ABSTRACT
Background
Trimethylamine-N-oxide (TMAO) is a compound that is present in seafood and produced through human gut microbial metabolism of its precursors. Previous studies have suggested that elevated TMAO concentrations are associated with an increased risk of cardiovascular events. However, the association between diet and TMAO concentrations in free-living adult populations has not been adequately described.
Objectives
The objective of this study was to identify dietary predictors of plasma TMAO concentrations.
Methods
TMAO concentrations were assessed in 2 fasting plasma samples collected 6 mo apart among 620 healthy men. Short-term and long-term dietary intakes were assessed during the same time-frame of blood collections via repeated 7-d dietary records (7DDRs) and a semiquantitative food-frequency questionnaire (SFFQ), respectively. We grouped individual food items into 21 groups and regressed against averaged TMAO concentrations. We also assessed the association between dietary scores and TMAO concentrations.
Results
In models adjusted for demographic characteristics and mutually adjusted for food groups, SFFQ-assessments of fish and egg intakes were significantly associated with increased TMAO concentration (β = 0.082; 95% CI: 0.021, 0.14; P = 0.009 for fish; β = 0.065; 95% CI: 0.004, 0.13; P = 0.039 for egg). The positive association between fish consumption and TMAO concentration was replicated in the 7DDR-assessments (β = 0.12; 95% CI: 0.060, 0.18; P < 0.001). There was no association between red meat intake and TMAO concentrations. The unhealthful plant-based diet index (uPDI) was inversely associated (β = −0.013; 95% CI: −0.021, −0.005; P = 0.001) and healthy dietary scores were positively correlated with TMAO concentration.
Conclusions
TMAO concentration was significantly associated with fish intake, but not with red meat consumption. uPDI, an unhealthy dietary pattern, was inversely related to TMAO concentration. As such, this study suggests that in free-living populations, higher circulating concentrations of TMAO cannot simply be interpreted as a marker of unhealthy food intake or an unhealthy dietary pattern.
Keywords: trimethylamine-N-oxide, semiquantitative food-frequency questionnaire, 7-d dietary records, plant-based diet index, fish, red meat
Introduction
Trimethylamine-N-oxide (TMAO) is a compound that is present in seafood and synthesized by hepatic oxidation of trimethylamine (TMA), which is produced by microorganisms comprising the human gastrointestinal microbiome (1). Previous clinical studies have reported an association between elevated concentrations of TMAO and increased risk of mortality and development of cardiovascular disease (CVD) (2). TMAO concentrations depend, in part, on diet and lifestyle choices (3–6), and therefore have been proposed as a target for behavioral interventions or therapeutic strategies aimed at preventing the development or progression of CVD (7). Thus, it is essential to identify dietary factors associated with the plasma concentration of TMAO.
Plasma TMAO originates from gut bacteria metabolism of dietary l-carnitine, betaine, choline, or phospholipids, or direct consumption of TMAO or TMA-rich products (3). Previous studies have explored the influence of certain dietary factors (such as red meat, egg, and fish) on the concentration of this biomarker in human adults (3–5, 8, 9). However, few studies have systematically investigated the interrelation of other dietary components in determining TMAO concentrations in free-living individuals. Furthermore, evidence from previous studies examining this topic is partially conflicting. For example, egg consumption was positively associated with TMAO concentrations in some, but not all, studies (8, 9). Also, many seafood products, higher in omega-3 fatty acids and being inversely associated with CVD, contain concentrations of preformed TMAO higher than those of any other individual foods (10, 11). The variance in the effects of diet on serum TMAO concentration is thought to be because TMAO concentrations may vary according to the dietary intake–blood sampling period, gut microbiome composition, permeability of the gut–blood barrier (12), the activity of liver flavin-containing monooxygenase-3 (3), demographics (13), and kidney function (14). Given the variety of factors influencing TMAO concentration, it is important to determine predictors of TMAO concentrations in free-living adults.
Finally, with a slow population shift away from red meat intake (15), it is important to consider the importance of new dietary patterns to TMAO concentration (6). The plant-based diet indexes (PDIs) have emerged as useful scores for quantifying the healthiness or quality of diets based on plant-based dietary habits (16, 17). A previous study reported associations between improved PDI or healthful PDI (hPDI) and lower risk of CVD mortality, and increased unhealthful PDI (uPDI) and higher risk (17). Given the potentially unfavorable relation of TMAO and CVD, TMAO concentration would be expected to be associated with the uPDI, although this hypothesis has not to our knowledge been examined.
In the present study, we utilized dietary intake data from both semiquantitative food-frequency questionnaires (SFFQs) and 7-d dietary records (7DDRs) to identify food groups and dietary patterns that were associated with TMAO concentration in a cohort of healthy free-living male adults.
Methods
Study population
The participants of the Men's Lifestyle Validation Study (MLVS) (18) were drawn from the Health Professionals Follow-up Study (HPFS) (19) and Harvard Pilgrim Health Care (HPHC) participants. 1) The HPFS started in 1986 with 51,529 male health professionals aged 40–75 y at baseline. Participants received a follow-up questionnaire every 2 y on lifestyle, health behaviors, and medical history, and every 4 y to assess dietary intake, with a response rate of ∼90% for each cycle. 2) The HPHC participants were recruited from HPHC enrollees who also receive their care at Harvard Vanguard Medical Associates.
For the MLVS, the age range was from 45 to 80 y. Participants with coronary artery disease, stroke, cancer, or major neurological disease or without internet access were ineligible for the MLVS. Because the goal of the MLVS is to evaluate the performance of multiple dietary instruments, MLVS participants were asked not to significantly change their diet and lifestyle during the study. A total of 796 men were enrolled in the MLVS. The MLVS and the present analysis were approved by the Human Subjects Committees of the Harvard TH Chan School of Public Health and the Brigham and Women's Hospital.
Participants of the MLVS were randomly assigned to 4 study groups. The timing of questionnaire administration and blood sample collection varied across each of the 4 groups in order to alternate the order of the measurements. Figure 1A details an example overview of the MLVS study design. The MLVS data collection consisted of 2 administrations of an SFFQ, two 1-wk food records (7DDRs) (administered 6 mo apart), 2 administrations of the modified Paffenbarger physical activity questionnaire (20), 2 fasting blood samples (collected 6 mo apart), and 2 weight measurements. At enrollment we collected information on year of birth, height, and ethnicity. Weight and height were used to calculate BMI as kg/m2.
FIGURE 1.
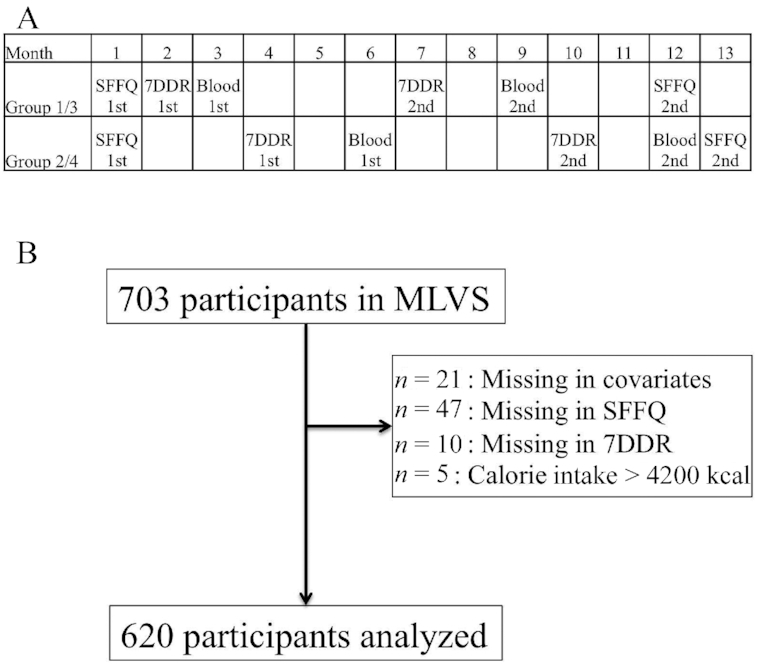
MLVS protocol. (A) Timeline of the MLVS, showing the timings of blood collection, SFFQs, and 7DDRs for each group (groups 1–4). (B) Study flow. MLVS, Men's Lifestyle Validation Study; SFFQ, semiquantitative food-frequency questionnaire; 7DDR, 7-d dietary record.
We excluded participants with SFFQ total daily energy intakes < 800 kcal or >4200 kcal or with >70 blank SFFQ questions. The present analysis included 620 participants, all of whom had complete data for the 2 SFFQs as well as the two 7DDRs and 2 measurements of TMAO (Figure 1B).
SFFQ
The 152-food-item SFFQ is an expanded version of a previously validated questionnaire (21). Respondents were asked how often, on average, they consumed the specified amount of each food or beverage during the preceding year; 9 possible frequency categories ranged from never/almost never to ≥6 times/d. Open-ended questions were used for usual brand and type of margarine, cooking oil, cold breakfast cereal, and multivitamins. We also collected detailed information regarding the type of fat used at the table and in food preparation. Total calorie intake was calculated by multiplying the weight proportional to the intake frequency by the calorie composition for the portion size specified for each food or vitamin supplement.
7DDR
Each participant received a Primo Multifunction Kitchen Scale (Model P115NB; Escali Corporation), a ruler (printed on the 7DDR booklet), an instructional DVD, and verbal instructions (via telephone) explaining how to implement the 7DDRs. A computerized system was used to send reminders and encouragement emails to participants on each day of diet recording. Participants measured and reported gram weights for foods before and after eating, and the amount consumed was computed by subtracting the after weight from the before weight. Participants provided recipes of all home-prepared foods, including the number of servings in the recipe and the portions of the recipes that they consumed. In addition, participants collected and returned labels of store-brand products. The Nutrition Coordinating Center at the University of Minnesota (22) used the Nutrition Data System for Research (NDSR 2011), primarily using USDA food composition sources, to analyze the 7DDRs (23). Intakes of >150 nutrient and dietary constituents were derived from this tool. The validity of using 7DDRs against SFFQs has been reported previously (24).
Food groups and dietary scores
For both the SFFQ and 7DDR, we grouped individual items into 21 broad food groups based on nutrient and culinary similarities (Supplemental Table 1). Dietary intake was counted based on serving sizes, which Supplemental Table 2 summarizes. Dietary scores including the alternate Healthy Eating Index-2010 (aHEI-2010) (25), the alternate-Mediterranean diet (aMED) (26), PDI, hPDI, and uPDI (16) were calculated from the 7DDR according to previously published protocols. For calculating PDIs, food groups were ranked into quintiles and given positive or reverse scores based on their quintile assignment (Supplemental Table 3). To derive the PDI, plant food groups were given positive scores, whereas animal food groups were given reverse scores. For the hPDI, positive scores were given to healthy plant food groups, and reverse scores were assigned to less healthy plant food groups and animal food groups. For the uPDI, positive scores were assigned to less healthy plant food groups, and reverse scores assigned to the healthy plant food groups and animal food groups. These individual food group scores were then summed to obtain the indexes.
Measurement of TMAO
Plasma TMAO concentration was analyzed by electrospray ionization (ESI)-LC-MS (TSQ QuantumTM mass spectrometer, Thermo Scientific Inc.) in positive mode using selected reaction monitoring at the University of Hawaii Cancer Center, Honolulu, HI (27). Ten-percent quality control samples were randomly inserted, in which the CV was 18.1%. Every participant provided 2 fasted blood samples, 1 in phase-1 and 1 in phase-2, the plasma from which was used to assess the circulating concentration of TMAO. The periods between the 7DDRs and the corresponding blood sampling were 5–6 mo in each phase. To reduce measurement error and random variation in concentrations, we compared the mean dietary intakes from the 2 SFFQs or 2 7DDRs with the average of the 2 TMAO concentrations. We also conducted phase-specific analyses using the phase-1 blood and phase-1 7DDR, and the phase-2 blood and phase-2 7DDR separately.
Statistical analysis
Depending on the normality of variables, continuous data were expressed as mean ± SD or median (IQR). Spearman rank correlation coefficients were calculated to reflect the correlation between SFFQ-derived and 7DDR-derived food groups, as well as among the food groups within each dietary assessment tool. Linear regression models were used to determine the association of food group intake (either SFFQ- or 7DDR-derived intakes) with the log-concentration of TMAO. The multivariate models included age, ethnicity, total physical activity [metabolic equivalents (METs) per week], total calorie intake, BMI, and simultaneous adjustment for each of the defined food groups. The same adjustments were performed in regressions where the exposures were specific food components. Models predicting log-concentration of TMAO by aHEI-2010, aMED, PDI, hPDI, or uPDI were also estimated. Coefficients in the regression models with food group were standardized, whereas dietary scores and fish/red meat components were assessed as per score and per serving per week, respectively. Model fit was evaluated by R2 values. All analyses were performed using SAS software version 9.2 (SAS Institute Inc.) or R 3.5.2 (The R Foundation), and statistical significance was set at a 2-tailed P value < 0.05. Bonferroni correction was used to adjust the P value thresholds for the multiple testing.
Results
Baseline characteristics
Table 1 summarizes baseline cohort characteristics of the analyzed 620 male participants. Mean age was 67.7 ± 7.7 y, and 2.1% were African Americans. Median total physical activity and total energy intake per day from the 2 measurements were 114 (82–144) METs/wk and 2075 (1724–2408) kcal/d, respectively. The median TMAO concentration over the 2 sample points was 4.1 (2.9–6.7) µM. Supplemental Table 2 summarizes the median intake of each food group and the correlation between food group intakes obtained from the SFFQ and 7DDR. Supplemental Figure 1 demonstrates the strong interrelations between the various food groups as assessed by the SFFQ and 7DDR. This highlights the importance of building models that simultaneously adjust for the intakes of the various food groups.
TABLE 1.
Baseline characteristics of 620 healthy adult males in the MLVS cohort1
| Characteristics | Values |
|---|---|
| Demographics | |
| Age, y | 67.7 ± 7.7 |
| African American | 13 (2.1) |
| BMI, kg/m2 | 25.5 [23.6–28.0] |
| Total activity, METs/wk | 114 [82–144] |
| Dietary intake | |
| Total calorie intake, kcal | 2075 [1724–2408] |
| aHEI-2010, units | 57 [49–66] |
| aMED, units | 4.5 [3.0–5.5] |
| PDI, units | 53 [49–57] |
| uPDI, units | 53 [49–58] |
| hPDI, units | 54 [49–59] |
| Biomarker | |
| TMAO, µM | 4.1 [2.9–6.7] |
1Values are means ± SDs, medians [IQRs], or n (%). aHEI-2010, alternate Healthy Eating Index-2010; aMED, alternate-Mediterranean diet; hPDI, healthful plant-based diet index; MET, metabolic equivalent; MLVS, Men's Lifestyle Validation Study; PDI, plant-based diet index; TMAO, trimethylamine-N-oxide; uPDI, unhealthful plant-based diet index.
Association of food group intake with TMAO concentration
Linear regression models were used to assess the relation of food group intake based on the SFFQ (Figure 2) and 7DDR (Figure 3) with the concentration of TMAO. The patterns of association were, in part, different between the SFFQ and the 7DDR.
FIGURE 2.
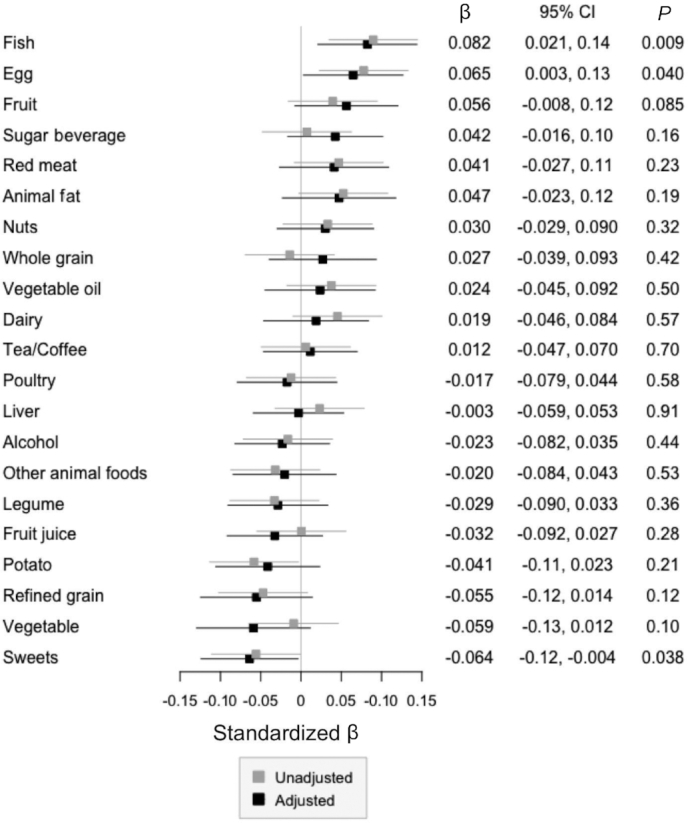
Association between SFFQ-defined food groups and TMAO concentration. Linear regression models for log-transformed TMAO concentrations by the SFFQ-based food groups in 620 healthy males were built. The results of the unadjusted (gray) and adjusted (black) models are presented. In the adjusted model, age, race, metabolic equivalents per week, total calories per day, BMI, and every food group were adjusted. The numbers indicate the standardized β (dots) and P value of each food group in the adjusted model. Bars indicate the 95% CIs. SFFQ, semiquantitative food-frequency questionnaire; TMAO, trimethylamine-N-oxide.
FIGURE 3.
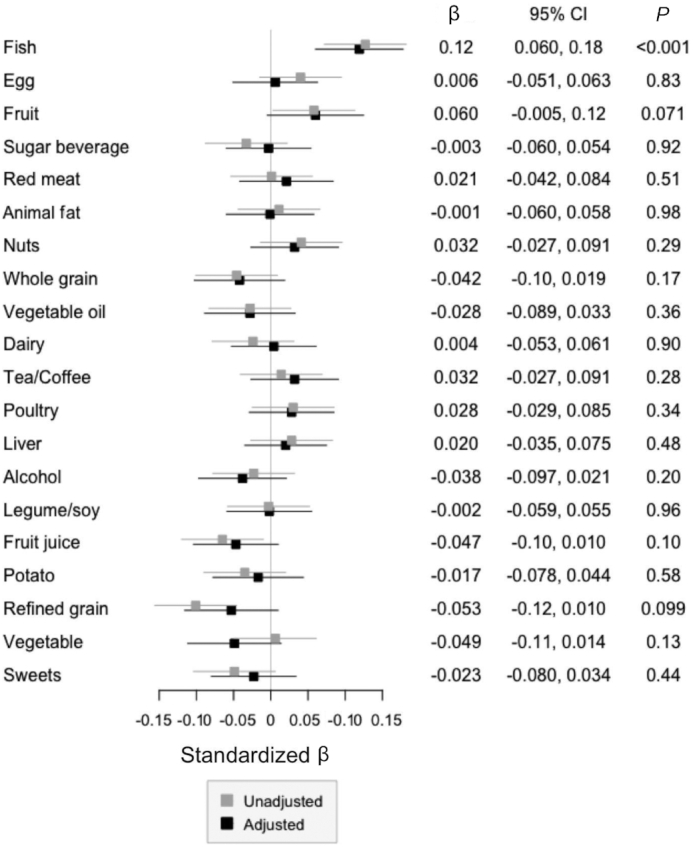
Association between 7DDR-defined food groups and TMAO concentration. Linear regression models for log-transformed TMAO concentrations by the 7DDR-based food groups in 620 healthy males were built. The results of the unadjusted (gray) and adjusted (black) models are presented. In the adjusted model, age, race, metabolic equivalents per week, total calories per day, BMI, and every food group were adjusted. The numbers indicate the standardized β (dots) and P value of each food group in the adjusted model. Bars indicate the 95% CIs. TMAO, trimethylamine-N-oxide; 7DDR, 7-d dietary record.
We observed that fish intake was significantly, positively, associated with TMAO concentration assessed using either the SFFQ (β = 0.082 per SD; 95% CI: 0.021, 0.14 per SD; P = 0.009) or the 7DDR (β = 0.12 per SD; 95% CI: 0.060, 0.18 per SD; P < 0.001). When we further investigated the association of specific food components of the fish food group (Table 2), SFFQ-assessments of dark meat fish (β = 0.073 per serving per week; 95% CI: 0.017, 0.13 per serving per week; P = 0.013) and shellfish intakes (β = 0.11 per serving per week; 95% CI: 0.025, 0.19 per serving per week; P = 0.011) and 7DDR-assessments of lean fish intake (β = 0.072 per 3 oz/wk; 95% CI: 0.042, 0.10 per 3 oz/wk; P < 0.001) were the strongest predictors of TMAO in the fully adjusted models. The adjusted P value threshold was 0.010.
TABLE 2.
Linear regression models for log-transformed TMAO concentration by detailed components of fish consumption1
| Univariable model | Multivariable model | |||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | |
| SFFQ-based assessment | ||||||
| Overall fish | 0.036 | 0.014, 0.058 | 0.001 | 0.033 | 0.008, 0.058 | 0.009 |
| Lean fish | ||||||
| Cod, haddock, halibut, etc. (3–5 oz.) | 0.056 | −0.009, 0.12 | 0.093 | 0.053 | −0.018, 0.12 | 0.14 |
| Breaded fish cakes/pieces (1 serving) | −0.040 | −0.20, 0.12 | 0.63 | −0.012 | −0.19, 0.16 | 0.89 |
| Fatty fish | ||||||
| Canned tuna fish (3–4 oz) | 0.030 | −0.021, 0.081 | 0.25 | 0.015 | −0.040, 0.070 | 0.58 |
| Dark meat fish (3–5 oz.)2 | 0.092 | 0.039, 0.14 | 0.001 | 0.073 | 0.017, 0.13 | 0.013 |
| Shellfish (1 main dish) | 0.11 | 0.029, 0.19 | 0.007 | 0.11 | 0.025, 0.19 | 0.011 |
| 7DDR-based assessment | ||||||
| Overall fish | 0.050 | 0.029, 0.071 | <0.001 | 0.047 | 0.024, 0.069 | <0.001 |
| Lean fish | ||||||
| Lean fish | 0.078 | 0.048, 0.11 | <0.001 | 0.072 | 0.042, 0.10 | <0.001 |
| Fried fish | −0.12 | −0.36, 0.13 | 0.35 | −0.051 | −0.30, 0.19 | 0.68 |
| Total fatty fish | 0.012 | −0.049, 0.073 | 0.70 | −0.016 | −0.079, 0.047 | 0.62 |
| Shellfish | ||||||
| Shellfish | 0.052 | −0.001, 0.10 | 0.055 | 0.050 | −0.004, 0.10 | 0.070 |
| Fried shellfish | −0.13 | −0.83, 0.58 | 0.72 | −0.26 | −0.96, 0.44 | 0.47 |
Coefficients are per serving per week for SFFQ-based assessment and per 3 oz/wk of each fish (1 oz = 28.3 g) for 7DDR-based assessment. Two measurements of TMAO and food assessment in the Men's Lifestyle Validation Study cohort were averaged and used for the regression. Data of 620 healthy males were used to build the models. Age, race, metabolic equivalents per week, total calories per day, BMI, and food groups other than fish (egg, fruit, sugar beverage, red meat, animal fat, nuts, whole grain, vegetable oil, dairy food, tea/coffee, poultry, liver, alcohol, other animal food, legume, fruit juice, potato, refined grain, vegetable, and sweet) were adjusted in the multivariable models. The adjusted P value threshold was 0.010. SFFQ, semiquantitative food-frequency questionnaire; TMAO, trimethylamine-N-oxide; 7DDR, 7-d dietary record.
Dark meat fish includes tuna steak, mackerel, salmon, sardines, bluefish, and swordfish.
There was no overall association between red meat intake and TMAO concentration, regardless of whether SFFQ (β = 0.041 per SD; 95% CI: −0.028, 0.11 per SD; P = 0.23) or 7DDR (β = 0.021 per SD; 95% CI: −0.042, 0.084 per SD; P = 0.51) estimates of red meat intake were used. Because red meat is implicated as being a major contributor to TMAO concentrations (4), we then sought to identify if any contributors to the red meat food group were associated with the concentration of TMAO. Supplemental Table 4 summarizes the association of individual food items comprising the red meat food group with TMAO concentration. When using the SFFQ to derive intake estimates, estimated mean consumption of lean meat hamburger was marginally associated with high TMAO concentration in the fully adjusted models (β = 0.026 per serving per week; 95% CI: 0.008, 0.044 per serving per week; P = 0.005; adjusted threshold for the P value was 0.0056). In 7DDR, lean veal consumption was significantly associated with high TMAO concentration (β = 0.65 per 3 oz/wk; 95% CI: 0.26, 1.05 per 3 oz/wk; P = 0.001; the adjusted P value threshold was 0.004).
Egg consumption was significantly positively related to TMAO when using the SFFQ to estimate intake (β = 0.065 per SD; 95% CI: 0.004, 0.13 per SD; P = 0.039), but not when using the 7DDR (β = 0.006 per SD; 95% CI: −0.051, 0.063 per SD; P = 0.83).
Next, we investigated the associations for phase-1 and -2 measurements of TMAO and 7DDR food groups separately. Supplemental Figure 2 indicates linear regression models predicting log TMAO from phase-1 (Supplemental Figure 2A) and -2 (Supplemental Figure 2B) measurements with the corresponding 7DDR closest to blood draw. In each fully adjusted model, only fish was consistently positively associated with higher concentrations of TMAO (β = 0.097 per SD; 95% CI: 0.036, 0.16 per SD; P = 0.002 in phase-1; and β = 0.086 per SD; 95% CI: 0.017, 0.15 per SD; P = 0.013 in phase-2).
The R2 of the adjusted models were 8.7% in phase-1 and 6.4% in phase-2. When the averaged 7DDR was used, the same fully adjusted model had an R2 of 10.3%. This implies that short-term dietary intake assessed by the 7DDR had similar, or even slightly less, discriminative power for TMAO compared with averaged dietary assessment of two 7DDRs and the mean of 2 TMAO measurements separated by 6 mo.
Dietary scores and TMAO concentration
Finally, to support our findings using the uPDI, we assessed the association of healthy dietary scores with the concentration of TMAO (Table 3). aHEI-2010, aMED, and hPDI were positively associated with the concentration of TMAO in models that included adjustment for potential confounders (β = 0.007 per score; 95% CI: 0.003, 0.011 per score; P = 0.001 for aHEI-2010; β = 0.045 per score; 95% CI: 0.014, 0.076 per score; P = 0.007 for aMED; β = 0.015 per score; 95% CI: 0.007, 0.023 per score; P < 0.001 for hPDI). The uPDI was inversely correlated with TMAO concentration (β = −0.013 per score; 95% CI: −0.021, −0.005 per score; P = 0.001). The adjusted P value threshold was 0.010.
TABLE 3.
Linear regression models for log-transformed TMAO concentration by dietary scores1
| Univariable model | Multivariable model | |||||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | |
| aHEI-2010 | 0.006 | 0.002, 0.010 | 0.005 | 0.007 | 0.003, 0.011 | 0.001 |
| aMED | 0.031 | 0.00, 0.062 | 0.054 | 0.045 | 0.014, 0.076 | 0.007 |
| PDI | −0.005 | −0.013, 0.003 | 0.28 | −0.002 | −0.01, 0.006 | 0.67 |
| Healthful PDI | 0.014 | 0.006, 0.022 | <0.001 | 0.015 | 0.007, 0.023 | <0.001 |
| Unhealthful PDI | −0.012 | −0.020, −0.004 | 0.002 | −0.013 | −0.021, −0.005 | 0.001 |
1Coefficients are per score. Two measurements of TMAO were averaged and used for the regression. Data of 620 healthy males were used to build the models. Age, race, metabolic equivalents per week, total calories per day, and BMI were adjusted in the multivariable model. The adjusted P value threshold was 0.010. aHEI-2010, alternate Healthy Eating Index-2010; aMED, alternate-Mediterranean diet; PDI, plant-based diet score; TMAO, trimethylamine-N-oxide.
Discussion
This is the first study to our knowledge that investigated the association of dietary factors and dietary habit assessed using 2 different well-validated dietary assessments with circulating TMAO concentration. Our results suggest that, irrespective of the tool used to assess the intake, regular consumption of fish, especially shellfish, was positively associated with TMAO concentration, and that healthy dietary scores were positively and uPDI was negatively correlated with TMAO concentrations.
In this study, we observed positive associations between seafood intake and circulating TMAO concentration. The associations for eggs and red meat were weaker and less consistent depending on the assessment method and the type of red meat. This discordance in associations across the different food groups may be because eggs are rich in choline (28) and red meat is abundant in both l-carnitine and choline (1), whereas fish and other seafood are rich in free TMAO (10). The TMAO precursors, l-carnitine and choline, from eggs and red meat are converted in the gastrointestinal tract by the microbiome to TMA, which is then absorbed and metabolized in the liver to TMAO (28). This is in stark contrast to the free TMAO from seafood, which is directly absorbed into the systemic circulation without the requirement for prior metabolism by the gastrointestinal microbiome (10). Our findings of a positive association of seafood intake with TMAO concentration are consistent with findings of a randomized controlled trial that found that lean-seafood consumption increased the postprandial concentration of serum TMAO (5) and those of recent cohort studies showing a positive association between fish intake and urinary TMAO (29, 30). We found a strong association with TMAO concentration especially in shellfish and lean fish, which has not been reported in detail previously and needs further validation.
We did not find a strong association between total red meat and TMAO despite results from a recent red meat trial. In this 3-way randomized crossover controlled trial of 113 healthy volunteers, the authors demonstrated that daily red meat consumption at 12% of total energy for 4 wk resulted in a significant elevation in the concentration of TMAO when compared with white meat or with plant- and dairy-based protein equivalents (4). The authors of the trial reported that red meat influenced microbial production of TMA from carnitine and affected renal excretion of TMAO. The trial population did differ from ours in many ways that could explain the weaker association in our study. The trial included a younger population that was 61% female and all with healthy renal function. We did not screen or exclude based on renal function although when we controlled for C-reactive protein our results did not substantially change (Supplemental Figure 3). The difference in age of the study population compared with others may be important in defining dietary predictors of TMAO. Hepatic TMAO production may be dysregulated in older populations which may weaken the correlations of dietary precursors to TMAO. Another explanation may be that the diets among participants in the intervention trials were low in fish so the large changes in circulating TMAO after the daily red meat intervention were on top of very low baseline concentrations. The trial demonstrated that TMAO is a sensitive marker of red meat intake in that setting, and our data showed that TMAO is not sensitive to red meat in free-living individuals. Also, TMAO may not be specific to red meat given the association with fish.
Our results from the analyses of the dietary scores do not support the notion that TMAO is a marker of poor diet quality; in turn, the findings indicate the opposite because healthy dietary scores are a consequence of the positive association with fish and fruit as opposed to negative influences of refined grain, fruit juice, and sweets. The consumption of refined grains and sugar might prevent microbiome-mediated conversion of l-carnitine/choline to TMA or directly restrict TMA/TMAO absorption; however, no studies have been performed to date to confirm this speculative hypothesis.
Our study does have several important limitations. Study participants were healthy males registered in either the HPFS or the HPHC and, as such, our results may not be generalizable to other populations, such as populations with large proportions of racial/ethnic minorities, younger subjects, subjects with diagnosed chronic diseases, or females. We cannot entirely rule out the possibility of unmeasured or unknown confounding factors that may account for the associations observed in this study, such as kidney function. However, the homogeneity of the study population and comprehensive lifestyle assessment minimized potential confounding. Dietary recalls and diet records both have their own sources of error, although we minimized these errors by averaging the assessments. Moreover, despite these different sources of errors, we observed consistent findings for our main results from both the SFFQ and 7DDR which provides strong internal validation. The study design is not optimal in investigating the temporal relation between dietary intake and TMAO concentration; for example, we did not assess postprandial blood samples that might reflect the direct influence of diet on TMAO concentration. However, the acute association and the long-term relation would be fundamentally different questions, and the repeated measures of diet and the biomarker reduced error in both diet and blood measures for assessing the long-term relation. Finally, details on the species of fish, such as whether they were freshwater or marine, could not be differentiated based on the current questionnaires. Further study is warranted to confirm which fish may contribute to TMAO concentration because in general freshwater fish do not produce TMAO.
In this cohort of healthy men, regular consumption of seafood, including dark meat fish and shellfish, was strongly positively associated with the circulating plasma concentration of TMAO. The association between red meat and TMAO was weak. An unhealthy dietary pattern measured by the uPDI had an inverse association with TMAO concentration, whereas healthy dietary patterns were positively associated with TMAO concentration. As such, this study adds further to the evidence that circulating concentrations of TMAO are not a simple marker of an unhealthy dietary pattern high in red meat. Future studies of the causal relations between diet, TMAO, and disease risk need to be rethought within the context of whole dietary patterns among free-living individuals.
Supplementary Material
Acknowledgments
RH, KLI, and EBR designed the study and wrote the first version of the manuscript; RH analyzed the data; MW consulted on the statistical analyses; DHL and JL consulted on the nutritional perspectives; AF, QS, and EBR collected clinical and biospecimen data; and all authors: read and approved the final manuscript.
EBR and KLI previously received funding from the US Highbush Blueberry Council for projects unrelated to this published work.
Notes
Supported by NIH grants U01CA167552, U54DE023798, U01CA152904, R01CA202704, UM1CA167552, R01HL035464, R01DK120870, and K24DK098311, the US Highbush Blueberry Council with oversight from the USDA, and the STARR Cancer Consortium. The activity of RH was supported by a scholarship from The Nakajima Foundation. The salary of KLI was supported by a National Health and Medical Research Foundation fellowship.
Supplemental Tables 1–4 and Supplemental Figures 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
Abbreviations used: aHEI-2010, alternate Healthy Eating Index-2010; aMED, alternate-Mediterranean diet; CVD, cardiovascular disease; hPDI, healthful PDI; HPFS, Health Professionals Follow-up Study; HPHC, Harvard Pilgrim Health Care; MET, metabolic equivalent; MLVS, Men's Lifestyle Validation Study; PDI, plant-based diet index; SFFQ, semiquantitative food-frequency questionnaire; TMA, trimethylamine; TMAO, trimethylamine-N-oxide; uPDI, unhealthful plant-based diet index; 7DDR, 7-d dietary record.
Contributor Information
Rikuta Hamaya, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Division of Preventive Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Cardiology, Tokyo Medical and Dental University, Tokyo, Japan.
Kerry L Ivey, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Microbiome and Host Health Programme, South Australian Health and Medical Research Institute, Adelaide, South Australia, Australia; Department of Nutrition and Dietetics, College of Nursing and Health Sciences, Flinders University, Adelaide, South Australia, Australia.
Dong H Lee, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Molin Wang, Department of Biostatistics, Harvard TH Chan School of Public Health, Boston, MA, USA.
Jun Li, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA.
Adrian Franke, University of Hawaii Cancer Center, Honolulu, HI, USA.
Qi Sun, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA.
Eric B Rimm, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, MA, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
References
- 1. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qi J, You T, Li J, Pan T, Xiang L, Han Y, Zhu L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22(1):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krüger R, Merz B, Rist MJ, Ferrario PG, Bub A, Kulling SE, Watzl B. Associations of current diet with plasma and urine TMAO in the KarMeN study: direct and indirect contributions. Mol Nutr Food Res. 2017;61(11):1700363. [DOI] [PubMed] [Google Scholar]
- 4. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40(7):583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmedes M, Balderas C, Aadland EK, Jacques H, Lavigne C, Graff IE, Eng O, Holthe A, Mellgren G, Young JF et al. The effect of lean-seafood and non-seafood diets on fasting and postprandial serum metabolites and lipid species: results from a randomized crossover intervention study in healthy adults. Nutrients. 2018;10(5):598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barrea L, Annunziata G, Muscogiuri G, Laudisio D, Di Somma C, Maisto M, Tenore GC, Colao A, Savastano S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: also a matter of sex?. Nutrition. 2019;62:7–17. [DOI] [PubMed] [Google Scholar]
- 7. Brown JM, Hazen SL. Microbial modulation of cardiovascular disease. Nat Rev Microbiol. 2018;16(3):171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DiMarco DM, Missimer A, Murillo AG, Lemos BS, Malysheva OV, Caudill MA, Blesso CN, Fernandez ML. Intake of up to 3 eggs/day increases HDL cholesterol and plasma choline while plasma trimethylamine-N-oxide is unchanged in a healthy population. Lipids. 2017;52(3):255–63. [DOI] [PubMed] [Google Scholar]
- 9. Miller CA, Corbin KD, da Costa KA, Zhang S, Zhao X, Galanko JA, Blevins T, Bennett BJ, O'Connor A, Zeisel SH. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr. 2014;100(3):778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landfald B, Valeur J, Berstad A, Raa J. Microbial trimethylamine-N-oxide as a disease marker: something fishy?. Microb Ecol Health Dis. 2017;28(1):1327309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seibel BA, Walsh PJ. Trimethylamine oxide accumulation in marine animals: relationship to acylglycerol storage. J Exp Biol. 2002;205(Pt 3):297–306. [DOI] [PubMed] [Google Scholar]
- 12. Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84. [DOI] [PubMed] [Google Scholar]
- 14. Missailidis C, Hallqvist J, Qureshi AR, Barany P, Heimburger O, Lindholm B, Stenvinkel P, Bergman P. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11(1):e0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neff RA, Edwards D, Palmer A, Ramsing R, Righter A, Wolfson J. Reducing meat consumption in the USA: a nationally representative survey of attitudes and behaviours. Public Health Nutr. 2018;21(10):1835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Satija A, Bhupathiraju SN, Spiegelman D, Chiuve SE, Manson JE, Willett W, Rexrode KM, Rimm EB, Hu FB. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70(4):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baden MY, Liu G, Satija A, Li Y, Sun Q, Fung TT, Rimm EB, Willett WC, Hu FB, Bhupathiraju SN. Changes in plant-based diet quality and total and cause-specific mortality. Circulation. 2019;140(12):979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ivey KL, Chan AT, Izard J, Cassidy A, Rogers GB, Rimm EB. Role of dietary flavonoid compounds in driving patterns of microbial community assembly. MBio. 2019;10(5):e01205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colditz GA, Rimm EB, Giovannucci E, Stampfer MJ, Rosner B, Willett WC. A prospective study of parental history of myocardial infarction and coronary artery disease in men. Am J Cardiol. 1991;67(11):933–8. [DOI] [PubMed] [Google Scholar]
- 20. Pereira MA, FitzerGerald SJ, Gregg EW, Joswiak ML, Ryan WJ, Suminski RR, Utter AC, Zmuda JM. A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc. 1997;29(6 Suppl):S1–205. [PubMed] [Google Scholar]
- 21. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26.; discussion 1127–36. [DOI] [PubMed] [Google Scholar]
- 22. Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30(1):47–57. [DOI] [PubMed] [Google Scholar]
- 23. Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88(10):1268–71. [PubMed] [Google Scholar]
- 24. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82(1):163–73. [DOI] [PubMed] [Google Scholar]
- 27. Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hazen SL, Brown JM. Eggs as a dietary source for gut microbial production of trimethylamine-N-oxide. Am J Clin Nutr. 2014;100(3):741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gibson R, Lau CE, Loo RL, Ebbels TMD, Chekmeneva E, Dyer AR, Miura K, Ueshima H, Zhao L, Daviglus ML et al. The association of fish consumption and its urinary metabolites with cardiovascular risk factors: the International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). Am J Clin Nutr. 2020;111(2):280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu D, Shu X-O, Rivera ES, Zhang X, Cai Q, Calcutt MW, Xiang Y-B, Li H, Gao Y-T, Wang TJ et al. Urinary levels of trimethylamine-N-oxide and incident coronary heart disease: a prospective investigation among urban Chinese adults. J Am Heart Assoc. 2019;8(1):e010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


