Abstract
Introduction
While the long-term oncologic safety of robot-assisted nipple sparing mastectomy (RNSM) remains to be elucidated, histologically detected residual breast tissue (RBT) can be a surrogate for oncologically sound mastectomy. The objective of this study is to determine the presence of RBT after RNSM.
Methods
Between August 2019–January 2020, we completed 5 cadaveric RNSMs. Full thickness biopsies from the mastectomy skin flap were obtained from predefined locations radially around the mastectomy skin envelop and nipple areolar complex to histologically evaluate for RBT.
Results
The first case was not technically feasible due to inability to obtain adequate insufflation. Five mastectomy flaps were analyzable. The average mastectomy flap thickness was 2.3 mm (range 2–3 mm) and the average specimen weight was 382.72 g (range 146.9–558.3 g). Of 70 total biopsies, RBT was detected in 11 (15.7%) biopsies. Most common location for RBT was in the nipple-areolar complex, with no RBT detected from the peripheral skin flaps.
Conclusions
In this cadaveric study, RNSM is feasible leaving minimal RBT on the mastectomy flap. The most common location for RBT is in the periareolar location consistent with previous published findings after open NSM. Clinical studies are underway to evaluate the safety of RNSM.
Keywords: Mastectomy, Robot-assisted mastectomy, Nipple sparing mastectomy, Robot-assisted nipple sparing mastectomy
Highlights
-
•
Robot-assisted nipple sparing mastectomy (RNSM) is technically feasible.
-
•
Residual breast tissue after RNSM is histologically detected only from the periareolar location.
-
•
Further clinical trials are underway to determine oncologic safety of RNSM.
Brief title: robot mastectomy
Financial and conflict of interest disclosure: The authors have no relevant financial disclosure. The authors declare that there are no competing interests.
Funding
The Ohio State University 2019 Intramural Research Program IDEA Award (46050–502730).
Author contribution
Study conception and design: Park, Skoracki. Acquisition of data: Park, Tozbikian, Ferry, Tsung, Chetta, Schulz, Skoracki. Analysis and interpretation of data: Park, Tozbikian. Drafting of manuscript: Park, Skoracki. Critical revision: Park, Tozbikian, Ferry, Tsung, Chetta, Schulz, Skoracki. Synopsis: Robot-assisted nipple sparing mastectomy (RNSM) is a surgical advancement for women undergoing removal of the breast for a variety of reasons. Utilizing cadavers we aimed to demonstrate procedure feasibility in completing an oncologically sound mastectomy by performing histologic analysis of the mastectomy flap after RNSM.
Introduction
With the advances in breast reconstruction after mastectomy for the treatment of breast diseases including breast cancer, surgical techniques have evolved to preserve the skin flaps and nipple areolar complex (NAC) to give better aesthetic outcome without compromising oncologic outcome [1,2]. The preservation of the nipple and areola complex during mastectomy can provide psychological and emotional benefits, and improve patient satisfaction [3,4]. Nipple sparing mastectomy (NSM) can provide major psychosocial benefits for patients but is technically demanding and has variable outcomes [[5], [6], [7]]. Total mammary glandular excision in NSM can be technically challenging, due to small size of the incision and poor visualization of the dissection plane. To help overcome the challenges in open NSM, use of the robot (da Vinci Surgical System, Intuitive Surgical, Sunnyvale, CA) for robot-assisted nipple sparing mastectomy (RNSM) is an emerging operation [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]].
There are limited number of centers throughout the world currently performing RNSM, thus the procedure is currently still under development and optimization. The use of the da Vinci surgical system is not FDA approved for use in breast surgery in the United States [21]. To study the technical feasibility and safety of RNSM, we performed a series of cadaveric studies using the robot. Our goal was to master the techniques of RNSM and to assess the potential oncologic safety by evaluating the mastectomy skin flaps for histologically detected residual breast tissue (RBT). We hypothesized that RNSM will be technically feasible, leaving minimal residual breast tissue on the mastectomy skip flap. The technique of RNSM is described and current literature is reviewed in detail in the discussion.
Methods
Under The Ohio State University institutional Ethics Committee approval (in lieu of Institutional Review Board) between August 2019–January 2020, 6 RNSM cadaveric cases were performed in three female fresh cadavers by a single surgeon (KUP). Two of the cadaveric surgeries were performed with the daVinci Si system (Intuitive Surgical, Sunnyvale, CA) and one was performed with daVinci Xi system. Robotic surgery certified breast surgical oncologist (KP) at the console performed all the surgeries with an experienced robotic certified first assistant (DF) at the bedside. After breast removal, the mastectomy flap was measured with a caliper to assess the thickness of the flap.
Variations in surgical technique
The cadaver was placed in the supine position with the ipsilateral arm to the operative site positioned out of the field. Prior to the start of the procedure, the midline, inframammary fold, extent of breast tissue, anterior axillary line, and horizontal axillary crease were marked and used to guide the position of the linear incision along the anterior axillary line. Next, the subcutaneous dissection was performed using scissors and electrocautery as far as the instruments would reach under direct visualization to create a ‘working space’. Cadaver # 1 and #3 were performed using the daVinci Si® system and cadaver #2 was performed using the daVinci Xi® system.
For the first cadaveric case, we used the entire linear incision to directly place the three robot ports adjacent to each other and secured the ports in place using suture and surgical skin stapler as previously described between ports [22]. Insufflation was difficult to achieve using this technique. Therefore, for the 2nd and 3rd cadaver cases, we modified the procedure and used the single port system (GelPOINT Mini, Applied Medical, Rancho Santa Margarita, CA). The single port system was inserted into the incision and the robot ports were placed into GelSeal Cap connected to an insufflator to keep the pressure at 8 mm Hg.
The robot was positioned at the cadaver’s head and docked. The 30-degree camera was placed in the middle port. Subcutaneous dissection was performed in the lateral to medial direction using the monopolar-curved scissors and bipolar grasping forceps for traction and exposure. The bedside assistant guided the surgeon at the console on the extent of the dissection as outlined by the skin markings prior to docking the robotic system. Using a similar technique of sharp and cautery dissection, the gland was separated from the pectoralis major muscle. The specimen was removed from the anterior axillary incision by manual gentle traction using the “waving flag technique” (move the gland back and forth gently until it is removed). If it is not feasible to remove the entire gland easily, the incision was extended to assure removal of the intact specimen.
Assessment of residual breast tissue
Prior to skin closure, approximately 1 cm [2] full thickness biopsies from the mastectomy skin flap were obtained using fourteen predefined locations radially around the mastectomy skin envelop and nipple areolar complex (Supplementary Fig. 1) as previously published [23]. Locations L, M, N, and O correspond to the area behind the NAC. The tissue was formalin-fixed, paraffin-embedded and stained with hematoxylin and eosin (H&E) as per standard institutional protocol. The tissue was assessed histologically by a board certified breast pathologist (GT) for presence of RBT.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper.
Results
Operative characteristics
Fresh non-formalin fixed female cadavers without previous history of breast cancer were used for the study. The cadavers were elderly and none had evidence of prior breast surgery (Table 1). There were no skin injuries (ie. button-hole) related to use of the robot (Table 2).
Table 1.
Characteristics of cadavers.
| Cadaver #1 | Cadaver #2 | Cadaver #3 | |
|---|---|---|---|
| Age | 76 | 71 | 85 |
| Race | White | Black | White |
| Cause of death | COPD | Retroperitoneal neoplasm | Dementia/Alzheimers |
| Estimated bra size | A | B | C |
| Ptosis of breast (grade) | 3 | 3 | 3 |
| Body Mass Index | 32.18 | 21.28 | 27.45 |
Table 2.
Operative characteristics of robot-assisted nipple sparing mastectomies.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Case completed (yes/no) | No | Yes | Yes | Yes | Yes | Yes |
| Mastectomy flap thickness (mm) | n/a | 2.0 | 2.0 | 2.5 | 3.0 | 2.0 |
| Skin injury (yes/no) | n/a | No | No | No | No | No |
| Weight of mastectomy specimen (grams) | n/a | 146.9 | 378.9 | 289.2 | 558.3 | 540.3 |
| Console time (minutes) | n/a | 46 | 70 | 80 | 247 | 75 |
Console time was measured from the time of docking the robot to undocking the system. The console time ranged from 46 to 247 min (Table 2). Case #5 was significantly longer as mid-case the robot was undocked to trial a different type of single-port system, GelPOINT Path Transanal access platform (Applied Medical, Rancho Santa Margarita, CA). Although this helped in acquiring a complete seal so that the CO2 did not leak at the skin-port junction, the self-retaining sleeve was too rigid and narrow, resulting in instrument collision.
Residual breast tissue
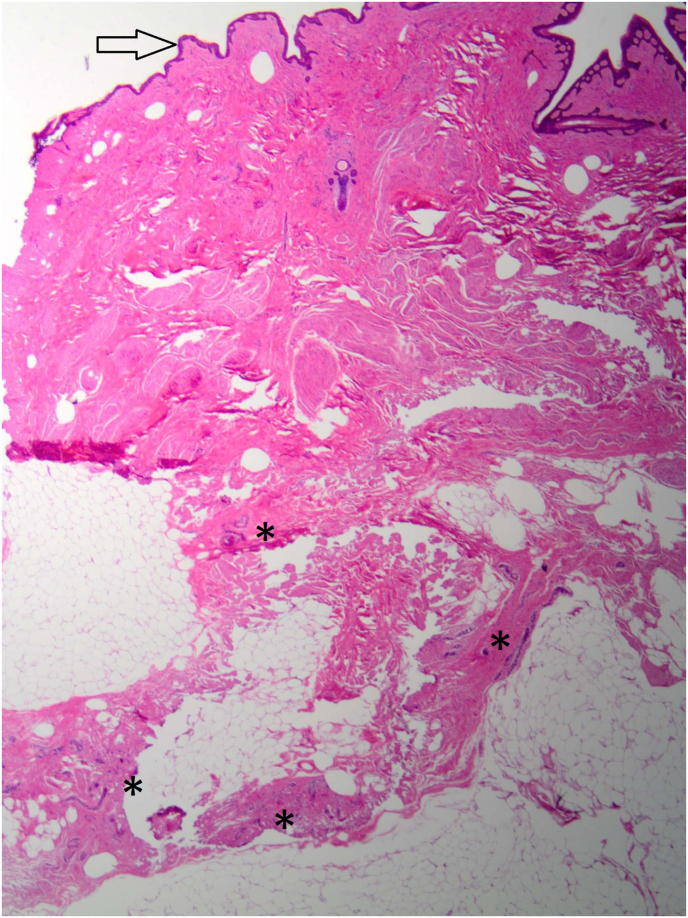
The average mastectomy flap thickness was 2.3 mm (range 2–3 mm) and the average specimen weight was 382.72 g (range 146.9–558.3 g) (Table 2). Of the 70 total biopsies, RBT was detected in 11 (15.7%) biopsies. All of the RBT were detected in tissue behind the NAC: in case 2 RBT was detected in location M,N, and O; in case 3 RBT in N, in case 4 in L,M,N, and O; in case 5 in N; and in case 6 in L, and N (Fig. 1). There were no RBT detected outside the NAC in the mastectomy skin flaps.
Fig. 1.
Representative slide of preiareolar skin, subcutaneous tissue, and residual breast parenchyma stained with hemoxylin and eosin. Arrow indicates periareolar skin and asterisk indicates terminal ductal lobular unit. Photo is taken with 40× magnification.
Discussion
In this cadaveric study, we demonstrate the technical reproducibility of robot-assisted nipple sparing (RNSM) and low residual breast tissue (RBT) after RNSM supporting our initial hypothesis. We detected at least one RBT-positive biopsy in all five RNSM but none were detected outside of the NAC. Although the cadavers in the present study were elderly with significant degree of ptosis and hence, not the typically patient who undergoes NSM, we were able to successfully complete five cadaveric RNSM procedures.
Residual breast tissue after mastectomy
Technical feasibility and reproducibility of RNSM have been demonstrated but previous studies did not evaluate for presence of RBT after mastectomy. In this study, we demonstrate that there were no histologically detectable RBT in the mastectomy flap outside of the NAC. The presence of residual breast tissue in the NAC is not surprising in this study. Presence of RBT after open NSM is not uncommon and is most common in tissue behind the NAC [23,24]. In a study using MRI to evaluate for RBT after mastectomy, the overall frequency of RBT was 77.8% and when the NAC region was excluded, the frequency of RBT was 51.6% in NSMs [24]. Papassotiropoulos et al. reported RBT was histologically detected in 18–28% of central biopsies around the NAC in open NSM cases [23]. This phenomenon may be due to lack of Cooper’s ligaments under the NAC, leading to more difficult precise dissection in the dermis-fibroglandular tissue junction [25]. More importantly, the incidence of local recurrence after open NSM is low despite the presence of RBT [2,[26], [27], [28]]. Nipple areolar complex recurrence after open NSM ranges from 0 to 7% in most recent series [25]. While the exact clinical relevance of RBT after mastectomy remains elusive, it is important to note that previous studies evaluating local recurrence and NAC recurrence demonstrate that tumor biology is a stronger risk factor than surgical technique.
Emergence of robotic-assisted nipple sparing mastectomy
Surgeons experience greater physical symptoms such as neck and lower back pain, mental strain, and fatigue from performing open NSM compared to skin sparing mastectomy [6,29]. Thus, a more ergonomically sound technique with greater visualization is needed to improve surgeon working conditions and to improve the ease of the operation during open NSM. Robot assisted surgery facilitates minimally invasive surgery through 3D vision with magnification, improved lighting, and seven degrees of motion of the instrument tip to allow for proper dissection of the surgical plane. Despite wide use in other surgeries, the use of the robot for breast surgery has been limited until recently.
One of the earliest reports of RNSM is from Dr.Antonio Toesca [30]. In this initial report of 3 consecutive successful cases, the authors report the surgical technique in detail. Through a 2.5 cm extra-mammary axillary line incision a subcutaneous flap is first developed. A single port access system (Access Transformer OCTO; Seoul, Korea) was used to dock the robotic arms and to keep the robotic arm elbows from clashing. CO2 insufflation created the necessary working space and retraction on the mastectomy flap. The dissection was performed using Maryland Bipolar Forceps and monopolar cautery.
Subsequently, few additional publications have described the use of RNSM (Table 3) [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. Reported complications include nipple areolar complex necrosis, mastectomy flap necrosis, temporary skin blistering, hematoma, seroma, infection, loss of implant from infection, delayed axillary wound healing, transient brachial plexus neurapraxia (due to arm positioning), transient common peronal nerve neurapraxia (due to patient positioning during surgery). Thus far, no local recurrences have been reported with a mean follow-up of 20 months, although longer follow up studies are pending [31]. The learning curve for RNSM ranged from 8 to 13 cases using the cumulative sum plot method [16,20].
Table 3.
Summary of published results on robot-assisted nipple sparing mastectomy as of December 2019 (case reports excluded).
| Author | Year of publication | Number of cases | Mastectomy flap or nipple areolar complex necrosis | Overall complication rate | Complication requiring reoperation |
|---|---|---|---|---|---|
| Toesca [8] | 2017 | 29 | 0 | 0 | 0 |
| Sarfati [12] | 2018 | 63 | 0 | 4.8% | 3.2% |
| Lai [16] | 2018 | 39 | 5.1% | 30.8% | 2.6% |
| Park [20] | 2019 | 12 | 25% | 25% | 0 |
| Houvenaghel [17] | 2019 | 27 | 3.7% | 40.7% | Not reported |
| Toesca [31] | 2019 | 94 | 1.1% | 22.3% | 4.3% |
Future studies in robotic mastectomy
Despite these early successes, the safety of robotic oncologic surgery has been called to question by the FDA and some of this may be due to inferior outcomes in minimally invasive surgery compared to open radical hysterectomy for cervical cancer (Laparoscopic Approach to Cervical Cancer [LACC] Trial) [32]. It is important to note that in this study there were no randomization between laparoscopic versus robotic approach and of the minimally invasive surgery cases only 15.6% were performed with robotic-assistance (N = 45). There are speculations that the use of uterine or cervical manipulators and carbon dioxide pneumoperitonium promoted tumor recurrence [33]. However, other abdominal robotic surgeries that require carbon dioxide such as minimally invasive pancreatectomy and proctectomy report comparable rates of overall survival compared to open surgery [34,35]. In response to the safety concerns, our institution has received FDA approval of an Investigational Device Exemption (IDE) to initiate a clinical study to evaluate the safety of RNSM (clinicaltrials.gov NCT04537312). This type of research is necessary to demonstrate the safety and feasibility of RNSM before this technique can be incorporated into routine practice.
Currently, there are numerous ongoing trials outside of the United States evaluating outcomes after RNSM [[36], [37], [38], [39], [40]], including a randomized study comparing open to robot-assisted NSM [41]. These ongoing international studies may help elucidate the long-term oncologic safety of RNSM.
Furthermore, future studies assessing the mastectomy skin flap viability may demonstrate low rates of flap complication and thus improved success of immediate breast reconstruction. Although current published number of cases are small, there is a trend for low skin flap necrosis [8,12,16,17,31]. While the same anatomic planes are adhered to during RNSM compared to open, the skin retraction with CO2 insufflation rather than physical retraction may be less traumatic through the duration of the surgery. Critical evaluation of the mastectomy skin flap in future studies is still needed.
Conclusion
Herein, we describe the first study evaluating the presence of RBT after robotic mastectomy. RNSM was technically feasible and presence of RBT was comparable to that reported in previous published literature on open NSM. The most common location of RBT, as expected, was centrally in the tissue behind the NAC. Further studies are underway to evaluate the safety and long-term oncologic outcomes of RNSM.
Acknowledgments
The authors would like to thank Angela Sarna and Heidi Pieper for their generous help with organizing the cadaver lab. We also thank the Pathology Core Shared Resource at The Ohio State University Comprehensive Cancer Center, Columbus, OH for preparation and interpretation of the slides.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.11.022.
Contributor Information
Ko Un Park, Email: Koun.park@ousmc.edu.
Gary H. Tozbikian, Email: Gary.Tozbikian@osumc.edu.
David Ferry, Email: David.Ferry@osumc.edu.
Allan Tsung, Email: Allan.Tsung@osumc.edu.
Mathew Chetta, Email: Matthew.Chetta@osumc.edu.
Steven Schulz, Email: Steven.Schulz@osumc.edu.
Roman Skoracki, Email: Roman.Skoracki@osumc.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Galimberti V., Morigi C., Bagnardi V. Oncological outcomes of nipple-sparing mastectomy: a single-center experience of 1989 patients. Ann Surg Oncol. 2018;25(13):3849–3857. doi: 10.1245/s10434-018-6759-0. [DOI] [PubMed] [Google Scholar]
- 2.Smith B.L., Tang R., Rai U. Oncologic safety of nipple-sparing mastectomy in women with breast cancer. J Am Coll Surg. 2017;225(3):361–365. doi: 10.1016/j.jamcollsurg.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Romanoff A., Zabor E.C., Stempel M., Sacchini V., Pusic A., Morrow M. A comparison of patient-reported outcomes after nipple-sparing mastectomy and conventional mastectomy with reconstruction. Ann Surg Oncol. 2018;25(10):2909–2916. doi: 10.1245/s10434-018-6585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei C.H., Scott A.M., Price A.N. Psychosocial and sexual well-being following nipple-sparing mastectomy and reconstruction. Breast J. 2016;22(1):10–17. doi: 10.1111/tbj.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopkash K., Novak K., Kuchta K. The “nipple whipple”?! A pilot study to assess the ergonomic effects of nipple-sparing mastectomy. Ann Surg Oncol. 2019;26(10):3216–3223. doi: 10.1245/s10434-019-07550-1. [DOI] [PubMed] [Google Scholar]
- 6.Jackson R.S., Sanders T., Park A. Prospective study comparing surgeons’ pain and fatigue associated with nipple-sparing versus skin-sparing mastectomy. Ann Surg Oncol. 2017;24(10):3024–3031. doi: 10.1245/s10434-017-5929-9. [DOI] [PubMed] [Google Scholar]
- 7.Park K.U. Nipple sparing mastectomy after neoadjuvant chemotherapy: identifying patients at high risk for complications. American Society of Breast Surgeons 19th Annual Meeting Poster Gallery. 2018 [Google Scholar]
- 8.Toesca A., Peradze N., Manconi A. Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study. Breast. 2017;31:51–56. doi: 10.1016/j.breast.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park H.S., Kim J.H., Lee D.W. Gasless robot-assisted nipple-sparing mastectomy: a case report. J Breast Cancer. 2018;21(3):334–338. doi: 10.4048/jbc.2018.21.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarfati B., Honart J.F., Leymarie N., Rimareix F., Al Khashnam H., Kolb F. Robotic da Vinci Xi-assisted nipple-sparing mastectomy: first clinical report. Breast J. 2018;24(3):373–376. doi: 10.1111/tbj.12937. [DOI] [PubMed] [Google Scholar]
- 11.Sarfati B., Struk S., Leymarie N. Robotic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: surgical technique. Plast Reconstr Surg. 2018;142(3):624–627. doi: 10.1097/PRS.0000000000004703. [DOI] [PubMed] [Google Scholar]
- 12.Sarfati B., Struk S., Leymarie N. Robotic prophylactic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a prospective study. Ann Surg Oncol. 2018;25(9):2579–2586. doi: 10.1245/s10434-018-6555-x. [DOI] [PubMed] [Google Scholar]
- 13.Lai H.W., Lin S.L., Chen S.T. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant. Plast Reconstr Surg Glob Open. 2018;6(6) doi: 10.1097/GOX.0000000000001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai H.W., Chen S.T., Lin S.L. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome. Ann Surg Oncol. 2018;26(1):42–52. doi: 10.1245/s10434-018-6704-2. [DOI] [PubMed] [Google Scholar]
- 15.Lai H.W., Lin S.L., Chen S.T. Robotic nipple sparing mastectomy and immediate breast reconstruction with robotic latissimus dorsi flap harvest - technique and preliminary results. J Plast Reconstr Aesthetic Surg. 2018;71(10):e59–e61. doi: 10.1016/j.bjps.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Lai H.W., Wang C.C., Lai Y.C. The learning curve of robotic nipple sparing mastectomy for breast cancer: an analysis of consecutive 39 procedures with cumulative sum plot. Eur J Surg Oncol. 2018;45(2):125–133. doi: 10.1016/j.ejso.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Houvenaeghel G., Bannier M., Rua S. Breast cancer robotic nipple sparing mastectomy: evaluation of several surgical procedures and learning curve. World J Surg Oncol. 2019;17(1):27. doi: 10.1186/s12957-019-1567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajappa S.K., Kumar R., Garg S., Ram D. Robotic nipple-sparing mastectomy: the first experience from Indian subcontinent. Breast J. 2018;24(6):1114–1115. doi: 10.1111/tbj.13146. [DOI] [PubMed] [Google Scholar]
- 19.Kuo W.-L., Huang J.-J., Huang Y.-T. Robot-assisted mastectomy followed by immediate autologous microsurgical free flap reconstruction: techniques and feasibility in three different breast cancer surgical scenarios. Clin Breast Canc. 2019;20(1):e1–e8. doi: 10.1016/j.clbc.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Park H.S., Lee J., Lee D.W. Robot-assisted nipple-sparing mastectomy with immediate breast reconstruction: an initial experience. Sci Rep. 2019;9(1):15669. doi: 10.1038/s41598-019-51744-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caution when using robotically-assisted surgical devices in women’s health including mastectomy and other cancer-related surgeries: FDA safety communication. Safety Communications. 2019 https://www.fda.gov/medical-devices/safety-communications/caution-when-using-robotically-assisted-surgical-devices-womens-health-including-mastectomy-and [Google Scholar]
- 22.Sarfati B., Honart J.F., Leymarie N., Kolb F., Rimareix F. Robotic-assisted Nipple Sparing Mastectomy: a feasibility study on cadaveric models. J Plast Reconstr Aesthetic Surg. 2016;69(11):1571–1572. doi: 10.1016/j.bjps.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Papassotiropoulos B., Güth U., Chiesa F. Prospective evaluation of residual breast tissue after skin- or nipple-sparing mastectomy: results of the SKINI-trial. Ann Surg Oncol. 2019;26(5):1254–1262. doi: 10.1245/s10434-019-07259-1. [DOI] [PubMed] [Google Scholar]
- 24.Giannotti D.G., Hanna S.A., Cerri G.G., Barbosa Bevilacqua J.L. Analysis of skin flap thickness and residual breast tissue after mastectomy. Int J Radiat Oncol Biol Phys. 2018;102(1):82–91. doi: 10.1016/j.ijrobp.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Smith B.L., Coopey S.B. Nipple-sparing mastectomy. Adv Surg. 2018;52(1):113–126. doi: 10.1016/j.yasu.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Giannotti D.G., Hanna S.A., Cerri G.G., Barbosa Bevilacqua J.L. Analysis of skin flap thickness and residual breast tissue after mastectomy. Int J Radiat Oncol Biol Phys. 2018;102(1):82–91. doi: 10.1016/j.ijrobp.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Jakub J.W., Peled A.W., Gray R.J. Oncologic safety of prophylactic nipple-sparing mastectomy in a population with brca mutations: a multi-institutional study. JAMA Surgery. 2018;153(2):123–129. doi: 10.1001/jamasurg.2017.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao K., Liederbach E., Tang R. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and Review of the literature. Ann Surg Oncol. 2015;22(2):370–376. doi: 10.1245/s10434-014-3883-3. [DOI] [PubMed] [Google Scholar]
- 29.Hallbeck M.S., Law K.E., Lowndes B.R. Workload differentiates breast surgical procedures: NSM associated with higher workload demand than SSM. Ann Surg Oncol. 2020;27(5):1318–1326. doi: 10.1245/s10434-019-08159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toesca A., Peradze N., Galimberti V. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg. 2017;266(2):e28–e30. doi: 10.1097/SLA.0000000000001397. [DOI] [PubMed] [Google Scholar]
- 31.Toesca A., Invento A., Massari G. Update on the feasibility and progress on robotic breast surgery. Ann Surg Oncol. 2019;26(10):3046–3051. doi: 10.1245/s10434-019-07590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramirez P.T., Frumovitz M., Pareja R. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. NEJM. 2018;379(20):1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 33.Fader A.N. Surgery in cervical cancer. NEJM. 2018;379(20):1955–1957. doi: 10.1056/NEJMe1814034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Hilst J., de Rooij T., Klompmaker S. Minimally invasive versus open distal pancreatectomy for ductal adenocarcinoma (DIPLOMA): a pan-European propensity score matched study. Ann Surg. 2019;269(1):10–17. doi: 10.1097/SLA.0000000000002561. [DOI] [PubMed] [Google Scholar]
- 35.Fleshman J., Branda M.E., Sargent D.J. Disease-free survival and local recurrence for laparoscopic resection compared with open resection of stage II to III rectal cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg. 2019;269(4):589–595. doi: 10.1097/SLA.0000000000003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NIH. ClinicalTrials.gov Robot assisted mastectomy via axillary way with areola conservation and immediate reconstruction (MARCI) https://clinicaltrials.gov/ct2/show/NCT02673268
- 37.NIH. ClinicalTrials.gov Robotic versus conventional or endoscopic nipple sparing mastectomy for breast cancer (RCENSM-R) https://clinicaltrials.gov/ct2/show/NCT04049305
- 38.NIH. ClinicalTrials.gov Robotic versus conventional or endoscopic nipple sparing mastectomy in the management of breast cancer-prospective study (RCENSM-P) https://clinicaltrials.gov/ct2/show/NCT04037852
- 39.NIH. ClinicalTrials.gov Surgical and patient reported outcomes of robotic nipple-sparing mastectomy (RNSM) https://clinicaltrials.gov/ct2/show/NCT04151368
- 40.NIH. ClinicalTrials.gov Surgical and oncologic outcomes after robotic nipple sparing mastectomy and immediate reconstruction (SORI) https://clinicaltrials.gov/ct2/show/NCT04108117
- 41.NIH. ClinicalTrials.gov Robotic nipple-sparing mastectomy vs conventional open technique. https://clinicaltrials.gov/ct2/show/NCT03440398
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper.