Abstract
Antimicrobial resistance (AMR) and persistence are associated with an elevated risk of treatment failure and relapsing infections. They are thus important drivers of increased morbidity and mortality rates resulting in growing healthcare costs. Antibiotic resistance is readily identifiable with standard microbiological assays, and the threat imposed by antibiotic resistance has been well recognized. Measures aiming to reduce resistance development and spreading of resistant bacteria are being enforced. However, the phenomenon of bacteria surviving antibiotic exposure despite being fully susceptible, so‐called antibiotic persistence, is still largely underestimated. In contrast to antibiotic resistance, antibiotic persistence is difficult to measure and therefore often missed, potentially leading to treatment failures. In this review, we focus on bacterial mechanisms allowing evasion of antibiotic killing and discuss their implications on human health. We describe the relationship between antibiotic persistence and bacterial heterogeneity and discuss recent studies that link bacterial persistence and tolerance with the evolution of antibiotic resistance. Finally, we review persister detection methods, novel strategies aiming at eradicating bacterial persisters and the latest advances in the development of new antibiotics.
Keywords: persistence, persistent infections, persisters, resistance, tolerance
Subject Categories: Microbiology, Virology & Host Pathogen Interaction; Molecular Biology of Disease
Bacteria that survive antibiotics challenges are one of the greatest threats to human health. While antibiotic resistance has been well studied, the survival and re‐growth of fully susceptible bacteria, called antibiotic persistence, is less well understood. This review discusses the mechanisms and implications of antibiotic resistance and persistence.

Glossary
- AACs
antibody–antibiotic conjugates
- ABDs
antibacterial drones
- AmpC
β‐lactamase
- AMPs
antimicrobial peptides
- AMR
antimicrobial resistance
- ATP
adenosine triphosphate
- blaZ
β‐lactamase
- C10
3‐[4‐(4‐methoxyphenyl)piperazin‐1‐yl] piperidin‐4‐yl biphenyl‐4‐carboxylate
- CA
community‐acquired
- CCCP
carbonyl cyanide m‐chlorophenyl hydrazine
- CDI
C. difficile infections
- CF
cystic fibrosis
- CFUs
colony‐forming units
- CIP
ciprofloxacin
- CRISPR
Clustered Regularly Interspaced Short Palindromic Repeats
- DNA
deoxyribonucleic acid
- dTMP
deoxythymidine monophosphate
- dUMP
deoxyuridine monophosphate
- ECDC
European Centre of Disease Prevention and Control
- EMBO
European Molecular Biology Organization
- ESBLs
extended‐spectrum β‐lactamases
- ESKAPE
E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa and Enterobacter species
- EU
European Union
- fabI/V
enoyl‐ACP reductase gene
- GltX
aminoacyl‐tRNA synthetase
- GyrA
DNA gyrase
- hip
high persister
- HR
heteroresistance
- IMP
imipenem
- KPC
K. pneumoniae carbapenamases
- MBC
minimal bactericidal concentration
- mcr
mobilized colistin resistance
- MDK
minimal duration of killing
- MDR
multidrug resistant
- MIC
minimum inhibitory concentration
- MLST
multilocus sequence type
- MRSA
methicillin‐resistant S. aureus
- NDM‐1
New Delhi metallo‐β‐lactamase 1
- nsSCs
non‐stable small colonies
- ParC
DNA topoisomerase IV
- PBP2a
penicillin binding protein 2a
- PJIs
prosthetic joint infections
- pmf
proton motive force
- QRDR
quinolone resistance determining region
- REPTIS
Replica Plating Tolerance Isolation System
- RNA
ribonucleic acid
- RND
resistance nodulation division
- ROS
reactive oxygen species
- RpoB
RNA polymerase beta‐subunit
- SaPIs
staphylococcal pathogenicity islands
- SCCmec
staphylococcal cassette chromosome mec
- SCVs
small colony variants
- TA
toxin/ antitoxin
- TCA
tricarboxylic acid
- TCS
two‐component systems
- TD
Tolerance Disk
- TMP‐SMX
trimethoprim‐sulfamethoxazole
- UN
United Nations
- UPEC
uropathogenic E. coli
- USA
United States of America
- UTIs
urinary tract infections
- UV
ultraviolet
- VRE
vancomycin‐resistant Enterococci
- VRSA
vancomycin‐resistant S. aureus
- WHO
World Health Organization
The antibiotic resistance crisis
Antibiotics have paved the way for today’s modern medicine. The mid‐20th century was even named the “antibiotic era”, and infectious diseases were believed to be eradicated by the end of the last century. Similarly, antibiotics have been fundamental for successful invasive and high‐end surgeries including organ transplantation, immunomodulatory treatments in rheumatology, oncology and many other medical disciplines (Ventola, 2015). Availability of antibiotic therapy has significantly reduced mortality in children resulting in increased life expectancy in general (Adedeji, 2016). Nevertheless, increasing numbers of bacteria are becoming resistant to multiple antibiotics currently in use resulting in multidrug‐resistant (MDR) bacteria (Tanwar et al, 2014). Sir Alexander Fleming, who was awarded the Nobel Prize in Medicine for discovering penicillin in 1945, warned about the potential risks of over‐ and misuse of antibiotics resulting in resistance development (Fleming, 1929; Aminov, 2010). Since new antimicrobial drugs are scarce and due to the increasing prevalence of MDR bacteria causing treatment failures, antibiotic‐resistant bacteria have become a major threat to modern health care (Spellberg et al, 2013).
Many governments together with the World Health Organization (WHO) and the United Nations (UN) have joined efforts to reduce and prevent resistance development, which is a fight against time. The latest report from the World Economic Forum 2019 in Davos, Switzerland, highlights the necessity of taking actions to fight the “rapid and massive spread of infectious diseases”, one of the greatest threats to human health (WorldEconomicForum, 2019). Infections by antibiotic‐resistant bacteria are responsible for around 700,000 deaths per year worldwide and estimated to be accountable for over 10 million deaths per year in 2050 (O’Neill, 2014; Aslam et al, 2018). Although AMR has been widely acknowledged as a pressing issue, the incidence of infections and spread of MDR bacteria is still rising. In addition, the increased use of implanted medical devices such as joint prostheses, artificial heart valves, vascular endoprostheses and pacemakers leads to a higher incidence of biofilm‐associated infections, which in turn leads to another important phenomenon: antibiotic tolerance (Davidson et al, 2019; Gollan et al, 2019). Antibiotic tolerance enables bacteria to survive antibiotic challenges even when they are fully susceptible in standard microbiological assays (Brauner et al, 2016). By using in vitro experiments and mathematical modelling, Balaban and co‐workers recently showed that tolerance can precede resistance and that tolerance mutations can lead to a rapid evolution of antibiotic resistance (Levin‐Reisman et al, 2017; Windels et al, 2019). In order to combat AMR, it is important to understand both antibiotic tolerance and resistance.
Antibiotic resistance mechanisms
Antibiotic resistance frequently results in delayed adequate antibiotic treatment, increasing morbidity and mortality. AMR is the inherited ability of microorganisms to grow at high antibiotic concentrations (Brauner et al, 2016). It is usually quantified by measuring the minimum inhibitory concentration (MIC) of a particular antibiotic wherein resistant bacteria are able to multiply and grow at concentrations of antibiotics, which are fatal to other strains of the same species. Antibiotic resistance comes in distinct flavours. On the one hand, there is natural intrinsic resistance due to lack or presence of certain structures resulting in ineffectiveness of antibiotics. On the other hand, bacteria can acquire resistance via mutations in chromosomal genes or via horizontal gene transfer of chromosomes or plasmids that lead to antibiotic resistance.
In Pseudomonas aeruginosa for example, antimicrobial substances such as triclosan, which inhibit fatty acid synthesis by targeting the enoyl‐ACP reductase, are ineffective, as the bacteria carry an insensitive homologue of fabI (enoyl‐ACP reductase), fabV, that encodes an enzyme that is not inhibited by triclosan (Zhu et al, 2010). In general, Gram‐negative bacteria are less permeable than Gram‐positive and show an intrinsic resistance to many antibacterial compounds such as the cell wall active glycopeptide vancomycin (Zgurskaya et al, 2015). These large molecules are unable to cross the outer bacterial membrane of Gram‐negative bacteria and thus cannot target the cell wall of Gram‐negative bacteria. In contrast, the Gram‐positive pathobiont Staphylococcus aureus is naturally susceptible to almost every antibiotic that has been developed, but it is well known for quickly acquiring antibiotic resistance by means of obtaining specific genetic modifications (mutations or horizontal gene transfer), causing infections that come in epidemic waves (Chambers & Deleo, 2009).
Several mechanisms can lead to resistance and have been investigated in detail. These molecular mechanisms are categorized in three main mechanistic groups of resistance: (i) reduction of intracellular antibiotic concentrations, (ii) modification of the antibiotic target and (iii) inactivation of the antibiotic (Blair et al, 2015).
Intracellular concentrations of antibiotics can be kept low via decreased permeability of the bacterial cell membrane, e.g. by reducing or mutating porins that would allow entry of antibiotics into the cell. This has been well described for several Gram‐negative pathogens such as carbapenem‐resistant Enterobacter species, Escherichia coli and Klebsiella pneumoniae that display reduced porin expression or mutated porins without expressing a carbapenemase (Wozniak et al, 2012; Baroud et al, 2013; Kong et al, 2018). Another way to decrease intracellular antibiotic concentrations is to increase the efflux of chemotherapeutics by using either substrate specific or MDR efflux pumps. Although many bacteria carry multiple genes that encode MDR efflux pumps on their chromosomes, some MDR efflux pump genes have been mobilized onto plasmids that can be transferred between bacteria (Lv et al, 2020). Genes coding for a novel tripartite resistance nodulation division (RND) pump were found to be carried on a plasmid together with genes encoding for the antibiotic‐targeting enzyme New Delhi metallo‐β‐lactamase 1 (NDM‐1) (Dolejska et al, 2013; Lv et al, 2020). This is especially worrying as it shows that different resistance mechanisms can be carried together on one plasmid and are thereby transmissible between bacteria, highlighting the importance of MDR efflux pumps as a major resistance mechanism. Many clinical isolates that overexpress drug efflux pumps, including P. aeruginosa and S. aureus, have been isolated from patients with systemic infections (Pumbwe & Piddock, 2000; Kosmidis et al, 2012).
Spontaneous mutations or post‐translational modifications of an antibiotic target molecule can lead to conformational changes that result in ineffective target binding and attenuation of antibiotic activity. Single amino acid changes in close proximity to the active site tyrosine of the bacterial type II topoisomerase enzymes, DNA gyrase GyrA or DNA topoisomerase IV ParC can cause ciprofloxacin resistance in S. aureus (Sreedharan et al, 1990; Hooper & Jacoby, 2015). Therefore, this domain has been named the quinolone resistance determining region (QRDR) (Yoshida et al, 1990). Although most of clinical methicillin‐resistant S. aureus (MRSA) isolates are susceptible to rifampicin (Moran et al, 2006), resistance development occurs rapidly if rifampicin is used as monotherapy. Point mutations in the gene encoding for the β‐subunit of the DNA‐dependent RNA polymerase (RpoB) cause decreased affinity for rifampicin and quickly confer resistance (Maranan et al, 1997).
Finally, resistance can be acquired by directly modifying antibiotics. Antibiotics can be inactivated by the transfer of chemical groups to vulnerable sites thus inhibiting target binding and function or directly destroyed by hydrolysis. Inactivation of antibiotics by addition of a chemical group, such as acyl, nucleotidyl, phosphate and ribitoyl groups, resulting in steric hindrance can cause antibiotic resistance. Especially, the large molecules of aminoglycosides with many exposed hydroxyl and amide groups are susceptible to such a inactivation mechanism. A genomic island found in Campylobacter coli isolated from chicken in China harbours genes for six aminoglycoside‐modifying enzymes and confers resistance to aminoglycosides, including gentamicin (Qin et al, 2012).
The development of new derivatives of already known antibiotic classes led to the evolution of a diverse range of enzymes that can degrade different antibiotics within the same class. For example, the early β‐lactamases were active against the 1st‐generation β‐lactams and were followed by extended‐spectrum β‐lactamases (ESBLs) that are able to hydrolyse extended‐spectrum oxyimino cephalosporins such as cefuroxime, cefotaxime, cefmenoxime and ceftriaxone (Johnson & Woodford, 2013). Together with the increasing numbers of bacteria carrying ESBL genes, the use of carbapenems has increased in the last two decades leading to the emergence of carbapenemase‐producing strains (Queenan & Bush, 2007). Carbapenemases can degrade a variety of β‐lactams, including extended‐spectrum cephalosporins, and many carbapenemase‐genes are now carried on plasmids and have been found in Enterobacteriaceae, K. pneumoniae, P. aeruginosa and Acinetobacter baumannii (Tzouvelekis et al, 2012) (Fig 1A).
Figure 1. Invasive bacterial isolates and resistance development over time in the European Union (EU) and European Economic Area (EEA).
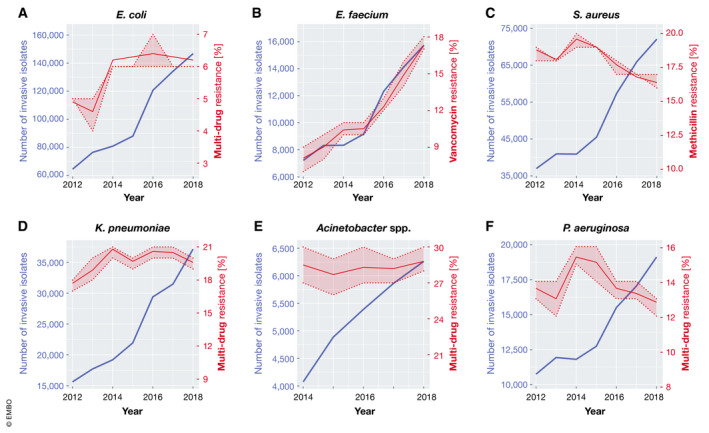
(A) Total number of invasive E. coli isolates tested and percentage with combined resistance to fluoroquinolones, 3rd‐generation cephalosporins and aminoglycosides, including 95% confidence intervals (95% CI, shaded area). (B) Total number of invasive E. faecium isolates tested and percentage with resistance to vancomycin, including 95% CI. (C) Total number of invasive S. aureus isolates tested and percentage with resistance to methicillin (MRSA), including 95% CI. (D) Total number of invasive K. pneumoniae isolates tested and percentage with combined resistance to fluoroquinolones, 3rd‐generation cephalosporins and aminoglycosides, including 95% CI. (E) Total number of invasive Acinetobacter spp., including most of the disease‐causing species (A. baumannii, A. pittii and A. nosocomialis) and the generally less pathogenic A. non‐baumannii group, isolates tested and percentage with combined resistance to fluoroquinolones, aminoglycosides and carbapenems, including 95% CI. (F) Total number of invasive P. aeruginosa isolates tested and percentage with combined resistance (resistance to three or more antimicrobial groups among piperacillin—tazobactam, ceftazidime, fluoroquinolones, aminoglycosides and carbapenems), including 95% CI. (A‐F) Data are derived from European Centre for Disease Prevention and Control’s yearly Surveillance of antimicrobial resistance in Europe (reports 2013‐2018 were used). ECDC collects data from invasive isolated reported to the European Antimicrobial Resistance Surveillance Network (EARS‐Net) by 30 EU and EEA (Iceland and Norway) countries.
ESKAPE pathogens
The acronym ESKAPE describes nosocomial pathogenic bacteria with growing levels of multidrug resistance and virulence. They place a significant burden on patient health, healthcare systems and economies. ESKAPE stands for the Gram‐positive bacterial species Enterococcus faecium and S. aureus and the Gram‐negative bacteria K. pneumoniae, A. baumannii, P. aeruginosa and Enterobacter species. They all commonly cause hospital‐acquired and potentially life‐threatening infections in critically ill and immunocompromised patients and are characterized by high levels of multidrug resistance (Rice, 2010). The WHO listed all ESKAPE pathogens among the twelve bacterial species against which new chemotherapeutics are desperately needed (Tacconelli et al, 2018).
E. faecium
The Gram‐positive commensal E. faecium has a low virulence but is difficult to eradicate from the hospital environment. Therefore and because of its potential to cause nosocomial infections and outbreaks, it is strongly feared in the healthcare system (Elsner et al, 2000). In the last decades, the incidence of drug‐resistant enterococcal infections increased, especially for vancomycin‐resistant Enterococci (VRE) reaching 14.9% in 2017 in the European Union (EU) (ECDC, 2018b) (Fig 1B) and 30% in the United States of America (USA) (CDC, 2019). Treatment of these nosocomial VRE infections is very difficult and sometimes impossible due to the resistance.
S. aureus
The Gram‐positive bacterium S. aureus is part of the normal skin microbiota, found especially in the nose. Carriage rates are high, with around 50% of the population intermittently or permanently colonized (Lowy, 1998). Nevertheless, S. aureus is a leading cause of infective endocarditis, osteomyelitis, skin and soft tissue infections as well as bacteraemia. Before the antibiotic era, mortality rates for S. aureus bacteraemia were dramatically high (over 80%) (Skinner & Keefer, 1941). With the introduction of penicillin in the 1940s, the prognosis of patients rapidly improved. Penicillin became the first choice of treatment for S. aureus infections, but extensive use quickly led to the evolution of β‐lactamases (blaZ)‐producing strains. Already in 1948, more than 80% of clinical isolates were resistant to penicillin G (Appelbaum, 2007; Brown & Ngeno, 2007; Wu et al, 2010). β‐lactamase, a primarily extracellular enzyme, hydrolyses the β‐lactam ring, rendering the β‐lactam inactive (Lacey, 1984). Already one year after the introduction of semi‐synthetic penicillins in 1961, MRSA strains were isolated and there is even evidence that S. aureus developed methicillin resistance even before its clinical use (Harkins et al, 2017; Turner et al, 2019). The widespread use of first‐generation β‐lactams, such as penicillin, prior to the introduction of methicillin, selected for strains carrying the mecA determinant that confers resistance to methicillin (Harkins et al, 2017). Nowadays, MRSA prevalence ranges from < 1 to more than 40% in Europe, with very low levels of invasive MRSA isolates in Scandinavian countries such as Iceland (0.0%) and Norway (0.9%), low levels, e.g. in the Netherlands (1.2%), in Switzerland (4.4%), in Germany (7.6%) and in France (12.1%) and up to 34% in Italy, 38% in Portugal and 43% in Romania (Hassoun et al, 2017; FOPH, 2018; ECDC, 2019) (Fig 1C). Methicillin resistance is mediated by the gene mecA that is spread via horizontal gene transfer of the mobile genetic element named staphylococcal cassette chromosome mec (SCCmec) (Katayama et al, 2000). The mecA gene encodes for a penicillin binding protein (PBP2a) that is responsible for crosslinking peptidoglycans during cell wall synthesis and the resistance arises through the low affinity of PBP2a to β‐lactams (Hartman & Tomasz, 1984) rendering them ineffective. One prominent pandemic and hypervirulent clonal lineage of community‐acquired (CA)‐MRSA is the multilocus sequence type (MLST) 8‐MRSA‐IV USA300 that started spreading in the USA 20 years ago and is a significant health threat and economic factor nowadays (Tenover & Goering, 2009).
Colonization with MRSA increases the MRSA infection risk, as 50–80% of isolated invasive strains were found to originate from colonizing strains as determined by molecular typing methods including whole genome sequencing (Benoit et al, 2018; Thomsen et al, 2019). It is a dynamic process, with strains persisting for long periods of time and others evolving or being replaced by another strain within the same host (Azarian et al, 2016). In addition, transmission of S. aureus commonly occurs via hospital coats, mobile phones and tablets, regularly used in clinics (Frey et al, 2019; Turner et al, 2019). In clinics, glycopeptide antibiotics such as vancomycin and teicoplanin, are used nowadays as first‐line treatment for MRSA infections. Shortly after the initiation of vancomycin therapy in the 1980s, MRSA strains with reduced sensitivity to teicoplanin appeared in Europe (Kaatz et al, 1990). In 2002, the first vancomycin‐resistant S. aureus (VRSA) was detected in the USA (Chang et al, 2003) and eleven years later in Europe (Melo‐Cristino et al, 2013). Effective alternatives to vancomycin include daptomycin, a lipopeptide bactericidal antibiotic, linezolid, tigecycline, trimethoprim–sulfamethoxazole (TMP‐SMX) or a 5th‐generation β‐lactam (e.g. ceftaroline or ceftobiprole). Similarly, if a reduced daptomycin susceptibility is detected together with a reduced vancomycin susceptibility, a combination or single use of the following is recommended; the streptogramin antibiotics quinupristin–dalfopristin, TMP‐SMX, linezolid or telavancin (Liu et al, 2011).
K. pneumoniae
K. pneumoniae is a Gram‐negative rod‐shaped pathogen that is often associated with nosocomial infections. Many K. pneumoniae strains have acquired a variety of β‐lactamases against penicillin, cephalosporins and carbapenems. Carbapenems are frequently used to treat infections with Gram‐negative bacteria, but the increase of K. pneumoniae carbapenamases (KPC)‐producing strains, carrying bla KPC, renders such infections more and more difficult to treat (Reyes et al, 2019) (Fig 1D). Additionally, some K.pneumoniae strains express the metallo‐β‐lactamase NDM‐1, encoded by bla NDM‐1 (Yong et al, 2009). The presence of NDM‐1 has increased the prevalence of carbapenem‐resistant K. pneumoniae strains, which has made the use of other antibiotics such as aminoglycosides and fluoroquinolones increasingly necessary. However, the more frequent use of these antibiotics may increase resistance against these drugs as well, making it even more difficult to treat these infections (Kumarasamy et al, 2010).
A. baumannii
The Gram‐negative bacillus A. baumannii is an opportunistic pathogen associated with nosocomial infections of primarily immunocompromised patients who have stayed in a hospital for more than 90 days (Montefour et al, 2008). A. baumannii causes a variety of infections including respiratory and urinary tract infections. Due to the high prevalence of antibiotic resistances, A. baumannii poses a significant threat to patients especially in intensive care units (Fig 1E). In addition, Carbapenem‐resistant A. baumannii strains carrying imipenem metallo‐β‐lactamases (bla IMP) and oxacillinase serine β‐lactamases (bla OXA) have been isolated. The combination of resistance genes enables them to evade the antimicrobial action of most conventional antibiotics (Vila et al, 2007; Boucher et al, 2009). Because of the increasing incidence of multidrug‐resistant A. baumannii strains globally, the WHO classified A. baumannii as critical (in the priority group 1) for the development of new antimicrobial agents (WHO, 2017).
P. aeruginosa
The Gram‐negative bacterium P. aeruginosa, also listed in the WHO priority group 1 of pathogens that urgently require new treatment options, intrinsically shows low susceptibility to many antimicrobial drugs and a high propensity to develop resistance. Reduced porin permeability together with AmpC overproduction lead to increased carbapenem resistance in P. aeruginosa (Fukuoka et al, 1993). In addition, P. aeruginosa can express KPCs, ESBLs and imipenem metallo‐β‐lactamases, resulting in high‐level carbapenem resistance (Fig 1F). The increasing prevalence of multidrug‐resistant isolates is challenging for successful treatments, and although the last‐resort antibiotic colistin is still effective in most cases, resistance was already reported due to previous widespread use of colistin in pig and poultry farming (Fukuoka et al, 1993; Pedersen et al, 2018; Hameed et al, 2019).
Enterobacter species
Enterobacter spp. are common Gram‐negative pathogens usually causing infections in immunocompromised, hospitalized patients and are well known for harbouring many antibiotic resistance genes, like ESBLs and carbapenamases. Enterobacter species show hyperproduction of AmpC β‐lactamase that can hydrolyse broad‐spectrum penicillins and cephalosporins (Schwaber et al, 2003). This overproduction is induced by β‐lactams and carbapenems leading to the occurrence of resistance during the course of the treatment and is associated with increased mortality rates (Schwaber et al, 2003; Huh et al, 2014). Due to this ability to rapidly develop resistances, many Enterobacter spp. strains are only susceptible to a few last‐resort antibiotics such as colistin and tigecycline (Pendleton et al, 2013). Recently, even resistance to colistin, either due to a chromosomal mutation in genes of two‐component systems (TCS) such as pmrA/B and phoP/Q and their regulators mgrB and pmrD or via plasmid‐mediated mobilized colistin resistance (mcr) genes, as well as to tigecycline have been reported, highlighting the urgent need for new antimicrobial drugs (Pournaras et al, 2016; Hong et al, 2018; Berglund, 2019).
Besides the ESKAPE pathogens, the Gram‐positive and spore‐forming bacterium Clostridioides difficile (until 2016: Clostridium difficile) is an emerging human pathogen with increasing resistance towards metronidazole (Dingsdag & Hunter, 2018). The European Centre of Disease Prevention and Control (ECDC) started in 2016 to monitor C. difficile infections (CDIs) and reported metronidazole resistance for 26/569 (4.6%) cases (ECDC, 2018a). The emergence of metronidazole resistance is of specific concern, since a new plasmid (pCD‐METRO) conferring metronidazole resistance has been found, occurring in both animal and human isolates of C. difficile (Boekhoud et al, 2020). CDIs should be carefully monitored worldwide, and the blind use of broad‐spectrum antibiotics has to be avoided, which should result in decreasing the incidence and spread of drug‐resistant C. difficile.
There are many excellent reviews already published focusing on antibiotic resistance (Turnidge & Christiansen, 2005; Spellberg et al, 2013; Blair et al, 2015; Foster, 2017; Rather et al, 2017; Zaman et al, 2017; Aslam et al, 2018; Jorge et al, 2019; Lopez‐Jacome et al, 2019). We would like to put our further focus on difficult‐to‐treat infections, phenotypic heterogeneity, antibiotic tolerance and persistence. We will discuss how they can cause treatment complications and future strategies to fight recurrent bacterial infections.
Antibiotic persistence—research then and today
Already at the beginning of the antibiotic era in 1942, Hobby and colleagues described a small subpopulation (< 1%) of S. aureus that survived treatment with penicillin despite being susceptible to the drug (Hobby et al, 1942). J. Bigger confirmed this finding two years later and named this newly discovered phenotype “antibiotic persistence” (Bigger, 1944). J. Bigger did not only show that S. aureus could survive penicillin treatment, but also that the surviving population displayed the heterogeneous survival phenotype of a rapidly dying population and a surviving subpopulation. The fact that the survivors did not grow under penicillin pressure clearly separated the phenomenon of “antibiotic persistence” from “antibiotic resistance”. The rise of antibiotic resistance caught much more attention, and the novel phenomenon of antibiotic persistence was almost forgotten for the next 39 years. After the discovery of the E. coli high‐persistence mutant hipA7 in 1983 by Moyed and Bertrand (Moyed & Bertrand, 1983), the scientific community refocused on the phenomenon of susceptible bacteria surviving antibiotic challenges. In the last 30 years, many mechanisms and characteristics underlying bacterial persistence have been revealed. However, research mainly focused on Gram‐negative bacteria, e.g. E. coli, P. aeruginosa and S. enterica ser. Typhimurium, and little is known about the mechanisms and influence of bacterial persistence in Gram‐positive bacteria especially S. aureus and coagulase‐negative staphylococci (S. epidermidis), which are often associated with chronic and recurrent, biofilm‐associated infections. The group of Kim Lewis was the first to link bacterial persistence to biofilms in P. aeruginosa (Brooun et al, 2000; Spoering & Lewis, 2001). Chronic infections are associated with bacterial biofilms, and the newly linked phenomenon of antibiotic persistence added a novel point of view to recurring and difficult‐to‐treat infections and attracted the interest of more and more scientists worldwide (Bjarnsholt, 2013; Lebeaux et al, 2014). In 2018 at the European Molecular Biology Organization (EMBO) Workshop “Bacterial Persistence and Antimicrobial Therapy”, 121 scientists studying antibiotic persistence and tolerance came together in Ascona, Switzerland, and laid the foundations for a “Consensus Statement” on the definitions and guidelines for research on antibiotic tolerance (Balaban et al, 2019a; Balaban et al, 2019b). Here, we follow the definitions published in the consensus statement (Balaban et al, 2019a).
Antibiotic persistence or heterotolerance is defined as the ability of a bacterial subpopulation to survive high bactericidal drug concentrations to which the bacteria are fully susceptible (no change in MIC). One major characteristic of antibiotic persistence is a biphasic killing, with a rapid killing of the susceptible population and a slower killing of the persister subpopulation. This phenomenon highlights the phenotypic heterogeneity in a bacterial culture and that not all bacteria in a clonal population are killed at the same rate. In contrast to antibiotic resistance and heteroresistance, where a small subpopulation transiently displays a higher MIC, persistent bacteria are not able to grow in the presence of antibiotics. Antibiotic persistence and tolerance are often used interchangeably, and both describe an increased survival in the presence of an antibiotic without a change in the MIC. However, persistence characterizes a bacterial subpopulation, whereas tolerance describes the ability of an entire bacterial population to survive antibiotic exposure. Thus, antibiotic persistence is a subcategory of tolerance, in which a small subpopulation of persisters tolerates antibiotic exposure, reflected by a biphasic killing curve.
Genetic basis of antibiotic persistence
In 1983, Moyed and Bertrand isolated mutants of E. coli that were able to form high amounts of persister cells, and seven years later, mutagenesis experiments lead to the isolation of the high persister (hip) mutant (Moyed & Bertrand, 1983; Wolfson et al, 1990). The hipA7 allele enhanced antibiotic persistence in E. coli K‐12 almost 1,000‐fold and gave the first evidence that antibiotic persistence levels can be increased by an inheritable mutation. The hipA gene encodes a eukaryotic‐like Ser/Thr kinase that inhibits translation via phosphorylation of aminoacyl‐tRNA synthetase GltX at its active site (Schumacher et al, 2009; Hansen et al, 2012; Germain et al, 2013). Inactivation of GltX leads to the formation of hungry codons, i.e. their cognate aminoacyl‐tRNAs are in short supply. This induces the production of the stringent response alarmone (p)ppGpp that inhibits DNA replication, and therefore, blockage of translation can indirectly result in additional tolerance towards fluoroquinolones (Schreiber et al, 1995; Korch & Hill, 2006; Kaspy et al, 2013; Wilmaerts et al, 2019). Next to hipA, hipB encodes an auto‐repressor of hipBA transcription (Black et al, 1991; Black et al, 1994). HipB directly inhibits HipA, which suggested that hipBA constitutes a toxin/ antitoxin (TA) locus (Korch et al, 2003). Today, numerous publications support the model of TA loci cumulatively regulating antibiotic persistence in E. coli (Vazquez‐Laslop et al, 2006; Dorr et al, 2010; Page & Peti, 2016).
Toxin/ Antitoxin modules and persistence
TA modules are composed of a stable toxin (always a protein) that intoxicates the bacterial cell by inhibiting essential processes, e.g. translation, transcription and DNA replication, and a labile antitoxin (either RNA or protein) that neutralizes the corresponding toxin (Harms et al, 2018). Up to now, there are six described classes of TA modules, defined by their neutralization mechanisms. Type I antitoxins are antisense RNAs that inhibit the translation of the mRNA of their corresponding toxins, therefore inhibiting toxin production (Thisted et al, 1994). In the largest and best studied group of TA modules, the type II TA systems, antitoxins are proteins that directly bind their cognate toxins thereby neutralizing the toxin (Pandey & Gerdes, 2005). Additionally, the antitoxin can be a toxin‐binding sRNA (type III) (Brantl & Jahn, 2015), which protects the toxin targets via binding to and directly inhibiting the toxin, instead of counteracting the toxins’ effect (type IV) (Masuda et al, 2012). The antitoxin can also be an endoribonuclease that degrades the toxins’ mRNA (type V) (Wang et al, 2012) or a proteolytic adapter of the toxin (type VI) (Aakre et al, 2013). Often, TA modules are described as stress‐responsive elements that are activated under specific stress conditions (Ronneau & Helaine, 2019). As described before, the type II TA system toxin HipA7 can induce antibiotic persistence by phosphorylation of its target GltX in E. coli (Moyed & Bertrand, 1983). Therefore, TA modules were proposed to be perfect candidates to trigger antibiotic persistence.
Environmental triggers of antibiotic persistence
Many environmental factors, such as nutrient limitation, antibiotic exposure, acidic pH, high cell numbers, heat shock and oxidative stress, have been shown to increase persister levels in an isogenic bacterial population (Fig. 2) (Dorr et al, 2010; Moker et al, 2010; Nguyen et al, 2011; Leung & Levesque, 2012; Vega et al, 2012; Amato et al, 2013; Bernier et al, 2013; Vulin et al, 2018; Rowe et al, 2020). The stringent response reprograms bacteria from rapid growth to metabolic homeostasis and is triggered under nutrient limitation. Mediated through the alarmone (p)ppGpp, found in almost all bacterial species, the stringent response plays a crucial role in triggering antibiotic persistence. In E. coli, (p)ppGpp is produced either by the synthetase RelA that is activated by a lack of amino acids and heat shock or by the bifunctional synthetase/ hydrolase SpoT during carbon, nitrogen, iron, phosphate and fatty acid starvation (Irving & Corrigan, 2018; Ronneau & Hallez, 2019). Accumulation of (p)ppGpp has been shown to upregulate or activate TA systems during stringent response. For example, isoleucine starvation of E. coli increases (p)ppGpp levels and leads to an upregulation of many type II TA modules, like MazEF, HicAB, RelBE and MqsRA as well as the type I toxin HokB (Fig. 2) (Traxler et al, 2008; Verstraeten et al, 2015; Shan et al, 2017; LeRoux et al, 2020). Mutations that affect (p)ppGpp synthesis significantly reduce persister levels in both Gram‐positive and Gram‐negative bacteria, indicating the prominent role and connections of stringent response with bacterial stress response and antibiotic persistence (Amato et al, 2013; Amato et al, 2014).
Figure 2. Environmental triggers of antibiotic persistence and bacterial response mechanisms.
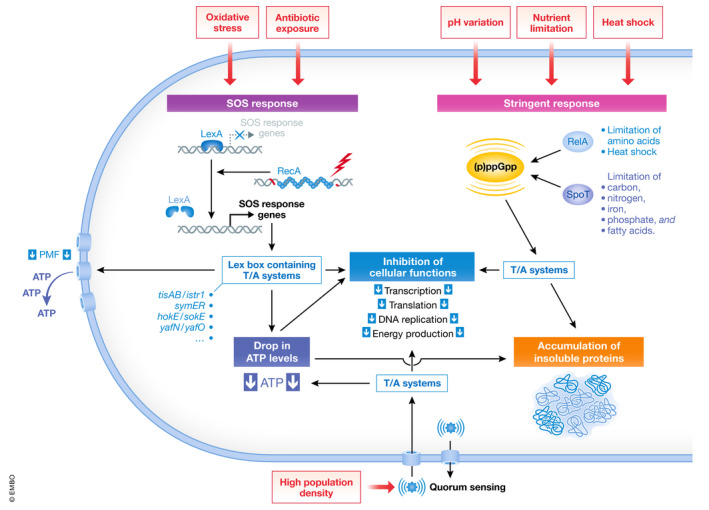
Environmental stressors such as oxidative stress and antibiotic exposure can trigger the bacterial SOS response via induction of RecA expression and the upregulation of the Lex box containing TA systems leading to a drop in ATP levels and a downregulation of essential cellular functions, e.g. transcription, translation, DNA replication and energy production. Other stressors such as pH changes, heat shock and starvation trigger the stringent response via production of (p)ppGpp, mainly by SpoT and RelA, and activation of toxin/antitoxin systems leading to the inhibition of essential cellular functions. Low intracellular ATP levels can favour the accumulation of insoluble proteins, a feature linked to increased antibiotic tolerance and dormancy. Additionally, high population densities can inhibit cellular functions via quorum sensing and T/A systems. External triggers/signals are shown in red boxes. Abbreviations: T/A, antitoxin/toxin; PMF, proton motive force.
DNA damage is caused by many factors including oxidative stress, UV light exposure and drugs and usually leads to the induction of the SOS response (Dorr et al, 2010; Baharoglu & Mazel, 2014). The SOS response not only activates DNA repair mechanism, but also upregulates the Lex box containing TA systems such as tisAB/istr1, symER, hokE/sokE and yafN/yafO (Pedersen & Gerdes, 1999; Fernandez De Henestrosa et al, 2000; Vogel et al, 2004; Kawano et al, 2007). The type I toxin TisB, a small 29 amino acid hydrophobic peptide, for example, binds to the bacterial cell membrane and disrupts the proton motive force (pmf), leading to a decrease in intracellular adenosine triphosphate (ATP) levels and a multidrug tolerant phenotype (Fig. 2) (Unoson & Wagner, 2008; Lewis, 2012). A drop in ATP levels has been associated with antibiotic tolerance in E. coli (Shan et al, 2017) and S. aureus (Conlon et al, 2016). In both studies, treatment of exponentially growing bacteria with arsenate, a drug known to inhibit ATP synthesis, resulted in elevated levels of antibiotic tolerance. Similarly, pretreatment with the metabolic poison carbonyl cyanide m‐chlorophenyl hydrazine (CCCP), an uncoupling agent that inhibits ATP synthesis, resulted in increased persister levels (Kwan et al, 2013). Congruently with stringent response‐mediated persistence, inhibition of translation by pretreatment with the antibiotic tetracycline yielded an increased fraction of persisters (Kwan et al, 2013). Recently, Conlon and co‐workers showed that inactivation of the tricarboxylic acid (TCA) cycle enzymes aconitase and succinate dehydrogenase by reactive oxygen species (ROS) produced by human macrophages during infection, decreased ATP levels and increased persister levels in S. aureus, further strengthening the link between reduced energy levels and antibiotic tolerance (Rowe et al, 2020).
Depletion or reduction of intracellular ATP levels could therefore be a general mechanism of downregulating central bacterial processes, such as transcription, translation and peptidoglycan synthesis, thereby rendering antibiotics ineffective as their respective targets are not active (Fig. 2).
In addition, accumulation of insoluble endogenous proteins in deep‐stationary phase, starved E. coli, promoted by decreased levels of ATP, has been linked to dormancy depth and antibiotic tolerance (Fig. 2) (Pu et al, 2019). Pu and co‐workers showed that the dormancy depth that correlates with antibiotic tolerance is linked to the formation of protein aggresomes. Resuscitation of these dormant cells leads to degradation of protein aggregates and requires DnaK and ClpB activity. The chaperone DnaK was previously identified to be involved in bacterial antibiotic persistence by analysing an E. coli mutant library further strengthening the involvement of protein aggregation in antibiotic persistence (Hansen et al, 2008).
Chronic and difficult‐to‐treat infections
There is a fundamental difference between the terms “persistent infection” and “antibiotic persistence”. Persistent infections are ongoing infections, which are not cleared by the host, whereas antibiotic persistence is used to describe a bacterial population that survives antibiotic exposure without being resistant (Balaban et al, 2019a). Nevertheless, antibiotic persistence is thought to be involved in persistent infections, potentially impeding successful treatment (Fauvart et al, 2011). Persistent infections are also known as chronic infections and are often associated with antibiotic treatment failure (Rhen et al, 2003; Fisher et al, 2017). These infections are not sufficiently cleared by the hosts' immune system. Many different factors, such as cell wall modification, capsule production, antigenic mimicry and inhibition of effector proteins, contribute to bacterial immune evasion (Hornef et al, 2002). Usually, the bacterial cell wall is the first target of the hosts’ immune system and the first line of defence of the bacteria. For example, many Gram‐negative pathogens such as E. coli and S. enterica acetylate lipid A molecules in their cell walls, which switches their negative surface charge to positive, repelling positively charged antimicrobial peptides (AMPs) from the host (Li et al, 2012). Others, like Borrelia spp. and Neisseria gonorrhoea, vary their surface antigens during infection and thus evade the hosts’ humoral immune response (Nataro et al, 2000; Norris, 2006). Some pathogens such as Listeria monocytogenes and Mycobacterium tuberculosis can trigger an anti‐inflammatory response by inducing the production of interleukin‐10 that suppresses interferon‐γ production by macrophages, which in general would inhibit bacterial growth (Redpath et al, 2001).
Another way in which bacteria evade host recognition is by forming biofilms. Secreted polysaccharides and extracellular DNA form a protective matrix that blocks complement‐killing (Domenech et al, 2013), recognition by immune cells (Leid et al, 2002; Jesaitis et al, 2003; Vuong et al, 2004), killing by many antibiotics (Jolivet‐Gougeon & Bonnaure‐Mallet, 2014) and contains persister cells (Spoering & Lewis, 2001; Kamble Ekta, 2020). The increasing age of the human population results in more tissue‐related biofilm‐associated infections such as osteomyelitis and endocarditis, but also in more foreign body‐associated biofilm infections due to the constantly ‐rising numbers of pacemaker and orthopaedic device implants. Biofilm formation has been linked to persistent infections, especially to difficult‐to‐treat foreign body infections such as pacemaker‐related infections and prosthetic joint infections (PJIs) (Jansen & Peters, 1993; Costerton et al, 1999; Dengler Haunreiter et al, 2019).
PJIs are often caused by S. aureus or coagulase‐negative staphylococci (e.g. S. epidermidis) and require prolonged antibiotic treatment and often surgical removal of the infected device (Tande & Patel, 2014). The majority of PJIs occur in the first year after implantation and are typically caused by commensal microorganisms that were inoculated during surgery (Del Pozo & Patel, 2009). Either physical contact or droplets lead to the contamination of the prosthesis surface and even very low inocula (< 102 CFUs for S. aureus) result in infection (Southwood et al, 1985). PJIs manifesting later than 2 years after surgery are typically caused by haematogenous spread during bacteraemia. A study including 14,378 patients with a primary knee or hip replacement showed that 3.8% (542/14,378) had at least one significant bacteremic episode and 8.3% (45/542) developed a PJI during bacteraemia. The most common cause of PJI was S. aureus, with 21% PJIs resulting from bacteraemia (Honkanen et al, 2019). Many studies investigating PJI caused by S. aureus and other staphylococcal species found small colony variants (SCVs) (Kahl et al, 2016). Proctor et al were the first to describe the occurrence of SCVs in a patient suffering from MRSA bacteraemia with a hip prosthesis infection (Proctor et al, 1995). However, SCVs are not only found in patients suffering from PJI, but there are many studies reporting SCVs in other persistent biofilm‐associated infections. Among these difficult‐to‐treat infections are pacemaker‐related infections (Baddour et al, 1990; von Eiff et al, 1999), endocarditis (Quie, 1969; Bhattacharyya et al, 2012), osteomyelitis (Proctor et al, 1995), airway infections (Kahl et al, 1998; Besier et al, 2007; Schneider et al, 2008) and skin and soft tissue infections (Abele‐Horn et al, 2000; Vulin et al, 2018).
Colony size heterogeneity in persistent and difficult‐to‐treat infections
Even before the introduction of antibiotics and the first description of bacterial persistence, SCVs were reported for S. aureus and several other coagulase‐negative staphylococci (Proctor et al, 2006). Similar to bacterial persisters, SCVs have been associated with recurrent and chronic infections (Proctor et al, 2006) and have been isolated from many different clinical specimens, e.g. abscesses and soft tissue, bones and joints, the respiratory tract and blood (Swingle, 1935; Goudie & Goudie, 1955; von Eiff et al, 1997a; Kahl et al, 1998; Seifert et al, 1999; von Eiff et al, 1999; Rolauffs et al, 2002).
SCVs are characterized by their small colony size, reduced virulence and slow growth. Most studies were done on genetically determined stable SCVs that display a small colony size because of genetic mutations (e.g. in hemB (von Eiff et al, 1997b), hemH (Schaaff et al, 2003), menD (Bates et al, 2003) or ctaA (Clements et al, 1999)), which either lead to an altered electron transport or defect thymidine metabolism (Proctor et al, 2006; Proctor, 2019) (Fig. 3A). Defective hemin or menadione synthesis causes a reduced electron transport and therefore decreased ATP levels. Low energy levels and reduced membrane potential lead to a slower growth rate of the bacteria and finally to smaller colonies. This defect can be overcome by supplementing with hemin or menadione leading to normal growing colonies. The second group of stable SCVs caused by a thymidine biosynthesis defect has been isolated mainly from cystic fibrosis (CF) patients that received a TMP‐SMX treatment (Gilligan et al, 1987; Kahl et al, 1998). Kahl and co‐workers showed that a thymidine‐auxotrophic clinical S. aureus could be complemented with thyA that encodes for a thymidylate synthase, enabling synthesis of dTMP from dUMP (Chatterjee et al, 2008; Kriegeskorte et al, 2014).
Figure 3. Characterization of colony radius heterogeneity and single‐cell growth dynamics of bacteria.
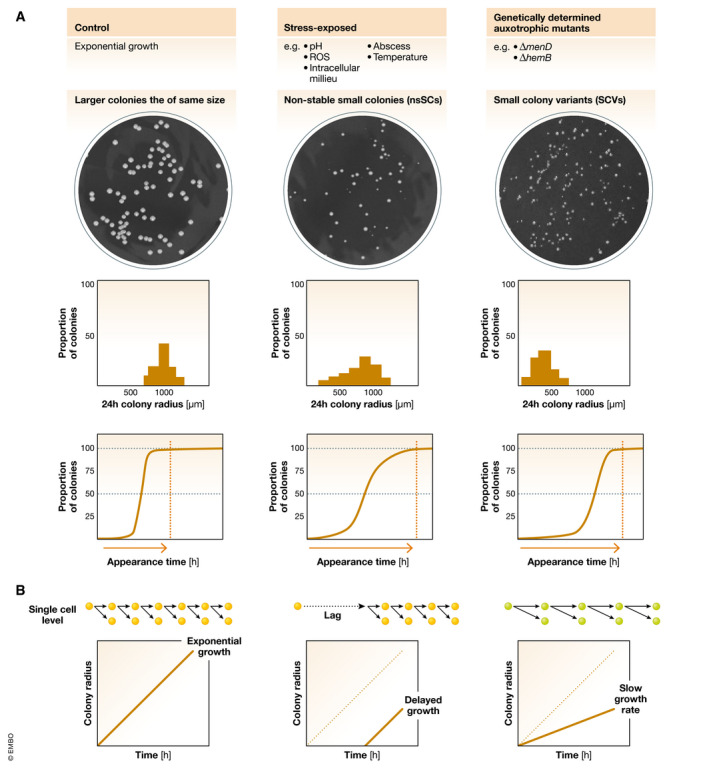
(A) Stress‐exposed S. aureus and genetically determined auxotrophic mutants show small colony phenotypes. Top: The stress‐exposed bacteria display colony radius heterogeneity on agar plates by forming non‐stable small colonies (nsSCs), whereas the auxotrophic mutants grow as genetically determined small colony variants (SCVs). Middle: The colony radius histograms for stress‐triggered bacteria show a broad distribution towards smaller colony sizes compared to exponentially growing bacteria (most colonies have the same size) and SCVs (most colonies are small and the same size). Bottom: Translated into colony appearance times, the exponentially growing bacteria almost all appear at the same time early after plating, whereas the stress‐exposed bacteria show a broad lag time distribution with some colonies appearing early and others later. In contrast, SCVs all appear later after a longer incubation time, but almost all at the same time. (B) The schemes show bacterial growth dynamics on a single‐cell level as well as different scenarios explaining the colony size at a certain time point. Short arrows indicate a fast division rate of wild type bacteria (yellow), whereas longer arrows represent a slower growth rate for SCV forming mutants (green). Colonies can be small because of a late onset of growth (lag time) or because of a slower growth rate.
However, most small colonies recovered in vitro, directly from patient material in clinical microbiology laboratories or from murine infection models revert to a normal colony size upon subcultivation (Tuchscherr et al, 2011; Vulin et al, 2018). Consequently, these small colonies are named non‐stable small colonies (nsSCs) (Fig 3A). Usually, these small colonies occur at a very low frequency in a bacterial population, but increase after stress exposure, e.g. acidic pH (Leimer et al, 2016; Vulin et al, 2018), exposure to an intracellular milieu in the host (Coffey & De Duve, 1968; Vesga et al, 1996; Tranchemontagne et al, 2016) or reactive oxygen species (Painter et al, 2015; Loss et al, 2019). NsSCs share many characteristics with bacterial persisters: (i) they both occur at a low frequency in a bacterial population; (ii) they display a revertible phenotype; and (iii) they show an increased tolerance towards antibiotics. A clear and meaningful definition of these small colonies is still missing. Usually, nsSCs are defined as colonies with an area 5 or 10 times smaller than the area of the most common colony type (Proctor et al, 2006). Instead of using a binary classification in “small” and “normal” colonies, more and more focus is put on phenotypic heterogeneity and colony size distributions that give a detailed overview on all recovered colony phenotypes.
Phenotypic heterogeneity can be beneficial for the survival of a bacterial population (Ackermann, 2015). Bacterial bet hedging via the formation of a subpopulation of persister cells in a clonal population of bacteria might be a great advantage in clinical infection settings. While the actively growing part of a clonal bacterial population is mostly responsible for host colonization and the successful establishment of an infection, the persister cell subpopulation ensures the survival of the genotype under changing environments, e.g. under antibiotic therapy (Claudi et al, 2014). Nevertheless, formation of these persisters is costly as they do not contribute to the growth of the bacterial population but serve as a back‐up plan in case of emergencies. Persister cells form in two ways, either spontaneously or triggered by environmental factors (Balaban et al, 2019a). The spontaneous formation of a small group of dormant bacterial cells in an overall growing population was previously described as type II‐persistence (Balaban et al, 2004). This spontaneous persistence leads to a constant fraction of persisters in a population (usually between 0.001% and 1%), ensuring bacterial survival in the presence of antibiotics, but seems to be less common than triggered persistence (previously type I‐persistence) (Balaban et al, 2019a; Balaban et al, 2004; Van den Bergh et al, 2017). Many factors have been reported to trigger antibiotic persistence in bacteria, including nutrient limitation (Gutierrez et al, 2017), high cell density (Vega et al, 2012), oxidative stress (Wu et al, 2012; Rowe et al, 2020), an acidic environment (Lewis, 2007; Vulin et al, 2018), immune factors (Manina et al, 2015) and exposure to immune cells (Helaine et al, 2014).
Phenotypic heterogeneity is a complex quality that cannot be detected when analysing a single bacterial cell at a certain time point. It needs the comparison to multiple other bacterial cells in a population or at least multiple observations of a single bacterium over time (Ackermann, 2015). In case of bacterial growth analysis and determination of lag phase, Balaban and co‐workers developed a method for E. coli to monitor the growth of many bacterial colonies and lag times in parallel, the so‐called ScanLag method (Levin‐Reisman et al, 2010; Levin‐Reisman et al, 2014). ScanLag generates a two‐dimensional distribution of the lag time and of the growth time of colonies on agar plates. We recently modified the ScanLag setup for the analysis of S. aureus lag time distributions after direct sampling from infection sites and in vitro stress exposure, named ColTapp (Vulin et al, 2018; Bär et al, 2020). Automated agar plate imaging as well as single‐cell microscopy revealed that the small colonies are the result of a prolonged bacterial lag phase and can be linked to an increased antibiotic persistence in S. aureus (Fig 3B). Therefore, these small colonies reflect a subpopulation of persisters and might serve as a read out for antibiotic persistence in clinical laboratories.
Antimicrobial heteroresistance
Another bacterial bet hedging strategy to survive antibiotic treatment is heteroresistance (HR). In contrast to persistence, HR describes the ability of a subpopulation of susceptible bacteria to grow despite antibiotic pressure. The exact mechanisms underlying HR are still unclear, but there is evidence that unstable amplification of antibiotic resistance genes is one way bacteria become heteroresistant (Hjort et al, 2016; Anderson et al, 2018; Nicoloff et al, 2019). In order to survive antibiotic challenges and to grow even in presence of antibiotics, the heteroresistant phenotype must be transferred to daughter cells to ensure growth. Antibiotic resistance gene amplification as the reason for HR can explain how this is achieved, at least for genetic HR. It is still not clear though how phenotypic HR that is not based on any genetic traits, is propagated in a population under antibiotic treatment. One possible explanation for the maintenance of the heteroresistant phenotype by bacteria could be epigenetic inheritance. There are several studies describing epigenetic trait propagation (inheritance) of phenotypic variations over generations (Veening et al, 2008; Ni et al, 2012; Ram & Goulian, 2013). This heteroresistant phenotype is not maintained indefinitely but is lost as soon as the antibiotic pressure is removed, resulting in a population consisting of antibiotic‐susceptible bacteria and a small fraction of heteroresistant bacteria creating the initial phenotypic heterogeneity.
The clinical relevance of heteroresistance and effect on treatment outcome is still under debate. Vancomycin HR in S. aureus has been suggested to cause vancomycin treatment failure (Hiramatsu et al, 1997; Moore et al, 2003), but there is also evidence that vancomycin treatment is still effective for treating vancomycin heteroresistant strains, depending on the antibiotic concentrations used (Khatib et al, 2011). Vancomycin heteroresistance might not be the only explanation for vancomycin treatment failure. In addition, intracellularly residing S. aureus (Dziewanowska et al, 1999; Jevon et al, 1999; Fraunholz & Sinha, 2012; Lehar et al, 2015) are protected from vancomycin and may be another reason for vancomycin treatment failure. Another study describes colistin HR in Enterobacter cloacae, where a subpopulation (1–10% of the entire population) displayed 1,000‐fold increased resistance to colistin (Napier et al, 2014). A murine infection model demonstrated that colistin therapy failed to successfully treat an infection with a heteroresistant strain of E. cloacae, whereas infection with a fully susceptible strain could be treated with colistin and the mice survived, highlighting the importance of HR in infection settings and the adverse effect on treatment outcome (Band et al, 2016).
Detection of antibiotic tolerance and persistence in the clinical setting
In the clinical microbiology laboratory, the options to test for antibiotic persistence and tolerance are limited and typically focus on the detection of antibiotic resistance. Additionally, prevalence and presence of bacterial persisters and their clinical relevance in infection per se are not well understood. One reason for this very limited testing for tolerance in clinical microbiology laboratories is that there is no quick and accurate testing protocol available so far. For resistance testing, the Kirby Bauer disc diffusion assay (Bauer et al, 1966) and the E‐test (Joyce et al, 1992) are used to measure increased MIC levels. These tests cannot be used for tolerance testing, as antibiotic tolerance is defined as the ability of a bacterial population to survive antibiotic exposure without any changes in the MIC, meaning without resistance.
Antibiotic tolerance can be determined by performing killing assays in form of time–kill curves that are characterized by a bimodal killing in the case of antibiotic persistence, but this is very labour‐intensive and shows high variability, which makes many repetitions necessary and undermines feasibility in routine clinical microbiology laboratories (Fig 4A). In addition, the levels of antibiotic persistence observed and evaluated in laboratories under in vitro conditions might not correspond to the levels of persistence occurring in the patient from whom the strain was isolated considering the complexities that contribute to this unstable phenomenon. Therefore, measurements of antibiotic persistence under laboratory conditions are only approximations of bacterial phenotypes of infections in vivo. Yet, they provide important additional information that might help physicians in their therapeutic decision‐making.
Figure 4. Different detection methods of antibiotic tolerance and persister cells.
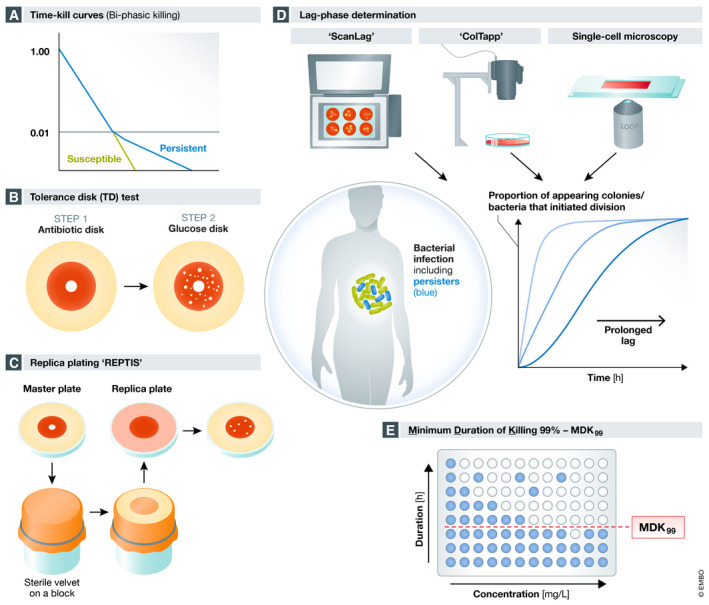
(A) The traditional method to test for antibiotic persistence is performing time–kill curves. Green line shows killing of a susceptible population and blue line displays the biphasic kill curve of a mixed population consisting of susceptible rapidly killed bacteria and persisters killed at a slower rate. (B) Tolerance Disk (TD) test allows the detection of bacteria that survive antibiotic exposure (persisters) by combining standard antibiotic disk assay (step 1) with regrowth after glucose addition (step 2). (C) The replica plating tolerance isolation system (REPTIS) allows detection of bacterial survivors on antibiotic plates (master plates). Colony‐forming units (CFUs) are transferred via a sterile velvet from a master plate on a fresh replica plate that does not contain antibiotics and allows growth of bacteria. Regrowth of bacteria indicates the presence of persister cells. (D) Detection of antibiotic persistence via lag phase determination. ScanLag, ColTapp and single‐cell microscopy determine colony and bacterial lag times that serve as a proxy for bacterial persistence (persistence by lag). (E) Determination of the minimum duration of killing 99% of a bacterial population (MDK99) can be used to measure antibiotic persistence. High MDK99 values indicate high antibiotic persistence.
Many studies used the minimal bactericidal concentration (MBC) of an antibiotic necessary to kill 99.9% of the bacterial population in 24 h to test for antibiotic tolerance (Handwerger & Tomasz, 1985). Although it was already known that tolerance is defined by a decreased rate of killing, for clinical reasons a strain is considered tolerant, when the MBC is at least 32 times higher than the MIC. An MBC/MIC ratio of > 32 is defined as tolerance (Handwerger & Tomasz, 1985; Jones, 2006; Gonzalez et al, 2013). However, the MBC/MIC ratio is not well suited to determine and measure antibiotic tolerance, and it especially fails to detect tolerance conferred by lag and by slow growth (Ishida et al, 1982; Keren et al, 2004; Lewis, 2019).
Balaban and co‐workers used the standard disk diffusion assay as a basis to develop a semi‐quantitative test for tolerance levels, the so‐called Tolerance Disk (TD) test (Gefen et al, 2017) (Fig 4B). The TD test uses the diffusion and degradation of antibiotics in the agar after removal of the antibiotic‐containing disc and enables the regrowth of tolerant and persistent bacteria that survived in the inhibition zone after addition of fresh glucose. This test allowed detection of high levels of tolerance in clinical isolates of E. coli and, most importantly, identified antibiotics that were able to kill these surviving bacteria. Therefore, the TD test might help tailoring the treatment regimens to treat persistent and recurrent infections. However, this test cannot detect triggered antibiotic tolerance/persistence as this requires exposure to certain stressors like acidic pH or nutrient limitation, as found in the host. Moreover, the standard antibiotic discs used in clinical microbiology laboratories contain very high concentrations of antibiotics leading to high residual drug concentrations in the agar inhibiting regrowth of bacteria. Ideally, discs should contain antibiotic concentrations high enough to create a large enough inhibition zone but low enough to fall below the MIC after 18 h. All these problematics make it difficult to standardize the TD test for clinical use.
Another way to test for antibiotic tolerance is to use the “Replica Plating Tolerance Isolation System” (REPTIS) developed by Hiramatsu and co‐workers to successfully identify and select ciprofloxacin (CIP)‐tolerant mutants in S. aureus (Matsuo et al, 2019) (Fig 4C). REPTIS does not require adjusting the antibiotic concentrations, overcoming this limitation of the TD test. Shortly, instead of replacing the antibiotic disc by a glucose disc, this method uses a sterile silk cloth to transfer colony‐forming units (CFUs) onto a fresh plate (replica plate) where surviving bacteria can regrow, and antibiotic tolerance is determined by the number of growing bacteria in the former inhibition zone. Again, this test cannot detect triggered antibiotic persistence but has the potential to be adapted for an automated use in diagnostic microbiology laboratories.
As mentioned above, the ScanLag method (Levin‐Reisman et al, 2010) and ColTapp (Bär et al, 2020) can be used to detect and measure colony growth heterogeneity and derive bacterial colonies’ lag times, which serve as a proxy for antibiotic tolerance (Fig 4D). To further validate and confirm the results from the colony analysis at the single‐cell level, single bacterial cell microscopy can be used. However, these methods need specific setups and equipment, laboratory space and are quite labour‐intensive, which limits their usage in routine clinical microbiology laboratories. For specific clinical cases of persistent and difficult‐to‐treat infections, where physicians suspect persister cell formation, these methods could be used to improve and enable individual patient‐tailored treatments, but a broad standard testing might not be suitable.
Balaban and colleagues introduced a metric and an automated experimental framework for measuring antibiotic tolerance and persistence, by determining the minimal duration of killing 99% of the population (MDK99) (Brauner et al, 2017) (Fig 4E). The MDK99 can be evaluated by a statistical analysis of measurements performed manually or automated using a robotic system. This makes it interesting for standard diagnostic testing. Additionally, the MDK99 evaluation has the inherent advantage of containing duration, an essential feature of persisters surviving stress over a period of time, is therefore superior to the MBC/MIC ratio evaluation, which is poorly linked to antibiotic tolerance (Keren et al, 2004).
Future treatment regimens and novel therapeutic approaches
Despite the increase in antimicrobial resistance and the growing awareness of antibiotic persistence as a clinically relevant issue, the development of new antimicrobial substances is declining. The reasons for this are complex but are associated with the current focus of the pharmaceutical industry on medication for non‐infectious chronic diseases such as cancer, metabolic and cardiovascular disorders and a lack of confidence in the profitability of new antibiotics. However, there are novel strategies and substances in basic research and preclinical studies that might offer treatment options in the near future (Fig 5).
Figure 5. New approaches to target multidrug‐resistant bacteria and bacterial persister cells.
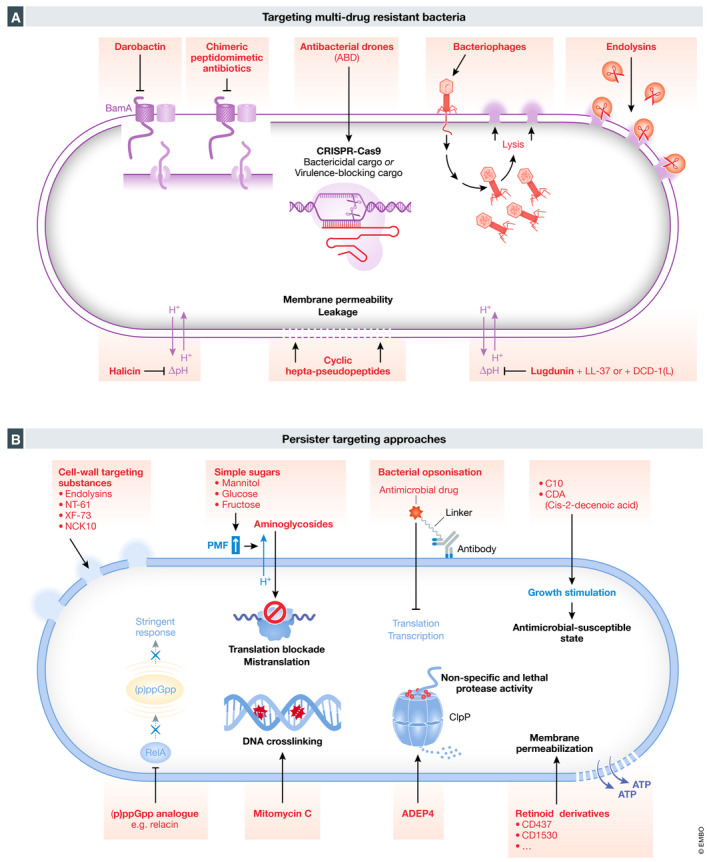
(A) Scheme showing novel strategies to target multidrug‐resistant Gram‐negative and Gram‐positive bacteria. New approaches to target multiresistant bacteria include darobactin, chimeric peptidomimetic antibiotics, antibacterial drones (ABD), bacteriophages, endolysins, halicin and cyclic hepta‐pseudopeptides and lugdunin. (B) Scheme of persister‐targeting approaches. Different strategies have been suggested to specifically target bacterial persister cells, e.g. addition of cell wall targeting substances like endolysins, NT‐61, XF‐73 or NCK10, simple sugars, bacterial opsonization by antibody–antibiotic conjugates (AACs), 3‐[4‐(4‐methoxyphenyl)piperazin‐1‐yl] piperidin‐4‐yl biphenyl‐4‐carboxylate (C10), cis‐2‐decenoic acid, (p)ppGpp analogues like relacin, mitomycin C, acyldeptipeptide (ADEP4) or retinoid acid derivatives (e.g. CD437 and CD1530).
Novel antimicrobials against a broad‐spectrum of multidrug‐resistant bacteria
The recently discovered chimeric peptidomimetic antibiotics possess a broad‐spectrum antimicrobial activity against Gram‐negative bacteria, including all Gram‐negative ESKAPE pathogens (Luther et al, 2019). Luther and colleagues showed that the bactericidal activity of these chimeric antibiotics involves binding to both lipopolysaccharide and the main component of the β‐barrel folding complex, BamA, that is required for the folding and insertion of β‐barrel proteins into the outer membrane of Gram‐negative bacteria, explaining the specific targeting of Gram‐negative bacteria while leaving eukaryotic cell membranes intact (Fig 5A). Yet, the exact mechanism driving the killing of Gram‐negative bacteria remains an open question. The leading candidate POL7306 underwent preclinical toxicology studies. Before moving into clinical trials, the focus is currently on a new formulation of POL7306 as well as optimizing peptide designs aiming to identify further candidates to broaden their therapeutic margins (Luther et al, 2019).
Generally, it is difficult to find new substances against Gram‐negative bacteria as they evolved an outer membrane that protects them from unwanted compounds (Li & Nikaido, 2004; Payne et al, 2007). Thus, Lewis and co‐workers reasoned that compounds against Gram‐negative bacteria would be present in microorganisms that need to compete with Gram‐negative bacteria. They screened isolates of Photorhabdus, symbionts of entomopathogenic nematode microbiomes, for antimicrobial substances against Gram‐negative bacteria and isolated a new compound named darobactin (Imai et al, 2019). In general, antimicrobial drugs target bacterial enzymes, but darobactin has been shown to stabilize the closed conformation of the chaperone BamA, inhibiting insertion of its substrates into the membrane (Imai et al, 2019) (Fig 5A). This newly discovered antimicrobial drug might provide novel treatment options for infections with multidrug‐resistant Gram‐negative pathogens and shows that there are diverse molecules against pathogens already available in nature.
Using a machine learning approach, Collins and co‐workers discovered completely new kinds of antibiotics, without using any previous human assumption (Stokes et al, 2020). Screening a pool of more than 100 million molecules led to the discovery of c‐Jun N‐terminal kinase inhibitor SU3327 (De et al, 2009), a preclinical nitrothiazole under investigation as a treatment for diabetes, exhibiting a strong antimicrobial activity. Halicin, renamed after HAL, the intelligent computer in the film “A Space Odyssey” from 2001, is structurally divergent from conventional antibiotics and exhibits bactericidal activity against a wide range of pathogens including M. tuberculosis, carbapenem‐resistant Enterobacteriaceae, C. difficile and pan‐resistant A. baumannii. Interestingly, halicin disrupts the flow of protons across the bacterial cell membrane (Fig 5A), a novel killing method compared to conventional antibiotics. Additionally, this study identified eight other antibacterial substances that are structurally different from conventional antibiotics and highlights the opportunities and impact machine learning can have on antibiotic discovery and prediction of properties of potential drugs while decreasing the cost of screening efforts (Stokes et al, 2020). However, these results will have to be carefully evaluated and tested in animal models including pharmacokinetic and toxicology studies before moving to clinical trials.
Based on the structure of a natural antibacterial peptide, Nicolas and colleagues generated a new family of peptidomimetics, the cyclic hepta‐pseudopeptides that show antibacterial activity against Gram‐positive and Gram‐negative multiresistant pathogens while having limited potential to lead to resistance development (Nicolas et al, 2019) (Fig 5A). They identified two peptide analogues that were active against MRSA and P. aeruginosa in severe sepsis and skin infection murine models and exhibited low nephrotoxicity. Despite these promising results, their efficacy in deep‐seated infections is still to be shown. Both compounds are considered potential candidates for drug development; however, extensive pharmacokinetic and toxicology studies will be necessary before starting phase I clinical trials (Nicolas et al, 2019).
Another approach taken towards the effort of clearing antibiotic‐resistant Gram‐positive and Gram‐negative bacteria is the use of bacteriophages (Fig 5A), viruses that infect and lyse bacteria (Twort, 1915; Salmond & Fineran, 2015). Due to the success of antibiotics, bacteriophage therapy was abandoned in Western countries, but is now being revived, because of increasing MDR bacteria and studies show that bacteriophage therapy successfully treats urinary tract infections (UTIs) (Leitner et al, 2017) and burn wound infections by P. aeruginosa (Jault et al, 2019). Nevertheless, there are several potential disadvantages of phage therapy: (i) not all phages are suitable for use as therapeutics. Temperate or toxin‐carrying phages and those with poor killing potential against target bacteria should be avoided. (ii) The narrow host spectrum, wherein different species or even different strains from the same species, might need specific phages for eliciting efficient killing. Although broadly acting phage cocktails are normally more selective in their spectrum of activity than conventional narrow‐spectrum antibiotics, it might be challenging to produce or at least provide all the needed phage cocktails. It might be even difficult to find the exact phage needed for therapy. (iii) Phages are protein‐based, biological agents that can actively replicate, evolve and interact with the hosts’ immune system. Especially, Western countries are not familiar with phages and there is still hesitancy to use such biological entities in humans (Loc‐Carrillo & Abedon, 2011).
Novel antimicrobials against a narrow‐spectrum of multidrug‐resistant bacteria
Because of the discussed disadvantages of phage therapy, research has focused more specifically on phage‐encoded peptidoglycan hydrolases, the endolysins, which lyse bacterial cells (Haddad Kashani et al, 2018) (Fig 5A). One of the biggest advantages of endolysins is their species specificity, avoiding selective pressure on commensal bacteria (Schmelcher et al, 2012). Another advantage is the low probability of bacteria evolving resistances against endolysins, as phages and host bacteria coevolved and endolysins target highly conserved structures in the bacterial cell wall (Borysowski et al, 2006). However, to overcome certain limitations, such as immunogenicity, short serum half‐lives, low penetration into eukaryotic cells to kill intracellular bacteria and toxicity, many research groups focus on engineering improved variants that might offer novel treatment options in the future (Schmelcher et al, 2012; Haddad Kashani et al, 2018; Röhrig et al, 2020).
Independent of discovering new antibiotic substances and phages, a new method using CRISPR‐Cas9, was recently developed to kill or block multidrug‐resistant S. aureus (Ram et al, 2018). In this study, they replaced the staphylococcal pathogenicity islands’ (SaPIs) toxin genes with antibacterial cargos to generate antibacterial drones (ABDs) that target S. aureus in the host (Fig 5A). By constructing ABDs with either a CRISPR–Cas9 bactericidal or a CRISPR–dCas9 virulence‐blocking module, bacteria are either killed or disarmed, leading to the abrogation of the infection. This was also shown in a murine abscess model, where mice were rescued by treatment with these ABDs (Ram et al, 2018). However, the ABD approach comes with some potential obstacles, e.g. simultaneous packaging of host genes, induction of or recombination with resident SaPIs or recombination with plasmids carrying virulence or resistance genes. Additionally, resistance to the ABDs can occur (Ram et al, 2018).
Taking a different approach, Krismer, Peschel and co‐workers identified the potent antimicrobial compound lugdunin from S. lugdunensis (Zipperer et al, 2016). Rather than treating an already existing infection, they suggest using lugdunin or lugdunin‐producing commensal staphylococci to prevent S. aureus colonization and invasive infections in high‐risk patients such as immunocompromised or before elective surgeries. The macrocyclic thiazolidine peptide antibiotic lugdunin is active against a wide range of Gram‐positive pathogens including MRSA and VRE with a high barrier to resistance by mutation (Zipperer et al, 2016) (Fig 5A). It exhibits immunomodulatory activity by inducing the expression of the host AMP LL‐37 and the pro‐inflammatory chemokines CXCL8 and MIP‐2 in human keratinocytes, which leads to the recruitment of monocytes and neutrophils potentially contributing to effective bacterial eradication (Bitschar et al, 2019). Moreover, lugdunin acts synergistically with the human AMPs LL‐37 and DCD‐1(L) in killing S. aureus. The antimicrobial activity against S. aureus is strongly associated with the disruption of the membrane potential without lysing the bacterial cells, demonstrating that the mode of action is translocation of protons (Schilling et al, 2019). Lugdunin could be used either directly as a topically applied drug to inhibit growth of S. aureus or indirectly applied with a safe probiotic strain such as an avirulent S. lugdunensis or an exclusively commensal species containing the lug genes (Zipperer et al, 2016).
Rather than directly targeting the pathogen, an alternative approach is to attenuate their pathogenicity by targeting specific virulence factors involved in the infection process. The blocking of virulence factors also helps avoiding resistance development by the bacteria, as less antibiotics would have to be used (Keyser et al, 2008). An important strategy is to hinder bacterial dissemination and tissue invasion by blocking bacterial attachment to host cells and translocation into host tissue. Mannosides have been shown to inhibit the FimH component of type I fimbriae of uropathogenic E. coli (UPEC) thereby significantly decreasing colonization of UPEC in a murine infection model (Klein et al, 2010).
The synthetic antibody ScFv‐Fc KP3 blocks the type 3 fimbrial subunit in K. pneumonia and has been shown to confer protection in murine pneumonia models (Wang et al, 2016). By neutralizing toxin B from C. difficile, the monoclonal antibody bezlotoxumab prevents recurrent C. difficile infections in combination with antibiotics and has already been approved for the use in humans (Mullard, 2016). Similarly, the monoclonal anti‐α‐toxin antibody, MEDI4893, has been shown to decrease the S. aureus burden and increase wound healing in diabetic foot ulcers and phase II clinical studies have just been concluded (ClinicalTrials.gov, 2019). Despite promising novel strategies and molecules targeting pathogens and their virulence factors, further research and alternative approaches are needed to ensure effective treatment regimens against multidrug‐resistant bacteria in the future.
Antipersister strategies
The recognition of the phenomenon of antibiotic persistence and its clinical importance in chronic and relapsing infections including its connection to resistance development has led to both basic and application‐oriented research. Today, there are three main strategies for treatment and prevention of infections associated with bacterial persisters: (i) directly targeting persister cells; (ii) inhibiting formation of bacterial persisters; and (iii) resuscitating persisters and sensitizing them to conventional antibiotic therapy.
Directly targeting persister cells
As mentioned above, persisters are often characterized by slow growth or dormancy rendering antibiotic targets inactive. One of the obvious targets for directly attacking persisters without the requirement of active targets is the bacterial membrane and cell wall.
Phage‐derived endolysins and other synthetic lysins degrade the peptidoglycans in the cell wall independent of the presence of metabolically active and growing bacteria, targeting both growing and non‐growing populations (Gutierrez et al, 2014; Rodríguez‐Rubio et al, 2016; Röhrig et al, 2020) (Fig 5B).
Synthetic retinoid acid derivatives, specifically CD437 and CD1530, have been shown to penetrate and disrupt the lipid bilayer of the cell membrane of MRSA thereby killing growing and non‐growing bacteria (Fig 5B). Additionally, CD437 alone or in combination with gentamicin exhibited high efficacy in a murine thigh infection model highlighting the potential of retinoid derivatives as a new class of antibiotics and antipersister drugs (Kim et al, 2018; Kim et al, 2019). Various other membrane‐active small molecules like NT‐61, a fluoroquinolone (Hu et al, 2010), XF‐73 (Ooi et al, 2010) or NCK10 (Ghosh et al, 2015) have shown activity against S. aureus persisters (Fig 5B). Another approach to eradicate persisters directly is to use compounds that interact with inactive targets in the bacterial cell. The acyldeptipeptide ADEP4, for example, activates the protease ClpP in S. aureus, leading to non‐specific and lethal protease activity (Conlon et al, 2013) (Fig 5B). In combination with conventional antibiotics, ADEP4 was able to clear a deep‐seated murine S. aureus biofilm infection (Conlon et al, 2013). Recently, repurposing of anticancer drugs leads to the discovery of their antipersister activity. Mitomycin C, for example, causes DNA crosslinking independently of bacterial growth and exhibits bactericidal activity against E. coli, P. aeruginosa and S. aureus growing and non‐growing cells (Kwan et al, 2015) (Fig 5B). As of their nature as anticancer drugs, these agents are quite cytotoxic for the host and certainly not specific to pathogens, making their use as antipersister compounds questionable.
Inhibition of persister formation
Ideally, we would be able to inhibit persister formation directly from the beginning and thereby limit the problem of difficult‐to‐treat and chronic infections. As described above, different cellular pathways, e.g. stringent response and SOS response, can lead to persister formation. Synthetic ppGpp analogues such as relacin, have been shown to efficiently inhibit Rel proteins, thereby decreasing long‐term survival potential of S. aureus (Wexselblatt et al, 2012) (Fig 5B). However, persister cell formation might happen already at the very beginning of an infection, when the bacteria encounter different stressors in the host, meaning that it requires an intervention at the same time as the infection happens or as a prophylactic treatment. For these reasons, the most promising approach, using the hosts’ first line of defence, might be vaccination.
Resuscitate and sensitize persisters
Awakening and stimulating the regrowth of persisters can render them again sensitive to conventional antibiotics that usually target actively growing bacteria. Some compounds that are able to wake up persister cells and resensitize them to conventional antibiotics have already been identified. Simple sugars like mannitol, fructose and glucose combined with gentamicin can decrease persister levels more than 1,000‐fold in E. coli and S. aureus not via activating bacterial growth, but by increasing the pmf that in turn increases the uptake of aminoglycosides (Allison et al, 2011) (Fig 5B). This effect was aminoglycoside‐specific, and this approach failed to reduce persister levels in combination with other classes of antibiotics like β‐lactams and fluoroquinolones. Kim and colleagues identified a compound, 3‐[4‐(4‐methoxyphenyl)piperazin‐1‐yl] piperidin‐4‐yl biphenyl‐4‐carboxylate (C10), that shortens the lag time of E. coli that survived in vitro ampicillin or ciprofloxacin exposure and facilitated their killing, suggesting that this compound is able to stimulate bacterial regrowth thus accelerating the reversion of persisters to sensitive cells (Kim et al, 2011) (Fig 5B). Similarly, the fatty acid signalling molecule cis‐2‐decenoic acid is able to activate E. coli and P. aeruginosa persisters growth and metabolism resensitizing them to antibiotics (Marques et al, 2014). In addition, cis‐2‐decenoic acid was shown to induce biofilm dispersion and might provide a new potential adjuvant drug to treat biofilm‐associated infections (Davies & Marques, 2009) (Fig 5B). However, no follow‐up studies for this molecule showing in vivo efficiency in reducing bacterial persisters were published and toxicity studies are missing. A different approach that targets bacteria irrespectively of their growth state and metabolic activity is to use antibody–antibiotic conjugates (AACs) (Fig 5B) (Lehar et al, 2015). Such constructs were designed to target and opsonize extracellular S. aureus directly promoting phagocytosis. The conjugated antimicrobial drug is only active and kills bacteria inside phagolysosome preventing the transfer of bacteria between host cells (macrophages) during infection. Additionally, the drug killed persister cells and targeted non‐dividing MRSA sequestered inside murine macrophages (Lehar et al, 2015). In summary, novel approaches and strategies will help boosting our capacity to treat microbial infections, especially therapy of persistent and chronic infections.
Concluding remarks
The rise of antibiotic resistance, as well as the increase of difficult‐to‐treat and biofilm‐associated infections caused by antibiotic‐susceptible pathogens, led to intensive research on new antimicrobial strategies including persister‐targeting approaches. Recent developments in antibiotic discovery give hope for new treatment options of infections caused by multiresistant bacteria. However, there is still a desperate need for further antibiotic research and discovery to overcome the imminent post‐antibiotic era. In addition, several mechanisms involved in persister formation in different bacterial species have been revealed. Global cellular dormancy and target inactivation, including reduced ATP levels, blocked translation, protein aggregation and arrested DNA replication, have been linked to bacterial survival despite antibiotic treatment. Although the field of antibiotic persistence research is growing and great advances have been made, we still do not fully understand how bacteria form persisters. Clearly, the interplay of multiple mechanisms and the resulting phenotypic heterogeneity is beneficial for the survival of a bacterial population during infection. Recent advances show novel therapeutic strategies aiming to eradicate such persisters, desperately needed to shorten treatment duration and thus reducing morbidity remarkably. However, further research and focus on modulating the host environment is needed to successfully tackle antibiotic resistance and persistence. Novel technologies including the development of cutting‐edge techniques such as single‐cell analysis, microfluidics, dual‐RNAseq and bacterial sorting as well as bacterial epigenetics are being used and will be used more and more to track, enrich and analyse rare, non‐growing bacteria that are able to survive antibiotic therapy without being resistant.
In need of answers.
How can the identification of resistance in diagnostics be accelerated to ensure a targeted therapy from the beginning? Moreover, can novel narrow‐spectrum antibiotics prevent microbiota disturbance and selection for resistance?
What is the persister status in an infected patient?
How homogeneous is a persister population during infection? Do we see differences in their transcriptome/ proteome on a single bacterial cell level?
How well do in vitro findings correlate with the in vivo situation?
How does the hosts’ immune system and milieu influence persister cell formation and interact with persisters?
Can a bacterial population including persisters be targeted and eradicated by combination treatments? Will a subpopulation of extremely dormant persisters survive?
How can we better implement testing for antibiotic persistence in clinical microbiology laboratories?
Will a better characterization of persisters in diagnostics allow predicting the clinical course, e.g. difficult‐to‐treat/ chronic or relapsing infections?
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
We thank the members of the Zinkernagel laboratory for feedbacks and proofreading of the manuscript, especially Judith Bergada Pijuan for her help with the presentation of the resistance data. The authors apologise to colleagues in the field for being unable to cite all relevant studies and thank the reviewers for their helpful comments. This work was funded by the SNSF project grant 31003A_176252 (to A.S.Z); by the Swedish Society for Medical Research (SSMF) foundation grant P17‐0179 (to S.M.S) and the Promedica Foundation 1449/M (to S.D.B).
EMBO Reports (2020) 21: e51034.
See the Glossary for abbreviations used in this article.
References
- Aakre CD, Phung TN, Huang D, Laub MT (2013) A bacterial toxin inhibits DNA replication elongation through a direct interaction with the beta sliding clamp. Mol Cell 52: 617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abele‐Horn M, Schupfner B, Emmerling P, Waldner H, Goring H (2000) Persistent wound infection after herniotomy associated with small‐colony variants of Staphylococcus aureus . Infection 28: 53–54 [DOI] [PubMed] [Google Scholar]
- Ackermann M (2015) A functional perspective on phenotypic heterogeneity in microorganisms. Nat Rev Microbiol 13: 497–508 [DOI] [PubMed] [Google Scholar]
- Adedeji WA (2016) The treasure called antibiotics. Ann Ib Postgrad Med 14: 56–57 [PMC free article] [PubMed] [Google Scholar]
- Allison KR, Brynildsen MP, Collins JJ (2011) Metabolite‐enabled eradication of bacterial persisters by aminoglycosides. Nature 473: 216–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato S, Fazen C, Henry T, Mok W, Orman M, Sandvik E, Volzing K, Brynildsen M (2014) The role of metabolism in bacterial persistence. Front Microbiol 5: 70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato SM, Orman MA, Brynildsen MP (2013) Metabolic control of persister formation in Escherichia coli . Mol Cell 50: 475–487 [DOI] [PubMed] [Google Scholar]
- Aminov RI (2010) A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol 1: 134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SE, Sherman EX, Weiss DS, Rather PN (2018) Aminoglycoside heteroresistance in Acinetobacter baumannii AB5075. mSphere 3: e00271‐18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelbaum PC (2007) Microbiology of antibiotic resistance in Staphylococcus aureus . Clin Infect Dis 45(Suppl 3): S165–170 [DOI] [PubMed] [Google Scholar]
- Aslam B, Wang W, Arshad MI, Khurshid M, Muzammil S, Rasool MH, Nisar MA, Alvi RF, Aslam MA, Qamar MU et al (2018) Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist 11: 1645–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarian T, Daum RS, Petty LA, Steinbeck JL, Yin Z, Nolan D, Boyle‐Vavra S, Hanage WP, Salemi M, David MZ (2016) Intrahost evolution of methicillin‐resistant Staphylococcus aureus USA300 among individuals with reoccurring skin and soft‐tissue infections. J Infect Dis 214: 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddour LM, Barker LP, Christensen GD, Parisi JT, Simpson WA (1990) Phenotypic variation of Staphylococcus epidermidis in infection of transvenous endocardial pacemaker electrodes. J Clin Microbiol 28: 676–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baharoglu Z, Mazel D (2014) SOS, the formidable strategy of bacteria against aggressions. FEMS Microbiol Rev 38: 1126–1145 [DOI] [PubMed] [Google Scholar]
- Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ et al (2019a) Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17: 441–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ et al (2019b) Publisher correction: definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17: 460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S (2004) Bacterial persistence as a phenotypic switch. Science 305: 1622–1625 [DOI] [PubMed] [Google Scholar]
- Band VI, Crispell EK, Napier BA, Herrera CM, Tharp GK, Vavikolanu K, Pohl J, Read TD, Bosinger SE, Trent MS et al (2016) Antibiotic failure mediated by a resistant subpopulation in Enterobacter cloacae . Nat Microbiol 1: 16053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bär J, Boumasmoud M, Kouyos RD, Zinkernagel AS, Vulin C (2020) Efficient microbial colony growth dynamics quantification with ColTapp, an automated image analysis application. Sci Rep 10: 16084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroud M, Dandache I, Araj GF, Wakim R, Kanj S, Kanafani Z, Khairallah M, Sabra A, Shehab M, Dbaibo G et al (2013) Underlying mechanisms of carbapenem resistance in extended‐spectrum beta‐lactamase‐producing Klebsiella pneumoniae and Escherichia coli isolates at a tertiary care centre in Lebanon: role of OXA‐48 and NDM‐1 carbapenemases. Int J Antimicrob Agents 41: 75–79 [DOI] [PubMed] [Google Scholar]
- Bates DM, von Eiff C, McNamara PJ, Peters G, Yeaman MR, Bayer AS, Proctor RA (2003) Staphylococcus aureus menD and hemB mutants are as infective as the parent strains, but the menadione biosynthetic mutant persists within the kidney. J Infect Dis 187: 1654–1661 [DOI] [PubMed] [Google Scholar]
- Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 45: 493–496 [PubMed] [Google Scholar]
- Benoit JB, Frank DN, Bessesen MT (2018) Genomic evolution of Staphylococcus aureus isolates colonizing the nares and progressing to bacteremia. PLoS One 13: e0195860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund B (2019) Acquired resistance to colistin via chromosomal and plasmid‐mediated mechanisms in Klebsiella pneumoniae . Infect Microb Dis 1 [Google Scholar]
- Bernier SP, Lebeaux D, DeFrancesco AS, Valomon A, Soubigou G, Coppee JY, Ghigo JM, Beloin C (2013) Starvation, together with the SOS response, mediates high biofilm‐specific tolerance to the fluoroquinolone ofloxacin. PLoS Genet 9: e1003144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besier S, Smaczny C, von Mallinckrodt C, Krahl A, Ackermann H, Brade V, Wichelhaus TA (2007) Prevalence and clinical significance of Staphylococcus aureus small‐colony variants in cystic fibrosis lung disease. J Clin Microbiol 45: 168–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Roy S, Mukhopadhyay P, Rit K, Dey J, Ganguly U, Ray R (2012) Small Colony variants of Staphylococcus aureus isolated from a patient with infective endocarditis: a case report and review of the literature. Iran J Microbiol 4: 98–99 [PMC free article] [PubMed] [Google Scholar]
- Bigger J (1944) Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 244: 497–500 [Google Scholar]
- Bitschar K, Sauer B, Focken J, Dehmer H, Moos S, Konnerth M, Schilling NA, Grond S, Kalbacher H, Kurschus FC et al (2019) Lugdunin amplifies innate immune responses in the skin in synergy with host‐ and microbiota‐derived factors. Nat Commun 10: 2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T (2013) The role of bacterial biofilms in chronic infections. APMIS 121: 1–58 [DOI] [PubMed] [Google Scholar]
- Black DS, Irwin B, Moyed HS (1994) Autoregulation of hip, an operon that affects lethality due to inhibition of peptidoglycan or DNA synthesis. J Bacteriol 176: 4081–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DS, Kelly AJ, Mardis MJ, Moyed HS (1991) Structure and organization of hip, an operon that affects lethality due to inhibition of peptidoglycan or DNA synthesis. J Bacteriol 173: 5732–5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ (2015) Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol 13: 42–51 [DOI] [PubMed] [Google Scholar]
- Boekhoud IM, Hornung BVH, Sevilla E, Harmanus C, Bos‐Sanders IMJG, Terveer EM, Bolea R, Corver J, Kuijper EJ, Smits WK (2020) Plasmid‐mediated metronidazole resistance in Clostridioides difficile . Nat Commun 11: 598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysowski J, Weber‐Dąbrowska B, Górski A (2006) Bacteriophage endolysins as a novel class of antibacterial agents. Exp Biol Med 231: 366–377 [DOI] [PubMed] [Google Scholar]
- Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J (2009) Bad bugs, no drugs: no ESKAPE! an update from the Infectious Diseases Society of America. Clin Infect Dis 48: 1–12 [DOI] [PubMed] [Google Scholar]
- Brantl S, Jahn N (2015) sRNAs in bacterial type I and type III toxin‐antitoxin systems. FEMS Microbiol Rev 39: 413–427 [DOI] [PubMed] [Google Scholar]
- Brauner A, Fridman O, Gefen O, Balaban NQ (2016) Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat Rev Microbiol 14: 320–330 [DOI] [PubMed] [Google Scholar]
- Brauner A, Shoresh N, Fridman O, Balaban NQ (2017) An experimental framework for quantifying bacterial tolerance. Biophys J 112: 2664–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooun A, Liu S, Lewis K (2000) A dose‐response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 44: 640–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Ngeno C (2007) Antimicrobial resistance in clinical isolates of Staphylococcus aureus from hospital and community sources in southern Jamaica. Int J Infect Dis 11: 220–225 [DOI] [PubMed] [Google Scholar]
- CDC (2019). Antibiotic resistance threats in the united states, 2019, in: Atlanta G.U.S.D.o.H.a.H.S. (Ed.). CDC.
- Chambers HF, Deleo FR (2009) Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 7: 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ et al (2003) Infection with vancomycin‐resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med 348: 1342–1347 [DOI] [PubMed] [Google Scholar]
- Chatterjee I, Kriegeskorte A, Fischer A, Deiwick S, Theimann N, Proctor RA, Peters G, Herrmann M, Kahl BC (2008) In vivo mutations of thymidylate synthase (encoded by thyA) are responsible for thymidine dependency in clinical small‐colony variants of Staphylococcus aureus . J Bacteriol 190: 834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudi B, Spröte P, Chirkova A, Personnic N, Zankl J, Schürmann N, Schmidt A, Bumann D (2014) Phenotypic variation of salmonella in host tissues delays eradication by antimicrobial chemotherapy. Cell 158: 722–733 [DOI] [PubMed] [Google Scholar]
- Clements MO, Watson SP, Poole RK, Foster SJ (1999) CtaA of Staphylococcus aureus is required for starvation survival, recovery, and cytochrome biosynthesis. J Bacteriol 181: 501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov (2019) Study of the efficacy and safety of MEDI4893 (SAATELLITE). https://clinicaltrials.gov/ct2/show/study/NCT02296320
- Coffey JW, De Duve C (1968) Digestive activity of lysosomes. I. The digestion of proteins by extracts of rat liver lysosomes. J Biol Chem 243: 3255–3263 [PubMed] [Google Scholar]
- Conlon BP, Nakayasu ES, Fleck LE, LaFleur MD, Isabella VM, Coleman K, Leonard SN, Smith RD, Adkins JN, Lewis K (2013) Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 503: 365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon BP, Rowe SE, Gandt AB, Nuxoll AS, Donegan NP, Zalis EA, Clair G, Adkins JN, Cheung AL, Lewis K (2016) Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat Microbiol 1: 16051 [DOI] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284: 1318–1322 [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Spratt D, Liddle AD (2019) Implant materials and prosthetic joint infection: the battle with the biofilm. EFORT Open Rev 4: 633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DG, Marques CNH (2009) A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol 191: 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Surya K, Stebbins JL, Chen LH, Riel‐Mehan M, Machleidt T, Dahl R, Yuan H, Emdadi A, Barile E, Chen V et al (2009) Design, synthesis, and structure‐activity relationship of substrate competitive, selective, and in vivo active triazole and thiadiazole inhibitors of the c‐Jun N‐terminal kinase. J Med Chem 52: 1943–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo JL, Patel R (2009) Clinical practice. Infection associated with prosthetic joints. N Engl J Med 361: 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler Haunreiter V, Boumasmoud M, Haffner N, Wipfli D, Leimer N, Rachmuhl C, Kuhnert D, Achermann Y, Zbinden R, Benussi S et al (2019) In‐host evolution of Staphylococcus epidermidis in a pacemaker‐associated endocarditis resulting in increased antibiotic tolerance. Nat Commun 10: 1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingsdag SA, Hunter N (2018) Metronidazole: an update on metabolism, structure‐cytotoxicity and resistance mechanisms. J Antimicrob Chemother 73: 265–279 [DOI] [PubMed] [Google Scholar]
- Dolejska M, Villa L, Poirel L, Nordmann P, Carattoli A (2013) Complete sequencing of an IncHI1 plasmid encoding the carbapenemase NDM‐1, the ArmA 16S RNA methylase and a resistance‐nodulation‐cell division/multidrug efflux pump. J Antimicrob Chemother 68: 34–39 [DOI] [PubMed] [Google Scholar]
- Domenech M, Ramos‐Sevillano E, Garcia E, Moscoso M, Yuste J (2013) Biofilm formation avoids complement immunity and phagocytosis of Streptococcus pneumoniae . Infect Immun 81: 2606–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorr T, Vulic M, Lewis K (2010) Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli . PLoS Biol 8: e1000317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziewanowska K, Patti JM, Deobald CF, Bayles KW, Trumble WR, Bohach GA (1999) Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect Immun 67: 4673–4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC (2018a) Healthcare‐associated infections: Clostridium difficile infections. In: ECDC. Annual epidemiological report for 2016. Stockholm: ECDC
- ECDC (2018b) Surveillance of antimicrobial resistance in Europe 2017.
- ECDC (2019) Surveillance of antimicrobial resistance in Europe 2018, Stockholm: European Centre for Disease Prevention and Control; [Google Scholar]
- Elsner HA, Sobottka I, Mack D, Claussen M, Laufs R, Wirth R (2000) Virulence factors of Enterococcus faecalis and Enterococcus faecium blood culture isolates. Eur J Clin Microbiol Infect Dis 19: 39–42 [DOI] [PubMed] [Google Scholar]
- Fauvart M, De Groote VN, Michiels J (2011) Role of persister cells in chronic infections: clinical relevance and perspectives on anti‐persister therapies. J Med Microbiol 60: 699–709 [DOI] [PubMed] [Google Scholar]
- Fernandez De Henestrosa AR, Ogi T, Aoyagi S, Chafin D, Hayes JJ, Ohmori H, Woodgate R (2000) Identification of additional genes belonging to the LexA regulon in Escherichia coli . Mol Microbiol 35: 1560–1572 [DOI] [PubMed] [Google Scholar]
- Fisher RA, Gollan B, Helaine S (2017) Persistent bacterial infections and persister cells. Nat Rev Microbiol 15: 453–464 [DOI] [PubMed] [Google Scholar]
- Fleming A (1929) On antibacterial action of culture of Penicillium, with special reference to their use in isolation of B. influenzae. Br J Exp Pathol 10: 226–236 [Google Scholar]
- FOPH (2018) Swiss Antibiotic Resistance Report 2018. Usage of Antibiotics and Occurrence of Antibiotic Resistance in Bacteria from Humans and Animals in Switzerland.
- Foster TJ (2017) Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol Rev 41: 430–449 [DOI] [PubMed] [Google Scholar]
- Fraunholz M, Sinha B (2012) Intracellular Staphylococcus aureus: live‐in and let die. Front Cell Infect Microbiol 2: 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey PM, Marti GR, Droz S, Derochevonarx M, Suter‐Riniker F, Aujesky D, Brugger SD (2019) Bacterial colonization of handheld devices in a tertiary care setting: a hygiene intervention study. Antimicrobial Resist Infect Control 8: 97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T, Ohya S, Narita T, Katsuta M, Iijima M, Masuda N, Yasuda H, Trias J, Nikaido H (1993) Activity of the carbapenem panipenem and role of the OprD (D2) protein in its diffusion through the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother 37: 322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen O, Chekol B, Strahilevitz J, Balaban NQ (2017) TDtest: easy detection of bacterial tolerance and persistence in clinical isolates by a modified disk‐diffusion assay. Sci Rep 7: 41284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain E, Castro‐Roa D, Zenkin N, Gerdes K (2013) Molecular mechanism of bacterial persistence by HipA. Mol Cell 52: 248–254 [DOI] [PubMed] [Google Scholar]
- Ghosh C, Manjunath GB, Konai MM, Uppu DS, Hoque J, Paramanandham K, Shome BR, Haldar J (2015) Aryl‐Alkyl‐lysines: agents that kill planktonic cells, persister cells, biofilms of MRSA and protect mice from skin‐infection. PLoS One 10: e0144094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan PH, Gage PA, Welch DF, Muszynski MJ, Wait KR (1987) Prevalence of thymidine‐dependent Staphylococcus aureus in patients with cystic fibrosis. J Clin Microbiol 25: 1258–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan B, Grabe G, Michaux C, Helaine S (2019) Bacterial persisters and infection: past, present, and progressing. Annu Rev Microbiol 73: 359–385 [DOI] [PubMed] [Google Scholar]
- Gonzalez N, Sevillano D, Alou L, Cafini F, Gimenez MJ, Gomez‐Lus ML, Prieto J, Aguilar L (2013) Influence of the MBC/MIC ratio on the antibacterial activity of vancomycin versus linezolid against methicillin‐resistant Staphylococcus aureus isolates in a pharmacodynamic model simulating serum and soft tissue interstitial fluid concentrations reported in diabetic patients. J Antimicrob Chemother 68: 2291–2295 [DOI] [PubMed] [Google Scholar]
- Goudie JG, Goudie RB (1955) Recurrent infections by a stable dwarf‐colony variant of Staphylococcus aureus . J Clin Pathol 8: 284–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez A, Jain S, Bhargava P, Hamblin M, Lobritz MA, Collins JJ (2017) Understanding and sensitizing density‐dependent persistence to quinolone antibiotics. Mol Cell 68: 1147–1154.e3 [DOI] [PubMed] [Google Scholar]
- Gutierrez D, Ruas‐Madiedo P, Martinez B, Rodriguez A, Garcia P (2014) Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS One 9: e107307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad Kashani H, Schmelcher M, Sabzalipoor H, Seyed Hosseini E, Moniri R (2018) Recombinant endolysins as potential therapeutics against antibiotic‐resistant Staphylococcus aureus: current status of research and novel delivery strategies. Clin Microbiol Rev 31: e00071‐00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed F, Khan MA, Muhammad H, Sarwar T, Bilal H, Rehman TU (2019) Plasmid‐mediated mcr‐1 gene in Acinetobacter baumannii and Pseudomonas aeruginosa: first report from Pakistan. Rev Soc Bras Med Trop 52: e20190237 [DOI] [PubMed] [Google Scholar]
- Handwerger S, Tomasz A (1985) Antibiotic tolerance among clinical isolates of bacteria. Rev Infect Dis 7: 368–386 [DOI] [PubMed] [Google Scholar]
- Hansen S, Lewis K, Vulic M (2008) Role of global regulators and nucleotide metabolism in antibiotic tolerance in Escherichia coli . Antimicrob Agents Chemother 52: 2718–2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Vulic M, Min J, Yen TJ, Schumacher MA, Brennan RG, Lewis K (2012) Regulation of the Escherichia coli HipBA toxin‐antitoxin system by proteolysis. PLoS One 7: e39185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins CP, Pichon B, Doumith M, Parkhill J, Westh H, Tomasz A, de Lencastre H, Bentley SD, Kearns AM, Holden MTG (2017) Methicillin‐resistant Staphylococcus aureus emerged long before the introduction of methicillin into clinical practice. Genome Biol 18: 130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms A, Brodersen DE, Mitarai N, Gerdes K (2018) Toxins, targets, and triggers: an overview of toxin‐antitoxin biology. Mol Cell 70: 768–784 [DOI] [PubMed] [Google Scholar]
- Hartman BJ, Tomasz A (1984) Low‐affinity penicillin‐binding protein associated with beta‐lactam resistance in Staphylococcus aureus . J Bacteriol 158: 513–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun A, Linden PK, Friedman B (2017) Incidence, prevalence, and management of MRSA bacteremia across patient populations‐a review of recent developments in MRSA management and treatment. Crit Care 21: 211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helaine S, Cheverton AM, Watson KG, Faure LM, Matthews SA, Holden DW (2014) Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 343: 204–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I (1997) Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350: 1670–1673 [DOI] [PubMed] [Google Scholar]
- Hjort K, Nicoloff H, Andersson DI (2016) Unstable tandem gene amplification generates heteroresistance (variation in resistance within a population) to colistin in Salmonella enterica . Mol Microbiol 102: 274–289 [DOI] [PubMed] [Google Scholar]
- Hobby GL, Meyer K, Chaffee E (1942) Observations on the mechanism of action of penicillin. Proc Soc Exp Biol Med 50: 281–285 [Google Scholar]
- Hong Y‐K, Lee J‐Y, Ko KS (2018) Colistin resistance in Enterobacter spp. isolates in Korea. Journal of Microbiology 56: 435–440 [DOI] [PubMed] [Google Scholar]
- Honkanen M, Jämsen E, Karppelin M, Huttunen R, Eskelinen A, Syrjänen J (2019) Periprosthetic Joint infections as a consequence of bacteremia. Open Forum Infecti Dis 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC, Jacoby GA (2015) Mechanisms of drug resistance: quinolone resistance. Ann N Y Acad Sci 1354: 12–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornef MW, Wick MJ, Rhen M, Normark S (2002) Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol 3: 1033–1040 [DOI] [PubMed] [Google Scholar]
- Hu Y, Shamaei‐Tousi A, Liu Y, Coates A (2010) A new approach for the discovery of antibiotics by targeting non‐multiplying bacteria: a novel topical antibiotic for staphylococcal infections. PLoS One 5: e11818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh K, Kang CI, Kim J, Cho SY, Ha YE, Joo EJ, Chung DR, Lee NY, Peck KR, Song JH (2014) Risk factors and treatment outcomes of bloodstream infection caused by extended‐spectrum cephalosporin‐resistant Enterobacter species in adults with cancer. Diagn Microbiol Infect Dis 78: 172–177 [DOI] [PubMed] [Google Scholar]
- Imai Y, Meyer KJ, Iinishi A, Favre‐Godal Q, Green R, Manuse S, Caboni M, Mori M, Niles S, Ghiglieri M et al (2019) A new antibiotic selectively kills Gram‐negative pathogens. Nature 576: 459–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving SE, Corrigan RM (2018) Triggering the stringent response: signals responsible for activating (p)ppGpp synthesis in bacteria. Microbiology 164: 268–276 [DOI] [PubMed] [Google Scholar]
- Ishida K, Guze PA, Kalmanson GM, Albrandt K, Guze LB (1982) Variables in demonstrating methicillin tolerance in Staphylococcus aureus strains. Antimicrob Agents Chemother 21: 688–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen B, Peters G (1993) Foreign body associated infection. J Antimicrobial Chemother 32(suppl A): 69–75 [DOI] [PubMed] [Google Scholar]
- Jault P, Leclerc T, Jennes S, Pirnay JP, Que YA, Resch G, Rousseau AF, Ravat F, Carsin H, Le Floch R et al (2019) Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double‐blind phase 1/2 trial. Lancet Infect Dis 19: 35–45 [DOI] [PubMed] [Google Scholar]
- Jesaitis AJ, Franklin MJ, Berglund D, Sasaki M, Lord CI, Bleazard JB, Duffy JE, Beyenal H, Lewandowski Z (2003) Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol 171: 4329–4339 [DOI] [PubMed] [Google Scholar]
- Jevon M, Guo C, Ma B, Mordan N, Nair SP, Harris M, Henderson B, Bentley G, Meghji S (1999) Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect Immun 67: 2677–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AP, Woodford N (2013) Global spread of antibiotic resistance: the example of New Delhi metallo‐beta‐lactamase (NDM)‐mediated carbapenem resistance. J Med Microbiol 62: 499–513 [DOI] [PubMed] [Google Scholar]
- Jolivet‐Gougeon A, Bonnaure‐Mallet M (2014) Biofilms as a mechanism of bacterial resistance. Drug Discov Today Technol 11: 49–56 [DOI] [PubMed] [Google Scholar]
- Jones RN (2006) Microbiological features of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin Infect Dis 42(Suppl 1): S13–24 [DOI] [PubMed] [Google Scholar]
- Jorge P, Magalhaes AP, Grainha T, Alves D, Sousa AM, Lopes SP, Pereira MO (2019) Antimicrobial resistance three ways: healthcare crisis, major concepts and the relevance of biofilms. FEMS Microbiol Ecol 95: fiz115 [DOI] [PubMed] [Google Scholar]
- Joyce LF, Downes J, Stockman K, Andrew JH (1992) Comparison of five methods, including the PDM Epsilometer test (E test), for antimicrobial susceptibility testing of Pseudomonas aeruginosa . J Clin Microbiol 30: 2709–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz GW, Seo SM, Dorman NJ, Lerner SA (1990) Emergence of teicoplanin resistance during therapy of Staphylococcus aureus endocarditis. J Infect Dis 162: 103–108 [DOI] [PubMed] [Google Scholar]
- Kahl B, Herrmann M, Everding AS, Koch HG, Becker K, Harms E, Proctor RA, Peters G (1998) Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J Infect Dis 177: 1023–1029 [DOI] [PubMed] [Google Scholar]
- Kahl BC, Becker K, Loffler B (2016) Clinical significance and pathogenesis of staphylococcal small colony variants in persistent infections. Clin Microbiol Rev 29: 401–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble Ekta PK (2020) Antibiotic tolerance in biofilm and stationary‐phase planktonic cells of Staphylococcus aureus . Microb Drug Resist 10.1089/mdr.2019.0425 [DOI] [PubMed] [Google Scholar]
- Kaspy I, Rotem E, Weiss N, Ronin I, Balaban NQ, Glaser G (2013) HipA‐mediated antibiotic persistence via phosphorylation of the glutamyl‐tRNA‐synthetase. Nat Commun 4: 3001 [DOI] [PubMed] [Google Scholar]
- Katayama Y, Ito T, Hiramatsu K (2000) A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus . Antimicrob Agents Chemother 44: 1549–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M, Aravind L, Storz G (2007) An antisense RNA controls synthesis of an SOS‐induced toxin evolved from an antitoxin. Mol Microbiol 64: 738–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K (2004) Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett 230: 13–18 [DOI] [PubMed] [Google Scholar]
- Keyser P, Elofsson M, Rosell S, Wolf‐Watz H (2008) Virulence blockers as alternatives to antibiotics: type III secretion inhibitors against Gram‐negative bacteria. J Internal Med 264: 17–29 [DOI] [PubMed] [Google Scholar]
- Khatib R, Jose J, Musta A, Sharma M, Fakih MG, Johnson LB, Riederer K, Shemes S (2011) Relevance of vancomycin‐intermediate susceptibility and heteroresistance in methicillin‐resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 66: 1594–1599 [DOI] [PubMed] [Google Scholar]
- Kim JS, Heo P, Yang TJ, Lee KS, Cho DH, Kim BT, Suh JH, Lim HJ, Shin D, Kim SK et al (2011) Selective killing of bacterial persisters by a single chemical compound without affecting normal antibiotic‐sensitive cells. Antimicrob Agents Chemother 55: 5380–5383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Zhu W, Hendricks GL, Van Tyne D, Steele AD, Keohane CE, Fricke N, Conery AL, Shen S, Pan W et al (2018) A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature 556: 103–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Zou G, Hari TPA, Wilt IK, Zhu W, Galle N, Faizi HA, Hendricks GL, Tori K, Pan W et al (2019) A selective membrane‐targeting repurposed antibiotic with activity against persistent methicillin‐resistant Staphylococcus aureus . Proc Natl Acad Sci USA 116: 16529–16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T, Abgottspon D, Wittwer M, Rabbani S, Herold J, Jiang X, Kleeb S, Lüthi C, Scharenberg M, Bezençon J et al (2010) FimH antagonists for the oral treatment of urinary tract infections: from design and synthesis to in vitro and in vivo evaluation. J Med Chem 53: 8627–8641 [DOI] [PubMed] [Google Scholar]
- Kong H‐K, Pan Q, Lo W‐U, Liu X, Law COK, Chan T‐f, Ho P‐L, Lau TC‐K (2018) Fine‐tuning carbapenem resistance by reducing porin permeability of bacteria activated in the selection process of conjugation. Sci Rep 8: 15248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korch SB, Henderson TA, Hill TM (2003) Characterization of the hipA7 allele of Escherichia coli and evidence that high persistence is governed by (p)ppGpp synthesis. Mol Microbiol 50: 1199–1213 [DOI] [PubMed] [Google Scholar]
- Korch SB, Hill TM (2006) Ectopic overexpression of wild‐type and mutant hipA genes in Escherichia coli: effects on macromolecular synthesis and persister formation. J Bacteriol 188: 3826–3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmidis C, Schindler BD, Jacinto PL, Patel D, Bains K, Seo SM, Kaatz GW (2012) Expression of multidrug resistance efflux pump genes in clinical and environmental isolates of Staphylococcus aureus . Int J Antimicrob Agents 40: 204–209 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte A, Block D, Drescher M, Windmüller N, Mellmann A, Baum C, Neumann C, Lorè NI, Bragonzi A, Liebau E et al (2014) Inactivation of thyA in Staphylococcus aureus attenuates virulence and has a strong impact on metabolism and virulence gene expression. mBio 5: e01447‐01414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S et al (2010) Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis 10: 597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan BW, Chowdhury N, Wood TK (2015) Combatting bacterial infections by killing persister cells with mitomycin C. Environ Microbiol 17: 4406–4414 [DOI] [PubMed] [Google Scholar]
- Kwan BW, Valenta JA, Benedik MJ, Wood TK (2013) Arrested protein synthesis increases persister‐like cell formation. Antimicrob Agents Chemother 57: 1468–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey RW (1984) Mechanisms of resistance to beta‐lactam antibiotics in Staphylococcus aureus . Scand J Infect Dis Suppl 42: 64–71 [PubMed] [Google Scholar]
- Lebeaux D, Ghigo JM, Beloin C (2014) Biofilm‐related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev 78: 510–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Vandlen R, DePalatis L, Raab H, Hazenbos WL, Morisaki JH et al (2015) Novel antibody‐antibiotic conjugate eliminates intracellular S. aureus . Nature 527: 323–328 [DOI] [PubMed] [Google Scholar]
- Leid JG, Shirtliff ME, Costerton JW, Stoodley P (2002) Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun 70: 6339–6345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leimer N, Rachmuhl C, Palheiros Marques M, Bahlmann AS, Furrer A, Eichenseher F, Seidl K, Matt U, Loessner MJ, Schuepbach RA et al (2016) Nonstable Staphylococcus aureus small‐colony variants are induced by low pH and sensitized to antimicrobial therapy by phagolysosomal alkalinization. J Infect Dis 213: 305–313 [DOI] [PubMed] [Google Scholar]
- Leitner L, Sybesma W, Chanishvili N, Goderdzishvili M, Chkhotua A, Ujmajuridze A, Schneider MP, Sartori A, Mehnert U, Bachmann LM et al (2017) Bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: a randomized, placebo‐controlled, double‐blind clinical trial. BMC Urol 17: 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoux M, Culviner PH, Liu YJ, Littlehale ML, Laub MT (2020) Stress can induce transcription of toxin‐antitoxin systems without activating toxin. Mol Cell 79: 280–292.e288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung V, Levesque CM (2012) A stress‐inducible quorum‐sensing peptide mediates the formation of persister cells with noninherited multidrug tolerance. J Bacteriol 194: 2265–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin‐Reisman I, Fridman O, Balaban NQ (2014) ScanLag: high‐throughput quantification of colony growth and lag time. J Vis Exp: 51456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin‐Reisman I, Gefen O, Fridman O, Ronin I, Shwa D, Sheftel H, Balaban NQ (2010) Automated imaging with ScanLag reveals previously undetectable bacterial growth phenotypes. Nat Methods 7: 737–739 [DOI] [PubMed] [Google Scholar]
- Levin‐Reisman I, Ronin I, Gefen O, Braniss I, Shoresh N, Balaban NQ (2017) Antibiotic tolerance facilitates the evolution of resistance. Science 355: 826–830 [DOI] [PubMed] [Google Scholar]
- Lewis K (2007) Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5: 48–56 [DOI] [PubMed] [Google Scholar]
- Lewis K (2012) Persister cells: molecular mechanisms related to antibiotic tolerance. Handb Exp Pharmacol 121–133 [DOI] [PubMed] [Google Scholar]
- Lewis K (2019) Persister cells and infectious disease. Springer: Springer; [Google Scholar]
- Li XZ, Nikaido H (2004) Efflux‐mediated drug resistance in bacteria. Drugs 64: 159–204 [DOI] [PubMed] [Google Scholar]
- Li Y, Powell DA, Shaffer SA, Rasko DA, Pelletier MR, Leszyk JD, Scott AJ, Masoudi A, Goodlett DR, Wang X et al (2012) LPS remodeling is an evolved survival strategy for bacteria. Proc Natl Acad Sci USA 109: 8716–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE et al (2011) Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin‐resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52: e18–55 [DOI] [PubMed] [Google Scholar]
- Loc‐Carrillo C, Abedon ST (2011) Pros and cons of phage therapy. Bacteriophage 1: 111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Jacome E, Franco‐Cendejas R, Quezada H, Morales‐Espinosa R, Castillo‐Juarez I, Gonzalez‐Pedrajo B, Fernandez‐Presas AM, Tovar‐Garcia A, Angarita‐Zapata V, Licona‐Limon P et al (2019) The race between drug introduction and appearance of microbial resistance. Current balance and alternative approaches. Curr Opin Pharmacol 48: 48–56 [DOI] [PubMed] [Google Scholar]
- Loss G, Simoes PM, Valour F, Cortes MF, Gonzaga L, Bergot M, Trouillet‐Assant S, Josse J, Diot A, Ricci E et al (2019) Staphylococcus aureus small colony variants (SCVs): news from a chronic prosthetic joint infection. Front Cell Infect Microbiol 9: 363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy FD (1998) Staphylococcus aureus infections. N Engl J Med 339: 520–532 [DOI] [PubMed] [Google Scholar]
- Luther A, Urfer M, Zahn M, Muller M, Wang SY, Mondal M, Vitale A, Hartmann JB, Sharpe T, Monte FL et al (2019) Chimeric peptidomimetic antibiotics against Gram‐negative bacteria. Nature 576: 452–458 [DOI] [PubMed] [Google Scholar]
- Lv L, Wan M, Wang C, Gao X, Yang Q, Partridge SR, Wang Y, Zong Z, Doi Y, Shen J et al (2020) Emergence of a plasmid‐encoded resistance‐nodulation‐division efflux pump conferring resistance to multiple drugs, including tigecycline, in Klebsiella pneumoniae . mBio 11: e02930‐19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manina G, Dhar N, McKinney JD (2015) Stress and host immunity amplify Mycobacterium tuberculosis phenotypic heterogeneity and induce nongrowing metabolically active forms. Cell Host Microbe 17: 32–46 [DOI] [PubMed] [Google Scholar]
- Maranan MC, Moreira B, Boyle‐Vavra S, Daum RS (1997) Antimicrobial resistance in staphylococci. Epidemiology, molecular mechanisms, and clinical relevance. Infect Dis Clin North Am 11: 813–849 [DOI] [PubMed] [Google Scholar]
- Marques CNH, Morozov A, Planzos P, Zelaya HM (2014) The fatty acid signaling molecule cis‐2‐decenoic acid increases metabolic activity and reverts persister cells to an antimicrobial‐susceptible state. Appl Environ Microbiol 80: 6976–6991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Tan Q, Awano N, Wu KP, Inouye M (2012) YeeU enhances the bundling of cytoskeletal polymers of MreB and FtsZ, antagonizing the CbtA (YeeV) toxicity in Escherichia coli . Mol Microbiol 84: 979–989 [DOI] [PubMed] [Google Scholar]
- Matsuo M, Hiramatsu M, Singh M, Sasaki T, Hishinuma T, Yamamoto N, Morimoto Y, Kirikae T, Hiramatsu K (2019) Genetic and transcriptomic analyses of ciprofloxacin‐tolerant Staphylococcus aureus isolated by the replica plating tolerance isolation system (REPTIS). Antimicrob Agents Chemother 63: e02019‐18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo‐Cristino J, Resina C, Manuel V, Lito L, Ramirez M (2013) First case of infection with vancomycin‐resistant Staphylococcus aureus in Europe. Lancet 382: 205 [DOI] [PubMed] [Google Scholar]
- Moker N, Dean CR, Tao J (2010) Pseudomonas aeruginosa increases formation of multidrug‐tolerant persister cells in response to quorum‐sensing signaling molecules. J Bacteriol 192: 1946–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefour K, Frieden J, Hurst S, Helmich C, Headley D, Martin M, Boyle DA (2008) Acinetobacter baumannii: an emerging multidrug‐resistant pathogen in critical care. Crit Care Nurse 28: 15–25; quiz 26 [PubMed] [Google Scholar]
- Moore MR, Perdreau‐Remington F, Chambers HF (2003) Vancomycin treatment failure associated with heterogeneous vancomycin‐intermediate Staphylococcus aureus in a patient with endocarditis and in the rabbit model of endocarditis. Antimicrob Agents Chemother 47: 1262–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA (2006) Methicillin‐resistant S. aureus infections among patients in the emergency department. N Engl J Med 355: 666–674 [DOI] [PubMed] [Google Scholar]
- Moyed HS, Bertrand KP (1983) hipA, a newly recognized gene of Escherichia coli K‐12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol 155: 768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullard A (2016) FDA approves antitoxin antibody. Nat Rev Drug Discov 15: 811 [DOI] [PubMed] [Google Scholar]
- Napier BA, Band V, Burd EM, Weiss DS (2014) Colistin heteroresistance in Enterobacter cloacae is associated with cross‐resistance to the host antimicrobial lysozyme. Antimicrob Agents Chemother 58: 5594–5597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro JP, Blaser MJ, Cunningham‐Rundles S (2000) Persistent bacterial infections. American Society of Microbiology; [Google Scholar]
- Nguyen D, Joshi‐Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, McKay G, Siehnel R, Schafhauser J, Wang Y et al (2011) Active starvation responses mediate antibiotic tolerance in biofilms and nutrient‐limited bacteria. Science 334: 982–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Decrulle AL, Fontaine F, Demarez A, Taddei F, Lindner AB (2012) Pre‐disposition and epigenetics govern variation in bacterial survival upon stress. PLoS Genet 8: e1003148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas I, Bordeau V, Bondon A, Baudy‐Floc'h M, Felden B (2019) Novel antibiotics effective against gram‐positive and ‐negative multi‐resistant bacteria with limited resistance. PLoS Biol 17: e3000337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoloff H, Hjort K, Levin BR, Andersson DI (2019) The high prevalence of antibiotic heteroresistance in pathogenic bacteria is mainly caused by gene amplification. Nat Microbiol 4: 504–514 [DOI] [PubMed] [Google Scholar]
- Norris SJ (2006) Antigenic variation with a twist–the Borrelia story. Mol Microbiol 60: 1319–1322 [DOI] [PubMed] [Google Scholar]
- O’Neill J (2014) Antimicrobial resistance: tackling a crisis for the health and wealth of nations. https://amr‐revieworg/sites/default/files/AMR%20Review%20Paper%20‐%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1pdf (last accessed online 10122019)
- Ooi N, Miller K, Randall C, Rhys‐Williams W, Love W, Chopra I (2010) XF‐70 and XF‐73, novel antibacterial agents active against slow‐growing and non‐dividing cultures of Staphylococcus aureus including biofilms. J Antimicrob Chemother 65: 72–78 [DOI] [PubMed] [Google Scholar]
- Page R, Peti W (2016) Toxin‐antitoxin systems in bacterial growth arrest and persistence. Nat Chem Biol 12: 208–214 [DOI] [PubMed] [Google Scholar]
- Painter KL, Strange E, Parkhill J, Bamford KB, Armstrong‐James D, Edwards AM (2015) Staphylococcus aureus adapts to oxidative stress by producing H2O2‐resistant small‐colony variants via the SOS. Response. 83: 1830–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey DP, Gerdes K (2005) Toxin‐antitoxin loci are highly abundant in free‐living but lost from host‐associated prokaryotes. Nucleic Acids Res 33: 966–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL (2007) Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6: 29–40 [DOI] [PubMed] [Google Scholar]
- Pedersen K, Gerdes K (1999) Multiple hok genes on the chromosome of Escherichia coli . Mol Microbiol 32: 1090–1102 [DOI] [PubMed] [Google Scholar]
- Pedersen MG, Olesen HV, Jensen‐Fangel S, Nørskov‐Lauritsen N, Wang M (2018) Colistin resistance in Pseudomonas aeruginosa and Achromobacter spp. cultured from Danish cystic fibrosis patients is not related to plasmid‐mediated expression of mcr‐1. J Cyst Fibros 17: e22–e23 [DOI] [PubMed] [Google Scholar]
- Pendleton JN, Gorman SP, Gilmore BF (2013) Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11: 297–308 [DOI] [PubMed] [Google Scholar]
- Pournaras S, Koumaki V, Spanakis N, Gennimata V, Tsakris A (2016) Current perspectives on tigecycline resistance in Enterobacteriaceae: susceptibility testing issues and mechanisms of resistance. Int J Antimicrob Agents 48: 11–18 [DOI] [PubMed] [Google Scholar]
- Proctor R (2019) Respiration and small colony variants of Staphylococcus aureus . Microbiol Spectr 7 10.1128/microbiolspec.GPP3-0069-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD (1995) Persistent and relapsing infections associated with small‐colony variants of Staphylococcus aureus . Clin Infect Dis 20: 95–102 [DOI] [PubMed] [Google Scholar]
- Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, Peters G (2006) Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol 4: 295–305 [DOI] [PubMed] [Google Scholar]
- Pu Y, Li Y, Jin X, Tian T, Ma Q, Zhao Z, Lin SY, Chen Z, Li B, Yao G et al (2019) ATP‐dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance. Mol Cell 73: 143–156.e4 [DOI] [PubMed] [Google Scholar]
- Pumbwe L, Piddock LJ (2000) Two efflux systems expressed simultaneously in multidrug‐resistant Pseudomonas aeruginosa . Antimicrob Agents Chemother 44: 2861–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Wang Y, Zhang Q, Chen X, Shen Z, Deng F, Wu C, Shen J (2012) Identification of a novel genomic island conferring resistance to multiple aminoglycoside antibiotics in Campylobacter coli . Antimicrob Agents Chemother 56: 5332–5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan AM, Bush K (2007) Carbapenemases: the versatile beta‐lactamases. Clin Microbiol Rev 20: 440–458, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quie PG (1969) Microcolonies (G‐variants) of Staphylococcus aureus . Yale J Biol Med 41: 394–403 [PMC free article] [PubMed] [Google Scholar]
- Ram G, Ross HF, Novick RP, Rodriguez‐Pagan I, Jiang D (2018) Conversion of staphylococcal pathogenicity islands to CRISPR‐carrying antibacterial agents that cure infections in mice. Nat Biotechnol 36: 971–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram S, Goulian M (2013) The architecture of a prototypical bacterial signaling circuit enables a single point mutation to confer novel network properties. PLoS Genet 9: e1003706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather IA, Kim BC, Bajpai VK, Park YH (2017) Self‐medication and antibiotic resistance: crisis, current challenges, and prevention. Saudi J Biol Sci 24: 808–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath S, Ghazal P, Gascoigne NR (2001) Hijacking and exploitation of IL‐10 by intracellular pathogens. Trends Microbiol 9: 86–92 [DOI] [PubMed] [Google Scholar]
- Reyes J, Aguilar AC, Caicedo A (2019) Carbapenem‐resistant klebsiella pneumoniae: microbiology key points for clinical practice. Int J Gen Med 12: 437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen M, Eriksson S, Clements M, Bergström S, Normark SJ (2003) The basis of persistent bacterial infections. Trends Microbiol 11: 80–86 [DOI] [PubMed] [Google Scholar]
- Rice LB (2010) Progress and challenges in implementing the research on ESKAPE pathogens. Infect Control Hosp Epidemiol 31(Suppl 1): S7–10 [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Rubio L, Chang W‐L, Gutiérrez D, Lavigne R, Martínez B, Rodríguez A, Govers SK, Aertsen A, Hirl C, Biebl M et al (2016) ‘Artilysation’ of endolysin λSa2lys strongly improves its enzymatic and antibacterial activity against streptococci. Sci Rep 6: 35382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhrig C, Huemer M, Lorgé D, Luterbacher S, Phothaworn P, Schefer C, Sobieraj AM, Zinsli LV, Mairpady Shambat S, Leimer N et al (2020) Targeting hidden pathogens: cell‐penetrating enzybiotics eradicate intracellular drug‐resistant Staphylococcus aureus . mBio 11: e00209–00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolauffs B, Bernhardt TM, von Eiff C, Hart ML, Bettin D (2002) Osteopetrosis, femoral fracture, and chronic osteomyelitis caused by Staphylococcus aureus small colony variants (SCV) treated by girdlestone resection–6‐year follow‐up. Arch Orthop Trauma Surg 122: 547–550 [DOI] [PubMed] [Google Scholar]
- Ronneau S, Hallez R (2019) Make and break the alarmone: regulation of (p)ppGpp synthetase/hydrolase enzymes in bacteria. FEMS Microbiol Rev 43: 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronneau S, Helaine S (2019) Clarifying the link between toxin‐antitoxin modules and bacterial persistence. J Mol Biol 431: 3462–3471 [DOI] [PubMed] [Google Scholar]
- Rowe SE, Wagner NJ, Li L, Beam JE, Wilkinson AD, Radlinski LC, Zhang Q, Miao EA, Conlon BP (2020) Reactive oxygen species induce antibiotic tolerance during systemic Staphylococcus aureus infection. Nat Microbiol 5: 282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond GP, Fineran PC (2015) A century of the phage: past, present and future. Nat Rev Microbiol 13: 777–786 [DOI] [PubMed] [Google Scholar]
- Schaaff F, Bierbaum G, Baumert N, Bartmann P, Sahl HG (2003) Mutations are involved in emergence of aminoglycoside‐induced small colony variants of Staphylococcus aureus . Int J Med Microbiol 293: 427–435 [DOI] [PubMed] [Google Scholar]
- Schilling NA, Berscheid A, Schumacher J, Saur JS, Konnerth MC, Wirtz SN, Beltran‐Belena JM, Zipperer A, Krismer B, Peschel A et al (2019) Synthetic lugdunin analogues reveal essential structural motifs for antimicrobial action and proton translocation capability. Angew Chem Int Ed Engl 58: 9234–9238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelcher M, Donovan DM, Loessner MJ (2012) Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7: 1147–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Muhlemann K, Droz S, Couzinet S, Casaulta C, Zimmerli S (2008) Clinical characteristics associated with isolation of small‐colony variants of Staphylococcus aureus and Pseudomonas aeruginosa from respiratory secretions of patients with cystic fibrosis. J Clin Microbiol 46: 1832–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber G, Ron EZ, Glaser G (1995) ppGpp‐mediated regulation of DNA replication and cell division in Escherichia coli . Curr Microbiol 30: 27–32 [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Piro KM, Xu W, Hansen S, Lewis K, Brennan RG (2009) Molecular mechanisms of HipA‐mediated multidrug tolerance and its neutralization by HipB. Science 323: 396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaber MJ, Graham CS, Sands BE, Gold HS, Carmeli Y (2003) Treatment with a broad‐spectrum cephalosporin versus piperacillin‐tazobactam and the risk for isolation of broad‐spectrum cephalosporin‐resistant Enterobacter species. Antimicrob Agents Chemother 47: 1882–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert H, von Eiff C, Fatkenheuer G (1999) Fatal case due to methicillin‐resistant Staphylococcus aureus small colony variants in an AIDS patient. Emerg Infect Dis 5: 450–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y, Brown Gandt A, Rowe SE, Deisinger JP, Conlon BP, Lewis K (2017) ATP‐dependent persister formation in Escherichia coli . mBio 8: e02267‐16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner D, Keefer CS (1941) significance of bacteremia caused by Staphylococcus aureus: a study of one hundred and twenty‐two cases and a review of the literature concerned with experimental infection in animals. Arch Intern Med 68: 851–875 [Google Scholar]
- Southwood RT, Rice JL, McDonald PJ, Hakendorf PH, Rozenbilds MA (1985) Infection in experimental hip arthroplasties. J Bone Joint Surg Br 67: 229–231 [DOI] [PubMed] [Google Scholar]
- Spellberg B, Bartlett JG, Gilbert DN (2013) The future of antibiotics and resistance. N Engl J Med 368: 299–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoering AL, Lewis K (2001) Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol 183: 6746–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan S, Oram M, Jensen B, Peterson LR, Fisher LM (1990) DNA gyrase gyrA mutations in ciprofloxacin‐resistant strains of Staphylococcus aureus: close similarity with quinolone resistance mutations in Escherichia coli . J Bacteriol 172: 7260–7262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes JM, Yang K, Swanson K, Jin W, Cubillos‐Ruiz A, Donghia NM, MacNair CR, French S, Carfrae LA, Bloom‐Ackerman Z et al (2020) A Deep Learning Approach to Antibiotic Discovery. Cell 180(688–702): e613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingle EL (1935) Studies on small colony variants of Staphylococcus aureus . J Bacteriol 29: 467–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y et al (2018) Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic‐resistant bacteria and tuberculosis. Lancet Infect Dis 18: 318–327 [DOI] [PubMed] [Google Scholar]
- Tande AJ, Patel R (2014) Prosthetic joint infection. Clin Microbiol Rev 27: 302–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanwar J, Das S, Fatima Z, Hameed S (2014) Multidrug resistance: an emerging crisis. Interdiscip Perspect Infect Dis 2014: 541340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover FC, Goering RV (2009) Methicillin‐resistant Staphylococcus aureus strain USA300: origin and epidemiology. J Antimicrob Chemother 64: 441–446 [DOI] [PubMed] [Google Scholar]
- Thisted T, Sorensen NS, Wagner EG, Gerdes K (1994) Mechanism of post‐segregational killing: Sok antisense RNA interacts with Hok mRNA via its 5'‐end single‐stranded leader and competes with the 3'‐end of Hok mRNA for binding to the mok translational initiation region. EMBO J 13: 1960–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen IP, Kadari P, Soper NR, Riddell S, Kiska D, Creech CB, Shaw J (2019) Molecular epidemiology of invasive Staphylococcus aureus infections and concordance with colonization isolates. J Pediatr 210: 173–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranchemontagne ZR, Camire RB, O'Donnell VJ, Baugh J, Burkholder KM (2016) Staphylococcus aureus strain USA300 perturbs acquisition of lysosomal enzymes and requires phagosomal acidification for survival inside macrophages. Infect Immun 84: 241–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler MF, Summers SM, Nguyen HT, Zacharia VM, Hightower GA, Smith JT, Conway T (2008) The global, ppGpp‐mediated stringent response to amino acid starvation in Escherichia coli . Mol Microbiol 68: 1128–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchscherr L, Medina E, Hussain M, Volker W, Heitmann V, Niemann S, Holzinger D, Roth J, Proctor RA, Becker K et al (2011) Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med 3: 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner NA, Sharma‐Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati M, Holland TL, Fowler VG Jr (2019) Methicillin‐resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol 17: 203–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnidge J, Christiansen K (2005) Antibiotic use and resistance‐proving the obvious. Lancet 365: 548–549 [DOI] [PubMed] [Google Scholar]
- Twort FW (1915) An investigation on the nature of ultra‐microscopic viruses. Lancet 186: 1241–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL (2012) Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 25: 682–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoson C, Wagner EG (2008) A small SOS‐induced toxin is targeted against the inner membrane in Escherichia coli . Mol Microbiol 70: 258–270 [DOI] [PubMed] [Google Scholar]
- Van den Bergh B, Fauvart M, Michiels J (2017) Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol Rev 41: 219–251 [DOI] [PubMed] [Google Scholar]
- Vazquez‐Laslop N, Lee H, Neyfakh AA (2006) Increased persistence in Escherichia coli caused by controlled expression of toxins or other unrelated proteins. J Bacteriol 188: 3494–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veening JW, Stewart EJ, Berngruber TW, Taddei F, Kuipers OP, Hamoen LW (2008) Bet‐hedging and epigenetic inheritance in bacterial cell development. Proc Natl Acad Sci USA 105: 4393–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega NM, Allison KR, Khalil AS, Collins JJ (2012) Signaling‐mediated bacterial persister formation. Nat Chem Biol 8: 431–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. P T 40: 277–283 [PMC free article] [PubMed] [Google Scholar]
- Verstraeten N, Knapen WJ, Kint CI, Liebens V, Van den Bergh B, Dewachter L, Michiels JE, Fu Q, David CC, Fierro AC et al (2015) Obg and membrane depolarization are part of a microbial bet‐hedging strategy that leads to antibiotic tolerance. Mol Cell 59: 9–21 [DOI] [PubMed] [Google Scholar]
- Vesga O, Groeschel MC, Otten MF, Brar DW, Vann JM, Proctor RA (1996) Staphylococcus aureus small colony variants are induced by the endothelial cell intracellular milieu. J Infect Dis 173: 739–742 [DOI] [PubMed] [Google Scholar]
- Vila J, Marti S, Sanchez‐Cespedes J (2007) Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii . J Antimicrob Chemother 59: 1210–1215 [DOI] [PubMed] [Google Scholar]
- Vogel J, Argaman L, Wagner EG, Altuvia S (2004) The small RNA IstR inhibits synthesis of an SOS‐induced toxic peptide. Curr Biol 14: 2271–2276 [DOI] [PubMed] [Google Scholar]
- von Eiff C, Bettin D, Proctor RA, Rolauffs B, Lindner N, Winkelmann W, Peters G (1997a) Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin Infect Dis 25: 1250–1251 [DOI] [PubMed] [Google Scholar]
- von Eiff C, Heilmann C, Proctor RA, Woltz C, Peters G, Gotz F (1997b) A site‐directed Staphylococcus aureus hemB mutant is a small‐colony variant which persists intracellularly. J Bacteriol 179: 4706–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eiff C, Vaudaux P, Kahl BC, Lew D, Emler S, Schmidt A, Peters G, Proctor RA (1999) Bloodstream infections caused by small‐colony variants of coagulase‐negative staphylococci following pacemaker implantation. Clin Infect Dis 29: 932–934 [DOI] [PubMed] [Google Scholar]
- Vulin C, Leimer N, Huemer M, Ackermann M, Zinkernagel AS (2018) Prolonged bacterial lag time results in small colony variants that represent a sub‐population of persisters. Nat Commun 9: 4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M (2004) Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 6: 269–275 [DOI] [PubMed] [Google Scholar]
- Wang Q, Chang C‐s, Pennini M, Pelletier M, Rajan S, Zha J, Chen Y, Cvitkovic R, Sadowska A, Heidbrink Thompson J et al (2016) Target‐agnostic identification of functional monoclonal antibodies against Klebsiella pneumoniae multimeric MrkA fimbrial subunit. J Infect Dis 213: 1800–1808 [DOI] [PubMed] [Google Scholar]
- Wang X, Lord DM, Cheng HY, Osbourne DO, Hong SH, Sanchez‐Torres V, Quiroga C, Zheng K, Herrmann T, Peti W et al (2012) A new type V toxin‐antitoxin system where mRNA for toxin GhoT is cleaved by antitoxin GhoS. Nat Chem Biol 8: 855–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexselblatt E, Oppenheimer‐Shaanan Y, Kaspy I, London N, Schueler‐Furman O, Yavin E, Glaser G, Katzhendler J, Ben‐Yehuda S (2012) Relacin, a novel antibacterial agent targeting the Stringent Response. PLoS Pathog 8: e1002925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2017) Prioritization of pathogens to guide discovery, research and development of new antibiotics for drug‐resistant bacterial infections, including tuberculosis. World Health Organization; [Google Scholar]
- Wilmaerts D, Windels EM, Verstraeten N, Michiels J (2019) General mechanisms leading to persister formation and awakening. Trends Genet 35: 401–411 [DOI] [PubMed] [Google Scholar]
- Windels EM, Michiels JE, Fauvart M, Wenseleers T, Van den Bergh B, Michiels J (2019) Bacterial persistence promotes the evolution of antibiotic resistance by increasing survival and mutation rates. ISME J 13: 1239–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson JS, Hooper DC, McHugh GL, Bozza MA, Swartz MN (1990) Mutants of Escherichia coli K‐12 exhibiting reduced killing by both quinolone and beta‐lactam antimicrobial agents. Antimicrob Agents Chemother 34: 1938–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WorldEconomicForum (2019) The Global Risks Report 2019. http://www3.weforum.org/docs/WEF_Global_Risks_Report_2019.pdf
- Wozniak A, Villagra NA, Undabarrena A, Gallardo N, Keller N, Moraga M, Roman JC, Mora GC, Garcia P (2012) Porin alterations present in non‐carbapenemase‐producing Enterobacteriaceae with high and intermediate levels of carbapenem resistance in Chile. J Med Microbiol 61: 1270–1279 [DOI] [PubMed] [Google Scholar]
- Wu D, Wang Q, Yang Y, Geng W, Wang Q, Yu S, Yao K, Yuan L, Shen X (2010) Epidemiology and molecular characteristics of community‐associated methicillin‐resistant and methicillin‐susceptible Staphylococcus aureus from skin/soft tissue infections in a children's hospital in Beijing, China. Diagn Microbiol Infect Dis 67: 1–8 [DOI] [PubMed] [Google Scholar]
- Wu Y, Vulic M, Keren I, Lewis K (2012) Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother 56: 4922–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR (2009) Characterization of a new metallo‐beta‐lactamase gene, bla(NDM‐1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 53: 5046–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Bogaki M, Nakamura M, Nakamura S (1990) Quinolone resistance‐determining region in the DNA gyrase gyrA gene of Escherichia coli . Antimicrob Agents Chemother 34: 1271–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman SB, Hussain MA, Nye R, Mehta V, Mamun KT, Hossain N (2017) A review on antibiotic resistance: alarm bells are ringing. Cureus 9: e1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zgurskaya HI, Lopez CA, Gnanakaran S (2015) Permeability barrier of gram‐negative cell envelopes and approaches to bypass it. ACS Infect Dis 1: 512–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Lin J, Ma J, Cronan JE, Wang H (2010) Triclosan resistance of Pseudomonas aeruginosa PAO1 is due to FabV, a triclosan‐resistant enoyl‐acyl carrier protein reductase. Antimicrob Agents Chemother 54: 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, Burian M, Schilling NA, Slavetinsky C, Marschal M et al (2016) Human commensals producing a novel antibiotic impair pathogen colonization. Nature 535: 511–516 [DOI] [PubMed] [Google Scholar]


