilized to account for time-invariant individual differences in cognitive performance at baseline, while random slopes were utilized to account for heteroscedasticity in longitudinal trajectories [20]. An unstructured covariance matrix was utilized to account for potential associations between individual cognitive performance at baseline and rate of change over time, when negative this association is attributable to regression to the mean [21]. Predicted results were used to provide model-estimated trajectories. The modeling strategy tested the assumption that age was associated with cognitive decline using age in years specified linearly; random intercepts and slopes were included in all modeling efforts. The beta coefficient for age in years was considered to capture the aging process. Multivariable adjusted models were then used to explain aging. These models included adjustments for Race/Ethnicity and sex as a base model while a second model further examined the impact of adjusting for Alzheimer’s disease and stroke pathology. The proposed method uses a non-linear pattern recognition software to differentiate causes of cognitive decline. Since prior work has identified quadratic change in aging as a potentially important component of the aging process, sensitivity analyses considered the role of a quadratic acceleration curve.
All analyses were completed using Stata 15/SE [StataCorp].
Results
The sample was in their 60s on average and made up predominantly of females (Table 1). Bivariate results revealed that individuals with any indication of pathological cognitive decline were older than those without, and that those with performance patterns consistent with Alzheimer’s or indeterminate patterns of decline were older than those with patterns consistent with stroke. Half of respondents lacked patterns indicative of either Alzheimer’s disease or stroke. Not shown in Table 1, age-standardized rates were aIR = 34.57 [29.38–39.77] /1,000 person-years for Alzheimer’s, and 22.73 [17.69–27.76] /1,000 person-years in stroke.
Table 1.
Descriptive characteristics of eligible participants and separated by cognitive pathology, Health & Retirement Study (N = 17,579)
| Whole Sample |
Normal (n = 6,601; 37.55%) |
Alzheimer’s Disease only (n=1,799; 10.23%) |
Stroke only (n = 4,661; 26.51%) |
Mixed or both (n = 4,518; 25.70%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Episodic Memory in words | 9.99 | 3.57 | 10.41 | 3.34 | 10.55 | 3.22 | 9.95 | 3.58 | 9.21 | 3.88 |
| Orientation | 12.67 | 2.48 | 12.86 | 2.31 | 12.66† | 2.46 | 12.91 | 2.21 | 12.39† | 2.73 |
| Age in years | 60.27 | 9.51 | 57.67 | 8.34 | 60.18† | 9.74 | 57.62 | 6.99 | 65.21† | 9.78 |
| Characteristic | % | % | % | % | % | |||||
| Race/Ethnicity | ||||||||||
| White | 75.84 | 75.03 | 73.51 | 75.46 | 79.09 | |||||
| Black | 13.50 | 14.07 | 14.63 | 13.32 | 12.23 | |||||
| Other | 2.11 | 2.06 | 2.44 | 2.13 | 1.88 | |||||
| Hispanic | 8.56 | 8.84 | 9.43 | 9.10 | 6.80 | |||||
| Male sex | 38.25 | 37.74 | 39.96 | 39.09 | 36.38 | |||||
Note: SD: standard deviation; %: percentage. Diagnostic categories were determined using pattern recognition protocols. P-values were derived from trend tests showing whether there were any differences at baseline between the diagnostic types:
P<1E-06;
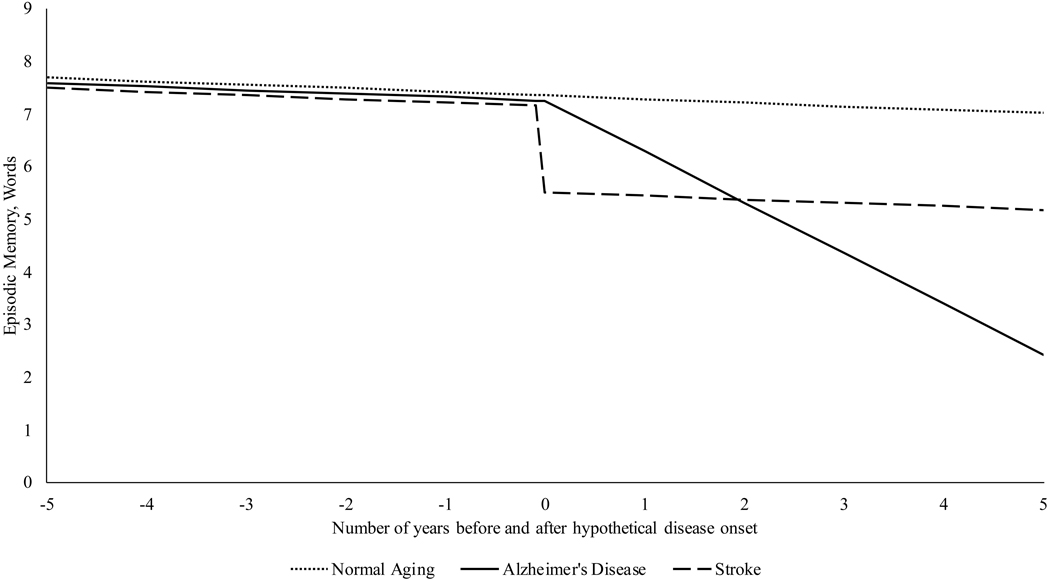
The unadjusted rate of decline in episodic memory was −0.146 (95% C.I. = [−0.144, −0.149] per year of decline after adjusting for random intercepts and slopes and their association (estimated: −0.434 [−0.412, −0.456]). After adjusting for cross-sectional differences in sex and race and for practice effects (Table 2, Model 1), the rate of age-related decline increased to −0.152, 95% C.I.=[−0.150, −0.155] words per year of age. Finally, adjusting for both stroke and Alzheimer’s pattern (Table 2, Model 2), associations decreased by 55.6% [53.29, 57.76] to −0.068 [−0.065, −0.070] words/year. Additionally, integration of stroke and Alzheimer’s pattern declines explained variance attributed to random slopes and negative associations found between random intercepts and slopes. Analyses suggested that while the effects of strokes are immediately evident (as represented in Figure 2), that the rate of progressive decline indicative of an Alzheimer’s-pattern overtook the effects of a single stroke after 1.82 [1.80–1.85] years. Alzheimer’s disease was associated with reductions in episodic memory of 1.5 SDs over the course of 5.98 years. In contrast, the rate of decline in normal cognitive aging shown here (B = −0.068) would take an equivalent of 13.17 years of normal aging to equate to the size of declines seen in only a single year of Alzheimer’s disease (0.896 words), and would cause only cause a decrement of 1.5 SDs in cognition (enough to be characterized a mildly cognitively impaired) over the course of 78.75 years. In other words, it is expected that cognitively normal older individuals aged 50 and older would need to more than double their current age to exhibit aging-related decrements in performance equivalent to those observed in the Alzheimer’s-pattern individuals.
Table 2.
Coefficients and standard deviations examining associations between age, stroke, and Alzheimer’s disease in explaining longitudinal declines in episodic memory
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| Fixed Effects | Mean | SE | Mean | SE |
| Male Sex | −0.947 | 0.035 | −1.098 | 0.037 |
| Age in years | −0.152 | 0.001 | −0.068 | 0.001 |
| Race/Ethnicity | ||||
| White | Reference | Reference | ||
| Black | −1.789 | 0.051 | −1.681 | 0.053 |
| Other | −1.238 | 0.119 | −1.007 | 0.125 |
| Hispanic | −1.862 | 0.061 | −1.711 | 0.064 |
| Stroke | −1.635 | 0.023 | ||
| Alzheimer’s Disease | −0.896 | 0.006 | ||
| Constant | 13.383 | 0.033 | 12.560 | 0.032 |
| Random Effects | ES | SE | ES | SE |
| Slopes, SD | 0.127 | 0.002 | 0.000 | 0.000 |
| Intercepts, SD | 2.342 | 0.018 | 2.262 | 0.014 |
| Corr(S,I) | −0.437 | 0.011 | 0.000 | 0.000 |
Note: SE: standard error; ES: estimate size.
All non-zero associations shown were statistically significant at p<1E-06 level. Analyses additionally adjusted for practice effects observed between the first and following waves.
Figure 2.
Predicted episodic memory trajectories for normal aging, stroke, and Alzheimer’s disease detected using pattern-recognition methodology, Health and Retirement Study
Note: Results were generated using posterior predictions from Table 2, Model 2
Analyses examining change in orientation provided similar overall results (Table 3), suggesting that episodic memory declines consistent with Alzheimer’s disease were characterized by the loss of 0.384 points/year in orientation, a decline that explained approximately 51.07 % [40.70–46.06] of aging-related declines in orientation. This loss was larger than the association with stroke, and equivalent to one-point loss in orientation every 2.60 years or one SD loss in orientation in 6.46 years. Integration of Alzheimer’s and stroke variables accounted for the negative association between individual random intercepts and slopes and even predicted an inverted association such that improved orientation was slightly associated with slower rates of cognitive decline.
Table 3.
Coefficients and standard deviations examining associations between age, stroke, and Alzheimer’s disease in explaining longitudinal declines in orientation
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| Fixed Effects | Mean | SD | Mean | SD |
| Male Sex | 0.450 | 0.028 | 0.403 | 0.027 |
| Age in years | −0.064 | 0.001 | −0.035 | 0.001 |
| Race/Ethnicity | ||||
| White | Reference | Reference | ||
| Black | −2.141 | 0.040 | −2.082 | 0.040 |
| Other | −1.213 | 0.096 | −1.141 | 0.094 |
| Hispanic | −2.085 | 0.049 | −2.016 | 0.048 |
| Stroke | −0.357 | 0.022 | ||
| Alzheimer’s Disease | −0.384 | 0.005 | ||
| Constant | 14.326 | 0.028 | 13.936 | 0.027 |
| Random Effects | ES | SE | ES | SE |
| Slopes, SD | 0.114 | 0.001 | 0.096 | 0.001 |
| Intercepts, SD | 1.544 | 0.013 | 1.462 | 0.013 |
| Corr(S,I) | −0.124 | 0.014 | 0.066 | 0.018 |
Note: SE: standard error; ES: estimate size.
All non-zero associations shown were statistically significant at p<1E-06 level. In addition to covariates shown, all analyses additionally adjusted for practice effect observed between the first and following waves.
Sensitivity analyses were conducted integrating a quadratic estimate of acceleration in cognitive decline. Results from this study suggested that quadratic age was associated with acceleration in decline (B= −0.0034) when included in models not adjusting for Alzheimer’s disease or stroke, but that this association was reduced by 61.76% in size (B =−0.0013) upon adjustment for Alzheimer’s disease and stroke. In those models, both AD and stroke retained 96.88% and 97.49% of their effect size estimates respectively. Additionally, estimates of model fit (~R2= 0.132 when including AD/stroke and quadratic versus 0.132 for models only including AD/stroke but 0.104 for models only incorporating quadratic forms) suggested that inclusion of the quadratic form was limited in impact. Finally, examinations of decline in the “mixed pathology” group were completed to characterize differences in this group. Results from analyses of this group suggested that declines attributed to both ADRD and stroke were both highly significant (p<1E-06) and that effect sizes were stronger in this group for Alzheimer’s disease (B=−0.80/year) and for stroke (B=−1.24) as compared to those presented in the models, potentially suggesting that this group is experiencing more severe cognitive decline than other groups.
Discussion
Clinical diagnosis of cognitively impairing conditions such as Alzheimer’s disease and stroke rely heavily on subjective reports to differentiate between potential root causes of precipitous cognitive decline. However, we propose that the earliest phases of vascular injury or Alzheimer’s disease can be detected using objective measurements in large longitudinal datasets. In this analysis, we utilized a pattern detection algorithm to account for Alzheimer’s disease and acute stroke events to differentiate patterns of clinical cognitive impairment from age-related cognitive decline evident in healthy older adults. We found that Alzheimer’s disease and stroke explained more than half of the association between age and verbal episodic memory as well as the association between age and orientation. Separating out individuals who exhibited longitudinal patterns of performance indicative of Alzheimer’s disease and stroke removed variation commonly attributed to random slopes in longitudinal multilevel modeling. Additionally, adjusting for AD and stroke explained most of the variation previously attributed to random slopes estimates in the multilevel model.
Improving the ability of clinicians to make diagnoses earlier in the disease course offers opportunities to lower patient risks, prevent complications, and may also help individuals to better prepare for their future [22]. Thus, there is a need to develop methods to reliably identify patterns associated with clinical levels of cognitive decline, and to accurately identify methods for reliably diagnosing the etiology of the impairment [23]. Years of preclinical cognitive decline in longitudinal data fits the natural history of Alzheimer’s disease, and in turn implies the possibility of utilizing population-based data mining techniques to improve identification. Distinct from models relying on neuropsychological test cutoffs and using random slopes methods, the current method reinterprets a longstanding debate emphasizing the critical importance of patterns of within-person change over time [24].
Results suggesting that Alzheimer’s disease and stroke can help to explain the association between age and cognitive function are supportive of work in the neuropathological field showing similar results. For example, recent work relying on the Mayo Clinic Study of Aging utilized positron emission tomography to show that 52% of the association between age and cognitive decline could be explained by cerebrovascular changes, amyloid deposition, and neurodegeneration [4]. Similarly, results using histopathological factors suggest that 41% of cognitive decline could be attributed directly to neuropathology including amyloid or Lewy bodies observed in the brain at autopsy [5]. However, less is known about the potential for mild cognitive dysfunction to emerge as a result of cerebrovascular disease, however, since a large amount of cerebrovascular disease is asymptomatic [25]. Further work is warranted to determine what types of cerebrovascular disease have cognitive profiles but lack symptoms of dementia.
The results of this study suggested that the rate of decline in episodic memory was similar in this group (−0.064 words or approximately −0.02 SDs in Table 3) as compared to previous reports of declines identified at rates of −0.024 in episodic memory [26], though other cognitive domains ranged from −0.003 to −0.096 SDs in size. The current study noted that −0.024 might be an over-estimate that was negatively biased due to the inclusion of a large number of participants with preclinical and undiagnosed Alzheimer’s disease and/or stroke. Future research is warranted to determine measures of cognition that are less sensitive to both stroke and Alzheimer’s disease neuropathology in order to better understand the rate of change attributable to aging alone.
Complementary evidence to the data presented here suggest that this might be a fruitful avenue to explore. For example, performance on an associative memory task has been shown to predict conversion rates from amnestic mild cognitive impairment to early-stage Alzheimer’s disease [27], but associative memory performance has been shown to be relatively spared in stroke patients [28] in comparison to age-matched healthy controls. Although extant data suggest that associative memory deficits are common even in healthy aging when compared to performance in younger cohorts [29], longitudinal assessment of performance over time in this domain may prove useful in differentiating cognitive profiles associated with healthy aging, Alzheimer’s disease, and stroke. Moving beyond the domain of memory, executive dysfunction is often observed even in very early stages of Alzheimer’s disease [30] and may serve as an early warning sign for risk of conversion to Alzheimer’s disease [31]. Executive dysfunction is also commonly observed in acute stroke patients [32]. However, longitudinal change in executive dysfunction should be examined to assess whether trajectories of change in this domain differ meaningfully at later stages of disease progression.
The specific pathologies that the pattern recognition method is designed to detect, while useful, are not well understood [33]. This lack of clarity represents a huge challenge to understanding both individual clinical diagnoses and diagnostic prevalence at the population level, because gold-standard diagnoses often rely on expensive neuroimaging or post-mortem evidence [34]. Lacking evidence from neuroimaging, prior efforts relied on individuals or their spouses to determine timing of symptoms in order to make a differential diagnosis [35] and, as a result, most cases of both Alzheimer’s disease [36] and stroke [37] may remain unobserved at death. Because these states are difficult to disentangle from processes that occur in the context of normal aging, studies rarely make a clear distinction between causes of functional decline [38]. This study is therefore unique in utilizing new methods to identify stroke and Alzheimer’s disease pathologies that have otherwise gone undetected, and in differentiating these trajectories from the rate of age-related cognitive decline observed in healthy older adult populations.
An increasing body of research has highlighted the importance of mixed Alzheimer’s and cerebrovascular disease as a crucial population of interest as many individuals lacking cerebrovascular disease do not progress in Alzheimer’s disease despite evidence of amyloidosis [39]. One explanation for this effect that has been offered is that microglia, which are activated in cerebrovascular disease [40], are also central to more rapid proliferation of protein-tau [41]. Additionally, researchers have posited that the amyloid cascade may require both the deposition of β−amyloid1–42 throughout the cerebrum alongside the inclusion of β−amyloid1–40 in the vasculature in order to cause neurodegeneration [42]. In either case, these results support a growing body of research suggesting that Alzheimer’s disease is worsened by the presence of cerebrovascular disease. Further research is warranted in this group to determine risk and protective factors and also to identify indicators of cognitive reserve and brain resilience in this group.
Limitations
This study is one of the first to specifically focus on modeling both healthy aging and different causes of cognitive decline using pattern recognition. Nevertheless, this method is designed to identify individuals with possible stroke and/or Alzheimer’s disease, rather than provide definitive diagnoses. Although this study has identified a potentially large cerebrovascular burden in older age, further work is needed to validate both patterns of cognitive decline in Alzheimer’s disease and stroke with biomarker data.
Although episodic memory is recognized as a central symptom for neuropathological injury, and to measure brain aging, strokes not fitting the pattern utilized for classification may be missed. Missing data were not used to inform pattern detection routines as they were deemed were beyond the scope of this study. Respondents in the current study included a wide array of American residents from disparate social and racial backgrounds. Nevertheless, further work should examine racial/ethnic subgroups to determine whether the efficacy of stroke detection efforts is modified by demographic characteristics. The current study was limited to including only individuals with a large number of follow-ups occurring over a relatively long period of time, biasing these results to favor individuals who are healthier since they had to continue responding after the onset of Alzheimer’s disease symptoms and, more importantly, stroke. Additionally, though individuals with the patterns identified are likely to fit the diagnostic criteria for Alzheimer’s disease and stroke-related cognitive decline, we did not incorporate information about the presence of neuropathological indicators to positively determine differential diagnosis.
Previous research has pointed to the possibility that stroke can produce subtle changes that may make diagnosis difficult [25], and therefore we may have missed cases of stroke that presented with subtle or atypical symptoms. Additionally, our algorithm was only designed to identify a single stroke event, thereby making it possible that individuals with multiple overlapping strokes were misclassified with the most likely end result being inappropriate attribution as rapid aging or as Alzheimer’s disease. Future work is warranted to increase sensitivity of the algorithm to identifying multiple strokes over time, and also to identifying other cognitively impairing diseases such as dementia due to Lewy Bodies. Bias due to misspecification of the period of onset was not quantified in this study; future work is warranted using simulation to examine the extent to which misspecification of timing influences model results. Finally, we only reported linear models for normal cognitive aging, in part because quadratic models were not highly informative after adjusting for Alzheimer’s and cerebrovascular disease burden.
Implications
Results from this study support prior work suggesting that approximately half of age-related decline in episodic memory and orientation in old age may be attributable directly to relatively common diseases including stroke and Alzheimer’s disease and even suggests that the modal experience of cognitive change in old age is neuropathologic. Episodic memory is important for maintaining quality of life, and being able to mentally replay past events is a key component of good episodic memory and serves to reinforce continuity and sense of self [43], improve quality of life and mental health [44], and reduce the impact of caregiver burden [45]. However, as more older adults struggle with cognitive decline in old age, these analyses support the increasing view that neuropathologic changes may be responsible for a large proportion of that decline.
Acknowledgements:
The HRS (Health and Retirement Study) is sponsored by the National Institute on Aging (grant number NIA U01AG009740) and is conducted by the University of Michigan. The authors would like to acknowledge the Integrative Analysis of Longitudinal Studies on Aging (NIH/NIA P01 AG043362) network of studies for supporting the development of this work, and to the National Institute on Aging for supporting this analysis in particular (NIH/NIA R01 AG058595).
Footnotes
Conflicts of Interest: None to report.
Contributor Information
Sean Clouston, Program in Public Health and Department of Family, Population, and Preventive Medicine, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA.
Lauren L. Richmond, Department of Psychology, Stony Brook University, Stony Brook, NY, USA.
Stacey B. Scott, Department of Psychology, Stony Brook University, Stony Brook, NY, USA.
Christian C. Luhmann, Department of Psychology, Stony Brook University, Stony Brook, NY, USA.
Ginny Natale, Program in Public Health, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA.
Douglas Hanes, Program in Public Health, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA.
Yun Zhang, Program in Public Health, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA.
Dylan M. Smith, Program in Public Health and Department of Family, Population, and Preventive Medicine, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA.
References
- 1.Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2016. April//;12(4):459–509. [DOI] [PubMed] [Google Scholar]
- 2.Association As. 2018 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2018;14(3):367–429. [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2011 September 1, 2011;42(9):2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vemuri P, Lesnick TG, Knopman DS, Przybelski SA, Reid RI, Mielke MM, et al. Amyloid, vascular, and resilience pathways associated with cognitive aging. Annals of Neurology.0(ja). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle PA, Wilson RS, Yu L, Barr AM, Honer WG, Schneider JA, et al. Much of late life cognitive decline is not due to common neurodegenerative pathologies. Annals of neurology. 2013;74(3):478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafson L, Nilsson L. Differential diagnosis of presenile dementia on clinical grounds. Acta psychiatrica Scandinavica. 1982;65(3):194–209. [DOI] [PubMed] [Google Scholar]
- 7.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia. 2011;7(3):263–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salthouse TA. Are individual differences in rates of aging greater at older ages? Neurobiology of Aging. 2012. 10//;33(10):2373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. International Psychogeriatrics. 2004;16(02):129–40. [DOI] [PubMed] [Google Scholar]
- 10.Richards M, Deary IJ. A life course approach to cognitive capability In: Kuh D, Cooper R, Hardy R, Richards M, Ben-Shlomo Y, editors. A Life Course Approach to Healthy Ageing. Oxford: Oxford University Press; 2014. p. 32–45. [Google Scholar]
- 11.Schneider BC, Gross AL, Bangen KJ, Skinner JC, Benitez A, Glymour MM, et al. Association of vascular risk factors with cognition in a multiethnic sample. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2014;70(4):532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clouston S. Determinants of cognitive pathology. In: North CS, editor. American Psychopathological Association; New York, NY: 2015. [Google Scholar]
- 13.Clouston SAP, Zhang Y, Smith DM. Pattern recognition to identify stroke in the cognitive profile: Secondary analyses of a prospective cohort study. Cerebrovascular Diseases Extra. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Health and Retirement Study (HRS). Health and Retirement Study, public use dataset. In: University of Michigan with funding from the National Institute on Aging (U01 AG 009740), editor. Ann Arbor, MI: 2014. [Google Scholar]
- 15.Backman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain. 2001. January;124(Pt 1):96–102. [DOI] [PubMed] [Google Scholar]
- 16.Dregan A, Wolfe CD, Gulliford MC. Does the influence of stroke on dementia vary by different levels of prestroke cognitive functioning? A cohort study. Stroke. 2013;44(12):3445–51. [DOI] [PubMed] [Google Scholar]
- 17.Clouston SAP, Glymour M, Terrera GM. Educational inequalities in aging-related declines in fluid cognition and the onset of cognitive pathology. Alzheimers Dement (Amst). 2015. September 1;1(3):303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clouston SAP, Zhang Y, Smith DM. Pattern Recognition to Identify Stroke in the Cognitive Profile: Secondary Analyses of a Prospective Cohort Study. Cerebrovascular Diseases Extra. 2019;9(3):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sliwinski MJ, Buschke H. Modeling intraindividual cognitive change in aging adults: Results from the Einstein aging studies. Aging, Neuropsychology, and Cognition. 2004;11(2):196–211. [Google Scholar]
- 20.Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata. STATA press; 2008. [Google Scholar]
- 21.Liu GF, Lu K, Mogg R, Mallick M, Mehrotra DV. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Statistics in medicine. 2009;28(20):2509–30. [DOI] [PubMed] [Google Scholar]
- 22.Doubal FN, Ali M, Batty GD, Charidimou A, Eriksdotter M, Hofmann-Apitius M, et al. Big data and data repurposing - using existing data to answer new questions in vascular dementia research. BMC Neurology. 2017. April 17;17(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011. May;7(3):270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg L, Hughes CP, Coben L, Danziger WL, Martin RL, Knesevich J. Mild senile dementia of Alzheimer type: research diagnostic criteria, recruitment, and description of a study population. Journal of Neurology, Neurosurgery & Psychiatry. 1982;45(11):962–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yousufuddin M, Young N. Aging and ischemic stroke. Aging. 2019;11(9):2542–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritchie SJ, Tucker-Drob EM, Cox SR, Corley J, Dykiert D, Redmond P, et al. Predictors of ageing-related decline across multiple cognitive functions. Intelligence. 2016;59:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irish M, Lawlor BA, Coen RF, O’Mara SM. Everyday episodic memory in amnestic mild cognitive impairment: a preliminary investigation. BMC neuroscience. 2011;12(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Geldorp B, Kessels RP, Hendriks MP. Single-item and associative working memory in stroke patients. Behavioural neurology. 2013;26(3):199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychology and aging. 2008;23(1):104. [DOI] [PubMed] [Google Scholar]
- 30.Lafleche G, Albert MS. Executive function deficits in mild Alzheimer’s disease. Neuropsychology. 1995;9(3):313. [Google Scholar]
- 31.Carlson MC, Xue Q-L, Zhou J, Fried LP. Executive decline and dysfunction precedes declines in memory: the Women’s Health and Aging Study II. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2009;64(1):110–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinn S, Bosworth HB, Hoenig HM, Swartzwelder HS. Executive function deficits in acute stroke. Archives of physical medicine and rehabilitation. 2007;88(2):173–80. [DOI] [PubMed] [Google Scholar]
- 33.Vitali P, Migliaccio R, Agosta F, Rosen HJ, Geschwind MD. Neuroimaging in Dementia. Seminars in neurology. 2008. October/08;28(4):467–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer’s disease. Lancet. 2016. July 30;388(10043):505–17. [DOI] [PubMed] [Google Scholar]
- 35.Pohjasvaara T, Mantyla R, Ylikoski R, Kaste M, Erkinjuntti T. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences. Stroke. 2000. December;31(12):2952–7. [DOI] [PubMed] [Google Scholar]
- 36.Boustani M, Peterson B, Hanson L, Harris R, Lohr KN. Screening for dementia in primary care: a summary of the evidence for the US Preventive Services Task Force. Annals of internal medicine. 2003;138(11):927–37. [DOI] [PubMed] [Google Scholar]
- 37.Ince P, Minett T, Forster G, Brayne C, Wharton S, Function MRCC, et al. Microinfarcts in an older population‐representative brain donor cohort (MRC CFAS): Prevalence, relation to dementia and mobility, and implications for the evaluation of cerebral Small Vessel Disease. Neuropathology and applied neurobiology. 2017;43(5):409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korczyn AD, Vakhapova V, Grinberg LT. Vascular dementia. Journal of the neurological sciences. 2012. May/08;322(1–2):2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mielke M, Rosenberg P, Tschanz J, Cook L, Corcoran C, Hayden K, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69(19):1850–58. [DOI] [PubMed] [Google Scholar]
- 40.Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–70. [DOI] [PubMed] [Google Scholar]
- 41.Pampuscenko K, Morkuniene R, Sneideris T, Smirnovas V, Budvytyte R, Valincius G, et al. Extracellular tau induces microglial phagocytosis of living neurons in cell cultures. Journal of Neurochemistry. 2019:e14940. [DOI] [PubMed] [Google Scholar]
- 42.Kritikos M, Clouston SAP, Diminich ED, Deri Y, Yang X, Carr M, et al. Pathway Analysis for Plasma β-Amyloid, Tau and Neurofilament Light (ATN) in World Trade Center Responders at Midlife. Neurology and Therapy. 2020 2020/April/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conway MA. Sensory–perceptual episodic memory and its context: Autobiographical memory. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2001;356(1413):1375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clouston SAP, Diminich ED, Kotov R, Pietrzak RH, Richards M, Spiro A 3rd, et al. Incidence of mild cognitive impairment in World Trade Center responders: Long-term consequences of re-experiencing the events on 9/11/2001. Alzheimers Dement (Amst). 2019. December;11:628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elif K, TAŞKAPILIOĞLU Ö, Bakar M. Caregiver burden in different stages of Alzheimer’s disease. Archives of Neuropsychiatry. 2017;54(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]