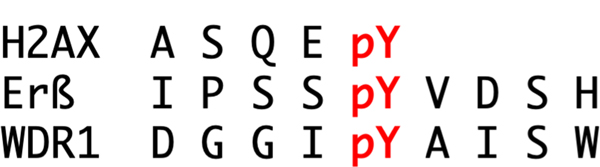Abstract
The Eyes Absent (EYA) proteins are the only known instance of a single polypeptide housing the following three separable biochemical activities: tyrosine phosphatase, threonine phosphatase, and transactivation. This uniquely positions the EYAs to participate in both transcriptional regulation and signal transduction pathways. But it also complicates the assignment of biological roles to individual biochemical activities through standard loss-of-function experiments. Nevertheless, there is an emerging literature linking developmental and pathological functions with the various EYA activities, and a growing list of disease states that might benefit from EYA-targeted therapeutics. There also remain multiple unresolved issues with significant implications for our understanding of how the EYAs might impact such ubiquitous signaling cascades as the MYC and Notch pathways.
This review will describe the unique juxtaposition of biochemical activities in the EYAs, their interaction with signaling pathways and cellular processes, emerging evidence of roles in disease states, and the feasibility of therapeutic targeting of individual EYA activities. We will focus on the phosphatase activities of the vertebrate EYA proteins and will examine the current state of knowledge regarding:
substrates and signaling pathways affected by the EYA tyrosine phosphatase activity
modes of regulation of the EYA tyrosine phosphatase activity
signaling pathways that implicate the threonine phosphatase activity of the EYAs including a potential interaction with PP2A-B55α
the interplay between the two phosphatase activities and the transactivation function of the EYAs
disease states associated with the EYAs and the current state of development of EYA-targeted therapeutics.
Keywords: Eyes Absent, EYA, PTP, threonine phosphatase, MYC, Notch, DNA damage repair, H2AX
INTRODUCTION
The reversible phosphorylation of proteins is among the most significant biochemical mechanisms through which cells respond to changing microenvironments. 75% of the human proteome is dynamically phosphorylated on either serine, threonine or tyrosine residues, with phosphotyrosines (pY) being the least frequent (accounting for <1% of the total phosphoproteome) (Hunter and Sefton 1980; Sharma et al. 2014). Protein phosphorylation is spatially and temporally regulated by the coordinated actions of protein kinases and protein phosphatases. Protein phosphatases are broadly divided into two groups – the Ser/Thr phosphatases (PSP) and the protein tyrosine phosphatases (PTP). PSPs are further divided into the phosphoprotein phosphatases (PPP e.g. PP2A, PP1, Calcineurin), metal-dependent phosphatases (PPMs e.g. PP2C), and the Asp-based phosphatases (defined by the DXDXT/V catalytic signature and comprised of the phosphoserine phosphatases responsible for dephosphorylating the C-terminal domain of RNA polymerase II (FCP1 and SCPs) and the cofilin phosphatase chronophin (Gohla et al. 2005)). The PTP superfamily is defined by the C[X5]R catalytic motif and includes enzymes that dephosphorylate only Tyr residues, Ser/Thr and Tyr residues (dual-specific phosphatases DSPs), and other substrates including mRNA and phosphoinositides (reviewed in (Alonso et al. 2004)). The Eyes Absent (EYA) proteins (schematized in Figure 1), the subject of this review, are particularly enigmatic phosphatases for several reasons. First, they have phosphotyrosine phosphatase activity in a domain that does not contain the C[X5]R catalytic motif. Second, a separate domain has been attributed with phosphothreonine (pT) phosphatase activity (Okabe et al. 2009; Sano and Nagata 2011) although there is no sequence homology with the PPP, PPM, DSP or Asp-based classes of pS/pT phosphatases. Further distinguishing the EYA proteins, they can also act as transcriptional activators when associated with a DNA-binding partner protein.
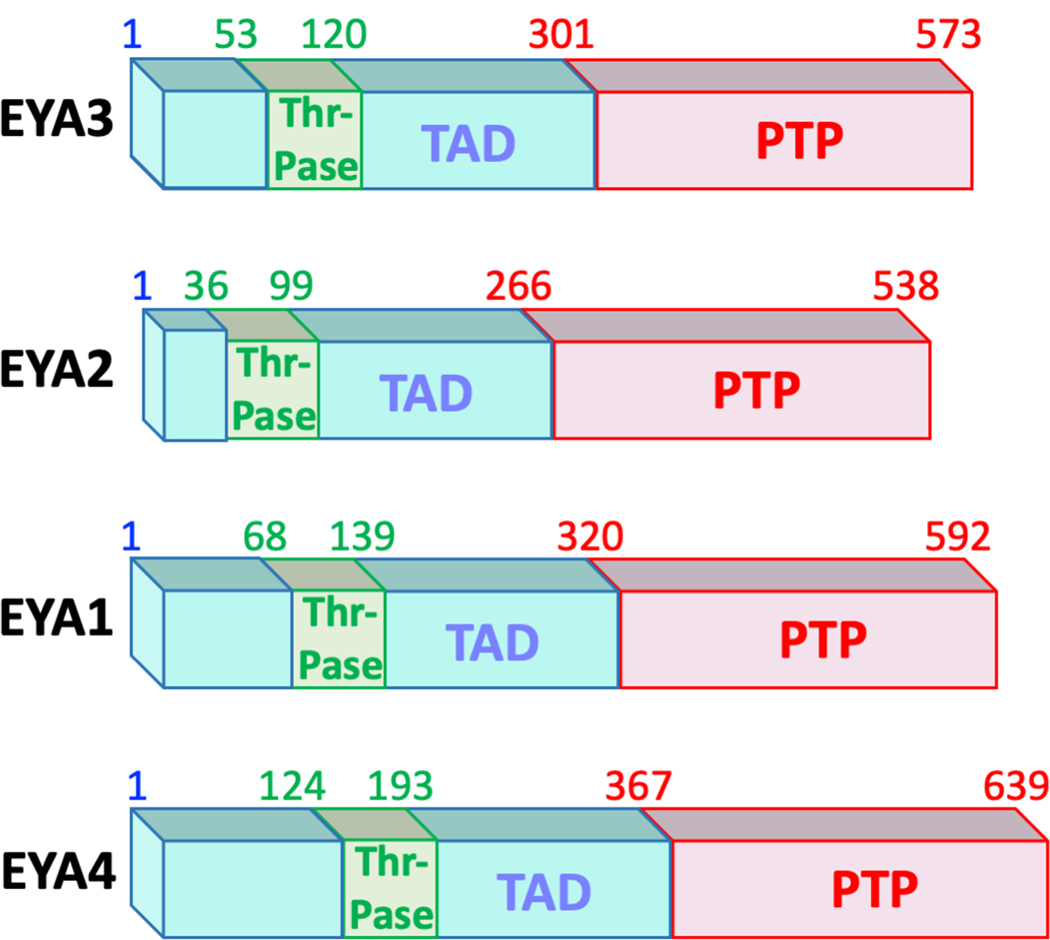
Fig. 1.
The domain structure of the four human EYA proteins. Amino acid numbering corresponding to the three domains is shown above each protein schematic. The most conserved domain is the C-terminal EYA-domain (ED) that houses the protein tyrosine phosphatase (PTP) activity (shown in red). The N-terminal domains (shown in blue) is poorly conserved both in terms of length and amino acid sequence and is relatively unstructured. A transactivation function (TAD) is present in this domain. A small 67-amino acid region within the N-terminal domain has been ascribed with Threonine phosphatase activity (green box), though it is possible that this activity is mediated through interaction with PP2A (see text). A color version of this figure is available online.
Since the initial reports in 2003 showing that the C-terminal domain of the EYA proteins belongs to the haloacid dehalogenase (HAD) structural family and use an Asp-based mechanism to execute its tyrosine phosphatase activity (Li X et al. 2003; Rayapureddi et al. 2003; Tootle et al. 2003) there have been extensive studies providing insights into the biochemical, structural, cellular, and biological roles of the EYA proteins. Notably there is a growing literature associating the EYA proteins with human disease states, and evidence that modulating specific EYA biochemical activities can have therapeutic benefits. These advances make it particularly essential that the associations between biochemical activity and biological functions of this unusual class of multi-functional proteins are clearly defined. This review presents the current state of understanding of each phosphatase activity of the vertebrate EYA proteins and their cellular and biological functions.
EYA PROTEIN TYROSINE PHOSPHATASE ACTIVITY
The C-terminal 273 amino-acid domain of the four vertebrate EYA proteins (EYA1, EYA2, EYA3, and EYA4) is the most conserved and is commonly referred to as the EYA domain (ED) (Figures 1, 2). Early studies showed that the ED domain mediated interaction with the sine-oculis (SO) family of homeodomain transcription factors (the SIX family) leading to the well-accepted proposal that EYA-SIX interaction translocates the intrinsically cytosolic EYA proteins to the nucleus where they could act as transcriptional activators (Ohto et al. 1999). The recognition that the ED domain housed the defining DXDXT/V (where X refers to any amino acid) motif found in the HAD family of phosphatases led to an initial search for enzymatic activity, resulting in the unexpected recognition that the ED domain was a tyrosine-specific phosphatase (Rayapureddi et al. 2003; Tootle et al. 2003; Rayapureddi et al. 2005). Interestingly, in one of these early studies full-length EYA3 protein was found to have phosphatase activity towards pS/pT and pY peptides, but mutation of the first conserved Aspartate in the DXDXT/V motif resulted in a complete loss of phosphatase activity towards the model substrate pNPP (Li X et al. 2003). While initially reported as evidence that the EYA proteins were dual-specificity phosphatases (Li X et al. 2003), studies with isolated ED domains suggested otherwise (Rayapureddi et al. 2003; Tootle et al. 2003; Rayapureddi et al. 2005). Nevertheless, these first observations with full-length EYA protein were an early indication that the N-terminal domain may have an independent catalytic activity (see discussion below). While the biochemical activities of the EYA N-terminal domain remain a subject of active debate, the tyrosine phosphatase activity of the ED domain has been validated in multiple in vitro and in vivo contexts over the years. Notably plant EYA proteins have only the C-terminal tyrosine phosphatase domain (Rayapureddi et al. 2005).
Fig. 2.
Amino acid sequence alignment of the four human EYA proteins using CLUSTAL Omega (Sievers et al. 2011). The transactivation domain (blue box), putative Threonine phosphatase domain (green shaded box) and the tyrosine phosphatase (PTP) domain (red box) are indicated. The degree of conservation is shown below the amino acid sequences. An asterisk (*) indicates a fully conserved residue, a colon (:) indicates conservation between groups of strongly similar properties, and a period (.) indicates a group exhibiting weak similarity. A color version of this figure is available online.
Fundamental to an understanding of the biological roles of the EYA family PTPs is the identification of substrates. The DNA damage sensing histone H2A variant H2AX (Cook et al. 2009; Krishnan et al. 2009), the tumor suppressor estrogen receptor ERß (Yuan et al. 2014), and the cytoskeleton modulating WD-repeat-containing protein WDR1 (Mentel et al. 2018) have all been proposed as EYA PTP substrates. The evidence supporting each of these, the kinase responsible for substrate phosphorylation, and the proposed biological relevance of each kinase – substrate - EYA-PTP module is briefly outlined below.
H2AX:
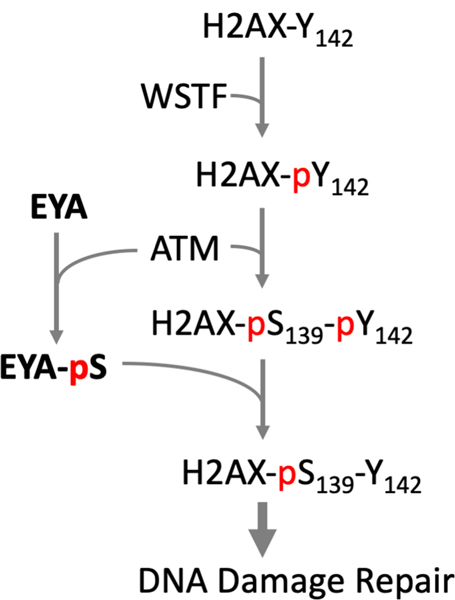
Phosphorylation of the minor histone protein H2AX on Ser139 (to form γH2AX) is one of the earliest responses to DNA damage and is essential for the assembly of DNA damage response (DDR) complexes at DNA double-strand break sites. H2AX is also constitutively phosphorylated at its C-terminal tyrosine residue (Y142) by the William-Beuren syndrome Transcription Factor WSTF (also known as BAZ1B), a component of the WICH ATP-dependent chromatin remodeling complex (Xiao et al. 2009). These authors postulated that Y142 phosphorylation is necessary for initial adjustment of the chromatin structure at sites of DNA breaks to permit Ser139 phosphorylation, and that subsequent dephosphorylation of Tyr142 would allow assembly of repair complexes containing MDC1. The EYA-PTPs play a role in this process. EYA1 and EYA3 are phosphorylated by the DNA-damage dependent ATM kinase (Cook et al. 2009) and can dephosphorylate H2AX-pY142 and thus promote DNA damage repair (Cook et al. 2009; Krishnan et al. 2009) (Fig. 3). In the absence of EYA, DNA damaged cells showed higher levels of H2AX-pY142, and apoptosis was favored over DNA damage repair and cell survival. ATM-dependent localization of a Zinc Finger protein ZNF506 has now been shown to recruit EYA proteins to double-strand breaks and regulate the dynamics of H2AX-pY142 dephosphorylation (Nowsheen et al. 2018). EYA-mediated H2AX-pY142 dephosphorylation is required for transcriptional silencing at transcribed active damage sites thus contributing to genomic integrity through coordination between the transcription machinery and DNA damage surveillance (Ji et al. 2019).
Fig. 3.
EYA PTPs promote DNA damage repair pathway by dephosphorylating Tyr142 of H2AX. H2AX is constitutively phosphorylated on Tyr142 by the WSTF kinase. Upon DNA damage the ATM kinase phosphorylates Ser139 of H2AX to form γH2AX, and also phosphorylates a Serine residue in the EYA proteins. EYA can dephosphorylate H2AX at Tyr142 (pY142). Doubly phosphorylated H2AX promotes apoptosis, while γH2AX dephosphorylated at pY142 permits assembly of a DNA damage repair complex (Cook et al. 2009). A color version of this figure is available online.
The role of EYA-promoted DNA damage repair in normal physiology has been investigated in a limited number of contexts. Local hypoxia during organ development is known to induce replication stress and DNA damage and EYA-promoted damage repair is seen during kidney development (Cook et al. 2009) and in limb regeneration (Sousounis et al. 2020). Angiogenesis is another developmental process in which an association between hypoxia, DNA damage and repair has been observed (Economopoulou et al. 2009). Loss of EYA3 in endothelial cells retards the post-natal development of the mouse retinal vasculature (Tadjuidje et al. 2012; Wang et al. 2016). Extensive DNA damage and meiotic recombination also occurs in male germ cells. EYA3 and EYA4 are highly expressed during spermatogenesis (Namekawa et al. 2006) and male infertility has been reported in Eya4−/− mice (Depreux et al. 2008) suggestive of a role for EYA4. Curiously, in germ cells phosphorylation of H2AX-pY142 occurs in the absence of WSTF/BAZ1B suggesting the existence of another as-yet-unidentified H2AX-Y142 phosphorylating kinase (Broering et al. 2015). Further investigation of the role of EYAs in germ cell meiosis could be revealing.
DNA damage repair also contributes to several pathologies. For instance, there is a complex interplay between DNA damage repair pathways and the length and degree of hypoxia in the tumor microenvironment (Scanlon and Glazer 2015; Riffle and Hegde 2017; Riffle et al. 2017). Similarly, pathological angiogenesis is driven by hypoxia-induced signaling cascades that include DNA damage response pathways (Economopoulou et al. 2009). A role for the EYA PTP activity has been noted in several instances of pathological angiogenesis: in a tumor xenograft model loss of EYA3 in tumor cells reduces the number of DNA repair foci in tumor tissue (Wang et al. 2018), and loss of endothelial EYA3 inhibits both γH2AX foci formation and retinal neovascularization in a classical model of hypoxia-induced pathological angiogenesis (Wang et al. 2016). However, one caveat in the interpretation of studies using loss of EYA proteins is that the EYA PTP activity also promotes cell migration (Pandey et al. 2010; Tadjuidje et al. 2012). Hence cell motility defects, in addition to defects in DNA damage repair, could contribute to any of the observed phenotypes.
Estrogen Receptor ERß:
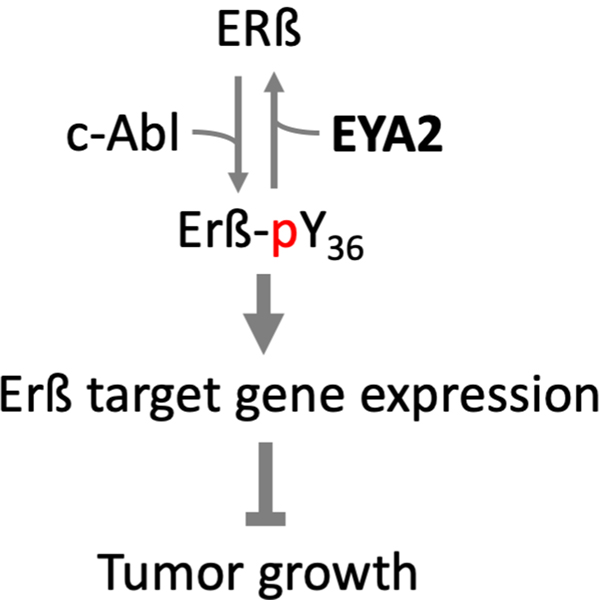
ERß acts as a tumor suppressor in multiple cancer types. The anti-proliferative and anti-invasive activity of ERß in breast cancer cells is dependent on phosphorylation of Tyr36 (Y36) by the c-Abl kinase (Yuan et al. 2014) (Fig. 4). EYA2 reverses this process by dephosphorylating ERß pY36, thus promoting tumor cell growth and opposing the action of c-Abl. This ligand-independent mechanism of ERß activation may be responsible for the tumor repressive effects of ERß in the absence of exogenous ligand, and when c-Abl/EYA2 ratios are high. It is also consistent with multiple reports associating high EYA2 levels with a worse prognosis in breast cancer (Farabaugh et al. 2012; Xu H et al. 2019).
Fig. 4.
EYA2 dephosphorylates Estrogen Receptor ß (ERß) at Tyr36. Phosphorylation of ERß by c-Abl converts it into a tumor suppressor. EYA2 dephosphorylates ERß reversing this process and promoting tumor growth (Yuan et al. 2014). A color version of this figure is available online.
WDR1:
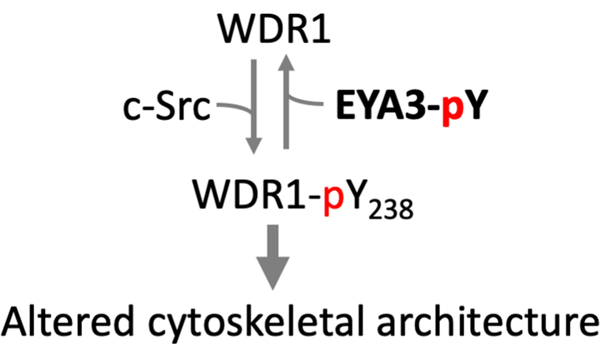
In a phosphopeptide array screen Mentel et al identified the WD repeat containing protein 1, WDR1, as an EYA3 PTP substrate (Mentel et al. 2018). WDR1 (also known as actin interacting protein 1 Aip1) promotes disassembly of actin-depolymerizing factor/cofilin-decorated actin filaments (F-actin), an essential step in the dynamic regulation of the actin cytoskeleton. Using in vitro studies, the authors showed that c-Src kinase phosphorylates WDR1, and EYA3 (but not EYA1) dephosphorylates WDR1. The phosphorylation state of WDR1 affects cytoskeletal architecture in over-expression studies conducted in 293T cells (Fig. 5). This is the second report linking the actin cytoskeleton with EYA3 PTP activity. A previous study showed that significant cytoskeletal changes occur in breast cancer cells upon over-expression of either wild-type or PTP-dead forms of EYA3, and that this was accompanied by changes in the levels of activation of the Rho/Rac/Cdc42 GTPases (Pandey et al. 2010); however no EYA3 PTP substrate was directly implicated. Whether there is a link between WDR1 dephosphorylation by EYA3 and changes in small GTPase activation remains to be established.
Fig. 5.
WDR1 is phosphorylated by c-Src and dephosphorylated by EYA3. The phosphorylation state of WDR1 influences the actin cytoskeletal architecture (Mentel et al. 2018). A color version of this figure is available online.
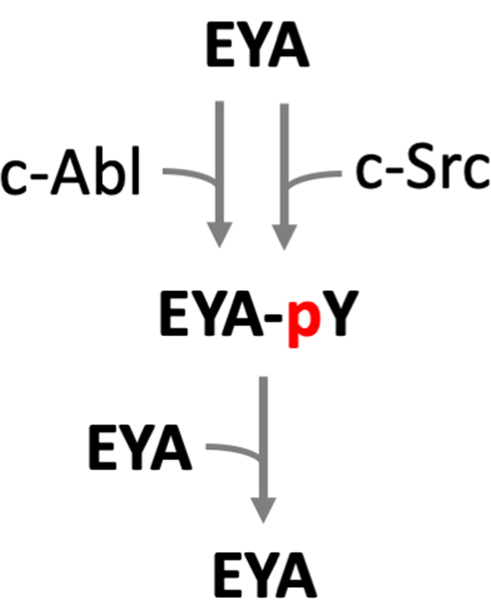
Auto-dephosphorylation of EYAs:
There is emerging evidence that tyrosine phosphorylation and auto-dephosphorylation of the EYA proteins themselves acts as a regulatory mechanism for EYA activity (Fig. 6). Early studies in cultured cells suggested that Drosophila Eya is tyrosine phosphorylated and that it has autocatalytic activity (Tootle et al. 2003). Subsequently the Abelson (Abl) tyrosine kinase was implicated in Eya tyrosine phosphorylation and in the cytoplasmic localization of Eya (Xiong et al. 2009). This Abl – Eya signaling circuitry plays a role in the development of the Drosophila visual system. There is also some evidence that EYA3 has auto-dephosphorylation activity when over-expressed in HEK293T cells (Ionescu et al. 2019). Whether tyrosine phosphorylation of EYA proteins affects it’s other biochemical activities (transactivation and putative threonine phosphatase) remains to be determined.
Fig. 6.
The kinases c-Abl and c-Src can both phosphorylate EYA proteins on as-yet unidentified tyrosine residues. EYA can auto-dephosphorylate thus setting up an auto-regulation loop. A color version of this figure is available online.
In the case of the classical PTPs there are generally accepted criteria for the assignment of PTP substrates (Tiganis and Bennett 2007): (1) interaction with a substrate-trapping form of the PTP, (2) modulation of the tyrosine phosphorylation level of the substrate in a cellular context, and (3) dephosphorylation of the substrate in vitro with recombinant, purified enzyme. Further, in vivo demonstration of an enzyme-substrate relationship as evidenced by altered phosphorylation patterns, downstream signaling events, and associated phenotypes provides the strongest validation of a bona-fide substrate. This level of substantiation is available for H2AX (Cook et al. 2009; Krishnan et al. 2009; Wang et al. 2016; Wang et al. 2018; Wang et al. 2019) and ERß (Yuan et al. 2014) as EYA PTP substrates. WDR1 as a substrate is supported by multiple lines of in vitro evidence (Mentel et al. 2018) but awaits in vivo validation.
REGULATION OF EYA PTP ACTIVITY.
EYA PTP activity is regulated at multiple levels including gene expression, sub-cellular localization, and post-translational modifications.
Differential expression of the four vertebrate EYA proteins during development in specific tissues and cell types represents the first line of regulation of EYA PTP activity. With a few exceptions Eya1 and Eya2 expression is restricted to developmental stages, Eya3 is expressed during development and in most adult tissues, while a more restricted pattern of expression is present for Eya4 in both developing and adult tissue. Elevated levels of various EYA proteins are present in pathological states such as cancer (Pandey et al. 2010; Farabaugh et al. 2012; Wu et al. 2013; Wang et al. 2018) and Pulmonary Arterial Hypertension (Wang et al. 2019).
Cell Cycle dependent regulation of EYA protein levels is reported in several contexts. EYA1 levels peak during mitosis and is significantly reduced as cells exit into the G1 phase both in vitro and in vivo (Sun et al. 2013); EYA1 degradation occurs through the ubiquitin-proteasome-mediated pathway. In turn, EYA1 can promote cell cycle progression via tyrosine-phosphatase activity dependent activation of the cyclin D1 promoter at the AP1 site (Wu et al. 2013). EYA2 is also degraded by the ubiquitin-proteasome pathway and this process is enhanced by interaction with CDK6 (Kohrt et al. 2014). Little is known thus far about cell-cycle associated fluctuations in EYA3 and EYA4 levels, but over-expression of EYA3 increases cell proliferation and this is linked to the phosphorylation of specific tyrosine residues in EYA3 (Ionescu et al. 2019).
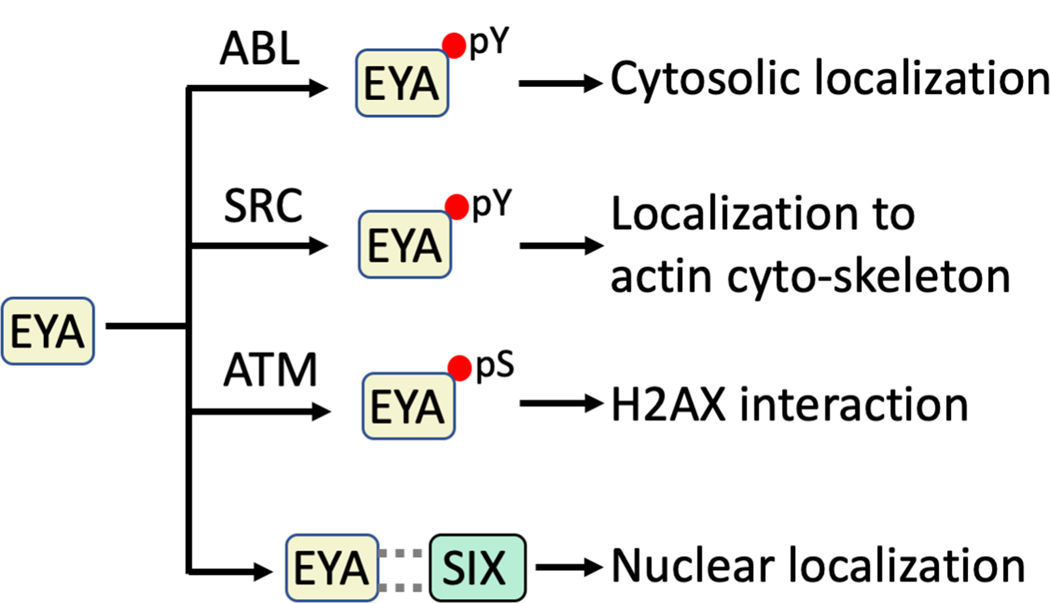
Sub-cellular localization coupled with post-translational modifications is emerging as a cellular mechanism of regulating the EYA phosphatase activity (Fig. 7). Early studies localized Drosophila Eya to the nucleoplasm (Bonini et al. 1998). Subsequent studies showed that vertebrate EYA proteins are intrinsically cytoplasmic and can be translocated into the nucleus via interaction with the SIX family of transcription factors (Ohto et al. 1999). Conversely phosphorylation of EYAs by c-Abl kinase increases cytoplasmic levels of Drosophila Eya (Xiong et al. 2009), and c-Src mediated phosphorylation of EYA3 and EYA1 localizes them to the actin cytoskeleton (Mentel et al. 2018). Notably, since both EYA3 and EYA1 have auto-tyrosine dephosphorylation activity (Mentel et al. 2018) they can auto-regulate their sub-cellular localization, possibly generating a negative feedback loop. An additional instance of phosphorylation-dependent regulation of EYA activity is the proposed requirement for ATM kinase-dependent serine phosphorylation before localization on damaged DNA via interaction with H2AX (Cook et al. 2009).
Fig. 7.
Mechanisms through which the sub-cellular localization of the EYA proteins is regulated. Sub-cellular localization of the EYA proteins is altered by tyrosine phosphorylation by the c-Abl and c-Src kinases and through interaction with the SIX family of transcription factors. Serine phosphorylation by the ATM kinase has been shown to precede interaction with H2AX. A color version of this figure is available online.
No evidence of redox regulation: An important mode of regulation of tyrosine-phosphorylation-dependent signal transduction occurs through the reversible oxidation of classical cysteine-based PTPs (Ostman et al. 2011). The EYA proteins have no reactive cysteine residue in their active site and hence their activity is not regulated by reactive oxygen species levels. Thus, the EYA PTPs could play a crucial role in signal transduction under microenvironmental conditions that inactivate other Cys-based PTPs.
Substrate specificity: Whether the intrinsic substrate-specificity differs among the four EYA proteins remains poorly investigated. Relevant to this question is the degree of sequence similarity (Fig. 2): all of the catalytic residues implicated in the tyrosine phosphatase activity are conserved among the EYA proteins. The PTP (ED) domain is highly conserved (between 83 – 95% sequence similarity), but the N-terminal domains are poorly conserved (between 45 – 69% sequence similarity) (Tadjuidje and Hegde 2013). Whether and how the N-terminal domain contributes to substrate specificity remains poorly understood. One instance of in vitro differences in EYA substrate specificity has been reported – EYA3 can dephosphorylate WDR1 but EYA1 cannot (Mentel et al. 2018). Most other studies using recombinant protein suggest that all the EYAs tested are able to similarly dephosphorylate substrate peptides presented to them.
Alignment of the amino acid sequences in the region of the target pY in EYA substrates (Fig. 8) does not reveal an obvious recognition motif. An analysis of the Arabidopsis thaliana homolog of EYA, which does not have an N-terminal domain (Rayapureddi et al. 2005), suggested a preference for acidic residues N-terminal to the phosphorylation site of substrates (Musharraf et al. 2008). But no similar analysis has yet been reported for the vertebrate EYA proteins, and known substrates (Fig. 8) do not strongly support this preference prediction.
Fig. 8.
Peptide sequence flanking the target phosphotyrosine residues (pY) in known EYA PTP substrates. A color version of this figure is available online.
In general, the issue of intrinsic EYA PTP target specificity has been inadequately assessed. Among the classical PTPs, substrate specificity usually arises as a consequence of some intrinsic sequence specificity of the catalytic domain that is fine-tuned through targeting domains and cellular localization (Tonks and Neel 2001). It is very likely that similar mechanisms are utilized by the EYAs. Knowledge of differences in substrate specificity among the EYAs would influence the interpretation of loss-of-function studies where compensation by other EYA proteins could alter the outcome of functional assays. It is also relevant in the context of therapeutic targeting of the EYAs.
THE EYA THREONINE PHOSPHATASE ACTIVITY.
Of the three independent biochemical activities present in the EYA proteins, the threonine phosphatase activity is the most controversial. Threonine phosphatase activity in the N-terminal domain of EYA4 and EYA3 was first reported in 2009 (Okabe et al. 2009). These authors used Flag-tagged EYA3 and EYA4 proteins purified from both 293T cells and a wheat germ cell-free system to show that they have both threonine and tyrosine phosphatase activities. They then performed deletion mutagenesis, assigning the threonine phosphatase activity to the N-terminal domain and the tyrosine phosphatase activity to the C-terminal ED domain of EYA. The threonine phosphatase activity was shown to play a role in the DNA-induced innate immune response, by stimulating the expression of IFN-ß and CXCL10 in response to undigested DNA of apoptotic cells. A similar role for Eya in the immune response to DNA was also documented in Drosophila (Liu et al. 2012). In a follow-up study it was shown that residues 53 – 120 of EYA3 were sufficient for the threonine phosphatase activity (Sano and Nagata 2011) (Figure 1). Importantly, no catalytic mechanism was proposed and there is no recognizable sequence motif in the EYA N-terminal domain that would provide homology with other threonine (or serine) phosphatases. Indeed, the EYA N-terminal domain lacks significant amino acid sequence homology with any known structural domain. In subsequent reports varying degrees of threonine phosphatase activity were reported for full-length EYA1, the N-terminal domain of EYA1, and the C-terminal ED domain of EYA1 (Li J et al. 2017). Mutation of the nucleophilic Asp residue (to Asn) in the HAD domain of EYA1 resulted in complete loss of tyrosine phosphatase activity as well as a reduction in threonine phosphatase activity, suggesting that the ED domain may make some contribution to the N-terminal threonine phosphatase activity or that such deletions perturb overall protein structure.
Provocatively, a recent study by the Ford lab proposes that the threonine phosphatase activity of EYA is mediated via an interaction with a B55α subunit containing PP2A holoenzyme complex (Zhang L et al. 2018). Specifically, they show that the B55α subunit of PP2A co-purifies with EYA3 when expressed in mammalian cells, and that E.coli purified EYA3 protein does not have intrinsic threonine phosphatase activity (or co-purifying PP2A-B55α). This model would require that the EYA3(53–120) minimal threonine phosphatase domain identified by Sano et al. (Sano and Nagata 2011) be able to bind and recruit B55α to the PP2A complex; this has yet to be established. Further, a recent (non-peer reviewed) preprint reports that EYA1 purified from S2 cells with no detectable co-purifying PP2A-B55α has dose-dependent Thr-phosphatase activity (Merk et al. 2019).
While the jury remains out regarding the precise molecular basis of threonine dephosphorylation by EYA proteins, several biologically relevant threonine dephosphorylation events have been associated with the EYA proteins. It will be of interest to see the results of future investigations that establish whether these are the direct consequences of EYA activity or are indirectly mediated through recruitment of a B55α-containing PP2A holoenzyme.
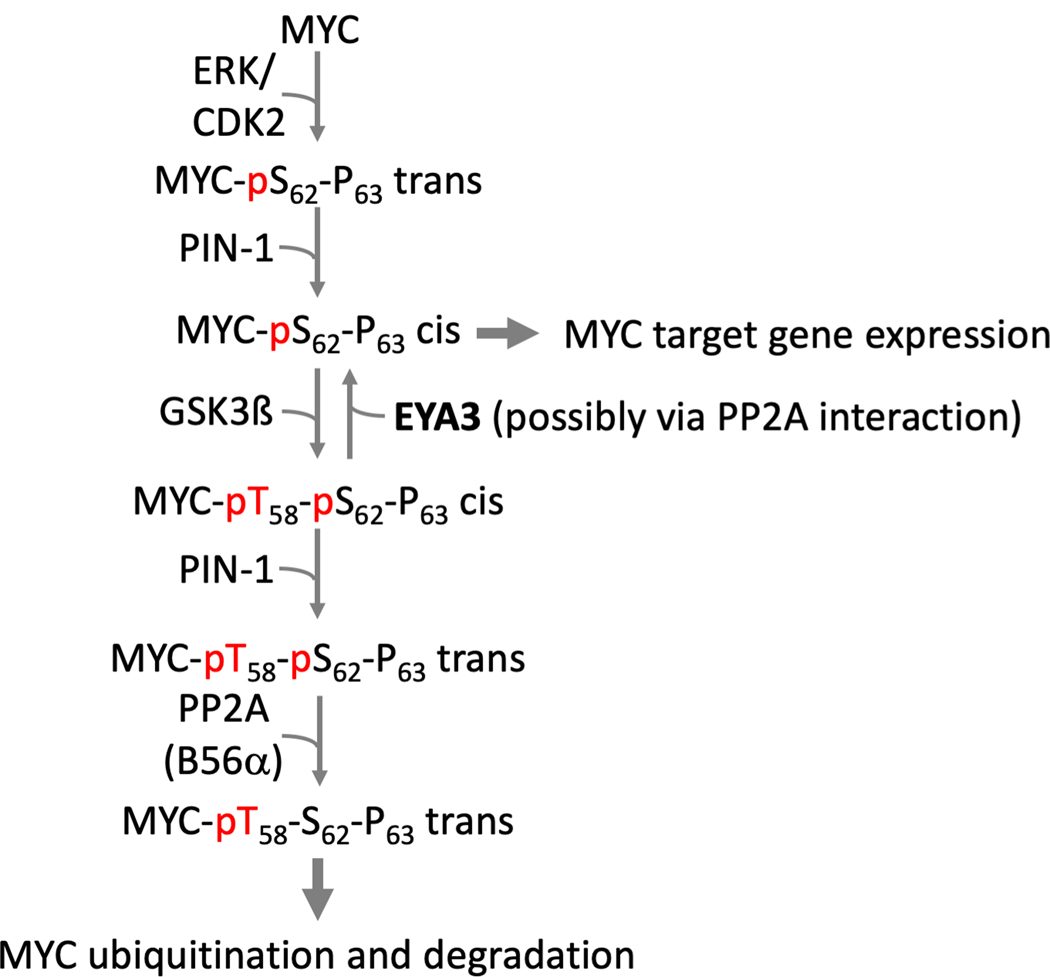
MYC stabilization:
Xu et al (Xu J et al. 2014) first reported that EYA1 dephosphorylates c-MYC at Thr58 thus reducing MYC degradation. They subsequently reported that MYC-pT58 dephosphorylation by EYA1 is inhibited by PIN1 (Li J et al. 2017), a prolyl isomerase that has an established role in activating PP2A-mediated MYC-pS62 dephosphorylation (and de-stabilization) (Yeh et al. 2004) (Fig. 9). While supporting the role of the EYA proteins in MYC stabilization, Zhang et al (Zhang L et al. 2018) propose that EYAs bind to the B55α subunit of PP2A thus targeting the PP2A-B55α threonine phosphatase activity to pT58 of c-MYC. This is an intriguing new mechanism of MYC regulation that awaits validation in other contexts as well as consolidation with other studies on the regulation of MYC levels. For instance, knockdown of B55α does not alter MYC stability while loss of B56α results in an increase in MYC levels (Arnold and Sears 2006).
Fig. 9.
Proposed role of the EYA threonine phosphatase activity in the stabilization of MYC. The signaling pathways that regulate MYC phosphorylation and accumulation are shown along with the proposed role of the EYA threonine phosphatase activity, either intrinsic or mediated through a PP2A-B55α interaction. A color version of this figure is available online.
Despite the fact that the mechanism of EYA-associated threonine phosphatase activity remains to be fully established, it is clear that EYA proteins increase the half-life of MYC. Given the myriad transcriptional networks linked to MYC, a change in the dynamics of MYC action can have wide-ranging biological consequences. When originally described, EYA-mediated MYC stabilization was linked to nephrogenesis (Xu J et al. 2014). An association between the EYA – MYC axis and tumor growth is a logical extension. The EYA proteins have long been implicated in breast cancer growth and metastasis. A role for EYA3 in breast cancer cell proliferation and migration was first reported by Pandey et al (Pandey et al. 2010). Subsequently elevated EYA2 was associated with breast cancer cell proliferation (Wu et al. 2013), and EYA2 was shown to mediate the pro-proliferative effects of SIX1 in breast cancer cells (Farabaugh et al. 2012). Elevated EYA3 levels are also seen in Ewing sarcoma (Robin et al. 2012), and elevated EYA4 has been reported in malignant peripheral nerve sheath tumors (MPNST) (Miller et al. 2010). These links between tumor growth and EYA levels are consistent with EYA-mediated MYC stabilization, but await further investigation. Interestingly, in triple negative breast cancer EYA3 threonine phosphatase-mediated MYC stabilization results in elevated PD-L1 levels and thus to immune suppression (Vartuli et al. 2018), opening up an entirely new avenue of investigation.
Notch Intracellular Domain (NICD) stabilization:
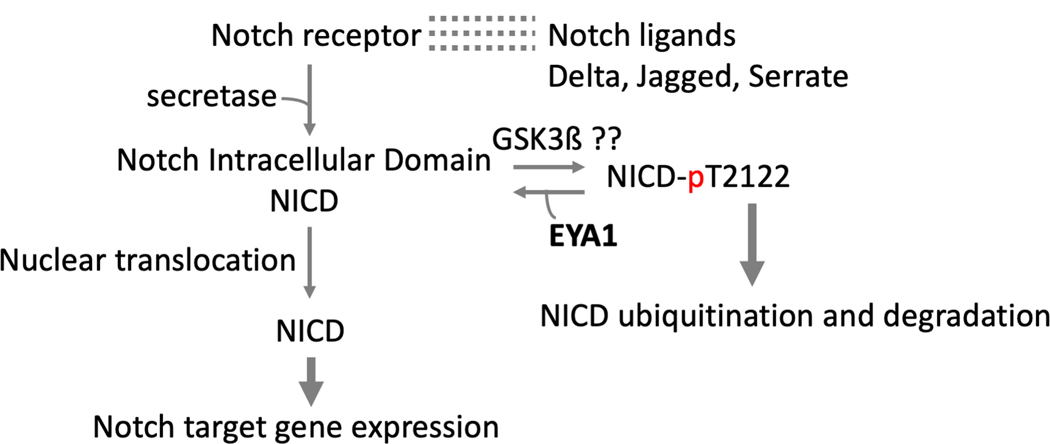
Recently EYA1 has been implicated in Notch signaling (Fig. 10) in the context of craniofacial development. Notch signaling is triggered by interaction between ligands (transmembrane Delta-Serrate-Lag-2 DSL proteins) and Notch receptors on adjacent cells (Hori et al. 2013). Upon activation, a series of intra-cellular proteolytic cleavages produce the NICD, which in turn translocates to the nucleus, binds to the DNA binding factor CSL (CBF1, Suppressor of Hairless, Lag-1) and activates transcription. Notch signaling is acutely sensitive to the nuclear concentration of NICD, which is regulated by NICD phosphorylation, ubiquitination, and degradation by the proteasome. Numerous kinases, including CDK8, GSK3ß, aPKC, PIM kinase and NEMO, phosphorylate Notch in the Cdc4 phospho-degron pThr-Pro-Pro-Xaa-pSer. Phospho-NICD is very labile. No phosphatases had been associated with NICD until the recent evidence that EYA1 dephosphorylates NICD at pT2122, thus increasing NICD stability and potentiating Notch signaling (Zhang H et al. 2017). This mechanism contributes to the maintenance of Notch signaling in the non-neuronal epibranchial placodes during craniofacial morphogenesis. But given the essential roles of the Notch signaling pathway in numerous cellular processes and cell fate decisions during embryonic development and tissue homeostasis, it is very likely that other biological contexts will emerge in which EYA-modulated Notch signaling plays a role.
Fig. 10.
EYA1 stabilizes the Notch intracellular domain by de-phosphorylating NICD at Thr2122, thus preventing ubiquitination and degradation. A color version of this figure is available online.
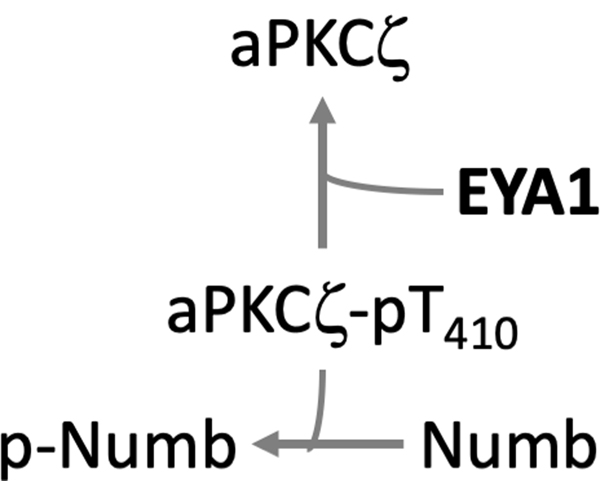
Atypical protein kinase C-zeta (aPKC-ζ). EYA1 dephosphorylates pThr410 in the active site loop of aPKC-ζ, a component of the PAR complex (aPKC-PAR3-PAR6 polarity complex) according to a report in a manuscript repository (Merk et al. 2019) (Fig. 11). aPKC-ζ in turn phosphorylates and de-stabilizes the cell fate determining protein Numb. Through this dephosphorylation event EYA1 plays a crucial role in the regulation of cell polarity, spindle orientation and cell fate determination. The EYA1-aPKC-Numb axis links the sonic hedgehog (Shh) pathway with cell polarity and the regulation of cell division. EYA1 and Shh signaling had been previously linked in hindbrain development (Eisner et al. 2015), lung morphogenesis (Lu et al. 2013), and fly eye development (Pappu et al. 2003). If EYA1 (either directly or through recruitment of PP2A) dephosphorylates pThr410 of aPKC-ζ, it would provide a mechanistic link between an EYA1-dependent dephosphorylation event, activation of the Shh pathway and a cellular output (spindle orientation).
Fig. 11.
A proposed role for EYA1 in inactivating aPKCζ through dephosphorylation at Thr410. As a consequence, levels of phospho-Numb (p-Numb) go down thus promoting SHH-driven symmetric cell division of granule cell precursors during cerebellar development (this model is based on an un-peer-reviewed preprint (Merk et al. 2019)). A color version of this figure is available online.
INTERACTION BETWEEN PHOSPHATASE AND TRANSACTIVATION ACTIVITIES OF THE EYA PROTEINS.
In addition to the phosphatase activities discussed above, the N-terminal domain of the EYA proteins can act as a transcriptional activator when tethered to DNA through interaction with the SIX family of protein. This was demonstrated in the context of mouse limb development and through a Gal4 reporter assay (Xu PX et al. 1997). Subsequently, direct molecular interaction between Drosophila Eya and Sine Oculis (the fly ortholog of the mammalian SIX proteins) was shown in a yeast two hybrid system, an interaction that plays a role in Drosophila eye development (Pignoni et al. 1997). Further it was demonstrated that EYA-SIX interaction results in translocation of the EYAs from the cytoplasm to the nucleus, thus facilitating transcription (Ohto et al. 1999)
The discovery of the C-terminal PTP activity in the EYA proteins revealed the first instance of phosphatase and transactivation functions in a single polypeptide and raised the possibility that these two activities were linked. An initial report showing that the PTP activity of EYA switched the function of the SIX-EYA transcriptional complex from a repressor to an activator suggested that the phosphatase activity of EYA influences it’s transactivation potential (Li X et al. 2003). More recent studies linking EYA1 and the sonic hedgehog pathway also suggest that the PTP activity of EYA1 is required for the EYA1-SIX-mediated transactivation of NRP1 and NRP2 transcription (Eisner et al. 2015). However, a microarray analysis using Drosophila Eya did not support any significant contribution of the PTP activity to transcriptional output (Jemc and Rebay 2007b).
The more recent description of a threonine phosphatase activity in the N-terminal domain has raised a whole new set of possibilities. While the exact mechanism of the threonine phosphatase activity and the specific residues involved in the process remain unclear, mutation of four residues in mouse EYA3 (Cys-56, Tyr-77, His-79, Tyr-90) impact the threonine phosphatase activity of mouse EYA3 produced in a wheat-germ cell-free system (Sano and Nagata 2011). At least one study showed that a single His-79>Ala replacement in mouse EYA3 abrogates it’s threonine phosphatase activity (Sano and Nagata 2011) without having any effect on either SIX interaction or transactivation in a luciferase reporter assay (Zhang L et al. 2018). This observation implies that the threonine phosphatase and transactivation activities of EYA3 are not linked.
In summary, as the evidence stands now, it appears that the C-terminal domain and specifically the PTP activity of EYA is required for (or at least enhances) EYA-SIX mediated transactivation in some contexts (although it has no effect on EYA-SIX complex formation). On the other hand, the threonine phosphatase activity of EYA has no impact on its transactivation potential. But a more systematic examination of the mechanism of regulation of EYA transactivation activity by EYA phosphatase activities remains to be conducted. The evidence for and against regulation of EYA transactivation by the PTP domain has been rigorously reviewed (Jemc and Rebay 2007a).
The question of whether the Thr-phosphatase and PTP activities of the EYAs interact is also unresolved. The isolated C-terminal ED domain of the EYAs has robust PTP activity and no Thr-phosphatase activity (Rayapureddi et al. 2003). The isolated N-terminal domain of EYA3 has threonine phosphatase activity (possibly via PP2A interaction?) but no PTP activity (Sano and Nagata 2011). A couple of reports (Xu J et al. 2014; Li J et al. 2017; Zhang H et al. 2017) suggest that PTP-activity disrupting point mutations in the C-terminal domain of the EYAs negatively impact the N-terminal threonine phosphatase activity. Whether this reflects a direct role for the C-terminal domain in the threonine phosphatase catalytic mechanism, a role in substrate binding, or a structural change that indirectly affects threonine phosphatase activity or PP2A-B55α interaction remains to be established. Importantly all of the conclusions regarding the EYA threonine phosphatase activity need to be qualified by the possibility that EYA3 threonine phosphatase activity is dependent on co-purified PP2A-B55α (Zhang L et al. 2018) while the EYA1 threonine phosphatase activity may not be dependent on PP2A-B55α according to a recent un-reviewed pre-print (Merk et al. 2019).
THERAPEUTIC TARGETING OF THE EYA PROTEINS.
Loss of EYA PTP activity or its transactivation potential is associated with several developmental disorders including branchio-oto-renal syndrome (Rayapureddi and Hegde 2006), congenital deafness DFNA10 (Zhang Y et al. 2004), and congenital cataracts (Azuma et al. 2000). But more relevant to therapeutic targeting with small molecules are conditions associated with EYA gain-of-function. There is a growing literature associating the EYA PTP activity with tumor growth (Miller et al. 2010; Pandey et al. 2010; Hansen et al. 2016), tumor metastasis (Pandey et al. 2010), and tumor angiogenesis (Tadjuidje et al. 2012; Wang et al. 2018). Furthermore, retinal neovascularization, as seen in Retinopathy of Prematurity (ROP) is also promoted by the EYA PTP activity (Wang et al. 2016). Most recently studies in rat and mouse models showed that vascular remodeling, the primary cause of increased pulmonary vascular resistance in Pulmonary Arterial Hypertension (PAH), is promoted by EYA3 PTP activity (Wang et al. 2019). Hence therapeutic targeting of the EYA PTP activity could be useful in a range of disease states.
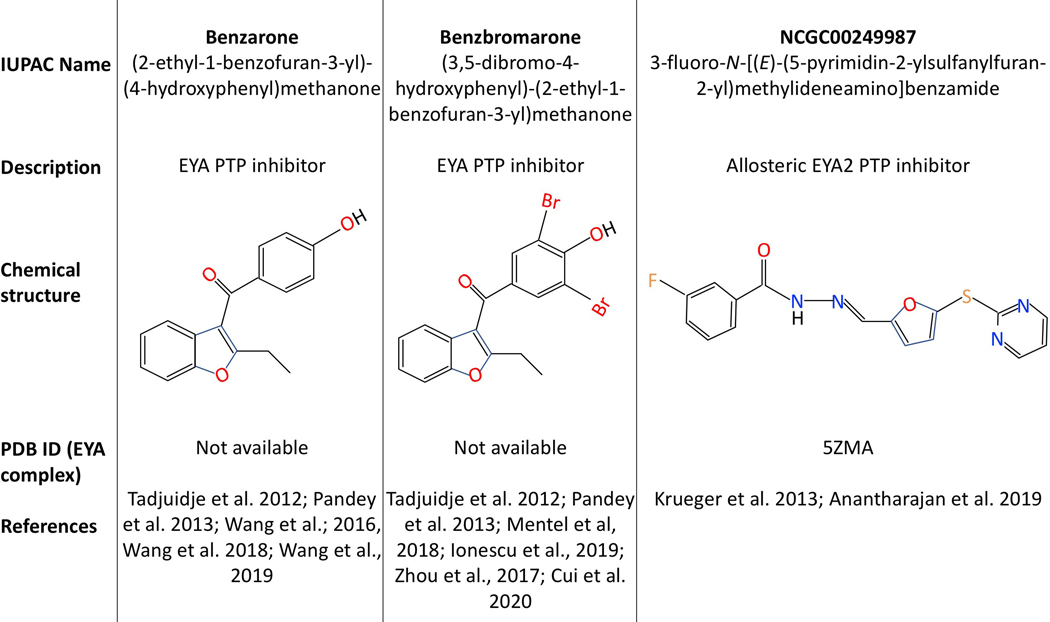
While the classical PTPs have been sought-after drug targets for diseases ranging from obesity to cancer, success has traditionally been difficult. This is generally attributed to the presence of a reactive active-site Cysteine that can confound high-throughput screens, the existence of over 100 PTPs with similar active-site stereo-chemistry making specificity challenging, and the fact that many identified PTP inhibitors tend to be charged mimetics of the substrate phospho-tyrosine. However, the outlook has become significantly better with recent progress in the identification of specific and cell-permeable inhibitors (reviewed in (Lazo et al. 2018)). The EYA PTPs have a unique advantage as they use a reaction mechanism that is different from that of the classical Cysteine-based PTPs; a nucleophilic Aspartate participates in a metal-dependent reaction similar to that carried out by the haloacid dehalogenases (Rayapureddi et al. 2003). As a result, targeting the EYA PTP active site has greater potential for specificity. Indeed, both active-site binding (Benzbromarone and Benzarone (Tadjuidje et al. 2012; Pandey et al. 2013)) and allosteric inhibitors (N-arylidenebenzohydrazide compounds (Krueger et al. 2013; Anantharajan et al. 2019)) of the EYA PTPs have been identified (Fig. 12), and in the case of Benzarone been validated in animal models of cancer (Wang et al. 2018), retinal neovascularization (Wang et al. 2016) and pulmonary vascular remodeling (Wang et al. 2019). Importantly, Benzarone treatment phenocopies the effect of a CRISPR-generated tyrosine phosphatase-dead form of EYA3 in an animal model of pulmonary vascular remodeling (Wang et al. 2019). Further attesting to the EYA PTP target specificity of Benzarone, tumor growth is retarded when tumor cells lack EYA3 and Benzarone has no effect on the growth of tumor xenografts established with tumor cells lacking EYA3 (Wang et al. 2018). Similarly, in vitro studies support the target specificity of an N-arylidenebenzohydrazide compound NCGC00249987; it has no effect on the migration of lung cancer cells with an EYA2 mutation that abolishes inhibitor binding (Anantharajan et al. 2019). Further, this inhibitor is EYA2-specific (does not inhibit EYA3). Benzbromarone has been used widely as a pharmacological tool in evaluating the role of EYA PTP activity both in vitro (Mentel et al. 2018; Ionescu et al. 2019) and in vivo (Zhou et al. 2017; Cui et al. 2020).
Fig. 12.
EYA PTP inhibitors. Chemical structures and related information as well as publications that describe the use of these compounds are shown. A color version of this figure is available online.
Since EYA proteins promote DNA damage repair, they would promote resistance to DNA damaging regimens in cancer treatment. Hence loss of the EYA tyrosine phosphatase activity could promote sensitivity to radiation and genotoxic chemotherapy. This has been documented both in vitro (Cook et al. 2009) and in vivo (Robin et al. 2012) by non-pharmacological methods. However, the ability of an EYA PTP-targeted inhibitor to potentiate DNA damaging therapeutics remains to be demonstrated.
Inhibiting the EYA threonine phosphatase activity (either intrinsic or via PP2A) could have therapeutic uses in the regulation of innate immunity or in the many contexts where MYC or Notch signaling play pathological roles. However, there are no reports yet of small molecule modulators. Much remains to be understood about this putative EYA activity, including a reaction mechanism and further investigation of the role of PP2A, before therapeutic targeting becomes feasible.
CONCLUSIONS.
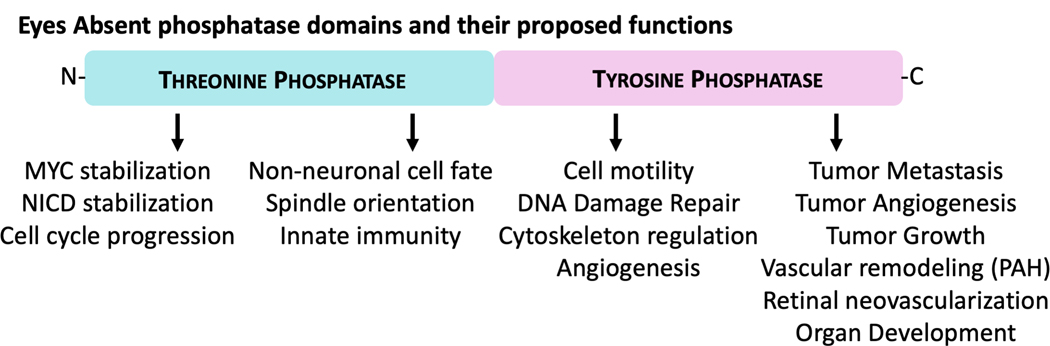
The unusual biochemical make-up of the EYA proteins uniquely positions them at the intersection of transcriptional regulation and signal transduction pathways. As a consequence, multiple physiological and pathological cellular processes are linked with EYA protein-associated molecular mechanisms (Fig. 13). The growing evidence that the EYA proteins contribute to pathological phenomena makes it particularly relevant to understand the molecular and cellular basis of EYA function. Excitingly, the EYA PTP activity has been successfully targeted by pharmacological agents, attesting to its druggability. While significant progress has occurred since the unexpected discovery that the EYA proteins were tyrosine phosphatases, much remains to be understood regarding the mechanisms of action and biological roles of this unusual family of multi-functional proteins in both normal development and in disease.
Fig. 13.
A schematic overview of the proposed cellular and biological roles for the two phosphatase activities of the EYA proteins. A color version of this figure is available online.
Acknowledgments
This work was supported by the National Institutes of Health under grants RO1-CA207068 and RO1-HL152094 to RSH.
Footnotes
Disclosure of Interest. The authors report no conflict of interest.
REFERENCES
- Alonso A, Sasin J, Bottini N, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. 2004. Protein tyrosine phosphatases in the human genome. Cell. 117(6):699–711. [DOI] [PubMed] [Google Scholar]
- Anantharajan J, Zhou H, Zhang L, Hotz T, Vincent MY, Blevins MA, Jansson AE, Kuan JWL, Ng EY, Yeo YK et al. 2019. Structural and Functional Analyses of an Allosteric EYA2 Phosphatase Inhibitor That Has On-Target Effects in Human Lung Cancer Cells. Mol Cancer Ther. 18(9):1484–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold HK, Sears RC. 2006. Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Molecular and cellular biology. 26(7):2832–2844. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma N, Hirakiyama A, Inoue T, Asaka A, Yamada M. 2000. Mutations of a human homologue of the Drosophila eyes absent gene (EYA1) detected in patients with congenital cataracts and ocular anterior segment anomalies. Human molecular genetics. 9(3):363–366. [DOI] [PubMed] [Google Scholar]
- Bonini NM, Leiserson WM, Benzer S. 1998. Multiple roles of the eyes absent gene in Drosophila. Developmental biology. 196(1):42–57. [DOI] [PubMed] [Google Scholar]
- Broering TJ, Wang YL, Pandey RN, Hegde RS, Wang SC, Namekawa SH. 2015. BAZ1B is dispensable for H2AX phosphorylation on Tyrosine 142 during spermatogenesis. Biol Open. 4(7):873–884. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG. 2009. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature. 458(7238):591–596. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Zhang D, Kong D, Tang J, Liao X, Yang Q, Ren J, Gong Y, Wu G. 2020. Co-transplantation with adipose-derived cells to improve parathyroid transplantation in a mice model. Stem Cell Res Ther. 11(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depreux FF, Darrow K, Conner DA, Eavey RD, Liberman MC, Seidman CE, Seidman JG. 2008. Eya4-deficient mice are a model for heritable otitis media. J Clin Invest. 118(2):651–658. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economopoulou M, Langer HF, Celeste A, Orlova VV, Choi EY, Ma M, Vassilopoulos A, Callen E, Deng C, Bassing CH et al. 2009. Histone H2AX is integral to hypoxia-driven neovascularization [Letter Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t]. Nature Medicine. 15(5):553–558. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner A, Pazyra-Murphy MF, Durresi E, Zhou P, Zhao X, Chadwick EC, Xu PX, Hillman RT, Scott MP, Greenberg ME et al. 2015. The Eya1 phosphatase promotes Shh signaling during hindbrain development and oncogenesis. Dev Cell. 33(1):22–35. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh SM, Micalizzi DS, Jedlicka P, Zhao R, Ford HL. 2012. Eya2 is required to mediate the pro-metastatic functions of Six1 via the induction of TGF-beta signaling, epithelial-mesenchymal transition, and cancer stem cell properties. Oncogene. 31(5):552–562. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohla A, Birkenfeld J, Bokoch GM. 2005. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat Cell Biol. 7(1):21–29. eng. [DOI] [PubMed] [Google Scholar]
- Hansen JN, Lotta LT Jr., Eberhardt A, Schor NF, Li X 2016. EYA1 expression and subcellular localization in neuroblastoma and its association with prognostic markers. J Cancer Res Ther (Manch). 4(2):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Sen A, Artavanis-Tsakonas S. 2013. Notch signaling at a glance. J Cell Sci. 126(Pt 10):2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Sefton BM. 1980. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proceedings of the National Academy of Sciences of the United States of America. 77(3):1311–1315. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu AE, Mentel M, Munteanu CVA, Sima LE, Martin EC, Necula-Petrareanu G, Szedlacsek SE. 2019. Analysis of EYA3 Phosphorylation by Src Kinase Identifies Residues Involved in Cell Proliferation. Int J Mol Sci. 20(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemc J, Rebay I. 2007a. The eyes absent family of phosphotyrosine phosphatases: properties and roles in developmental regulation of transcription. Annu Rev Biochem. 76:513–538. eng. [DOI] [PubMed] [Google Scholar]
- Jemc J, Rebay I. 2007b. Identification of transcriptional targets of the dual-function transcription factor/phosphatase eyes absent. Developmental biology. 310(2):416–429. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JH, Min S, Chae S, Ha GH, Kim Y, Park YJ, Lee CW, Cho H. 2019. De novo phosphorylation of H2AX by WSTF regulates transcription-coupled homologous recombination repair. Nucleic Acids Res. 47(12):6299–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrt D, Crary J, Zimmer M, Patrick AN, Ford HL, Hinds PW, Grossel MJ. 2014. CDK6 binds and promotes the degradation of the EYA2 protein. Cell Cycle. 13(1):62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan N, Jeong DG, Jung SK, Ryu SE, Xiao A, Allis CD, Kim SJ, Tonks NK. 2009. Dephosphorylation of the C-terminal tyrosyl residue of the DNA damage-related histone H2A.X is mediated by the protein phosphatase eyes absent. The Journal of biological chemistry. 284(24):16066–16070. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger AB, Dehdashti SJ, Southall N, Marugan JJ, Ferrer M, Li X, Ford HL, Zheng W, Zhao R. 2013. Identification of a selective small-molecule inhibitor series targeting the eyes absent 2 (Eya2) phosphatase activity. J Biomol Screen. 18(1):85–96. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo JS, McQueeney KE, Burnett JC, Wipf P, Sharlow ER. 2018. Small molecule targeting of PTPs in cancer. The international journal of biochemistry & cell biology. 96:171–181. [DOI] [PubMed] [Google Scholar]
- Li J, Rodriguez Y, Cheng C, Zeng L, Wong EY, Xu CY, Zhou MM, Xu PX. 2017. EYA1’s Conformation Specificity in Dephosphorylating Phosphothreonine in Myc and Its Activity on Myc Stabilization in Breast Cancer. Molecular and cellular biology. 37(1). eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW et al. 2003. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 426(6964):247–254. [DOI] [PubMed] [Google Scholar]
- Liu X, Sano T, Guan Y, Nagata S, Hoffmann JA, Fukuyama H. 2012. Drosophila EYA regulates the immune response against DNA through an evolutionarily conserved threonine phosphatase motif. PLoS One. 7(8):e42725. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Reddy R, Berika M, Warburton D, El-Hashash AH. 2013. Abrogation of Eya1/Six1 disrupts the saccular phase of lung morphogenesis and causes remodeling. Developmental biology. 382(1):110–123. eng. [DOI] [PubMed] [Google Scholar]
- Mentel M, Ionescu AE, Puscalau-Girtu I, Helm MS, Badea RA, Rizzoli SO, Szedlacsek SE. 2018. WDR1 is a novel EYA3 substrate and its dephosphorylation induces modifications of the cellular actin cytoskeleton. Sci Rep. 8(1):2910. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merk DJ, Zhou P, Cohen SM, Pazyra-Murphy MF, Hwang GH, Rehm KJ, Alfaro J, Zhao X, Park E, Xu P-X et al. 2019. The Eya1 phosphatase mediates Shh-driven symmetric cell division of cerebellar granule cell precursors bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SJ, Lan ZD, Hardiman A, Wu J, Kordich JJ, Patmore DM, Hegde RS, Cripe TP, Cancelas JA, Collins MH et al. 2010. Inhibition of Eyes Absent Homolog 4 expression induces malignant peripheral nerve sheath tumor necrosis. Oncogene. 29(3):368–379. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musharraf A, Markschies N, Teichmann K, Pankratz S, Landgraf K, Englert C, Imhof D. 2008. Eyes absent proteins: characterization of substrate specificity and phosphatase activity of mutants associated with branchial, otic and renal anomalies. Chembiochem. 9(14):2285–2294. [DOI] [PubMed] [Google Scholar]
- Namekawa SH, Park PJ, Zhang LF, Shima JE, McCarrey JR, Griswold MD, Lee JT. 2006. Postmeiotic sex chromatin in the male germline of mice. Curr Biol. 16(7):660–667. [DOI] [PubMed] [Google Scholar]
- Nowsheen S, Aziz K, Luo K, Deng M, Qin B, Yuan J, Jeganathan KB, Yu J, Zhang H, Ding W et al. 2018. ZNF506-dependent positive feedback loop regulates H2AX signaling after DNA damage. Nat Commun. 9(1):2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, Kawakami K. 1999. Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Molecular and cellular biology. 19(10):6815–6824. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, Sano T, Nagata S. 2009. Regulation of the innate immune response by threonine-phosphatase of Eyes absent. Nature. 460(7254):520–524. eng. [DOI] [PubMed] [Google Scholar]
- Ostman A, Frijhoff J, Sandin A, Bohmer FD. 2011. Regulation of protein tyrosine phosphatases by reversible oxidation. J Biochem. 150(4):345–356. [DOI] [PubMed] [Google Scholar]
- Pandey RN, Rani R, Yeo EJ, Spencer M, Hu S, Lang RA, Hegde RS. 2010. The Eyes Absent phosphatase-transactivator proteins promote proliferation, transformation, migration, and invasion of tumor cells [Research Support, N.I.H., Extramural]. Oncogene. 29(25):3715–3722. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey RN, Wang TS, Tadjuidje E, McDonald MG, Rettie AE, Hegde RS. 2013. Structure-Activity Relationships of Benzbromarone Metabolites and Derivatives as EYA Inhibitory Anti-Angiogenic Agents. PLoS One. 8(12):e84582. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu KS, Chen R, Middlebrooks BW, Woo C, Heberlein U, Mardon G. 2003. Mechanism of hedgehog signaling during Drosophila eye development. Development (Cambridge, England). 130(13):3053–3062. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. 1997. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development [published erratum appears in Cell 1998 Feb 20;92(4):following 585]. Cell. 91(7):881–891. [DOI] [PubMed] [Google Scholar]
- Rayapureddi JP, Hegde RS. 2006. Branchio-oto-renal syndrome associated mutations in Eyes Absent 1 result in loss of phosphatase activity. FEBS Lett. 580(16):3853–3859. [DOI] [PubMed] [Google Scholar]
- Rayapureddi JP, Kattamuri C, Chan FH, Hegde RS. 2005. Characterization of a plant, tyrosine-specific phosphatase of the aspartyl class. Biochemistry. 44(2):751–758. [DOI] [PubMed] [Google Scholar]
- Rayapureddi JP, Kattamuri C, Steinmetz BD, Frankfort BJ, Ostrin EJ, Mardon G, Hegde RS. 2003. Eyes absent represents a class of protein tyrosine phosphatases. Nature. 426(6964):295–298. [DOI] [PubMed] [Google Scholar]
- Riffle S, Hegde RS. 2017. Modeling tumor cell adaptations to hypoxia in multicellular tumor spheroids. J Exp Clin Cancer Res. 36(1):102. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riffle S, Pandey RN, Albert M, Hegde RS. 2017. Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids. BMC Cancer. 17(1):338. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin TP, Smith A, McKinsey E, Reaves L, Jedlicka P, Ford HL. 2012. EWS/FLI1 regulates EYA3 in Ewing sarcoma via modulation of miRNA-708, resulting in increased cell survival and chemoresistance. Mol Cancer Res. 10(8):1098–1108. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Nagata S. 2011. Characterization of the threonine-phosphatase of mouse eyes absent 3. FEBS Lett. 585(17):2714–2719. eng. [DOI] [PubMed] [Google Scholar]
- Scanlon SE, Glazer PM. 2015. Multifaceted control of DNA repair pathways by the hypoxic tumor microenvironment. DNA Repair (Amst). 32:180–189. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, D’Souza RC, Tyanova S, Schaab C, Wisniewski JR, Cox J, Mann M. 2014. Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep. 8(5):1583–1594. eng. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J et al. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 7:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousounis K, Bryant DM, Martinez Fernandez J, Eddy SS, Tsai SL, Gundberg GC, Han J, Courtemanche K, Levin M, Whited JL. 2020. Eya2 promotes cell cycle progression by regulating DNA damage response during vertebrate limb regeneration. Elife. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Karoulia Z, Wong EY, Ahmed M, Itoh K, Xu PX. 2013. The phosphatase-transcription activator EYA1 is targeted by anaphase-promoting complex/Cdh1 for degradation at M-to-G1 transition. Molecular and cellular biology. 33(5):927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjuidje E, Hegde RS. 2013. The Eyes Absent proteins in development and disease. Cell Mol Life Sci. 70(11):1897–1913. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjuidje E, Wang TS, Pandey RN, Sumanas S, Lang RA, Hegde RS. 2012. The EYA Tyrosine Phosphatase Activity Is Pro-Angiogenic and Is Inhibited by Benzbromarone. PLoS One. 7(4):e34806. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiganis T, Bennett AM. 2007. Protein tyrosine phosphatase function: the substrate perspective. The Biochemical journal. 402(1):1–15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks NK, Neel BG. 2001. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol. 13(2):182–195. [DOI] [PubMed] [Google Scholar]
- Tootle TL, Silver SJ, Davies EL, Newman V, Latek RR, Mills IA, Selengut JD, Parlikar BE, Rebay I. 2003. The transcription factor Eyes absent is a protein tyrosine phosphatase. Nature. 426(6964):299–302. [DOI] [PubMed] [Google Scholar]
- Vartuli RL, Zhou H, Zhang L, Powers RK, Klarquist J, Rudra P, Vincent MY, Ghosh D, Costello JC, Kedl RM et al. 2018. Eya3 promotes breast tumor-associated immune suppression via threonine phosphatase-mediated PD-L1 upregulation. J Clin Invest. 128(6):2535–2550. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pandey RN, Riffle S, Chintala H, Wikenheiser-Brokamp KA, Hegde RS. 2018. The Protein Tyrosine Phosphatase Activity of Eyes Absent Contributes to Tumor Angiogenesis and Tumor Growth. Mol Cancer Ther. 17(8):1659–1669. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pandey RN, York AJ, Mallela J, Nichols WC, Hu YC, Molkentin JD, Wikenheiser-Brokamp KA, Hegde RS. 2019. The EYA3 tyrosine phosphatase activity promotes pulmonary vascular remodeling in pulmonary arterial hypertension. Nat Commun. 10(1):4143. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tadjuidje E, Pandey RN, Stefater JA 3rd, Smith LE, Lang RA, Hegde RS 2016. The Eyes Absent Proteins in Developmental and Pathological Angiogenesis. The American journal of pathology. 186(3):568–578. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Li Z, Cai S, Tian L, Chen K, Wang J, Hu J, Sun Y, Li X, Ertel A et al. 2013. EYA1 Phosphatase Function Is Essential to Drive Breast Cancer Cell Proliferation through Cyclin D1. Cancer research. 73(14):4488–4499. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao A, Li H, Shechter D, Ahn SH, Fabrizio LA, Erdjument-Bromage H, Ishibe-Murakami S, Wang B, Tempst P, Hofmann K et al. 2009. WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature. 457(7225):57–62. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Dabbouseh NM, Rebay I. 2009. Interactions with the abelson tyrosine kinase reveal compartmentalization of eyes absent function between nucleus and cytoplasm. Dev Cell. 16(2):271–279. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Jiao Y, Yi M, Zhao W, Wu K. 2019. EYA2 Correlates With Clinico-Pathological Features of Breast Cancer, Promotes Tumor Proliferation, and Predicts Poor Survival. Front Oncol. 9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wong EY, Cheng C, Li J, Sharkar MT, Xu CY, Chen B, Sun J, Jing D, Xu PX. 2014. Eya1 interacts with Six2 and Myc to regulate expansion of the nephron progenitor pool during nephrogenesis. Dev Cell. 31(4):434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Cheng J, Epstein JA, Maas RL. 1997. Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proceedings of the National Academy of Sciences of the United States of America. 94(22):11974–11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida T et al. 2004. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 6(4):308–318. eng. [DOI] [PubMed] [Google Scholar]
- Yuan B, Cheng L, Chiang HC, Xu X, Han Y, Su H, Wang L, Zhang B, Lin J, Li X et al. 2014. A phosphotyrosine switch determines the antitumor activity of ERbeta. J Clin Invest. 124(8):3378–3390. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang L, Wong EYM, Tsang SL, Xu PX, Lendahl U, Sham MH. 2017. An Eya1-Notch axis specifies bipotential epibranchial differentiation in mammalian craniofacial morphogenesis. Elife. 6. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhou H, Li X, Vartuli RL, Rowse M, Xing Y, Rudra P, Ghosh D, Zhao R, Ford HL. 2018. Eya3 partners with PP2A to induce c-Myc stabilization and tumor progression. Nat Commun. 9(1):1047. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Knosp BM, Maconochie M, Friedman RA, Smith RJ. 2004. A comparative study of Eya1 and Eya4 protein function and its implication in branchio-oto-renal syndrome and DFNA10. J Assoc Res Otolaryngol. 5(3):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JJ, Huang Y, Zhang X, Cheng Y, Tang L, Ma X. 2017. Eyes absent gene (EYA1) is a pathogenic driver and a therapeutic target for melanoma. Oncotarget. 8(62):105081–105092. [DOI] [PMC free article] [PubMed] [Google Scholar]