Abstract
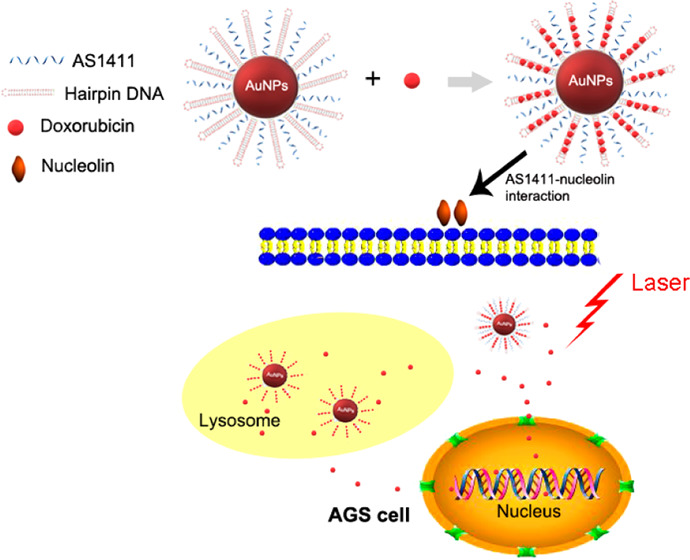
Gastric cancer therapy is still a big challenge, and nanomedicines bring much more hope. It is essential to develop multifunctional nanoparticles, especially those with high targeted capacity and antitumor effects, to improve gastric cancer therapy. In this study, we constructed AS1411 aptamer-based gold nanoparticles with appropriate size facilitating endocytosis and actively targeted drug delivery for gastric cancer cells via the specific AS1411–nucleolin interaction. The AS1411-based nanoparticles showed obviously increased targeted capacity towards AGS cells compared to random ssDNA-based nanoparticles. Meanwhile, compared to L929 cells, the AS1411-based nanoparticles showed selective drug uptake and delivery for AGS cells. Importantly, the AS1411-based nanoparticles exhibited significantly stronger antitumor effects on AGS cells under laser irradiation compared to chemotherapy alone. Our nanoparticles combined targeted drug delivery and efficient antitumor effects within one single nanoplatform, which are promising to be applied as targeted nanomedicines against gastric cancer.
Introduction
Currently, gastric cancer has the second highest mortality rate worldwide.1 Gastric cancer therapy is still a big challenge despite the progress in medical therapy methods.2−5 It is imperative to develop multifunctional nanoparticles with targeted and efficient cytotoxicity to overcome the side effects of nontargeted drugs.
Nanotechnology is promising for cancer treatment. The problem of targeting special localization and delivery needs to be solved to overcome the difficulty of transporting larger-sized nanoparticles across the cell membrane, more likely through nonspecific macropinocytosis.6 Besides, it has been reported that the nanoparticles show significantly greater uptake by gastrointestinal mucosal tissue when the sizes are about 100 nm.7 To increase the targeting efficiency, nanoparticles modified with a DNA or RNA aptamer are widely studied for their smaller size, low immunogenicity, and active targeted property not relying on the passive enhanced permeability and retention (EPR) effect of tumor tissues.8,9 Although some aptamers targeted to gastric cancer cells had been selected by SELEX,10,11 they were mainly used for diagnosis in the absence of antitumor studies in vitro or vivo. Nucleolin on the cell surface is a new molecular target for gastric cancer treatment. Disordered accumulation of nucleolin can promote the proliferation of gastric cancer cells12 and is associated with a worse prognosis for gastric cancer patients.13 It has been demonstrated that the AS1411 aptamer can strongly bind to nucleolin with high specificity.14−17 Therefore, it is worth developing AS1411-based nanoparticles for targeted gastric cancer therapy via the AS1411–nucleolin interaction.
Only a few studies have utilized AS1411 to guide gastric cancer therapy. For instance, a dendrimer-based pharmaceutical system guided by AS1411 had been designed to transfer 5-FU to gastric cancer cells.18 However, the oversized diameter might limit the specific absorption and endocytosis by targeted cells because the receptor-mediated pinocytosis always occurs at a size of about 100–120 nm.19 Multifunctional carbon nanotubes tagged with the AS1411 aptamer were designed to achieve gene and drug delivery into human gastric cancer cells.20 However, we need to emphasize its biological toxicity. Gold nanoparticles have gained much attention for use in gastric cancer theranostics in recent years.21−23 Among the diverse shapes of nanoparticles, spheres or stars exhibit the highest uptake rates compared with other shapes like flower, cube, rod, or disk.24
Spherical-gold nanoparticles (AuNPs) have become more attractive because of their highly efficient internalization and least cytotoxicity in biological safety.25 More importantly, AuNPs, with controllable sizes, are easy to be modified with targeting ligands or anticancer drugs on the surface and can convert optical energy into thermal energy with high efficiency.26 Near-infrared ray (NIR) light has been used for gastrointestinal cancer therapy because of its minimal absorption by human tissue and deeper tissue penetration.27,28 There was a recent study that reported a laser (785 nm, 1.2 W/cm2)-responsive gold nanoshell combining hyperthermia and siRNA gene therapy and immunotherapy for gastric cancer treatment.29 However, the laser power was limited because of a possible thermal damage to normal cells owing to the lack of specificity to cancer cells.
Herein, we performed the synthesis and application of AuNPs modified with the AS1411 aptamer and DNA rich in GC (named hairpin DNA). The double-stranded DNA or hairpin DNA rich in GC could load doxorubicin (DOX) by intercalation. The AS1411-based nanoparticles actively targeted AGS cells (nucleolin-abundant on cytomembrane12) mediated by the AS1411–nucleolin interaction and then entered into cells through endocytosis. Our nanosystem showed tumor-specific targeting as well as laser and pH dual-sensitive drug release, and exhibited excellent antitumor effects on AGS cells. Our multifunctional nanosystem may be potentially used as targeted therapy for gastric cancer in the future.
Results
Characterization of Aptamer-Based Gold Nanoparticles
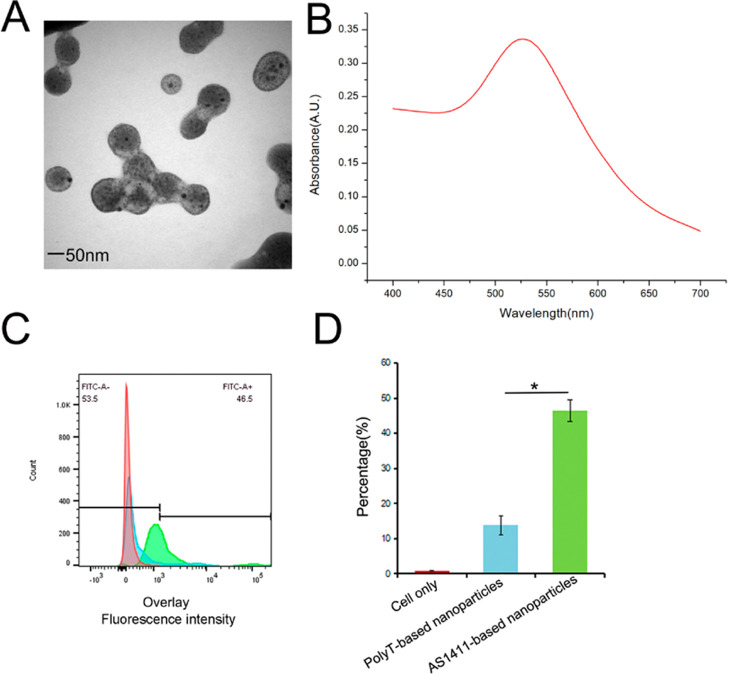
Transmission electron microscopy (TEM) and UV–vis results of AS1411-based nanoparticles are shown in Figure 1A,B. A higher fluorescence intensity of fluorescein amidite (FAM) in the AS1411-based nanoparticles group was observed in AGS cells compared to that in the random DNA (PolyT)-based nanoparticles group with about threefold higher binding (Figure 1C,D). This result indicated a stronger binding ability guided by the AS1411–nucleolin interaction, which contributed to targeted therapy.
Figure 1.
(A) TEM. (B) UV–vis. (C) Flow cytometric assay in AGS cells. Fluorescence from AGS cells labeled with FAM-AS1411 or FAM-PolyT. (D) Quantitative analysis of flow cytometry. *P < 0.05.
Increased Cellular Drug Delivery and Uptake
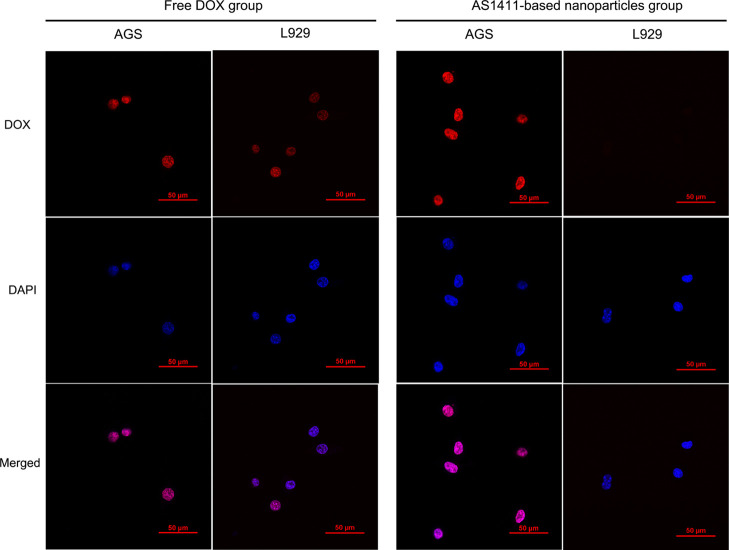
To evaluate the selective cytotoxicity to AGS cells, we detected fluorescent signal (red)-reflected intracellular drug delivery and uptake using L929 cells (low nucleolin expressing) as controls (Figure 2). The red fluorescence was almost parallel in both AGS and L929 cells incubated with free DOX, suggesting nonspecific uptake and side effects. When cells were treated with AS1411-based nanoparticles, stronger red fluorescent intensity was observed in the AGS cells, but less in the L929 cells, indicating the excellent targeted property.
Figure 2.
Confocal imaging (×600) of intracellular drug from free DOX or AS1411-based nanoparticles in AGS or L929 cells. The fluorescence signal represents DOX (red) or DAPI (blue).
Internalization and Drug Release of Nanoparticles
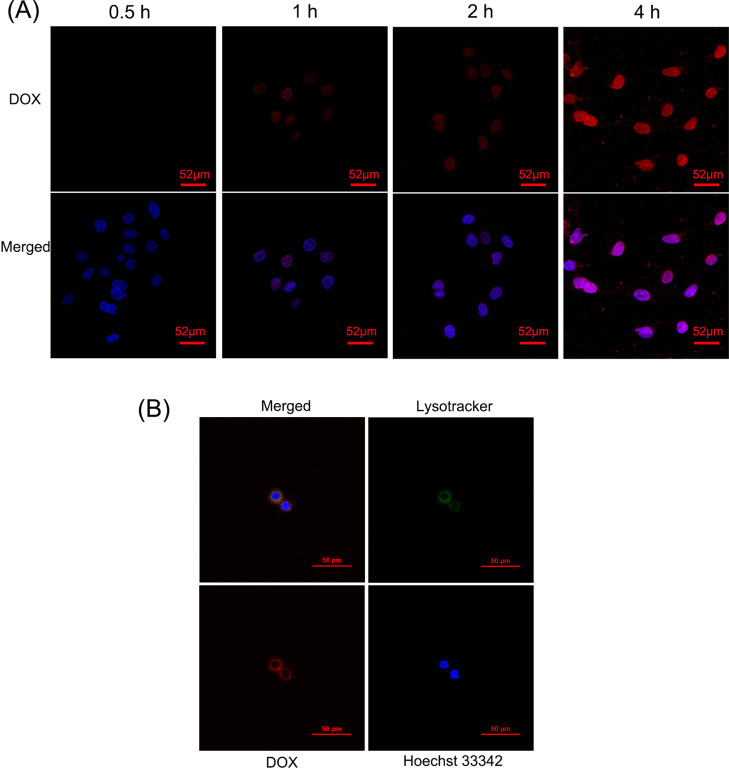
It has been reported that nanoparticles enter the cell mainly by endocytosis and then fuse with the early endosome, where the low pH induces fast degradation and drug release.19,30 The cellular internalization and cellular drug release course are shown in Figure 3A. To confirm the endocytosis process, the colocalization tested by the lysotracker showed that AS1411-based nanoparticles efficiently internalized into lysosomes (Figure 3B).
Figure 3.
(A) Confocal imaging (×400) of cellular uptake and cellular drug release course in AGS cells incubated with AS1411-based nanoparticles for different time periods. The fluorescence signal is represented by DOX (red) or DAPI (blue). (B) Colocalization confocal imaging (×600) of AS1411-based nanoparticles in AGS cells. The fluorescence signal represented the colocalization overlap color (yellow), Lysotracker (green), DOX (red), or Hoechst 33342 (blue).
Increased Cellular Drug Release by Laser Irradiation
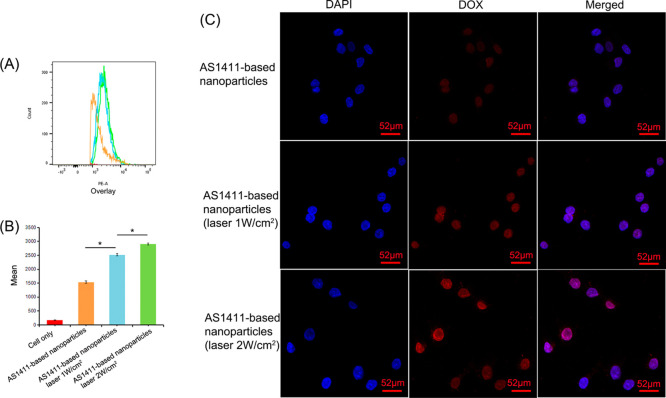
As shown in Figure 4, the mean fluorescence intensity of intracellular DOX was much stronger with the AS1411-based nanoparticles under the 808 nm laser irradiation than that without laser irradiation in the flow cytometry tests and confocal imaging. In particular, the fluorescent signal under laser irradiation at 2 W/cm2 was higher than that at 1 W/cm2, suggesting more laser-triggered drug release inside the cells and better chemotherapy effects.
Figure 4.
Laser-responsive DOX release. (A) Flow cytometry histograms displaying the fluorescence signals of DOX in AGS cells exposed to an 808 nm laser (1 or 2 W/cm2) for 10 min. (B) Quantitative analysis of the mean fluorescence intensity. *P < 0.05. (C) Confocal imaging of the AGS cells incubated by AS1411-based nanoparticles for 2 h and processed under 808 nm laser irradiation (1 or 2 W/cm2). The fluorescence signal is represented by DOX (red) or DAPI (blue).
Targeted Cytotoxicity Mediated by Aptamer-Guided Therapy
The laser caused slight heat damage to cells in the AuNPs + AS1411 + hairpin DNA group without DOX (Figure 5). Importantly, the AS1411-based nanoparticles group showed stronger cytotoxicity compared to the PolyT-based nanoparticles group under the same conditions. These results revealed that the AS1411–nucleolin interaction played an important role in increasing targeted cytotoxic effects on the AGS cells. In addition, the AS1411-based nanoparticles group displayed a remarkable reduction of cell viability when exposed to the laser at 2 or 2.5 W/cm2 compared to the free DOX group. These observations suggested the therapeutic advantage of AS1411-based nanoparticles for targeted and efficient cytotoxicity towards gastric cancer cells, which could be appropriate for gastric cancer cells treatment.
Figure 5.
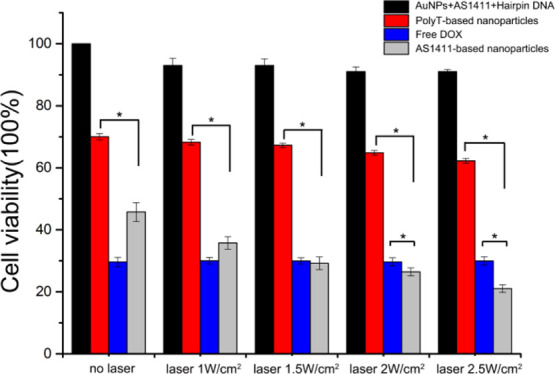
CCK-8 assay for AGS cells. Cytotoxicity of different nanoparticles under 808 nm laser irradiation at different powers (1, 1.5, 2, or 2.5 W/cm2) for 10 min or without laser. *P < 0.05.
Discussion
At present, targeted drug delivery through nanotechnology is the research focus for malignant tumor therapeutics. Doxorubicin (DOX) is one of the most commonly used traditional chemotherapy drugs.31 Nanoformulations of doxorubicin designed through nanotechnology are focused on achieving targeted drug delivery and overcoming the systemic toxicity of conventional cancer treatment.32 The aim of this study was to perform the synthesis and application of targeted nanoparticles based on AuNPs guided by the AS1411 aptamer and functional hairpin DNA loaded with DOX, which could realize precisely targeted chemotherapy and increased antitumor effects under laser irradiation.
In our study, we chose gold nanoparticles as nanocarriers because their large surface area binds more aptamers to increase the targeting affinity.33 TEM results showed the appropriate size of the AS1411-based nanoparticles, facilitating transport across gastric tumor cell membranes or endocytosis. Flow cytometry results showed the good targeting capacity of AS1411, which indeed showed about threefold higher binding ability to AGS cells compared with the random DNA. The targeting capacity was further demonstrated by confocal imaging in the in vitro experiment. When incubated with the AS1411-based-NPs, more intracellular DOX fluorescence signals were observed in AGS cells than in L929 cells.
In a recent study, Zhang et al. reported that a zinc-tetraphenylethene dual-targeted gold nanoprism provided strong photothermal therapy against SGC-7901 human gastric cancer cell growth.34 Gold nanomaterials had been demonstrated to absorb NIR light and then induce DNA dehybridization.35 It is worth noting that gold nanoshells absorb NIR light energy and increase their surface temperature to achieve ssDNA release at or near 37 °C with little or no temperature increase of the local environment.36 In our study, we detected the DOX release from hairpin DNA on the gold surface induced by laser irradiation. We observed that laser obviously promoted DOX release from the AS1411-based nanoparticles with an increase of power and extension of the irradiation time. Importantly, under laser irradiation, the AS1411-based nanoparticles released more drug at pH 5.0. These results suggested that the AS1411-based nanoparticles further released DOX after endocytosis into the lysosomes or in the acid intracellular environment of tumor cells and manifested increased chemotherapy effects under laser irradiation. Besides, we observed that there was almost no drug release at pH 7.4 (physiological condition) without laser, indicating the stability and safety of the AS1411-based nanoparticles.
To investigate the targeted antitumor effect, we measured the cell cytotoxicity of the AS1411-based nanoparticles using the CCK-8 assay using PolyT-based nanoparticles or free DOX as the control. To find the maximum anticancer effect with minimum power, we tested the cytotoxicity under different laser irradiation conditions. We observed that the cytotoxicity of the AS1411-based nanoparticles to AGS cells was always stronger than that of PolyT-based nanoparticles, further indicating the targeted chemotherapy capacity. It was more advantageous that the AS1411-based nanoparticle group exhibited more efficient cytotoxicity (about 22%) after laser exposure at 2.5 W/cm2 than the free DOX group (about 30%). Besides, there was minimal cell damage caused by AuNPs only modified with DNA. These results indicated that the AS1411-based nanoparticles could effectively target and kill the AGS cells guided by AS1411 under laser irradiation and were safer than traditional chemotherapy.
The preliminary results showed the advantage of the AS1411-based nanosystem with good safety, specifically targeted drug delivery capacity, and a favorable antitumor effect, which may be promising for targeted therapy of gastric cancer cells.
Conclusions
In summary, our nanosystem exhibited multiple attractive advantages, including appropriate size, stability, and safety, specifically targeted capacity, and potent antitumor effect. This multifunctional nanosystem may be promising for gastric cancer cells treatment.
Experimental Section
AS1411 (5′ SH C6-GGTGGTGGTGGTTGTGGTGGTGGTGG-3′ 6-FAM), random ssDNA PolyT (5′ SH C6-TTTTTTTTTTTTTTTTTTTTTTTTTT-3′ 6-FAM), and hairpin DNA (5′ SH C6-TTTTTTGCGTACGCGTACGCGATCTTTTGATCGCGTACGCGTACGC-3′ 6-FAM) were all purchased from Sangon Biotech. Hydrogen tetrachloroaurate hydrate (HAuCl4) was purchased from Sigma-Aldrich. DOX was purchased from Sangon Biotech. The DAPI and cell counting kit-8 (CCK-8) were purchased from Beyotime Biotechnology.
Preparation of AuNPs
AuNPs were synthesized following the literature procedures.37 Transmission electron microscopy (TEM) (OPTON EM 10C, Germany) was used to confirm the synthesis of AuNPs. The concentration of AuNPs was calculated by UV–vis measurements via Beer’s law (A = εbc).38
Immobilization of DNA on AuNPs
The equal molar DNA (AS1411 or hairpin DNA) was reacted with AuNPs through the Au–S bond. The loading of FAM-labeled DNA onto the AuNPs was dividedly determined by fluorescence measurements using an F2700 fluorescence spectrophotometer (Hitachi, Japan) at λexc = 492 nm, λem = 518 nm. Standard curves were dividedly made using known molar concentrations of FAM-labeled DNA. The supernatant containing free DNA was collected to calculate molar concentrations by a standard curve and the molar concentrations of nanoparticles were then calculated.
DOX Loading on the Nanoparticles
The AuNPs modified with DNA were dividedly incubated in free DOX (0.3 mg/L). The fluorescence signal of free DOX was quenched when it intercalated into the hairpin DNA rich in the GC sequence.39,40 After overnight incubation, the solutions were centrifuged at 18 000 rpm for 15 min to remove excess DOX. The supernatant containing unbound free DOX was collected to calculate concentrations by a DOX standard curve constructed by a fluorescence measurement at λexc = 480 nm, λem = 590 nm. The DOX concentrations of nanoparticles were calculated by subtracting them from the total free DOX before incubation. To obtain the DOX incorporation capacity of nanoparticles, the nanoparticles were incubated with DOX under different molar ratios (from 1:10 000 to 1:200), and the quenching of fluorescence intensity change of DOX was recorded.
Drug Release Test
The AS1411-based nanoparticles were irradiated under an 808 nm laser using the NIR laser equipment (Changchun New Industries Electronics Tech Co., Ltd., China) (2, 4, 6 W/cm2) or no laser (dark) at different time periods (0, 5, 10, 15, 30 min) at pH 7.4 or 5.0, respectively, at a room temperature of 25 °C; the fluorescence of DOX (λ = 590 nm) was recorded from the supernatant.
Cell Culture
AGS cells (Shanghai Branch of Chinese Academy of Science) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with high glucose containing 10% qualified fetal calf serum (FB15015; Clark Biosciences, Richmond, VA). L929 cells (Shanghai Branch of the Chinese Academy of Science) were cultured in DMEM containing 10% qualified horse serum (iCell Bioscience Inc, Shanghai, China). Cells were cultured in sterile flasks (Thermo Fisher Scientific, USA) in a humidified atmosphere containing 5% CO2 at a temperature of 37 °C.
Flow Cytometry
Before flow cytometric analysis (BD LSRFortessaTM, BD Bioscience, USA), cells were treated in ethylenediaminetetraacetic acid (EDTA) with no trypsin. Moreover, AGS cells were incubated with AS1411-based nanoparticles or equal molar PolyT-based nanoparticles for 1 h at 37 °C and then treated with or without 808 nm laser irradiation at 1 or 2 W/cm2 for 10 min. The mean fluorescence intensity of FAM or intracellular DOX was analyzed and calculated using Flow Jo 10.0.
Confocal Imaging
The AGS or L929 cells were cultured for 24 h and then treated with free DOX or AS1411-based nanoparticles at an equivalent DOX concentration (5 μg/mL) for different time periods. After AGS cells were treated with AS1411-based nanoparticles for 2 h, the cells were stained with LysoTracker Green DND-26 (Thermo Fisher Scientific) at 75 nM for 1 h, stained with Hoechst 33342, and then images were taken. To monitor drug release influenced by laser irradiation, after AGS cells were treated with AS1411-based nanoparticles for 2 h, the cells were irradiated by the 808 nm laser and fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 for 10 min, and then stained with DAPI. Images (×600) were taken using a confocal laser scanning microscope (Nikon Intensilight C-HGFIE).
CCK-8 Test
AGS cells were plated at a density of 10 000 cells per well for 24 h. The cells were treated with free DOX or AS1411-based nanoparticles at a DOX concentration (2.5 μg/mL) for 48 h. Meanwhile, the AGS cells were treated with nanoparticles (AuNPs + AS1411 + hairpin DNA, without DOX) at the same concentration of AS1411-based nanoparticles. AGS cells were irradiated under the 808 nm laser at different powers (1, 1.5, 2, or 2.5 W/cm2) for 10 min or without laser and then were given aforementioned arrangement. After 48 h, cells were treated with 10 μL of CCK-8 for 2 h. The percentage viability of the cells was calculated by the quantification of CCK-8 measured from the optical densities at a wavelength of 450 nm using a microplate reader (Model 550; Bio-Rad, Hercules, CA).
Statistical Analysis
All experiments were repeated at least three times. All quantitative data analyzed by SPSS 22.0 were expressed as means ± standard deviation. Values of P < 0.05 were considered statistically significant.
Acknowledgments
The authors would like to thank Prof. Jie Liu, Prof. Guo jie Liu, Dr. Hong jiu Ren, and Dr. Yao Zhang for their gracious guidance in the experimental design and execution.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04605.
TEM images of AuNPs, AuNPs + DNA ( AS1411 + Hairpin DNA ), AuNPs + DNA (AS1411 + Hairpin DNA) + DOX are shown in Figure S1. The molar ratio of per nanoparticle/DOX and drug release results are shown in Figures S2 and S3 (PDF)
Author Contributions
Y.Z., J.T., X.S., J.L., Y.M., and L.Z. contributed to the formulation of the research idea and participated in the preparation of the manuscript. Design, Y.Z. and Y.M.; conducting of experiments, Y.Z.; writing and editing, Y.Z.; funding, Y.M. and L.Z. All authors have read and approved the final manuscript.
This research was funded by the National Natural Science Foundation of China (Grant No. 21372262) and the Basic Research Program of Higher Education of Liaoning Province of China (Grant No. LQNK201742).
The authors declare no competing financial interest.
Supplementary Material
References
- Song Z.; Wu Y.; Yang J.; Yang D.; Fang X. Progress in the Treatment of Advanced Gastric Cancer. Tumor Biol. 2017, 39, 1010428317714626 10.1177/1010428317714626. [DOI] [PubMed] [Google Scholar]
- Adashek J. J.; Martinez Y. A.; Menta A. K.; Kurzrock R.; Kato S. Therapeutic Implications of Epidermal Growth Factor Receptor (EGFR) in the Treatment of Metastatic Gastric/GEJ Cancer. Front. Oncol. 2020, 10, 1312 10.3389/fonc.2020.01312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilson D. H. Advances in the treatment of gastric cancer. Curr. Opin. Gastroenterol. 2020, 36, 525–529. [DOI] [PubMed] [Google Scholar]
- de Guillebon E.; Roussille P.; Frouin E.; Tougeron D. Anti Program death-1/anti Program Death-Ligand 1 in Digestive Cancers. World J. Gastrointest. Oncol. 2015, 7, 95–101. 10.4251/wjgo.v7.i8.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabernero J.; Hof P. M.; Shen L.; Ohtsu A.; Shah M. A.; Cheng K.; Song C.; Wu H.; Eng-Wong J.; Kim K.; Kang Y. K. Pertuzumab Plus Trastuzumab and Chemotherapy for HER2-positive Metastatic Gastric or Gastro-Oesophageal Junction Cancer (JACOB): Final Analysis of a Double-Blind, Randomised, Placebo-Controlled Phase 3 Study. Lancet Oncol. 2018, 19, 1372–1384. 10.1016/S1470-2045(18)30481-9. [DOI] [PubMed] [Google Scholar]
- Gratton S. E. A.; Ropp P. A.; Pohlhaus P. D.; Luft J. C.; Madden V. J.; Napier M. E.; DeSimone J. M. The effect of particle design on cellular internalization pathways. Proc. Natl Acad. Sci. U.S.A. 2008, 105, 11613–11618. 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M. P.; Labhasetwar V.; Amidon G. L.; Levy R. J. Gastrointestinal Uptake of Biodegradable Microparticles: Effect of Particle Size. Pharm. Res. 1996, 13, 1838–1845. 10.1023/A:1016085108889. [DOI] [PubMed] [Google Scholar]
- Liao J.; Liu B.; Liu J.; Zhang J.; Chen K.; Liu H. Cell-specific Aptamers and Their Conjugation With Nanomaterials for Targeted Drug Delivery. Expert Opin. Drug Delivery 2015, 12, 493–506. 10.1517/17425247.2015.966681. [DOI] [PubMed] [Google Scholar]
- Zhu G.; Chen X. Aptamer-based Targeted Therapy. Adv. Drug Delivery Rev. 2018, 134, 65–78. 10.1016/j.addr.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H. Y.; Yuan A. H.; Shi X. S.; Chen W.; Miao Y. Evolution of a Gastric Carcinoma Cell-Specific DNA Aptamer by Live cell-SELEX. Oncol. Rep. 2014, 32, 2054–2060. 10.3892/or.2014.3411. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Zhang J.; Ma Y.; Pei X.; Liu Q.; Lu B.; Jin L.; Wang J.; Liu J. A Cell-Based Single-Stranded DNA Aptamer Specifically Targets Gastric Cancer. Int. J. Biochem. Cell Biol. 2014, 46, 1–8. 10.1016/j.biocel.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Watanabe T.; Hirano K.; Takahashi A.; Yamaguchi K.; Beppu M.; Fujiki H.; Suganuma M. Nucleolin on the Cell Surface as a New Molecular Target for Gastric Cancer Treatment. Biol. Pharm. Bull. 2010, 33, 796–803. 10.1248/bpb.33.796. [DOI] [PubMed] [Google Scholar]
- Qiu W.; Zhou F.; Zhang Q.; Sun X.; Shi X.; Liang Y.; Wang X.; Yue L. Overexpression of Nucleolin and Different Expression Sites Both Related to the Prognosis of Gastric Cancer. APMIS 2013, 121, 919–925. 10.1111/apm.12131. [DOI] [PubMed] [Google Scholar]
- Jia W.; Yao Z.; Zhao J.; Guan Q.; Gao L. New Perspectives of Physiological and Pathological Functions of Nucleolin (NCL). Life Sci. 2017, 186, 1–10. 10.1016/j.lfs.2017.07.025. [DOI] [PubMed] [Google Scholar]
- Jiang F.; Liu B.; Lu J.; Li F.; Li D.; Liang C.; Dang L.; Liu J.; He B.; Badshah S. A.; Lu C.; He X.; Guo B.; Zhang X. B.; Tan W.; Lu A.; Zhang G. Progress and Challenges in Developing Aptamer-Functionalized Targeted Drug Delivery Systems. Int. J. Mol. Sci. 2015, 16, 23784–23822. 10.3390/ijms161023784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdian-Robati R.; Bayat P.; Oroojalian F.; Zargari M.; Ramezani M.; Taghdisi S. M.; Abnous K. Therapeutic applications of AS1411 aptamer, an update review. Int. J. Biol. Macromol. 2020, 155, 1420–1431. 10.1016/j.ijbiomac.2019.11.118. [DOI] [PubMed] [Google Scholar]
- Reyes-Reyes E. M.; Teng Y.; Bates P. J. A New Paradigm for Aptamer Therapeutic AS1411 Action: Uptake by Macropinocytosis and Its Stimulation by a Nucleolin-Dependent Mechanism. Cancer Res. 2010, 70, 8617–8629. 10.1158/0008-5472.CAN-10-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzegar Behrooz A.; Nabavizadeh F.; Adiban J.; Ardestani M. S.; Vahabpour R.; Aghasadeghi M. R.; Sohanaki H. Smart Bomb AS1411 aptamer-functionalized/PAMAM Dendrimer Nanocarriers for Targeted Drug Delivery in the Treatment of Gastric Cancer. Clin. Exp. Pharmacol. Physiol. 2017, 44, 41–51. 10.1111/1440-1681.12670. [DOI] [PubMed] [Google Scholar]
- Sahay G.; Alakhova D. Y.; Kabanov A. V. Endocytosis of Nanomedicines. J. Controlled Release 2010, 145, 182–95. 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi S.; Nia A. H.; Abnous K.; Ramezani M. Polyethylenimine-functionalized Carbon Nanotubes Tagged With AS1411 Aptamer for Combination Gene and Drug Delivery Into Human Gastric Cancer Cells. Int. J. Pharm. 2017, 516, 301–312. 10.1016/j.ijpharm.2016.11.027. [DOI] [PubMed] [Google Scholar]
- Li C. F.; Wang Y. M.; Zhang H. F.; Li M.; Zhu Z. Y.; Xue Y. W. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized gold nanoparticles using Cardiospermum halicacabum on AGS gastric carcinoma cells. Int. J. Nanomed. 2019, 14, 951–962. 10.2147/IJN.S193064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Arunachalam C.; Sulaiman A. A.; Zhu J. Biosynthesis of gold nanoparticles using Vetex negundo and evaluation of pro-apoptotic effect on human gastric cancer cell lines. J. Photochem. Photobiol., B 2020, 203, 111749 10.1016/j.jphotobiol.2019.111749. [DOI] [PubMed] [Google Scholar]
- Zhang A.; Pan S. J.; Zhang Y. H.; Chang J.; Cheng J.; Huang Z. C.; Li T. L.; Zhang C. L.; Fuentea J. M. D. L.; Zhang Q.; Cui D. X. Carbon-gold hybrid nanoprobes for real-time imaging, photothermal/photodynamic and nanozyme oxidative therapy. Theranostics. 2019, 9, 3443–3458. 10.7150/thno.33266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisca F. V.; Andrés C. L.; Octavio T. R.; Laura A. P. Understanding Cellular Interactions With Nanomaterials: Towards a Rational Design of Medical Nanodevices. Nanotechnology 2020, 31, 132002 10.1088/1361-6528/ab5bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckiewicz K. P.; Ewelina B.; Anna M.; Agata Z. P.; Grzegorz N.; Adriana Z. M.; Iwona I. S. Impact of Gold Nanoparticles Shape on Their Cytotoxicity Against Human Osteoblast and Osteosarcoma in in Vitro Model. Evaluation of the Safety of Use and Anti-Cancer Potential. J. Mater. Sci. Mater. Med. 2019, 30, 22 10.1007/s10856-019-6221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeta Rama Raju G.; Benton L.; Pavitra E.; Yu J. S. Multifunctional Nanoparticles: Recent Progress in Cancer Therapeutics. Chem. Commun. 2015, 51, 13248–13259. 10.1039/C5CC04643B. [DOI] [PubMed] [Google Scholar]
- Weissleder R.; Ntziachristos V. Shedding Light Onto Live Molecular Targets. Nat. Med. 2003, 9, 123–128. 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- Singh M.; Harris-Birtill D. C. C.; Markar S. R.; Hanna G. B.; Elson D. S. Application of Gold Nanoparticles for Gastrointestinal Cancer Theranostics: A Systematic Review. Nanomedicine 2015, 11, 2083–2098. 10.1016/j.nano.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Zhao T.; Han F.; Hu Y.; Li Y. Photothermal and Gene Therapy Combined With Immunotherapy to Gastric Cancer by the Gold Nanoshell-Based System. J. Nanobiotechnology 2019, 17, 80 10.1186/s12951-019-0515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou L.; Sun J.; Zhai Y.; He Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian. J. Pharm. Sci. 2013, 8, 1–10. 10.1016/j.ajps.2013.07.001. [DOI] [Google Scholar]
- Scartozzi M.; Galizia E.; Verdecchia L.; Berardi R.; Antognoli S.; Chiorrini S.; Cascinu S. Chemotherapy for advanced gastric cancer: across the years for a standard of care. Expert Opin. Pharmacother. 2007, 8, 797–808. 10.1517/14656566.8.6.797. [DOI] [PubMed] [Google Scholar]
- Borišev I.; Mrđanovic J.; Petrovic D.; Seke M.; Jović D.; Srđenović B.; Latinovic N.; Djordjevic A. Nanoformulations of doxorubicin: how far have we come and where do we go from here?. Nanotechnology 2018, 29, 332002 10.1088/1361-6528/aac7dd. [DOI] [PubMed] [Google Scholar]
- Dam D. H. M.; Lee R. C.; Odom T. W. Improved in Vitro Efficacy of Gold Nanoconstructs by Increased Loading of G-quadruplex Aptamer. Nano Lett. 2014, 14, 2843–2848. 10.1021/nl500844m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Ding X.; Cheng H.; Yin C.; Yan J.; Mou Z.; Wang W.; Cui D.; Fan C.; Song D. Dual-Targeted Gold Nanoprism for Recognition of Early Apoptosis, Dual-Model Imaging and Precise Cancer Photothermal Therapy. Theranostics 2019, 9, 5610–5625. 10.7150/thno.34755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A. M.; Hogan N. J.; Gottheim S.; Li C.; Clare S. E.; Halas N. J. Understanding Resonant Light-Triggered DNA Release from Plasmonic Nanoparticles. ACS Nano. 2017, 11, 171–179. 10.1021/acsnano.6b06510. [DOI] [PubMed] [Google Scholar]
- Huschka R.; Barhoumi A.; Liu Q.; Roth J. A.; Ji L.; Halas N. J. Gene Silencing by Gold Nanoshell-Mediated Delivery and Laser-Triggered Release of Antisense Oligonucleotide and siRNA. ACS Nano 2012, 6, 7681–7691. 10.1021/nn301135w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frens G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. 10.1038/physci241020a0. [DOI] [Google Scholar]
- Haiss W.; Thanh N. T.; Aveyard J.; Fernig D. G. Determination of Size and Concentration of Gold Nanoparticles From UV–vis Spectra. Anal. Chem. 2007, 79, 4215–4221. 10.1021/ac0702084. [DOI] [PubMed] [Google Scholar]
- Yi Y.; Wang H.; Wang X.; Liu Q.; Ye M.; Tan W. A Smart, Photocontrollable Drug Release Nanosystem for Multifunctional Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 5847–5854. 10.1021/acsami.6b15414. [DOI] [PubMed] [Google Scholar]
- Li Z. H.; Chen Y. J.; Yang Y.; Yu Y.; Zhang Y. H.; Zhu D. H.; Yu X. P.; Ouyang X. X.; Xie Z. Y.; Zhao Y. L.; Li L. J. Recent Advances in Nanomaterials-Based Chemo-Photothermal Combination Therapy for Improving Cancer Treatment. Front. Bioeng. Biotechnol. 2019, 7, 293 10.3389/fbioe.2019.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.