ABSTRACT
Background
Red meat is a rich source of nutrients but is typically high in saturated fats. Carcinogenic chemicals can be formed during cooking and processing. Little is known about the relation of red meat consumption to mortality in African Americans (AAs), a group with excess mortality and high consumption of red meat relative to whites.
Objective
Our objective was to assess the association between red meat consumption and mortality in AA women.
Methods
The Black Women's Health Study (BWHS) is a prospective cohort study of AA women across the USA who completed health questionnaires at enrollment in 1995 (median age 38 y, median BMI 27.9 kg/m2) and every 2 y thereafter. The analyses included 56,314 women who completed a validated FFQ and were free of cardiovascular disease and cancer at baseline in 1995. Exposures were total red meat, processed red meat, and unprocessed red meat consumption. Outcomes were all-cause and cause-specific mortality. Cox proportional hazards models with control for age, socioeconomic status, lifestyle factors, medical history, and dietary factors were used to estimate HRs with 95% CIs.
Results
During 22 y of follow-up through to 2017, we identified 5054 deaths, which included 1354 cardiovascular deaths and 1801 cancer deaths. The HR for all-cause mortality was 1.47 (95% CI: 1.33, 1.62) for the highest quintile of total red meat consumption relative to the lowest. Each 1 serving/d increase in red meat consumption was associated with a 7% (95% CI: 5%, 9%) increased risk of all-cause mortality. Red meat consumption was also associated with increased cardiovascular mortality, but not with cancer mortality. Results were similar for the consumption of processed and unprocessed red meat.
Conclusions
Red meat consumption is associated with increased all-cause and cardiovascular mortality among AA women.
Keywords: red meat, processed red meat, unprocessed red meat, mortality, African American, women
Introduction
Red meat is a widely consumed food group worldwide and consumption continues to rise, with the USA as the leading consumer (1–3). For Americans, red meat accounts for 58% of total meat consumption and processed red meat accounts for 22% (1, 2). Red meat is a rich source of protein, B vitamins, zinc, and iron, but it is usually high in saturated fats. Furthermore, many carcinogenic chemicals, such as N-nitroso compounds, heterocyclic amines, and polycyclic aromatic hydrocarbons, can form during the meat processing and cooking process (4). The health implications of red meat consumption are of great public interest (5–8). Based on evidence predominantly from white populations (9–17), the WHO stated that red meat in general is “probably carcinogenic” to humans and processed red meat is “carcinogenic” (18). The 2015 committee for US dietary guidelines recommended that Americans cut down on red meat and processed red meat consumption (19), but some argue that lean beef can be part of a healthy diet (20).
In 2017, African-American (AA) women had a life expectancy of 78.5 y at birth, 2.7 y shorter than that of US white women (21). It is unknown whether red meat consumption contributes to the excess mortality among AAs. AAs consume more red meat and the trend has increased in recent years (22). AAs are also less likely to consume lean beef compared with whites (23), and they obtain more of their total fat intake from processed luncheon meats, bacon, and fried poultry (24). Even among AAs who consume a more plant-based dietary pattern, the consumption of red and processed red meat is still high and above the guideline recommended level (25, 26). Within the AA community, there is a wide range of culinary traditions, from the American South, the Caribbean, South America, and Africa. Soul food, which has deep historical and cultural connections with the AA community, may account, in part, for the greater preference for fried meat.
There has been little study of the relation of red meat consumption to all-cause and cause-specific mortality in an AA population. Zhong et al. (27) reported an association of processed meat and unprocessed red meat measured at baseline with increased all-cause mortality for non-Hispanic blacks. The study did not differentiate processed red meat from other types of processed meat, such as poultry, and did not examine cause-specific mortality. In the present study, with data from a large long-term cohort study, the Black Women's Health Study (BWHS), we assessed red meat consumption, processed and unprocessed, measured at 2 time points in relation to all-cause and cause-specific mortality in AA women.
Methods
Data described in this article, code book, and analytic code will not be made publicly available. Information on the procedure to obtain and access data from the BWHS is described at http://www.bu.edu/bwhs under the information for researchers.
Study population
The BWHS is a prospective cohort study of 59,000 AA women (age 21–69 y with a median age of 38 y at baseline), enrolled from across the continental USA in 1995 (28). Since 1995, participants have provided information on biennial mailed and web health questionnaires on demographic characteristics, socioeconomic factors, medical conditions, and lifestyle factors. Follow-up has been successful for 85% of potential person-years through 11 completed biennial rounds of follow-up. All but 5% of BWHS participants were born in the USA. The Boston University Medical Campus Institutional Review Board approved the study. Study participants gave informed consent.
Dietary information
In 1995 and 2001, participants completed modified versions of the National Cancer Institute Block FFQ, providing information on usual dietary intake in the past year (29). Questionnaire items on processed red meats included sausage, bacon, hot dogs, ham, bologna, salami, and other lunch meats. Questionnaire items on unprocessed red meats included hamburgers, and main dish or mixed dish of beef, beef stew, liver, and pork. For each food item, participants were asked, on average, how frequently they consumed the specific food item and the serving size. Responses ranged from “never or <1 per month” to “2 or more per day.” Serving sizes were “small,” “medium,” and “large” in the 1995 FFQ, with “super” added to the 2001 FFQ. Small was specified as about one-half of medium, large was about one and one/half times medium, and super was about twice the medium size. The medium serving sizes were 113 g (4 ounces) for unprocessed red meat, 28 g (2 slices) for bacon, 90 g (2 pieces) for hot dogs, 56 g (2 slices or 2 ounces) for sausage, salami, bologna, and other processed red meats. Total red meat consumption was calculated as the sum of processed and unprocessed red meat consumption.
In a validation study of 408 BWHS participants with 3 nonconsecutive 24-h recalls and 3-d food diaries over a 1-y period (29), the deattenuated correlation coefficients (correlation after measurement error correction) (30, 31) were 0.45 for fat, 0.53 for saturated fat, and 0.58 for protein. Meat intake was not assessed separately.
Outcome assessment
The outcomes assessed were all-cause and cause-specific mortality. Deaths after baseline in 1995 through to 2017 were identified by searching the National Death Index for study participants who did not return the biennial questionnaire and were not previously known to be deceased. Reports of deaths were also obtained from next of kin, the Social Security Administration Death Master File, and the US Postal Service. Cause of death information (underlying and immediate cause) was obtained from the National Death Index Plus or a state-issued death certificate. Women were classified as having died from cancer if cancer was listed as the underlying cause of death based on the International Classification of Diseases codes (C00–C97 for cancer) and as having died from cardiovascular disease (CVD) if the underlying cause of death was codes I00–I09, I11, I13, I20–I51. The 5 leading causes of cancer death in the BWHS were breast cancer (26%), lung cancer (21%), pancreatic cancer (7.6%), colon cancer (6.7%), and ovarian cancer (4.6%).
Covariate assessment
Covariates were chosen a priori based on the literature and were adjusted as time varying. We included age (continuous), questionnaire cycle (continuous), BMI category (<23, 23–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), education level (≤12, 13–15, 16, ≥17 y), geographic region (Northeast, South, Midwest, West), neighborhood socioeconomic status (quintiles) (32), physical activity level (<7.5, 7.5 to <15, 15 to <30, ≥30 h of metabolic equivalent tasks [MET] per week), cigarette smoking (cigarettes/d, continuous), family history of myocardial infarction (yes, no), family history of cancer (yes, no), history of diabetes (yes, no), history of hypertension (yes, no), history of hypercholesterolemia (yes, no), total energy intake (quintiles, kcal/d), and modified Alternative Healthy Eating Index (AHEI) without red meat (quintiles) (33, 34). All covariates were modeled as time varying, updating from report at baseline to report at the 2001 questionnaire cycle, which was the last cycle the FFQ was administered. Information on vigorous exercise and walking for exercise was obtained at baseline in 1995 and on 7 subsequent biennial questionnaires (35). We estimated energy expended per activity by multiplying the MET value by the number of hours per week spent for that activity and summing to estimate total energy expenditure in MET hours per week (MET-h/wk) from walking and vigorous activity. Hypertension was identified from biennial questionnaires as physician-diagnosed hypertension together with use of an antihypertensive medication or diuretic, or use of an antihypertensive alone. In a prior validation study, 99% of hypertension cases were confirmed by medical records (36, 37). Type 2 diabetes was self-report of physician-diagnosed diabetes at age 30 y or older; in a validation study, 95% of the women who reported diabetes were confirmed by their physicians to have type 2 diabetes (38).
Statistical analyses
The analytic sample consisted of 56,314 participants who provided information on their dietary intake and were free of CVD and cancer at baseline in 1995, with ≤9 blank responses on the 1995 FFQ and without implausible energy intake (<400 or >3800 kcal/d). For each study participant, follow-up was from baseline in 1995 until the date of death, loss to follow-up, or the end of study follow-up (31 Dec, 2017), whichever came first. We calculated the cumulative average of red meat consumption over the 2 cycles of dietary assessment to better reflect women's usual red meat intake and minimize within-person variation (39).
We used Cox proportional hazards models to examine associations of cumulative average red meat consumption with mortality. To assess dose-response relations, we used the restricted cubic spline method (40). We examined associations of total red meat, processed red meat, and unprocessed red meat intake with all-cause and cause-specific mortality. The proportional hazards assumption was tested by modeling multiplicative interaction terms between time and exposure variables. We repeated our analyses modeling red meat in grams/day using the Willett residual method (30). We also updated red meat exposure rather than assessing cumulative average. We repeated our analyses using the Fine-Gray method to account for competing risk (41). In subgroup analyses, we tested for potential effect modification by age, BMI, education, neighborhood socioeconomic status, smoking status, physical activity level, diet quality, and alcohol consumption using a likelihood ratio test.
Using a substitution modeling approach, we examined the change in the risk of death by replacing 1 serving of red meat per day with 1 serving of poultry, fish, whole grains, or vegetables. We used the differences in the β-coefficients for red meat and the substituted food to estimate the HR, and estimated the 95% CI using corresponding variances and covariances (42). We calculated population attributable risk fraction for the association between total red meat and all-cause mortality.
We grouped causes of cancer death into 6 categories (breast cancer [C50], lung cancer [C33–C34], pancreatic cancer [C25], colon cancer [C18–C21], ovarian cancer [C56], and other). The 5 leading causes of cancer death in the BWHS were breast cancer (26%), lung cancer (21%), pancreatic cancer (7.6%), colon cancer (6.7%), and ovarian cancer (4.6%). We examined associations of red meat with site-specific cancer mortality.
To examine potential biases, we performed several sensitivity analyses: 1) we modeled red meat consumption using baseline 1995 data only; 2) because undiagnosed major diseases could influence red meat intake, we conducted analyses excluding the first 2 y of follow-up; 3) alcohol consumption is highly associated with red meat consumption. In addition to adjusting alcohol as a component of diet quality score, we also included alcohol consumption as a covariate in the multivariable model and simultaneously adjusted for diet quality (AHEI derived without alcohol and red meat components). All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
We followed 56,314 BWHS participants who reported red meat consumption and were free of chronic conditions at baseline. During 22 y of follow-up, we identified 5054 deaths, which included 1354 cardiovascular deaths, 1801 cancer deaths, and 1899 deaths from other causes. Participants were followed from 1995 until death or the end of follow-up (December 2017). Median follow-up was 17 y (IQR = 13–19 y).
Among the 56,314 women followed, 40,920 (73%) reported their red meat consumption again on the 2001 FFQ. Changes in red meat consumption were small. The mean intake of red meat intake was 0.76 (SD = 1.1) servings per day in 1995 and 0.80 (SD = 1.0) servings per day in 2001. The comparable values reported in 1995 and 2001 were 0.41 (SD = 0.67) servings per day and 0.46 (SD = 0.69) servings per day for processed red meat, and 0.35 (SD = 0.53) servings per day and 0.34 (SD = 0.53) servings per day for unprocessed red meat.
AA women who ate more red meat tended to be younger, have higher BMI, live in neighborhoods of lower socioeconomic status, live in the South or Midwest, smoke more, be less physically active, drink more alcohol, have high blood pressure or diabetes, consume a lower quality diet, and have higher total energy intake (Table 1). Similar characteristics were observed for unprocessed and processed red meat.
TABLE 1.
Age-standardized baseline characteristics in Black Women's Health Study participants by quintile of total red meat consumption, processed red meat consumption, and unprocessed red meat consumption
| Total red meat | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| N | 11,268 | 11,220 | 11,272 | 11,291 | 11,263 |
| Total red meat, servings/d | 0.04 ± 0.04 | 0.2 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.1 | 2.1 ± 1.6 |
| Age, y | 39.5 ± 10.9 | 39.6 ± 10.7 | 38.9 ± 10.5 | 38.1 ± 10.3 | 36.6 ± 10.1 |
| Neighborhood SES: highest quintile, % | 22.9 | 21.7 | 18.9 | 15.7 | 12.0 |
| Geographic region, % | |||||
| Northeast | 33.5 | 29.0 | 26.2 | 24.8 | 23.4 |
| South | 25.7 | 29.5 | 31.6 | 33.2 | 33.4 |
| Midwest | 18.7 | 21.1 | 23.6 | 24.6 | 27.7 |
| West | 21.9 | 20.1 | 18.4 | 17.1 | 15.4 |
| BMI, kg/m2 | 26.3 ± 5.5 | 27.2 ± 6.0 | 27.9 ± 6.4 | 28.6 ± 6.9 | 29.6 ± 7.8 |
| Pack years | 3.2 ± 7.9 | 3.5 ± 8.2 | 3.9 ± 8.8 | 4.4 ± 9.5 | 5.3 ± 10.4 |
| Physical activity, MET-h/wk | 21.2 ± 18.9 | 18.1 ± 17.6 | 16.4 ± 16.9 | 15.2 ± 16.3 | 13.5 ± 15.6 |
| Alcohol: current, % | 23.7 | 26.7 | 28.3 | 30.5 | 34.9 |
| Hypertension, % | 19.1 | 21.9 | 23.5 | 24.7 | 27.4 |
| Diabetes, % | 2.6 | 3.5 | 4.1 | 4.7 | 6.8 |
| Hyperlipidemia, % | 20.8 | 21.8 | 20.3 | 20.4 | 20.7 |
| Total caloric intake, kcal/d | 1243 ± 747 | 1245 ± 722 | 1375 ± 738 | 1581 ± 857 | 2220 ± 1518 |
| Diet quality, AHEI diet score without red meat component | 33.5 ± 7.7 | 30.7 ± 6.9 | 29.0 ± 6.7 | 27.4 ± 6.7 | 26.0 ± 7.6 |
| Vegetables, servings/d | 2.6 ± 2.1 | 2.1 ± 1.6 | 2.2 ± 1.5 | 2.4 ± 1.7 | 3.5 ± 2.9 |
| Fruits, servings/d | 2.6 ± 2.1 | 2.2 ± 1.8 | 2.2 ± 1.8 | 2.3 ± 1.8 | 2.7 ± 2.2 |
| Whole grains, servings/d | 0.9 ± 0.8 | 0.8 ± 0.7 | 0.8 ± 0.7 | 0.9 ± 0.7 | 1.1 ± 1.0 |
| Total fat, % kcal | 29.8 ± 7.1 | 32.2 ± 6.4 | 34.1 ± 6.1 | 35.6 ± 5.8 | 38.2 ± 5.7 |
| Fiber, g/d | 13.9 ± 8.5 | 11.7 ± 7.3 | 12.1 ± 7.1 | 13.2 ± 7.2 | 16.7 ± 8.5 |
| Processed red meat | |||||
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| n | 12,218 | 9978 | 11,742 | 11,119 | 11,257 |
| Processed red meat, servings/d | 0.01 ± 0.01 | 0.08 ± 0.03 | 0.2 ± 0.05 | 0.4 ± 0.09 | 1.2 ± 1.0 |
| Age, y | 38.9 ± 11.0 | 39.1 ± 10.6 | 38.9 ± 10.5 | 38.3 ± 10.2 | 37.4 ± 10.3 |
| Neighborhood SES: highest quintile, % | 22.7 | 20.9 | 18.8 | 16.6 | 12.0 |
| Geographic region, % | |||||
| Northeast | 31.7 | 27.5 | 27.3 | 25.7 | 23.9 |
| South | 25.6 | 29.5 | 30.5 | 34.1 | 34.1 |
| Midwest | 20.3 | 22.0 | 23.5 | 23.3 | 26.7 |
| West | 22.2 | 20.7 | 18.4 | 16.6 | 15.1 |
| BMI, kg/m2 | 26.5 ± 5.7 | 27.4 ± 6.1 | 27.9 ± 6.6 | 28.4 ± 6.9 | 29.3 ± 7.6 |
| Pack years | 3.2 ± 8.0 | 3.6 ± 8.6 | 3.9 ± 8.7 | 4.3 ± 9.3 | 5.1 ± 10.1 |
| Physical activity, MET-h/wk | 20.7 ± 18.8 | 17.5 ± 17.4 | 16.6 ± 16.9 | 15.5 ± 16.4 | 13.9 ± 15.9 |
| Alcohol: current, % | 24.1 | 27.1 | 28.3 | 30.0 | 34.9 |
| Hypertension, % | 20.3 | 22.3 | 23.1 | 24.0 | 26.9 |
| Diabetes, % | 2.8 | 3.6 | 3.8 | 4.9 | 6.3 |
| Hyperlipidemia, % | 20.6 | 21.7 | 21.0 | 20.3 | 20.8 |
| Total caloric intake, kcal/d | 1305 ± 804 | 1298 ± 768 | 1390 ± 785 | 1572 ± 867 | 2100 ± 1517 |
| Diet quality, AHEI diet score without red meat component | 32.9 ± 8.0 | 30.3 ± 7.2 | 28.9 ± 6.9 | 27.6 ± 6.8 | 26.6 ± 7.4 |
| Vegetables, servings/d | 2.7 ± 2.2 | 2.2 ± 1.7 | 2.2 ± 1.6 | 2.4 ± 1.6 | 3.2 ± 2.8 |
| Fruits, servings/d | 2.6 ± 2.1 | 2.2 ± 1.8 | 2.2 ± 1.8 | 2.3 ± 1.8 | 2.7 ± 2.2 |
| Whole grains, servings/d | 0.9 ± 0.8 | 0.8 ± 0.7 | 0.8 ± 0.7 | 0.9 ± 0.7 | 1.1 ± 0.9 |
| Total fat, % kcal | 30.3 ± 7.0 | 32.4 ± 6.6 | 33.9 ± 6.1 | 35.4 ± 5.9 | 38.0 ± 5.9 |
| Fiber, g/d | 14.3 ± 8.5 | 12.0 ± 7.5 | 12.2 ± 7.2 | 13.1 ± 7.2 | 15.9 ± 8.3 |
| Unprocessed red meat | |||||
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| n | 11,513 | 10,962 | 11,592 | 10,913 | 11,334 |
| Unprocessed red meat, servings/d | 0.01 ± 0.02 | 0.1 ± 0.03 | 0.2 ± 0.04 | 0.3 ± 0.07 | 1.0 ± 0.8 |
| Age, y | 39.6 ± 10.8 | 39.8 ± 10.7 | 39.2 ± 10.6 | 37.8 ± 10.1 | 36.2 ± 9.9 |
| Neighborhood SES: highest quintile, % | 22.4 | 20.7 | 18.2 | 16.1 | 13.8 |
| Geographic region, % | |||||
| Northeast | 33.7 | 29.1 | 26.9 | 24.5 | 22.7 |
| South | 25.8 | 30.5 | 32.5 | 32.4 | 31.8 |
| Midwest | 18.8 | 20.6 | 23.2 | 25.3 | 27.9 |
| West | 21.3 | 19.5 | 17.1 | 17.5 | 17.3 |
| BMI, kg/m2 | 26.3 ± 5.6 | 27.3 ± 6.1 | 27.9 ± 6.5 | 28.5 ± 6.9 | 29.5 ± 7.6 |
| Pack years | 3.2 ± 8.1 | 3.4 ± 8.1 | 4.1 ± 9.1 | 4.5 ± 9.5 | 5.1 ± 10.2 |
| Physical activity, MET-h/wk | 20.8 ± 18.7 | 17.9 ± 17.6 | 16.5 ± 17.0 | 15.1 ± 16.1 | 13.9 ± 15.8 |
| Alcohol: current, % | 24.1 | 26.5 | 28.8 | 31.1 | 33.6 |
| Hypertension, % | 19.3 | 22.1 | 23.6 | 24.8 | 27.1 |
| Diabetes, % | 3.0 | 3.7 | 4.0 | 4.9 | 6.0 |
| Hyperlipidemia, % | 20.8 | 21.7 | 20.7 | 19.9 | 20.7 |
| Total caloric intake, kcal/d | 1274 ± 790 | 1256 ± 739 | 1392 ± 786 | 1590 ± 899 | 2158 ± 1478 |
| Diet quality, AHEI diet score without red meat component | 33.2 ± 7.7 | 30.5 ± 6.9 | 28.8 ± 6.8 | 27.7 ± 6.8 | 26.4 ± 7.8 |
| Vegetables, servings/d | 2.6 ± 2.1 | 2.1 ± 1.6 | 2.1 ± 1.6 | 2.5 ± 1.6 | 3.6 ± 2.9 |
| Fruits, servings/d | 2.6 ± 2.1 | 2.2 ± 1.8 | 2.2 ± 1.8 | 2.3 ± 1.8 | 2.7 ± 2.2 |
| Whole grains, servings/d | 0.9 ± 0.8 | 0.8 ± 0.7 | 0.8 ± 0.7 | 0.9 ± 0.7 | 1.1 ± 0.9 |
| Total fat, % kcal | 30.7 ± 7.2 | 32.5 ± 6.7 | 34.1 ± 6.4 | 35.5 ± 5.9 | 37.4 ± 5.7 |
| Fiber, g/d | 14.0 ± 8.5 | 11.8 ± 7.2 | 12.0 ± 7.0 | 13.4 ± 7.2 | 16.6 ± 8.5 |
AHEI, Alternative Healthy Eating Index; MET, metabolic equivalent of task; MET-h/wk, MET hours per week; Q: quintile; SES, socioeconomic status.
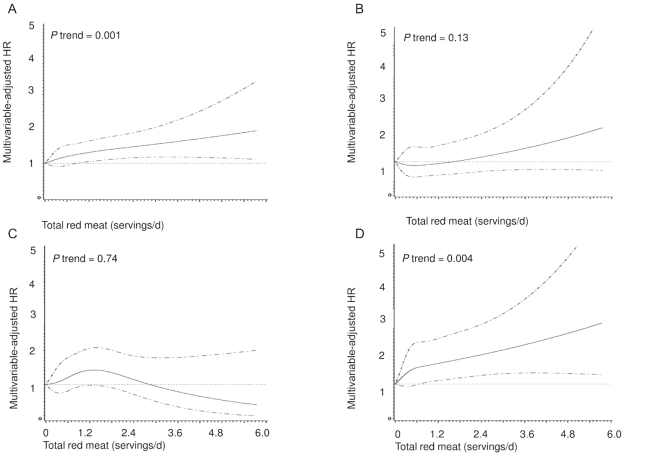
In multivariable-adjusted models, total red meat consumption was associated with higher all-cause mortality (Figure 1, Table 2). Elevated mortality was observed for both processed and unprocessed red meat (Supplemental Figures 1and2, Table 2). Compared with the lowest quintile of total red meat consumption, the HR for all-cause mortality in the highest quintile was 1.47 (95% CI: 1.33, 1.62, P-trend = 0.001); 9% (95% CI: 6%, 11%) of all-cause deaths were attributable to high total red meat consumption. The comparable HRs for cardiovascular and other cause mortality were 1.70 (95% CI: 1.40, 2.06) and 1.73 (95% CI: 1.46, 2.04), respectively. For cancer mortality, the HR for the highest quintile of total red meat consumption relative to the lowest was 1.09 (95% CI: 0.92, 1.29, P-trend = 0.72). Physical activity and smoking appeared to be the strongest confounders for the association with cancer mortality. Similarly, no associations were observed for processed and unprocessed red meat with cancer mortality (Figure 1, Table 2). No violations of the proportional hazard assumption were observed (P = 0.32).
FIGURE 1.
Splines of the associations of total red meat consumption with all-cause (A), cardiovascular disease (B), cancer (C), and other-cause (D) mortality among 56,314 African-American women. Multivariable adjusted model adjusted for age (continuous), questionnaire cycle (continuous), BMI (<23, 23–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), educational level (≤12, 13–15, 16, ≥17 y), geographic region (Northeast, South, Midwest, West), neighborhood SES (socioeconomic status) (quintiles), physical activity level (<7.5, 7.5 to <15, 15 to <30, ≥30 h of metabolic equivalent tasks per week), cigarette smoking (cigarettes/d, continuous), family history of myocardial infarction (yes, no), family history of cancer (yes, no), history of diabetes (yes, no), history of hypertension (yes, no), history of hypercholesterolemia (yes, no), total energy intake (quintiles, kcal/d), and modified 2010 Alternative Healthy Eating Index without red meat (quintiles, components include alcohol consumption).
TABLE 2.
Associations of total red meat, processed red meat, and unprocessed red meat consumption with all-cause and cause-specific mortality among 56,314 African-American women
| Total red meat | Processed red meat | Unprocessed red meat | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/person-y | IR | Model 11 HR (95% CI) | Model 22 HR (95% CI) | n/person-y | IR | Model 11 HR (95% CI) | Model 22 HR (95% CI) | n/person-y | IR | Model 11 HR (95% CI) | Model 22 HR (95% CI) | |
| Quintiles of red meat intake in servings/d | ||||||||||||
| All-cause mortality | ||||||||||||
| Q1 | 826/239,359 | 3.45 | Ref | Ref | 835/240,680 | 3.47 | Ref | Ref | 815/234,848 | 3.47 | Ref | Ref |
| Q2 | 955/239,864 | 3.98 | 1.15 (1.05,1.26) | 1.05 (0.96,1.16) | 895/233,738 | 3.83 | 1.09 (0.99,1.20) | 1.02 (0.93,1.12) | 1045/240,503 | 4.35 | 1.25 (1.14,1.37) | 1.15 (1.05,1.27) |
| Q3 | 996/239,211 | 4.16 | 1.30 (1.18,1.42) | 1.15 (1.04,1.26) | 1004/244,039 | 4.11 | 1.24 (1.13,1.36) | 1.11 (1.01,1.22) | 1029/238,699 | 4.31 | 1.35 (1.23,1.48) | 1.18 (1.07,1.29) |
| Q4 | 1057/238,664 | 4.42 | 1.50 (1.37,1.64) | 1.25 (1.14,1.38) | 1008/238,757 | 4.22 | 1.32 (1.21,1.45) | 1.13 (1.02,1.24) | 1031/239,151 | 4.31 | 1.50 (1.37,1.64) | 1.25 (1.14,1.38) |
| Q5 | 1220/237,265 | 5.14 | 2.02 (1.85,2.20) | 1.47 (1.33,1.62) | 1299/236,143 | 5.50 | 1.89 (1.73,2.06) | 1.40 (1.28,1.55) | 1105/235,121 | 4.70 | 1.97 (1.80,2.16) | 1.45 (1.32,1.61) |
| P-trend | <0.0001 | 0.001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| per 1 serving/d | 1.07 (1.05,1.09) | 1.12 (1.08,1.15) | 1.10 (1.05,1.15) | |||||||||
| Cardiovascular mortality | ||||||||||||
| Q1 | 209/239,810 | 0.87 | Ref | Ref | 203/241,150 | 0.84 | Ref | Ref | 205/235,299 | 0.87 | Ref | Ref |
| Q2 | 263/240,390 | 1.09 | 1.25 (1.04,1.50) | 1.12 (0.93,1.34) | 232/234,235 | 0.99 | 1.17 (0.96,1.41) | 1.05 (0.87,1.28) | 286/241,068 | 1.19 | 1.36 (1.14,1.63) | 1.22 (1.02,1.47) |
| Q3 | 239/239,774 | 0.99 | 1.23 (1.02,1.48) | 1.07 (0.88,1.29) | 267/244,584 | 1.09 | 1.37 (1.14,1.64) | 1.18 (0.98,1.42) | 261/239,272 | 1.09 | 1.38 (1.15,1.65) | 1.17 (0.97,1.41) |
| Q4 | 279/239,244 | 1.16 | 1.58 (1.32,1.89) | 1.28 (1.06,1.54) | 262/239,319 | 1.09 | 1.42 (1.18,1.70) | 1.18 (0.98,1.43) | 278/239,711 | 1.16 | 1.64 (1.37,1.96) | 1.31 (1.08,1.58) |
| Q5 | 364/237,899 | 1.53 | 2.44 (2.06,2.90) | 1.70 (1.40,2.06) | 385/236,817 | 1.63 | 2.34 (1.97,2.78) | 1.66 (1.38,2.00) | 318/235,709 | 1.35 | 2.35 (1.97,2.81) | 1.62 (1.33,1.97) |
| P-trend | <0.0001 | 0.001 | <0.0001 | <0.0001 | <0.0001 | 0.04 | ||||||
| per 1 serving/d | 1.07 (1.03,1.11) | 1.14 (1.07,1.21) | 1.09 (1.00,1.18) | |||||||||
| Cancer mortality | ||||||||||||
| Q1 | 350/239,708 | 1.46 | Ref | Ref | 348/241,041 | 1.44 | Ref | Ref | 333/235,198 | 1.42 | Ref | Ref |
| Q2 | 351/240,319 | 1.46 | 1.00 (0.87,1.16) | 0.96 (0.83,1.12) | 347/234,155 | 1.48 | 1.01 (0.87,1.17) | 0.99 (0.85,1.15) | 410/240,980 | 1.70 | 1.20 (1.04,1.39) | 1.16 (1.01,1.35) |
| Q3 | 397/239,663 | 1.65 | 1.22 (1.06,1.41) | 1.13 (0.98,1.32) | 375/244,499 | 1.53 | 1.11 (0.96,1.28) | 1.05 (0.90,1.22) | 358/239,209 | 1.50 | 1.15 (0.99,1.33) | 1.07 (0.91,1.24) |
| Q4 | 362/239,182 | 1.51 | 1.21 (1.05,1.41) | 1.10 (0.94,1.28) | 355/239,250 | 1.48 | 1.12 (0.97,1.30) | 1.02 (0.87,1.19) | 366/239,643 | 1.53 | 1.29 (1.11,1.50) | 1.18 (1.01,1.38) |
| Q5 | 341/237,917 | 1.43 | 1.33 (1.14,1.54) | 1.09 (0.92,1.29) | 374/236,827 | 1.58 | 1.30 (1.13,1.51) | 1.09 (0.93,1.28) | 325/235,702 | 1.38 | 1.41 (1.21,1.64) | 1.20 (1.01,1.42) |
| P-trend | <0.0001 | 0.72 | <0.0001 | 0.61 | 0.003 | 0.92 | ||||||
| per 1 serving/d | 1.01 (0.96,1.06) | 1.02 (0.95,1.10) | 1.00 (0.91,1.11) | |||||||||
| Other mortality | ||||||||||||
| Q1 | 267/239,765 | 1.11 | Ref | Ref | 284/241,085 | 1.18 | Ref | Ref | 277/235,244 | 1.18 | Ref | Ref |
| Q2 | 341/240,320 | 1.42 | 1.26 (1.08,1.48) | 1.13 (0.96,1.33) | 316/234,169 | 1.35 | 1.13 (0.97,1.33) | 1.04 (0.88,1.22) | 349/241,020 | 1.45 | 1.23 (1.05,1.43) | 1.10 (0.94,1.29) |
| Q3 | 360/239,691 | 1.50 | 1.44 (1.23,1.69) | 1.24 (1.05,1.45) | 362/244,517 | 1.48 | 1.31 (1.12,1.53) | 1.13 (0.97,1.32) | 410/239,155 | 1.71 | 1.57 (1.35,1.83) | 1.31 (1.12,1.53) |
| Q4 | 416/239,151 | 1.74 | 1.79 (1.53,2.09) | 1.42 (1.21,1.67) | 391/239,225 | 1.63 | 1.49 (1.28,1.74) | 1.20 (1.02,1.41) | 387/239,630 | 1.61 | 1.62 (1.39,1.90) | 1.30 (1.10,1.52) |
| Q5 | 515/237,786 | 2.17 | 2.56 (2.20,2.97) | 1.73 (1.46,2.04) | 540/236,703 | 2.28 | 2.27 (1.97,2.62) | 1.53 (1.31,1.79) | 462/235,611 | 1.96 | 2.34 (2.01,2.72) | 1.59 (1.35,1.87) |
| P-trend | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| per 1 serving/d | 1.10 (1.07,1.13) | 1.16 (1.10,1.22) | 1.16 (1.09,1.23) | |||||||||
Model 1: adjusted for age (continuous), questionnaire cycle (continuous).
Model 2: further adjusted for BMI (<23, 23–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), educational level (≤12, 13–15, 16, ≥17 y), geographic region (Northeast, South, Midwest, West), neighborhood SES (socioeconomic status) (quintiles), physical activity level (<7.5, 7.5 to <15, 15 to <30, ≥30 h of metabolic equivalent tasks per week), cigarette smoking (cigarettes/d, continuous), family history of myocardial infarction (yes, no), family history of cancer (yes, no), history of diabetes (yes, no), history of hypertension (yes, no), history of hypercholesterolemia (yes, no), total energy intake (quintiles, kcal/d), and modified 2010 Alternative Healthy Eating Index without red meat (quintiles, components include alcohol consumption).
IR, incidence rate per 1000 person-years; n, case number; per 1 serving/d, 1 serving per day increment; Ref, reference; person-y, person-years; Q, quintiles.
For other mortality (Table 2), comparing the highest with lowest quintiles, HRs were 1.73 (95% CI: 1.46, 2.04) for total red meat, 1.53 (95% CI: 1.31, 1.79) for processed red meat, and 1.59 (95% CI: 1.35, 1.87) for unprocessed red meat. Results remained the same after using the Fine-Gray method to account for competing risks (41).
In sensitivity analyses, we modeled red meat as a time-varying exposure rather than as a cumulative average, and we also used a residual method (Supplemental Tables 1and2); the results were similar to those in our main analysis shown in Table 2. For the substitution of 1 serving of red meat per day with poultry, there was an 18% reduction in mortality (HR = 0.82, 95% CI: 0.74, 0.91). Restriction to substitution with nonfried chicken yielded a similar finding (HR = 0.81, 95% CI: 0.73, 0.92). There was a 6% reduction in mortality (HR = 0.94, 95% CI: 0.90, 0.98) when vegetables were substituted for red meat, and an 11% reduction in mortality (HR = 0.89, 95% CI: 0.83, 0.95) when whole grains were substituted for red meat. Substituting 1 serving of red meat with fish was associated with a 9% reduction in mortality (HR = 0.91, 95% CI: 0.81, 1.01).
We examined the association of red meat with mortality among several subgroups (Table 3). The elevated mortality associated with high consumption of red meat was present in all subgroups, including “low risk” groups such as women with BMI <25 kg/m2 and women aged <50 y. Null associations with cancer mortality were consistently observed for all subgroups. Results were unchanged with additional control for more detailed categories of alcohol consumption instead of adjusting alcohol as a component of diet score. Results were unchanged in analyses that either used baseline diet data only or excluded the first 2 y of follow-up (Supplemental Table 3).
TABLE 3.
Associations of total red meat consumption with all-cause mortality in African-American women by BMI, age, neighborhood Socioeconomic Status (SES), education, smoking, physical activity, alcohol consumption, and AHEI score. SES, socioeconomic status
| All-cause mortality | P for interaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Person-years | HR1 (95% CI) | n | Person-years | HR1 (95% CI) | n | Person-years | HR1 (95% CI) | ||
| Quintiles of total red meat intake in servings/d | ||||||||||
| BMI <25 | 25 ≤BMI <30 | BMI ≥30 | ||||||||
| Q1 | 242 | 97,353 | Ref | 279 | 79,082 | Ref | 268 | 55,040 | Ref | 0.72 |
| Q2 | 250 | 79,370 | 1.16 (0.97, 1.39) | 300 | 80,505 | 1.00 (0.85, 1.18) | 370 | 72,221 | 1.01 (0.87, 1.17) | |
| Q3 | 247 | 70,221 | 1.30 (1.08, 1.56) | 308 | 80,055 | 1.06 (0.90, 1.25) | 406 | 81,437 | 1.03 (0.89, 1.20) | |
| Q4 | 247 | 64,534 | 1.43 (1.18, 1.73) | 317 | 74,111 | 1.22 (1.03, 1.45) | 456 | 92,129 | 1.04 (0.90, 1.21) | |
| Q5 | 276 | 59,978 | 1.64 (1.34, 2.01) | 298 | 65,242 | 1.35 (1.12, 1.62) | 576 | 102,931 | 1.29 (1.11, 1.50) | |
| Age <50 | 50 ≤ Age <65 | Age ≥65 | ||||||||
| Q1 | 128 | 126,040 | Ref | 291 | 85,391 | Ref | 407 | 27,928 | Ref | 0.61 |
| Q2 | 164 | 127,075 | 1.18 (0.94, 1.49) | 369 | 85,218 | 1.15 (0.98, 1.34) | 422 | 27,572 | 0.95 (0.83, 1.09) | |
| Q3 | 194 | 133,216 | 1.26 (1.01, 1.59) | 357 | 81,933 | 1.08 (0.92, 1.26) | 445 | 24,062 | 1.13 (0.98, 1.29) | |
| Q4 | 247 | 140,588 | 1.43 (1.14, 1.80) | 380 | 77,256 | 1.11 (0.95, 1.31) | 430 | 20,820 | 1.20 (1.04, 1.38) | |
| Q5 | 326 | 151,624 | 1.48 (1.17, 1.86) | 523 | 70,203 | 1.46 (1.24, 1.72) | 371 | 15,438 | 1.32 (1.13, 1.54) | |
| Neighborhood SES Q1, Q2 | Neighborhood SES Q3 | Neighborhood SES Q4, Q5 | ||||||||
| Q1 | 286 | 61,483 | Ref | 161 | 39,481 | Ref | 267 | 94,754 | Ref | 0.06 |
| Q2 | 385 | 69,631 | 1.09 (0.94, 1.27) | 170 | 39,256 | 0.97 (0.77, 1.20) | 287 | 88,813 | 1.05 (0.89, 1.24) | |
| Q3 | 406 | 74,586 | 1.18 (1.01, 1.38) | 158 | 40,578 | 0.92 (0.73, 1.16) | 298 | 82,196 | 1.15 (0.97, 1.37) | |
| Q4 | 500 | 80,454 | 1.39 (1.20, 1.62) | 158 | 39,769 | 1.01 (0.80, 1.28) | 247 | 74,993 | 1.12 (0.94, 1.35) | |
| Q5 | 628 | 95,263 | 1.60 (1.37, 1.87) | 206 | 39,004 | 1.33 (1.05, 1.68) | 195 | 57,656 | 1.23 (1.00, 1.51) | |
| Education <16 | Education 16 | Education >16 | ||||||||
| Q1 | 182 | 25,348 | Ref | 388 | 126,830 | Ref | 253 | 86,504 | Ref | 0.07 |
| Q2 | 249 | 35,874 | 1.02 (0.84, 1.24) | 458 | 133,245 | 1.04 (0.91, 1.19) | 235 | 70,167 | 1.06 (0.88, 1.26) | |
| Q3 | 274 | 37,872 | 1.17 (0.97, 1.42) | 469 | 138,199 | 1.08 (0.94, 1.24) | 238 | 62,332 | 1.16 (0.97, 1.40) | |
| Q4 | 297 | 42,249 | 1.22 (1.01, 1.48) | 545 | 140,639 | 1.27 (1.11, 1.46) | 209 | 55,011 | 1.17 (0.96, 1.42) | |
| Q5 | 431 | 55,334 | 1.52 (1.25, 1.84) | 584 | 139,211 | 1.43 (1.24, 1.66) | 184 | 41,864 | 1.33 (1.08, 1.65) | |
| Smoking current | Smoking past | Smoking never | ||||||||
| Q1 | 131 | 19,753 | Ref | 271 | 51,999 | Ref | 422 | 167,222 | Ref | 0.48 |
| Q2 | 237 | 29,865 | 1.11 (0.89, 1.37) | 268 | 48,028 | 1.00 (0.85, 1.19) | 438 | 161,392 | 1.00 (0.87, 1.15) | |
| Q3 | 269 | 35,515 | 1.04 (0.84, 1.29) | 270 | 45,294 | 1.13 (0.95, 1.34) | 446 | 157,716 | 1.12 (0.97, 1.28) | |
| Q4 | 319 | 40,126 | 1.12 (0.90, 1.38) | 299 | 43,003 | 1.37 (1.15, 1.63) | 436 | 155,078 | 1.14 (0.99, 1.31) | |
| Q5 | 447 | 50,936 | 1.31 (1.06, 1.62) | 269 | 39,896 | 1.43 (1.18, 1.73) | 488 | 145,690 | 1.37 (1.18, 1.59) | |
| Physical activity Q1, Q2 | Physical activity Q3 | Physical activity Q4, Q5 | ||||||||
| Q1 | 424 | 77,648 | Ref | 125 | 35,626 | Ref | 275 | 125,854 | Ref | 0.70 |
| Q2 | 570 | 95,635 | 1.05 (0.93, 1.19) | 122 | 37,032 | 1.03 (0.80, 1.33) | 254 | 106,957 | 1.07 (0.90,1.28) | |
| Q3 | 653 | 104,953 | 1.20 (1.06, 1.36) | 116 | 37,206 | 0.95 (0.73, 1.24) | 217 | 96,685 | 1.08 (0.90, 1.30) | |
| Q4 | 687 | 112,208 | 1.22 (1.08, 1.39) | 133 | 36,902 | 1.22 (0.94, 1.58) | 235 | 89,271 | 1.37 (1.14, 1.65) | |
| Q5 | 819 | 123,322 | 1.45 (1.27, 1.65) | 134 | 33,817 | 1.33 (1.00, 1.76) | 253 | 79,677 | 1.65 (1.36, 2.01) | |
| Alcohol, never drinker | Alcohol, past drinker | Alcohol, current drinker | ||||||||
| Q1 | 227 | 55,888 | Ref | 169 | 33,817 | Ref | 415 | 147,407 | Ref | 0.13 |
| Q2 | 277 | 64,084 | 0.95 (0.79, 1.13) | 196 | 33,748 | 1.08 (0.87, 1.33) | 464 | 139,907 | 1.11 (0.97, 1.27) | |
| Q3 | 328 | 68,154 | 1.08 (0.91, 1.28) | 189 | 32,548 | 1.13 (0.91, 1.40) | 461 | 136,725 | 1.19 (1.04, 1.37) | |
| Q4 | 375 | 72,512 | 1.17 (0.98, 1.40) | 232 | 32,873 | 1.48 (1.20, 1.83) | 444 | 131,600 | 1.18 (1.03, 1.36) | |
| Q5 | 480 | 82,731 | 1.35 (1.13, 1.61) | 226 | 33,426 | 1.57 (1.25, 1.97) | 484 | 119,225 | 1.45 (1.25, 1.68) | |
| AHEI Q1, Q2 | AHEI Q3 | AHEI Q4, Q5 | ||||||||
| Q1 | 129 | 43,792 | Ref | 102 | 32,602 | Ref | 406 | 118,507 | Ref | 0.09 |
| Q2 | 197 | 62,488 | 1.04 (0.83, 1.30) | 138 | 43,503 | 0.96 (0.74, 1.24) | 392 | 95,462 | 1.08 (0.94, 1.24) | |
| Q3 | 261 | 80,848 | 1.21 (0.97, 1.50) | 185 | 45,025 | 1.23 (0.96, 1.58) | 367 | 77,357 | 1.17 (1.01, 1.35) | |
| Q4 | 345 | 100,024 | 1.26 (1.02, 1.57) | 200 | 43,538 | 1.35 (1.05, 1.73) | 310 | 59,745 | 1.24 (1.06, 1.46) | |
| Q5 | 452 | 113,391 | 1.43 (1.15, 1.78) | 197 | 35,024 | 1.46 (1.11, 1.91) | 298 | 47,903 | 1.61 (1.36, 1.92) | |
Multivariable model adjusted for age (continuous), questionnaire cycle (continuous), BMI (<23, 23–24.9, 25–29.9, 30–34.9, ≥35 kg/m2), educational level (≤12, 13–15, 16, ≥17 y), geographic region (Northeast, South, Midwest, West), neighborhood SES (socioeconomic status) (quintiles), physical activity level (<7.5, 7.5 to <15, 15 to <30, ≥30 h of metabolic equivalent tasks per week), cigarette smoking (cigarettes/d, continuous), family history of myocardial infarction (yes, no), history of diabetes (yes, no), history of hypertension (yes, no), history of hypercholesterolemia (yes, no), total energy intake (quintiles, kcal/d), and modified 2010 Alternative Healthy Eating Index without red meat (quintiles, components include alcohol consumption).
AHEI, Alternative Healthy Eating Index; n, case numbers; Q: quintiles of total red meat intake in servings/d.
For site-specific cancer mortality, HRs for highest versus lowest quintiles of red meat consumption were 1.11 (95% CI: 0.74, 1.64, Supplemental Table 4) for lung cancer mortality (n = 391 deaths) and 0.77 (95% CI: 0.52, 1.14) for breast cancer mortality (n = 407 deaths). Small numbers prohibited investigation of other specific cancer sites.
Discussion
In 22 y of follow-up in a prospective cohort of 56,314 AA women, women who ate ∼2 servings of total red meat per day had an estimated 42% increase in all-cause mortality relative to women who consumed almost no red meat. Mortality increased 6% per 1 serving/d increment of red meat intake. Associations were similar for processed and unprocessed red meat intake. Red meat intake was also associated with higher cardiovascular mortality, but the results provide little or no support for the hypothesis that higher red meat consumption is associated with increased cancer mortality.
Currently, we are aware of only 1 other study that has reported on unprocessed red meat and mortality among AAs. Zhong et al. (27) reported a HR of 1.05 (95% CI: 1.02, 1.09) for each additional 2 servings of unprocessed red meat consumed per week with all-cause mortality for non-Hispanic blacks based on 1862 deaths. Diet was measured at baseline. The study did not differentiate processed red meat from other types of processed meat, such as poultry, and did not examine cause-specific mortality. Almost all of the existing evidence from follow-up studies of red meat and mortality is from whites, from the USA (10–12, 17, 27, 43, 44) and Europe (9, 14, 16, 45). There was 1 study of Asians (46). The NIH-AARP Diet and Health Study cohort contains AA participants (<4%) but race-specific results were not reported (10). It is possible that, compared with whites, AAs may have a different response to sodium intake (47, 48), and different iron (49), fatty acid (50–52), and lipid metabolisms (53–55). However, our findings on red meat and overall mortality are generally consistent with associations observed in studies of whites.
Our findings on red meat with CVD mortality are also consistent with the increased risk found for white Americans, Europeans, and Asian populations (56). A meta-analysis of red meat and CVD mortality reported a pooled HR of 1.19 (95% CI: 1.14, 1.25) (56). For CVD mortality, high concentrations of saturated fats and cholesterol from red meat partially explain the red meat and CVD mortality association (57, 58). Dietary iron, particularly heme iron, has been suggested to be associated with risk of CVD (59–62). Further, high sodium and nitrite concentrations in processed red meat have been associated with endothelial dysfunction, impaired insulin response, as well as risk of atherosclerosis (63, 64).
We found little evidence of an association of red meat intake with cancer mortality whereas studies of whites have found increased risk. For processed red meat in relation to cancer mortality in the present study, the HR was 1.02 (95% CI: 0.95, 1.10) for a 1 serving/d increase (Table 2). A meta-analysis of red meat intake and cancer mortality in whites reported a HR of 1.09 (1.06, 1.12) for US participants and 1.06 (1.02, 1.10) for European participants per 1 serving/d increase (56). For unprocessed red meat, the HR for per 1 serving/d increment was 1.00 (95% CI: 0.91, 1.11) in our cohort of AA women (Table 2), whereas it was 1.17 (1.09, 1.25) for US whites, 1.09 (0.81, 1.46) for Europeans, and 0.87 (0.79, 0.94) for Asians. It will be of interest to determine the association of red meat with cancer mortality in other studies of AAs.
Studies based on predominantly white populations reported a stronger association of processed red meat with mortality than for unprocessed meat with mortality (56). In our study, associations with mortality were similar for both processed and unprocessed meats. A difference between white and AA populations could be due to differences in types of processed and unprocessed red meat consumed or differences in cooking methods. For example, AAs are less likely to consume lean beef compared with whites (23) and more likely to use frying as the cooking method (24). In the BWHS, we did not have sufficient information to examine this possibility. In the study of Zhong et al. (27), HRs for all-cause mortality were similar for unprocessed red meat and processed meat, with 1.05 (95% CI: 1.02, 1.09) for unprocessed red meat and 1.03 (95% CI: 1.00, 1.05) for processed meat (per 2 servings/wk increment). However, this study did not differentiate processed red meat from processed poultry or other types of meat. Future studies of AAs with detailed information on cooking methods and types of unprocessed and processed red meat are needed.
Our study is the largest providing evidence on red meat consumption with mortality for AA women, a population that has a high consumption of red meat and high mortality. BWHS participants were from several different geographic regions across the USA and live in neighborhoods of varying socioeconomic level. The strengths of the study were the large sample size, long-term follow-up, detailed information collected repeatedly on participants’ lifestyle and medical conditions, and dietary information measured at 2 time points for both processed and unprocessed red meat.
Although we measured dietary intake at 2 time points, more measurements over time would have been desirable. Study participants typically underreport total caloric intake when responding to a FFQ, and that was likely the case in our study (30). We calculated a cumulative average to represent average red meat intake and to reduce random error. Misclassification due to measurement error could not be completely avoided. Although we conducted a dietary validation study, we did not examine correlation coefficients specifically for red meat. Frying and grilling are popular cooking methods among AAs and may contribute to higher contents of N-nitroso compounds, polycyclic aromatic hydrocarbons, and heterocyclic amines. We did not have detailed information on cooking methods and were not able to examine whether the associations differed by preparation or cooking procedures. Although we controlled numerous potential confounders, unmeasured confounding cannot be ruled out. We performed several sensitivity analyses to assess the influence of potential residual confounding and our results were consistent across different analytic strategies. We had no information on heme iron or nitrate concentration and therefore could not further evaluate the extent to which red meat and mortality association was mediated through these factors.
Red meat is a highly consumed food group worldwide, with Americans having the highest per capita intake. Investigation of the potential harms and benefits of red meat consumption among AAs is needed because of cultural differences in dietary intake and possible biological differences in terms of iron and lipid metabolisms. Given the disproportionately high mortality burden among AAs and the alarming progressive Westernization of diet across Africa, evidence from this investigation may have direct impact on AAs’ perception of a healthy diet and everyday dietary choice. Results from our study provide direct evidence for AAs and could inform policymakers and community leaders both in the USA and Africa.
In conclusion, red meat consumption was associated with elevated all-cause and cardiovascular mortality among AA women. The lack of association observed with cancer mortality was unexpected; additional research in other AA populations is needed.
Supplementary Material
Acknowledgments
We thank the staff and participants in the BWHS study.
The authors’ contributions were as follows—study concept and design: SS, JRP, and LR; acquisition of data: LR and JRP; analysis and interpretation of data: SS, LR, and JRP; drafting of the manuscript: SS; critical revision of the manuscript for important intellectual content: SS, LR, and JRP; statistical analysis: SS; administrative, technical, or material support: LR and JRP; study supervision: LR and JRP; SS had primary responsibility for final content; and all authors: read and approved the final manuscript. SS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Notes
This study was funded by NIH, R01CA058420, UM1CA164974, U01CA164974, and R01MD015085.
Author disclosures: The authors report no conflicts of interest.
The sole role of the funders was to support data collection. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supplemental Tables 1–4 and Supplemental Figures 1 and 2 are available from the ‘‘Supplementary data’’ link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AA, African American; AHEI, Alternative Healthy Eating Index; BWHS, Black Women's Health Study; CVD, cardiovascular disease; MET, metabolic equivalent tasks.
Contributor Information
Shanshan Sheehy, Slone Epidemiology Center, Boston University School of Medicine , Boston, MA, USA.
Julie R Palmer, Slone Epidemiology Center, Boston University School of Medicine , Boston, MA, USA.
Lynn Rosenberg, Slone Epidemiology Center, Boston University School of Medicine , Boston, MA, USA.
References
- 1. Daniel CR, Cross AJ, Koebnick C, Sinha R. Trends in meat consumption in the USA. Public Health Nutr. 2011;14:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zeng L, Ruan M, Liu J, Wilde P, Naumova EN, Mozaffarian D, Zhang FF. Trends in processed meat, unprocessed red meat, poultry, and fish consumption in the United States, 1999–2016. J Acad Nutr Diet. 2019;119:1085–98.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ritchie H, Roser M. Meat and seafood production & consumption. Global Data Change Lab; University of Oxford, UK: 2018. [Internet] Available at: https://ourworldindata.org/meat-production. Accessed 2020 Jul 30. [Google Scholar]
- 4. Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. [DOI] [PubMed] [Google Scholar]
- 5. The New York Times Red Meat is Not The Enemy. https://www.nytimes.com/2015/03/31/upshot/red-meat-is-not-the-enemy.html. Published2015; [Internet]. Accessed 2020 Jan 10. [Google Scholar]
- 6. The New York Times Eat Less Red Meat, Scientists Said. https://www.nytimes.com/2019/09/30/health/red-meat-heart-cancer.html. Published2019; [Internet]. Accessed 2020 Jan 10. [Google Scholar]
- 7. The New York Times Eat Less Meat, Live Longer?. https://www.nytimes.com/2019/04/11/well/eat/eat-less-meat-live-longer.html. Published2019; [Internet]. Accessed 2020 Jan 10. [Google Scholar]
- 8. BBC. Should I eat meat? The big health dilemma. BBC2014-2015. https://www.telegraph.co.uk/culture/tvandradio/tv-and-radio-reviews/11042113/Horizon-Should-I-Eat-Meat-BBC-Two-review-a-meaty-dilemma.html [Internet]. Accessed 2020 Jan 10.
- 9. Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjonneland A, Nailler L, Boutron-Ruault MC, Clavel-Chapelon F, Krogh V et al. Meat consumption and mortality – results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009;169(6):562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Etemadi A, Sinha R, Ward MH, Graubard BI, Inoue-Choi M, Dawsey SM, Abnet CC. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ. 2017;357:j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172(7):555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr. 2014;112(5):762–75. [DOI] [PubMed] [Google Scholar]
- 14. Bellavia A, Stilling F, Wolk A. High red meat intake and all-cause cardiovascular and cancer mortality: is the risk modified by fruit and vegetable intake?. Am J Clin Nutr. 2016;104(4):1137–43. 10.3945/ajcn.116.135335. [DOI] [PubMed] [Google Scholar]
- 15. Larsson SC, Orsini N. Red meat and processed meat consumption and all-cause mortality: a meta-analysis. Am J Epidemiol. 2014;179(3):282–9. [DOI] [PubMed] [Google Scholar]
- 16. Whiteman D, Muir J, Jones L, Murphy M, Key T. Dietary questions as determinants of mortality: the OXCHECK experience. Public Health Nutr. 1999;2(4):477–87. 10.1017/s136898009900066x. [DOI] [PubMed] [Google Scholar]
- 17. Kappeler R, Eichholzer M, Rohrmann S. Meat consumption and diet quality and mortality in NHANES III. Eur J Clin Nutr. 2013;67(6):598–606. 10.1038/ejcn.2013.59. [DOI] [PubMed] [Google Scholar]
- 18. Bouvard V, Loomis D, Guyton KZ, Grosse Y, Ghissassi FE, Benbrahim-Tallaa L, Guha N, Mattock H, Straif K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16(16):1599–600. [DOI] [PubMed] [Google Scholar]
- 19. U.S. Department of Health and Human Services and U.S. Department of Agriculture 2015–2020 Dietary Guidelines for Americans. 8th Edition [Internet]. Accessed 2020 Jan 10 2015. Available at: https://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 20. Li D, Siriamornpun S, Wahlqvist ML, Mann NJ, Sinclair AJ. Lean meat and heart health. Asia Pac J Clin Nutr. 2005;14:113–9. [PubMed] [Google Scholar]
- 21. National Center for Health Statistics. Trends in cancer and heart disease death rates among adults aged 45–64: United States, 1999–2017. National Vital Statistics Reports. Vol 68: No. 5, 2019. U.S. Department of Health and Human Services; Available at: https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_05-508.pdf [Internet]. Accessed 2020 Jan 10.
- 22. Wang Y, Beydoun MA, Caballero B, Gary TL, Lawrence R. Trends and correlates in meat consumption patterns in the US adult population. Public Health Nutr. 2010;13(9):1333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. An R, Nickols-Richardson S, Alston R, Shen S, Clarke C. Total, fresh, lean, and fresh lean beef consumption in relation to nutrient intakes and diet quality among U.S. adults, 2005–2016. Nutrients. 2019;11(3):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Popkin BM. Understanding global nutrition dynamics as a step towards controlling cancer incidence. Nat Rev Cancer. 2007;7(1):61–7. [DOI] [PubMed] [Google Scholar]
- 25. Judd SE, Letter AJ, Shikany JM, Roth DL, Newby PK. Dietary patterns derived using exploratory and confirmatory factor analysis are stable and generalizable across race, region, and gender subgroups in the REGARDS study. Front Nutr. 2015;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sterling S, Judd S, Bertrand B, Carson TL, Chandler-Laney P, Baskin ML. Dietary patterns among overweight and obese African-American women living in the rural south. J Racial Ethn Health Disparities. 2018;5(1):141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhong VW, Van Horn L, Greenland P, Carnethon MR, Ning H, Wilkins JT, Lloyd-Jones DM, Allen NB. Associations of processed meat, unprocessed red meat, poultry, or fish intake with incident cardiovascular disease and all-cause mortality. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2019.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women's Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc. 1995;50(2):56–8. [PubMed] [Google Scholar]
- 29. Kumanyika SK, Mauger D, Mitchell DC, Phillips B, Smiciklas-Wright H, Palmer JR. Relative validity of food frequency questionnaire nutrient estimates in the Black Women's Health Study. Ann Epidemiol. 2003;13(2):111–8. [DOI] [PubMed] [Google Scholar]
- 30. Willett W. Nutritional Epidemiology. 3rd ed Oxford: Oxford University Press; 2013. [Google Scholar]
- 31. Horn-Ross PL, Lee VS, Collins CN, Stewart SL, Canchola AJ, Lee MM, Reynolds P, Clarke CA, Bernstein L, Stram DO. Dietary assessment in the California Teachers Study: reproducibility and validity. Cancer Causes Control. 2008;19(6):595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bethea TN, Palmer JR, Rosenberg L, Cozier YC. Neighborhood socioeconomic status in relation to all-cause, cancer, and cardiovascular mortality in the Black Women's Health Study. Ethn Dis. 2016;26(2):157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li S, Chiuve SE, Flint A, Pai JK, Forman JP, Hu FB, Willett WC, Mukamal KJ, Rimm EB. Better diet quality and decreased mortality among myocardial infarction survivors. JAMA Intern Med. 2013;173(19):1808–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carter-Nolan PL, Adams-Campbell LL, Makambi K, Lewis S, Palmer JR, Rosenberg L. Validation of physical activity instruments: Black Women's Health Study. Ethn Dis. 2006;16(4):943–7. [PubMed] [Google Scholar]
- 36. Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR, Rosenberg L. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125(6):767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. [DOI] [PubMed] [Google Scholar]
- 38. Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168(14):1487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149(6):531–40. [DOI] [PubMed] [Google Scholar]
- 40. Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Statist Med. 2007;26(20):3735–52. [DOI] [PubMed] [Google Scholar]
- 41. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. 1999;94:496–509. [Google Scholar]
- 42. Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alshahrani SM, Fraser GE, Sabate J, Knutsen R, Shavlik D, Mashchak A, Lloren JI, Orlich MJ. Red and processed meat and mortality in a low meat intake population. Nutrients. 2019;11(3):622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zheng Y, Li Y, Satija A, Pan A, Sotos-Prieto M, Rimm E, Willett WC, Hu FB. Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ. 2019;365:l2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van den Brandt PA. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in The Netherlands Cohort Study. Eur J Epidemiol. 2019;34(4):351–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee JE, McLerran DF, Rolland B, Chen Y, Grant EJ, Vedanthan R, Inoue M, Tsugane S, Gao YT, Tsuji I et al. Meat intake and cause-specific mortality: a pooled analysis of Asian prospective cohort studies. Am J Clin Nutr. 2013;98(4):1032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weinberger MH, Luft FC, Bloch R, Henry DP, Pratt JH, Weyman AE, Rankin LI, Murray RH, Willis LR, Grim CE. The blood pressure-raising effects of high dietary sodium intake: racial differences and the role of potassium. J Am Coll Nutr. 1982;1(2):139–48. [DOI] [PubMed] [Google Scholar]
- 48. Wilmot B, Voruganti VS, Chang Y-PC, Fu Y, Chen Z, Taylor HA, Wilson JG, Gipson T, Shah VO, Umans JG et al. Heritability of serum sodium concentration: evidence for sex- and ethnic-specific effects. Physiol Genomics. 2012;44(3):220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gordeuk VR, Brannon PM. Ethnic and genetic factors of iron status in women of reproductive age. Am J Clin Nutr. 2017;106(Suppl 6):1594S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Oliveira Otto MC, Wu JHY, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR Jr, Mozaffarian D. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. JAHA. 2013;2(6):e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lemaitre RN, Tanaka T, Tang W, Manichaikul A, Foy M, Kabagambe EK, Nettleton JA, King IB, Weng LC, Bhattacharya S et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet. 2011;7(7):e1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chilton FH, Murphy RC, Wilson BA, Sergeant S, Ainsworth H, Seeds MC, Mathias RA. Diet-gene interactions and PUFA metabolism: a potential contributor to health disparities and human diseases. Nutrients. 2014;6(5):1993–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393–423. [DOI] [PubMed] [Google Scholar]
- 54. Keebler ME, Deo RC, Surti A, Konieczkowski D, Guiducci C, Burtt N, Buxbaum SG, Sarpong DF, Steffes MW, Wilson JG et al. Fine-mapping in African Americans of 8 recently discovered genetic loci for plasma lipids: the Jackson Heart Study. Circ Cardiovasc Genet. 2010;3(4):358–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang X, Lin X, Ouyang YY, Liu J, Zhao G, Pan A, Hu FB. Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016;19(5):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121(21):2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ley SH, Sun Q, Willett WC, Eliassen AH, Wu K, Pan A, Grodstein F, Hu FB. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am J Clin Nutr. 2014;99(2):352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ascherio A, Willett WC, Rimm EB, Giovannucci EL, Stampfer MJ. Dietary iron intake and risk of coronary disease among men. Circulation. 1994;89(3):969–74. [DOI] [PubMed] [Google Scholar]
- 60. Kaluza J, Wolk A, Larsson SC. Heme iron intake and risk of stroke: a prospective study of men. Stroke. 2013;44(2):334–9. [DOI] [PubMed] [Google Scholar]
- 61. Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86(3):803–11. [DOI] [PubMed] [Google Scholar]
- 62. Tzonou A, Lagiou P, Trichopoulou A, Tsoutsos V, Trichopoulos D. Dietary iron and coronary heart disease risk: a study from Greece. Am J Epidemiol. 1998;147(2):161–6. [DOI] [PubMed] [Google Scholar]
- 63. Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40(2):295–302. [DOI] [PubMed] [Google Scholar]
- 64. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.